 Open Access
Open Access
ARTICLE
Effect of Senescence Retardants on the Quality of Persian Lime Fruits
1 National Institute of Forestry, Agriculture and Livestock Research (INIFAP), South Pacific Regional Center, Santo Domingo Barrio Bajo, Villa de Etla, Oaxaca, 68200, México
2 National Institute of Forestry, Agriculture and Livestock Research (INIFAP), Zacatepec Research Station, Zacatepec, Morelos, 62780, México
3 National Institute of Forestry, Agriculture and Livestock Research (INIFAP), Central Chiapas Research Station, Ocozocoautla, Chis, 29140, México
4 National Institute of Forestry, Agriculture and Livestock Research (INIFAP), Oaxaca Central Valley Research Station, Santo Domingo Barrio Bajo, Villa de Etla, Oaxaca, 68200, México
* Corresponding Author: Miguel A. Cano-García. Email:
Phyton-International Journal of Experimental Botany 2024, 93(8), 1805-1818. https://doi.org/10.32604/phyton.2024.050093
Received 27 January 2024; Accepted 11 June 2024; Issue published 30 August 2024
Abstract
The objective of this study consisted of evaluating the effect of the application of chitosan (Q), 1-methylcyclopropene (1-MCP) under two controlled temperatures on some physical, physiological, and chemical parameters contributing to the quality of Persian lime Citrus latifolia fruits. Eight treatments were evaluated, resulting from the combination of four senescence retardant applications (Q, 1-MCP, 1-MCP + Q, and without application) on fruits stored at two temperatures (12/20°C). Epidermis color (luminosity, chromaticity, and hue), fruit appearance, respiration, weight loss, total juice content, total soluble solids, and titratable acidity were registered during the study. The results indicate that the 1-MCP and Q combination favored fruit postharvest quality conditions such as reducing respiration rate; maintaining fruit good appearance, turgidity, and brighter fruit chromaticity; fruits treated with chitosan and stored at 12°C, favored total soluble solids. On the other hand, Variables such as weight loss, juice content, acidity, phenols, flavonoids, antioxidant activity, and hue values were favored by a 12°C storing temperature regardless of the treatment. In general, the use of modified atmospheres, senescence retardants, and refrigeration as postharvest methods, can increase Persian lime shelf life.Keywords
Mexico is one of the main world citrus producers ranking fifth in lemon and lime production of the Mexican lime (51%), Persian lime (45%), and Italian lemon (4%). The United States is the main exportation destiny with lesser proportions exported to Europe and Japan [1]. At postharvest, deficient harvesting, processing, transportation, and storing practices can produce Persian lime losses causing the presence of diseases, weight, and brightness loss, and changes in quality attributes such as total soluble solids and titratable acidity [2].
Because of its acidity in flavor, high nutrient content, and availability, Persian lime is a worldwide popular fruit. However, it is prone to become physically, physiologically, and microbiologically deteriorated after postharvest [3]; for instance, the loss of green color limits their quality as the fruits turn yellowish in postharvest [4]. Additionally, water loss has major implications concerning weight loss and appearance. After harvesting, it has been determined that water loss is four to six times greater on the peduncle than in other parts of the fruits, so it is advisable to use wax or modified atmospheres to reduce the water loss [5].
There are techniques used to increase the fruit’s postharvest life to reduce the oxygen and carbon dioxide partial pressure with modified atmospheres, and decreasing the temperature by storing fruits in cold chambers [4]. The use of edible films forms a continuous macromolecule matrix around the fruits to create modified atmospheres with macromolecules such as pectins, cellulose and collagen byproducts, and chitosan (Q) [4]. This latter is a crustacean by-product that forms a semipermeable barrier to keep fruits pathogenic-free in postharvest.
Ethylene, abscisic acids, and gibberellins are involved in chlorophyll and carotenoid metabolism in citrus flavedo which has a significant role in fruit pigmentation [6]. The use of ethylene, exogenous to harvested fruits induces chlorophyll expression and specific genes within carotenoid genesis that match up with chlorophyll deterioration and carotenoid accumulation [6]. Exogenous ethylene application to harvested fruits induces chlorophyllase and specific gene expression in carotene genesis, which correlate with chlorophyll degradation and carotenoid accumulation [6]. Likewise, produced ethylene in fruit maturation even in low levels or sensitivity changes, could be needed for external changes in pigmentation [6]. Application of 1-Methylcyclopropene (1-MCP) and chitosan (Q) was reported to improve banana’s postharvest life increasing fruit shelf life and suggesting that the combination of these products is more effective [7]. Methylcyclopropane (MCP) is a competitor for ethylene’s action sites and for that reason, it has been used to slow down some ethylene-induced processes. MCP effect on fruit quality has been reported on mango [8,9], apple [10], climacteric fruits [11] and cut rose [12].
Finally, there are reports that Persian lime produced under tropical climates can be stored under suitable conditions for about 4–8 weeks at 10°C to 12°C and relative humidity of 85% to 95%. In general, fruits require 30 days of shelf life [13] by using suitable transportation means that contribute to diminishing costs and increase competitiveness in international markets [13]. However, under these conditions and using adequate handling postharvest techniques, growers and exporters are still facing problems with fruit turgidity and losses in green color, which are key features for the final consumer [13].
As previously mentioned, this work evaluated the effect of chitosan, Methylcyclopropane, and two storage temperatures on some physical, physiological, and chemical parameters that contribute to Persian lime quality in order to propose the ideal combinations to increase Persian lime shelf life.
Persian lime fruits were collected between 8:00 and 9:00 h from five-year-old trees of an orchard located in the village of Cajones in the municipality of Amacuz, Morelos under a dry warm climate. The harvest was done when the fruits had a smooth and intense green epidermis on more than 90% of the surface. The fruits were manually harvested and transported to the lab where they were washed with running water and a 1% chlorine solution to eliminate dust and pathogens that could harm the fruit; then they were air-dried at room temperature and the treatments were applied later.
The fruits were stored at 22°C and 12°C because 22°C is close to the environment temperature and the 12°C is applied to avoid burn damage of this tropical fruit. The eight treatments are shown in Table 1 and the data were taken at the beginning and every ten days, ending at thirty days. Data were taken on ten fruits at the beginning and at the end of the study and on six fruits at ten and twenty days.
Chitosan application was done by dissolving 164 kDa in acetic acid and 1% distilled water. In addition, 1-MCP application was done placing the fruits in a 10 L capacity box and with a bag containing the product in a buffer for its liberation. Both products were used as immersion or epidermis coating as these treatments can be considered senescence retardants by reducing gas exchange.
A completely randomized experimental design was used with one fruit being the experimental unit. The fruit was marked to be analyzed with five, six, and ten replications for respiration, destructive, and color-weight loss measurements, respectively, on each treatment sampling. The following variables were evaluated.
Epidermis color. Ten fruits per treatment were selected to evaluate epidermis color and equatorial cross-section of the fruit with the evaluation of L, a, and b parameters that were obtained with a sphere spectrophotometer (X-rite, USA). Subsequently, a and b values were used to estimate chromaticity
Appearance. In order to describe fruit appearance, photographs of the fruits were taken at the beginning of the study and every ten days, ending after thirty days. When more than 50% of the fruits had an appearance inadequate for the consumer, the treatments were not evaluated anymore.
Respiration. Measurements were taken daily, placing two fruits in hermetic recipients for two hours. After that, 1 mL of headspace was extracted with a hypodermic needle and then injected into a gas chromatograph (Agilent, USA), equipped with a flame ionization detector and thermal conductivity. Gasses were quantified using a 460 ppm CO2 standard [15].
Weight loss. This variable was measured daily by weighing the fruits in a digital balance the following equation was applied: %PP = ((initial weight − Final weight)/Initial weight)) × 100.
Total juice. Every ten days, the juice of six fruits was extracted with an industrial juicer extractor (Turmix, México), and the total fruit weight was obtained to have the percentage of juice per fruit.
Total soluble solids (TSS). Three juice drops were placed in a refractometer (ATAGO PAL-1, Japan) to determine the percentage of total soluble solids.
Titratable acidity was obtained from 5 mL of juice fruits, adding, twenty milliliters of distilled water, and then in a 5 mL aliquot three drops of phenolphthalein were added to titrate at 0.1N of sodium hydroxide [5].
Phenols content, total flavonoids, and antioxidant activity. These variables were obtained by the ABTS, DPPH, and FRAP methods by colorimetry, using the methodologies described by Brand-Williams et al. [16], Singleton et al. [17], Arvouet-Grand et al. [18] and Re et al. [19].
One-way ANOVA and Tukey’s test means comparison were used to analyze the results as reported by [7]. Data were submitted to variance homogeneity and normalized distribution principles. When these principles were not fulfilled, data analysis went through a range test. The analyses were carried out with Sigma Plot V14.0 software [20].
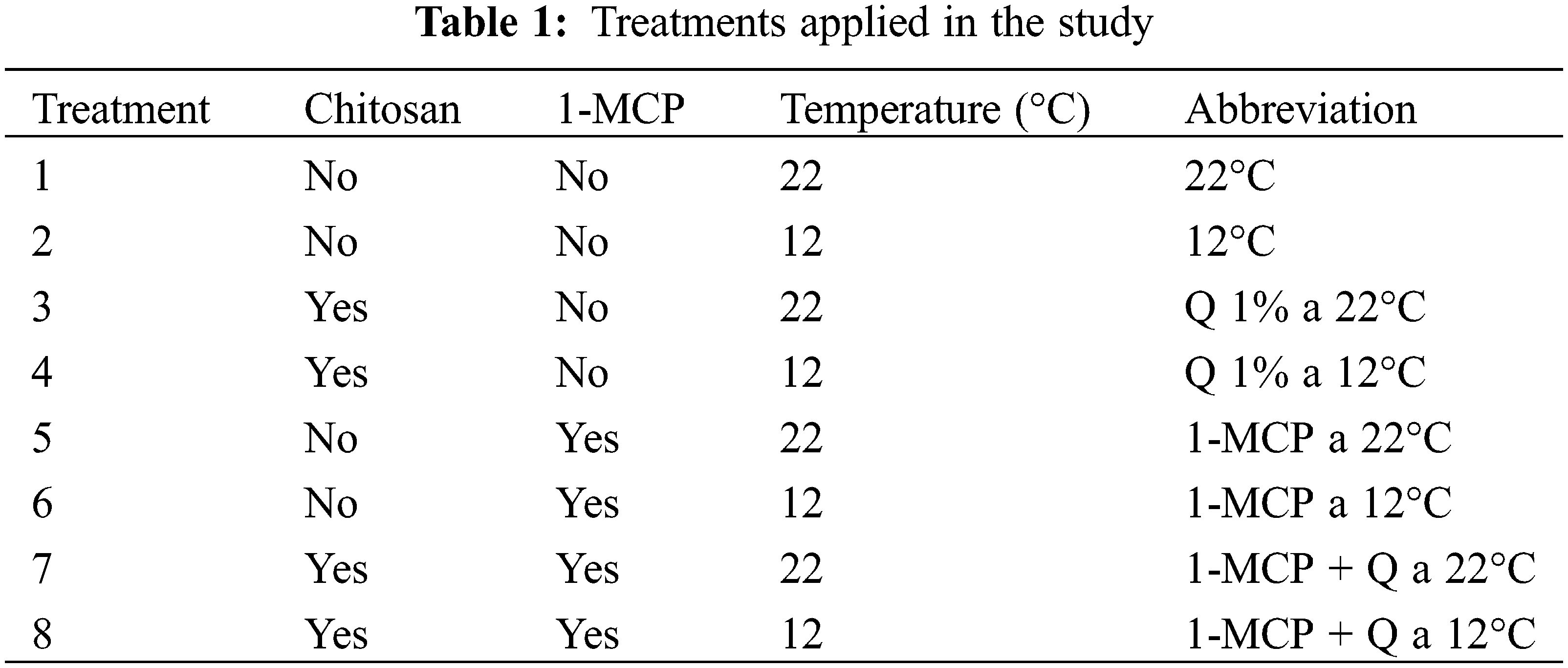
Respiration. All of the treatments had a respiration rate of 1.2 mL kg−1h−1 at the beginning of the study. After ten days, this value increased to 5.1 and 7.9 mL kg−1h−1 for fruits stored at 22°C, treated with chitosan, and untreated. It is important to point out that fruits on these treatments plus those treated with 1-MCP + Q and stored at 22°C presented inadequate conditions for the consumer before 20 days. In the rest of the treatments, the fruits had respiration values less than 4.0 mL kg−1h−1 and after 20 days, this value decreased slightly to less than 3.2 mL kg−1h−1. All the fruits stored at 12°C had respiration rates lower than 3.0 and even this value decreased to less than 2.5 for fruits treated with 1-MCP+Q and only with 1-MCP (Fig. 1).
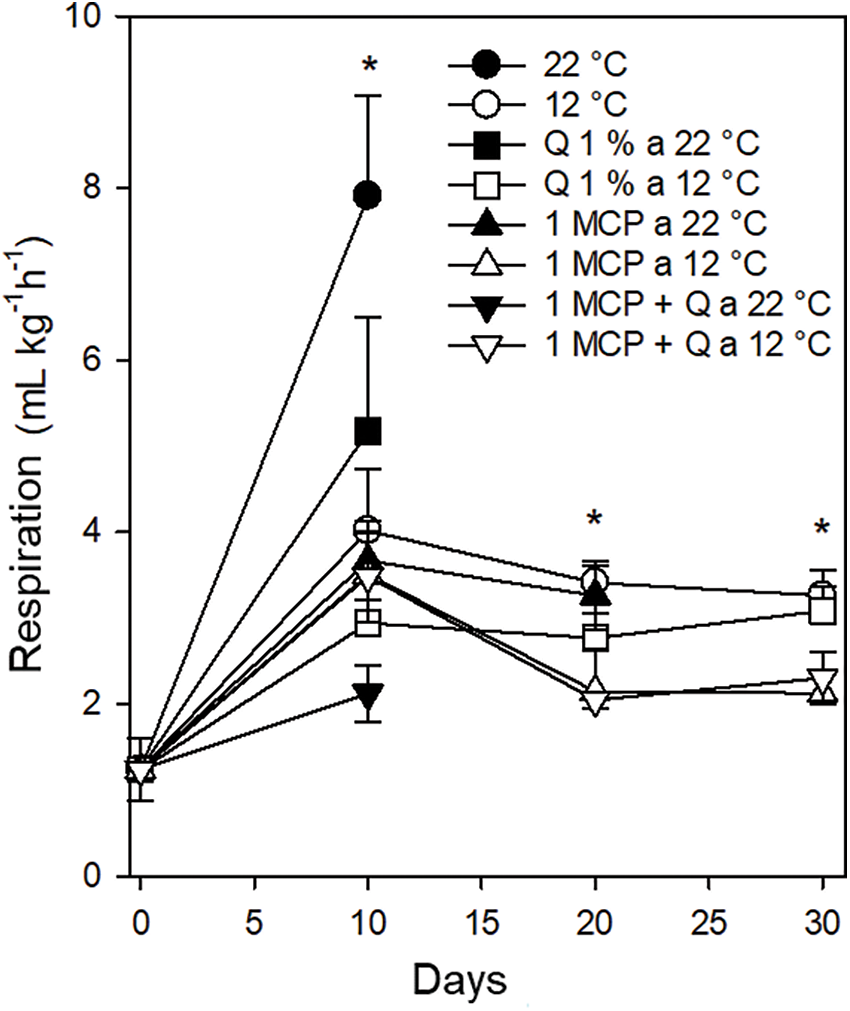
Figure 1: Respiration velocity in Persian lime fruits treated with senescence retardants, stored at two temperatures. Each point represents the mean from 6 to 10 observations and its standard error. *: Significant at 0.05 probability
During postharvest, Persian lime fruits go under stress because of harvest and postharvest handling, processes associated with respiration velocity increase [21,22]. Laureth et al. [3] observed that Persian lime fruits increase respiration velocity after three days of harvest. A similar behavior was observed in fruits covered up with various films of nanocomposites and some polymers, suggesting that plastic films are less effective in decreasing respiration. On the other hand, applications of 1-MCP reduce respiration velocity because it reduces ethylene production indirectly, a hormone known as a respiration promotor [23]. Chitosan as a film helps decrease gas exchange and for that reason can be considered to reduce respiration rate. Finally, refrigeration contributed to respiration rate as reported by Jomori et al. [23], who observed a significant respiration rate decrease by storing Persian lime fruits at 5°C, compared to fruits stored at 10°C. In this study, fruits stored at 12°C showed this decreasing respiration rate trend.
Weight loss. All fruits stored at 22°C, regardless of the treatment, lost more than 16% weight after ten days of treatment with those fruits treated with 1-MCP + Q reaching up to 22% weight loss. Fruits stored at 12°C, regardless of the treatment, lost less than 8% weight after ten days this value was between 10.5% and 13.5% after 20 days increasing to a range between 14.0% and 17.5% after 30 days of treatment. It is important to highlight that all fruits stored at 12°C reached 30 days with conditions adequate for the consumer (Fig. 2).
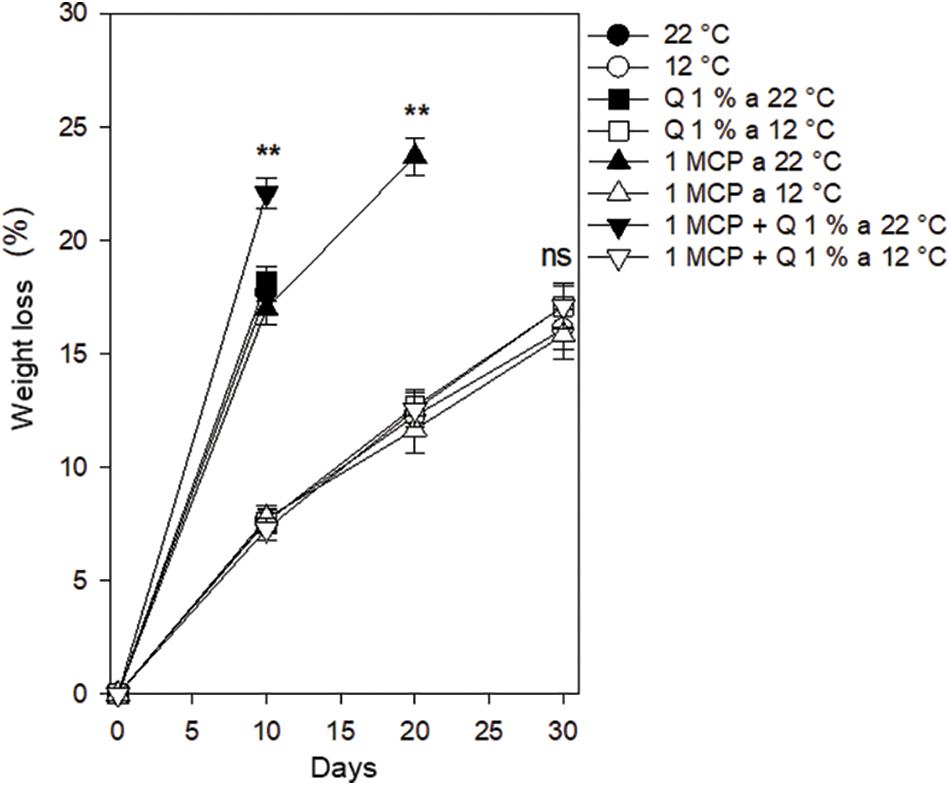
Figure 2: Weight loss in Persian lime fruits treated with senescence retardants, stored at two temperatures. Each point represents the mean from 6 to 10 observations and its standard error. **: Significant at 0.01 probability. ns: Not significant
Caron et al. [13] reported fruit daily weight losses of 1.33%. In addition, Bassan et al. [2] indicated that weight loss in Persian lime is attributed to mechanical damage causing higher respiration and water loss. In this experiment, storing temperature caused a lesser weight loss by decreasing physiological and metabolic processes drastically.
Color parameters (L*, c, and h). Luminosity (L*). Luminosity values started between 34 and 39 and after ten days, this value increased slightly, particularly for the control and 1-MCP treatments, both stored at 22°C. A similar trend was observed for the control and Q treatments stored at 12°C. After 20 days only those fruits stored at 12°C and the ones treated with 1-MCP and stored at 22°C, kept conditions adequate to the consumer but the late treatment lost good conditions before 30 days. All the fruit stored at 12°C, regardless of the treatment had good consumer conditions with luminosity values between 38 and 43 after 30 days but the treatments were not statistically different (Fig. 3).
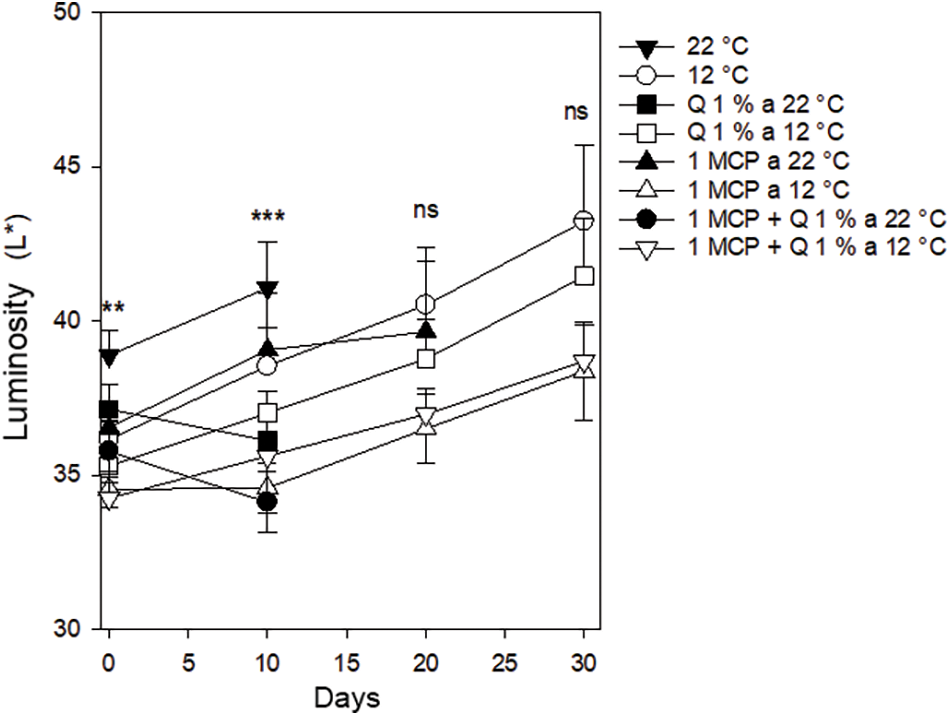
Figure 3: Luminosity in Persian lime fruits treated with senescence retardants, stored at two temperatures. Each point represents the mean from 6 to 10 observations and its standard error. ** and ***: Significant at 0.01 and 0.001 probability. ns: Not significant
Chromaticity (c). This parameter showed differences among treatments during the complete evaluation period. Results using 1-MCP, alone or in combination with Q and stored at 12°C, presented a brighter color than those in the other treatments (Fig. 4). This information suggests that 1-MCP, at lower temperatures, helped the fruits to maintain their green color achieving greater purity color in Persian lime. This is attributed that during postharvest, ethylene is produced in small amounts and this substance triggers chlorophyll deterioration and carotenoids synthesis [6].
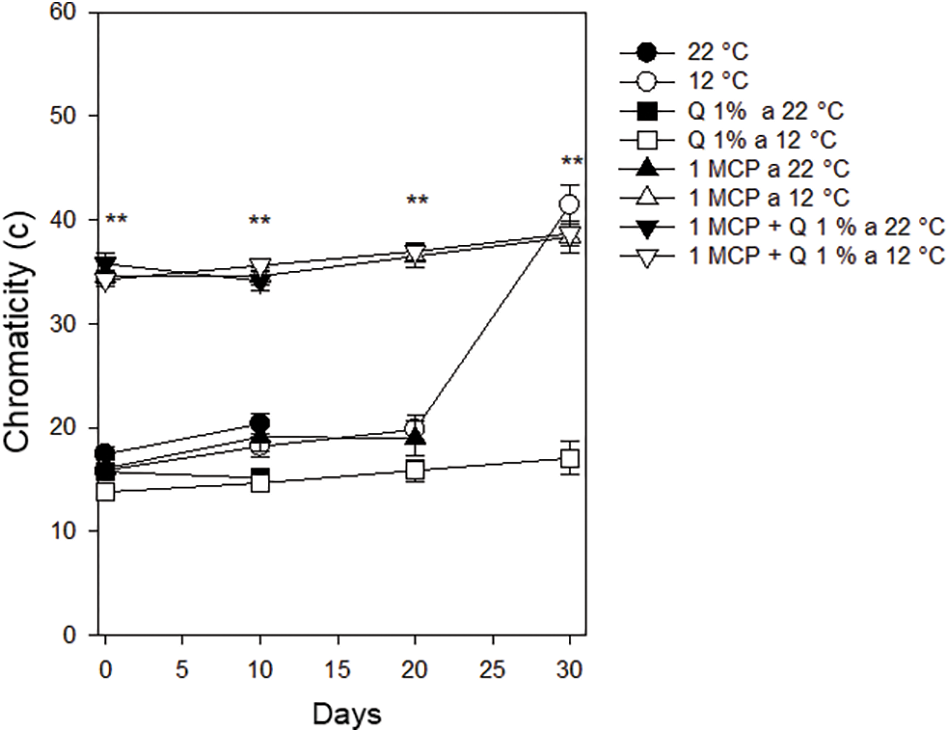
Figure 4: Chromaticity in Persian lime fruits treated with senescence retardants, stored at two temperatures. Each point represents the mean from 6 to 10 observations and its standard error. **: Significant at 0.01 probability
Hue (h). Values of this variable started between 115 and 120 with statistical differences among treatments. After ten days these values remained similar except for those stored at 22°C and after 20 days only those fruits stored at 12°C and the one treated with 1-MCP at 22°C had good conditions for the consumer. However, only those treatments stored at 12°C kept adequate conditions for the consumer although there was no statistical difference among treatments after 30 days (Fig. 5). Blum et al. [24] reported that applications of 1-MCP decrease the loss of green.
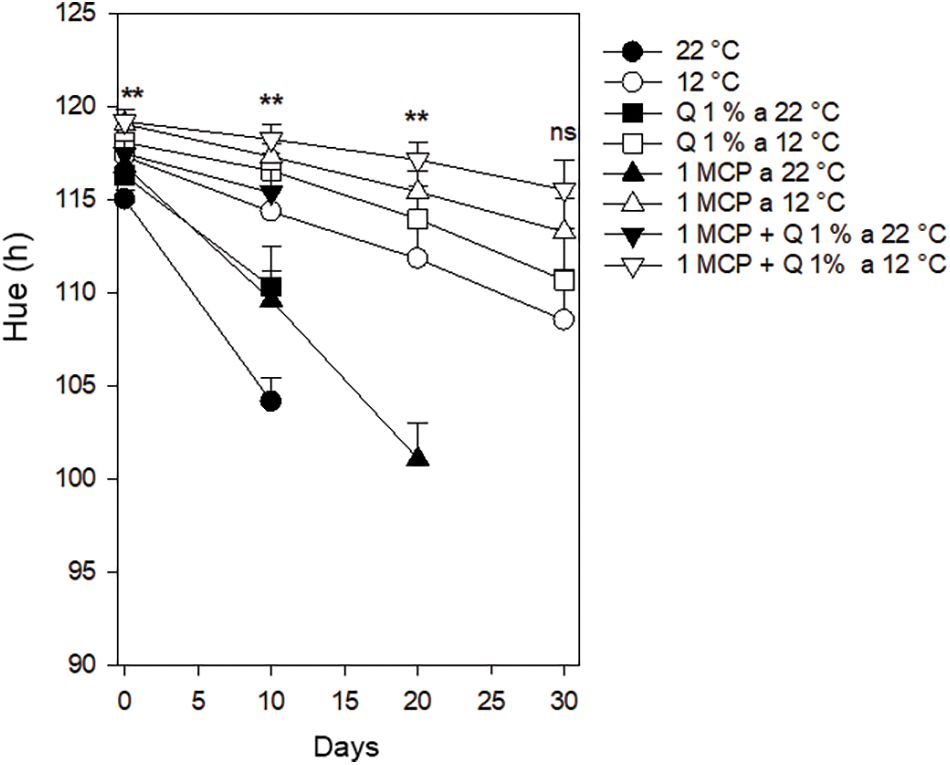
Figure 5: Hue in Persian lime fruits treated with senescence retardants, stored at two temperatures. Each point represents the mean from 6 to 10 observations and its standard error. **: Significant at 0.01 probability. ns: Not significant
Juice content. All the fruits had an initial value of 50% juice content and after ten days this value increased in the fruits treated with 1-MCP and stored at 12°C and those treated with Q and without treatment, both stored at 22°C. The juice content percentage diminished in the fruits treated with 1-MCP and 1-MCP + Q, both stored at 22°C. This last treatment had a juice content percentage of 31% after ten days and did not keep good conditions for the consumer before 20 days. The remaining treatments showed values similar to the initial juice content percentage. After 20 days the fruits showed an increase in the juice content percentage with values between 55% and 66%, except for the fruits treated with 1-MCP and stored at 12°C, which had values very close to the initial value. Finally, after 30 days, all the fruits stored at 12°C, regardless of the treatment, had juice content percentage values between 51% and 60% (Fig. 6). Juice content percentage is an important Persian lime quality characteristic. There are reports that for those fruits with a dark green epidermis, the juice content percentage is about 55.6% [25], even though Khan et al. [26] indicated that Persian lime can be harvested when having more than 30% juice. The higher juice content percentage is attributed to the weight loss occurring during postharvest.
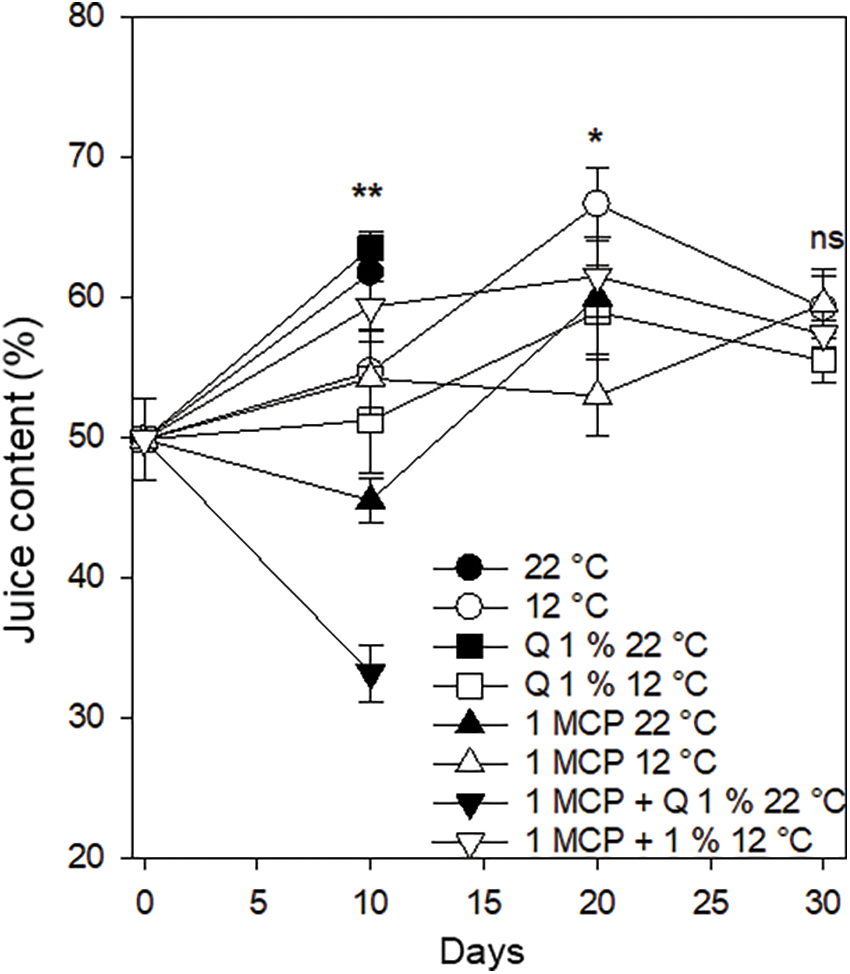
Figure 6: Juice content percentage in Persian lime fruits treated with senescence retardants, stored at two temperatures. Each point represents the mean from 6 to 10 observations and its standard error. * and **: Significant at 0.05 and 0.01 probability. ns: Not significant
Appearance. Control treatment fruits, stored at 22°C, showed a good appearance on days seven and eight, but on the 10th day, they turned yellowish with brown-color spots and wilting epidermis (Fig. 7). Fruits stored at 22°C with applications of Q or 1-MCP + Q reached a shelf life of ten days; moreover, fruits treated with 1-MCP and stored at 22°C had a good appearance after 20 days (Fig. 7). Fruits stored at 12°C had a good appearance until day 20; when Q or 1-MCP were applied, fruit shelf life reached up to day 20 and fruits using 1-MCP + Q showed less yellowish and had a more turgid appearance (Fig. 7).
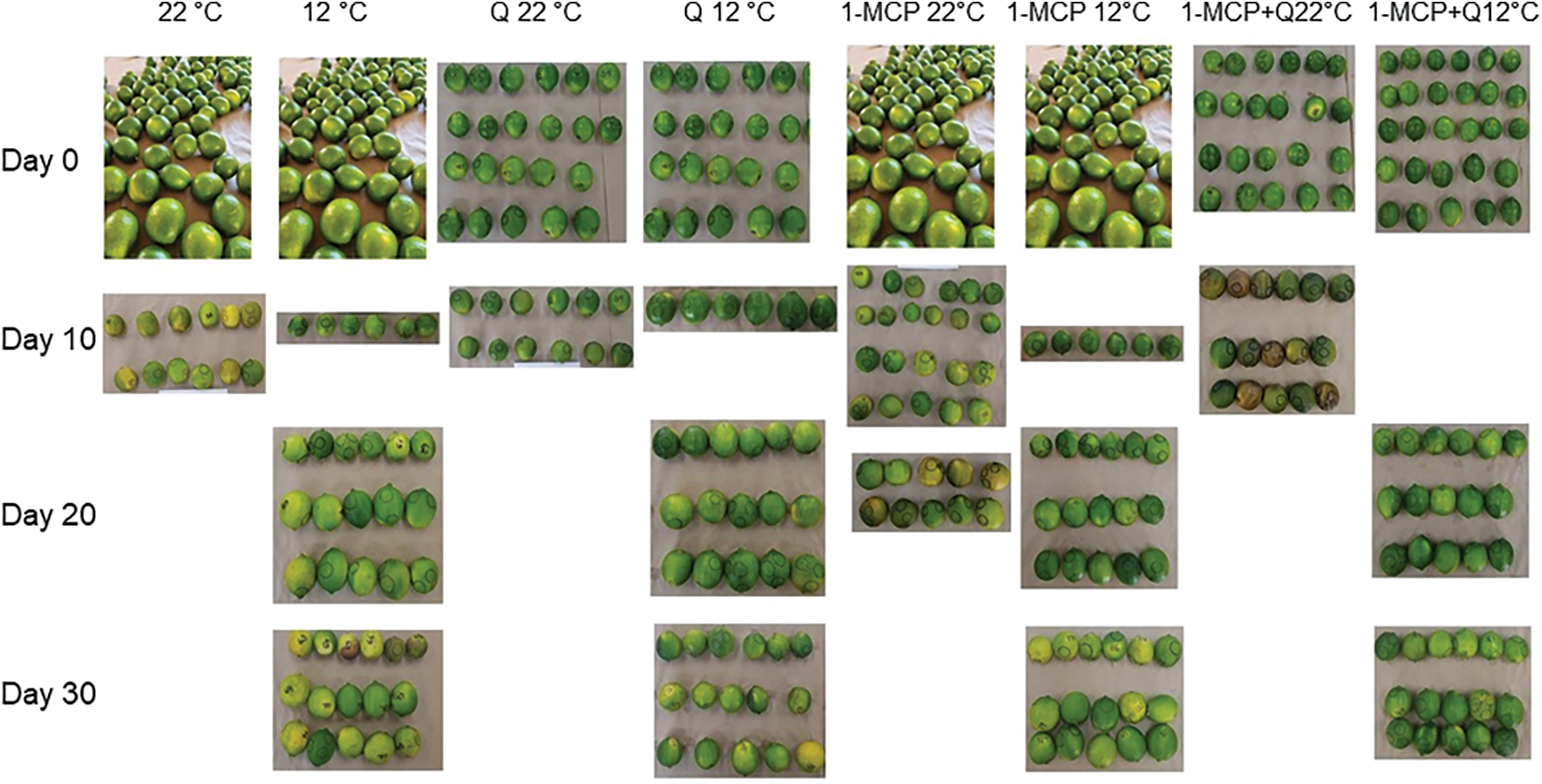
Figure 7: Appearance in Persian lime fruits treated with senescence retardants, stored at two temperatures
Total soluble solids (TSS). The value of this variable started at 7.2°Brix and changed to values between 7.5 and 8.6°Brix for those treatments that included chitosan or 1-MCP, with the exception of fruits treated with chitosan and stored at 12°C. This treatment experienced a constant increase from the initial value to 11°Brix after 20 days and a decrease to 9.9°Brix after 30 days. This treatment (Q – 12°C), was statistically different than the rest of the treatments whose fruits were stored at 12°C and only reached a value of 8.1°Brix (Fig. 8). During postharvest, total soluble solids increase According to Maftoonazad et al. [27] who stored lime fruits at temperatures between 5°C and 25°C and the accumulation was between 11.5 and 12.7°Brix after 8 to 32 storing days.
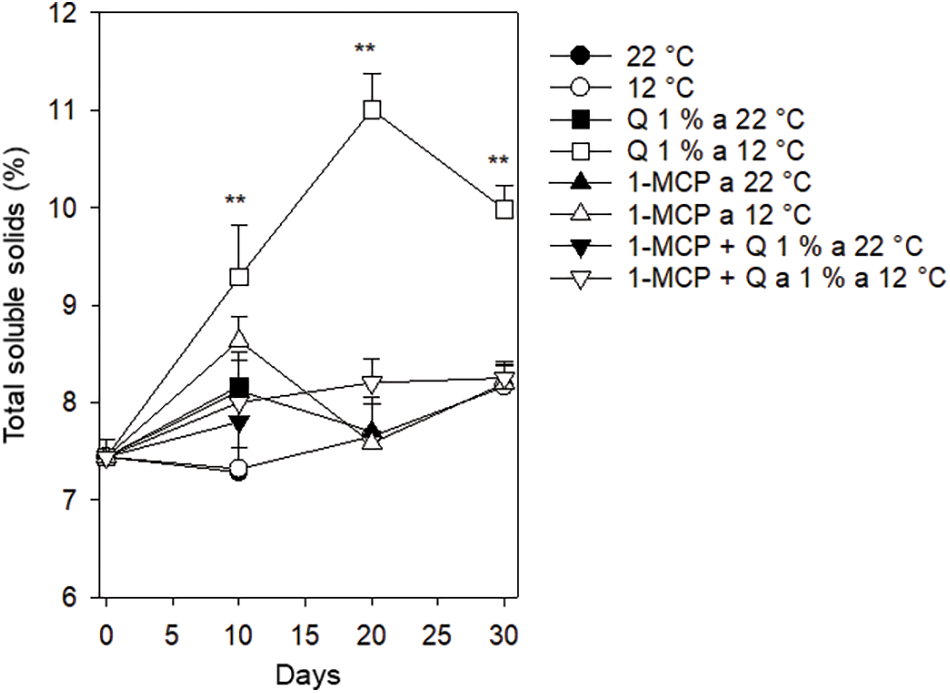
Figure 8: Total soluble solids in Persian lime fruits treated with senescence retardants, stored at two temperatures. Each point represents the mean from 6 to 10 observations and its standard error. **: Significant at 0.01 probability
Titratable acidity. The value of this variable initiated at 5.1% and after ten days its behavior was variable but the lowest value was for the fruits treated with chitosan and stored at 22°C. On the other hand, the control at 22°C had the highest value with 6.7%. These two treatments did not have adequate conditions for the consumer before 20 days. Treatments that reached the 20 days (those with storing temperature of 12°C and 1-MCP at 22°C) had values between 6.0% to 7.0% and this range decreased to 6.1% to 6.7% after 30 days (Fig. 9). Khan et al. [26] indicated that the main quality of lime is acidity, reporting values between 8% and 9%. During postharvest storage, acidity decreases because of respiration, which is part of the fruits’ normal maturation process [27]. Blum et al. [24] observed that titratable acidity had a lineal increment in Persian lime stored at 24°C with 70% of HR, While accumulation was quadratic in fruits treated with 1-MCP, with an initial reduction and posterior-increment.
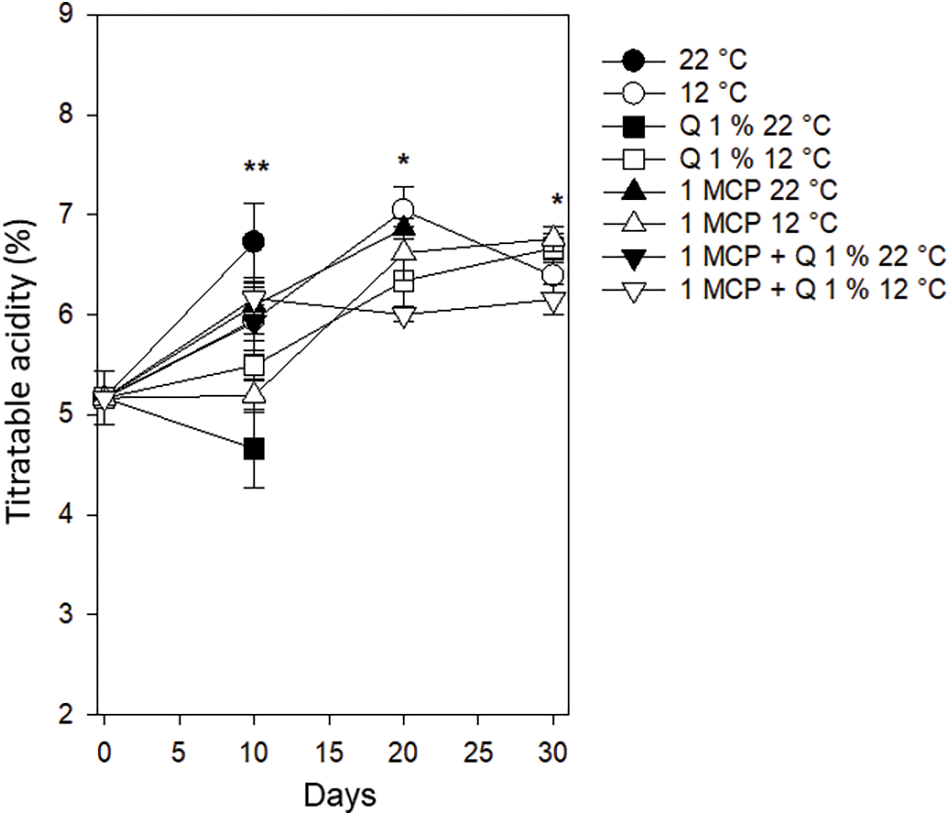
Figure 9: Titratable acidity in Persian lime fruits treated with senescence retardants, stored at two temperatures. Each point represents the mean from 6 to 10 observations and its standard error. * and **: Significant at 0.05 and 0.01 probability
Total phenols. Total phenols started for all the fruits with a value of 816 mg EAG 100 g−1 and after ten days, their behavior was variable with values between 748 and 1071 mg EAG 100 g−1. For all fruits stored at 12°C, regardless of the treatment, these values decreased to a range between 561 and 718 mg EAG 100 g−1 after 20 days and increased to a range between 789 and 844 mg EAG 100 g−1, after 30 days, without statistical difference. According to this, lower temperatures keep good total phenol levels. The presence of 112 mg 100 g−1 of dry-weight total phenols is reported when they are extracted in maceration but reaches up to 580 mg 100 g−1 when extracted by assisted ultrasound [28]. Studies suggest that phenols in citrus are great candidates for nutraceutical and functional foods development, oriented to cancer and diabetes control [29]. Refrigeration is a key method to retard phenols deterioration in Persian lime fruits (Fig. 10).
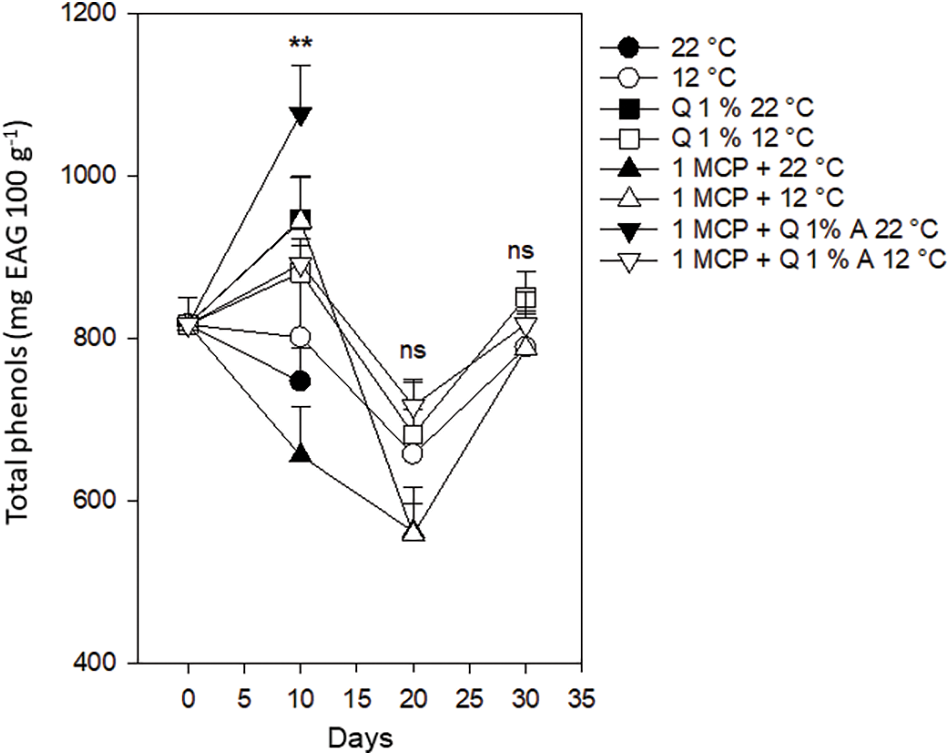
Figure 10: Total phenols in Persian lime fruits treated with senescence retardants, stored at two temperatures. Each point represents the mean from 6 to 10 observations and its standard error. **: Significant at 0.01 probability. ns: Not significant
Flavonoids. Flavonoid content followed a similar trend to total phenols, starting at 16.1 mg EQ 100 g−1 and showing a variable behavior (14.4 to 22 mg EQ 100 g−1) after ten days. Fruits stored at 12°C regardless of the treatment had flavonoid values between 17 and 18 mg EQ 100 g−1 after 20 days and between 17.7 and 20.4 mg EQ 100 g−1 after 30 days (Fig. 11). According to this, lowering storing temperatures, favor flavonoids conservation. Flavonoids and phenolic acids are the most prominent citrus components of total phenols, whose antioxidant activity protects cells against oxidative stress caused by free radicals [30]. Medina-Torres et al. [28] reported the presence of eriocitrin, diosmin, hesperid, sinapic acid, catechin, p-camaric acid, neohesperidin, and naringenin in Persian lime.
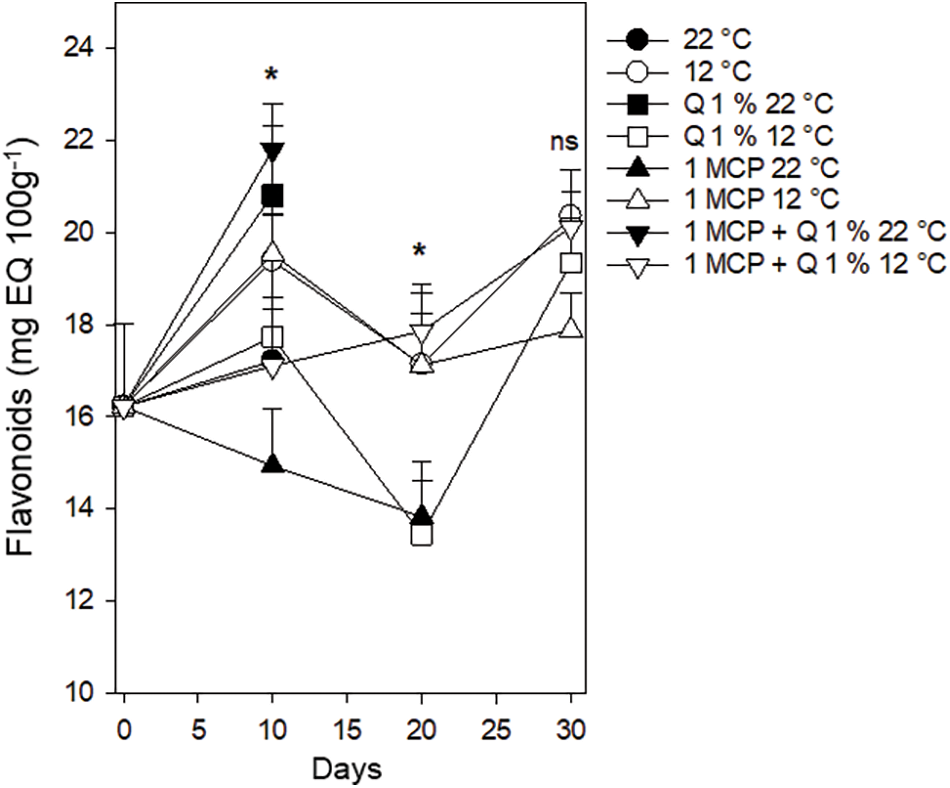
Figure 11: Flavonoid content in Persian lime fruits treated with senescence retardants, stored at two temperatures. Each point represents the mean from 6 to 10 observations and its standard error. *: Significant at 0.05 probability. ns: Not significant
Antioxidant activity (DPPH, ABTS, and FRAP methods). The antioxidant activity showed a variable behavior when the DPHH and ABTS methods were used. However, when the FRAP method was used, fruits treated with chitosan and stored at 12°C antioxidant activity after 30 days, had values higher than 12,000 mg EAA 100 g−1 and the remaining treatments with 12°C storing temperature had values between 8600 and 10,200 mg EAA 100 g−1 with statistical difference between these treatments (Fig. 12). The results indicate that low temperatures contribute to keeping antioxidant activity lasting more time.
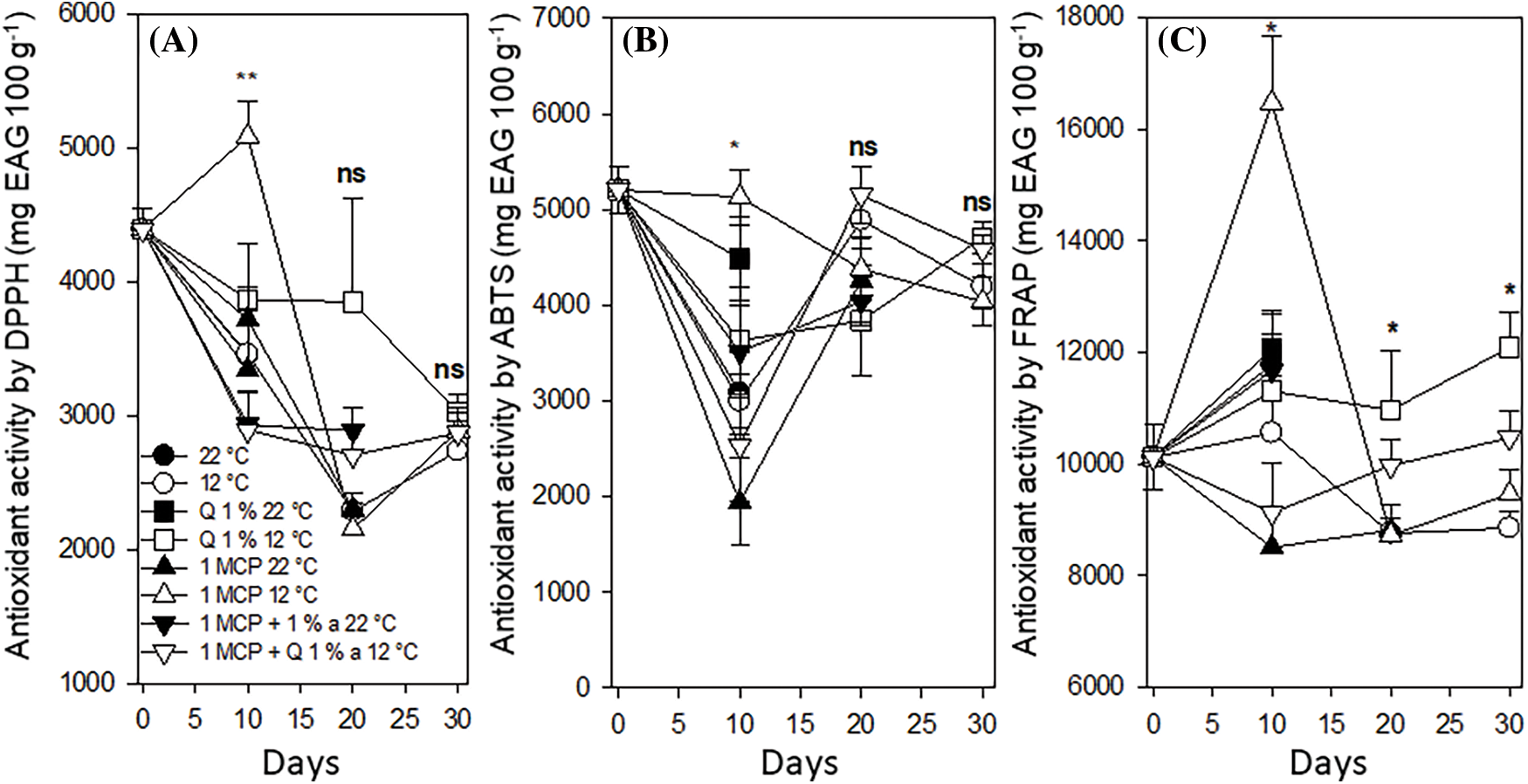
Figure 12: Antioxidant activity by DPPH (A), ABTS (B) and FRAP (C) methods in Persian lime fruits treated with senescence retardants, stored at two temperatures. Each point represents the mean from 6 to 10 observations and its standard error. * and **: Significant at 0.05 and 0.01 probability. ns: Not significant
Fruits stored at 12°C, regardless of the treatment, reached 30-day shelf life meaning that only storing the fruits at a temperature lower than room temperature, helps to lengthen the time fruits are in good condition for the consumer. In total soluble solids, fruits treated with chitosan and stored at 12°C, were statistically superior to the rest of the treatments, showing a high increase trend from the beginning of the study to the 20 days after treatment. Fruits stored at 22°C and treated with 1-MCP, reached 20 days shelf life when evaluated for respiration, weight loss, juice content, TSS, and flavonoids. According to this treatment with MCP represents an alternative when it is not possible to lower temperature below 22°C. Even though in some variables such as respiration, TSS, and Titratable acidity there were statistical differences among treatments, in general, the effect of reducing temperature from 22°C to 12°C was enough for fruit shelf life to reach 30 days. The combination of 1-MCP and Q favors not only better physical and physiological responses but also some biochemical parameters that define quality. On the other hand, lowering the storing temperature to 12°C helps to keep functional molecules and their antioxidant activity for a longer time. Generally, the use of modified atmospheres, senescence retardants, and cold storage, are postharvest methods that help to increase Persian lime shelf life.
Acknowledgement: None.
Funding Statement: This study was funded by the National Institute of Forestry, Agriculture and Livestock Research (INIFAP) of México. INIFAP provided the funds for research Project Number 14574034553 “Evaluation of Rootstocks and Physiology of Citrus Production in Morelos, Guerrero and Oaxaca”.
Author Contributions: Study conception and design: Rafael Ariza-Flores; data collection: Rafael Ariza-Flores and Rafael Ambriz-Cervantes; analysis and interpretation of results: Rafael Ariza-Flores, Luis A. Gálvez-Marroquín and Miguel A. Cano-García; draft manuscript preparation and review: Rafael Ariza-Flores, Luis A. Gálvez-Marroquín, Miguel A. Cano-García and Pedro Cadena-Iñiguez. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data included in this work are available upon request by contact with the corresponding author.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Herrera-Aguilar I, Sandoval-González OO, Malagón-González F, Águila-Rodríguez G, González-Sánchez BE, Flores-Cuautle JJA. Sistema automático de selección de limón (Citrus latifolia Tanaka) basado en discriminación por color. Agroproductividad. 2017;10(10):73–8 (In Spanish). [Google Scholar]
2. Bassan MM, Filho FAAM, Caron VC, Couto HTZ, Jacomino AP. The harvesting system affects the quality and conservation of ‘Tahiti’ acide lime. Sci Hortic. 2013;155:72–7. doi:10.1016/j.scienta.2013.03.008. [Google Scholar] [CrossRef]
3. Laureth JCU, Moraes AJ, Franca DLB, Fluzino-Neto WP, Braga GC. Physiology and quality of ‘Tahiti’ acid lime coated with nanocellulose based nanocomposites. Food Sci Technol. 2018;38(Suppl. 1):327–32. doi:10.1590/fst.21717. [Google Scholar] [CrossRef]
4. Dotto M, Pirola K, Júnior AW, Radaelli JC, Danner MA. Biofilmes e embalagens na conservacao pós-colheita de lima ácida Tahiti. Agrária-Revista Brasileira de Ciencias Agrárias. 2015;10(3):365–9 (In Spanish). doi:10.5039/agraria.v10i3a38351. [Google Scholar] [CrossRef]
5. Ladaniya MS. Citrus fruits. Biology, technology and evaluation. Sandiego, USA: Academic Press-Elsevier; 2008. p. 558. [Google Scholar]
6. Tadeo FR, Terol J, Rodrigo MJ, Licciordello C, Sadka A. Fruits growth and development. In: Talón M, Caruso M, Gmitter FG, editors. The genus citrus. Duxford, UK: Elsevier; 2020. p. 245–69. [Google Scholar]
7. Mubarok S, Rahman IM, Kamaluddin NN, Solihin E. Impact of 1-methylcyclopropene combined with chitosan on postharvest quality of tropical banana ‘Lady Finger’. Int J Food Prop. 2022;25(1):1171–85. doi:10.1080/10942912.2022.2074028. [Google Scholar] [CrossRef]
8. Abu-Goukh AA, Elzubeir MM, Osman OA. Effect of 1-methylcyclopropene (1-MCP) and waxing on quality and shelf-life of mango fruits. Gezira J Agric Sci. 2019;17(1):67–87. [Google Scholar]
9. Manigo BI, Antibo NP. 1-methylcyclopropene and ethephon effects on ‘Carabao’ mango fruits harvested at different stages of fruit maturity. Indonesian J Agric Res. 2022;5(1):11–28. doi:10.32734/injar.v5i01.7409. [Google Scholar] [CrossRef]
10. Win NM, Yoo J, Kwon JG, Kang IK. Effects of 1-methylcyclopropene treatment on fruit quality and antioxidant activities of ruby-S apples during cold storage. Korean J Food Preserv. 2022;29(4):590–600. [Google Scholar]
11. Satekge TK, Magwaza LS. Postharvest application of 1-methylcyclopropene (1-MCP) on climacteric fruits: factors affecting efficacy. Int J Fruit Sci. 2022;22(1):595–607. doi:10.1080/15538362.2022.2085231. [Google Scholar] [CrossRef]
12. Mubarok S, Maunah M, Kusumiyati, Farida, Rufaidah F, Suminar E, et al. Dataset on the combination effects of 1-methylcyclopropene and salicylic acid on postharvest quality of cut roses ‘Peach Avalanche’ and ‘Sexy Red’. Data in Brief. 2020;33(1):106600. doi:10.1016/j.dib.2020.106600. [Google Scholar] [PubMed] [CrossRef]
13. Caron VC, Jacomino AP, Sarantópoulos CIGL, Miguel ACA. Carnauba wax and modified atmosphere in refrigerated preservation of ‘Tahiti’ acid limes. Packag Technol Sci. 2015;28(7):647–56. doi:10.1002/pts.2133. [Google Scholar] [CrossRef]
14. Neguerula AI.Is the color measured in food the color that we see? In: Caivano JL, Buera M del P, editors. Color in food. Technological and psychophysical aspects. Buenos Aires, Argentina: CRC Press; 2012. p. 81–91. [Google Scholar]
15. Salveit ME. Respiratory metabolism. In: Pareek S, editor. Postharvest ripening physiology of crops. USA: CRC Press-Taylor & Francis Group; 2016. p. 139–56. [Google Scholar]
16. Brand WW, Culivier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci Technol. 1995;28(1):25–30. doi:10.1016/S0023-6438(95)80008-5. [Google Scholar] [CrossRef]
17. Singleton VL, Orthofer R, Lamuela RRM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299(10):152–78. doi:10.1016/S0076-6879(99)99017-1. [Google Scholar] [CrossRef]
18. Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardisation dun extrait de propolis et identification des principaux constituents. J Pharmacie Belgique. 1994;49(6):462–8 (In Spanish). [Google Scholar]
19. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;9(26):1231–337. doi:10.1016/S0891-5849(98)00315-3. [Google Scholar] [PubMed] [CrossRef]
20. Inpixon. SigmaPlot (v14). Palo Alto, Ca, United States of America: Inpixon Systat Software Inc.; 2022. https://systatsoftware.com/. [Accessed 2023]. [Google Scholar]
21. Dhital R, Joshi P, Becerra-Mora N, Umagiliyage A, Chai T, Kohli P, et al. Integrity of edible nano-coatings and its effects on quality of strawberries subjected to simulated in-transit vibrations. Lebensmittel-Winssenschaft & Technologie. 2017;80(1):257–64. doi:10.1016/j.lwt.2017.02.033. [Google Scholar] [CrossRef]
22. Mditshwa A, Magwaza LS, Tesfay SZ, Opara UL. Postharvest factors affecting vitamin C content of citrus fruits: a review. Sci Hortic. 2017;218(1):95–104. doi:10.1016/j.scienta.2017.02.024. [Google Scholar] [CrossRef]
23. Jomori MLL, Kluege RA, Jacomino AP. Cold storage of ‘Tahiti’ lime treated with 1-methylcyclopropene. Scientia Agricola. 2003;60(4):785–8. doi:10.1590/S0103-90162003000400027. [Google Scholar] [CrossRef]
24. Blum J, Ayub RA. Conservacao pós-colheita da lima ácida “Tahiti” tratada com 1-metilciclopropeno. Biotemas. 2008;21(2):27–31. [Google Scholar]
25. Ziena HMS. Quality attributes of bears seedless lime (Citrus latifolia Tan) juice storage. Food Chem. 2000;71(2):167–72. doi:10.1016/S0308-8146(00)00064-9. [Google Scholar] [CrossRef]
26. Khan AS, Singh Z. Harvesting and postharvest management. In: Khan MM, Al-Yahyai R, Al-Said F, editors. The lime: botany, production and uses. Wallingford, Ox, UK: CABI; 2017. p. 186–204. [Google Scholar]
27. Maftoonazad N, Ramaswamy HS. Application and evaluation of a pectin-based edible coating process for quality change kinetics and shelf-life extensión of lime fruits (Citrus aurantifolium). Coatings. 2019;9(5):285. doi:10.3390/coatings9050285. [Google Scholar] [CrossRef]
28. Medina-Torres N, Espinosa-andrews H, Trombotto S, Ayora-Talavera T, Patrón-Vázquez J, González-Flores T, et al. Ultrasound-assisted extraction optimization of phe81nolic compounds from citrus latifolia for chitosan bioactive nanoparticles development. Molecules. 2019;24(19):3541. doi:10.3390/molecules24193541. [Google Scholar] [PubMed] [CrossRef]
29. Bahorum T, Ramful-Baboolall D, Neergheen-Bhuun V, Aruoma OI, Kumar A, Verma S, et al. Phytophenolic nutrients in citrus: biochemical and molecular evidences. In: Srivastava AK, editor. Advances in citrus nutrition. New York, NY, USA: Springer; 2012. p. 25–40. [Google Scholar]
30. Xu G, Liu D, Ma Y, Ding T. Phenolic compounds and bioactive agents in citrus fruits. In: Ye X, editor. Phytochemicals in citrus. Application in functional foods. Boca Raton, Fl, USA: CRC Press; 2017. p. 169–209. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools