

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.016862
ARTICLE
Light Intensity Affects the Coloration and Structure of Chimeric Leaves of Ananas comosus var. bracteatus
College of Landscape Architecture, Sichuan Agricultural University, Chengdu, 611130, China
*Corresponding Author: Jun Ma. Email: junma365@hotmail.com
Received: 02 April 2021; Accepted: 29 June 2021
Abstract: Ananas comosus var. bracteatus is an important ornamental plant because of its green/white chimeric leaves. The accumulation of anthocyanin makes the leaf turn to red especially in the marginal part. However, the red fades away in summer and winter. Light intensity is one of the most important factors affecting leaf color along the seasons. In order to understand the effects of light intensity on the growth and coloration of the chimeric leaves, Ananas comosus var. bracteatus was grown under full sunlight, 50% shade and 75% shade for 75 days to evaluate the concentration of pigments, the color parameters (values L*, a*, b*) and the morpho-anatomical variations of chimeric leaves. The results showed that a high irradiance was beneficial to keep the chimeric leaves red. However, prolonged exposure to high irradiance caused a damage, some of the leaves wrinkled and even burned. Shading instead decreased the concentration of anthocyanin and increased the concentration of chlorophyll, especially in the white marginal part of the leaves. Numerous chloroplasts were observed in the mesophyll cells of the white marginal part of the chimeric leaves under shading for 75 days. The increase in chlorophyll concentration resulted in a better growth of plants. In order to balance the growth and coloration of the leaves, approximately 50% shade is suggested to be the optimum light irradiance condition for Ananas comosus var. bracteatus in summer.
Keywords: Ananas comosus var. bracteatus; light intensity; leaf color; anatomy
Variegated leaf chimeric plants are important ornamental plants because of their unique colorful characteristics. The high diversity of color in chimeric plants is due to the changes of spatial distribution of pigment types, concentrations and proportions in the leaves. These changes are the result of the interaction between genetic and environmental factors [1–3]. Climate, soil nutrients, and water have long been understood to be primary factors influencing plant growth, and the light intensity is an important environmental factor [4,5]. In green leaves, chlorophyll dominates in pigments causing a green appearance, and chlorophyll deficiency can make leaves become white or yellow. Light intensity can affect the concentration of chlorophyll, carotenoid, and anthocyanin in the leaves, leading to variation in leaf color [6]. It has been shown that different plants have different acclimation potential to light intensity. Photinia × fiaseri grown under full light was more densely foliated than shaded plants [7]. Approximately 67% of natural light (the irradiance intensity of sunlight was about 2500 μmol m2 s−1 at noon during September in Jinhua) was concluded to be the optimum light irradiance conditions for Tetrastigma hemsleyanum Diels et Gilg [4]. The research conducted by Kong et al. [8] showed that 30% and 50% of sunlight (the irradiance intensity of sunlight was about 2000 ± 20 μmol photons m−2 s−1 at noon) were the most suitable growth environment for Mahonia bodinieri (Gagnep.) Laferr, obtaining higher biomass and improving the plant economic value. In addition, light intensity can tightly regulate color changes. Galax urceolata leaves had a transition from red to green along with the decrease of light intensity [9]; Kalamegh leaf changed its color from green to red from time to time under varying intensities of light and shade [10]. Similarly, shading was reported to improve foliar color of Kalmia latifolia L from yellow-green to dark green in the first year of production [11]. The leaves of both ‘Royal Glissade’ and ‘UF06-1-6’ gradually changed from green to red under different light environments, the anthocyanin concentration increased while chlorophyll concentration decreased [12]. A study in Arabidopsis thaliana shown that light intensity can affect the type of anthocyanin [13]. Anthocyanins accumulate in the form of glycosides in plant vacuoles. In plants, they can provide colorations in flowers, fruits, stems, and leaves of plants ranging in color from red to purple. Sugars are structural components, energy sources and signals regulating the expression of various genes in the primary and secondary metabolisms. Some studies showed that sugar concentration is one of the factors affecting anthocyanin synthesis. For example, sugars promoted the accumulation of anthocyanins in radish hypocotyls and grape berries [14,15], and the anthocyanin synthesis was enhanced by exogenous sugar in several plant species. Furthermore, the balance of biosynthesis and degradation determines the anthocyanin concentration. The anthocyanin biosynthetic pathway has been extensively studied in many plants. Phenylalanine ammonia lyase (PAL), chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), dihydroflavonol 4-reductase (DFR), and anthocyanidin synthase (ANS) are the main enzymes in this pathway [16]. Anthocyanin concentration was also reported to be related to the polyphenol oxidase (POD) and peroxidase (PPO) activities. However, the mechanism of its degradation is complicated and there are only few studies about it [17,18].
Plants develop specific changes to acclimate to different light conditions for their survival, these changes in morphology, physiology, structure of leaves and chloroplasts can be adaptable [19,20]. For instance, the changes in anatomical structure can maximize photosynthetic efficiency and keep internal temperatures at optimal levels [21,22]. Ultrastructure of chloroplasts in plants change significantly with increased irradiance, and the number of chloroplasts under high light is lower than under low light. Moreover, plants grown under high irradiance can use the energy of many photons and maintain a high photosynthetic net rate, but chronic photo-inhibition may occur upon a too long prolonged exposure to high light conditions. Therefore, it is very important to investigate the characteristics of leaf morphology and anatomy of plants under different light conditions for the cultivation of Ananas comosus var. bracteatus.
Ananas comosus var. bracteatus, belonging to the family Bromeliaceae, is a herbaceous perennial monocot. It has a high ornamental value all year round due to its green/white chimeric leaves. During spring and autumn, the leaves are bright red, which improves a lot the ornamental value of A. comosus var. bracteatus [23]. However, in summer and winter, the redness of the leaves gradually fades, and the leaf becomes green/white. How to maintain the red coloration of leaves is an important way to improve the ornamental and economic value of A. comosus var. bracteatus. Therefore, we have carried out a lot of studies on the molecular mechanism of chimera traits formation of on this species [24–28]. Recently, we conducted a study on pigments metabolome in the leaves of A. comosus var. bracteatus [29]. When the leaf turned red, the chlorophyll concentration in the green central part could reach 57 times higher than that in the red marginal part, while the anthocyanins accounted for 93.2% of all pigments in red marginal part with a small proportion of chlorophyll and carotenoid. According to the results, we determined that anthocyanin and cyanidin derivatives presenting different degrees of red color, accounted for 97% of total anthocyanin concentration. Cyanidin-3,5-O-diglucoside, accounting for 98% of cyanidin derivatives in red marginal part, was the most important anthocyanin for the red phenotype. The genetic characteristics and physiological properties of other variegated leaf chimeric plants have been systematically studied [30–33]. However, few studies have addressed how chimeric plants respond to varying light intensity, and the mechanism under it is still unknown.
The objective of our study was to determine the optimum light intensity for the growth and high ornamental value of A. comosus var. bracteatus by investigating the effects of different light intensities on the coloration change, pigments concentration and proportion, and internal anatomy of chimeric leaves. Our findings would provide a reference for the production application of A. comosus var. bracteatus, and be beneficial to the nursery and landscape industries.
2.1 Plants Materials and Culture Condition
Two-year-old Ananas comosus var. bracteatus plants with red chimeric leaves were collected from the experimental field at Sichuan Agricultural University, located in Chengdu, China. All of the samples were healthy and of the same age. The temperature is 18.4~32.3°C between June and September. The highest light intensity in daytime at noon is approximately 2500 μmol m2 s−1 during the summer. Annual average rainfall is 957.05 mm. Plants were grown in pots containing a mixture of coconut bran and river sand (1:2). Row spacing between plants was set 30 cm × 30 cm. Plants were divided into three treatments: natural sun-light condition (CK), 50% shading condition (T1), and 75% shading conditions (T2). Shading was accomplished by using one or two layers of commercial black cloth shade. Each treatment had 30 pots, there were a total of 90 pots for all three treatments. In order to ensure each individual received on average an equal irradiance under each treatment, the positions of pots were changed randomly every 15 days. The other conditions were uniform during the experimental period. We analyzed three independent biological replicates, and each replicate from three randomly selected plants. Each sample from the fourth or fifth leave of randomly selected plants was collected every 15 days from June 16 to September 01 in 2018. The middle parts of these leaves were divided into two types of samples based on the color: the green parts and the red parts (Fig. 1). The marginal parts from the same leaves were mixed up for weighing. After washing with distilled water, the samples were collected for further color parameters and pigments detection.
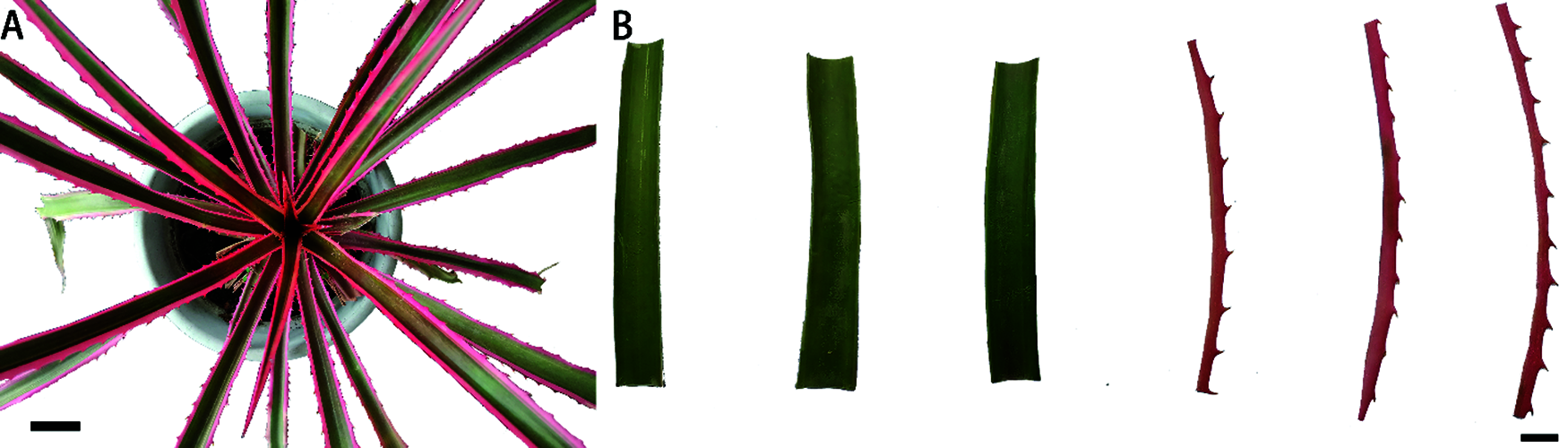
Figure 1: (A) Ananas comosus var. bracteatus chimeric plant, Bar = 5 cm. (B) Green central parts and red edges from the middle parts of chimeric leaves, Bar = 1 cm
Color parameters—L*(Lightness), a*(Redness) and b*(Yellowness) values of leaves were measured by an automatic color meter (Konica Minolta CM-2600d, Japan). The measurement was taken on the surface of middle typical parts of chimeric leaves, and both the red and green parts of each sample were analyzed for three times. The detection was based on the Commission Internationale de l’Éclairage (CIE D65/10°) scale [34]. The CIE color system defines the range of color using a CIE brightness parameter (L*) and CIE chromaticity coordinates (a* and b*). L* values from 0 to 100 indicate that the color ranges from black to white. Values from −a* to +a* indicate the change from green to red in the leaf color. Positive values of b* indicate more yellow, and negative values of b* indicate more blue. With the data, the following formula was used to calculate the color index:
2.3 Pigments Concentration Detection
Both the green central parts and red marginal parts of three fresh leaves were cut into pieces separately, and weighed. Leaf material corresponding to 0.1 g was then put into centrifuge tubes with 5 ml 95% ethanol (v/v). Samples were kept in the dark at room temperature for 48 h until the leaf turned completely colorless; the centrifuge tube was shaken several times during the extraction period. We used 95% ethanol as blank, and measured the absorbances of samples at 649, 665, and 470 nm respectively based on three replicates by a UV spectrophotometer (UV1901 s/UV1901PCS, Shanghai Youke Instrument Co., Ltd., China). The pigment concentration was calculated using the following formulas [36]:
where Chl is the total concentration of chlorophyll, Car is total the concentration of carotenoid. A665nm, A649nm and A470nm are the absorbance of the extracts at 665, 649, 470 nm, respectively. V is the total volume of extract, W is the fresh weight.
Total anthocyanin concentrations of red and green samples were measured based on a pH differential method [30]. 0.1 g of frozen samples was immersed in 5 ml of methanol acidified with 1% HCl, and kept in the dark with shaking for 4 h. Then, it was centrifuged at 15,000× g for 10 min. Absorptions at 510 and 700 nm of e supernatant in buffers at pH 1.0 (A510., A710) and pH 4.5 (
To observe the pigments distribution in the leaf tissues of leave, four types of leaves (the untreated leaf, the leaf under full light, 50% and 75% of full light for 75 days, respectively) were chosen for freehand cross sectioning. Middle parts of typical leaf samples from each treatment were sliced by two overlapping blades; a section from the gap between the blades were mounted in water, and then put onto a glass slide sealed with a cover glass. The sections were viewed immediately by a light microscope. In order to furtheobserve the histological changes, paraffin sections were conducted on the four types of leaves using the method of Liu et al. [37]. The green and red parts of middle parts were cut into small cubes. They were fixed with FAA solution, dehydrated by increasing concentrations of ethanol, made transparent in xylene, embedded in paraffin and sliced. Then, after being fixed on microslides and subjected to toluidine bule staining, the sections were sealed with resins and photographed at the microscope (Leica DM1000, Leica Microsystems, Ltd., Germany).
We conducted every analytical determination at least three times, and processed preliminary experimental data and tables in Microsoft Excel. SPSS 26.0 software (IBM Crop., Armonk, NY, USA) was used to analyze the data, and the correlations were performed using Duncan’s single factor analysis of variance (ANOVA). Differences showed by the ANOVA were considered statistically significant at p < 0.05. Different lowercase letters indicate significance differences among three treatments in the same day. All values are mean ± SE.
3.1 Morphological Changes of the Leaves under Different Light Conditions
Shading treatment had a significantly effect on leaf morphology of A. comosus var. bracteatus. The morphological changes of the leaves under different light conditions are shown in Fig. 2. Plants under 75% and 50% shade grew well throughout the experimental period while sunburn was observed in the leaves of plants under full light; the degree of leaf sunburn increased with time of exposure. The marginal parts of the chimeric leaves under 75% shade firstly lost the red color and became white (about 45 days), then turned to green. The marginal parts of leaves under both full light and 50% shade maintained the red color during the period. However, sunburn spots occurred on the leaves under full light during about 60 days. According to previous studies, light damage was caused by the interaction of excess light and high leaf temperatures and as a consequence plant growth was affected [38,39]. Indeed, shading usually reduces plant growth, but some plants, especially many evergreens, benefit from shading in the warm areas. For Euonymus japonica Hand. -Mazz. ‘Aureo-marginata’, plant growth was optimized with 50% shading [40]. Shading increased the growth of Rhododendron × ‘Pink Ruffles’ [41].
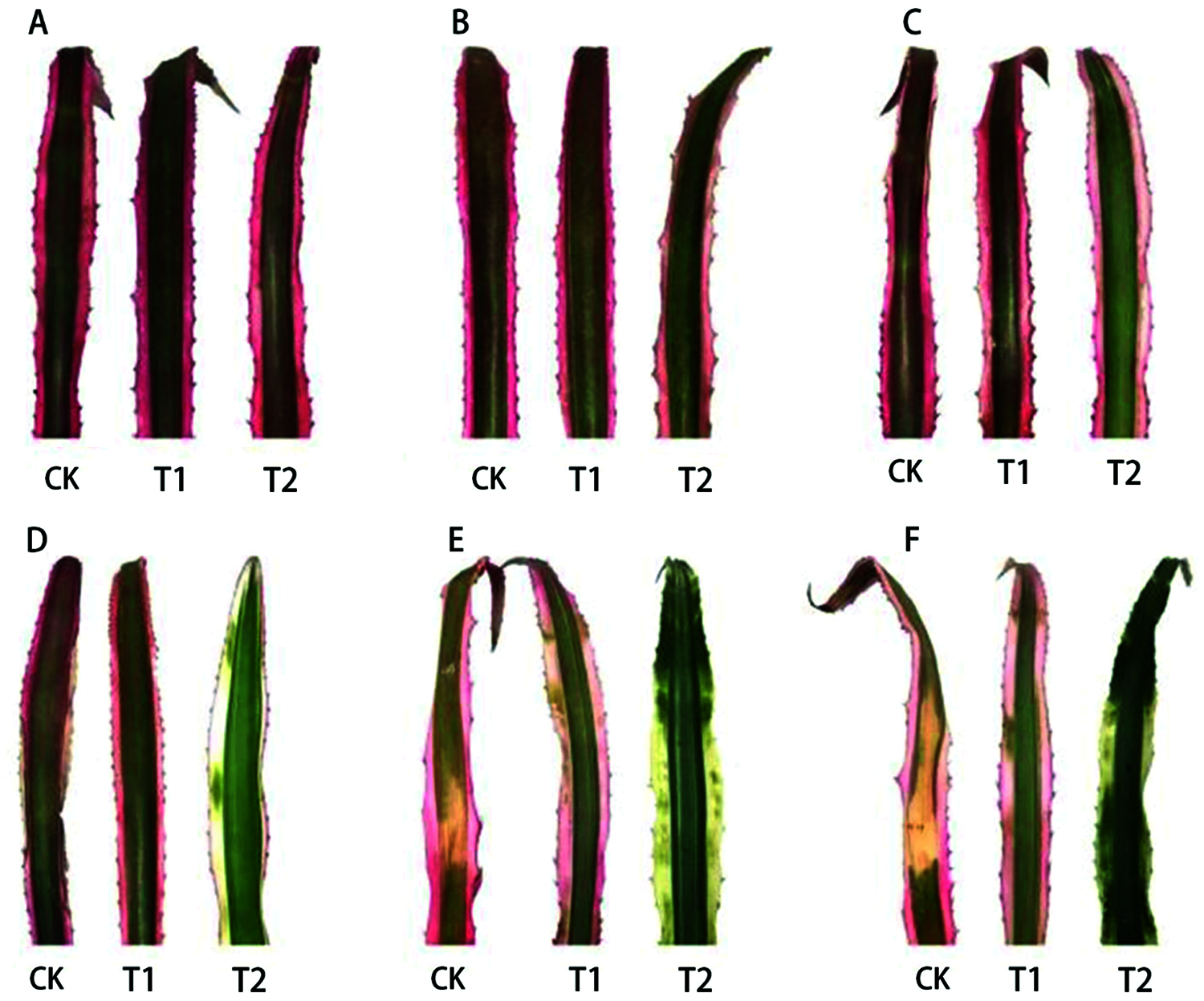
Figure 2: Visual foliage color changes of Ananas comosus var. bracteatus leaves under different light intensities. (CK) full light, (T1) 50% shade, (T2) 75% shade, (A) day 0, (B) day 15, (C) day 30, (D) day 45, (E) day 60, (F) day 75
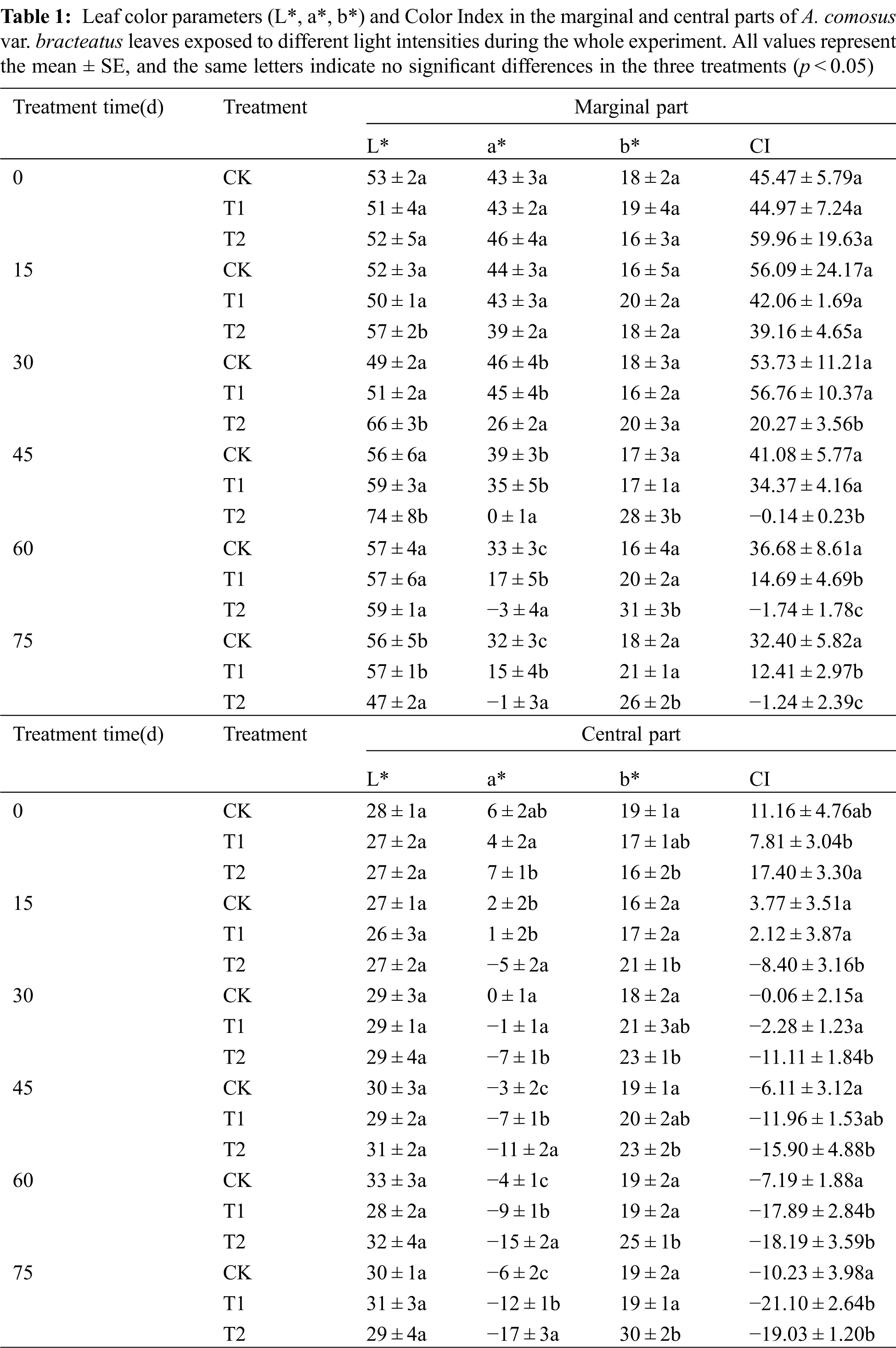
The color parameter and the color index of leaves varied among the experimental period (Tab. 1). For treatments T1 and T2, the redness (a*) of the marginal part gradually decreased. The lightness (L*) of the central part increased slightly as shade increased for all treatments indicating that the marginal part of leaves had a gradual transition from red to green, and the central parts of leaves became lighter for all treatments during our experiment. The redness (a*) and yellowness (b*) of marginal parts of leaves decreased faster than the central parts, which indicated that marginal parts had a greater anthocyanin degradation than the central part. The change in color can be quantified by the color index CI, which was reported to be related to the pigment composition in plant tissues [42,43]. Positive values indicate a yellow or red coloration, while negative values represent green color. In our study, it was observed that higher light intensity gave the leaves higher CI values with higher redness, which indicated that high irradiance stimulated the biosynthesis of anthocyanins, protecting against the damage due to excess light. Similar results have been found in Galax urceolata [9], where changes in pigments are related to light intensity. The higher redness (a*) in the leaves under the three treatments enhanced the red leaf formation with higher color index because of the accumulation of anthocyanins in the tissues. Therefore, the chimeric leaves of the three treatments kept red in the first 30 days. However, with the increase in shading time, the CI values became lower and the leaf quickly lost its red color. This result showed that only few anthocyanins were in tissues, which was related to the anthocyanin degradation in leaves. After 75 days of 75% shading treatment, the leaf color of marginal parts changed from red to green with the decreased CI, which showed that shading influenced the leaf color of the marginal part apparently by decreasing the red color and increasing the green color [11]. Similar results were observed in the central part and the CI became negative, because more chlorophylls were present in the tissues of the central parts. From our results, light intensity had a great influence on the color parameters of A. comosus var. bracteatus leaves.
3.2 Pigments Concentration Changes in Ananas comosus var. bracteatus Chimeric Leaves
The leaf color is directly related to the pigments concentration of the leaves. In order to reveal the material basis of the leaf color changes, the pigment concentration of the marginal and central parts of chimeric leaves of A. comosus var. bracteatus were analyzed.
3.2.1 Pigments Changes in the Center Green Part of the Chimeric Leaves
Shading had significant influences on the accumulation of chlorophyll, carotenoid and anthocyanin in the green center parts of A. comosus var. bracteatus leaves (Figs. 3A–3C). The chlorophyll concentration in the central parts of chimeric leaves under full sunlight and 50% shading treatments kept decreasing during 75 days, and the chlorophyll concentration under full light treatment decreased faster than that under 50% shading. This finding may be related to the result of the interaction between long-term adaptions in plants and a greater degradation caused by high solar radiation. High light stress affects the photosynthetic apparatus and its functioning in a direct or indirect way. There exists short and long-term responses in plants to acclimate themselves to various light intensities. Plants can suffer from the inhibition of photosynthetic quantum conversion and electron transport under too high irradiance, which results in short-term declines in photosynthetic function [44]. Long-term high light stress, often accompanied by temperature and water stresses, leads to a decline in chlorophyll concentration [45]. Leaves also under go many changes such as the formation of sun-type chloroplasts with lower amounts of high-harvesting pigments, less LHC-II (LHCPs) and a higher capacity for photosynthetic quantum conversion, which helps plants to avoid damage or photoinhibition [46,47]. In addition, chlorophyll degradation often occurs in the process of plants acclimation to light stress. Light was reported to enhance the degradation of chlorophyll in Camellia sinensis L. cultivar ‘Huangjinya’ [48]. Furthermore, it was reported that prolonged exposure to high light intensities may be harmful to the photosynthetic apparatus [49]. Chlorophyll was usually synthesized and photo-oxidized in the presence of light, so the concentration of chlorophyll was greatly affected by shading [4,50]. Moreover, the degree of photosynthetic damage increased with the time of exposure to high solar radiation, and severe photo-inhibition was followed by leaf death [40]. It is also important to point out that there was a remarkable increase in chlorophyll concentration under 75% shade for 45 days, which demonstrated the plant’s ability to develop mechanisms for improving light harvesting [51]. The carotenoid concentration decreased apparently under full light and 50% shading treatments during the 75 days. But under 75% shading it decreased during the first 45 days, and then increased apparently in the subsequent period (Fig. 3B). Increased carotenoids concentration under shading can enhance light absorption and transfer to chlorophyll for photosynthesis [52]. During the experiment time, anthocyanins concentration of the green center part of the leaves all decreased consequently. When the light intensity decreased, the concentration of anthocyanin decreased more. The highest anthocyanin concentration was observed under full light (Fig. 3C). Previous studies showed that anthocyanins have the ability to protect chlorophyll pigments from damage under high solar radiation conditions [53].
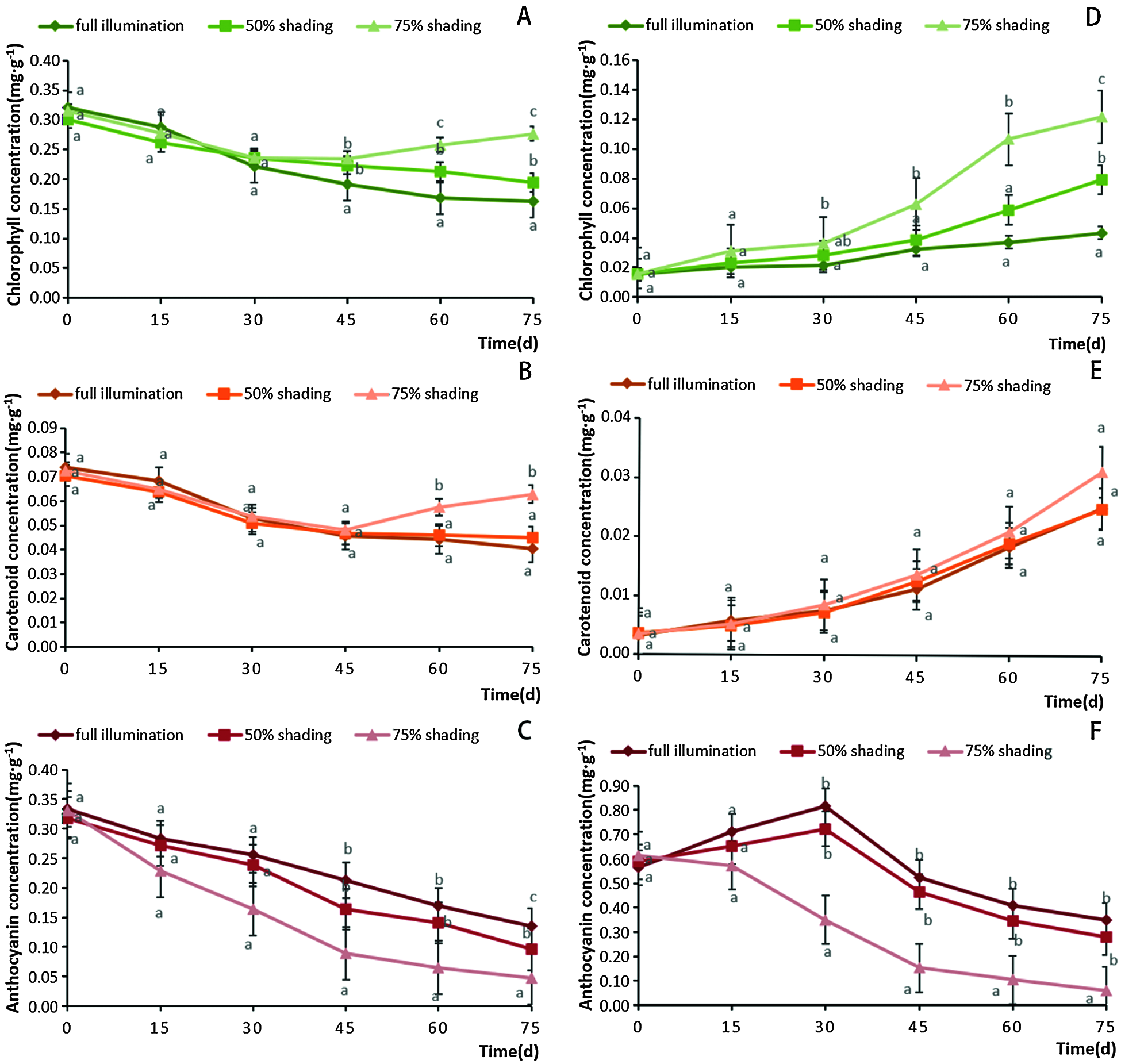
Figure 3: Changes of chlorophyll (A), carotenoid (B) and anthocyanin (C) concentrations in the central parts of Ananas comosus var. bracteatus leaves, and the changes of chlorophyll (D), carotenoid (E) and anthocyanin (F) concentrations in the marginal parts under different shading treatments. All values are means ± SE. Different letters mark statistical differences among shade treatments in the same day (p < 0.05)
3.2.2 Pigments Changes in the Red Margin Parts of the Chimeric Leaves
During the treatment period, the chlorophyll concentrations of the red margin part of chimeric leaves increased in all three treatments. There were apparent differences in the increasing chlorophyll concentration among the three treatments. Under 75% shading, the chlorophyll concentration was the highest and increased the fastest, especially after 45 days of treatment (Fig. 3D). Along the treatment procedure, the carotenoid concentration of marginal parts of the leaves increased consequently under the three treatments. But there were no significant differences in carotenoid concentration among the three treatments (Fig. 3E). However, shading decreased the anthocyanin concentration in the red margin parts of the leaves (Fig. 3F). The concentration of anthocyanin decreased more when the shading intensity and time increased. At the first 30 days of full light and 50% shading, the anthocyanin concentration increased, and then decreased quickly in the following period. Under 75% shading, the anthocyanin content decreased consequently along the 75 days. It has been shown that the light exposure was a prerequisite for significant anthocyanin synthesis, and a high level of solar radiation promoted the anthocyanin synthesis [9,10]. In addition, anthocyanin in epidermal tissues can provide some protection from UV solar radiation [54]. In order to maintain plant growth, chlorophyll gradually accumulated in the red part while the anthocyanin concentration decreased after day 30.
3.2.3 Proportion of Pigments in Ananas comosus var. bracteatus Chimeric Leaves
The leaf color was determined by the concentration and relative proportion of the chlorophyll, carotenoid and anthocyanin concentrations (Fig. 4). In the green center parts of the chimeric leaves, chlorophyll and anthocyanin were the dominant pigments sharing about 45% of the total pigments, respectively, during the first 15 days, and the central parts of the leaves were dark green. This was the result of the interaction between the reflectance of green chlorophyll and red anthocyanin. From days 30 to 75, the proportion of chlorophyll concentration increased apparently. The increase in the chlorophyll proportion was more apparent with the increase of shading intensity. After 75 days of 75% shading, the proportion of chlorophyll increased to about 70% and the proportion of anthocyanin decreased to about 10%. The proportion of carotenoid increased slightly along the shading time and shading intensity (Fig. 4A). According to the increase of chlorophyll and decrease of anthocyanin, the color of the central parts of the chimeric leaves changed from dark green to green.
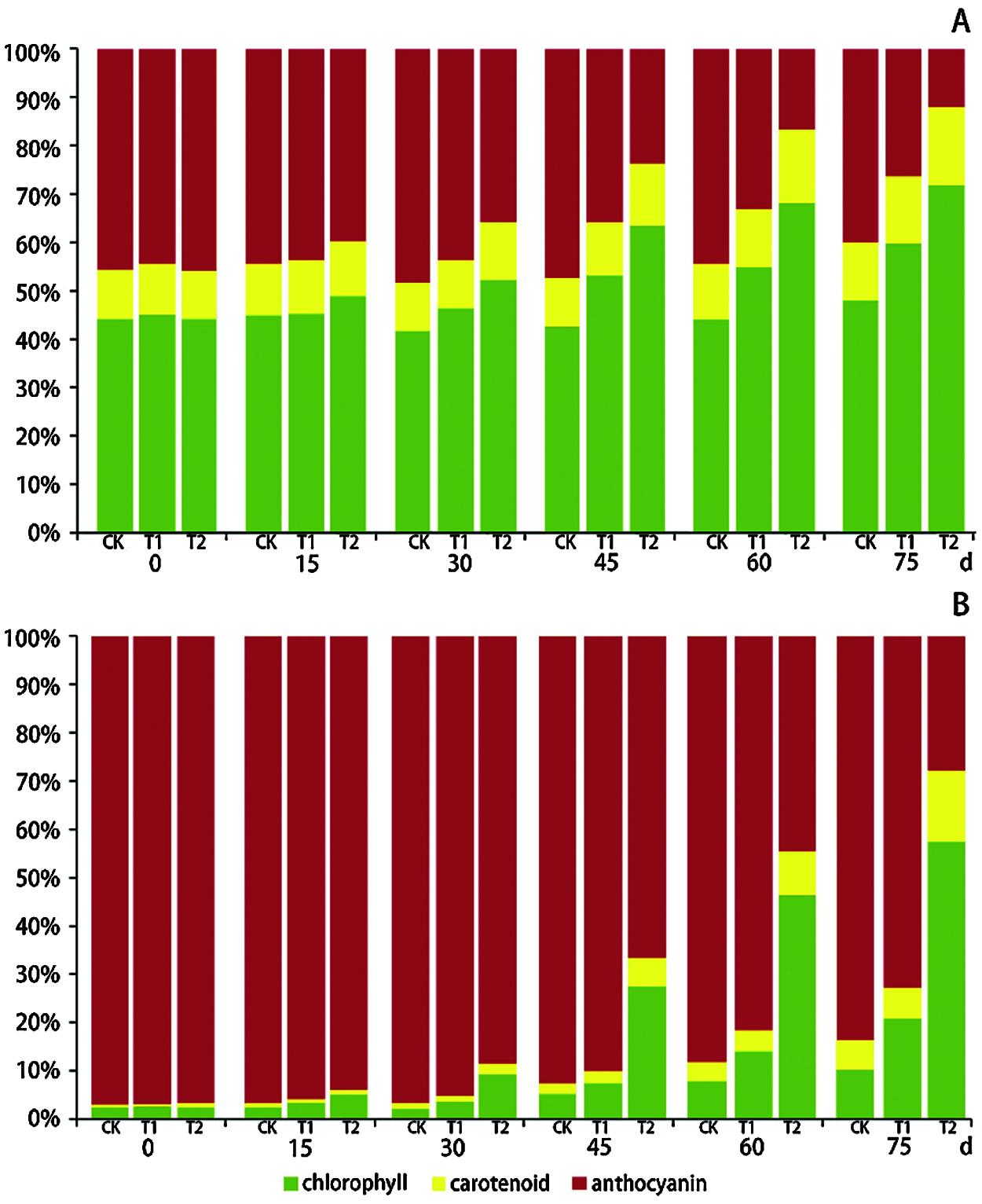
Figure 4: The changes of relative proportion of the chlorophyll, carotenoid and anthocyanin concentrations in the green center parts (A) and red margin parts (B) of Ananas comosus var. bracteatus chimeric leaves under different light intensities
In the red margin parts of the chimeric leaves, anthocyanin was the dominant pigment. At the first 30 days, anthocyanin accounted for more than 90% of the total pigment concentration. After 30 days of treatment, the proportion of chlorophyll and carotenoid increased and that of anthocyanin decreased (Fig. 4B). As the shading intensity and time increased, the chlorophyll proportion increased more, and the anthocyanin proportion decreased more. Under full light treatment for 75 days, the proportion of anthocyanin concentration remained more than 80% of the total pigments concentration, while under 75 days of 75% shading treatment the proportion of anthocyanin decreased to 28%. Under 50% shading for 75 days, the anthocyanin proportion was more than 70%. The changes in pigments relative proportions resulted in the morphology changes we observed. It is notable that the proportion of carotenoid in the red margin parts of the leaves increased more apparently than that in the green center parts of leaves, especially under 75% of shading treatment. There were different pigment ratios in plants grown under different light conditions, and the ratios of pigments could reflect the light adaptability of plants. Plants grown under high light tended to produce more anthocyanin, which enhanced the ornamental character of plants [9,12,54]. However, plants tended to produce more chlorophyll under low light condition to maintain sufficient photosynthetic antennas. This response allowed the plant to capture the required light energy [8,10]. In addition, the different colorations between the central and marginal parts of the chimeric leaves were the result of the different concentration and ratio between anthocyanin and chlorophyll. Shading can influence the pigment ratios in A. comosus var. bracteatus, which agrees with other studies [10,12].
3.3 Pigments Location and Leaf Structure Changes of Chimeric Leaves
In order to reveal the distribution of pigments in the chimeric leaves and the effect of shading on the structure of the leaf tissues, anatomic observations of the leaves were carried out (Fig. 5). The red chimeric leaves used in this experiment consisted of the red and green tissues (Fig. 5A). In the red margin part, no chloroplast was observed in the mesophyll cells, and all the mesophyll cells were white (Figs. 5B1, 5C1, 5E1). In the green central part of the leaf, many chloroplasts were observed in the mesophyll cells, which gave this leaf part of its green color (Figs. 5B1, 5D1, 5F1). Red cells containing anthocyanin were located in the upper and lower epidermis of the cells and the 1–2 cell layer beneath the epidermis of the leaf (Figs. 5C1, 5D1). Anthocyanins were located in the vacuoles of the cells, which made the almost entire cells appear red. Under full light for 75 days, the red cells with anthocyanins always existed in/under the epidermis in both red margin and green center parts of the leaves, but were less than those in leaves at 0 day (Figs. 5A2, 5B2, 5C2, 5D2). Almost no green cells with chloroplasts were observed in the mesophyll cells of marginal part under full light (Figs. 5B2, 5C2). However, a few green spots were observed in the red margin of the leaf under 50% shading for 75 days (Fig. 5A3) because some green cells with chloroplasts existed in the red margins (Figs. 5B3, 5C3). Red cells of the leaf under 50% shading existed in/under the upper and lower epidermis of red margin and green center part of the leaf, but were lower than those of the leaf in the 0 day (Figs. 5B3, 5C3, 5D3). After treated with 75% shading for 75 day, the marginal parts of the leaf lost the red coloration and then turned green (Fig. 5A4). Red cells were seldom observed in/under the epidermis of the leaf. However, green cells with chloroplasts apparently accumulated in the marginal parts of the leaf (Figs. 5B4, 5C4, 5D4). It is also important to point out that there was no coexistence of chlorophyll and anthocyanin in a single cell.
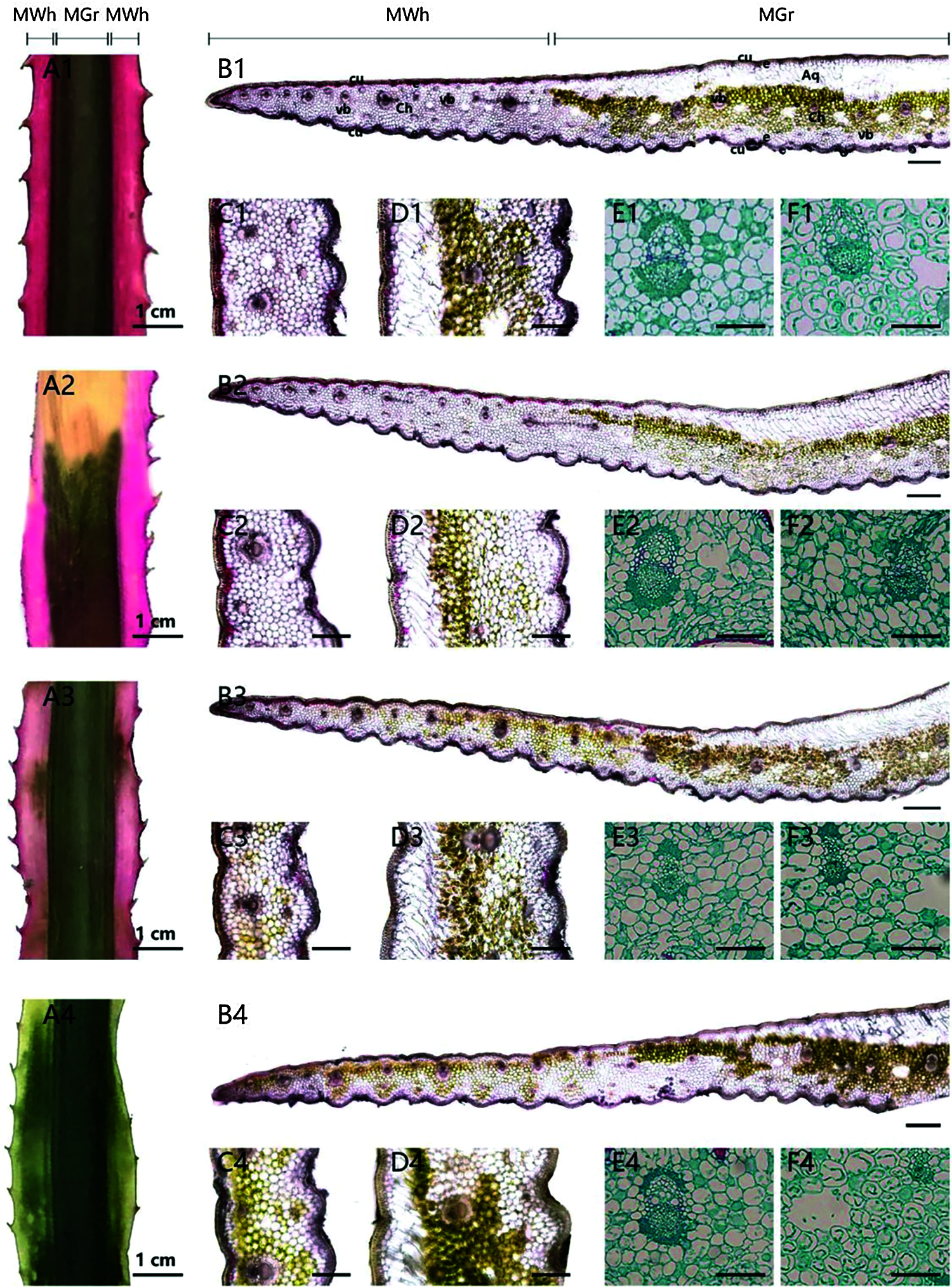
Figure 5: The pigments location and anatomic character of the central part and marginal parts of the chimeric leaves. (A1–F1) The anatomic structure of the leaf at 0 day. (A2–F2) The anatomic structure of the leaf under full light (CK) for 75 days (A3–F3) The anatomic structure of the leaf under 50% shading (T1) for 75 days. (A4–F4) The anatomic structure of the leaf under 75% shading (T2) for 75 days. (A) Leaf sample (Scale bars = 1 cm). (B) Leaf cross-section (Scale bars = 400 μm). (C) The distribution of pigments in marginal tissues (Scale bars = 200 μm). (D) The distribution of pigments in the central tissues (Scale bars = 200 μm). (E) The mesophyll parenchyma cell in the marginal parts of the chimeric leaves (Scale bars = 100 μm). (F) The mesophyll parenchyma cell in the central part of the chimeric leaves (Scale bars = 100 μm). MWh, white tissue; Mgr, green tissue; Cu, cuticle; e, epidermis; Ch, mesophyll parenchyma; Aq, water storage parenchyma; vb, vascular bundle
Light intensity is an important factor for the regulation of the photosynthetic mechanism. However, plants absorb more light than they need to saturate photosynthesis and consequently a high light intensity may cause the damage of the photosynthetic machinery. With increasing time of exposure to high light conditions, the degree of damage will increase too and the leaf death can occur [39]. In our present study, full light was probably too much for A. comosus var. bracteatus. As is already known, light can promote structural changes in leaf tissues. The mesophyll cells in the marginal and central tissues of the leaves under full light were reduced in size, and some of them were wrinkled in transverse section (Figs. 5E2, 5F2). It could be that excessive light and high leaf temperature resulted in water loss from the cells. However, the mesophyll cells under 75% shading were oval and turgid (Figs. 5E4, 5F4). Under the cuticle endowed epidermis of the leaf central green part, large parenchyma cells have also a function for water storage, an important structural feature of A. comosus var. bracteatus as a drought-enduring plant adapted to drought environments. This is an important structural feature of the green center part of chimeric leaves.
In this study, we showed that light intensity affects the leaf color; color parameters; pigment accumulation, distribution and ratio; mesophyll cells, and chloroplast occurrence in the marginal and central parts of chimeric leaves of A. comosus var. bracteatus. 75% shading boosted the decrease in anthocyanin concentration, the increase in chlorophyll concentration, and the number of chloroplasts in the mesophyll cells, and the leaves changed their color from red to green reducing their plant ornamental value. However, high irradiance promoted the accumulation of anthocyanins in the mesophyll cells, the relative proportion of anthocyanin concentration and, consequently, the redness (a*) of the marginal parts of chimeric leaves. The plants exposed to full light and 50% shade could maintain red and had a higher ornamental value. In addition, long exposure to full light contributed to light damage or even leaf death, the occurrence of wrinkled and smaller mesophyll cells in A. comosus var. bracteatus. Fifty percent shade could prevent the light damage caused by full light. In order to balance its growth and ornamental value, 50% shading is proposed as a good cultivation condition for A. comosus var. bracteatus in summer.
Acknowledgement: We are thankful to Sichuan Agricultural University, China, for providing support for the present investigation.
Funding Statement: This work was funded by the National Natural Science Foundation of China (Grant Nos. 31971704; 31770743).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |