 Open Access
Open Access
ARTICLE
Evaluation of Resistance of Different Kiwifruit Varieties (Lines) to Canker Disease and Brown Spot Disease
1 Institute of Mountain Resources of Guizhou Province, Guizhou Academy of Sciences, Guiyang, 550001, China
2 Guizhou Botanical Garden, Guizhou Academy of Sciences, Guiyang, 550001, China
3 Key Laboratory of Plant Resource Conservation and Germplasm Innovation in Mountainous Region (Ministry of Education), Institute of Agro-Bioengineering/College of Life Sciences, Guizhou University, Guiyang, 550025, China
* Corresponding Author: Weijie Li. Email:
Phyton-International Journal of Experimental Botany 2024, 93(6), 1249-1261. https://doi.org/10.32604/phyton.2024.051935
Received 19 March 2024; Accepted 10 May 2024; Issue published 27 June 2024
Abstract
Kiwifruit canker and brown spot are significant diseases affecting kiwis, caused by Pseudomonas syringae pathogenic variations (Pseudomonas syringae pv. Actinidiae (Psa)) and Corynesporapolytica (Corynespora cassiicola). At present, the research on canker disease and brown spot disease mainly focuses on the isolation and identification of pathogenic bacteria, drug control, resistance gene mining and functional verification. Practice has proved that breeding disease resistant varieties are an effective method to control canker disease and brown spot disease. However, most existing cultivars lack genes for canker and brown spot resistance. Wild kiwifruit resources in nature exhibit extensive genetic diversity due to prolonged natural selection, containing numerous resistance genes. But, due to insufficient understanding of the resistance of most kiwifruit varieties (lines) to canker disease and brown spot disease, some high-quality resources have not been fully utilized. The incidence of canker and brown spot of 18 kiwifruit cultivars (lines) was measured by inoculating isolated branches and leaves, and their resistance to canker and brown spot was analyzed according to the length, disease index, mean diameter, and systematic clustering. The results were as follows: Among 18 different kiwifruit varieties (lines) for canker disease, there were two highly resistant materials, eight disease-resistant materials, four disease-susceptible materials, and two highly susceptible materials. Moreover, regarding brown spot disease, there were one highly resistant material, five disease-resistant materials, four susceptible materials, and three highly susceptible materials. Furthermore, four resources were resistant to both diseases. The outcomes provided a theoretical basis for breeding kiwifruit against canker and brown spot.Keywords
The kiwifruit is a deciduous fruit tree belonging to the Actinidia genus of the Actinidiaceae family. Consumers prefer it due to its distinctive flavor, abundant nutrients, and high ascorbic acid levels [1,2]. Kiwifruit has become the primary source of income for numerous farmers in major kiwi-producing countries such as China, New Zealand, and Italy [3]. China has been the leading country in kiwifruit cultivation and production since 2009, holding the top position for 10 years in a row. In 2019, China’s kiwifruit harvest area and annual output represented 67.9% and 50.5% of the global total, respectively, as reported by the Food and Agriculture Organization of the United Nations (FAO) [4].
However, as the kiwifruit business continues to grow, the problem of kiwifruit disease becomes increasingly significant. Pseudomonas syringae pv. actinidiae (Psa) causes cankerous disease, whereas Corynespora cassiicola causes brown patch, both devastating diseases affecting kiwifruit [5,6]. Canker is a type of bacterial disease that affects kiwifruit; and spread fast, and it may cause the branches to ulcerate and produce bacterial pus. Concurrently, the leaves develop necrotic spots, buds are damaged and fail to open, cankers are harmful, and prevention and control are challenging, making it a devastating disease in the global kiwifruit cultivation area, severely hindering the kiwifruit industry’s growth [7,8]. As a fungal airborne disease, brown spot disease has become one of the three major diseases endangering kiwifruit in Guizhou Province, second only to canker disease and soft rot [9,10]. Brown spot disease mainly leads to early deciduous fall of kiwifruit, slight germination in autumn, consumption of tree nutrients, affecting the flowering fruit in the next year, and seriously affecting the yield and quality of kiwifruit [11,12].
At present, chemical agents are mainly used in production for the prevention and control of brown spot and canker disease. Research has shown that the use of large amounts of chemical agents can harm human health, pose a threat to the environment, and develop drug resistance [13]. Practice has proved that breeding kiwifruit varieties with good resistance and fruit quality is one of the most effective ways to control canker and brown spot [14,15]. However, the varieties of kiwi with good commercial property cultivated at present are easily infected by Psa and Corynespora cassiicola, and it is difficult to prevent and control the infection [16]. Therefore, it is difficult to breed kiwifruit varieties with good resistance through existing varieties [17–19]. There are abundant wild kiwifruit resources in Guizhou, but different kiwifruit resources have significant differences in terms of disease resistance. Due to long-term growth in natural conditions, wild kiwifruit has many genes with strong adaptability and good resistance after long-term natural selection and rich genetic variation. Therefore, excellent resources with good resistance and good fruit quality should be identified. They can be cultivated, crossed, or backcrossed with existing varieties to improve the resistance of existing varieties and achieve the purpose of breeding resistant varieties [20–22].
In this study, 18 different kiwi varieties (lines) were studied for five years to assess their resistance to canker disease and brown spots. The goal was to identify varieties with better resistance to these diseases, and establish a foundation for breeding kiwi varieties that are resistant to canker disease and brown spots.
Test strain: The pathogenic variant of Psa from kiwifruit canker was stored in 25% glycerol at –20°C in the refrigerator for future use, provided by Zhao Zhibo Laboratory of Guizhou University [23]. Corynespora cassiicola, the pathogen of kiwifruit brown spot, was isolated from the leaves of kiwifruit production, education, and research base in Miluo Town, Shuicheng District. After detection using PCR-specific primers and molecular identification, it was stored in the refrigerator with a PDA slope at –20°C for further use [24].
Kiwi varieties (lines) for test: These 18 kiwi fruit resources were collected from different counties in Guizhou Province, including Hongyang, Guichang, Xuxiang, CH-1, GH-1, GH-2, HS-8, HS-10, HS-13, HS-2, QJ-1, JK-5, XY-6, DD-8, GD-1, LL-4, HS-12, and DS-4. The above resources were stored in the millet town of ShuiCheng District, which serves as a kiwi production and research base.
2.2.1 Strain Activation and Culture
Activation of canker strains: The Psa was inoculated on LB medium using the streak-plate procedure and cultured at 25°C for 48 h. Single colonies were selected for detection and verification with PSA-specific primers. The positive single colonies were selected and cultured in liquid LB medium and used after the concentration of bacterial solution reached OD600 nm = 0.1.
Brown spot strain activation: The fungal mycelia stored on the inclined surface were selected and cultured on PDA medium at 28°C for seven days before use.
2.2.2 Identification of Resistance of Isolated Branches Inoculated with Canker Strains
The collected healthy kiwifruit branches (annual) with consistent growth were cut into 15 cm stem segments. The surface was disinfected with 75% alcohol, then washed with sterile water three times, and placed on a super-clean work table to dry naturally. Subsequently, the branches were sealed with paraffin wax. Five branches per resource were inoculated, each branch at two inoculation points. Moreover, a sterilized surgical blade was used to scratch the bark on the branch to the xylem (depth 2–3 mm). The injured site was inoculated with 10 µL spore suspension using a pipette and sterile water as the control. After the bacterial solution penetrated the branches, it was placed in an artificial climate box with a photoperiod of 16 h/8 h, diurnal temperature of 10°C/4°C, and relative humidity of 95%. After 25 days, the length of the branches lesions was measured, and the disease index was calculated to evaluate the resistance of different kiwifruit resources to canker disease, the experiment was repeated three times.
2.2.3 Identification of Resistance of Isolated Leaves Inoculated with Brown Spot Strain
Kiwifruit leaves with healthy growth and uniform size were collected in the field. Subsequently, the surface was disinfected with 75% alcohol for 30 s, washed repeatedly with sterile water three times, and then placed on a super-clean work table to dry naturally. Then, the petiole was moistened with sterilized moistening cotton and put on a porcelain plate. Cultured pathogens were taken along the edge of the colony with bacteria cakes with a diameter of 5 mm, and 2–3 bacteria cakes were inoculated on one side of each leaf. In contrast, PDA was inoculated on the other side as a control, and three leaves were inoculated on each resource. After inoculation, the porcelain plate with treated leaves was cultured in an artificial climate chamber with a photocycle of 16 h/12 h, temperature of 27°C, and relative humidity of 80%. After inoculation for seven days, the diameter of the spot was measured using the crisscrossing method to evaluate the resistance of different kiwifruit resources to brown spots, the experiment was repeated three times.
2.2.4 Resistance Evaluation Method
The classification standard of kiwifruit ulcer disease was divided according to Song’s method (Table 1) [14], evaluation of different kiwifruit resources for resistance to ulcer disease by calculating disease indexes based on the disease class. Brown spot grading criteria refer to the method of Huang et al. [25] for classification (Table 2), measurement of lesion diameters after inoculation and evaluation of the level of resistance of different kiwifruit resources to brown spot on the basis of lesion diameters. The specific resistance grading criteria are listed in Table 2. Meanwhile, cluster analysis was conducted with reference to the research methods of resistance evaluation and cluster analysis of kiwifruit germplasm by Li et al. [26], Pei et al. [27], and Huang et al. [25].
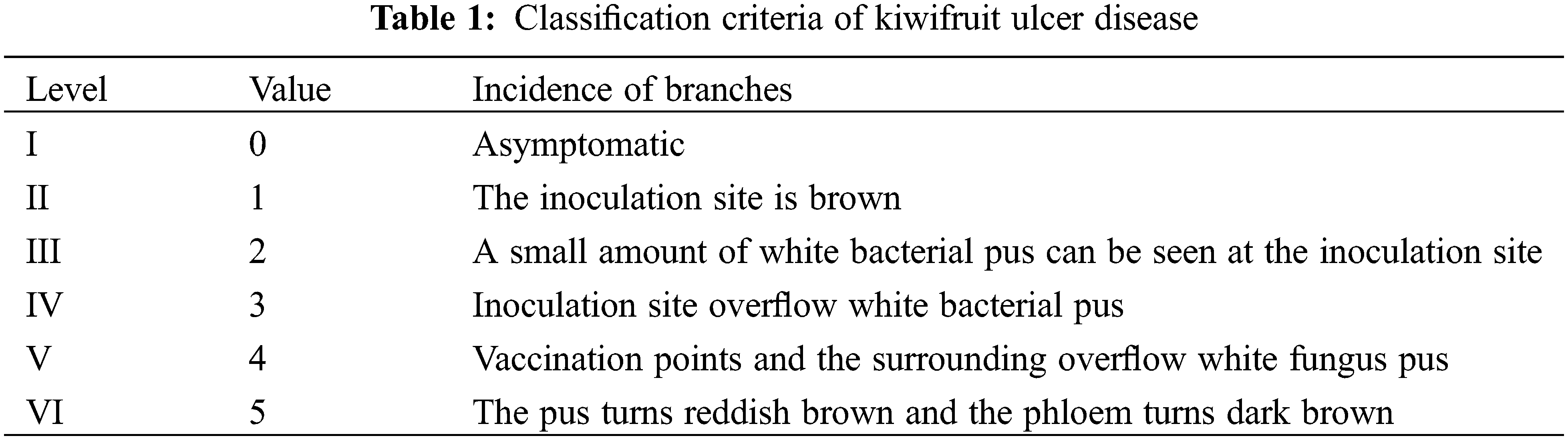
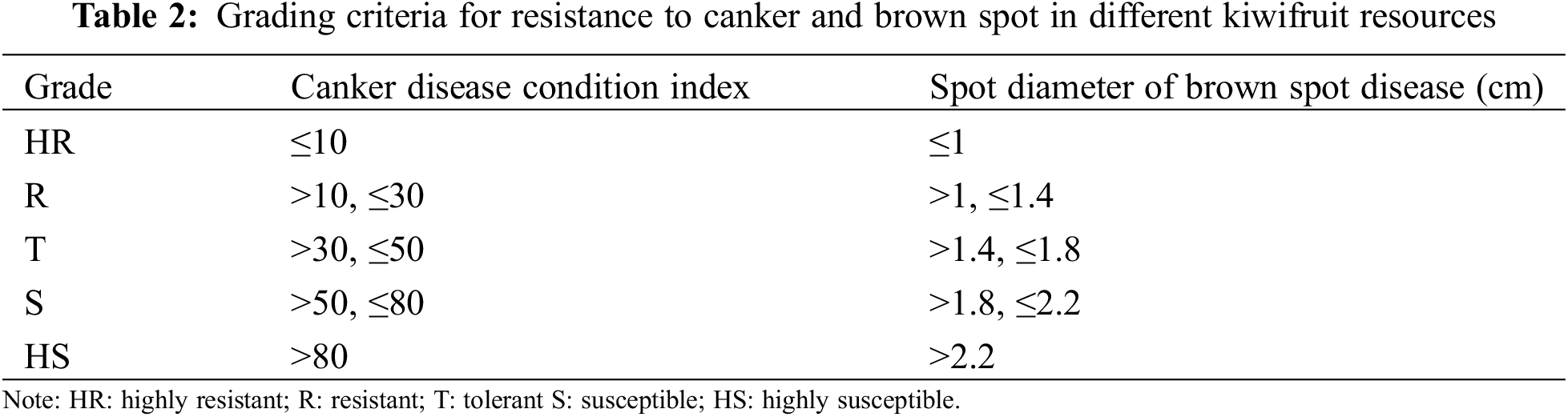
The disease index is calculated as follows:
Disease index = 100 × ∑ (number of vaccinations in disease grade × number of representative grades)/(total number of vaccinations in the test × number of the highest representative grade of vaccination incidence).
Using SPSS21.0 for data processing and cluster analysis, all data were analyzed by one-way ANOVA.
3.1 Evaluation of Resistance of Different Kiwi Varieties (Lines) to Canker Disease
3.1.1 Symptoms of Isolated Branches Inoculated with Psa in Different Varieties (Lines)
After the isolated branches of kiwi were inoculated with Psa, the symptoms were observed every three days. On the seventh day after inoculation, some varieties (lines) began to develop the disease, the inoculation spots turned red-brown, and some branches had alpinia pus over flowing. With the increase of culture time, the length of disease spots gradually increased, and the symptoms appeared after 25 days of inoculation (Fig. 1).
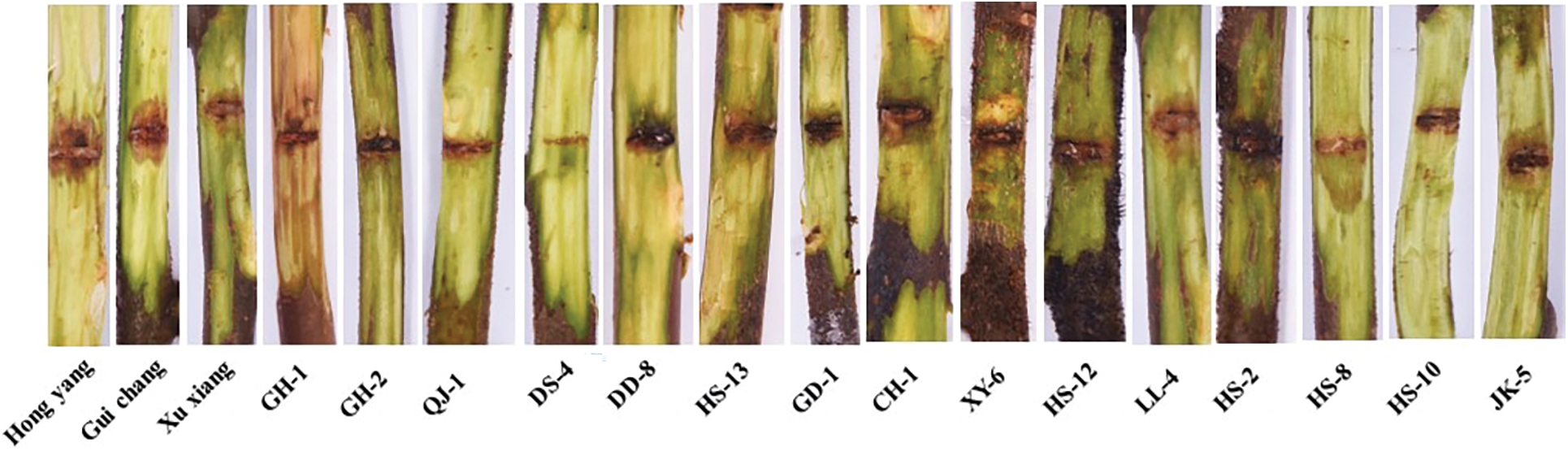
Figure 1: Symptoms of Psa inoculated branches of different varieties (lines)
3.1.2 Classification of Resistance to Canker in Different Kiwifruit Varieties (Lines)
According to the incidence of isolated branches of kiwifruit, the disease index of different varieties (lines) was calculated 25 days after inoculation with pathogenic varieties of Pseudomonas syringae. As per the classification standard of kiwifruit resistance to canker (Table 3), among the 18 varieties (lines) tested, Hongyang and GH-1 showed high susceptibility, CH-1, GH-2, HS-8 and HS-10 showed susceptibility, HS-13 and HS-2 showed resistance; QJ-1, JK-5, Guichang, XY-6, DD-8, Xuxiang, GD-1, LL-4 showed resistance to canker; and HS-12 and DS-4 showed high resistance to canker.
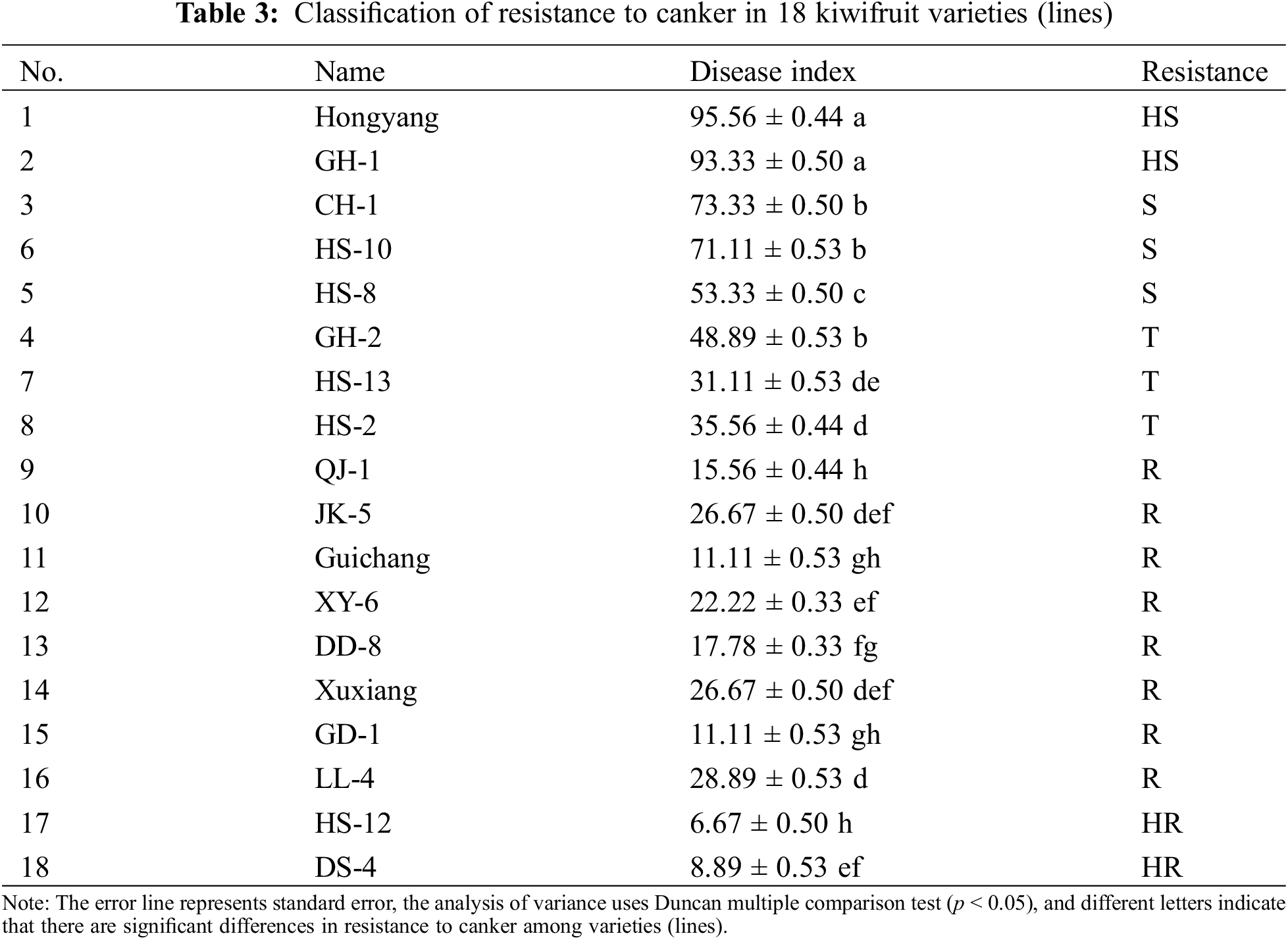
3.2 Classification of Resistance to Canker in Different Kiwifruit Varieties (Lines)
3.2.1 Symptoms of Isolated Leaves Inoculated with Corynespora cassiicola of Different Varieties (Lines)
After inoculation with Corynespora cassiicola, kiwi leaves were subjected to daily moisturizing observation. On the third day after inoculation, some varieties (lines) began to develop the disease, and brown lesions appeared on the leaves at the site of the disease. With the increase in culture time, the diameter of the lesions gradually increased. Fig. 2 demonstrates the symptoms seven days after inoculation.
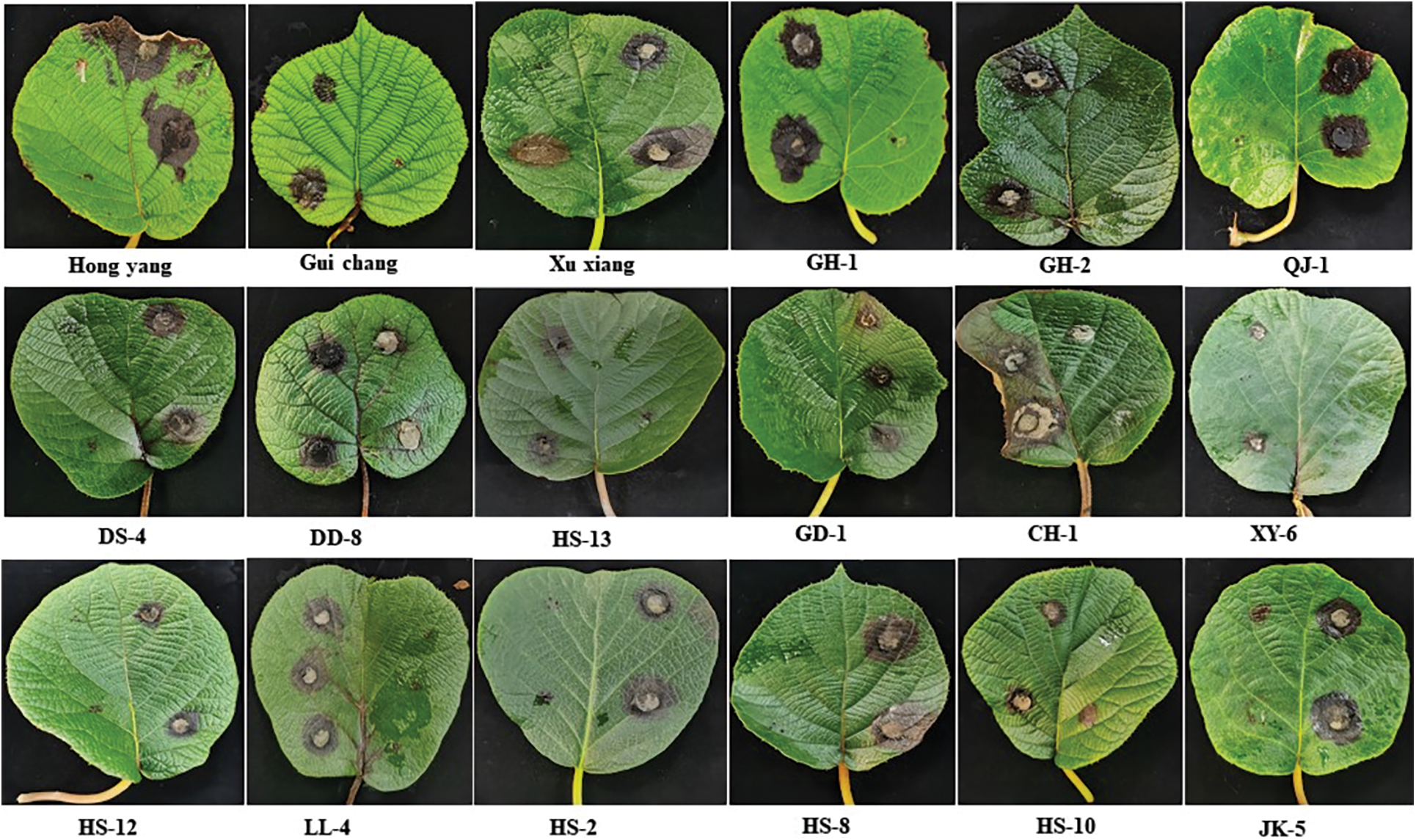
Figure 2: Symptoms of Corynespora cassiicola inoculated with isolated leaves of different varieties (lines)
Note: The infected side of the same leaf is the result of inoculation with Corynespora cassiicola, and the other side is the result of inoculation with PDA.
3.2.2 The Resistance Level of Different Kiwi Varieties (Lines) to Brown Spot Disease
The resistance of different kiwi varieties (lines) was graded according to the measurement of the disease spot diameter of kiwi fruit inoculated with Corynespora cassiicolafor sevendays. In Fig. 3, Hongyang, CH-1, and JK-5 showed high susceptibility; GH-1, HS-8, QJ-1, and DS-4 showed susceptibility; and CH-2, HS-2, Guichang, Xuxiang, and LL-4 showed resistance. HS-13, HS-12, DD-8, XY-6, and GD-1 exhibited disease resistance, while HS-10 exhibited high resistance.
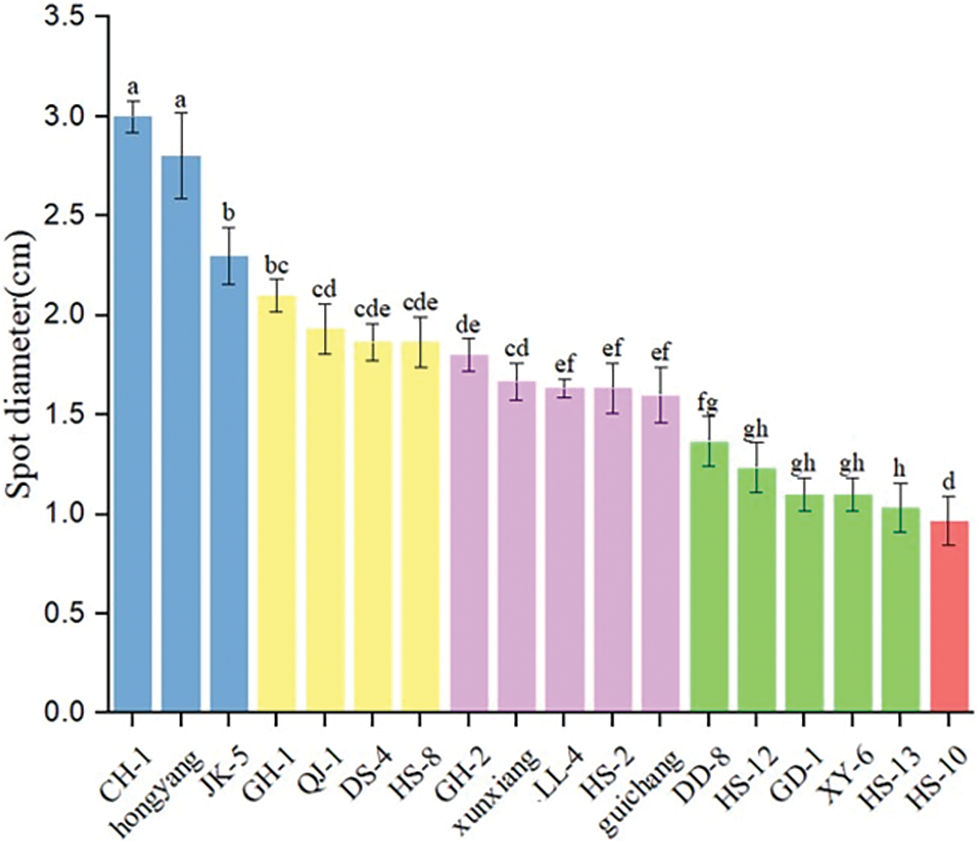
Figure 3: Diameter of diseased spots in 18 kiwi cultivars (lines) inoculated with Corynespora cassiicola
Note: The error line represents standard error, the analysis of variance uses Duncan multiple comparison (p < 0.05), and different letters indicate that there are significant differences in resistance to brown spot disease among varieties (lines).
3.3 Comprehensive Resistance of 18 Kiwi Cultivars (Lines) to Canker Disease and Brown Spot Disease
The resistance of 18 varieties to the two diseases was comprehensively evaluated by measuring the length of the disease spot of the branches infected by the canker strain. The diameter of the disease spot of the leaf infected by the brown spot strain (Figs. 4 and 5). It was clear that Hongyang was highly susceptible to the two diseases. GH-1, CH-1, and HS-8 exhibited susceptibility to two diseases; HS-2 and GH-2 demonstrated resistance to two diseases; XY-6, DD-8, GD-1, and HS-12 showed resistance to two diseases. Moreover, the resistance of the same variety (line) to different diseases varies significantly.
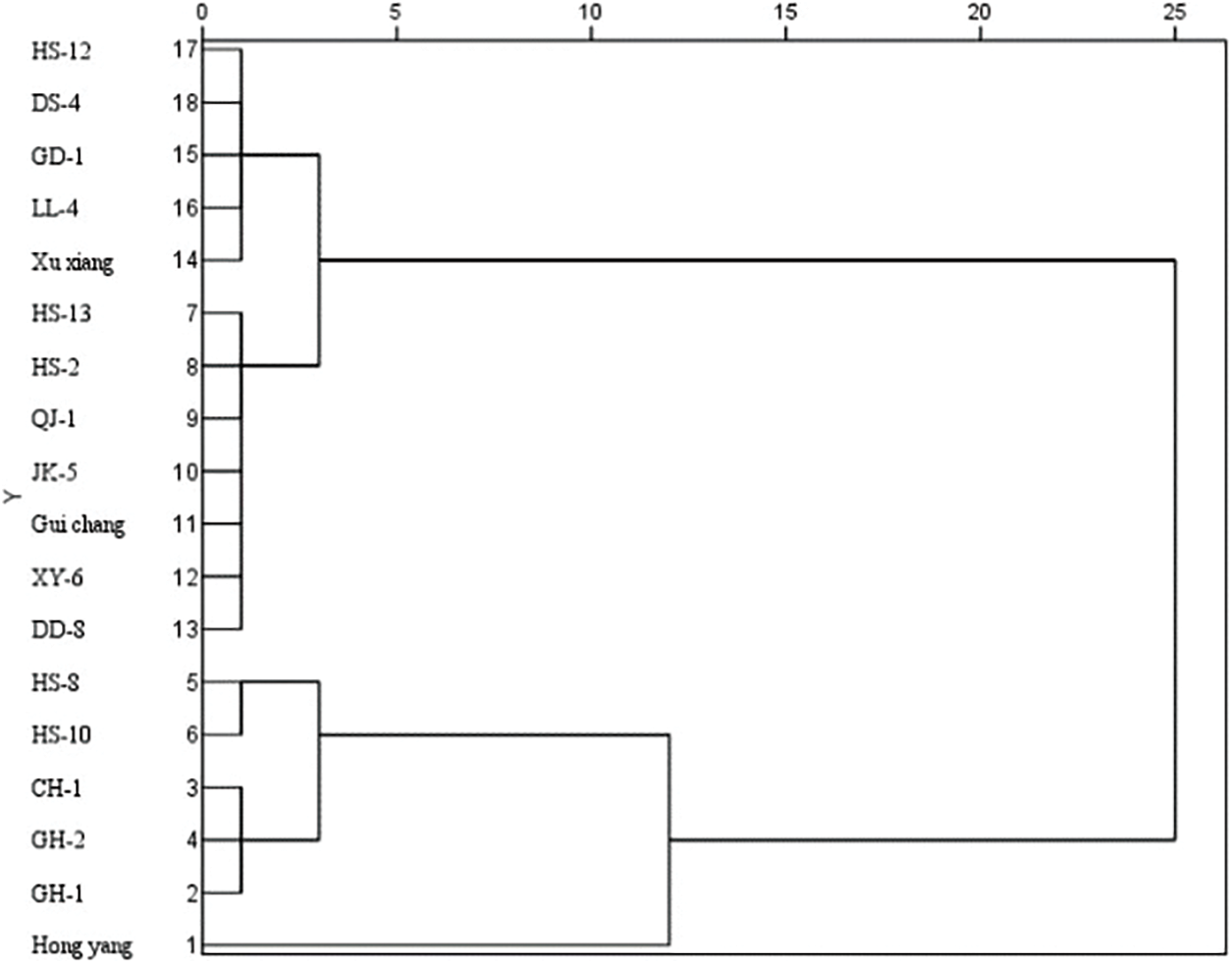
Figure 4: Cluster analysis of resistance to canker in 18 kiwifruit varieties (lines)
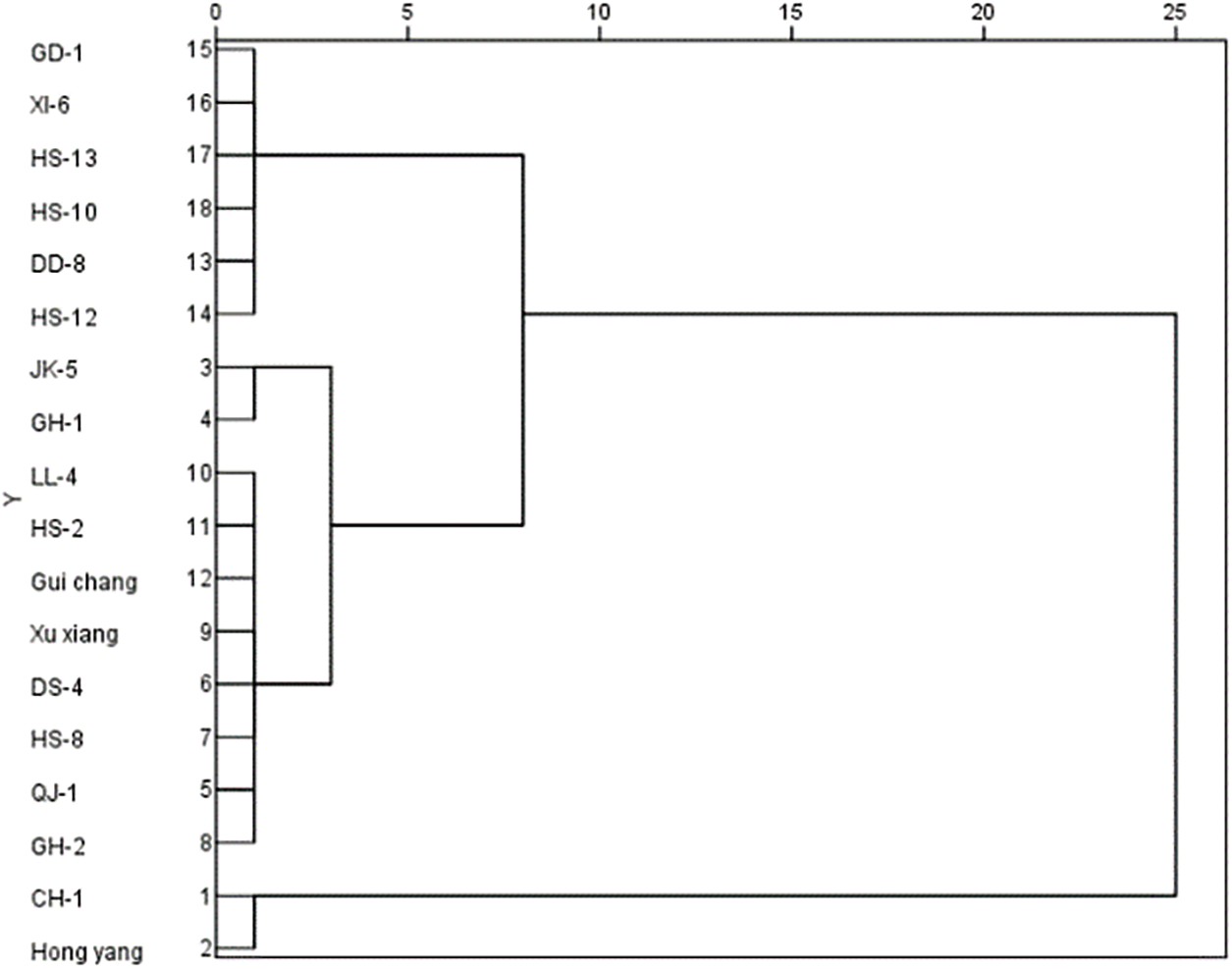
Figure 5: Cluster analysis of resistance to brown spot of 18 kiwi varieties (lines)
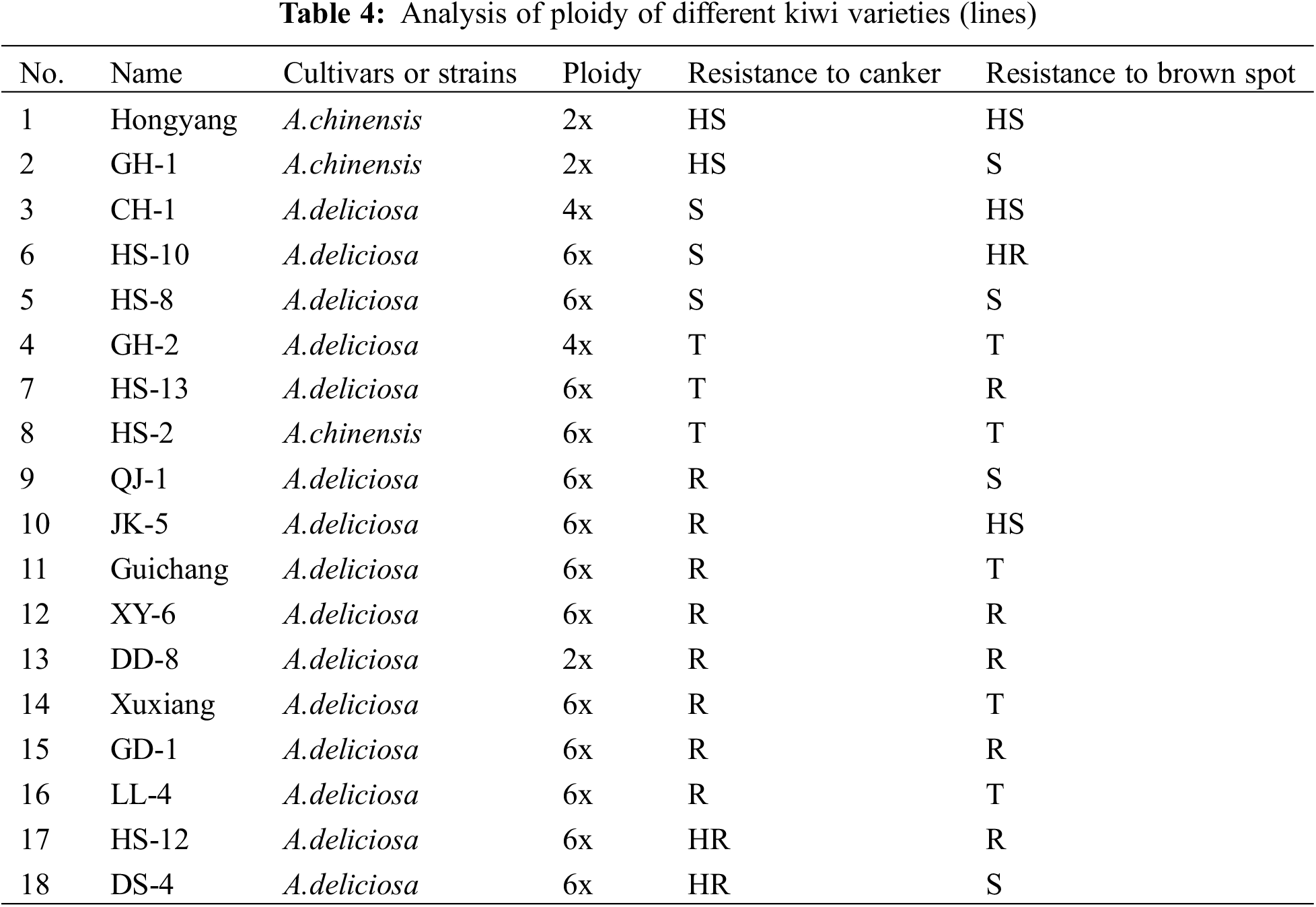
3.4 Analysis of Ploidy and Disease Resistance of Different Kiwi Varieties (Lines)
Hongyang and XY-20 were used as the 2X and 6X standard samples, respectively, for the ploidy detection of the collected samples. The prominent peaks appearing at the horizontal axis 100 and 300 represented 2X and 6X, respectively (Figs. 6A and 6B). The outcomes of the ploidy detection of different kiwi fruits revealed the following. In the 18 kiwifruit tested, 2X accounted for 16.67%, 4X represented 11.11%, 6X constituted 72.22%, most of them were A.deliciosa (Table 4). In A.chinensis, the resistance to canker and brown spot was HS-2> GH-1>Hongyang. The higher the ploidy, the stronger the resistance. Furthermore, no significant correlation exists between the resistance and ploidy of different kiwifruit varieties (lines) to canker and brown spot.
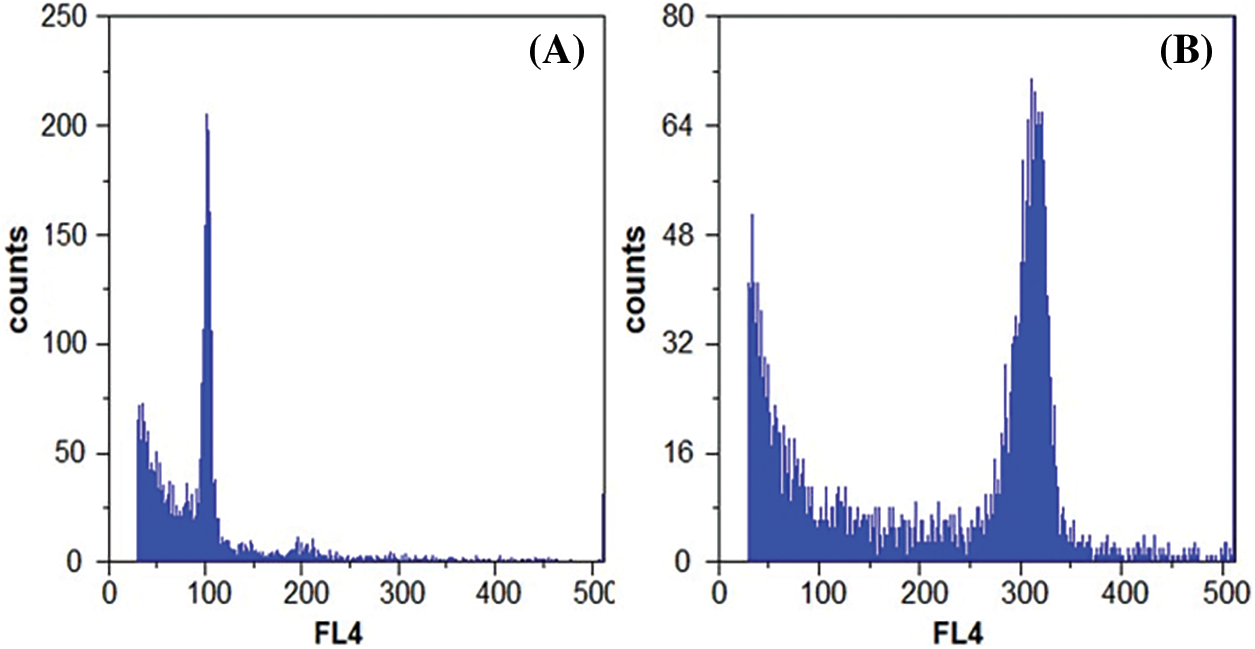
Figure 6: Flow cytometry of control materials
Kiwifruit canker disease and brown spot disease spread rapidly in the field, impact inga wide range and causing significant pathogenicity. The pathogenic bacteria are easy to mutate and develop drug resistance, seriously endangering the production of the macaque peach orchard and resulting in a sharp decline in the yield and fruit quality of the macaque peach orchard [28,29]. Therefore, effective control of kiwifruit canker and brown spot disease has been a particular concern of scholars at home and abroad. Researchers primarily focused on isolating and identifying pathogenic bacteria of kiwifruit canker and brown spot [30–32], exploring the regularity of disease occurrence [33]. And indoor and field drug prevention and control [34,35] to find effective methods in order to prevent and control kiwifruit canker and brown spot. However, recently, more efforts have been made to identify variety resistance, screen resistant germplasm, and study the mechanism of resistance [36–38], with the aim of preventing and controlling diseases by breeding new resistant varieties. However, most of the research focuses on the existing commercial planting varieties, which is difficult to break through.
Field identification is the most intuitive method for disease resistance identification of fruit trees. However, it is susceptible to many factors, such as climate, geographical environment, and management measures. Identification requires a long observation period, and there is a risk of pathogen transmission in field identification, causing huge losses to the industry. At present, a large number of studies have confirmed that the incidence of diseases through laboratory inoculation in vitro is significantly positively correlated with the investigation results of field inoculation. Therefore, in vitro inoculation is widely used in the identification of disease resistance of fruit trees [39,40]. However, indoor identification differs significantly from field identification, making it suitable only as a supplementary method for identifying resistance in numerous materials.
Various types of kiwi fruit exhibit notable variations in disease resistance. Shi et al. [41] graded the resistance to ulcer disease of 24 kiwifruit varieties based on the length of diseased spots in isolated branches, and proved that the resistance of Xuxiang to ulcer disease was obviously better than that of Hongyang. Huang et al. [25] evaluated the resistance of 42 kiwi fruits to brown spot and determined that Xuxiang resistance to brown spot was better than that of Hongyang. At the same time, the study showed that both cake inoculation and spore suspension inoculation could be used for rapid identification of disease resistance in laboratory. However, in indoor culture, Corynespora cassiicola is not easy to produce spores, and the inoculation effect of bacteriosis is better. In this study, we evaluated 18 different kiwifruit cultivars (lines) for resistance to canker and brown spot. The results showed that Corynespora cassiicola was difficult to produce spores in the room. And Hongyang kiwifruit showed high sensitivity to canker and brown spot, the resistance of Xuxiang was better than that of Hongyang, these are consistent with the results of previous studies. By analyzing the relationship between different ploidy kiwifruit resources and ulcer resistance. Song et al. [14] and Li et al. [26] clarified that within the same species, the higher the ploidy, the greater the resistance. However, for delicious kiwis, DD-8 and LL-4 resources were diploid. And their resistance to canker and brown spots was higher than that of hexaploid HS-8, which was not consistent with the previous results. The relationship between ploidy and resistance needs further study.
In the process of plant response to pathogen infection. Firstly, cell signaling related substances, such as salicylic acid and ethylene, first change. Followed by changes in nitrogen oxides, methyl jasmonic acid, methyl salicylic acid and other systemically acquired resistance related substances. Finally changes in primary metabolism and secondary metabolites, such as carbohydrates, organic acids, amino acids and lipids. And then resist the invasion of pathogenic bacteria [42]. The response ability of different kiwi varieties to pathogen infection was also different. Huo et al. studied the antibacterial activity and induction activity of pHBA on Aspergillus flavus of kiwi fruit. The application of pHBA promoted the accumulation of endogenous pHBA and induced oxidative stress in A. flavus infected kiwifruit, resulting in an increase in H2O2 content and catalase (CAT) and superoxide dismutase (SOD) activities [43]. Qin evaluated the resistance of different kiwifruit resources to canker disease, and conducted transcriptome and proteome analysis after psa inoculation of resistant and susceptible germplasm. The results showed that the differential metabolites increased gradually after Psa inoculation in resistant and susceptible germplasm. However, the metabolites of susceptible germplasm were more than those of resistant germplasm [44]. A large number of studies have shown that the mechanism of kiwifruit response to pathogen infection is very complex, which can not only induce the change of enzyme activity. But also stimulate the plant to produce a large number of metabolites to resist pathogen infection. This study evaluated the resistance of canker disease and brown spot disease from different resources, multi-omics analysis can be combined through resources for resistance. Exploring the relationship between metabolites and resistance of different varieties to canker and brown spot, and then provide the corresponding experimental materials for the research of resistance breeding by molecular biological means in the later stage.
In this experiment, the resistance of different kiwifruit varieties (lines) to canker disease and brown spot disease was evaluated comprehensively using the in vitro inoculation method. Moreover, the difference in resistance of different varieties (lines) to canker disease and brown spot disease was identified. Ten anti-canker materials were screened: HS-12 and DS-4 were highly resistant materials; HS-10 was a highly resistant material; XY-6, DD-8, GD-1, and HS-12 were resistant to canker and brown spots. At the same time, the results laid a foundation for breeding resistant varieties from molecular perspective. In this study, in vitro, inoculation was carried out indoors. Although there was a certain positive correlation between indoor inoculation and field inoculation results, field experiments were easily affected by environment, climate, and management measures. Therefore, it is necessary to conduct field identification of the selected high-quality resources in the future, to provide theoretical guidance for breeding.
Acknowledgement: We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Funding Statement:: This work was jointly supported by the following grants: Science and Technology Support Plan of Guizhou Province: Breeding Research and Demonstration of all-Red Bud Transformation of “GH-1” Clone of “Hong yang” Kiwifruit (Guizhou Family Combination Support [2021] General 234), the National Key Research and Development Program “Quality and Efficiency Improvement Technology Integration and Demonstration of Advantageous Characteristic Industries in Guizhou Karst Mountain Area (2021YFD1100300)” Post-Subsidy Fund, Task 3 of National Key Research and Development Program, Green Prevention and Control Technology Integration and Demonstration of Main Diseases and Insect Pests of Kiwifruit in Shuicheng City, China (2022YFD1601710-3). All the authors reviewed the findings and approved the final version of the manuscript.
Author Contributions: Wenwen Su and Chunguang Ren conceived and designed the experiment. Zhencheng Han and Di Wu collected the test data; Wenwen Su, Yi Yang and Tao Li analyzed and interpreted the test data. Wenwen Su and Chongpei Zheng wrote the manuscript. Weijie Li guided the article writing.
Availability of Data and Materials: The data that support the findings of this study are available on request from the corresponding author, Weijie Li, upon reasonable request.
Ethics Approval: This article has not been conducted on humans or animals, so it does not involve any ethical issues.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Huang HW. Actinidia germplasm resources in China. Beijing, China: China Forestry Publishing House; 2013. p. 25–6 (In Chinese). [Google Scholar]
2. Ran XY, Huang WJ, Zhong CH. Advance in starch metabolism research of kiwifruit. J Fruit Sci. 2024;41(2):325–37 (In Chinese). [Google Scholar]
3. Wang KL, Li M, Wang YX, Liu ZH, Ni YY. Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021;110:106162–72. doi:10.1016/j.foodhyd.2020.106162. [Google Scholar] [CrossRef]
4. Zhong CH, Huang WJ, Li DW, Zhang Q, Li L. World kiwifruit industry development and fresh fruit trade dynamic analysis. China Fruits. 2021;7:101–8 (In Chinese). [Google Scholar]
5. Ma JB, Yang MM, Zhao YJ, Tan YX, Chang BS, Wang NN, et al. Identification and characterization of PilZ domain containing proteins in pathogenicity and motility of Pseudomonas syringae pv. Actinidiae. Acta Phytopathologica Sin. 2023;10:1–16 (In Chinese). [Google Scholar]
6. Pan LS, Ren CG, Su WW, Han ZC, Li WJ. The effect of different disease resistant inducers on the field control of kiwi brown spot disease and their effects on fruit quality. Non-wood For Res. 2023;41(1):154–64. [Google Scholar]
7. Gyoung HK, Jae SJ, Young JK. Occurrence and epidemics of bacterial canker of kiwifruit in Korea. Plant Pathol J. 2017;33(4):351–61. doi:10.5423/PPJ.RW.01.2017.0021. [Google Scholar] [PubMed] [CrossRef]
8. Carla P, Pedro C, Larindja P, Victor M, Adelaide A. Kiwifruit bacterial canker: an integrative view focused on biocontrol strategies. Planta. 2021;253(2):49. doi:10.1007/s00425-020-03549-1. [Google Scholar] [PubMed] [CrossRef]
9. Yuan GQ, Xie YL, Tan DC, Li QQ, Lin W. First report of leaf spot caused by Corynespora cassiicola on kiwifruit (Actinidia chinensis) in China. Plant Dis. 2014;98(11):1586. doi:10.1094/PDIS-06-14-0604-PDN. [Google Scholar] [PubMed] [CrossRef]
10. Cui YL, Gong GG, Yu XM, Xu J, Wen XW, Zhang M, et al. First report of brown leaf spot on kiwifruit caused by Corynespora cassiicola in Sichuan. China Plant Dis. 2015;99:725. doi:10.1094/PDIS-08-14-0808-PDN. [Google Scholar] [CrossRef]
11. Zhu Y, Yao K, Ma M, Cui Y, Xu J. Occurrence regionalization of kiwifruit brown spot in Sichuan. J Fungi. 2023;9(9):899. doi:10.3390/jof9090899. [Google Scholar] [PubMed] [CrossRef]
12. Gong GS, Li Q, Zhang M, Cui YL. Primary color map and control technology of kiwifruit pests and diseases. Beijing, China: Science Press; 2020. p. 21–9 (In Chinese). [Google Scholar]
13. Chu HJ, Zhang C, Wang MC, Gouda M, Wei XH, He Y, et al. Hyperspectral imaging with shallow convolutional neural networks (SCNN) predicts the early herbicide stress in wheat cultivars. J Hazard Mater. 2022;421:126706. doi:10.1016/j.jhazmat.2021.126706. [Google Scholar] [PubMed] [CrossRef]
14. Song YL, Lin MM, Zhong YP, Chen JY, Qi XJ, Sun LM, et al. Evaluation of resistance of kiwifruit varieties (strains) against bacterial canker disease and correlation analysis among evaluation indexes. J Fruit Sci. 2020;37:900–8 (In Chinese). [Google Scholar]
15. Wu JH. Cultivar, environment and integration of horticultural practices will determine the future of the kiwifruit industry. In: Scripta Horticulturae #20: Global Kiwifruit Industrial Development Conference; 2019. p. 171–8. [Google Scholar]
16. Qin HY, Zhao Y, Chen XL, Zhang BX, Wen X, Li CY, et al. Pathogens identification and resistance evaluation on bacterial canker in Actinidia arguta germplasm. J Plant Pathol. 2023;105:973–85. doi:10.1007/s42161-023-01417-x. [Google Scholar] [CrossRef]
17. Li DW, Zhong CH, Liu YF, Huang HW. Correlation between ploidy level and fruit characters of the main kiwifruit cultivars in China: implication for selection and improvement. New Zeal J Crop Hort. 2010;38:137–45. doi:10.1080/01140671.2010.482966. [Google Scholar] [CrossRef]
18. Scortichini M, Marcelletti S, Ferrante P, Petriccione M, Firrao G. Pseudomonas syringae pv. actinidiae: a re-emerging, multi-faceted, pandemic pathogen. Mol Plant Pathol. 2012;13:631–40. doi:10.1111/mpp.2012.13.issue-7. [Google Scholar] [CrossRef]
19. Wang FM, Li JW, Ye KY, Gong HJ, Liu PP, Hu YK, et al. Preliminary report on the improved resistance towards Pseudomonas syringae pv. actinidiae of cultivated kiwifruit (Actinidia chinensis) when grafted onto wild Actinidia guilinensis rootstock in vitro. J Plant Pathol. 2021;103(1):51–4 (In Chinese). doi:10.1007/s42161-020-00719-8. [Google Scholar] [CrossRef]
20. Li Y, Wang L. Genetic resources, breeding programs in china, and gene mining of peach: a review. Hortic Plant J. 2020;6:205–15. doi:10.1016/j.hpj.2020.06.001. [Google Scholar] [CrossRef]
21. Wang FM, Mo QH, Ye KY, Gong HJ, Jiang QS, Liu PP, et al. An in vitro Actinidia bioassay to evaluate the resistance to Pseudomonas syringae pv. actinidiae. Plant Pathol J. 2019;35(4):372–80 (In Chinese). doi:10.5423/PPJ.OA.02.2019.0035. [Google Scholar] [PubMed] [CrossRef]
22. Lei YH, Jing ZB, Li L. Selection and evaluation of a new kiwifruit rootstock hybrid for bacterial canker resistance. Agric Food Sci. 2015;1096:413–20 (In Chinese). [Google Scholar]
23. Zhao ZB, Gao XN, Huang QL, Huang LL, Kang ZS. Identification and characterization of the causal agent of bacterial canker of kiwifruit in the Shaanxi Province of China. J Plant Pathol. 2013;95(1):155–62. [Google Scholar]
24. W.W. SU, Ren CG, Pan LS, Wu D, Han ZC, Wang JG, et al. Identification of pathogen of brown spot disease of actinidia hongyang and screening of laboratory agents. South China Fruits. 2022;51(4):119–24 (In Chinese). [Google Scholar]
25. Huang XL, Cui YL, Xu J, Zhu YH, Chen HB. Resistance evaluation of kiwifruit germplasm materials to brown leaf spot caused by Corynespora cassiicola. Acta Phytophylacica Sin. 2018;48(5):711–5. [Google Scholar]
26. Li L, Pan H, Li WY. Screening of wild Actinidia germplasms resistant to bacterial canker disease in China. Plant Sci J. 2022;40(6):801–9 (In Chinese). [Google Scholar]
27. Pei YG, Zhu YH, Sui LY, Tao QJ, Gong GS. Detection of the resistance of Botrytis cinerea from kiwifruit to four fungicides in Sichuan. Plant Protect. 2021;47(4):180–5 (In Chinese). [Google Scholar]
28. Li BJ, Gao W, Shi YX, Xie XW. Progress in researches on Corynespora leaf spot. Acta Phytophylacica Sin. 2021;39(2):171–6 (In Chinese). [Google Scholar]
29. Wen X, Qin HY, Ai J, Wang Y, Han XY, Li CY, et al. Establishment and evaluation of resistance identification method for Pseudomonas syringae pv. actinidiae disease in Actinidia arguta germplasm resources. Plant Protection. 2020;47:193–9 (In Chinese). [Google Scholar]
30. Tontou R, Giovanardi D, Ferrari M, Stefani E. Isolation of bacterial endophytes from Actinidia Chinensis and preliminary studies on their possible use as antagonists against Pseudomonas syringae pv. actinidiae. J Berry Res. 2016;6(4):395–406. doi:10.3233/JBR-160118. [Google Scholar] [CrossRef]
31. Chen J, Ran F, Shi J, Chen T, Zhao Z, et al. Identification of the causal agent of brown leaf spot on kiwifruit and its sensitivity to different active ingredients of biological fungicides. Pathogens. 2022;11(6):673. [Google Scholar] [PubMed]
32. Froud KJ, Beresford RM, Cogger N. Risk factors for kiwifruit bacterial canker disease development in ‘Hayward’ kiwifruit blocks. Australas Plant Path. 2017;46:421–31. doi:10.1007/s13313-017-0504-1. [Google Scholar] [CrossRef]
33. Yang GQ, Mo FX, Chen TT, Wang K, Wang DH, Huang DH, et al. Screening and application of drug combinations to control kiwifruit ulcer disease. China Plant Protect. 2020;40(11):73–6 (In Chinese). [Google Scholar]
34. Lin S, Lu XL, Zhao JP, Liu Y, Luo JD, Luo W, et al. Meteorological conditions and comprehensive control of kiwifruit canker in Sichuan Province. Jiangsu Agric Sci. 2020;48(9):123–6 (In Chinese). [Google Scholar]
35. Cui LH, Song JQ, Huang W. Effect of six kinds of medicine on control of brown spot disease of kiwifruit in Western Hunan. Heilongjiang Agric Sci. 2023;2:51–4 (In Chinese). [Google Scholar]
36. Ran F, Zhang RQ, Yuan T, Ma JL, Yin XH, Li WZ, et al. Isolation and identification of the pathogen of ‘Hongyang’ kiwifruit brown spot and toxicity of fungicides to the pathogen. China Fruits. 2021;6:27–32+111 (In Chinese). [Google Scholar]
37. Kaji R, Yariuchi R, Fujii Y, Taniguchi S, Uji Y, Suzuki G, et al. Expression analysis of defense-related genes in wild kiwifruit (Actinidia rufa) tolerant to bacterial canker. J Gen Plant Pathol. 2021;87:361–5. doi:10.1007/s10327-021-01024-7. [Google Scholar] [CrossRef]
38. Hoyte S, Reglinski T, Elmer P, Mauchline N, Stannard K. Developing and using bioassays to screen for Psa resistance in New Zealand kiwifruit. Acta Hortic. 2015;1095:171–80. [Google Scholar]
39. Zhang HQ, Mao XQ, Xiao JP, Zhang Z, Xie M. Rapid molecular identifcation of Actinidia Bacterial canker and preliminary screening of resistant materials in kiwifruit. J Nuclear Agric Sci. 2014;28(7):1181–7 (In Chinese). [Google Scholar]
40. Zhang MX, Zhai LF, Hu HJ, Zhang BX, Hong N. Laboratory evaluation for the resistance of pyrus germplasm accession to valsa canke. Acta Hortic Sin. 2014;41(7):1297–306. [Google Scholar]
41. Shi ZJ, Zhang HQ, Xiao JP, Yang LQ, Sun ZW, Xie M, et al. The resistance evaluation of different kiwifruit varieties to canker. Acta Agric Zhejiangensis. 2014;26(3):752–9. [Google Scholar]
42. Wurms KV, George MP, Lauren DR. Involvement of phenolic compounds in host resistance against Botrytis cinereain leaves of the two commercially important kiwifruit (Actinidia chinensis and A. deliciosa) cultivars. New Zeal J Crop Hort. 2003;31(3):221–33. doi:10.1080/01140671.2003.9514256. [Google Scholar] [CrossRef]
43. Huo ZY, Shi XC, Wang YX, Jiang YH, Zhu GY, Wang SY, et al. Antifungal and elicitor activities of p-hydroxybenzoic acid for the control of aflatoxigenic Aspergillus flavus in kiwifruit. Food Res Int. 2023;173:113331. doi:10.1016/j.foodres.2023.113331. [Google Scholar] [PubMed] [CrossRef]
44. Qin HY. Physiological response and multi-omics analysis of Actinidia arguta to bacterial canker (Psa) infection (Ph.D. Thesis). Chin Acad Agric Sci: China; 2022. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools