 Open Access
Open Access
ARTICLE
Selenium Regulates Antioxidant Capacities and Diterpenoid Biosynthesis in the Medicinal Plant Isodon rubescens
1 Anhui Provincial Engineering Laboratory for Efficient Utilization of Featured Resource Plants, College of Life Sciences, Huaibei Normal University, Huaibei, 235000, China
2 Huaibei Key Laboratory of Efficient Cultivation and Utilization of Resource Plants, College of Life Sciences, Huaibei Normal University, Huaibei, 235000, China
* Corresponding Author: Yongbo Duan. Email:
Phyton-International Journal of Experimental Botany 2024, 93(7), 1705-1716. https://doi.org/10.32604/phyton.2024.052287
Received 28 March 2024; Accepted 11 June 2024; Issue published 30 July 2024
Abstract
Dōng líng căo, the dried aboveground parts of Isodon rubescens (Hemls.) Hara., is commonly consumed as a medicinal decoction or tea beverage. Natural beverages can be an important source of human dietary selenium (Se). However, how I. rubescens plants respond to exogenous Se remains unknown. In this study, a pot cultivation experiment was employed to investigate the phenotypic and physiological responses of I. rubescens plants exposed to Se. Fifteen days after applying different concentrations of sodium selenate to the soil, the Se enrichment capacity, growth indices, antioxidant capacities, and the content of flavonoids and diterpenoids were measured in the plants. Further, the oridonin content was quantified using the high-performance liquid chromatography method, and the expression levels of key diterpenoid synthesis genes were analyzed by quantitative real-time PCR (qRT-PCR). I. rubescens plants efficiently accumulated Se, with the Se content increasing proportionally to the applied dose, reaching levels of nearly 200 mg·kg dry leaves as Se concentration increased. None of the three Se treatments significantly altered the phenotypic indices, except a longer root length occurred in the 3 μM·kg Se group. Among three Se doses, 6 μM·kg Se gave the highest accumulation of flavonoids, diterpenoids, and oridonin, with the increase of 2.0-, 1.8-, and 1.9-fold in aboveground parts, respectively. Selenium application boosted the activities of antioxidant enzymes and antioxidant capacities according to 2,2-Diphenyl-1-picrylhydrazyl (DPPH), ferric reducing/antioxidant power, and tea brewing color experiments. Four key synthase genes were upregulated significantly by 6 μM·kg Se treatment, notably 1-deoxy-D-xylulose 5-phosphate reductoisomerase (IrDXR), with a 5-fold increase, and kaurene synthase-like 4 (IrKSL4), with a 6-fold increase. Thus, Se application in I. rubescens cultivation may be a potential biofortification method to supplement Se while increasing flavonoid and diterpenoid contents.Keywords
Supplementary Material
Supplementary Material FileSince first discovered in the human body in 1817 [1], selenium (Se) has been extensively studied for its importance to human health [2−4]. Humans mainly acquire Se from foods and natural beverages [5,6]. Plants can absorb inorganic Se from the soil and metabolize it into organic forms [7]. The magnitude of Se enrichment by plants is directly associated with the Se abundance and forms in the soil [8−10]. However, under anticipated climate change and Se soil decreases, deficient dietary Se uptake is expected in approximately 0.7 billion people [11]. Accordingly, irrigation [12], foliar spraying [13], and seed priming [14] with exogenous Se are efficient ways to biofortify plant-based foods.
When exposed to exogenous Se, plants manifest multifaceted responses, including phenotypic, biochemical, and stress-resistance responses [15,16]. In addition, Se enrichment has been shown to modulate the secondary metabolisms of plants [17,18]. In recent years, as concerns regarding dietary nutrients have increased, the secondary metabolites in plants treated with Se have garnered broad research attention. In particular, Se can regulate flavonoid and terpenoid accumulation in plants [19,20]. However, these studies have mostly been conducted in crops, and the associated response mechanisms have not been elucidated.
Medicinal plants are usually consumed as decoctions or tea beverages for the uptake of bioactive compounds, providing another approach to supplement human dietary Se uptake. Isodon rubescens (Hemls.) Hara., with its aboveground parts called dōng líng căo in Mandarin, is a plant in the family Labiatae with medicinal and food value. This plant has a long history of being used as a wild vegetable and tea beverage in China [21,22]. Dōng líng căo has been proven effective in treating various diseases, including sore throat, tonsillitis, bronchitis, chronic hepatitis, joint rheumatism, and various cancers [23]. Owing to its high efficacy and broad usage, dōng líng căo-derived products have an annual commercial value reaching nearly $100 million in China [22]. Chromatographic analysis revealed that diterpenoids, phenolic acids, and flavonoids are the major components of dōng líng căo [24]. The ent-kaurane diterpenoid oridonin has been identified as a particularly important monomeric compound due to its anti-cancer and anti-inflammatory activities [22]. Oridonin biosynthesis in I. rubescens is regulated by methyl jasmonate [25], suggesting that it responds to external stimuli. Previous research has mostly focused on field crops, with a limited understanding of how Se enrichment affects secondary metabolisms in medicinal plants like Isodon rubescens. As a pillar-like medicinal plant, it is still unclear whether I. rubescens plants could take up and become enriched in Se as field crops. Whether Se enrichment is involved in diterpenoid metabolism regulation also needs to be investigated. Thus, the influence of external Se on I. rubescens plants may facilitate the creation of Se-biofortified dōng líng căo with more bioactive compounds, further improving the cultivation efficiency of this medicinal plant.
The hypothesis of this study is that I. rubescens accumulates more secondary metabolites while becoming enriched from exogenous Se. Thus, the present study investigated the response of I. rubescens plants to Se by measuring the resulting biochemical indicators and content of flavonoids, terpenoids, and oridonin. In addition, the mRNA levels of key synthase genes for terpenoid biosynthesis were quantified to explain the possible regulation mechanisms involved in Se enrichment. The results will help to develop high-quality dōng líng căo with high added value.
I. rubescens seeds were purchased from Xiaxian County Shuanglian Traditional Chinese Herb Cultivation Specialized Cooperative, Yuncheng City, Shanxi Province, China.
Sodium selenate and standard Se were purchased from companies as described in our previous study [14]. Standard references for oridonin and rutin were obtained from Chengdu Purechem-Standard Co., Ltd. (Chengdu, China). Unless otherwise mentioned, all reagents used were the products of China National Pharmaceutical Industry Co., Ltd. (Beijing, China).
2.2 Experimental Design and Culture Conditions
The experiment was performed in the greenhouse facilities of Anhui Provincial Engineering Research Center for Efficient Utilization of Featured Resource Plants, Anhui, China (E116.8, N34.0). The surface-sterilization of seeds were carried out with 0.1% aqueous mercuric chloride solution (w/v). Then the seeds were spread on a seedling tray filled with nutrient soil (turf: coir: sandy loam: perlite: vermiculite, 5:3:3:1:1 [v:v:v:v:v], Huai’an Zhonghe Agriculture Co., Ltd., Huai’an, China). The culture conditions were set at 25°C and 60% relative humidity. The illumination condition was 16 h light/8 h dark, with a light intensity of 50 μmol photons m−2s−1 provided by cool white fluorescent lights.
At an approximate height of 10 cm, the seedlings were moved to 4 L-pots (20 cm in height) with commercial nutrient soil. Each pot contained 25 seedlings. Two weeks later, Se treatment was conducted by adding Na2SeO4 solution (150 mL) to the soil to reach final soil Se contents of 3, 6, and 9 μM·Kg−1 Se, with other seedlings irrigated with an equal volume of water as controls. The Se concentration range was set according to previous studies [12,15]. Plants were given 150 mL of running water to prevent Se from leaching out of the soil. After 15 d of growth, the plant materials were analyzed. After measuring the lengths of shoots and roots, some of the samples were vacuum-dried at 50°C to determine the dry weight and flavonoid and diterpenoid contents. The other samples were immediately used for measurement or stored at −80°C for physiological and gene expression analysis.
2.3 Assay of Antioxidant Capacities
The activities of antioxidant enzymes were determined as described previously [26]. The fresh leaf powder was extracted with an ice-cold extraction buffer to obtain a plant enzyme extract, and the protein content was quantified following the Bradford method [27]. Enzymatic activity was measured in the plant extract solution. Antioxidant capacities were assayed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing/antioxidant power (FRAP) as described previously [28].
A tea brewing experiment was further conducted to visualize the antioxidant capacity. For each treatment, 0.2-g samples of dried leaves were immersed in 100 mL of drinking water for 12 h. Then, the final color obtained was observed and imaged using a Nikon D700 camera (Nikon, Tokyo, Japan).
2.4 Measurement of Total Flavonoids
Flavonoid content was measured following a previously published method [29]. The flavonoids were extracted by mixing 100 mg of the powder derived from the vacuum-dried aboveground parts with methanol. After 10 min of extraction at room temperature with vortexing (100 r·min−1), the mixture was centrifuged to isolate the extract supernatants. The pellets were subjected to a second extraction with methanol. Two replicates were combined for flavonoid analysis. The extracts were reacted with NaNO2 and AlCl3 to measure total flavonoids, and the reaction was terminated with NaOH before recording the absorbance of the sample at 510 nm (A510) with a spectrophotometer. The content was calculated by substituting A510 into a calibration curve for rutin based on a gradient concentration series, and the results were recorded as mg rutin equivalents per 1 g sample (mgRE·g−1DW).
2.5 Quantification of Diterpenoids
Total diterpenoids were measured by ultraviolet spectrophotometry [30]. For each sample, diterpenoids were extracted from 200 mg of powder obtained from the vacuum-dried aboveground parts by dissolving it in 3 mL of 95% ethanol. The absorbance of the extract solution at 238 nm was determined to calculate the total diterpenoid content against a calibration curve based on the oridonin standard.
High-performance liquid chromatography (HPLC) was used to quantify oridonin content based on our previously published method for measuring diterpenoids [31]. The measurement was done with an Agilent Technologies 1260 Infinity Series HPLC system (Agilent Technologies, Santa Clara, CA, USA). Solvent A was 0.1% acetic acid in water (w:w), and solvent B was 100% methanol. The gradient elution was adjusted linearly from 40% to 45% B from 0–6 min, held at 45% B from 6–35 min, and adjusted linearly from 45% to 95% B from 35–45 min. The oridonin content was calculated by comparing the peak area with that of the oridonin standard by linear regression and presented as a percentage of dry weight (DW). Each treatment contained three parallel replicates.
2.6 Determination of Se Content by Atomic Fluorescence Spectroscopy
The Se content in the roots, stems, and leaves of I. rubescens plants was determined following a previously published method [14]. Plant Se content was obtained by comparing the fluorescence intensity of the plant extract with a Se standard curve.
The mRNA levels of key diterpenoid synthase genes, 1-deoxy-d-xylulose 5-phosphate synthase (IrDXS), 1-deoxy-d-xylulose 5-phosphate reductoisomerase (IrDXR), copalyl diphosphate synthase 5 (IrCPS5), and kaurene synthase-like 4 (IrKSL4), were quantified by real-time quantitative PCR (qRT-PCR). mRNA isolation, cDNA synthesis, and qRT-PCR were carried out according to a previously published method [31]. The actin gene was used to normalize the expression levels. The primers and gene sequences listed in Table S1 were obtained from previously published references [32,33].
The experiment contained three parallel replicates. Statistical analyses to identify differences between the control treatment without Se (CK) and Se treatments were conducted by analysis of variance (ANOVA) in IBM SPSS Statistics 20.0 (IBM Corp., Armonk, NY, USA) using Duncan’s multiple-range test (p < 0.05).
3.1 Isodon rubescens Plants Accumulate Exogenous Se
When treated with 3–9 μM·Kg−1 Se, I. rubescens plants differentially accumulated Se in the roots, stems, and leaves. A two-way ANOVA showed a significant interaction between Se dose and plant tissue (Table S2). Se-treated I. rubescens plants had 10.8–23.1-fold, 27.3–45.8-fold, and 13.5–27.2-fold increases in Se content in the roots, stems, and leaves, respectively, compared with control (p < 0.05) (Fig. 1). Concerning various tissues, Se accumulation capacity was greatest in the roots, which was higher than that of leaves and stems (p < 0.05). While the relative Se content was not significantly different between stems and leaves treated with CK and 3 μM·Kg−1 Se (p > 0.05), it was higher in leaves than stems treated with high doses of Se (p < 0.05). Se accumulation increased with increases in Se treatment concentrations, with the relative Se content reaching 194.7 mg·kg−1 DW in leaves treated with 6 μM·Kg−1 Se and 247.7 mg·kg−1 DW with 9 μM·Kg−1 Se treatment. It suggests that exogenous Se application during I. rubescens cultivation is a potential approach to increasing human dietary Se uptake.
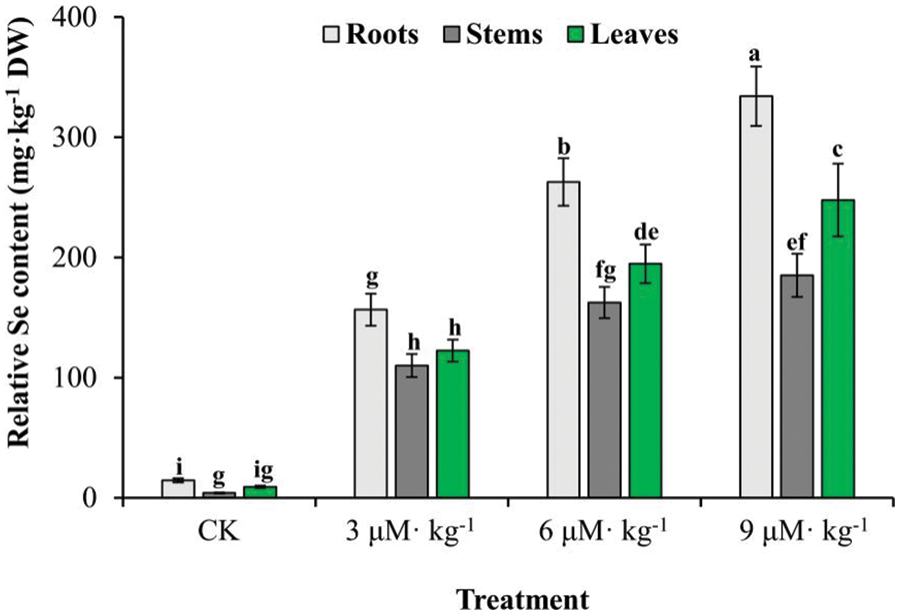
Figure 1: Se accumulation in various tissues of Se-treated and non-treated (CK) Isodon rubescens plants. Error bars indicate the standard error of the mean (n = 3). Values marked by different lowercase letters differ significantly using Duncan’s multiple range test (p < 0.05)
3.2 Se Does not Affect the Phenotype of I. rubescens
Isodon rubescens plants treated with Se grew normally (Fig. 2A), except scattered grayish spots were observed on the leaves of plants treated with 9 μM·kg−1 Se. Se treatment did not change the plant height (Fig. 2B), shoot weight (Fig. 2D), or root weight (Fig. 2E) (p > 0.05). However, increased root length was observed under 9 μM·kg−1 Se treatment (Fig. 2C) (p < 0.05) compared with the controls. As leaf spots appeared under 9 μM·kg−1 Se treatment, plants were inferred to be impaired by the highest level of exogenous Se tested. Thus, a suitable Se concentration would not compromise I. rubescens plant growth but might improve plant vigor by increasing root length.
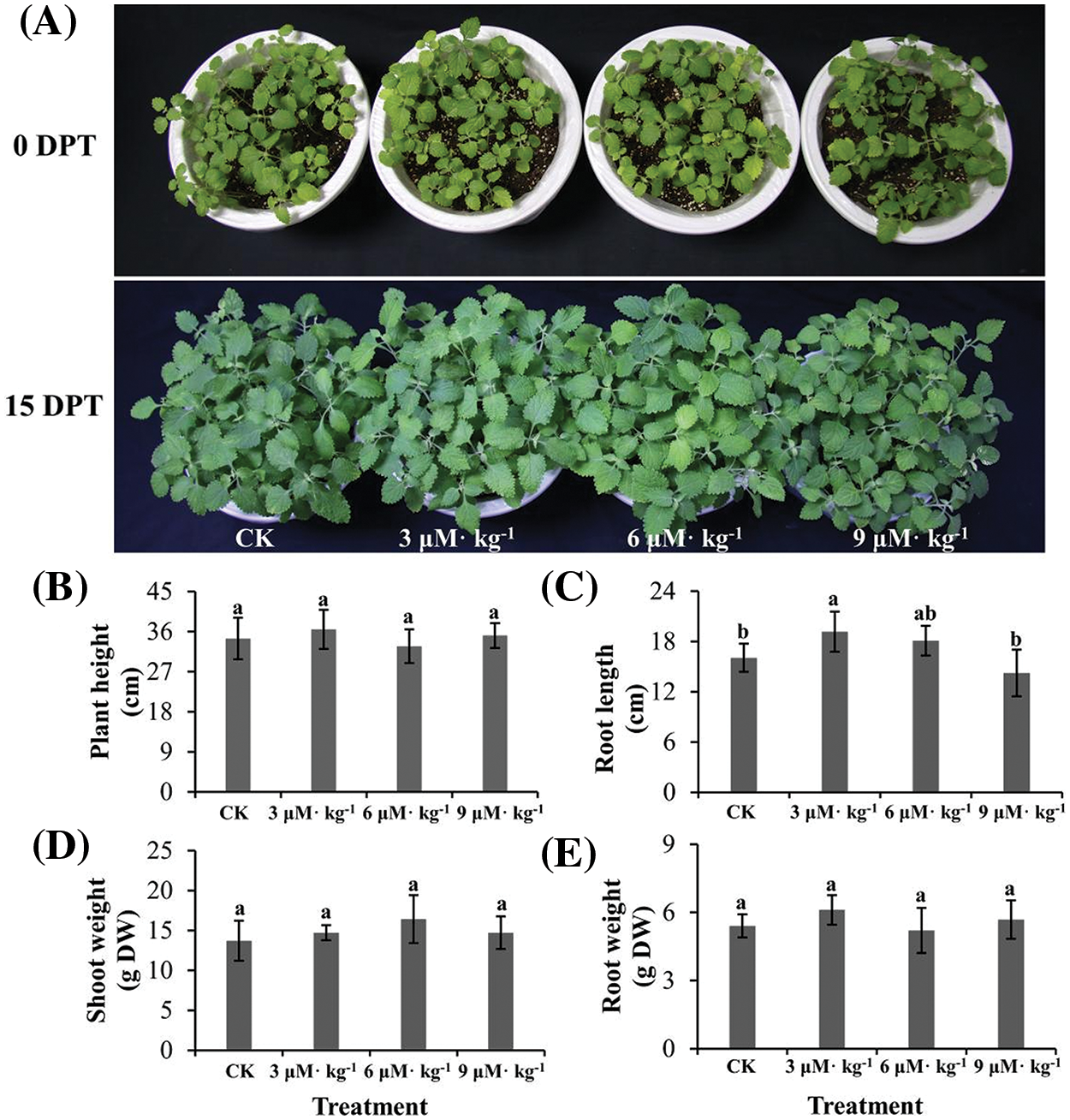
Figure 2: Phenotypic analysis of Se-treated and non-treated (CK) Isodon rubescens plants. (A) Plants without Se treatment or treated with 3, 6, or 9 μM·kg−1 Se. DPT, days post-treatment. (B) Plant height. (C) Root length. (D) Shoot weight. (E) Root weight. The data were collected on day 15 of treatment. Error bars indicate the standard error of the mean (n = 3). Values marked by different lowercase letters differ significantly within each panel (p < 0.05)
3.3 Se Improves Flavonoid and Diterpenoid Accumulation in I. rubescens
Selenium treatment significantly increased total flavonoid accumulation in I. rubescens plants, with increases of 1.8-, 2.0- and 1.5-fold by 3, 6, and 9 μM·kg−1 Se treatments, respectively (p < 0.05) (Fig. 3A). This indicates that Se regulates flavonoid metabolism in I. rubescens plants.
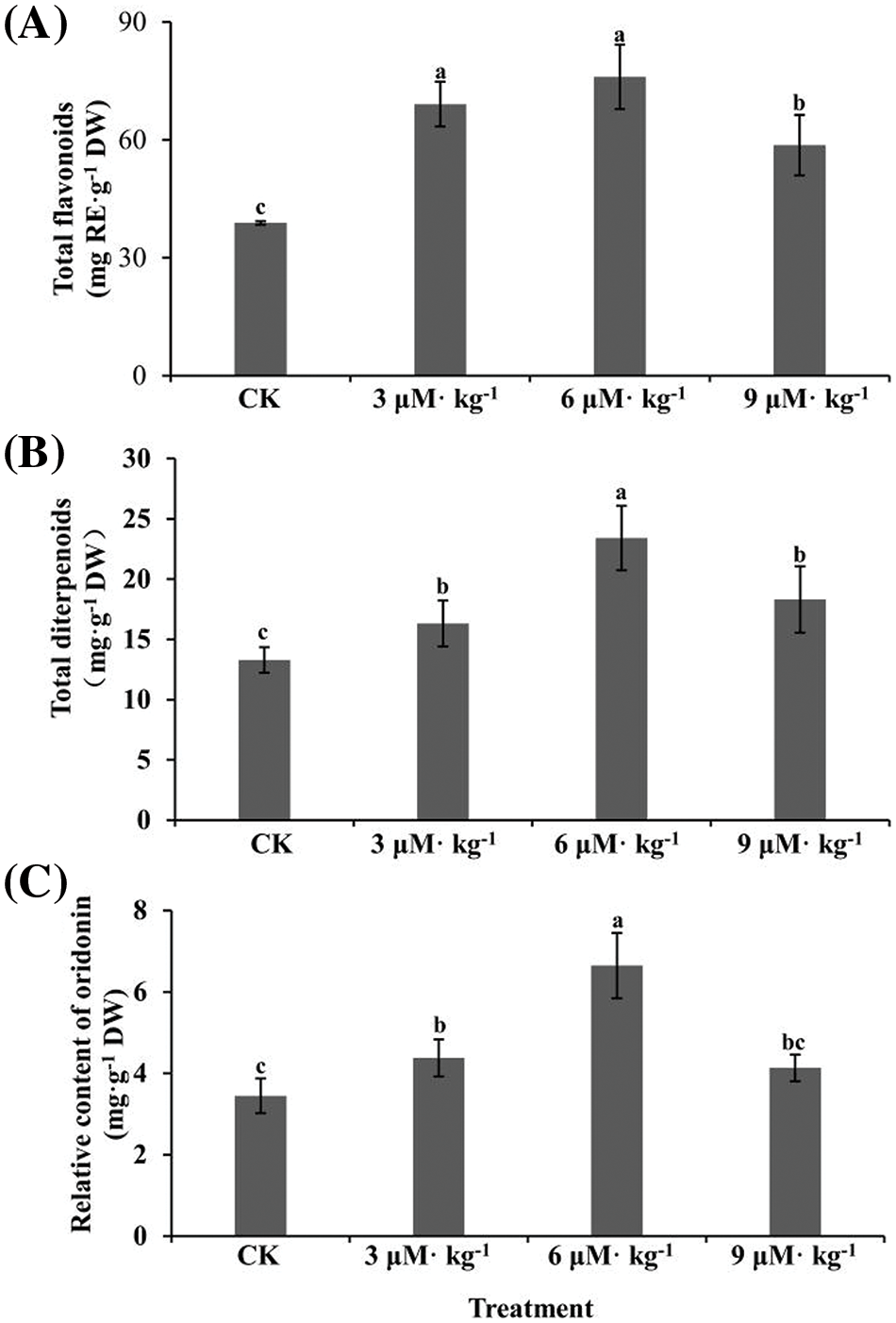
Figure 3: Total flavonoids (A), total diterpenoids (B), and relative oridonin content (C) in the aboveground parts of Se-treated and non-treated (CK) Isodon rubescens plants. Error bars indicate the standard error of the mean (n = 3). Values marked by different lowercase letters differ significantly within each plot (p < 0.05)
Similarly, the total diterpenoid content was also enhanced by all three Se treatments (p < 0.05) (Fig. 3B), with a 1.8-fold increase by 6 μM·kg−1 treatment. The content of the major diterpenoid oridonin was quantified by HPLC (Fig. S1). Treatment with 3 and 6 μM·kg−1 Se significantly promoted oridonin accumulation (Fig. 3C), with a 1.9-fold increase by 6 μM·kg−1 Se treatment but not 9 μM·kg−1 Se treatment. Thus, suitable concentrations of exogenous Se could modulate the content of flavonoids and diterpenoids in I. rubescens.
3.4 Se Enrichment Increases Antioxidant Capacity in I. rubescens
SOD activity was increased by all three Se treatments (p < 0.05) by 1.3–2.0-fold compared to CK treatment (Fig. 4A). Similar patterns were observed in POD (Fig. 4B) and CAT activities (Fig. 4C). The antioxidant capacity under all three Se treatments increased significantly compared with CK treatment, as indicated by the DPPH (Fig. 5A) and FRAP assays (Fig. 5B). The tea brewing color under all three Se treatments was lighter than that of CK treatment, particularly that of 6 μM·kg−1 Se treatment (Fig. 5C).
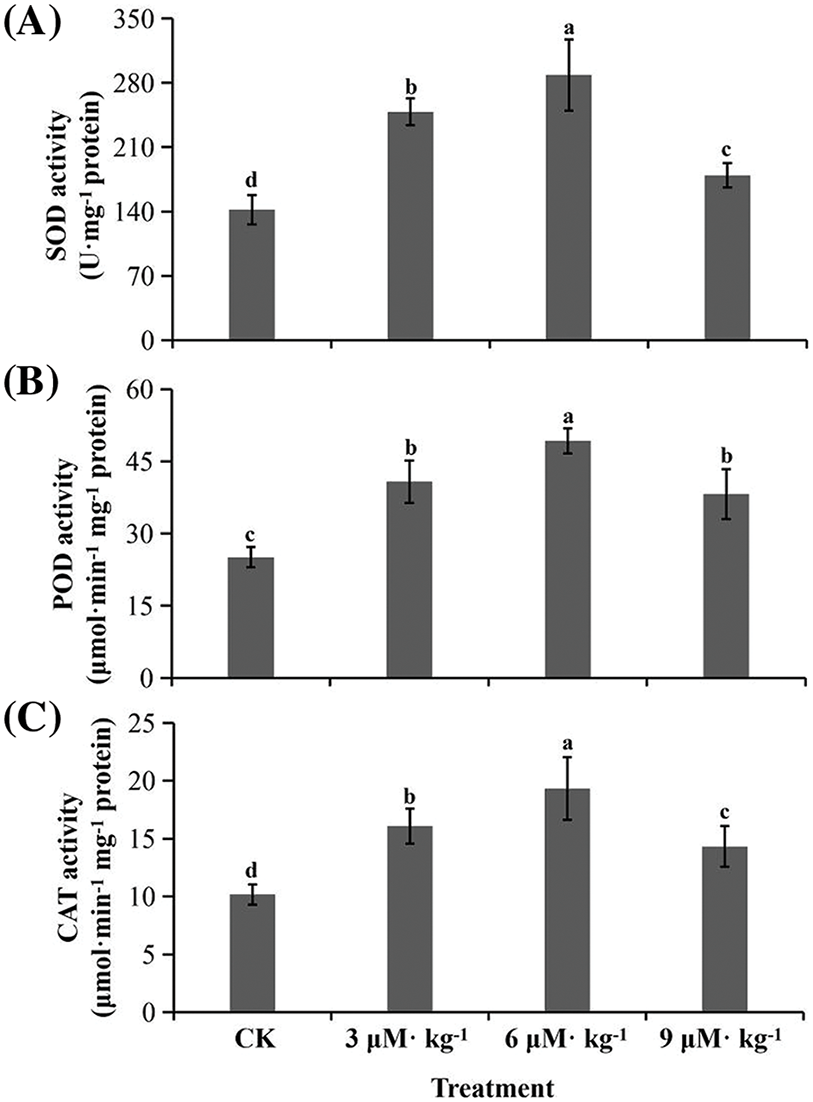
Figure 4: Total flavonoids and antioxidant enzymatic activity in Se-treated and non-treated (CK) Isodon rubescens plants. (A) Superoxide dismutase (SOD) activity. (B) Peroxidase (POD) activity. (C) Catalase (CAT) activity. Error bars indicate the standard error of the mean (n = 3). Values marked by different lowercase letters differ significantly within each plot (p < 0.05)
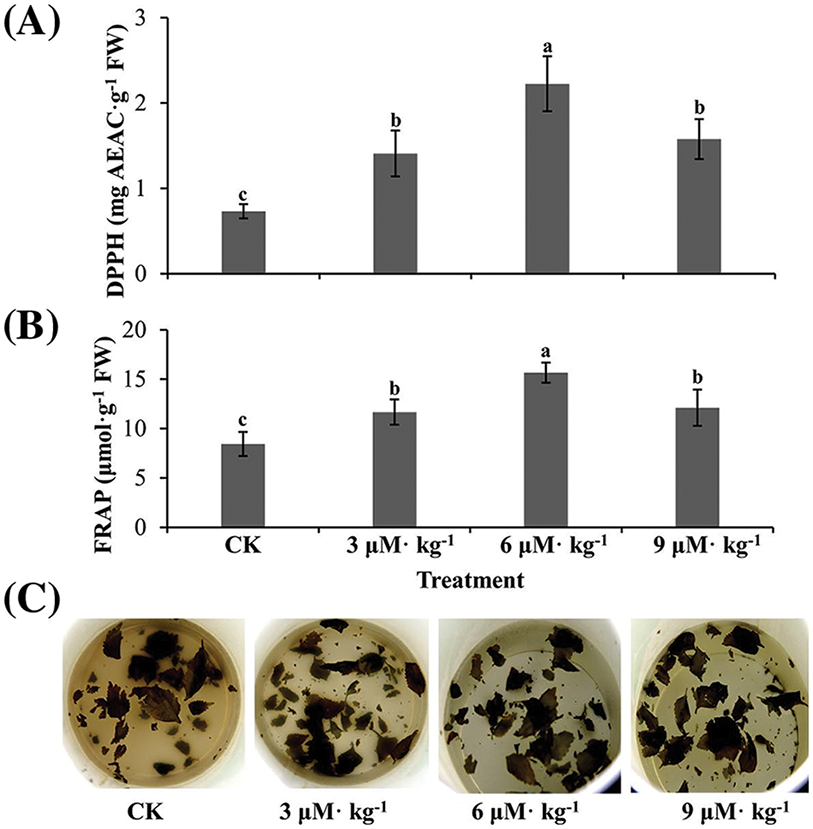
Figure 5: Antioxidant capacity assay of Se-treated and non-treated (CK) Isodon rubescens plants. (A) 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay results. (B) Ferric reducing/antioxidant power (FRAP) assay results. (C) Tea brewing after 12 h. Error bars indicate the standard error of the mean (n = 3). Values marked by different lowercase letters differ significantly within each plot (p < 0.05)
3.5 Se-Caused Diterpenoid Accumulation Is Associated with Expression of Key Synthase Genes
The biosynthetic pathway of diterpenoids is generally understood, although the functions of some key synthases remain unclear (Fig. 6A) [22,32,33]. Except for IrDXR under 3 μM·kg−1 Se treatment and IrCPS5 under 3 and 9 μM·kg−1 Se treatments, all four genes were upregulated in Se-treated I. rubescens plants, particularly IrDXS by 5-fold under 9 μM·kg−1 Se treatment, IrDXR by 5-fold under 6 μM·kg−1 Se treatment, and IrKSL4 by 6-fold under 6 μM·kg−1 Se treatment (p < 0.05) (Fig. 6B). Thus, expression increases induced in these genes may have contributed to the enhanced accumulation of diterpenoids observed in Se-treated I. rubescens.
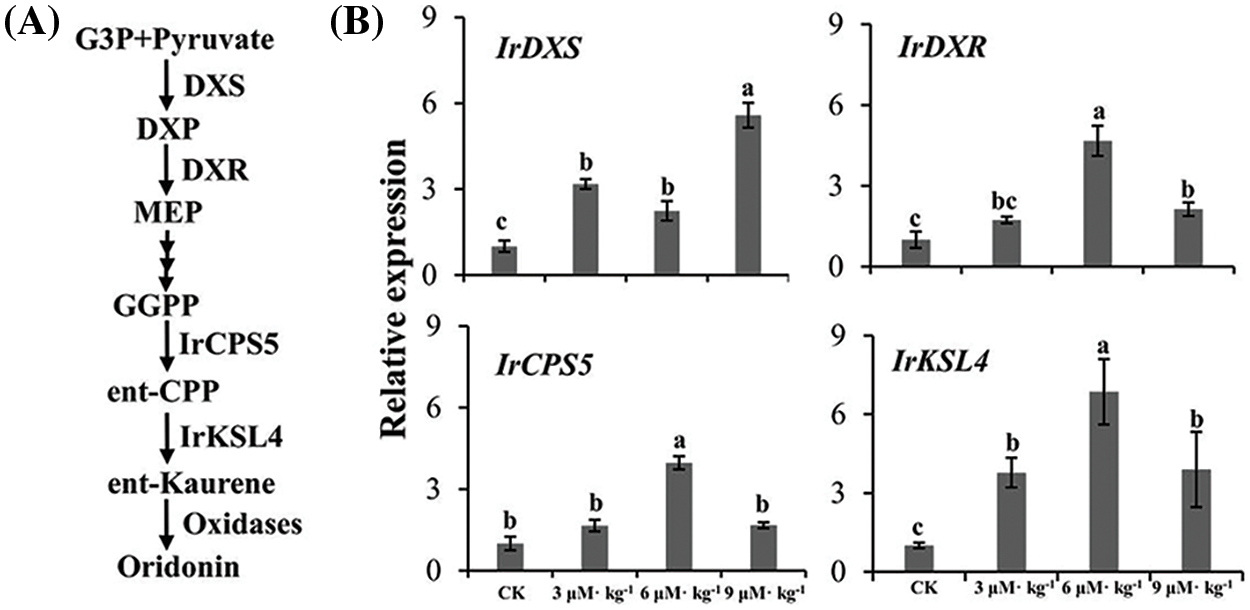
Figure 6: Expression of key biosynthase genes for diterpenoid accumulation in Isodon rubescens as determined by qRT-PCR. (A) A proposed pathway of diterpenoid biosynthesis. (B) Relative expression levels of genes based on qRT-PCR results. Different lowercase letters show significant differences within each plot (p < 0.05). IrDXS, I. rubescens 1-deoxy-d-xylulose 5-phosphate synthase; IrDXR, I. rubescens 1-deoxy-d-xylulose 5-phosphate reductoisomerase; IrCPS5, I. rubescens copalyl diphosphate synthase 5; IrKSL4, I. rubescens kaurene synthase-like 4
The exogenous application of Na2SeO4 promoted the accumulation of Se, flavonoids, and diterpenoids (particularly oridonin) in I. rubescens but did not decrease the plant biomass. This promoting effect was likely attributable to the upregulated expression of key diterpenoid biosynthesis genes.
The safe Se range in humans is extremely narrow, with an intake of <40 μg·d−1 as deficient and an intake of >400 μg·d−1 as toxic [17]. The recommended daily allowance of Se is 60–70 µg [34], particularly close to the deficiency threshold, highlighting the importance of clarifying the Se sources and content in foods and beverages. The medicinal plant I. rubescens has clear application potential for Se supplementation in human diets since it is already consumed in the form of wild vegetables, medicinal decoctions, and tea beverages. Previous studies reported the successful preparation of Se polysaccharides based on the structural modification of polysaccharides extracted from I. rubescens [35], implying the value of utilizing I. rubescens for Se supplementation. When exposed to Na2SeO4, I. rubescens plants accumulated at least 17-fold more Se in their leaves than control plants, reaching nearly 200 mg·kg−1 DW. The Se accumulation capacity was similar to that of other medicinal and edible plants, including Plantago ovata [36], Cardamine hupingshanesis [37], Brassica oleracea, and Triticum aestivum [38]. None of the three Se doses tested affected the phenotypic indices or biomass of I. rubescens, in contrast to P. ovata and Brassica rapa, which were both phenotypically influenced by Se treatment [36,39]. This discrepancy is likely due to differences in Se tolerance among these plant species. The present results suggest that the exogenous Se application to I. rubescens allows Se supplementation in human diets without compromising dōng líng căo yield.
Selenium enrichment, like environmental stress, activates plant instincts to protect their biological systems [21,40,41]. This process occurs through enzymatic and non-enzymatic approaches, thus helping plants to scavenge excess reactive oxygen species. In this study, Se treatments boosted the activity of three antioxidant enzymes, SOD, CAT, and POD, and enhanced the antioxidant capacity. Se accumulators usually have a higher antioxidant capacity than Se non-accumulators [42], consistent with the Se-regulated antioxidant capacity observed in the present study.
When absorbing exogenous Se, plants are invaded by a foreign matter that may disrupt their intrinsic metabolic systems. This imbalance may influence the secondary metabolism of plants, thus regulating the accumulation of secondary metabolites. Se treatment has been observed to regulate secondary metabolites in many other plants [18]. In the present study, when I. rubescens plants were treated with Na2SeO4, total flavonoids and diterpenoids were increased, consistent with the results of previous studies [14,42,43]. Moreover, higher antioxidant capacity was observed in I. rubescens plants treated with exogenous Se, which was likely explained by the increased antioxidant enzymatic activities and secondary metabolites observed. Since the diterpenoid oridonin is the major pharmaceutically active compound in dōng líng căo, it was further assayed by HPLC. The oridonin content increased by 1.9-fold in I. rubescens leaves treated with 6 μM·kg−1 Se. In contrast to previous studies that mostly focused on the content of total essential oils, our study revealed that exogenous Se induced the accumulation of oridonin, an important anti-cancer compound.
The expression levels of four genes encoding proteins involved in the diterpenoid biosynthesis pathway were quantified to explain why Se promoted the accumulation of diterpenoids (particularly oridonin) in I. rubescens plants. The four genes tested have each been functionally characterized [32,33], and all were upregulated by treatment with Na2SeO4, particularly exhibiting a 5-fold increase in IrDXR and a 6-fold increase in IrKSL4 by 6 μM·kg−1 Se treatment. DXR is the second protein involved in the terpenoid synthesis pathway, while KSL catalyzes the last several steps of oridonin biosynthesis [32,33]. Thus, the genes encoding these proteins may contribute to the increased biosynthesis of diterpenoids and oridonin, in particular, in I. rubescens plants treated with Se. This study revealed key synthase genes involved in diterpenoid biosynthesis, but the response mechanism to Se needs further study. Transcriptomic analysis may provide insight into the response mechanism of plants to Se treatment [44]. Thus, combining transcriptomic analysis with the functional characterization of key synthase genes/transcription factors could reveal these molecular mechanisms, thus providing breeding targets for selecting high-quality I. rubescens germplasms.
The present results suggest that dōng líng căo consumed as a wild vegetable, medicinal decoction, or tea beverage has promising potential as a dietary source of supplemental Se, especially when plants are treated with exogenous Se. The observed Se-induced diterpenoid accumulation was explained by the upregulated expression of key synthase genes, particularly IrDXR and IrKSL4. These results advance the understanding and exploration of the full value of dōng líng căo as a medicinal resource and dietary supplement.
Acknowledgement: None.
Funding Statement: This study was supported by the Key Project of Natural Science Research for Colleges and Universities in Anhui Province (2023AH050345, KJ2021A0533) and the Excellent Scientific Research and Innovation Team of Universities in Anhui Province (2022AH010029).
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Yongbo Duan and Fenglan Zhao; data collection: Fenglan Zhao, Shuwen Wu, and Xue Meng; analysis and interpretation of results: Jianping Xue; and manuscript writing: Fenglan Zhao and Yongbo Duan. All authors reviewed the results and approved the final version of the manuscript for submission.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/phyton.2024.052287.
References
1. Berzelius J. Letter from Mr. Berzelius to Mr. Berthollet on two new metals. Ann Chim Phys. 2021;7:199–206. [Google Scholar]
2. Dinh QT, Cui ZW, Huang J, Tran TAT, Wang D, Yang WX, et al. Selenium distribution in the Chinese environment and its relationship with human health: a review. Environ Int. 2018;112:294–309. doi:10.1016/j.envint.2017.12.035. [Google Scholar] [PubMed] [CrossRef]
3. Filippini T, Fairweather-Tait S, Vinceti M. Selenium and immune function: a systematic review and meta-analysis of experimental human studies. Am J Clin Nutr. 2023;117:93–110. doi:10.1016/j.ajcnut.2022.11.007. [Google Scholar] [PubMed] [CrossRef]
4. Sun Y, Wang Z, Gong P, Yao W, Ba Q, Wang H. Review on the health-promoting effect of adequate selenium status. Front Nutr. 2023;10:1136458. doi:10.3389/fnut.2023.1136458. [Google Scholar] [PubMed] [CrossRef]
5. Reich HJ, Hondal RJ. Why nature chose selenium. ACS Chem Biol. 2016;11:821–41. doi:10.1021/acschembio.6b00031. [Google Scholar] [PubMed] [CrossRef]
6. Teixeira JLDP, Rebellato AP, Fioravanti MIA, Milani RF, Morgano MA. Selenium in plant-based beverages: total content, estimated bioaccessibility and contribution to daily intake. J Trace Elem Med Bio. 2024;81:127329. doi:10.1016/j.jtemb.2023.127329. [Google Scholar] [PubMed] [CrossRef]
7. Gupta M, Gupta S. An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci. 2017;7:2074. [Google Scholar] [PubMed]
8. Terry N, Zayed AM, De Souza MP, Tarun AS. Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Bio. 2009;51:401–32. [Google Scholar]
9. Zhou F, Peng Q, Wang M, Xue M, Zhai H, Liu N, et al. Advances in the evaluation of selenium bioavailability in soil-plant system. Chin Sci Bull. 2022;67(6):461–72. doi:10.1360/TB-2020-1268. [Google Scholar] [CrossRef]
10. Qu L, Xu J, Dai Z, Elyamine AM, Huang W, Han D, et al. Selenium in soil-plant system: transport, detoxification and bioremediation. J Hazard Mater. 2023;452:131272. doi:10.1016/j.jhazmat.2023.131272. [Google Scholar] [PubMed] [CrossRef]
11. Jones GD, Droz B, Greve P, Gottschalk P, Poffet D, McGrath SP, et al. Selenium deficiency risk predicted to increase under future climate change. Proc Natl Acad Sci U S A. 2017;114:2848–53. doi:10.1073/pnas.1611576114. [Google Scholar] [PubMed] [CrossRef]
12. Cunha MLO, de Oliveira LCA, Mendes NAC, Silva VM, Vicente EF, dos Reis AR. Selenium increases photosynthetic pigments, flavonoid biosynthesis, nodulation, and growth of soybean plants (Glycine max L.). J Soil Sci Plant Nut. 2023;23:1397–407. [Google Scholar]
13. Xu J, Zhang Y, Zhang M, Wei X, Zhou Y. Effects of foliar selenium application on Se accumulation, elements uptake, nutrition quality, sensory quality and antioxidant response in summer-autumn tea. Food Res Int. 2024;175:113618. doi:10.1016/j.foodres.2023.113618. [Google Scholar] [PubMed] [CrossRef]
14. Zhao FL, Jin JZ, Yang M, Santiago FEM, Xue JP, Duan YB. Selenium differentially regulates flavonoid accumulation and antioxidant capacities in sprouts of twenty diverse mungbean (Vigna radiata (L.) Wilczek) genotypes. Phyton. 2024;93(3):611–25. doi:10.32604/phyton.2024.048295. [Google Scholar] [CrossRef]
15. Lanza MGDB, Silva VM, Montanha GS, Lavres J, Pereira DC, Hudson W, et al. Assessment of selenium spatial distribution using μ-XFR in cowpea (Vigna unguiculata (L.) Walp.) plants: integration of physiological and biochemical responses. Ecotox Environ Safe. 2021;207:111216. doi:10.1016/j.ecoenv.2020.111216. [Google Scholar] [PubMed] [CrossRef]
16. Li Q, Xian L, Yuan L, Lin Z, Chen X, Wang J, et al. The use of selenium for controlling plant fungal diseases and insect pests. Front Plant Sci. 2023;14:1102594. doi:10.3389/fpls.2023.1102594. [Google Scholar] [PubMed] [CrossRef]
17. D’Amato R, Regni L, Falcinelli B, Mattioli S, Benincasa P, Dal Bosco A, et al. Current knowledge on selenium biofortification to improve the nutraceutical profile of food: a comprehensive review. J Agric Food Chem. 2020;68(14):4075–97. doi:10.1021/acs.jafc.0c00172. [Google Scholar] [PubMed] [CrossRef]
18. Skrypnik L, Feduraev P, Golovin A, Maslennikov P, Styran T. The integral boosting effect of selenium on the secondary metabolism of higher plants. Plants. 2022;11:3432. doi:10.3390/plants11243432. [Google Scholar] [PubMed] [CrossRef]
19. Skrypnik L, Novikova A, Tokupova E. Improvement of phenolic compounds, essential oil content and antioxidant properties of sweet basil (Ocimum basilicum L.) depending on type and concentration of selenium application. Plants. 2019;8:458. doi:10.3390/plants8110458. [Google Scholar] [PubMed] [CrossRef]
20. Rao S, Gou Y, Yu T, Cong X, Gui J, Zhu Z, et al. Effects of selenate on Se, flavonoid, and glucosinolate in broccoli florets by combined transcriptome and metabolome analyses. Food Res Int. 2021;146:110463. doi:10.1016/j.foodres.2021.110463. [Google Scholar] [PubMed] [CrossRef]
21. Chen Q, Chen C, Li SH, Ning FJ, Xiong H, Zhao Q. Preparation, characterization, and in vitro antioxidant activities of natural selenium-enriched peanut protein fractions. Food Biosci. 2022;49:101923. doi:10.1016/j.fbio.2022.101923. [Google Scholar] [CrossRef]
22. Sun Y, Shao J, Liu H, Wang H, Wang G, Li J, et al. A chromosome-level genome assembly reveals that tandem-duplicated CYP706V oxidase genes control oridonin biosynthesis in the shoot apex of Isodon rubescens. Mol Plant. 2023;16:517–32. doi:10.1016/j.molp.2022.12.007. [Google Scholar] [PubMed] [CrossRef]
23. Chen X, Dai X, Liu Y, He X, Gong G. Isodon rubescens (Hemls.) Hara.: a comprehensive review on traditional uses, phytochemistry, and pharmacological activities. Front Pharmacol. 2022;13:766581. doi:10.3389/fphar.2022.766581. [Google Scholar] [PubMed] [CrossRef]
24. Du Y, Liu P, Yuan Z, Jin Y, Zhang X, Sheng X, et al. Simultaneous qualitative and quantitative analysis of 28 components in Isodon rubescens by HPLC-ESI-MS/MS. J Sep Sci. 2010;33:545–57. doi:10.1002/jssc.v33:4/5. [Google Scholar] [CrossRef]
25. Lian C, Zhang F, Yang H, Zhang X, Lan J, Zhang B, et al. Multi-omics analysis of small RNA, transcriptome, and degradome to identify putative miRNAs linked to MeJA regulated and oridonin biosynthesis in Isodon rubescens. Int J Biol Macromol. 2024;258:129123. doi:10.1016/j.ijbiomac.2023.129123. [Google Scholar] [PubMed] [CrossRef]
26. Tian C, Li XY, Huang Y, Xu JJ, Liu Z, Xiang ZM, et al. Functional characterization of the Pinellia ternata cytoplasmic class II small heat shock protein gene PtsHSP17.2 via promoter analysis and overexpression in tobacco. Plant Physiol Bioch. 2022;177:1–9. doi:10.1016/j.plaphy.2022.02.017. [Google Scholar] [PubMed] [CrossRef]
27. Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;2:248–54. [Google Scholar]
28. Paula LC, Lemes AC, Valencia-Mejía E, Moreira BR, Oliveira TS, Campos ITN, et al. Effect of extrusion and autoclaving on the biological potential of proteins and naturally-occurring peptides from common beans: antioxidant and vasorelaxant properties. Food Chem X. 2022;13:100259. doi:10.1016/j.fochx.2022.100259. [Google Scholar] [PubMed] [CrossRef]
29. Duan YB, Santiago FEM, dos Reis AR, de Figueiredo MA, Zhou SP, Thannhauser TW, et al. Genotypic variation of flavonols and antioxidant capacity in broccoli. Food Chem. 2021;338:127997. doi:10.1016/j.foodchem.2020.127997. [Google Scholar] [PubMed] [CrossRef]
30. Ke Y, Yuan DD, Xu YG, Liu HM. Determination of total diterpenoids in Rabdosia rubescens by ultra violet spectrophotometry. Lishizhen Med Mat Med Res. 2010;21(4):828–9. [Google Scholar]
31. Zhao FL, Sun MC, Zhang WJ, Jiang CL, Teng JT, Sheng W, et al. Comparative transcriptome analysis of roots, stems and leaves of Isodon amethystoides reveals candidate genes involved in Wangzaozins biosynthesis. BMC Plant Biol. 2018;18:272. doi:10.1186/s12870-018-1505-0. [Google Scholar] [PubMed] [CrossRef]
32. Zhu Y, Su X, Dong C, Chen S, Shao Y, Zhang F. Cloning and expression analysis of 1-deoxy-D-xylulose 5-phosphate synthase gene in Isodon rubescens. Guihaia. 2016;36(12):1476–82. [Google Scholar]
33. Jin BL, Cui GH, Guo J, Tang JF, Duan LX, Lin HX, et al. Functional diversification of kaurene synthase-like genes in Isodon rubescens. Plant Physiol. 2017;174:943–55. doi:10.1104/pp.17.00202. [Google Scholar] [PubMed] [CrossRef]
34. Duborská E, Šebesta M, Matulová M, Zverina O, Urík M. Current strategies for selenium and iodine biofortification in crop plants. Nutrients. 2022;14(22):4717. doi:10.3390/nu14224717. [Google Scholar] [PubMed] [CrossRef]
35. Cheng S, He F, Fu L, Zhang Y. Preparation of selenium polysaccharide from Rabdosia rubescens and analysis of its antioxidant activity. Fine Chem. 2021;38(10):2064–71. [Google Scholar]
36. Dey S, Raychaudhuri SS. Selenium biofortification improves bioactive composition and antioxidant status in Plantago ovata Forsk., a medicinal plant. Genes Environ. 2023;45:38. doi:10.1186/s41021-023-00293-2. [Google Scholar] [PubMed] [CrossRef]
37. Yuan L, Zhu Y, Lin ZQ, Banuelos G, Li W, Yin X. A novel selenocystine-accumulating plant in selenium-mine drainage area in Enshi, China. PLoS One. 2013;8(6):e65615. doi:10.1371/journal.pone.0065615. [Google Scholar] [PubMed] [CrossRef]
38. Viltres-Portales M, Sánchez-Martín MJ, Llugany M, Boada R, Valiente M. Selenium biofortification of microgreens: influence on phytochemicals, pigments and nutrients. Plant Physiol Biochem. 2024;206:108283. doi:10.1016/j.plaphy.2023.108283. [Google Scholar] [PubMed] [CrossRef]
39. Hussain S, Ahmed S, Akram W, Li G, Yasin NA. Selenium seed priming enhanced the growth of salt stressed Brassica rapa L. through improving plant nutrition and the antioxidant system. Front Plant Sci. 2023;13:1050359. doi:10.3389/fpls.2022.1050359. [Google Scholar] [PubMed] [CrossRef]
40. Zhang JR, Lu SL, Luo RF, Ma XN, Wang GD. Separation, purification and antioxidant activity of selenium polysaccharide from selenium-enriched grapes. Sci Technol Food Ind. 2023;44(8):116–24. [Google Scholar]
41. Wang H, Cong X, Qin K, Yan MK, Xu XF, Liu MK, et al. Se-enriched Cardamine violifolia improves laying performance and regulates ovarian antioxidative function in aging laying hens. Antioxidants. 2023;12(2):450. doi:10.3390/antiox12020450. [Google Scholar] [PubMed] [CrossRef]
42. Santiago FEM, Silva MLDS, Cardoso AADS, Duan YB, Guilherme LRG, Liu JP, et al. Biochemical basis of differential selenium tolerance in arugula (Eruca sativa Mill.) and lettuce (Lactuca sativa L.). Plant Physiol Biochem. 2020;157:328–38. doi:10.1016/j.plaphy.2020.11.001. [Google Scholar] [PubMed] [CrossRef]
43. Mezeyová I, Hegedusova A, Andrejiová A, Hegedus O, Golian M. Phytomass and content of essential oils in Ocimum basilicum after foliar treatment with selenium. J Int Sci Publ. 2016;4:19–27. [Google Scholar]
44. Çakır Ö, Turgut-Kara N, Arı Ş, Zhang B. De novo transcriptome assembly and comparative analysis elucidate complicated mechanism regulating Astragalus chrysochlorus response to selenium stimuli. PLoS One. 2015;10(10):e0135677. doi:10.1371/journal.pone.0135677. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools