 Open Access
Open Access
ARTICLE
Morphometry and Mineral Content in the Seeds and Soil of Two Species of Argemone L. (Papaveraceae) in the Central Part of the Chihuahuan Desert
1 Unidad Regional Universitaria de Zonas Áridas, Bermejillo, Universidad Autónoma Chapingo, Durango, 35230, México
2 Facultad de Ciencias Biológicas, Universidad Juárez del Estado de Durango, Gómez Palacio, Durango, 35010, México
* Corresponding Author: Fabián García-González. Email:
Phyton-International Journal of Experimental Botany 2024, 93(2), 371-386. https://doi.org/10.32604/phyton.2024.048338
Received 05 December 2023; Accepted 15 January 2024; Issue published 27 February 2024
Abstract
The genus Argemone L. (Papaveraceae) is found widely distributed in Mexico’s Chihuahuan Desert (CD). Some species of this genus are of phytochemical or ethnobotanical interest. They are inedible plants considered as scrubs. To date they have not been broadly studied; thus, their ecology is, to our knowledge, unknown. The present work was centered around carrying out a morphometric analysis and the determination of minerals in the soil and seeds of the wild populations of Argemone at sites belonging to two ecoregions of the CD in Mexico. In April 2021 and April 2022, seeds of Argemone spp., and soil samples were collected at 10 sites of the CD. The seeds were selected under a randomized design, and weight, length, diameter, thickness, buoyancy, and mineral content were determined. The soil samples were obtained under the Mexican regulation NOM-021-RECNAT-2000, and determinations of mineral content, electrical conductivity, apparent density, and soluble anions were performed. The information obtained was grouped by variable, species, and place of precedence. The statistical tests consisted of an ANOVA, Tukey means tests considering p ≤ 0.05, and a Principal Components Analysis. Argemone pleiacantha exhibited differences in terms of weight (F = 54.79, p = 0.001), length (F = 90.83, p = 0.001), thickness (F = 104.89, p = 0.001), and diameter (F = 155.82, p = 0.001), and the differences in Argemone mexicana were in weight (F = 46.71, p = 0.001), thickness (F = 187.49, p = 0.001), length (F = 191.56, p = 0.001), and diameter (F = 215.83, p = 0.001). The evaluated seeds reached their maximal imbibition velocity at 24 h of evaluation. The content of the micro- and macro-nutrients analyzed in the seeds and soil suggest a tight relation with the morphometric characteristics of the seeds.Keywords
The national Mexican territory includes approximately, 50%–70% of arid- and semi-arid regions [1]. The Mexican National Commission of Arid Zones (CONAZA) estimates that of the total population of Mexico, 18% inhabit regions such as these [2]. The arid Chihuahuan zone is one of the arid ecosystems of greatest territorial extension. This kind of ecosystem is one of the most important in North America.
The Chihuahuan Desert (CD) is considered one of the most biologically diverse arid regions of the world, occupying an approximate area of 647,500 km2 [3]. This geographic zone is situated in the Northern-Central part of Mexico, between the Sierra Madre Oriental and the Sierra Madre Occidental [4]. The CD comprises extensions of the Mexican states of Chihuahua (Chih), Coahuila (Coah), Durango (Dgo), Zacatecas (Zac), Nuevo León (NL), and San Luis Potosí (SLP); as well as areas of the U.S. in the states of Arizona, New Mexico, and Texas [5]. The CD has been subdivided into different regions and ecoregions as follows: Trans-Pecos (Ecoregion I: Apachean, and Ecoregion II: Northern Chihuahua) considered as the northernmost zone, which includes approximately 40% of the CD surface, and, also, comprehends sections belonging to the U.S. The mid-region comprises the Bolsón of Mapimí, and part of the Eastern regions of the Mexican states of Chih, Coah, and Dgo (Ecoregion III: Chihuahuense Central). The third region, Saladan, is limited to the Southern zone of the desert, covering areas of the states of Zac and SLP (Ecoregion IV: Central Meseta) [6,7].
Semi-arid ecosystems are characterized by scarce plant covering and low biomass [8]; however, the CD can be distinguished by three basic types of vegetation that make up a xerophilic scrub ecosystem [9]. Microphyll desert scrub is distributed in alluvial soils where small-leaf arbustive elements predominate (e.g., Vachellia (=Acacia), Neltuma (=Prosopis), Larrea) [9,10]. The rosetophyllous desert scrub grows in calcium-rich soils, in those with good drainage, and those that are characterized by being composed of succulent plants, spinescent, with rosette-shaped leaves (e.g., Agave, Yucca) [11]. Crasicaule desert scrub is linked with rocky sites and with igneous soils in nature in which there predominate large Cactaceae with flat or cylindrical stems (e.g., Opuntia) [11]. The xerophytic shrubs can be found under diverse topographical conditions and do not demonstrate a great demand in terms of edaphic properties, although these factors tend to exert an influence on the physiognomy of plant communities [11].
The long-lived perennial plants can form high-biomass patches in arid zones [8]. Wet periods tend to be registered between the months of June and September. The months of October through December experience light precipitations [5], which tend to increase the average biomass values due to the exuberant growth of annual plants and others that are short-lived and opportunistic [8]. The latter are plants that provide environmental services such as nutrient regulation, the contribution of organic material, and biological control [9]. Between the annual or ephemeral vegetation widely distributed in semi-arid regions are found the species belonging to Papaveraceae Family.
The Papaveraceae Family comprises 44 genera and approximately 760 species [12]. The genus Argemone L. (Papaveraceae) is made up of approximately 60 species, among which 28 are native to the American Continent [13]. In South America, at least, five taxa of the genus have been identified as follows: Argemone, in Chile, Argentina, Uruguay, Paraguay, Bolivia, and Panama [14]. Argemone is distributed from the coastal areas of the U.S. Southeast to Texas, as well as in desert regions, Northern California, and Southeastern Oregon. Argemone is present throughout Mexico [15] and in Central America, except in very high mountains [14]. Plants of the genus are found principally distributed in the Northern states of Mexico [15]. The species of this genus are found in regions with low precipitation, in elevations near sea level or even up to 2,400 masl, rarely at higher altitudes, in areas of moderately strong rains, and are localized in soils with a low water-retention capacity [14].
Human activity has played an important role in the distribution of Argemone. This role has probably been passive in the majority of cases, or the seeds have been transported as contaminants in the seeds of cultivated similar-sized plants [14]. The seeds of Argemone mexicana L. and Argemone ochroleuca Sweet are frequent contaminants of grains [16]. However, abiotic factors such as wind and water are also efficient in the dispersion of Argemone seeds [14]. Such plants can be found along drains, at the edges of highways, in cultivated or abandoned fields, and are considered scrubs [16–18].
Argemone seeds are contained in septicidal capsules with four to six locules [19]. Within the capsules, the seeds remain joined to the intralocular septum by the placenta [20]. During the development and desiccation, the coat of the seed thickens and becomes transparent-to-brownish yellow and black in color with the appearance of a concave depression on the surface; this conferring upon it a reticulated aspect [19].
Species of the genus Argemone are of phytochemical and ethnobotanical interest due to their content of alkaloids [18,19]. Plants of the genus have been studied at different structural levels, for example, the leaves were evaluated for their laxative and diuretic effect [21], the extracts of the complete plants were evaluated for their effect against phytopathogens [21,22], analysis of the distribution of alkaloids in seeds that indicate elevated concentrations of sanguinarine [19], and the characterization of the ontogeny of the ovule and the anatomy of the seeds of Argemone aurantica [20]. The obtention of biodiesel from the seeds has been reported for Argemone pleiacantha Greene and A. mexicana [23,24]. The species considered in this study were selected because they are the main species distributed in the central part of the Chihuahuan Desert. Nonetheless, plants belonging to the genus Argemone do not have a direct use in the nutrition of humans or domestic animals, and are considered scrubs; therefore, they have not been broadly studied, and many aspects concerning their ecology are unknown, to or knowledge, to date [25]. Knowing the morphometric characteristics of the seeds and the physical-chemical properties of the soil where these species are distributed could facilitate the domestication process as these are species with medicinal use in the region and the country. The present investigation had as its objective to carry out a morphometric and mineral analysis of the seed; as well as the evaluation of the soil where wild populations of Argemone spp. grow, within the Central Chihuahuan and Central Meseta ecoregions of the CD.
Argemone spp. seeds were gathered during the months of April 2021 and April 2022 in different sites that form part of the Chihuahuan Desert (CD) (Table 1). The areas of Argemone were selected randomly according to their presence at the edge of highways or in agricultural areas. In total, we registered 10 areas of interest (patches of Argemone spp.). In each of these areas, we randomly selected six individuals, as in Pozo-Gómez et al. [26], for their identification and herborization [26]. The botanical material was employed for the taxonomic identification using dichotomous keys [14] and was safeguarded in the Jorge Arturo Alba Avila Herbarium (HJAAA-FCB) of the Universidad Juárez del Estado de Durango, with registration N° HJAAA/FCB-UJED-1123-00035. From each specimen, five fruits were collected. The total amount of seeds was hand-extracted from these fruits at each studied site. Only five fruits were collected because the collection permit No. SGPA/DGVS/04321/22 stipulates so. Seeds per fruit were not quantified because they were in the process of dehiscence. The seeds were stored in paper envelopes at room temperature for four weeks, as in Sánchez-Salas et al. [27]. Afterward, the seeds were revised in a stereomicroscope to discard those with anomalies or physical damage in the coat, such as fractures or malformations [28].
The morphological analysis was conducted randomly in 30 seeds in each of the selected populations, without damage to the seed coat. The variables analyzed included weight, length, diameter, and thickness. Weight was determined on an ADAM® analytical scale (Changzhou, Jiangsu, China), and the measurements of length, diameter, and thickness were performed in a Zeiss Discovery V8 Stereomicroscope (Göttingen, Germany) using AxioVision SE64 4.8 software (Informer Technologies, Inc.) to the nearest millimeter. The analysis also included an evaluation of the external structure of the seeds according to Barthlott and Hunt [29] and color classification according to the Royal Horticulture Society (RHS) [29].
2.2 Imbibition Velocity in the Seeds
Determination of the imbibition rate in seeds consisted of placing two plastic containers with a capacity of 500 ml (experimental units) each, to which we added 300 ml of tap water. Into each experimental unit, 20 seeds were placed, one of A. mexicana (20 seeds) and the other of A. pleiacantha (20 seeds), from which the initial weight was previously obtained to subsequently record absorption values. Seeds were selected and placed “de visu” in similar sizes to ensure reliable results as in Sánchez-Salas et al. [30]. The experiment was set up on a fixed laboratory table against vibrations that could alter the evaluation process. Weight recording was carried out at 8, 12, 24, 32, 36, 48, 56, 60, 72, 360, and 720 h to record weight changes in the seeds as in Sánchez-Salas et al. [28].
The data was analyzed as in Sánchez-Salas et al. [28]. The imbibition velocity was calculated according to the Eq. (1) [31]:
The buoyancy tests consisted of preparing two plots of 50 seeds of A. pleiacantha and A. mexicana, respectively. The lot was divided into 10-seed groups in plastic containers with a 500 ml capacity. To each container, 300 ml of water was added directly from the tap, and proceeded to place the seeds above the water with a plastic spoon to avoid manual manipulation of the seeds [30]. Afterward, every 24 h the treatments were supervised, registering the amount of seeds that were submerged until the completion at day 60 as in Sánchez-Salas et al. [32]. It is worth mentioning that the evaluation time for buoyancy was extended to 60 days due to the absence of sinking.
2.4 Analysis of the Micro- and Macro-Nutrients in Seeds
Determination of the minerals was performed from the seed samples obtained from the 10 sampled populations. Obtention of the ashes of each sample was conducted according to the Official Methods of Analysis of the Official Association of Analytical Chemists (AOAC) [33]. The ashes were submitted to a process of acid digestion for the extraction of micro- and macro-nutrients. The elements determined were copper (Cu), manganese (Mn), iron (Fe), zinc (Zn), calcium (Ca), potassium (K), sodium (Na), and magnesium (Mg). The determinations were carried out by atomic absorption spectrophotometry (PerkinElmer®, Model-AAnalyst 200) (Singapur) using lamps and calibration curves, with specific standards, according to the AOAC [33]. Analysis of the content of nitrogen (N) was performed using the micro-Kjeldahl [34] method. The concentration of phosphorus (P) was quantified by UltraViolet (UV)-Visible spectrophotometry.
2.5 Analysis of Micro- and Macro-Nutrients, pH, Electrical Conductivity, Apparent Density, and Soil-Soluble Anions on the Soil
The soil analysis was performed according to the statutes indicated by the Mexican NOM-021-RECNAT-2000 [34]. The tests conducted were the following: Soil-extractable phosphorus (AS-11); Content of available micronutrients (AS-14); Capacity of cation exchange and interchangeable cations (AS-12); pH in a water medium (AS-02); Apparent Density (AD) (AS-03); Electrical Conductivity (EC) (AS-18); Inorganic Nitrogen (AS-08), and bicarbonates, and the determination of soluble anions (AS-20), which includes bicarbonates, chlorides, and sulfates [34].
The data were statistically analyzed using tests of normality and one-way ANOVA with a significance level of p ≤ 0.05 to define possible differences between variables. Later, the Tukey means test was considered with a p ≤ 0.05. The Principal Components Analysis (PCA) was carried out in the correlation matrix analysis. The data employed for the building of such a matrix were the means of N, P, Cu, Mn, Fe, Zn, Ca, K, Na, Mg, length, diameter, and seed thickness, as well as pH, EC, AD), bicarbonates, chlorides, sulfates, N, P, Cu, Mn, Fe, Zn, Ca, K, Na, and Mg in soil for the 10 sites evaluated. The dendrogram was constructed utilizing the method of Ward, and distance is expressed as Euclidian distance. The information was processed in the Minitab® 17 statistical software program (Minitab, LLC), PASW Statistics version 18.0.0 (SPSS, Inc., Chicago, IL, USA).
3.1 Argemone pleiacantha Greene
The seeds of the analyzed sites showed significant differences in weight, length, diameter, and thickness, as presented in Table 2. The seeds from the site of Ramón Corona, Dgo., exhibited the highest values in all the measured variables. In contrast, the seeds from Ciudad Juárez, Dgo., demonstrated fewer morphometric characteristics. The determinations performed in the sites of El Derrame, Dgo., and El Diamante, Dgo., did not evidence statistically significant differences for weight, length, and diameter. The analyzed seeds of the two previously mentioned sites only showed differences in thickness.
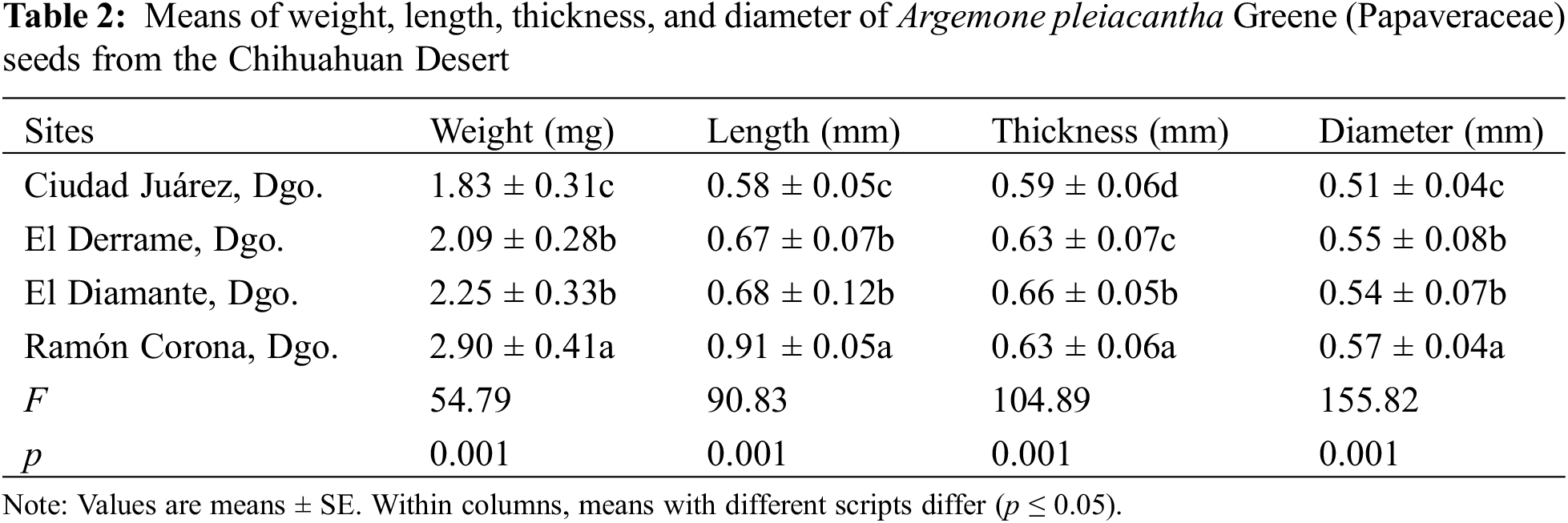
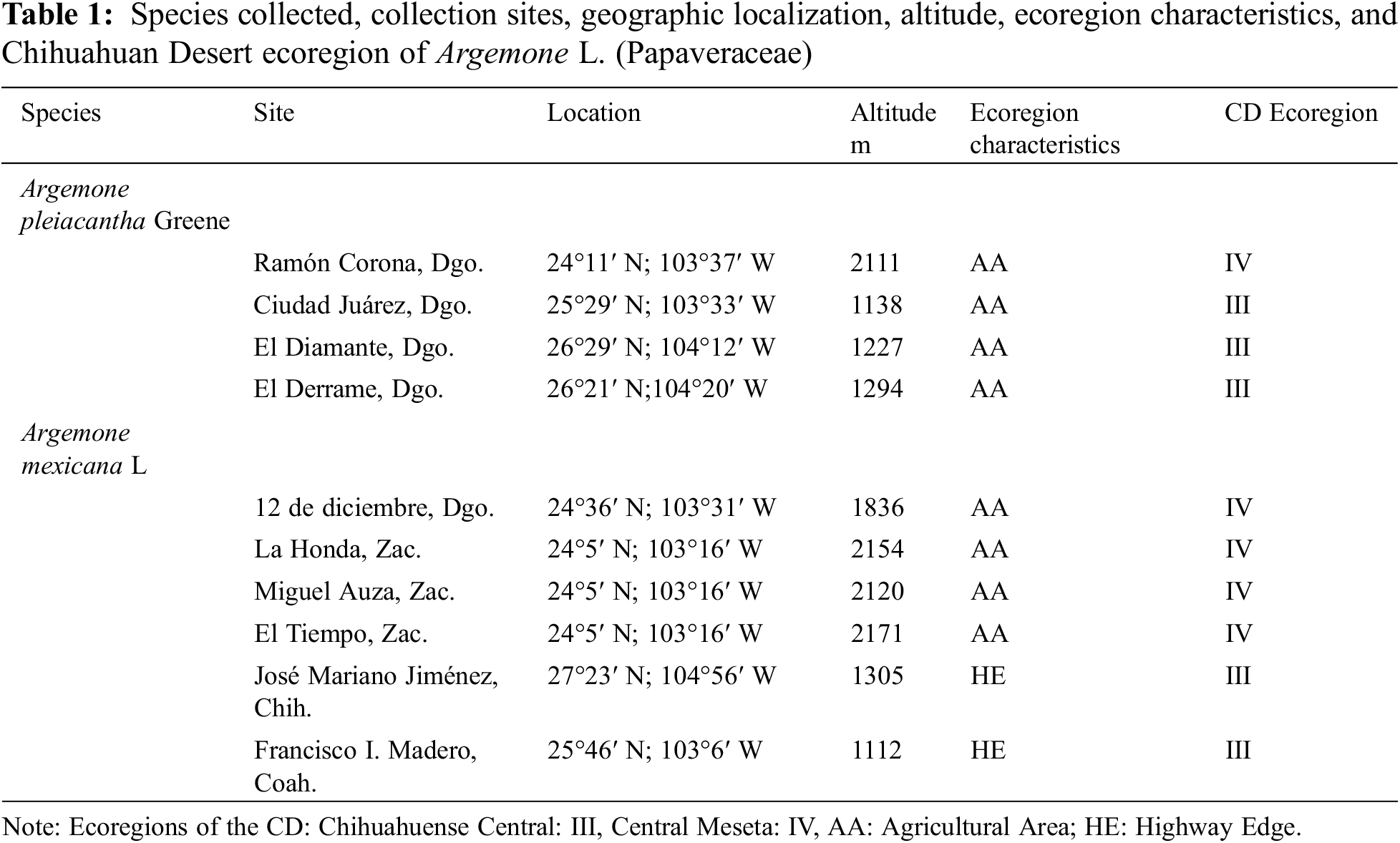
The analyzed seeds of A. pleiacantha exhibited a general sub-globular and apiculate structure. The periphery of the seeds was aquiline in shape. The cells of the seed presented an irregular hexagonal arrangement with straight channeled, and anticlinal limits. The estrophil was crested and the hilus has a superficial and oblique configuration; in addition to possessing a disjunctive micropyle (Fig. 1). The seeds, at first glance, were dark brown in color No. RHS 166A, and on stereoscopy were of a medium yellowish-brown coloration No. RHS 165C; these characteristics did not vary in the seed lot reviewed.
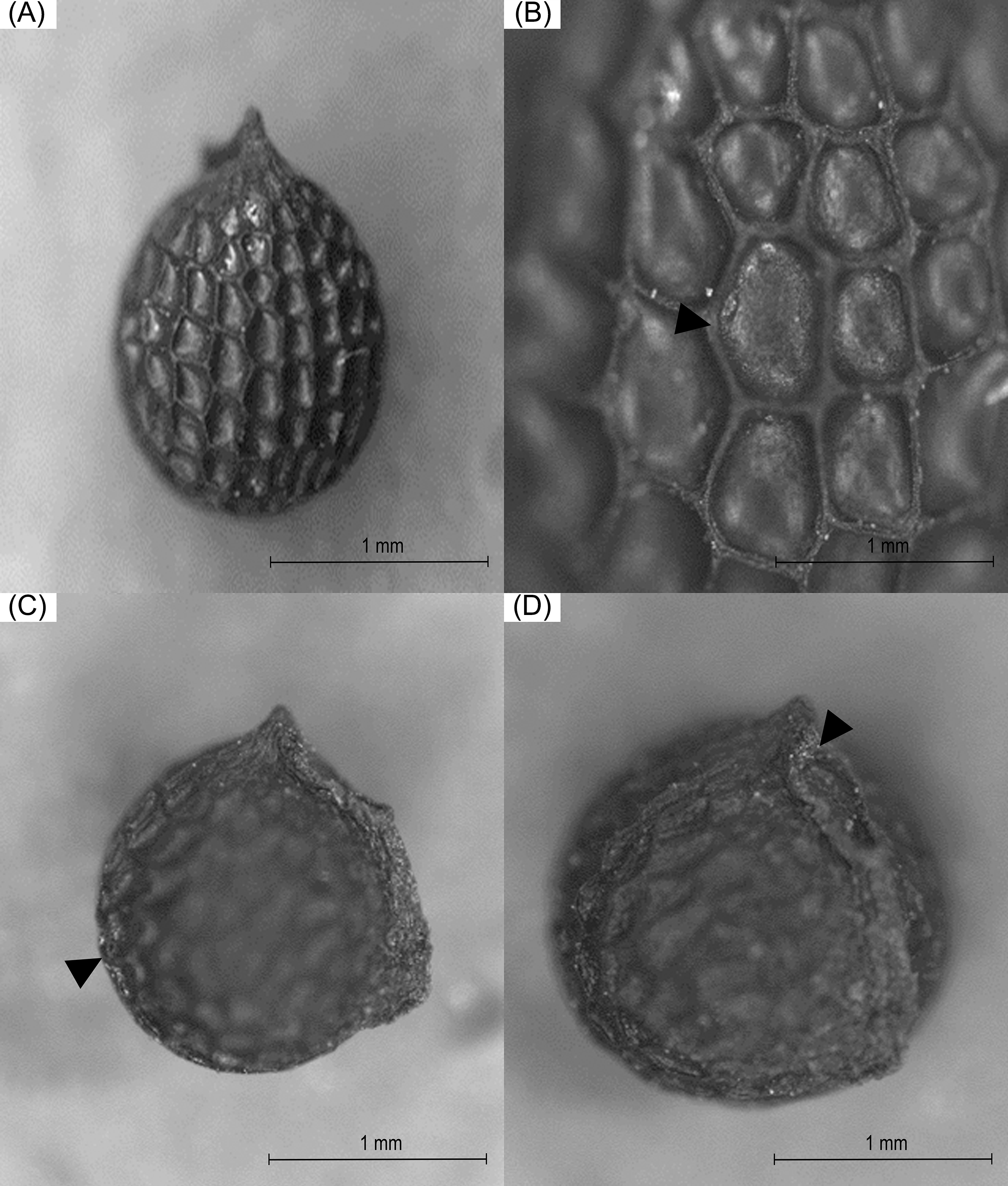
Figure 1: Morphological analysis through stereoscopy in which the general structure of A. pleiacantha seeds was shown (A), grooves originating at the edges between the epidermal cells (B), the aquiline periphery (C), and the hilus (D)
The seeds of the analyzed sites showed significant differences in all variables measured. The evaluations were conducted at the site of Francisco I. Madero, Coah., had higher values (Table 3). In contrast, the seeds at the site of 12 de diciembre, Dgo, had smaller values. The lots from the sites of Miguel Auza, Zac. and El Tiempo, Zac., did not exhibit statistical differences in weight, length, and thickness; however, they showed differences in diameter.
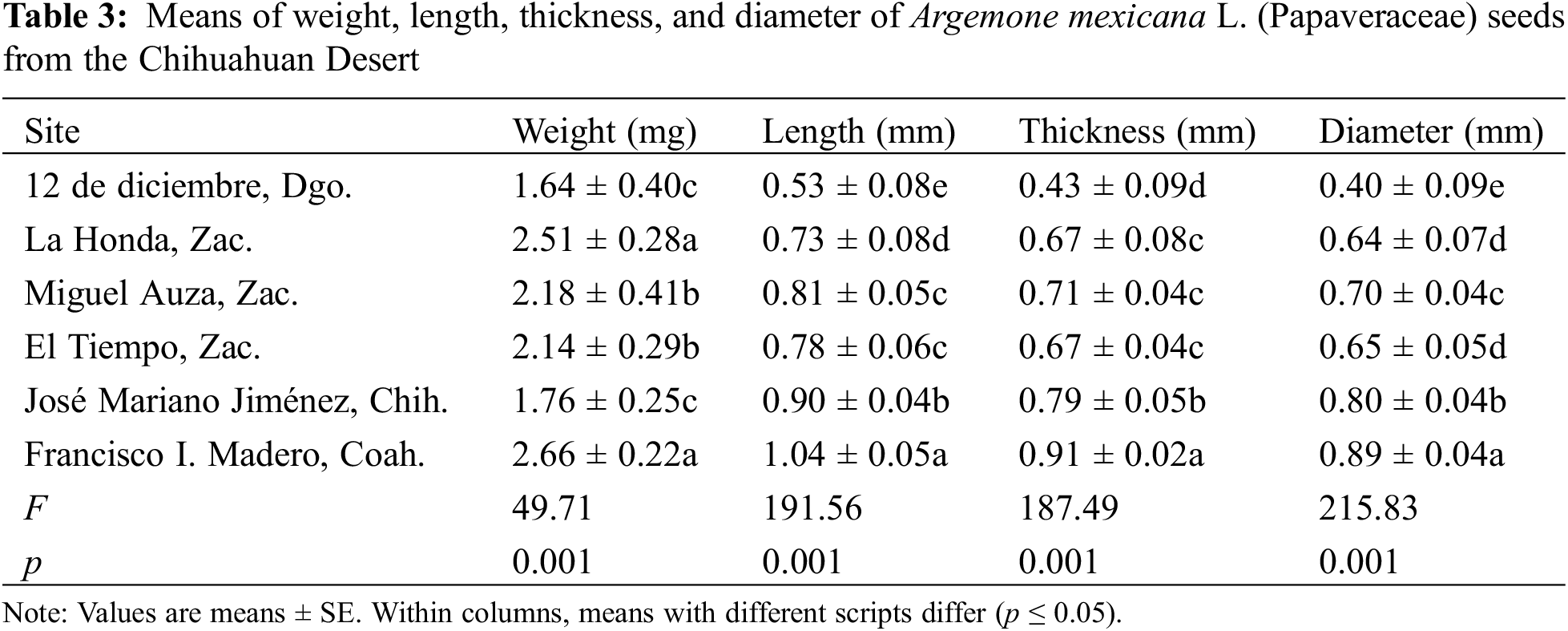
The seeds of A. mexicana included in this study, exhibited a general apiculate and sub-globular structure. The periphery was aquiline in form. The cells presented an irregular hexagonal arrangement with anticlinal channeled and straight limits. The estrophil was crested and the hilus presented a superficial and oblique configuration, in addition to having a disjunctive microphyll (Fig. 2). The seeds, at a simple glance, were dark brown in color (No. RHS 166A), and on stereoscopy presented a medium yellowish-brown coloration (No. RHS 165C), the characteristics of which did not vary in the analyzed lot.
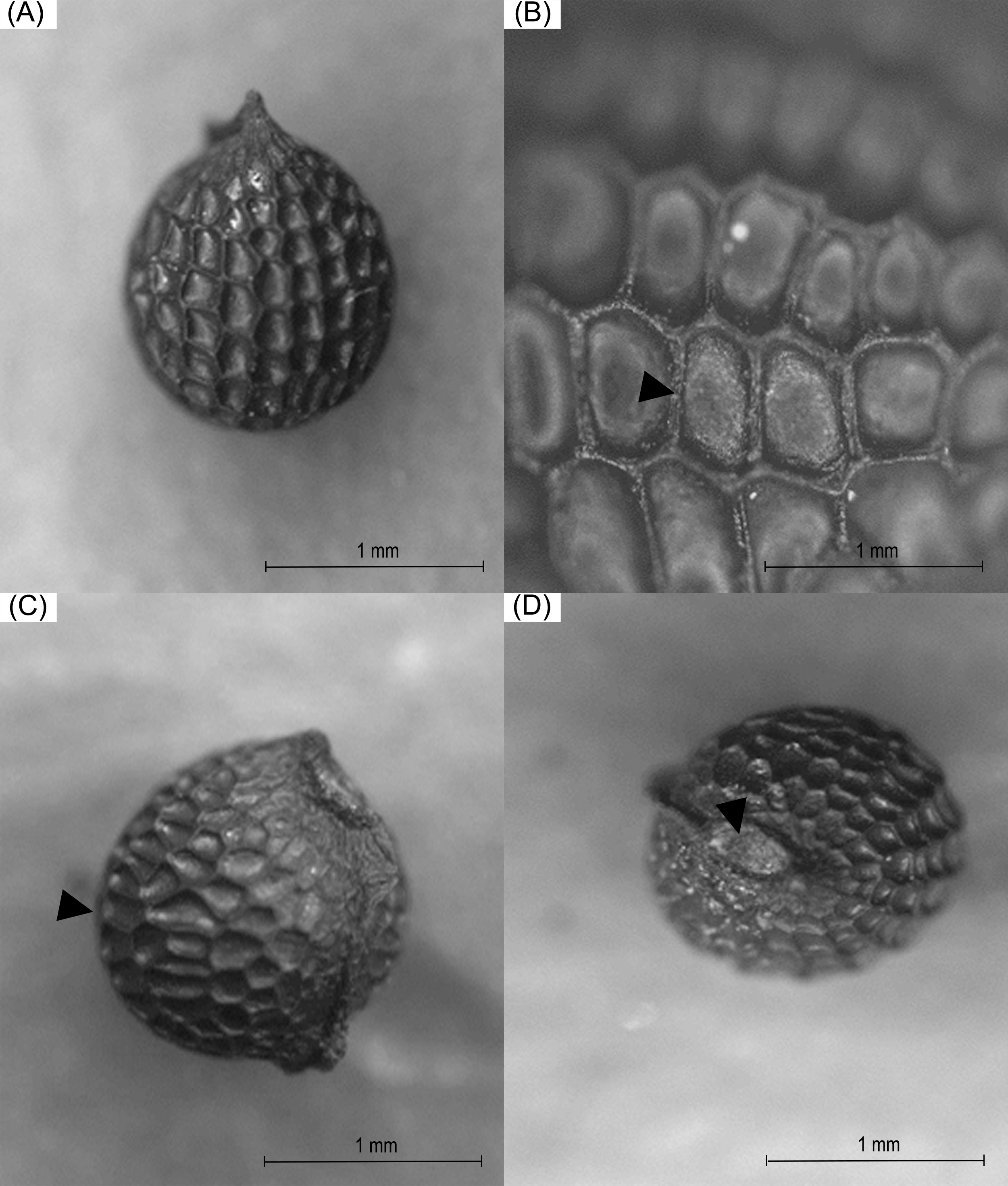
Figure 2: Morphological analysis through stereoscopy in which the general structure of A. mexicana seeds was shown (A), grooves originating at the edges between the epidermal cells (B), the aquiline periphery (C), and the hilus (D)
3.3 Imbibition Velocity in Seeds
The imbibition velocity was similar for the two species evaluated (Fig. 3). The seeds of A. mexicana presented the highest imbibition velocity at 24 h with 0.094 mg h−1. The specimens of A. pleiacantha also presented their maximal velocity at 24 h of imbibition with 0.090 mg h−1. The two groups of seeds diminished progressively their imbibition velocity from 32 to 720 h afterward.
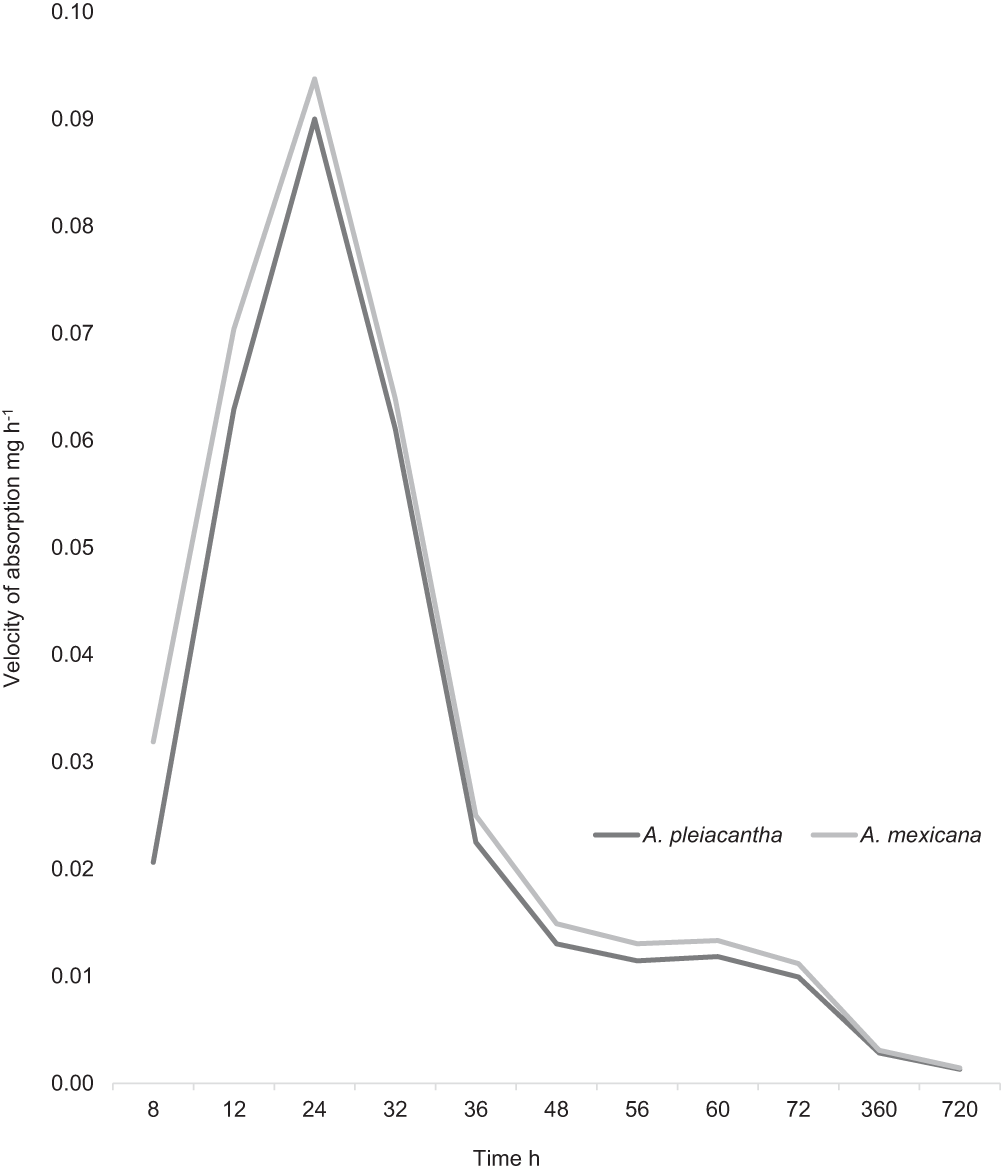
Figure 3: Imbibition velocity in the seeds of Argemonepleiacantha Greene and Argemone mexicana L. (Papaveraceae) from the Chihuahuan Desert
3.4 Evaluation of the Buoyancy of Argemone pleiacantha and Argemone mexicana
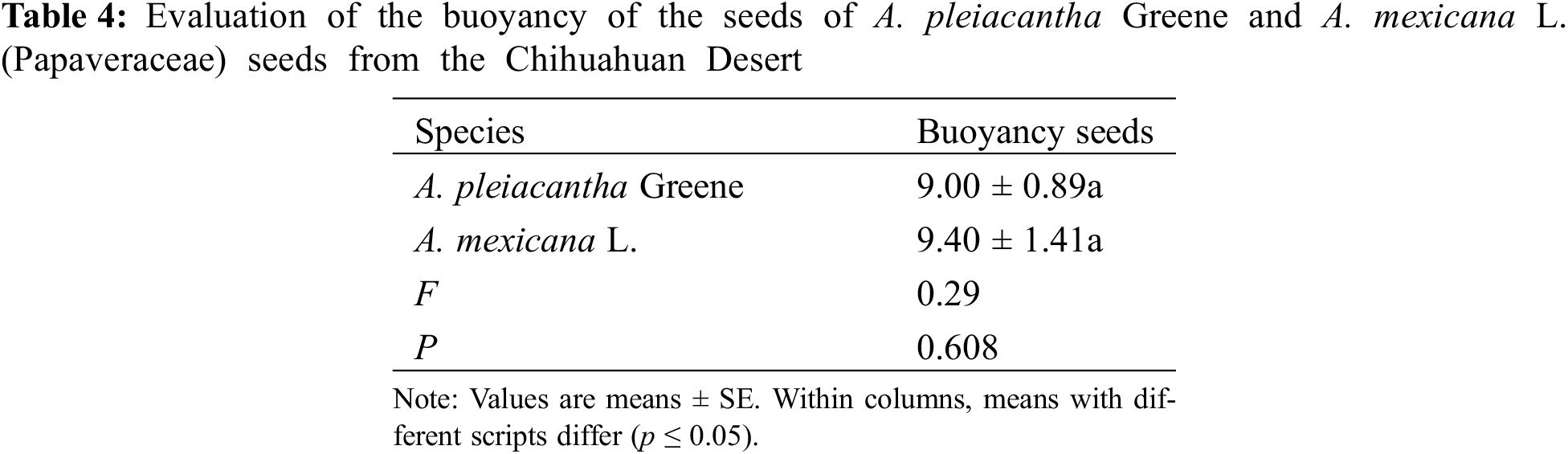
The test for the buoyancy of the seeds did not demonstrate significant differences between the species evaluated (Table 4). The sinking occurred during the first 24 h of the experiment. After this time, sinkings were not registered again along the test despite extending the evaluation period to 60 days to see how the seeds behave to determine possible ecological implications for their dispersal.
3.5 Principal Components Analysis (PCA)
The description of the results was carried out depending on the first three Principal Components (PC). PC 1 explained 28.40% of the total variation of the data set; while 20.99% of the variance was explained by PC 2 and 14%, by PC 3. Therefore, these three PCs explained 63.39% of the variance of the means of the 31 variables analyzed in the seed and soil of the populations of Argemone.
The loading matrix indicates that PC 1 was highly correlated with the morphological variables of the seeds and their contents of macro-nutrients (Table 5). PC 2 was importantly influenced by some of the variables that measured the content of micro-nutrients in seed (Fe, Zn, and K), as well as some variables determined in the evaluated soil samples as follows: sulfates; Zn; Na; Mg, and EC. The third component, PC 3, was in its majority influenced by the bicarbonates, chlorides, P, and Fe in soil. The contents shown for minerals in seed and soil were obtained from the PCA (Table 6).
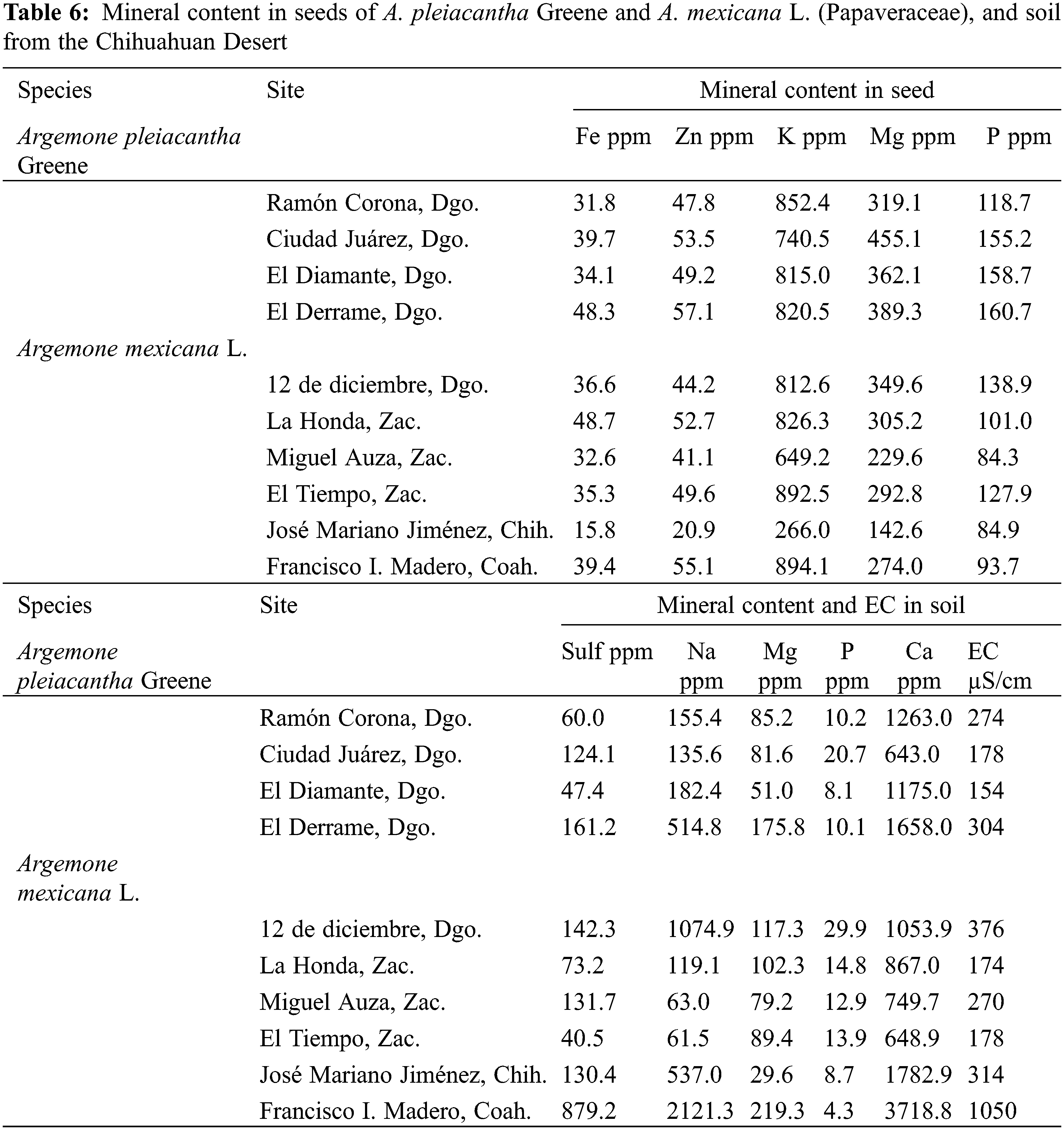
The dispersion graph (Fig. 4) permits distinguishing the groupings of vectors with close interaction, as well as that the analyzed populations are distributed in different quadrants; thus, each population entertains a particular association with the PCA. The vector that represented weight in seeds was related to the following characteristics of the soil: EC Ca; Na; Mg; K; sulfates, and Zn. On the other hand, length, diameter, and thickness were correlated with the macro-nutrients P, Ca, and Mg present in the seed, as well as with P in the soil. The seeds belonging to the towns of 12 de diciembre, Dgo., La Honda, Zac., and El Derrame, Dgo., denote having a greater content of micro-nutrients (Zn, Fe, and Mn) and macro-nutrients (K, Ca, Mg, and P).
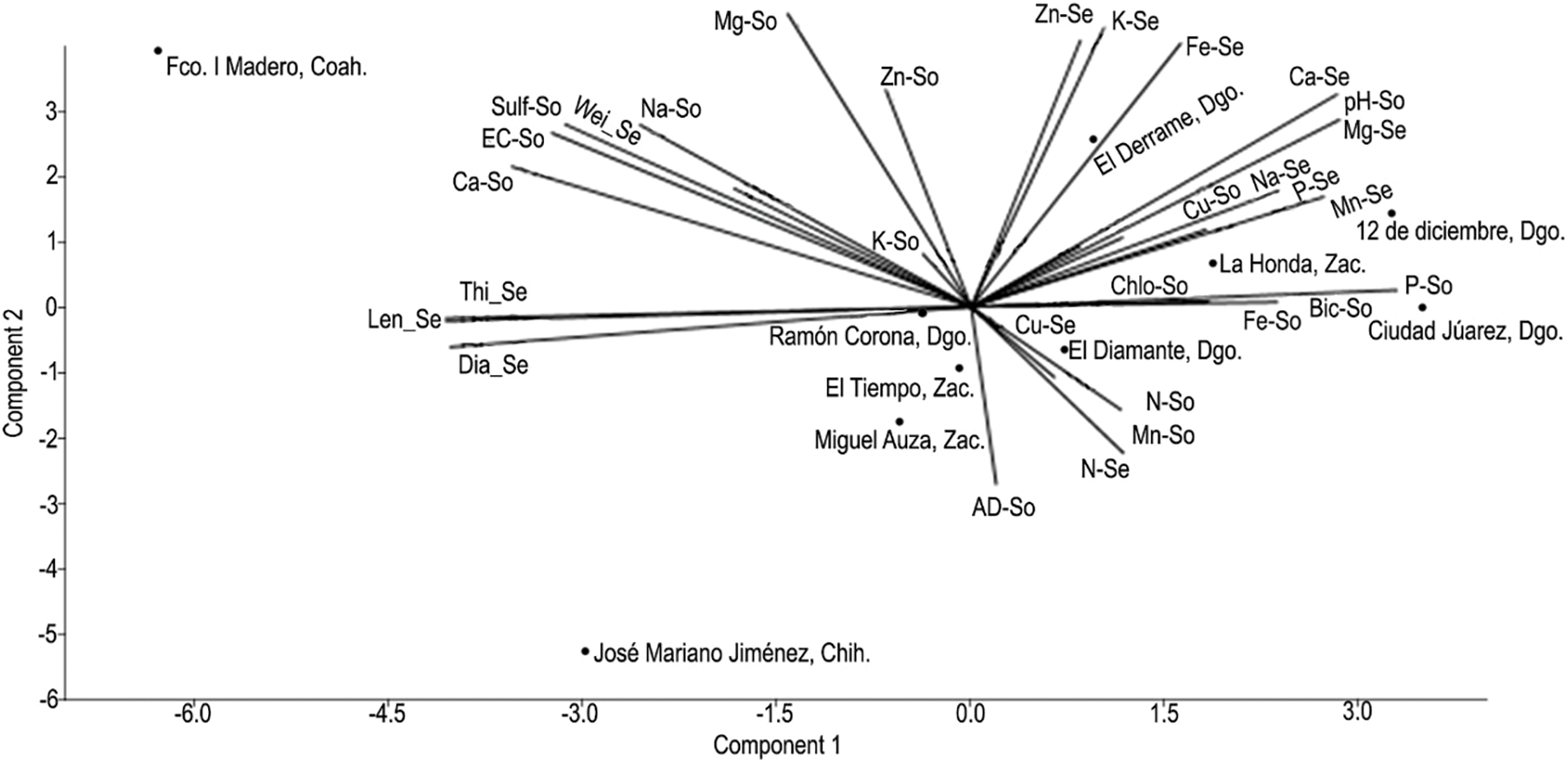
Figure 4: Dispersion graph of the principal component values (PC 1 and PC 2) for the populations of Argemone pleiacantha Greene and Argemone mexicana L. (Papaveraceae) from the Chihuahuan Desert
The dendrogram represented in Fig. 5 denoted the formation of three main groups separated by length, diameter, thickness, and micro- and macro-nutrients in seeds and soil. Group I was comprised of the sites of the towns of 12 diciembre, Dgo., El Derrame, Dgo., and José Mariano Jiménez, Chih., sharing 71.87% similarity corresponding to the species A. mexicana. Group II was that which demonstrated the greatest similarities in the towns analyzed, with 75.62% of similarity (La Honda, Zac., Ramón Corona, Dgo., El Diamante, Dgo., El Tiempo, Zac., Ciudad Juárez, Dgo., and Miguel Auza, Zac.) and which includes the populations of A. mexicana and A. pleiacantha. This is in contrast with Group III, which exhibited a greater dissimilarity (6.25%), including only one observation (Fco. I Madero, Coah.) of A. mexicana.
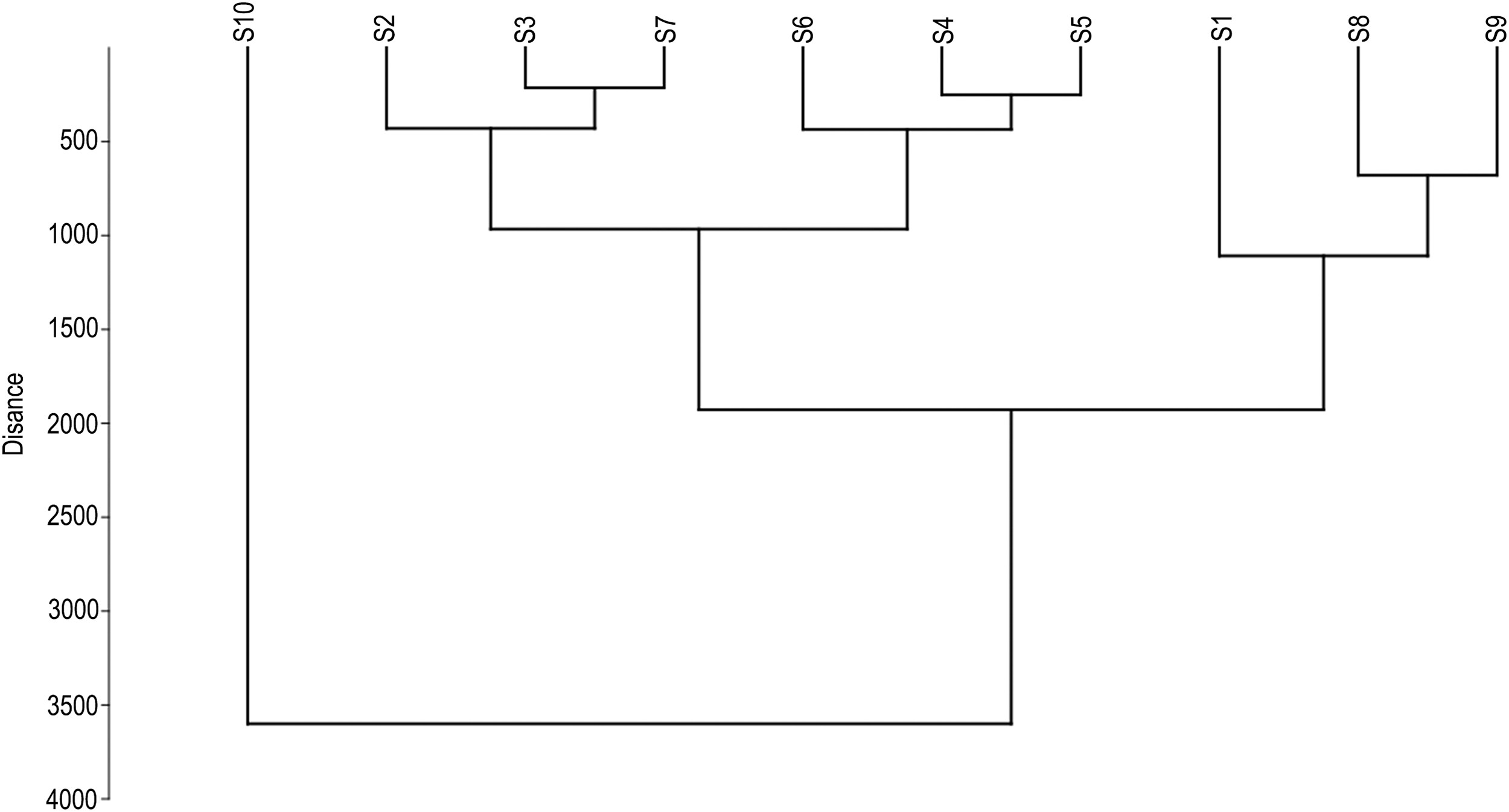
Figure 5: Dendrogram of different sampled populations of Argemone pleiacantha Greene and Argemone mexicana (Papaveraceae) from the Chihuahuan Desert according to the method of Ward, and distance is expressed as Euclidian distance as follows: S1: 12 de diciembre, Dgo; S2: La Honda, Zac; S3: Ramón Corona, Dgo; S4: Ciudad Juárez, Dgo; S5: Miguel Auza, Zac; S6: El Tiempo, Zac; S7: El Diamante, Dgo; S8: El Derrame, Dgo; S9: José Mariano Jiménez, Chih; and S10: Fco. I Madero, Coah
Variability in the morphology and size of the seeds exerts an influence on the biological capacities of survival, such as dispersion and germination [35]. The differences in length and weight in the rate of germination, as has been reported in the Family Cactaceae, where 1 mg implies changes in germinative capacity [27].
In arid zones, the biggest seeds, such as Astrophytum myriostigma Lem. (Cactaceae), possess advantages in germination in a certain manner [27,36]; the latter can be attributed to a large reserve of nutrients [37]. The dispersion and establishment of seeds can be considered factors that exert selective pressure [36], which tends to set the seeds in opportune physical areas that allow for diminishing intraspecific competition. The seeds of A. pleiacantha and A. mexicana presented a difference in weight, length, diameter, and thickness; therefore, it can be considered that dispersion as well as the establishment, could be favored in A. mexicana, due to its greater size in the previously mentioned variables.
The process of imbibition is the mechanism of capturing water by the seed [38]. Such a process permits the seeds to achieve, in a quick way, the same level of humidity, and they activate the metabolic apparatus related to the pre-germinative process [39]. The imbibition tests suggest that Argemone seeds are considered permeable during the initial hours of their contact with the water. The rapid imbibition orients toward an adaptation to take advantage of the space hydric resource available during the rainy season. The wet seasons tend to be concentrated during the seasons of summer–autumn and during short periods of winter rains associated with the cold fronts forming during this time of the year [5]. The latter rainy conditions are present at sites in Northern Mexico, where diverse species of the genus are widely distributed [15]. The absorption of the water is directly influenced by the presence of the seed coat and the permeability that the species possesses [31,40]. The seeds can present elevated levels of permeability when they possess external structural arrangements that allow the capture and storage of environmental humidity. The irregular hexagonal cellular arrangement that the seeds of Argemone present could permit the retention of water on the external surface.
The duration of the buoyancy of the seeds is an adaptative mechanism that increases the efficiency of the dispersion [41]: it has been reported that seeds of the Families Taxodiaceae and Cactaceae can remain buoyant for more than one month without losing their bioavailability, in that they are dispersed through run-offs [32,42]. The content of oils in the Argemone seed [21] could proffer a lesser density than the water, permitting longer buoyancy. Argemone seeds can remain buoyant for more than one month. However, it is necessary to conduct tests to determine their viability and to elucidate whether they present morphological and internal anatomical adaptations for their hydrodispersion.
The edaphic properties tend to influence the physiognomy of plant communities [11]. The present study revealed a close interaction between the weight of the seeds and the content of the macronutrients present in the soil at the sampled sites, particularly in those of Fco. I. Madero, Coah., El Tiempo, Zac., and José Mariano Jiménez, Chih. The latter correspond to populations of A. mexicana, which can indicate that this species is found better adapted to saline and calcareous soils. This is in contrast with the majority of the populations of A. pleiacantha, which are indicated as being adapted to and distributed more widely in soils with higher mineral content.
On the other hand, the variables of length, diameter, and thickness were related to the amount of micro-and macronutrients present in the seed most significantly with P, which is attributed to that because P constitutes part of the phytin, the main manner of the storage of P in seeds. The size, number, and variability of the seeds can be affected by the content of P in the soil [43].
In this study, the variables measured in soil and seed indicate that the sites evaluated share similarities, despite corresponding to different ecoregions (Chihuahuense Central and Meseta Central). The hierarchical cluster analysis grouped greater similarities in some of the sites that corresponded to the following agricultural areas: La Honda, Zac.; Ramón Corona, Dgo.; Ciudad Juárez, Dgo.; Miguel Auza, Zac.; El Tiempo, Zac., and El Diamante, Dgo. The previously mentioned sites share the following ecological characteristics necessary for the development of Argemone: low precipitation; elevations no higher than 2,400 masl and soils with a low water-retention capacity [14]. The Chihuahuense Central ecoregion is characterized by having soils deriving from limestone [7], which was highly reflected in one of the sites belonging to this ecoregion with an elevated interaction with Ca and Mg in the soil.
The genus Argemone responds to environmental characteristics; thus, it exhibits conditions of adaptability in the morphology of its seeds. The seeds’ morphological characteristics confer on Argemone a rapid imbibition velocity and long buoyancy; these characteristics suggest an adaptation to their being disseminated using hydrochory during the wet seasons. The content of micro- and macro-nutrients analyzed in seed and soil suggest a close relation with the morphological characteristics of the seeds and a better adaptation of A. mexicana to saline and calcareous soils.
Acknowledgement: We thank the Facultad de Ciencias Biológicas (Gómez Palacio, Dgo, México) for the technical support, and we are sincerely thankful for the help from Biologist Arturo Salcido Adame.
Funding Statement: The authors did not receive specific funds for this study.
Author Contributions: Conception and study design: Ochoa-García Perla Patricia, Sánchez-Salas Jaime; data collection: Ochoa-García Perla Patricia; analysis of interpretation of the results: Ochoa-García Perla Patricia, Sánchez-Salas Jaime, Trejo-Calzada Ricardo, Quezada-Rivera Jesús Josafath, García-González Fabián; preparation of the manuscript draft: Ochoa-García Perla Patricia. All of the authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable, temporally.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they do not have conflicts of interest to inform concerning the present study.
References
1. Pontifes P, García-Meneses P, Gómez-Aíza L, Monterroso-Rivas A, Caso-Chávez M. Land use/land cover change and extreme climatic events in the arid and semi-arid ecoregions of Mexico. Atmósfera. 2018;31(4):355–372. [Google Scholar]
2. CNF. Las zonas áridas son más que desierto. Available from: https://www.gob.mx/conafor/es/articulos/las-zonas-aridas-son-mas-que-desierto?idiom=es. [Accessed 2018]. [Google Scholar]
3. Hoyt CA. The Chihuahuan Desert: diversity at risk. Endalger Species Bull. 2002;19(6):16–7. [Google Scholar]
4. NPS. Chihuahuan Desert inventory and monitoring network. Available from: https://www.nps.gov/im/chdn/ecoregion.htm. [Accessed 2022]. [Google Scholar]
5. Service UF. 321 Chihuahuan Desert Province. Available from: https://www.fs.usda.gov/land/ecosysmgmt/colorimagemap/images/321.html#:~:text=In%20July%2C%20summer%20rains%20usually,typical%20of%20the%20Chihuahuan%20Desert. [Accessed 2024]. [Google Scholar]
6. Brown DE. Chihuahuan Desert scrub. Desert Plants. 1982;4(1–4):169–79. [Google Scholar]
7. Dinerstein E, Olson D, Montoya J, Loucks C, Contreras-Balderas S, Abell R, et al. Ecoregion-based conservation in the Chihuahuan Desert: a biological assessment. 2nd edWorld Wildlife Fund, Mexico; 2001. p. 1–122. [Google Scholar]
8. Huenneke LF, Clason D, Muldavin E. Spatial heterogeneity in Chihuahuan Desert vegetation: implications for sampling methods in semi-arid ecosystems. J Arid Environ. 2001;47:257–70. [Google Scholar]
9. NACOBIO. Thickets. Available from: https://www.biodiversidad.gob.mx/ecosistemas/Matorral. [Accessed 2022]. [Google Scholar]
10. Josse C. Central mexican mixed desert scrub. Available from: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.938188/Matorral_Crasicaule_de_Mexico_Central. [Accessed 2022]. [Google Scholar]
11. Rzedowski J. Vegetación de México Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. 1978. [Google Scholar]
12. Granados-Echegoyen CA, Chan-Bacab MJ, Ortega-Morales BO, Vásquez-López A, Lagunez-Rivera L, Fidel Diego-Nava F, et al. Argemone mexicana (Papaverales: papavaraceae) as an alternative for mosquito control: first report of larvicidal activity of flower extract. J Med Entomol. 2018;56(1):261–7. [Google Scholar]
13. Hernández-Ruiz J, Bernal J, Gonzales-Castañeda J, Ruiz-Nieto J, Mireles-Arriaga A. Argemone ochroleuca: (Papaveraceaealkalois potential source for agricultural and medical uses. Trop Subtrop Agroecosyt. 2020;23(2):1–13. [Google Scholar]
14. Ownbey GB. Monograph of the genus Argemone for North America and the West Indies. Bull Torrey Bot Club. 1958;21(1):1–159. [Google Scholar]
15. Villaseñor JL. Checklist of the native vascular plants of Mexico Catálogo. Rev Mex Biodivers. 2016;87:559–902. [Google Scholar]
16. Jimoh F, Adedapo A, Aliero A, Afolayan A. Polyphenolic and biological activities of leaves extracts of Argemone subfusiformis (Papaveraceae) and Urtica urens (Urticaceae). Rev Biol Trop. 2010;58(4):1513–31. [Google Scholar]
17. Alemayehu K. Prevalence and effects of Argemone mexicana (Papaveraceae) on biodiversity in Ethiopia. Afr J Ecol. 2011;50(2):160–6. [Google Scholar]
18. Rajvaidhya S, Nagori BP, Singh GK, Dubey BK, Desai P, Sanjay J. A review on Argemone mexicana Linn. An Indian medicinal plant. IJPRS. 2012;3(8):2494–501. [Google Scholar]
19. Loza-Muller LJ, Laines-Hidalgo JI, Monforte-González M, Vázquez-Flota F. Alkaloid distribution in seeds of Argemone mexicana L. (Papaveraceae). J Mex Chem. 2021;65(4):501–6. [Google Scholar]
20. Cresson RA, Schneider EL. Ovule and seed structure in Argemone aurantiaca (Papaveraceae). Bull Torrey Bot Club. 1988;115(2):108–12. [Google Scholar]
21. Granados-Echegoyen C, Ortega-Morales BO, Chan-Bacab MJ, Reyes-Estébanez MMJ, Camacho-Chab JC. Efecto del extracto etanolico de partes vegetales de Argemone mexicana (Papaveraceae) sobre larvas y pupas del mosquito Culex quinquefasciatus (Say) (Diptera: culicidae). Entomología mexicana. 2016;3:436–40. [Google Scholar]
22. Vetal D, Pardeshi A. Larvicidal potential of Argemone mexicana L. plant extracts against Spodoptera litura fab. J Pharm Innov. 2019;8(6):698–702. [Google Scholar]
23. Rao R, Zubaidha P, Kondhare D, Reddy N, Deshmukh S. Biodiesel production from Argemone mexicana seed oil using crystalline manganese carbonate. Pol J Chem Tech. 2012;14(1):65–70. [Google Scholar]
24. Sáez-Bastante J, Carmona-Cabello M, Villarreal-Ornelas E, Trejo-Calzada R, Pinzi S, Pilar Dorado M. Feasibility of the production of Argemone pleiacantha ultrasound-assisted biodiesel for temperate and tropical marginal areas. Energies. 2023;16(2588):1–14. [Google Scholar]
25. Karnawat M, Malik CP. Phylogenetic relationships of Argemone species based on seed and leaf protein polymorphism revealed by SDS-PA. Jour Pl Sci Res. 2011;27(2):211–4. [Google Scholar]
26. Pozo-Gómez DM, Orantes-García C, Rioja-Paradela TM, Moreno-Moreno RA, Farrera-Sarmiento O. Diferencias en morfometría y germinación de semillas de Croton guatemalensis (Euphorbiaceaeprocedentes de poblaciones silvestres de la Selva Zoque, Chiapas, México. Acta Bot Mex. 2019;126:1–12. [Google Scholar]
27. Sánchez-Salas J, Flores J, Martínez-García E. Efecto del tamaño de semilla en la germinación de Astrophytum myriostigma Lemaire. (Cactaceaeespecie amenazada de extinción. Interciencia. 2006;31:371–5. [Google Scholar]
28. Sánchez-Salas J, Flores J, Jurado E, Sáenz-Mata J, Orozco-Figueroa P, Muro-Pérez G. Hidrocoria en semillas de Agave victoriae-reginae T. Moore, especie en peligro de extinción: morfología y anatomía como facilitadores de la hidro-dispersión y germinación. Gayana Bot. 2017;74:251–61. [Google Scholar]
29. Barthlott W, Hunt D. Descriptors and descriptive terminology. England: Remous Ltd.; 2000. [Google Scholar]
30. Sánchez-Salas J, Flores J, Muro-Pérez G, Arias S, Jurado E. Morfometría de semillas en la cactácea amenazada de extinción Astrophytum myriostigma lemaire. Polibotánica. 2015;39:119–131. [Google Scholar]
31. Méndez-Natera JR, Merazo-Pinto JF, Montaño-Mata NJ. Relación entre la tasa de imbibición y el porcentaje de germinación en semillas de maíz (Zea mays L.caraota (Phaseoulus vulgaris L.) y quinchoncho (Cajanum cajan (L.) Mill.) UDO Ag. 2008,8(1):61–6. [Google Scholar]
32. Sánchez-Salas J, Jurado E, Flores J, Estrada-Castillón E, Muro-Pérez G. Desert species adapted for dispersal and germination during floods: experimental evidence in two Astrophytum species (Cactaceae). Flora–Morphol Distrib Funct Ecol Plants. 2012;207:707–11. [Google Scholar]
33. AOAC. Official methods of analysis of the association of official analytical chemists. Washington DC, USA: Association of Official Analytical Chemist; 1990. [Google Scholar]
34. NOM-021-RECNAT-2000. Mexico: DOF; 2002. p. 1–73. [Google Scholar]
35. Gutterman Y. Strategies of seed dispersal and germination in plants inhabiting deserts. Bot Rev. 1994;60(4):373–425. [Google Scholar]
36. Gutterman Y. Environmental factors and survival strategies of annual plant species in the Negev Desert. Israel Plant Species Biol. 2000;15(2):113–25. [Google Scholar]
37. Leishman M, Wright IJ, Moles AT, Westoby M. The ecology of regeneration in plant communities. In: The evolutionary ecology of seed size. Wallingford, RU: CABI Publishing; 2000. p. 31–57. [Google Scholar]
38. Civan F. Instrumental and laboratory techniques for characterization of reservoir rock. In: Civan F, editor. Reservoir formation damage. 4th edUSA: Gulf Professional Publishing; 2023. p. 615–44. [Google Scholar]
39. Burgas R, Powell A. Evidence for repair processes in the invigoration of seeds by hydration. Ann Bot. 1984;53(5):753–7. [Google Scholar]
40. Moreno F, Plaza GA, Magnitskiy SV. Effect of the seed coats on germination of rubber (Hevea brasiliensis Muell.) seeds. Agron Colom. 2006;24(2):290–5. [Google Scholar]
41. López OR. Seed flotation and postflooding germination in tropical terra firme and seasonally flooded forest species. Funct Ecol. 2001;15(6):763–71. [Google Scholar]
42. Boedeltje G, Bakker JP, Ten Brinke A, van Groenendael JM, Soesbergen M. Dispersal phenology of hydrochorous plants in relation to discharge, seed release time and buoyancy of seeds: the flood pulse concept supported. J Ecol. 2004;92(5):786–96. [Google Scholar]
43. Kołodziejek J. Effect of seed position and soil nutrients on seed mass, germination and seedling growth in Peucedanum oreoselinum (Apiaceae). Sci Rep. 2017;7(1959):1–11. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools