 Open Access
Open Access
ARTICLE
Effects of Flowering Mode and Pollinator Sharing on Reproductive Success in Natural Hybrid of Two Epimedium (Berberidaceae) Species
1 College of Life Sciences, China West Normal University, Nanchong, 637000, China
2 School of Ecology and Environment, Tibet University, Lasha, 850000, China
3 College of Environment Science and Engineering, China West Normal University, Nanchong, 637000, China
* Corresponding Author: Qiumei Quan. Email:
Phyton-International Journal of Experimental Botany 2024, 93(3), 551-566. https://doi.org/10.32604/phyton.2024.048103
Received 28 November 2023; Accepted 06 February 2024; Issue published 28 March 2024
Abstract
Mediated by pollen flow, natural hybridization is deemed a crucial factor that propels speciation in floral plants. Despite the fact that the diversity of Epimedium species is concentrated mainly in Southwestern China, the potential impact of interspecific pollination on natural hybridization has yet to be empirically scrutinized. To explore this, we studied the flowering period and the visitors of flowers in three frequently overlapping Epimedium species at Jinchengshan National Forest Park, located in Nanchong, Sichuan Province. Additionally, we performed a series of pollination experiments to examine breeding systems and hybrid compatibility. Morphologically, Epimedium pubescens and Epimedium wushanense are clearly distinct and easily recognizable by flower morphology, while the putative hybrid is morphologically intermediate between them. Our observation revealed that E. pubescens and E. wushanense had 15 days of overlapping anthesis. Both species are self-incompatible and require pollinator services for successful reproduction. Although their pollinators differ in quantity and species, Lasioglossum sp. (Hymenoptera: Halictidae) visited all three species. Artificial pollination revealed no breeding barriers among the three species, and the resulting hybrid seeds exhibited fertility. The overlapping time of flowering, common floral visitors, and compatibility of hybrids may be important factors contributing to successful interspecific pollination and speciation for natural hybrid species of E. pubescens and E. wushanense. Consequently, the role of potential interspecific pollination is crucial for hybrid speciation and the evolutionary dynamics of Epimedium species.Keywords
Hybridization in nature is important for the evolutionary processes of flowering plants, leading to increased diversity and the emergence of new species [1,2]. Even self-pollinating plants like soybean and barley experience hybridization events to some extent [3–5]. While interspecific hybridization is estimated to be low in animals (approximately 10%), it can reach up to 25% or even more than 50% in plants [6]. In flowering plants, where pollen transfer relies on biotic vectors such as pollinators, the behavior of these pollinators can influence the formation and dynamics of plant hybrid zones. Consequently, pollen movement affects genetic combinations and adaptation [7–9]. However, due to limited research on hybrid formation, maintenance, and adaptation, the role of hybridization in evolution remains poorly understood. The relationship between flowering mode, pollination contribution, and reproductive success among different species also lacks comprehensive understanding [10,11]. To understand the process of natural hybrid species formation, the stability of plant hybrid breeding systems is crucial [12]. Over the years, various research techniques have been used to study natural hybrids. Initially, morphological and pollination ecology analyses were the primary methods for detecting natural hybrids until the advent of molecular markers and biochemical markers [13–15]. Additionally, artificial hybridization and molecular biology techniques have been employed [16–18].
Epimedium, a genus within the Berberidaceae family, contains more than 60 species that are widely distributed globally and include popular medicinal plants, as well as potential ground covers and ornamental herbs [19,20]. Variations and interspecific transitions of the original species have been observed in western Sichuan Province [21–24]. Morphological differences among Epimedium species have been used for interspecific classification [8,25,26]. Based on petal size, Stearn [27] classified Epimedium into two series: Brachycerae and Dolichocerae. Suzuki [25] successfully hybridized Epimedium species through artificial pollination, such as E. diphyllum and E. grandiflorum in the large-flowered section. However, natural hybrids between the two series have not been reported. Du et al. [28] established RAPD fingerprints and a phylogenetic map of seventeen Epimedium species using Random Amplified Polymorphic DNA (RAPD) and Polymerase Chain Reaction-Restriction Fragment Length Polymorphisms (PCR-RFLP) techniques. Chinese Epimedium species can be grouped into the large-flower taxa and the small-flower taxa, with E. pubescens belonging to the small-flower taxa and E. wushanense to the large-flower taxa [24,28,29].
Previous studies have shown that Epimedium species can readily hybridize through manual manipulation [25,28]. However, there is limited understanding of the pollination ecology of naturally occurring hybrids among Epimedium species [24,25]. E. pubescens and E. wushanense are frequently distributed allopatrically in forests or forest edges of Sichuan Province, but they coexist sympatrically at Jinchengshan National Forest Park, Nanchong of Sichuan Province [24,30]. To explore the possibility of hybridization between E. wushanense and E. pubescens, we conducted investigations of floral biology, breeding systems, and pollination ecology in these Epimedium species, along with various artificial pollination experiments. This study aimed to achieve the following objectives: (i) understanding the breeding system in E. pubescens and E. wushanense and elucidating how the flowering mode affects reproductive success and interspecific hybridization; (ii) investigating pollinator diversity and resource sharing in sympatric Epimedium species; (iii) examining the compatibility of interspecific hybridization and the potential for hybrid backcrossing with parental species.
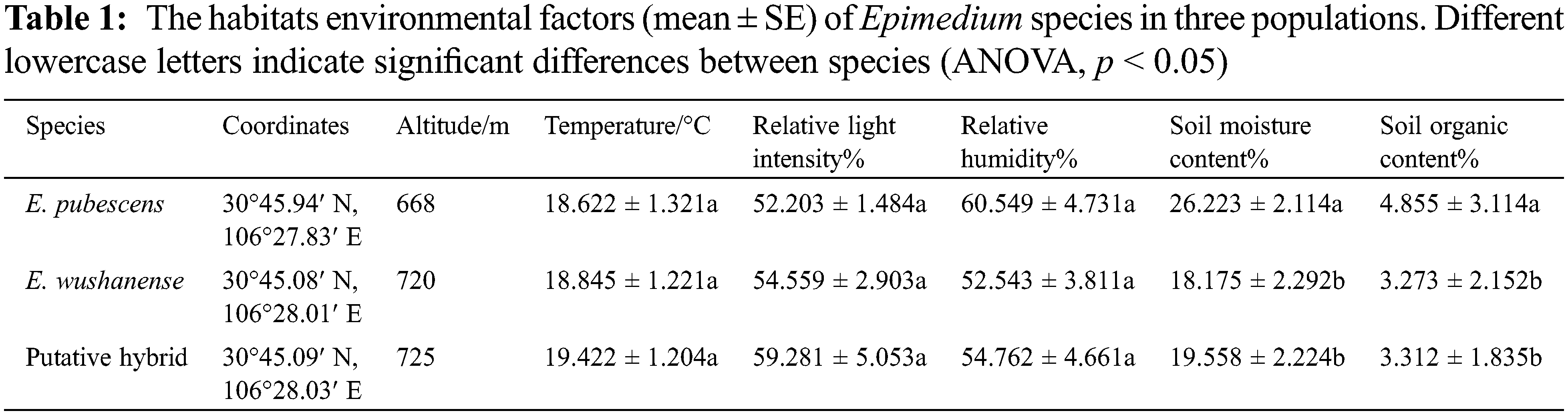
From March 2014 to May 2017, wild populations of E. pubescens, E. wushanense, and putative hybrid were studied in the Jinchengshan National Forest Park, Nanchong of Sichuan Province, China (1128 m a.s.l., 30°45′N, 106°28′E), an area considered the forefront of the ecological barrier on the upper reaches of the Yangtze River. The region experiences a mean annual temperature of 18.6°C, with average temperatures ranging from 5.4°C in January to 21.7°C in July. Annual precipitation and evaporation average 1063 mm and 705.8 mm, respectively. The soil in this area is red loam soil at a depth of 0–30 cm. The tree and shrub layers of the plots were similar, dominated by secondary conifer and broad-leaf mixed forests, which succeeded the evergreen broad-leaf forest [31]. The habitats of E. pubescens, E. wushanense, and the putative hybrid populations exhibited slight differences (Table 1).
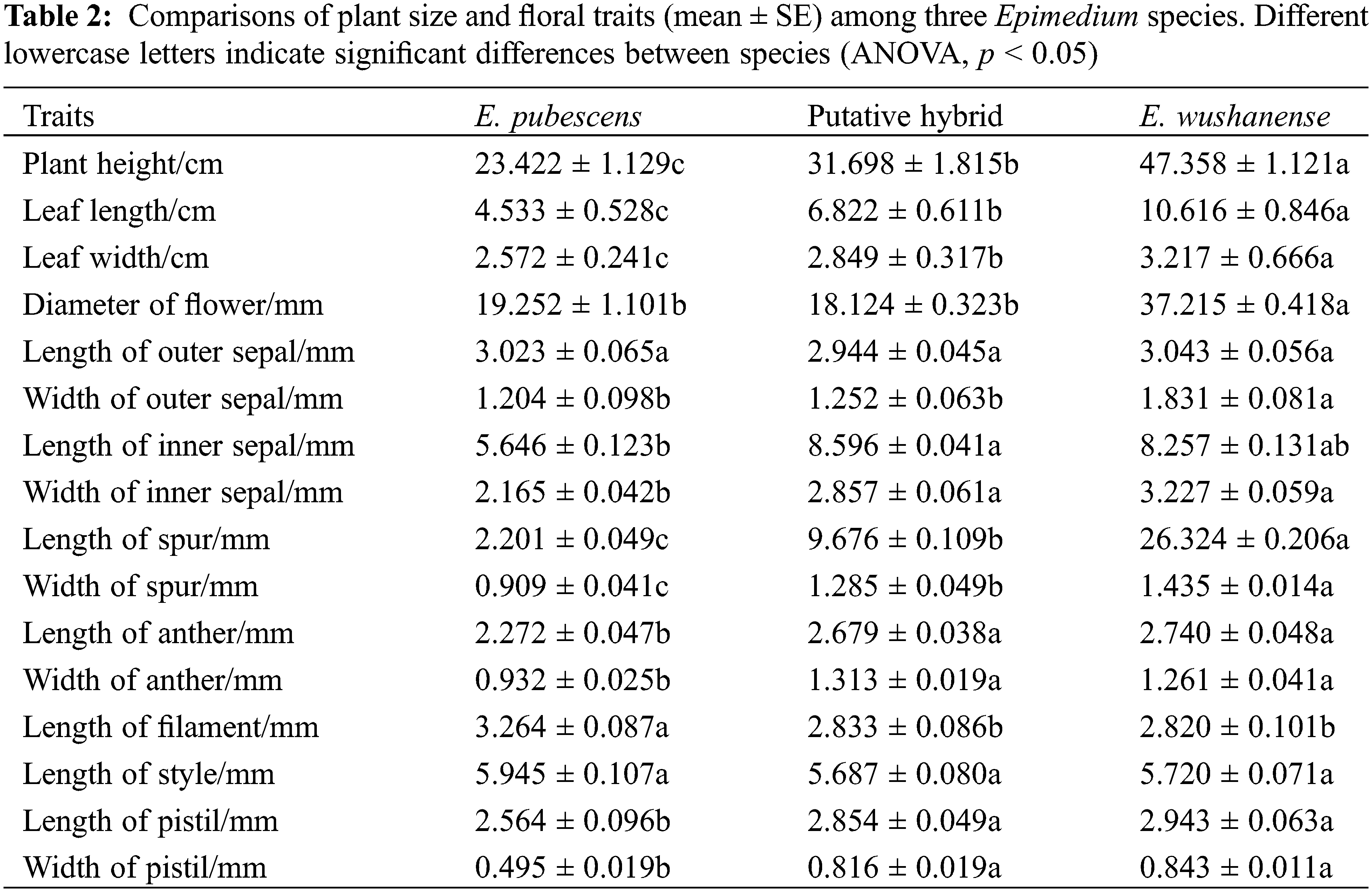
Epimedium species typically have a flowering period from March to May, during which they produce terminal inflorescences. Each panicle generally contains 10–50 flowers. The flowers consist of two layers, with four sepals in each layer, as well as four exterior conical spurs and four stamens surrounding the persistent pistil inside. The outer sepals may exhibit pale green, purple, or yellow colors and shed as the buds open, while the inner sepals are white. The four petals are pale yellow and have a spur-like shape. Nectar is produced by nectaries located at the tip of the spur and stored within the spurs. Each anther contains two chambers, and green or yellow pollen is present upon dehiscence. Detailed floral parameters of the Epimedium species studied at these sites can be found in Table 2.
2.3 Flowering Phenology Monitoring
During the months of March to May in both 2014 and 2016, the flowering phenology of each Epimedium species was meticulously observed and recorded at three subplots, both at the population and individual levels. The parameters observed in this study included the first flowering date, peak flowering date, last flowering date, the flowering amplitude curves, and the flowering intensity. The determination of the first flowering date, peak flowering date, and last flowering date followed the statistical methods described by Dafni [32]. Before the anthesis stage of the Epimedium plants, 30 buds were randomly selected from three populations within each Epimedium subplot. These buds were carefully labeled with notice plates to record the total number of buds per inflorescence. The number of open flowers was subsequently recorded every two days until the end of the flowering period. The first flowering date was defined as the point when approximately 25% of the plants had started to flower. The peak flowering date was reached when over 50% of the plants were in bloom. The last flowering date was considered the point at which over 75% of the plants had faded, marking the end of the flowering period. The flowering amplitude curve and flowering intensity were calculated using the statistical methods developed by Herrera [33]. The flowering amplitude curve represents the number of opening flowers per day and per unit time (from 4:30–18:30 h) at the individual plant level. Flowering intensity, on the other hand, is the ratio of the maximum number of opening flowers produced by a plant on the peak flowering date.
2.4 Measurements of Plant Size and Floral Morphology
To assess plant height, leaf morphology, and floral morphology, over 30 flowering plants were randomly chosen from three populations within each Epimedium subplot. Various measurements were taken during the peak blooming period of each species (n = 30), including floral diameter, spur length and height, entrance height and width of the spur, stamen length, pistil length, style length, as well as anther length and width. Vernier calipers from Guanglu, Guilin, China were used for these measurements. To determine the volume of nectar produced, a minimum of 40 flower buds from 10 plants in each population were randomly selected during the peak blooming period, labeled, and bagged. Ten flowers were carefully removed from different plants at 9:00 AM on the first, second, third, and fourth days of flowering. The nectar volume of each flower was immediately measured using calibrated 5 μL capillary tubes from Hirschmann Laborgeräte, Germany.
During the peak blooming period, pollination observations were conducted for a duration of 5 days in all three populations of each Epimedium species. The observations involved documenting the type of pollinator, their pollination behavior, visit frequency, and pollination time. Furthermore, images of the pollinators were captured using a Nikon DSLR D7000 camera with 16.2 megapixels. All flower visitors were captured and later identified and measured in the laboratory. To compare the effectiveness of pollinators in each species, the removal and deposition of pollen by a single efficient floral visitor per plant were examined. Sixty flower buds from 10 plants (6 flowers per plant) were randomly chosen at the peak blooming period in each population, labeled, and placed in bags. These bagged flowers were then divided into two groups randomly, with 30 flowers in each group. In one group, the flowers were gently opened and emasculated using tweezers before the anthers disappeared, after which the bags were carefully covered again. The other group did not undergo any treatment. On the first day of flowering, when floral visitors were observed, the bags were opened. Whenever a flower was visited by a potential insect pollinator, it was promptly removed and preserved in a Formalin Aceto-Alcohol (FAA) solution. To determine the mean number of pollen grains per unvisited flower per Epimedium species, we collected 20 unvisited flowers per species, just before anthesis. These were also fixed using FAA and the pollen grains in each were counted in the laboratory using a Leica DM500 binocular microscope (Heerbrugg, Switzerland). By counting the pollen removal from captured insects, they were placed in a high-speed centrifuge to separate the pollen grains from other tissues through centrifugal force. Subsequently, the separated pollen grains were counted. Using these data, a pollen-removal ratio per insect visitor was calculated. Pollen deposition on stigmas by pollinators of the Epimedium species was assessed per insect visitor and reported as the number of individual pollen grains deposited [34].
2.6 Manipulated Pollination Experiments
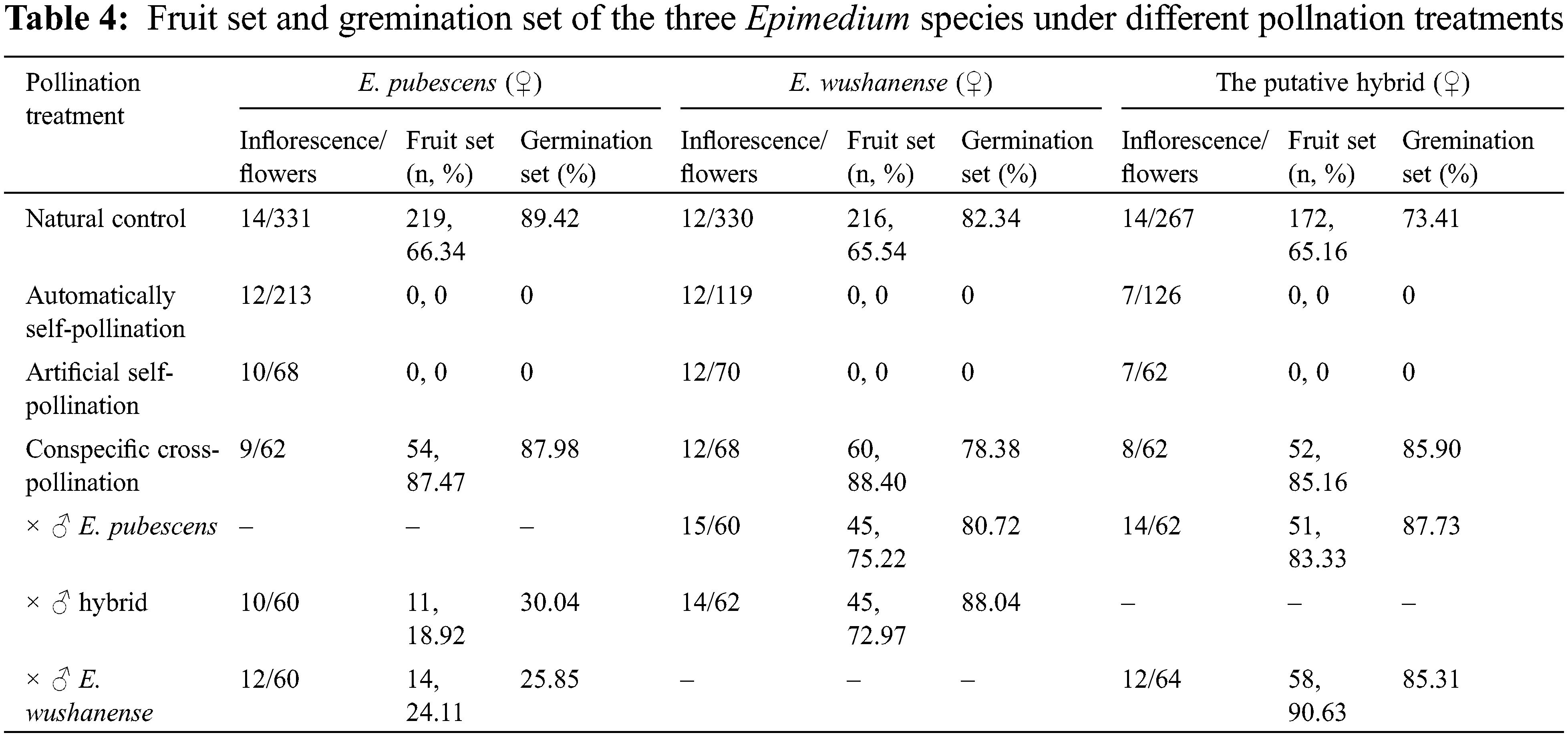
During the peak blooming period of the three Epimedium species, we conducted five pollination treatments from 2016 to 2017. These treatments included: (1) Natural control: 30 buds from different panicles were labeled and left open for pollination without any treatment until the fruits ripened. (2) Automatic self-pollination: Thirty buds from different panicles were labeled and covered with fine-mesh nylon bags to prevent insect visitors. No other manipulations were performed. (3) Artificial self-pollination: Thirty buds from different panicles were labeled and covered with fine-mesh nylon bags. The stigmas were manually pollinated with pollen from the same flower after the anthers had decreased. The bags were then used to cover the panicles. (4) Conspecific cross-pollination: Thirty buds from different panicles were labeled and covered with fine-mesh nylon bags. Before the flowers opened, the anthers were manually removed using tweezers. Then, the stigmas were pollinated with pollen from other individuals of the same species that were located at least 200 meters away. These panicles were bagged after pollination. (5) Interspecific artificial pollination: Sixty buds from each Epimedium species were labeled and covered with fine-mesh nylon bags. Before the anthers dehisced, the flowers were opened and emasculated using tweezers. Then, the stigmas were pollinated as follows: (a) E. pubescens stigmas were pollinated with pollen from E. wushanense and putative hybrid, (b) E. wushanense stigmas were pollinated with pollen from E. pubescens and putative hybrid, and (c) putative hybrid stigmas were pollinated with pollen from E. pubescens and E. wushanense. These panicles were bagged after pollination. The harvested Epimedium seeds from that year were sown directly in the seedbeds of the study sites, which had humus-rich soil. The seeds were covered with approximately 1 cm of soil and kept moist on the surface. By the beginning of the second spring, the germination rate was counted.
All the collected data, which included the flowering phenology, floral parameters, volume of secreted nectar, fruit set, germination set, and the efficiency of effective pollinators, were subjected to statistical analysis using SPSS v22.0 (SPSS Inc., Chicago, Illinois, USA). The normality and homoscedasticity of variables were evaluated using the Shapiro-Wilk and Levene tests, respectively. Normal and normalizable data were compared using a one-way analysis of variance (ANOVA) with Duncan’s multiple-range test. Furthermore, regression analysis (LSD) was employed to compare the visit frequencies among different Epimedium species. To minimize experimental errors and enhance result reliability, it is important to incorporate randomization and repetition in sampling and experimental design.
Phenological studies of Epimedium sites indicate that the flowering season lasts for 20–30 days, from early March to mid-May annually. However, the first, peak and last flowering dates of each species vary, depending on the plant’s habitat and climate (Fig. 1). The flowering amplitude curves of E. pubescens and E. wushanense exhibit bell curves that peak on the 15th and 26th days of flowering, respectively (Fig. 1a). At the population level, the flowering periods of E. pubescens and E. wushanense overlap for approximately 15 days period. E. pubescens and E. wushanense are largely characterized by a single peak in their flowering amplitude curves, while the putative hybrid tends to bloom gradually in batches. The relative flowering intensities are consistently over 50%, suggesting that the flowering period of these Epimedium species is short and centralized (Fig. 1b).
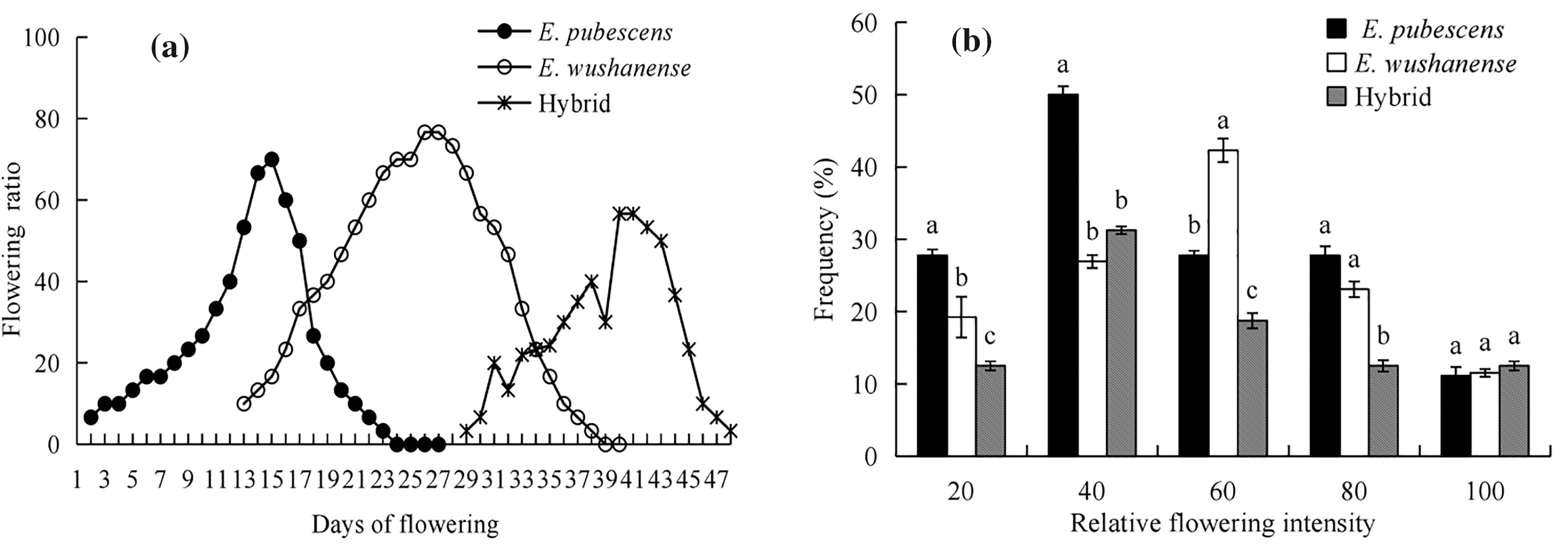
Figure 1: Flowering phenology of Epimedium species. (a) presents flowering amplitudes curves, and (b) frequency distributions of the flowering intensity (mean ± SE). Different lowercase letters indicate significant differences between species (ANOVA, p < 0.05)
The longevity of a single flower of each species is 5–7 days, bisexual, zygomorphic, inodorous, and grows invertedly. Intuitively, the floral features of the putative hybrid exhibit intermediate forms between E. pubescens and E. wushanense (Fig. 2). The leaves of E. pubescens are oval-acuminate, with finely spined toothed edges and brown patches, thinly leathery; while E. wushanense leaves are acutely pointed, with toothed edges, fine spines, and a dark green, glossy appearance. The putative hybrid produces acutely pointed, light green leaves with brown patches and the leaf margins have toothed edges with fine spines. The stems may be hairy or glabrous. The flowers of all species display inner and outer sepals, with each layer containing 4 sepals that grow in a symmetrical manner. The outer sepals exhibit a pale purple or green color and gradually shed during the bud stage. Following the large-bud stage, the inner white sepals envelop the petals, as well as the male and female organs, along with the outer sepals, and progressively expand throughout the blooming process (Figs. 2a–2i). Four pale yellow-spurred petals grow symmetrically. The petals of E. pubescens are small and vertical (Fig. 2c), whereas those of E. wushanense (Fig. 2f) and the putative hybrid (Fig. 2i) are either straight or curved, and the nectaries are located at the tip of the spur. From the large-bud stage until the first day of flowering, the pistil is slightly longer than the stamen (Figs. 2b, 2f, 2g). The anthers decrease in length and enclose the stigma on the initial day of flowering. After successful pollination, the milky white stigma transitions to a pale yellow hue and gradually elongates. E. pubescens possesses 4–5 ovules in one ovary, whereas E. wushanense and the putative hybrid have 10–12 ovules.
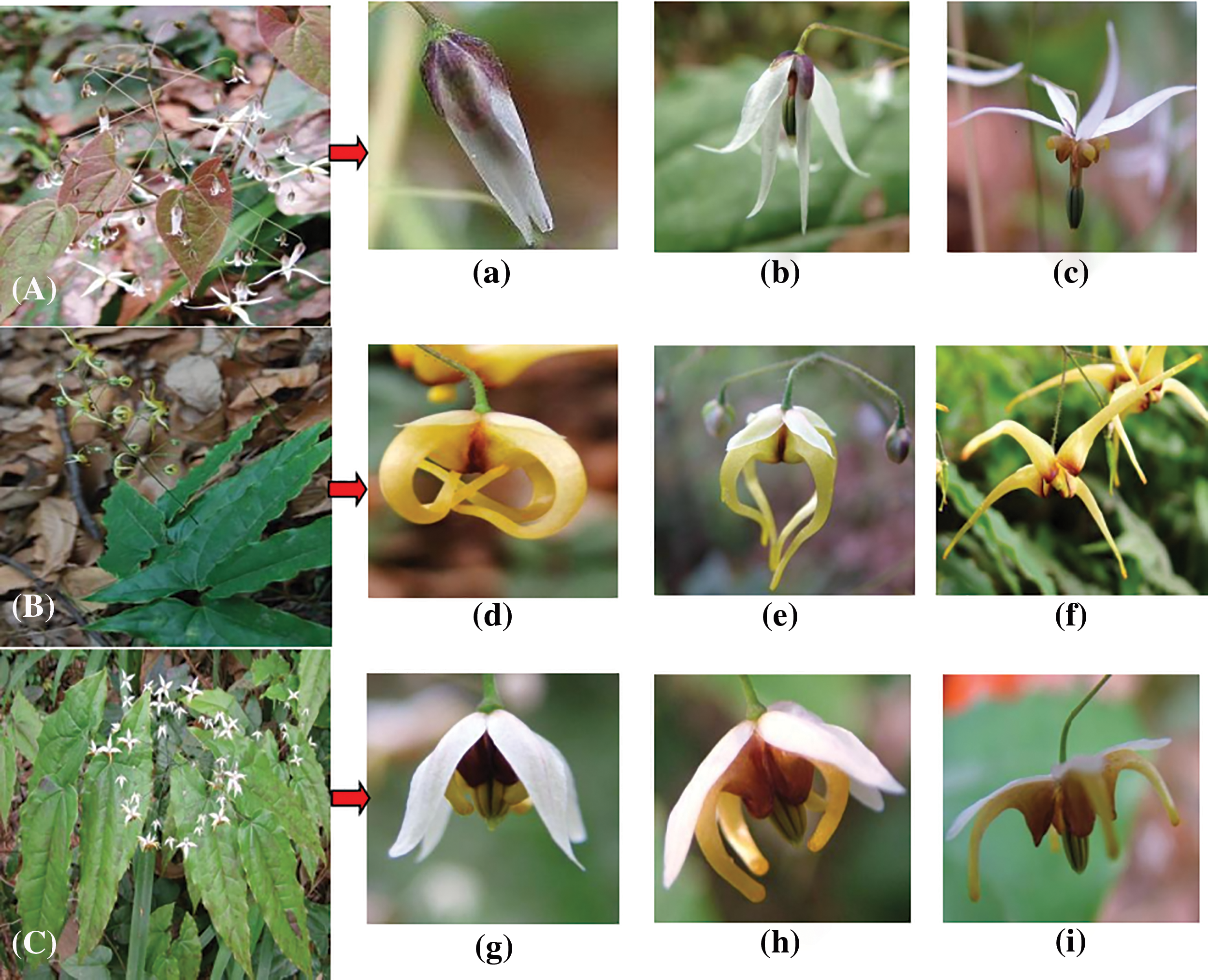
Figure 2: Plant morphology A is E. pubescens, B is E. wushanense, C is putative hybrid showing stages of floral development (a~c is E. pubescens, d~f is E. wushanense, g~i is putative hybrid)
3.2 Plant Size and Floral Features
Differences in morphological parameters among the Epimedium species include plant size, leaf shapes (leaf length and width), and floral features, which are summarized in Table 1. Plant height was significantly different between E. wushanense (47.358 ± 1.121 cm), the putative hybrid (31.698 ± 1.815 cm), and E. pubescens (23.422 ± 1.129 cm) (Table 2, p < 0.05), while leaf length and width were also significantly different between the two species and putative hybrid (Table 2, p < 0.05). The diameter of E. wushanense flowers (37.215 ± 0.418 mm) was significantly greater than that of E. pubescens (19.252 ± 1.101 mm) and the putative hybrid (18.124 ± 0.323 mm) (Table 2, p < 0.05), although the difference was not significant between E. pubescens and the putative hybrid (Table 2, p > 0.05). The length and width of the spur were significantly different between E. wushanense, the putative hybrid, and E. pubescens, respectively (Table 2, p > 0.05). The length and width of the anthers in E. wushanense and the putative hybrid are significantly greater than in E. pubescens (Table 2, p > 0.05), with no significant differences found between E. wushanense and the putative hybrid. Evaluations of plant height, leaf length, leaf width, length of outer sepal, length of filament, length of style, and other floral characters demonstrated that E. wushanense was significantly larger than E. pubescens and the putative hybrid. The morphology of the putative hybrid showed an apparent transitional state between E. pubescens and E. wushanense. The secretion of nectar in the three species took place daily from the large-bud stage until the withering of the flowers, and the volume of nectar in the bagged flowers gradually increased. However, on the second day, the rate of increase slowed Fig. 3. The mean nectar volume per flower between the three study subjects was significantly different, with E. wushanense having ± 38.6 times more nectar than E. pubescens and ± 5.9 times more nectar than the putative hybrid (Fig. 3, p < 0.05).
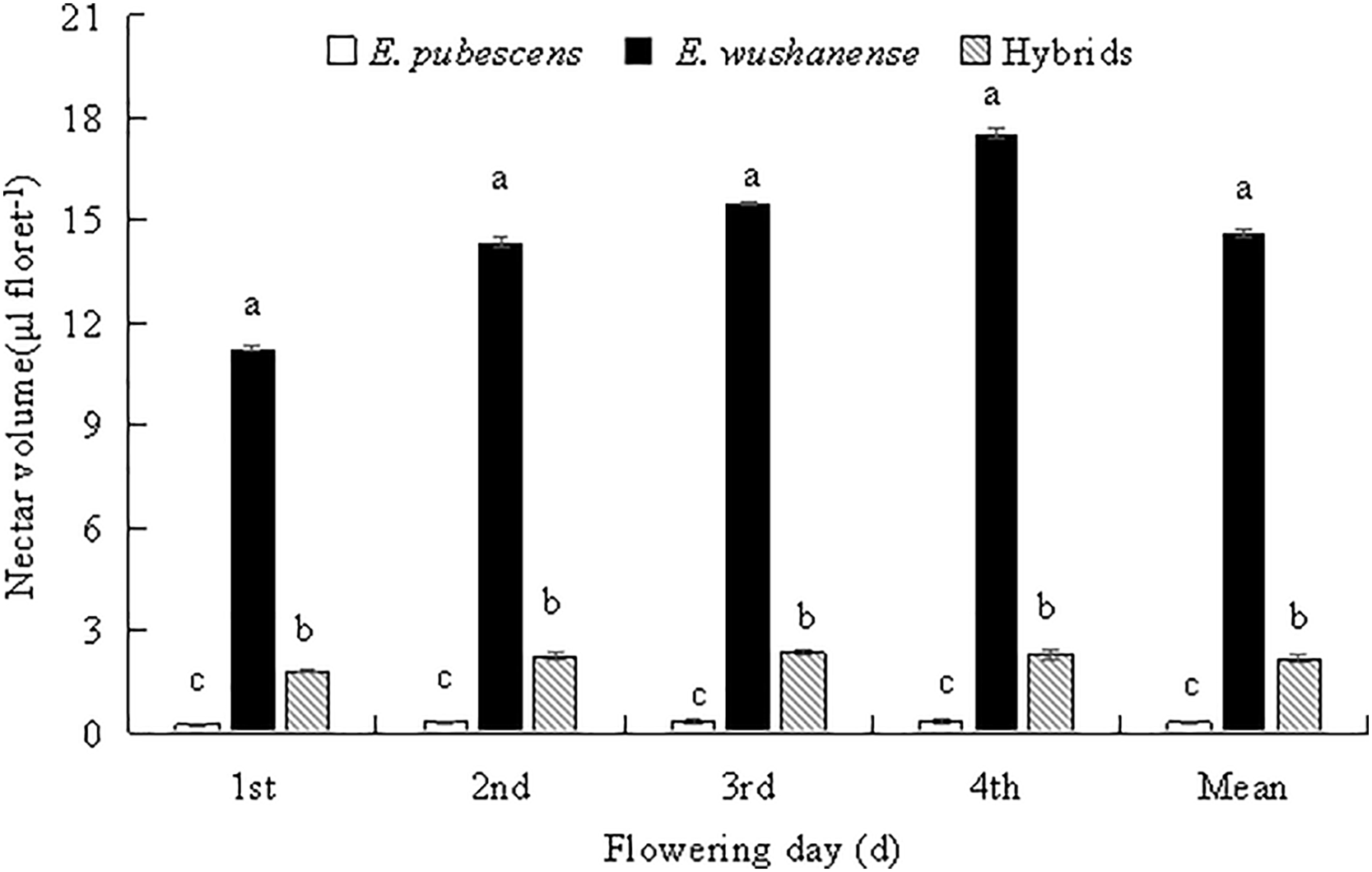
Figure 3: Mean nectar volume of E. pubescens, E. wushanense and the putative hybrid over the first four days of flowering. Different lowercase letters indicate significant differences between species (ANOVA, p < 0.05)
3.3 Observations of Pollination Characteristics
Insect visitation to the three Epimedium species was more frequent within 2–3 days of a flower opening. Few insects visited plants during rainy weather. At least sixteen different species of insects, mainly Hymenoptera and Diptera, were recorded during the field observation period for the three Epimedium species. By counting the number of pollen grains on each captured insect specimen’s body using a binocular microscope. It was found that various bee species carried more pollen and were therefore arguably effective pollinators (Table 3). Lasioglossum sp. (Hymenoptera: Halictidae) were the most efficient pollinators of E. pubescens in terms of the pollen grain count they carried, visiting flowers for both pollen and nectar (Figs. 4a, 4b). The pollen grains on the stamens of E. pubescens were collected and transported by the legs of bees, which packed them into their pollen baskets. During flower visitation, these bees also deposited pollen grains from their baskets onto the stigma. Apis sp. (Hymenoptera: Apidae) were opportunistic visitors with a low frequency of visits and were occasionally seen on E. pubescens (Fig. 4c). The nectar-feeding Bombus trifasciatus (Hymenoptera: Apidae) and Bombus grahami (Hymenoptera: Apidae) frequently visited the flowers of E. wushanense, and distinct visiting behaviors were observed between the two species of bumblebees. B. trifasciatus showed legitimate pollination behavior when visiting E. wushanense by tightly grasping a spur with their front legs, inserting their proboscis into the spur to extract nectar, pollen grains adhering to neck hairs during visitation (Fig. 4d). When B. trifasciatus visited flowers on separate plants of E. wushanense, neck hair pollen was deposited on stigmas during flower visitation. B. grahami did not display legitimate floral visitation to E. wushanense by piercing holes in the middle of spurs to consume nectar, bypassing the anthers (Fig. 4e). Microscopic examination revealed no pollen on the bodies of these bees. Lasioglossum sp. species showed legitimate flower visitation to E. wushanense, pollen being deposited onto their pollen basket (Fig. 4f). By pollen grain counts, the most efficient visitors to the putative Empodium hybrid were Lasioglossum sp. and Anthophora sp. (Hymentoptera: Anthophoridae. Figs. 4g–4h). Anthophora sp. were only observed visiting the hybrid Epimedium, collecting nectar. B. grahami were opportunistic visitors to the hybrid to collect nectar (Fig. 4i), having a low visitation frequency and with no pollen found on their bodies during microscopic examination. Our results show that in terms of pollen grains removed from anthers and pollen grains deposited onto stigmas, Lasioglossum sp. moved significantly more pollen grains than B. trifasciatus and Anthophora sp. (Table 3, p < 0.05).
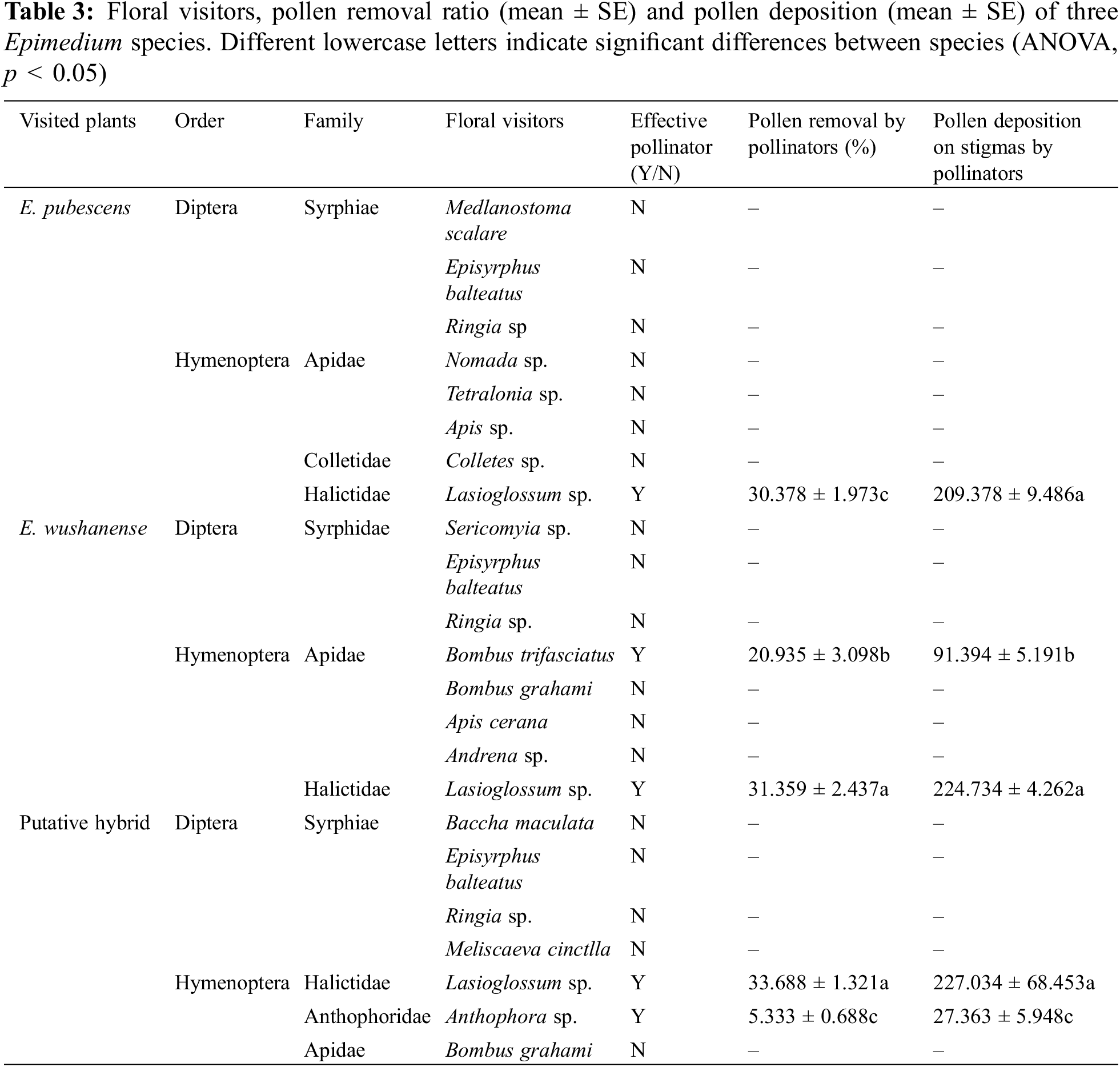
Figure 4: The pollinators of Epimedium species. (a) and (b) are Lasioglossum sp. of E. pubescens, (c) is Apis sp. of E. pubescens, (d) is B. trifasciatus of E. wushanense, (e) is Lasioglossum sp. of E. wushanense, (f) is B. grahami of E. wushanense, (g) is Lasioglossum sp. of the putative hybrid, (h) is Anthophora sp. of putative hybrid, (i) is B. grahami of putative hybrid
The visiting frequency of effective pollinators of the three Epimedium species (with mean visiting frequencies of E. wushanense at 3.08%, E. pubescens at 4.63%, and the hybrid at 1.49%, respectively) was relatively low (Fig. 5). There were significant variations in the visitation rate of effective pollinators throughout the day. Lasioglossum sp. had a higher visit frequency than B. trifasciatus and Anthophora sp., with the peak period for effective pollinator visits occurring from 12:00 to 14:00 (Fig. 5).
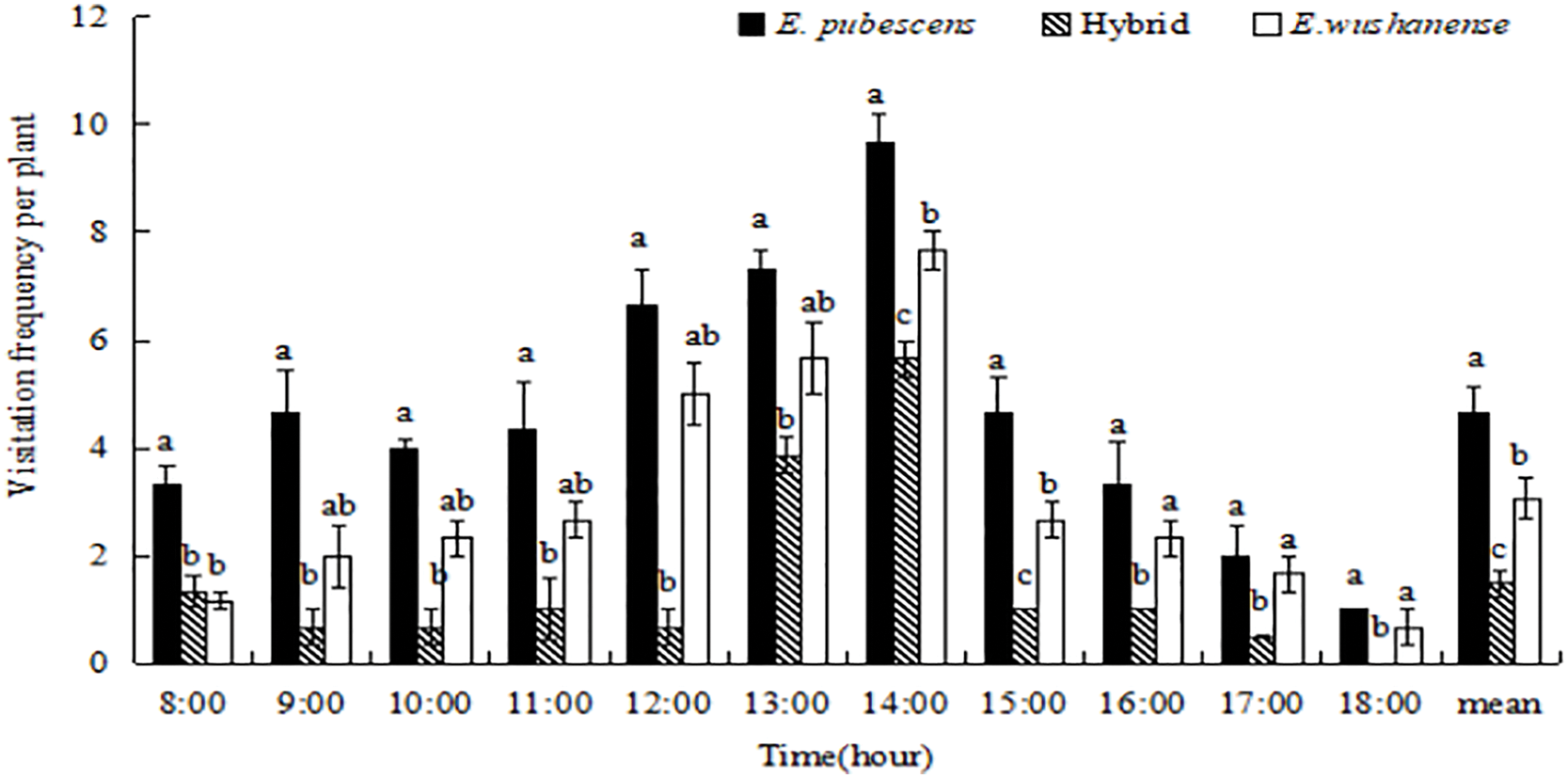
Figure 5: The comparison of visitation frequency (mean ± SE) of effective pollinators of three Epimedium species. Different lowercase letters indicate significant differences between species (ANOVA, p < 0.05)
3.4 Manipulated Pollination Experiments
The results of the artificial pollination combinations and the corresponding fruit-setting rates and germination-setting rates are presented in Table 4. All three Epimedium species in this study were found to have an outcrossing breeding system. Cross-pollination and open pollination treatments led to the production of fruits, with fruit set rates surpassing 50% and germination setting rates exceeding 70%. Conversely, treatments involving autonomous and artificial self-pollination did not result in any fruit production. When interspecies artificial pollination was conducted among these three Epimedium species, fruit production was observed. When E. pubescens was used as the female parent (♀) and E. wushanense and putative hybrid were used as the male parent (♂), the fruit set obtained was 18.92% and 24.11%, respectively. The seeds obtained from the hybrid also exhibited a certain germination setting rate of 30.04% and 25.85%, respectively. When E. wushanense was treated as the female parent (♀) and E. pubescens and putative hybrid were used as the male parent (♂), the fruit set reached 75.22% and 72.97%, while the germination set reached 80.72% and 88.04%, respectively. When the putative hybrid was used as the female parent (♀) and E. pubescens and E. wushanense were used as the male parent (♂), the fruit set reached 83.33% and 90.63%, with germination set rates as high as 87.73% and 85.31%, respectively.
4.1 Conditions for the Formation of a Natural Hybrid
Floral morphology and pollination biology are essential considerations in the study of floral evolution and plant reproduction. The pollination system serves as a model for understanding the interaction between natural selection and evolution [35,36]. Having a comprehensive understanding of how pollination influences the reproductive success of hybrids is essential for their conservation and recovery. This is particularly important because certain hybrids may give rise to unique genotypes through intraspecific hybridization, potentially enhancing their ability to adapt to the environment [37–39].
The occurrence of hybridization among species is primarily influenced by their geographical distributions [40]. The occurrence of spontaneous hybridization between closely related species in sympatric populations frequently leads to gene introgression, which significantly impacts the genetic structure of natural populations [41]. Epimedium species, categorized as belonging to the Old World temperate distribution type, have a widespread and sporadic distribution within a narrow strip spanning from Japan in Asia to Algeria in North Africa (25°~48°N, 5°~143°E) with each species usually having a narrow distribution [42,43]. Both E. pubescens and E. wushanense have small population sizes and are fragmented in their distribution within the Jincheng Mountain National Forest Park in Sichuan. The population of E. wushanense is significantly smaller than that of E. pubescens [23,24]. The coexistence of Epimedium species in the Jinchengshan National Forest Park, along with the relatively narrow ecological niche of E. wushanense, provides conditions for the possibility of hybridization. Pollen movement also contributes to the potential hybridization of different species [44]. The synchronization of flowering affects the flow of genes within species and the interactions between plants and other trophic levels, such as pollinators [45]. The relative flowering intensity indicates the spatial distribution of plant flower resources, which can influence the movement patterns of pollen gene flow in plants [46]. Although the first flowering dates, peak flowering dates, and last flowering dates of E. pubescens and E. wushanense differ, they both exhibit a short and centralized flowering period that overlaps for approximately 15 days at the population level (Fig. 1), indicating the potential for hybridization.
Hybridization facilitates ecological divergence as hybrids display a mixture and transition of parental traits, and the new gene combinations generated are speculated to contribute to ecological divergence [47]. Suzuki found that once hybrids are formed, they maintain distinctive traits between their genetic parents and inherit the dominant genes [48]. Breeding system methods can easily determine the parent of the hybrid in the sexual reproduction of Epimedium plants, as clonal plants do not form hybrids [49]. The obvious differences between parents are revealed through the morphology of the hybrid, such as inflorescence height, inflorescence number, ovule number, and flower characteristics [28,29]. The morphology of the Epimedium flower has been widely studied in previous research. The evolutionary trend of Epimedium corolla morphology was originally a reduction in the number of petals from five to four and three, and a decrease in the size of the innermost petal to form spurs [25,50]. This trend is thought to be related to the long-tongued pollination syndrome that evolved in insects, allowing more efficient transfer of pollen to the stigma. According to previous research, the floral aromas in the Epimedium genus are usually small and generally do not have a noticeable fragrance. However, some varieties of flowers may have a subtle and mild aroma. Overall, the rewards that attract insects to Epimedium are primarily pollen or nectar [51]. The nectar reward and morphology of the spur play important roles in the attraction of pollinators. The putative hybrids have intermediate traits between their parents in terms of the corolla shape, spur length, and stamen length, which are associated with the reproductive fitness of plants. The putative hybrid had longer corolla tubes and spurs than E. pubescens but shorter than E. wushanense, potentially attracting different pollinators and increasing the efficiency of pollinator-mediated gene flow. The putative hybrid also had an intermediate number of stamens, which may affect its pollen production and transfer. These traits contribute to the adaptability of the putative hybrid and provide a potential mechanism for its successful establishment in nature.
4.2 Pollination Biology and Adaptability of Natural Hybridization
It has been reported that the majority of Epimedium species exhibit self-incompatibility as a mechanism to prevent self-fertilization. This is achieved through dichogamy, herkogamy, and self-incompatibility mechanisms [24]. Pollination experiments with three Epimedium species (Table 4) showed that they belong to an outcrossing breeding system, relying on insect pollination and excluding self-pollination. The continuity of populations is reliant on pollination services, as approximately two-thirds of crops worldwide depend on insect pollination to some degree, with wild pollinators typically contributing to crop yields [52]. Plants attract insects for pollination through color, size, pollen, or nectar reward, influencing pollinator activity, efficiency, and reproductive efficiency of flowers [53,54]. Through the observation of pollinators, it was found that E. pubescens and E. wushanense share the pollinator Lasioglossum sp., indicating the possibility of natural hybridization between them. Furthermore, Anthophora spp. was observed only on the putative hybrid and not on its parents, creating isolation conditions between the hybrid and its parents. Effective pollinator species and their visiting behaviors are crucial for the successful reproduction of plants [55]. The quantity of conspecific pollen grains present on a stigma is frequently indicative of seed production and is utilized to measure the effectiveness of pollinators in plants [56]. In this study, the pollen removal and deposition by effective pollinators varied among Lasioglossum sp. was the most effective pollinator, contributing to the reproductive fitness of the putative hybrid in fragmented habitats. The flower-visiting behaviors of Lasioglossum sp. primarily involved pollen collection, while B. trifasciatus and Anthophora sp. were rewarded with nectar due to the feedback mechanism of the sender on the change of flower characteristics. The diversity of Epimedium species is significantly impacted by pollinator-mediated gene flow.
The experimental results from artificial pollination combinations indicate that different species of Epimedium can pollinate each other successfully and obtain fertile seeds. However, the fruit setting rates and seed germination rates of different combinations vary (Table 3). Hybrid incompatibility and hybrid sterility can prevent hybridization due to the various isolation mechanisms between species [12]. Previous studies have shown that E. pubescens and E. wushanense have a relatively close genetic relationship based on ISSR and RAPD markers and hybridization is not restricted [28]. Suzuki and Du et al. have produced hybrids between large-flower taxa and small-flower taxa Epimedium species under positive and reverse hybridization artificial pollination, showing that large-flower taxa Epimedium species are widely affinitive pollen receptors and are suitable as female parents for interspecific hybridization, while small-flower type Epimedium species have good pollen affinity and are suitable as male parents in crossbreeding [25,57]. The seed germination rate of E. pubescens as the female parent (♀) and E. wushanense and putative hybrid as the male parent (♂) were only 30.04% and 25.85%, respectively, while the other hybrid combinations were above 70.00%, with the highest rate of 88.04%. This indicates that there is a possibility of natural hybridization between them to form species. It further proves that E. wushanense grouped into large-flower taxa is suitable for the female parent in interspecific hybridization, and E. pubescens grouped into small-flower taxa is suitable for the male parent. Moreover, it is possible that putative hybrids can backcross with their parents, providing continuity in the formation of putative hybrid species [16,58].
In summary, natural hybridization occurs in fragmented populations of E. pubescens and E. wushanense due to their overlapping flowering periods, shared pollinators, and compatibility with artificial pollination. The putative hybrid exhibits intermediate characteristics between its parents in terms of floral morphology and pollination biology, which may contribute to its successful establishment in the natural environment. The formation of the putative hybrid provides an example of how hybridization can contribute to the ecological divergence and adaptation of plant species. Future research will focus on investigating the population as a unit, conducting in-depth studies of morphology and molecular biology to obtain sufficient evidence proving the formation of natural hybrid species of E. pubescens and E. wushanense. The insights gained from this study have implications for the conservation and management of Epimedium populations and provide a foundation for further research on the mechanisms and consequences of natural hybridization in plants.
Acknowledgement: We thank the Jinchengshan National Forest Park of Sichuan Province for their support and help during the fieldwork. Prof. Shi Aiming’s team for identification of flower-visiting insects. The authors wish to extend their thanks to EditSprings (https://www.editsprings.com/) for the professional linguistic support rendered.
Funding Statement: This research was supported by the Natural Science Foundation of Sichuan Province (No. 2023NSFSC1282), the National General Cultivation Project of China West Normal University (No. 19B029), Youth Foundation Specialization of West China Normal University (No. 22KB004).
Author Contributions: Qiumei Quan and Yunxiang Li were involved in the study’s conception and design. Lanying Chen conducted the data collection and experiments, while Qiumei Quan and Lanying Chen analyzed the data. Lanying Chen drafted the initial manuscript, which was subsequently reviewed by all authors. All authors read and approved the final manuscript.
Availability of Data and Materials: All data included in this study are available upon request by contact with the corresponding author.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Rieseberg LH, Ellstrand NC, Arnold M. What can molecular and morphological markers tell us about plant hybridization? Crit Rev Plant Sci. 1993;12(3):213–41. doi:10.1080/713608045. [Google Scholar] [CrossRef]
2. Yakimowski SB, Barrett SCH. The role of hybridization in the evolution of sexual system diversity in a clonal, aquatic plant. Evol. 2016;70(6):551–6. doi:10.1111/evo.12941. [Google Scholar] [PubMed] [CrossRef]
3. Wang XT, Chen LY, Ma JX. Genomic introgression through interspecific hybridization counteracts genetic bottleneck during soybean domestication. Genome Biol. 2019;20(1):1–15. doi:10.1186/s13059-019-1631-5. [Google Scholar] [PubMed] [CrossRef]
4. Kim HJ, Kim DY, Moon YS, Pack IS, Park KW, Chung YS, et al. Gene flow from herbicide resistant transgenic soybean to conventional soybean and wild soybean. Appl Biol Chem. 2019;62(1):1–8. doi:10.1186/s13765-019-0461-1. [Google Scholar] [CrossRef]
5. Kilian B, Dempewolf H, Guarino L, Werner P, Coyne C, Warburton ML. Crop science special issue: adapting agriculture to climate change: a walk on the wild side. Crop Sci. 2021;61(1):32–6. doi:10.1002/csc2.20418. [Google Scholar] [CrossRef]
6. Mallet J. Hybridization as an invasion of the genome. Trends Ecol Evol. 2005;20(5):229–37. doi:10.1016/j.tree.2005.02.010. [Google Scholar] [PubMed] [CrossRef]
7. Huang SQ, Guo YH. New advances in pollination biology. Chin Sci Bull. 2000;45(16):225–37. doi:10.1007/BF02898884. [Google Scholar] [CrossRef]
8. Shwe EE, Wang B, Huang SQ. Both small and large plants are likely to produce staminate (male) flowers in a hermaphrodite lily. Plant Divers. 2020;42(3):142–7. doi:10.1016/j.pld.2020.01.004. [Google Scholar] [PubMed] [CrossRef]
9. Rita J, Capo M, Moragues E, Bota J, Cursach J. Hybridization processes in an introduced subpopulation of an endangered plant: management strategies to guarantee the conservation of Helosciadium bermejoi (Apiaceae). J Nat Conserv. 2018;41(11):26–34. doi:10.1016/j.jnc.2017.10.006. [Google Scholar] [CrossRef]
10. Wesselingh A. Pollinator behaviour and the evolution of Louisiana iris hybrid zones. J Evol Biol. 2010;13(12):171–80. doi:10.1046/j.1420-9101.2000.00153.x. [Google Scholar] [CrossRef]
11. Emms SK, Arnold ML. Site-to-site differences in pollinator visitation patterns in a Louisiana iris hybrid zone. Oikos. 2000;91(3):568–78. doi:10.1034/j.1600-0706.2000.910319.x. [Google Scholar] [CrossRef]
12. Wendt T, Canela MBF, De Faria APG, Rios R. Reproductive biology and natural hybridization between two endemic species of Pitcairnia (Bromelliceae). Am J Bot. 2001;88(10):1760–7. doi:10.2307/3558350. [Google Scholar] [CrossRef]
13. Edward DA, Tracey C. Sex-specific effects of developmental environment on reproductive trait expression in Drosophila melanogaster. Ecol Evol. 2012;2(7):1362–70. doi:10.1002/ece3.243. [Google Scholar] [PubMed] [CrossRef]
14. Choi K, Kim JS, Pak JH. Natural hybridization between Pseudostellaria davidii and Pseudostellaria palibiniana (Caryophyllaceae). Plant Spec Biol. 2001;16(1):39–47. doi:10.1046/j.1442-1984.2001.00051.x. [Google Scholar] [CrossRef]
15. Li C, Tian D, Li X. Morphological and molecular identification of natural hybridization between Begonia hemsleyana and B. macrotoma. Sci Hortic. 2015;192:357–60. doi:10.1016/j.scienta.2015.06.031. [Google Scholar] [CrossRef]
16. Motley TJ, Carr GD. Artificial hybridization in Hawaiian endemic genus Labordia (Logonniaceae). Am J Bot. 1998. 1998;85(5):650–60. doi:10.2307/2446534. [Google Scholar] [CrossRef]
17. Saitou N, Nei M. The neighbor-joining method a new method for reconstructing Phylogenetic trees. Mol Biol Evol. 1987;47(1):406–25. doi:10.1016/j.ympev.2008.01.019. [Google Scholar] [PubMed] [CrossRef]
18. Stankiewicz E, Guo T, Mao X, Lu YJ. Fluorescence in situ hybridization and rehybridization using bacterial artificial chromosome probes. Methods Mol Biol. 2019;2054:243–61. doi:10.1016/j.ymeth.2011.002. [Google Scholar] [CrossRef]
19. Teo YL, Cheong WF, Cazenave-Gassiot A, Ji S, Logan S, Lee ZX, et al. Pharmacokinetics of prenylflavonoids following oral ingestion of standardized Epimedium extract in humans. Planta Med. 2019;85(4):347–55. doi:10.1055/a-0806-7673. [Google Scholar] [PubMed] [CrossRef]
20. Lee W, Nam JH, Cho HJ, Lee JY, Cho WK, Kim Y, et al. Epimedium koreanum Nakai inhibits PMA-induced cancer cell migration and invasion by modulating NF-κB/MMP-9 signaling in monomorphic malignant human glioma cells. Oncol Rep. 2017;38(6):3619–31. doi:10.3892/or.2017.6043. [Google Scholar] [PubMed] [CrossRef]
21. Stearn WT. New Chinese taxa of Epimedium (Berberidaceae) from Sichuan. Curtis’s Bot Mag. 1995;12(1):15. doi:10.1111/j.1467-8748.1995.tb00481.x. [Google Scholar] [CrossRef]
22. Xu YQ, Li RQ, Zhang HY, Li FQ, Jiang Y, Huang XF. Distribution of Epimedium sagittatum and its research progress on quality characteristics. Chin Tradit Herb Drugs. 2020;51(23):6119–32. [Google Scholar]
23. Guo MY, Ren L, Xu YQ, Liao BS, Song JY, Li Y, et al. Development of plastid genomic resources for discrimination and classification of Epimedium wushanense (Berberidaceae). Int J Mol Sci. 2019;20(16):4003. doi:10.1166/jctn.2016.5073. [Google Scholar] [CrossRef]
24. Chen LY, Xiao X, Xiao J. Floral traits and reproductive characters of different large-flowered taxa Epimedium (Berberidaceae). Bull Bot Res. 2019;39(6):808–16. doi:10.7525/j.issn.1673-5102.2019.06.002. [Google Scholar] [CrossRef]
25. Suzuki K. Biosystematic studies of Japanese Epimedium (Berberidaceae) variation of the populations in Shikoku. Shokubutsu Kenkyu Zasshi. 1987;7:203–12. [Google Scholar]
26. Stearn WT. The genus Epimedium and other herbaceous berberidaceae including the genus podophyllum. European: The Bath Press; 2002. p. 49. [Google Scholar]
27. Stearn WT. Epimedium and Vancouveria (berberidaceaea monograph. Bot J Linn Soc. 1938;51(340):409–535. doi:10.1111/j.1095-8339.1937.tb01914.x. [Google Scholar] [CrossRef]
28. Du MF, Li MJ, Chen QF. PCR-RFLP genetic diversity of plants in Epimedium L. Chin Herb Med. 2012;43(3):562–7. [Google Scholar]
29. Sheng MY, Gao MD, Wang LJ. Heterochromatin banding and rDNA physical mapping in 22 Epimedium species and two Vancouveria species: implications for evolution in Epimedium. Bot J Linn Soc. 2020;194(4):480–97. doi:10.1093/botlinnean/boaa051. [Google Scholar] [CrossRef]
30. Zhang YJ, Li JQ, Wang Y, Liang Q. Taxonomy of Epimedium (Berberidaceae) with special reference to Chinese species. Chin Herb Med. 2022;14(1):20–35. doi:10.1016/j.chmed.2021.12.001. [Google Scholar] [PubMed] [CrossRef]
31. Qian YF, Li YX, Zhang XM, Quan QM. Yuhina nigrimenta Blyth (Zosteropidae) as a bird pollinator of Brandisia hancei Hook.f. (Scrophulariaceae) during winter. Turk J Bot. 2017;41(5):476–85. doi:10.3906/bot-1610-48. [Google Scholar] [CrossRef]
32. Dafni A. Pollination ecology, a practical approach. New York: Oxford University Press; 1992. p. 155–6. [Google Scholar]
33. Herrera J. Flowering and fruiting phenology in the coastal shrublands of Doñana, South Spain. Vegetatio. 1986;68(2):91–8. doi:10.1007/BF00045059. [Google Scholar] [CrossRef]
34. Russell AL, Fetters AM, James EI, Ashman TL. Pollinator effectiveness is affected by intraindividual behavioral variation. Oecologia. 2021;197(1):189–200. doi:10.1007/s00442-021-05016-4. [Google Scholar] [PubMed] [CrossRef]
35. Husband BC, Schemske DW. Evolution of the magnitude and timing of inbreedingdepression in plants. Evol. 1996;50(1):54–70. doi:10.1111/j.1558-5646.1996.tb04472.x. [Google Scholar] [PubMed] [CrossRef]
36. Pansarin ER, Ferreira AWC. Evolutionary disruption in the pollination system of Vanilla (Orchidaceae). Plant Biol. 2022;24(1):157–67. doi:10.1111/plb.13356. [Google Scholar] [PubMed] [CrossRef]
37. Arnold ML. Natural hybridization as an evolutionary process. Annu Rev Ecol Syst. 1992;23(1):237–61. doi:10.1146/annurev.es.23.110192.001321. [Google Scholar] [CrossRef]
38. George JP, Theroux-Rancourt G, Rungwattana K, Scheffknecht S, Momirovic N, Neuhauser L, et al. Assessing adaptive and plastic responses in growth and functional traits in a 10-year-old common garden experiment with pedunculate oak (Quercus robur L.) suggests that directional selection can drive climatic adaptation. Evol Appl. 2020;13(9):2422–38. doi:10.1111/eva.13034. [Google Scholar] [PubMed] [CrossRef]
39. Erickson KD, Pratt PD, Rayamajhi MB, Horvitz CC. Seedling maturation drives spatial variability in demographic dynamics of an invader with multiple introductions: insights from an LTRE analysis. Biol Invasions. 2020;22(7):2185–203. doi:10.1007/s10530-020-02249-x. [Google Scholar] [CrossRef]
40. Aizawa M, Iwaizumi MG. Natural hybridization and introgression of Abies firma and Abies homolepis along the altitudinal gradient and genetic insights into the origin of Abies umbellata. Plant Species Biol. 2020;35(2):147–57. doi:10.1111/1442-1984.12269. [Google Scholar] [CrossRef]
41. Efraín TS, Oyama K. Natural hybridization and hybrid zones between Quercus crassifolia and Quercus crassipes (Fagaceae) in Mexico: morphological and molecular evidence. Am J Bot. 2004;91(9):1352–62. doi:10.3732/ajb.91.9.1352. [Google Scholar] [PubMed] [CrossRef]
42. Ying JS, David EB, Anthony RB. Epimedium L. In: Peter HR, Zhang LB, Al-Shehbaz IA et al., editors. Flora of China. Beijing and St. Louis: Science Press and Missouri Botanical Garden Press; 2011. p. 787–99. [Google Scholar]
43. Ying JS. Petal evolution and distribution patterns of Epimedium Linn. (Berberidaceae). J Syst Evol. 2002;40(6):481–9. [Google Scholar]
44. Kulbaba MW, Worley AC. Patterns of pollen removal and deposition in Polemonium brandegeei (Polemoniaceaethe role of floral visitors, floral design and sexual interference. Plant Biol. 2014;16(6):1087–95. doi:10.1111/plb.12163. [Google Scholar] [PubMed] [CrossRef]
45. Nagahama A, Yahara T. Quantitative comparison of flowering phenology traits among trees, perennial herbs, and annuals in a temperate plant community. Am J Bot. 2019;106(12):1545–57. doi:10.1002/ajb2.1387. [Google Scholar] [PubMed] [CrossRef]
46. Burd M. Offspring quality in relation to excess flowers in Pultenaea gunnii (Fabaceae). Evol. 2004;58(10):2371–6. doi:10.1111/j.0014-3820.2004.tb01610.x. [Google Scholar] [PubMed] [CrossRef]
47. Rieseberg LH. Hybrid speciation in wild sunflowers. Ann Mo Bot Gard. 2006;93(1):34–48. Available from: https://www.jstor.org/stable/40035044. [Accessed 2023]. [Google Scholar]
48. Suzuki K. Adaptive shifts in the regulation system of seed yield in Japanese species of Epimedium (Berberidaceae). Plant Species Biol. 1986;1(2):195–205. doi:10.1111/j.1442-1984.1986.tb00027.x. [Google Scholar] [CrossRef]
49. Sheng MY, Chen QF, Wang LJ, Tian XJ. Hybridization among Epimedium (Berberidaceae) species native to China. Sci Hortic. 2011;128(3):342–51. doi:10.1016/j.scienta.2011.01.020. [Google Scholar] [CrossRef]
50. Guan P, Liu X, Peng CQ, Yang P, Chen XY, Li MD, et al. Analysis of the genetic relationship of 11 Epimedium plants by ITS sequence and ISSR markers. Mol Plant Breed. 2019;17(6):1958–64. doi:10.13271/j.mpb.017.001958. [Google Scholar] [CrossRef]
51. Qian YF, Du W, Chen LY, Quan QM, Li YX. Floral traits and pollination biology of Epimedium chlorandrum Stearn (Berberidaceae). J Plant Ecol. 2023;16(4):106–19. doi:10.1093/jpe/rtad003. [Google Scholar] [CrossRef]
52. Montoya JE, Arnold MA, Rangel J, Stein LR, Palma MA. Pollinator-attracting companion plantings increase crop yield of cucumbers and habanero peppers. HortSci. 2020;55(2):1–6. doi:10.21273/Hortsci14468-19. [Google Scholar] [CrossRef]
53. Foeldesi R, Kovacs-Hostyanszki A, Koroesi A, Somay L, Elek Z, Marko V, et al. Relationships between wild bees, hoverflies and pollination success in apple orchards with different landscape contexts. Agric Forest Entomol. 2016;18(1):68–75. doi:10.1111/afe.12135. [Google Scholar] [CrossRef]
54. Noman A, Aqeel M, Qasim M, Haider I, Lou YG. Plant-insect-microbe interaction: a love triangle between enemies in ecosystem. Sci Total Environ. 2020;699:134181. doi:10.1016/j.scitotenv.2019.134181. [Google Scholar] [PubMed] [CrossRef]
55. Chakraborty P, Chatterjee S, Smith BM, Basu P. Seasonal dynamics of plant pollinator networks in agricultural landscapes: how important is connector species identity in the network? Oecologia. 2021;196(3):825–37. doi:10.1007/s00442-021-04975-y. [Google Scholar] [PubMed] [CrossRef]
56. Page ML, Ison JL, Bewley AL, Holsinger KM, Kaul AD, Koch KE, et al. Pollinator effectiveness in a composite: a specialist bee pollinates more florets but does not move pollen farther than other visitors. Am J Bot. 2019;106(11):1487–98. doi:10.1002/ajb2.1383. [Google Scholar] [PubMed] [CrossRef]
57. Du MF, Chen QF. Studies on morphology of F1 hybrid and systematic evolution of Epimedium species. Seed. 2013;32(8):562–7. [Google Scholar]
58. Buchanan AL. Effects of damage and pollination on sexual and asexual reproduction in a flowering clonal plant. Plant Ecol. 2015;216(2):273–82. doi:10.1007/s11258-014-0434-8. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools