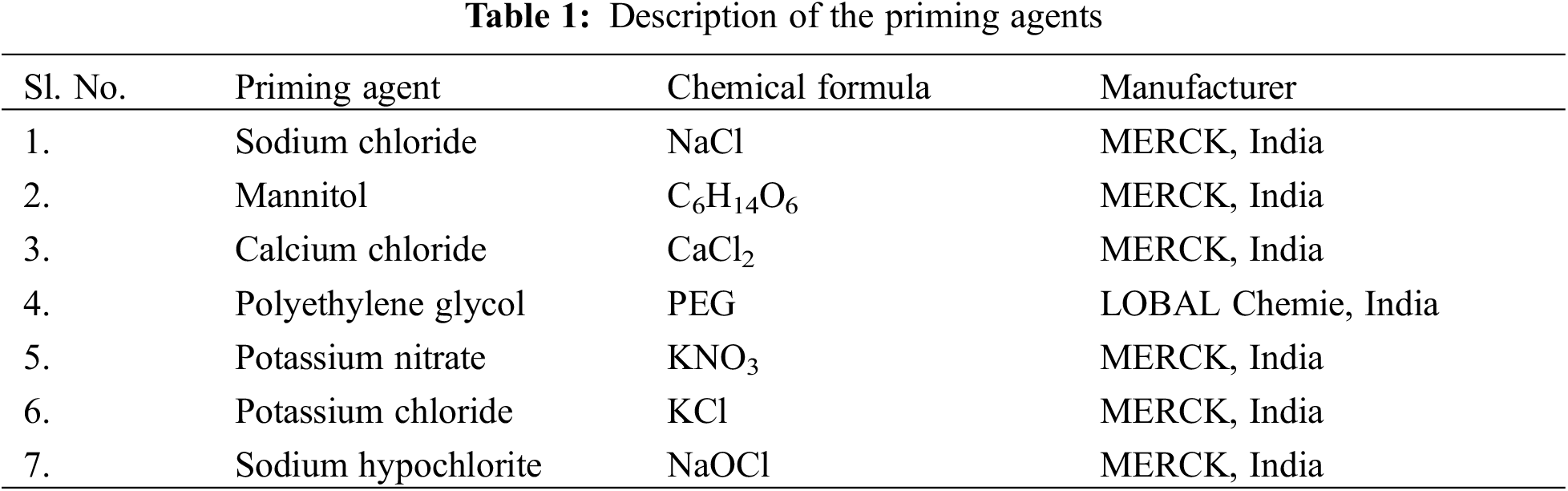
 Open Access
Open Access
ARTICLE
Relationship between Seed Priming Mediated Seedling Vigor and Yield Performance of Spring Wheat
Department of Agronomy, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
* Corresponding Authors: Md. Parvez Anwar. Email: ; A. K. M. Mominul Islam. Email:
Phyton-International Journal of Experimental Botany 2024, 93(6), 1159-1177. https://doi.org/10.32604/phyton.2024.049073
Received 27 December 2023; Accepted 20 May 2024; Issue published 27 June 2024
Abstract
Seed priming is a pre-germinated technique that can enhance seed germination percentage, faster and synchronized germination, better seedling growth, and yield under stress conditions. To ascertain the most effective seed priming method that would ensure the potential yield of wheat in Bangladesh, two experiments were carried out from December 2021 to March 2022 at the Department of Agronomy, Bangladesh Agricultural University. Two wheat varieties namely BARI Gom-28 and BWMRI Gom-1 were subjected to a range of priming chemicals in both lab and pot tests. These compounds included the following: control (no priming), hydropriming (distilled water), 10000 ppm KNO, 15000 ppm KNO, 40000 ppm Mannitol, 60000 ppm Mannitol, 10000 ppm NaCl, 20000 ppm NaCl, 100 ppm PEG, 150 ppm PEG, 500 ppm NaOCl, 1000 ppm NaOCl, 10000 ppm CaCl, 20000 ppm CaCl, 10000 ppm KCl and 20000 ppm KCl. A complete randomized design (CRD) with three replications was used to set up the experiments. The results showed that BARI Gom-28 and BWMRI Gom-1 responded best to KCl priming in terms of rapid seed germination and strong seedling development. On the other hand, the best priming agents for plant growth and productivity turned out to be CaCl and KCL. The results of this study support the possibility of using seed priming as a technique to improve wheat plant development and output by raising seed emergence and survival rates.Keywords
Wheat (Triticum aestivum L.) is the most prominent crop and is considered one of the “big three” cereals in terms of both area and production [1]. It is anticipated that by 2050, the developing world’s need for wheat will have increased by 60% [2]. In Bangladesh, wheat has a major role in creating job opportunities, food and nutritional security, and economic growth. Wheat has the second-highest economic and dietary importance in the country, next to rice. Only 20% of Bangladesh’s national wheat needs could be satisfied by its 1.15 million tons of production, which takes up 0.33 million hectares of land [3]. To feed its expanding population and ensure future food security, the government of Bangladesh imports wheat, which is likely to supply around 88% of the country’s consumption needs [4]. To be more specific, Bangladesh imported 5.8 million MT of wheat in FY 2021 compared to 1.08 million MT produced, and throughout the next five years, there is predicted to be an approximately 10 million MT rise in local consumption [5].
Agronomic crops are heavily dependent on meteorological factors and seasonal traits including temperature, humidity, rainfall, and length of day [6]. Bangladesh’s temperature regimes have already significantly changed as a result of climate change-induced global warming, which has a substantial effect on wheat output [7]. There are also some other factors influencing wheat output other than climate change [7]. Following the 2016 wheat blast, the country’s wheat area has decreased or remained steady due to certain geophysical variables [8]. In Bangladesh, the best period to plant wheat is from the middle of November until the first week of December. Too early seeding results in weak plants with inadequate root systems, inconsistent germination, frequent embryo mortality, and endosperm degradation from bacterial or fungal activity because the temperature is above optimal. According to reports, after December 1st, there is a 1.3% decline in grain yield for every day that passes. Despite these obstacles, more wheat has to be produced to fulfill domestic demand and lessen reliance on imports by using appropriate adaptation techniques [9].
An alternate method to get over these restrictions is seed priming, which is a mechanism to help plants become more resilient to stress [10]. The practice of seed priming can help to speed up germination, guarantee steady germination, and boost crop production. This pre-sowing therapy enhances the benefits of seed germination by inducing a physiological condition. In addition, priming entails a straightforward process that involves hydrating the seed in a regulated setting and drying back to prevent radical appearances during the germination process [11]. According to Moosavi et al. [12], primed seeds considerably increased root length, seed vigor, germination rate, and germination percentage. For summer-grown maize, priming produced improved crop emergence, growth, early flowering, and higher yields [13]. With seed priming, farmers have observed early blooming (7–10 days) and maturity (8–10 days) in rice and chickpeas [14]. The best methods for boosting seed viability and enhancing germination were hydropriming, priming with growth regulators, and priming with different calcium and potassium salts [15]. In direct-seeded rice, the use of potassium chloride (KCl) or calcium chloride (CaCl2) as a priming agent increased seedling development, germination, and yield [16]. Strong establishment, yield performance, and seedling development have also been documented in wheat using CaCl2 priming, most likely under late planting circumstances [17]. In late circumstances, osmoprimed wheat had the highest biological yield, grain yield, yield index, and less productive tillers per unit area [17]. It is evident from earlier research that primed seeds have demonstrated faster and more consistent emergence, more robust seedlings, and improved performance even under stressful situations as compared to unprimed seeds.
Short winter spells, relatively high temperatures, and soil moisture stress at the reproductive stage are the major causes behind the low productivity of wheat under Bangladesh conditions. Also, late sowing and low seed germination due to very high soil moisture content during sowing results in lower germination, poor stand establishment, and low wheat yield. As reported in many previous studies, seed priming could be a prospective approach to address lower seed germination and high-temperature issues in wheat to ensure potential wheat yield. Therefore, the present study was designed to evaluate the effect of seed priming on seed germination and seedling vigor of wheat and to recognize their subsequent effect on wheat yield.
The two experiments that made up this study were carried out from December 2021 to March 2022 at the Department of Agronomy, Bangladesh Agricultural University, one at the Agro Innovation Laboratory (lab experiment) and the other in the net house (pot experiment). In this study, the effectiveness of primed wheat seeds was assessed in terms of plant growth, wheat production, seed germination, and seedling vigor and establishment. The test location was in the humid subtropical monsoon environment of the Old Brahmaputra floodplain (AEZ-9) at 23°77′ N latitude and 90°33′ E longitude. The experimental soil was neutral in reaction (pH value 6.8), low in organic matter content (1.37%), and the general fertility level of the soil was low (1.1% total N, 25 ppm available P and 0.16 me % exchangeable K, 22.2 ppm available sulfur and 0.43 ppm zinc).
For lab and pot experiments, two wheat varieties, BARI Gom-28 and BWMRI Gom-1, were exposed to various priming agents, including the following, Control (no priming), Hydropriming (distilled water), 10000 ppm KNO3, 15000 ppm KNO3, 40000 ppm Mannitol, 60000 ppm Mannitol, 10000 ppm NaCl, 20000 ppm NaCl, 100 ppm PEG, 150 ppm PEG, 500 ppm NaOCl, 1000 ppm NaOCl, 10000 ppm CaCl2, 20000 ppm CaCl2, 10000 ppm KCl and 20000 ppm KCl. Different seed priming agents and their concentrations were selected based on their performances as reported by previous studies of the authors [17–21]. The experiment employed only laboratory-grade priming chemicals. Table 1 provides specifics on the priming agents. Four replications and a complete randomized design (CRD) were used to set up the experiments.
2.3 Conduction of the Experiment
Sufficient seeds (considering 50 seeds for each replication) of both wheat varieties were steeped for six hours at room temperature (25°C ± 2°C) in various priming agent solutions that had been previously prepared using distilled water. There was a 1:5 (g mL−1) seed weight to solution volume ratio. After taking the seeds out of the priming agent solution, they were repeatedly washed with distilled water to get rid of any remaining chemical residue. Then, the seeds were dried back to the original moisture content by forced air. Dried seeds were put in polythene bags and stored in a refrigerator at 5°C ± 1°C until used. While control treatment received no prior seed priming.
2.4 Preparation of Germination Media and Seed Placement
Sterilized sand (to avoid any pathogenic contamination inhibiting seed germination or damaging seedlings) was used as germination media, and plastic-made petri dishes of 90 mm diameter with 15 mm depth were used as containers. The moisture content of the media was maintained at around 80% of the field capacity by watering with distilled water as necessary following the protocol developed by Mim et al. [18]. Fifty seeds were placed manually on moist soil maintaining more or less equal distance in each petri dish. Petri dishes were put on the desk of the laboratory at a room temperature of 25°C ± 2°C.
A plastic pot of size 25 cm in height and 22 cm in diameter was used as the experimental unit. Each pot was filled up to
Data were collected on germination, seedling vigor, and seedling growth.
Germination percentage (GP)
The number of germinated seeds was counted on the 7th day. The appearance of normal seedlings over the sand layer was considered germination. The GP was calculated as per AOSA [19] using the following equation:
Mean germination time (MGT)
MGT was calculated as per Islam et al. [20] with slight modification using the following equation:
where n is the number of seeds germinated on the day and D is the number of days counted from the beginning of germination.
Germination index (GI)
GI was calculated as per AOSA [19] with slight modification using the following equation:
Seedling vigor index (SVI)
After seed placement for germination, on the 7th day, ten seedlings from each replicate were randomly selected. Root and shoot lengths were measured and then oven-dried at 70°C for 72 h. to record the root and shoot dry weight of seedlings. The seedling vigor index (SVI) was calculated as per AOSA [19] using the following equation:
where Seedling length = Root length + Shoot length
Germination coefficient (GC)
The coefficient of germination was calculated as per Copeland [21] using the following formula:
where A is the number of seeds germinated, T is the time corresponding to A, and n is the number of days to the final count.
Seedling growth
On the 7th day of seed placement for germination, 10 seedlings from each replicate were randomly selected. Root length, shoot length, root-shoot ratio (R:S), root dry weight, and shoot dry weight were measured. The dry weight was measured after drying in an oven at 70°C for 72 h.
Data were collected on plant height, spike length, number of spikelets spike−1, number of grains spikelets−1, number of grains spike−1, 1000-grain weight, grain weight plant−1, and grain yield. For collecting data on plant characters, all six plants were uprooted from each pot after maturity. Yield contributing characters and yield were recorded taking all the plants of each pot and the average number was recorded.
The recorded data were compiled and tabulated for statistical analysis. Analysis of variance (ANOVA) was done at a 1% level of significance with the help of the computer package MSTAT-C (statistical software). The mean differences among the treatments were adjudged by Duncan’s Multiple Range Test [22]. The correlation matrix and principal component analysis were done with R.
3.1.1 Germination and Seedling Vigor of Wheat
The final germination percentage, mean germination time, germination index, seedling vigor index, and germination coefficient were used to assess the germination and seedling vigor of two wheat cultivars. On the ultimate germination %, germination index, and seedling vigor index, variety had no discernible impact. Conversely, variety had a considerable impact on the mean germination time and germination coefficient. Compared to BARI Gom-28, BWMRI Gom-1 demonstrated quicker germination and a better germination coefficient (Table 2).
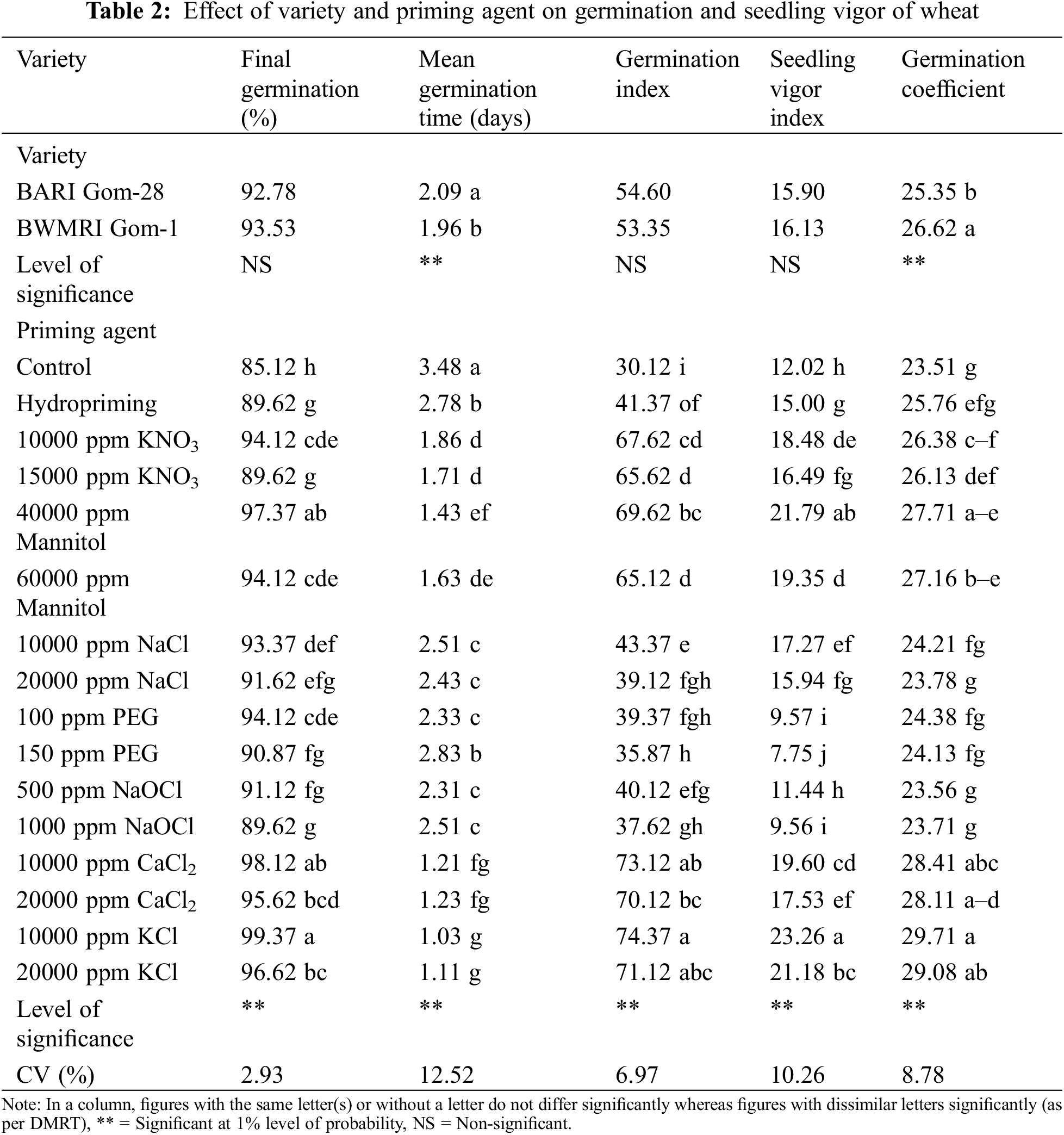
The seed germination rate and seedling vigor of seedlings were significantly impacted by the priming agent. It was observed that in comparison to the control, the germination percentage rose by around 5.29% to 16.74% as a result of priming with different agents. The most effective methods for increasing the germination rate were hydropriming, priming with 10000 ppm KCl, priming with 10000 ppm KNO3, and priming with 1000 ppm NaOCl. As compared to the control, Table 2 shows that the priming agent reduces the mean germination time. The least amount of time was needed for mean germination when the seed was primed with 10000 ppm KCl, while the most time was needed when the seed was primed with 150 ppm PEG. The germination index varied from 30.12 to 74.37 where no priming treatment had the lowest index. Priming with KCl at any concentration and priming with 10000 ppm CaCl2 produced the best germination index, in addition to that priming with 10000 ppm KCl and priming with 40000 ppm Mannitol resulted in the most vigorous seedlings. However, priming with 150 ppm PEG had the lowest vigor index (Table 2).
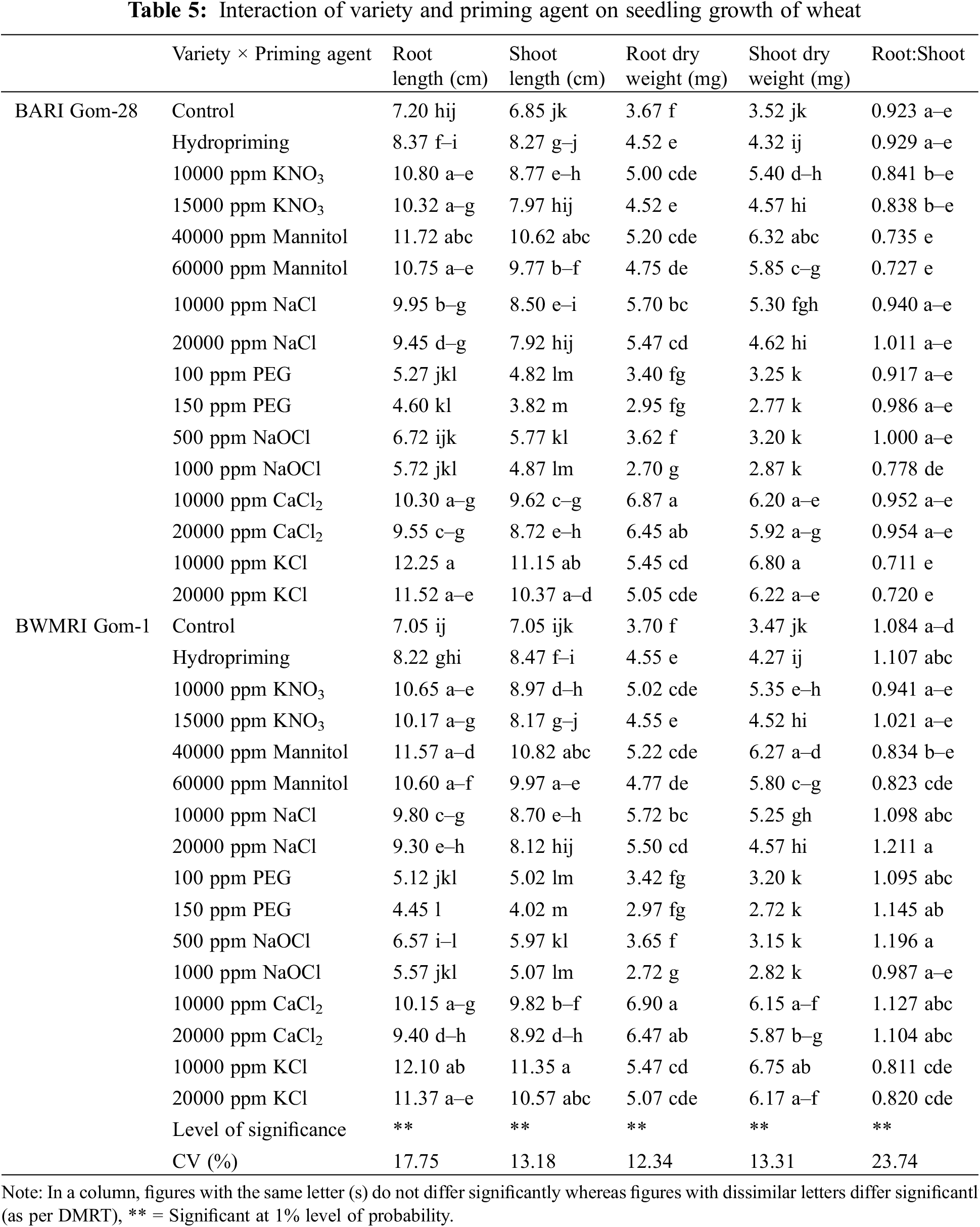
All of the parameters related to germination and seedling vigor were significantly impacted by interactions between the variety and the priming agent (Table 3). Due to different priming agents, the germination percentage increased by around 5.31% to 16.81% in BARI Gom-28 and by roughly 5.26% to 16.67% in BWMRI Gom-1 when compared to the control. When primed with 10000 ppm KCl, both varieties exhibited the greatest germination percentages; however, BWMRI Gom-1 exhibited a higher germination rate than BARI Gom-28. In contrast, the germination rate was lowered by hydropriming, 15000 ppm KNO3, and 1000 ppm NaOCl priming. Compared to the control, as shown in Table 4, seed priming shortened the seed germination period of wheat. While BARI Gom-28 took 3.55 days and BWMRI Gom-1 took 3.42 days to germinate in the control treatment, both the varieties required the least amount of days to germinate with 10000 ppm KCL among the priming agents, and BWMRI Gom-1 took the shortest days with this treatment. A similar pattern was also observed in the case of germination index, seedling vigor index, and germination coefficient. The germination index varied from 30.75 to 75.00 in BARI Gom-28 and 29.50 to 73.75 in BWMRI Gom-1 where no priming treatment had the lowest index; the highest index was found with 10000 ppm KCl in both the varieties. In both BARI Gom-28 and BWMRI Gom-1, priming with 10000 ppm KCl also resulted in robust seedlings with the highest germination coefficient (Table 3).
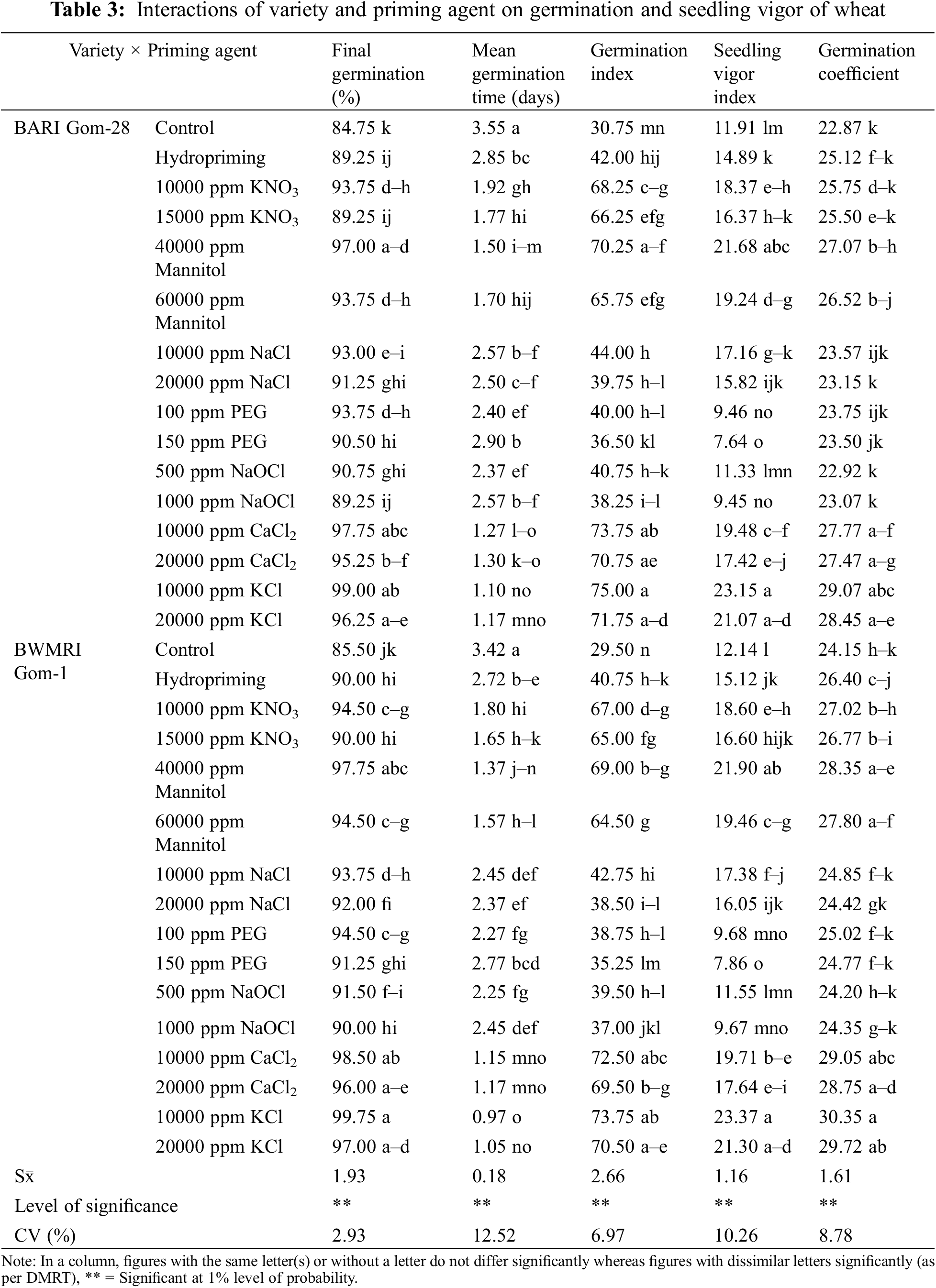
3.1.2 Seedling Growth of Wheat
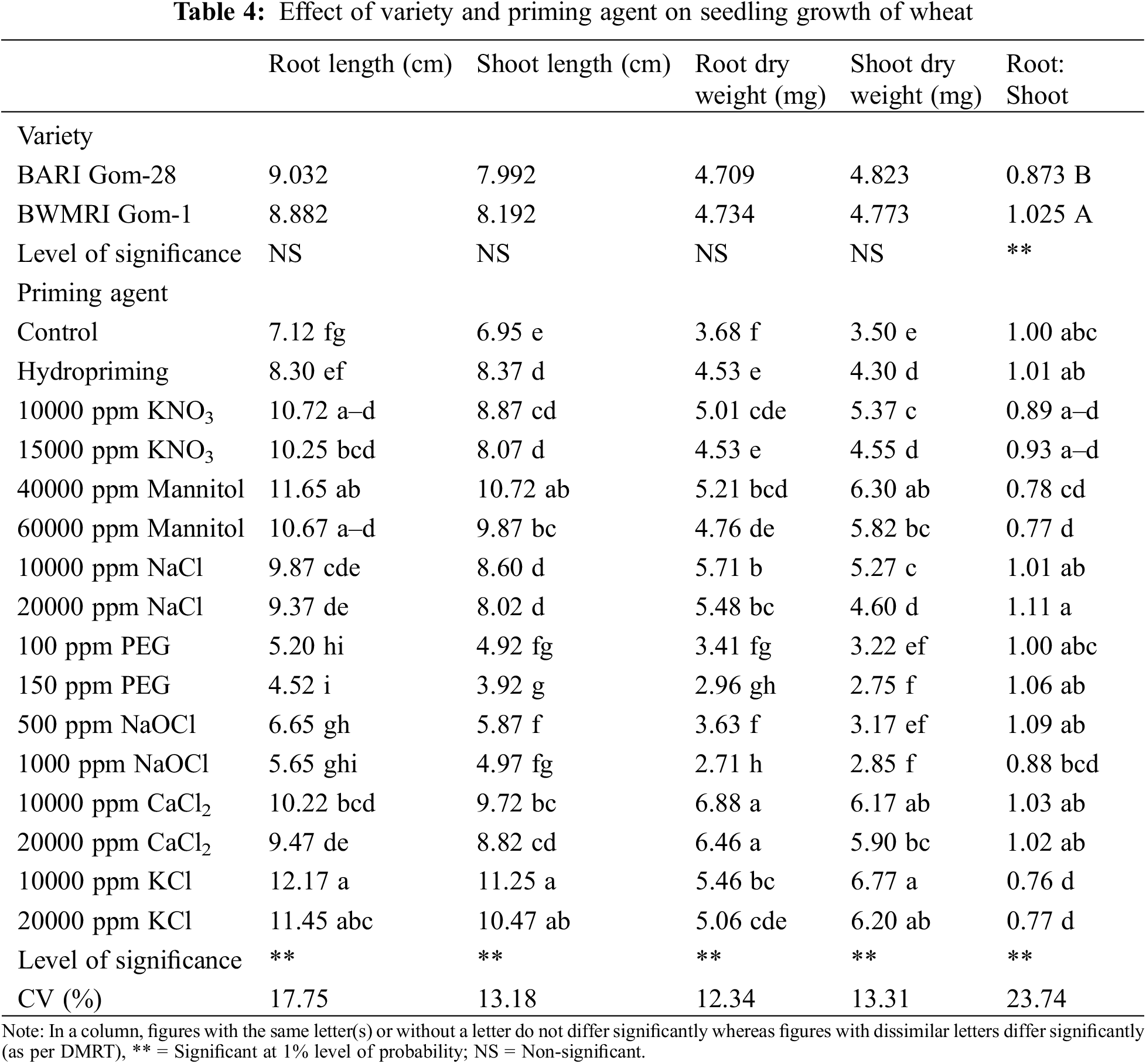
Except for the root shoot ratio, variety had no significant impact on the metrics related to wheat seedling development. Table 4 shows that compared to BARI Gom-28, BWMRI Gom-1 displayed a larger root-shoot ratio.
All the parameters related to the growth of seedlings were significantly impacted by the priming agent and the interaction of variety and priming agent. In terms of root and shoot length, seed priming with KCl, Mannitol, and 10000 ppm KNO3 worked the best, whereas PEG and 1000 ppm NaOCl priming performed the poorest, even lower than the control treatment. Priming with CaCl2 produced the highest root dry weight and seed priming with KCl, 10000 ppm CaCl2, and 40000 ppm Mannitol produced the maximum shoot dry weight. On the contrary, no advantage of PEG and NaOCL was observed since they produced statistically lower root and shoot dry weight compared to no priming control. The root-shoot ratio varied between 0.77 and 1.11; the highest root-shoot ratio was obtained by priming with 20000 ppm NaCl, and the lowest root-shoot ratio was created by 10000 ppm KCl which was even lower than the unprimed seeds (Table 4). Different priming agents caused variations in root and shoot length of both varieties. As can be seen from Table 3, PEG had no discernible benefit since it produced statistically less shoot and root length than no priming. In both kinds, a decrease of about 7.65 and 7.33 cm in shoot and root length was seen with 150 ppm PEG priming, as opposed to 10000 ppm KCL priming. In the case of root dry weight of wheat, priming with 10000 ppm CaCl2 resulted in the highest values but for shoot dry weight priming with 10000 ppm CaCl2 performed the best for both varieties. In those cases also PEG and NaOCl priming produced the lowest values than that of control. In BWMRI Gom-1, the root-shoot ratio was the highest when primed with 20000 ppm NaCl, and this was followed by several additional interactions. However, BARI Gom-28 primed with 10000 ppm KCl had the lowest root-shoot ratio (Table 5).
3.2.1 Plant Height, Yield Contributing Characters, and Yield of Wheat
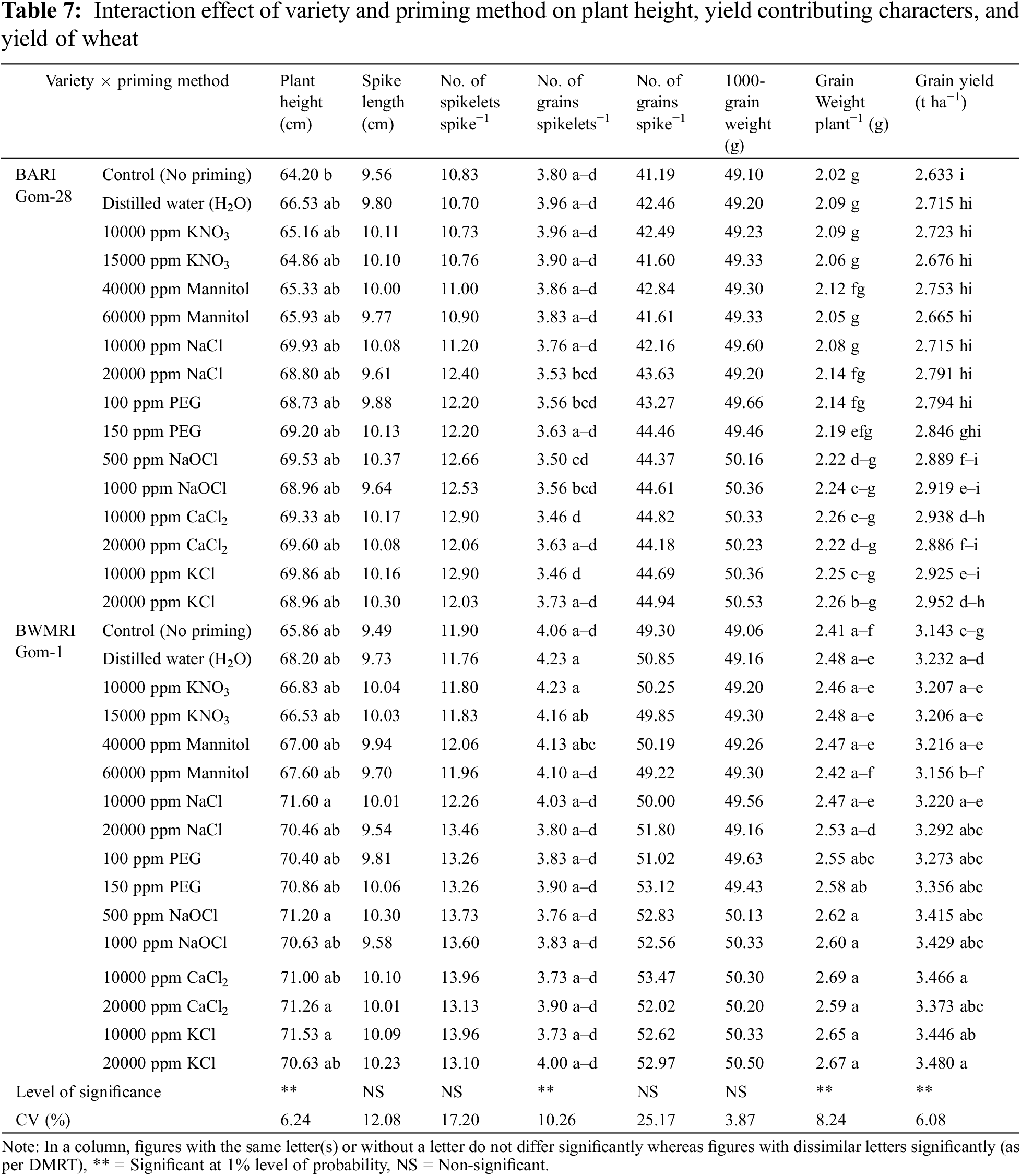
The effects of variety on wheat grains’ spikelet−1, spike−1, weight, and yield were noteworthy. Taking into account the factors that contribute to yield, it was found that BWMRI Gom-1 performed better than BARI Gom-28, yielding an 18% higher grain yield (Table 6).
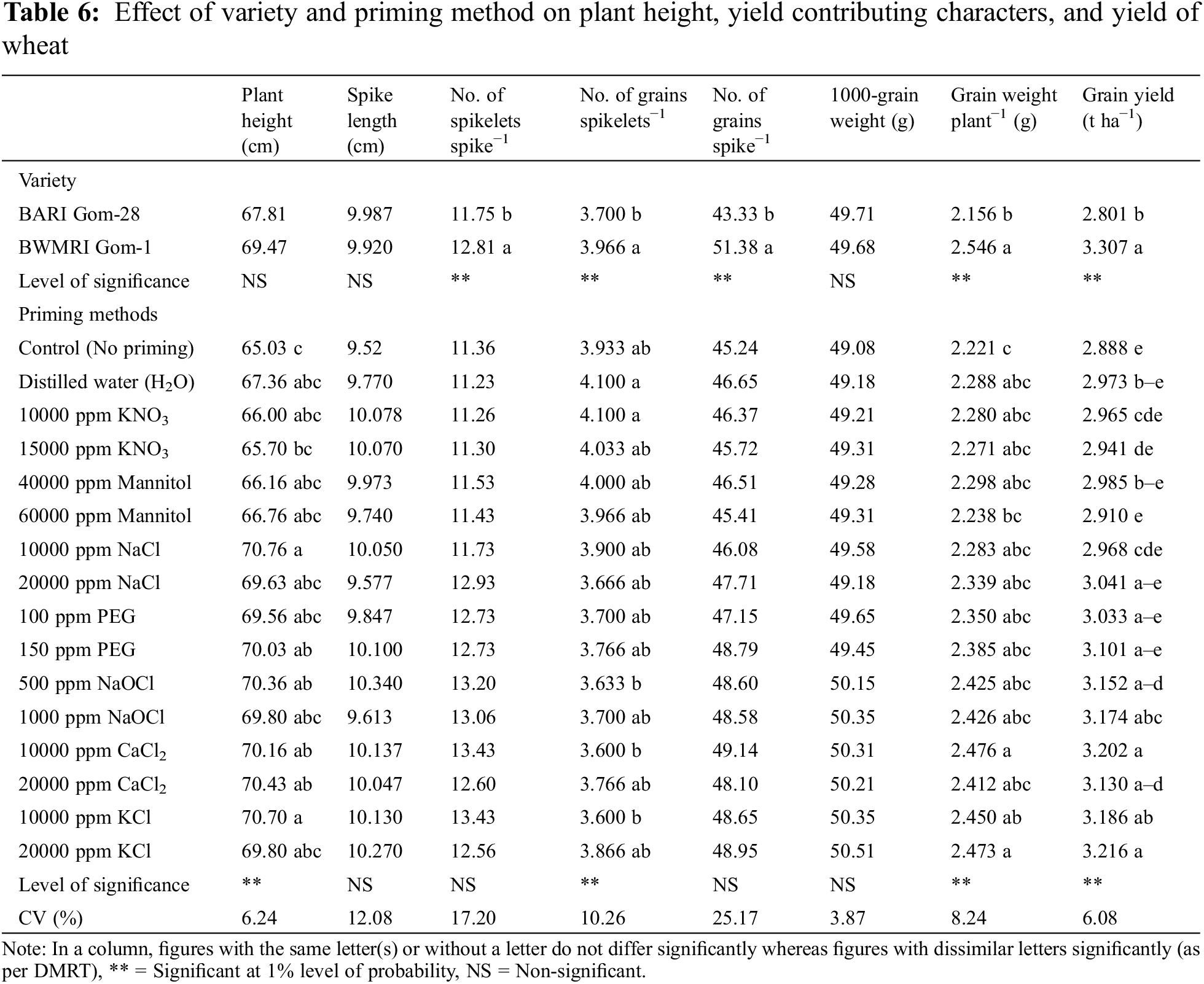
The effects of the priming treatment were seen in the following parameters: plant height, grain weight, grains spikelet−1, and yield. Different priming agents increased plant height of wheat by approximately 1.03% to 8.82% over control. Plant height was increased by 5 cm due to 10000 ppm NaCl over control resulting in the highest plant height statistically followed by 10000 ppm KCl. Plant height was least affected by priming with KNO3 among the priming agents. When wheat seeds were primed with distilled water and 10000 ppm KNO3 it helped to produce more number of grains spikelets−1 while priming with 10000 ppm KCl and CaCl2 performed the worst, and the rest of the methods had a statistically similar effect on the number of grains spikelets−1. Grain weight plant−1 varied from 2.21 to 2.476 g where priming with 10000 ppm CaCl2 resulted in the highest grain weight plant−1 statistically followed by 20000 ppm KCl and many others while no priming method produced the lowest grain weight plant−1 of wheat. Grain yield was also increased due to the priming agent and it ranged from 0.76% to 11.35%. Seed priming with 20000 ppm KCl showed the best yield performance statistically followed by 10000 ppm CaCl2. No priming method produced the lowest grain yield (Table 6).
The combination of variety and priming technique resulted in a considerable increase in plant height for both varieties. BARI Gom-28 and BWMRI Gom-1 had plant heights ranging from 64.2 to 69.93 cm and 65.98 to 71.60 cm, respectively. Different priming agents were found to provide an approximately 1.03%–8.92% and 1.01%–8.71% increase in plant height in BARI Gom-28 and BWMRI Gom-1, respectively. In this instance, BWMRI Gom-1 produced the tallest plant when seeds were primed with 10000 ppm NaCl. However, in the case of no priming, BARI Gom-28 yielded the shortest plant. When taking into account the number of grains spikelet−1, the interaction between BWMRI Gom-1 and either distilled water or 10000 ppm KNO3 yielded the highest results, whereas the interaction between BARI Gom-28 and either 10000 ppm KCl or KNO3 produced the worst results. For BARI Gom-28 and BWMRI Gom-1, priming had a major impact on grain weight. The grain weight increased by 1.48%–11.88% and 0.41%–8.71% in BARI Gom-28 and BWMRI Gom-1, respectively, among different priming agents. However, once seeds were primed with 10000 ppm CaCl2, the cultivar BWMRI Gom-1 produced the highest grain weight plant−1. Conversely, in the case of no priming, BARI Gom-28 yielded the minimal grain weight plant−1. Additionally, grain yield improved by 1.33% to 12.24% in BARI Gom-28 and 0.51% to 10.83% in BWMRI Gom-1. In BWMRI Gom-1, the highest grain production was recorded when seeds were primed with 20000 ppm KCl, statistically followed by several other interactions. In contrast, BARI Gom-28 yielded the lowest grain when not priming (Table 7).
3.3 Relationship between Studied Yield and Contributing Parameters
The correlation matrix of the measured attributes is shown in Fig. 1, which enables us to look into the connections between them. The germination and seedling growth parameters like GP, GT, GI, VI, GC, SL, RL, SDW, and RDW are all more or less positively correlated with each other but the negative correlation of these parameters was noticed with GT. Here only R:S was positively correlated with GT. However, most of these parameters had no significant correlation with growth, yield, and yield contributing characters. In that case, the only positive relationship of GP with TGW and R:S with PH, SS, GS, GW, and GY was noticed. But also a negative correlation of GT with TGW and R:S with SL was noticed. The growth, yield, and yield contributing characters PH, SPL, SS, GS, TGW, GW, and GY showed different correlations. GY is mostly correlated with PH, SS, GS, and GW (Fig. 1).
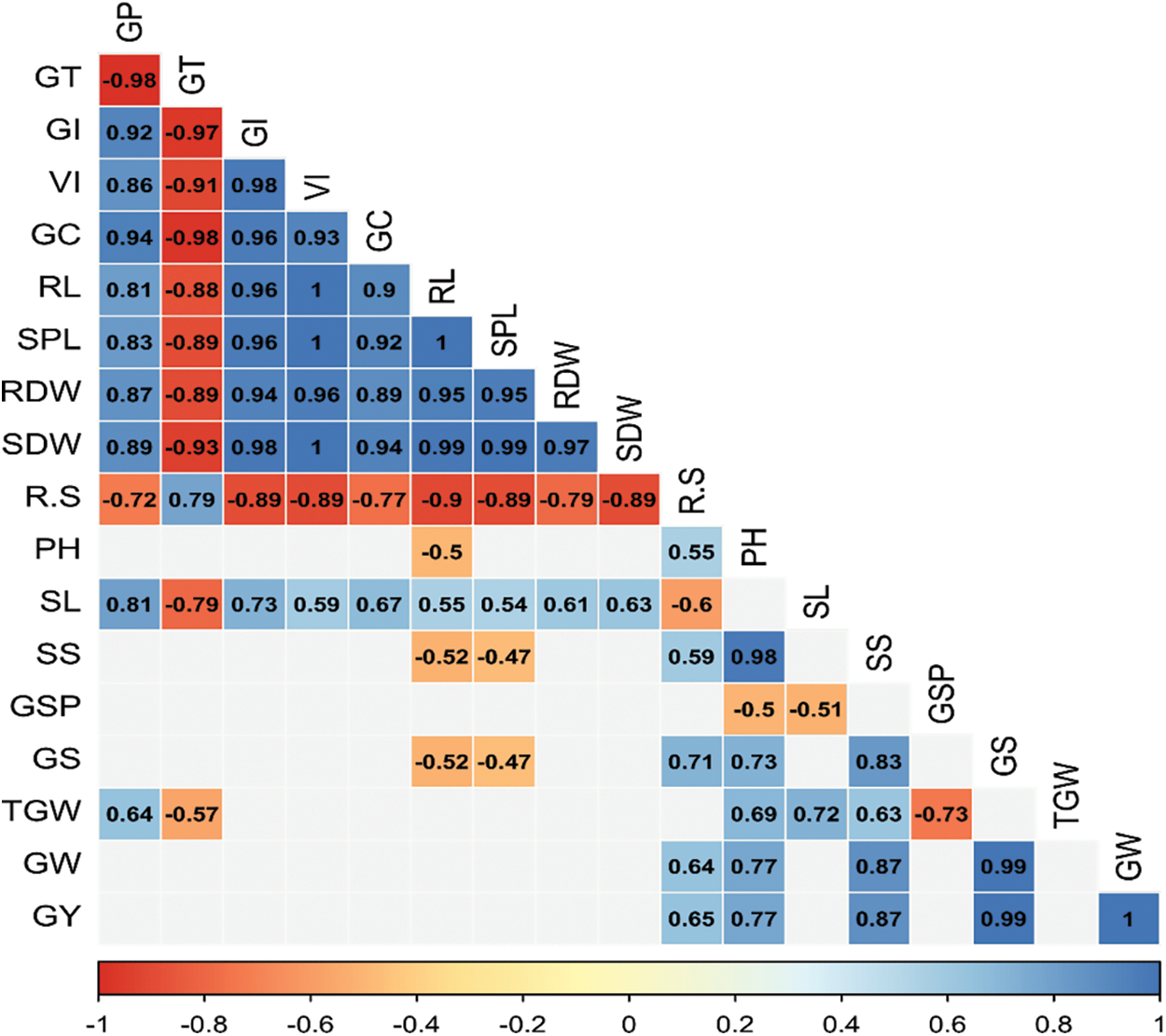
Figure 1: Correlation matrix of assessed traits. The range between the highest and lowest values is 1 to −1. Blue and red ellipses represent positive and negative associations, correspondingly. The greater color intensity reflects a stronger co-efficient, whilst the lower coefficient is reflected by lower color intensity. Here, GP = Germination percentage, GT = Germination time, GI = Germination index, VI = Vigour index, GC = Germination coefficient, SL = Shoot length, RL = Root length, SDW = Shoot dry weight; RDW = Root dry weight; PH = Plant height; SPL = Spike length; SS = No. of spikelets spike−1; GSP = No. of grains spikelets−1; GS = No. of grains spike−1; TGW = 1000-grain weight; GW = Grain Weight plant−1; GY = Grain yield
3.4 Principal Component Analysis
Principal component analysis (PCA) was applied to the trial dataset, which contained two wheat varieties, 16 priming agents, and 18 distinct parameters, to decrease data heterogeneity and discover possible correlations between varieties, priming agents, and measured parameters (Fig. 2). A PCA biplot was constructed with only the first two components. The PCA found that the first two principal components (PCs) described 72.8% of the overall heterogeneity. Because the first and second PCs generated 45.1% and 27.7% of the entire divergence. From Fig. 2, it was clear that SS, GS, and GW were closely related to GY, and these parameters were found slightly higher in V2P12, V2P13, V2P14, and V2P15. On the other hand, GI, SDW, VI, RL, GI, GP, and GC were closely related to each other and were found higher in V1P14, V1N16, and V1P12.
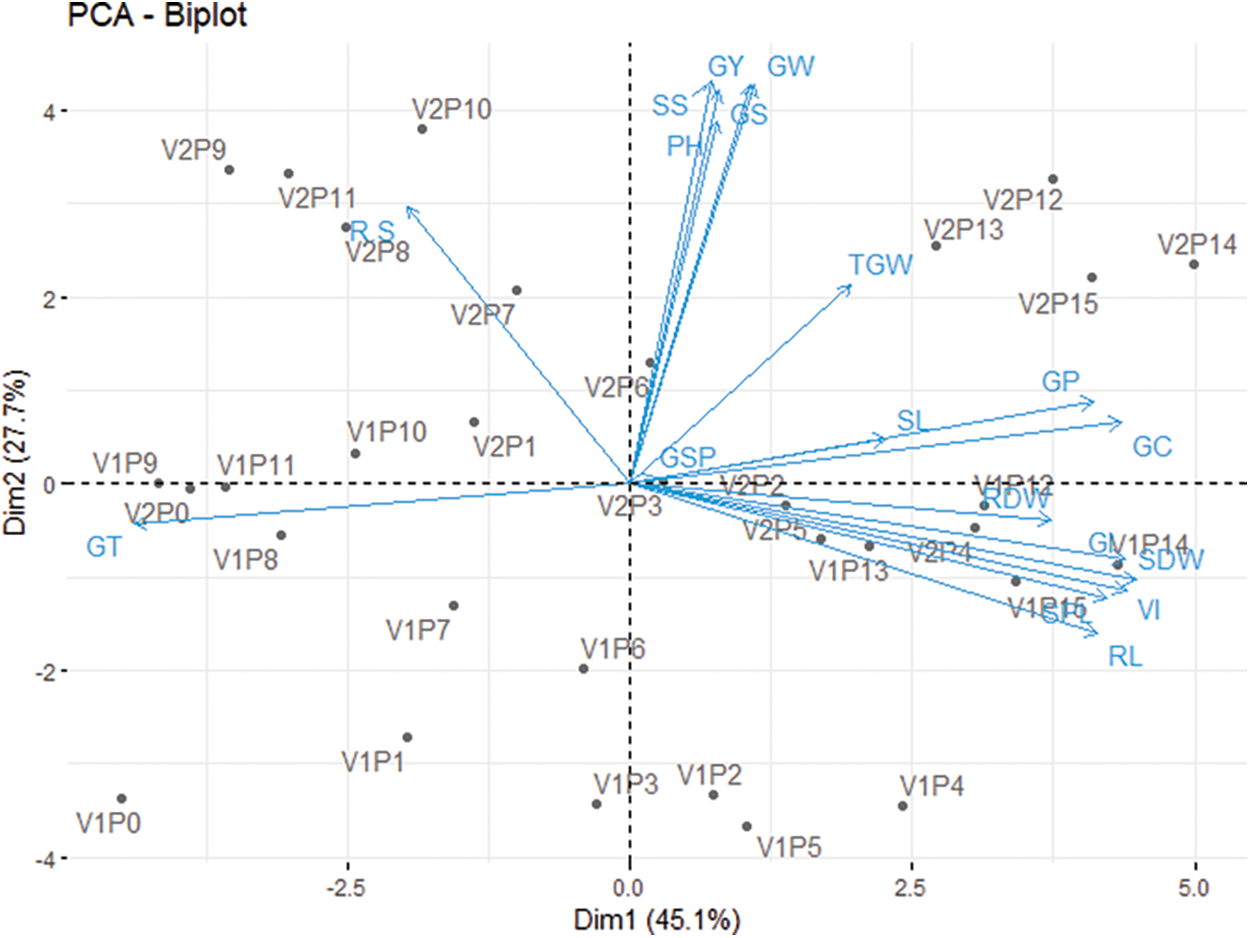
Figure 2: Principal component analysis. V1 = BARI Gom-28, V2 = BWMRI Gom-1 P0 = Control (No priming), P1 = Hydropriming (distilled water), P2 = 10000 ppm KNO3, P3 = 15000 ppm KNO3, P4 = 40000 ppm Mannitol, P5 = 60000 ppm Mannitol, P6 = 10000 ppm NaCl, P7 = 20000 ppm NaCl, P8 = 100 ppm PEG, P9 = 150 ppm PEG, P10 = 500 ppm NaOCl, P11 = 1000 ppm NaOCl, P12 = 10000 ppm CaCl2, P13 = 20000 ppm CaCl2, P14 = 10000 ppm KCl, P15 = 20000 ppm KCl; GP = Germination percentage, GT = Germination time, GI = germination index, VI = Vigour index, GC = Germination coefficient, SL = Shoot length, RL = Root length, SDW = Shoot dry weight; RDW = Root dry weight; PH = Plant height; SPL = Spike length; SS = No. of spikelets Spike−1; GSP = No. of grains spikelets−1; GS = No. of grains spike−1; TGW = 1000-grain weight; GW = Grain Weight plant−1; GY = Grain yield
It was proposed that using a pre-sowing seed treatment might boost germination, raise the rate of germination, and improve the vigor and development of seedlings. The technique of seed priming can be used to improve the viability and vigor of wheat seeds [23]. These factors could aid wheat seedlings in overcoming various challenges, which are presently prevalent in Bangladesh. This study assessed the germination rate, viability of the seedlings, growth, and yield of primed wheat seeds.
In this study, it was discovered that KCl priming outperformed PEG and other priming agents in terms of favorable effects on the germination percentage of wheat seeds. To improve Poaceae plant germination, emergence, and growth, potassium chloride has been used as the osmoticum [24] which can increase water uptake that is important for germination. Seed priming causes the seed to undergo several biochemical changes, including hydrolysis, enzyme activation, and dormancy breaking, all of which are necessary to start the germination process [25] resulting in early seedling development, especially under less-than-ideal circumstances [26]. Seed priming improved germination rates by 20%, germination index by 78%, shoot, and root length by 79% and 21%, dry weight, and vigor index by 64%, and germination time by 9% in comparison to unprimed seeds [27]. Early DNA replication [28], quicker embryo development [29], and the restoration of damaged seed components [30,31] are all made possible by priming. McDonalds [32] asserts that primed seeds may quickly absorb and revive the metabolism of the seed, hence boosting the rate of germination. This investigation verified that seedling development was hindered by PEG priming and increased by KCl priming. In this study, the maximum seedling dry weight was achieved by priming with KCl. Conversely, no benefit of PEG was noted since the seedling dry weight it produced was statistically lower than the control. But Osmo priming with PEG improved the germination and seedling vigor of wheat seeds [33]. These findings could be comparable to the finding that KCl is a major stressor that affects the weight of wheat plants [34]. The osmotic advantage that K+ possesses in increasing cell water saturation and functioning as a co-factor in the activities of many enzymes may account for the increased efficiency of seed priming with KCl [35]. Enzyme activation, osmotic control in cells, and the equilibrium of membrane potential and turgor all depend on K+ [36]. Activating alpha-amylase improves the K+ balance, which is the basis for the vitality of seeds. In a similar experiment, rice seed stimulated with 5% CaCl2 and 3% KCl for 24 h, yielded the greatest shoot dry mass of 0.8 mg [37]. A positive impact of seed priming with different agents including CaCl2 and KCl on seed germination and seedling vigor has been reported in many studies [38–42].
The results of this study support the possibility of using seed priming as a technique to boost seedling vigor and increase wheat seed germination. Therefore, these results will open up new possibilities for improving seed priming to boost wheat germination and seedling vigor. This study unequivocally demonstrates that the seed priming technique positively affects plant height, productivity, and yield-enhancing traits in wheat. These results corroborate previous research showing that primed seeds often result in higher plant height, root weight, and dry matter output when compared to control [43]. Plant phenological traits enhanced as a result of seed priming; plants that were primed had a better start, grew more quickly, and reached maturity sooner than their unprimed counterparts. A shorter imbibition time and quick emergence might be the causes of priming’s improved phenology [14]. Regarding plant height, both cultivars in this study responded well to seed priming with KCl and NaCl. Potassium’s primary job in the cytoplasm is to maintain the proper ionic environment for metabolic activities. As a result, it regulates several different processes, including growth control. The most common source of potassium (K) for crops is potassium chloride (KCl), and chloride (Cl) is regarded as a necessary micronutrient for the best possible development [44]. Taller wheat plants were seen when seeds primed with KCl were used, as reported by Farooq et al. [45].
The yield contributing characteristics and wheat yield were positively impacted by the seed priming method in this study, with KCl or CaCl2 demonstrating the highest performance. Better seedlings from primed seeds may be able to acquire resources earlier and more effectively than weaker seedlings from unprimed seeds, which might lead to an increase in grain output [46]. To achieve high yield and early maturity, seed priming has the potential to improve the viability and vigor of seeds above control, which are critical components of germination and the establishment of robust seedlings. Others also verified that primed wheat seeds had considerably longer spike length, spikelet count (spike−1), grain count (spike−1), and 1000-grain weight than non-primed wheat seeds [47]. According to Ugale et al. [47], KCl-treated seeds had a high potential to increase wheat yield and yield-attributing components, while Farooq et al. [16] found that osmohardening with CaCl2 and KCl was the most effective treatment for enhancing rice seedling development and productivity. This ultimately leads to robust seedling growth, improved plant growth, increased seed output, and higher-quality rice. Research on dry direct seeded rice with KCl priming and seeds exposed to CaCl2 osmohardening has shown improvements in the number of viable tillers, kernel yield, and harvest index [48]. Similar findings were made by Toklu et al. [49], who discovered that hydro-priming, KCl, and PEG treatments raised wheat grain yields above the control. According to Ramamurthy et al. [50], seed priming in wheat produced a noticeably greater grain yield (17%) compared to non-primed wheat and also enhanced wheat grain production in late conditions. Wheat yield was shown to increase by 15% when wheat seed was soaked in 2.5% potassium chloride (KCl) for 12 h before planting, according to Misra et al. [23]. Similar to this, Suryakant et al. [51] found that the best wheat grain yields came from sowing sprouted seeds, which was followed by priming treatments with IAA, KCI, water, and ZnSO4, while the lowest yields came from sowing dry seeds (control). In a field experiment, Sarlach et al. [41] observed that 12 h. seed priming with 15 μg mL−1 CoCl2 and 2.0% KNO3 gave significantly higher grain yield in wheat. Overall, the outcomes validate our theory that seed priming affects germination and seedling development, and as a result, seed priming can assist in boosting grain output.
The present study confirms that pre-sowing seed priming can ensure higher, faster, and more uniform germination along with vigorous seedlings and a higher survival rate in wheat. Seed priming also resulted in higher wheat yield as the consequence of better performance of yield parameters. However, no relationship between seed priming-mediated seedling vigor and wheat growth and yield was found. Among the priming agents tested CaCl2 and KCl were found promising for higher seed germination, seedling vigor, and better productivity of wheat. Therefore, seed priming strategy should be further explored as a tool to boost wheat productivity in a sustainable way. Further in-depth research counting other priming agents including different botanicals and growth regulators/hormones could be helpful to identify better candidates for priming wheat seed and reveal the mechanism behind it.
Acknowledgement: None.
Funding Statement: The authors are very much grateful to Bangladesh Agricultural University Research System (BAURES), Bangladesh Agricultural University, Mymensingh-2202, Bangladesh for the financial support through the research project entitled “Induction of Heat and Drought Tolerance in Wheat through Seed Priming” (Project No. 2021/35/BAU) to carry out the research work.
Author Contributions: Study conception and design: Md. Parvez Anwar; data collection: Masuma Akhter and Sharmin Aktar; analysis and interpretation of results: Md. Parvez Anwar and Sinthia Afsana Kheya; draft manuscript preparation: Md. Parvez Anwar, A. K. M. Mominul Islam, Sabina Yeasmin and Sinthia Afsana Kheya, reviewing and editing: Ahmed Khairul Hasan and Md. Harun Or Rashid. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data will be available on request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Costa R, Pinheiro N, Ameida AS, Gomes C, Coutinho J, Coco J, et al. Effect of sowing date and seeding rate on bread wheat yield and test weight under Mediterranean conditions. Emirates J Food Agric. 2013;25(12):951–61. doi:10.9755/ejfa.v25i12.16731. [Google Scholar] [CrossRef]
2. Siddiqui MH, Iqbal MA, Naeem W, Hussain I, Khaliq A. Bio-economic viability of rainfed wheat (Triticum aestivum L.) cultivars under integrated fertilization regimes in Pakistan. Custos e Agronegocio. 2019;15(3):81–96. [Google Scholar]
3. Islam AKMM, Uddin MN, Yeasmin S, Kheya SA, Islam MS, Ahmed S, et al. Preliminary report on the comparative weed suppressibility of Bangladeshi wheat varieties. Heliyon. 2023;9(4):e14942. doi:10.1016/j.heliyon.2023.e14942. [Google Scholar] [PubMed] [CrossRef]
4. Nazu SB, Khan MA, Saha SM, Hossain ME, Rashid MHA. Adoption of improved wheat management practices: an empirical investigation on conservation and traditional technology in Bangladesh. J Agric Food Res. 2021;4(1):100–43. doi:10.1016/j.jafr.2021.100143. [Google Scholar] [CrossRef]
5. Statistical year book of Bangladesh. Bangladesh: Bangladesh Statistics Division, Ministry of Planning, Govt. People’s Republic of Bangladesh; Bangladesh bureau of statistics. 2012. [Google Scholar]
6. Sawan ZM. Climatic variables: evaporation, sunshine, relative humidity, soil and air temperature and its adverse effects on cotton production. Inf Process Agric. 2018;5(1):134–48. doi:10.1016/j.inpa.2017.09.006. [Google Scholar] [CrossRef]
7. Hossain A, Teixeira da Silva JA. Wheat production in Bangladesh: its future in the light of global warming. AoB Plants. 2013;5:pls042. doi:10.1093/aobpla/pls042. [Google Scholar] [PubMed] [CrossRef]
8. Mottaleb KA, Govindan V, Singh PK, Sonder K, He X, Singh RP, et al. Economic benefits of blast-resistant biofortified wheat in Bangladesh: the case of BARI Gom 33. Crop Prot. 2019;123:45–58. doi:10.1016/j.cropro.2019.05.013. [Google Scholar] [PubMed] [CrossRef]
9. Wood SA, Jina AS, Jain M, Kristjanson P, DeFries RS. Smallholder farmer cropping decisions related to climate variability across multiple regions. Glob Environ Change. 2014;25(1):163–72. doi:10.1016/j.gloenvcha.2013.12.011. [Google Scholar] [CrossRef]
10. Sen A, Puthur JT. Influence of different seed priming techniques on oxidative and antioxidative responses during the germination of Oryza sativa varieties. Physiol Mol Biol Plants. 2020;26(3):551–65. doi:10.1007/s12298-019-00750-9. [Google Scholar] [PubMed] [CrossRef]
11. Paparella S, Araújo SS, Rossi G, Wijayasinghe M, Carbonera D, Balestrazzi A. Seed priming: state of the art and new perspectives. Plant Cell Rep. 2015;34(8):1281–93. doi:10.1016/B978-0-12-818032-7.00006-0. [Google Scholar] [CrossRef]
12. Moosavi A, Afshari RT, Sharif-Zadeh F, Aynehband A. Effect of seed priming on germination characteristics, polyphenoloxidase, and peroxidase activities of four amaranth cultivars. J Food Agric Environ. 2009;7:353–8. [Google Scholar]
13. Harris D, Raghuwanshi BS, Gangwar JS, Singh SC, Joshi KD, Rashid A, et al. Participatory evaluation by farmers of on-farm seed priming in wheat in India, Nepal and Pakistan. Exp Agric. 2001;37(3):403–15. doi:10.1017/S0014479701003106. [Google Scholar] [CrossRef]
14. Harris D, Joshi A, Khan PA, Gothkar P, Sodhi S. On farm seed priming in semi-arid agriculture: development and evaluation in maize, rice and chickpea in India using participatory methods. Exp Agric. 1999;35(1):15–29. doi:10.1017/S0014479799001027. [Google Scholar] [CrossRef]
15. Basra SMA, Farooq M, Khaliq A. Comparative study of pre-sowing seed enhancement treatments in fine rice (Oryza sativa L.). Pak J Life Soc Sci. 2003;1:5–9. [Google Scholar]
16. Farooq M, Basra SMA, Hafeez K. Rice seed invigoration by osmo hardening in coarse and fine rice. Seed Sci Technol. 2006a;34(1):181–6. doi:10.15258/sst.2006.34.1.19. [Google Scholar] [CrossRef]
17. Farooq M, Aziz T, Cheema MA, Hussain M, Khaliq A. Activation of antioxidant system by KCl improves the chilling tolerance in hybrid maize. J Agron Crop Sci. 2008;194:438–48. doi:10.1111/j.1439-037X.2008.00334.xAnwar. [Google Scholar] [CrossRef]
18. Mim TF, Anwar MP, Ahmed M, Sriti N, Moni EH, Hasan AK, et al. Competence of different priming agents for increasing seed germination, seedling growth and vigor of wheat. Fundam Appl Agric. 2021;6(4):444–59. doi:10.5455/faa.46026. [Google Scholar] [CrossRef]
19. AOSA (Association of Official Seed Analysis). Seed vigour testing handbook. Contribution No. 32 to the handbook on seed testing. Springfield, IL: AOSA; 1983. [Google Scholar]
20. Islam AKMM, Kato-Noguchi H. Phytotoxic activity of Ocimum tenuiflorum extracts on germination and seedling growth of different plant species. Sci World J. 2014;2014. doi:10.1155/2014/676242. [Google Scholar] [PubMed] [CrossRef]
21. Copeland LO. Principles of seed science and technology. Minneapolis, Minnesota: Burgess Pub. Com; 1976. p. 164–5. [Google Scholar]
22. Gomez K, Gomez A. Statistical procedure for agricultural research. New York, USA: John Wiely and Sons; 1984. [Google Scholar]
23. Misra NM, Dwivedi DP. Effects of pre-sowing seed treatments on growth and dry-matter accumulation of high yielding wheat under rain-fed conditions. Indian J Agron. 1980;25:230–4. [Google Scholar]
24. Hamza JH. Seed priming of bread wheat to improve germination under drought stress. Iraqi J Agric Sci. 2012;43(2):100–7. [Google Scholar]
25. Farooq M, Wahid A, Basra SM, Rehman A, Siddique KH. Improving crop resistance to abiotic stresses through seed invigoration. In: Handbook of plant and crop stress. USA: CRC Press; 2010. p. 1031–50. [Google Scholar]
26. Farooq MS, Basra SMA, Saleem BA, Nafees M, Chishti SA. Enhancement of tomato seed germination and seedling vigor by osmopriming. Pak J Agric Sci. 2005;42:3–4. [Google Scholar]
27. El-Sanatawy AM, El-Kholy AS, Ali MM, Awad MF, Mansour E. Maize seedling establishment, grain yield and crop water productivity response to seed priming and irrigation management in a Mediterranean arid environment. Agronomy. 2021;11(4):756. doi:10.3390/agronomy11040756. [Google Scholar] [CrossRef]
28. Bray CM, Davison PA, Ashraf M, Taylor R. Biochemical changes during osmopriming of leek seeds. Ann Bot. 1989;63(1):185–93. doi:10.1093/oxfordjournals.aob.a087722. [Google Scholar] [CrossRef]
29. Dahal P, Bradford KJ, Jones RA. Effects of priming and endosperm integrity on seed germination rates of tomato genotypes. II. Germination at reduced water potential. J Exp Bot. 1990;41:1441–53. [Google Scholar]
30. Saha R, Mandal AK, Basu RN. Physiology of seed invigoration treatments in soybean (Glycine max L.). Seed Sci Technol. 1990;18:269–76. [Google Scholar]
31. McDonald MB. Seed priming, in seed technology and its biological basis. England: Sheffield Academic Press; 2000. p. 287–325. [Google Scholar]
32. Salehjade H, Shishavan MI, Ghiyasi M, Forouzin F, Siyahjani AA. Effect of seed priming on germination and seedling growth of wheat (Triticum aestivum L.). Res J Biol Sci. 2009;4(5):629–31. [Google Scholar]
33. Natasha K, Khalid S, Haq SIU, Jilani NS, Khan SA, Wali S. Comparative effect of sodium chloride, potassium chloride and combined salt stress on germination and growth of Triticum aestivum L. (Var. Atta. Habib). Pure Appl Biol. 2021;10(4):1450–65. doi:10.19045/bspab.2021.100151. [Google Scholar] [CrossRef]
34. Taiz L, Zeiger E, Møller IM, Murphy A. Plant physiology and Development. 2015;2015(6):761. [Google Scholar]
35. Cherel I. Regulation of K+ channel activities in plants: from physiological to molecular aspects. J Exp Bot. 2004;55(396):337–51. doi:10.1093/jxb/erh028. [Google Scholar] [PubMed] [CrossRef]
36. Hasan MN, Salam MA, Chowdhury MMI, Sultana M, Islam N. Effect of osmopriming on germination of rice seed. Banglad J Agric Res. 2016;41(3):451–60. doi:10.22004/ag.econ.209260. [Google Scholar] [CrossRef]
37. Yousof F. Effect of rice seed priming with calcium chloride (CaCl2) on germination and seedlings vigor under salinity stress. J Plant Prod. 2013;4(4):523–35. doi:10.21608/jpp.2013.72394. [Google Scholar] [CrossRef]
38. Baque A, Nahar M, Yeasmin M, Quamruzzaman M, Rahman A, Azad MJ, et al. Germination behavior of wheat (Triticum Aestivum L.) as influenced by polyethylene glycol (PEG). Univers J Agric Res. 2016;4(3):86–91. doi:10.13189/ujar.2016.040304. [Google Scholar] [CrossRef]
39. Joshi A, Kaur S, Dharamvir K, Nayyar H, Verma G. Multi-walled carbon nanotubes applied through seed-priming influence early germination, root hair, growth and yield of bread wheat (Triticum aestivum L.). J Sci Food Agric. 2018. doi:10.1002/jsfa.8818. [Google Scholar] [PubMed] [CrossRef]
40. Asaduzzaman M, Huqe M, Uddin M, Hossain M, Haque M. Seed priming improves germination and early seedling growth in wheat under control and drought condition. J Bangladesh Agric Univ. 2021;19:184–91. doi:10.5455/jbau.73529. [Google Scholar] [CrossRef]
41. Sarlach RS, Sharma A, Bains NS. Seed priming in wheat: effect on seed germination, yield parameers and grain yield. Prog Res. 2013;8(1):109–12. [Google Scholar]
42. Farooq M, Bramley H, Patla JA, Siddique KHM. Heat stress in wheat during reproduction and grain filling phases. Crit Rev Plant Sci. 2011;30(6):491–507. doi:10.1080/07352689.2011.615687. [Google Scholar] [CrossRef]
43. Fixen PE. Crop responses to chloride. Adv Agron. 1993;50:107–50. doi:10.1016/S0065-2113(08)60833-0. [Google Scholar] [CrossRef]
44. Asadujjaman M, Anwar MP, Hossain A, Kheya SA, Hasan AK, Yeasmin S, et al. Augmenting spring wheat productivity through seed priming under late-sown condition in Bangladesh. Res Agric Sci. 2023;54(2):57–67. doi:10.5152/AUAF.2023.23112. [Google Scholar] [CrossRef]
45. Farooq MA, Wahid O, Lee DJ, Siddique KHM. Advances in drought resistance of rice. Crit Rev Plant Sci. 2009;28(4):199–217. doi:10.1080/07352680902952173. [Google Scholar] [CrossRef]
46. Meena RP, Sendhil R, Tripathi SC, Chander S, Chhokar RS, Sharma RK. Hydropriming of seed improves the water use efficiency of grain yield and economic return of wheat under different moisture regime. SAARC J Agric. 2013;11(2):149–59. doi:10.3329/sja.v11i2.18410. [Google Scholar] [CrossRef]
47. Ugale RV, Mungse HB. Effect of pre-sowing seed hardening on yield and yield components of wheat. J Maharashtra Agric Univ. 2001;26(1):103–4. [Google Scholar]
48. Farooq M, Tabassum R, Afzal I. Enhancing the performance of direct seeded fine rice by seed priming. Plant Prod Sci. 2006b;9(4):446–56. doi:10.1626/pps.9.446. [Google Scholar] [CrossRef]
49. Toklu F, Shehzad F, Baloch Karaköy T, Ozkan H. Effects of different priming applications on seed germination and some agro morphological characteristics of bread wheat (Triticum aestivum L.). Turk J Agric. 2015;39:1005–13. doi:10.3906/tar-1404–1441. [Google Scholar] [CrossRef]
50. Ramamurthy V, Venugopalan MV, Parhad VN, Prasad J. Effect of seed priming on emergence and yield of late sown wheat (Triticum aestivum L.) on typic haplusterts of Central India. Indian J Agric Res. 2015;49(3):245–9. doi:10.5958/0976-058X.2015.00038.4. [Google Scholar] [CrossRef]
51. Suryakant, Pahuja SS, Verma SS. Productivity of late sown wheat (Triticum aestivum) as influenced by different seed priming treatments. Haryana J Agron. 2000;16(1,2):35–9. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools