 Open Access
Open Access
ARTICLE
Variation in the Composition of the Essential Oil of Commercial Salvia officinalis L. Leaves Samples from Different Countries
1 Institute of Pharmacy, Faculty of Medicine, University of Tartu, Tartu, 50411, Estonia
2 Institute of Chemistry, Tallinn University of Technology, Tallinn, 12618, Estonia
3 The Department of Pharmaceutical Management, Drug Technology and Pharmacognosy, Ivano-Frankivsk National Medical University, Ivano-Frankivsk, 76018, Ukraine
4 The Department of Pharmacognosy, National University of Pharmacy, Kharkiv, 61002, Ukraine
5 The Department of Clinical Laboratory Diagnostics, Kharkiv National Medical University, Kharkiv, 61022, Ukraine
* Corresponding Authors: Ain Raal. Email: ; Oleh Koshovyi. Email:
Phyton-International Journal of Experimental Botany 2024, 93(8), 2051-2062. https://doi.org/10.32604/phyton.2024.052790
Received 15 April 2024; Accepted 19 July 2024; Issue published 30 August 2024
Abstract
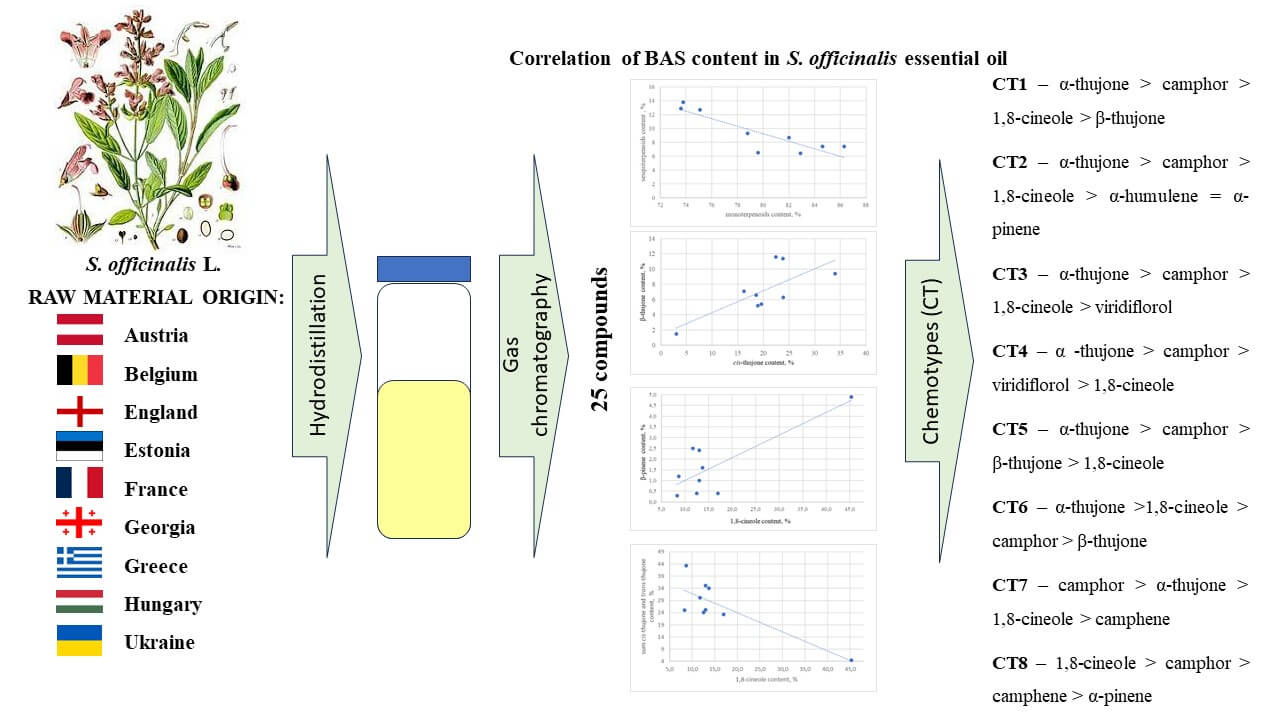
Salvia officinalis L. (Lamiaceae) leaves and its essential oil is used for mouth and throat disorders, skin disorders, minor wounds, and gastrointestinal disorders, and is widely used worldwide. The research aimed to conduct a comparative study of the composition of S. officinalis essential oils from commercial samples, and their main chemotypes. The volatile constituents from S. officinalis leaves were investigated using gas chromatography (GC). The commercial samples of sage leaves were obtained from retail pharmacies in nine mainly European countries. The yield of essential oil in S. officinalis commercial leaves was between 10.0 and 24.8 mL/kg. The principal components (>5%) among the main identified 25 compounds were 1,8-cineole (8.3%–45.3%), α-thujone (3.0%–34.0%), сamphor (11.3%–29.3%), β-thujone (1.5%–12.9%), viridiflorol (1.1%–10.4%), camphene (2.6%–7.1%), and α-pinene (1.3%–5.8%). In seven (Estonia, England, France, Hungary, Belgium, Ukraine, Georgia) samples α-thujone dominated. Four samples (Estonia, Georgia, England, Hungary) belong to the most common chemotype α-thujone > camphor > 1,8-cineole. Eight chemotypes of S. officinalis essential oils have been found. Toxic thujones are widespread compounds among them.Graphic Abstract
Keywords
The genus Salvia is the largest genus in the Lamiaceae family, including over 900 species spread all over the world. All organs of the Salvia plants contain essential oils (EO), the main components of which are cyclic, acyclic, and aromatic monoterpenoids with the predominance of one or several components [1,2]. Salvia officinalis L. (Common sage, Lamiaceae) leaves are used for diseases of the throat and mouth disorders, minor wounds, skin disorders, and gastrointestinal disorders. Sage has been shown to have significant antibacterial and anti-inflammatory effects [3]. Salvia officinalis essential oil has been implemented to treat diseases like the respiratory, digestive and nervous systems, heart and blood circulation, endocrine, and metabolic diseases. In addition, sage EO has been shown to have antioxidant, carminative, antispasmodic, antiseptic, and astringent properties [4–6].
The herbal drug of European Pharmacopoeia Salvia officinalis folium contains more than 15 ml/kg of EO for the whole leaves and not less than 10 mL/kg for the cut raw material in calculation to the anhydrous drug [7,8]. Salvia officinalis EO content has been varied from 0.1% to 2.8% [9–14]. In aerial parts of S. officinalis more than 120 components of the EO have been discovered. The raw material contains up to 3% EO, the dominant components of which are monoterpenoids: 1,8-cineole (1%–15%), camphor (5%–20%), α-thujone (10%–60%) and β-thujone (4%–36%); sesquiterpenes: β-caryophyllene, α-humulene, and viridiflorol [7]. In addition, borneol, pinene, camphor, elemene, ledene, were found in the Salvia EO [15–20].
Its pharmacological activity largely depends on the composition of the EO, which is inherent in the chemotype of the plant. Adapting to various environmental conditions, sage S. officinalis synthesizes different groups of biologically active substances that help it survive, forming stable characteristics of the chemical composition of the plant, the so-called chemotypes. First of all, adaptive substances are represented by terpenoids and phenolic compounds. EO has a very variable composition depending on the harvesting time, genetics, climate, seasonality, environment, and other factors [21–24]. Phenolic substances, amino acids, and monosaccharides in the composition of S. officinalis were also studied [2,25,26].
The effect of drought on the accumulation of cineole, α-thujone, β-thujone, and camphor in the sage leaves was established (the content of monoterpenes in plants that received a sufficient amount of moisture was compared with those that were in conditions of limited water supply—70% of the optimal). Studies have shown that in arid conditions, sage leaves accumulate a significantly higher concentration of monoterpenes (approximately 33%) than those plants cultivated under optimal irrigation conditions [27].
One of the key terpenes in the S. officinalis EO is thujone, whose contents due to its toxicity should be regulated. Thujone is a neurotoxic terpen and chemotypes with its low content should be preferred. The amount of thujone has to be specified in the given product and its daily exposure has to be below 6.0 mg [8]. The S. officinalis aerial parts have been used in traditional medicine and cookery for centuries. Its leaves are approved for use in the European Union as a coloring, category N2, with preliminary restrictions on the content of α- and β-thujones in the product (0.5 mg/kg) [28]. In the USA, Sage leaves are permitted for use in food and are recognized as safe (21 CFR 182.10 and 182.20) [29].
Also, S. officinalis leaves contain diterpene bitter principles, triterpenes, steroids, rosmarinic acid (up to 3.3%), flavonoids, and tannins [3,21,30–32].
Previously we studied the content of S. officinalis EO from several countries [33]. The purpose of this work is to determine the EO composition in commercial samples of S. officinalis leaves from nine countries to establish the variability of the content of their components and to identify possible chemotypes of this species with a focus on toxic thujone.
The S. officinalis L. leaves were obtained as commercial samples from retail pharmacies or health shops in different countries: Austria (AUT), Belgium (BEL), England (ENG), Estonia (EST), France (FRA), Georgia (GEO), Greece (GRC), Hungary (HUN), and Ukraine (UKR) from 2007 to 2020. We used the samples only of local production, which usually are grown by local farms. All the samples were marked accordingly. They were stored at a room temperature (22 ± 2°C) in their commercial packaging and analyzed as soon as possible after acquisition within four months, and all had a valid “best before” date when studied. The EO from the dried raw materials (20.0 g for a one experiment) were obtained using the method of distillation according to the European Pharmacopoeia requirements [8]. The EO were analyzed as soon as possible after the distillation, but not later than within 1–2 days. They were collected into glass vials for chromatography and were kept in a freezer (−17 ± 2°C).
2.2 Capillary Gas Chromatography
GC analysis was carried out using a Chrom-5 chromatograph (Laboratorni Pristroe Prague, Czech Republic) with FID on two fused silica capillary columns with a bonded stationary phase: poly(5%-diphenyl-95%-dimethyl) siloxane SPB-5 (30 m × 0.25 mm, Supelco) and polyethyleneglycol SW-10 (30 m × 0.25 mm, Supelco). Film thickness of both stationary phases was 0.25 µm. Carrier gas was helium with a split ratio 1:150, and the flow rate 35–40 (SPB-5) and 30–35 (SW-10) cm/s was applied. The temperature was from 50°C to 250°C at 2°C/min, and the injector temperature was 200°C. A Hewlett-Packard Model 3390A integrator was used for data processing.
The identification of the EO components was carried out by comparing their retention indices (RI) using as standards n-alkanes C6–C24, on two columns with the RI values of reference standards, both our RI data bank and literature data [5,8,9]. GC/MS confirmed the results obtained. The percentage composition of the EOs was established in peak areas (nonpolar column) using the normalization method without correction factors. The relative standard deviation of percentages of EO components of three repeated GC analyses of a single oil sample didn’t exceed 5% [20,30–32].
The identified constituents in the leaves EO of the nine S. officinalis samples from different countriesare gained in Table 1. The EO yields in the studied samples were 10.0–24.8 mL/kg (Table 2), which corresponded in all cases to the minimum standard (10 mL/kg) of European Pharmacopoeia for the cut drug [8].


High variation coefficients of the predominant compounds (>1) demonstrated that their content strongly differs from samples to samples. Low variation coefficients (0.56–0.75) are typical for p-cymene, α-terpinene, γ-terpinene, terpinolene, (E)-β-caryophyllene and caryophyllene oxide. Trace amounts (<0.05%) of α-thujene, (E)-β-caryophyllene, and caryophyllene oxide were detected in one sample, (Z)-β-ocimene in three samples, myrtenol in seven samples, and thymol and spathulenol in five samples of the studied EOs.
Twenty-five compounds, representing 80.1%–93.6% of the total EO, were identified in the nine studied sage EO. Such a rather large range presented in Table 1 indicates variability in the EO composition of S. officinalis. All identified components have been previously found in the S. officinalis EO [17,19,22,23]. In all the studied samples, monoterpenoids dominate (70.1%–85.4%), much less sesquiterpenoids (6.9%–13.6%) and the least aromatic compounds (0.3%–1.7%). All EOs are characterized by a strong negative correlation (r = −0.88) between the content of monoterpenoids and sesquiterpenoids (Fig. 1), where symbols are individual. To perform scatter plots (or correlation fields), the application package Statistica of Microsoft Excel was used.

Figure 1: Correlation of monoterpenoids and sesquiterpenoids content in nine commercial samples of Salvia officinalis essential oil
The amount of α-thujone (18.0%–43.0%) and β-thujone (3.0%–8.5%), 1,8-cineole (5.5%–13.0%), bornyl acetate (≤2.5%), camphene (1.5%–7.0%) and camphor (4.5%–24.5%), α-humulene (≤12.0%), α-pinene (1.0%–6.5%), limonene (0.5%–3.0%), and linalool+linalyl acetate (≤1.0%) in EOs for medicinal uses are regulated by ISO 9909:1997 [34]. This standard covers EO production methods, quality requirements and evaluation criteria to ensure its purity and potency. The requirements for the content of α-pinene, camphene and α-humulene are met by all the studied samples (Table 2). The content of 1,8-cineole is significantly higher than the upper limit of normalization in the EO sample from Greece (45.3%) and slightly higher in Ukraine (13.7%). At the same time, the sample of oil from Greece differs from others in its low content of α-thujone (3%) and β-thujone (1.5%), which is significantly below the lower limit of normalization of the content of these compounds. The content of β-thujone exceeds the upper limit of normalization in EO samples from France, Ukraine, and Georgia and is 9.4%, 11.6%, and 11.4%, respectively. In the sample of EO from Austria, the content of camphor and bornyl acetate is above the norm—29.3% and 2.7%, respectively. Limonene, linalool, and linalyl acetate are absent in the studied samples.
The main components in the nine studied EOs were 1,8-cineole (8.3%–45.3%), α-thujone (3.0%–34.0%), сamphor (11.3%–29.3%), β-thujone (1.5%–11.6%), viridiflorol (1.1%–10.4%), camphene (2.6%–6.8%), α-pinene (1.3%–6.4%), borneol (1.8%–5.0%), β-pinene (0.3%–4.9%), (E)-β-caryophyllene (tr.–4.9%), myrcene (0.7%–4.2%), α-humulene (0.4%–6.4%), bornyl acetate (0.1%–2.7%) (Table 2).
In the seven studied EO samples from Estonia, England, France, Hungary, Belgium, Ukraine and Georgia α-thujone (18.6%–34.0%) is the main component. Previously scientific publications also indicate that α-thujone is the dominant component in S. officinalis EOs from Turkey [23], Bulgaria [14], Mexico and California [20], Georgia [35], Romania [19,36], Albania [37], Algeria [38], France and Hungary [33,36], Brazil [39], Ukraine, Belgium, Moldova, and Estonia [33]. High concentrations of β-thujone were reported in EO samples from Turkey [40], Sudan [41], Uzbekistan [42], Portugal and Czech Republic [37], but in the studied EOs there were less amount of it. The high concentrations of β-thujone were just observed in the EOs from Ukraine, Geogia and France. So, it is common for variations in these main chemical components in analyzes of EOs from the same plant species that were cultivated in different countries. Thujones are neurotoxic and their amount are key points in the standardization of the S. officinalis EO [43–45]. The European Union, the USA and other countries have restrictions on the content of α- and β-thujones in products [28,29]. In the USA the addition of pure thujone to food is prohibited and its content must be less than 6.0 mg per day [8]. Therefore, for farms cultivating medicinal plants, it is advisable to recommend Salvia spp. seeds from chemotypes with a low thujone content.
In the studied EO from Greece 1,8-cineole (45.3%) dominates. The dominance of 1,8-cineole in the essential oil from Greece is close to the literature data in EO samples from Jordan [9], Egypt [12], Albania [20], Iran [46], Greece [33], and Poland [47].
In the studied EO from Austria сamphor (29.3%) is the predominant component. Previously the camphor dominance in S. officinalis EOs from Morocco [10,11], Tunisia [16], Romania [19] and Sudan [4] is confirmed.
A high content of camphor (19.2%–19.3%) was found in the EO samples from France, Estonia, and Belgium; 1,8-cineole (13.0%–13.7%)–samples from Hungary, Georgia and Ukraine; β-thujone (9.4%–11.6%)–samples from France, Ukraine and Georgia; α-pinene (5.1%–6.4%)–samples from England, Estonia, Hungary, Belgium and Greece; camphene (5.9%–6.8%)–samples from Austria, Belgium and Greece; β-pinene (2.4%–4.9%)–samples from Estonia, Hungary and Greece; mircene (4.2%)–sample from Greece; borneol (4.7%–5.0%)–samples from Hungary, England and Austria; bornyl acetate (2.1%–2.7%)–samples from Estonia, England and Austria; (E)-β-caryophyllene (2.7%–4.9%)–samples from Ukraine, Hungary and Greece; α-humulene (5.3%–6.4%)–samples from Estonia and England; viridiflorol (7.9%–10.4%)–samples from Hungary, Belgium and Ukraine. Viridiflorol was a principal compound in many samples published previously [48,49].
Our results indicate strong positive correlations between the content of α- and β-thujone (r = 0.73) (Fig. 2); between the content of 1,8-cineole and β-pinene (r = 0.81) (Fig. 3) and a negative correlation between 1,8-cineole and the sum of α- and β-thujone (r = −0.82) (Fig. 4). In these diagrams all symbols are individual. Having received the analysis diagram, we did not determine the value that was far from the totality of data and that needed to be removed. In biology and other natural sciences, a significant (strong) correlation is considered to be a value between 0.3 and 1.0 [50,51].

Figure 2: Correlation of α-thujone and β-thujone content in nine commercial samples of Salvia officinalis essential oil from different countries

Figure 3: Correlation of β-pinene and 1,8-cineole content in nine commercial samples of Salvia officinalis essential oil from different countries

Figure 4: Correlation of 1,8-cineole and sum α-thujone and β-thujone content in nine commercial samples of Salvia officinalis essential oil
The statistics show a strong correlation between content of the biologically active substances and Pearson coefficients, which confirms this. The positive strong correlation is evidenced the conjugated biosynthesis and accumulation of these substances in S. officinalis leaves. Our research shows genotypic connections of these substances. There are some publications about correlations between terpenoids, phenolic compounds and ecological minds of production, that testify about their adaptation powers [52–55].
The results obtained by us (Table 2) show that if we take into account the content of four components, the samples we studied correspond to 8 chemotypes (CT): CT1 – α-thujone > camphor > 1,8-cineole > β-thujone (samples from Estonia and Georgia); CT2 – α-thujone > camphor > 1,8-cineole > α-humulene = α-pinene (sample from England); CT3 – α-thujone > camphor > 1,8-cineole > viridiflorol (sample from Hungary); CT4 – α-thujone > camphor > viridiflorol > 1,8-cineole (sample from Belgium); CT5 – α-thujone > camphor > β-thujone > 1,8-cineole (sample from France); CT6 – α-thujone > 1,8-cineole > camphor > β-thujone (sample from Ukraine); CT7 – camphor > α-thujone > 1,8-cineole > camphene (sample from Austria); CT8 – 1,8-cineole > camphor > camphene > α-pinene (sample from Greece). Previously according to the content of dominant components, S. officinalis EOs can be divided into different chemotypes. Tucker and Maciarello described five groups of sage chemotypes based on four principal constituents: (1) camphor > α-thujone > 1,8-cineole > β-thujone; (2) camphor > α-thujone > β-thujone > 1,8-cineole; (3) β-thujone > camphor > 1,8-cineole > α-thujone; (4) 1,8-cineole > camphor > α-thujone > β-thujone; and (5) α-thujone > camphor > β-thujone > 1,8-cineole [32].
Jug-Dujaković et al. [39] divided sage leaves by chemotypes, based on the content of 8 main components (α-thujone, β-thujone, camphene, borneol and bornyl acetate, camphor, 1,8-cineole, β-pinene). The authors concluded that the first major component separates populations high in thujone from populations rich in camphor, while the second component separates populations rich in α-thujone from populations rich in β-thujone. They distinguish three chemotypes of S. officinalis populations: (A) α-thujone > camphor > 1,8-cineole > β-thujone; (B) β-thujone > α-thujone > camphor ≈ 1,8-cineole; and (C) camphor > α-thujone > 1,8-cineole > camphene ≈ borneol. The results of our research show that none of the studied EO samples can be attributed to the β-thujone chemotype.
Craft et al. [20] used the content of 26 EO components for cluster analysis and established the presence of 5 main sage chemotypes based on the content of two dominant compounds. They believe the most typical is the α-thujone > camphor > 1,8-cineole chemotype of sage. Of the samples studied by us, CT4 (samples from Estonia, Georgia, Hungary and England) correspond to this type.
In European countries, EO raw materials, in particular S. officinalis, are cultivated for the needs of industry (pharmaceutical, food, etc.,) and are usually supplied by specialized farms for the cultivation of medicinal herbs. This raw material is grown according to strictly regulated conditions (GAСP) [56–59]. The collection period and cultivation conditions are regulated, so the seeds are the main and key factor that affect the quality of the raw material. It is usually standardized, thus the information about their chemotypes is especially important. The farmer is responsible for the quality of raw materials, for compliance with the regulatory document, but they, of course, do not analyse the EO composition, which was done in our work. Depending on the size of the country, the number of such farms may vary, but the issue of seed supply is not so varied. Therefore, taking this into account, the obtained data are of practical importance and will allow to make a targeted choice regarding chemotypes with low content of thujone and high concentration of other target terpenes.
The EO yields in the studied commercial sage leaves from nine countries corresponded to the minimum standard of European Pharmacopoeia for the cut drug. S. officinalis EOs were rich in thujones, camphor, 1,8-cineole, viridiflorol, α-humulene, camphene, and α-pinene. Toxic thujones are found in almost all analyzed samples. Based on these results eight chemotypes of S. officinalis were established. Considering the three components, the samples from Estonia, Georgia, Hungary, and England correspond to the most typical chemotype of 1,8-cineole, camphor, and α-thujone. The obtained results create prospects for purposeful choice of the chemotypes with low concentrations of toxic thujone and high content of other target terpenes.
Acknowledgement: The authors of the study thank all pharmacy students who helped to obtain commercial samples studied and performed hydrodistillations of EOs. The authors sincerely thank all the defenders who are fighting for the independence of Ukraine. The authors sincerely appreciate the support of the partners who stand with Ukraine.
Funding Statement: This work was carried out in the MSCA4 Ukraine project “Design and Development of 3D-Printed Medicines for Bioactive Materials of Ukrainian and Estonian Medicinal Plants Origin” (ID Number 1232466) and financed by the European Union.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Ain Raal, Anne Orav, Tetiana Ilina, Alla Kovalyova, Oleh Koshovyi; data collection: Anne Orav, Taras Koliadzhyn, Yuliia Avidzba; analysis and interpretation of results: Ain Raal, Anne Orav, Tetiana Ilina, Alla Kovalyova, Oleh Koshovyi; draft manuscript preparation: Ain Raal, Anne Orav, Tetiana Ilina, Alla Kovalyova, Oleh Koshovyi. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the author and/or corresponding author on reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Jakovljević M, Jokić S, Molnar M, Jašić M, Babić J, Jukić H, et al. Bioactive profile of various Salvia officinalis L. preparations. Plants. 2019;8(3):55. doi:10.3390/plants8030055. [Google Scholar] [PubMed] [CrossRef]
2. Koshovyi O, Raal A, Kovaleva A, Myha M, Ilina T, Borodina N, et al. The phytochemical and chemotaxonomic study of Salvia spp. growing in Ukraine. JABB. 2020;8(3):29–36. doi:10.7324/JABB.2020.80306. [Google Scholar] [CrossRef]
3. Ghorbani A, Esmaeilizadeh M. Pharmacological properties of Salvia officinalis and its components. J Tradit Complement Med. 2017;7(4):433–40. doi:10.1016/j.jtcme.2016.12.014. [Google Scholar] [PubMed] [CrossRef]
4. Loizzo MR, Tundis R, Menichini F, Saab AM, Statti GA, Menichini F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res. 2007;27(5A):3293–9. [Google Scholar] [PubMed]
5. Radulescu V, Chiliment S, Oprea E. Capillary gas chromatography-mass spectrometry of volatile and semi-volatile compounds of Salvia officinalis. J Chromatogr A. 2004;1027(1–2):121–6. doi:10.1016/j.chroma.2003.11.046. [Google Scholar] [PubMed] [CrossRef]
6. Hussain A, Anwar F, Iqbal T, Bhatti I. Antioxidant attributes of four Lamiaceae essential oils. Pak J Bot. 2011;43:1315–21. [Google Scholar]
7. Assessment report on Salvia officinalis L., folium and Salvia officinalis L., aetheroleum; EMA/HMPC/150801/2015. Committee on Herbal Medicinal Products (HMPC). [Google Scholar]
8. European Pharmacopoeia. 11th edStrasbourg: Council of Europe; 2022. [Google Scholar]
9. Abu-Darwish MS, Cabral C, Ferreira IV, Gonçalves MJ, Cavaleiro C, Cruz MT, et al. Essential oil of common sage (Salvia officinalis L.) from Jordan: assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. Biomed Res Int. 2013;2013(7):1–9. doi:10.1155/2013/538940. [Google Scholar] [PubMed] [CrossRef]
10. El Hadri A, del Río MÁG, Sanz J. Cytotoxic activity of α-humulene and transcaryophyllene from Salvia officinalis in animal and human tumor cells. An R Acad Nac Farm. 2010;76:343–56. [Google Scholar]
11. Maache S, Zbadi L, Ghouizi AE, Soulo N, Saghrouchni H, Siddique F, et al. Antioxidant and antimicrobial effects of essential oils from two Salvia species with in vitro and in silico analysis targeting 1AJ6 and 1R4U proteins. Sci Rep. 2023;13(1):14038. doi:10.1038/s41598-023-41178-2. [Google Scholar] [PubMed] [CrossRef]
12. Mohammed HA, Eldeeb HM, Khan RA, Al-Omar MS, Mohammed SAA, Sajid MSM, et al. Sage, Salvia officinalis L., constituents, hepatoprotective activity, and cytotoxicity evaluations of the essential oils obtained from fresh and differently timed dried herbs: a comparative analysis. Molecules. 2021;26(19):5757. doi:10.3390/molecules26195757. [Google Scholar] [PubMed] [CrossRef]
13. El Euch SK, Hassine DB, Cazaux S, Bouzouita N, Bouajila J. Salvia officinalis essential oil: chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S Afr J Bot. 2019;120:253–60. doi:10.1016/j.sajb.2018.07.010. [Google Scholar] [CrossRef]
14. Damyanova S, Mollova S, Stoyanova A, Gubenia O. Chemical composition of Salvia officinalis L. essential oil from Bulgaria. Ukr Food J. 2016;5(4):695–700. doi:10.24263/2304-974X-2016-5-4-8. [Google Scholar] [CrossRef]
15. Badiee P, Nasirzadeh AR, Motaffaf M. Comparison of Salvia officinalis L. essential oil and antifungal agents against candida species. J Pharm Technol Drug Res. 2012;1(1):7. doi:10.7243/2050-120X-1-7. [Google Scholar] [CrossRef]
16. Hayouni EA, Chraief I, Abedrabba M, Bouix M, Leveau JY, Mohammed H, et al. essential oils: their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int J Food Microbiol. 2008;125(3):242–51. doi:10.1016/j.ijfoodmicro.2008.04.005. [Google Scholar] [CrossRef]
17. Couladis M, Koutsaviti A. Chemical composition of the essential oils of Salvia officinalis, S. fruticosa, Melissa officinalis, and their infusions. Ratar i povrt. 2017;54(1):36–41. doi:10.5937/ratpov54-12365. [Google Scholar] [CrossRef]
18. Mitic-Culafic D, Vukovic-Gacic B, Knezevic-Vukcevic J, Stankovic S, Simic D. Comparative study on the antibacterial activity of volatiles from Sage (Salvia officinalis L.). Arch Biol Sci. 2005;57(3):173–8. doi:10.2298/ABS0503173M. [Google Scholar] [CrossRef]
19. Mot MD, Gavrilaş S, Lupitu AI, Moisa C, Chambre D, Tit DM, et al. Salvia officinalis L. essential oil: characterization, antioxidant properties, and the effects of aromatherapy in adult patients. Antioxidants. 2022;11(5):808. doi:10.3390/antiox11050808. [Google Scholar] [PubMed] [CrossRef]
20. Craft J, Satyal P, Setzer W. The chemotaxonomy of common sage (Salvia officinalis) based on the volatile constituents. Medicines. 2017;4(3):47. doi:10.3390/medicines4030047. [Google Scholar] [PubMed] [CrossRef]
21. Bradley P. British herbal compendium. vol. 2. 2006. [Google Scholar]
22. Perry NB, Anderson RE, Brennan NJ, Douglas MH, Heaney AJ, McGimpsey JA, et al. Essential oils from Dalmatian sage (Salvia officinalis L.variations among individuals, plant parts, seasons, and sites. J Agric Food Chem. 1999;47(5):2048–54. doi:10.1021/jf981170m. [Google Scholar] [PubMed] [CrossRef]
23. Kulak M, Gul F, Sekeroglu N. Changes in growth parameter and essential oil composition of sage (Salvia officinalis L.) leaves in response to various salt stresses. Ind Crops Prod. 2020;145(2):112078. doi:10.1016/j.indcrop.2019.112078. [Google Scholar] [CrossRef]
24. Stešević D, Ristić M, Nikolić V, Nedović M, Caković D, Šatović Z. Chemotype diversity of indigenous dalmatian sage (Salvia officinalis L.) populations in montenegro. Chem Biodivers. 2014;11(1):101–14. doi:10.1002/cbdv.201300233. [Google Scholar] [PubMed] [CrossRef]
25. Koshevoi ON. Amino acid and monosaccharide compositions of Salvia officinalis leaves. Chem Nat Compd. 2011;47(3):92–493. doi:10.1007/s10600-011-9976-3. [Google Scholar] [CrossRef]
26. Koshovyi O, Vovk G, Akhmedov Ey, Komissarenko AN. The study of the chemical composition and pharmacological activity of Salvia officinalis leaves extracts getting by complex processing. Azerbaijan Pharm Pharmacotherapy J. 2015;15(1):30–4. [Google Scholar]
27. Dzhumaev KK, Tkachenko KG. Essential oils of inflorescences and leaves of Salvia. Rastit Resour. 1989;25(3):410–4. [Google Scholar]
28. Salvia officinalis L., herb, leaf. Strasbourg: Natural Sources of Flavourings, Council of Europe; 2007. [Google Scholar]
29. Sage. Food and Drugs. USA Code of Federal Regulations; 2000. [Google Scholar]
30. Francik S, Francik R, Sadowska U, Bystrowska B, Zawiślak A, Knapczyk A, et al. Identification of phenolic compounds and determination of antioxidant activity in extracts and infusions of Salvia leaves. Materials. 2020;13(24):5811. doi:10.3390/ma13245811. [Google Scholar] [PubMed] [CrossRef]
31. Lu Y, Yeap Foo L. Polyphenolics of Salvia–a review. Phytochemistry. 2002;59(2):117–40. doi:10.1016/S0031-9422(01)00415-0. [Google Scholar] [PubMed] [CrossRef]
32. Tucker AO, Maciarello MJ. Essential oils of cultivars of Dalmatian sage (Salvia officinalis L.). J Essential Oil Res. 1990;2(3):139–44. doi:10.1080/10412905.1990.9697844. [Google Scholar] [CrossRef]
33. Raal A, Orav A, Arak E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat Prod Res. 2007;21(5):406–11. doi:10.1080/14786410500528478. [Google Scholar] [PubMed] [CrossRef]
34. ISO 9909. Oil of Dalmatian sage (Salvia officinalis L.). 1997. [Google Scholar]
35. Sagareishvili TG, Grigolava BL, Gelashvili NE, Kemertelidze EP. Composition of essential oil from Salvia officinalis cultivated in Georgia. Chem Nat Compd. 2000;36(4):360–1. doi:10.1023/A:1002884612229. [Google Scholar] [CrossRef]
36. Chalchat JC, Michet A, Pasquier B. Study of clones of Salvia officinalis L. yields and chemical composition of essential oil. Flavour Fragr J. 1998;13(1):68–70. doi:10.1002/(SICI)1099-1026(199801/02)13. [Google Scholar] [CrossRef]
37. Hasa E, Duka S, Lika E, Mançe S. A study on differences of Albanian Salvia officinalis L. essential oils depending on geographical position. Int J Ecosyst Ecol Sci. 2020;10(4):609–16. doi:10.31407/ijees10.405. [Google Scholar] [CrossRef]
38. Dob T, Berramdane T, Dahmane D, Benabdelkader T, Chelghoum C. Chemical composition of the essential oil of Salvia officinalis from Algeria. Chem Nat Compd. 2007;43(4):491–4. doi:10.1007/s10600-007-0173-3. [Google Scholar] [CrossRef]
39. Jug-Dujaković M, Ristić M, Pljevljakušić D, Dajić-Stevanović Z, Liber Z, Hančević K, et al. High diversity of indigenous populations of Dalmatian sage (Salvia officinalis L.) in essential-oil composition. Chem Biodivers. 2012;9(10):2309–23. doi:10.1002/cbdv.201200131. [Google Scholar] [PubMed] [CrossRef]
40. Karik U, Cinar O, Tuncturk M, Sekeroglu N, Gezici S. Essential oil composition of some sage (Salvia spp.) species cultivated in İzmir (Turkey) ecological conditions. IJPER. 2018;52(4s):s102–7. doi:10.5530/ijper.52.4s.83. [Google Scholar] [CrossRef]
41. Mohamed A, Mustafa A. Gas chromatography-mass spectrometry (GC-MS) analysis of essential oil Salvia officinalis in Sudan. J Multidis Res Rev. 2019;1:43–5. [Google Scholar]
42. Gad HA, Mamadalieva RZ, Khalil N, Zengin G, Najar B, Khojimatov OK, et al. GC-MS chemical profiling, biological investigation of three Salvia species growing in Uzbekistan. Molecules. 2022;27(17):5365. doi:10.3390/molecules27175365. [Google Scholar] [PubMed] [CrossRef]
43. Gandhi GR, Hillary VE, Antony PJ, Zhong LLD, Yogesh D, Krishnakumar NM, et al. A systematic review on anti-diabetic plant essential oil compounds: dietary sources, effects, molecular mechanisms, and safety. Crit Rev Food Sci Nutr. 2024;64(19):6526–45. doi:10.1080/10408398.2023.2170320. [Google Scholar] [PubMed] [CrossRef]
44. Jaffar S, Lu Y. Toxicity of some essential oils constituents against oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: tephritidae). Insects. 2022;13(10):954. doi:10.3390/insects13100954. [Google Scholar] [PubMed] [CrossRef]
45. Stojanović NM, Ranđelović PJ, Simonović M, Radić M, Todorović S, Corrigan M, et al. Essential oil constituents as anti-inflammatory and neuroprotective agents: an insight through microglia modulation. Int J Mol Sci. 2024;25(10):5168. doi:10.3390/ijms25105168. [Google Scholar] [PubMed] [CrossRef]
46. Kazemi M. Essential oil of the aerial parts of Salvia officinalis (Lamiaceae) from Iran. J Essent Oil Bearing Plants. 2015;18(3):725–7. doi:10.1080/0972060X.2014.985737. [Google Scholar] [CrossRef]
47. Rzepa J, Wojtal L, Staszek D, Grygierczyk G, Labe K, Hajnos M, et al. Fingerprint of selected Salvia species by HS-GC-MS analysis of their volatile fraction. J Chromatogr Sci. 2009;47(7):575–80. doi:10.1093/chromsci/47.7.575. [Google Scholar] [PubMed] [CrossRef]
48. Đurović S, Micić D, Pezo L, Radić D, Bazarnova JG, Smyatskaya YA, et al. The effect of various extraction techniques on the quality of sage (Salvia officinalis L.) essential oil, expressed by chemical composition, thermal properties and biological activity. Food Chem X. 2022;13(1):100213. doi:10.1016/j.fochx.2022.100213. [Google Scholar] [PubMed] [CrossRef]
49. Assaggaf HM, Naceiri Mrabti H, Rajab BS, Attar AA, Alyamani RA, Hamed M, et al. Chemical analysis and investigation of biological effects of Salvia officinalis essential oils at three phenological stages. Molecules. 2022;27(16):5157. doi:10.3390/molecules27165157. [Google Scholar] [PubMed] [CrossRef]
50. Afifi AA, Azen SP. Statistical Analysis. A computer oriented approach. 2nd edNew York: San Francisco: London: Academic Press; 1979. [Google Scholar]
51. Moumni S, Elaissi A, Trabelsi A, Merghni A, Chraief I, Jelassi B, et al. Correlation between chemical composition and antibacterial activity of some Lamiaceae species essential oils from Tunisia. BMC Complement Med Ther. 2020;20(1):103. doi:10.1186/s12906-020-02888-6. [Google Scholar] [PubMed] [CrossRef]
52. Gouyon PH, Vernet Ph, Guillerm JL, Valdeyron G. Polymorphisms and environment: the adaptive value of the oil polymorphisms in Thymus vulgaris L. Heredity. 1986;57(1):59–66. [Google Scholar]
53. Lauranson J, Lebreton PH. Flavonoid variability within and between natural populations of Pinus uncinate. Biochem Syst Ecol. 1991;19(8):659–64. [Google Scholar]
54. Kou YN, Zhang X, Kong FK, Dong HJ, Yang J, Wang WH, et al. Content of secondary metabolites and antioxidant activities of Lonicera japonica flowers at different developmental stages from Shandong province. Zhongguo Zhong Yao Za Zhi. 2024;49(10):2654–65 (In Chinese). doi:10.19540/j.cnki.cjcmm.20240129.101. [Google Scholar] [PubMed] [CrossRef]
55. Tang YM, Liu YZ, Zhang YH, Cao YN, Song PP, Hou LM, et al. A comparative analysis of the nutrient and phytochemical richness among different varieties of quinoa in China. Food Sci Nutr. 2024;12(6):4473–85. doi:10.1002/fsn3.4113. [Google Scholar] [PubMed] [CrossRef]
56. Chen XY, He C, Yan BB, Li WB, Geng YY, Hou JL, et al. Investigation and analysis on difference of cultivation technique situation of Salvia miltiorrhiza. Zhongguo Zhong Yao Za Zhi. 2019;44(7):1314–20 (In Chinese). doi:10.19540/j.cnki.cjcmm.20190129.010. [Google Scholar] [PubMed] [CrossRef]
57. Zhang R, Zhang MX, Chen Y, Wang CC, Zhang CH, Heuberger H, et al. Future development of good agricultural practice in China under globalization of traditional herbal medicine trade. Chin Herb Med. 2021;13(4):472–9. doi:10.1016/j.chmed.2021.09.010. [Google Scholar] [PubMed] [CrossRef]
58. Mykhailenko O, Buydin Y, Ivanauskas L, Krechun A, Georgiyants V. Innovative GACP approaches for obtaining the quality Iris hybrida leaves for the pharmaceutical industry. Chem Biodivers. 2022;19(4):e202200149. doi:10.1002/cbdv.202200149. [Google Scholar] [PubMed] [CrossRef]
59. Mykhailenko O, Saidov NB, Ivanauskas L, Georgiyants V. Model implementation of the legal regulation on medicinal plant cultivation for pharmaceutical purposes. Case study of Crocus sativus cultivation in Ukraine. Botanica. 2022;28(1):27–38. doi:10.35513/botlit. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools