 Open Access
Open Access
ARTICLE
Morphometric Attributes of Two Native Forage Species According to Water Source Distance in Semiarid Central Grasslands of Argentina
1 Laboratorio de Ecología en Ambientes Semiáridos, Ecología Vegetal, Facultad de Agronomía, UNLPam, Santa Rosa, 6300, Argentina
2 Facultad de Ciencias Veterinarias, UNLPam, General Pico, 6360, Argentina
3 Becaria Doctoral CONICET, APN, Facultad de Agronomía, UNLPam, Santa Rosa, 6300, Argentina
* Corresponding Author: Carla Etel Suárez. Email:
(This article belongs to the Special Issue: Ecology of Rangelands in Argentina)
Phyton-International Journal of Experimental Botany 2024, 93(8), 2063-2074. https://doi.org/10.32604/phyton.2024.053193
Received 26 April 2024; Accepted 26 July 2024; Issue published 30 August 2024
Abstract
The semiarid grasslands of Argentina’s central region have been modified by domestic livestock grazing, both in their composition and structure. The increase in the proportion of woody and non-forage species and the decrease in forage species are some of the most evident results of this process. There is limited available information about the effect of differential grazing pressures on morphometric attributes of native species, and it also depends on the life histories of the species in this grassland. The objective of this work was to evaluate some morphometric aspects in the grasses Poa ligularis Nees ex Steud and Piptochaetium napostaense (Speg.). Hack according to distance from the water source in communities in the central semiarid region of Argentina. The study area included areas of low grassland, golden forests, and secondary forests in grasslands (6 fields with 9 paddocks). Grazing pressure was established based on the distance to the water source, so sampling areas were designated near the water source (grazing pressure is greater) and far from the water source (grazing pressure light) in each of the pastures. In both species, specimens were selected at random, and the following attributes were measured: crown diameter at ground level (cm), burial depth (cm), average tiller weight per plant (g. Marcello−1), and the density of tillers per unit of crown surface (tillers.cm−2 crown). The morphometric attributes evaluated showed differences according to the distance to the watering hole and in the different pastures. Both species presented similar behaviors for the attributes. There were significant differences in the depth of burial and the average weight of tillers per plant, being greater in the areas close to the water source. Regarding the distance to the watering hole, there were no significant differences in crown diameter and tiller density, but the highest values were recorded for the former near the watering hole and for the second far from the watering hole. In pastures with low grassland or secondary forest on the plain, the greatest depth of burial and weight of tillers was recorded closer to the water. In forest areas, the highest density of tillers was found far from the water. For crown diameter, although there was no interaction, the largest diameters were found in plain grassland areas near the waterhole. In general, both species had a differential behavior depending on the grazing pressure that was evident along the physiognomic gradient from plain grassland to forest and that could be interpreted as a strategy to perpetuate themselves against herbivory.Keywords
The plant communities of the central semiarid region of Argentina have been modified by domestic livestock grazing, both in their composition and structure [1–3]. Processes such as a woody species increase in the intermediate strata of the golden woodland, the advance of these species in the low grasslands, a non-forage species increase in the grassy-herbaceous layer and different desertification levels are the result of a non-conservative use history [1,4–7]. The most notable consequence was a livestock receptivity loss of the system, with a strong decrease in the forage species productivity in the grassy-herbaceous stratum [8].
In these semi-arid environments, water availability is an important limitation of livestock production that has conditioned the paddock size depending on the location of the water source [3,9]. The technologies associated with grazing management (fencing, water sources, firebreaks) have also contributed to a heterogeneous use of grasslands, concentrating pressure in areas near the water sources [10–13]. In addition, inadequate stocking rate has been another factor that has affected the production of these systems due to excessive or insufficient grazing [1,14]. In this way, two of the main forage species such as Poa ligularis Nees ex Steud and Piptochaetium napostaense (Speg.) Hack have had different degrees of grazing pressure according to their location in the paddock [15,16]. These C3 species, characteristic of winter grasslands, allow also for use in autumn and early spring and represent two of the species with the highest forage value in the region [17,18].
New management proposals aimed at rehabilitating grasslands have led to the use of smaller paddocks, with a better distribution of water sources, high-density rotational grazing, and extended no-grazing periods for grassland species [19].
There is limited available information about the effect of differential grazing pressures on morphometric attributes of native species, and it also depends on the life histories of the species in this grassland. In the field, it is common to observe differences in the structure of plants in grazed areas compared to non-grazed ones [20,21]. In this sense, it is known that the grasses of this region have not evolved with a strong herbivory interaction [22–25].
Therefore, when thinking about this interaction, trade-offs appear in the allocation of resources towards aerial parts (intercalary, axillary, and apical meristems) and underground parts (roots and modified leaves) [26–31] in response to a recent story. In this regard, an essential part of grasses is the crown that gathers the buds that will give rise to tillering. The degree of grazing could then determine whether there is a mobilization of photoassimilates from them to the aerial meristems or whether there is a contribution to the root system [16,32]. The distance to the water source would then be a gradient that expresses differential grazing pressure in these systems, so it would be expected to find differences in the architecture of the forage species throughout it.
The objective of this work was to evaluate some morphometric aspects of two winter foraging species in communities of the central semiarid region of Argentina in areas near and far from the water source. This was achieved from the evaluation of the crown diameter, weight, and density of tillers and the depth of crown burial of Poa ligularis and Piptochaetium napostaense in low grasslands, and the grassy-herbaceous layer of golden woodland.
This study was carried out in representative sites of the calden woodland and low grasslands, located in the Central-Northern of La Pampa Province in Central Argentina (Fig. 1). It occupies the subregion of hills and valleys in the Eastern physiographic region The region’s climate is temperate-semi-arid with rainfall ranging from 400 to 600 mm mainly concentrated in the summer-autumn season. The average annual temperature ranges around 15°C. As for the relief, it ranges from undulating to hilly with predominantly Entic Haplustollsoils and Typic Ustipsamments [21].
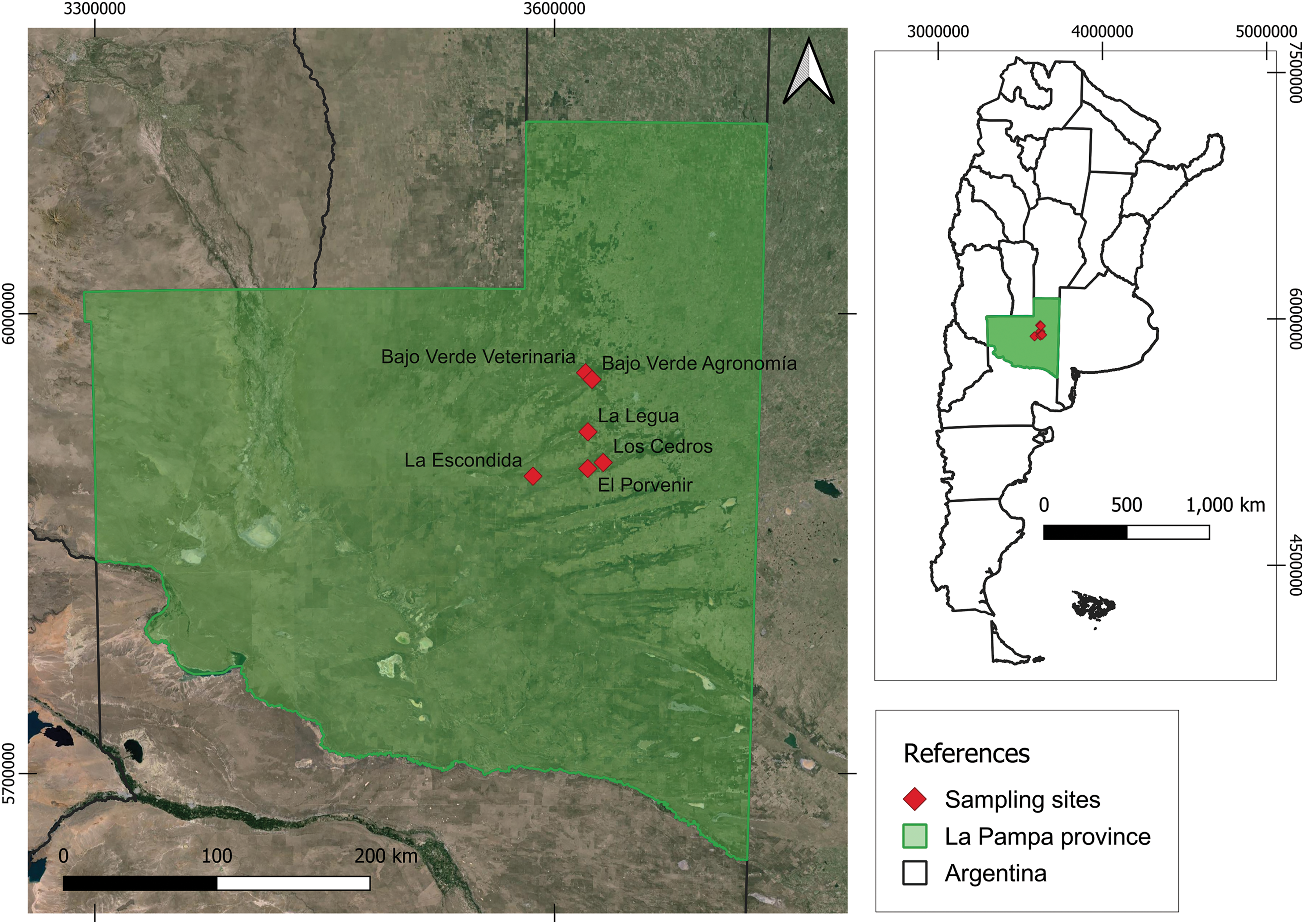
Figure 1: Study area. Location of the establishments where the sampling was carried out. Note: La Legua 1 and 2: LG1 and 2; Bajo Verde Agronomía 1 and 2: BVA1 and 2; Bajo Verde Veterinaria 1 and 2: BVV1; El Porvenir: EP; Los Cedros: LC and La Escondida: LE
The study area included low grassland areas (La Legua 1-LG1; La Legua 2-LG2), calden woodlands (Bajo Verde Agronomía 2-BVA2; Bajo Verde Veterinaria 1 and 2-BVV1 and 2; El Porvenir-EP) and secondary woodlands on grasslands (Bajo Verde Agronomía 1-BVA1; Los Cedros-LC; La Escondida-LE) (Table 1). These communities share practically the same species with profound changes mainly in the vertical structure. The grassy-herbaceous layer in general was characterized by forage species such as the P. napostaense, P. ligularis, and non-forage them as Nassella tenuissima (Trin.) Barkworth, Jarava ichu Ruiz & Pav. and Amelichloa brachychaeta Godr. Regarding the woodland physiognomy, the main species is Neltuma caldenia (Burkart) C.E. Hughes & G.P. Lewis and other accompanying species are Geoffroea decorticans Burkart, Schinus fasciculatus (Griseb.) I.M. Johnst and Jodina rhombifolia (Hook. & Arn.) Reissek, with variants in the development of the vertical structure and greater contribution of the intermediate woody layer in the secondary woodlands on the grasslands where juvenile N. caldenia trees predominate.
2.2 Location of the Sampling Areas
The study was conducted across 6 establishments (Fig. 1) where 9 paddocks of over 50–100 hectares were selected with a similar use history (rotational grazing in the last 15 years). Grazing pressure was established based on the distance to the water source; thus, sampling areas were designated near the water source (grazing pressure is higher), close, approximately 75 meters from the water source and far, approximately 1000 meters or more from the water source, where livestock rarely come to graze. We worked with two species P. ligularis (Pli) and P. napostaense (Pna) due to their importance in terms of forage quality and contribution to the grass-herbaceous stratum. The 6 establishments were dedicated to raising cattle and the average stocking rate of each of the fields was around 0.2 UG.ha−1.
In each paddock and for each situation (near and far from the watering hole), 21 specimens of each species were randomly collected (n = 42; N = 378). At the beginning of autumn 2017, the crown diameter (CD) at ground level (cm) and burial depth (CBD) being the distance from the base where the roots are inserted to the point where the plant emerges from the soil surface (cm) were measured in the field [32]. The collected material was prepared in the laboratory for the determination of the tillers weight average (TW) per plant (g.tillers−1) and the tillers density (TD) per unit of crown surface (tillers.cm−2 crown) for each specimen.
The data were analyzed using Generalized Linear Mixed Models with a comparison of means using the LSD method. For the selection of the models, the AIC value was considered, using the model that minimizes said index. The assumptions of normality and homoscedasticity were checked; when necessary, heteroskedastic models (TD and CD) were adjusted. Principal component analysis (PCA) and cluster analysis (average linkage, euclidean distance) were performed for both species according to sampling sites and attributes. The statistical packages Infostat version 2018 [33].
The morphometric attributes evaluated showed differences according to the distance to the water source and in the different paddocks. In terms of the distance to the water source, there were significant differences for both species in the CBD (pPliv < 0.0001; pPna < 0.0001) and in the TW average per plant (pPli = 0.023; pPna = 0.01), being greater in nearby areas (Table 2). Although there were no significant differences for CD (pPli = 0.1686; pPna = 0.518) and TD (pPli = 0.3470; pPna = 0.232), the highest values were recorded for the first one near the water source and for the second far from it.
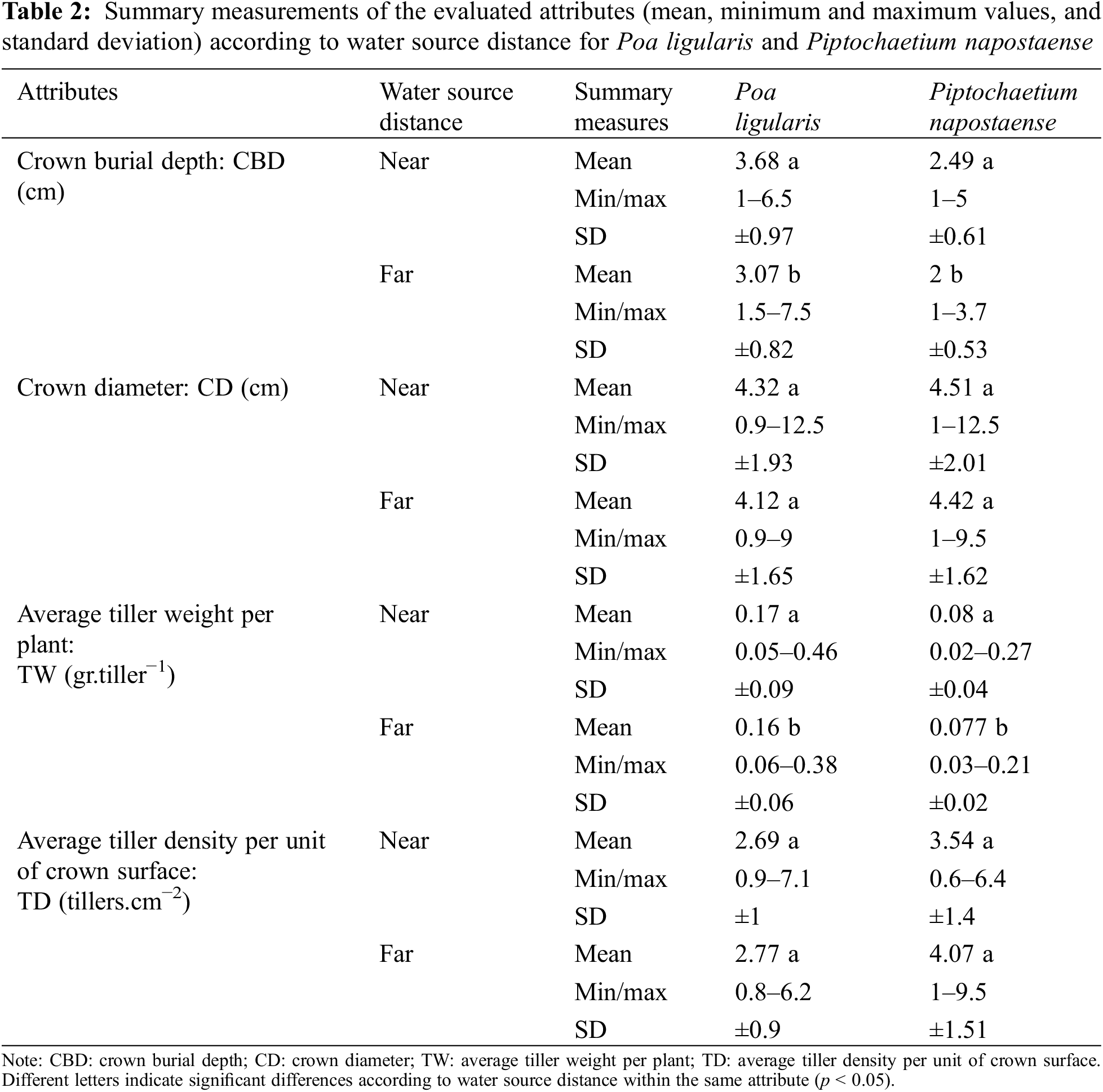
Both species presented similar behaviors for the attributes, however Pli presented a greater depth of CBD and a greater TW average both near and far from the water source. While Pna presented higher TD also for near and far de water source.
Regarding the paddocks, all the measured attributes presented significant differences for both species (pPli < 0.0001; pPna < 0.0001). There was an interaction between paddocks and the water source distance in the TW average, CBD and TD per plant (pPli < 0.0001; pPna < 0.0001). Specifically, in paddocks with low grassland or secondary woodland in grasslands, the greatest CBD (Pli = 4.83 cm; Pna = 3.40 cm) and TW (Pli = 0.31 g. tiller−1; Pna = 0.16 g.tiller−1) were found near the water source. In woodlands areas, the highest TD (Pli = 3.71 tiller.cm−2; Pna = 4.54 tiller.cm−2) was found far from the water source. For CD, although there was no interaction (pPli = 0.1919; pPna = 0.2437), the largest diameters were found in areas of low grassland near the water source (Pli = 6.75 cm; Pna = 7.03 cm).
From the cluster classification analysis, two groupings were identified (Fig. 2). A first group contemplates the paddocks with physiognomy of low grassland and secondary woodlands on the grasslands in areas near the water source and the second group includes the secondary woodlands on the grasslands far from the waterhole and the rest of the paddocks with woodland physiognomy.
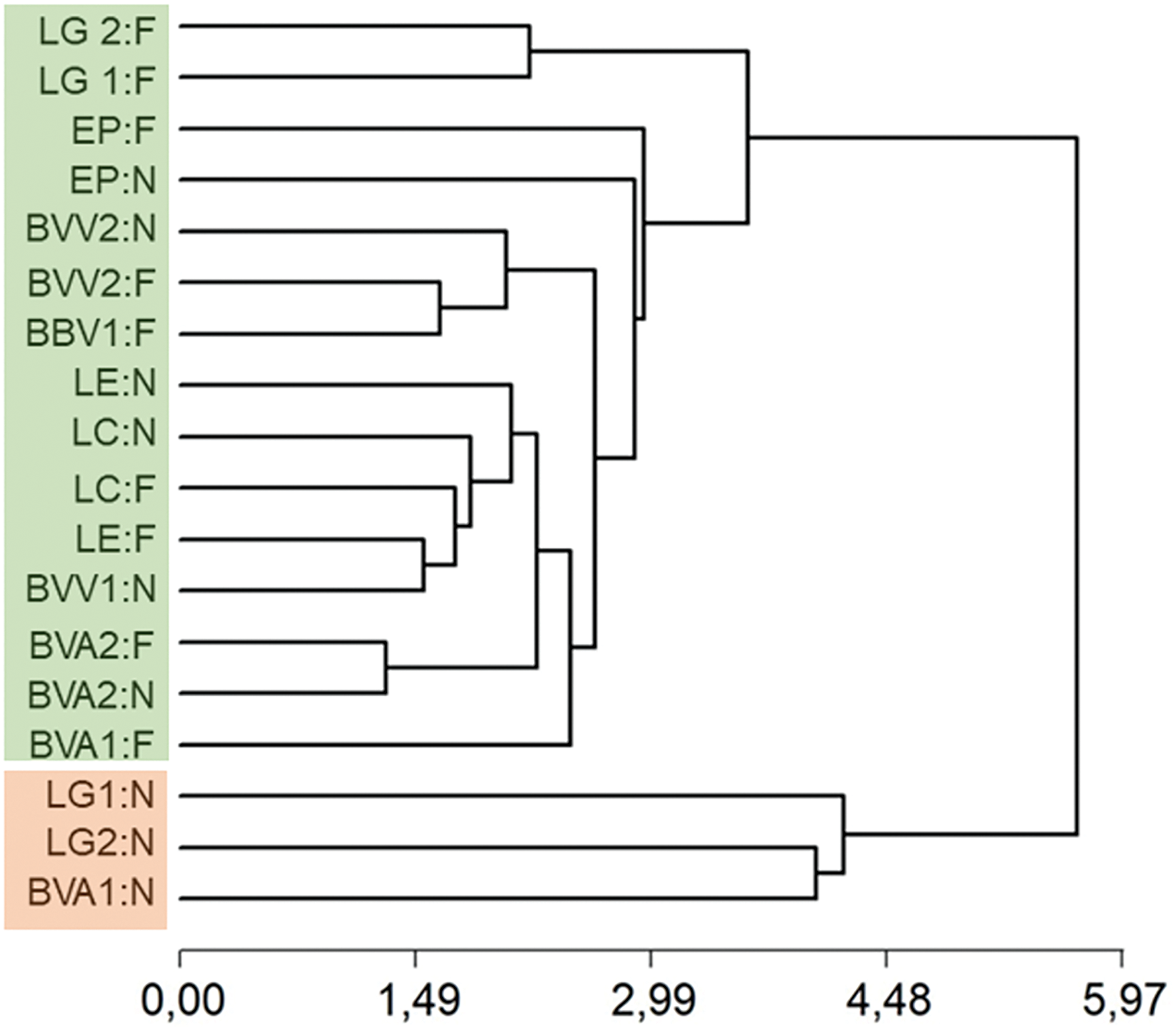
Figure 2: Cluster analysis. Cluster with the morphometric attributes crown burial depth; average tiller weight per plant; average tiller density per unit of crown surface of Poa ligularis and Piptochaetium napostaense together according to water source distance and pastures. Cophenetic correlation: 0.887. Note: LG1 and 2: La Legua 1 and 2 (Low grassland); BVA2: Bajo Verde Agronomía 2; BVV1 and 2: Bajo Verde Veterinaria 1 and 2; EP: El Porvenir (Calden woodland); BVA1: Bajo Verde Agronomía 1; LC: Los Cedros; LE: La Escondida (Secondary calden woodland on grassland areas). N: near water source and F: far from the water source. The two groups are indicated in different colours
The PCA showed the distribution and relationship between the attributes of both species and the paddocks according to the water source distance (explained 75.9% of the variance; axis 1: 58.4% and axis 2: 17.5%). The first axis allowed to distinguish the paddocks with the physiognomy of low grassland and secondary woodland on grasslands from those with a physiognomy of woodland (Table 1). The second axis provided information regarding the distance to the water source (Fig. 3). In this way, the paddocks with low grassland and some secondary woodlands on grasslands near the water source were characterized by the greatest CBD, TW and CD; while the remaining paddocks with secondary woodland on grassland and woodlands had higher TD.
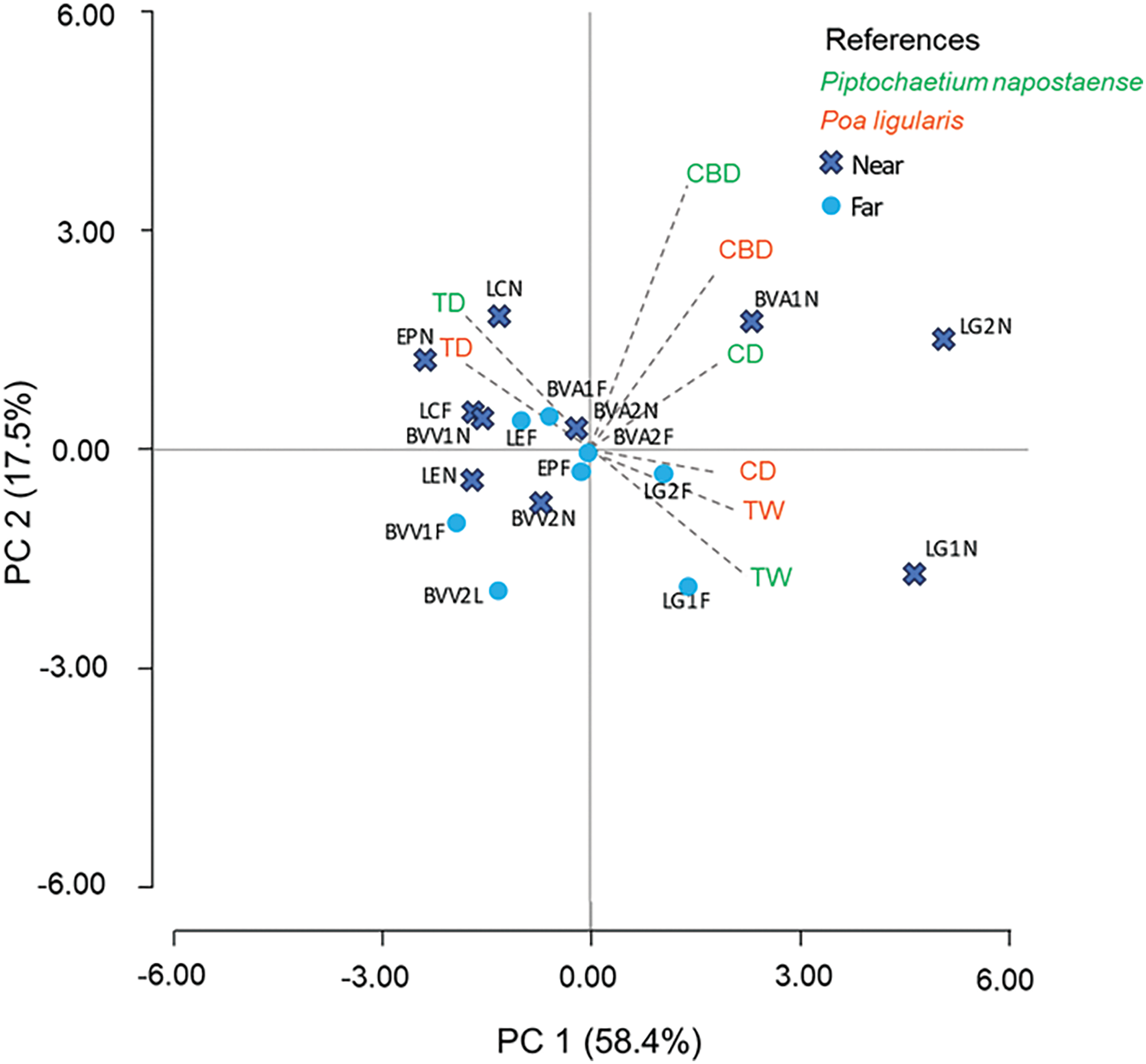
Figure 3: Principal component analysis for different attributes of Poa ligularis and Piptochaetium napostaense. Note: LG1 and 2: La Legua 1 and 2 (Low grassland); BVA2: Bajo Verde Agronomía 2; BVV1 and 2: Bajo Verde Veterinaria 1 and 2; EP: El Porvenir (Calden woodland); BVA1: Bajo Verde Agronomía 1; LC: Los Cedros; LE: La Escondida (Secondary calden woodland on grassland areas). N: near water source; F: far water source. CBD: crown burial depth; CD: crown diameter; TW: average tiller weight per plant; TD: average tiller density per unit of crown surface (Explained 75.9% of the variance)
The grasslands forage species of the central semiarid region of Argentina, unlike what occurs in other grasslands in the world, have evolved with low grazing pressure exerted by a few species of wild herbivores [22,23]. In the last century, the introduction of domestic livestock has increased grazing pressure with effects on species of these grasslands that are still unclear.
Somehow these species persist in many of these systems although with variable coverage. The results obtained in this work show changes in the architecture of the species in response to grazing pressure in the different systems.
Near the water source, the widespread effect of herbivory is observed not only in overgrazing but also in greater trampling, excrement, and nutrient concentration [11,12,34,35] that generate differential environments. In this regard, in these situations, plants rarely achieve the complete life cycle and are in almost permanent tissue regeneration. Grazing causes the mobilization of reserves within the plant from the underground part to the aerial part for a short time [36], long enough to initiate self-sufficiency in photoassimilates. This produces an initial vigor in the plants that could lead to local extinction depending on how recurrent the grazing is, the intensity and the accompanying environmental factors. Pli and Pna are two species highly selected by domestic livestock that tend to disappear under conditions of high grazing under grazing pressure [37–42].
In this work, it was observed that in places with greater grazing pressure, these species were found more buried, results that coincide with others carried out in the same region [15,32]. Furthermore, this greater depth coincided with a greater tiller weight. In some way, this highlights that individuals must choose between investing in continuous regrowth or improving root growth and bud protection to avoid extinction, at least locally.
The existing information on the physiological aspects accompanying trade-offs in resource allocation is scant and sometimes contradictory. For example, Souto et al. [39] mention for Pli that a high frequency of defoliation can increase the death of buds at the base of the stems, which could explain the low density of tillers that was found in areas near the water source areas. However, authors such as Gastal et al. [43] and Assuero et al. [31] mention a higher density of tillers in grass species with a high rate of leaf renewal or with greater grazing pressure, respectively. This demonstrates the variability that exists in terms of the response to grazing depending on the species considered.
In terms of the crown burial depth in response to frequent defoliation, it could be interpreted as a storage strategy for reallocating reserves to a few tillers capable of reaching reproductive stages [44] serving as a last chance for self-perpetuation. This is supported by the tiller weight observed in this study, which was greater in individuals with lower tiller density. In this regard, authors have reported higher growth rates and biomass production per tiller in those with greater weight and lower tiller density per plant [45–48].
Regarding crown diameter, its variability can be attributed to the effect that recent grazing (time elapsed) has on this attribute, which masks the effect of the water source distance. The tillering dynamics in these grasses is centrifugal with the presence of senescent material in the center and active tillers on the periphery. This is true for both grazed and ungrazed plants, however, in the former, the action of the recurring cut causes different stages of separation to occur at the same diameter until they become independent plants [29].
Finally, overall, both species exhibited differential behavior in response to grazing pressure, which was evident across the physiognomic gradient from low grasslands to woodland (Fig. 4). In this sense, changes in the communities’ vertical structure seem to be an important component in the livestock behavior reflected in grazing pressure [6]. In this way, grassland environments have a greater stocking rate concentration around the water, with limited movement and use of the most distant areas [11,13]. In contrast, in communities with woody cover and good accessibility, the spatial heterogeneity given by forage and non-forage patches would promote livestock dispersion in the paddock. All this would translate into greater pressure on the plants near the water [3,49]. In these areas, a greater crown burial depth with heavier and less dense tillers would respond to an acquired phenotypic plasticity [47] that would allow greater grazing tolerance of these species.
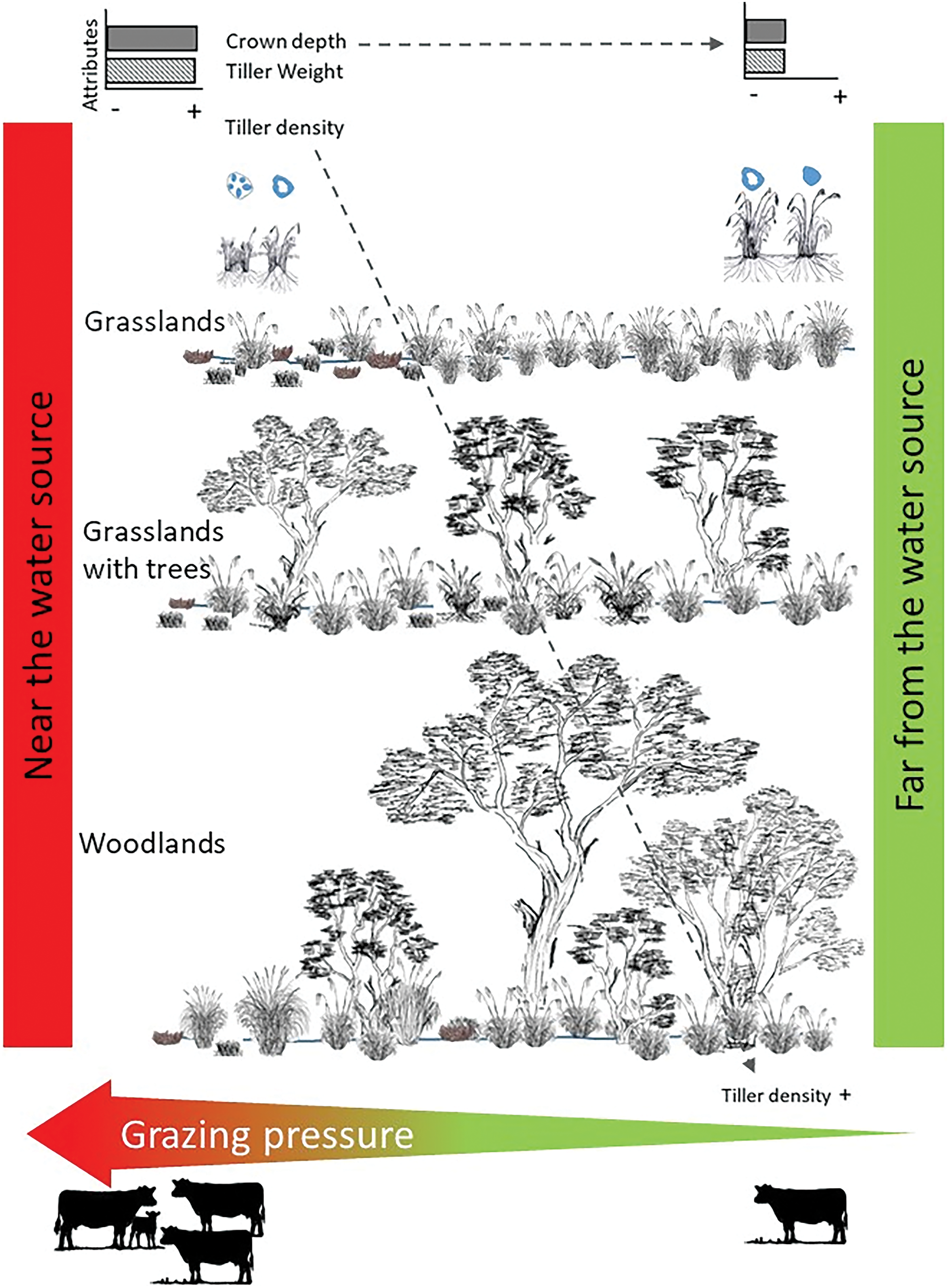
Figure 4: Conceptual model on the morphometric attributes of Poa ligularis and Piptochaetium napostaense according to distance from the water source
Future work should consider whether these adjustments constitute a long-term survival strategy or a short-term morphophysiological response to face different grazing pressures. As well as delve into other aspects of the system such as accessibility, quality of light, and water interception marked by the structural complexity that occurs in the forest systems of this region and that may influence these attributes.
Acknowledgement: The authors thank the anonymous reviewers, whose comments improved the quality of the paper. Furthermore, the authors thank to Melissa Wilson, Berenice Cerrato, María Angelica Elizalde and Ricardo Ernst for their collaboration in field sampling and treatment of plant material.
Funding Statement: This work was supported by Facultad de Agronomía, UNLPam (308/13, 343/2013, 231/17-CD-FA-UNLPam, HDE).
Author Contributions: Study conception and design: Héctor Daniel Estelrich; data collection: Héctor Daniel Estelrich; analysis and interpretation of results: Carla Etel Suárez, María Sol Rossini; draft manuscript preparation: Carla Etel Suárez, María Sol Rossini; review and editing: Carla Etel Suárez, María Sol Rossini, Ernesto Francisco Atilio Morici, Héctor Daniel Estelrich. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Partial data used for this study is available within the text, more information can be requested by contacting the corresponding authors.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Estelrich D, Chirino C, Morici EFA, Fernández B. Modelo conceptual de funcionamiento de áreas naturales cubiertas por bosque y pastizal en la región semiárida central de Argentina. In: Oesterheld M, Aguiar M, Ghersa CM, Paruelo J, editors. La heterogeneidad de la vegetación de los agroecosistemas. Un homenaje a Rolando León. Buenos Aires: Editorial Facultad de Agronomía; 2005. p. 351–64 (In Spanish). [Google Scholar]
2. Rauber RB, Cendoya MA, Arroyo DN, Bogino SM. Composición florística y funcional del pastizal natural del centro de la Argentina: efecto del pastoreo bovino y el fuego. Ecología Austral. 2023;33(1):13–9 (In Spanish). doi:10.25260/EA.23.33.1.0.2054. [Google Scholar] [CrossRef]
3. Scaglia JA, Flores DG, Tapia R, Martinelli M. Effects of geomorphology and distribution of water sources for livestock on the floristic composition and livestock receptivity of the Arid Chaco. Revista FCA UNCuyo. 2024;56(1):12–25. doi:10.48162/rev.39.119. [Google Scholar] [CrossRef]
4. Estelrich HD, Suárez CE, Morici EFA, Ernst RD. Capítulo 7: intervenciones combinadas en áreas de bosque con renoval-arbustal y fachinales. In: Estelrich HD, Suárez CE, editors. El bosque de caldén: un abordaje multidisciplinario para su manejo y conservación. 1st edSanta Rosa: Editorial de la Universidad Nacional de La Pampa; 2022. p. 97–110 (In Spanish). [Google Scholar]
5. Peinetti HR, Bestelmeyer BT, Chirino CC, Vivalda FL, Kin AG. Thresholds and alternative states in a Neotropical dry forest in response to fire severity. Ecol Appl. 2024;34(2):54. doi:10.1002/eap.2937. [Google Scholar] [PubMed] [CrossRef]
6. Coria RD, Bravo S, Kunst C. Un aporte al entendimiento de la lignificación de los pastizales/sabanas del Chaco Semiárido sudamericano. Ecología Austral. 2021;31(3):390–574 (In Spanish). doi:10.25260/EA.21.31.3.0.1615. [Google Scholar] [CrossRef]
7. Coria RD, Kunst C, Anriquez A, Bravo S. Manejo de pastizales/sabanas lignificados del Chaco Semiárido Sudamericano: restauración ecológica vs. implantación de pasturas africanas. Ecología Austral. 2023;33(2):489–506 (In Spanish). doi:10.25260/EA.23.33.2.0.2156. [Google Scholar] [CrossRef]
8. Estelrich HD, Castaldo A. Receptividad y carga ganadera en distintas micro regiones de la provincia de La Pampa (Argentina) y su relación con las precipitaciones. SEMIÁRIDA Rev Fac Agron, UNLPAM. 2014;24:7–19 (In Spanish). [Google Scholar]
9. Vázquez PM, Rojas MC, Fernández EA, González AC. Cuantificación de la disponibilidad de agua en la región criadora de la provincia de La Pampa. Bol Prod An. 2021(3):19–23 (In Spanish). Available from: http://hdl.handle.net/20.500.12123/10766. [Accessed 2024]. [Google Scholar]
10. Cheli GH, Pazos GE, Flores GE, Corley JC. Efecto de los gradientes de pastoreo ovino sobre la vegetación y el suelo en Península Valdés, Patagonia Argentina. Ecol Austral. 2016;26(2):200–11 (In Spanish). doi:10.25260/EA.16.26.2.0.237. [Google Scholar] [CrossRef]
11. Hess B, Dreber N, Liu Y, Wiegand K, Ludwig M, Myer H, et al. PioLaG: un generador de paisajes de piosferas para el modelado de pastizales de sabana. Landscape Ecol. 2020;35(9):2061–82 (In Spanish). doi:10.1007/s10980-020-01066-w. [Google Scholar] [CrossRef]
12. Shahriary E, Langford RP, Gill TE, Hussein M, Hargrove WL, Golding P. Variación de la distribución en las comunidades vegetales alrededor de la piosfera de Lajaneh, Irán. Arid Land Res Manage. 2020;35(1):32–54 (In Spanish). doi:10.1080/15324982.2020.1783025. [Google Scholar] [CrossRef]
13. Shezi T, O’Connor T, Witkowski E. Impacto de la intensidad del pastoreo del ganado en la diversidad vegetal de los pastizales montañosos en el norte de Drakensberg. Sudáfrica. Revista Africana de Ciencias de los Pastizales y los Forrajes. 2021;38(1):67–79 (In Spanish). doi:10.2989/10220119.2020.183795. [Google Scholar] [CrossRef]
14. Siyabulela S, Tefera S, Wakindiki I, Keletso M. Comparison of grass and soil conditions around water points in different land use systems in semi-arid South African rangelands and implications for management and current rangeland paradigms. Arid Land Res Manage. 2020;34(2):207–30. doi:10.1080/15324982.2019.1670279. [Google Scholar] [CrossRef]
15. Morici EFA, Kin AG, Mazzola MB, Ernst R, Poey MS. Efecto del pastoreo sobre las gramíneas perennes Piptochaetium napostaense y Poa ligularis en relación con la distancia a la aguada. SEMIÁRIDA Rev Fac Agron. 2006;17:1–13 (In Spanish). [Google Scholar]
16. Martin M, Morici EFA, Petruzzi H. Efecto del tiempo de pastoreo sobre el banco de semillas y los parámetros estructurales de Piptochaetium napostaense. SEMIÁRIDA Rev Fac Agron. 2018;28(2):9–15 (In Spanish). doi:10.19137/semiarida.2018(02).915. [Google Scholar] [CrossRef]
17. Rabotnikof C, Sáenz A, Cerqueira E. Intensidad de defoliación y floración en Poa ligularis en dos condiciones del bosque de caldén y en dos años con distinta precipitación invierno primaveral. Revista argentina de producción animal. 1998;18(1):167–8 (In Spanish). [Google Scholar]
18. Frasinelli CA, Terenti OA, Del Castello E. Consumo, digestibilidad in vivo y calidad de Poa ligularis Ness. Ap.Steudel durante el invierno. Revista argentina de producción animal. 2000;20(1):206–7 (In Spanish). [Google Scholar]
19. Hernández AP, Bautista CM, Vite RAM, Cortes JGB, Paulino AE. Pastoreo Racional Voisin como una herramienta para la ganaderia sustentable. Brazil J Dev. 2024;10(1):1402–19 (In Spanish). doi:10.34117/bjdv10n1-091. [Google Scholar] [CrossRef]
20. Sala OE. The effect of herbivory on vegetation structure. In: Werger MJA, van der Aart PJM, During HJ, Verhoeven JTA, editors. Plant form and vegetation structure. Amsterdam: SPB Academic Publishing; 1986. p. 317–30. [Google Scholar]
21. Cano E, Casagrande G, Conti H, Salazar Lea Plaza J, Peña Zubiate C, Maldonado Pinedo D, et al. Inventario integrado de los recursos naturales de la provincia de La Pampa. Clima, Geomorfología, Suelo y Vegetación INTA; 1980. p. 19–88 (In Spanish). Available from: https://repo.unlpam.edu.ar/handle/unlpam/5808. [Accessed 2024]. [Google Scholar]
22. Bucher EH. Herbivory in arid and semiarid regions of Argentina. Rev Chil Hist Natural. 1987;60:265–73. [Google Scholar]
23. Deregibus VA. Importancia de los pastizales naturales en la República Argentina: situación presente y futura. Rev Arg Prod Anim. 1988;8:67–78 (In Spanish). [Google Scholar]
24. Milchunas DG, Sala OE, Lauenroth WK. A generalized model of the effects of grazing by large herbivores on grassland community structure. Am Nat. 1988;132(1):87–106. doi:10.1086/284839. [Google Scholar] [CrossRef]
25. Cingolani AM, Noy-Meir I, Díaz S. Grazing effects on rangeland diversity: a synthesis of contemporary models. Ecol Appl. 2005;15(2):757–73. doi:10.1890/03-5272. [Google Scholar] [CrossRef]
26. Briske DD. Developmental morpholory and physiology of grasses. In: Heitschmitdt RK, Stuth JW, editors. Grazing management: an ecological perspective. Portland: Timber Press; 1991. p. 11–26. [Google Scholar]
27. Xia JX, Hodgson J, Chu ACP. Effects of severity of grazing on tissue turnover in Matua prairie grass dairy pasture. New Zeal J Agr Res. 1994;37(1):41–50. doi:10.1080/00288233.1994.9513039. [Google Scholar] [CrossRef]
28. Matthew C, Lemaire G, Sackville Hamilton NR, Hernández Garay A. A modified self-thinning equation to describe size/density relationships for defoliated swards. Ann Bot. 1995;76(6):579–87. doi:10.1006/anbo.1995.1135. [Google Scholar] [CrossRef]
29. Briske DD. Strategies of plant survival in grazed systems: a functional interpretation. In: Hordgson J, Illius AW, editors. The ecology and management of grazing systems. New York: CAB International; 1996; p. 37–66. [Google Scholar]
30. Briske DD, Derner JD. Clonal biology of caespitose grasses. In: Cheplick GP, editor. Population biology of grasses. UK: Cambridge University Press; 1998. p. 106–35. [Google Scholar]
31. Assuero SG, Tognetti JA. Tillering regulation by endogenous and environmental factors and its agricultural management. Am J Plant Sci Biotechnol. 2010;4(1):35–48. [Google Scholar]
32. Estelrich HD, Martin F, Ernst RD. Posición de las coronas como mecanismo para tolerar el pastoreo en especies forrajeras del pastizal bajo en la región semiárida central de Argentina. Arch zootec. 2016;65:381–8 (In Spain). [Google Scholar]
33. Di Rienzo JA, Casanoves F, Balzarini MG, González L, Tablada M, Robledo CW. Centro de Transferencia InfoStat. Argentina: FCA, Universidad Nacional de Córdoba; 2018 (In Spanish). Available from: http://www.infostat.com.ar. [Accessed 2024]. [Google Scholar]
34. Andrew MH. Grazing impact in relation to livestock watering points. Trends Ecol Evol. 1988;3(12):336–9. doi:10.1016/0169-5347(88)90090-0. [Google Scholar] [PubMed] [CrossRef]
35. Rajper AM, Willing BP, Cahill JF, Bork EW, Chang SX, Carlyle CN. Drought and defoliation affect soil extracellular enzyme activity in northern temperate grasslands. J Arid Envir. 2024;8(223):105197. doi:10.1016/j.jaridenv.2024.105197. [Google Scholar] [CrossRef]
36. Busso CA, Richards JH, Chatterton NJ. Nonstructural carbohydrates and spring regrowth of two cool-season grasses: interaction of drought and clipping. J Range Manag Arch. 1990;43(4):336–43. [Google Scholar]
37. Anderson VJ, Briske DD. Herbivore-induced species replacement in grasslands: is it driven by herbivory tolerance or avoidance? Ecol Appl. 1995;5(4):1014–24. doi:10.2307/2269351. [Google Scholar] [CrossRef]
38. Saint Pierre C. Capacidad competitiva y tolerancia a la defoliación en Stipa clarazii, Stipa tenuis y Stipa ambigua (M.Sc. Thesis). Universidad Nacional del Sur: Bahía Blanca, Argentina; 2002 (In Spanish). Available from: http://www.scielo.org.ar/scielo.php?. [Accessed 2024]. [Google Scholar]
39. Souto CP, Becker GF, Siffredi GL, Busso CA, Sternberg M. Axillary bud viability and dry matter production of Poa ligularis in Patagonian grasslands. Phyton-Int J Exp Bot. 2004;73:39–51. [Google Scholar]
40. Dalgleish HJ, Hartnett DC. The effects of fire frequency and grazing on tallgrass prairie productivity and plant composition are mediated through bud bank demography. Plant Ecol. 2009;201(2):411–20. doi:10.1007/s11258-008-9562-3. [Google Scholar] [CrossRef]
41. Peláez DV, Bóo RM, Mayor MD, Elia OR, Cardona NM. Effect of post-fire defoliation on bud viability and plant mortality of Piptochaetium napostaense (Speg.) Hack. and Poa ligularis Ness. J Arid Environ. 2009;73:708–12. [Google Scholar]
42. N’Guessan M, Hartnett DC. Differential responses to defoliation frequency in little bluestem (Schizachyrium scoparium) in tallgrass prairie: implications for herbivory tolerance and avoidance. Plant Ecol. 2011;212(8):1275–85. doi:10.1007/s11258-011-9904-4. [Google Scholar] [CrossRef]
43. Gastal F, Lemaire G. Defoliation, shoot plasticity, sward structure and herbage utilization in pasture: review of the underlying ecophysiological processes. Agriculture. 2015;5(4):1146–71. doi:10.3390/agriculture5041146. [Google Scholar] [CrossRef]
44. Cerrato BE, Elizalde MA, Ernst RD, Estelrich HD, Suarez CE, Morici EFA. Cambios en aspectos morfométricos de forrajeras nativas en diferentes situaciones de pastoreo. Un modelo conceptual. Revista de Divulgación Técnica Agropecuaria. Agroindustrial y Ambiental Facultad de Ciencias Agrarias. 2016;3(4):64 (In Spanish). [Google Scholar]
45. Ganderats S, Hepp C. Mecanismos de crecimiento de Lolium perenne, Festuca arundinacea y Dactylis glomerata en la zona intermedia de Aysén. Agricultura Técnica. 2003;63(3):259–65 (In Spanish). doi:10.4067/S0365-28072003000300005. [Google Scholar] [CrossRef]
46. Colabelli M, Agnusdei M, Mazzanti A, Labreveux M. El proceso de crecimiento y desarrollo de gramíneas forrajeras como base para el manejo de defoliación. Sitio argentino de producción animal. Boletín Técnico N° 148; 1998 (In Spanish). Disponible en: https://www.produccion-animal.com.ar/produccion_y_manejo_pasturas/pastoreo%20sistemas/01-proceso_crecimiento.pdf [Accessed 2023]. [Google Scholar]
47. Duchini PG, Guzatti GC, Ribeiro Filho HMN, Sbrissia AF. Tiller size/density compensation in temperate climate grasses grown in monoculture or in intercropping systems under intermittent grazing. Grass Forage Sci. 2014;69(4):655–65. doi:10.1111/gfs.12095. [Google Scholar] [CrossRef]
48. Silva LS, Silva VJ, Yasuoka JL, Sollenberger LE, Pedreira CGS. Tillering dynamics of ‘Mulato II’ brachiariagrass under continuous stocking. Crop Sci. 2020;60(2):1105–12. doi:10.1002/csc2.20008. [Google Scholar] [CrossRef]
49. Melak Y, Angassa A, Abebe A. Effects of grazing intensity to water source on grassland condition, yield and nutritional content of selected grass species in Northwest Ethiopia. Ecol Process. 2019;8(1):8–12. doi:10.1186/s13717-019-0162-z. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools