 Open Access
Open Access
ARTICLE
Melatonin Alleviates Abscisic Acid Deficiency Inhibition on Photosynthesis and Antioxidant Systems in Rice under Salt Stress
1 School of Life Sciences and Food Engineering, Huaiyin Institute of Technology, Huai’an, 223003, China
2 Jianghuai Plain Crop Industry Engineering Research Institute, Huaiyin Institute of Technology, Huai’an, 223003, China
* Corresponding Author: Guoliang Zhang. Email:
Phyton-International Journal of Experimental Botany 2024, 93(7), 1421-1440. https://doi.org/10.32604/phyton.2024.053914
Received 13 May 2024; Accepted 02 July 2024; Issue published 30 July 2024
Abstract
Melatonin and abscisic acid, as major plant hormones, play important roles in the physiological and biochemical activities of crops, but the interaction between the two under salt stress is not yet clear. This study investigated the endogenous levels of melatonin and abscisic acid in rice by using exogenous melatonin, abscisic acid, and their synthetic inhibitors, and examined their interactions under salt stress. The research results indicate that melatonin and abscisic acid can improve rice salt tolerance. Melatonin alleviated the salt sensitivity caused by abscisic acid deficiency, increased antioxidant enzyme activity and antioxidant content in rice treated with abscisic acid synthesis inhibitors, and reduced total reactive oxygen species content and thiobarbituric acid reactive substance accumulation. Melatonin also increased the activity of key photosynthetic enzymes and the content of photosynthetic pigments, maintaining the parameters of photosynthetic gas exchange and chlorophyll fluorescence. In summary, melatonin alleviated the effects of abscisic acid deficiency on photosynthesis and antioxidant systems in rice and improved salt tolerance. This study is beneficial for expanding the understanding of melatonin regulation of crop salt tolerance.Keywords
With the rapid growth of the global population in the past few decades, food demand has rapidly increased [1]. With expanding agriculture and irrigation, soil salinity poses a significant risk to the efficiency of crop production. At present, around 20% of the world’s cultivable land is impacted by salinity, and soil salinization is exacerbated globally owing to improper irrigation methods, excessive fertilizer use, and increasing industrial pollution [2,3]. Salinization mainly affects crops through ionic, osmotic, and oxidative stresses. When plants experience salt stress, their physiological, biochemical, and molecular processes are impacted. This is evident in the reduction of photosynthesis and respiration, decreased function of associated metabolic enzymes, slowed or halted cell growth and expansion, and the buildup of a significant quantity of reactive oxygen species (ROS) within cells [4,5]. Due to the influence of physiological and biochemical processes, the growth, development, yield, and quality of crops are significantly hindered [6]. Rice is one of the most important food crops. It is estimated that salt affects 30% of rice-growing areas [7]. Rice is a salt-sensitive crop, and the period when it is in the seedling stage is one of the most sensitive growth periods to salt. When rice seedlings are subjected to high salt stress, it is difficult for them to complete the entire growth cycle, and the yield of the surviving rice can decrease by more than 50%. Hence, it is essential to enhance the salt resistance of rice in order to guarantee food safety [8].
The use of exogenous hormones to enhance crop resistance to abiotic stressors has been widely studied. In the year 1958, the hormone melatonin (MT) was initially found within the pineal gland of animals. In 1995, MT was first discovered in plants [9]. In recent years, the regulation and mechanisms of MT in crop stress resistance have become important topics. Through ongoing investigation, MT has been recognized as a widely applicable regulatory factor in the growth, maturation, and response to different forms of stress in crops [10–12]. Melatonin plays an important role in seed germination, seedling growth, and root structure development of crops [13]. Under stress conditions, MT exhibits regulatory functions in crop antioxidant enzyme systems, non-enzymatic antioxidant systems, ion homeostasis, transcription factors, polyamine metabolism, hormone metabolism, and other physiological and biochemical activities [13–15]. The plant hormone abscisic acid (ABA) is derived from isoprene and plays a crucial role in regulating a variety of physiological processes, including stomatal opening and gene expression. It also helps crops adapt to environmental stresses such as cold, salt, and drought. Additionally, ABA acts as a signal mediator and regulates plants’ adaptive responses to different stress conditions [16,17].
Several studies have indicated that the interaction between MT and ABA is crucial for plant regulation during abiotic stress. Their interconnected signaling pathways create a sophisticated regulatory system, which enables plants to withstand challenging environments [18–20]. Studies have shown that MT selectively regulates genes related to ABA synthesis and decomposition, leading to a decrease in ABA levels under drought stress [19]. Similarly, under salt stress, MT regulates ABA metabolism-related genes and key enzymes in cucumber, thereby reducing ABA content [21]. Melatonin alleviates tomato aging and seed germination by antagonizing ABA [22,23]. These results suggest that MT and ABA have opposing effects on the regulation of plant stress resistance genes; however, other research findings indicate the opposite. Several studies under drought conditions have shown that MT promotes ABA-induced stomatal closure, which enhances crop drought resistance [19,24,25]. In the process of crop response to salt stress, MT improves the regulation of ABA-mediated ion channels and transport proteins, leading to enhanced ion homeostasis [21]. Furthermore, MT has an impact on the regulation of genes that respond to ABA [26]. Melatonin has been demonstrated to increase the activity of different ABA-responsive genes and control the ABA signaling pathway [27,28]. Recent research has indicated that ABA promotes the production of MT in plants [29]. In summary, there is a complex and subtle relationship in plants between MT and ABA that includes both synergistic and antagonistic effects. This indicates the complex roles of MT and ABA in the regulation of crop stress resistance. There remains a large gap in the existing understanding of the interaction between MT and ABA, which provides a rich avenue for further exploration.
In this study, we examined the operational network and connection between MT and ABA in controlling rice photosynthesis under saline conditions, and starting from one of the main functions of MT, antioxidant, we elucidated the physiological mechanisms behind the complex relationship between MT and ABA in regulating salt stress in rice. The results of this study contribute to a deeper understanding of the complex relationship between MT and ABA regulation of crop stress and provide new insights and ideas for MT regulation of salt tolerance in rice.
2.1 Planting of Plant Materials
Japonica rice Ningjing 7 was used as the plant material. After disinfection, rice seeds were sown in a hydroponic box filled with Kimura B nutrient solution [30]. Rice was cultivated in an artificial climate chamber (day/night temperatures: 28°C/25°C, 14 h of light (150 μM/m2/s photosynthetic photon flux density)).
2.2 Melatonin, Abscisic Acid, and Their Synthetic Inhibitorstreatment; Salt Stress Treatment
Chemical reagent treatment was applied to the rice during the three-leaf stage, and chemical reagent spraying was carried out at 17:00 every day for three consecutive days. One day after the final chemical reagent spray, the nutrient solution in the hydroponic box was replaced with a nutrient solution containing 150 mM NaCl to induce salt stress. Samples were collected on days 0 and 5 of salt stress to determine relevant physiological and biochemical indicators. The chemical reagents and NaCl added for each treatment are listed in Table 1. The concentration of chemical reagents is based on our previous research [31,32].
2.3 Endogenous MT and ABA Content
A plant MT ELISA assay kit (DG91543Q, Dogesce Inc., Beijing, China) was used to measure endogenous MT content, and a plant ABA ELISA assay kit (DG90626Q, Dogesce Inc.) was used to measure endogenous ABA content.
2.4 Fresh Weight, Dry Weight, Water Content and Relative Water Content
Collect rice plants for fresh weight measurement, then soaked the samples in distilled water to obtain turgid weight. After drying the samples to a constant weight, obtain dry weight, and calculate the water content and relative water content (RWC) using conventional methods.
2.5 Sucrose and Starch Content
Samples were dried and extracted three times with 80% ethanol, collect the supernatant after filtration and centrifugation. 2 M NaOH was introduced into the supernatant, and the combination was heated to boiling. Next, the mixture was supplemented with 30% HCl and 0.1% C6H6O2, followed by measuring the absorbance at 480 nm. A standard curve was established using a sucrose standard solution [33]. After extracting sucrose with ethanol, the residue of the plant sample was released starch by boiling water bath for 30 min. After the solution was heated and brought to a boil, 9.2 M of HClO4 was introduced to break down the starch. Following that, Anthrone reagent was used to determine the absorbance of the solution at a wavelength of 620 nm [34]. F A standard curve was generated by utilizing a glucose standard solution.
2.6 Photosynthetic Pigment Content
A ball mill was used to crush the leaves, which were then placed in an extraction solution composed of acetone and anhydrous ethanol at a 1:1 volume ratio. The extraction process was carried out at a temperature of 25°C without exposure to light for a duration of 24 h. After centrifuging the extract, the supernatant was collected and the absorbance was measured at wavelengths of 663, 645, and 470 nm [35,36].
2.7 Leaf Gas Exchange and Chlorophyll Fluorescence Parameters
The leaves were collected on the 5th day following exposure to salt stress for the measurement of photosynthetic gas exchange parameters and chlorophyll fluorescence. The LI-6400 (Li-Cor Inc., Lincoln, NE, USA) was used to measure the photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) 5 h after the start of lighting. Each treatment involved measuring 10 rice plants, with two leaves selected for each plant and three detection points selected for each leaf.
The Hexagon-Imaging-Pam portable chlorophyll fluorescence analyzer (Walz Inc., Effeltrich, Germany) was used to measure chlorophyll fluorescence parameters. The following parameters were recorded: minimum chlorophyll fluorescence yield (F0), maximum chlorophyll fluorescence yield (Fm), maximum photochemical quantum yield of photosystem II (Ymax), effective photochemical quantum yield of photosystem II (Fv/Fm), actual quantum yield (Y), non-photochemical fluorescence quenching (NPQ), coefficient of non-photochemical fluorescence quenching (qN), coefficient of photochemical fluorescence quenching (qP) and fluorescence attenuation rate (Rfd).
2.8 Total Reactive Oxygen Species and Thiobarbituric Acid Reactive Substances
The levels of total reactive oxygen species (T-ROS) were measured using a kit from Bestbio Co., Ltd. (Nanjing, China). For the determination of thiobarbituric acid reactive substances (TBARS), rice samples were homogenized with 10% trichloroacetic acid and the supernatant was collected after centrifugation. The supernatant was then mixed with an equal amount of thiobarbituric acid and heated in water for 15 min. After centrifugation, the absorbance values at 532, 600, and 450 nm were measured [37].
2.9 Rubisco and Rubisco Activase Activity
Rice samples were ground with polyethylene polypyrrolidone, and then homogenized with precooled buffer at 4°C. The buffer consisted of 1 mM EDTA, 50 mM HCl, 10 mM MgCl2, 12% glycerol, 1% polyethylene pyrrolidone 40% and 0.1% b-mercaptoethanol. The supernatant was extracted from the homogenate after low-temperature centrifugation to determine the enzyme activity. The change in absorbance at 340 nm caused by NADH oxidation can be used to determine Rubisco activity [38]. The Rubisco activase (RCA) activity was determined based on changes in the absorbance values of the reaction system at 340 nm after ATP-dependent ADP production [39].
2.10 Antioxidant Enzymes Activities
Rice samples and extracts were homogenized and centrifuged in a precooled phosphate saline buffer (pH 7.5) containing 0.1 mM ethylenediaminetetraacetic acid and 5.0% cross-linked polyvinylpyrrolidone. The supernatant was collected for enzyme activity measurements. Superoxide dismutase (SOD) activity was determined using the nitro blue tetrazolium method [40]. Peroxidase (POD) activity was determined using the guaiacol method [41]. C Catalase (CAT) activity was measured by monitoring the absorbance of CAT-catalyzed hydrogen peroxide decomposition under 240 nm ultraviolet light [42]. Glutathione reductase (GR) activity was measured by detecting the absorption peak of NADPH at 340 nm after the reaction between GSSG and NADPH catalyzed by GR [43]. Ascorbate peroxidase (APX) activity was detected by measuring the absorbance of AsA at 290 nm after its catalytic oxidation by APX [44]. Glutathione peroxidase (GPX) activity was measured based on the increase in absorbance at 436 nm caused by the oxidation of guaiacol at 25°C [45].
In order to quantify flavonoids, rice samples were mixed with cold methanol and then spun in a centrifuge to collect the liquid portion. The resulting filtrate was treated with 5% NaNO2, 10% AlCl3, and 1 M NaOH. Absorbance levels were recorded at a wavelength of 510 nm [46]. T Total phenols were assessed by adding 5% sodium carbonate and 10% Folin-Ciocalteu solution to the filtrate, followed by the addition of 20% Na2CO3 after a 5-min reaction period; absorbance was measured at a wavelength of 765 nm. To measure ascorbic acid (AsA), plant samples were blended with cold HClO4 and then separated into supernatant after centrifugation. An equal volume of AsA oxidase was incubated with the supernatant, and absorbance levels were recorded at wavelengths of both before and after incubation [47]. To measure reduced glutathione (GSH), a homogenized mixture containing 3% trichloroacetic acid was added to the rice sample, spun in a centrifuge, and then separated into supernatant. A combination of potassium phosphate (50 mM), DTNB (0.2 mM), NADPH (0.2 mM), and three units of glutathione reductase was added to the supernatant. Changes in absorbance values at a wavelength of 412 nm over 1 min were detected [48].
The data was presented as mean ± SD (three independent biological replicates, with three technical replicates for each biological replicate). SPSS 22.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for multiple comparisons at the p < 0.05 level (Duncan method) to analyze differences between treatments. GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA) was utilized to create the figures.
Under normal conditions (0d salt stress), the use of p-chlorophenylalanine (CPA; an MT synthesis inhibitor) and fluridone (FLU) resulted in inhibitory effects on fresh weight, dry weight, and RWC. While MT alleviated the inhibitory effect of FLU, ABA did not alleviate the inhibitory effect of CPA on rice growth. Additionally, both CPA and FLU led to reductions in fresh weight, dry weight, water content, and RWC in salt-stressed rice. Melatonin was effective in alleviating the inhibitory effects of both CPA and FLU, while ABA only alleviated the inhibitory effect of FLU (Fig. 1).
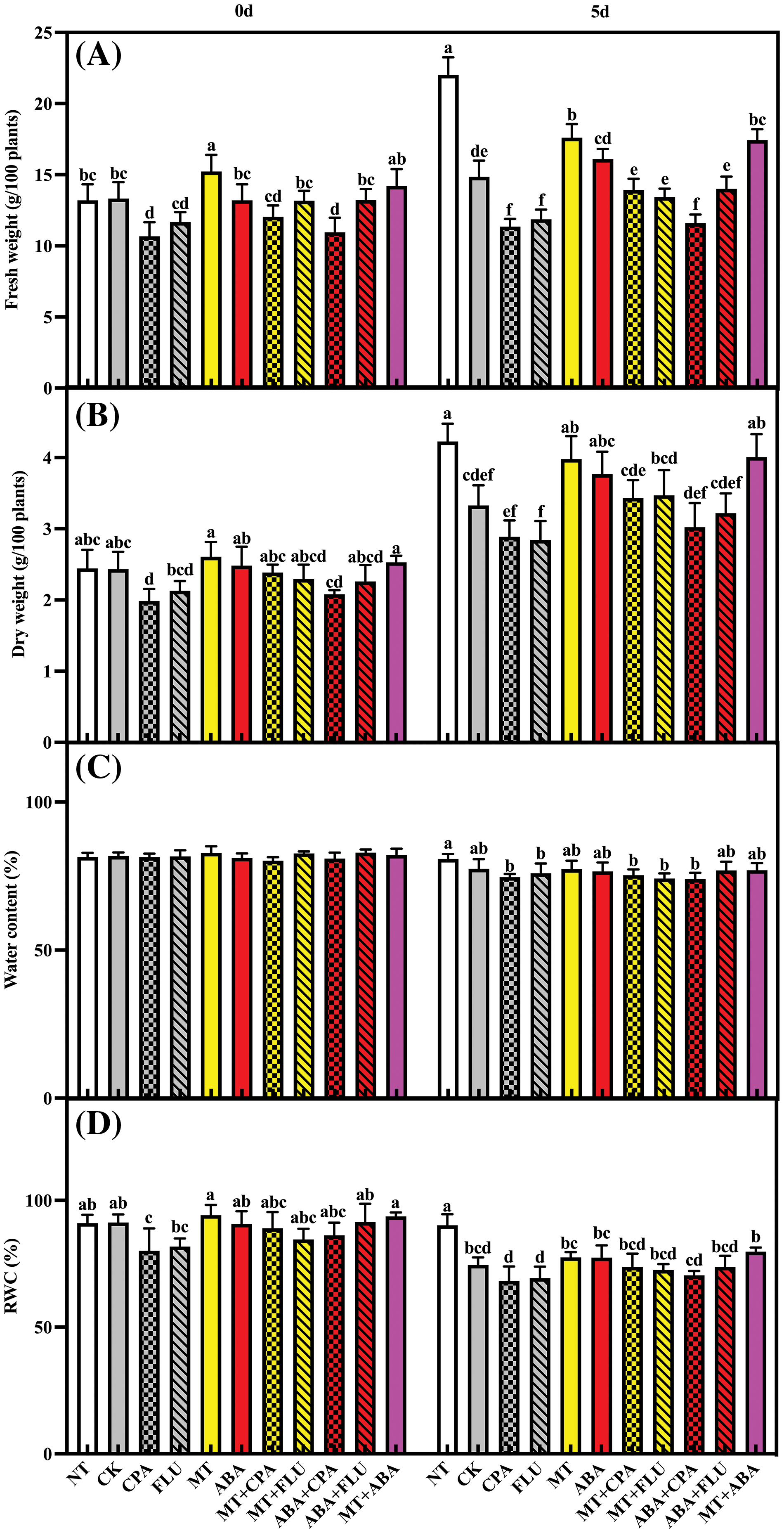
Figure 1: The effects of different treatments on the fresh weight (A), dry weight (B), water content (C), and RWC (D) of rice after 0 and 5 days of salt stress
Note: NT: No salt stress and no chemicals; CK: 150 mM salt stress and no chemicals; CPA: 150 mM salt stress and 50 μM p-chlorophenylalanine; FLU: 150 mM salt stress and 50 μM fluridone; MT: 150 mM salt stress and 200 μM melatonin; ABA: 150 mM salt stress and 100 μM abscisic acid; MT+CPA: 150 mM salt stress and 200 μM melatonin and 50 μM p-chlorophenylalanine; MT+FLU: 150 mM salt stress and 200 μM melatonin and 50 μM fluridone; ABA+CPA: 150 mM salt stress and 100 μM abscisic acid and 50 μM p-chlorophenylalanine; ABA+FLU: 150 mM salt stress and 100 μM abscisic acid and 50 μM fluridone; MT+ABA: 150 mM salt stress and 200 μM melatonin and 100 μM abscisic acid. 0d: 0 days after salt stress; 5d: 5 days after salt stress. Different letters indicate significant differences according to Duncan’s multiple range test (p < 0.05). Data represent means ± SD of three replicate samples.
The levels of endogenous MT and ABA in rice were significantly increased by the treatments of MT and ABA, and their effects remained consistent under both normal and salt stress conditions. It is important to note that, under salt stress, the MT treatment led to a significant increase in ABA content in rice, mitigating the inhibitory impact of FLU on ABA content. This resulted in the restoration of plant ABA content to levels similar to those in the control group (Fig. 2).
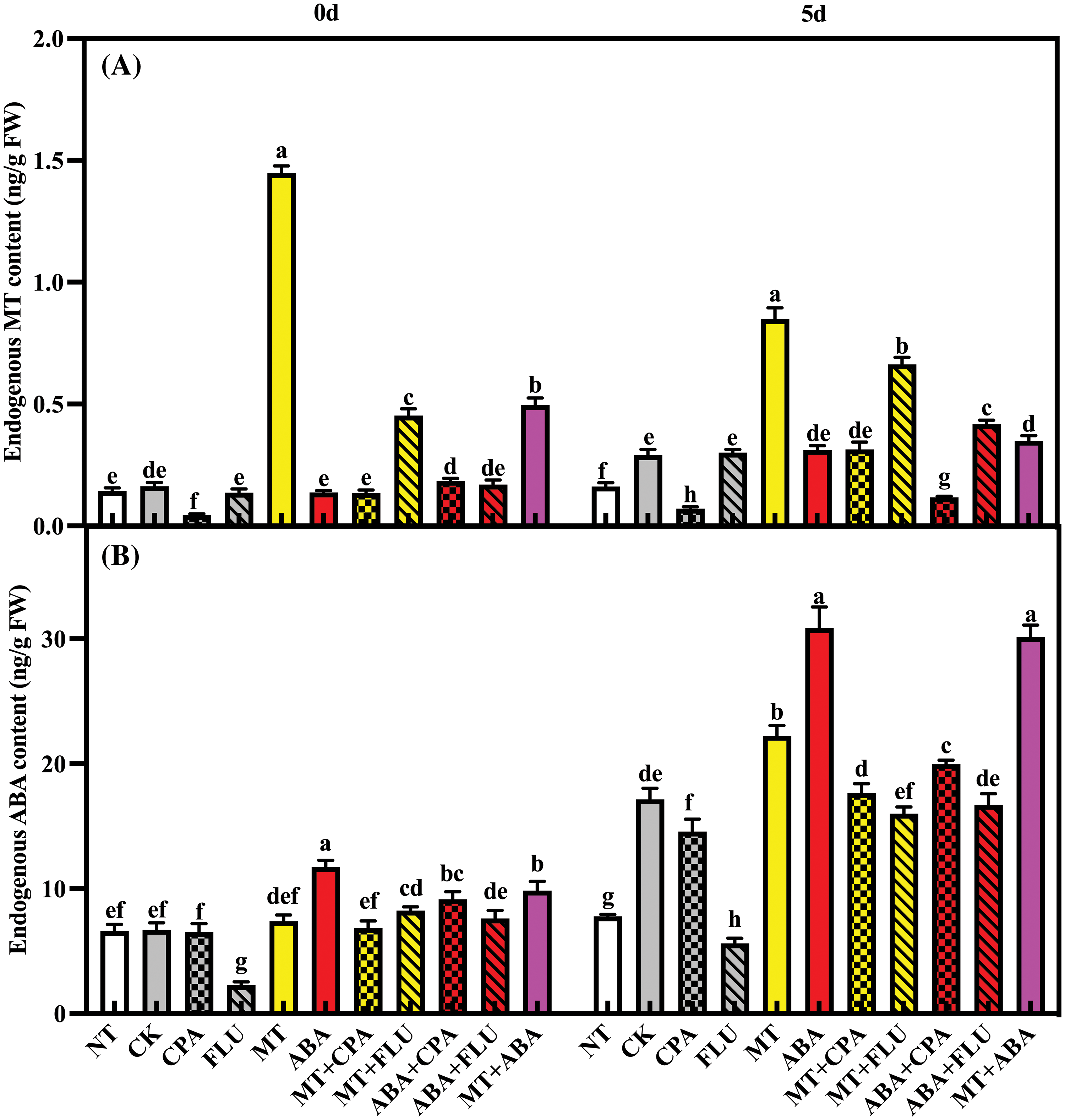
Figure 2: The effects of different treatments on the endogenous MT (A) and endogenous ABA (B) of rice after 0 and 5 days of salt stress
Note: Same as Fig. 1.
Melatonin and ABA alleviate salt stress-induced inhibition of chlorophyll and carotenoid content in rice plants. Fluridone significantly inhibited chlorophyll and carotenoid content, and this inhibitory effect was even stronger under salt stress conditions. Both the ABA and MT treatments alleviated this inhibitory effect (Fig. 3).
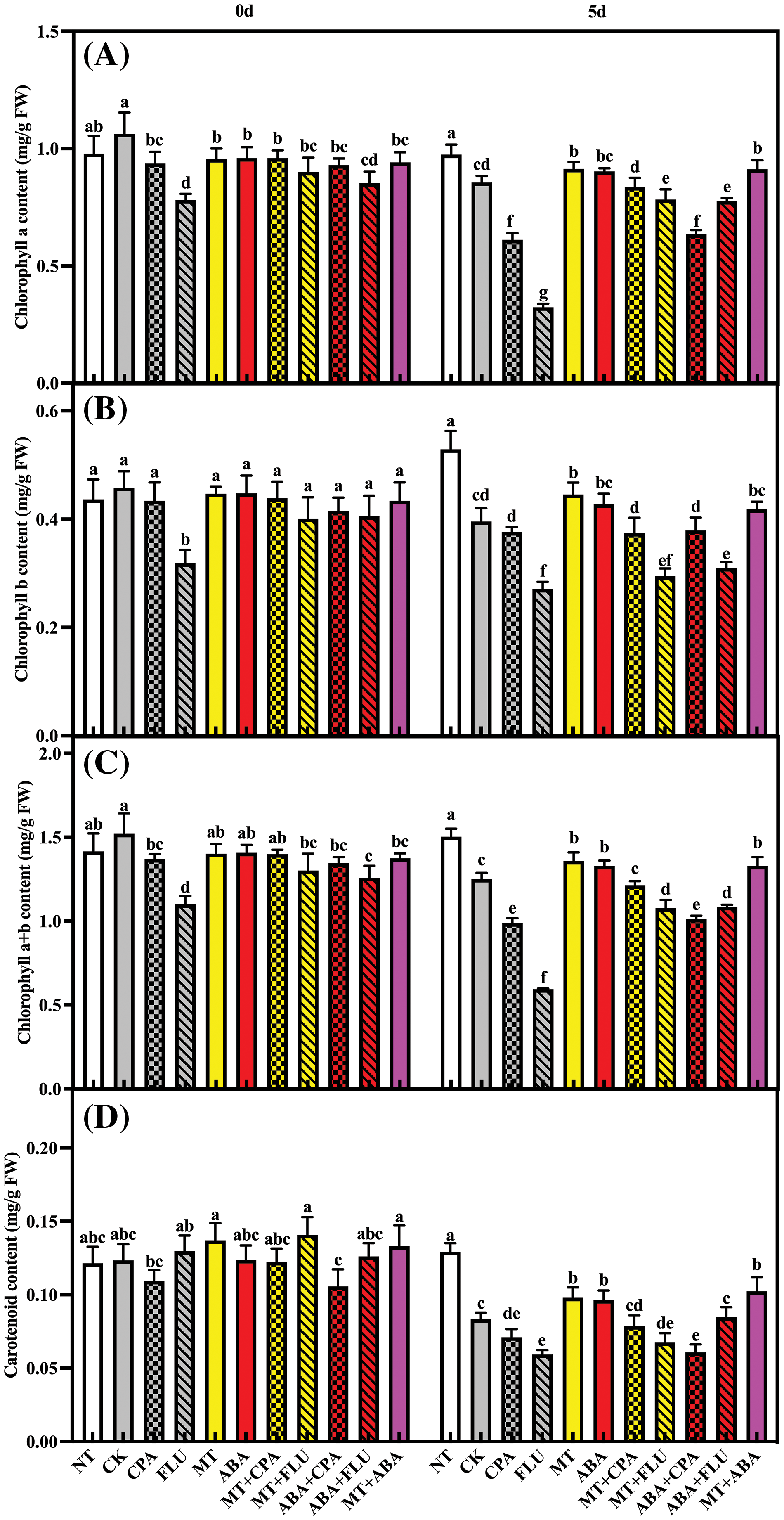
Figure 3: The effects of different treatments on the chlorophyll a content (A), chlorophyll b content (B), chlorophyll a+b content (C) and carotenoid content (D) of rice after 0 and 5 days of salt stress
Note: Same as Fig. 1.
The levels of Pn, Tr, Gs, and Ci were significantly reduced under salt stress, with further exacerbation by CPA and FLU. Melatonin, ABA, and MT+ABA helped alleviate the decrease in photosynthetic gas exchange parameters caused by salt stress. MT also mitigated the negative effects of CPA and FLU on photosynthetic gas exchange parameters (Fig. 4).
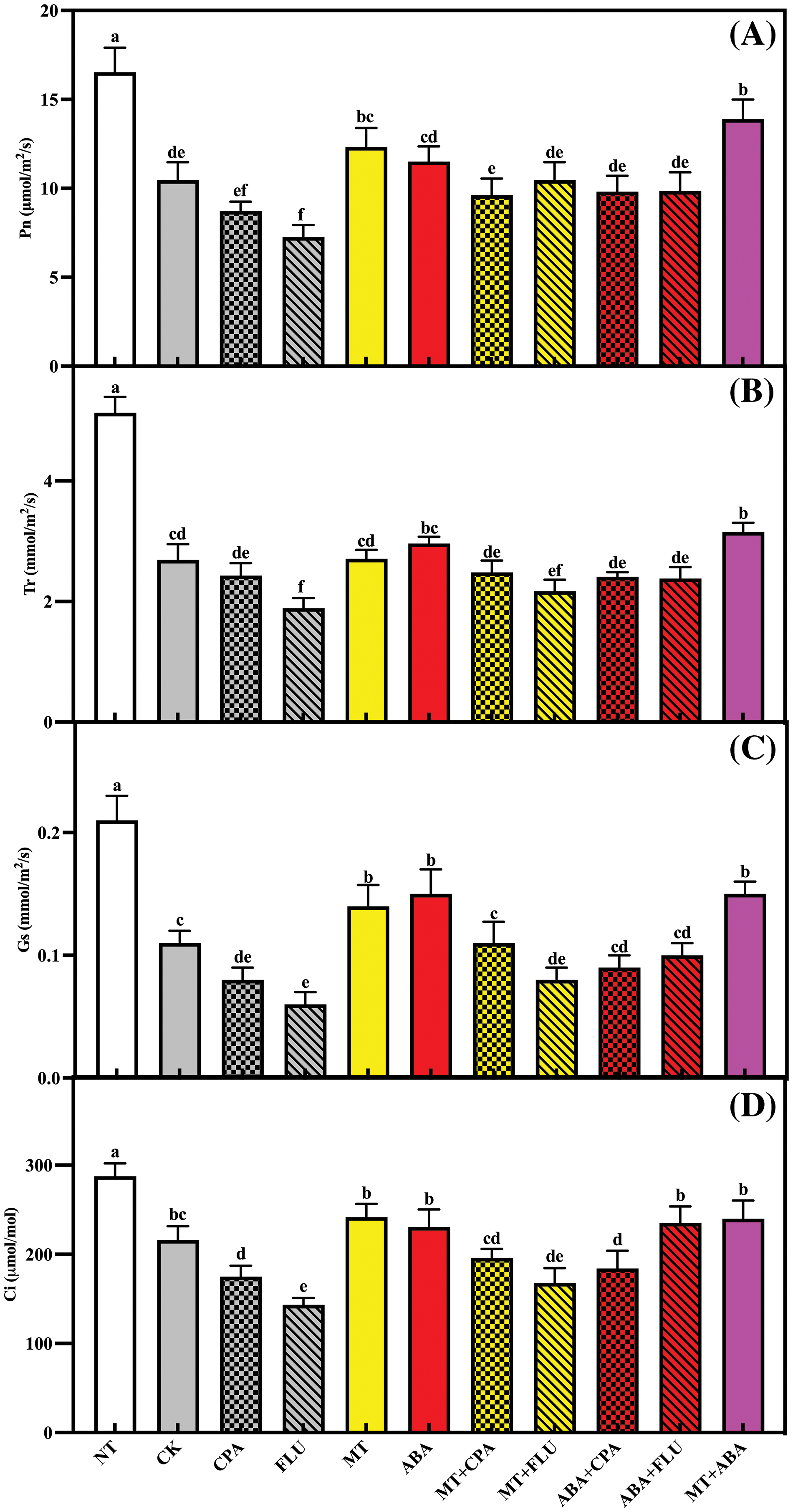
Figure 4: The effects of different treatments on the Pn (A), Tr (B), Gs (C) and Ci (D) of rice after 5 days of salt stress
Note: Same as Fig. 1.
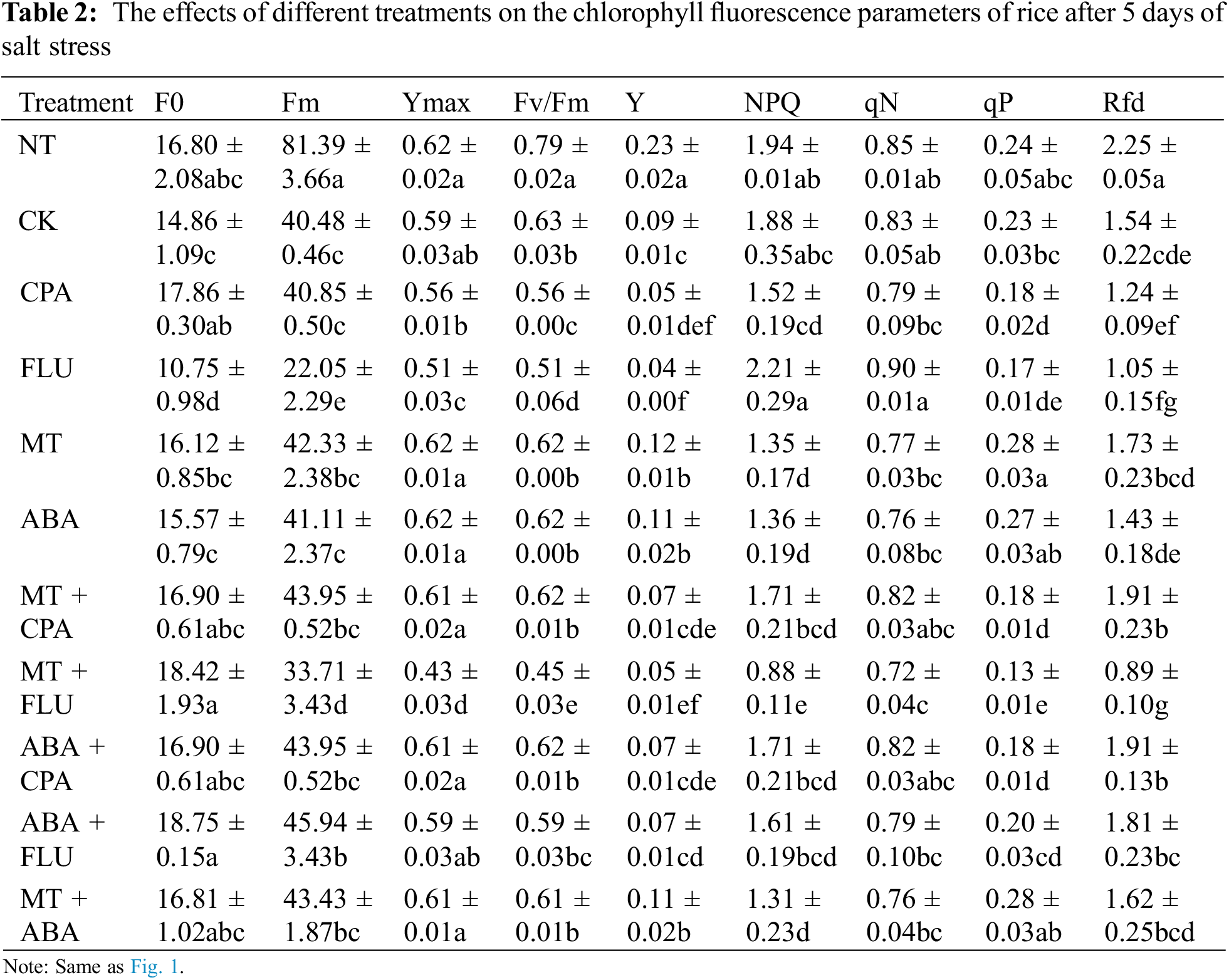
Compared to a non-saline environment, the presence of salt stress resulted in a decrease in the Fv/Fm, Ymax, Fv/Fm, and qP of rice seedlings. The application of melatonin and ABA treatments mitigated the impact of salt stress on these parameters. Additionally, p-chlorophenylalanine and FLU had a combined effect with salt stress on these parameters (Table 2).
Under normal conditions, the treatment of MT and ABA led to a significant increase in starch levels, while not having a notable impact on sucrose levels. When exposed to salt stress, rice plants experienced a significant decrease in both sucrose and starch content. However, the application of MT and ABA resulted in a significant increase in sucrose levels in rice plants. The use of p-chlorophenylalanine and FLU had a noticeable inhibitory effect on sucrose content in rice plants under salt stress. Although MT and ABA helped alleviate this inhibition, they did not fully restore the levels to those seen under normal conditions (Fig. 5).
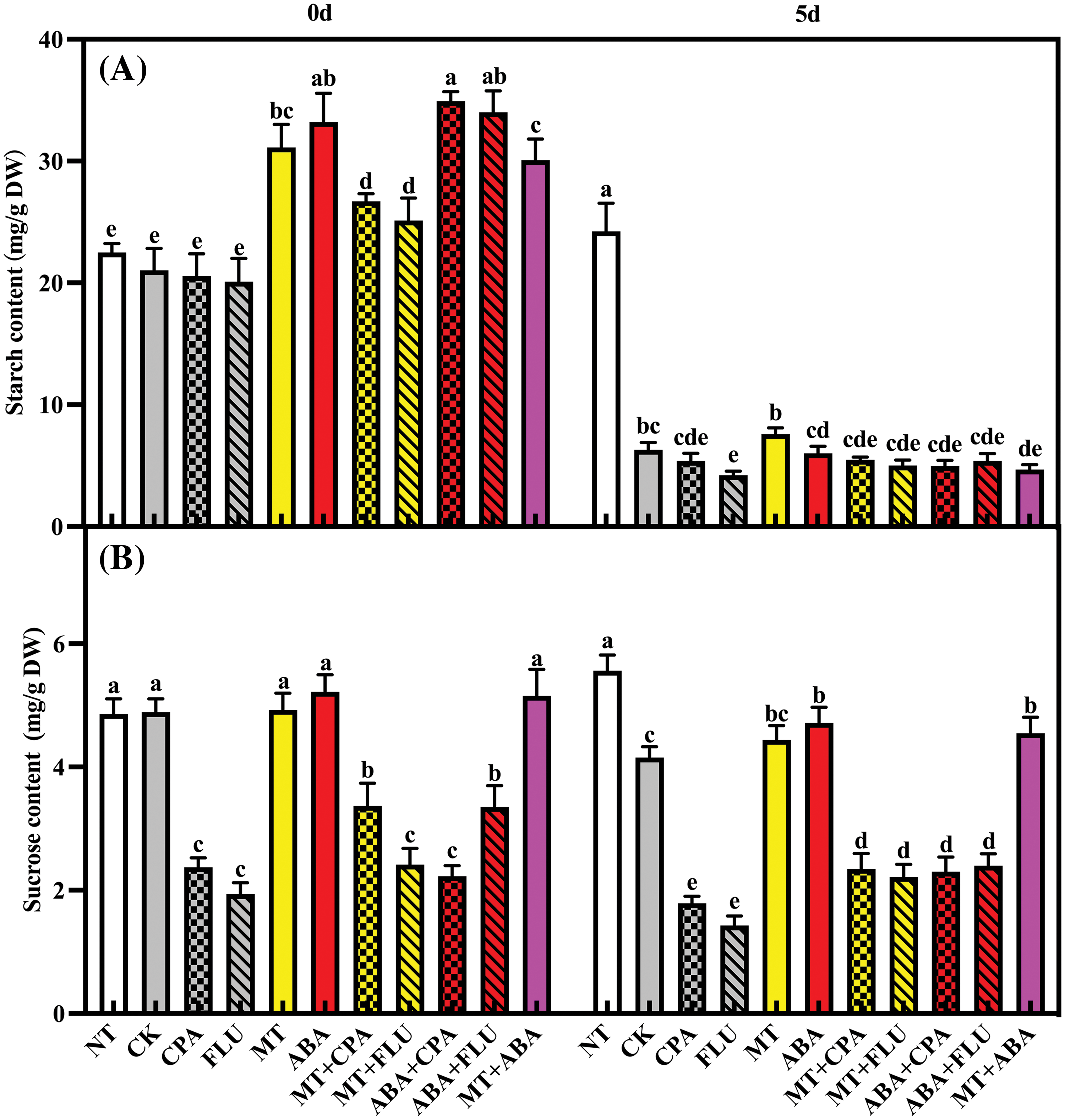
Figure 5: The effects of different treatments on the starch (A) and sucrose (B) contents of rice after 0 and 5 days of salt stress
Note: Same as Fig. 1.
Salt stress inhibited Rubisco and RCA activities, and this inhibitory effect became more severe after treatment with CPA and FLU. Melatonin and ABA alleviated the adverse effects of salt stress on Rubisco and RCA activities. Melatonin alleviated FLU-induced inhibition of Rubisco and RCA activities caused by FLU (Fig. 6).
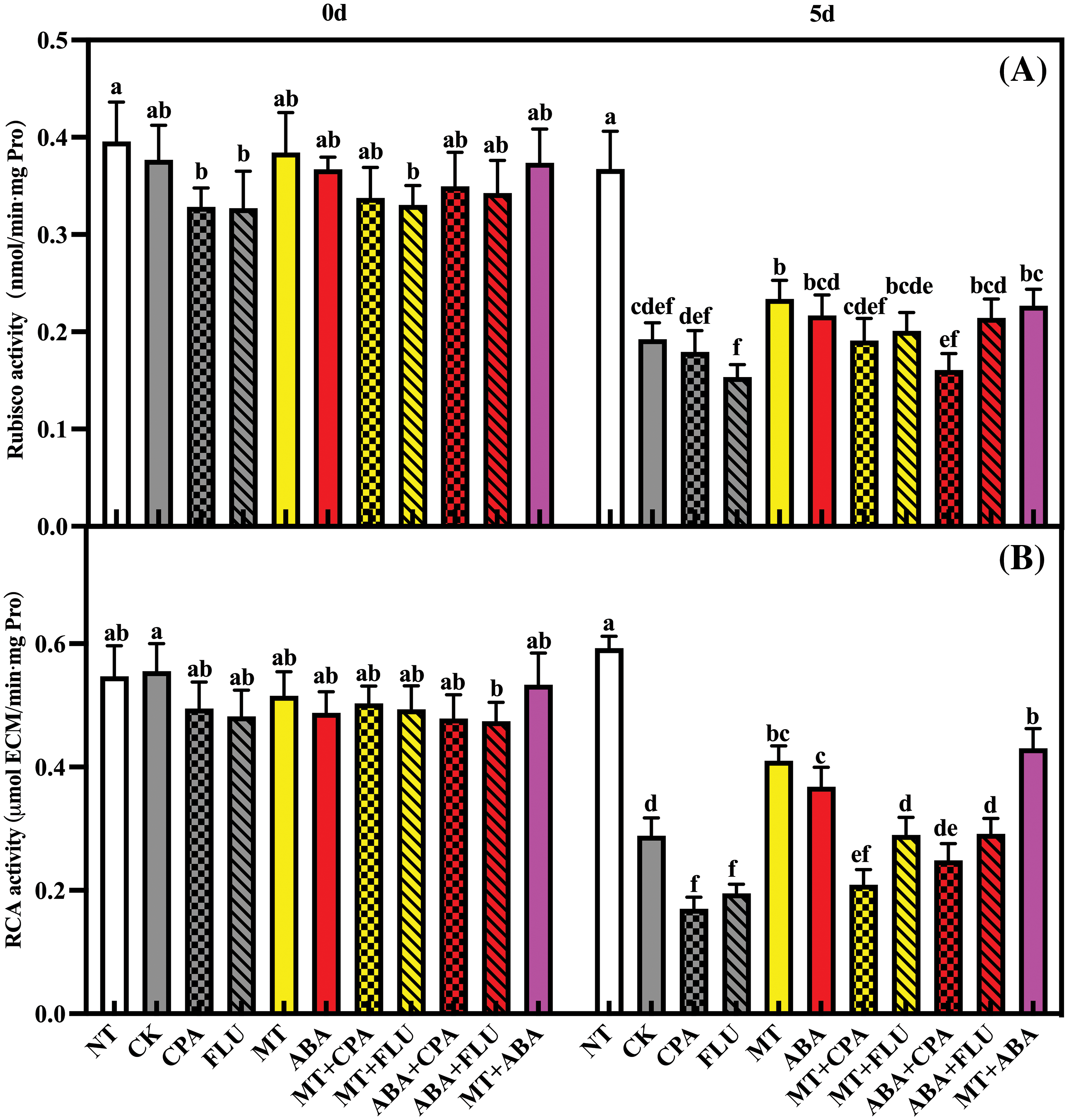
Figure 6: The effects of different treatments on the Rubisco (A) and RCA (B) activities of rice after 0 and 5 days of salt stress
Note: Same as Fig. 1.
In normal conditions, the application of CPA and FLU resulted in a notable rise in TBARS and T-ROS levels in rice plants. When exposed to salt stress, there was a significant increase in TBARS and T-ROS content. Melatonin and ABA effectively decreased the accumulation of these substances, with melatonin showing a stronger effect (Fig. 7).
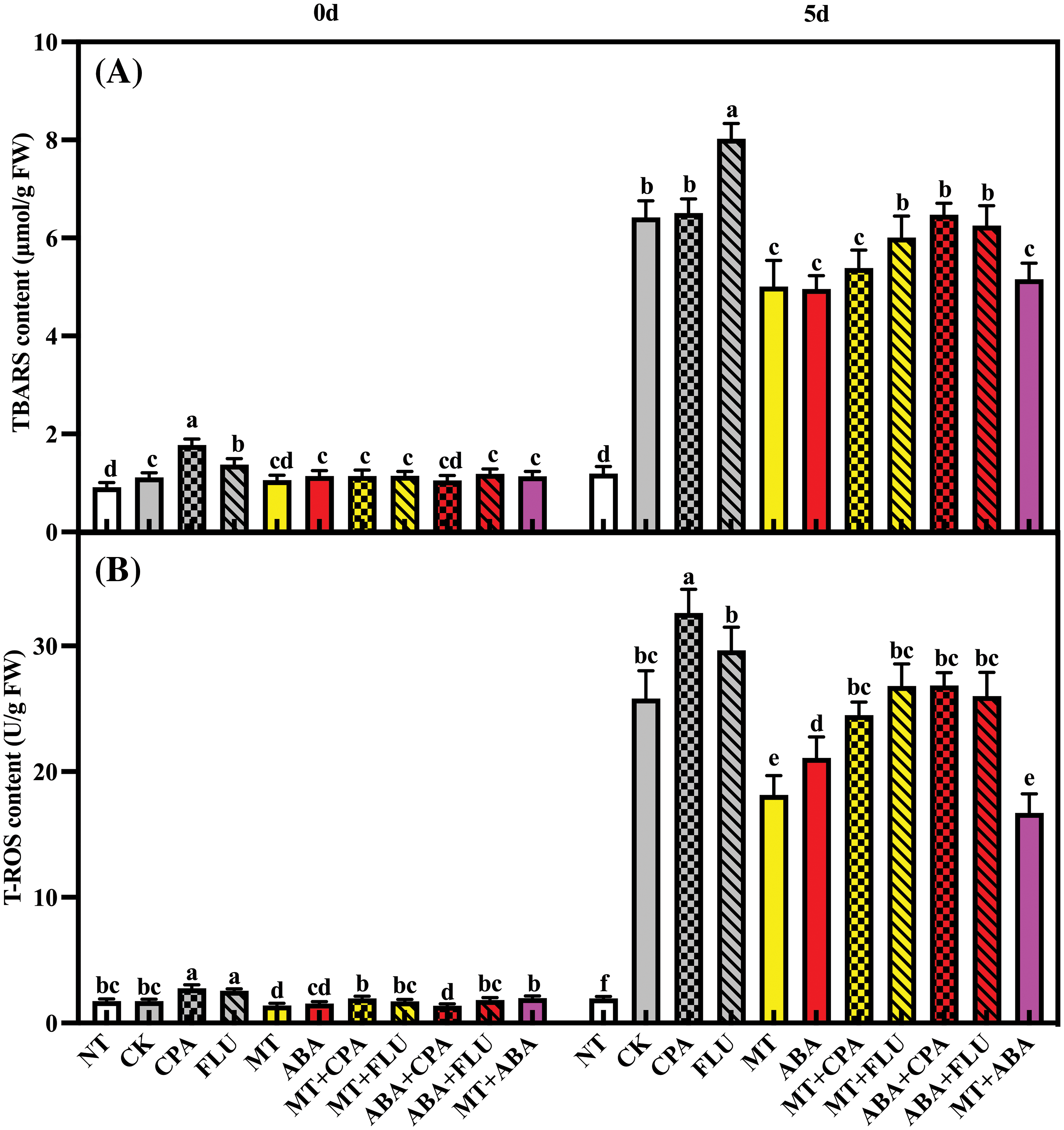
Figure 7: The effects of different treatments on the TBARS (A) and T-ROS (B) contents of rice after 0 and 5 days of salt stress
Note: Same as Fig. 1.
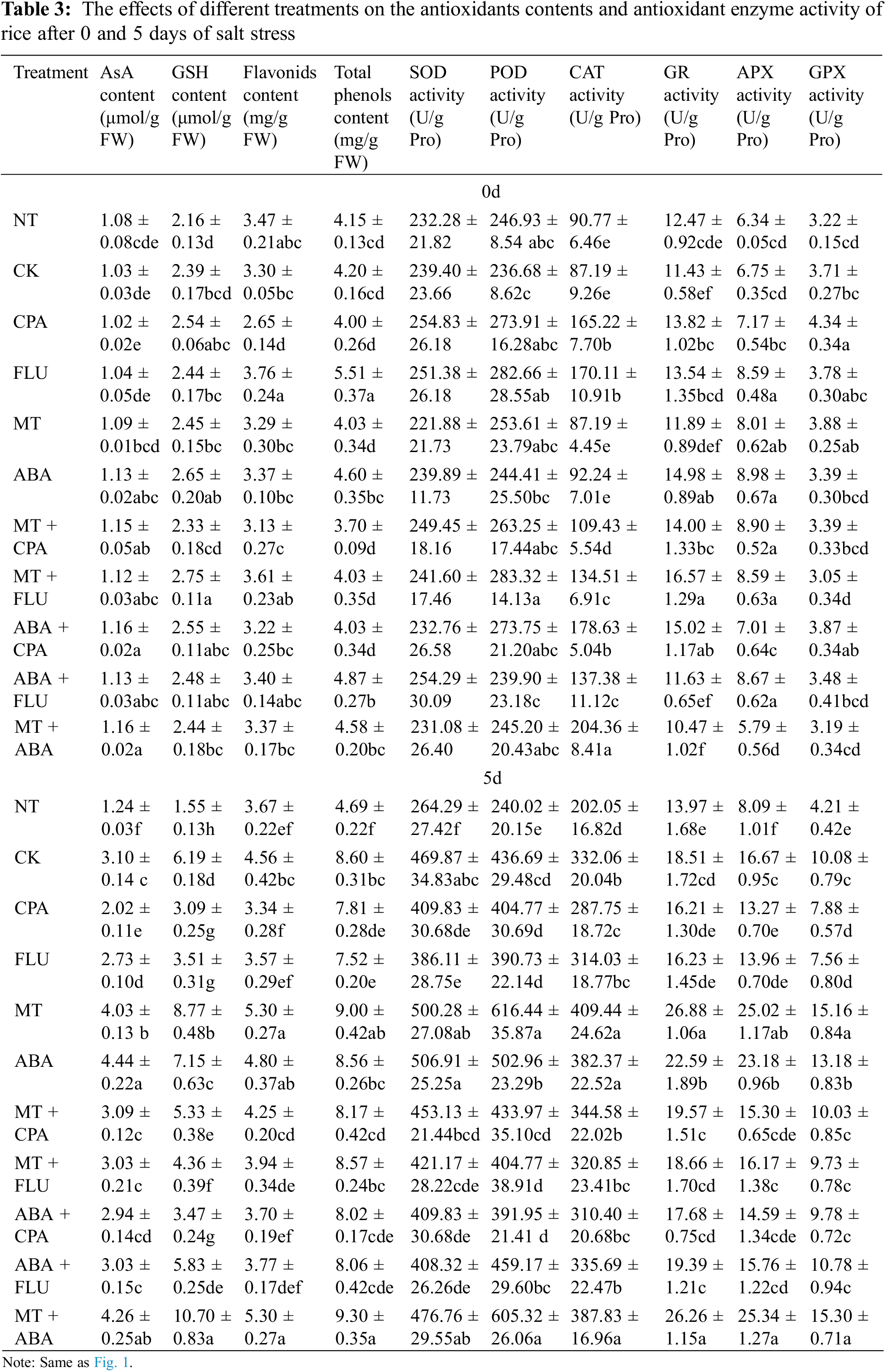
During exposure to salt stress, rice plants exhibited increased activity of antioxidant enzymes and a significant rise in the level of antioxidants. p-chlorophenylalanine and FLU significantly inhibited antioxidant enzyme activity and antioxidant content, whereas MT and ABA significantly increased these two indicators. Regarding the adverse effects caused by CPA, ABA can restore some indicators to control levels but not others. Similar phenomena were observed in the interactions between FLU and MT (Table 3).
4.1 Melatonin and ABA Alleviated the Inhibitory Effect of Salt Stress on Rice Growth
Salt stress can seriously inhibit crop growth, which manifests as a decrease in dry and fresh weights, and in water content, as well as a slowdown or stagnation of development [5]. This phenomenon was also observed in the present study. Melatonin and ABA play important roles in crop stress resistance [49,50]. Our findings suggest that the application of exogenous MT and ABA can enhance the ability of rice to tolerate salt stress, as evidenced by an increase in dry weight, fresh weight, and RWC (see Fig. 1). This result is in line with previous research. Melatonin has been widely documented to enhance salt tolerance, as well as drought and heat resistance in rice, wheat, and corn; ABA has been extensively reported to improve salt and cold tolerance in cucumber, wheat, and barley [14,51]. However, it is worth noting that under non-salt stress conditions, MT exhibited a significant promoting effect on the fresh weight of rice, whereas ABA did not. This may be because of the structural similarity between MT and auxins [52], which promote crop growth. Another interesting finding was that, regardless of salt stress, the use of CPA and FLU to clear MT and ABA inhibited rice growth. Exogenous MT supplementation partially relieved the inhibitory effects of CPA and FLU, while ABA only partially alleviated the inhibitory effects of FLU, as observed in measurements of fresh and dry weight. These findings suggest that MT may act upstream of ABA in regulating salt stress in rice, and that MT may be able to substitute for ABA to some extent. The results for endogenous MT and ABA provide a clear basis for this speculation (Fig. 2). Exogenous MT treatment restored the endogenous ABA in rice treated with FLU to the control level; however, ABA was not able to restore the endogenous MT in rice treated with CPA to the control level. In summary, our research indicates that MT can improve salt tolerance in rice and that this regulatory effect involves the regulation of ABA by MT.
4.2 Melatonin Mediates ABA Regulation of the Antioxidant System to Enhance Photosynthesis in Rice under Salt Stress
Photosynthesis is a fundamental physiological process involved in material accumulation and energy production in plants [53]. Suffering salt stress may result in the closure of stomata, reduction in photosynthetic rate, and inhibition of key photosynthetic enzyme activity as well as the accumulation of photosynthetic products [54]. These effects ultimately result in reduced dry matter accumulation and insufficient energy sources in crops, leading to arrested growth or death [55,56]. It exerts extensive regulatory effects on various processes of photosynthesis [57]. Studies have indicated that the application of MT can enhance the chlorophyll levels in crops experiencing drought and salt stress [58]. Additionally, MT treatment has been found to elevate the Pn and quantum yield of photosystem II, as well as the electron transfer rate and NPQ in plants facing salt, low temperature, and drought stress [57,59,60].
In the present study, we also observed the promoting effects of MT on chlorophyll content (Fig. 3), photosynthetic gas exchange parameters (Fig. 4), accumulation of photosynthetic products (Fig. 5), and chlorophyll fluorescence parameters in rice under salt stress (Table 2). It is worth noting that ABA also exhibits a positive regulatory effect on the above indicators. Previous studies have also shown that ABA can increase photosynthetic pigment content, net photosynthetic rate, and sucrose-converting enzyme activity in rice, wheat, and corn under salt, drought, and heat stresses [61–64]. Our findings also suggested that the addition of exogenous MT can mitigate the inhibition of photosynthesis-related indicators caused by the reduction in endogenous ABA content resulting from FLU. In contrast to growth indicators, exogenous ABA mitigated the decrease in sucrose content and chlorophyll fluorescence parameters caused by insufficient endogenous MT due to CPA (Fig. 5) (Table 2). When plants are under salt stress, their stomatal conductance decreases, leading to a reduction in effective carbon dioxide. Light capture exceeds the requirements for photosynthesis, causing the electron transport chain to become overloaded and generating ROS through the Mehler reaction at the antenna pigment [65]. Reactive oxygen species exhibit strong oxidative activity in the membrane structure and are key photosynthetic enzymes in chloroplasts [66]. Melatonin has the unique advantage of alleviating oxidative stress in crops. It is not only an efficient antioxidant but also enhances the antioxidant capacity of crops by stimulating the upregulation of antioxidant enzyme activity, the AsA-GSH cycle, and antioxidant content [67,68]. The study showed that MT decreased the build-up of T-ROS and TBARS in rice plants when exposed to salt stress (Fig. 7), primarily due to its enhancement of antioxidant enzyme activity and content (Table 3). This is beneficial for clearance of excess ROS and has a protective effect on the membrane system, thereby maintaining stable physiological and biochemical activities. In the present study, it was observed that MT treatment led to an increase in the activity of crucial enzymes involved in photosynthesis under salt stress (see Fig. 6). Additionally, it was noted that MT mitigated the suppressive impact of FLU on antioxidant enzyme activity and content in rice under salt stress. Conversely, ABA did not alleviate the inhibitory effect of CPA on antioxidant enzyme activity and content (refer to Table 3). These findings suggest that MT has the potential to mitigate the inhibitory effects caused by ABA deficiency on rice’s antioxidant systems.
4.3 The Interaction between MT and ABA and Their Effects on Rice Photosynthesis and the Antioxidant System
Extensive research has explored the complex relationship between MT and ABA in different crops, and there is no consistent pattern of increase or decrease in endogenous ABA content after MT treatment [69]. In cucumber seeds, MT downregulates ABA synthesis genes and promotes their metabolism, leading to a decrease in endogenous ABA content [21]. Research has indicated that MT enhances the germination of melon seeds by counteracting the effects of ABA and controlling the equilibrium between ABA and gibberellic acid [23]. In research on heat stress, MT slowed leaf senescence by reducing ABA content [70]. These results indicated that MT and ABA exert antagonistic effects. Our research showed that MT significantly increases ABA content under salt stress (Fig. 2), and the same phenomenon has been observed in studies on watermelon, grape berries, and apples [26,71,72]. Research has shown that MT enhances crop drought resistance by promoting ABA regulation in stomata [73]. Melatonin can also increase ABA and ethylene content to promote fruit ripening [26]. Our findings suggest that MT may mitigate the inhibitory impacts of ABA deficiency (FLU treatment) on rice photosynthesis and antioxidant systems under salt stress. Additionally, ABA can counteract the negative effects of MT deficiency (CPA treatment), indicating a synergistic relationship between MT and ABA. These results reveal a complex interplay between MT and ABA in regulating crop stress responses. We regulated the levels of MT and ABA through various treatments and studied the photosynthetic and antioxidant systems, deepening our understanding of this complex interaction.
This study manipulated the endogenous levels of MT and ABA in rice using exogenous MT, ABA, and their synthetic inhibitors and explored their synergistic or antagonistic effects under salt stress. The results indicate that MT alleviates the salt sensitivity caused by ABA deficiency in rice through synergistic effects. This is mainly manifested as MT increasing antioxidant enzyme activity and antioxidant content under ABA deficiency conditions, reducing oxidative damage, and alleviating the inhibition of photosynthesis.
Acknowledgement: None.
Funding Statement: This study was supported by National Programs for Coordinated Promotion of Major Agricultural Technologies (Grant No. 2021-ZYXT-02–1), Key Projects of Key research and Development Programs of Jiangsu Province (Grant No. BE2021323), the “333 Project” Scientific Research Project of Jiangsu Province (Grant No. 70), Rural Revitalization Project of Huai’an (Grant No. HAN202312), Talent Introduction Research Project of Huaiyin Institute of Technology (Z301B22504).
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Guoliang Zhang; methodology, Feiyu Yan; software, Feiyu Yan, Xin Chen, Zhenzhen Wang, Yuxuan Xia, Dehui Zheng, Sirui Xu; validation, Feiyu Yan; formal analysis, Feiyu Yan, Hongliang Zhao; investigation, Feiyu Yan, Zhiwei Huang; resources, Feiyu Yan, Yuan Niu; data curation, Xin Chen, Zhenzhen Wang, Yuxuan Xia, Dehui Zheng, Sirui Xue; writing-original draft preparation, Feiyu Yan; writing-review and editing, Guoliang Zhan, Feiyu Yan; visualization, Feiyu Yan; supervision, Guoliang Zhang; funding acquisition: Guoliang Zhang, Feiyu Yan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data supporting this article can be found within the text.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors confirm that there are no competing interests to disclose with respect to the current study.
References
1. Savary S, Akter S, Almekinders C, Harris J, Korsten L, Roetter R, et al. Mapping disruption and resilience mechanisms in food systems. Food Secur. 2020;12(4):695–717. doi:10.1007/s12571-020-01093-0. [Google Scholar] [PubMed] [CrossRef]
2. Zhao C, Zhang H, Song C, Zhu J, Shabala S. Mechanisms of plant responses and adaptation to soil salinity. The Innovation. 2020;1(1):100017. doi:10.1016/j.xinn.2020.100017. [Google Scholar] [PubMed] [CrossRef]
3. Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59(1):651–81. doi:10.1146/annurev.arplant.59.032607.092911. [Google Scholar] [PubMed] [CrossRef]
4. Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P. Regulation of plant responses to salt stress. Int J Mol Sci. 2021;22(9):4609. doi:10.3390/ijms22094609. [Google Scholar] [PubMed] [CrossRef]
5. Zulfiqar F, Ashraf M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol Biochem. 2021;160(7141):257–68. doi:10.1016/j.plaphy.2021.01.028. [Google Scholar] [PubMed] [CrossRef]
6. Yang Y, Guo Y. Unraveling salt stress signaling in plants. J Integr Plant Biol. 2018;60(9):796–804. doi:10.1111/jipb.v60.9. [Google Scholar] [CrossRef]
7. Takehisa H, Shimodate T, Fukuta Y, Ueda T, Yano M, Yamaya T, et al. Identification of quantitative trait loci for plant growth of rice in paddy field flooded with salt water. Field Crops Res. 2004;89:85–95. doi:10.1016/j.fcr.2004.01.026. [Google Scholar] [CrossRef]
8. Razzaq A, Ali A, Bin Safdar L, Zafar MM, Rui Y, Shakeel A, et al. Salt stress induces physiochemical alterations in rice grain composition and quality. J Food Sci. 2020;85(1):14–20. doi:10.1111/jfds.v85.1. [Google Scholar] [CrossRef]
9. Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, et al. Melatonin in edible plants identified by radioimmunoassay and by high-performance liquid chromatography-mass spectrometry. J Pineal Res. 1995;18(1):28–31. doi:10.1111/jpi.1995.18.issue-1. [Google Scholar] [CrossRef]
10. Calvo JR, Gonzalez-Yanes C, Maldonado MD. The role of melatonin in the cells of the innate immunity: a review. J Pineal Res. 2013;55(2):103–20. doi:10.1111/jpi.2013.55.issue-2. [Google Scholar] [CrossRef]
11. Nawaz MA, Huang Y, Bie Z, Ahmed W, Reiter RJ, Niu M and Hameed S. Melatonin: current status and future perspectives in plant science. Front Plant Sci. 2016;6:1230. doi:10.3389/fpls.2015.01230. [Google Scholar] [PubMed] [CrossRef]
12. Gao T, Liu X, Tan K, Zhang D, Zhu B, Ma F, et al. Introducing melatonin to the horticultural industry: physiological roles, potential applications, and challenges. Hortic Res. 2022;9:uhac094. doi:10.1093/hr/uhac094. [Google Scholar] [PubMed] [CrossRef]
13. Zhao J, Hu J. Melatonin: current status and future perspectives in horticultural plants. Front Plant Sci. 2023;14:1140803. doi:10.3389/fpls.2023.1140803. [Google Scholar] [PubMed] [CrossRef]
14. Colombage R, Singh MB, Bhalla PL. Melatonin and abiotic stress tolerance in crop plants. Int J Mol Sci. 2023;24(8):7447. doi:10.3390/ijms24087447. [Google Scholar] [PubMed] [CrossRef]
15. Ahmad I, Zhu G, Zhou G, Liu J, Younas MU, Zhu Y. Melatonin role in plant growth and physiology under abiotic stress. Int J Mol Sci. 2023;24(10):8759. doi:10.3390/ijms24108759. [Google Scholar] [PubMed] [CrossRef]
16. Sah S, Reddy K, Li J. Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci. 2016;7(15013):571. doi:10.3389/fpls.2016.00571. [Google Scholar] [PubMed] [CrossRef]
17. Xie G, Xu R, Chong L, Zhu Y. Understanding drought stress response mechanisms in tomato. Veg Res. 2024;4(1):e001. [Google Scholar]
18. Hu E, Liu M, Zhou R, Jiang F, Sun M, Wen J, et al. Relationship between melatonin and abscisic acid in response to salt stress of tomato. Sci Hortic. 2021;285(6):110176. doi:10.1016/j.scienta.2021.110176. [Google Scholar] [CrossRef]
19. Li C, Tan D, Liang D, Chang C, Jia D, Ma F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J Exp Bot. 2015;66(3):669–80. doi:10.1093/jxb/eru476. [Google Scholar] [PubMed] [CrossRef]
20. Zhang H, Qiu Y, Ji Y, Wu X, Xu X, Wu P. Melatonin promotes seed germination via regulation of ABA signaling under low temperature stress in cucumber. J Plant Growth Regul. 2023;42(4):2232–45. doi:10.1007/s00344-022-10698-y. [Google Scholar] [CrossRef]
21. Zhang H, Zhang N, Yang R, Wang L, Sun QQ, Li DB, et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis Sativus L.). J Pineal Res. 2014;57(3):269–79. doi:10.1111/jpi.2014.57.issue-3. [Google Scholar] [CrossRef]
22. Jahan MS, Li G, Xie D, Farag R, Hasan MM, Alabdallah NM, et al. Melatonin mitigates salt-induced growth inhibition through the regulation of carbohydrate and nitrogen metabolism in tomato seedlings. J Soil Sci Plant Nutr. 2023;23(3):4290–308. doi:10.1007/s42729-023-01348-7. [Google Scholar] [CrossRef]
23. Li H, Guo Y, Lan Z, Zhang Z, Ahammed GJ, Chang J, et al. Melatonin antagonizes aba action to promote seed germination by regulating Ca2+ efflux and H2O2 accumulation. Plant Sci. 2021;303:110761. doi:10.1016/j.plantsci.2020.110761. [Google Scholar] [PubMed] [CrossRef]
24. Dai L, Li J, Harmens H, Zheng X, Zhang C. Melatonin enhances drought resistance by regulating leaf stomatal behaviour, root growth and catalase activity in two contrasting rapeseed (Brassica napus L.) genotypes. Plant Physiol Biochem. 2020;149:86–95. doi:10.1016/j.plaphy.2020.01.039. [Google Scholar] [PubMed] [CrossRef]
25. Yoon YH, Kim M, Park WJ. Foliar accumulation of melatonin applied to the roots of maize (Zea mays) seedlings. Biomolecules. 2019;9(1):26. doi:10.3390/biom9010026. [Google Scholar] [PubMed] [CrossRef]
26. Xu L, Yue Q, Xiang G, Fe B, Yao Y. Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Hortic Res. 2018;5:41. doi:10.1038/s41438-018-0045-y. [Google Scholar] [PubMed] [CrossRef]
27. Tan X, Fan Z, Kuang J, Lu W, Reiter RJ, Lakshmanan P, et al. Melatonin delays leaf senescence of chinese flowering cabbage by suppressing ABFS-mediated abscisic acid biosynthesis and chlorophyll degradation. J Pineal Res. 2019;67(1):e12570. doi:10.1111/jpi.2019.67.issue-1. [Google Scholar] [CrossRef]
28. Hardeland R. Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions. J Exp Bot. 2015;66(3):627–46. doi:10.1093/jxb/eru386. [Google Scholar] [PubMed] [CrossRef]
29. Guo Y, Zhu J, Liu J, Xue Y, Chang J, Zhang Y, et al. Melatonin delays aba-induced leaf senescence via H2O2-dependent calcium signalling. Plant Cell Environ. 2023;46(1):171–84. doi:10.1111/pce.v46.1. [Google Scholar] [CrossRef]
30. Yoshida S, Forno DA, Cock JH, Gomez KA. Laboratory manual for physical studies of rice. In: Yoshida S, Forno DA, Cock JH, Gomez KA, editors. Routine procedure for growing rice plants in culture solution. 3rd EditionLos Banõs, Laguna, Philippines: International Rice Research Institute; 1976. vol. 17, p. 61–6. [Google Scholar]
31. Yan F, Wei H, Li W, Liu Z, Tang S, Chen L, et al. Melatonin improves K+ and Na+ homeostasis in rice under salt stress by mediated nitric oxide. Ecotoxicol Environ Saf. 2020;206:111358. doi:10.1016/j.ecoenv.2020.111358. [Google Scholar] [PubMed] [CrossRef]
32. Yan F, Wei H, Ding Y, Li W, Chen L, Ding C, et al. Melatonin enhances Na+/K+ homeostasis in rice seedlings under salt stress through increasing the root H+-pump activity and Na+/K+ transporters sensitivity to ROS/RNS. Environ Exp Bot. 2021;182:104328. doi:10.1016/j.envexpbot.2020.104328. [Google Scholar] [CrossRef]
33. Hendrix DL. Rapid extraction and analysis of nonstructural carbohydrates in plant-tissues. Crop Sci. 1993;33(6):1306–11. doi:10.2135/cropsci1993.0011183X003300060037x. [Google Scholar] [CrossRef]
34. Seifter S, Dayton S, Novic B, Muntwylar E. The estimation of glycogen with the anthrone reagent. Arch Biochem Biophys. 1950;20:190–200. [Google Scholar]
35. Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1–15. doi:10.1104/pp.24.1.1. [Google Scholar] [PubMed] [CrossRef]
36. Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans. 1983;11:591–2. doi:10.1042/bst0110591. [Google Scholar] [CrossRef]
37. Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189–98. [Google Scholar] [PubMed]
38. Racker E. Ribulose diphosphate carboxylase from spinach leaves. Method Enzymol. 1962;5:266–70. doi:10.1016/S0076-6879(62)05216-7. [Google Scholar] [CrossRef]
39. Robinson SP, Portis AR. Adenosine-triphosphate hydrolysis by purified rubisco activase. Arch Biochem Biophys. 1989;268(1):93–9. doi:10.1016/0003-9861(89)90568-7. [Google Scholar] [PubMed] [CrossRef]
40. Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–87. doi:10.1016/0003-2697(71)90370-8. [Google Scholar] [PubMed] [CrossRef]
41. Nickel KS, Cunningham BA. Improved peroxidase assay method using leuco 2,3′,6-trichloroindophenol and application to comparative measurements of peroxidatic catalysis. Anal Biochem. 1969;27(2):292–9. doi:10.1016/0003-2697(69)90035-9. [Google Scholar] [PubMed] [CrossRef]
42. Bergmeyer HU. Methoden der enzymatischen analyse. Arch Pharm. 1970;295:863–4. [Google Scholar]
43. Foster JG, Hess JL. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980;66(3):482–7. doi:10.1104/pp.66.3.482. [Google Scholar] [PubMed] [CrossRef]
44. Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–80. [Google Scholar]
45. Fernández-García N, Carvajal M, Olmos E. Graft union formation in tomato plants: peroxidase and catalase involvement. Ann Bot. 2004;93(1):53–60. doi:10.1093/aob/mch014. [Google Scholar] [PubMed] [CrossRef]
46. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. doi:10.1016/S0308-8146(98)00102-2. [Google Scholar] [CrossRef]
47. Logan BA, Grace SC, Adams WW, Demmig-Adams B. Seasonal differences in xanthophyll cycle characteristics and antioxidants in mahonia repens growing in different light environments. Oecologia. 1998;116(1–2):9–17. [Google Scholar] [PubMed]
48. Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106(1):207–12. doi:10.1016/0003-2697(80)90139-6. [Google Scholar] [PubMed] [CrossRef]
49. Yu Z, Duan X, Luo L, Dai S, Ding Z, Xia G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020;25(11):1117–30. doi:10.1016/j.tplants.2020.06.008. [Google Scholar] [PubMed] [CrossRef]
50. Zhan H, Nie X, Zhang T, Li S, Wang X, Du X, et al. Melatonin: a small molecule but important for salt stress tolerance in plants. Int J Mol Sci. 2019;20(3):709. doi:10.3390/ijms20030709. [Google Scholar] [PubMed] [CrossRef]
51. Yoon Y, Seo DH, Shin H, Kim HJ, Kim CM, Jang G. The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants. Agronomy. 2020;10(6):788. doi:10.3390/agronomy10060788. [Google Scholar] [CrossRef]
52. Wang Y, Reiter RJ, Chan Z. Phytomelatonin: a universal abiotic stress regulator. J Exp Bot. 2018;69(5):963–74. doi:10.1093/jxb/erx473. [Google Scholar] [PubMed] [CrossRef]
53. Sinclair TR, Rufty TW, Lewis RS. Increasing photosynthesis: unlikely solution for world food problem. Trends Plant Sci. 2019;24(11):1032–9. doi:10.1016/j.tplants.2019.07.008. [Google Scholar] [PubMed] [CrossRef]
54. Zahra N, Al Hinai MS, Hafeez MB, Rehman A, Wahid A, Siddique KHM, et al. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol Biochem. 2022;178:55–69. doi:10.1016/j.plaphy.2022.03.003. [Google Scholar] [PubMed] [CrossRef]
55. Wungrampha S, Joshi R, Singla-Pareek SL, Pareek A. Photosynthesis and salinity: are these mutually exclusive? Photosynthetica. 2018;56(1):366–81. [Google Scholar]
56. Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 2009;103(4):551–60. doi:10.1093/aob/mcn125. [Google Scholar] [PubMed] [CrossRef]
57. Yang S, Zhao Y, Qin X, Ding C, Chen Y, Tang Z, et al. New insights into the role of melatonin in photosynthesis. J Exp Bot. 2022;73(17):5918–27. doi:10.1093/jxb/erac230. [Google Scholar] [PubMed] [CrossRef]
58. Ren J, Yang X, Ma C, Wang Y, Zhao J. Melatonin enhances drought stress tolerance in maize through coordinated regulation of carbon and nitrogen assimilation. Plant Physiol Biochem. 2021;167:958–69. doi:10.1016/j.plaphy.2021.09.007. [Google Scholar] [PubMed] [CrossRef]
59. Muhammad I, Shalmani A, Ali M, Yang Q, Ahmad H, Li FB. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front Plant Sci. 2021;11:615942. doi:10.3389/fpls.2020.615942. [Google Scholar] [PubMed] [CrossRef]
60. Altaf MA, Shahid R, Altaf MM, Kumar R, Naz S, Kumar A, et al. Melatonin: first-line soldier in tomato under abiotic stress current and future perspective. Plant Physiol Biochem. 2022;185:188–97. doi:10.1016/j.plaphy.2022.06.004. [Google Scholar] [PubMed] [CrossRef]
61. Iqbal N, Sehar Z, Fatma M, Umar S, Sofo A, Khan NA. Nitric oxide and abscisic acid mediate heat stress tolerance through regulation of osmolytes and antioxidants to protect photosynthesis and growth in wheat plants. Antioxidants. 2022;11(2):372. doi:10.3390/antiox11020372. [Google Scholar] [PubMed] [CrossRef]
62. Wang W, Wang X, Huang M, Cai J, Zhou Q, Dai T, et al. Hydrogen peroxide and abscisic acid mediate salicylic acid-induced freezing tolerance in wheat. Front Plant Sci. 2018;9:1137. doi:10.3389/fpls.2018.01137. [Google Scholar] [PubMed] [CrossRef]
63. Mohammed AR, Cothren JT, Tarpley L. High night temperature and abscisic acid affect rice productivity through altered photosynthesis, respiration and spikelet fertility. Crop Sci. 2013;53(6):2603–12. doi:10.2135/cropsci2013.01.0060. [Google Scholar] [CrossRef]
64. Chater CCC, Oliver J, Casson S, Gray JE. Putting the brakes on: abscisic acid as a central environmental regulator of stomatal development. New Phytol. 2014;202(2):376–91. doi:10.1111/nph.2014.202.issue-2. [Google Scholar] [CrossRef]
65. Ozgur R, Uzilday B, Sekmen AH, Turkan I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct Plant Biol. 2013;40(8–9):832–47. [Google Scholar] [PubMed]
66. Mansoor S, Ali Wani O, Lone JK, Manhas S, Kour N, Alam P, et al. Reactive oxygen species in plants: from source to sink. Antioxidants. 2022;11(2):225. doi:10.3390/antiox11020225. [Google Scholar] [PubMed] [CrossRef]
67. Gu Q, Xiao Q, Chen Z, Han Y. Crosstalk between melatonin and reactive oxygen species in plant abiotic stress responses: an update. Int J Mol Sci. 2022;23(10):5666. doi:10.3390/ijms23105666. [Google Scholar] [PubMed] [CrossRef]
68. Bose SK, Howlader P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: a review. Environ Exp Bot. 2020;176:104063. doi:10.1016/j.envexpbot.2020.104063. [Google Scholar] [CrossRef]
69. Ali M, Pan Y, Liu H, Cheng Z. Melatonin interaction with abscisic acid in the regulation of abiotic stress in solanaceae family plants. Front Plant Sci. 2023;14:1271137. doi:10.3389/fpls.2023.1271137. [Google Scholar] [PubMed] [CrossRef]
70. Jahan MS, Shu S, Wang Y, Hasan MM, El-Yazied AA, Alabdallah NM, et al. Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA- and GA-mediated pathways. Front Plant Sci. 2021;12:650955. doi:10.3389/fpls.2021.650955. [Google Scholar] [PubMed] [CrossRef]
71. Li H, Mo Y, Cui Q, Yang X, Guo Y, Wei C, et al. Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and-susceptible watermelon genotypes. Plant Sci. 2019;278:32–43. doi:10.1016/j.plantsci.2018.10.016. [Google Scholar] [PubMed] [CrossRef]
72. Mao J, Niu C, Li K, Chen S, Tahir MM, Han M, et al. Melatonin promotes adventitious root formation in apple by promoting the function of MdWOX11. BMC Plant Biol. 2020;20(1):536. doi:10.1186/s12870-020-02747-z. [Google Scholar] [PubMed] [CrossRef]
73. Gong B, Shi Q. Review of melatonin in horticultural crops. Sci Agric Sin. 2017;50:2326–37. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools