 Open Access
Open Access
ARTICLE
Various Organic Nutrient Sources in Combinations with Inorganic Fertilizers Influence the Yield and Quality of Sweet Corn (Zea mays L. saccharata) in New Alluvial Soils of West Bengal, India
1 Department of Agronomy, School of Agricultural Sciences, JIS University, Kalyani, Nadia, West Bengal, 741235, India
2 Department of Agronomy, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, West Bengal, 741252, India
3 Department of Agronomy, Uttar Banga Krishi Viswavidyalaya, Coochbehar, West Bengal, 736101, India
4 Office of The Assistant Director of Agriculture, Gosaba Block, Arampur, South 24 Parganas, West Bengal, 743370, India
5 Department of Agricultural Statistics, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, West Bengal, 741252, India
6 Zoology Department, College of Science, King Saud University, Riyadh, 11451, Saudi Arabia
7 Department of Botany, Hindu College Moradabad (Mahatma Jyotiba Phule Rohilkhand University, Bareilly), Moradabad, Uttar Pradesh, 244001, India
8 Institute of Plant and Environmental Sciences, Slovak University of Agriculture, Tr. A. Hlinku 2, Nitra, 949 01, Slovak
9 Division of Soil Science, Bangladesh Wheat and Maize Research Institute, Dinajpur, 5200, Bangladesh
* Corresponding Author: Akbar Hossain. Email:
(This article belongs to the Special Issue: Integrated Nutrient Management in Cereal Crops)
Phyton-International Journal of Experimental Botany 2024, 93(4), 763-776. https://doi.org/10.32604/phyton.2024.049473
Received 09 January 2024; Accepted 19 March 2024; Issue published 29 April 2024
Abstract
Nutrient management plays a crucial role in the yield and quality of sweet corn. A field experiment was conducted in consecutive two kharif seasons in 2018 and 2019 to investigate the effect of various organic sources of nutrients in combination with inorganic sources on the yield and quality of sweet corn under new alluvial soils of West Bengal, India. Treatments were: T: Control (without fertilizers); T: 100% recommended dose (RDF) of chemical fertilizers (CF) (RDF CF); T: 100% recommended dose of N (RDN) through vermicompost (VC) (RDN VC); T: 50 RDN through CF + 50% RDN through VC (RDN CF + RDN VC); T: 50% RDF through CF + 50% RDN through organic source (OS)1, Soligro (Ascophyllum nodosum) granular (RDN CF + RDN OS); T: 50% RDN through CF + 50% RDN through OS 2, Bioenzyme (liquid) (RDN CF + RDN OS); T: 50% RDN through CF + 50% RDN through OS 3, Opteine (Ascophyllum nodosum) filtrate [RDN CF + RDN OS]; T: 50% RDN through VC + 50% RDF through OS 1, Soligro (Ascophyllum nodosum) granular (RDN VC + RDN OS). The OS of fertilizers were VC, SoliGro Gr (OS1) (Ascophyllum nodosum), Bioenzyme liquid (OS2), and Opteine (Ascophyllum nodosum) filtrate (OS3). The inorganic source was traditional CF applied at the RDF (150:75:75 kg ha of N: PO:KO). The VC was used to supply 100% RDN as one source or 50% RDN when combined with CF or OS. Maximum fruit yield (10.75 and 10.79 t ha in 2018 and 2019, respectively) was recorded when RDF was substituted through CF only, being statistically at par with 50% CF + 50% VC on a nitrogen equivalent basis (9.92 and 10.00 t ha in 2018 and 2019, respectively) and 100% VC (8.22 and 8.32 t ha in 2018 and 2019, respectively). Compared to chemical sources of nutrients, VC-based treatments produced a larger percentage of large-size cob (>25 cm). The 100% VC increased antioxidant (8.35 and 8.45 mg g), carotenoid (0.59 and 0.61 mg/100 g), and phenol (55.06 and 55.02 mg 100 g) content compared with its 50% dose in combination with other sources. The study revealed the potentiality of organic sources towards achieving improved cob quality of sweet corn.Keywords
Sweet corn (Zea mays L. saccharata), frequently referred to as sugar corn, is a hybrid type of maize (Zea mays L.) that was specially bred to increase the sugar content and was obtained from the USA. It is cultivated mainly for human consumption as a vegetable instead of a grain. The amount of sugar and starch in the endosperm controls a crop’s sweetness, which is its primary determining factor [1]. The internal conversion of sugar to starch in the kernel is mostly regulated by recessive gene mutations. Sweet corn is not just a staple grain; it may be consumed fresh as a dessert item. Sweet corn is mostly consumed as a vegetable instead of a grain and needs to be plucked during the milking stage, unlike field corn varieties [2]. Additionally, the nutritional value of this crop has increased the demand among consumers. Kernals contain 18%–20% carbohydrates, 5%–6% free sugars, 2.1%–4.5% proteins, 70% water, vitamin A and many other minerals [3]. The sweetness, color, odor, and tenderness of sweet corn are the main attributes perceived by the consumer and are therefore the most important attributes to be preserved [4].
Sweet corn is becoming increasingly popular in modern cooking as a foundation for salads, soups, syrups, sweets, jams, creams, and other delicious dishes. Its feed is also very succulent, flavourful, and easy to digest. Considering the potential to become one of the largest producers in the country, recently, the local government has targeted increasing the area of maize crops. Sweet corn may be a suitable option for inclusion in the system due to its shorter duration and industrial importance. To date, no such agronomic research has been performed on this crop in the state of West Bengal. As an exhaustive crop, it requires more nutrients to flourish. Therefore, maintaining the production of sweet corn requires careful management of plant nutrients. Moreover, the use of chemical fertilizers at high rates can hamper soil productivity and environmental quality [5]. In this context, the slow release of nutrients from organic sources is very useful for preserving the soil nutrient balance and maintaining soil fertility [6]. This is why the integration of organic and inorganic sources of nutrients helps to coordinate nutrient release with crop needs and reduce the extent of chemical load due to environmental pollution [7]. A wealth of research suggests that the use of nitrogenous chemical fertilizers under integrated nutrient management speeds up mineralization by reducing the large C:N ratio of carbon-rich, low-nitrogen organic matter [8]. Additionally, this integration supports the long-term improvement of soil quality, the accumulation of soil carbon, and the repair of secondary and micronutrient deficiencies, thus leading to sustainability [9,10]. Hence, in situations where horizontal land intensification is not possible, overall productivity must be increased through the methodical application of environmentally sustainable techniques [10]. In this regard, even environmentally benign biostimulants such as biozymes and seaweed extracts are becoming increasingly important for maintaining agricultural productivity globally. Extracts from the seaweed Ascophyllum nodosum (ANE) have been reported to exhibit growth-stimulating activities when applied repeatedly at very low doses [11]. These extracts increase stress tolerance, nutrient uptake, growth, and yield; reduce seed dormancy; enhance root systems, flowering [12], fruit quality, and taste [13]; and significantly improve phytochemical activity and antioxidant content [14]. Seaweed extracts are complex mixtures that include proteins, osmoprotectants, different types of carbohydrates, amino acids, and a tiny amount of phytohormones [15,16]. The extract has potential applications as a foliar spray and presowing seed-soaking agent [17]. Integration of these biostimulants either through soil or foliar application with organic and inorganic sources of primary nutrients might be more effective in supplying the nutrient demands of an exhaustive crop such as sweet corn. Therefore, the present study was designed to evaluate the impact of biostimulants combined with organic and inorganic nutrients on sweet corn productivity and quality parameters.
The field experiment was conducted during the kharif seasons of 2018 and 2019 at the C block farm, Kalyani, under Bidhan Chandra Krishi Vishwavidyalaya (BCKV), Nadia, West Bengal, India (22°57" N, 88°2" E, elevation 9.75 meters above the MSL). The climate at the trial site was subtropical, with maximum and minimum temperatures ranging between 35.4°C and 11.70°C and between 36.1°C and 13.90°C during 2018 and 2019, respectively (Fig. 1). During both the experimental years of 2018 and 2019, the total rainfall was 1054.8 and 490.2 mm, respectively. The relative humidity ranged from 39.9% to 95.2% and 43% to 91.6% in 2018 and 2019, respectively (Fig. 1). The maximum and minimum bright sunshine hours observed were 7.5 to 4 h (2018) and 7.4 to 3.4 (2019) h day−1, respectively. The suitable temperature for the growth of corn is 20°C, with 500–700 mm of rainfall during the crop growth period. Poor grain formation may arise from the exceptionally high temperature and low humidity during flowering, but based on the meteorological parameters prevailing during the period of experimentation, it was evident that both years were favourable for sweet corn growth and development.
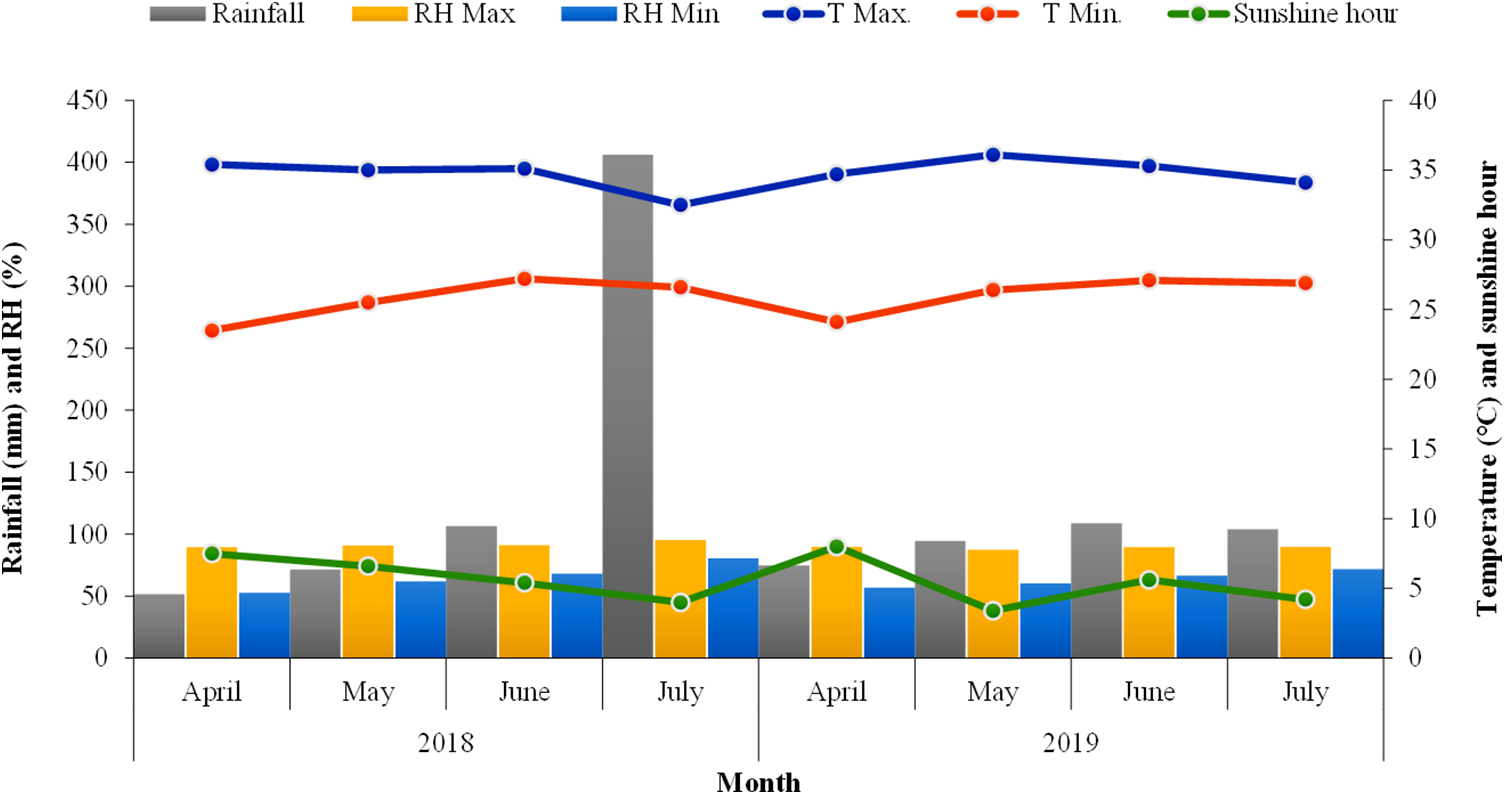
Figure 1: Monthly rainfall, temperature, relative humidity, and sunshine hours for different months during the crop growth periods of 2018 and 2019
For NPK analysis, initial soil samples (0–30 cm depth) were taken to provide a general understanding of the experimental field’s fertility state. The effective rhizosphere of the crop was mostly confined to the top 30 cm layer, for which we collected soil samples from the top 0–30 cm soil layer. The nutrient concentrations may be lower at subsurface depths. The amount of accessible soil N was estimated using the hot alkaline permanganate method [18]. The soil was extracted with 0.5 M NaHCO3, pH 8.5 [19], and the amount of accessible phosphorus (P) was measured using a UV–VIS spectrophotometer. Using neutral normal ammonium acetate as an extracting reagent, the amount of accessible potassium (K) in the soil was measured, and a flame photometer was used to determine the final amount [20]. The initial physicochemical properties of the experimental soil are summarized in Table 1.
Eight treatments, each of which was replicated three times, were arranged in a randomized block design (RBD) experiment. The details of the treatments are as follows:
T1: Control (without fertilizers); T2: 100% recommended dose of chemical fertilizers (RDF CF100%); T3: 100% recommended dose of N through vermicompost (RDN VC100%); T4: 50% recommended dose of N through chemical fertilizers + 50% recommended dose of N through vermicompost (RDN CF50% + RDN VC50%); T5: 50% recommended dose through chemical fertilizers + 50% recommended dose of N through organic source 1, Soligro (Ascophyllum nodosum) granular (RDN CF50% + RDN OS150%); T6: 50% recommended dose of N through chemical fertilizers + 50% recommended dose of N through organic source 2, Bioenzyme (liquid) [RDN CF50% + RDN OS250%]; T7: 50% recommended dose of N through chemical fertilizers + 50% recommended dose of N through organic source 3, Opteine (Ascophyllum nodosum) filtrate [RDN CF50% + RDN OS350%]; T8: 50% RDN through VC + 50% RDF through OS 1, Soligro (Ascophyllum nodosum) granular [RDN VC50% + RDN OS150%].
The overall fertilizer recommendation for sweet corn is 150-75-75 kg N-P2O5-K2O ha−1. Urea (46% N), single superphosphate (16% P2O5), and muriate potash (60% K20) were used as chemical fertilizer sources. For organic source 1, SoliGro Grp was applied as a basal application in the soil, while for organic sources 2 and 3, enzymes and opteine were applied as foliar sprays @ 625 ml ha−1 at 35 days after sowing (DAS). The sweet corn variety Sugar-75, which is a short-duration (75–80 days) hybrid variety of maize with a potential yield of up to 20 t ha−1 depending on the season, geography, and cultural practices (Syngenta, India), was used as the test variety. The seeds were manually sown at 10 kg/ha during the first fortnight of April. The plots were 5 m long and 4 m wide and had a 1 m long irrigation channel. The sowing was performed at a distance of 40 cm row to row, followed by 20 cm plant to plant by the dibbling method, and the seeds were placed at a depth of 2 cm and finally covered with soil. Presowing irrigation and light irrigation were applied immediately after sowing at 5-day intervals according to the rainfall, especially during the tasselling and silking stages.
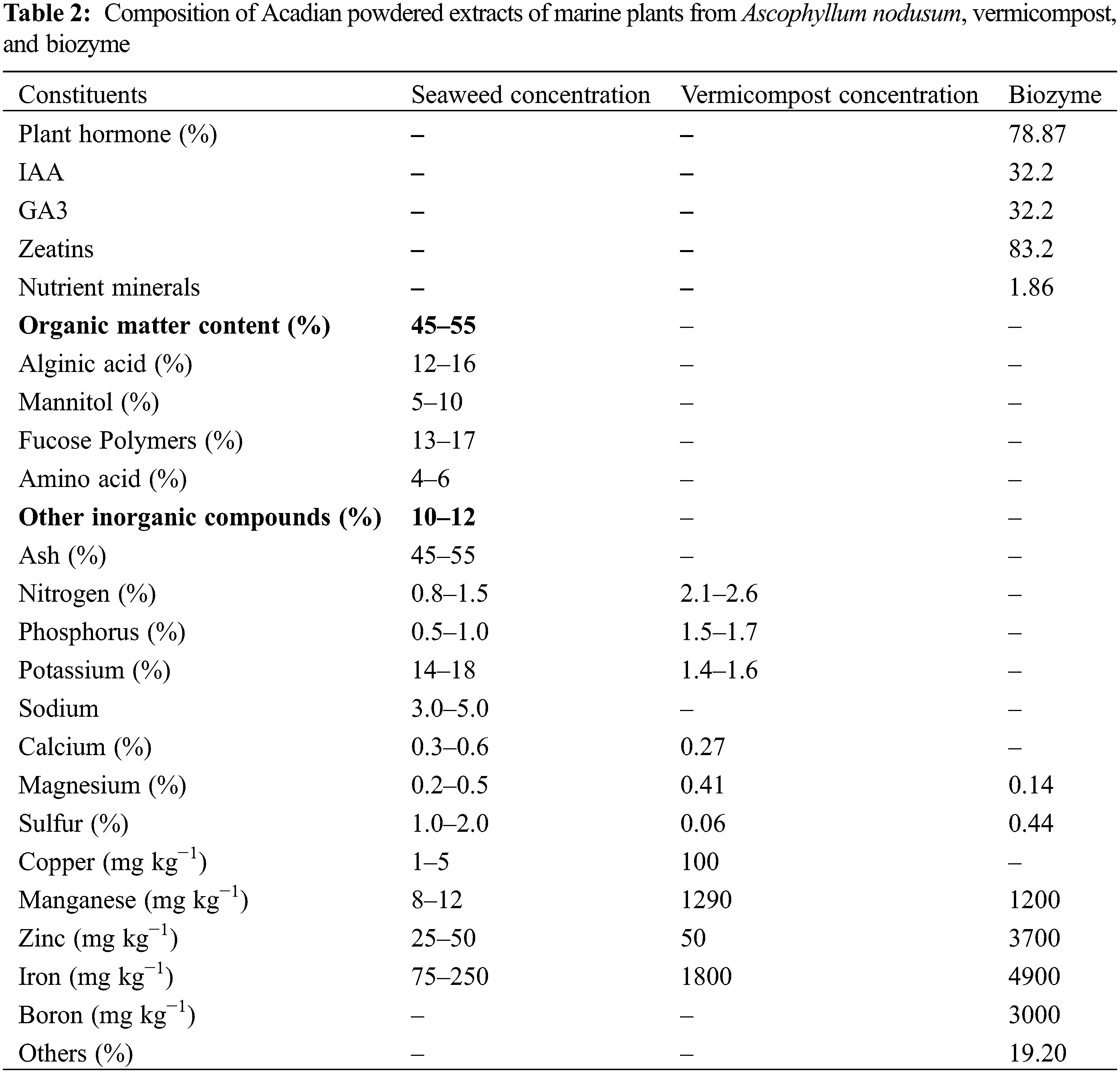
Earthing was performed manually with a spade at 30 DAS to provide support to the plant. Preemergence application of atrazine (Atratraf 50 WP) at 1.0–1.5 kg a.i. ha−1 in 600 litres of water at 2 DAS was used to treat weeds. Two manual weeding sessions were conducted at 20 and 40 DAS. At 35 DAS, 2 ml/L Solomon water was applied to combat autumn armyworms and other insects. Dithane M-45 (Mancozeb) 75 WP @ 2.5 g/L of water was used uniformly against both northern and southern maize blight at intervals of 15 days. In both growing seasons, sweet corn was manually harvested in the third week of July using a sickle. The organic sources of nutrients that were used in the experiment are available in Table 2. The cob yield was estimated based on a net plot (1 m × 1 m) area, and the fresh weight of the harvested cob was expressed in kg/ha.
2.3 Preparation of Seaweed Sap
The use of an organic source of seaweed sap as a source of plant nutrients helps to lessen the negative effects of chemical fertilizers by reducing their amount of use. For foliar applications, the preparation of seaweed sap is essential. Alkaline hydrolysis, arguably the most popular industrial hydrolysis technique, was utilized to prepare the Ascophyllum nodusum extract. Using this approach, biomass from A. nodosum was extracted at “relatively low” temperatures—between 70 and 100°C—in NaOH or KOH solutions. Complex polysaccharides were broken down into smaller, lower molecular weight oligomers via this process. When the A. nodosum biomass was treated with alkali, new chemicals were created that were not previously present in the seaweed biomass. Hydrolysis chemicals (KOH) and components of brown seaweed tissues interact to produce these compounds, which are the end products of condensation, rearrangement, breakdown, and base-catalyzed synthesis processes [24]. Additionally, polyphenols in the tissue are affected by alkali treatments of brown seaweed biomass, leading to a complicated range of reaction products that rely on the original polyphenol hydroxylation pattern [24]. The alkali extract of Ascophyllum is used in the production of three major commercial biostimulants: Acadian (Canada), Seasol (Australia), and Maxicrop (United States).
Following the estimation of yield, the cobs were ready for quality examination. The skin and membranes were removed by removing the grains, blending them using a homogenizer, and filtering them through gauze. A slurry of sweet corn weighing between 10 and 20 g was made and stored in plastic bags independently for every replication and treatment. After the bags were sealed, they were promptly kept at −70°C to facilitate the study of sweet corn quality characteristics, including total sugar, total soluble solids (TSS), protein, carbohydrate, ascorbic acid, carotenoid, and phenol contents.
The cobs were run through grading plates with a known diameter as soon as they were harvested. Next, five distinct sizes of the fruits were graded: 25, 25–20, 20–15, 15–10 and 10 cm. The total sugar content was determined by the Lane and Eynon method [25]. The total soluble solids (TSS) in sweet corn grain were analysed at the milky stage of sweet corn by using a hand refractometer and expressed in °Brix 0.1%. Using the phenol sulphuric acid method, the total carbohydrate content of sweet maize grain was determined [26]. Using a modified micro Kjeldahl method, the wet digestion method was used to assess the total nitrogen content of sweet maize grain [22]. The nitrogen content (%) in the grains was then multiplied by a factor of 6.25 to determine the protein content in the grains for each treatment, which was then represented as a percentage. Based on early determinations, the average N content of proteins was found to be approximately 16%, which led to the use of a factor of 6.25 (100/16). N × 6.25 should be used because proteins are composed of chains of amino acids joined by peptide bonds. This protein is sometimes referred to as a “true protein”. A spectrophotometric approach, as described by Sadasivam et al. [25], was used to estimate the beta-carotene content. By combining 2,4-dinitrophenyl hydrazine (DNPH) with the ketonic groups of dehydroascorbic acid by the oxidation of ascorbic acid by 2,6 dichlorophenolindophenol (DCPIP), which forms a yellowish orange color under acidic conditions, the total ascorbic acid content was determined [27]. The total phenol content was determined spectrophotometrically [28]. Two grams of sample was ground in a mortar and pestle in a 10 × volume of 80% ethanol. Extracts (100 µl) were diluted in 2.9 ml of distilled water, 0.5 ml of Folin-Ciocalteu reagent, and 20% Na2CO3, and the mixture was allowed to stand for 90 min. The absorption was measured against the reagent blank in a UV–VIS spectrophotometer at 760 mm. The total phenol content was calculated by using a standard curve and is expressed as mg/100 g. A calibration curve was drawn with standard catechol, and the results are expressed as catechol equivalents (mg CE/100 g fresh weight).
The data for each parameter from the 2-year experiment were subjected to statistical analysis using analysis of variance (ANOVA), and the effect of treatments was evaluated by standard procedures as described by Gomez et al. [29]. Post hoc Duncan’s multiple range test (DMRT) was carried out at a 0.05% probability (p ≤ 0.05) level.
3.1 Yield of Sweet Corn as Influenced by Various Organic and Inorganic Sources of Nutrients
Various treatments have a substantial impact on the cob production of sweet corn (Fig. 2). The maximum corn yield (10.75 and 10.79 t ha−1 in 2018 and 2019, respectively) was noted in the treatment comprising 100% RD supplied through CF, representing an approximately 150% increase in yield. It was noted that the application of nutrients through a suboptimal dose of chemical fertilizer with seaweed extract always produced a lower yield than a full dose of nutrients either through the sole application of organic and inorganic fertilizer or their combination. However, the corn yield of soliGro Gr in combination with vermicompost was lower (5.31 and 5.48 t ha−1 in 2018 and 2019, respectively) than that of the other organic sources (OS) with chemical fertilizer.
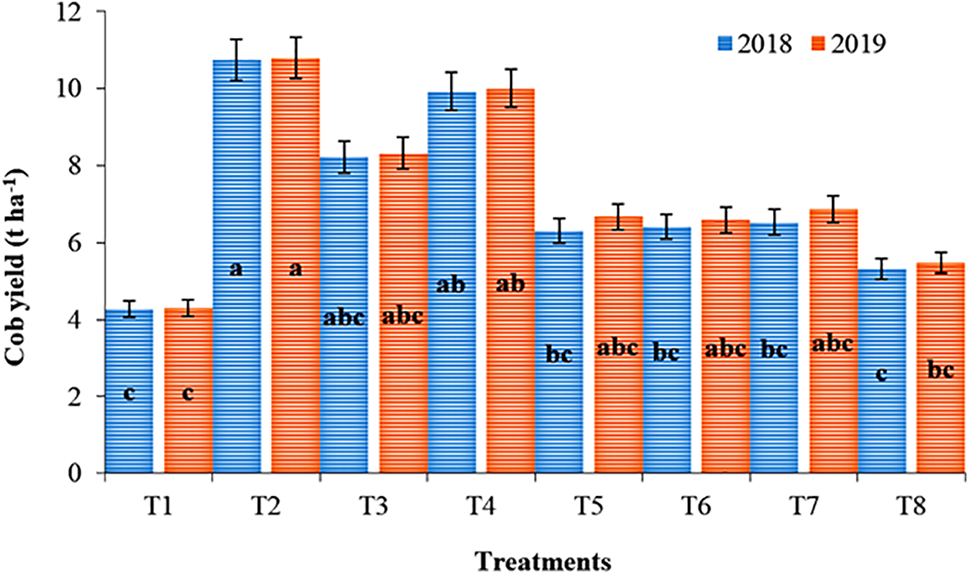
Figure 2: Yield of sweet corn influenced by various organic sources of nutrients in combination with inorganic fertilizers during the crop growing periods of 2018 and 2019. T1: Control plot where no fertilizers were applied; T2: 100% recommended dose through chemical fertilizers (RDF CF100%); T3: 100% recommended N dose through vermicompost (RDN VC100%); T4: 50% recommended N dose through chemical fertilizers + 50% recommended N dose through vermicompost (RDN CF50% + RDN VC50%); T5: 50% recommended dose through chemical fertilizers + 50% recommended N dose through organic source 1, Soligro (Ascophyllum nodosum) granular (RDN CF50% + RDN OS150%); T6: 50% recommended N dose through chemical fertilizers + 50% recommended N dose through organic source 2, Bioenzyme (liquid) [RDN CF50% + RDN OS250%]; T7: 50% recommended N dose through chemical fertilizers + 50% recommended N dose through organic source 3, Opteine (Ascophyllum nodosum) filtrate [RDN CF50% + RDN OS350%]; T8: 50% recommended dose N through vermicompost + 50% recommended N dose through organic source 1, Soligro (Ascophyllum nodosum) granular [RDN VC50% + RDN OS150%]. The means followed by the same letters in a column are not significantly different at the p ≤ 0.05 level. The ±SE (standard error) in each treatment was estimated from three replications
3.2 The Quality of Sweet Corn as Influenced by Various Organic and Inorganic Sources of Nutrients
For all the treatments, the maximum proportion of cobs was in the 15–20 cm layer. Concerning RDN, the percentage of cob sizes larger than 25 cm was greatest in the VC100% treatment group, followed by the VC50% + OS1 and VC50% + CF50% RDN treatment groups (Fig. 3).
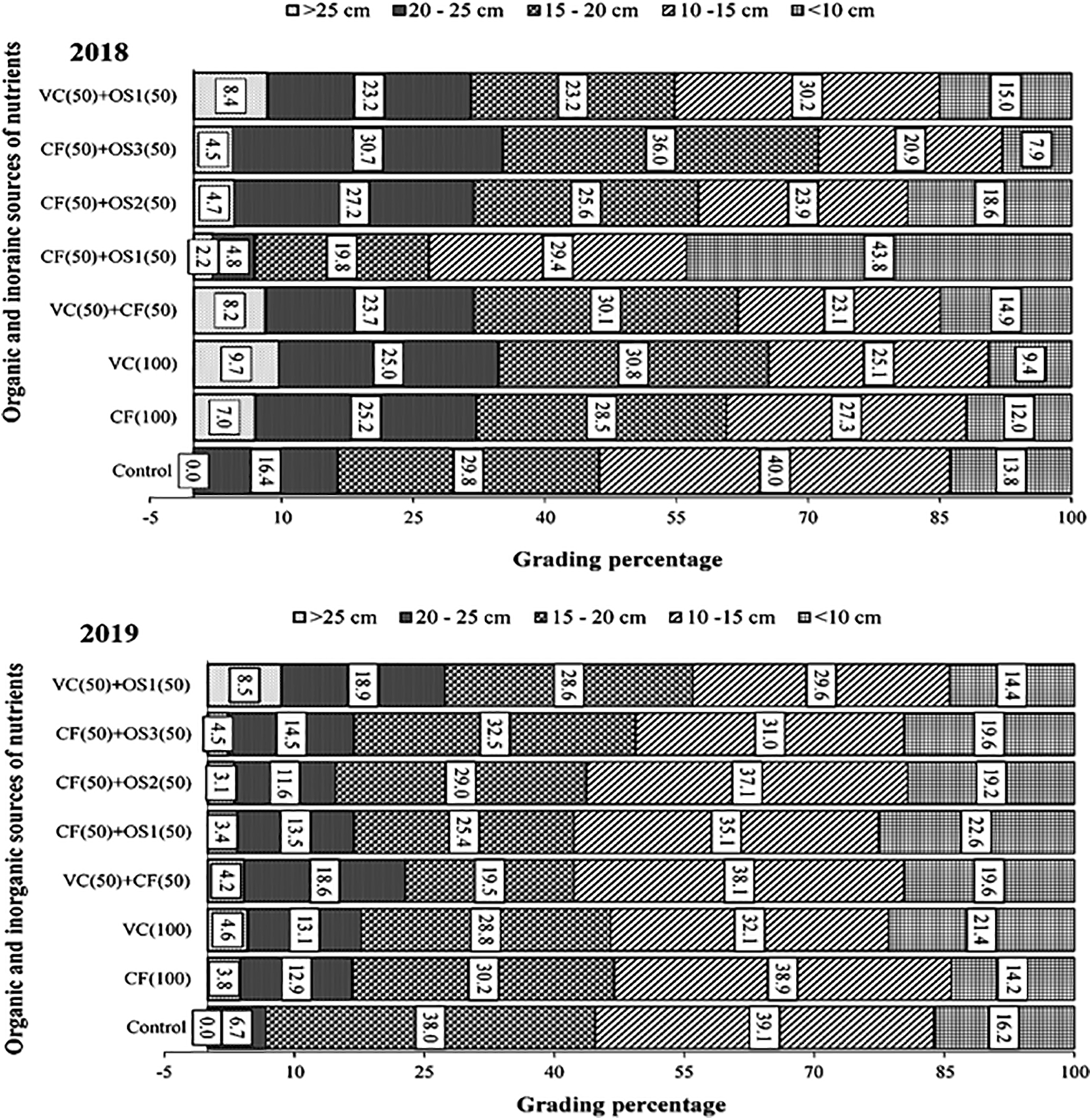
Figure 3: Effect of fertilizer sources on sweet corn cob grade during 2018 and 2019. T1: Control plot where no fertilizers were applied; T2: 100% recommended dose through chemical fertilizers (RDF CF100%); T3: 100% recommended N dose through vermicompost (RDN VC100%); T4: 50% recommended N dose through chemical fertilizers + 50% recommended N dose through vermicompost (RDN CF50% + RDN VC50%); T5: 50% recommended dose through chemical fertilizers + 50% recommended N dose through organic source 1, Soligro (Ascophyllum nodosum) granular (RDN CF50% + RDN OS150%); T6: 50% recommended N dose through chemical fertilizers + 50% recommended N dose through organic source 2, Bioenzyme (liquid) [RDN CF50% + RDN OS250%]; T7: 50% recommended N dose through chemical fertilizers + 50% recommended N dose through organic source 3, Opteine (Ascophyllum nodosum) filtrate [RDN CF50% + RDN OS350%]; T8: 50% recommended dose N through vermicompost + 50% recommended N dose through organic source 1, Soligro (Ascophyllum nodosum) granular [RDN VC50% + RDN OS150%]
During the first year (2018), the control treatment yielded the greatest percentage of cobs that were smaller in size (10–15 cm). However, in the second year, the RDN VC50% + OS1 treatment produced the highest percentage of large fruits (>25 cm) among all the treatments. When a half dose of N was applied through chemical fertilizers along with organic sources, a greater percentage of large-size cobs were noted, particularly in the RDN VC50% + OS1 treatment. The different quality parameters of sweet corn, namely, total sugar content (%), TSS (°Brix), total carbohydrate (%), protein (%), carotenoid (mg/100 g), ascorbic acid (mg g−1), and phenol (mg/100 g), were significantly influenced by different organic and inorganic fertilizer sources and their combinations; however, the lowest value was obtained in the control plot only (Tables 3 and 4).
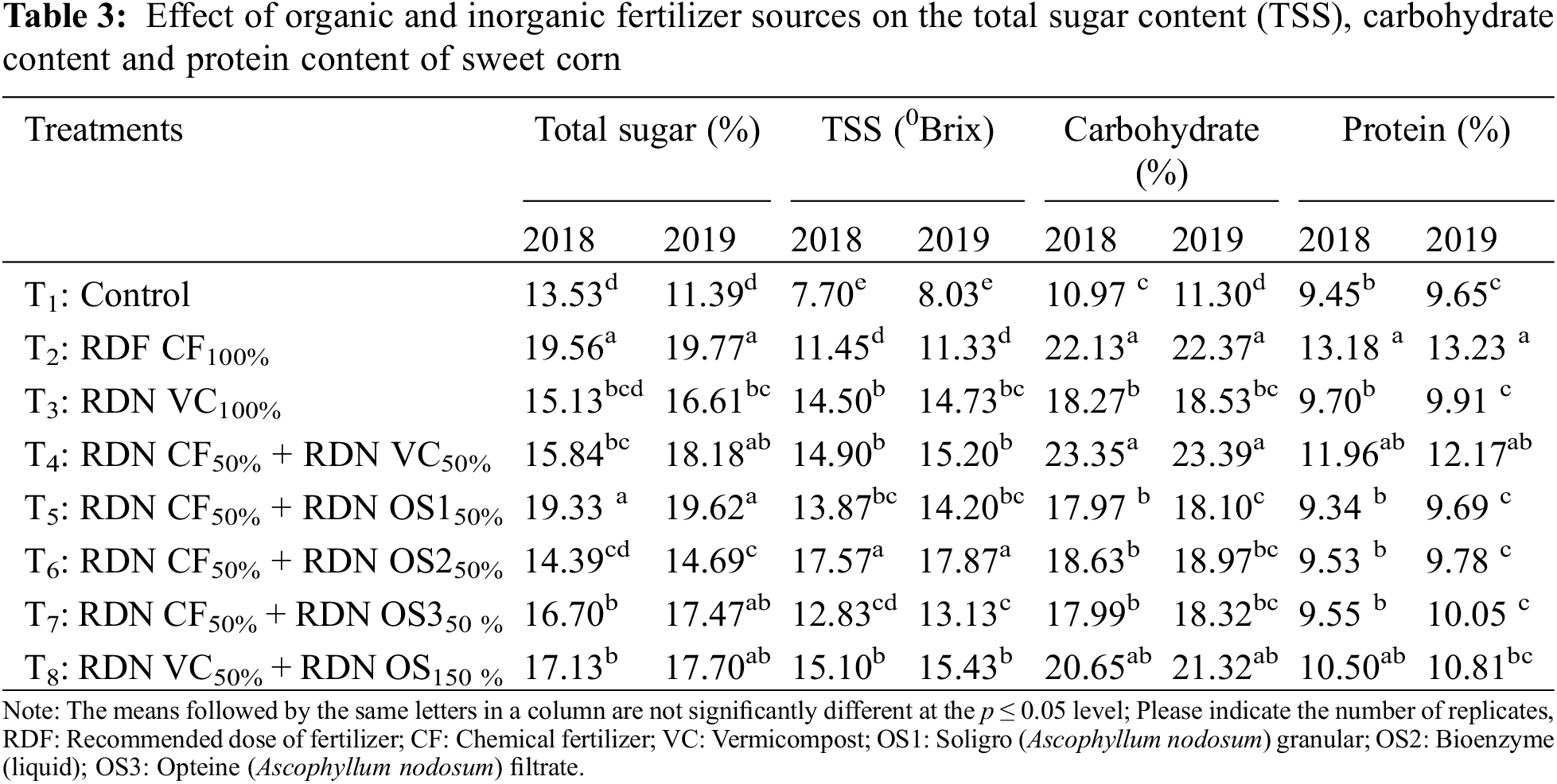
The maximum corn sugar content (19.56% and 19.77% in 2018 and 2019, respectively) was recorded after the application of the entire dose of recommended chemical fertilizer (RDF CF100%), which was on par with the 50% of the recommended dose of chemical fertilizer and 50% of the recommended dose of soliGro on a nitrogen-equivalent basis (19.33% and 19.62% in 2018 and 2019, respectively), with 44.56% and 73% of the total sugar enhancement occurring in 2018 and 2019, respectively.
During the experimental period, the total soluble solid (TSS) content was significantly influenced by different treatment combinations. The maximum TSS values (17.57 and 17.870 Brix in 2018 and 2019, respectively) were observed for corn receiving 50% of the recommended dose of chemical fertilizer along with 50% of the recommended dose of bioenzymes (liquid) on a nitrogen equivalent basis (RDN CF50% + RDN OS250%), which was significantly greater than that of the other treatments. Interestingly, the full dose of the recommended fertilizer (RDF CF100%) resulted in less TSS among all the treatment combinations.
The highest carbohydrate content (23.35% and 23.37% in 2018 and 2019, respectively) was obtained when sweet corn was treated with a 50% recommended dose of chemical fertilizer along with a 50% recommended dose of vermicompost on a nitrogen equivalent basis (RDN CF50% + RDN VC50%), which was statistically at par with RDFCF100%. It was also observed that different seaweed combinations resulted in significantly good results.
The maximum corn protein content (13.18% and 13.23% in 2018 and 2019, respectively) was obtained with the application of an entire dose of the recommended chemical fertilizer, i.e., RDFCF100%, which was statistically at par with the 50% recommended dose of chemical fertilizer along with the 50% recommended dose of vermicompost on a nitrogen-equivalent basis (RDN CF50% + RDN VC50%). Concerning this trait, we observed that different seaweed applications combined with the recommended chemical fertilizer failed to influence the protein content of sweet corn.
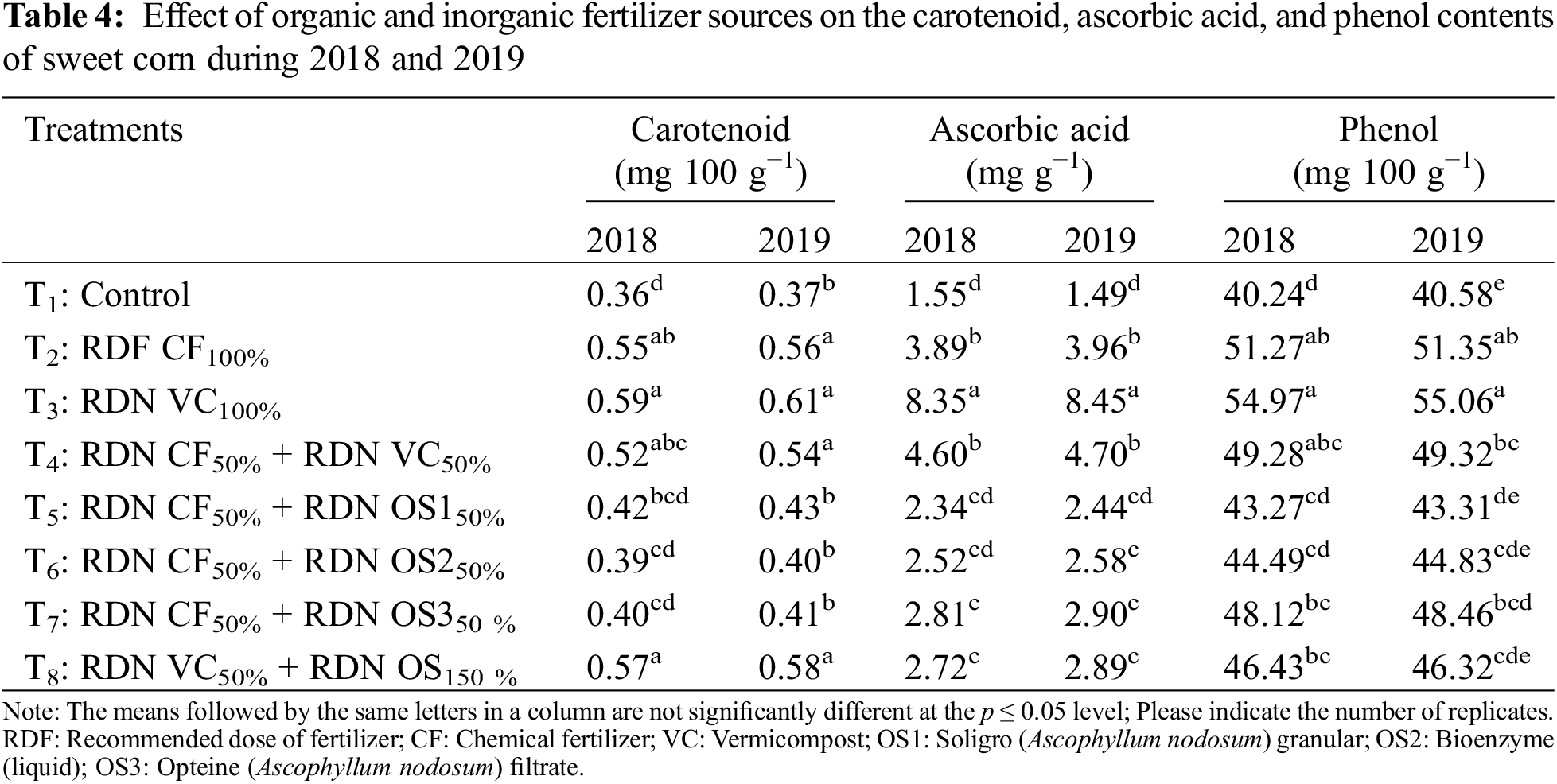
A significant maximum carotenoid content (0.59 and 0.61 mg/100 in 2018 and 2019, respectively) was obtained with the application of an entire dose of vermicompost in nitrogen equivalent, i.e., RDN VC100%, which was statistically at par with the values obtained from the corn treated with RDF CF100% and RDN VC50% + RDN OS150%.
The ascorbic acid content of sweet corn varied under the different fertilizer treatments. The maximum ascorbic acid (8.35 and 8.45 mg g−1 under 2018 and 2019, respectively) content was obtained when the entire dose of the recommended vermicompost (RDN VC100%) was applied, which was found to be significantly superior to the other treatments.
The optimal dose of fertilizer supplied either through chemical or organic sources produced more phenolic compounds than did the suboptimal dose or control treatments. The maximum corn phenol content (54.97 and 55.06 mg 100 g−1 in 2018 and 2019, respectively) was recorded following the application of a full dose of vermicompost on a nitrogen-equivalent basis (RDN VC100%).
4.1 Yield of Sweet Corn as Influenced by Various Organic and Inorganic Sources of Nutrients
The entire dose of either vermicompost or chemical fertilizer or their combined application at 50% was superior for achieving higher sweet corn cob yields. In this experiment, the maximum cob yield was observed when chemical fertilizers were applied at the full dose. The increase in cob yield under the recommended dose of nutrients applied through chemical fertilizers might be attributed to the faster rate of mineralization, which could increase the availability of nutrients for crops compared to organic sources. Thus, the yield advantage of the application of a full dose of chemical fertilizer was mainly due to its ability to supply essential plant nutrients, which in turn resulted in increased nutrient uptake. Xiao et al. [30] also reported quick nutrient release from chemical fertilizers over organic sources in supporting short-duration crops. Furthermore, the increase in yield with the integrated application of chemical and vermicompost shows the role of organic manure in crop growth and development in the reproductive phase. In terms of providing nutrient components in the available form, the combined application of chemical and vermicompost was also shown to be promising. In addition to vital minerals and growth-promoting compounds, there was a gradual release of nutrients from organic sources with faster release from the CF. This finding conformed to the findings of other workers [31], who reported higher yields with VC in combination with CF. During mineralization, the essential nutrients were released and utilized for proper synthesis, which ultimately resulted in enhanced plant growth and grain setting in sweet corn. This resulted in better yield components for which we obtained higher crop yields.
4.2 The Quality of Sweet Corn as Influenced by Various Organic and Inorganic Sources of Nutrients
In this experiment, full doses of chemical fertilizer yielded more cobs vis-à-vis the total production of sweet corn than either a suboptimal or no amount of fertilizer. When a full dose of fertilizer was applied through organic and/or chemical sources, as opposed to no fertilizer application, the number of larger-sized sweet corn cobs increased due to higher levels of available nutrients within the soil and their greater uptake. Large fruits under organic cultivation have been reported previously [32].
The higher crude protein content of chemically fertilized crops was associated with greater nitrogen availability in the soil from the chemical fertilizer, which in turn resulted in greater N uptake by the crop. The increased availability of N from chemical sources plays an important role in the synthesis of nucleic acids and proteins. Similar findings were also reported by previous workers [33,34], who reported higher crude protein in conventionally grown sweet corn than in organic corn. The increased availability of soil N in the chemically treated plot may have also contributed to the higher sugar and carbohydrate contents in the fully chemically treated corn. This, in turn, may have led to a greater accumulation of assimilates (sugars and starch) in the sink (sweet corn kernels). Similar results of increased sugar and carbohydrate contents with increasing levels of inorganic fertilizers were reported by Bala et al. [35]. Additionally, the abundance of various plant nutrients found in the ANE, including potassium, phosphorus, zinc, copper, and others, combined with other growth regulators, helps to speed up the buildup of photosynthates, or sugar and starch, in corn. The results of our experiments were consistent with those of Pramanik et al. [36], who reported that foliar spraying of seaweed along with chemical fertilizer resulted in increased sugar content. However, vermicompost combined with chemical fertilizer also improved the sugar, carbohydrate, and protein contents of sweet corn. This could be due to greater solubilization of nutrients in the root zone by the organic acids produced during vermicompost decomposition, which increases the uptake of nutrients and improves photosynthetic and metabolic activities, resulting in better partitioning of photosynthates to sinks [35]. According to our present study, Obaid et al. [37] achieved a greater TSS content after foliar spraying of bioenzyme-treated muskmelon crops, which was also in line with our results. Compared to chemical fertilizers, vermicompost added secondary nutrients as well as micronutrients to the soil. Micronutrients such as Cu, Mn, and Zn play a significant role in many vital metabolic processes and are cofactors of antioxidant enzymes.
The major secondary metabolites of sweet corn are ascorbic acid and total phenolics. The C/N balance theory states that when inorganic fertilizers are applied, nitrogen is easily available to plants and results in the production of compounds with high N content, such as protein for growth; however, when organic sources of nutrients are applied, the availability of N is limited due to slow release, and plant metabolism triggers the production of carbon-containing compounds, such as starch and cellulose, and non-N-containing secondary metabolites, such as phenolics and terpenoids [38]. In our experiment, VC application to sweet corn crops resulted in low soil available N and increased ascorbic acid and phenolic compound levels. These findings were in line with the findings of other investigators [39] who reported the abundance of phenolic phytochemicals in organically cultivated broccoli, blueberries, grapefruits, peppers, plums, potatoes, strawberries, and tomatoes. Murmu et al. [40] also reported greater amounts of ascorbic acid with a full dose of vermicompost. However, ANE did not show any desirable performance in the presence of ascorbic acid, and the phenol content might be due to the higher nitrogen content in vermicompost than in ANE. A greater carotenoid content might result from the slow and steady addition of both macro- and micronutrients to the soil, which increases plant absorption of these nutrients. This might have happened either through the complete dosage of vermicompost and seaweed or from their combination [41]. A full dose of vermicompost increased the amount of carotenoids. Micronutrients such as zinc, copper, and magnesium are important for metabolic activities and work with antioxidant enzymes as cofactors [42].
From the findings of this two-year study, it can be concluded that the application of 100% of the recommended dose of vermicompost or 50% of the recommended dose of vermicompost + 50% of the recommended chemical source resulted in a greater yield of sweet corn. However, 100% of the vermicompost had greater antioxidant (8.35 and 8.45 mg g−1), carotenoid (0.59 and 0.61 mg 100 g−1), and phenol (55.06 and 55.02 mg 100 g−1) contents than did 50% of the other treatments in combination with other sources. In addition, Ascophyllum nodosum extract increased the total sugar and TSS contents.
Acknowledgement: This project was supported by Researchers Supporting Project Number (RSP2024R7), King Saud University, Riyadh, Saudi Arabia
Funding Statement: This study was funded by the Department of Agronomy, School of Agricultural Sciences, JIS University, Kalyani, Nadia, West Bengal 741235, India. This project was also supported by Researchers Supporting Project Number (RSP2024R7), King Saud University, Riyadh, Saudi Arabia.
Author Contributions: Conceptualization, A.D., K.M., B.M., P.B., R.K., and M.R.; methodology, A.D., K.M., B.M., P.B., R.K., and M.R.; software, M.B., M.J.A., S.A., A.H. and A.D.; validation, A.D., K.M., B.M., P.B., R.K., and M.R.; formal analysis, M.B., M.J.A., S.A., A.H. and A.D.; investigation and resources, A.D., K.M., B.M., P.B., R.K., and M.R.; data curation, M.B., M.J.A., S.A., A.D., and A.H.; writing—original draft preparation, A.D., K.M., B.M., P.B., R.K., and M.R.; writing—review and editing, M.B., M.J.A., S.A., A.H., B.M., and P.B.
Availability of Data and Materials: The data may be available upon request to the corresponding author.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no competing interests.
References
1. Tracy WF, Hallauer AR. Sweet corn in ‘Speciality Corns’. Boca Raton, USA: SRC Press Inc.; 1994. p. 148–87. [Google Scholar]
2. Lahay RR, Sipayung R, Sabrina T. The growth and yield of sweet corn (Zea mays saccharata Sturt.) with inorganic and organo-bio fertilizer. IOP Conf Series: Earth Environ Sci. 2019;260(1):012156. [Google Scholar]
3. Khan AA, Hussain A, Ganai MA, Sofi NR, Hussain ST. Yield, nutrient uptake and quality of sweet corn as influenced by transplanting dates and nitrogen levels. J Pharmacogn Phytochem. 2018;7(2):3567–71. [Google Scholar]
4. Becerra-Sanchez F, Taylor G. Reducing post-harvest losses and improving quality in sweet corn (Zea mays L.challenges and solutions for less food waste and improved food security. Food Energy Secur. 2021;10(3):e277. [Google Scholar]
5. Babu S, Singh R, Yadav D, Rathore SS, Raj R, Avasthe R, et al. Nanofertilizers for agricultural and environmental sustainability. Chemosphere. 2022;292:133451. [Google Scholar] [PubMed]
6. Shaji H, Chandran V, Mathew L. Organic fertilizers as a route to controlled release of nutrients. Control Release Fertil Sustain Agric. 2021;17(6):231–45. doi:10.1016/B978-0-12-819555-0.00013-3. [Google Scholar] [CrossRef]
7. Ghosh D, Brahmachari K, Skalický M, Roy D, Das A, Sarkar S, et al. The combination of organic and inorganic fertilizers influences the weed growth, productivity and soil fertility of monsoon rice. PLoS One. 2022;17(1):e0262586. [Google Scholar] [PubMed]
8. Marzi M, Shahbazi K, Kharazi N, Rezaei M. The influence of organic amendment source on carbon and nitrogen mineralization in different soils. J Soil Sci Plant Nutr. 2020;20(1):177–91. [Google Scholar]
9. Padbhushan R, Rakshit R, Das A, Sharma RP. Assessment of long-term organic matter amendments effect on some sensitive indicators of carbon under subtropical climatic condition. The Bioscan. 2015;10:1237–40. [Google Scholar]
10. Koch M, Naumann M, Pawelzik E, Gransee A, Thiel H. The importance of nutrient management for potato production Part I: plant nutrition and yield. Potato Res. 2019;63:97–119. [Google Scholar]
11. van Oosten MJ, Pepe O, de Pascale S, Silletti S, Maggio A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem Biol Tech Agric. 2017;4(1):1–12. [Google Scholar]
12. Ali O, Ramsubhag A, Jayaraman J. Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS One. 2019;14(5):e0216710. [Google Scholar] [PubMed]
13. Kapur B, Sarıdaş MA, Çeliktopuz E, Kafkas E, Karg SP. Health and taste related compounds in strawberries under various irrigation regimes and biostimulant application. Food Chem. 2018;263:67–73. [Google Scholar] [PubMed]
14. Vasantharaja R, Abraham LS, Inbakandan D, Thirugnanasambandam R, Senthilvelan T, Jabeen SA, et al. Influence of seaweed extracts on growth, phytochemical contents and antioxidant capacity of cowpea (Vigna unguiculata L. Walp). Biocatal Agric Biotechnol. 2019;17:589–94. [Google Scholar]
15. Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, et al. Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul. 2009;28:386–99. [Google Scholar]
16. du Jardin P. Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic. 2015;196:3–14. [Google Scholar]
17. Mahmoud SH, Salama DM, El-Tanahy AM, Abd El-Samad EH. Utilization of seaweed (Sargassum vulgare) extract to enhance growth, yield and nutritional quality of red radish plants. Ann Agric Sci. 2019;64(2):167–75. [Google Scholar]
18. Subbaiah BV, Asija GL. A rapid procedure for estimation of available nitrogen in soil. Curr Sci. 1956;25:259–60. [Google Scholar]
19. Olsen SR, Cole CV, Watanabe FS, Dean LA. Estimation of available phosphorus in soils by extraction with NaHCO3. USDA Circ. 1954;939:1–19. [Google Scholar]
20. Warncke D, Brown JR. Potassium and other basic cations. Recommended chemical soil tests procedures for the north central region. North Central Reg Res Publ. 1988;221:31–3. [Google Scholar]
21. Bouyoucos GJ. Hydrometer method improved for making particle size analyses of soils. Agron J. 1962;54(5):464–5. doi:10.2134/agronj1962.00021962005400050028x. [Google Scholar] [CrossRef]
22. Jackson ML. Soil chemical analysis. New Delhi, India: Pentice hall of India Pvt. Ltd.; 1973. vol. 498, p. 151–4. [Google Scholar]
23. Walkley AJ, Black IA. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934;37:29–38. [Google Scholar]
24. Craigie JS. Seaweed extract stimuli in plant science and agriculture. J Appl Phycol. 2011;23:371–93. [Google Scholar]
25. Sadasivam S, Manickam A. Biochemical methods for agricultural sciences. New Delhi: Wiley Eastern Ltd.; 1992. [Google Scholar]
26. Krishnaveni S, Balasubramanian T, Sadasivam S. Sugar distribution in sweet stalk sorghum. Food Chem. 1984;15(3):229–32. [Google Scholar]
27. Pelletier O. Vitamin C (L-ascorbic and dehydro-L-ascorbic acids). In: Augustin J, Klein BP, Becker DA, Venugopal PB, editors. Methods of vitamin assay. 4th edNew York (NYWiley; 1985. p. 303–47. [Google Scholar]
28. Malick CP, Singh MB. Plant enzymology and histoenzymology. New Delhi: Kalyani Publishers; 1980. p. 286. [Google Scholar]
29. Gomez KA, Gomez AA. Statistical procedures for agricultural research, an International Rice Research Book. 2nd edition (illustrated)New York, USA: John Wiley & Sons; 1984. p. 704. [Google Scholar]
30. Xiao L, Sun Q, Yuan H, Lian B. A practical soil management to improve soil quality by applying mineral organic fertilizer. Acta Geochim. 2017;36:198–204. [Google Scholar]
31. Kumar R, Jha S, Singh SP, Kumar M, Kumari N, Padbhushan R. Combined application of chemical fertilizer and enriched household vermicompost influences maize yield and soil quality under calcareous soil. J Pharm Innov. 2022;8(11):2069–75. [Google Scholar]
32. Doan TT, Henry-des-Tureaux T, Rumpel C, Janeau JL, Bouquet P. Impact of compost, vermicompost, and biochar on soil fertility, maize yield and soil erosion in Northern Vietnam: a three-year mesocosm experiment. Sci Total Environ. 2015;514:147–54. [Google Scholar] [PubMed]
33. Lockeretz W, Shearer G, Kohl DH. Organic farming in the corn belt. Science. 1981;211(4482):540–7. [Google Scholar] [PubMed]
34. Magkos F, Arvaniti F, Zampelas A. Organic food: nutritious food or food for thought? A review of the evidence. Int J Food Sci Nutr. 2003;54:357–71. [Google Scholar] [PubMed]
35. Bala J, Srivastava LK, Mishra VN, Patel J, Jatav G, Banwasi RK. Effect of integrated plant nutrient management on the quality parameters of sweet corn in a vertisol of Chhattisgarh. J Pharm Innov. 2023;12(1):1282–90. [Google Scholar]
36. Pramanick B, Brahmachari K, Mahapatra BS, Ghosh A, Ghosh D, Kar S. Growth, yield and quality improvement of potato tubers through the application of seaweed sap derived from the marine alga Kappaphycus alvarezii. J Appl Phycol. 2017;29:3253–60. [Google Scholar]
37. Obaid AA, Al-Alawy HH, Hassan KD, Hamdi GJ. Effect of shading net, planting methods and bioextract on production of muskmelon. J Agric Sci. 2021;2:284–8. [Google Scholar]
38. Haukioja E, Ossipov V, Koricheva J, Honkanen T, Larsson S, Lempa K. Biosynthetic origin of carbon-based secondary compounds: cause of variable responses of woody plants to fertilization? Chemoecology. 1998;8:133–9. [Google Scholar]
39. Vinha AF, Barreira SV, Costa AS, Alves RC, Oliveira MBP. Organic versus conventional tomatoes: influence on physicochemical parameters, bioactive compounds and sensorial attributes. Food Chem Toxicol. 2014;67:139–44. [Google Scholar] [PubMed]
40. Murmu K, Ghosh BC, Swain DK. Yield and quality of tomato grown under organic and conventional nutrient management. Arch. Agron. Soil Sci. 2013;59(10):1311–21. [Google Scholar]
41. Das A, Murmu K, Bandopadhyay P, Roy M. Seaweed and its role in enhancing yield and antioxidant properties in sweet corn. Res Square. 2022. doi: 10.21203/rs.3.rs-1545375/v1. [Google Scholar] [CrossRef]
42. Grotz N, Guerinot ML. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim Biophys Acta (BBA)-Mol Cell Res. 2006;1763(7):595–608. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools