 Open Access
Open Access
ARTICLE
Heterosis Analysis in Endogenous Substances in Root Bleeding Sap of Sorghum
1 College of Agriculture, Jilin Agricultural University, Changchun, 130118, China
2 Jilin Academy of Agricultural Sciences, Changchun, 130033, China
3 Jilin Engineering Vocational College, Siping, 136001, China
* Corresponding Authors: Zhian Zhang. Email: ; Ziyang Zhou. Email:
Phyton-International Journal of Experimental Botany 2024, 93(8), 1963-1980. https://doi.org/10.32604/phyton.2024.053072
Received 23 April 2024; Accepted 09 July 2024; Issue published 30 August 2024
Abstract
Despite hybrid dominance contributing to the genetic improvement of crops, little is known about heterosis and inheritance patterns of endogenous substances in sorghum (Sorghum bicolor (L.) Moench) root bleeding sap. In this study, six sterile and six restorer lines of sorghum and 36 hybrid sorghum combinations formulated as incomplete double-row crosses were selected as test materials, and heterosis, combining ability, heritability, and their interrelationships of root bleeding sap endogenous substances in different hybrid sorghum combinations and their parents were investigated. The results showed that the root bleeding sap of the F1 generation of hybrid sorghum had a high heterosis in both soluble sugar content and amino acid content at the flowering stage, and the average high-parent heterosis was 129.34% and 74.57%, respectively. Indole-3-acetic acid (IAA), cytokinins (CTK), gibberellic acid (GA3), abscisic acid (ABA), soluble sugar, amino acid, and root bleeding intensity were mainly affected by non-additive genetic effects of the genes. Soluble protein was affected by additive genetic effects of the genes and had a high narrow heritability (75.50%), which could be selected at low generations in breeding. The combining ability analyses showed that the sterile lines 521A and 170A, and the restorer lines Ji318R and 0–30 were promising parents with high general combining ability. Correlation analysis showed that all endogenous substances of root bleeding sap were positively correlated with the sum of parental general combining ability (GCA) at highly significant levels, and IAA, CTK, GA3, ABA, soluble sugar, amino acid, and root bleeding intensity were positively correlated with male GCA at significant or highly significant levels. Therefore, the GCA of the restorer lines root bleeding sap endogenous material or the sum of both parents’ GCA can be used to predict the performance of wounding endogenous material in the F1 generation of hybrid sorghum. Overall, this study results can help elucidate heterosis mechanisms of root bleeding sap endogenous material and improve sorghum quality.Keywords
Sorghum (Sorghum bicolor (L.) Moench) and its products are integral to human life and are extensively studied by researchers worldwide [1,2]. Although numerous studies have focused on optimizing plant morphology and enhancing leaf photosynthetic capacity to boost sorghum yield, the crucial role of the transport mechanism of the root system as plant source stores has often been overlooked [3,4]. The plant root system absorbs minerals and water from the soil to support plant growth and synthesizes essential plant nutrients [5]. The root system is one of the three major nutrient metabolism organs in plants, performing vital functions such as nutrient absorption, plant immobilization, information transmission, and organic matter synthesis [6–8]. As a control center and sensory organ in crop growth and development, the life processes of the root system are intricately linked to those of the entire plant [9,10].
Endogenous plant hormones play important regulatory roles at various stages of plant growth and development. These hormones include indole-3-acetic acid (IAA), abscisic acid (ABA), gibberellic acid (GA3), cytokinins (CTK), and ethylene (ETH), which significantly affect plant growth and development at low concentrations [11,12]. The main functions of endogenous plant hormones include promoting or inhibiting cell division, elongation, and differentiation, and influencing processes such as germination, rooting, flowering, fruiting, sex determination, dormancy, and abscission [13,14]. Although these hormone levels in plants are very low, their physiological effects are complex and diverse, thereby achieving fine regulation of plant growth and development [15]. Soluble sugars, proteins, and free amino acids play important roles in plant metabolic activities [16]. Soluble sugars are energy sources and structural materials in plants and play key regulatory roles in seed germination, growth, development, maturation, and senescence. Soluble proteins are gene expression products and play a role in plant metabolic activities, and their levels indicate the intensity of physiological and biochemical organ functions [17,18]. Amino acids are the basic units of proteins, and the nitrogen absorbed by plant roots is mainly transported as amino acids and amides. Therefore, determining different endogenous substance levels in the root wounding fluid is necessary for root physiological studies [19,20]. Furthermore, heterosis in root physiological traits requires further research to improve plant variety.
Nie et al. found that as the yield increases in sorghum varieties, the intensity of root bleeding sap also increases [21]. Hoecker et al. observed heterosis during early maize root development shortly after germination [22]. Zhang et al. discovered heterosis in the diversity and composition of root microorganisms in hybrid rice compared to its parents [23]. Karaağaç found that root length and weight inheritance were mainly influenced by additive genes, while grafting success was controlled by both additive and non-additive genes in cucurbit rootstock studies [24]. Zhang revealed that genetic enhancements in sorghum genes are the primary drivers of yield growth, with root physiological characteristics undergoing significant changes as the yield increases [25]. Moreover, sorghum varieties can improve their mineral and water absorption abilities from the soil by enhancing the intensity of the root bleeding sap, ultimately leading to enhanced photosynthesis, increased biomass, and higher yields. Liu noted that both root bleeding sap volume and aboveground biomass in soybeans increased over successive breeding years, showing a strong positive correlation between the two, suggesting that genetic improvements in soybean varieties boost aboveground biomass and strengthen root vigor [26]. Deng et al. study demonstrated a gradual increase in soluble sugar content in soybean root bleeding sap as breeding years progressed [27]. However, currently, there is limited research on root physiological characteristics regarding sorghum genetic improvement, and the heterosis and inheritance patterns of sorghum root bleeding sap intensity and endogenous substance content remain unclear. Understanding the genetic characteristics of hybrid sorghum combinations and their parent varieties is important for the breeding of sorghum species. Therefore, further exploration of heterosis and inheritance patterns of root physiological characteristics in hybrid sorghum combinations and their parent varieties is crucial for advancing sorghum breeding practices.
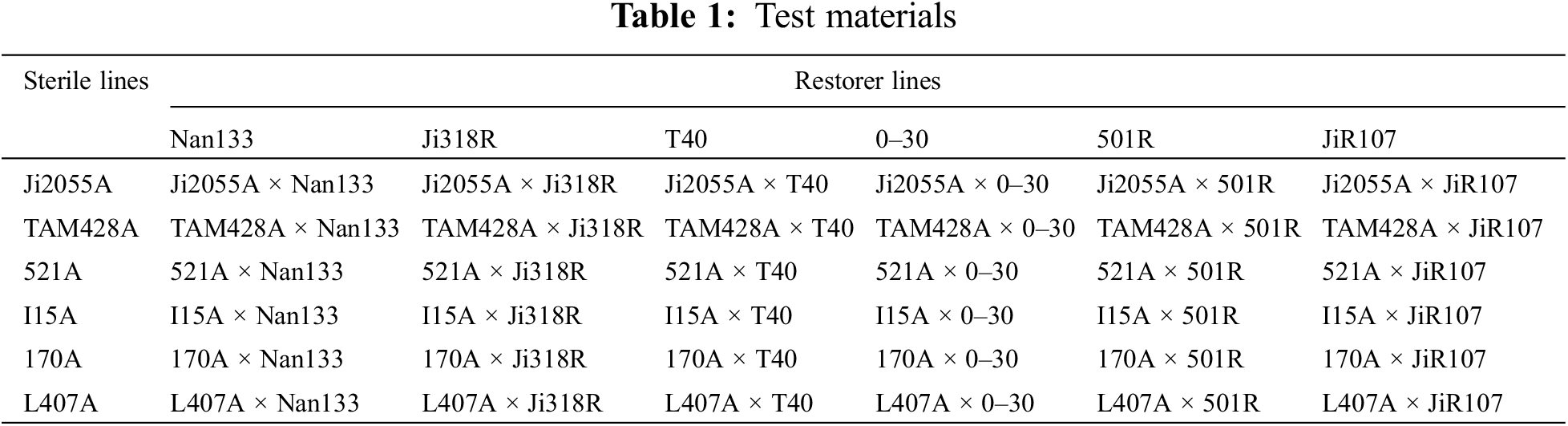
2.1 Experimental Materials and Design
Six sterile sorghum lines (Ji2055A, TAM428A, 521A, I15A, 170A, and L407A) and six restorer lines (Nan133, Ji318R, T40, 0–30, 501R, and JiR107) were selected to create 36 hybrid combinations using the NCII incomplete biallelic genetic mating design method (Table 1). These hybrids, along with their parents, were planted separately in the F1 generation on 13 May 2023, at the Sorghum Germplasm Innovation Experimental Base in the Gongzhuling Campus of the Jilin Academy of Agricultural Sciences (latitude: 43°29′N; longitude: 124°48′E). The field experiment followed a randomized block design with six rows, row spacing of 0.65 m, row length of 5 m, and three replications, totaling 144 plots, with each plot covering an area of 19.5 m2. Seedlings were transplanted at the 5-leaf stage at a density of 120,000 per hectare and harvested on 29 September 2023 under standard field cultivation and management.
In 2023, the experimental site had black soil, and the previous crop grown was soybeans. The soil in the 0–40 cm tillage layer had an organic matter content of 22.40 g/kg, quick-acting nitrogen content of 75.70 mg/kg, quick-acting phosphorus content of 42.10 mg/kg, quick-acting potassium content of 120.30 mg/kg, and a pH value of 6.43. The effective cumulative temperature (≥10°C) in 2023 was 3087.50°C. The average daily temperature and total precipitation during the reproductive period (May to October) were 20.18°C ± 4.59°C and 660.20 mm, respectively. Fig. 1 shows the average daily temperature and precipitation. The meteorological data for the experimental area were sourced from an automatic meteorological monitoring system at the study site.
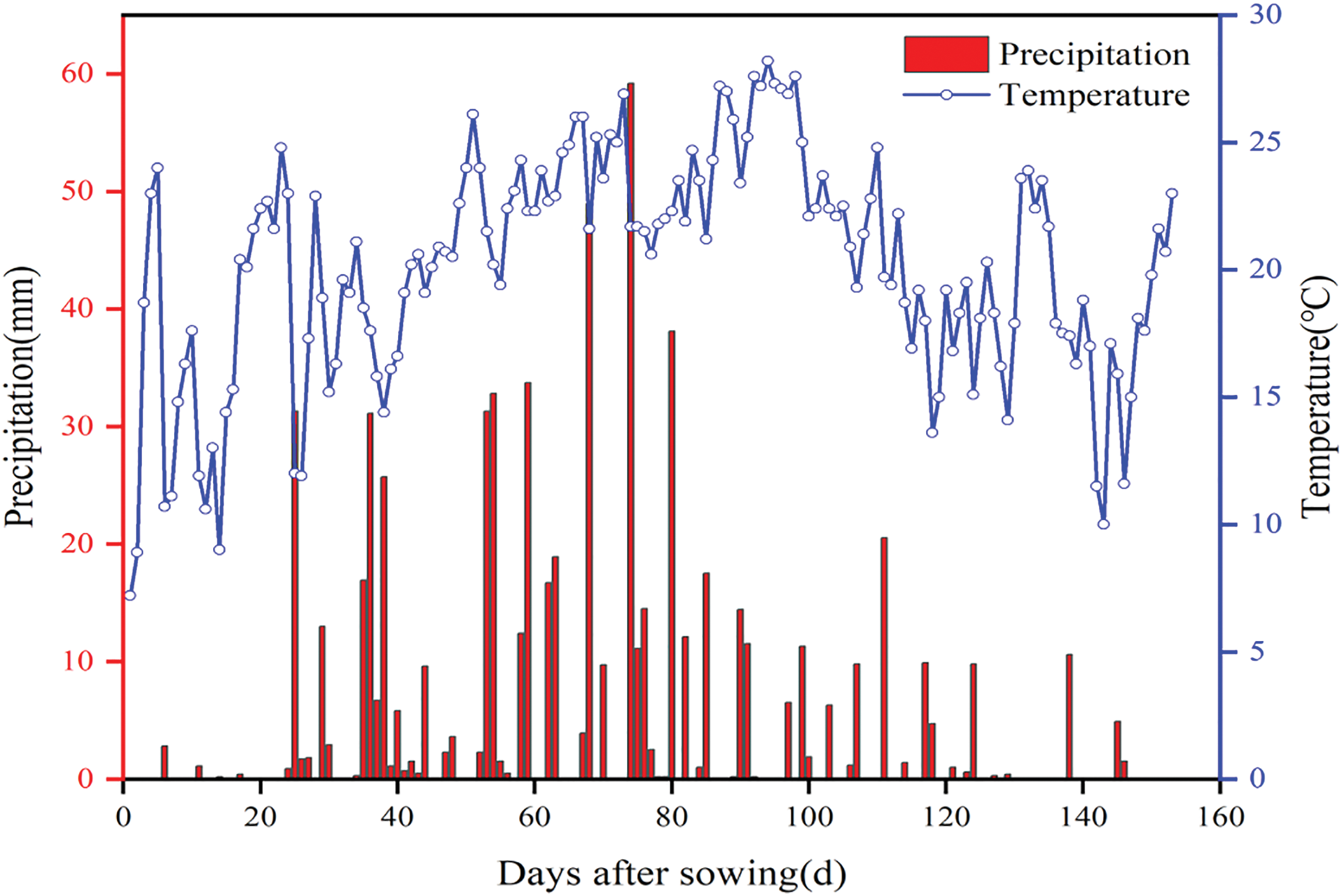
Figure 1: Average daily temperature and precipitation in the whole growth period of sorghum
2.3.1 Determination of Root Bleeding Intensity
Root bleeding sap was collected in the morning on sunny days at different growth stages of sorghum: jointing, flowering, and ripening. Cotton, plastic bags, and rubber sleeves were assembled, weighed, and recorded as W1 (g). Three sorghum plants with similar growth rates were selected from each sample. Plants were cut 5 cm above the ground, cleaned, and dried using distilled water and blotting paper. Pre-weighed cotton was placed against the cuts, sealed in a plastic bag, and tied with a rubber sleeve to prevent sap volatilization. After 2 h, the cotton was removed, weighed as W2 (g), and transferred to a laboratory. The root bleeding sap was stored at −70°C for further analysis. The formula used to calculate bleeding intensity was: bleeding intensity (g·h−1·p−1) = (W2 − W1)/2.
2.3.2 Determination of Osmotic Adjustment Substance and Hormone Content in Root Bleeding Sap
IAA, GA3, CTK, and ABA levels were determined using an enzyme-linked immunoassay (ELISA) [28]. The soluble sugar content of the root bleeding sap was determined using the anthrone-sulfuric acid method described by Quan et al. [29]. Soluble protein content was measured using the Coomassie Brilliant Blue G-250 method described by Guzel et al. [30]. Free amino acid content was determined using the ninhydrin colorimetry method described by Sun et al. [31].
Raw data collation, analysis of differences in root traits between hybrid sorghum combinations and their parents, and heterosis analyses were performed using Excel 2016. Heterosis was calculated using the following formula:
Medium parental dominance (MPH) = (F1 − MP)/MP × 100%
High parental dominance (HPH) = (F1 − HP)/HP × 100%
where F1 is the mean value of hybrid combination traits, MP is the mean value of biparental traits, and HP is the mean value of optimal parental traits.
Statistical analysis of the genetic mating design was performed using DPS 7.05 software to estimate the general combining ability (GCA) of the parents, the special combining ability (SCA) of each cross combination, and their genetic parameters. Analysis of variance (ANOVA) and significance tests were performed using Origin 2021 (Originlab Corp., Northampton, MA, USA) and SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
3.1 Changes in Endogenous Substances in Sorghum Root Bleeding Sap at Different Fertility Periods
The results illustrated in Fig. 2 shows that IAA and GA3 levels in the root bleeding sap of sorghum exhibited an increasing pattern, followed by a decrease throughout the reproductive stages, peaking at flowering and declining at ripening. Moreover, the F1 generation of the hybrids displayed higher levels of IAA and GA3 than the parent plants across all three periods. In contrast, CTK content showed a steady increase, with no significant differences between the F1 generation at the jointing and flowering stages, although the former had lower CTK levels than its parents. This suggests that positive heterosis was not observed in CTK content at the jointing and flowering stages. Additionally, ABA levels in the hybrid F1 generation peaked at flowering, slightly decreased at ripening, and did not exhibit significant differences compared with its parents at the jointing and ripening stages. However, at the flowering stage, the ABA content of the F1 generation showed heterosis, which was notably higher than that of the parent plants. Soluble sugars, soluble proteins, and amino acids gradually increased, peaking during the ripening stage. The synthesis of these compounds in the root bleeding sap is primarily observed during the middle and late reproductive stages. The hybrid F1 generation displayed 196.37% and 131.58% higher soluble sugar contents than the mother and father, respectively, at the flowering stage. However, soluble proteins and amino acids did not exhibit heterosis at different reproductive stages. Interestingly, the F1 hybrid generation showed a higher heterosis in soluble protein content at the jointing and flowering stages. Moreover, the amino acid content of the hybrid F1 generation displayed the highest heterosis at the ripening stage, particularly at the flowering stage. As the reproductive period of sorghum progressed, root bleeding intensity increased and then decreased, peaking at the flowering stage suggesting that during the flowering stage, the sorghum root system is more robust and transports more nutrients to the aboveground parts. In conclusion, further studies on the heterosis of endogenous substances in the root bleeding sap during the flowering stage of sorghum are required.
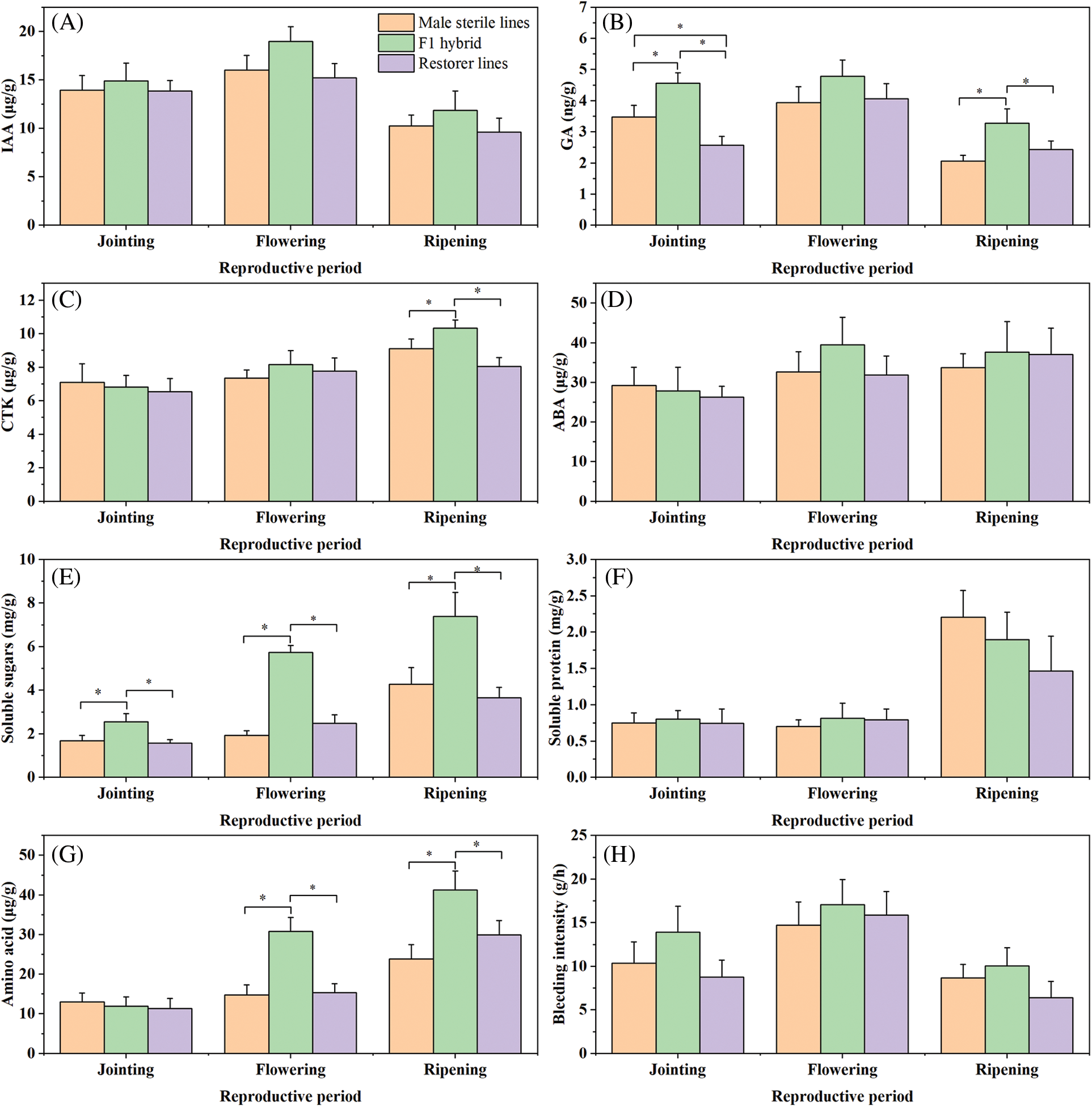
Figure 2: Changes of IAA (A), GA3 (B), CTK (C), ABA (D), soluble sugar (E), soluble protein (F), amino acid (G), bleeding intensity (H) in root bleeding sap of sorghum at different fertility periods. *Significant at p < 0.05
3.2 Differences in Endogenous Substances in Flowering Stage Sorghum Root Bleeding Sap
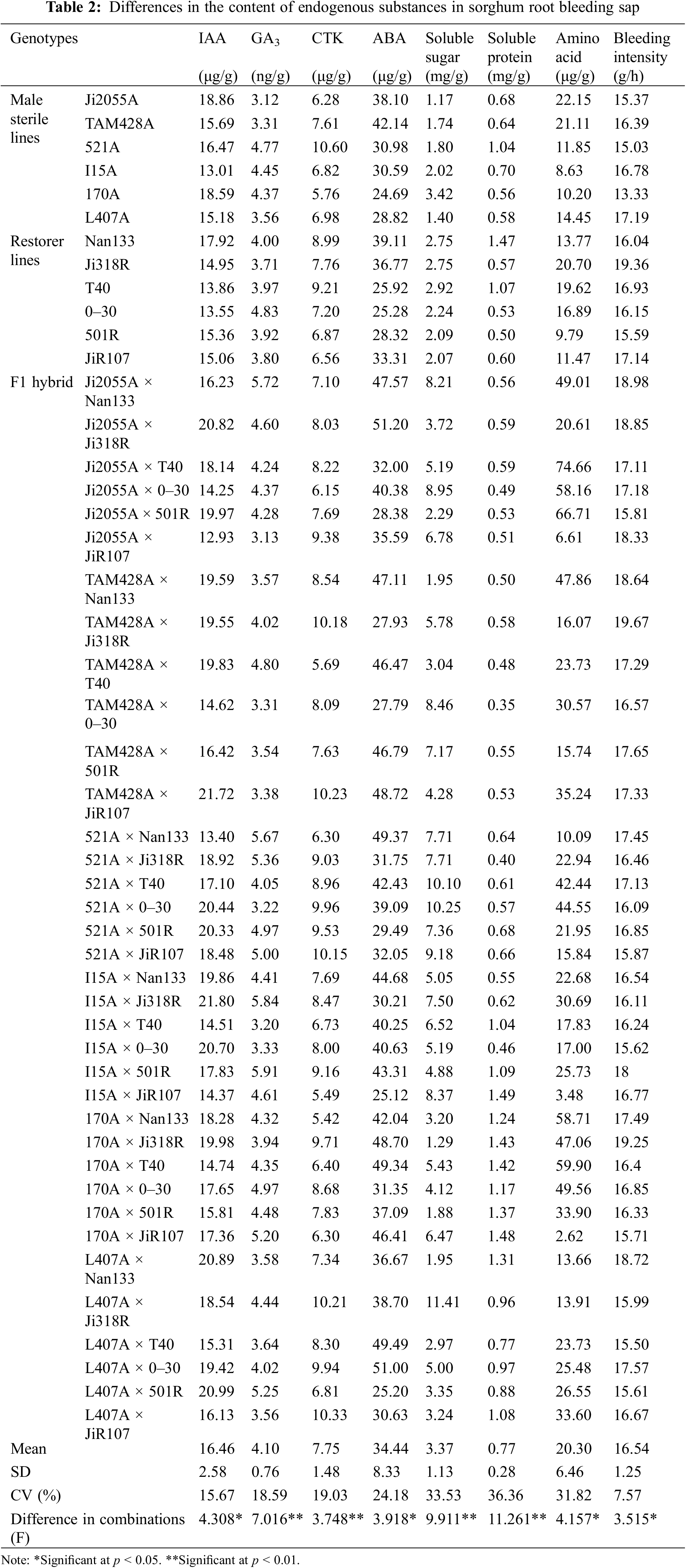
As shown in Table 2, there were differences between the groups at significant or highly significant levels (F-values) regarding endogenous substances content in the 12 parents, as well as in the eight root bleeding sap samples of the 36 hybrid sorghum combinations that were grouped together. The coefficient of variation (CV) was highest for soluble protein (36.36%) and soluble sugar (33.53%), and lowest for root bleeding intensity (7.57%). Moreover, this study revealed that the inheritance of these traits is complex and not solely based on additive effects. Notably, the F1 generation of hybrids did not always exhibit the lowest or highest content compared with the parental groups. Furthermore, the mean values of endogenous substances in the root-bleeding sap were higher in the hybrid sorghum combinations than in their parents, indicating the presence of heterosis.
3.3 Heterosis Analysis of Endogenous Substances in Root Bleeding Sap of Hybrid Sorghum Combinations
Mid-parent heterosis of the eight root bleeding sap components in the 36 sorghum hybrid combinations is illustrated in Fig. 3A. Among the traits, soluble sugars and amino acids exhibited significantly higher mean mid-parent heterosis than other traits. Specifically, the mean mid-parent heterosis for soluble sugars in sorghum hybrid root bleeding sap was 172.06%, ranging from −58.07% to 449.88%. Similarly, the mean mid-parent heterosis for amino acids in the hybrid root bleeding sap was 106.47%, ranging from −75.81% to 389.67%. For IAA, GA3, CTK, ABA, soluble sugar, and root bleeding intensity, the mean mid-parent heterosis in the hybrid root-bleeding sap was 15.22%, 9.62%, 9.37%, 24.62%, 17.95%, and 5.18%, respectively. Consequently, it was observed that the mid-parent heterosis of soluble sugar and amino acid was more pronounced compared with other endogenous substances in root bleeding sap, with a greater variation among materials.
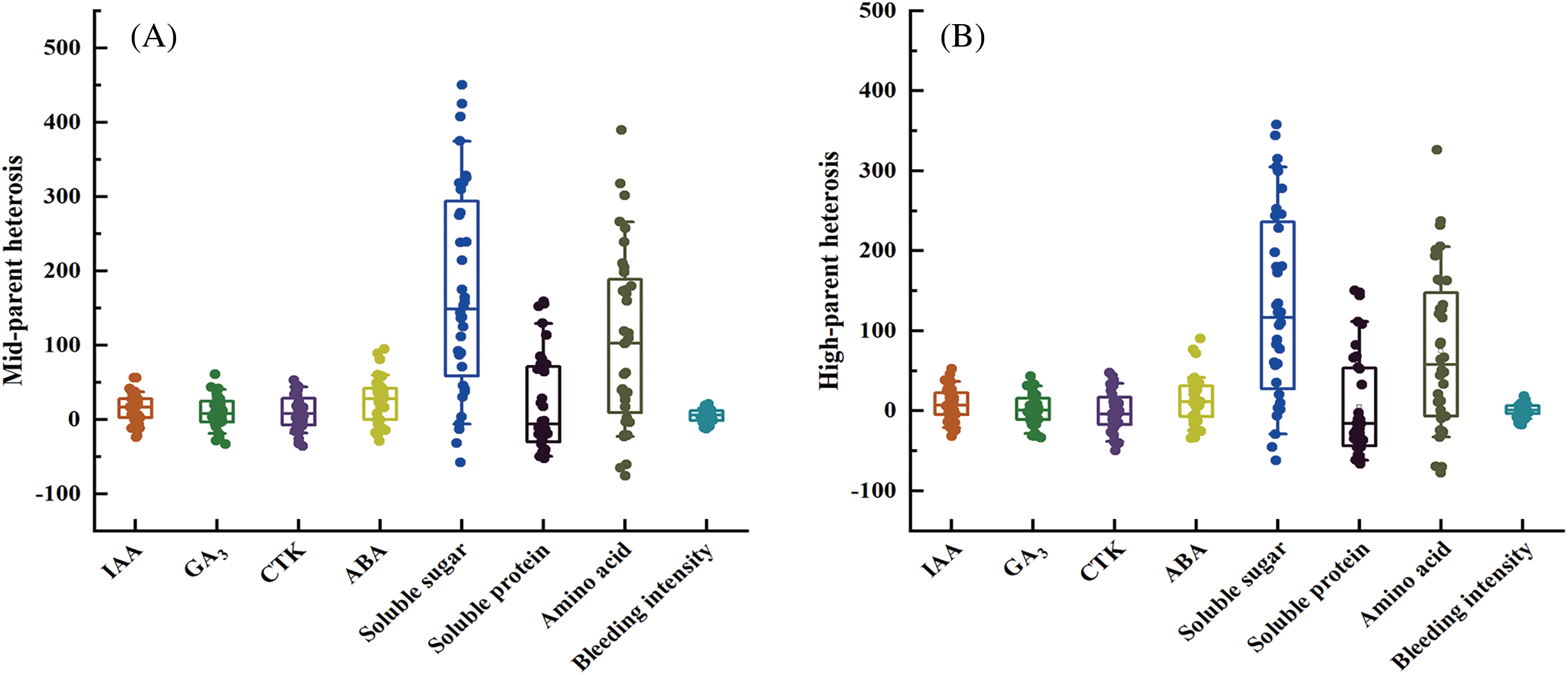
Figure 3: Heterosis analysis of each endogenous substance in sorghum root bleeding sap. (A) Mid-parent heterosis of hybrids with different parameters; (B) High-parent heterosis of hybrids with different parameters
The high parent heterosis of the eight root bleeding sap components in the 36 sorghum hybrid combinations is illustrated in Fig. 3B. It is evident that the high-parent heterosis of the hybrid combinations exhibited a trend similar to that of mid-parent heterosis. The average high-parent heterosis values for soluble sugars and amino acids in the root bleeding sap of the hybrids were 129.34% and 74.57%, respectively, which were significantly higher than those for other root bleeding sap endogenous substances. The mean high-parent heterosis values for IAA, GA3, CTK, ABA, soluble sugar, and root bleeding intensity in the root bleeding sap were 7.93%, 1.28%, −0.82%, 13.15%, 4.89%, and 0.22%, respectively. Therefore, the soluble sugar and amino acid contents in the root bleeding sap exhibited more pronounced high-parent heterosis, showing greater variability among the materials.
3.4 Relationship of Hybrid Sorghum Combinations to Various Endogenous Substances of the Parental Root Bleeding Sap
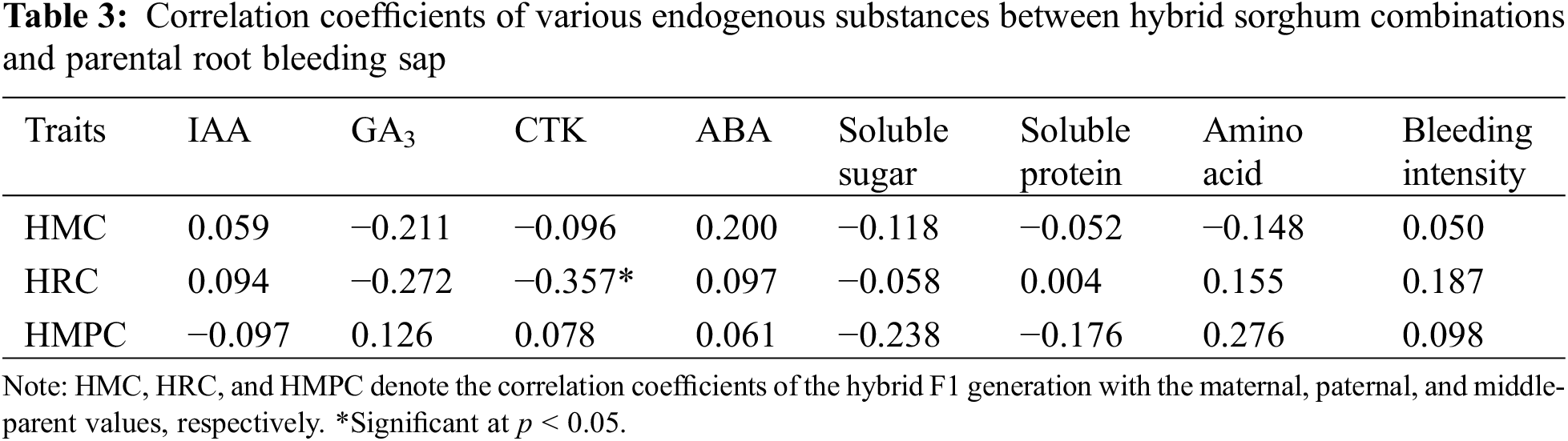
As shown in Table 3, CTK reached a significant level of negative correlation with its paternal trait, while all other traits did not reach a significant level with either parent, as well as with both parents’ means.
3.5 ANOVA Measured the Combining Ability of Each Endogenous Substance in Hybrid Sorghum Root Bleeding Sap
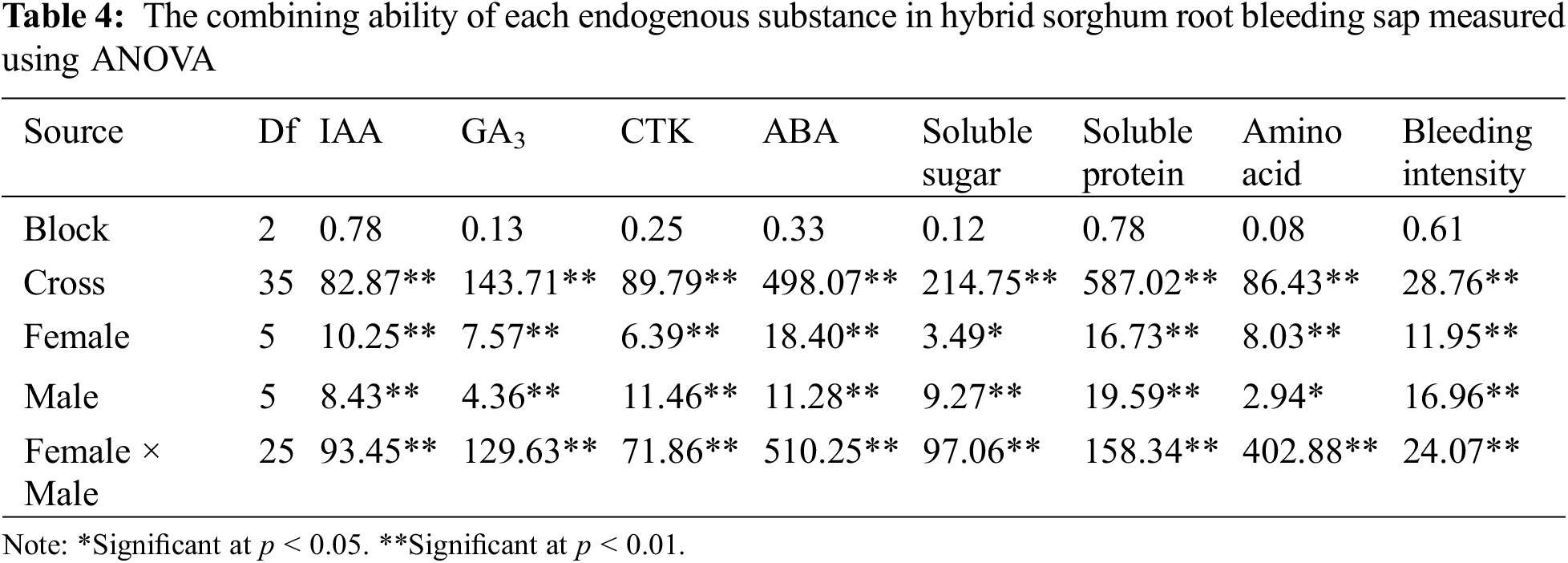
As shown in Table 4, the 36 tested hybrid sorghum combinations of root bleeding sap had significant or highly significant levels of inter-combination variance for each endogenous substance, and the differences between the zones were not significant. Furthermore, the combined ability measured by ANOVA showed that the variance in GCA for each endogenous substance in the root bleeding sap of both parents of hybrid sorghum combinations reached a significant or highly significant level of difference. The differences in the SCA variance for each endogenous substance in the root bleeding sap of the hybrid sorghum combinations were also highly significant. It can be indicated that there are real differences in the F1 generation of hybrid sorghum root bleeding sap for each endogenous substance, and these differences may be caused by genetic factors, so that the relative effect values of GCA of the parents and SCA among combinations can be further estimated.
3.6 GCA Analysis of Each Endogenous Substance in the Root Bleeding Sap of Hybrid Sorghum Parents
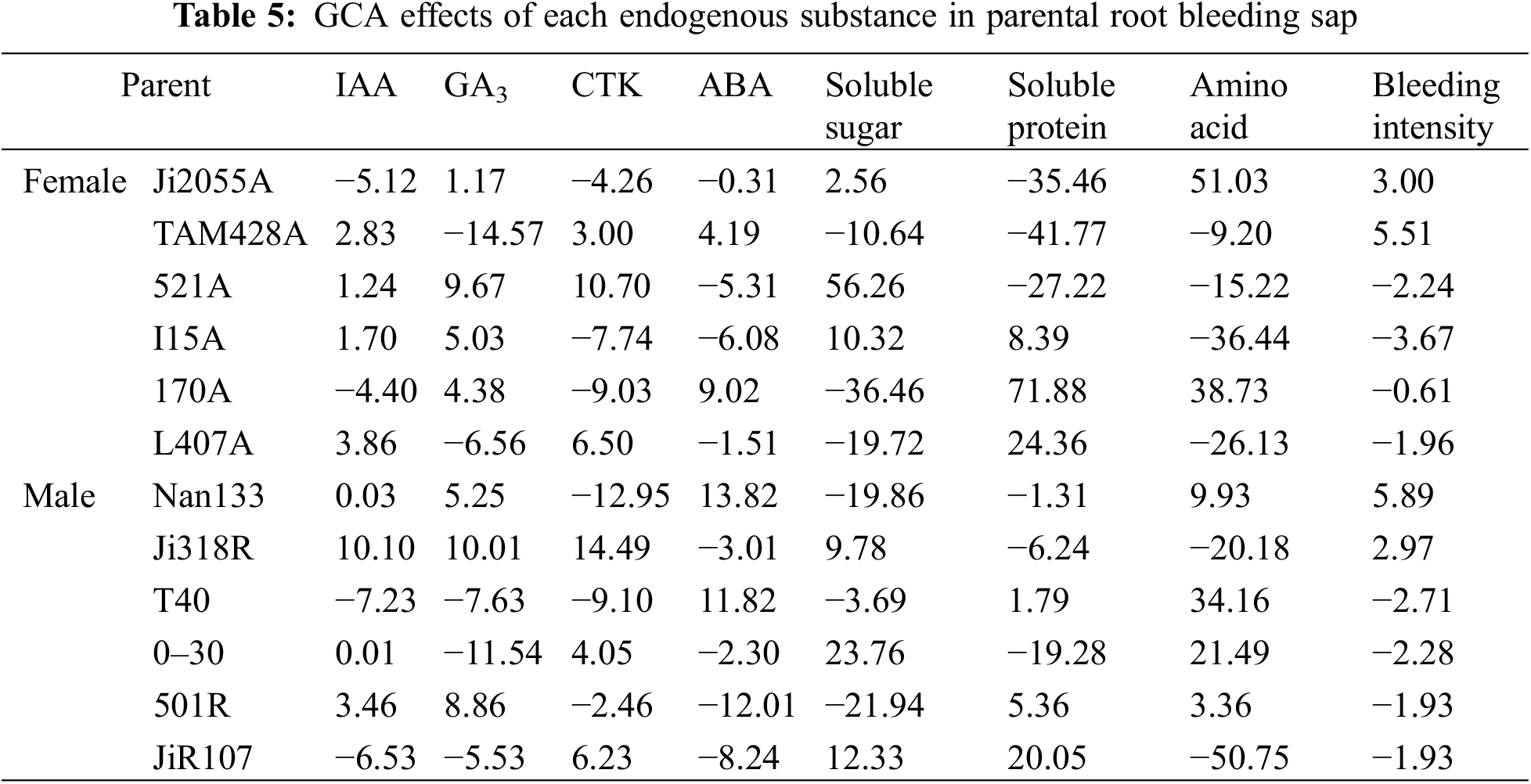
The genotypic effects of each endogenous substance, GCA, on the root-bleeding sap of hybrid sorghum parents varied among different parameter traits within the same parents and among different parents within the same parameter traits (Table 5). This suggests that the magnitude of the additive effects on the genetic effects of different endogenous substances in the hybrid sorghum parents differed. Among the sterile lines, 521A and 170A were the top performers, whereas Ji318R and 0–30 stood out among the restored lines.
3.7 SCA Analysis of Each Endogenous Substance in Hybrid Sorghum Root Bleeding Sap
The results of the SCA analysis for each endogenous substance in the root bleeding sap of the 36 sorghum combinations are presented in Table 6. The results show that the SCA values for each endogenous substance varied significantly among sorghum combinations with the same hybrid sorghum parents compared with those with different root bleeding sap from the same hybrid sorghum combinations. This suggests diverse interactions among genes controlling each endogenous substance in hybrid sorghum root bleeding sap. The top-performing combinations were TAM428A × JiR107, TAM428A × T40, TAM428A × 0–30, L407A × Ji318R, L407A × JiR107, I15A × JiR107, and I15A × 501R. Further comparison of the general combining ability effects of the hybrid sorghum parents with the specific combining ability effects of the combinations revealed no direct correlation between the general combining ability effects and the specific combining ability effects for each endogenous substance in the hybrid sorghum root bleeding sap.
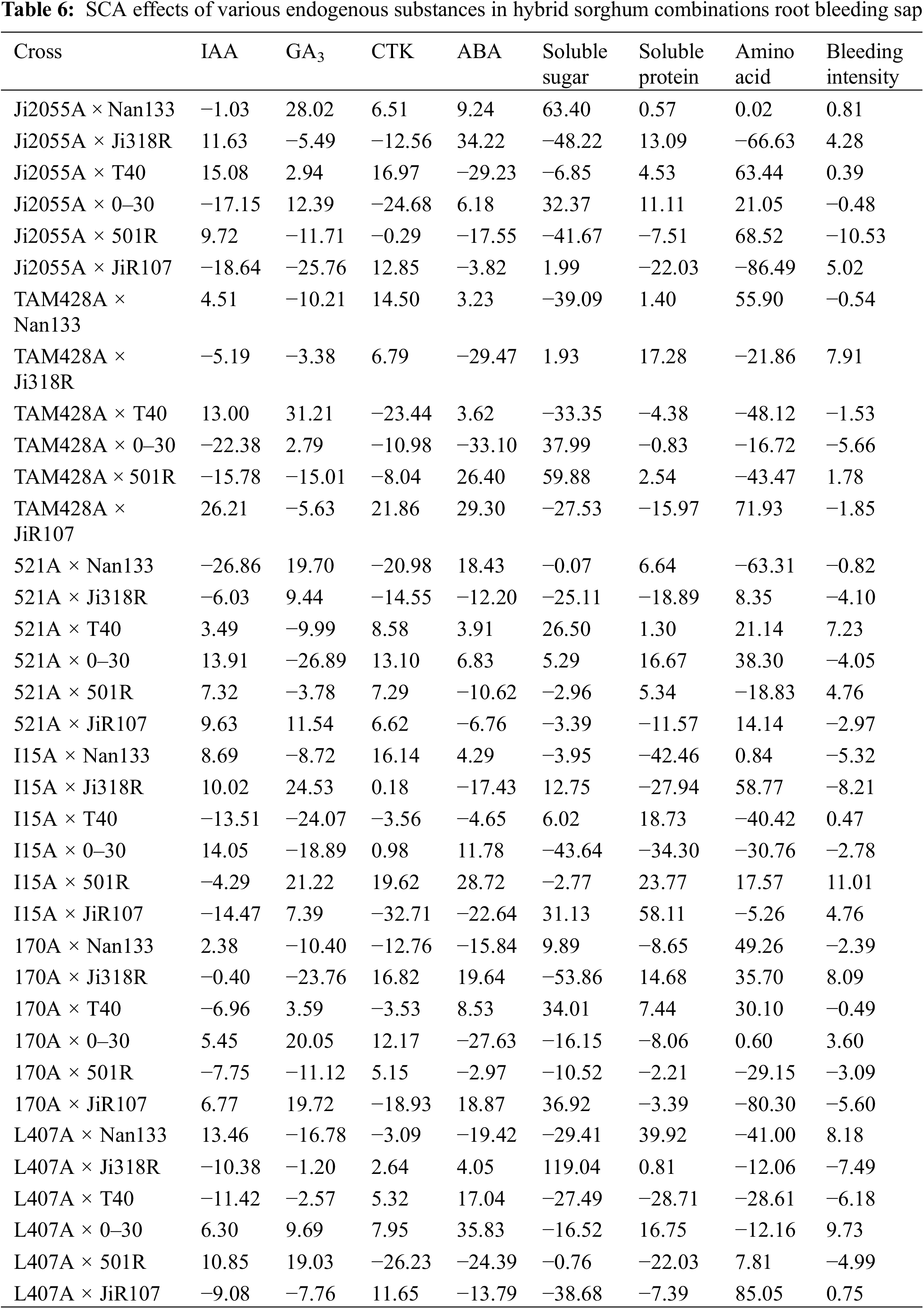
3.8 Correlation Analysis of Various Endogenous Substances with GCA in Hybrid Sorghum Parents Root Bleeding Sap
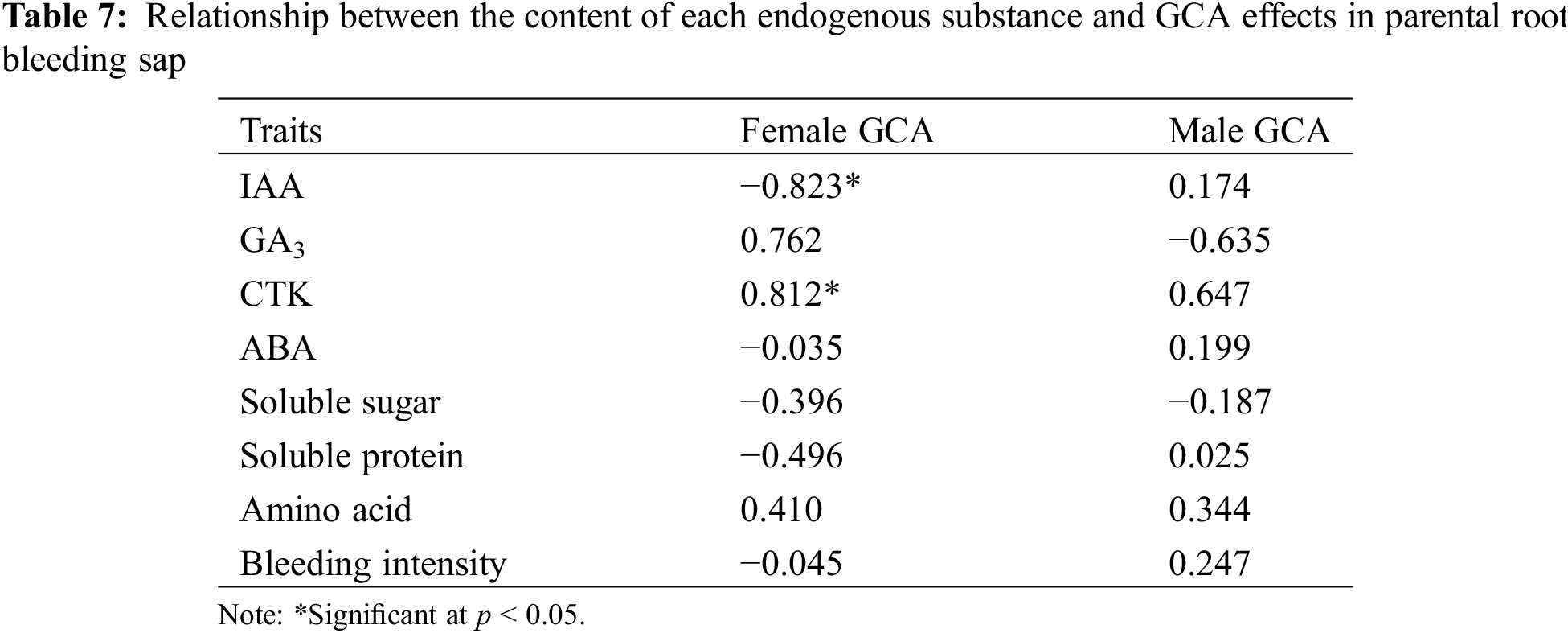
The relationship between the content of each endogenous substance in the root bleeding sap of hybrid sorghum parents and general combining ability effects is shown in Table 7, which shows that the IAA content of sterile lines in the hybrid sorghum parents was significantly negatively correlated with general combining ability effects; the correlation coefficient was −0.823, whereas the CTK content of the sterile lines and the general combining ability effects were significantly positively correlated, with a correlation coefficient of 0.812. This indicates that the general combining ability effects of hybrid sorghum sterile lines with high IAA content would be reduced accordingly, but the general combining ability effects would increase with high CTK content. Notably, no significant correlation was found between other endogenous substances in the root bleeding sap and the general combining ability effects. Therefore, when selecting and breeding hybrid sorghum combinations, it is crucial to consider the content of endogenous substances in the root bleeding sap and the level of general combining ability of each substance.
3.9 Correlation Analysis of Various Endogenous Substances with Combining Ability in F1 Generation of Hybrid Sorghum Root Bleeding Sap
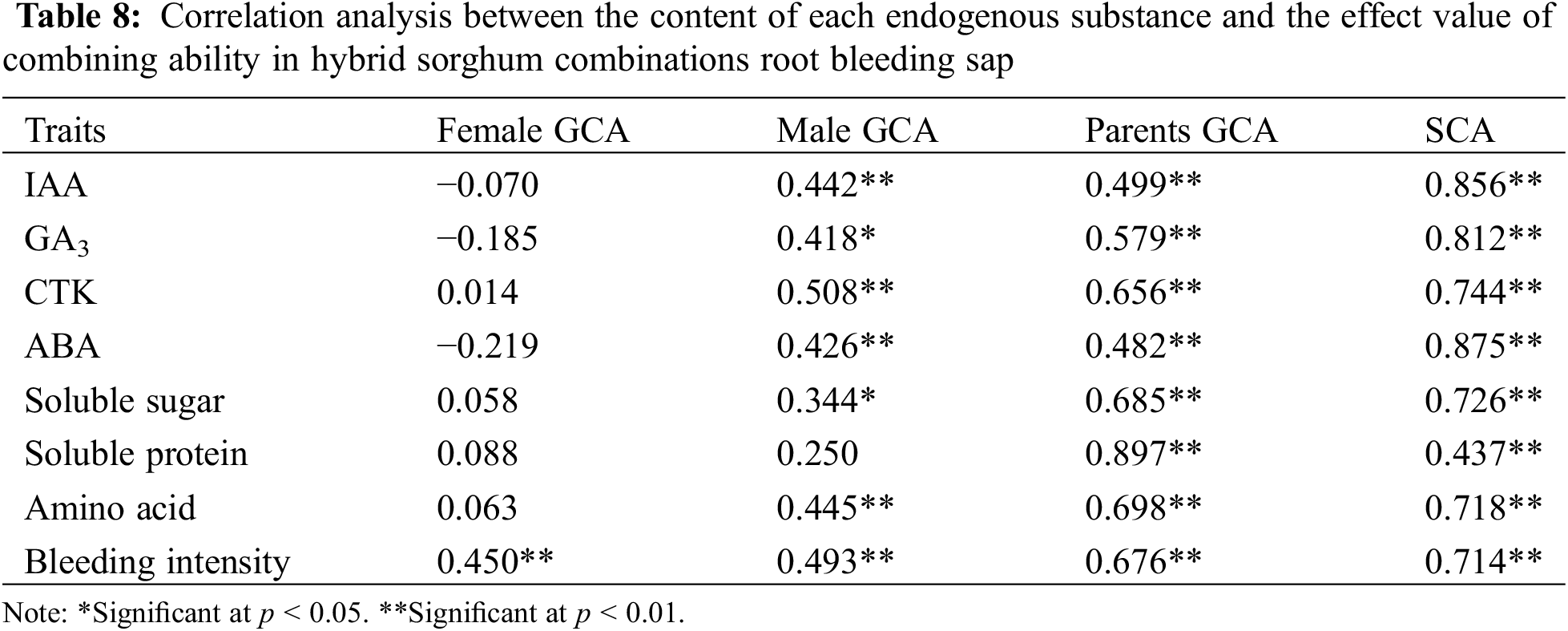
The correlation coefficients between the content of endogenous substances in the F1 generation of hybrid sorghum root-blended sap and the sum of maternal, paternal, and parental GCA, as well as the SCA between each hybrid combination, are shown in Table 8, which shows that the endogenous substances content in the root blending sap of hybrid sorghum combinations is highly significantly positively correlated with the sum of parental GCA and SCA, with a variation range of 0.482–0.897 and 0.437–0.875, respectively. Except for soluble protein, endogenous substances contents in the root-blended sap of the hybrid sorghum group were significantly or extremely significantly positively correlated with the GCA of the male parent. The root blending intensity of the hybrid sorghum combinations showed a highly significant positive correlation with the GCA of the maternal parent, whereas the correlation between other parameters and the maternal parent was not significant. Therefore, regarding the root blending sap of sorghum, the content of endogenous substances in the F1 hybrid root blending sap can be inferred using male GCA or parent GCA and SCA.
3.10 Analysis of Genetic Parameters of Each Endogenous Substance in Hybrid Sorghum Root Bleeding Sap
To further analyze the impact of endogenous substances in the root bleeding sap of hybrid sorghum parents and gene interactions on hybrid combinations, we assessed the genotypic variance, environmental variance, GCA variance, SCA variance, broad-sense heritability, and narrow-sense heritability of each endogenous substance. Table 9 shows that the proportion of SCA variance for most endogenous substances, except soluble proteins, which was over 60%, significantly higher than the GCA variance, indicating a significant contribution of non-additive gene effects to the genetic variation in these substances. Conversely, the GCA variance for soluble protein was notably higher at 75.85%, suggesting a predominant role of additive genetic effects in determining its content in root bleeding sap.
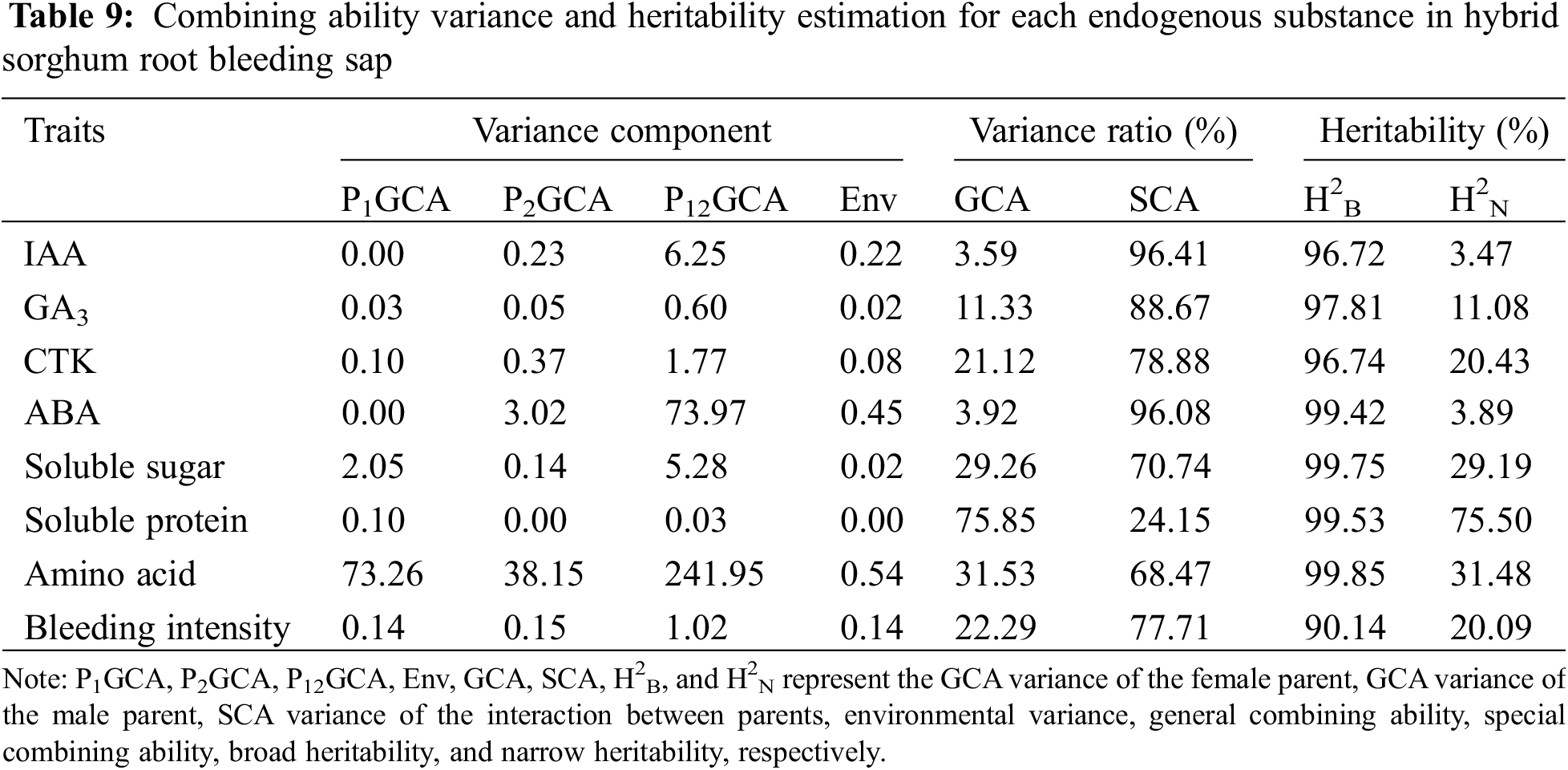
Based on the results of the combined ability ANOVA, the broad and narrow heritability of endogenous substances in the hybrid sorghum root blended sap was further estimated. The results showed that the narrow heritability of soluble proteins was as high as 75.50%, which was much higher than the narrow heritability of endogenous substances in other root-blended sap samples. Therefore, during breeding, selection can be performed on the lower generations.
The root system is a vital organ for crop growth and development, plays a crucial role in controlling plant life activities, and is key to regulating plant hormones [32]. Root bleeding sap is a manifestation of the active absorption of minerals and water by the root system. The sap in the xylem overflows from the stem base conduction tissues under root pressure, which is closely related to the growth status of the root system, strength of vital metabolism, and other intrinsic factors. This is closely related to intrinsic factors, such as root growth and the strength of vital metabolism. The components of root bleeding intensity and root bleeding sap can also characterize crop growth and the strength of physiological activity of the root system, and their performances differ during different reproductive periods [33–35]. Liu et al. reported that the soluble sugar content of maize root wound sap was low at the seedling stage and high at the nodulation, tassel, and grouting stages, whereas the free amino acid content was high at the seedling stage, low at the nodulation and tassel stages, and elevated again at the grouting stage [36]. Here, soluble sugars and amino acids in sorghum root bleeding sap were at higher levels at the anthesis and grouting stages, which may be related to the fact that sorghum has more amino acid or protein synthesis at the anthesis and grouting stages. Soluble protein was lower at the flowering stage and higher at the grouting stage because amino acids were synthesized in large quantities as proteins after the flowering stage, resulting in elevated soluble protein, whereas amino acids were synthesized in large quantities at the grouting stage, which resulted in higher levels of soluble sugar content in these two periods. Overall, all types of endogenous substances synthesized and transported by the root system were related, and the synthesis of nutrients resulted from the joint participation of various indicators.
Further analysis of variance of endogenous substances in the root-bleeding sap showed that there were significant differences in the measured traits, and the mean values of each index in the hybrid combinations were higher than those of their parents, indicating that there was significant heterosis of endogenous substances in the root-bleeding sap of hybrid combinations, with the most obvious heterosis of soluble sugar reaching 129.34%. Heterosis of endogenous substances in the hybrid combinations was significantly higher than that of the other endogenous substances, with the most obvious heterosis being soluble sugars, with an average high-parent heterosis of 129.34%, which was significantly higher than that of the other endogenous substances. Windpassinger et al. discovered heterosis in sorghum root parameters at low temperatures [37].
The GCA effect is the most important indicator designating the average potential value of a parent used in a series of hybrid combinations and is based on additive genes, whereas the SCA effect is the expression of the variability of the GCA effect of the parent used in different hybrid combinations and is associated with nonadditive genes [38]. In the present study, there was a significant difference in the GCA and SCA variance of each endogenous substance of the root bleeding sap, indicating the co-existence of both additive and non-additive gene effects. Sharma et al. reported that additive gene action contributes more to trait expression than non-additive gene action [39]. Furthermore, this study showed that additive gene action has a significant effect on the expression of soluble proteins. The prioritization of additive genetic variation suggests that parental selection can be made based on the GCA values of soluble proteins in the wounding fluid. The priority of additive genetic variation for a trait also implies that opportunities exist for genetic advancement through the selection of suitable genes besides crossbreed breeding [40]. Conversely, non-additive gene action had equally significant effects on the expression of traits other than soluble proteins. Narrow heritability (H2N) is the ratio of additive genetic variation to phenotypic variation [41] and Robinson et al. [42] categorised H2N into three classes as low (<30%), medium (30%–60%) and high (>60%). Thus, the H2N content of the soluble proteins was high H2N (75.50%), suggesting that soluble proteins could be used in early breeding selection programs. These results suggest that non-additive effects should not be neglected when focusing on parental GCA in sorghum breeding. Correlation analysis showed that all endogenous substances in the hybrid combinations were positively correlated with the sum of the parental GCA at significant levels. Except for soluble proteins, other endogenous substances were positively correlated with the parental GCA at a highly significant level. Root bleeding intensity positively correlated with parental GCA at a highly significant level. This indicated that the parent had a greater effect on root vigor, whereas the parent had a greater effect on endogenous substances. Therefore, parental selection should be considered comprehensively when mating hybrid sorghum.
Hybrid dominance plays an important role in accelerated sorghum breeding because of the highly additive gene and GCA effects of the parents [43]. In the present study, the highest GCA values were recorded for soluble sugar in the sterile line 521A and restorer lines 0–30, and the highest GCA values were recorded for soluble protein in the sterile line 170A and restorer line JiR107. Since soluble sugar has the highest hybrid dominance and soluble protein is mainly controlled by additive genes, the sterile lines 521A, 170A, and restorer lines 0–30, JiR107 can be selected as good parents for sorghum breeding. SCA helps in identifying different hybrids with desired traits [44]. Similarly, different hybrid combinations showed different (positive or negative) SCA effects on these traits. Here, the hybrid combinations TAM428A × JiR107, TAM428A × T40, TAM428A × 0–30, L407A × Ji318R, L407A × JiR107, I15A × JiR107, and I15A × 501R exhibited significant SCA effects. These hybrid combinations can be used in sorghum breeding programs to improve root traits based on different SCA performance. For example, the hybrid combination I15A × 501R, which performed better in terms of root bleeding intensity, could be used to further improve root vigor. Although GCA effects were more important than SCA effects for all traits, high hybrid dominance values indicated that non-additive gene effects were also important in influencing the sorghum root system. It has been reported that, in this case, hybrid dominance and fitness should be evaluated in combination [45]. For example, I15A × JiR107 and its parents showed good performance and significant SCA for soluble proteins with high hybrid dominance.
To the best of our knowledge, this is the first study to report hybrid dominance, fitness, and heritability of endogenous substances in the root wound sap of sorghum hybrids and their parents. GCA enables breeders to identify parents of high breeding value to be retained in the hybridization process or to discard them while following the cyclic selection process used to develop genotypes. Additive and non-additive gene actions were present in this study, and non-additive gene action was the dominant type of gene action controlling all traits except soluble proteins. However, significant GCA variance and higher H2N for soluble proteins indicated that a selection strategy with a stronger response to additive effects in sorghum breeding favored the genetic improvement of soluble proteins in the early generations. Endogenous soluble sugars and amino acids had higher hybrid dominance, suggesting that this trait can be successfully improved using plant breeding methods. The results of this study showed that the sterile lines 521A and 170A and the restorer lines 0–30, JiR107 are excellent parents for improving soluble sugar and soluble protein and that a high GCA value is associated with high selection efficiency, high SCA effect, and high value of utilization in breeding for hybrid dominance in sorghum.
Acknowledgement: We are grateful to the editors and reviewers for their valuable suggestions on improving the manuscript. We also thank the Journal Editor Board for their help and patience.
Funding Statement: This research was funded by the Jilin Province Science and Technology Development Plan Project (20210202001NC) of Ziyang Zhou, the Jilin Agricultural Science and Technology Innovation Project (CXGC2021TD011) of Ziyang Zhou.
Author Contributions: Conceptualization, Renjie Zhao and Zhian Zhang; methodology, Zhian Zhang and Ziyang Zhou; software, Renjie Zhao and Yueqiao Li; validation, Renjie Zhao, Yihan Zhou and Zexin Qi; formal analysis, Chen Xu; investigation, Renjie Zhao, YueqiaoLi, Chen Xu, Yihan Zhou and Zexin Qi; resources, Renjie Zhao and Ziyang Zhou; data curation, Renjie Zhao and Yueqiao Li; writing—original draft preparation, Renjie Zhao; writing—review and editing, Zhian Zhang and Ziyang Zhou; visualization, Renjie Zhao and Zhian Zhang; supervision, Zhian Zhang and Ziyang Zhou; project administration, Zhian Zhang and Ziyang Zhou; funding acquisition, Zhian Zhang and Ziyang Zhou. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Tomobe H, Tsugawa S, Yoshida Y, Arita T, Tsai AYL, Kubo M, et al. A mechanical theory of competition between plant root growth and soil pressure reveals a potential mechanism of root penetration. Sci Rep. 2023;13(1):7473. doi:10.1038/s41598-023-34025-x. [Google Scholar] [PubMed] [CrossRef]
2. Moon S, Chandran AKN, Gho YS, Park SA, Kim SR, Yoo YH, et al. Integrated omics analysis of root-preferred genes across diverse rice varieties including Japonica and indica cultivars. J Plant Physiol. 2018;220:11–23. doi:10.1016/j.jplph.2017.10.003. [Google Scholar] [PubMed] [CrossRef]
3. Cao XY. Effect of different nitrogen application rates on quality and nitrogen utilisation in sorghum. Shanxi University: China; 2024. [Google Scholar]
4. Zhang RD, Gao MY, Yue ZX, Zhou YF, Cao X. Effect of different stages of drought on starch accumulation in sorghum grains during the filling period. Crops. 2021;4:172–7. [Google Scholar]
5. Han LJ, Cai HW. Progress in genetic transformation of sorghum. Sci Agric Sin. 2024;57(3):454–68. [Google Scholar]
6. Deinlein U, Weber M, Schmidt H, Rensch S, Trampczynska A, Hansen TH, et al. Elevated nicotianamine levels in arabidopsis halleri roots play a key role in zinc hyperaccumulation. Plant Cell. 2012;24(2):708–23. doi:10.1105/tpc.111.095000. [Google Scholar] [PubMed] [CrossRef]
7. Mulkey TJ, Kuzmanoff KM, Evans ML. Promotion of growth and hydrogen ion efflux by auxin in roots of maize pretreated with ethylene biosynthesis inhibitors. Plant Physiol. 1982;70(1):186–8. [Google Scholar] [PubMed]
8. Sharon FP, Saul B, Yaacov O. Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. Fems Microbiol Lett. 2012;326(2):99–108. [Google Scholar]
9. Li W. Progress in the study of the effects of drought on the root fertility of soybean and the mitigating effects of irrigation. Acta Pratac Sin. 2019;28(4):192–202. [Google Scholar]
10. Xu C, Zhao RJ, Liu XL, Bian SF, Zhao HX, Yan WP, et al. Effects of different drip irrigation quotas on post-flowering leaf photosynthetic traits and kernel growth and development in maize. Acta Agric Boreali-Sin. 2024;39(1):83–94. [Google Scholar]
11. Hu Y, Javed HH, Du YL, Liao QW, Ye W, Zhou J, et al. Improving lignin metabolism, lodging resistance, and yield of rapeseed by applying straw-fermented fertilizer. J Soil Sci Plant Nutr. 2023;23:2832–48. [Google Scholar]
12. Chin HF, Neales TF, Wilson JH. The effects of cotyledon excision on growth and leaf senescence in soya-bean plants. Ann Bot. 1977;41(4):771–7. doi:10.1093/oxfordjournals.aob.a085352. [Google Scholar] [CrossRef]
13. Hussner A, Meyer C, Busch J. The influence of water level and nutrient availability on growth and root system development of myriophyllum aquaticum. Weed Res. 2009;49(1):73–80. doi:10.1111/j.1365-3180.2008.00667.x. [Google Scholar] [CrossRef]
14. Xie AQ, Sun LM, Zhang DL, Li Y, Liu ZM, Li X, et al. Changes in the root system of the herbaceous peony and soil properties under different years of continuous planting and replanting. Horticult Plant J. 2023;9(4):801–10. doi:10.1016/j.hpj.2021.12.006. [Google Scholar] [CrossRef]
15. Feng S. Changes of nitrogen metabolising enzymes in wounded liquid nitrogen in soybean roots of different evolutionary types and their relationship with leaf photosynthesis. Jilin Agricultural University: China; 2023. [Google Scholar]
16. Sun QQ, Hu CH, Dong S. Studies on the evolution of root characteristics of maize varieties of different ages throughout their reproductive life in China. Acta Agron Sin. 2003;5:641–5. [Google Scholar]
17. Oliveira SS, Kátia APC, Souza WFD, Santos CBD, Teixeira DAA, Silva VCE. Production and quality of the silage of sorghum intercropped with Paiaguas palisadegrass in different forage systems and at different maturity stages. Anim Prod Sci. 2020;60(5):694–704. doi:10.1071/AN17082. [Google Scholar] [CrossRef]
18. Yang WQ, Lin RC, Rui MDQ, Li L, Lu CM, Ou YM, et al. Some important research advances in the field of photosynthesis in the last 10 years. Plant Physiol J. 2024;60(2):211–47. [Google Scholar]
19. Zhang QQ. Study on the Development of dryland sorghum industry cluster in Shanxi Province. Shanxi Agricultural University: China; 2022. [Google Scholar]
20. Wei LC. Analysis of key constraints to the development of sorghum industry in Zhejiang Province and countermeasures research. Guangxi University: China; 2023. [Google Scholar]
21. Nie M, Ning N, Liang D, Zhang H, Li S, Li S, et al. Seed priming with selenite enhances germination and seedling growth of Sorghum under salt stress. Acta Agric Scand Sect B Soil Plant Sci. 2023;73(1):42–53. [Google Scholar]
22. Hoecker N, Keller B, Piepho HP, Hochholdinger F. Manifestation of heterosis during early maize root development. Theor Appl Genet. 2006;112(3):421–9. doi:10.1007/s00122-005-0139-4. [Google Scholar] [PubMed] [CrossRef]
23. Zhang M, Wang Y, Hu Y, Wang H, Liu Y, Zhao B, et al. Heterosis in root microbiota inhibits growth of soil-borne fungal pathogens in hybrid rice. J Integr Plant Biol. 2023;65(4):1059–76. doi:10.1111/jipb.13416. [Google Scholar] [PubMed] [CrossRef]
24. Karaağaç O. Combining ability and heterosis for root structure and graft-related traits of interspecific cucurbita rootstocks. Euphytica. 2021;217(8):166. doi:10.1007/s10681-021-02884-y. [Google Scholar] [CrossRef]
25. Zhang HW. Physiological mechanisms of sorghum in response to differential inter-root salt distribution. Shenyang Agricultural University: China; 2020. [Google Scholar]
26. Liu GN. Studies on changes in photosynthesis and root vigour of soybean varieties of different ages in Jilin province. Jilin Agricultural University: China; 2013. [Google Scholar]
27. Deng HZ, Li X, Xu KZ. Changes in soluble sugar content in root wound sap of soybean varieties of different ages and its relation to leaf photosynthesis. J South China Agric Univ. 2013;34:197–202 (In Chinese). [Google Scholar]
28. Zhang ZA, Chen ZY. Experimental technique of plant physiology. China: Jilin University Press; 2006. p. 43–73 (In Chinese). [Google Scholar]
29. Quan RD, Shang M, Zhang H, Zhao YX, Zhang J. Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci. 2004;166(1):141–9. [Google Scholar]
30. Guzel S, Terzi R. Exogenous hydrogen peroxide increases dry matter production, mineral content and level of osmotic solutes in young maize leaves and alleviates deleterious effects of copper stress. Bot Stud. 2013;54:26. [Google Scholar] [PubMed]
31. Sun SW, Lin YC, Weng YM, Chen MJ. Efficiency improvements on ninhydrin method for amino acid quantification. J Food Compos Anal. 2006;19(2):112–7. [Google Scholar]
32. Henkes GJ, Thorpe MR, Minchin PEH, Schurr U, Rose USR. Jasmonic acid treatment to part of the root system is consistent with simulated leaf herbivory, diverting recently assimilated carbon towards untreated roots within an hour. Plant Cell Environ. 2008;31:1229–36. [Google Scholar] [PubMed]
33. Xiao WX, Wang YB, Zhao HY, Ye YS, Wang DW, Zhang Y. Evolution of morphological, physiological and functional properties of maize roots in different ages in China. J Liaoning Agric Sci. 2022;3:45–9 (In Chinese). [Google Scholar]
34. Zhang FY, Ping JA, Jiao XY. Current status and prospects of research on barrenness tolerance and efficient nutrient use in sorghum. Crops. 2023;6:26–34. [Google Scholar]
35. Pfeiffer BK, Pietsch D, Schnell RW, Rooney WL. Long-term selection in hybrid sorghum breeding programs. Crop Sci. 2019;59(1):150–64. doi:10.2135/cropsci2018.05.0345. [Google Scholar] [CrossRef]
36. Liu SQ, Zhang TZ, Yan XL, Song FB, Zhou X, Zhu XC, et al. Analysis of the contents of carbon and nitrogen metabolism-related components in wounding streams and wounding fluids of maize from different drought-tolerant genotypes. J Soil Crop. 2012;1(1):10–4. [Google Scholar]
37. Windpassinger S, Friedt W, Deppé I, Werner CH, Snowdon R, Wittkop B. Towards enhancement of early-stage chilling tolerance and root development in sorghum F1 hybrids. J Agron Crop Sci. 2017;203(2):146–60. doi:10.1111/jac.12171. [Google Scholar] [CrossRef]
38. Musembi KB, Githiri SM, Yencho GC, Sibiya J. Combining ability and heterosis for yield and drought tolerance traits under managed drought stress in sweetpotato. Euphytica. 2015;201(3):423–40. doi:10.1007/s10681-014-1230-1. [Google Scholar] [CrossRef]
39. Sharma RC, Smith EL. Combining ability analysis for harvest index in winter wheat. Euphytica. 1991;55(3):229–34. doi:10.1007/BF00021243. [Google Scholar] [CrossRef]
40. Feyzian E, Dehghani H, Rezai AM, Javaran MJ. Diallel cross analysis for maturity and yield-related traits in melon. Euphytica. 2009;168(2):215–23. doi:10.1007/s10681-009-9904-9. [Google Scholar] [CrossRef]
41. Sharma HC, Dhillon MK, Pampapathy G, Reddy BVS. Inheritance of resistance to spotted stem borer, Chilo partellus, in sorghum, Sorghum bicolor. Euphytica. 2007;156(1–2):117–28. doi:10.1007/s10681-007-9358-x. [Google Scholar] [CrossRef]
42. Robinson HF, Comstock RE, Harvey PH. Estimates of heritability and the degree of dominance in corn. J Agron. 1949;41(8):353–9. doi:10.2134/agronj1949.00021962004100080005x. [Google Scholar] [CrossRef]
43. Su J, Zhang F, Yang X, Feng Y, Yang X, Wu Y, et al. Combining ability, heterosis, genetic distance and their intercorrelations for waterlogging tolerance traits in chrysanthemum. Euphytica. 2017;213(2):1–15. doi:10.1007/s10681-017-1837-0. [Google Scholar] [CrossRef]
44. Rukundo P, Shimelis H, Laing M, Gahakwa D. Combining ability, maternal effects, and heritability of drought tolerance, yield and yield components in sweet potato. Front Plant Sci. 2017;7:1981. [Google Scholar] [PubMed]
45. Kenga R, Alabi SO, Gupta SC. Combining ability studies in tropical sorghum. Field Crops Res. 2004;88(2):251–60. doi:10.1016/j.fcr.2004.01.002. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools