 Open Access
Open Access
ARTICLE
Comparative Analysis of the Essential Oil of the Underground Organs of Valeriana spp. from Different Countries
1 Institute of Pharmacy, University of Tartu, Tartu, 50411, Estonia
2 Zaporizhzhia State Medical and Pharmaceutical University, Zaporizhzhia, 69035, Ukraine
3 Institute of Chemistry, Tallinn University of Technology, Tallinn, 12618, Estonia
4 National University of Pharmacy, Kharkiv, 61002, Ukraine
* Corresponding Author: Oleh Koshovyi. Email:
Phyton-International Journal of Experimental Botany 2024, 93(7), 1365-1382. https://doi.org/10.32604/phyton.2024.053754
Received 09 May 2024; Accepted 13 June 2024; Issue published 30 July 2024
Abstract
Valeriana officinalis L. is a plant from the Caprifoliaceae family, which is widely distributed in various parts of the world, especially in Europe and Asia. All species of Valeriana are distinguished by their ability to synthesize essential oil, which has a powerful effect on the physiological and mental aspects of the human body. The aim was to study the qualitative and quantitative composition of essential oil from valerian roots, collected in different countries, using the gas chromatography method, and to establish marker compounds for valerian species. 13 samples of commercial roots with rhizomes of V. officinalis from nine countries of the world and a sample of Valeriana pratensis and Valeriana stolonifera growing in Ukraine were selected for the study. The essential oil was obtained from dried valerian roots by the distillation method described in the European Pharmacopoeia. To determine the component composition of the essential oils of the selected samples, the methods of gas chromatography with a mass spectrometric detector and capillary gas chromatography were used. The commercial samples of V. officinalis from different countries yielded 0.21%–1.03% of essential oil. Only six of 13 samples contained essential oil in an amount that satisfies the requirement of the European Pharmacopoeia standard (not less than 4 mL/kg). 150 compounds were identified in the essential oils of 13 samples of V. officinalis essential oils. The range and average content and coefficients of variation of the identified compounds were determined. The principal compounds of V. officinalis essential oils were bornyl acetate (1.6%–27.1%), valeranone (0.5%–17.9%), valerenal (0%–14.7%), camphene (0%–14.6%), α-fenchene (0%–10.6%), and valerenic acid (0%–8.5%). The samples of V. pratensis and V. stolonifera yielded rather high levels of essential oil (1.18% and 0.93%, respectively). Three chemotypes of V. officinalis samples were determined-bornyl acetate/valerenal, valeranone, and isovaleric acid. The composition of the three essential oils compared was rather similar. Based on the study results, we propose the following marker compounds for V. officinalis consistently present in all 16 examined samples: bornyl acetate (1.6%–27.1%), limonene (0.2–2.3), and valeranone (0.5%–17.9%). The study of samples from Ukraine indicates the prospects of using these species of the genus Valeriana with the aim of expanding the raw material base and creating potential herbal preparations with a sedative effect, which are extremely necessary for the population of the country in the war and post-war periods.Keywords
Valerian (Valeriana officinalis L.) is the common name for a perennial herb plant belonging to the family Caprifoliaceae, a family of herbaceous plants, that contains 13 genera and about 400 species and is native to various regions of America, Europe, and Asia [1–4]. This plant is notable for its ability to synthesize essential oil, which exerts a powerful influence on the physiological and mental aspects of the human body [3–5].
The roots and rhizomes of Valeriana spp. are rich in essential oil [6–8], iridoids [9,10], flavonoids, alkaloids, lignanoids, and other bioactive compounds [11–13]. This raw material is utilized in medicine for its diverse properties, serving as an antidepressant [10], sedative [4,14], hypnotic [15,16], and hypotensive agent and displaying antiarrhythmic [17], antimicrobial [18] and antioxidant [19] activity [11,20].
In Ukraine, the collective species V. officinalis L. encompasses 13 distinct species, including V. dioica L., V. tuberosa L., V. tripteris L., V. transsilvanika Schur, V. sambucufolia Mikan, V. rossica P.Smirn., V. tanaitica Worosch., V. pratensis Dierb. (syn. V. collina Wallr.), V. simplicifolia (Reichenb.) Kabath, V. exaltata Mikan, V. nitida Kreyer, V. grossheimii Worosch., and V. stolonifera Czern. [3]. We focused on V. pratensis and V. stolonifera, alongside V. officinalis, as they are widespread in Ukraine and have large reserves These species hold promise for further exploration, offering potential expansion of the raw material base and the development of phytomedicines.
Previous studies from Bulgaria [8,21], Romania [7], and Italy [2] revealed that V. officinalis essential oils from specimens in Ukraine [5] were rich in bornyl acetate, spatulenol, valeranone, and valerenal. The principal compounds of essential oil of V. officinalis roots from Estonia and several other European [6,22–24] countries were bornyl acetate (up to 33.7%), α-fenchene (28.3%), valerianol (18.2%), valerenal (15.6%), isovaleric acid (13.1%), camphene (11.1%) and valeranone (10.9%). The essential oil yield was between 0.19% and 1.16%.
According to the European Pharmacopoeia, the minimum essential oil concentration should be 4 mL/kg, and valerenic acid should be at a minimum of 0.17% (m/m) [25]. Among the most prevalent constituents in the essential oil of V. officinalis L. s.l. are monoterpenes [2], specifically borneol and its esters bornyl acetate and bornyl isovalerate. These compounds interact with various receptors in the central nervous system, inducing changes in neurotransmitter activity and other biochemical processes that influence the mental and physiological state of an individual [11,17].
Using the gas chromatography method, our research aims to study the qualitative and quantitative composition of essential oil from valerian underground parts collected in different countries. The aim is to establish marker compounds to further develop standardization parameters for medicinal plant raw materials, specifically V. pratensis and V. stolonifera. This will not only help reveal the features of the plant’s chemical profile depending on the conditions of its growth but also expand our understanding of the possibilities and limitations of valerian as a powerful therapeutic agent. To the best of our knowledge, we have investigated the essential oil composition of V. stolonifera for the first time.
To study the composition of the essential oil, we selected 13 samples of raw materials (roots with rhizomes) of V. officinalis from different countries worldwide: Armenia (1), Austria (2), Belgium (3), England (4), Estonia (5–8), France (9), Georgia (10 and 11), Hungary (12) and Netherlands (13). Commercial valerian samples were obtained from various countries’ community pharmacies or grocery and health stores. As well in Ukraine the raw materials were collected in such locations: V. stolonifera (Kantserivska balka, Zaporizhsky District, Zaporizhzhia Region, near the village of Baburka, 47.8383330, 34.9733330), V. pratensis (the right bank of the Dnipro River, Zaporizhzhia, 47.8239122, 35.0622869), V. officinalis (v. Shiroke, Vasylivskyi District, Zaporizhzhia Region, 47.4240243, 35.4110803). The preliminary identification of the species V. pratensis and V. stolonifera was carried out using the Identifier of Plants of Ukraine [26], and after that confirmation of the species was provided by the Research Station of Medicinal Plants of the Institute of Agroecology and Nature Management of the National Academy of Agrarian Sciences of Ukraine.
2.2 Extraction of Essential Oil
The essential oil of V. officinalis, V. stolonifera and V. pratensis was obtained from dried valerian roots using the distillation method described in the European Pharmacopoeia [25]. The process involved 150 g of crushed roots, a 3000 mL round-bottom flask, and 1000 mL of water as the distillation. Additionally, 0.5 mL of xylene was added (in a graduated tube). Distillation was carried out for 4 h at a rate of 3–4 mL/min.
2.3 Gas Chromatography/Mass Spectrometry (Samples 1–13)
GC/MS analysis was conducted using a Chrom-5 chromatograph (Laboratorni Pristroe, Prague, Czech Republic) with FID on two fused silica capillary columns with a bonded stationary phase: poly (5%-diphenyl-95%-dimethyl) siloxane SPB-5 (30 m × 0.25 mm, Supelco) and polyethyleneglycol SW-10 (30 m × 0.25 mm, Supelco). The film thickness of both stationary phases was 0.25 µm. Carrier gas was He with a split ratio of 1:150, and the flow rate 35–40 (SPB-5) and 30–35 (SW-10) cm/s was applied. The temperature program was 50°C–250°C at 2 °C/min; Oven and transfer line temperatures were 50°C–250°C; the injector temperature was 200°C. A Hewlett-Packard Model 3390A integrator was used for data processing [6,27].
The essential oil components were identified by comparing their retention indices (RI) using n-alkanes C6–C24 as standards on two columns with the RI values of reference standards, our RI data bank and literature data [27,28]. GC/MS confirmed the results obtained. The percentage composition of the EOs was calculated in peak areas using normalization method without correction factors. The relative standard deviation of percentages of oil components of three repeated GC analyses of a single EO sample did not exceed 5%.
2.4 Gas Chromatography/Mass Spectrometry (Samples V. officinalis, V. pratensis and V. stolonifera from Ukraine)
GC/MS analysis was conducted using an Agilent 7890B with a 5977B mass spectrometric detector and a Gerstel CIS 4 cooled injection system. The chromatography column used was DB-5ms ((5%-phenyl)-methylpolysiloxane), with dimensions 30 m × 250 µm × 0.25 µm. Helium was used as the carrier gas with a 1.3 mL/min speed. The injection volume was 0.5 μl, and a standard injection with a flow split ratio of 1:10 was applied. The temperature of the sample input unit was 270°C. The thermostat temperature followed a programmed sequence: 45°C (1 min delay) → 5 °C/min → 200°C (10 min delay), then from 200°C to 250°C at 5 °C/min with a 10 min delay. The total chromatography time was 57 min. The GC/MS interface temperature was 275°C, ion sources were set at 230°C, and the quadrupole mass analyzer at 150°C. Electron impact ionization at an electron energy of 70 eV was employed, and the mass number range scanned was 30–700 m/z. Components were identified using the NIST14 mass spectrum library [29].
The V. officinalis commercial samples from different countries yield 0.21%–1.03% of essential oil; some of them (from Armenia (1), Estonia (5), and Georgia (10)) contained less oil than was possible to detect (Table 1). The highest oil level has been found in two samples (4 and 5) from Estonia (1.02% and 1.03%, respectively). Only six of 13 samples contained essential oil in an amount that satisfies the requirement of the European Pharmacopoeia [25] standard (a maximum of 4 mL/kg). According to the literature, the oil concentration of V. officinalis ranges from 0.1% to 2% [11,22,28,30].
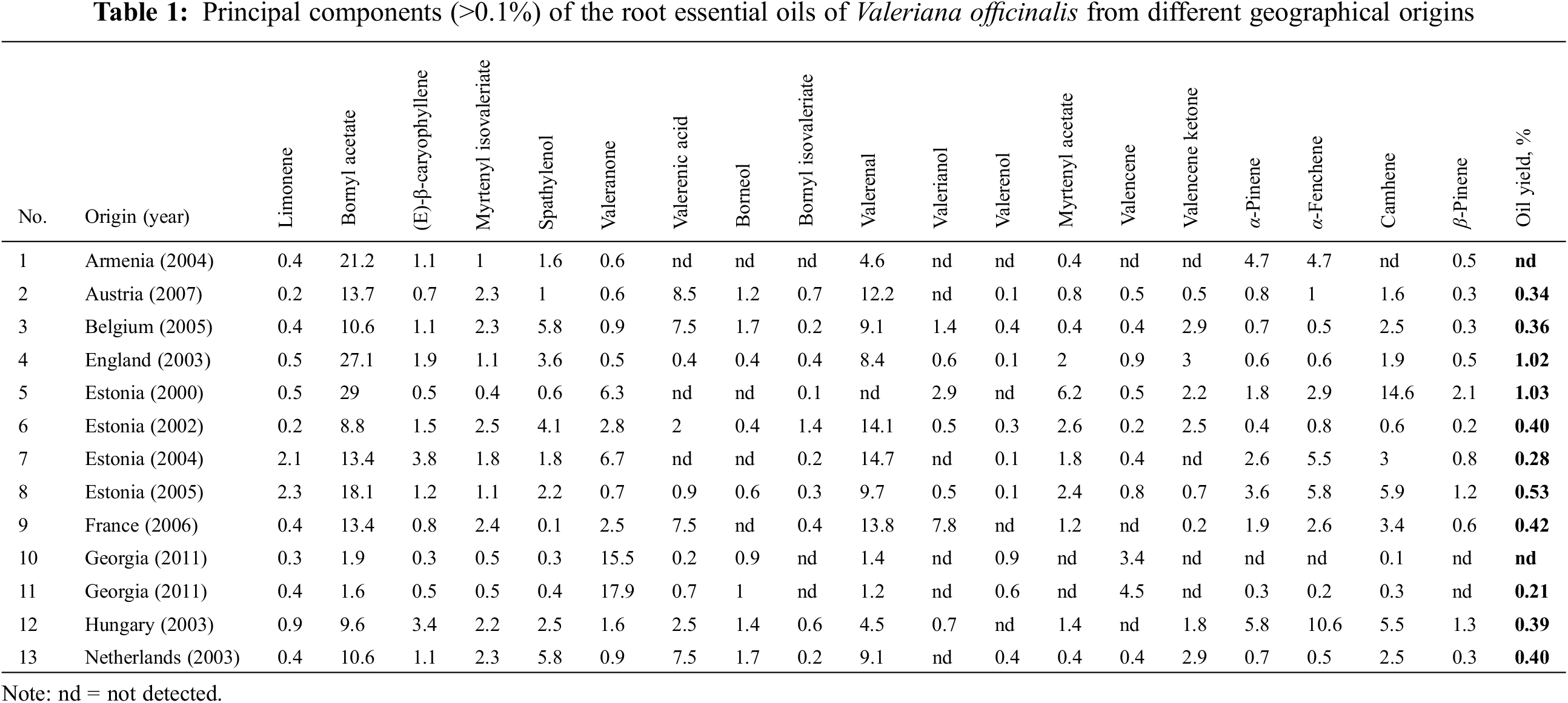
In total 150 compounds were identified in the essential oils of 13 samples of valerian essential oils. Eight compounds were identified solely from mass spectrum data, utilizing the [M+] value and haracteristic m/z peak values. The range and mean concentrations (%) and coefficients of variation of compounds found in the 13 V. officinalis samples are presented in Table 2. High coefficients of variation for most compounds (>1) indicate that the concentrations of these compounds varied from sample to sample. Low coefficients of variation (0.52–1.00) are observed for myrtenyl isovaleriate, kessyl alcohol, bornyl acetate, terpinen-4-ol, valerenal, terpinyl acetate, (Е)-β-caryophyllene, spathylenol, borneol, viridiflorol, germacrene D, tricyclene, p-cymene, kessane, β-pinene, valencene ketone, limonene, α-pinene.
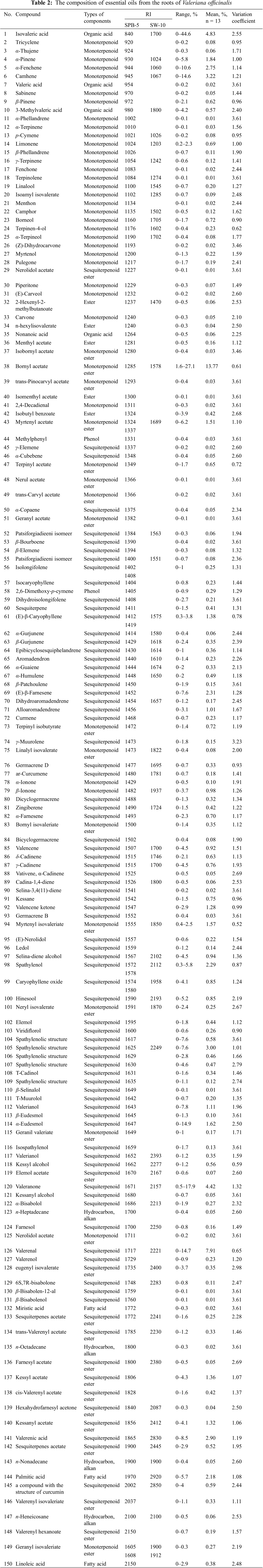
The main components of the essential oil in the studied samples were isovaleric acid (0%–44.6%), bornyl acetate (1.6%–27.1%), valeranone (0.5%–17.9%), valerenal (0%–14.7%), camphene (0%–14.6%), α-fenchene (0%–10.6%), and valerenic acid (0%–8.5%) (Table 2, Fig. 1).
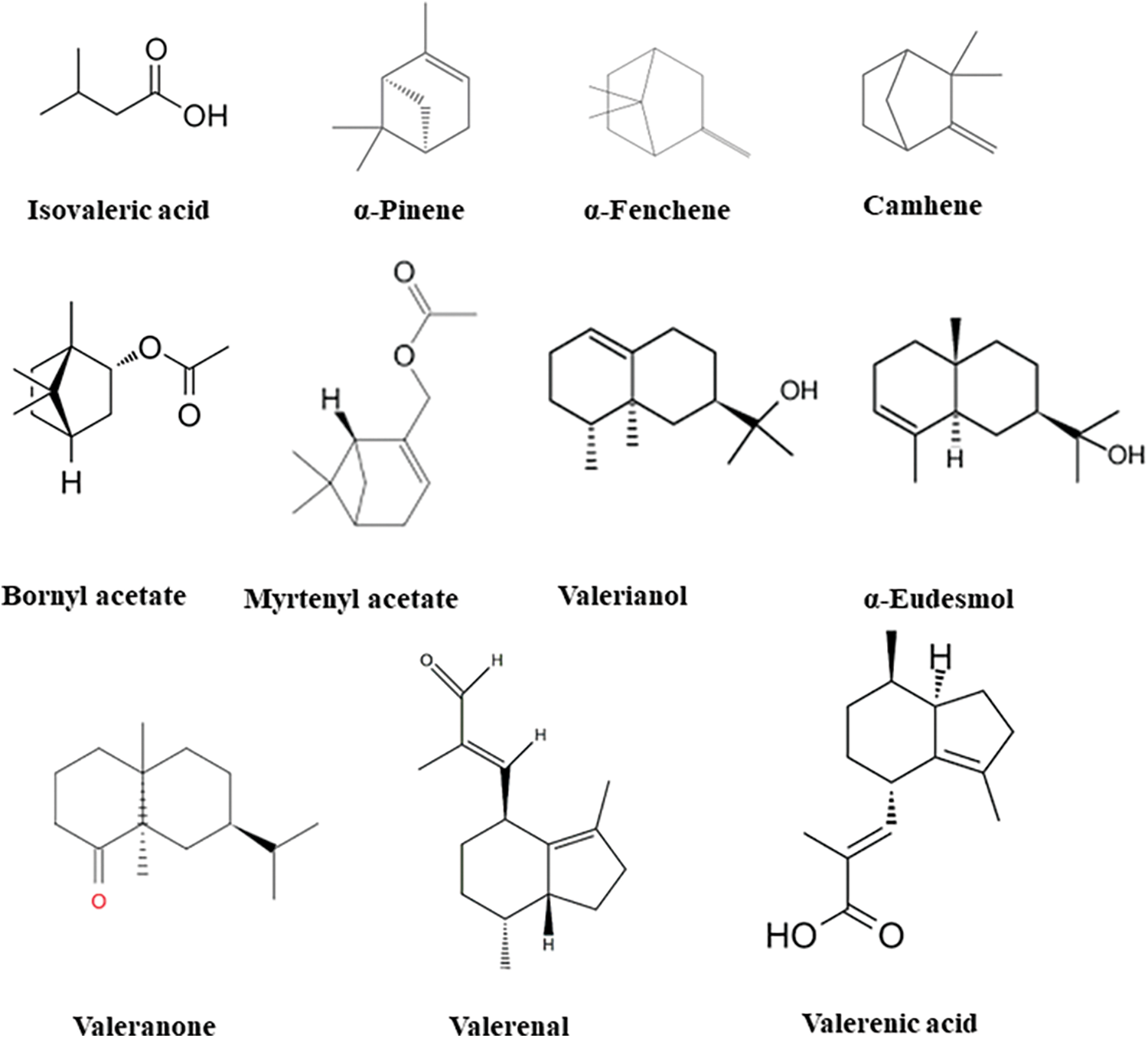
Figure 1: Structures of the main components (more than 5%) in the V. officinalis essential oils
In nature, besides V. officinalis, there are also other species, which can be impurities of this raw material or its analogues. If the composition of their main active compounds is very similar to the official specie, in this case they can also be recommended for use in medical practice as an alternative raw material after further research. Thus, we conducted a study of the two most common valerian species in Ukraine: V. stolonifera and V. pratensis. The chemical composition of their essential oils is presented in the Table 3. The essential oil concentrations of V. officinalis, V. stolonifera and V. pratensis were 0.70%, 0.93% and 1.18%, respectively. All samples contained essential oil in an amount that satisfies the requirement of the European Pharmacopoeia [25] standard (a maximum of 4 mL/kg).
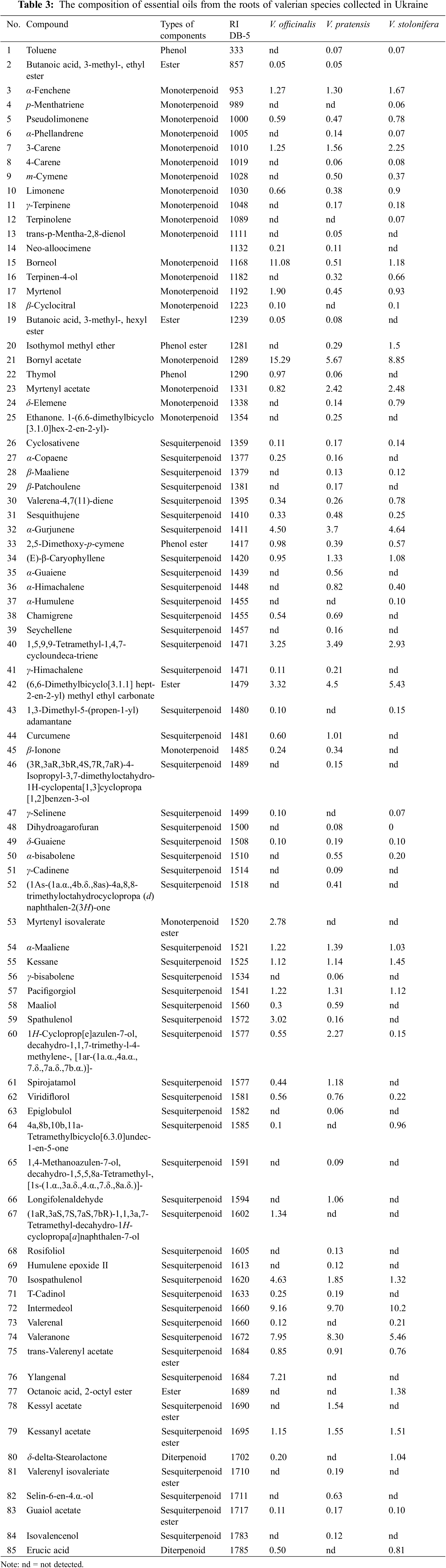
85 compounds were identified in three samples of valerian essential oil collected on the territory of Ukraine. The main components of the essential oil in all studied samples were bornyl acetate (5.46%–15.29%), borneol (0.51%–11.08%), valeranone (7.95%–8.85%), (6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl) methyl ethyl carbonate (3.32%–5.43%), and α-gurjunene (3.7%–4.64%) (Table 3). The concentrations of essential oils of compared three Valeriana species was rather similar; V. officinalis oil contained more borneol, bornyl acetate, and pathulenol, other two oils do not contain ylangenal (7.21% in V. officinalis oil).
Previously, in 1970th just iridoids and flavonoids were studied in V. stolonifera [31]. Therefore, the obtained data about essential oil composition in V. stolonifera are generally novel. The chemical composition of the essential oil of V. pratensis has been published only in two articles. Bos et al. studied the composition of the essential oil of V. pratensis (syn. V. officinalis ssp. collina) ‘Shipka’ cultivated in Bulgaria in 1995 and 1998 [21]. Valeranone (12%), valeranal (9%), valerena-4,7(11)-diene (7%), and bornyl acetate (6%) were found as the principal compounds of the oil. In the second study [32] also valeranone and valerenal (10% and 20%, respectively) were mentioned as the main compounds of the V. pratensis oil. In our study, the principal constituents in the essential oil of V. pratensis were intermedeol (9.2%), valeranone (8.3%), and bornyl acetate (5.7%), but valerenal was not found.
Based on the study results, we can propose the following V. officinalis marker compounds consistently present in all 16 examined samples: bornyl acetate (1.6%–27.1%), limonene (0.2%–2.3%), and valeranone (0.5%–17.9%). Thus, in both the species V. stolonifera and V. pratensis the principal constituents (valeranone, α-gurjunen, and bornyl acetate) are similar to V. officinalis marker compounds. According to literature data, these markers are associated with various pharmacological effects, including sedative [31], inflammatory [32,33] antispasmodic, antidepressant [34], tranquillizer [34], and anti-ulcer properties [11,35–37].
In previous studies, such compounds as markers were established borneol, bornyl acetate, α-terpinyl acetate, spathulenol, ar-curcumene, β-caryophyllene, allo-aromadendrene, kessane, myrtenyl isovalerate, sesquiterpene alcohol, viridiflorol, β-bisabolol and valerenal [6], α-pinene, α-fenchene, camphene, bornyl acetate, myrtenyl acetate, alloaromadendrene, myrtenyl isovalerate, spathulenol, valerianol, valeranone [22].
The accumulation of the two main components, bornyl acetate and valeranone, in the underground organs of valerian is directly influenced by the climatic conditions of the raw material’s growth [38,39]. A high concentration of bornyl acetate (1.6%–29%) requires a moderate climate typical of northern and western Europe, with average summer temperatures ranging from +16°C to +23°C. Conversely, a high concentration of valeranone (3.6%–17.9%) is observed in Valeriana species that thrive in the warm climates of southern Europe, where average summer temperatures in Ukraine reach +30°C. In Armenia and Georgia, they soar to +32°C. The influence of collection sites on the nutraceutical composition and potential biological activity of plant products is well known [23,35,36], given that a variety of eco-geographical features can differentially modulate gene expression and the activity of biochemical pathways involved in the production of secondary metabolites [40,41].
The compound of essential oil of V. officinalis with the highest concentration was isovaleric acid (44% in a sample from Armenia), followed by bornyl acetate (up to 29%), valerenal (14.7%), valeranone (17.9%), α-fenchene (10.6%). In our previous work [6], where the samples of valerian from different countries were analyzed, the concentrations of these substances were 13.1%, 33.7%, 13.1%, 10.9%, and 28.35%, respectively. Valerenal (9.7% and 10.2%, respectively), bornyl acetate (5.7% and 8.9%), and valeranone (5.5% and 8.3%) were also predominant in the samples of V. pratensis and V. stolonifera from Ukraine.
Several samples did not detect valerenic acid (Armenia, Estonia (5 and 7)), and isovaleric acid (Belgium, England, Estonia (5 and 6), France, Hungary, and the Netherlands). The presence of isovaleric acid, α-pinene, camphene, farnesene, valerianol, and valerenic acid was not detected in the essential oils of V. pratensis and V. stolonifera. It is unclear how significantly this may affect the effects of these valerian species on the central nervous system.
From previous studies, several valerian root oil chemotypes are known-valerianol, valeranone, cryptofauronol and valerenal, bornyl acetate/valerenal [6,22]. According to the research results, three chemotypes of V. officinalis species can be distinguished. The most typical, bornyl acetate/valerenal chemotype was characteristic to samples from Austria, Belgium, England, Estonia (5–8), France, Netherlands and the Ukraine (V. officinalis, V. pratensis and V. stolonifera); valeranone chemotype was distinct to both samples from Georgia, and isovaleric acid chemotype in a sample from Armenia.
Analysis of the 13 commercial samples of dried valerian roots with rhizomes from different countries shows the three main chemotypes of V. officinalis: bornyl acetate/valerenal, valeranone, and isovaleric acid. The commercial samples of V. officinalis yield 0.21%–1.03% of essential oil. Totally 150 compounds were identified in the essential oils of V. officinalis essential oils. The principal compounds of V. officinalis essential oils were bornyl acetate (up to 27.1%), valeranone (up to 17.9%), valerenal (up to 14.7%), camphene (up to 14.6%), and α-fenchene (up to 10.6%). In both V. stolonifera and V. pratensis essential oils the principal constituents (valeranone, α-gurjunen, and bornyl acetate) are similar to V. officinalis marker compounds. Thus, analysis of V. pratensis and V. stolonifera roots with rhizomes growing in Ukraine show the perspectives of their use for expanding the raw material base for developing potential remedies with a sedative activity like V. officinalis ones.
Acknowledgement: The authors of the study thank for obtaining valerian commercial samples, distillation of essential oils and analysis of the results Mariana Džaniašvili, Maris Ellram, Olga Kamp, Margit Karileet, Maria Konsap, Marita Tõnissoo, and other pharmacy students of the Institute of Pharmacy, University of Tartu, Estonia. The authors sincerely thank all of the defenders of Ukraine who made the performance of this study possible. The authors sincerely appreciate the support of the partners who stand with Ukraine.
Funding Statement: This work was also supported by the European Union in the MSCA4Ukraine Project “Design and Development of 3D-Printed Medicines for Bioactive Materials of Ukrainian and Estonian Medicinal Plants Origin” (ID Number 1232466).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Ain Raal, Vira Odyntsova, Oleh Koshovyi; data collection: Anne Orav, Valeriia Kokitko; analysis and interpretation of results: Anne Orav, Ain Raal, Valeriia Kokitko, Vira Odyntsova, Oleh Koshovyi; draft manuscript preparation: Ain Raal, Valeriia Kokitko, Vira Odyntsova, Oleh Koshovyi. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the author and/or corresponding author on reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Chen HW, Wei BJ, He XH, Liu Y, Wang J. Chemical components and cardiovascular activities of Valeriana spp. Evid-Based Complement Altern Med. 2015;2015:947619. [Google Scholar] [PubMed]
2. Coassini Lokar L, Moneghini M. Geographical variation in the monoterpenes of Valeriana officinalis leaf. Biochem Syst Ecol. 1989;17(7–8):563–7. [Google Scholar]
3. Feng YX, Wang Y, Geng ZF, Zhang D, Almaz B, Du SS. Contact toxicity and repellent efficacy of Valerianaceae spp. to three stored-product insects and synergistic interactions between two major compounds camphene and bornyl acetate. Ecotoxicol Environ Saf. 2020;190:110106. doi:10.1016/j.ecoenv.2019.110106. [Google Scholar] [PubMed] [CrossRef]
4. Marcucci C, Rademacher M, Kamecki F, Pastore V, Bach HG, Ricco RA, et al. Biological evaluation of Valeriana extracts from Argentina with potent cholinesterase inhibition for the treatment of neurodegenerative disorders and their comorbidities—the case of Valeriana carnosa Sm. (Caprifoliaceae) studied in mice. Pharmaceuticals. 2023;16(1):129. doi:10.3390/ph16010129. [Google Scholar] [PubMed] [CrossRef]
5. Boguslavska NY, Kornievska VG, Kornievskiy Y, Panchenko SV. Valerian medicinal. Zaporizhzhia: ‘Karat’ LLC; 2014. [Google Scholar]
6. Wang W, Wang Y, Guo Q, Li H, Wang Z, Li J, et al. Valerian essential oil for treating insomnia via the serotonergic synapse pathway. Front Nutr. 2022;9:927434. doi:10.3389/fnut.2022.927434. [Google Scholar] [PubMed] [CrossRef]
7. Kenda M, Kočevar Glavač N, Nagy M, Sollner Dolenc M. Medicinal plants used for anxiety, depression, or stress treatment: an update. Molecules. 2022;27(18):6021. doi:10.3390/molecules27186021. [Google Scholar] [PubMed] [CrossRef]
8. Raal A, Arak E, Orav A, Kailas T, Müürisepp M. Variation in the composition of the essential oil of commercial Valeriana officinalis L. roots from different countries. J Essent Oil Res. 2008;20(6):524–9. doi:10.1080/10412905.2008.9700079. [Google Scholar] [CrossRef]
9. Pop MR, Sand C, Bobit D, Antofie MM, Pavel PB, Mircea S. Studies concerning the production of volatile oil, rhizomes and roots, to different genotypes of Valeriana officinalis L. An Univ Oradea, Fasc Biol. 2010;17:332–5. [Google Scholar]
10. Georgiev EV, St. Stojanova A, At. Tchapkanov V. On the Bulgarian valerian essential oil. J Essent Oil Res. 1999;11(3):352–4. doi:10.1080/10412905.1999.9701152. [Google Scholar] [CrossRef]
11. Zhang XM, Zhu JL, Sun Y, Dai YL, Chen X, Cao JH, et al. Anxiolytic potency of iridoid fraction extracted from Valeriana jatamansi Jones and its mechanism: a preliminary study. Nat Prod Res. 2018;32(17):2071–5. doi:10.1080/14786419.2017.1360881. [Google Scholar] [PubMed] [CrossRef]
12. Zhang L, Wang L, Huang L, Zhao Y, Ding H, Li B, et al. Antidepressant effects of total iridoids of Valeriana jatamansi via the intestinal flora-blood-brain barrier pathway. Pharmaceutical Biology. 2021;59(1):910–9. doi:10.1080/13880209.2021.1944222. [Google Scholar] [PubMed] [CrossRef]
13. Nandhini S, Narayanan KB, Ilango K. Valeriana officinalis: a review of its traditional uses, phytochemistry and pharmacology. Asian J Pharm Clin Res. 2018;11(1):36. doi:10.22159/ajpcr.2018.v11i1.22588. [Google Scholar] [CrossRef]
14. Li J, Li X, Wang C, Zhang M, Ye M, Wang Q. The potential of Valeriana as a traditional Chinese medicine: traditional clinical applications, bioactivities, and phytochemistry. Front Pharmacol. 2022;13:973138. doi:10.3389/fphar.2022.973138. [Google Scholar] [PubMed] [CrossRef]
15. Sen-Utsukarci B, Taskin T, Goger F, Tabanca N, Estep AS, Kessler SM, et al. Chemical composition and antioxidant, cytotoxic, and insecticidal potential of Valeriana alliariifolia in Turkey. Arh Hig Rada Toksikol. 2019;70(3):207–18. doi:10.2478/aiht-2019-70-3273. [Google Scholar] [PubMed] [CrossRef]
16. Korczak M, Pilecki M, Granica S, Gorczynska A, Pawłowska KA, Piwowarski JP. Phytotherapy of mood disorders in the light of microbiota-gut-brain axis. Phytomedicine. 2023;111:154642. doi:10.1016/j.phymed.2023.154642. [Google Scholar] [PubMed] [CrossRef]
17. Valente V, Machado D, Jorge S, Drake CL, Marques DR. Does valerian work for insomnia? An umbrella review of the evidence. Eur Neuropsychopharmacol. 2024;82:6–28. doi:10.1016/j.euroneuro.2024.01.008. [Google Scholar] [PubMed] [CrossRef]
18. Wang C, Lin XW, Wu LG, Fu C, Jin LJ, Wang J, et al. Impact of acupuncture on sleep and comorbid symptoms for chronic insomnia: a randomized clinical trial. Nat Sci Sleep. 2021;13:1807–22. doi:10.2147/NSS.S326762. [Google Scholar] [PubMed] [CrossRef]
19. Marcucci C, Relats JMA, Bach HG, Kamecki F, Varela BG, Wagner ML, et al. Neurobehavioral evaluation and phytochemical characterization of a series of argentine valerian species. Heliyon. 2020;6(12):e05691. doi:10.1016/j.heliyon.2020.e05691. [Google Scholar] [PubMed] [CrossRef]
20. Letchamo W, Ward W, Heard B, Heard D. Essential oil of Valeriana officinalis L. cultivars and their antimicrobial activity as influenced by harvesting time under commercial organic cultivation. J Agric Food Chem. 2004;52(12):3915–9. doi:10.1021/jf0353990. [Google Scholar] [PubMed] [CrossRef]
21. Thusoo S, Gupta S, Sudan R, Kour J, Bhagat S, Hussain R, et al. Antioxidant activity of essential oil and extracts of Valeriana jatamansi roots. Biomed Res Int. 2014;2014:614187. [Google Scholar] [PubMed]
22. Tyagi T, Sharma S, Sharma R. Pharmacological actions of Valeriana Wallichii (Tagaraa fundamental analysis supporting traditional benefits. Int J Ayu Pharm Res. 2022;10(1):1–7. [Google Scholar]
23. Bos R, Hendriks H, Pras N, St. Stojanova A, Georgiev EV. Essential oil composition of Valeriana officinalis ssp. collina cultivated in Bulgaria. J Essent Oil Res. 2000;12(3):313–6. doi:10.1080/10412905.2000.9699524. [Google Scholar] [CrossRef]
24. Agaphonov VA, Jusubov MV, Shkrobotko PJu, Tkachev AV, Belousov MV, Fursa NS. Component composition of essential oil from rhizomes with roots of Valeriana officinalis L. s. str. in the surroundings of Yaroslavl and Valeriana collina Wallr. in the surroundings of Zaporizhie. Bull VSU. 2009;2:190–7. [Google Scholar]
25. Arak E, Kailas T, Müürisepp M, Orav A, Raal A. Variation in the composition of the essential oil of Valeriana officinalis L. roots from Estonia. Proc Est Acad Sci Chem. 2007;56(2):67–74. [Google Scholar]
26. European Pharmacopoeia. 11th ed. Strasbourg: Council of Europe; 2022. [Google Scholar]
27. Bączek KB, Kosakowska O, Boczkowska M, Bolc P, Chmielecki R, Pióro-Jabrucka E, et al. Intraspecific variability of wild-growing common valerian (Valeriana officinalis L.). Plants. 2022;11(24):3455. doi:10.3390/plants11243455. [Google Scholar] [PubMed] [CrossRef]
28. Pavlovic M, Kovacevic N, Tzakou O, Couladis M. The essential oil of Valeriana officinalis L. s.l. growing wild in Western Serbia. J Essent Oil Res. 2004;16(5):397–9. doi:10.1080/10412905.2004.9698753. [Google Scholar] [CrossRef]
29. Barbarych AI, Bradys EM, Visyulin OD, Kotov MI. Identifier of plants of Ukraine: study guide. 2nd ed. Kyiv: Urozhai; 1965. [Google Scholar]
30. Huzio N, Grytsyk A, Raal A, Grytsyk L, Koshovyi O. Phytochemical and pharmacological research in Agrimonia eupatoria L. herb extract with anti-inflammatory and hepatoprotective properties. Plants. 2022;11(18):2371. doi:10.3390/plants11182371. [Google Scholar] [PubMed] [CrossRef]
31. Safaralie A, Fatemi S, Sefidkon F. Essential oil composition of Valeriana officinalis L. roots cultivated in Iran. J Chromatogr A. 2008;1180(1–2):159–64. [Google Scholar] [PubMed]
32. Lin S, Ye J, Liang X, Zhang X, Su J, Fu P, et al. Mass spectrometric profiling of valepotriates possessing various acyloxy groups from Valeriana jatamansi. J Mass Spectrom. 2015;50(11):1294–304. doi:10.1002/jms.v50.11. [Google Scholar] [CrossRef]
33. Koshovyi O, Heinämäki J, Raal A, Laidmäe I, Topelius NS, Komisarenko M, et al. Pharmaceutical 3D-printing of nanoemulsified eucalypt extracts and their antimicrobial activity. Eur J Pharm Sci. 2023;187:106487. doi:10.1016/j.ejps.2023.106487. [Google Scholar] [PubMed] [CrossRef]
34. Sermukhamedova O, Ludwiczuk A, Widelski J, Głowniak K, Sakipova Z, Ibragimova L, et al. Chemical comparison of the underground parts of Valeriana officinalis and Valeriana turkestanica from Poland and Kazakhstan. Open Chem. 2017;15(1):75–81. doi:10.1515/chem-2017-0010. [Google Scholar] [CrossRef]
35. Kornievs’kiĭ II, Nikolaeva AG, Koroshchuk KE. Chemistry of valeriana stolonifera. Farm Zh. 1972;27(1):81–2. [Google Scholar] [PubMed]
36. Jun ZZ, Long SY, Fen RX. Bornyl acetate: a promising agent in phytomedicine for inflammation and immune modulation. Phytomedicine. 2023;114:154781. doi:10.1016/j.phymed.2023.154781. [Google Scholar] [PubMed] [CrossRef]
37. Yang H, Zhao R, Chen H, Jia P, Bao L, Tang H. Bornyl acetate has an anti-inflammatory effect in human chondrocytes via induction of IL-11. IUBMB Life. 2014;66(12):854–9. doi:10.1002/iub.v66.12. [Google Scholar] [CrossRef]
38. Wu X, Xiao F, Zhang Z, Li X, Xu Z. Research on the analgesic effect and mechanism of bornyl acetate in volatile oil from amomum villosum. Zhong Yao Cai. 2005;28(6):505–7 (In Chinese). [Google Scholar] [PubMed]
39. Rücker G, Tautges J, Sieck A, Wenzl H, Graf E. Isolation and pharmacodynamic activity of the sesquiterpene valeranone from Nardostachys jatamansi DC. Arzneimittelforschung. 1978;28(1):7–13 (in Germany). [Google Scholar]
40. de Rossi S, di Marco G, D’Agostino A, Braglia R, Mecca G, Canini A, et al. Influence of environmental conditions on the production of nutraceuticals in Italian edible plant landraces. Food Res Int. 2023;165(6):112483. doi:10.1016/j.foodres.2023.112483. [Google Scholar] [PubMed] [CrossRef]
41. Chrysargyris A, Mikallou M, Petropoulos S, Tzortzakis N. Profiling of essential oils components and polyphenols for their antioxidant activity of medicinal and aromatic plants grown in different environmental conditions. Agronomy. 2020;10(5):727. doi:10.3390/agronomy10050727. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools