 Open Access
Open Access
ARTICLE
Effect of Ecotype and Gender on the Variation of Leaf Morphological, Epidermal and Stomatal Traits among Pistacia atlantica Desf.
1 Centre de Recherche en Agropastoralisme (CRAPAST), Djelfa, 17000, Algérie
2 Faculty of Nature and Life Sciences, University of Djelfa. P.O. Box 3117, Djelfa, 17000, Algeria
3 Scientific and Technical Research Center on Arid Regions, Biskra, 07000, Algeria
4 Aix Marseille Université, Avignon Université, CNRS, IRD, IMBE, Marseille, 13331, France
5 Aix Marseille Université, CNRS, Centrale Marseille, FSCM, PRATIM, Marseille, 13331, France
6 Conservation, Management and Improvement of Forest Ecosystems Laboratory, National Higher Agronomic School, Algiers, 16004, Algeria
7 Faculté des Sciences Biologiques et Agronomiques, Université Mouloud Mammeri, Tizi-Ouzou, 15000, Algérie
8 Plant Production Department, College of Food and Agriculture Sciences, King Saud University, P.O. Box 2460, Riyadh, 11451, Saudi Arabia
* Corresponding Author: Abdelghafour Doghbage. Email:
Phyton-International Journal of Experimental Botany 2024, 93(9), 2383-2413. https://doi.org/10.32604/phyton.2024.055528
Received 29 June 2024; Accepted 23 August 2024; Issue published 30 September 2024
Abstract
The Atlas pistachio tree is a typically Mediterranean species, which represents an important forest heritage in the arid and semi-arid regions of Algeria. It is deeply rooted in the local population’s culture, making it essential to better understand this species for its conservation and valorization. Through our work on 7 provenances of Pistacia atlantica distributed across different bioclimates in Algeria and based on 28 quantitative and qualitative leaf, trichome, and stomatal traits, it was revealed that the Atlas pistachio tree exhibits significant ecotypic variability linked to its habitat and a high adaptability to extreme conditions in its environments (aridity and altitude). Indeed, statistical analyses indicate a substantial heterogeneity in the studied characteristics among different ecotypes of P. atlantica. Genetic factors undoubtedly play a primary role in this variability, but environmental factors also exert a remarkable impact on this heterogeneity. Gender also plays a crucial role in this variability. Microphotographs of leaf samples taken under scanning electron microscopy (SEM), such as the density and type of trichomes, and form and position of stomates in the epidermis, can provide an important taxonomic tool for identifying Pistacia species and valuable insights into their adaptation to xeric conditions, thus enabling their use in desertification control projects and the rehabilitation of highly degraded forest environments such as those found in the “Green Dam” initiative.Keywords
The Mediterranean climate is characterized by a dry period, varying in length depending on the latitude. Plants develop numerous morphological adaptations to cope with these periods of water scarcity [1]. The genus Pistacia is part of this vegetation, which includes xeric species with significant ecological and economic potential.
The species of the genus Pistacia exhibit a highly complex taxonomy. Therefore, the taxonomic relationships among its different species remain a subject of controversy and are not yet well-defined. The number of recognized species varies among authors, ranging from 9 to 11 species [2–6].
The Atlas Pistachio tree (Pistacia atlantica Desf.) is a fascinating deciduous tree that holds a significant place in the Algerian steppe and Saharan ecosystems. Its ecological range stretches from the heart of the Sahara to the margins of humid bioclimates [7]. It adapts to diverse climatic conditions, and its contribution to Algerian ecosystem biodiversity is invaluable. However, this plant heritage is endangered, with its degradation resulting from several factors such as habitat loss, climate change, overexploitation, and diseases that threaten the survival of this species protected by Algerian law [8] (Fig. 1). Therefore, a comprehensive understanding of this species is essential to establish and develop effective and sustainable conservation strategies. Hence, studying intraspecific and intersex variability in the species is valuable for explaining and predicting population adaptation to climate change and understanding mechanisms for conserving and restoring endangered species. However, research focusing on inter-population variability and between-sex differences in Pistacia species is considered negligible compared to interspecific variability. Notable works in this area include studies by [9–14].
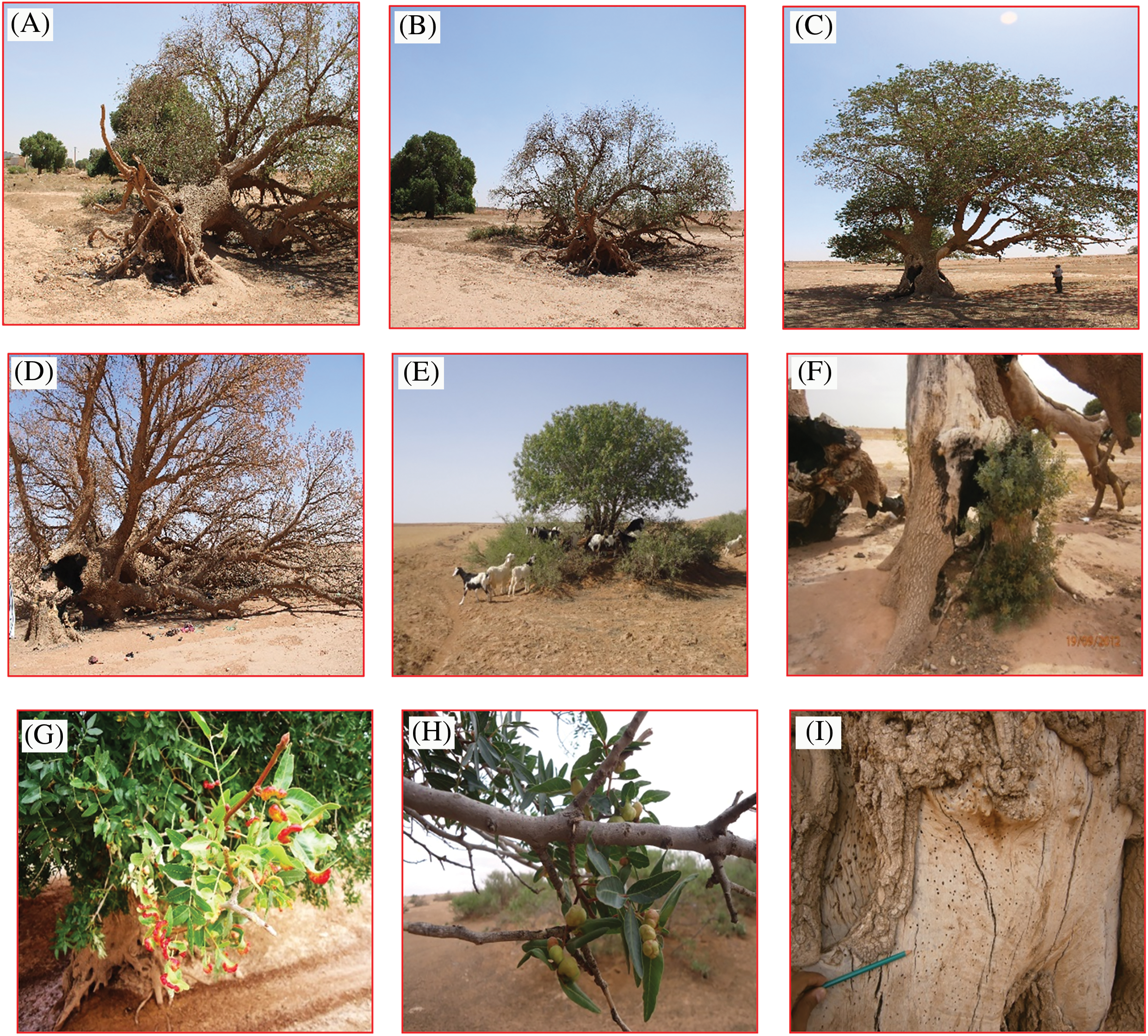
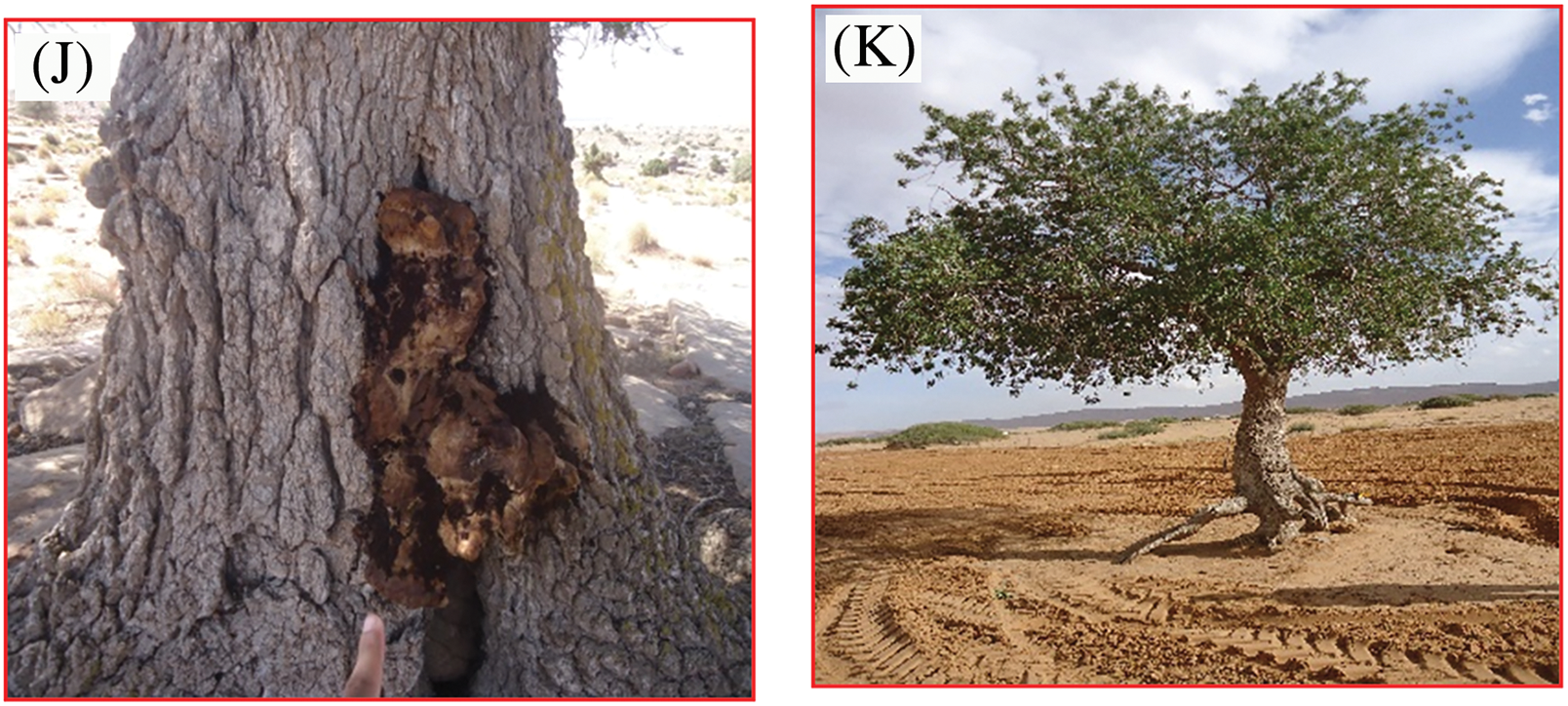
Figure 1: Illustration of some degradation factors affecting pistachio trees. Uprooting due to water and wind erosion (A, B), aging of trees (C, D), grazing (E), unauthorized cutting pests and diseases (F–J), illegal plowing (K) (Original, photography: (A) Doghbage, from 2013 to 2024)
In this context, given the complexity of taxonomic affinities within the genus Pistacia and their behaviors in response to understudied environmental heterogeneity, the present study contributes to the investigation of diversity and variability among 7 ecotypes of P. atlantica sampled from various bioclimates. The study aims to explore intra-specific micro and macromorphological leaf variability and even inter-sex variability to understand how this species adapts to extreme conditions in its environment (aridity and altitude). This is achieved by examining morphological and ultrastructural leaf traits, particularly using scanning electron microscopy (SEM) for leaf epidermal characteristics (density, size, distribution, and position of stomata, type and density of trichomes, etc.), and potentially providing criteria for its systematic classification. Our study will also enhance our understanding of Pistacia species, which are largely understudied and therefore underutilized in afforestation and desertification control programs such as the “Green Dam,” despite their ecological and economic significance.
2.1 Sampling and Bioclimatic Study
Leaf sampling of Pistacia atlantica was conducted at a total of 7 sites, selected along a North-South and East-West transect (Table 1). The majority of the stations are located in areas characterized by a semi-arid to arid climate with low precipitation, exhibiting significant intra-monthly and interannual variability. They are generally situated between 150 mm on the Saharan border and over 500 mm on the Tellian border.
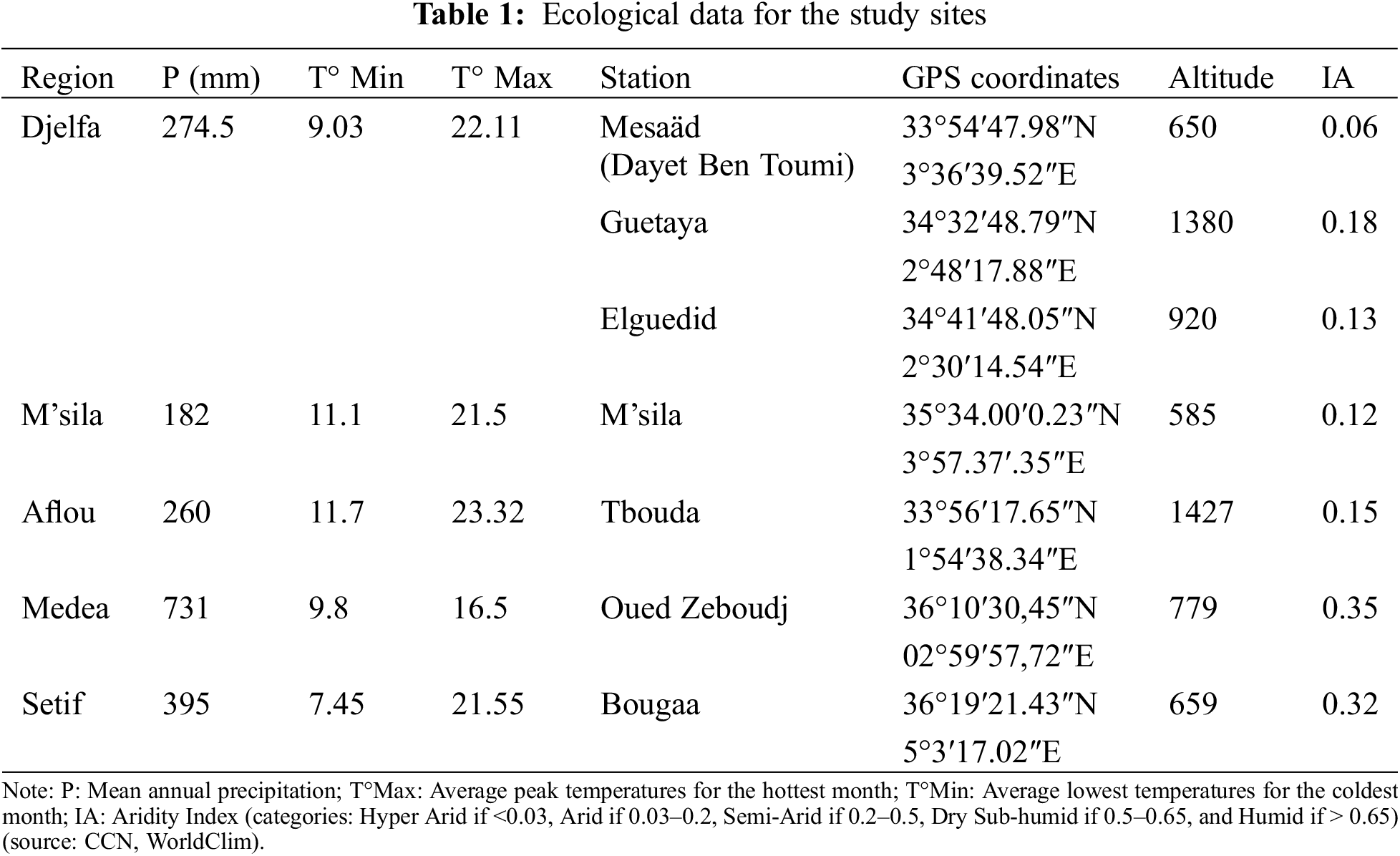
Leaves were randomly collected from each tree at a rate of 30 leaves per tree. Ten (10) repetitions were performed for each population, with 5 male trees (MT) and 5 female trees (FT) of approximately the same age chosen.
For the creation of maps showing the Global Aridity Index and bioclimates, we utilized geo-spatial data available on the Consultative Group for International Agriculture Research/Consortium for Spatial Information (CGIAR-CSI) website (https://cgiarcsi.community) (accessed on 18 April 2024) [15]. The data were downloaded in raster format and processed using ArcGIS 10.5 software. The calculation of the Global Aridity Index_ET0 (Global-AI_ET0) was performed using the following equation: Global-Aridity_ET0 (AI_ET0) = MA-Pr/MA-ET0 (Fig. 2). Where: MA-Pr = Mean Annual Precipitation; MA-ET0 = Mean Annual Reference Evapotranspiration.
Figure 2: Bioclimate of study sites
2.2 Macro-Morphological Study of Leaves
For leaf macro-morphology, 15 quantitative and qualitative traits were measured (Fig. 3, Table A1 in Appendix A). To conduct this part of the study, we relied on the guidelines provided by [16] (a manual or descriptors for different species of the Pistacia genus developed by IPGRI).
Figure 3: Macro-morphological leaf traits measured and observed according to the IPGRI manual (1998)
2.3 Micro-Morphological Study of Leaves: Epidermis, Stomata, and Trichomes
Light microscopy: To observe stomata, a leaf impression method was utilized. Initially, a thin coating of clear nail varnish was applied to the upper (adaxial) and lower (abaxial) surfaces of the leaves. This layer was allowed to dry for 5 to 10 min. Following this, a strip of transparent adhesive tape (sellotape) was pressed onto the dried varnish to capture an imprint. The tape, now carrying the leaf impression, was carefully removed and placed onto a glass microscope slide for observation. The replicas were examined using an optical microscope (OPTICA AXIOM 7000) at magnifications of ×100 and ×400. Digital images were captured for analysis. Measurements of stomatal length and width were taken from the abaxial surface of ten stomata per leaf. Furthermore, stomatal counts were performed in ten adaxial and ten abaxial areas across five leaves from each population.
Scanning electron microscopy: Remaining leaves were treated with 90% ethanol to remove any external debris. Standard procedures for Scanning Electron Microscopy (SEM) were then followed to investigate the epidermal surfaces of the leaves. Three specimens from each location were examined. A 5 mm2 section of the dried leaf, including both adaxial and abaxial surfaces, was affixed to a labeled stub. The samples were then gold-coated and scanned using a Philips XL 30 ESEM (Philips Electronic Instruments Co., Mahwah, NJ, USA). SEM images were captured at varying magnifications.
Observation and measurement parameters: Thirteen characteristics related to stomata and trichomes were evaluated, both quantitatively and qualitatively, including:
Qualitative characteristics: Stomatal shape, distribution, and positioning on the epidermis; trichome distribution and density on leaf margins, adaxial and abaxial surfaces, and along the main midrib; and trichome types.
Quantitative characteristics: Stomatal length and width on the abaxial surface (µm) and stomatal density on both adaxial and abaxial surfaces (stomata/mm2).
2.4 Statistical Analyses of Data
Descriptive statistics, correlation coefficients between the measured variables and environmental parameters (altitude and aridity), and a two-way ANOVA (considering ecotype and gender) were conducted to assess the impact of these factors on the variability of the measured traits. The Newman-Keuls (HSD) test was subsequently applied to identify homogeneous groups (p < 0.05), where groups sharing the same letter (a, b, c, d, e) are not significantly different, and “***” denotes significant differences; results are presented with Avg (Average), SD (Standard Deviation), Range (Min-Max), and C.V. (Coefficient of Variation, %).
3.1 Quantitative Macromorphological Characteristics of the Leaf and Terminal Leaflet
3.1.1 Effect of Ecotype on the Leaf
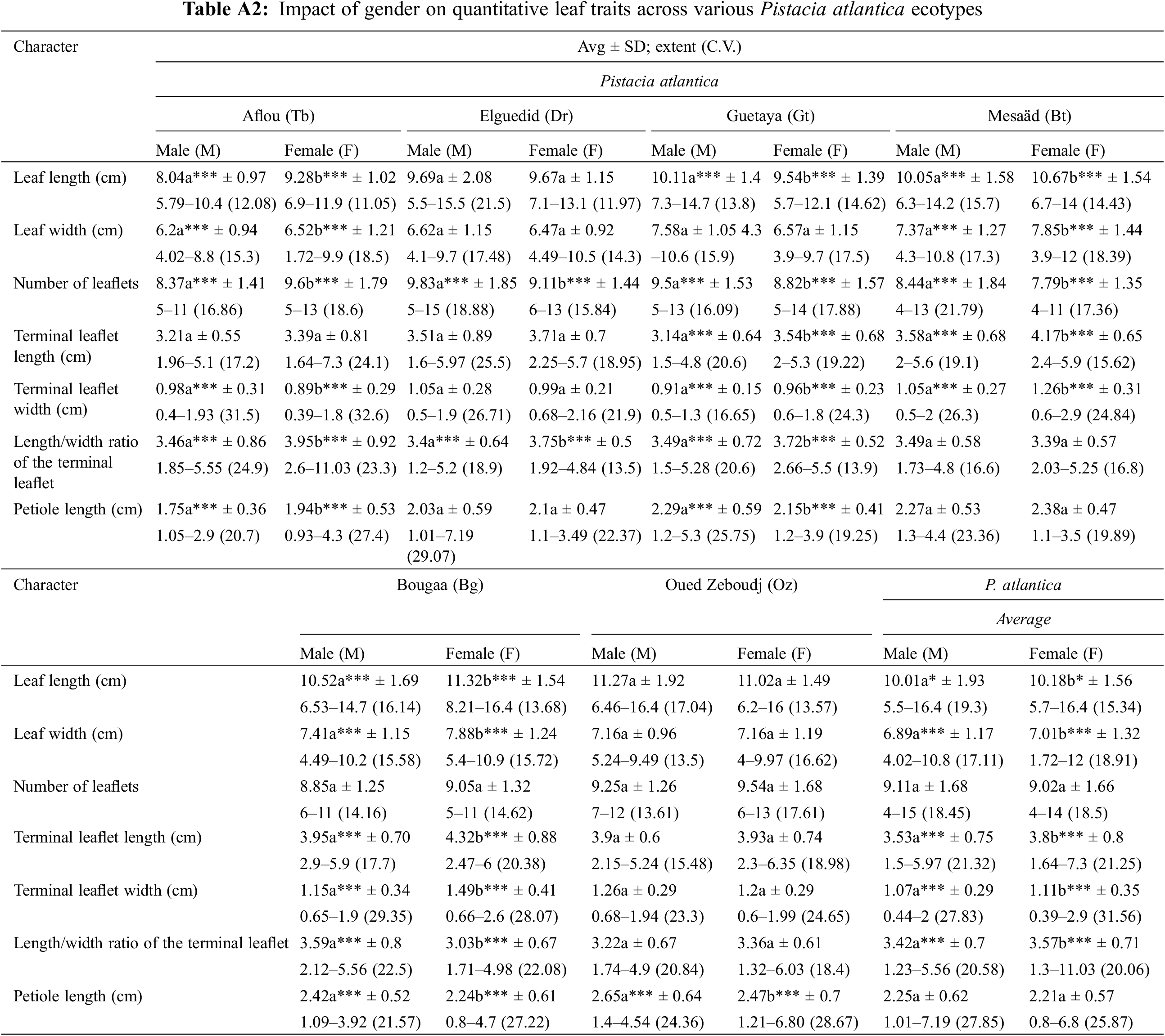
For the lengths of P. atlantica leaves recorded at different sites, the variation is distinguished by an average of 10.1 ± 1.74 cm, ranging from a minimum of 5.5 ± 1.74 cm to a maximum of 16.40 ± 1.74 cm. Regarding width, the recorded values range from 1.72 ± 1.24 cm to 12 ± 1.24 cm, with an average of 6.95 ± 1.24 cm.
P. atlantica leaves have between 4 ± 1.66 and 15 ± 1.66 leaflets, with an average of 9.04 leaflets. The most common number of leaflets is 9 (41.06%). The length of the petiole varies from a minimum of 0.8 ± 0.59 cm to a maximum of 7.19 ± 0.59 cm, with an average of 2.24 ± 0.59 cm and a coefficient of variation of 26.63% (Table 2).
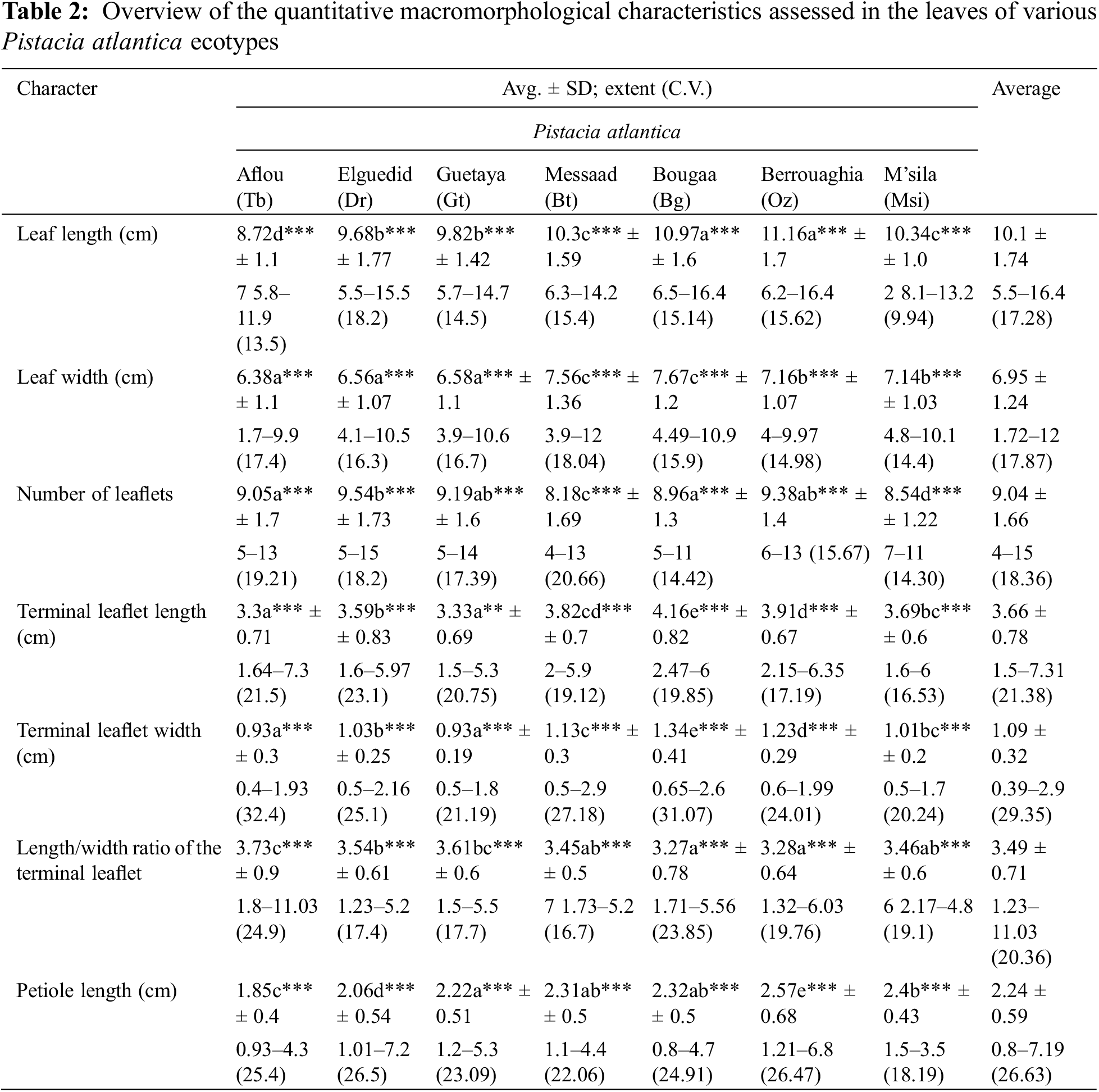
The leaves are longer at Oued Zeboudj (Oz) (11.16 cm) and Bougaa (Bg) (10.97 cm), while shorter at Aflou (Tb) (8.72 cm) and Guetaya (Gt) (9.82 cm). However, they are wider at (Bg) (7.67 cm) and Messaäd (Bt) (7.56 cm), and narrower at (Tb) (6.38 cm), Elguedid (Dr) (6.56 cm), and (Gt) (6.58 cm). The number of leaflets varies among populations, with the highest average recorded at (Dr) (9.54) and (Oz) (9.38), while the smallest number of leaflets was observed at (Bt) (8.18). The petiole is longer at (Oz) (2.57 cm) and M’sila (Msi) (2.40 cm), whereas smaller values were recorded at (Tb) (1.85 cm) (Table 2, Fig. 4).
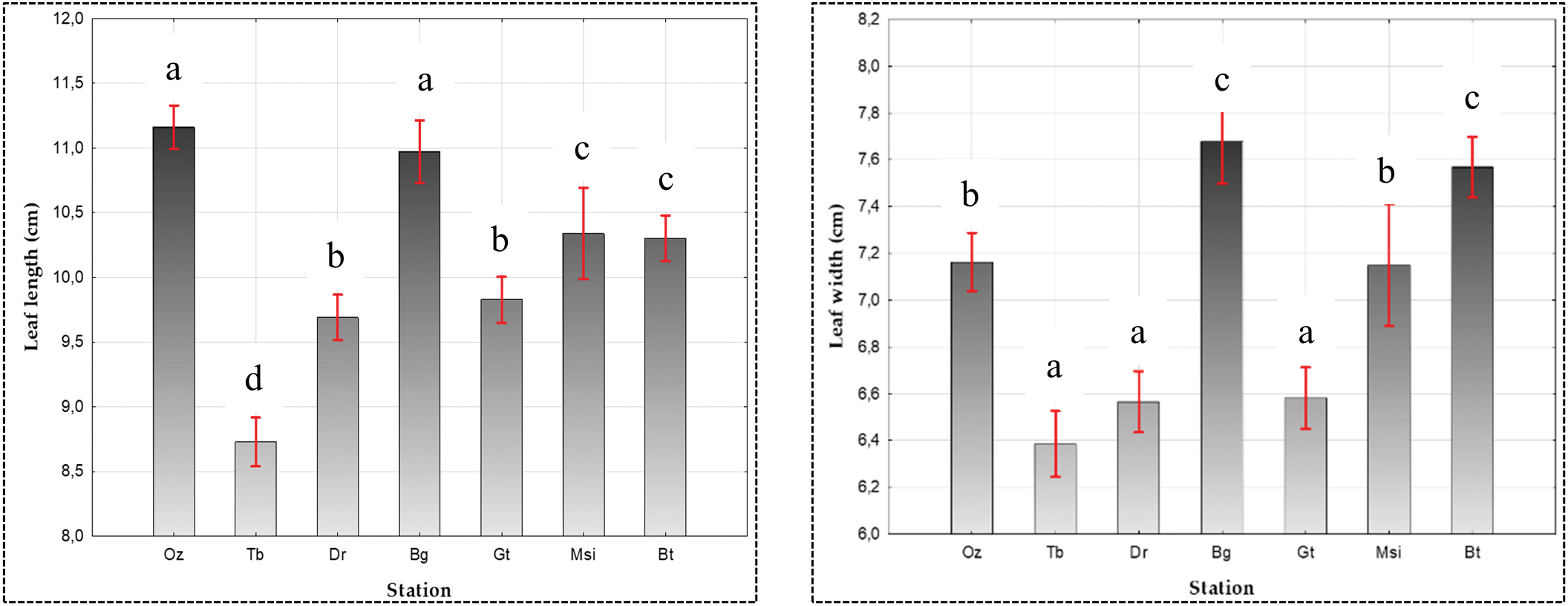
Figure 4: Influence of ecotype on leaf and terminal leaflet biometrics in Pistacia atlantica as analyzed by ANOVA (α = 0.05)
The analysis of variance (ANOVA) for leaf dimensions (length/width), number of leaflets, and petiole length reveals a significant difference (α = 0.05) among P. atlantica populations with p < 0.05 (Fig. 4). Multiple comparison of means using the Newman-Keuls test at a risk level α = 0.05 reveals the presence of 4 distinct groups for leaf length: Group 1 (Bg and Oz), Group 2 (Dr and Gt), Group 3 (Msi and Bt), and finally Group 4 (Tb). Similarly, 3 distinct groups are identified for leaf width: Group 1 (Tb, Gt, and Dr), Group 2 (Msi and Oz), and Group 3 (Bt and Bg). 4 distinct groups are recorded for the number of leaflets: Group 1 (Tb, Bg, Gt, and Oz); Group 2 (Gt, Oz, and Dr); Group 3 (Bt); and Group 4 (Msi). Regarding petiole length, 5 distinct groups are noted: Group 1 (Gt, Bg, and Bt); Group 2 (Bg, Bt, and Msi); Group 3 (Tb); Group 4 (Dr); and Group 5 (Oz) (Table 2).
3.1.2 Effect of Ecotype on the Terminal Leaflet
An average length of 3.66 ± 0.78 cm was recorded for the terminal leaflet (TL), ranging from a minimum of 1.5 ± 0.78 cm to a maximum of 7.31 ± 0.78 cm. As for the width of the TL, recorded values varied between 0.39 ± 0.32 cm and 2.9 ± 0.32 cm, with an average of 1.1 ± 0.32 cm. The length-to-width ratio averaged at 3.49 ± 0.71, with values ranging from a minimum of 1.23 ± 0.71 to a maximum of 11.03 ± 0.71. The coefficient of variation was 21.38% for the length of the terminal leaflet and 29.35% for the width of the TL (Table 2).
ANOVA, for the dimensions of TL and its length-to-width ratio, revealed a significant difference at the α = 0.05 threshold among the various populations of P. atlantica with p < 0.05 (Fig. 4). The results obtained for the TL biometry through multiple mean comparisons, using the Newman-Keuls test at an α = 0.05 risk, revealed the presence of:
- 05 distinct groups for the length of the TL: Group 1: Tb (3.30 ± 0.71 cm) and Gt (3.33 ± 0.69 cm); Group 2: Dr (3.59 ± 0.83 cm) and Msi (3.69 ± 0.61 cm); Group 3: Msi (3.69 ± 0.61 cm) and Bt (3.82 ± 0.7 cm); Group 4: Bt (3.82 ± 0.7 cm) and Oz (3.91 ± 0.67 cm); and Group 5: Bg (4.16 ± 0.82 cm).
- 05 groups for the width of the TL: Group 1: Tb (0.93 ± 0.30 cm) and Gt (0.93 ± 0.19 cm); Group 2: Dr (1.03 ± 0.25 cm) and Msi (1.09 ± 0.22 cm); Group 3: Msi (1.09 ± 0.22 cm) and Bt (1.13 ± 0.3 cm); Group 4: Oz (1.23 ± 0.29 cm); and Group 5: Bg (1.34 ± 0.41 cm).
- 03 groups for the length-to-width ratio of the TL: Group 1: Bg (3.27 ± 0.78), Oz (3.28 ± 0.64), Bt (3.45 ± 0.57), and Msi (3.46 ± 0.66); Group 2: Bt (3.45 ± 0.57), Msi (3.46 ± 0.66), Dr (3.54 ± 0.61), and Gt (3.61 ± 0.63); Group 3: Gt (3.61 ± 0.63) and Tb (3.73 ± 0.92) (Table 2).
3.1.3 Effect of Gender on the Leaf
Across all populations of P. atlantica, we observed that female trees (FT) have longer leaves compared to male trees (MT), with an average length of 10.18 ± 1.56 cm for FT and 10.01 ± 1.93 cm for MT. The coefficient of variation is 15.34% for FT and 19.30% for MT. t-tests revealed a significant difference between the leaves of MT and FT (α = 0.05) (Table A2 in Appendix A, Fig. 5).
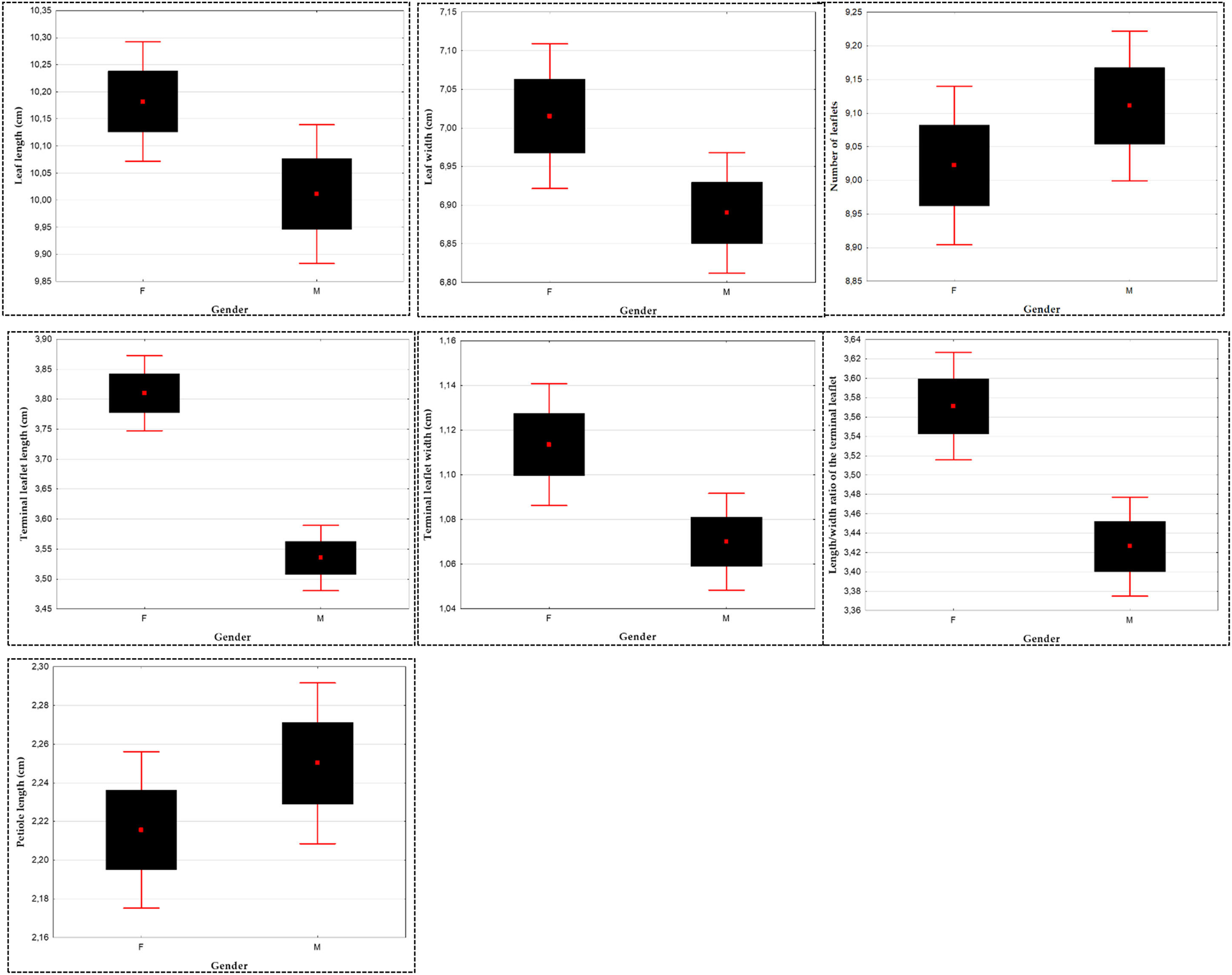
Figure 5: Analysis of gender influence on leaf biometrics and terminal leaflet (TL) in Pistacia atlantica using t-tests (α = 0.05)
For stations (Oz) and (Dr), no significant difference was recorded between MT and PF, with p > 0.05. In the majority of P. atlantica populations studied, FT leaves are longer than MT leaves, as seen in Tb (8.04 cm ♂; 9.28 cm ♀), Bg (10.52 cm ♂; 11.32 cm ♀), and Bt (10.05 cm ♂; 10.67 cm ♀). However, they are shorter than MT leaves at Gt (10.12 cm ♂; 9.54 cm ♀) (Table A2).
Across all populations of P. atlantica, the width of FT leaves ranges from a minimum of 3.72 ± 1.31 cm to a maximum of 12 ± 1.31 cm, with an average of 7.02 cm; while for MT leaves, it ranges from 4.02 ± 1.17 cm to 10.8 ± 1.17 cm, with an average of 6.89 cm. t-tests shows a significant difference (α = 0.05) in leaf width between MT and FT (Table A2, Fig. 5).
Significant differences (p < 0.05) were observed between MT and FT leaves in the Tb (6.20 cm ♂; 6.52 cm ♀), Bg (7.41 cm ♂; 7.89 cm ♀), and Bt (7.37 cm ♂; 7.86 cm ♀) populations. MT leaves are narrower (Table A2).
Number of Leaflets and Petiole Length
Regarding the number of leaflets, the t-tests show that there is no significant difference between MT and FT leaves (p > 0.05) (0.28 > 0.05), across all P. atlantica leaves (9.11 ♂; 9.02 ♀) (Table A2, Fig. 5). However, we observed in the Dr, Gt, and Bt stations that the number of leaflets in MT leaves is higher. Only in the Tb station, the number of leaflets in FT leaves is higher than in MT leaves (Table A2). For the length of the petiole, p > 0.05 (0.24 > 0.05), indicating that there is no significant difference between MT and FT leaves. However, in the Oz, Bg, and Gt stations, the length of the petiole in MT leaves is higher. In the Tb station, the length of the petiole in FT leaves is higher than in MT leaves (Table A2).
3.1.4 Effect of Gender on the Terminal Leaflet
Overall, FT have longer terminal leaflets (3.80 ± 0.80 cm) compared to MT (3.53 ± 0.75 cm). The coefficient of variation is 21.25% for FT and 21.32% for MT. The t-tests show a significant difference between MT and FT for this variable at the α = 0.05 level (Table A2, Fig. 5).
On some sites, we did not record any significant difference between MT and FT, especially in the Oz, Tb, and Dr stations, with p > 0.05. However, FT terminal leaflets are longer in populations from Bg (3.95 cm ♂; 4.32 cm ♀), Gt (3.14 cm ♂; 3.54 cm ♀), and Bt (3.58 cm ♂; 4.17 cm ♀) (Table A2).
In all studied populations of P. atlantica, the width of the TL of the FT ranges from 0.39 ± 0.35 cm to 2.9 ± 0.35 cm, with an average of 1.11 cm, and from 0.44 ± 0.29 cm to 2 ± 0.29 cm for MT terminal leaflets, with an average of 1.07 cm. t-tests reveal a significant difference at the α = 0.05 level (p = 0.03 < 0.05) between MT and FT terminal leaflets (Table A2, Fig. 5).
In the majority of the studied sites (Bg, Gt, and Bt), MT terminal leaflets are narrower than FT ones, except for the Tb station where we observed the opposite. For this variable, significant differences (p < 0.05) are recorded between MT and FT terminal leaflets for the Tb (0.98 cm ♂; 0.89 cm ♀), Bg (1.15 cm ♂; 1.49 cm ♀), Gt (0.91 cm ♂; 0.96 cm ♀), and Bt (1.05 cm ♂; 1.26 cm ♀) populations (Table A2).
Regarding this variable, the values range from 1.32 to 11.03 with a mean of 3.57 ± 0.71 for FT and from 1.23 to 5.56 with a mean of 3.42 ± 0.70 for MT. t-tests reveal a significant difference at the α = 0.05 level (p < 0.05) between MT and FT across the different studied populations (Table A2, Fig. 5). Depending on the stations, it was observed that the length/width ratio values of the TL are higher in FT than in MT for the populations of Tb (3.46 ♂; 3.95 ♀), Dr (3.40 ♂; 3.75 ♀), and Gt (3.49 ♂; 3.72 ♀), with the only population showing the opposite trend being Bg (3.59 ♂; 3.03 ♀) (Table A2).
3.2 Qualitative Macromorphological Characteristics of the Leaf and Terminal Leaflet
3.2.1 Effect of Ecotype on the Leaf
In our study, we observed that almost half of the leaves of P. atlantica have narrow green wings along the rachis to the petiole (55.12%), while the other half has wings only along the rachis (44.88%). All samples have entire margins. Regarding the color of the leaves, they are green (53%) and dark green (41.66%), but we observed light green leaves (5.33%). The leaves of P. atlantica have rounded and flattened petioles on the upper surface with a high percentage (92.49%); however, 5.89% are flattened and 1.61% are rounded (Table 3).
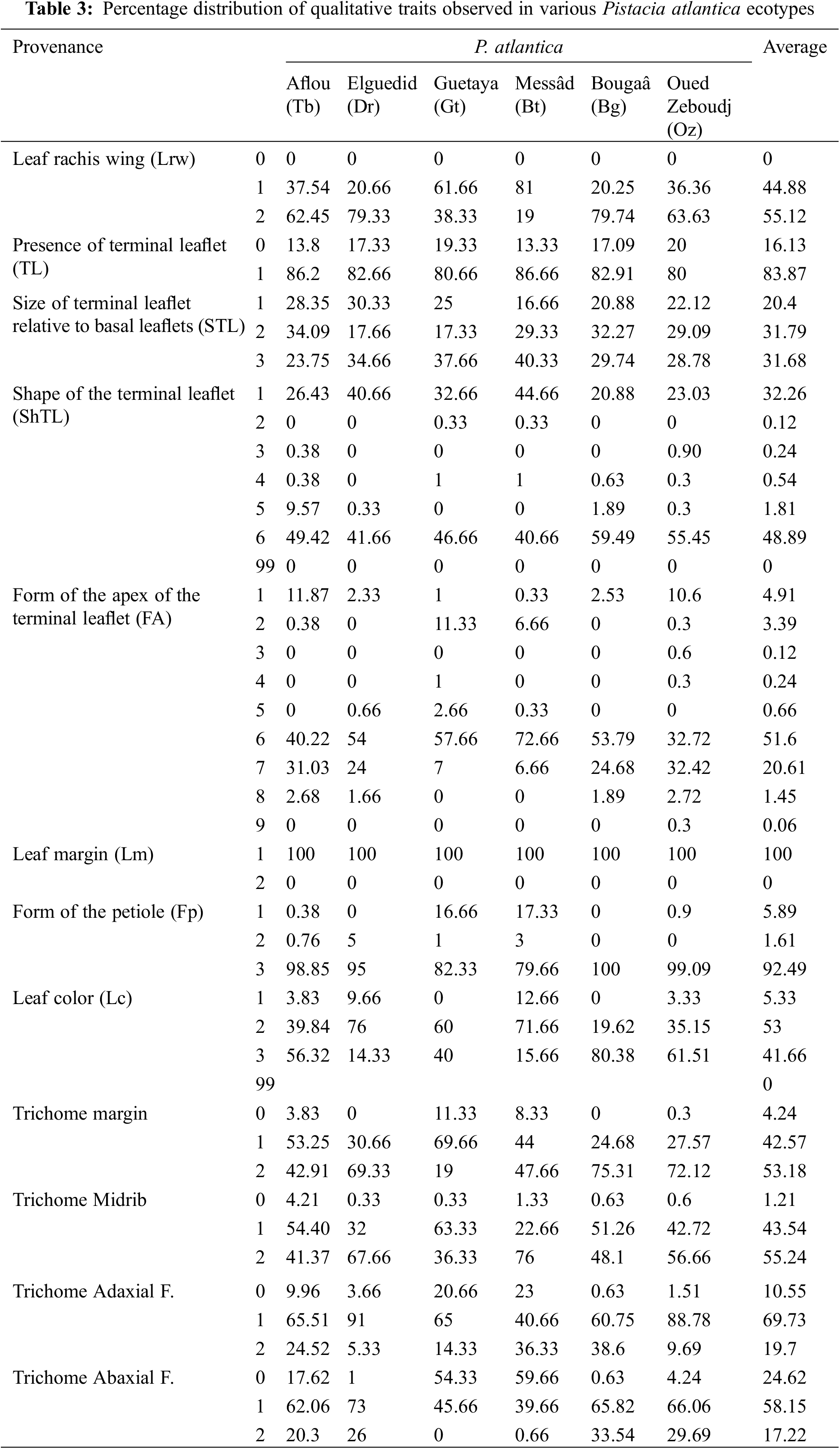
By ecotype, the majority of P. atlantica leaves exhibit narrow leaf wings along the rachis and petiole at varying rates, ranging from 62.45% (Tb), 63.63% (Oz), 79.33% (Dr), and 79.74% (Bg); however, these rates are lower in (Gt) at 38.33% and (Bt) at 19%. Regarding leaf color, leaves are mostly green and dark green. Stations (Oz) (61.51%) and (Bg) (80.38%) have darker-colored leaves compared to other stations, while the green color dominates in (Dr) (76%), (Gt) (60%), and (Bt) (71.66%). Light green color was observed with lower percentages in stations (Bt) (2.66%), (Dr) (9.66%), (Tb) (3.83%), and (Oz) (3.33%). Concerning the petiole, leaves from different studied populations have a rounded petiole on their lower surface, with very high rates in (Oz) (99.09%), (Tb) (98.85%), (Dr) (95%), (Bg) (Msi) (100%), (Gt) (82.33%), and (Bt) (79.66%) (Table 3).
3.2.2 Effect of Ecotype on the Terminal Leaflet
The majority of leaves (83.87%) have a TL. The results show that the rates of TL wider or the same size as the basal leaflets are very close, at 31.68% and 31.79%, respectively, while 20.40% have a smaller TL than the basal leaflets. Regarding the shape of this leaflet, it varies between narrow elliptical (48.89%) and lanceolate (32.26%). Elliptical shape (1.81%) was also observed in P. atlantica leaves. The apex of TLs comes in different shapes, with acute shape being the most distinguished (51.6%) followed by obtuse (20.61%). Other shapes were recorded at lower rates: mucronulate (4.91%), acuminated (3.39%), and retuse (1.45%) (Table 3).
By ecotype, the absence of TL was reported across all stations at varying rates: 20% (Oz)-(Gt), 13.79% (Tb), 17.33% (Dr), 17.08% (Bg), and 14.66% (Bt). Populations (Oz) (29.09%), (Tb) (34.09%), (Bg) (32.27%), and (Msi) (65.33%) have TL and basal leaflets of the same size, while stations (Bt) (40.33%), (Gt) (37.66%), and (Dr) (34.66%) present TL larger than basal leaflets. However, when the TL is smaller than the basal leaflets, variable rates are recorded: Oz (22.12%), Tb (28.35%), Dr (17.66%), Bg (20.88%), Gt (25%), Msi (9.33%), and Bt (16.66%) (Table 3).
Regarding the shape of TL, narrow elliptical and lanceolate shapes are the most common, with varying proportions: (Bg) (59.49%; 20.88%), (Oz) (55.45%; 23.03%), (Tb) (49.42%; 26.43%), (Gt) (46.66%; 32.66%), (Dr) (41.66%; 40.66%), and Bt (40.66%; 44.66%). The elliptical shape was recorded for (Tb) with 9.57%. For the apex of this leaflet, acute and obtuse shapes are found at high rates across all stations. Other shapes such as mucronate, mucronulate, acuminated, and retuse were also recorded in most origins but at lower occurrences. The emarginate shape was observed only in station (Oz) (Table 3).
3.2.3 Effect of Gender on the Leaf
By gender, almost half of MT leaves (55.07%) exhibit leaf wings along the rachis and petiole, a proportion also observed in FT (55.18%). The presence of leaf wings only on the rachis was observed in 44.92% of MT leaves and almost the same rate (44.82%) for FT. The color of leaflets among FT varies between green (52.20%), dark green (44.81%), and light green (2.97%), similarly for MT, green color is most frequent (53.70%), followed by dark green (38.88%), and light green (7.41%). For the petiole, the proportions of the rounded shape on the lower surface are similar between the two genders (91.44% ♂; 92.22% ♀).
3.2.4 Effect of Gender on the Terminal Leaflet
By gender, the presence rates of TL in males and females are very close (83.23% ♂ and 82.51% ♀). The results show that females have a higher rate (37.56%) of TL larger than basal leaflets compared to males (28.73%). TL smaller than basal leaflets was ob-served at a rate of 22.46% in males and 20.85% in females. The proportion of TL the same size as basal leaflets is higher in males than in females (32.04% ♂ and 24.09% ♀).
Regarding the shape of this leaflet, the percentage of narrow elliptical shape is higher in males (51.99% ♂ and 43.65% ♀). Meanwhile, the lanceolate shape is more frequent in females (28.5% ♂ and 36.52% ♀). The results for the shape of the apex show that the acute shape is slightly dominant in males (52.33%) than in females (50.77%), while the obtuse shape was observed in 19.72% of males and 21.63% of females. The emarginate shape was observed only in female individuals but with a very low occur-rence (0.12%).
3.3 Micromorphological Characteristics of the Leaf
In P. atlantica, leaves exhibit hairs at the leaf margin (95.75%), on the midrib (98.78%), on the adaxial surface of the leaf (89.44%), and on abaxial surface of the leaf (75.37%) (Table 3). Considering the different ecotypes, all of them show the presence of hairs at the margin (Oz) (99.69%), (Tb) (96.16%), (Dr) (100%), (Bg) (100%), (Gt) (88.66%), and (Bt) (91.66%), on the midrib (Oz) (99.39%), (Tb) (95.78%), (Dr) (99.66%), (Bg) (99.36%), (Gt) (99.66%), and (Bt) (98.66%), on the upper surface (Oz) (98.48%), (Tb) (90.03%), (Dr) (96.33%), (Bg) (99.36%), (Gt) (79.33%), and (Bt) (76.99%), and the lower surface (Oz) (95.75%), (Tb) (82.37%), (Dr) (99%), (Bg) (99.36%), (Gt) (45.66%), and (Bt) (40.34%) (Table 3). However, their density varies from one station to another and from one part of the leaf to another. Generally, they are denser at the margin and midrib and less dense on the leaf blade of both leaf surfaces (Figs. 6 and 7).
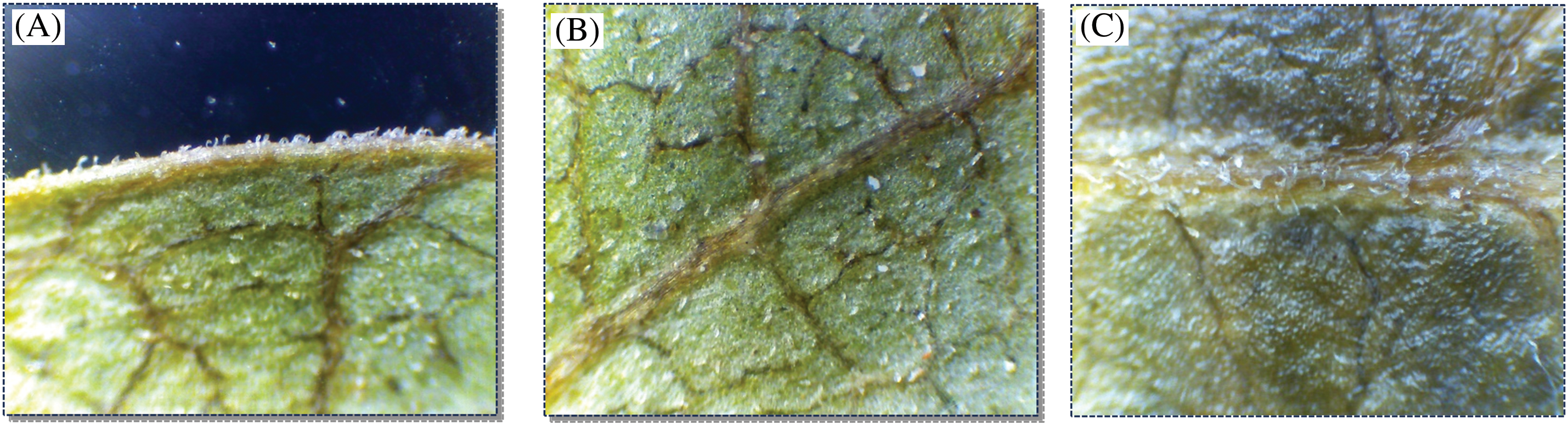
Figure 6: Examination of leaf pilosity in different sections of the Pistacia atlantica leaf using a binocular magnifying glass: (A) Margin, (B) Abaxial surface, (C) Central midrib (40× magnification)
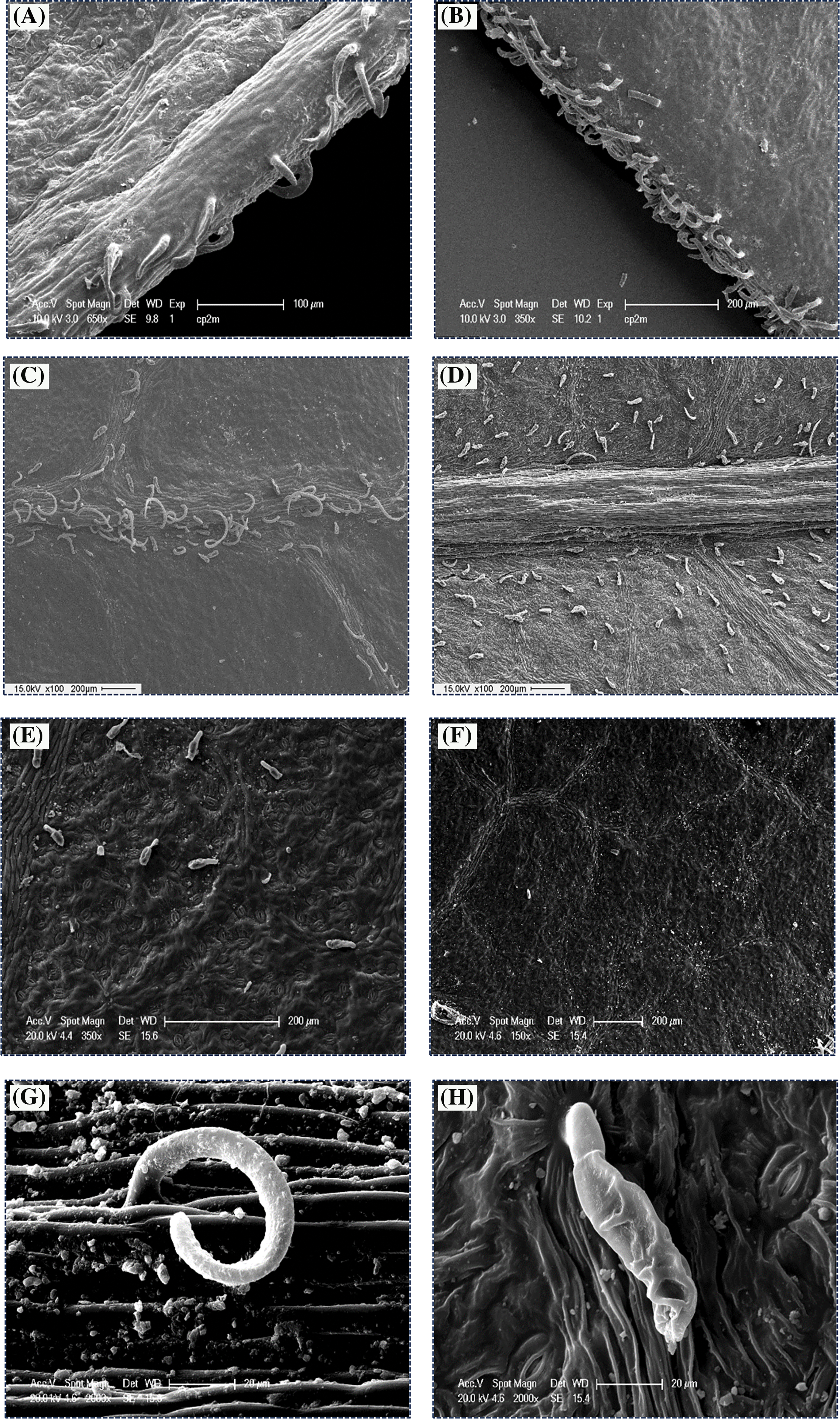
Figure 7: SEM micrographs showing leaf trichomes (Tr) of P. atlantica, (A, B) at the margin, (C, D) at the midrib, (E) abaxial surface, (F) adaxial surface, (G) non-glandular trichome (ciliate), (H) glandular trichome (G-Tr)
For all stations, 2 types of trichomes were distinguished under the SEM: glandular trichomes (peltate type) distributed over the entire leaf surface and non-glandular trichomes (ciliate type) observed along the midrib and at the margin (Fig. 7).
We noticed homogeneity between the two sexes of individuals regarding the presence of hairs at the margin (95.21% ♂; 96.37% ♀), on the midrib (98.63% ♂; 98.96% ♀), and on the lower part (74.57% ♂; 76.29% ♀). However, on the adaxial surface, we observed a slight difference between males and females (86.77% ♂; 92.48% ♀).
The length of stomata varies between 12.57 ± 2.9 µm–35.74 ± 2.9 µm with an average of 24.58 ± 2.9 µm and a coefficient of variation of 11.8%, while the width values range from 9.53 ± 1.9 µm–22.95 ± 1.9 µm with an average of 15.48 ± 1.9 µm (Table 4). Across different ecotypes, the values range between 23.72 ± 2.76 µm (Oz) and 25.68 ± 3.19 µm (Tb) for length, and between 14.47 ± 1.78 µm (Gt) and 16.29 ± 1.87 µm (Dr) for width (Table 4). ANOVA revealed a significant difference at the α = 0.05 level between P. atlantica populations with p < 0.05 for these two variables (Fig. 8). Regarding the comparison of means (Newman-Keuls test) at the risk level α = 0.05, we recorded 3 groups for stomatal length (Group 1: (Bg), (Oz), and (Gt); Group 2: (Msi), (Dr), and (Bt); and Group 3: (Tb)); 4 groups for stomatal width (Group 1: (Msi), (Tb), and (Oz), Group 2: (Bg) and (Bt), Group 3: (Gt), and Group 4: (Dr)) (Table 4; Fig. 8).

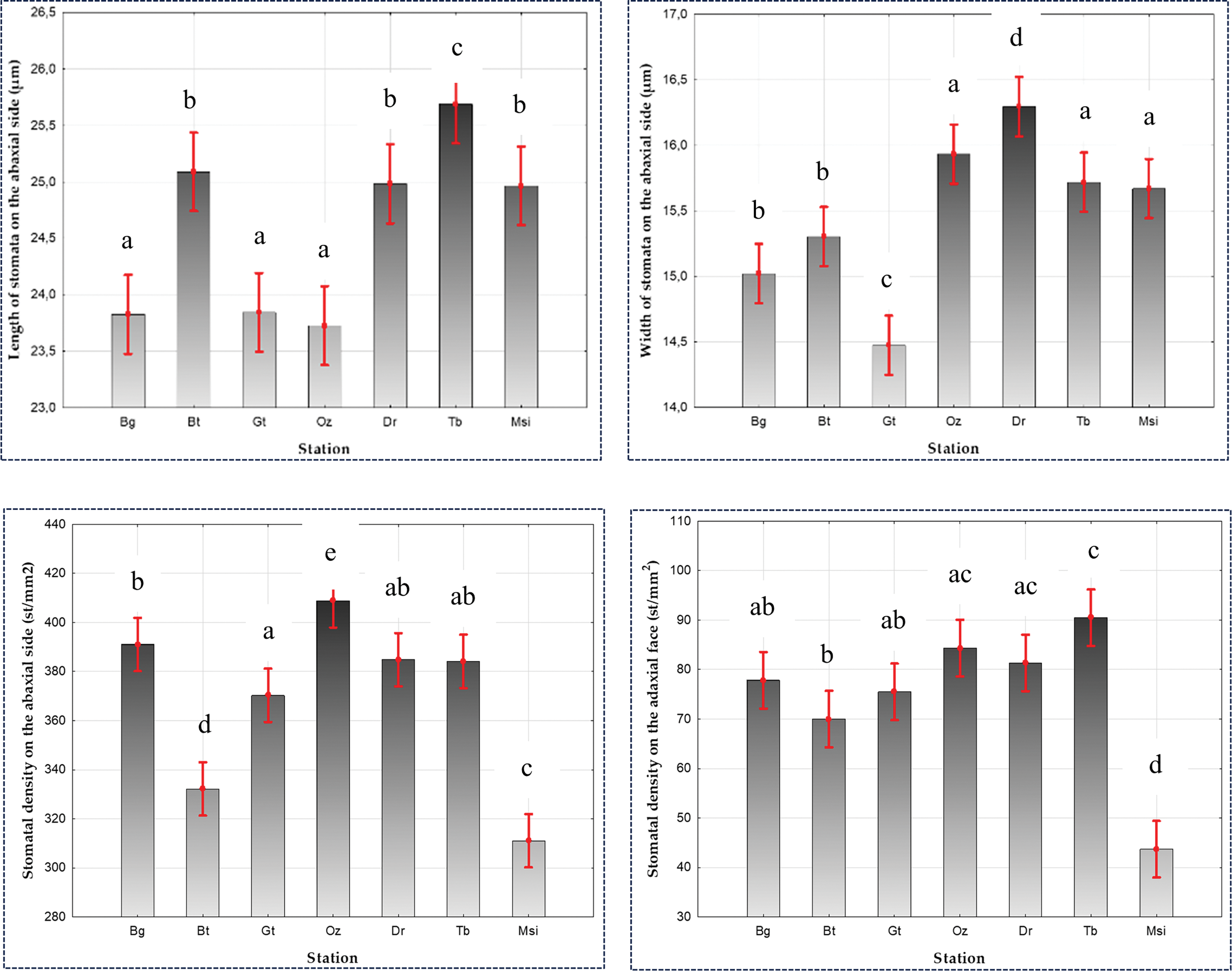
Figure 8: Impact of ecotype on the size and density of stomata on the abaxial and adaxial surfaces of Pistacia atlantica, analyzed using ANOVA (α = 0.05)
Stomatal density varies between 125 and 916.66 st/mm2 on the abaxial surface with an average of 368.91 st/mm2, while on the adaxial surface of the leaf, the number of stomata is lower, clustering near the main veins (Fig. 9) where values range between 0 and 416.66 st/mm2 with an average of 74.74 st/mm2 and a coefficient of variation of 64.29% (Table 4).
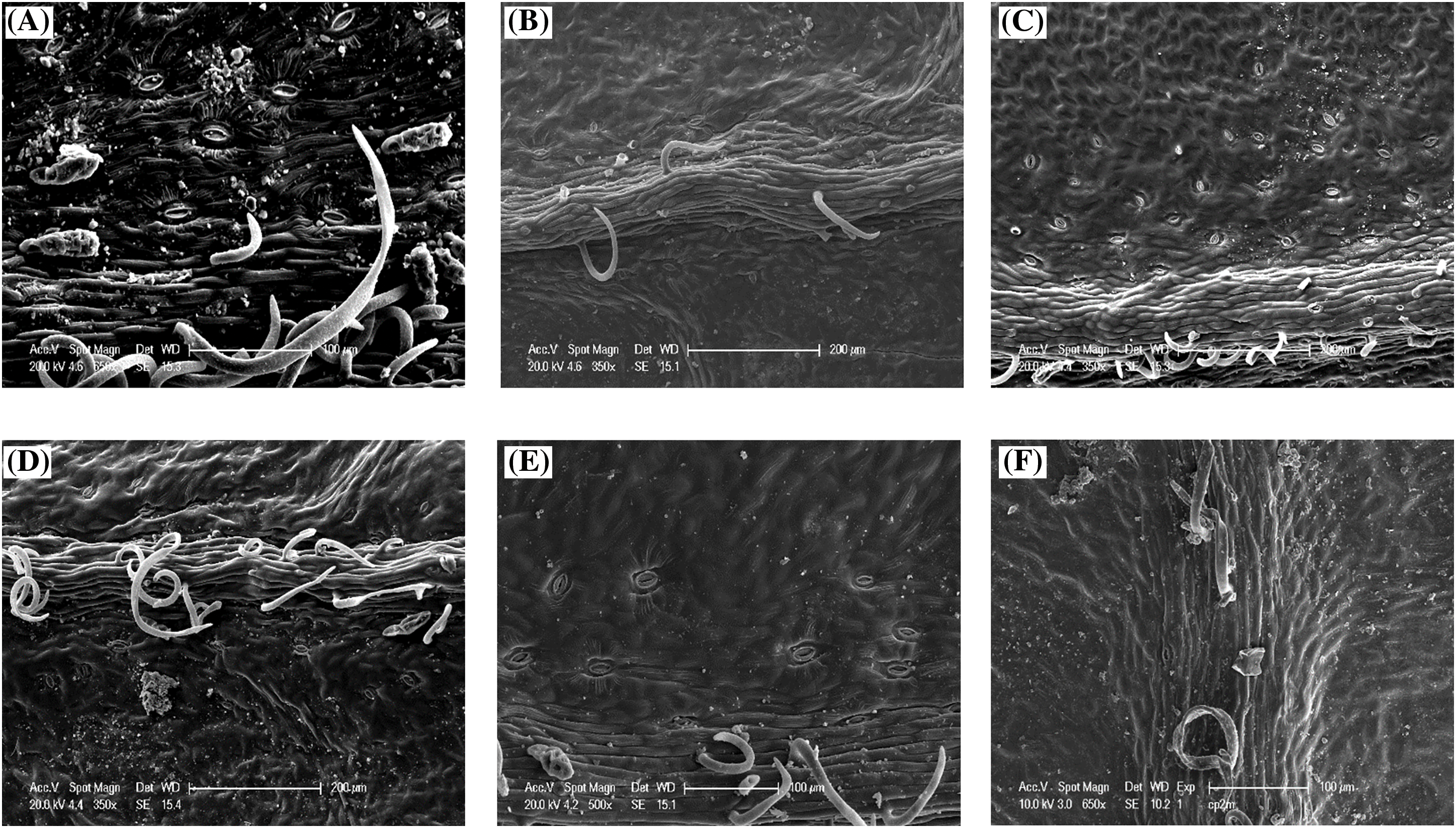
Figure 9: Scanning electron microscopy (SEM) images displaying stomatal density and distribution on the main drip of the adaxial leaf surface across various ecotypes of P. atlantica: (A) Messaad, (B) Guettia, (C) Elguedid, (D) Berrouaghia, (E) Aflou, (F) Bougaa
Leaves exhibit different densities among the 07 studied populations (Fig. 10). They are higher on the abaxial surface in Oz (408.83 st/mm2) and Bg (391 st/mm2), while stations (Msi) and (Bt) have the lowest values at 311.06 st/mm2 and 332.16 st/mm2, respectively. For the adaxial surface, the highest values are recorded at Tb (90.5 st/mm2) and Oz (84.33 st/mm2), whereas stations Bt (70 st/mm2) and Msi (43.70 st/mm2) have the lowest values (Table 4). ANOVA shows a significant difference at the α = 0.05 level between P. atlantica populations with p < 0.05 (Fig. 8), for stomatal density on the lower and upper surfaces. Similarly, the comparison of means at the α = 0.05 risk level reveals 5 distinct groups for stomatal density on the lower surface (Group 1: (Gt), (Tb), and (Dr), Group 2: (Tb), (Dr), and (Bg), Group 3: (Msi), Group 4: (Bt), and Group 5: (Oz)) and 4 groups for stomatal density on the upper surface (Group 1: (Gt), (Bg), (Dr), and (Oz), Group 2: (Bt), (Gt), and (Bg), Group 3: (Dr), (Oz), and (Tb), and Group 4: (Msi)) (Table 4).
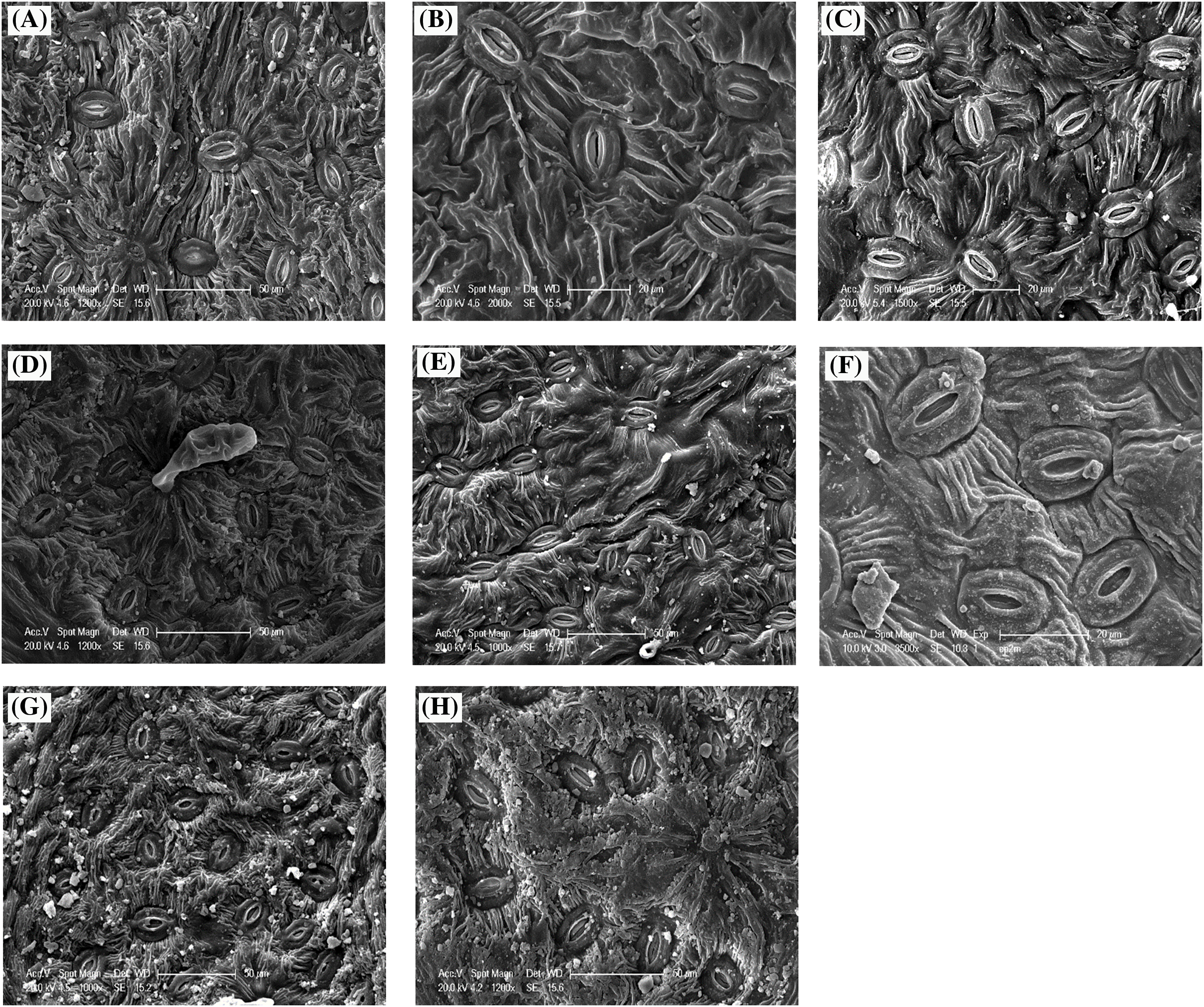
Figure 10: SEM micrographs illustrating the shape, distribution, and density of stomata on the abaxial leaf epidermis across various ecotypes of P. atlantica, (A) Messaad, (B) Guettia, (C) Elguedid, (D) Berrouaghia, (E) M’sila, (F) Bougaa, (G, H) Aflou
The values for stomatal length and width are higher in MT: length (24.72 ± 2.89 µm ♂; 24.32 ± 2.89 µm ♀) and width (15.65 ± 2.01 µm ♂; 15.25 ± 1.75 µm ♀). A signif-icant difference was recorded between MT and FT for these variables at the α = 0.05 level (Table 5; Fig. 11).
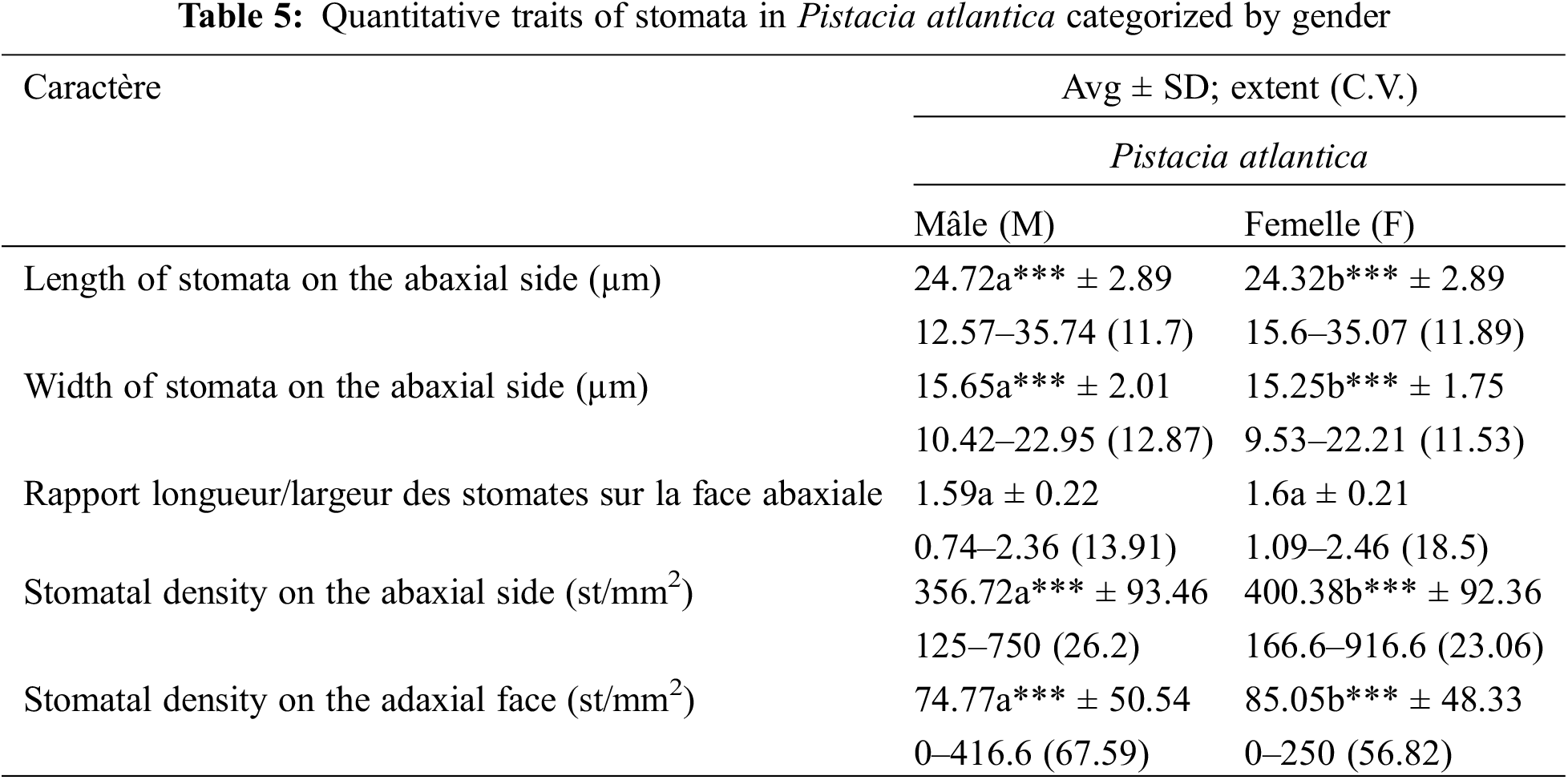
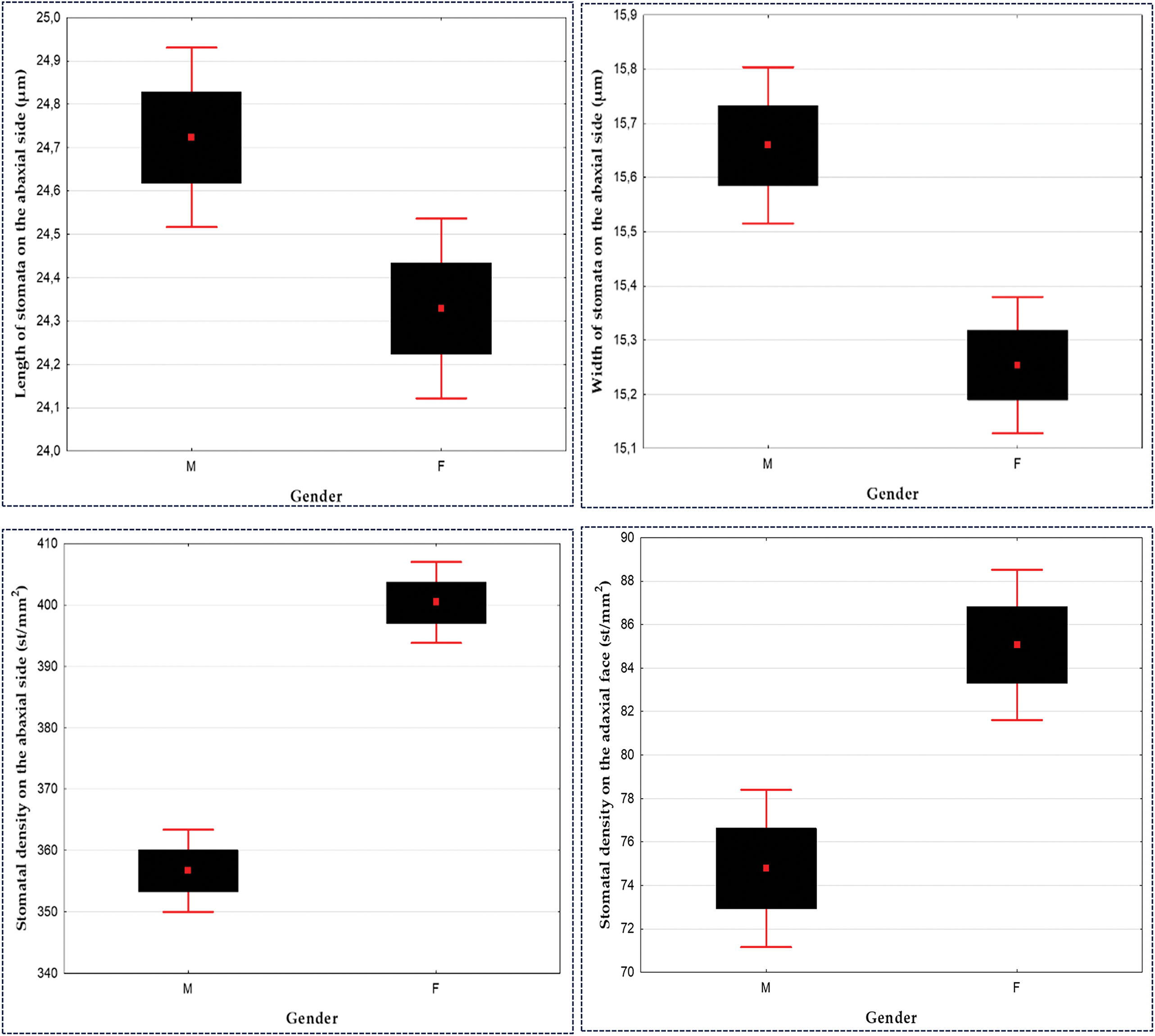
Figure 11: Influence of gender on stomatal size and density on the abaxial and adaxial surfaces of Pistacia atlantica, assessed using t-tests (α = 0.05)
Our results show that the leaves of FT have a higher stomatal density on the ab-axial surface (356.72 st/mm2 ♂ and 400.38 st/mm2 ♀) and adaxial surface (74.77 st/mm2 ♂ and 85.05 st/mm2 ♀) compared to male individuals. The t-test reveals a significant difference between MT and FT for stomatal density, at the α = 0.05 level (Table 5; Fig. 11).
Plant responses to drought vary depending on the adaptive mechanisms expressed by the plants, as they have adapted by implementing stress-adaptive strategies favoring drought tolerance and/or survival [17–20]. Most of these characteristics manifest in leaves as the primary organ for regulating water loss through transpiration.
At the aerial level, reducing water loss can be achieved through the regulation of stomatal opening or leaf surface area [18,21]. Thus, modifying the number of stomata [22,23] and/or reducing stomatal conductance [24–26] allows for the control of water fluxes. Additionally, reducing leaf size decreases the plant’s evaporative surface area [27]. Some species or populations develop leaf adaptations to protect leaf surfaces from intense solar radiation, creating a moister environment around stomata that slows water loss. These adaptations include wax accumulation on leaf cuticles [28], dense trichomes as seen in Olea europaea [29], or leaf rolling as observed in Oryza sativa [30]. Analyzing leaf morphology, especially epidermal features, has thus been necessary to better understand plant adaptive responses to fluctuating environmental conditions.
This study aimed to enhance our understanding of Pistacia species, particularly Pistacia atlantica, which remains poorly defined in terms of taxonomy and ecology. We focused on macro-and micro-morphological leaf traits to explore potential criteria for Pistacia species’ taxonomy and understand the observed intraspecific and inter-sexual heterogeneity and the adaptive processes they reveal among different P. atlantica populations located in various bioclimatic regions in Algeria. Unfortunately, intraspecific variability is often considered negligible compared to interspecific variability across broad species pools. However, numerous studies highlight the importance of considering intraspecific variability in functional traits to improve our understanding of plant community structure and distribution [31–36], which can be highly significant among populations of the same species along ecological gradients [37]. Similar studies regarding inter-population variability in Pistacia species have been reported for populations of different origins studied by various authors, in P. atlantica [38–41], and in P. lentiscus [11,13].
Regarding quantitative leaf macro-morphological traits, a strong correlation was observed between leaf and terminal leaflet biometrics for all P. atlantica populations. These results align with those of [42] in a study on morphological diversity among three Pistacia species in Turkey and [43] for P. atlantica. Indeed, they note strong correlations between leaf and leaflet dimension variables. Our results also reveal a positive correlation between leaf and terminal leaflet dimensions with the aridity index of collection sites; however, they are negatively correlated with altitude. Reference [44] similarly noted strong correlations between leaf dimensions, terminal leaflet length, and altitude, while the number of leaflets is negatively correlated with altitude.
In the present study, remarkable variability in quantitative leaf traits was observed among different ecotypes of P. atlantica. This could be due to individual sexes or environmental parameters (aridity and altitude). Indeed, in P. atlantica, leaves are longer at Oz (11.16 cm) (IA: 0.35; Altitude 779 m) and Bg (10.97 cm) (IA: 0.32; Alt. 659 m), and shorter at Aflou (Tb) (8.72 cm) (IA: 0.15; Alt. 1427 m) and Guetaya (Gt) (9.82 cm) (IA: 0.18; Alt. 1380 m). Therefore, the reduction in leaf size could be explained by the effect of aridity and altitude on plants, leading to a truly adaptive limitation of transpiring surfaces (leaves being the first organs exposed to water deficit) [45]. Altitude also impacts plant leaf surface, with climate changes as it rises. The air becomes colder and drier, affecting plant life accordingly. Many plants have adapted strategies to survive at high altitudes by reducing leaf surface to minimize water loss through transpiration and maintain a stable internal temperature. Our results align with [14], indicating that the smallest P. atlantica leaves were reported at higher stations rather than at the most arid ones.
We also found a significant difference between male and female individuals for the majority of quantitative leaf traits. Indeed, leaf and terminal leaflet dimensions are higher in female trees than in males for all P. atlantica populations.
However, no significant difference between the sexes in P. atlantica was observed for the number of leaflets and petiole length. This sex-related variability was also ob-served within P. atlantica populations. This sex-related variability was demonstrated in our study on the effect of individual sex on the morphological diversity of P. lentiscus leaves in a previous work [9]. This aligns with [46] results, who noted that leaf and terminal leaflet dimensions of female trees are slightly higher than those of males in P. atlantica. However, opposite trends are reported by [47] for P. lentiscus. These differences between male and female trees may be due to the energy needs of reproduction, as during fruit formation season, females require more energy than males [48]. According to [49], females develop adaptive strategies by keeping older leaves longer, as seen in P. lentiscus, or by increasing leaf surface area as in Siparuna grandiflora [50].
Regarding the macromorphological qualitative leaf traits, our study shows significant intraspecific heterogeneity for most studied variables. Leaf wings are a crucial character in identifying species of the Pistacia genus [2]. In this study, we observed that nearly half of P. atlantica leaves have green wings (narrow) along the rachis to the petiole (55.12%), and the other half only on the rachis (44.88%), with variable rates among populations (narrowly winged rachis and petiole) 62.45% (Tb), 63.63% (Oz), 79.33% (Dr), and 79.74 (Bg). However, the lowest percentage (19%) was found at station (Bt) with a lower aridity index (0.06). Our results for this variable are consistent with those of [10,39,40,43] for P. atlantica. According to [51], trees known as P. terebinthus in California could be a variety of P. atlantica, due to their high vigor and presence of a winged rachis.
Regarding leaf color, it ranges from green to dark green in P. atlantica, as confirmed by previous studies [14,42,52]. Depending on the origins, Oz (61.51%) and Bg (80.38%) with aridity indices (0.35, 0.32) and altitudes (779, 659 m) close to each other, have darker-colored leaves compared to other stations, while green color dominates in Djelfa Dr (76%), Gt (60%), and Bt (71.66%). Light green color was observed at very low rates. In terms of sex, the percentage of green color is nearly identical between male and female individuals, while the percentage of dark green color is higher in female leaves. The literature [14,42,52] reports a color range from green to dark green for this species, consistent with our results. Reference [53] demonstrated that under optimal conditions, the photosynthetic capacity of female Pistacia lentiscus is similar, if not higher, than that of males. However, under stress conditions, their photosynthetic capacity decreases compared to males. Therefore, Reference [54] noted that a high content of essential oils is present in female plants.
The majority of populations have petioles with a rounded and flattened shape on the adaxial surface (92.49%). Flat (5.89%) and round (1.61%) shapes were also observed. We found that the (Bt) origin had the lowest rate (79.66%) compared to other P. atlantica populations. These results are similar to those obtained by [41] in P. atlantica. Contrary to the flattened form reported by other authors [2,42,52,55], no difference was recorded between males and females for this species.
In our study, the size of the terminal leaflet compared to basal leaflets shows significant intraspecific variability. Indeed, its size relative to basal leaflets is either smaller, the same size, or larger. According to [2,42,52], the terminal leaflet is larger than basal leaflets in P. atlantica. However, Reference [43] found variable results for the same species, which align with our findings. As for its shape, it remains quite variable both between sexes and depending on the origin. Various shapes were observed, including narrow elliptical and lanceolate. These results are consistent with those recorded by [43] and [44], who observed a dominance of the lanceolate and narrow elliptical shapes in P. atlantica.
The apex of the leaf remains an important character for distinguishing species within the genus Pistacia [2]. In P. atlantica, it varies from acute, obtuse, mucronulate to Emarginate form (observed only in the Oz station). Eight forms of leaf apex in P. atlantica were observed in the study by [44]; for our study, six apex forms were recorded, among which (obtuse, acute, mucronulate, and acuminate) have been previously described [14,42,56]. The other forms (emarginate, retuse) have not been observed before, which constitutes new information for this species.
On the micromorphological level, all populations of P. atlantica present hairs with a remarkable density at the leaf margin (a line of microscopic cilia curved toward the apex); on the midrib, they are less dense on both leaf surfaces. We noticed a slight difference between males and females, with females having a higher adaxial hairiness. Additionally, observation using SEM allowed us to distinguish the presence of glandular capitate hairs across the leaf surface, as well as non-glandular trichomes of the ciliate type. Systematically speaking, the morphology and distribution of trichomes can provide important clues in the classification of species within the genus Pistacia [2,40,43,57]. According to [38], trichome distribution differs among varieties in P. atlantica, for example, they are present on both surfaces at the main vein level for the atlantica variety and on the entire lower surface for the cabulica variety, while they are absent in the kurdica variety [42,57]. According to [57], leaflets have a ciliate margin in the mutica variety and rarely ciliate in the kurdica variety.
Trichome density is also a characteristic influenced by ecological conditions. Altitude and minimum temperatures can play an important role in their distribution and density on leaves [43]. In arid environments, xeromorphic plants often have leaves covered with trichomes and wax. In this regard, [58] indicate that trichomes and waxes reduce the sedimentation rate of aphids and thus discourage herbivores in the presence of attractive food sources. Some hairs may also play a role in water absorption in semi-desert habitats [59–61]. This confirms our results regarding the presence of trichomes with very high intensity on the leaves of P. atlantica, which plays an important role in Algerian steppe and Saharan ecosystems.
Observation using SEM of the leaves allowed us to observe wax deposits of varied structure from one species to another [12]. In P. atlantica, a fine structure with flake deposits is observed (granular film with a powdery structure). In many xeromorphic plants, wax particles cover stomata to prevent water loss due to high radiation [62].
Stomata can also be an important character in species identification and taxonomy [63]. Our results showed that P. atlantica leaves are hypostomatic. They are more abundant on the abaxial surface; however, on the adaxial surface, we recorded rare stomata along the main and secondary veins. Reference [64] suggested that hypostomy is an evolutionary trait of Pistacia species and is considered a strongly xeromorphic trait.
Our results show a significant difference among the 7 populations studied for stomatal density on both surfaces, with the Oz (IA = 0.35), Bg (IA = 0.32), and Tb (Alt. 1427 m) stations having the highest abaxial stomatal density (408.83 st/mm2), (391 st/mm2), and (90.5 st/mm2) on the adaxial surface. On the other hand, the lowest abaxial and adaxial densities were observed at Msi (IA = 0.12) and Bt (IA = 0.06) with 311.06 and 332.16 st/mm2 for the abaxial surface and 43.70 and 70 st/mm2 for the adaxial surface, respectively. Our results also show that high-altitude stations have a high stomatal density on both surfaces for this species. Our data are in line with those of [65], who stated that increased stomatal density and reduced stomatal size correspond to better adaptation to water economy. Reference [64] suggested that this variation could be related to the ecological plasticity of Pistacia species across a wide range of environmental conditions. Indeed, our samples were collected from different sites with different climatic conditions (aridity and altitude). This demonstrates remarkable plasticity of this species, enabling it to survive in a very wide geographic distribution range. Similarly, significant variation was observed among the 10 provenances of P. lentiscus regarding this variable [13].
Regarding stomatal size on the abaxial surface, intra-specific and inter-sex variability was recorded. Indeed, the values of stomatal length and width on the abaxial surface vary from one population to another, with the Oz population (IA = 0.35) having the smallest length value and Tb (Alt. 1427 m and IA = 0.15) having the largest. Reference [66] indicated that adaptation to drought involves a decrease in stomatal size.
In this study, a significant difference between males and females was recorded for abaxial and adaxial stomatal size and density. We noticed that stomatal size is slightly higher in males, while females have the highest densities.
Studying the presence of Pistacia atlantica under different bioclimates in Algeria has led us to investigate its behavior and adaptive processes developed by this species in response to environmental constraints, particularly altitude, and aridity, through various macromorphological and micromorphological leaf traits.
This study has allowed us to highlight new characteristics in the studied species, such as the occurrence of waxes and glandular hairs on leaflets, sunken stomata in the epidermis, and stomatal shape. These criteria provide an important taxonomic tool for identifying species within the Pistacia genus. Additionally, highlighting certain xerophytic characteristics is an important asset considering the potential use of these species in new plantations to combat desertification, given their ability to withstand particularly harsh water-related conditions. Therefore, their rehabilitation and conservation are necessary to contribute to the sustainable development of arid areas.
Our study reveals a very significant divergence in the studied traits among different ecotypes of Pistacia atlantica. Genetic factors undoubtedly play a crucial role in this variability, but we have observed that environmental factors also have a remarkable impact on the heterogeneity of most measured leaf traits. Gender also plays a crucial role in this variability. We can summarize the main characteristics revealed for this species as follows:
- Leaf dimensions: 10.1 cm × 6.95 cm; 10.01 cm × 6.89 cm ♂; 10.18 cm × 7.01 cm ♀;
- Higher number of leaflets (9.04); imparipinnate leaves (83.87%);
- Terminal leaflet size: 3.66 cm × 1.09 cm and length/width ratio: 3.49;
- Petiole length: 2.24 cm; rounded and flattened on the upper surface;
- Leaf wings on the rachis only (44.88%), up to the petiole (55.12%);
- Entire leaf margin;
- Leaf color: green (53%) (53.70% ♂; 52.20% ♀), dark green (41.66%) (38.88% ♂; 44.81% ♀); narrow elliptical shape (48.89%) (51.99% ♂; 43.65% ♀) and lanceolate (32.26%) (28.5% ♂; 36.52% ♀);
- Terminal leaflet apex: acute shape (32.72%) (52.33% ♂; 50.77% ♀), obtuse (32.42%) (19.72% ♂; 21.63% ♀), or mucronulate (10.60%);
- Leaf pilosity concentrated at the margin and midrib of the adaxial surface, less concentrated on the abaxial and adaxial surfaces;
- Presence of 02 types of trichomes: glandular and non-glandular (ciliate type);
- Wax deposits with a finer structure;
- Hypostomatic leaves, with rare stomata on the adaxial surface located along the main and secondary veins;
- Abaxial stomatal density: (368.91 st/mm2); (356.72 st/mm2 ♂ and 400.38 st/mm2 ♀);
- Adaxial stomatal density: (74.74 st/mm2); (74.77 st/mm2 ♂ and 85.05 st/mm2 ♀);
- Stomatal size: (24.58 µm × 15.48 µm); length/width ratio (1.6); elliptical shape; slightly sunken in the epidermis.
Acknowledgement: The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R390), King Saud University, Riyadh, Saudi Arabia and la Direction Générale de la Recherche Scientifique et du Développement Technologique (DGRSDT), Algeria.
Funding Statement: This Research was funded by la Direction Générale de la Recherche Scientifique et du Développement Technologique (DGRSDT), CRAPAST, Algeria and the Researchers Supporting Project number (RSP2024R390), King Saud University, Riyadh, Saudi Arabia.
Author Contributions: Conceptualization: Abdelghafour Doghbage, Safia Belhadj, Arezki Derridj; Data curation: Abdelghafour Doghbage; Formal analysis: Abdelghafour Doghbage; Funding acquisition: Abdelghafour Doghbage, Fathi Abdellatif Belhouadjeb, Walid Soufan; Investigation: Abdelghafour Doghbage, Jean Philippe Mevy, Thierry Gauquelin, Alain Tonetto, Benbader Habib, Zahra Roba Bouabdelli; Methodology: Abdelghafour Doghbage, Safia Belhadj, Fathi Abdellatif Belhouadjeb; Project administration: Abdelghafour Doghbage, Safia Belhadj, Fathi Abdellatif Belhouadjeb, Arezki Derridj; Resources: Abdelghafour Doghbage, Safia Belhadj, Fathi Abdellatif Belhouadjeb, Hassen Boukerker, Jean Philippe Mevy, Thierry Gauquelin, Alain Tonetto, Benbader Habib, Zahra Roba Bouabdelli; Software: Abdelghafour Doghbage, Alain Tonetto; Supervision: Abdelghafour Doghbage, Safia Belhadj, Fathi Abdellatif Belhouadjeb, Arezki Derridj; Validation: Abdelghafour Doghbage, Safia Belhadj, Fathi Abdellatif Belhouadjeb, Hassen Boukerker, Jean Philippe Mevy, Thierry Gauquelin, Alain Tonetto, Arezki Derridj, Walid Soufan; Visualization: Abdelghafour Doghbage; Writing—original draft: Abdelghafour Ddoghbage, Safia Belhadj, Fathi Abdellatif Belhouadjeb; Writing—review & editing: Abdelghafour Doghbage, Fathi Abdellatif Belhouadjeb, Hassen Boukerker, Walid Soufan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All the data supporting the findings of this study are included in this article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Vincent WF. Effects of climate change on lakes. In: Likens GE, editor. Encyclopedia of Inland waters. Amsterdam: Elsevier; 2009. p. 55–60. doi:10.1016/B978-012370626-3.00233-7. [Google Scholar] [CrossRef]
2. Zohary MA. Monographical study of the genus Pistacia. Palest J Bot. 1952;5:187–228. [Google Scholar]
3. Yi T, Wen J, Golan-Goldhirsh A, Parfitt DE. Phylogenetics and reticulate evolution in Pistacia (Anacardiaceae). Am J Bot. 2008;95:241–51. doi:10.3732/ajb.95.2.241. [Google Scholar] [PubMed] [CrossRef]
4. AL-Saghir MG. Phylogenetic analysis of the genus Pistacia (Anacardiaceae). USA: Virginia Polytechnic Institute and State University; 2006. [Google Scholar]
5. AL-Saghir MG, Porter DM, Nilsen ET. Leaf anatomy of Pistacia species (Anacardiaceae). J Biol Sci. 2006;6:242–4. doi:10.3923/jbs.2006.242.244. [Google Scholar] [CrossRef]
6. Al-Saghir MG, Porter DM. Taxonomic revision of the genus Pistacia L. (Anacardiaceae). Am J Plant Sci. 2012;3:12–32. doi:10.4236/ajps.2012.31002. [Google Scholar] [CrossRef]
7. Ifticene-Habani N, Messaoudene M. Radial growth and sensitivity to climate of the Mount Atlas mastic tree, Pistacia atlantica Desf., in Algeria. Bois et Forêts des Trop. 2016;329:3–15. [Google Scholar]
8. Journal Officiel. Décret executif N°12-03 fixant la liste des espèces végétales non cultivés protégées [Executive decree No. 12-03 establishing the list of protected non cultivated plant species]. Available from: https://www.joradp.dz/FTP/JOFRANCAIS/2012/F2012003.pdf [Accessed 2023]. [Google Scholar]
9. Doghbage A, Belhadj S, Derridj A. Effect of gender on the leaf morphological diversity in Pistacia lentiscus. Options Méd Série A. 2016;119:193–6. [Google Scholar]
10. Doghbage A, Belhadj S, Boukerker H. Analyse éco-botanique comparative de deux populations du pistacia atlantica en Algérie par le biais de marqueurs morphologiques foliaires. Co Sav. 2018;25:119–26. [Google Scholar]
11. Doghbage A, Belhadj S, Derridj A, Mevy JP, Gauquelin T, Merdas S, et al. Comparative eco-botanical analysis of Pistacia lentiscus L. in Algeria through morphological and ultra-structural markers related to leaves and stomata. Agrobiologia. 2020;10(1):1826–36. [Google Scholar]
12. Doghbage A, Belhadj S, Gauquelin T, Greff S, Tonetto A, Derridj A, et al. Wax chemical composition and morphology in four Pistacia species from Algeria. Alg J Arid Env. 2021;11(2):17. [Google Scholar]
13. Doghbage A, Belhadj S, Belhouadjeb FA, Boukerker H, Mevy JP, Gauquelin T, et al. Leaf morphological and epidermal traits variability along an environmental gradients in ten natural populations of Pistacia lentiscus. Life. 2023;13(7):1617. doi:10.3390/life13071617. [Google Scholar] [PubMed] [CrossRef]
14. Belhadj S, Derridj A, Auda Y, Gers C, Gauquelin T. Analyse de la variabilité morphologique chez huit populations spontanées de Pistacia atlantica en Algérie. Botany. 2008;86:520–32. doi:10.1139/B08-008. [Google Scholar] [CrossRef]
15. Trabucco A, Zomer RJ. Global aridity index and potential evapotranspiration (ET0) climate database v2. CGIAR Consort Spat Inf. 2018;10:m9. [Google Scholar]
16. IPGRI. Descriptors for Pistacia spp. (excluding Pistacia vera L.). Rome, Italy: International Plant Genetic Resources Institute; 1998. [Google Scholar]
17. Bandurska H. Drought stress responses: coping strategy and resistance. Plants. 2022;11(7):922. doi:10.3390/plants11070922. [Google Scholar] [PubMed] [CrossRef]
18. Pamungkas SST, Farid N. Drought stress: responses and mechanism in plants. Rev Agr Sci. 2022;10:168–85. doi:10.7831/ras.10.0_168. [Google Scholar] [CrossRef]
19. Hasanuzzaman M, Tanveer M. Salt and drought stress tolerance in plants: signaling networks and adaptive mechanisms. Switzerland: Springer Nature; 2020. [Google Scholar]
20. Hashem HA, Mohamed AH. Strategies for drought tolerance in xerophytes. In: Plant ecophysiology and adaptation under climate change: mechanisms and perspectives I. Singapore: Springer; 2020. p. 269–93. [Google Scholar]
21. Gamez AL, Soba D, Zammareno AM, García-Mina JM, Aranjuelo I, Morales F. Effect of water stress during grain filling on yield, quality and physiological traits of Illpa and Rainbow Quinoa (Chenopodium quinoa Willd.) cultivars. Plant. 2019;8(173):1–15. [Google Scholar]
22. Driesen E, Van Den Ende W, De Proft M, Saeys W. Influence of environmental factors light, CO2, temperature and relative humidity on stomatal opening and development: a review. Agron J. 2020;10(1975):1–28. [Google Scholar]
23. Robertson BC, Han Y, Li C. A comparison of different stomatal density phenotypes of Hordeum vulgare under varied watering regimes reveals superior genotypes with enhanced drought tolerance. Plants. 2023;12(15):2840. doi:10.3390/plants12152840. [Google Scholar] [PubMed] [CrossRef]
24. Liao Q, Gu S, Kang S, Du T, Tong L, Wood JD, et al. Mild water and salt stress improve water use efficiency by decreasing stomatal conductance via osmotic adjustment in field maize. Sci Total Env. 2022;805:150364. doi:10.1016/j.scitotenv.2021.150364. [Google Scholar] [PubMed] [CrossRef]
25. Taiwo AF, Daramola O, Sow M, Semwal VK. Ecophysiology and responses of plants under drought. In: Plant ecophysiology and adaptation under climate change: mechanisms and perspectives I: general consequences and plant responses. Singapore: Springer; 2020. p. 231–68. doi:10.1007/978-981-15-2156-0_8. [Google Scholar] [CrossRef]
26. Bashir SS, Hussain A, Hussain SJ, Wani OA, Zahid Nabi S, Dar NA, et al. Plant drought stress tolerance: understanding its physiological, biochemical and molecular mechanisms. Biotech Biotech Equi. 2021;35(1):1912–25. doi:10.1080/13102818.2021.2020161. [Google Scholar] [CrossRef]
27. Sacita AS, June T, Impron I. Soybean adaptation to water stress on vegetative and generative phase. Agrotech J. 2018;3(2):1–11. [Google Scholar]
28. Li H, Mo Y, Cui Q, Yang X, Guo Y, Wei C, et al. Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and-susceptible watermelon genotypes. Plant Sci. 2019;278:32–43. doi:10.1016/j.plantsci.2018.10.016. [Google Scholar] [PubMed] [CrossRef]
29. Bacelar EA, Correia CM, Moutinho-Pereira JM, Gonçalves BC, Lopes JI, Torres-Pereira JM. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree phys. 2004;24(2):233–9. doi:10.1093/treephys/24.2.233. [Google Scholar] [PubMed] [CrossRef]
30. O’Toole JC, Cruz RT. Response of leaf water potential, stomatal resistance, and leaf rolling to water stress. Plant Physiol. 1980;65:428–32. doi:10.1104/pp.65.3.428. [Google Scholar] [PubMed] [CrossRef]
31. Jung V, Violle C, Mondy C, Hoffmann L, Muller S. Intraspecific variability andvtrait-based community assembly. J Ecology. 2010;98:1134–40. doi:10.1111/jec.2010.98.issue-5. [Google Scholar] [CrossRef]
32. Albert CH, Grassein F, Schurr FM, Vieilledent G, Violle C. When and how should intraspecific variability be considered in trait-based plant ecology? Perspect Plant Ecol, Ev Sys. 2011;13:217–25. doi:10.1016/j.ppees.2011.04.003. [Google Scholar] [CrossRef]
33. Violle C, Enquist BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, et al. The return of the variance: intraspecific variability in community ecology. Trends Eco Evol. 2012;27:244–52. doi:10.1016/j.tree.2011.11.014. [Google Scholar] [PubMed] [CrossRef]
34. Violle C, Reich PB, Pacala SW, Enquist BJ, Kattge J. The emergence and promise of functional biogeography. Proc Nat Aca Sci. 2014;111:13690–6. doi:10.1073/pnas.1415442111. [Google Scholar] [PubMed] [CrossRef]
35. Escudero A, Valladares F. Trait-based plant ecology: moving towards a unifying species coexistence theory: features of the special section. Oecologia. 2016;180:919–22. doi:10.1007/s00442-016-3578-5. [Google Scholar] [PubMed] [CrossRef]
36. Shipley B, De Bello F, Cornelissen JHC, Lalibert E, Laughlin DC, Reich PB. Reinforcing loose foundation stones in trait-based plant ecology. Oecologia. 2016;180:923–31. doi:10.1007/s00442-016-3549-x. [Google Scholar] [PubMed] [CrossRef]
37. Fajardo A, Piper FI. Intraspecific trait variation and covariation in a widespread tree species (Nothofagus pumilio) in southern Chile. New Phyto. 2011;189:259–71. doi:10.1111/nph.2010.189.issue-1. [Google Scholar] [CrossRef]
38. Alyafi J. Approches Systématiques et Ecologiques du Genre Pistacia dans la Région Méditerranéenne (Ph.D. Thesis). Faculté des Sciences et Techniques de Saint-Jérôme: Marseille, France; 1979. [Google Scholar]
39. Zohary M. The genus Pistacia L. In: Padulosi S, Caruso T, Barone E, editors. Taxonomy, distribution, conservation and uses of Pistacia genetic resources. In: Proceedings of the IPGRI Workshop, 29–30 June 1995; Palermo, Italy. 1996. p. 1–11. [Google Scholar]
40. Monjauze A. Connaissance du « betoum » Pistacia atlantica Desf. Biologie et forêt Rev For Fran. 1980;4:357–63. [Google Scholar]
41. Kafkas S, Perl-Treves R. Morphological and molecular phylogeny of Pistacia species in Turkey. Theor App Gen. 2002;102:908–15. [Google Scholar]
42. Kafkas S, Kafkas E, Perl-Treves R. Morphological diversity and a germplasm survey of three wild Pistacia species in Turkey. Genet Resour Crop Evol. 2002;49:261–70. doi:10.1023/A:1015563412096. [Google Scholar] [CrossRef]
43. Kafkas E. Etude Eco-Botanique de P. atlantica Desf. (Anacardiaceae) en Algérie Préalable à la Conservation des Ressources Génétiques de L’espèce et Sa Valorisation. (Ph.D. Thesis). Université Mouloud Mammeri de Tizi Ouzou: Algeria; 2007. [Google Scholar]
44. El Zerey-Belaskri E, Benhassaini H. Morphological leaf variability in natural populations of Pistacia atlantica Desf. subsp. atlantica along climatic gradient: new features to update Pistacia atlantica subsp. atlantica key. Int J Bio. 2016;60(4):577–89. doi:10.1007/s00484-015-1052-4. [Google Scholar] [PubMed] [CrossRef]
45. Rini DS, Budiarjo, Gunawan I, Agung RH, Munazar R. The mechanism of plant response to drought stress. J Ilmu-Ilmu Hay LIP. 2020;19(3B):373–84. [Google Scholar]
46. Mehdeb D. Etude de la variabilité morphologique du pistachier de l’atlas (Pistacia atlantica Desf.) dans la région de Tiaret (Mémoire de Magister). Université d’Oran1: Algeria; 2012. [Google Scholar]
47. Barazani O, Dudai N, Golan-Goldhirsh A. Comparison of mediterranean Pistacia Lentiscus genotypes by random amplified polymorphic DNA, chemical, and morphological analyses. J Chem Eco. 2003;29(8):1939–52. doi:10.1023/A:1024862614345. [Google Scholar] [PubMed] [CrossRef]
48. Zluvova J, Zak J, Janousek B, Vyskot B. Dioecious Silene latifolia plants show sexual dimorphism in the vegetative stage. BMC Plant Biol. 2010;10(1):1–5. [Google Scholar]
49. Jonasson S, Medrano H, Flexas J. Variation in leaf longevity of Pistacia lentiscus and its relationship to sex and drought stress inferred from leaf δ13C. Funct Eco. 1997;11(3):282–9. doi:10.1046/j.1365-2435.1997.00090.x. [Google Scholar] [CrossRef]
50. Nicotra AB, Chazdon RL, Montgomery RA. Sexes show contrasting patterns of leaf and crown carbon gain in a dioecious rainforest shrub. Am J Bot. 2003;90(3):347–55. doi:10.3732/ajb.90.3.347. [Google Scholar] [PubMed] [CrossRef]
51. Ayfer M, Serr EF. Effects of gibberellin and other factors on seed germination and early growth in Pistacia species. Proc Ame Soc Hort Sci. 1961;77:308–15. [Google Scholar]
52. Yaltirik F. Pistacia L. In: Davis PH, editor. Flora of Turkey and the east Aegean Islands. Scotland: Edinburgh University Press; 1967. vol. 2, p. 542–8. [Google Scholar]
53. Correia O, Diaz Barradas MC. Ecophysiological differences between male and female plants of Pistacia lentiscus L. Plant Eco. 2000;149(2):131–42. doi:10.1023/A:1026588326204. [Google Scholar] [CrossRef]
54. Gourine N, Sifi IM, Gaydou E, Yousfi M. Chemical composition of the essential oil of unripe galls of Pistacia atlantica Desf. from Algeria. Nat Prod J. 2011;1(2):125–7. [Google Scholar]
55. Choulak S, Chatti K, Rhouma S. Recent advances in genomics, conservation, and breeding of pistachio. Tree Genet Genome. 2023;19(5):40. doi:10.1007/s11295-023-01615-9. [Google Scholar] [CrossRef]
56. Quezel P, Santa S. Nouvelle flore de l’Algérie et des régions désertiques méridionales. Paris, France: National Centre for Scientific Research (CNRS); 1963. p. 571–1170 (In Spanish). [Google Scholar]
57. Behboodi BS. Pistacia atlantica Desf. 1800 in Iran. NUCIS Newsl. 2004;12:27–9. [Google Scholar]
58. Agrawal AA. Plant defense and density dependence in the population growth of herbivores. Am Nat. 2004;164(1):113–20. doi:10.1086/420980. [Google Scholar] [PubMed] [CrossRef]
59. Hernandez JO, Park BB. The leaf trichome, venation, and mesophyll structural traits play important roles in the physiological responses of oak seedlings to water-deficit stress. Int J Mol Sci. 2022;23(15):8640. doi:10.3390/ijms23158640. [Google Scholar] [PubMed] [CrossRef]
60. Steyn HM, Van Wyk AE. Taxonomic significance of trichomes in the genus Acanthopsis Harv (Acanthaceae, tribe Acantheae). Adanso. 2021;43(14):163–76. [Google Scholar]
61. Waseem M, Nie ZF, Yao GQ, Hasan M, Xiang Y, Fang XW. Dew absorption by leaf trichomes in Caragana korshinskii: an alternative water acquisition strategy for withstanding drought in arid environments. Physi Plant. 2021;172(2):528–39. doi:10.1111/ppl.v172.2. [Google Scholar] [CrossRef]
62. Peguero-Pina JJ, Vilagrosa A, Alonso-Forn D, Ferrio JP, Sancho-Knapik D, Gil-Pelegrín E. Living in drylands: functional adaptations of trees and shrubs to cope with high temperatures and water scarcity. Forests. 2020;11(10):1028. doi:10.3390/f11101028. [Google Scholar] [CrossRef]
63. Butt MA, Zafar M, Ahmad M, Kayani S, Bahadur S, Ullah F, et al. The use of taxonomic studies to the identification of wetlands weeds. Adv Weed Sci. 2021;2021(39):e222645. [Google Scholar]
64. Al-Saghir M, Porter DM. Stomatal distribution in Pistacia species (Anacardiaceae). Int J Bot. 2005;1:183–7. doi:10.3923/ijb.2005.183.187. [Google Scholar] [CrossRef]
65. Aussenac G. Effets de conditions microclimatiques différentes sur la morphologie et la structure anatomique des aiguilles de quelques résineux. Ann Sci For. 1973;30(4):375–92 (In Spanish). doi:10.1051/forest/19730401. [Google Scholar] [CrossRef]
66. De Micco V, Aronne G. Morpho-anatomical traits for plant adaptation to drought. In: Plant responses to drought stress: from morphological to molecular features. Berlin/Heidelberg, Germany: Springer; 2012. p. 37–61. doi:10.1007/978-3-642-32653-0_2. [Google Scholar] [CrossRef]
Appendix A

Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools