 Open Access
Open Access
ARTICLE
Comparative Chemical Research in Essential Oils from Six Apiaceae Species Growing in the Northern Region of Vietnam
1 Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology (VAST), Cau Giay, Ha Noi, 100000, Viet Nam
2 Graduate University of Science and Technology, Vietnam Academy of Science and Technology (VAST), Cau Giay, Ha Noi, 100000, Viet Nam
3 Department of Pharmacognosy, Faculty of Pharmacognosy and Traditional Medicine, Hanoi University of Pharmacy, Hoan Kiem, Ha Noi, 100000, Viet Nam
4 Sa Pa Research Center of Medicinal Materials, National Institute of Medicinal Materials, Dien Bien Phu, Sa Pa, Lao Cai, 330000, Viet Nam
5 Institute of Pharmacy, Faculty of Medicine, University of Tartu, Tartu, 50411, Estonia
* Corresponding Author: Ain Raal. Email:
Phyton-International Journal of Experimental Botany 2024, 93(7), 1677-1687. https://doi.org/10.32604/phyton.2024.053624
Received 06 May 2024; Accepted 11 June 2024; Issue published 30 July 2024
Abstract
Our study aimed to compare the essential oil (EO) concentration and composition of several Apiaceae species growing in the Northern region of Vietnam. The yields of EOs from materials ranged from 0.03% (root EO of Angelica acutiloba and aerial parts EO of Heracleum bivittatum)−0.27% (leaf EO of Xyloselinum vietnamense). Gas chromatography-mass spectrometry (GC-MS) allowed the identification of 74 components in the EOs of six Apiaceae species, making up 94.4%–100.0% of the oils. In EO from Angelica acutiloba, (Z)-ligustilide accounted for an extremely large proportion (94.9%). EO of Angelica pubescens was dominated by six characteristic components including α-pinene (21.5%), β-phellandrene (18.1%), p-cymene (12.2%), 3-methylnonane (8.7%), o-cymene (8.1%), and D-sylvestrene (6.2%). The EO from Cryptotaenia japonica was characterized by high amounts of α-selinene (48.7%), β-selinene (23.7%), and trans-β-farnesene (5.4%). The EOs from leaves and stems of Xyloselinum vietnamense were characterized by high concentrations of sabinene (69.8% and 33.8%), 4-terpineol (8.7% and 7.4%) and β-pinene (4.0% and 6.5%) while EOs from aerial parts and root of Xyloselinum leonidii comprise four characteristic monoterpenes including α-pinene (28.2% and 52.8%), β-pinene (7.9% and 10.3%), β-phellandrene (7.6% and 15.3%), and sabinene (3.0% and 4.1%). Additionally, cryptone is also one of the major components in the EO of Xyloselinum leonidii (13.2% in the aerial parts oil and 2. 8% in the root oil). In the EOs isolated from the aerial parts and root of Heracleum bivittatum, α-pinene (22.5% and 70.2%) and β-pinene (43.2% and 20.0%) were the predominant monoterpenes. Sabinene appeared in the EO from aerial parts of Heracleum bivittatum with a relatively high concentration (13.5%) while bornyl acetate (5.1%) was also one of the main components in the EO from its aerial parts but was not detected in other Apiaceae species in the present study. These databases help identify and control the quality of plant material studied from the family Apiaceae growing in Vietnam.Keywords
Family Apiaceae Lindl. consists of 3931 accepted species belonging to 448 genera [1]. These species are widely distributed in the temperate zone of both hemispheres, mainly in the Eurasian continent, especially in Central Asia [2]. Several studies have revealed the chemical composition of different Apiaceae species [3–5]. These species seem rich sources of phenolic compounds such as caffeic acid, chlorogenic acid, some aglycone flavonoids, and their glucosides. Besides, many studies have investigated the chemical composition of EOs from Apiaceae species worldwide [6–8].
In Vietnam, the family Apiaceae comprises numerous species containing bioactive components utilized in treating several diseases in traditional medicine. Many species of the family were introduced to Vietnam and cultivated as medicinal plants such as Angelica pubescens Maxim. and Angelica acutiloba (Siebold & Zucc.) Kitag. On the other hand, there are some Apiaceae species that native to Vietnam such as Cryptotaenia japonica Hassk., Heracleum bivittatum H.Boissieu (syn. Tetrataenium bivittatum (H.Boissieu) Manden.), Xyloselinum leonidii Pimenov & Kljuykov. and Xyloselinum vietnamense Pimenov & Kljuykov. The roots of H. bivittatum possessed hemostatic and tonic effects, while the leaves and stems of C. japonica were used to treat scabies, toxic pimples, and skin and reduce inflammation [9].
Among the above-mentioned species, some species have previously been studied regarding EO chemical composition. A. pubescens Maxim. EO with high concentrations of ostiole and eugenol, known for their anti-inflammatory and antioxidant effects [10] while A. acutiloba (Siebold & Zucc.) Kitag. possessed EO with the main components being (Z)-ligustilide and butylidene phthalide [11,12]. Besides, many species of the family Apiaceae have not been studied in terms of EO composition such as H. bivittatum H.Boissieu. The EO composition of leaves and stems of two Xyloselinum endemic in Vietnam was investigated previously [13] but the EO composition of their root is still unclear.
Our study aimed to compare the EO composition of several Apiaceae species encompassing Angelica acutiloba (Siebold & Zucc.) Kitag., Angelica pubescens Maxim., Cryptotaenia japonica Hassk., Heracleum bivittatum H.Boissieu., Xyloselinum leonidii Pimenov & Kljuykov, and Xyloselinum vietnamense Pimenov & Kljuykov and find possible fingerprints to different species. To the best of our knowledge, this is the first time the EO composition of Heracleum bivittatum and root of Xyloselinum leonidii were investigated using GC-MS.
The plant materials used in the study could be wild-growing or cultivated species of the family Apiaceae. The wild-growing plants of Cryptotaenia japonica (1.2 kg), Heracleum bivittatum (0.4 kg), Xyloselinum leonidii (0.7 kg), Xyloselinum vietnamense (5.0 kg), and two cultivated species including Angelica acutiloba (0.4 kg) and Angelica pubescens (0.3 kg) were collected (Table 1). The plant species were identified by Dr. Nguyen Quang Hung, Department of Plant Resources, Institute of Ecology and Biological Resources (IEBR), Vietnam Academy of Science and Technology (VAST) and MsC. Nghiem Duc Trong, Department of Botany, Hanoi University of Pharmacy. The voucher specimens (Vs) were deposited at the Department of Plant Resources, IEBR, VAST (Table 1). Based on the amount of collected materials, each species could be divided into different plant parts or used whole aerial parts; after that, they were dried in the shade until reaching the compatible humidity and then used to study chemical composition.
Chloroform and n-hexane used for the GC-MS analyses are of analytical grade and were obtained, as well as the alkane standard solution C8–C20, from Merck (Darmstadt, Germany).
All the air-dried materials were chopped into small pieces (5–10 mm) and submitted to the steam distillation of EO using a Clevenger-type apparatus according to Vietnam Pharmacopoeia [14] without adding an amount of organic solvent to the apparatus. The obtained oils were added sodium sulfate to absorb small amounts of water in the oils.
2.4 Gas Chromatography–Mass Spectrometry Analysis of Essential Oils
The EOs from six species of the family Apiaceae were diluted to 0.1% (v/v) in n-hexane. GC-MS analysis was performed using an In tuvo 9000 GC system equipped with a mass spectrometer detector MSD 5977B (Agilent, Frederick, CO, USA) and a non-polar DB-5MS fused silica capillary column (30 m × 0.25 mm × 0.25 μm). The oven temperature was set at 50°C, then ramped up to 200°C at a rate of 5°C/min, further raised to 280°C at a rate of 8°C/min, and maintained for 10 min, with an inlet temperature of 150°C and a split ratio of 300:1. Helium was used as the carrier gas with a flow rate of 1 ml/min. Ionization energy was set at 70 eV, and the scan range was from 45 to 450 amu.
The ratio of essential oil components relied on peak areas. Retention indices (RI) were determined by analyzing an n-alkanes chain (C8–C20) under identical GC conditions. Volatile component identification was based on comparison with mass spectra and RI-values from the NIST 08 mass spectral library, NIST Chemistry WebBook, and Adams book [15].
Multivariate analysis was employed to measure the distances between groups based on the composition of the aforementioned Apiaceae EOs. The overall similarity among the units of measurement was assessed using the Pearson distance in the UPGMA clustering method (Unweighted Pair Group Mean Association), considering all identified components in the studied Apiaceae EOs. Statistical analyses were conducted using R-Studio tools.
The yields of EOs from materials shown in Table 1, ranging from 0.03% (root EO of A. acutiloba and aerial parts EO of H. bivittatum)–0.27% (leaf EO of X. vietnamense).
The results of the GC-MS analysis are given in Table 2. 74 components were identified in the EOs of 6 Apiaceae species, making up 94.4%–100.0% of the oils.
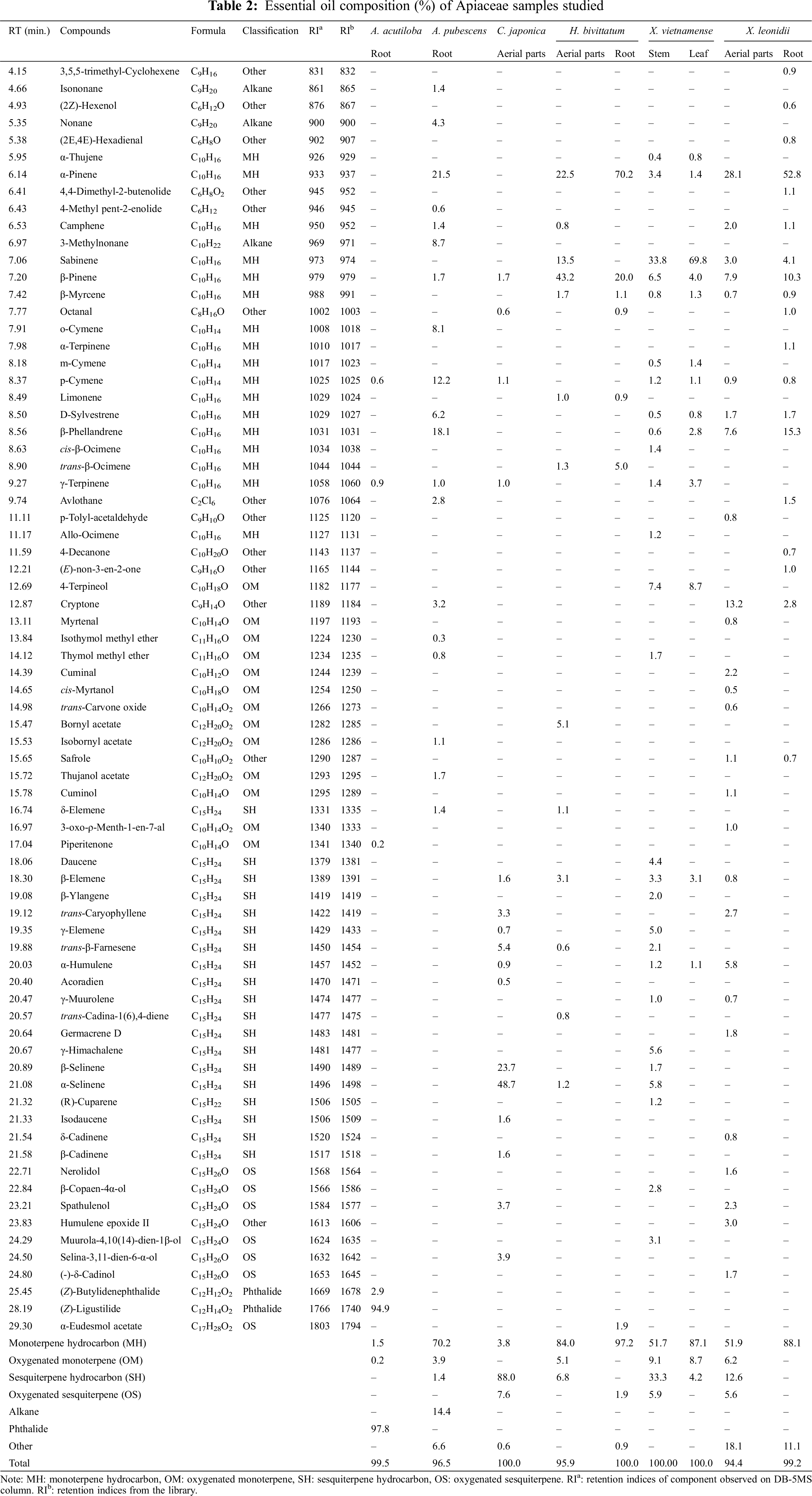
Monoterpenes were predominant in the EOs of A. pubescens (70.2%), H. bivittatum (84.0% in aerial parts oil, 97.2% in root oil), X. vietnamense (51.7% in stem oil, 87.1% in leaf oil) and X. leonidii (51.9% in aerial parts oil, 88.1% in root oil), while sesquiterpenes were only dominant in EO of C. japonica (88.0%).
In EO from A. acutiloba, (Z)-ligustilide accounted for an extremely large proportion (94.9%), followed by (Z)-butylidenephthalide (2.9%). Meanwhile, the remaining components constitute only a minute fraction, each contributing less than 1.0% to the overall composition. (Z)-ligustilide is known for its antioxidant and anti-inflammatory activities [16]. Additionally, it may offer potential benefits in cancer treatment [17]. However, the practical applications of (Z)-ligustilide are limited due to its physicochemical properties, including poor water solubility, thermolability, and weak photostability [16]. One study indicated that the growth and yield of A. acutiloba plants cultivated in Hokkaido varied with different nitrogen (N) levels. The concentration of (Z)-ligustilide increased with increased nitrogen supply. Therefore, it is necessary to use optimal nitrogen levels for the healthy growth of A. acutiloba in Hokkaido [18].
The EO of A. pubescens was dominated by monoterpenes (70.2%), with five characteristic components: α-pinene (21.5%), β-phellandrene (18.1%), p-cymene (12.2%), o-cymene (8.1%), and D-sylvestrene (6.2%). Additionally, some alkanes, such as 3-methylnonane (8.7%) and nonane (4.3%) also constituted a significant portion of the EO of A. pubescens, which were exclusive to A. pubescens and not found in other species. These results differ from previous studies, in which osthole was the predominant component in the EO of A. pubescens (44.6%) [10,19]. This could lead to the conclusion that the biological effects of the EO of A. pubescens cultivated in Vietnam may differ from those of A. pubescens cultivated in China.
The EO from C. japonica was characterized by high amounts of α-selinene (48.7%), β-selinene (23.7%) and trans-β-farnesene (5.4%). The concentrations of the three above-stated components were quite similar to the previous study in which α-selinene (13.2%–39.1%), β-selinene (4.8%–15.5%), and trans-β-farnesene (9.0%–11.1%) were major constituents in the EO from three kinds of C. japonica used in Japan [20]. This means that there is not so much difference between the EO composition of C. japonica growing in Vietnam and the three kinds of this species used in Japanese food.
The EOs from leaves and stems of X. vietnamense studied by us were quite similar to those in a previous study [13], with sabinene (69.8% and 33.8%, respectively) as the commanding compound. However, 4-terpineol was detected in relatively high concentration in both stem oil and leaf oil (7.4% and 8.7%, respectively) in our study, while this compound only appeared in stem oil (10.3%) in the previous study [13]. Besides, the EO from stems also had some components with high concentrations such as β-pinene (6.5%), α-selinene (5.8%), γ-elemene (5.0%), daucene (4.4%), α-pinene (3.4%) and β-elemene (3.3%). In previous study, these components possessed much lower concentrations (0%–0.8%) [13]. In contrast, cis-β-ocimene accounted for quite a high amount of stem oil (9.7%) in a previous study [13] but was in low concentration in X. vietnamense stem oil studied by us (1.4%). For the steam-distilled EO from the leaves of X. vietnamense, our study revealed that β-pinene (4.0%), γ-terpinene (3.7%) and β-elemene (3.1%) are components present in relatively high proportions, slightly higher than in previous research (1.9%–2.5%) [13]. On the other hand, santalon at a proportion of 5.1% was one of the main components in the EO from the leaves of X. vietnamense in previous studies [13], but was not present in our study.
Monoterpenes dominated the EOs of X. leonidii, including aerial parts oil (51.9%) and the root oil (88.1%). Both EOs comprise four characteristic monoterpenes: α-pinene (28.2% and 52.8%, respectively), β-pinene (7.9% and 10.3%, respectively), β-phellandrene (7.6% and 15.3%, respectively), and sabinene (3.0% and 4.1%, respectively). Additionally, cryptone is also one of the major components in the EOs of X. leonidii (13.2% in the aerial parts oil and 2.8% in the root oil). Furthermore, the EO from aerial parts contains a considerable amount of α-humulene (5.8%). Previously, the composition of the EO of the aerial parts has been studied [13], and our results are quite similar. In previous studies, α-pinene (7.6%–9.8%), sabinene (10.0%–29.3%), β-pinene (2.5%–13.7%), and β-phellandrene (9.5%–17.8%) were also the main monoterpenes in the EO of the aerial parts. Additionally, there were β-myrcene (2.2%–12.9%), (Z)-β-ocimene (2.5%–12.9%), and terpinen-4-ol (3.5%–4.1%) as characteristic components [13].
In the EO isolated from the aerial parts of H. bivittatum, α-pinene (22.5%) and β-pinene (43.2%) were the predominant monoterpenes. These two components were also identified in the root oil, comprising 70.2% and 20.0%, respectively. Sabinene, the main component in the EO of X. vietnamense, also appeared in the EO from aerial parts of H. bivittatum with relatively high concentration (13.5%) but was absent in the root oil of H. bivittatum. Bornyl acetate (5.1%) was also one of the main components in the EO from its aerial parts but was not detected in other Apiaceae species in the present study. Besides, trans-β-ocimene accounted for a high proportion in the root EO (5.0%), and a lower proportion in the aerial parts EO (1.3%), but it was also absent in the EO of other Apiaceae species studied.
Through a cluster analysis utilizing the concentrations of all components, distinct groupings of EOs were identified, as illustrated in Table 1. The chemical composition of the EO from the aerial parts of X. leonidii and the EO of A. pubescens is quite similar, with α-pinene, β-phellandrene, and cryptone being the main common components. Therefore, these two EOs form a distinct cluster. The aerial parts oil of H. bivittatum is quite like the cluster of the two X. leonidii and A. pubescens EOs. The root oils of both H. bivittatum and X. leonidii are also quite similar and form a cluster, with common main components such as α-pinene, sabinene, and β-pinene. The cluster of these two root EOs forms a group with the cluster of the three EOs mentioned above, forming a cluster of five EOs. On the other hand, the EOs from the leaves and stems of X. vietnamense form a separate cluster due to sharing some main components such as sabinene, 4-terpineol, β-pinene, and β-elemene. The composition of the EO of C. japonica is quite like the cluster of the two parts of X. vietnamense, thus forming a cluster of three EOs. This cluster of three EOs differs from the cluster of five EOs mentioned above. Finally, the EO of A. acutiloba is the most different from the others, with the main component being (Z)-ligustilide.
Cluster analysis has helped us identify the diversity and similarity in the chemical composition of the EOs among Apiaceae species. The oil clusters may reflect correlations between the chemical composition and environmental factors such as climate conditions, geography, and habitat. Furthermore, cluster analysis can aid in identifying oil groups with similar biological properties and effects. This helps in understanding and fully optimizing the utilization of these natural resources. Utilizing the UPGMA clustering method, it was observed that the studied EOs were divided into three distinct groups. A. acutiloba has the most distinctive essential oil composition compared to the other species. A. pubescens root EO, EOs from H. bivittatum aerial parts and roots, and EOs from aerial parts and roots of X. leonidii form a distinct cluster, separate from another cluster comprising C. japonica aerial parts EO and EOs from stem and leaves of X. vietnamense.
Research conducted on EOs and extracts from various Heracleum species has revealed a spectrum of diverse biological properties. For instance, Heracleum sibiricum has been noted for its cytotoxic effects [21], while H. nepalense has demonstrated antioxidant and antimicrobial activities [22]. Additionally, H. maximum has immunostimulant properties [23], and H. persicum exhibits an anticonvulsant effect [24]. We synthesized and compared the differences in the main components of the EO of H. bivittatum with the EOs of previously studied Heracleum species, including H. pastinacifolium C. Koch, H. persicum Desf. Ex Fischer, H. rechingeri Manden, H. transcaucasicum Manden, H. sphondylium L. and H. rawianum C.C.Towns.
We can observe that the composition of the EO of H. bivittatum differs significantly from that of other Heracleum species. While α-pinene was the main component in the EO of H. bivittatum, this compound was only present in the EO of H. sphondylium (3.8%) and absent in the other species. Similarly, β-pinene, the other major component of H. bivittatum, was found only in H. pastinacifolium with an average concentration of (3.1%). In contrast, the other species have very little or none of it. The remaining main compounds, such as sabinene (13.5%), bornyl acetate (5.1%), trans-β-ocimene (5.0%), and β-elemene (3.1%) are completely absent in other Heracleum species. This result confirmed that the chemical composition of the EOs of genus Heracleum L. can vary depending on cultivar and species variation [25–27].
The GC-MS methods were employed to analyze the essential oils extracted from six Apiaceae species growing in the Northern region of Vietnam. 74 volatile components were identified, accounting for 94.4%–100.0% of the total oils. The differences in the essential oil composition of the studied Apiaceae species may aid in identifying plant materials of this family and the essential oils obtained from them, and the products containing them.
Acknowledgement: Not applicable.
Funding Statement: This research was supported by a grant from the Vietnam Academy of Science and Technology, Project Code CSCL09.03/23-24.
Author Contributions: Study conception and design: Nguyen Quang Hung, Nguyen Thanh Tung; sample collection: Nguyen Quang Hung, Nguyen Phuong Hanh, Luong Van Hao, data collection: Nguyen Thanh Tung, Chu Thi Thu Ha, Nguyen Thi Nhung; analysis and interpretation of results: Nguyen Quang Hung, Nguyen Thi Nhung, Nguyen Thai An, Vu Xuan Giang, draft manuscript preparation: Nguyen Thanh Tung, Nguyen Thi Nhung, Oleh Koshovyi, Ain Raal. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. WFO. Family apiaceae. Available from: https://wfoplantlist.org/taxon/wfo-7000000036-2023-12?page=1. [Accessed 2024]. [Google Scholar]
2. Zehui P, Watson MF, Cannon JFM, Holmes-Smith I, Kljuykov EV, Phillippe LR, et al. Apiaceae (Umbelliferae). In: Wu Z, Raven P, Hong D, editors. Flora of China. China & USA: Science Press (China) & Missouri Botanical Garden (USA); 2005. vol. 14. [Google Scholar]
3. Zengin G, Sinan KI, Ak G, Mahomoodally MF, Paksoy MY, Picot-Allain C, et al. Chemical profile, antioxidant, antimicrobial, enzyme inhibitory, and cytotoxicity of seven Apiaceae species from Turkey: a comparative study. Ind Crops Prod. 2020;153:112572. doi:10.1016/j.indcrop.2020.112572. [Google Scholar] [CrossRef]
4. Shawky E, Abou El Kheir RM. Rapid discrimination of different Apiaceae species based on HPTLC fingerprints and targeted flavonoids determination using multivariate image analysis. Phytochem Anal. 2018;29(5):452–62. doi:10.1002/pca.v29.5. [Google Scholar] [CrossRef]
5. Trifan A, Bostănaru AC, Luca SV, Grădinaru AC, Jităreanu A, Aprotosoaie AC, et al. Antifungal potential of Pimpinella anisum, Carum carvi and Coriandrum sativum extracts. A comparative study with focus on the phenolic composition. Farmacia. 2020;68(1):22–7. doi:10.31925/farmacia. [Google Scholar] [CrossRef]
6. Gladikostić N, Ikonić B, Teslić N, Zeković Z, Božović D, Putnik P, et al. Essential oils from apiaceae, asteraceae, cupressaceae and lamiaceae families grown in serbia: comparative chemical profiling with in vitro antioxidant activity. Plants. 2023;12(4):745. doi:10.3390/plants12040745. [Google Scholar] [PubMed] [CrossRef]
7. Miclea V, Donca I, Culea M, Fiţ N, Podea P. Comparative study on essential oils of selected apiaceous seeds cultivated in Transylvania. Studia Universitatis Babes-Bolyai Chemia. 2019;64(2 T1):127–38. doi:10.24193/subbchem.2019.2.11. [Google Scholar] [CrossRef]
8. Campana R, Tiboni M, Maggi F, Cappellacci L, Cianfaglione K, Morshedloo MR, et al. Comparative analysis of the antimicrobial activity of essential oils and their formulated microemulsions against foodborne pathogens and spoilage bacteria. Antibiotics. 2022;11(4):447. doi:10.3390/antibiotics11040447. [Google Scholar] [PubMed] [CrossRef]
9. National Institute of Medical Materials. Checklits of Vietnamese medicinal plants. Vietnam: Science and Technology Publishing House; 2016 (In Vietnamese). [Google Scholar]
10. Chen D, Du Z, Lin Z, Su P, Huang H, Ou Z, et al. The chemical compositions of Angelica pubescens oil and its prevention of UV-B radiation-induced Cutaneous photoaging. Chem Biodivers. 2018;15(10):e1800235. doi:10.1002/cbdv.v15.10. [Google Scholar] [CrossRef]
11. Du L, Wang X, Cai C, Wang T. Constituent analysis of essential oils from radix of Angelica acutiloba. Zhong Yao Cai. 2002;25(7):477–8 (In Chinese). [Google Scholar] [PubMed]
12. Roh J, Lim H, Shin S. Biological activities of the essential oil from Angelica acutiloba. Nat Prod Sci. 2012;18(4):244–9. [Google Scholar]
13. Thai TH, Khang NS, Hien NT, Hoi TM, Dat NT. Chemical compositions of essential oils from Xyloselinum vietnamense and Xyloselinum leonidii. NPC Nat Prod Commun. 2012;7(10):1373–4. [Google Scholar]
14. Vietnam Pharmacopoeia V. The Ministry of Health. Vietnam: Medical Publishing House; 2017 (In Vietnamese). [Google Scholar]
15. Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. USA: Allured Pub Corp; 2007. [Google Scholar]
16. Song X, Liu C, Zhang Y, Xiao X, Han G, Sun K, et al. Sustainable extraction of ligustilide and ferulic acid from Angelicae sinensis radix, for antioxidant and anti-inflammatory activities. Ultrason Sonochem. 2023;94:106344. doi:10.1016/j.ultsonch.2023.106344. [Google Scholar] [PubMed] [CrossRef]
17. Yin L, Ying L, Guo R, Hao M, Liang Y, Bi Y, et al. Ligustilide induces apoptosis and reduces proliferation in human bladder cancer cells by NFκB1 and mitochondria pathway. Chem Biol Drug Des. 2023;101(6):1252–61. doi:10.1111/cbdd.v101.6. [Google Scholar] [CrossRef]
18. Igarashi M, Fuchino H, Sakurai M, Matsuba T, Hishida A. Efficient fertilization in the cultivation of Angelica acutiloba (Siebold & Zucc.) Kitag. in Hokkaido: effect of amount of supplied nitrogen on growth, yield, and quality of A. acutiloba. J Nat Med. 2022;76(1):298–305. doi:10.1007/s11418-021-01573-3. [Google Scholar] [PubMed] [CrossRef]
19. Li C, Cai Q, Wu X, Tan Z, Yao L, Huang S, et al. Anti-inflammatory study on the constituents of Angelica sinensis (Oliv.) Diels, Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav., Angelica pubescence Maxim and Foeniculum vulgare Mill. essential oils. J Oleo Sci. 2022;71(8):1207–19. doi:10.5650/jos.ess22031. [Google Scholar] [PubMed] [CrossRef]
20. Okuno Y, Marumoto S, Miyazawa M. Comparison of essential oils from three kinds of Cryptotaenia japonica Hassk (Kirimitsuba, Nemitsuba, and Itomitsuba) used in Japanese food. J Oleo Sci. 2017;66(11):1273–6. doi:10.5650/jos.ess17133. [Google Scholar] [PubMed] [CrossRef]
21. Bogucka-Kocka A, Smolarz HD, Kocki J. Apoptotic activities of ethanol extracts from some Apiaceae on human leukaemia cell lines. Fitoterapia. 2008;79(7–8):487–97. [Google Scholar] [PubMed]
22. Dash S, Nath LK, Bhise S. Antioxidant and antimicrobial activities of Heracleum nepalense D don root. Trop J Pharm Res. 2007;4(1):341–7. [Google Scholar]
23. Webster D, Taschereau P, Lee TDG, Jurgens T. Immunostimulant properties of Heracleum maximum Bartr. J Ethnopharmacol. 2006;106(3):360–3. doi:10.1016/j.jep.2006.01.018. [Google Scholar] [PubMed] [CrossRef]
24. Sayyah M, Moaied S, Kamalinejad M. Anticonvulsant activity of Heracleum persicum seed. J Ethnopharmacol. 2005;98(1–2):209–11. [Google Scholar] [PubMed]
25. Firuzi O, Asadollahi M, Gholami M, Javidnia K. Composition and biological activities of essential oils from four Heracleum species. Food Chem. 2010;122(1):117–22. doi:10.1016/j.foodchem.2010.02.026. [Google Scholar] [CrossRef]
26. Matejic JS, Dzamic AM, Mihajilov-Krstev T, Ristic MS, Randelovic VN, Krivošej Z, et al. Chemical composition, antioxidant and antimicrobial properties of essential oil and extracts from Heracleum sphondylium L. J Essent Oil Bear Plants. 2016;19(4):944–53. doi:10.1080/0972060X.2014.986538. [Google Scholar] [CrossRef]
27. Hasheminya SM, Dehghannya J. Chemical composition, antioxidant, antibacterial, and antifungal properties of essential oil from wild Heracleum rawianum. Biocatal Agric Biotechnol. 2021;31(2):101913. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools