 Open Access
Open Access
ARTICLE
Novel Insights into the Conservation Physiology and Ex situ Conservation of the Threatened and Rare Semi-Aquatic Moss Drepanocladus lycopodioides (Amblystegiaceae)
1 Institute of Botany and Botanical Garden “Jevremovac”, Faculty of Biology, University of Belgrade, Belgrade, RS-11000, Serbia
2 Center of Plant Biotechnology and Conservation (CPBC), Belgrade, RS-11000, Serbia
3 Department of Botany, Hungarian Natural History Museum, Budapest, HU-1476, Hungary
4 Department of Plant Biology, Institute of Biology and Ecology, Faculty of Science, Pavol Jozef Šafárik University in Košice, Košice, SK-040 01, Slovakia
* Corresponding Author: Marko S. Sabovljević. Email:
Phyton-International Journal of Experimental Botany 2024, 93(11), 3039-3054. https://doi.org/10.32604/phyton.2024.058469
Received 13 September 2024; Accepted 01 November 2024; Issue published 30 November 2024
Abstract
The rare and threatened semi-aquatic moss Drepanocladus lycopodioides (Amblystegiaceae) was the subject of growth optimization under ex situ axenic laboratory conditions. The positioning of the plantlets on media, media types as well as selected growth regulators and sugars were parameters tested in optimizing growth promotion of this species in captivity. Out of the tested media types, the KNOP medium and the upright positioning of the explants were the best for propagation and biomass production of D. lycopodioides. The addition of sugars had no significant effect on this moss development axenically, while exogenously applied Benzylaminopurine (BAP) at a concentration of 3 µM induced the development of archegonia on the sterile gametophores and can be used as a marker for confirmation of the female sex, by inducing archegonia on the sterile female plants or clones. This study aimed to contribute to the conservation of this threatened moss species, by massive propagation for reintroduction and population straitening in natural and semi-natural habitats, in scenarios of rapid climate change and water shortage. Laboratory experiments were carried out to study the biological features of the species itself, as due to its rarity empirical data are lacking. This research contributed to the conservation and multiplication of D. lycopodioides and suggested an elegant method to prevent the extinction of this species from its natural habitats. This enables experimental investigation in this species and directs a plan of action for species survival both in captivity (ex situ) and in nature (in situ).Keywords
Mosses (along with other bryophyte groups) are a group of higher plants that receive not so much conservation attention like tracheophytes. However, due to their small size and adaptation to narrow habitat types, i.e., micro-habitats with specific micro-niches, they often severely suffer small environmental changes. In an era of a rapidly changing environment and climate crisis, the active conservation effort is rather needed and inevitable for many bryophyte species to enable their survival [1]. Moss species that are particularly sensitive to environmental changes include those belonging to the semi-aquatic life forms, not only due to changes in water chemistry and physical properties of water but also due to prolonged periods of drought and the inability to cope with such prolonged dry periods. Among such species, Drepanocladus lycopodioides (Brid.) Warnst. (Amblystegiaceae) is assessed as threatened with extinction risk both regionally and at the European level. It is a European sub-endemic moss, that occurs in western, northern, and central Europe, but also east to the Urals mountains, the Caucasus, and Turkey, as well as in Georgia and western Siberia in Russia [2].
Its habitat types include wetlands like seasonally flooded calcareous dune slacks and wet, calcareous mesotrophic fens where often Caricetum communities are present. The species is dioecious and capsules are rarely reported [3]. It can be seen along a wide altitudinal range up to 2200 m above the sea, but it cannot fully dry out of the habitat during the summertime. Shallowly submerged during high water levels is well survived but the complete drying out of habitat is excluded since at least surface moist underneath has to be kept during dry summer months to maintain this species present in such sites. In its habitat, high calcium levels (26–218 mg/L) and circum-neutral to basic habitat (pH 6.0–8.2) are often characteristic [4]. Dryness, drainage, and eutrophication of its habitats are the main reasons for the rapid decline of this species. Infrastructure development and low rate of sexual propagation remain additional problems in species survival. This species is regarded as vulnerable (IUCN: VU) in Europe [3], and it is also red-listed in many national and regional lists of threatened species. Thus, it is critically endangered (IUCN: CR) in Slovakia, endangered (IUCN: EN) in Hungary, Norway, and Romania, vulnerable (IUCN: VU) in Finland, Slovenia, Switzerland and Ukraine [3–6]. It is also considered in national categorization under threat in Belgium, Britain, Latvia, Lithuania, the Netherlands, and Poland [3]. In Ireland, it is near threatened (IUCN: NT) [3], while in Italy its status is insufficiently known (IUCN: DD) [7]. In Serbia, its presence was rejected in the revision of previously reported material and was therefore not assessed as extinct in the recent Red List of Mosses [8]. The rapid decline of this species’ European population, with a decrease of more than 30% within the last three generations [3], makes this species one of those with urgent needs for integrative conservation. The idea of the paper is to study morpho-genetical features of D. lycopodioides and to find out the best way for its propagation and multiplication procedures. To learn more about the biological features of this rare and threatened moss and to optimize growth and biomass production ex situ for conservation purposes, we conducted a series of experiments that led to morphogenesis understanding. All these activities led to achieving enough material for potential reintroduction and propagation in natural and semi-natural sites.
The novel discipline of conservation physiology in bryophytes protection [9] considers apart from conservation measures and active approach, also the studies of species biology which are, in fact, highly valuable since due to its rarity in nature there are no empirical data on many species features as in more common species, which can be used in adequate conservation. Additionally, axenic, ex situ, and/or in vitro bryophyte cultivation is a challenge with many problems, but rather a good approach where one can control many variables and study the direct or joint effect. Such data can be extremely worthy in good protection measure definition and thus species survival.
In the conservation of bryophytes, mainly passive methods are currently used [3,4], but ex situ methods are often proposed as a survival measure, including tests and application of biotechnology in addition to biotechnical approaches to increase the establishment probability and survival rate of the target taxon.
Therefore, integrative conservation measures (i.e., experimental studies) are required for many bryophyte species, and this is the approach we use in this study for the welfare and survival of D. lycopodioides.
The plant material originated from Hungary. It was collected and identified by Beata Papp following Hedenäs [10], from one of its known sites in Veszprém County, a wet meadow between Gyepükaján and Szentimrefa, N47°02′49,9″, E17°17′02,0″, 160 m, 24.04.2017. Herbarium vouchers: BP192626 and BEOU-Bryo7235.
The rather fresh sample 10 mm apical tips (two weeks after field collection) were taken to establish in vitro axenic culture. Young tips were surface sterilized using 3% NaDCC (sodium dichloroisocyanurate, Sigma Aldrich, Vienna, Austria) for 4 min and placed on a minimal KNOP basal medium. The sterilization did not make the plant axenic thus cutting the newly developed tips and transferring to a new sterile medium occurred at least 6 times before getting fully axenic cultures. This time and NaDCC concentration were chosen while longer time exposure did not harm the associated algal co-habitants but was lethal for plant tips, while higher concentrations of NaDCC were lethal for the washed and fully hydrated plant material even if the exposure time was lower. Thus, the subsequent transfer to fresh media of new tips led to moss material in vitro fully disposed of bacterial, fungal, or algal contaminants i.e., axenic. Such plants were tested for contaminant organisms on a minimal KNOP medium enriched with sugar (1% sucrose added) to provoke contaminant blossoming if any. The crucial step in this procedure is to test as many as possible combinations of time and sterilizing agency, since the prosperous approach, apart from species, can be depending also on the state of the start material, i.e., plant fitness (old, young, fresh, partly dried, wet, fully rehydrated, plants originated from in optimal/suboptimal or sublethal conditions, etc.).
After establishing the axenic in vitro culture, the plants were further propagated on a minimal KNOP sugar-free medium [11] until sufficient material for the experiments was obtained. The pH of the media was adjusted to 5.8 before autoclaving at 121°C for 45 min. For all experiments, 10 mm long, decapitated explants were used. Decapitation (tip removal) aims to remove the dividing cell(s) and avoid potential artifacts in measurements of regeneration and reproduction time. Thus, each explant had the same probability to dedifferentiate and start production of the new meristem and tip(s).
Each treatment consisted of 5 Petri dishes with 4 explants, resulting in 20 replicates per treatment (n = 20). The experiment was carried on in axenic conditions free from uncontrolled effects of cohabitants or unpredicted abiotic condition variations [12]. The plant material was grown in sterile chambers at a constant temperature (18 ± 2°C), humidity of 60%–70%, and a long-day light regime (16 h light/8 h dark). The light source was fluorescent tubes (Tesla Pancevo) with a flux density of 50 µmol m−2 s−1.
Three types of experiments were carried out in this study (Table 1).
Influence of media type
The influence of different types of media on the growth and development of Drepanocladus lycopodioides was examined in Experiment type I. The explants were placed upright (erect) and prostrate on minimal KNOP medium, half-strength MS medium (referred to as MS/2), and BCD medium [13–15].
Influence of plant growth regulators
After choosing the best media type for growth and development, the effects of different concentrations of exogenously added plant growth regulators (PGR) were investigated in Experiment type II. The explants were placed upright and prostrate on KNOP medium supplemented with different concentrations (0.03, 0.3 or 3 µM) of the auxin indole-3-butyric acid (IBA) or the cytokinin 6-benzylamino purine (BAP).
Influence of the carbon source
To examine the influence of different sugar concentrations and test them as signal molecules and/or carbon sources, the third type of experiment was applied. Upright-positioned explants were placed on a minimal KNOP medium supplemented with different types of sugar (fructose, glucose, maltose, and sucrose) at two different concentrations (0.04 or 0.08 M). The primary sugar concentration was chosen to be used as half-strength sucrose (15 g/L equivalent to 0.04 M) for all four tested sugars since the usual concentration of sugars for the cultivation of vascular plants is at full strength 30 g/L. Therefore to equalize molarity and enable comparison, a concentration of 0.04 M was chosen for maltose and sucrose (disaccharides), while 0.08 M was chosen for fructose and glucose (monosaccharides). Thus, both concentrations of each sugar were applied for all types of experiments to be able to compare the results.
In all three types of experiments, after 4 weeks, morphological parameters such as the index of multiplication (IM), which represents newly formed shoots that originated from the newly induced buds on the initial explant [9,15,16], secondary protonemal diameter and percentage of survival were measured. The explants were photographed using a Leica MZ stereomicroscope (Leica MZ 7.5 Bi-Optic Inc. Santa Clara, CA, USA).
The concentrations of plant growth regulators or sugars have been determined on the basis of previous experiments with bryophytes (e.g., [9, 15–17] and the referenes therein), i.e., we have chosen the concentrations that have proven to be effective with other bryophyte species.
2.4 Methodology for the Establishment of Ex Situ Population of Laboratory-Born Drepanocladus lycopodioides
The moss material grown in laboratory-controlled conditions was used for acclimation in two steps. It was grown xenically with an application of rainwater in controlled laboratory conditions (temperature (18 ± 2°C), humidity over 80%, and a long-day light regime (16 h light/8 h dark). The light source was fluorescent tubes (Tesla Pancevo) with a flux density of 50 µmol m−2 s−1. The plantlets were placed on filter paper soaked in rain unsterilized water previously collected in the area of Botanical Garden “Jevremovac”, University of Belgrade. Such plants were adapted to xenic organisms in water and were able to deal with cohabitants from the air and water. The next step was to place them in semi-shade inclined plastic basins (30° inclined) in the fall (02 November 2021). They were positioned at the edge of the water (transition area in the inclined basin) and covered with the inert plastic net to keep them attached to the wet filter paper. The plants were green and controlled moist, and in spring they began to develop rather well. During one year, including the winter and summer periods, the plants were completely covered by water on rainy days, but they were never allowed to dry out completely and in such conditions, they could cover 4 dm2 of basin surface.
Complete statistical analysis was performed using the R programming language (v. 4.3.1) [18]. In all experiments for the index of multiplication (IM) parameter nonparametric, factorial ANOVA was implemented through the Aligned Rank Transform (ART) procedure [19,20]. Utilizing the “ARTool” R package [21] factorial models were built using the “art” function, and the significance of main effects and interactions was evaluated with the “anova” function. Contrast tests were carried out using the “art. con” function from the same R package.
3.1 The Effects of Different Media Types and Explant Orientation on the Morphogenesis of Drepanocladus lycopodioides
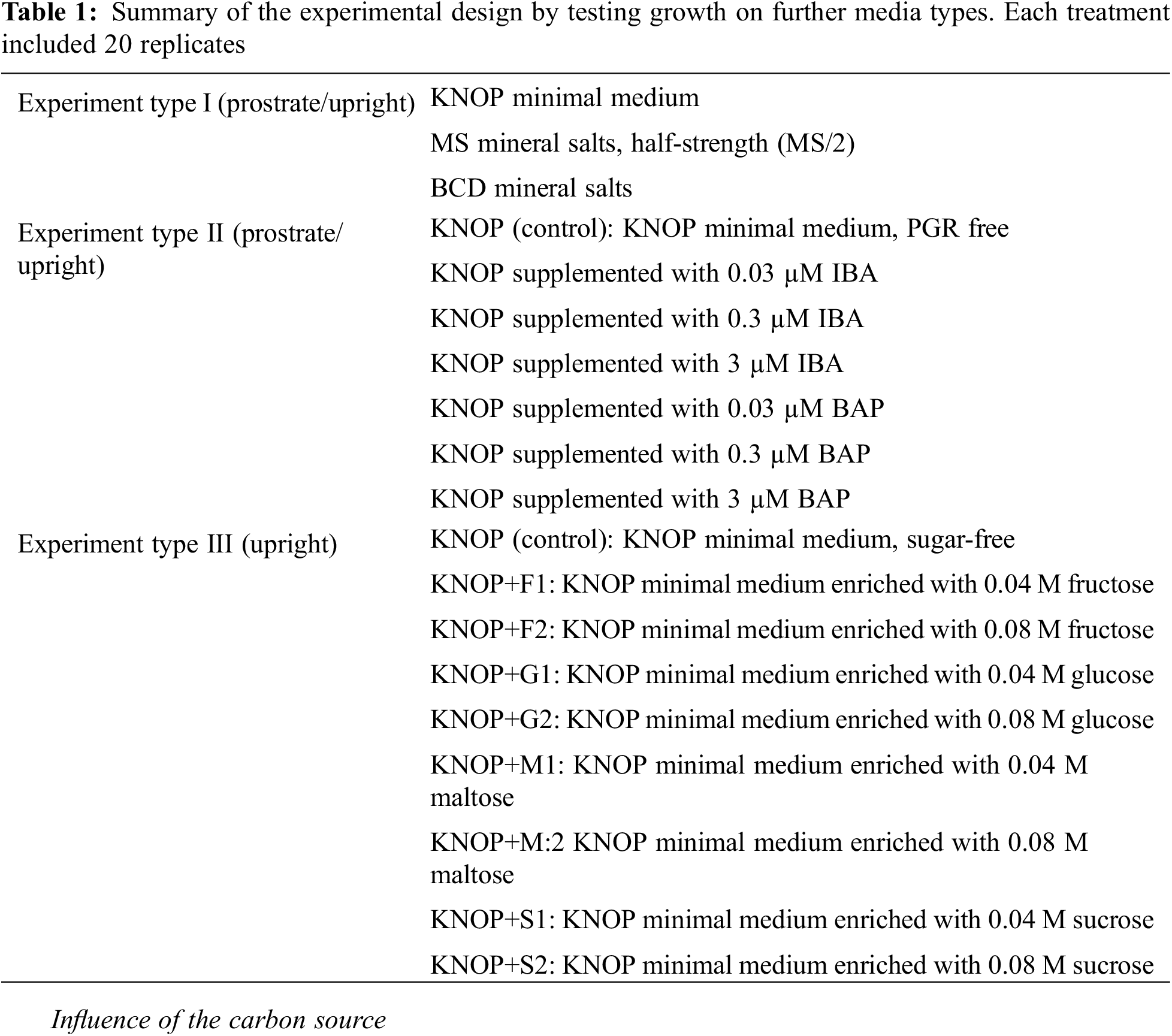
In Experiment type I, the main effects of explant position and medium type significantly affected the index of multiplication (IM) (p < 0.01 and p < 0.001, respectively) (Table 2). Furthermore, a significant interaction was observed between explant position and medium type (p < 0.001), indicating that the explant position alters the way medium type influences the index of multiplication (IM) of D. lycopodioides.
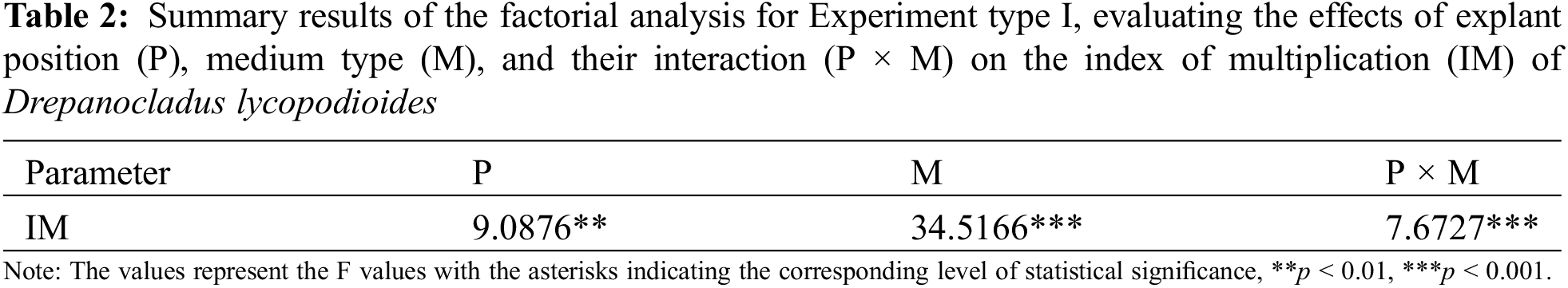
The highest index of multiplication (IM) was observed for plants grown on KNOP minimal medium and upright explant position (Fig. 1), significantly distinguishing this micropropagation strategy from all the others tested in Experiment type I (p < 0.05). Explants inoculated in different positions (prostrate and upright) displayed significantly different indexes of multiplication (IM) when grown on KNOP and BCD media (p < 0.05). However, such observation was not documented for plants grown on the MS/2 medium (Fig. 1). Furthermore, the multiplication index (IM) of the upright position micropropagation strategy was significantly higher compared to the prostrate position in both KNOP and BCD media (p < 0.05), although this effect was not observed when plants were propagated on the MS/2 medium.
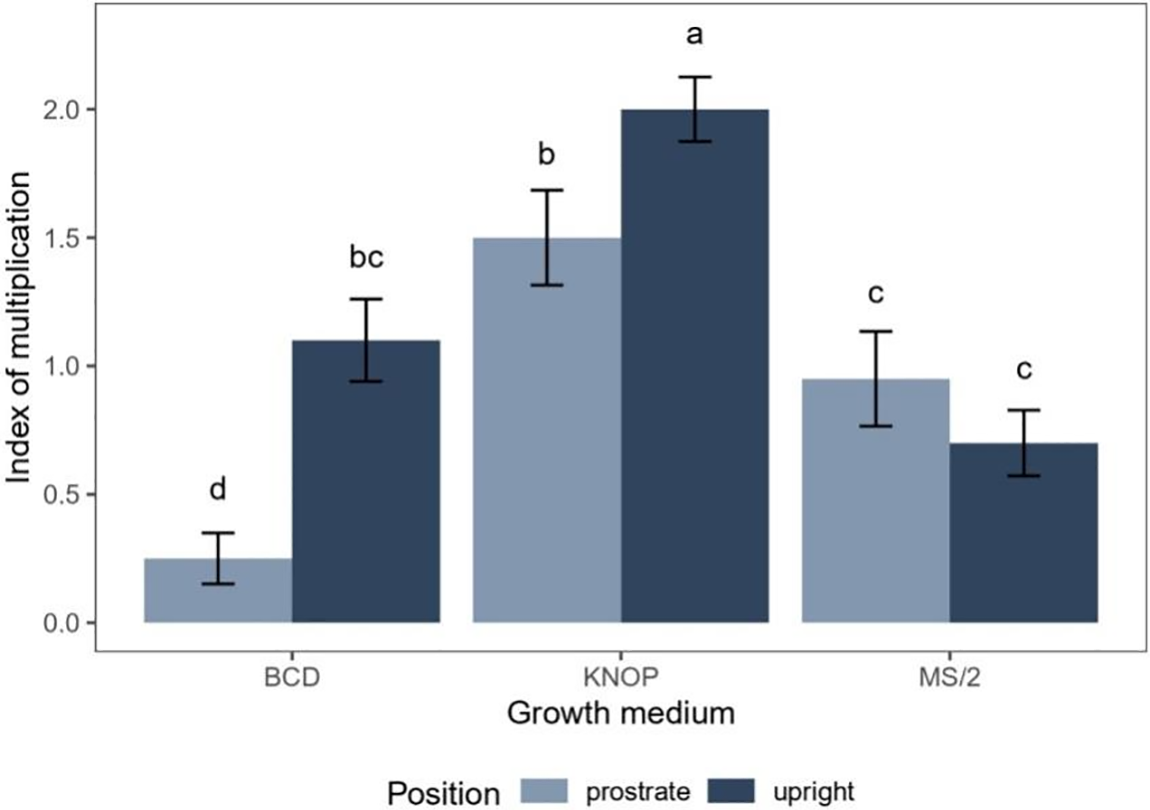
Figure 1: The effects of different growth media types and explant position on the index of multiplication (IM) of Drepanocladus lycopodioides in Experiment type I. Data are presented as the mean ± standard error. Different letters above the bars denote statistically significant differences (p < 0.05) among the experimental groups
Based on the morphological traits represented in Fig. 2, it was evident that plants grown on KNOP and BCD media developed normally both when positioned prostrate (Fig. 2A and B) and upright (Fig. 2D and E), i.e., green gametophores with new branches were documented. On the contrary, plants grown on the MS/2 medium were small, underdeveloped, and with depigmented phylloids independent of the explant position (Fig. 2C and F). Therefore, according to the obtained results in Experiment type I, KNOP appeared to be the most suitable for the micro-propagation of D. lycopodioides when plantlets were positioned upright in the medium.
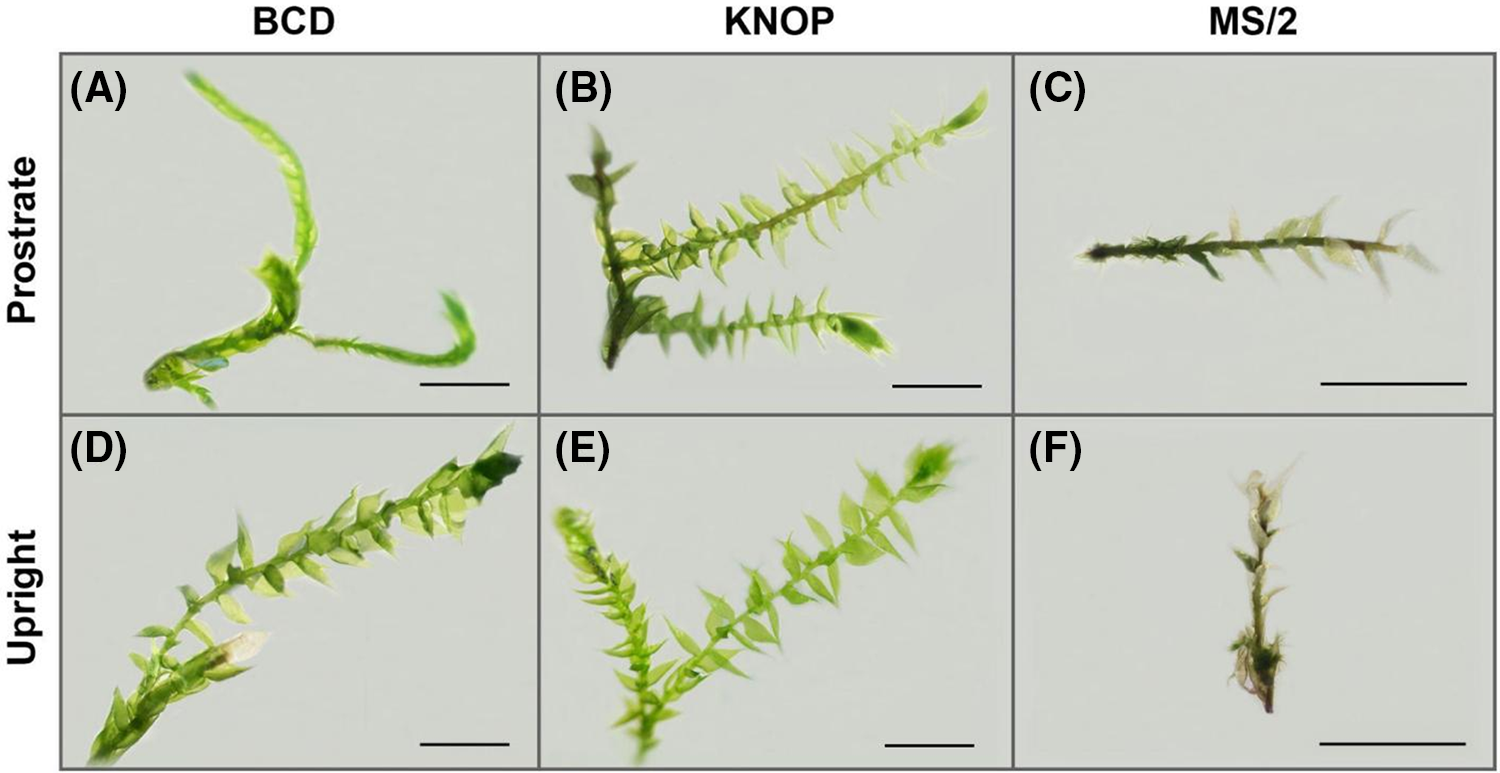
Figure 2: The appearance of Drepanocladus lycopodioides explants grown on different media types in the Experiment type I. The bars represent a size of 2 mm in accordance with magnification (0.63× A, B, D, and E; 1× C and F)
3.2 The Effects of Plant Growth Regulators and Explant Orientation on the Morphogenesis of Drepanocladus lycopodioides
In Experiment type II, the main effects of explant position significantly affected the index of multiplication (IM) of the species for both IBA (p < 0.05) and BAP (p < 0.01) (Table 3; Fig. 3A,B). Similarly, PGR concentration had a significant effect (p < 0.001). Additionally, significant interactions were observed between the explant position and PGR concentration (p < 0.001), indicating that the explant position modifies the effect of PGR concentration on the index of multiplication (IM) of D. lycopodioides.
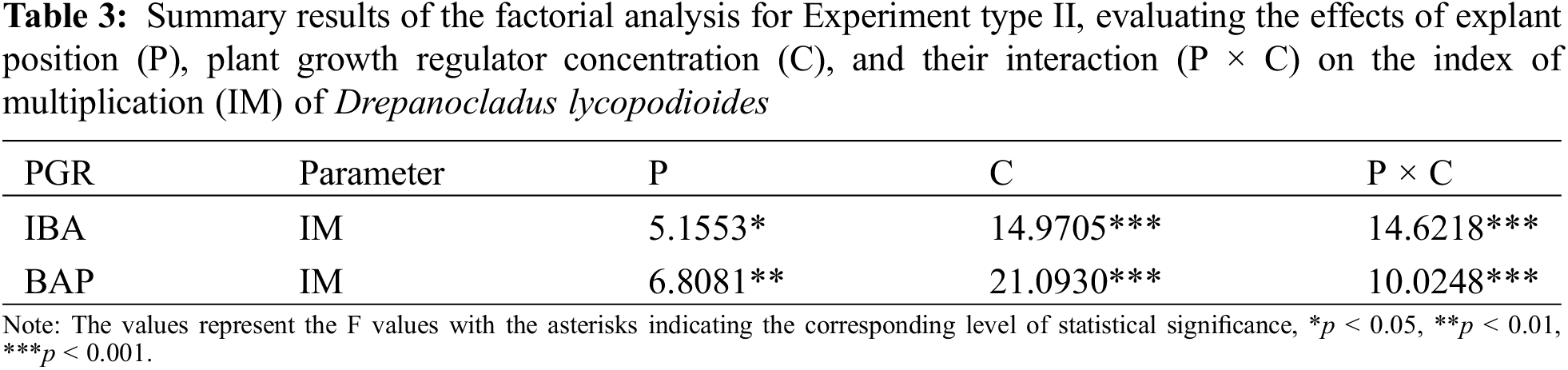
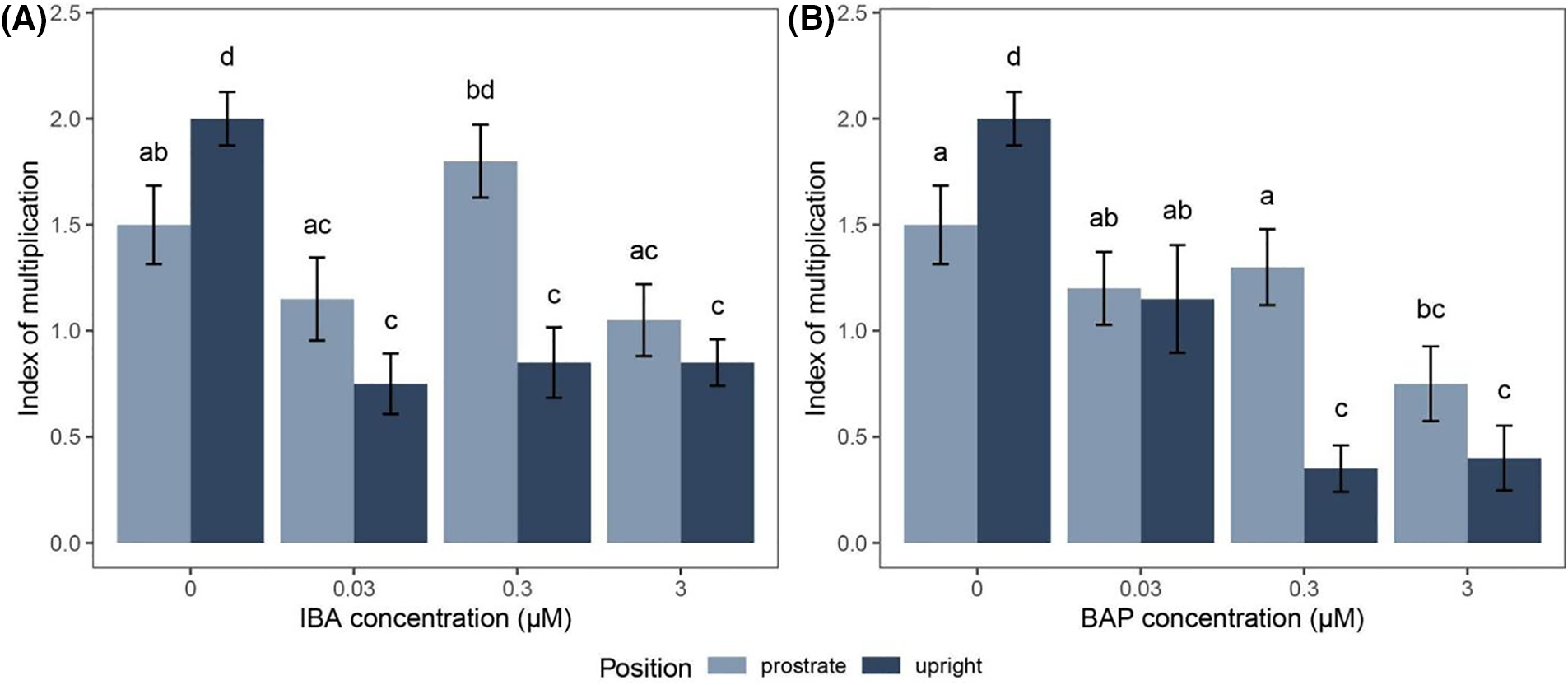
Figure 3: The effects of explant position and KNOP growth media supplemented with different concentrations of IBA (A) and BAP (B) on the index of multiplication (IM) of D. lycopodioides in Experiment II. Data are presented as the mean ± standard error. Different letters above the bars denote statistically significant differences (p < 0.05) among the experimental groups
Different concentrations of IBA significantly reduced the index of multiplication (IM) of D. lycopodioides grown in the upright position compared to the control group (p < 0.05) (Fig. 3A). However, in this case, no significant differences were observed between the individual IBA concentrations (Fig. 3A). In contrast, when grown in the prostrate position, none of the IBA treatments significantly influenced the index of multiplication (IM) compared to the control group (Fig. 3A), suggesting different absorption rates and/or transduction paths of IBA depending on the position in which the plant is grown. However, the auxin biology within the bryophytes remains obscured, and further studies are needed.
Exogenously added IBA led to the decrease of the new lateral shoots in plants grown both in the prostrate and upright position (Fig. 4) as compared to the control group, i.e., plants grown on PGR-free KNOP medium (Fig. 2B). Plants grown on the KNOP medium supplemented with different IBA concentrations appeared smaller, especially when a higher IBA concentration (3 µM) was applied (Fig. 4C and F). However, no great differences were documented between explants grown on the media containing lower IBA concentrations (Fig. 4A,B,D, and E). Nevertheless, IBA led to the decrease of IM, all plants developed normally, although they were smaller in size and slightly less green than the plants in the control group.
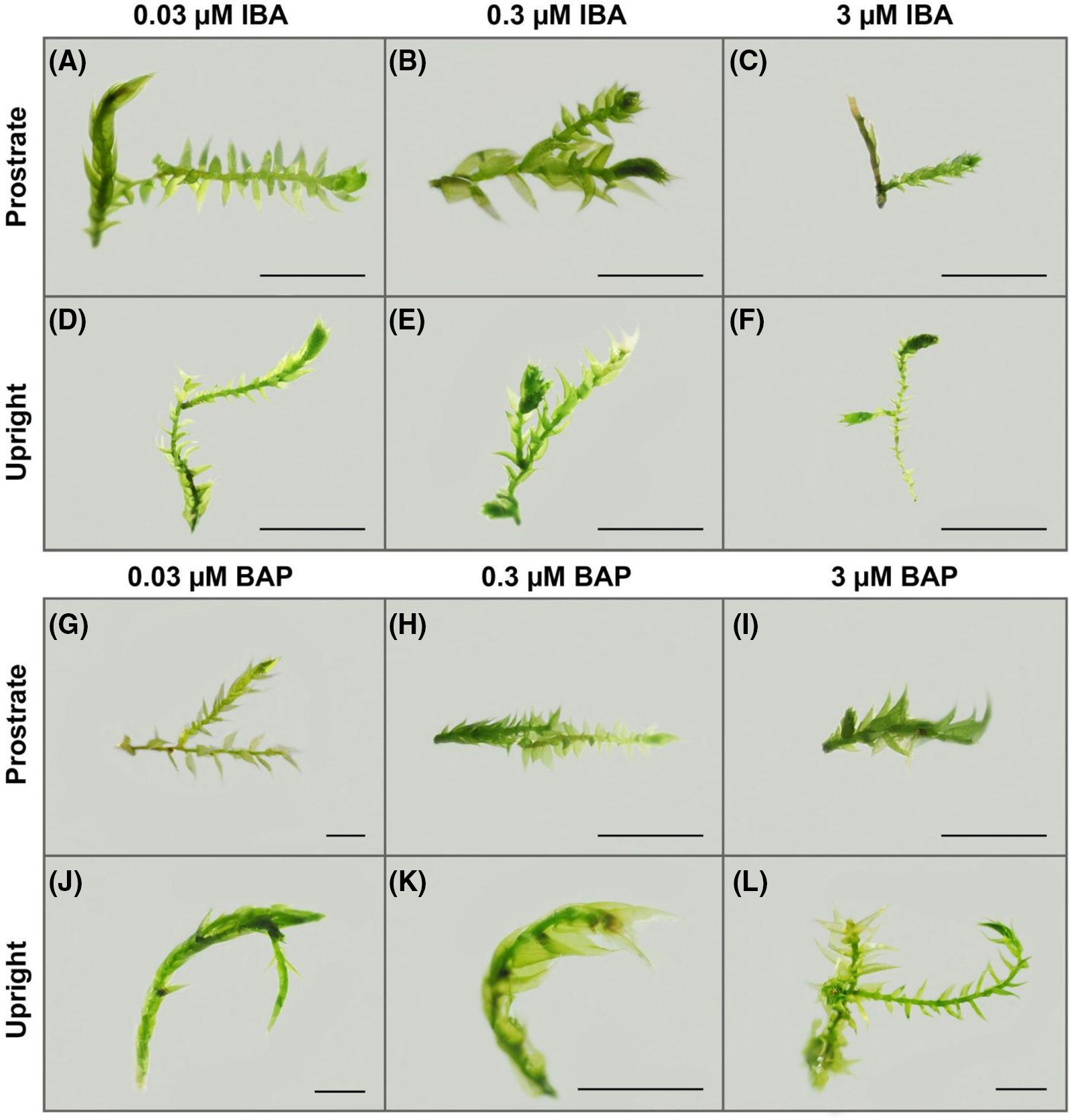
Figure 4: The appearance of Drepanocladus lycopodioides explants grown on KNOP media supplemented with different IBA and BAP concentrations in the Experiment type II. The bars represent a size of 2 mm in accordance with magnification (0.63× G; 0.8× J and L; 1× A–F, H–I; 1.25× K). Control group plants are equal to the plants shown in micrographs in Fig. 2B,E
Regarding BAP treatments, a significant decrease in the index of multiplication (IM) was observed for all BAP concentrations when plants were grown in the upright position (p < 0.05), with the lowest concentration (0.03 µM) exhibiting a smaller decrease compared to the two higher concentrations (0.3 and 3 µM) (p < 0.05) (Fig. 3B). In the prostrate position, this decrease was significant when the highest BAP concentration (3 µM) was applied (p < 0.05) (Fig. 3B), indicating a higher extent of BAP uptake in the upright position, similar to the observed differences in PGR absorption rate between the growth positions in the case of IBA treatments.
Although BAP treatment significantly affected the decrease of IM, plants developed normally in appearance when grown in the upright position (Fig. 4J–L), but slightly less compared to the control group (Fig. 2E). Interestingly, the highest BAP concentration (3 µM) induced the formation of archegonia at the tip of the gametophores grown in the upright position (Figs. 4L and 5) indicating that our material is a female clone which was not possible to diagnose in sterile material from herbarium or collected patch, bearing no any sexual organ nor sporophytes. Therefore, BAP treatment could be used in the case of obtaining the sex organs of D. lycopoidoides (i.e., determination of female plants), although it is not suitable for the gametophore multiplication in vitro (Fig. 5). Such a result was not observable when plants were positioned prostrated (Fig. 4G–I). When BAP (0.3 and 3 µM) was applied in combination with the prostrate explant position, plants were underdeveloped and failed to produce new lateral shoots (Fig. 4H and I) as compared with the control group (Fig. 2B).

Figure 5: The appearance of archegonia in perichaetium at the newly developed branch tip of the D. lycopodioides gametophores when grown in the upright position is induced by the highest applied BAP concentration (3 µM). The arrow points at the single isolated archegonium (magnified 2.5×)
3.3 The Effects of Different Sugar Types on the Morphogenesis of Drepanocladus lycopodioides
In Experiment type III, the main effects of sugar type and sugar concentration significantly influenced the index of multiplication (p < 0.05 and p < 0.001, respectively) (Table 4). Additionally, a significant interaction between these factors was observed (p < 0.001), indicating that the effect of sugar concentration on the index of multiplication of D. lycopodioides varies depending on the type of sugar used.
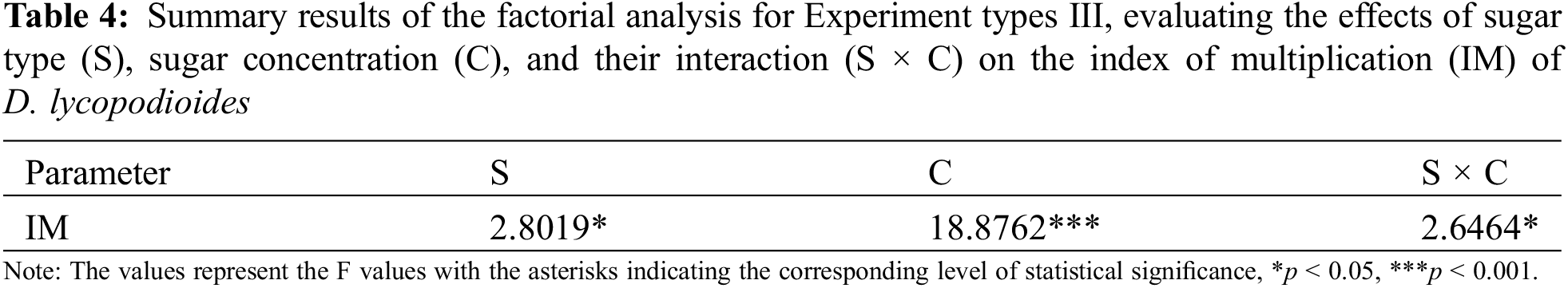
When different sugar types (fructose, glucose, maltose, or sucrose) were exogenously added to KNOP minimal media at concentrations of 0.04 and 0.08 M, no significant differences in the index of multiplication (IM) of upright-grown D. lycopodioides were observed as compared to the control moss group, except in the case of lower glucose concentration (0.04 M), which significantly reduced it (p < 0.05) (Fig. 6). When applied in higher concentration (0.08 M) monosaccharides (fructose and glucose) and disaccharides (maltose and sucrose) treated plants exhibited similar indexes of multiplication (IM) within their respective groups. However, disaccharides-treated plants seem to have a lower index of multiplication (IM) compared to those treated with monosaccharides, although this difference was not statistically significant. Such results could possibly be explained by the different osmotic potential of the two tested sugar groups. No signaling effect was observed.
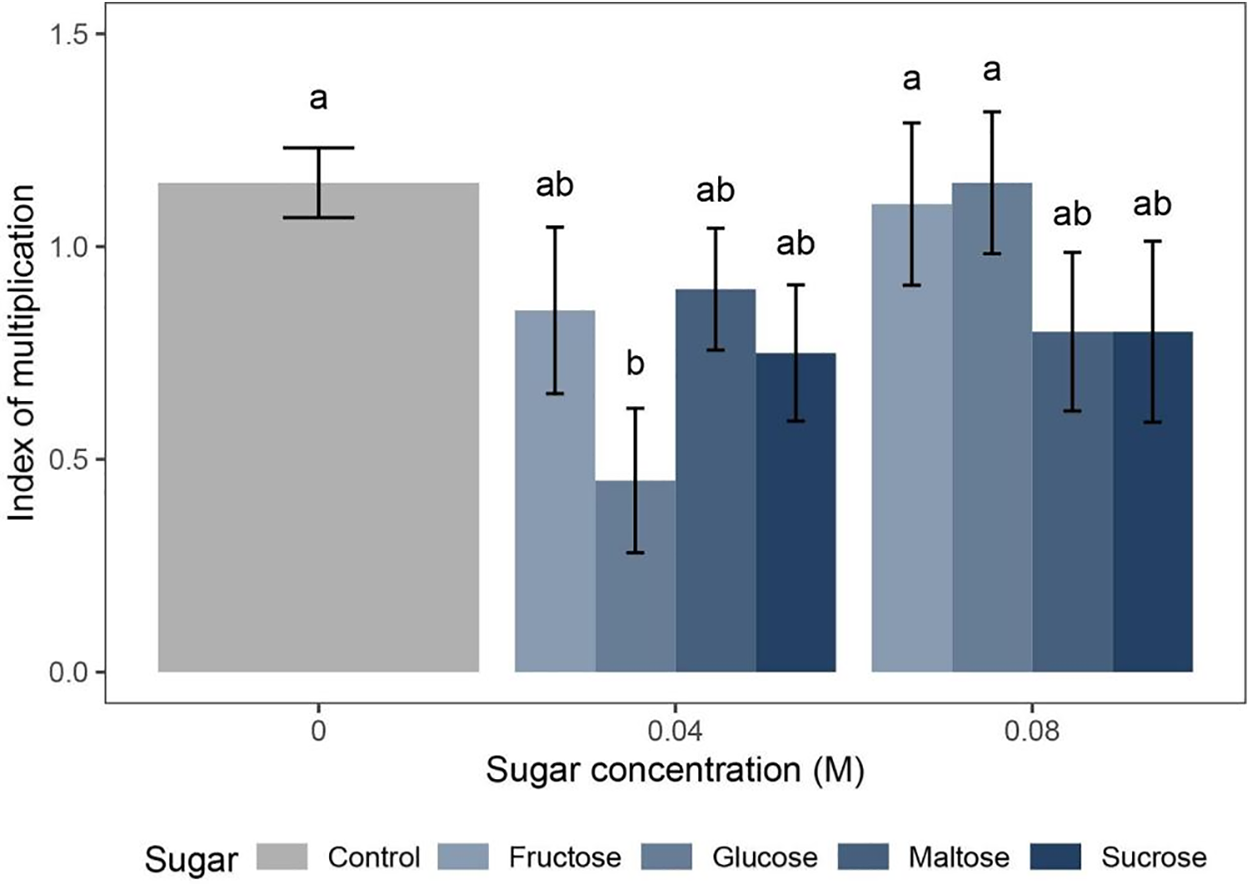
Figure 6: The effect of KNOP growth media supplemented with varying concentrations of different sugar types on the index of multiplication (IM) of D. lycopodioides in Experiment type III. Data are presented as the mean ± standard error. Different letters above the bars denote statistically significant differences (p < 0.05) among the experimental groups
Exogenously added sugars seem not to affect greatly the formation of new shoots in D. lycopoidoides when grown in the upright position (Fig. 7). Plants developed normally as compared to the control group (Fig. 2E), although plants grown on the media enriched with 0.04 M glucose failed to develop new lateral shoots (Fig. 7B). When the higher concentration of sugars was applied (0.08 M), different results were observed, i.e., disaccharides sucrose and maltose decreased the index of multiplication (IM) (Fig. 7G and H), although such a result was not statistically significant, as previously explained (Fig. 6).

Figure 7: The appearance of D. lycopodioides explants grown on KNOP media supplemented with different sugar types and sugar concentrations in the Experiment type III. The bars represent a size of 2 mm in accordance with magnification (1× A–H). Control group plants are equal to the plants shown in micrograph in Fig. 2E
3.4 Establishment of Ex Situ Population of Laboratory-Born Drepanocladus Lycopodioides
In the late autumn period (02 November 2021), the moss plants previously developed in in vitro conditions and then acclimatized were placed outside in the botanical garden condition (Fig. 8A). They started to develop significantly the next spring (Fig. 8B). During the one year including winter and summer periods, the plants were completely covered by the water on rainy days, but they were never left to dry out completely and in such conditions, they could cover 4 dm2 basin surface completely (Fig. 8C).
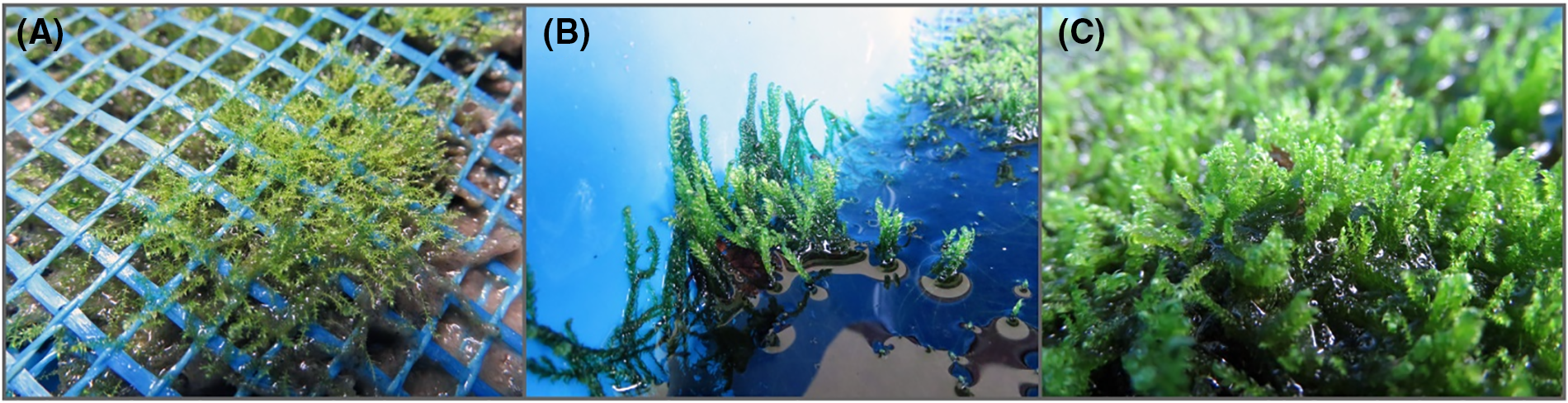
Figure 8: Drepanocladus lycopodioides growing in the plastic pools with rainwater in ex situ condition of outer space of Botanical Garden “Jevremovac” of the Belgrade University, Serbia. (A) placement of in vitro plant with decaying filter paper under the plastic net (November 2021); (B) spring growth (March 2022) and (C) fully covered space of plastic basin (October 2022)
There was no need to remove other spontaneous plants, but the leaf litter releasing phenolic compounds needs to be removed at least twice a year.
4.1 The Effects of Growth Medium Type and Explant Orientation on the Morphogenesis of Drepanocladus Lycopodioides
Even though studied rare and threatened species belong to the pleurocarps and have a prostrate growth form, the highest index of multiplication (IM) was documented both in upright and prostrate positioned test plants on KNOP minimal medium type (Fig. 1).
However, explants grown on a BCD medium showed a significant difference between upright and prostrate orientation with upright plants exhibiting a higher index of multiplication (IM). This indicates the existence of some kind of cell-to-cell transport of minerals, and that the percentage of moss surface attached to media did not significantly increase or speed up the plant structure development. Based on the results presented in Fig. 2, gametophores were properly developed with green phylloids and exhibited new branches on both KNOP and BCD media. On the other hand, the plants grown on MS/2 medium appear underdeveloped and slightly de-pigmented, regardless of their orientation, showing this type of medium was not suitable for the propagation of the selected species.
Given that D. lycopodioides thrives in humid, swampy areas and fens rich in nitrogen and calcium, it was not surprising why KNOP media is the most suitable for growth. The KNOP medium is often referred to as a minimal medium which contains nitrogen and calcium sources in lower concentrations that may be in the conditions of the natural habitat of D. lycopodioides. Moreover, the KNOP medium is one of the most commonly used media for numerous bryophyte species cultivated in vitro [9,12,14–17], especially those with no high nutrient requirements. This one is certainly not the best medium for all the bryophyte species tested up to date, and specific nutritional requirements surely need to be further studied especially for species whose biological features, like rare and threatened ones, are blurred or poorly known. Conversely, while the BCD medium contains nitrogen sources, the higher concentration of potassium nitrate (KNO3) may have rendered this medium less suitable compared to the KNOP medium (Fig. 1). Nevertheless, previous studies have demonstrated that the BCD medium is highly effective for the development of peculiar bryophyte species including Thamnobryum alopecurum (Hedw.) Gangulee, Molendoa hornschuchiana (Hook.) Lindb. ex Limpr., as well as the bryo-halophytes Hennediella heimii (Hedw.) R.H. Zander [15]. Due to its specific composition and high salt content, the MS/2 medium has proven to be unsuitable for the cultivation of here tested species (Figs. 1 and 2C,F). Additionally, the composition of the half-strength MS/2 medium does not provide optimal nutrient concentrations necessary for optimal growth of investigated species. In research done to date, MS/2 was suitable for the cultivation of several other bryophyte species in vitro but rarely better than KNOP medium [9]. There is no universal nutrient formula for bryophytes since these species are very micro-habitat-specific and often very phylogenetically distant. Thus further tests are needed, but a good point is to look into empirical knowledge on its habitat conditions when one wants to establish and cultivate newly studied bryophyte species for any purpose.
Based on the collected and processed data, it was evident that using an upright-oriented explant placed on the KNOP medium was the most effective method for mass multiplication of D. lycopoidoides. One of the possible explanations why explants positioned upright might show better growth is that the upright position facilitates more even airflow around the plant compared to a plant placed prostrate in the medium, and thus respiration and carbon source supply. Additionally, this can probably be explained by better absorption of plant growth regulators and nutrients through the wounded ends of explants and the polarity [22]. Another significant consideration is that the photosynthetic rates of poikilohydric plants may decline if they are carbon dioxide over-saturated [23].
4.2 The Effects of Plant Growth Regulators and Explant Orientation on the Morphogenesis of Drepanocladus lycopodioides
The influence of randomly chosen and exogenously applied growth regulators on the growth and development of bryophytes in vitro has been documented in numerous studies to date. Von Schwartzenberg [24] demonstrated that while some species, including the model organism Physcomitrella patens (Hedw.) Mitt., can spontaneously produce numerous new shoots under axenic conditions, other species require the addition of growth regulators to achieve similar results. Regarding this, it is necessary to examine how growth regulators affect individual species to reveal patterns of their effects, which is crucial for mass micro-propagation and the conservation of rare and endangered bryophytes.
The plants tested developed quite well on the KNOP medium, which was free of plant growth regulators. The addition of IBA had the opposite effect to our expectations and led to a suppression of the formation of new buds (Fig. 3), but did not affect the morphology of our plants, especially not the difference in size (Fig. 4A–F). Similar results were previously documented for moss Entosthodon pulchellus (H. Philib.) Brugues [16] where IBA and BAP individually applied led to a decrease in new shoot formation. Low concentrations of IBA, as observed in moss Pterygoneurum sibiricum Otnyukova [12], and high concentrations, as shown in Physcomitrella patens [25], negatively affected the development of new buds. When it comes to treatments with exogenously added BAP, significant inhibition of new bud development in D. lycopodioides was observed in all upright-oriented explants. In contrast, for prostrate-oriented explants, inhibition of new bud development compared to the control was only evident when the highest BAP concentration was applied (Fig. 3B).
Similar to the IBA treatment, the plants showed normal development when treated with BAP (Fig. 4G–L). Results from research on P. sibiricum and Atrichum undulatum (Hedw.) P. Beauv. indicated that low concentrations of BAP had a stimulating effect on the development of gametophores (e.g., [12] and the references therein). These results are not entirely unexpected, as cytokinins are known to play a significant role in bud formation and are also localized in the caulonema [26]. High concentrations of BAP can influence the reduction in growth and inhibition of bud development which was documented for other moss species such as Hennediella heimii [15], and Bryum argenteum Hedw. [16], and P. sibiricum [12]. Although auxins and cytokinins are necessary for proper plant growth and development, their addition to the media for the mass production of gametophores in D. lycopodioides was mostly inadequate at the concentration tested. However, it was documented that certain concentrations of exogenously added PGR may positively contribute to better development in some mosses [27]. Therefore, it would be useful to conduct more experiments including a wider range of PGR concentrations and more types of PGR individually and/or combined to find the optimal concentrations that can be used for massive propagation of certain species.
Although high BAP concentrations often lead to a decreased index of multiplication (IM), this PGR can induce the formation of certain sex organs in some species, and such was the case in this research. Interestingly, the highest concentration of BAP in upright explants triggered the development of female reproductive organs, with the presence of archegonia (perichaetium) observed at the tips of the gametophores (Fig. 5).
Considering that D. lycopodioides is a dioecious species and the formation of sex organs is extremely rare in nature, this result could potentially facilitate the methodology of sex determination of sterile plants, sexual reproduction for this species under axenic conditions, even though it does not contribute to increased biomass production. This aspect of tests enables us to confirm that our clone is female and this can be used in tests of various clones. The addition to this would be to develop special sex molecular markers [9] and this can be used as a compatible approach in population management both ex situ and in situ.
4.3 The Effects of Different Sugar Types on the Morphogenesis of Drepanocladus lycopodioides
The third type of experiment aimed to examine whether exogenously added sugars can influence the increase in the multiplication index (IM) of D. lycopodioides. For this setup, the KNOP medium was selected based on the results from Experiment type I (Fig. 1), and the explants were placed on the sugar-supplemented media in an upright position according to the results obtained in other experimentations. Sugars can serve as an energy source for plantlet growth but also as signal molecules. Based on the results obtained (Fig. 6), there were no significant differences between the two sugar concentrations and the control group (i.e., explants grown on medium without sugar), except in the case of glucose at 0.04 M, where the index of multiplication (IM) decreased.
When applied at higher concentrations (0.08 M), both monosaccharides (fructose and glucose) and disaccharides (maltose and sucrose) resulted in similar indexes of multiplication (IM) indicating that both groups of tested sugars can be used as supplements with similar effectiveness. This observation could potentially be explained by the differing osmotic potentials of the two sugar groups rather than physiological influence. Morphologically, plants grown with different types and concentrations of sugar do not differ significantly from those grown on medium without added sugar (Fig. 7). Hence, based on the documented data, it was clear that exogenous sugars are not necessary to improve the growth and development of the D. lycopoidoides under in vitro laboratory conditions. Hence, this confirms that most of the mosses in vitro keep photoautotrophy, in comparison to vascular plants that need additional carbon sources and behave as photomixotrophs [28].
On the other hand, sugars (e.g., sucrose) can be important for improving moss growth as was documented for Atrichum undulatum, Dicranum scoparium Hedw., and Pogonatum urnigerum (Hedw.) P. Beauv. (e.g., [9] and the references therein). It is also interesting to note that some species, such as Leptobryum pyriforme (Hedw.) Wilson and Barbula gregaria (Mitt.) A. Jaeger [29] does not develop buds in the absence of sucrose in the medium indicating that for certain species exogenous carbon source is necessary for proper growth and development. Based on previous studies, fructose was also tested in this experiment. Prior results indicate that this sugar stimulates the development of protonemal patches and sex organs in Bryum argenteum [9,17], but this effect was not observed in D. lycopodioides. Such contradictory results in the literature for up-to-date examined bryophyte taxa indicate species-specific responses and different developmental strategies that occur in bryophytes that are often effected by their mineral requirements [12]. It has also been shown that the addition of sucrose and glucose to the nutrient medium promotes the formation of new buds in Pohlia nutans (Hedw.) Lindb. [30] and Funaria hygrometrica Hedw. [31]. Hoffman [31] showed that the addition of maltose to the medium can induce spore germination in Funaria hygrometrica Hedw. Interestingly, experiments with glucose, fructose, maltose, and sucrose have shown that F. hygrometrica and L. pyriforme can grow in the dark when one of these carbon sources is available [32]. Ultimately, it can be concluded from the results available regarding the role of sugars that their effect is species-specific. Therefore, more sugar types and a wider concentration range need to be investigated to find the optimal concentration in D. lycopoidoides development, if any.
KNOP medium and upright explant position was the best considering D. lycopodioides multiplication. Plants grown on KNOP and BCD media were fully developed both when positioned prostrate and upright, while plants grown on MS/2 medium were smaller.
Plant growth regulators did not have a significantly positive effect on the index of multiplication (IM). All tested IBA concentrations induced decreased lateral shoot development, both in the prostrate and upright position. BAP induced a significant decrease in the index of multiplication (IM) when plants were grown both in prostrate and upright positions. The highest applied BAP concentration (3 µM) induced the development of archegonia at the tip of the gametophores grown in the upright position. However, this phenomenon was not observed when plants were positioned prostrated.
Tested sugars, such as fructose, glucose, maltose, and sucrose applied in two concentrations (0.04 and 0.08 M) did not have a positive effect on D. lycopodioides gametophore multiplication. Plants were fully developed when grown on KNOP media enriched with sugars, but the index of multiplication (IM) was the highest when plants were grown on basal KNOP medium, which is the best one when dealing with biomass production for further investigation or conservation purposes.
The material produced in laboratory conditions was suitable for establishing ex situ populations in controlled xenic conditions within the outer space of the Botanical Garden “Jevremovac”, University of Belgrade, Serbia.
Acknowledgement: The authors acknowledge the independent and anonymous reviewers for constructive and useful suggestions for the improvement of the first version of the manuscript.
Funding Statement: The project is supported by the Serbian Ministry of Science, Technological Development and Innovations, contract nos. 451-03-65/2024-03/200178 and 451-03-66/2024-03/200178.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Marko S. Sabovljević, Aneta D. Sabovljević, Beata Papp; data collection: Bojana Z. Jadranin, Djordje P. Božović, Marija V. Ćosić, Milorad M. Vujičić; analysis and interpretation of results: Bojana Z. Jadranin, Djordje P. Božović, Marko S. Sabovljević; draft manuscript preparation: Bojana Z. Jadranin, Djordje P. Božović, Marija V. Ćosić. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Slate ML, Antoninka A, Bailey L, Berdugo MB, Callaghan DA, Cárdenas M, et al. Impact of changing climate on bryophyte contributions to terrestrial water, carbon, and nitrogen cycles. New Phytol. 2024;242(6):2411–29. doi:10.1111/nph.19772. [Google Scholar] [PubMed] [CrossRef]
2. Blockeel TL, Bosanquet SDS, Hill MO, Preston CD. Atlas of British & Irish bryophytes. Newbury: Pisces Publications; 2014. [Google Scholar]
3. Hodgetts N, Blockeel T, Konstantinova N, Lönnell N, Papp B, Schnyder N, et al. Drepanocladus lycopodioides (Europe assessment). The IUCN Red List of Threatened Species; 2019. Available from: https://www.iucnredlist.org/species/88278428/88382536. [Accessed on 15 November 2024]. [Google Scholar]
4. Hodgetts N, Cálix M, Englefield E, Fettes N, García Criado M, Patin L, et al. A miniature world in decline: European red list of mosses, liverworts and hornworts. Brussels, Belgium: IUCN; 2019b. doi:10.2305/IUCN.CH.2019.ERL.2.en. [Google Scholar] [CrossRef]
5. Martinčič A. New checklist and the red list of the mosses (Bryophyta) of Slovenia. Hacquetia. 2024;23(1):69–118. doi:10.2478/hacq-2023-0006. [Google Scholar] [CrossRef]
6. Mišikova K, Godovičova K, Širka P, Šoltes R. Checklist and red list of mosses (Bryophyta) of Slovakia. Biologia. 2020;75(1):21–37. doi:10.2478/s11756-019-00349-1. [Google Scholar] [CrossRef]
7. Puglisi M, Campisi P, Aleffi M, Bacilliere G, Bonioni I, Cogoni A, et al. Red-list of Italian bryophytes. 2. Mosses. Plant Biosyst. 2024;158(5):1031–56. doi:10.1080/11263504.2024.2386330. [Google Scholar] [CrossRef]
8. Sabovljević MS, Pantović JP, Širka P, Vujičić MM, Sabovljević AD, Papp B. Red-list of moss species of Serbia: 2024 assessment. Bot Serb. 2024;48(2):207–22. doi:10.2298/BOTSERB2402207S. [Google Scholar] [CrossRef]
9. Sabovljević MS, Ćosić MV, Jadranin BZ, Pantović JP, Giba ZS, Vujičić MM, et al. The conservation physiology of bryophytes. Plants. 2022;11(10):1282. doi:10.3390/plants11101282. [Google Scholar] [PubMed] [CrossRef]
10. Hedenäs L. The European species of the Calliergon-Scorpidium-Drepanocladus complex, including some related or similar species. Meylania. 2003;28:1–116. [Google Scholar]
11. Reski R, Abel WO. Induction of budding on chloronemata and caulonemata of the moss Physcomitrella patens, using isopentenyladenine. Planta. 1985;165(3):354–8. doi:10.1007/BF00392232. [Google Scholar] [PubMed] [CrossRef]
12. Jadranin BZ, Ćosić MV, Božović DP, Vujičić MM, Ignatov MS, Ignatova EA, et al. An insight into the biology of the rare and peculiar moss Pterygoneurum sibiricum (Pottiaceaea conservation physiology approach. Plants. 2023;12(6):1359. doi:10.3390/plants12061359. [Google Scholar] [PubMed] [CrossRef]
13. Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–97. doi:10.1111/j.1399-3054.162.tb08052.x. [Google Scholar] [CrossRef]
14. Sabovljević A, Sabovljević M, Jocković N. In vitro culture and secondary metabolite isolation in bryophytes. Methods Mol Biol. 2009;547:117–28. doi:10.1007/978-1-60327-287-2_10. [Google Scholar] [PubMed] [CrossRef]
15. Ćosić MV, Sabovljević MS, Papp B, Giba ZS, Šinžar-Sekulić JB, Sabovljević AD, et al. Micropropagation of rare bryo-halophyte Hennediella heimii. Bot Serb. 2022;46(2):187–95. doi:10.2298/BOTSERB2202187C. [Google Scholar] [CrossRef]
16. Ćosić MV, Božović DP, Ignatov MS, Ignatova EA, Vujičić MM, Sabovljević AD, et al. Micropropagation and optimisation of in vitro production of the rare and threatened moss Entosthodon pulchellus (Funariaceae). Acta Bot Croat. 2025;84(2):1–16. doi:10.37427/botcro-2025-012. [Google Scholar] [CrossRef]
17. Liang SF, Sun Y, Zhu RL. In vitro micropropagation of Bryum argenteum Hedw. Cryptogam Bryol. 2010;31(3):233–9. [Google Scholar]
18. R Core Team. R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. Available from: https://www.R-project.org/. [Accessed 2022]. [Google Scholar]
19. Elkin L, Kay M, Higgins J, Wobbrock J. An aligned rank transform procedure for multifactor contrast tests. In: Proceedings of the 34th Annual ACM Symposium on User Interface Software and Technology, 2021; New York, NY, USA: Association for Computing Machinery; p. 10–4. [Google Scholar]
20. Wobbrock J, Findlater L, Gergle D, Higgins J. The aligned rank transform for nonparametric factorial analyses using only ANOVA procedures. In: Proceedings of the ACM Conference on Human Factors in Computing Systems (CHI 2011), 2011 May 7–12; Vancouver, BC, Canada: ACM Press; p. 143–6. [Google Scholar]
21. Kay M, Elkin L, Higgins J, Wobbrock J. mjskay/ARTool: ARTool 0.11.0 (v0.11.0). Zenodo; 2021. Available from: https://zenodo.org/records/4721941. [Accessed 2022 Dec 20]. [Google Scholar]
22. Papafotiou M, Martini AN. Effect of position and orientation of leaflet explants with respect to plant growth regulators on micropropagation of Zamioculcas zamiifolia Engl. (ZZ). Scientia Horticulturae. 2009;120(1):115–20. doi:10.1016/j.scienta.2008.09.023. [Google Scholar] [CrossRef]
23. Wang Z, Bader MY. Associations between shoot-level water relations and photosynthetic responses to water and light in 12 moss species. AoB Plants. 2018;10(3):ply034. doi:10.1093/aobpla/ply034. [Google Scholar] [PubMed] [CrossRef]
24. von Schwartzenberg K, Knight C, Perroud P-F, Cove D. Hormonal regulation of development by auxin and cytokinin in moss. In: The moss Physcomitrella patens. Hoboken, NJ, USA: Wiley Online Library; 2009. vol. 36, p. 246–81. doi:10.1002/9781119312994.apr0393. [Google Scholar] [CrossRef]
25. Ashton NW, Cove DJ, Chopra RN, Bahtla SC. Mutants as tools for the analytical dissection of cell differentiation in Physcomitrella patens gametophytes. In: Bryophyte development: physiology and biochemistry. Boca Raton: CRC Press; 1990. p. 17–31. [Google Scholar]
26. Szweykowska A, Dornowska E, Cybulska A, Wasiek G. The cell division response to cytokinins in isolated cell cultures of the protonema of Funaria hygrometrica and its comparison with the bud induction response. Biochem Physiol Pflanz. 1971;162(6):514. doi:10.1016/S0015-3796(17)31185-X. [Google Scholar] [CrossRef]
27. Sarla, Chopra RN. Effect of some auxins and antiauxins on protonemal growth and bud formation in Bryum pallescens Schleich. ex Schwaegr. grown in vitro. Plant Sci. 1987;51:251–6. doi:10.1016/0168-9452(87)90200-7. [Google Scholar] [CrossRef]
28. Švečikova H, Lhotakova Z, Hamet J, Lipavska H. Mixotrophic in vitro cultivations: the way to go astray in plant physiology. Physiol Plant. 2018;16(3):365–77. doi:10.1111/ppl.12893. [Google Scholar] [PubMed] [CrossRef]
29. Chopra RN, Kumra PK. Protonemal differentiation and bud formation in mosses. In: Biology of bryophytes. New Delhi, India: Wiley Eastern Limited; 1988. [Google Scholar]
30. Mitra G, Allsopp A. Effects of kinetin, gibberellic acid and certain auxins on the development of shoot buds on the protonema of Pohlia nutans. Nature. 1959;183:974–5. [Google Scholar]
31. Hoffman GR. The effects of certain sugars on spore germination in Funaria hygrometrica Hedw. Bryologist. 1964;67(3):321. doi:10.1639/0007-2745(1964)67[321:TEOCSO]2.0.CO;2. [Google Scholar] [CrossRef]
32. Simola LK. The effect of various mono-and disaccharides on the growth of Sphagnum nemoreum thalli in sterile cultures. Physiol Plant. 1969;22(5):1079–84. doi:10.1111/j.1399-3054.1969.tb07469.x. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools