 Open Access
Open Access
ARTICLE
Phytochemical and Pharmacological Research in Galenic Remedies of Solidago canadensis L. Herb
1 Department of Pharmaceutical Management, Drug Technology and Pharmacognosy, Ivano-Frankivsk National Medical University, Ivano-Frankivsk, 61002, Ukraine
2 Institute of Pharmacy, Faculty of Medicine, University of Tartu, Tartu, 50411, Estonia
3 Department of Pharmacognosy and Nutriciology, National University of Pharmacy, Kharkiv, 61002, Ukraine
4 Institute of Chemistry, Faculty of Science and Technology, University of Tartu, Tartu, 50411, Estonia
5 Institute of Pharmaceutical Technologies, Lithuanian University of Health Sciences, Kaunas, 44307, Lithuania
* Corresponding Author: Ain Raal. Email:
Phyton-International Journal of Experimental Botany 2024, 93(9), 2303-2315. https://doi.org/10.32604/phyton.2024.055117
Received 17 June 2024; Accepted 23 August 2024; Issue published 30 September 2024
Abstract
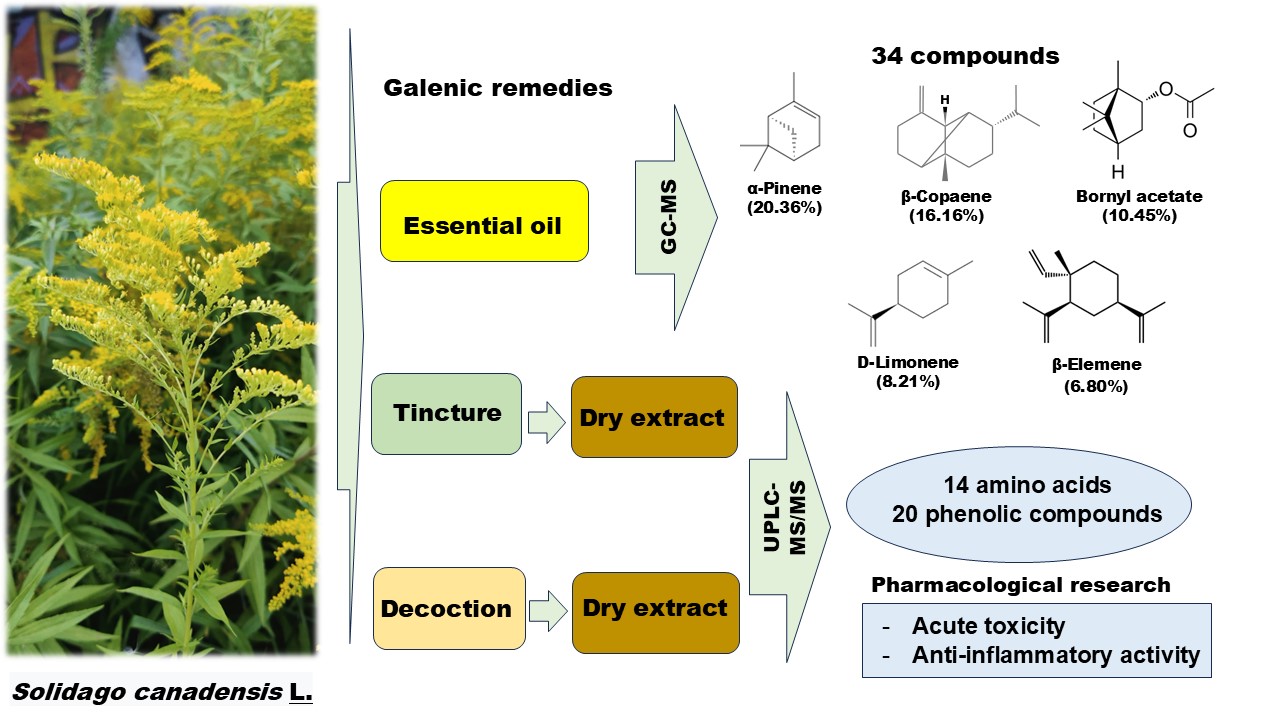
Canadian goldenrod (Solidago canadensis L.) is a rhizomatous plant of the Asteraceae family. In folk medicine, Solidago galenic remedies are used for diseases of the kidneys, urinary tract, liver, etc. Externally, goldenrod is used to treat purulent wounds, furunculosis, and gum abscesses as washes and compresses. The aims of this research were to study the yield and chemical composition of essential oil (EO), the anti-inflammatory activity of S. canadensis dry extracts based on its decoction and tincture. In EO (2.8 mL/kg) of S. canadensis were identified and quantified 34 compounds. The principal compounds of the EO from flowering tops of S. canadensis were α-pinene (20.36%), β-copaene (16.16%), bornyl acetate (10.45%), D-limonene (8.21%), and β-elemene (6.80%). In the S. canadensis dry extracts were identified and quantified 20 phenolics (10 flavonoids, 8 hydroxycinnamic acids and 2 phenolic acids) and 14 amino acids, 7 of which are essential. The dominant hydroxycinnamic acids were neochlorogenic and chlorogenic acids, and 4,5-dicaffeoylquinic, 3,5-dicafeylquinic and 3,4-dicafeylquinic acids. The main flavonoids were rutin and isoquercitrin. The main amino acids (more than 1 mg/g) were proline, histidine, serine, alanine, aspartic acid, lysine and glutamic acid. The extracts of S. canadensis were characterized as practically non-toxic substances (toxicity class V). The extracts act on the exudative phase of inflammation. The antiexudative effect of the dry aqueous-alcohol S. canadensis extract was 23.59%, and for the aqueous one –19.26%. The dry aqueous-alcohol S. canadensis extract showed promising anti-inflammatory activity.Graphic Abstract
Keywords
Solidago canadensis L. is a perennial rhizomatous plant that belongs to Asteraceae family. In the Solidago genus there are about 190 species and 330 taxons [1]. In Europe, both S. canadensis L. and S. virgaurea L. are the most widely spread species and have a long tradition of use in folk and official medicine [2,3]. They are considered as invasive weeds [2,4].
Thus, S. canadensis is a very common species and can be found everywhere because of its wide and rapid distribution. It is undemanding to soils but develops better on relatively heavy and rich soils with average moisture. It is also easily cultivated, so the raw material base of this plant is significant. Considering the long and successful use of folk medicine in many countries, it is interesting to expand scientific knowledge, and its wider use in official medicine is promising.
The Canadian goldenrod’s remedies are implemented for the treatment of cystitis, chronic nephritis, and urolithiasis, and as an antiphlogistic drug [5,6] and as a mouth rinse in the treatment of inflammations of the mouth and throat [7,8]. The antibacterial, antioxidative, anti-inflammatory and antimutagenic effects have been proved for Solidago remedies and are connected to flavonoids, terpenoids, and saponins [9,10].
S. canadensis essential oil (EO) were studied previously and their chemical composition was presented in some publications [5,7,11,12]. Germacrene D, α-pinene, β-elemene, limonene, and bornyl acetate predominated [13,14].
The European Pharmacopoeia monograph “Solidago herba” regards flavonoids as quality markers, and there must be at least 0.5% and a maximum of 1.5% in the calculation to hyperoside [15]. The Solidago flavonoids are represented by quercetin and kaempferol glycosides [16–18]. The S. canadensis herb also contains polyphenolic acids (vanillic, gallic, ferulic, caffeic, and chlorogenic acids) [19] and oleanane-type triterpene saponins [1,5]. Considering the rich composition of phenolic compounds, especially flavonoids and hydrocinnamic acids, using S. canadensis raw material in inflammatory urinary tract diseases, possibly in prostate adenoma and chronic prostatitis, is promising.
In Ukraine dry extracts of S. canadensis are part of complex medicines: Marelin, Phytolysin, Prostamed [20]. In folk medicine, Solidago galenic remedies are used for diseases of the urinary tract, kidneys, liver, etc. Externally, it also helps to treat purulent wounds, gum abscesses and furunculosis [21,22]. Considering this, it is advisable to investigate the chemical composition and pharmacological activity of the main galenic remedies of S. canadensis herb to establish the prospects of their use for developing modern dosage forms and implementation in medical and pharmaceutical practice.
This research aimed to study the chemical composition of essential oil, phenolic compounds, amino acids and the anti-inflammatory activity of S. canadensis dry extracts based on its decoction and tincture for use as promising agents in medicinal and pharmaceutical practice. As S. virgaurea is the most well-studied species, S. canadensis can be considered as its analogue after obtaining this new knowledge. To the best of our knowledge, this work is the first to analyze the amino acid content of S. canadensis herb and investigate the anti-inflammatory activity of this plant extract.
The flowering tops of S. canadensis were harvested in Tartu (58.36277085085124, 26.747175570884128) in July 2023 and dried for 14 days at room temperature in a well-ventilated area. The plant species were identified by Professor Andriy Grytsyk according to the botanical catalogue [23]. Voucher specimens No. 455–457 were deposited at the Department of Pharmaceutical Management, Drug Technology and Pharmacognosy, Ivano-Frankivsk National Medical University (IFNMU, Ivano-Frankivsk, Ukraine). It was stored in paper bags.
The EO hydrodistilled from the dried flowering tops of S. canadensis using the method described in the European Pharmacopoeia [15]. It lacks a verb in the yellow-shaded sentence.
The flowering tops of S. canadensis (20 g) with 400 mL of purified water were distilated in a 1000 mL round-bottom flask during 2 h (3–4 mL/min). Hexane (0.5 mL) was added to a graduated tube to remove the distilled oil.
100.0 g of the S. canadensis herb was filled with 1000 mL of water, heated to 100°C for 15 min and macerated overnight. The liquid extract was filtrated and evaporated with a vacuum rotary evaporator and finally dried in the lyophilic dryer Scanvac Coolsafe 55-4 Pro (LaboGene ApS, Lillerød, Denmark).
100.0 g of the S. canadensis herb was filled with 70% ethanol (1000 mL) and macerated over 7 days at room temperature. The liquid extract was separated by filtration and evaporated with a vacuum rotary evaporator and finally dried with the same lyophilic dryer.
2.2 Gas Chromatography/Mass Spectrometry
The samples of EO were analyzed by gas chromatography with mass detections (GC/MS), using an Agilent 6890/5973 GCMS system controlled by mass spectrometry detectors (MSD) Chemstation. 1 µL of the sample was injected at an injector temperature of 280°C in split mode (150:1), using He as the carrier gas onto Agilent HP-5MSI column (30 m length, 0.25 mm inner diameter, 0.25 µm film thickness). The carrier gas was held at the constant flow rate of 1 mL/min. The oven was held at 50°C for 2 min, followed by a ramp of 4 °C/min to a final temperature of 280°C and held at 280°C for 5 min.
The MSD was operated in EI mode at 70 eV. Mass spectra were recorded in the range of 29–400 m/z with a delay time of 4 min and a scan speed of 3.8 scans per second. The data were analyzed by the deconvolution algorithm of Agilent Masshunter Software package using different window size factors. Obtained compounds were identified by using NIST20 library with Match Factor ≥90 and by retention indexes (relative to n-alkanes C8–C20) either made available in the literature [7,14] or obtained by the analysis of the reference compounds. The area percentages of each peak were calculated from the total areas in the chromatograms without using correction factors.
2.3 Spectrophotometric Analysis of Phenolic Compounds
The Spectrophotometric assay of sum of hydroxycinnamic acids, polyphenols, and flavonoids in the dry S. canadensis herb extracts was conducted with Shimadzu UV-1800 (Shimadzu Corporation, Kyoto, Japan). The sum of hydroxycinnamic acids was measured in calcilation to chlorogenic acid after a reaction with sodium molybdate and sodium nitrite at 525 nm [15]. The sum of flavonoids was determined regarding rutin after a reaction with aluminium chloride at 417 nm [24]. The content of total polyphenolic compounds was assayed in terms of gallic acid at 270 nm [25]. The repeatability of the experiments was three times for statistical validity.
2.4 UPLC-MS/MS Analysis of Phenolic
Identification and quantification of phenolic compounds in the S. canadensis extracts was established by UPLC-MS/MS. on Acquity H-class UPLC chromatograph (Waters, Milford, MA, USA) with column YMC Triart C18 (100 mm × 2.0 mm 1.9 µm) at such conditions: column temperature –40°C; the mobile phase flow rate 0.5 mL/min.
Solvent A was formic acid (0.1% aqueous solution). Gradient elution was: solvent B (pure acetonitrile) from 0 to 1 min at 5%, 1 to 5 min. to 30% of solvent B, 5 to 7 min., linear decrease to 50%, 7.5 to 8 min. just solvent B, and 8.1 to 10 min balance column to initial conditions of 5% of solvent B. The chemical structure analysis of phenolics was performed with a triple quadrupole tandem mass spectrometer (Xevo, Waters, USA). Negative electrospray ionization [ESI] was used to create ions and receive MS/MS data. The MS/MS analysis were carried out at such conditions: a capillary voltage 2 kV, the nitrogen gas temperature 400°C, a flow rate 700 L/h, the gas flow rate –20 L/h, and the ion source temperature 150°C. The phenolic compounds was determined by comparing their MS/MS data and retention times with the standards and using standard dilution method and linear regression fit models [26,27].
2.5 UPLC-MS/MS Analysis of Amino Acids
Aa assay of amino acids in the S. canadensis extracts was conducted on Acquity H-class (Waters, Milford, MA, USA) UPLC system with mass spectrometerXevo TQD (Waters, Milford, MA, USA) using a BEH Amide (150 mm × 2.1 mm, 1.7 µm) column (Waters, Milford, MA, USA) at 25°C. The mobile phase consist of eluent A (10 mmol ammonium formate with 0.125% formic acid) and eluent B (acetonitrile) and was used at such conditions: 0 to 1 min. 95% B; 1–3.9 min, 70% B; 3.9–5.1 min, 30% B; 5.1–6.4 min, the column was washed with eluent A, 70%; at 6.5 to 10 min. the initial composition was used. The mobile phase flow rate was 0.6 mL/min. For the analysys 1 µL of the extracts were used. The MS/MS analysis were carried out at such conditions: positive electrospray ionization + 3.5 kV, cone voltage 30 V, desolvation gas flow 800 L/h, and temperature 400°C, the ion source temperature 120°C. The assay of amino acids was determined by comparing their MS/MS data and retention times with the standards and using linear regression fit models [28].
2.6 The Acute Toxicity of the Extracts
A study of the acute toxicity of the S. canadensis extracts herb was carried out according to the methodology of preclinical studies of the harmlessness of medicinal products [29]. The mice were divided into 3 groups of 6 animals each: Group 1–the intact group of mice, who consumed purified water; Group 2–the mice consumed the dry aqueous extract of S. canadensis; Group 3–the mice consumed the dry extract of S. canadensis, obtained with 70% ethanol solution. Animals were under surveillance for 14 days. The degree of extracts’ toxicity was considered based on the mortality rate and changes in the general condition of animals. The toxicity class was established according to the generally accepted classification [29]. The study used non-linear white mice that were born and raised in the IFNMU’s vivarium.
2.7 Anti-Inflammatory Activity of the Extracts
The anti-inflammatory activity of the S. canadensis extracts was determined using a model of formalin oedema on rats [24,29]. The diclofenac sodium was a reference drug and its recommended dose is 0.8 mg [29]. The concentration of phenolic substances in the S. canadensis extract is around 12%, so, considering this, 10 mg of the extracts were used for analysis, as a comparable dose to the reference medicine. The study used non-linear white rats that were born and raised in the IFNMU’s vivarium.
All practical data is processed using variational statistics, which calculates the arithmetic mean and standard deviation. The reliability of the compared values was assessed according to the Student’s test, and the probability level was accepted as p ≤ 0.05. Statistical processing of the obtained results was carried out using a package of Windows application programs-MS Excel 2007 according to the methodology of the State Pharmacopoeia of Ukraine [30,31].
S. canadensis EO was an oily liquid with a slightly yellowish tint, yielding 2.80 mL/kg on dry mass. The yield of the dry aqueous extract was 22.77% and the dry aqueous-alcohol extract was 16.82%. The dry S. canadensis aqueous extract was a light brown powder with a specific smell.
Using GC-MS, the S. canadensis EO was analyzed (Table 1, Fig. 1).
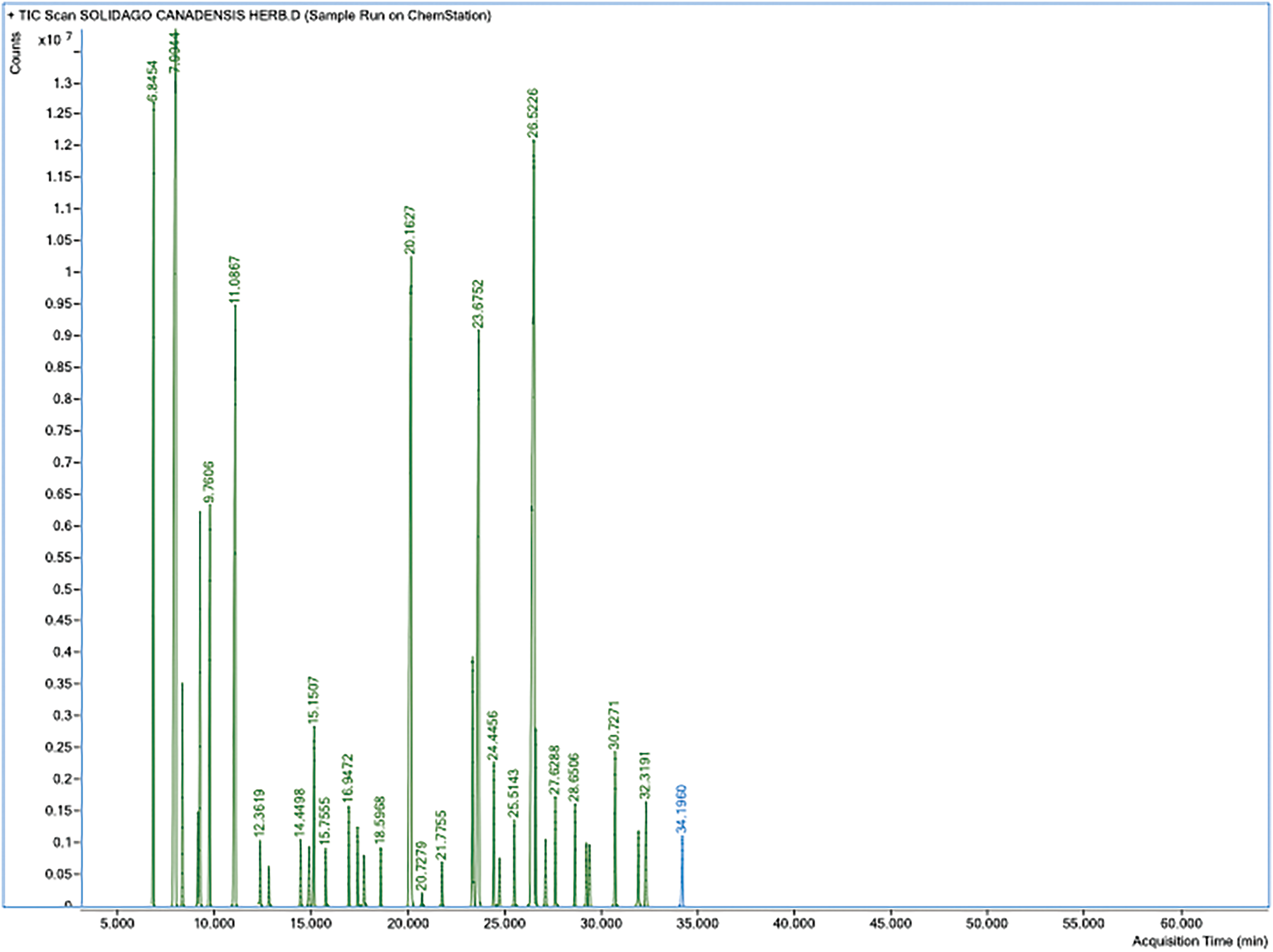
Figure 1: The GC-MS chromatogram of the essential oil from S. canadensis flowering herb with retention times. The main compounds (>5%): 7.99–α-pinene, 11.09–D-limonene, 20.16–bornyl acetate, 23.67–β-elemene, 26.52–β-copaene isomer (other in Table 1)
In total, 34 components were isolated in the studied EO, which makes up 86.01% of all components found in the oil studied. The concentrations of most unidentified components were only in the 0.01%–0.20% range. The principal compounds of the EO from flowering tops of S. canadensis were α-pinene (20.36%), β-copaene (16.16%), bornyl acetate (10.45%), D-limonene (8.21%), and β-elemene (6.80%). Pinene and limonene have been mentioned among the main constituents of S. canadensis EO in previous studies [5,11,33]. At the same time, the contents of β-cubenene and germacrene D showed the highest concentrations, respectively. Germacrene D is also found to be one of the main compounds in the oil of S. virgaurea [4]. In the comparative study of four Solidago species, α-pinene, germacrene D, bornyl acetate, and E-verbenol were mentioned as principal compounds of EOs [7]. The contents of S. canadensis and S. virgaurea oils showed more similar concentrations of main compounds than the EOs distilled from S. × niederederi and S. gigantea. Thus, the results of our study are rather similar to the already published papers mentioned below.
The research in phenolics was carried out by UPLC-MS/MS and spectrophotometry (Table 2, Fig. 2).
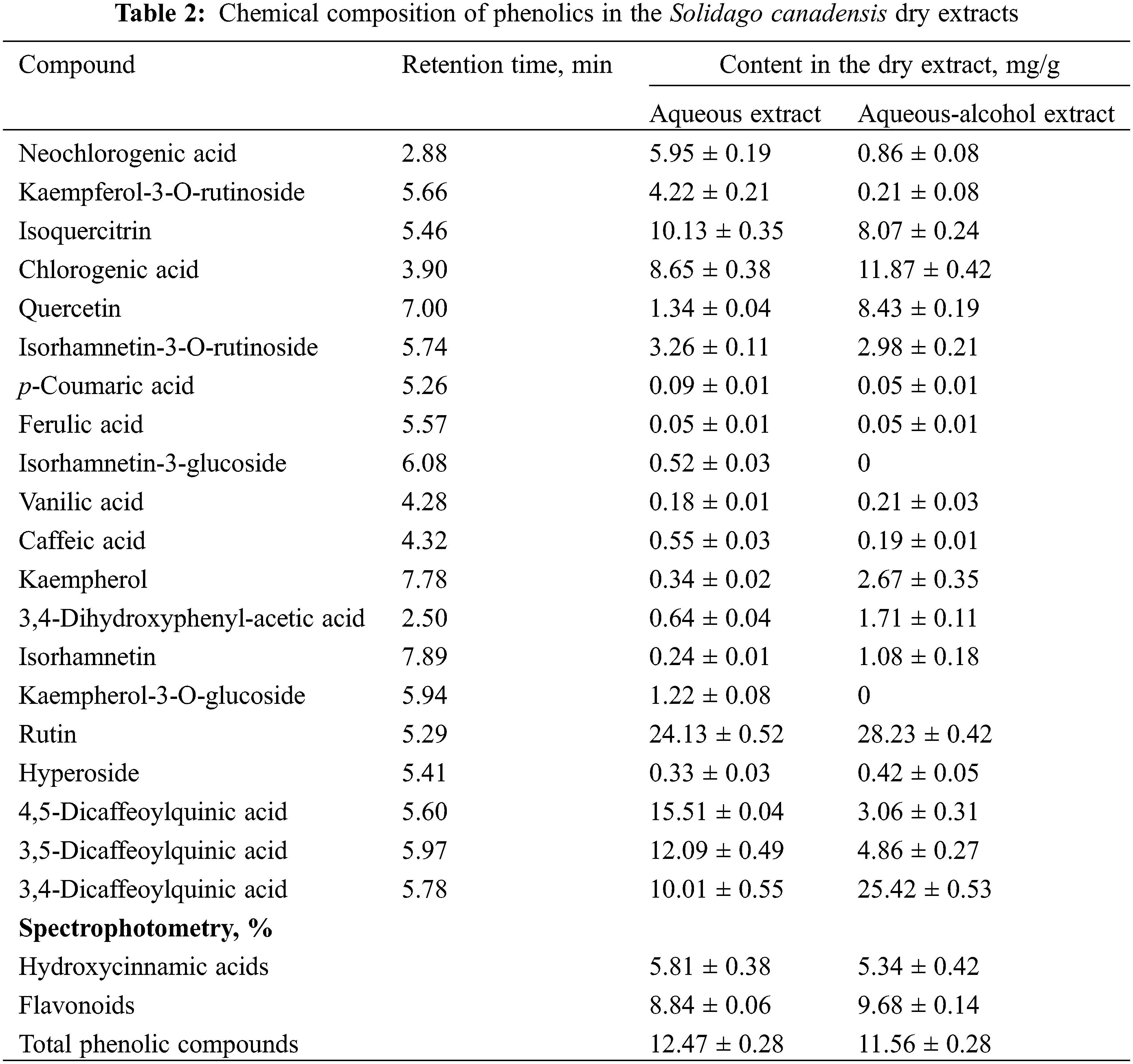
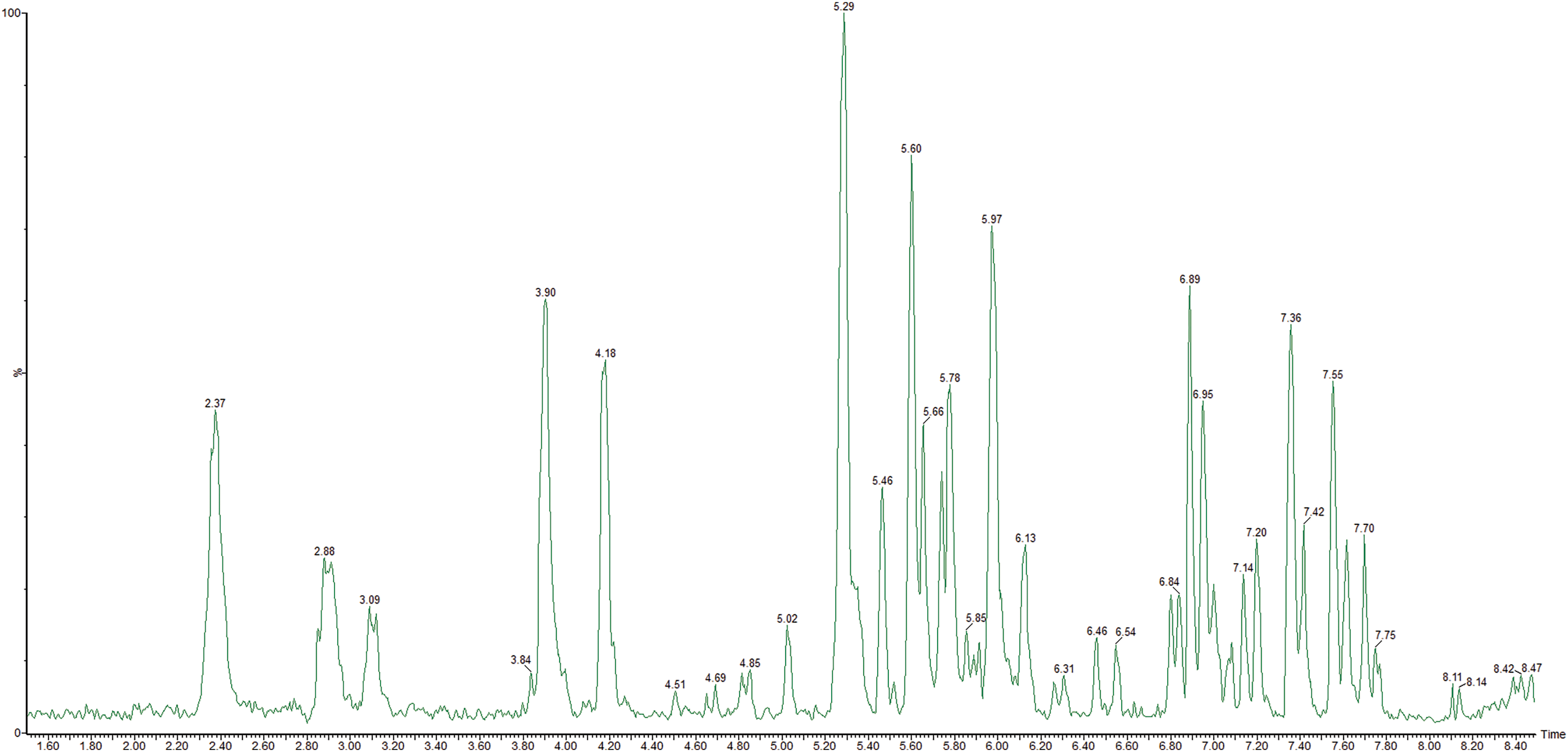
Figure 2: UPLC-MS negative scan chromatogram of Solidago herb sample (Aqueous-alcohol extract). The main compounds (>10%): 5.29–rutin, 5.46–isoquercitrin, 5.60–4,5-dicaffeoylquinic acid, 5.78–3,4-dicaffeoylquinic acid, 5.97–3,5-dicaffeoylquinic acid (other in Table 2)
Totally in the S. canadensis, dry extracts were identified and quantified 20 phenolics (2 phenolic and 8 hydroxycinnamic acids, and 10 flavonoids): 20 compounds in the aqueous extract and 19 in the aqueous-alcohol extract. The dominant hydroxycinnamic acids are neochlorogenic and chlorogenic acids, 4,5-dicaffeoylquinic, 3,5-dicaffeoylquinic and 3,4-dicaffeoylquinic acids. The main flavonoids were rutin and isoquercitrin. Previously, it was reported that in S. canadensis predominated flavonols such as quercetin and its glycosides, kaempferol and its glycosides were also found in a significant amount [16]; the data about quercetin compounds corresponded to our results, but the content of kaempferol derivatives is quite lower. It was reported that caffeoylquinic acid esters such as 5-O-caffeoylquinic acid (neochlorogenic acid) accompanied by several mono-, di-caffeoylquinic and feruoylquinic acids also predominated among phenolic compounds [16]. Still, in our extracts, the major compounds were 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, chlorogenic acid (in order of increasing concentrations), and ferulic acid derivatives were not detected. The European Pharmacopoeia monograph for Solidago herba regards flavonoids in terms of hyperoside as quality markers [15], but rutin is predominated, and a high content of hydroxycinnamic acids is observed. Therefore, in the standardising of the dry extracts, it’s advisable to consider these two groups of biologically active substances.
The study results on the amino acid composition of the S. canadensis dry extracts are presented in Table 3 and Fig. 3.
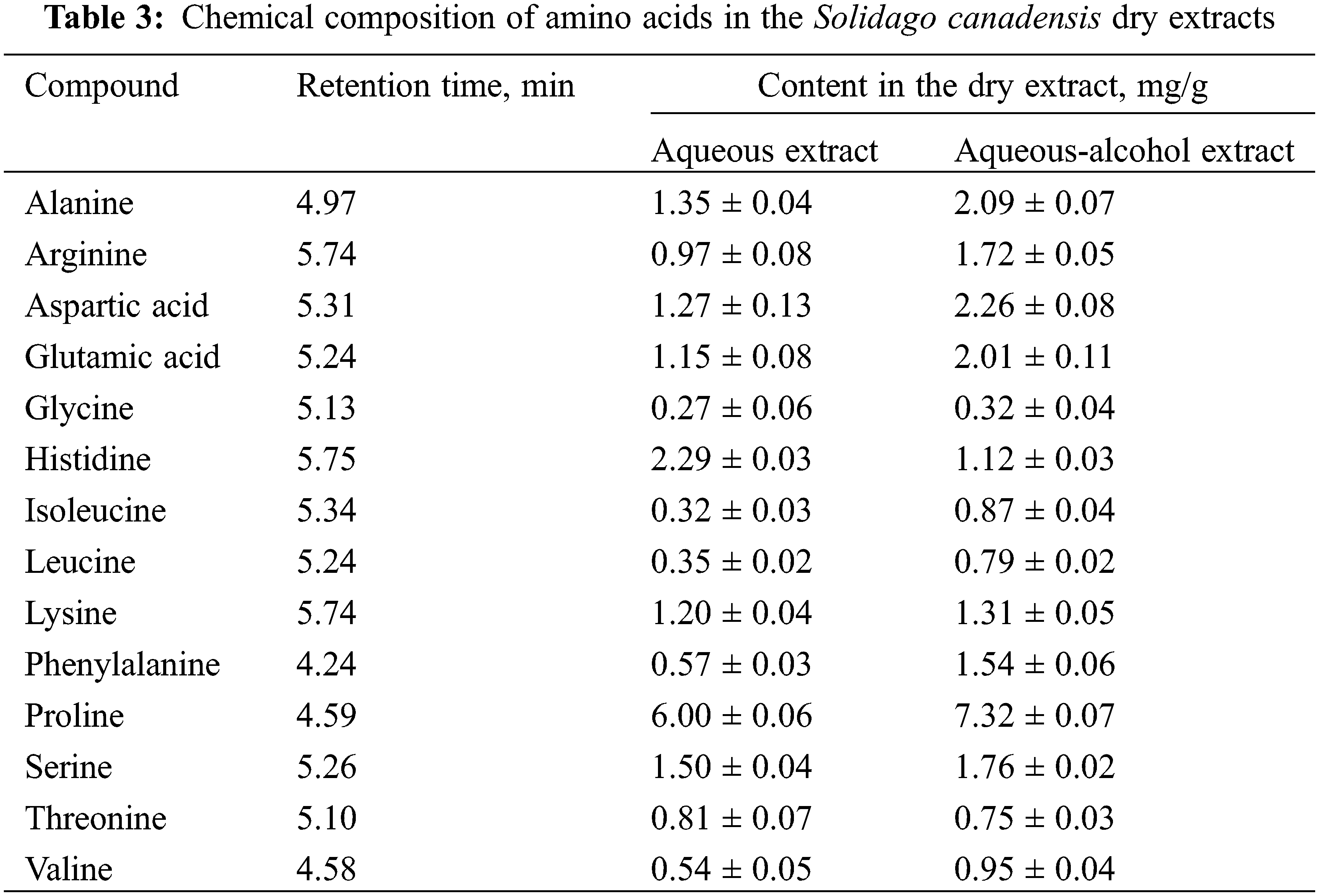

Figure 3: UPLC-MS/MS chromatograms of amino acids of Solidago herb sample (Aqueous-alcohol extract)
In the S. canadensis dry extracts were identified and quantified 14 amino acids, 7 of which are essential. The main amino acids (more than 1 mg/g) are proline, histidine, serine, alanine, aspartic acid, lysine and glutamic acid. There is no available data about the amino acid composition of S. canadensis raw material and its extracts in scientific literary sources, so these data might be considered novel.
The study of acute toxicity of the S. canadensis dry extracts was carried out on white non-linear sexually mature male mice weighing 19–21 g, grown in the nursery of the vivarium of the IFNMU. The conducted studies showed that after the intragastric administration of the extracts of S. canadensis herb at a dose of 6000 mg/kg, no deaths were observed: the animals were tidy, had a satisfactory appetite, reacted normally to light and sound stimuli, the processes of defecation and urination were normal, breathing disorders and seizures were not observed. Thus, the extracts were characterized as practically non-toxic (toxicity class V, LD50 > 5000 mg/kg).
The anti-inflammatory activity of the studied extracts was assessed by their ability to inhibit the development of formalin-induced oedema in the paw of rats compared to animals of the control group (Table 4).
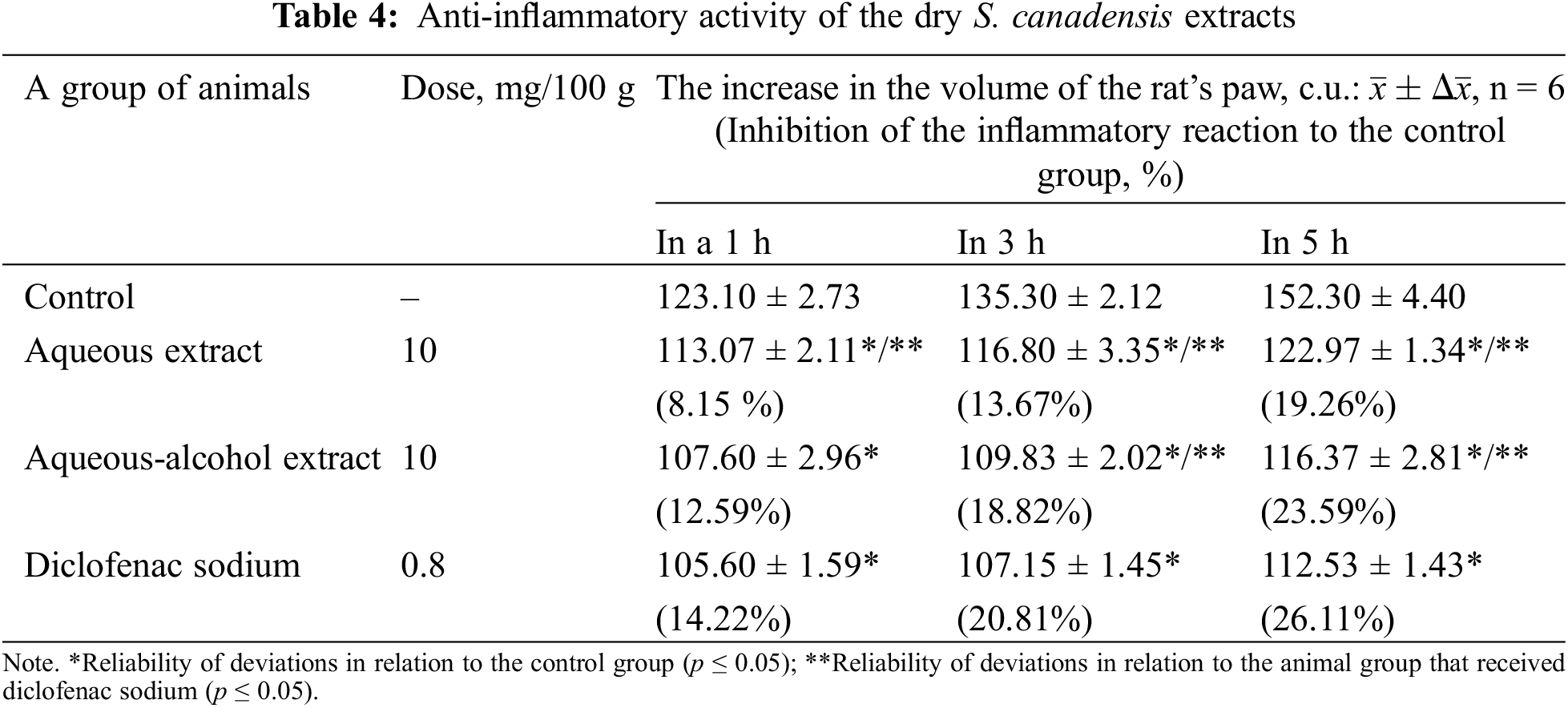
The obtained data indicate the effect of the extracts on the exudative phase of inflammation. The antiexudative effect of sodium diclofenac in 5 h was 26.11%. At the same level acted the dry aqueous-alcohol S. canadensis extract (23.59%), and a little bit less was the effect of the aqueous extract (19.26%). It’s interesting and advisable to establish the dose-effect relationship of S. canadensis extract in the future, but we took only a single dose, as it was preliminary research. A comparable dosage of the extracts to diclofenac sodium (0.8 mg) was 10 mg, as the concentration of phenolic substances in the extract is around 12%. Anyway, this single dose has proven the anti-inflammatory activity of the extracts and galenic remedies from which they originated. The anti-inflammatory effect of the S. canadensis extracts is due to both the phenolic compounds and the EO terpenes. Thus, pronounced antioxidant and anti-inflammatory activity is inherent in phenolics of the S. canadensis, such as isoquercitrin and rutin, chlorogenic and dicaffeoylquinic acids. Isoquercitrin also displays several chemoprotective effects against oxidative stress, cardiovascular disorders, diabetes, allergic reactions and cancer [34]. Rutin also has anti-carcinogenic, cardioprotective, anti-thrombotic, and neuroprotective activities [35]. Chlorogenic acid exhibits strong antioxidant and anti-inflammatory effects [36]. Previously, it was only reported about the anti-inflammatory activity of S. virguarea extracts and pure substances, such as a a triterpene saponin fraction (1.25–2.5 mg/kg) [37]. In the experiment of a carrageenan-induced oedema model in rats, S. virguarea extract showed the anti-exudative effect at the level of 27% (after 2 h) and 54% (after 5 h) [38]. Aqueous and ethanolic extracts of S. virgaurea reduced paw oedema and arthritic paw volume in rat models [39]. Also it was proved that Solidago hydroalcoholic extract can inhibit dihydrofolate reductase and contribute S. virgaurea extracts anti-inflammatory activity [1,40]. However, there is no data about the anti-inflammatory activity of S. canadensis extract in available scientific literary sources, so these data might be considered quite novel.
It is important to interrelate results and the composition of the S. canadensis extracts. The anti-exudative effect was stronger in the aqueous-alcohol S. canadensis extract than in the aqueous one. Caffeic, ferulic and p-coumaric acids exhibit anti-inflammatory activity [24] but their content in S. canadensis extracts was low (less than 0.6 mg/g). The aqueous-alcohol extract contained more caffeic acid than the aqueous extract (0.55 and 0.19 mg/g, respectively). The main polyphenol of S. canadensis extract, rutin, has antioxidant activity [41], and its concentration in both extracts was similar.
Phytochemical and pharmacological research in the galenic remedies of S. canadensis L. herb indicates possible perspectives of their use for developing novel dosage forms. 34 compounds of EO, 20 phenolics (10 flavonoids, 8 hydroxycinnamic acids and 2 phenolic acids) and 14 amino acids were found and quantified in S. canadensis galenic remedies. The extracts of S. canadensis were characterized as practically non-toxic substances (toxicity class V). The dry aqueous-alcohol S. canadensis extract shows promising anti-inflammatory activity. The mechanisms of action of pharmacological effect need to be elucidated in further work, as does the relationship of the biological activities to the chemical composition of the plant material.
Acknowledgement: The authors sincerely thank the Armed Forces of Ukraine for defending Ukrainian statehood and independence, and the partners who stand with Ukraine.
Funding Statement: This work was also supported by the European Union in the MSCA4Ukraine project “Design and development of 3D-printed medicines for bioactive materials of Ukrainian and Estonian medicinal plants origin” (ID number 1232466).
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Ain Raal, Oleh Koshovyi, Valdas Jakštas; data collection: Yurii Hrytsyk, Martin Lepiku, Vaidotas Žvikas, Tetiana Matus, Mariia Melnyk, Lyubov Grytsyk; analysis and interpretation of results: Ain Raal, Oleh Koshovyi, Yurii Hrytsyk, Martin Lepiku, Valdas Jakštas, Vaidotas Žvikas, Lyubov Grytsyk; draft manuscript preparation: Ain Raal, Oleh Koshovyi, Yurii Hrytsyk. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the author and/or corresponding author on reasonable request.
Ethics Approval: Pharmacological research was carried out considering ethical and moral-legal principles that guarantee humane treatment of experimental animals for scientific and educational purposes (protocol of the Ethics Committee of IFNMU No. 139/23 dated 16 November 2023).
Conflicts of Interest: The authors declare that there are no conflicts of interest to report regarding the present study.
References
1. Fursenco C, Calalb T, Uncu L, Dinu M, Ancuceanu R. Solidago virgaurea L.: a review of its ethnomedicinal uses, phytochemistry, and pharmacological activities. Biomolecules. 2020;10(12):1619. doi:10.3390/biom10121619. [Google Scholar] [PubMed] [CrossRef]
2. WHO Global Centre for Traditional Medicine. Geneva: World Health Organization; 2023. Available from: https://www.who.int/initiatives/who-global-centre-for-traditional-medicine. [Accessed 2023]. [Google Scholar]
3. Walck JL, Baskin JM, Baskin CC. Relative competitive abilities and growth characteristics of a narrowly endemic and a geographically widespread Solidago species (Asteraceae). Am J Bot. 1999;86(6):820–8. doi:10.2307/2656703. [Google Scholar] [CrossRef]
4. Šutovská M, Capek P, Kocmálová M, Fraňová S, Pawlaczyk I, Gancarz R. Characterization and biological activity of Solidago canadensis complex. Int J Biol Macromol. 2013 Jan;52:192–7. doi:10.1016/j.ijbiomac.2012.09.021. [Google Scholar] [PubMed] [CrossRef]
5. Elshafie HS, Gruľová D, Baranová B, Caputo L, De Martino L, Sedlák V, et al. Antimicrobial activity and chemical composition of essential oil extracted from Solidago canadensis L. growing wild in Slovakia. Molecules. 2019;24(7):1206. doi:10.3390/molecules24071206. [Google Scholar] [PubMed] [CrossRef]
6. Apáti P, Szentmihályi K, Kristó Sz.T, Papp I, Vinkler P, Szoke É, et al. Herbal remedies of Solidago-correlation of phytochemical characteristics and antioxidative properties. J Pharm Biomed Anal. 2003;32(4–5):1045–53. [Google Scholar]
7. Nkuimi Wandjou JG, Quassinti L, Gudžinskas Z, Nagy DU, Cianfaglione K, Bramucci M, et al. Chemical composition and antiproliferative effect of essential oils of four Solidago species (S. canadensis, S. gigantea, S. virgaurea and S. × niederederi). Chem Biodivers. 2020;17(11):e2000685. doi:10.1002/cbdv.v17.11. [Google Scholar] [CrossRef]
8. Kołodziej B. Antibacterial and antimutagenic activity of extracts aboveground parts of three Solidago species: Solidago virgaurea L., Solidago canadensis L. and Solidago gigantea Ait. J Med Plants Res. 2011;5(31):6770–9. [Google Scholar]
9. Abdel Motaal A, Ezzat SM, Tadros MG, El-Askary HI. In vivo anti-inflammatory activity of caffeoylquinic acid derivatives from Solidago virgaurea in rats. Pharmaceut Biol. 2016;54(12):2864–70. doi:10.1080/13880209.2016.1190381. [Google Scholar] [PubMed] [CrossRef]
10. Kraujalienė V, Pukalskas A, Venskutonis PR. Biorefining of goldenrod (Solidago virgaurea L.) leaves by supercritical fluid and pressurized liquid extraction and evaluation of antioxidant properties and main phytochemicals in the fractions and plant material. J Funct Foods. 2017;37:200–8. doi:10.1016/j.jff.2017.07.049. [Google Scholar] [CrossRef]
11. Huang B, Lei Y, Qin L, Liu J. Chemical composition and cytotoxic activities of the essential oil from the inflorescences of Solidago canadensis L., an invasive weed in Southeastern China. J Essent Oil Bearing Plants. 2012;15(4):667–71. doi:10.1080/0972060X.2012.10644103. [Google Scholar] [CrossRef]
12. Mishra D, Joshi S, Bisht G, Pilkhwal S. Chemical composition and antimicrobial activity of solidago canadensis linn. Root essential oil. J Basic Clin Pharm. 2010 Jun;1(3):187–90. [Google Scholar] [PubMed]
13. El-Sherei M, Khaleel A, Motaal AA, Abd-Elbaki P. Effect of seasonal variation on the composition of the essential oil of Solidago canadensis cultivated in Egypt. J Essent Oil Bear Plants. 2014 Sep 3;17(5):891–8. doi:10.1080/0972060X.2014.901612. [Google Scholar] [CrossRef]
14. Shelepova O, Vinogradova Y, Zaitchik B, Ruzhitsky A, Grygorieva O, Brindza J. Constituents of the essential oil in Solidago canadensis L. from Eurasia. Potr S J F Sci. 2018;12(1):20–5. doi:10.5219/847. [Google Scholar] [CrossRef]
15. European pharmacopoeia. 11th edStrasbourg: Council of Europe; 2022. [Google Scholar]
16. Woźniak D, Ślusarczyk S, Domaradzki K, Dryś A, Matkowski A. Comparison of polyphenol profile and antimutagenic and antioxidant activities in two species used as source of Solidaginis herba-Goldenrod. Chem Biodivers. 2018;15(4):e1800023. doi:10.1002/cbdv.v15.4. [Google Scholar] [CrossRef]
17. Dobjanschi L, Păltinean R, Vlase L, Babotă M, Fritea L, Tămaş M. Comparative phytochemical research of Solidago genus: S. graminifolia. Note I. flavonoids. Acta Biologica Marisiensis. 2018;1(1):18–26. doi:10.2478/abmj-2018-0003. [Google Scholar] [CrossRef]
18. Dobjanschi L, Fritea L, Patay EB, Tamas M. Comparative study of the morphological and phytochemical characterization of Romanian Solidago species. Pak J Pharm Sci. 2019;32(4):1571–9. [Google Scholar] [PubMed]
19. Thiem B, Wesołowska M, Skrzypczak L, Budzianowski J. Phenolic compounds in two Solidago L. species from in vitro culture. Acta Pol Pharm. 2001;58(4):277–81. [Google Scholar] [PubMed]
20. Kovalenko VN. Compendium 2020. In: Medicines. Kyiv, Ukraine: MORION; 2020. [Google Scholar]
21. Grodzinsky AM. Medicinal plants. In: Encyclopedic guide. Kyiv, Ukraine: Ukrainian Encyclopedia named after M. P. Bazhana; 1990. [Google Scholar]
22. Fedotova VV, Chelombytko VA. Species of the genus Solidago: significance for medical practice, study prospects. Sci Bull BelSU. 2012;16:136–45. [Google Scholar]
23. Dobrochaeva DN, Kotov MI, Prokudin YN, Barbarich AI. Key to higher plants of Ukraine. Kyiv, Ukraine: Naukova Dumka; 1999. [Google Scholar]
24. Huzio N, Grytsyk A, Raal A, Grytsyk L, Koshovyi O. Phytochemical and pharmacological research in Agrimonia eupatoria L. herb extract with anti-inflammatory and hepatoprotective properties. Plants. 2022;11(18):2371. doi:10.3390/plants11182371. [Google Scholar] [PubMed] [CrossRef]
25. Vlasova I, Gontova T, Grytsyk L, Zhumashova G, Sayakova G, Boshkayeva A, et al. Determination of standardization parameters of Oxycoccus macrocarpus (Ait.) Pursh and Oxycoccus palustris Pers. Leaves. ScienceRise: Pharmaceutil Sci. 2022;3(37):48–57. doi:10.15587/2519-4852.2022.260352. [Google Scholar] [CrossRef]
26. Vilkickyte G, Raudone L, Petrikaite V. Phenolic fractions from Vaccinium vitis-idaea L. and Their antioxidant and anticancer activities assessment. Antioxidants. 2020;9(12):1261. doi:10.3390/antiox9121261. [Google Scholar] [PubMed] [CrossRef]
27. Marzullo L, Ochkur O, Orlandini S, Renai L, Gotti R, Koshovyi O, et al. Quality by design in optimizing the extraction of (poly)phenolic compounds from Vaccinium myrtillus berries. J Chromatogr A. 2022;1677:463329. doi:10.1016/j.chroma.2022.463329. [Google Scholar] [PubMed] [CrossRef]
28. Uminska K, Gudžinskas Z, Ivanauskas L, Georgiyants V, Kozurak A, Skibytska M, et al. Amino acid profiling in wild Chamaenerion angustifolium populations applying chemometric analysis. J App Pharm Sc. 2023;13(5):171–80. [Google Scholar]
29. Stefanov OV. Preclinical studies of drugs. Kyiv, Ukraine: Avicenna; 2001. [Google Scholar]
30. State pharmacopoeia of Ukraine. 2nd edKharkiv, Ukraine: Ukrainian Scientific Pharmacopoeial Center of Drugs Quality; 2015. [Google Scholar]
31. Lapach SN, Chubenko AV, Babich PN. Statistical methods in biomedical research using Excel. Kyiv, Ukraine: MORION; 2000. [Google Scholar]
32. Tkachev AV, Korolyuk EA, Letchamo W. Volatile oil-bearing flora of Siberia VIII: essential oil composition and antimicrobial activity of wild Solidago virgaurea L. from the Russian Altai. J Essent Oil Res. 2006;18(1):46–50. doi:10.1080/10412905.2006.9699382. [Google Scholar] [CrossRef]
33. Kalemba D. Constituents of the essential oil of Solidago virgaurea L. Flavour Fragr J. 1998;13(6):373–6. doi:10.1002/(ISSN)1099-1026. [Google Scholar] [CrossRef]
34. Valentová K, Vrba J, Bancířová M, Ulrichová J, Křen V. Isoquercitrin: pharmacology, toxicology, and metabolism. Food Chem Toxicol. 2014;68:267–82. doi:10.1016/j.fct.2014.03.018. [Google Scholar] [PubMed] [CrossRef]
35. Akash SR, Tabassum A, Aditee LM, Rahman A, Hossain MI, Hannan MDA, et al. Pharmacological insight of rutin as a potential candidate against peptic ulcer. Biomed Pharmacother. 2024;177:116961. doi:10.1016/j.biopha.2024.116961. [Google Scholar] [PubMed] [CrossRef]
36. Ziółkiewicz A, Niziński P, Soja J, Oniszczuk T, Combrzyński M, Kondracka A, et al. Potential of chlorogenic acid in the management of metabolic dysfunction-associated steatotic liver disease (MASLD): animal studies and clinical trials—a narrative review. Metabolites. 2024;14(6):346. doi:10.3390/metabo14060346. [Google Scholar] [PubMed] [CrossRef]
37. Jacker HJ, Voigt G, Hiller K. Antiexudative behavior of various triterpene saponins. Pharmazie. 1982;37(5):380–2. [Google Scholar] [PubMed]
38. Metzner J, Hirschelmann R, Hiller K. Antiphlogistic and analgesic effects of leiocarposide, a phenolic bisglucoside of Solidago virgaurea L. Pharmazie. 1984;39(12):869–70. [Google Scholar] [PubMed]
39. el-Ghazaly M, Khayyal MT, Okpanyi SN, Arens-Corell M. Study of the anti-inflammatory activity of Populus tremula, Solidago virgaurea and Fraxinus excelsior. Arzneimittelforschung. 1992;42(3):333–6. [Google Scholar] [PubMed]
40. Strehl E, Schneider W, Elstner EF. Inhibition of dihydrofolate reductase activity by alcoholic extracts from Fraxinus excelsior, Populus tremula and Solidago virgaurea. Arzneimittelforschung. 1995;45(2):172–3. [Google Scholar] [PubMed]
41. Negahdari R, Bohlouli S, Sharifi S, Dizaj SM, Saadat YR, Khezri K, et al. Therapeutic benefits of rutin and its nanoformulations. Phytother Res. 2021;35(4):1719–38. doi:10.1002/ptr.v35.4. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF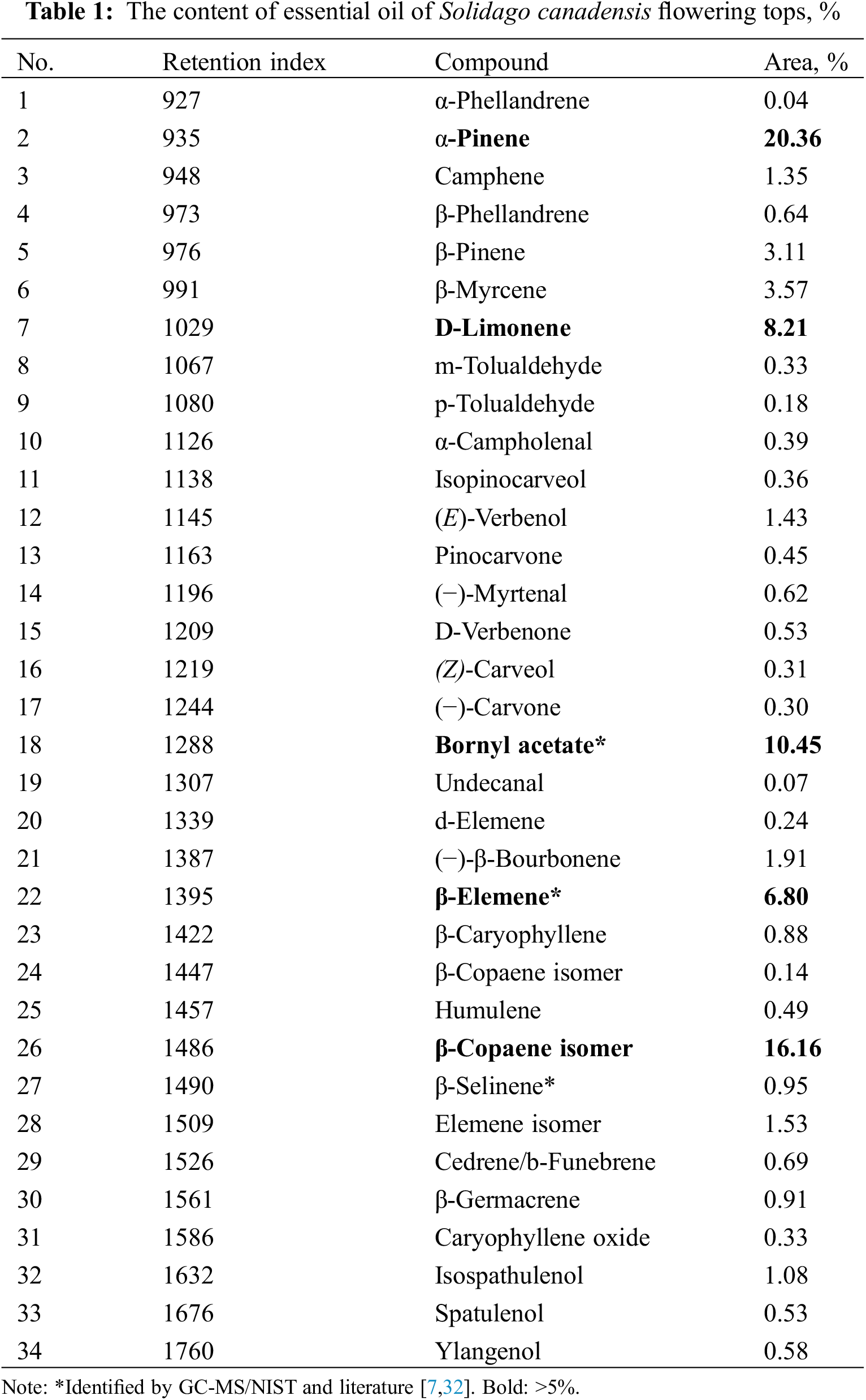
 Downloads
Downloads
 Citation Tools
Citation Tools