 Open Access
Open Access
ARTICLE
Uniting the Role of Entomopathogenic Fungi against Rhizoctonia solani JG Kühn, the Causal Agent of Cucumber Damping-Off and Root Rot Diseases
1 Research Laboratory of Agricultural Production Systems and Sustainable Development (LR03AGR02), Carthage University, Regional Centre of Agricultural Research of Sidi Bouzid, CRRA, Sidi Bouzid, 9100, Tunisia
2 Science College, Biology Department, Taibah University, Almadina, 42317, Saudi Arabia
3 Department of Plant Pathology and Microbiology, Mahatma Phule Krishi Vidyapeeth, Rahuri, 413722, Maharashtra, India
4 School of Biology Science, Universiti Sains Malaysia, Minden, 11800, Malaysia
* Corresponding Authors: Lobna Hajji-Hedfi. Email: ,
; Nahla Alsayd Bouqellah. Email:
Phyton-International Journal of Experimental Botany 2024, 93(11), 2857-2881. https://doi.org/10.32604/phyton.2024.057591
Received 21 August 2024; Accepted 16 October 2024; Issue published 30 November 2024
Abstract
Beauveria bassiana and Metarhizium spp. are entomopathogenic fungi with potential applications beyond insect pest control, including plant disease suppression, plant growth promotion, and rhizosphere colonization. This study investigated the plant growth-promoting characteristics and extracellular enzyme activities of Metarhizium spp. and B. bassiana in relation to phytopathogen interactions and plant growth. Additionally, the efficacy of these fungi in mitigating damping-off and root rot caused by Rhizoctonia solani on cucumber plants was evaluated in vitro and in vivo. Results indicate that B. bassiana and M. anisopliae produce indole-3-acetic acid, hydrocyanic acid, and hydrolytic enzymes. Seed treatment with these fungi significantly reduced disease severity (3.85%–1.86%, respectively) and enhanced germination parameters [germination percentage (85.33%–86%, respectively), germination index (10.67–12.29, respectively), seedling length vigor index (86.41–109.44, respectively), and seedling weight vigor index (30.24–37.57, respectively)] compared to the control positive. Both fungi demonstrated high inhibition rates of R. solani mycelial growth (93.90%–90.46%, respectively). Greenhouse trials revealed that preventive treatments using B. bassiana and M. anisopliae increased catalase (104.40–105.52 units/mg protein/min, respectively), (4.58–5.77 units/mg protein/min, respectively), superoxide dismutase (40.65–41.74 units/mg protein/min, respectively), and polyphenol oxidase (0.539–0.559 units/mg protein/min, respectively) activities, as well as total phenolic (2.60–2.65 mg/g, respectively) and total sugar content (2.23–2.16 mg/g, respectively) in cucumber plants. Consequently, disease severity (9.51–6.99%, respectively) was reduced, and plant height (93.76–98.76 cm, respectively) increased compared to the positive control. These findings suggest that B. bassiana and M. anisopliae can enhance plant growth, stimulate plant defense mechanisms, and effectively control damping-off and root rot diseases, making them promising candidates for biological control strategies.Keywords
Based on scientific projections, the global population is expected to reach between 9.4 and 10.1 billion by 2050 [1]. This significant human population growth, coupled with ongoing climate change and dwindling natural resources, presents a substantial global challenge: ensuring sufficient food supplies for the future [2]. To meet the nutritional demands of this expanding population, total food production will need to increase by an estimated 70% [3]. However, a critical constraint on achieving this goal is the documented scarcity of essential agricultural resources. Multiple scientific publications highlight the diminishing availability of freshwater in many regions [4]. Similarly, agricultural land is becoming increasingly scarce, as reported by various studies [5,6]. Furthermore, supplies of crucial mineral resources for fertilizer production, such as rock phosphate [7] and potassium [8], are also declining. Adding to these challenges is the concern about the rise in the spread of pests and diseases that threaten crop yields [2]. In response to these pressing issues, researchers emphasize the urgent need to adopt sustainable agricultural practices that can minimize plant disease severity [9]. Additionally, experts propose strategies to improve food production through enhanced efficiency in natural resource utilization and a reduction in the environmental impact of fungicides currently used in agriculture [10–12]. Cucumber (Cucumis sativus L.) is one of the most widely cultivated and consumed vegetable crops globally. Cucumbers rank among the top four most cultivated vegetables in the world, following only tomatoes, brassicas, and onions [13].
Cucumbers (Cucumis sativus L.) are susceptible to a range of fungal diseases, with root rot and damping-off being particularly destructive on a global scale [14]. These diseases are caused by numerous fungal genera, including Macrophomina phaseolina (Maublanc) S. Ashby, Fusarium oxysporum Schlechtendal, F. solani (von Martius) Saccardo, Phytophthora spp. De Bary, Rhizoctonia solani JG Kühn, and Sclerotinia rolfsii Saccardo, as documented in various studies [15–21]. Among these, R. solani (teleomorph: Thanatephorus cucumeris (AB Frank) Donk) stands out as a major root rot pathogen affecting numerous plant species worldwide [17,19]. While the specific symptoms caused by R. solani can vary depending on the host plant, it primarily targets subterranean tissues [22]. Damping-off is the most common symptom associated with Rhizoctonia infection, characterized by the inhibition of seed germination and the destruction of seedlings either before or after emergence from the soil [22]. This destructive pathogen is responsible for significant yield losses in both horticultural and crops [17,19,21]. R. solani is a destructive soil-borne fungal pathogen responsible for significant crop yield losses globally. Unlike many fungi, R. solani lacks asexual spores (conidia) for reproduction. Instead, it relies on sclerotia, hardened survival structures, for propagation and persistence in the soil [19,23]. These sclerotia are considered a primary source of R. solani inoculum, facilitating disease spread and infection in susceptible crops [19,23].
The persistent challenge of R. solani management stems from its long-lived sclerotia that remain viable in the soil for extended periods, hindering effective control efforts [24]. Despite the implementation of various management strategies, including cultural practices, chemical application, and physical controls, for the management of R. solani-induced root rot diseases, overall success has been limited [21]. While crop rotation is a common strategy for managing some plant diseases, the non-specific nature of some R. solani strains renders this approach only partially effective [22]. Fungicide use, another common control method, raises concerns about human health risks, environmental pollution, and the potential for pathogen resistance development [9,25]. In recent decades, biological control using various microbes, particularly fungal and bacterial strains, has emerged as a promising alternative for controlling both airborne and soil-borne plant diseases, offering a potential solution for R. solani management [26,27].
Beauveria spp. Vuillemin and Metarhizium spp. Sorokin, entomopathogenic fungi, have gained significant recognition for their potential in managing various plant pathogens, including R. solani [26,28–32]. Beyond their direct antagonistic effect on pathogens, research suggests that incorporating these fungi into crop production systems can promote overall plant health and growth. Studies by [30] and [33] have shown that Beauveria spp. and Metarhizium spp. can enhance plant yield and disease resistance. Notably, these fungi have been successfully employed against R. solani in a wide range of host plants [27,34]. The mechanisms underlying their antagonistic activity involve both mycoparasitism, where they directly parasitize the pathogen, and the induction of plant resistance [33–36]. Besides, Beauveria spp. and Metarhizium spp. can colonize the root system without causing harm, while simultaneously stimulating the plant’s defense system by increasing the activity of peroxidase and chitinase enzymes, thereby enhancing plant resistance to R. solani [27,34]. Additionally, these fungi exhibit faster growth rates compared to fungal plant pathogens. They can further suppress various soil-borne pathogens through the secretion of antifungal compounds. Their application in biological control offers a promising approach to activate plant defense systems and promote growth [37,38]. The present study aimed to investigate the efficacy of Beauveria bassiana, Metarhizium anisopliae, and Metarhizium sp. Me351, in controlling damping-off and root rot diseases in cucumber plants. Additionally, the study sought to evaluate the impact of these entomopathogenic fungi on cucumber seedlings and plant growth, as well as their ability to enhance the plant’s defense mechanisms. To achieve these objectives, both in vitro and in vivo experiments were conducted.
2.1 Fungal Inoculums Preparation
R. solani was chosen as the target pathogen for both in vitro and in vivo studies. Three entomopathogenic fungi (B. bassiana, M. anisopliae, and Metarhizium sp. Me351) were evaluated as potential biocontrol agents. All cultures originated from the Culture Collection of the Laboratory of Plant Protection (CRRA, Sidi Bouzid, Tunisia). The R. solani isolate was obtained from symptomatic cucumber plants, while the entomopathogenic fungi were isolated from cucumber rhizosphere soil collected in agricultural fields of Regueb, Sidi Bouzid. Fungal inocula were prepared from seven-day-old cultures. Briefly, each culture plate was flooded with sterile distilled water. Spores were carefully dislodged using a glass spreader, and the resulting suspension was then filtered with a muslin cloth to eliminate mycelial fragments. The filtered spore suspension was subsequently diluted with sterile distilled water and adjusted to a concentration of 107 spores mL−1 using a hemocytometer.
2.2 In Vitro Plant-Growth-Promoting and Extracellular Enzymes
This study was conducted under laboratory conditions to assess the potential of three entomopathogenic fungi to promote plant growth. The evaluation focused on essential properties known to benefit plants, including atmospheric nitrogen fixation (N), hydrocyanic acid production (HCN), and indole-3-acetic acid production (IAA). Additionally, the capacity of these fungi to produce extracellular enzymes, namely β-1,3-glucanase (Glu), pectinase (Pec), catalase (Cat), protease (Pro), cellulase (Cell), chitinase (Chi), amylase (Amy), and lipase (Lip) was investigated.
Nitrogen fixation: The atmospheric nitrogen fixation assay employed a Norris Glucose Nitrogen Free (N-free) medium to assess the ability of fungal isolates to utilize atmospheric nitrogen as a nitrogen source. Isolates capable of N fixation were expected to grow and use atmospheric nitrogen for their metabolic needs. The entomopathogenic fungi were incubated at 30°C for 5 days. This growth manifested as a visible film on the surface of the N-free medium [39].
Hydrocyanic acid production: HCN production by the entomopathogenic fungi was evaluated using a qualitative assay adapted from [9]. This method assesses the cyanogenic potential of the fungi, which refers to their ability to produce HCN. Agar plates containing 15 mL of PDA supplemented with 4.4 g/L glycine were inoculated with a one-disc plug. The lids of these dishes are modified to include a filter paper (diameter 9 cm) impregnated with an alkaline picrate solution placed underneath. The plates were incubated at 28°C ± 2°C for 4 days. HCN production by the fungi, if present, would cause a color change in the filter paper from its original yellow to a reddish-brown hue. This color change is a positive indicator of HCN production by the fungi [9].
Indole-3-acetic acid production: IAA production by the entomopathogenic fungi was evaluated using a qualitative colorimetric assay adapted from [9]. This assay assesses the ability of the fungi to synthesize IAA, a plant growth hormone. The method involves placing a one-disc plug of fungi on the Luria-Bertani medium. Following incubation (28°C for 48 h), a Whatman paper disk (diameter 5 cm) pre-treated with Salkowski’s reagent is placed on the culture surface. Salkowski’s reagent reacts with IAA to produce a color change. If the fungi produce IAA, the filter paper will change from yellow to a pinkish-brown hue. This color shift is a positive indicator of IAA production by the fungi [9].
β-1,3-glucanase activity: The ability of the entomopathogenic fungi to produce Glu was evaluated using a clear zone formation assay based on the method described by [40]. This assay assesses the presence and activity of Glu enzymes, which can degrade β-1,3-glucan polysaccharides. The assay utilizes solidified agar plates containing laminarin (1 g/L), a β-1,3-glucan substrate, along with peptone (0.5 g/L) and yeast extract (0.1 g/L) for fungal growth. A fungal plug was inoculated onto the agar surface and incubated at 28°C. If the fungi produce Glu, the enzyme will diffuse from the colony and hydrolyze the surrounding laminarin. This degradation creates a clear halo zone around the fungal colony. The absence of a stainable substrate in this zone allows for easy visualization upon observation. The size of the clear zone can be correlated with the level of Glu activity produced by the fungi [40].
Pectinase production: The pectinase activity of the entomopathogenic fungi was assessed using a zone-formation assay based on the method described by [41]. Pectinase enzymes degrade pectin, a major component of plant cell walls. The assay employs solidified agar plates containing pectin as the substrate for potential pectinase activity. A fungal plug was inoculated onto these plates and incubated at 28°C for five days. Following incubation, the plates are flooded with a 0.05% ruthenium red solution. Ruthenium red acts as a specific stain for pectin, binding to it and causing the medium to turn red. After staining, the plates are thoroughly rinsed with distilled water to remove unbound ruthenium red. A clear halo zone surrounding the fungal colonies after washing signifies pectinase production by the isolates. This clear zone represents the area where pectin has been degraded by the secreted pectinases. The formation of this halo zone demonstrates the enzymatic activity of the fungi and their potential role in plant tissue maceration, facilitating nutrient acquisition from the degraded plant material [41].
Catalase production: Catalase activity of the entomopathogenic fungi was assessed using a qualitative slide test adapted from [39]. Catalase is an enzyme that decomposes hydrogen peroxide (H2O2) into water and oxygen. This assay employs a simple and rapid method to determine the presence of catalase activity in the fungal isolates. A small drop of H2O2 solution is mixed with a sample of the fungal colony and placed on a microscope slide. If the fungi possess catalase activity, the H2O2 will be rapidly broken down, resulting in the production of visible gas bubbles. The formation of these air bubbles serves as a positive indicator of catalase activity. This assay provides a quick and easy method for preliminary screening of catalase activity in fungal isolates [39].
Protease production: The protease activity of the entomopathogenic fungi was assessed using a zone-formation assay on skim milk agar medium. Skim milk agar is a commonly employed medium for detecting protease activity as it contains casein, a milk protein that serves as a substrate for proteases. A fungal plug was inoculated onto the solidified plates and incubated at 28°C for five days. Following incubation, the plates were observed for a clear halo zone surrounding the fungal colonies. The formation of this clear zone is indicative of protease production by the entomopathogenic fungi. This zone represents the area where casein has been hydrolyzed (broken down) by the secreted proteases. The observed enzymatic activity demonstrates the ability of the fungi to degrade proteins, which can be beneficial in various applications [42].
Cellulase production: The cellulase activity of the entomopathogenic fungi was evaluated using a zone-formation assay on carboxymethylcellulose (CMC) agar medium. CMC serves as a surrogate substrate for cellulose, a major component of plant cell walls that can be degraded by cellulase enzymes. The assay employs solidified CMC agar plates inoculated with one-disc plug of fungi and incubated at 28°C for five days. Following incubation, the plates are flooded with a 1% Congo red solution. Congo red is a dye with a high affinity for cellulose, staining it red. However, cellulase activity disrupts this binding. After staining, the plates are thoroughly washed with distilled water to remove unbound Congo red. The formation of a clear halo zone surrounding the fungal colonies after washing signifies cellulase production by the isolates. This clear zone represents the area where cellulose has been degraded by the secreted cellulases, preventing Congo red from binding. This reduction in stain binding demonstrates the enzymatic activity of the fungi and their potential role in cellulose breakdown [43].
Chitinase production: The chitinase activity of the entomopathogenic fungi was assessed using a zone-formation assay based on the method described by [44]. Chitinase enzymes break down chitin, a polysaccharide that is a major structural component of phytopathogenic fungi and bacteria cell walls and the exoskeletons of insects. The assay utilizes a solidified agar medium containing colloidal chitin as the substrate for potential chitinase activity. A fungal plug was inoculated onto these plates and incubated at 28°C for 5 days. Following incubation, the plates are observed for a clear halo zone surrounding the fungal colonies. The formation of this clear zone signifies chitinase production by the fungal isolates. This zone represents the area where chitin has been degraded by the secreted chitinases. The observed enzymatic activity demonstrates the potential of the fungi to weaken the cell walls of phytopathogenic fungi and bacteria or decompose insect remains through chitin breakdown [44].
Amylase production: The amylase activity of the entomopathogenic fungi was evaluated using a zone-formation assay on a soluble starch-containing agar medium. Starch is a complex carbohydrate found in many organisms and can be broken down into simpler sugars by amylase enzymes. The assay employs solidified agar plates containing soluble starch as the substrate for potential amylase activity. A fungal plug was inoculated onto these plates and incubated at 28°C for 5 days. Following incubation, the plates are flooded with an iodine solution. Iodine has a high affinity for starch, readily binding and forming a characteristic blue-black complex. However, amylase activity disrupts this interaction. If the fungi produce amylase, the enzyme will hydrolyze the surrounding starch into simpler sugars that cannot bind iodine. After staining with iodine, the plates are observed for a clear halo zone surrounding the fungal colonies. The formation of this clear zone signifies positive amylase production by the entomopathogenic fungi. This zone represents the area where starch has been degraded by the secreted amylases, preventing iodine binding. The observed enzymatic activity demonstrates the ability of the fungi to break down starch, which can play a role in their carbohydrate metabolism and nutrient acquisition [45].
Lipase production: The lipase activity of the entomopathogenic fungi was evaluated using a zone-formation assay on a solid medium containing Tween 80. Tween 80 is a synthetic substrate commonly used in lipase assays as it mimics the structure of natural fats and oils. A fungal plug was inoculated onto the solidified plates and incubated at 28°C. Lipase enzymes, if produced by the fungi, can hydrolyze (break down) Tween 80 into simpler components. Following incubation, the plates were observed for the formation of an opaque halo zone surrounding the fungal colonies. The presence of this opaque zone signifies positive lipase production by the isolates. This zone represents the area where Tween 80 has been hydrolyzed by the secreted lipases. The hydrolysis process produces fatty acids, which are insoluble in the agar medium. These insoluble fatty acids precipitate and scatter light, resulting in the opaque appearance of the halo zone [9].
2.3 Cucumber Seed Germination Assay
To assess the effects of three entomopathogenic fungi on cucumber seed germination and their potential interaction with the fungal pathogen R. solani, a germination assay was conducted. Healthy cucumber seeds were surface-sterilized using a 2% sodium hypochlorite solution for 3 min, rinsed thoroughly with distilled water, and dried under laminar airflow on sterilized blotting paper. Subsequently, the sterilized seeds were treated by submersion in flasks containing conidial suspensions of each entomopathogenic fungus for 30 min. After treatment, all seeds were placed in a moist chamber at 98% relative humidity and 25°C–28°C for 24 h, followed by air-drying. After 24 h incubation, seeds were individually inoculated with R. solani. Each seed received a single 10 μL droplet of the R. solani inoculum. Two positive controls were established: one inoculated with only the pathogen and another treated solely with the entomopathogenic fungi suspension (10 mL). Distilled water served as the negative control. The experiment employed eight treatments: T1—untreated seeds (negative control), T2—seeds treated only with R. solani (positive control), T3—seeds treated only with B. bassiana, T4—seeds treated only with M. anisopliae, T5—seeds treated with Metarhizium sp. Me351, T6—seeds treated with B. bassiana and inoculated with R. solani, T7—seeds treated with M. anisopliae and inoculated with R. solani, and T8—seeds treated with Metarhizium sp. Me351 and inoculated with R. solani. Each treatment was replicated four times, resulting in 160 Petri dishes [8 treatments × 4 replicates (each replicate containing 5 Petri dishes)]. Each Petri dish (9 cm) contained 10 cucumber seeds placed on a layer of absorbent cotton wool and sterilized blotting paper. For germination, the dishes were incubated at 25°C under a controlled light regime of 16 h light and 8 h dark.
Seed germination was assessed based on radicle emergence and a minimum length exceeding 2.0 mm. Fifteen days post-inoculation (dpi), seedlings were collected from each treatment group to evaluate various parameters. These parameters included disease progression [disease severity index (DSI) and percent of infected seeds (PIS)] as outlined by [46] and [47], and germination metrics [germination percentage (GP), germination index (GI), seedling length vigor index (SLVI), and seedling weight vigor index (SWVI)] as described by [48]. Disease index was assessed using a 0–4 scale adapted from [46], where 0 indicates no visible damage, 1 signifies minor discoloration of the hypocotyl, 2 represents discoloration combined with small necrotic lesions (less than 1 mm diameter) on the hypocotyl, 3 indicates discoloration with larger necrotic lesions (greater than or equal to 1 mm diameter) on the hypocotyl, and 4 signifies seedling death. Disease severity data were then processed using McKinney’s formula [49] to generate a numerical DSI: DSI (%) = (Σvn)/(NV) × 100. In this formula, Σvn represents the sum of the product obtained by multiplying the disease index score (v) by the number of plants (n) assigned to that score. N represents the total number of plants in the experiment, and V represents the highest numerical value on the disease index scale. The efficacy of each entomopathogenic fungi treatment was subsequently rated based on the calculated DSI using the classification system established by [25]. This classification system categorizes treatment efficacy as follows: EE: Extremely effective (DSI = 0%), HE: Highly effective (DSI = 0.1% to 5%), E: Effective (DSI = 5.1% to 25%), I: Ineffective (DSI = 25.1% to 50%), and HI: Highly Ineffective (DSI = 50.1% to 100%). PIS = Total number of infected seedlings/Total seeds sown × 100 [47]. Germination% = Total number of germinated seeds/Total seeds sown × 100 [48]. GI = Total number of germinated seeds/Total number of days [48]. SLVI = [seedling length (cm) × seed germination (%)] [48]. SWVI = [seedling DW (mg) × seed germination (%)] [48].
2.4 Antagonistic Action of Entomopathogenic Fungi toward Rhizoctonia solani
The antagonistic interaction between the entomopathogenic fungi and the R. solani pathogen was evaluated using a dual culture assay on potato dextrose agar (PDA) plates. Two agar plugs (0.5 cm) were obtained: one containing a four-day-old culture of the entomopathogenic fungus and another containing the R. solani pathogen. These plugs were placed on opposing sides of a single 9-cm diameter PDA plate, maintaining a distance of 2 cm from the plate edge towards the center for the antagonist plug and a distance of 5 cm between the two plugs. A control plate included only a PDA plug on one side and the R. solani plug on the opposite side. Each treatment was replicated three times, with five plates per replicate. All plates were incubated at 28°C ± 2°C for 7 days. After incubation, the percent inhibition of R. solani radial growth was calculated using the formula established by [50]: I (%) = (1 − Cn/C0) × 100. In this formula, Cn represents the radial growth of the pathogen colony in the presence of the antagonist fungus, and C0 represents the radial growth of the control pathogen colony. Mycelial growth was assessed in cm as described in [49].
2.5 Greenhouse Evaluation of Entomopathogenic Fungi against Rhizoctonia solani
The in vivo experiment investigated the potential of entomopathogenic fungi for the preventive control of R. solani in cucumber plants. Healthy cucumber seeds were sown in nursery trays containing cells with a 250 mL volume. Each treatment consisted of 15 plants, further divided into three replicates with five plants each. The substrate for the experiment was a sterilized 1:1 mixture of peat and vermiculite, achieved by autoclaving twice at 120°C. Thirty-day-old cucumber seedlings were then root-dipped in flasks containing conidial suspensions of the different entomopathogenic fungi for 30 min as a preventive treatment. Twenty-four hours after this treatment, the seedlings were challenged with R. solani by watering each plant with 10 mL of the pathogen’s conidial suspension. Each experimental block included two control groups: a positive control, where plants were inoculated only with the R. solani pathogen, and a negative control, where plants were treated solely with sterile distilled water. The experiment employed a randomized complete block design with eight treatments: T1 (negative control)—untreated seedlings; T2 (positive control)—seedlings inoculated only with R. solani; T3—seedlings treated only with B. bassiana, T4—seedlings treated only with M. anisopliae, T5—seedlings treated only with Metarhizium sp. Me351, T6—seedlings treated with B. bassiana and inoculated with R. solani, T7—seedlings treated with M. anisopliae and inoculated with R. solani, and T8—seedlings treated with Metarhizium sp. Me351 and inoculated with R. solani. After treatment, the pots were incubated in a greenhouse for 60 days (at 25°C). Post-experimental, the tomato plants were carefully removed from their pots. The root systems were subsequently rinsed thoroughly with tap water. The entire experiment was repeated twice to ensure data robustness [49,51].
Disease assessment was conducted at 60 dpi using a DSI using McKinney’s formula [49]. The efficacy of each entomopathogenic fungi treatment was subsequently rated based on the calculated DSI using the classification system established by [50] and further refined by [52]. Plant growth was assessed by measuring plant length (PL) [25].
To elucidate the biochemical alterations induced in cucumber plants by the preventive application of B. bassiana, M. anisopliae, and Metarhizium sp. Me351, a series of biochemical parameters were evaluated. Enzyme activity assays were conducted on five cucumber root samples collected per treatment and block, at 60 dpi. These assays measured the activity of catalase (CAT), peroxidase (POX), superoxide dismutase (SOD), and polyphenol oxidase (PPO). Additionally, total phenolic content (TPC) and total sugar (TS) levels were quantified in the root tissues.
To prepare enzyme extracts for activity assays, 0.1 g root samples from each treatment were flash-frozen in liquid nitrogen to inhibit proteolytic activity. The frozen samples were then homogenized in a chilled extraction buffer (0.1 M phosphate buffer with 0.5 mM EDTA, pH 7.5) at 1:5 (buffer:tissue weight). The homogenate was centrifuged at 15,000× g for 20 min at 4°C. The resulting supernatant was collected in Eppendorf tubes for subsequent enzyme activity assays. CAT activity was measured according to the method described by [53], monitoring the decrease in absorbance at 240 nm. POX activity was assayed following the protocol established by [54]. The reaction mixture for POX activity consisted of 0.5 mL guaiacol, 1 mL phosphate buffer, 0.5 mL H2O2, 0.1 mL enzyme extract, and 0.9 mL distilled water. The change in absorbance was measured at 470 nm. SOD activity was determined using the method of [55]. The reaction mixture for SOD activity contained 130 μM L-methionine, 13 μM riboflavin, 50 mM phosphate buffer (pH 7.0), 630 μM NBT (nitro blue tetrazolium), EDTA disodium salt (EDTAna2), and enzyme extract. The absorbance of this mixture was monitored at 560 nm. PPO activity was evaluated according to the method of [56], measuring the increase in absorbance at 408 nm. The reaction mixture for PPO activity comprised 2.7 mL of 14 mg mL−1 catechol solution in 0.1 M phosphate buffer and 0.3 mL of enzyme extract [56].
TPC and TS levels were determined in cucumber root tissues. TPC was measured using the Folin-Ciocalteu method, where the absorbance of the reaction mixture was measured at 765 nm. TPC was then expressed as milligrams of gallic acid equivalent per gram of fresh weight (mg GAE/g FW) according to the method established by [57]. For TS quantification, the anthrone method was employed, with absorbance readings taken at 625 nm. The TS content was expressed based on the extrapolation from a linear standard curve prepared using glucose [58].
Statistical analysis was performed using a one-way analysis of variance (ANOVA) implemented in SPSS version 20.0 software to evaluate potential variations among the different treatment groups. Before conducting the ANOVA, data from replicate samples were averaged to obtain representative mean values for each treatment. The assumptions of normality and homogeneity of variance were verified using appropriate tests before proceeding with the ANOVA. Subsequently, Duncan’s Multiple Range Test was employed to identify statistically significant differences (p ≤ 0.05) between the treatment means. This post-hoc test allowed for a nuanced comparison of the treatment effects and identifying important variations in the measured parameters across the different entomopathogenic fungi treatments and control groups.
3.1 In Vitro Plant-Growth-Promoting Traits and Extracellular Enzymes Activities
An assessment of extracellular enzyme production and plant growth promotion capabilities was conducted for three entomopathogenic fungi. Table 1 presents the detailed results of enzyme activity and plant growth effects. Both B. bassiana and M. anisopliae exhibited a wide range of extracellular enzyme activities. These two fungi showed positive activities for Glu, Pec, Cat, Pro, Cell, Chi, Amy, and Lip. However, Metarhizium sp. Me351 showed a more selective enzyme profile. It demonstrated positive activities for Glu, Pec, Cat, and Cell but lacked Pro, Chi, Amy, and Lip (Table 1). B. bassiana, M. anisopliae, and Metarhizium sp. Me351 showed positive traits for HCN and IAA production; crucial for plant growth promotion. However, none of the isolates exhibited atmospheric nitrogen fixation ability, as indicated by the negative results in the N-free medium (Table 1).

3.2 Cucumber Seed Germination Assay
The effectiveness of entomopathogenic fungi on the progression of disease caused by R. solani in cucumber seeds is summarized in Table 2. Seeds without any treatment (negative control) and those treated with just entomopathogenic fungi (B. bassiana, M. anisopliae, and Metarhizium sp. Me351) showed no signs of disease (DSI = 0%). This indicates they are extremely effective (EE) at preventing the disease. In contrast, seeds inoculated only with the disease-causing fungus (positive control) displayed severe symptoms (DSI = 97.33%), making them highly ineffective (HI). When these beneficial fungi (B. bassiana and M. anisopliae) were combined with the harmful fungus, they significantly reduced disease severity (DSI = 3.85% and 1.86%, respectively), making them highly effective (HE). Metarhizium sp. Me351, while effective (E) at reducing disease (DSI = 27.77%), provided less protection compared to the other two beneficial fungi (Table 2).

The negative control group, representing seeds without treatment, had a 0% infection rate, as expected. Interestingly, treatments containing only entomopathogenic fungi also resulted in a 0% infection rate. This suggested that these fungal strains themselves are not harmful to the seeds and might even possess some protective properties. In contrast, the positive control group, representing seeds inoculated with R. solani, exhibited a high infection rate of 96.33%. When either B. bassiana or M. anisopliae was combined with R. solani, the seed infection rate dropped significantly. The combination with B. bassiana resulted in a 3.67% infection rate, while the combination with M. anisopliae achieved an even lower infection rate of 1.67%. These significant reductions in infection rate compared to the positive control group demonstrated the effectiveness of this combined approach. However, Metarhizium sp. Me351, when combined with R. solani, resulted in a higher infection rate (22%) than other combinations. While this infection rate is still significantly lower than the positive control, it suggests that Metarhizium sp. Me351 might be less effective in this specific context (Table 2).
Table 3 delved into the effects of entomopathogenic fungi on seed germination and seedling development after exposure to R. solani. The experiment was conducted under controlled conditions in a growth chamber for 15 days after inoculation. The negative control group, representing seeds without treatment, displayed a high GP (85.67%) and GI (8.57), indicating successful seed development. The seedling length vigor index (SLVI = 106.85) and seedling weight vigor index (SWVI = 32.12) further confirm healthy seedling growth in the negative control. When compared to the negative control group, seeds in the positive control group exhibited significantly lower values for all measured parameters: GP (42.33%), GI (2.19), SLVI (74.70), and SWVI (27.99). These findings suggested that R. solani infection disrupted physiological processes for successful seed germination and seedling development. When applied alone, B. bassiana or M. anisopliae did not negatively affect germination, with germination metrics similar to the negative control. Thus, both entomopathogenic fungi maintained high GP exceeding 88% and GI above 11.04. Moreover, B. bassiana and M. anisopliae exhibited a significantly higher value of SLVI (111.69 and 109.07, respectively), and SWVI (34.25 and 31.62, respectively), suggesting robust seedling development. However, Metarhizium sp. Me351 displayed a slight decrease in all germination metrics (GP = 80.33%, GI = 8.93, SLVI = 100.02, SWVI = 32.19) compared to the other entomopathogenic fungi. Moving on to the combination treatments, both B. bassiana and M. anisopliae partially mitigated the negative effects of R. solani on germination. They achieved GP (85.33% and 86%, respectively), GI (10.67 and 12.29, respectively), SLVI (86.41 and 109.44, respectively), and SWVI (30.24 and 37.57, respectively) closer to the negative control compared to the positive control. However, Metarhizium sp. Me351, in the presence of R. solani, offered less protection for germination metrics (Table 3).

3.3 Antagonistic Action of Entomopathogenic Fungi toward Rhizoctonia solani
The experiment investigated the impact of entomopathogenic fungi on the mycelial growth of R. solani. Significant effects (p < 0.01) were observed on both R. solani growth and the percentage of inhibition of its radial growth (Figs. 1 and 2). Among the entomopathogenic fungi tested, B. bassiana and M. anisopliae displayed the strongest inhibition of R. solani growth, with radial growth values of 0.31 cm and 0.49 cm, respectively. Conversely, the control group exhibited the highest radial growth at 5.13 cm (Fig. 1). Similarly, B. bassiana and M. anisopliae resulted in the greatest percentage inhibition of R. solani mycelial growth, achieving values between 93.90% and 90.46% (Fig. 2).
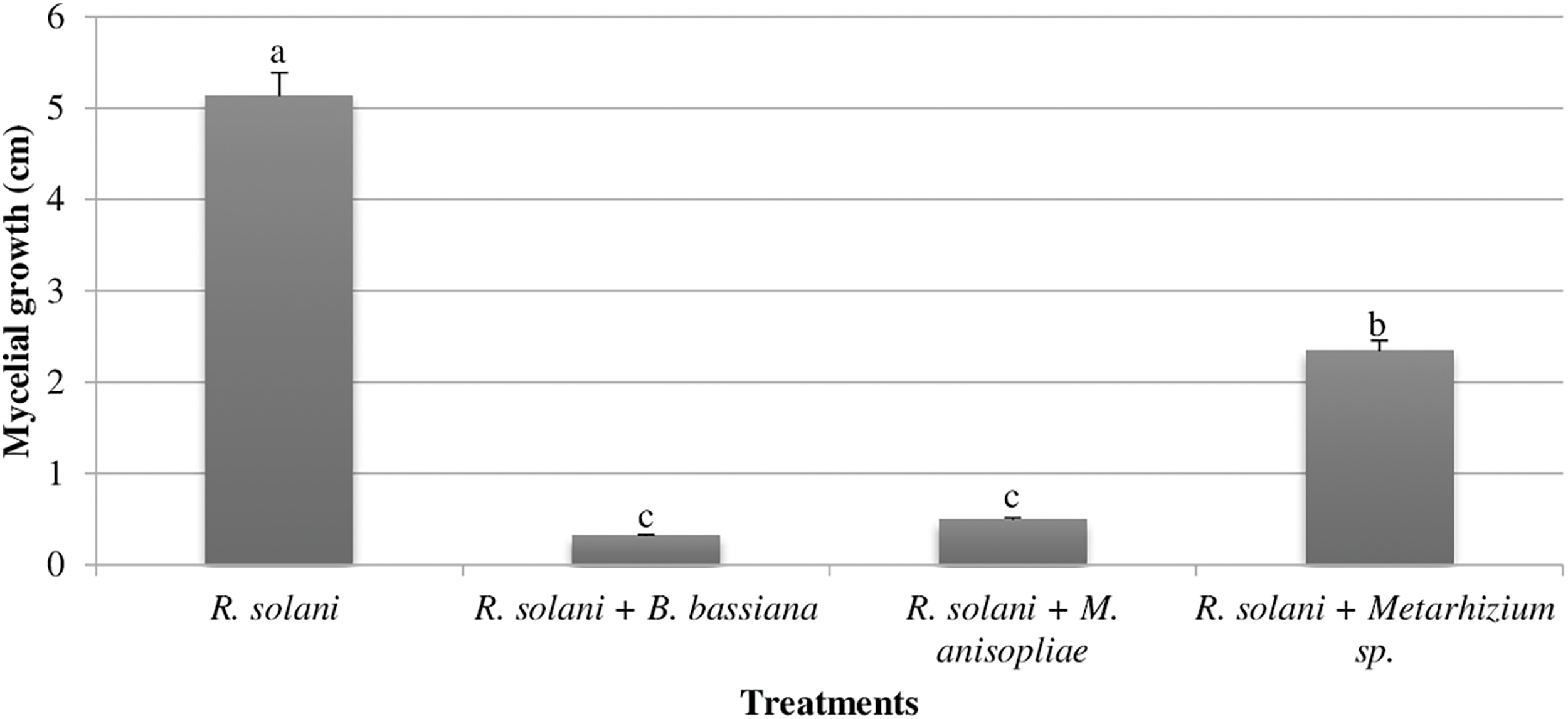
Figure 1: Effect of entomopathogenic fungi (B. bassiana, M. anisopliae, and Metarhizium sp. Me351) on mycelial growth of R. solani after 7 days of incubation at 28 ± 2°C under laboratory conditions. Potato Dextrose Agar medium solidified in 9 cm diameter Petri dishes served as the growth substrate for the fungal isolates. Different letters above bars indicate statistically significant differences between treatments within the experiments (p ≤ 0.01) according to Duncan’s multiple range tests

Figure 2: Effect of entomopathogenic fungi (B. bassiana, M. anisopliae, and Metarhizium sp. Me351) on the percent inhibition of R. solani radial growth after 7 days of incubation at 28 ± 2°C under laboratory conditions. Potato Dextrose Agar medium solidified in 9 cm diameter Petri dishes served as the growth substrate for the fungal isolates. Different letters above bars indicate statistically significant differences between treatments within the experiments (p ≤ 0.01) according to Duncan’s multiple range tests
3.4 Greenhouse Evaluation of Entomopathogenic Fungi against Rhizoctonia solani
The efficacy of entomopathogenic fungi in controlling R. solani was evaluated in vivo by assessing the DSI in cucumber roots. The results revealed a significant suppressive effect of B. bassiana against R. solani, with a DSI of 9.51% compared to the positive control group experiencing a severe disease level (DSI = 99.33%). Furthermore, M. anisopliae demonstrated even greater efficacy, achieving a remarkably low DSI of 6.99%. Metarhizium sp. Me351, although exhibiting a less effect (DSI = 40.19%) compared to the other two fungi, still displayed a statistically significant reduction in disease severity compared to the untreated control group. These findings suggest that all three entomopathogenic fungi possess potential for the biological control of R. solani in cucumber, with M. anisopliae demonstrating the most promising results in this study (Fig. 3).
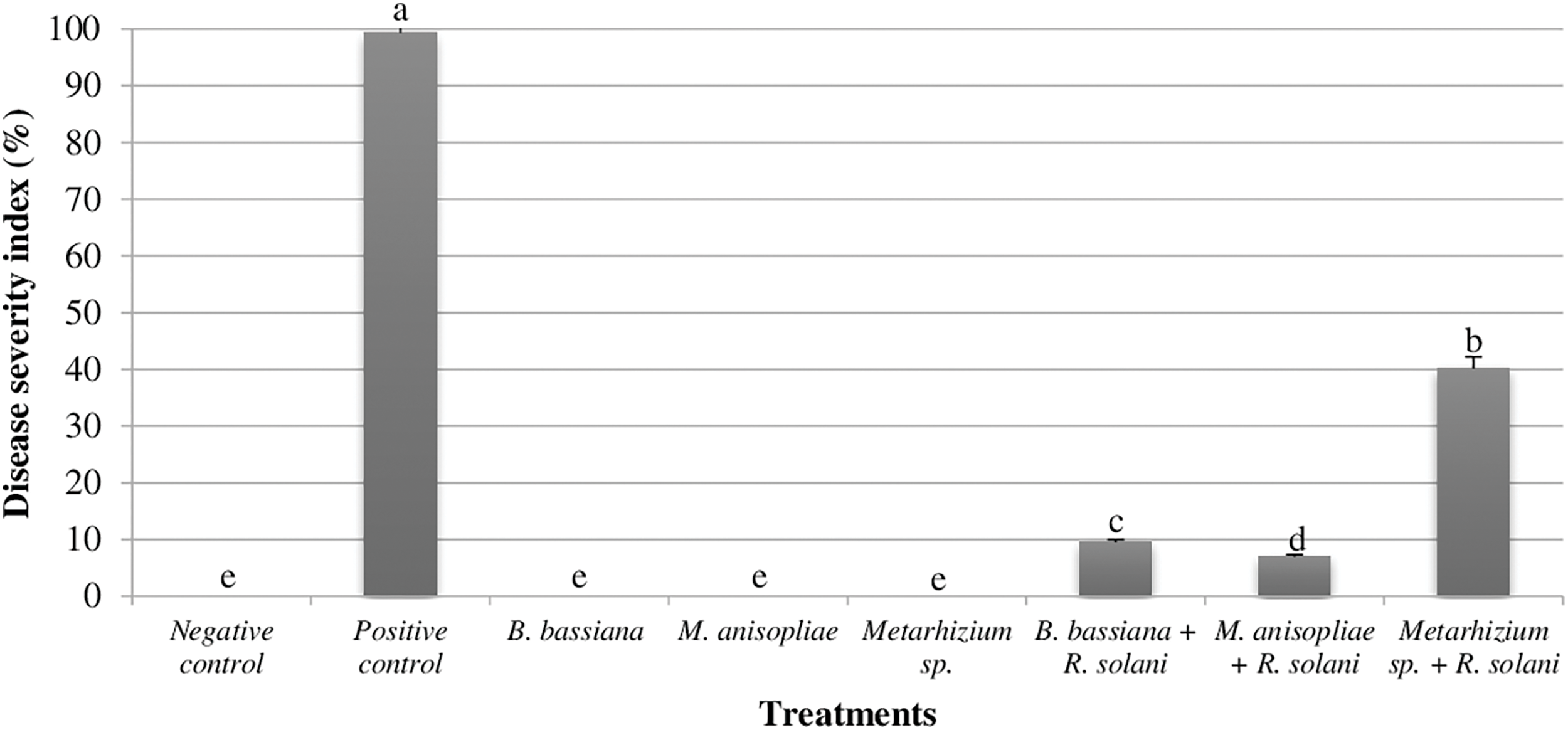
Figure 3: In vivo evaluation of entomopathogenic fungi (B. bassiana, M. anisopliae, and Metarhizium sp. Me351) on the disease severity index in cucumber roots inoculated with R. solani after 60 days of growth under greenhouse conditions at 25°C. Different letters above bars indicate statistically significant differences between treatments within the experiments (p ≤ 0.01) according to Duncan’s multiple range tests
This study also evaluated the effects of entomopathogenic fungi on cucumber plant growth against R. solani. After 60 days of controlled greenhouse growth, plant length was measured. Uninoculated and untreated plants (negative control) exhibited moderate growth (83.15 cm). However, plants inoculated with R. solani alone (positive control) displayed the most severe growth reduction (35.33 cm). Thus, treatment with B. bassiana resulted in the greatest plant height (140.79 cm), indicating significant growth promotion. Metarhizium treatments (Me351: 140.77 cm and M. anisopliae: 139.94 cm) also significantly enhanced plant growth with lengths comparable to B. bassiana. When co-inoculated with R. solani, M. anisopliae (98.76 cm) and B. bassiana (93.76 cm) treatments displayed reduced plant length compared to entomopathogenic fungi-only treatments but remained significantly taller than controls. Metarhizium sp. Me351 co-inoculation led to the lowest plant height within the fungi-treated groups (59.66 cm) but surpassed the positive control (Fig. 4).

Figure 4: In vivo evaluation of entomopathogenic fungi (B. bassiana, M. anisopliae, and Metarhizium sp. Me351) on the plant length of cucumber plants inoculated with R. solani after 60 days of growth under greenhouse conditions at 25°C. Different letters above bars indicate statistically significant differences between treatments within the experiments (p ≤ 0.01) according to Duncan’s multiple range tests
Table 4 revealed the effect of three entomopathogenic fungi in the presence of R. solani on CAT, POX, SOD, PPO, TPC, and TS. B. bassiana, M. anisopliae, and Metarhizium sp. Me351 treatments displayed an average CAT activity of 70.03, 70.77, and 69.41 units/mg protein/min, respectively, compared to the negative (14.94 units/mg protein/min) and positive (23.60 units/mg protein/min) controls. Similarly, POX activity more than tripled in the presence of entomopathogenic fungi, with an average of 3.24, 3.45, and 3.33 units/g/mL/min for B. bassiana, M. anisopliae, and Metarhizium sp. Me351, respectively, compared to the negative (1.13 units/mg protein/min) and positive (1.52 units/mg protein/min) controls. Furthermore, the result revealed an interaction between the entomopathogenic fungi and R. solani. When these fungi were co-inoculated with R. solani, CAT activity witnessed a further significant increase compared to the treatments with entomopathogenic fungi alone. Interestingly, M. anisopliae + R. solani exhibited the highest CAT activity (105.52 units/mg protein/min) among all treatments. A similar trend was observed for POX activity, with the M. anisopliae + R. solani treatment showing the highest value (5.77 units/mg protein/min) (Table 4). The combination of entomopathogenic fungi and R. solani (B. bassiana + R. solani, M. anisopliae + R. solani) resulted in the highest SOD (40.65 and 41.74 units/mg protein/min, respectively) and PPO (0.539 and 0.559 units/mg protein/min, respectively) activity, while Metarhizium sp. + R. solani showed an intermediate effect (34.96 units/mg protein/min and 0.383 units/mg protein/min, respectively) (Table 4). All fungal treatments significantly (p < 0.01) increased TPC compared to the negative (1.33 mg/g) and positive (1.64 mg/g) controls. The highest TPC levels were observed in plants treated with M. anisopliae (2.65 mg/g) and B. bassiana (2.60 mg/g) in the presence of R. solani (Table 4). The fungal treatments generally resulted in increased TS compared to the controls. Moreover, the combination of B. bassiana (2.23) and M. anisopliae (2.16) with R. solani showed an increase in TS (Table 4).

Table 5 detailed the correlation coefficients between various indicators of plant growth, stress response, and disease severity measured after 60 days of controlled greenhouse growth. The table highlighted significant positive correlations (r > 0.8) between the activities of four antioxidant enzymes (CAT, POX, SOD, and PPO) and TPC. This strong positive correlation suggests that under these growth conditions, plants responded to potential stress by simultaneously increasing their production of antioxidant enzymes and accumulating phenolic compounds. Phenolic compounds are known to play a role in plant defense mechanisms, and their rise alongside the activities of antioxidant enzymes indicates a coordinated stress response strategy. Interestingly, the disease severity index exhibited a statistically significant negative correlation with TPC and the activities of antioxidant enzymes. This implies that plants with higher levels of these defensive elements experienced lower disease severity. In contrast, PL showed a negative correlation with DSI (r = −0.816) (Table 5).

Metarhizium spp. and Beauveria spp., well-established entomopathogenic fungi, play a significant ecological role within agricultural ecosystems by regulating insect populations [59,60]. Their recognized value as biocontrol agents (myco-insecticides) has led to their widespread application in sustainable insect pest management strategies [60,61]. However, recent research suggests these fungi offer even broader potential benefits for plant health. Studies indicated they may function as dual-purpose agents, biostimulants promoting plant growth, and bioprotectants enhancing plant defense mechanisms [62,63]. Notably, research has demonstrated their efficacy in protecting cucumber plants from phytopathogens, including the damaging R. solani fungus [63]. This multifaceted ecological role underscores the potential of Metarhizium spp. and Beauveria spp. as valuable tools for integrated disease management strategies in cucumber production systems [64]. This present study investigated this dual functionality in the context of cucumber cultivation. We examined the potential of Metarhizium sp. Me351, B. bassiana, and M. anisopliae act as biocontrol agents against damping-off and root rot diseases while simultaneously assessing their ability to promote plant growth in cucumber. B. bassiana and M. anisopliae demonstrated superior efficacy as biological control agents against R. solani in cucumber plants. These entomopathogenic fungi not only suppressed the phytopathogen but also improved plant growth and enhanced defense mechanisms.
Extracellular enzyme profiles of B. bassiana and M. anisopliae isolates revealed a broad spectrum of enzymatic activity. These fungi exhibited positive results for β-1,3-glucanase, pectinase, catalase, protease, cellulase, chitinase, amylase, and lipase enzymes. Existing research suggests these enzymes play a crucial role in degrading the cell walls of various hosts, including fungi, nematodes, and insects [65]. Additionally, they may facilitate nutrient acquisition by utilizing host proteins [65]. The activity of these enzymes is hypothesized to contribute to the inhibition of pathogen growth through mechanisms such as fungal cell wall degradation and limiting nutrient availability [65–67]. Notably, Metarhizium sp. Me351 displayed a more selective enzyme profile, lacking protease, chitinase, amylase, and lipase activity. This observed variation in enzyme activity profiles might be linked to the previously reported differences in biocontrol efficacy observed among various fungal isolates [60,65,66].
B. bassiana and Metarhizium spp. add another mechanism to their biocontrol arsenal through their ability to produce HCN. Plant growth-promoting fungi (PGPF) are recognized for producing low levels of HCN, which is believed to offer a unique advantage in biocontrol and plant growth promotion [9,68,69]. By maintaining low HCN levels, these PGPF strains may prevent the targeted fungi from developing resistance, ensuring the effectiveness of other antifungal metabolites they produce. This combined action enhances the biocontrol activity of the PGPF strain [9,69]. Furthermore, HCN exhibits antifungal properties by inhibiting the fungal electron transport system. These responses may improve plant tolerance to various environmental stresses, including abiotic and biotic stresses. Ultimately, this translates to improved plant growth and overall health [70,71].
Evaluation of the three fungal isolates revealed a positive capacity for IAA production. The ability of diverse soil microorganisms to synthesize this plant growth hormone, particularly auxin, significantly influences plant growth and development [72–74]. IAA acts as a regulator in stimulating root elongation and branching, ultimately leading to an increased root surface area. This enhanced root system facilitates improved nutrient and water uptake from the surrounding soil [75,76]. Furthermore, research suggests that IAA plays a role in influencing the biosynthesis of diverse secondary metabolites within plants. These metabolites may contribute to plant resistance against fungal diseases and strengthen their defense responses [59].
Evaluation of cucumber seed germination revealed significant biocontrol efficacy of B. bassiana and M. anisopliae against R. solani. This conclusion is supported by the observed substantial reduction in DSI following treatment with these fungal isolates. Notably, the calculated DSI values categorized these treatments as “highly effective,” indicating a statistically significant decrease in disease symptoms compared to the positive control group. The percentage of infected seeds followed a similar pattern, with B. bassiana and M. anisopliae treatments demonstrating the lowest infection rates. Additionally, the fungal treatments positively impacted germination metrics, including parameters like germination rate and seedling vigor, particularly B. bassiana and M. anisopliae. These findings suggested that these fungi may promote seed germination and enhance seedling vigor in cucumber plants. This observed growth promotion could be attributed to the enzyme activities and plant growth-promoting traits documented for these fungal isolates [32,63,77].
B. bassiana and M. anisopliae have been explored for their potential application in controlling plant pathogenic fungi. Evidence suggests their efficacy against a diverse range of plant pathogens, including R. solani, Pythium myriotylum, Sphaerotheca fuliginea, Botrytis cinerea, F. oxysporum, Colletotrichium spp., Phytophthora spp., and Plasmopara viticola [37,60,77–85]. Furthermore, entomopathogenic fungi, such as Metarhizium spp. and Beauveria spp., are known to produce protease enzymes, which play a significant role in their ability to control insect pests. References [37,60,77–85] showed that a similar mechanism might be at play in the observed inhibition of plant pathogenic fungi. Previous studies support this hypothesis, demonstrating a significant increase in healthy seedlings when seeds are cultivated in soil inoculated with Metarhizium spp. and contaminated with the plant pathogen Pythium aphanidermatum, compared to seedlings grown solely in pathogen-contaminated soil [64]. The observed increase in healthy seedlings is attributed to the potential inhibition of P. aphanidermatum by the co-inoculated entomopathogenic fungus [37,77,84–86].
Studies investigating the effect of entomopathogenic fungi on seed germination have yielded promising results, comparatively in chili [87] a nearly 10% increase of seed germination was observed when inoculated with B. bassiana and M. anisopliae compared to the control groups. Similarly, Reference [88] reported a significant enhancement in corn seed germination following B. bassiana inoculation, with inoculated plants exhibiting an 89% germination rate compared to the 77% observed in the control group. Reference [89] further demonstrated that seed treatment with B. bassiana not only increased germination rates but also accelerated and synchronized germination compared to untreated seeds. Soaking chili seeds in B. bassiana suspensions for 9–12 h resulted in the highest germination rates compared to shorter soaking durations [89]. Reference [90] investigated the influence of entomopathogenic fungi strains on Vicia faba plant growth. Their study revealed that inoculation with M. brunneum and B. bassiana significantly enhanced growth parameters, including seedling emergence, plant height, number of leaf pairs, and fresh root weight. Furthermore, extending the seed treatment duration with these fungi resulted in a positive effect on plant growth, as measured by plant height, number of leaf pairs, fresh shoot weight, and fresh root weight. This finding suggests that increased seed treatment duration may promote a higher plant colonization rate by the beneficial fungi, ultimately leading to enhanced growth responses [90]. Reference [91] reported that endophytic strains of B. bassiana and M. brunneum improved iron availability, chlorophyll content, root length, and fine root abundance in sorghum. Further studies by [30] and [88] demonstrated enhanced plant growth parameters, including stem height, root length, shoot and root weight, and yield in wheat and soybean, respectively, when inoculated with B. bassiana. Furthermore, research by [92] and [93] suggested that spraying maize seeds with B. bassiana and M. anisopliae can promote plant growth, evidenced by increased plant height, leaf number, and cob development.
Research suggests that these entomopathogenic fungi may exert their growth-promoting effects through direct and indirect pathways. One potential mechanism involves the production of secondary metabolites by the fungi. These metabolites may include phytohormones that directly stimulate seed germination and root formation in plants. Enhanced root development can ultimately lead to increased nutrient uptake by the plant. Supporting this concept, studies by [94,95], and [96] have shown that Metarhizium spp. and Beauveria spp. can invade plant root tissues, triggering the formation of root hairs and lateral roots. This increased root development can significantly enhance the plant’s ability to acquire nutrients from the surrounding soil. Furthermore, recent research revealed that Metarhizium spp. and Beauveria spp. may play an indirect role in promoting plant growth by acting as facilitators for nutrient transfer. These entomopathogenic fungi may function as bridges, facilitating the movement of nutrients from the rhizosphere to the plant roots, as evidenced by the work of [64] and [97]. These combined findings provide a compelling framework for understanding the multifaceted mechanisms by which Metarhizium spp. and Beauveria spp. can promote plant growth [64].
This study examined the antagonistic potential of two entomopathogenic fungi, B. bassiana and M. anisopliae, against the plant pathogenic fungus R. solani under laboratory conditions. Both fungal species significantly inhibited the radial and mycelial growth of R. solani, with B. bassiana demonstrating a superior inhibitory effect. These results corroborate previous findings by [27,98], and [63] who reported similar suppression of R. solani by B. bassiana. However, the biocontrol efficacy of M. anisopliae may vary depending on the specific strain employed. While this study focused on growth inhibition, it is important to acknowledge the broader biocontrol potential of M. anisopliae as evidenced by previous research. References [36,60,66], and [99] reported that M. anisopliae strains can additionally suppress the formation and germination of sclerotia and conidia in R. solani, suggesting a multifaceted mode of action. The antagonistic interaction between B. bassiana and M. anisopliae with R. solani involves the production of secondary metabolites. These diffusible compounds function as antibiosis agents, documented by [27,36,60,63,66,98], and [99]. The formation of inhibition zones and potential mycelial collapse of R. solani can occur before physical contact with the antagonistic fungi. Studies by [27,63,66,100], and [101] identified specific secondary metabolites produced by B. bassiana and M. anisopliae, including alkaloids, non-peptide pigments, cyclodepsipeptides, and cyclopeptides. These metabolites possess a range of bioactivities, including insecticidal, antimicrobial, and antioxidant properties. In another interaction mode, physical proximity between R. solani and entomopathogenic fungi can induce the formation of a distinct deep brown mycelial barrier at the interface. This phenomenon, observed in studies of [36] and [99] is accompanied by aging, browning of R. solani mycelia, and increased sclerotial production. The release of beauvericin, a secondary metabolite known to inhibit cell proliferation and induce apoptosis through the mitochondrial pathway, is a potential mechanism for these observed effects. Future research could explore the detailed impact of different secondary metabolites on R. solani and their contribution to the overall biocontrol efficacy [102,103].
The current study demonstrated the efficacy of B. bassiana and M. anisopliae, possessing antimicrobial properties, in mitigating damping-off and root rot diseases in cucumber when applied as a seedling root dip. Entomopathogenic fungi have been shown to reduce plant damage caused by various phytopathogens, including R. solani [26,63,64,76,79,85,104]. Pre-planting treatment with Metarhizium spp. and B. bassiana decreased Rhizoctonia disease symptoms, including damage to sprouts, stems, stolons, and sclerotium formation on tubers throughout the growing season [27]. Although Metarhizium spp. exhibited greater in vitro activity against R. solani, field studies demonstrated superior Rhizoctonia disease suppression by B. bassiana on sprouts, stems, and stolons, while both fungi equally reduced sclerotium index on tubers [27]. Endophytic colonization of tomato and cotton by B. bassiana following treatment significantly reduced damping-off disease caused by R. solani, as reported in studies by [105] and [106]. Reference [63] reported a reduction in sheath blight disease incidence following B. bassiana application. Additionally, Reference [107] observed a decreased disease incidence percentage in plants treated with Metarhizium sp. and R. solani compared to R. solani treatment alone. A 58.33% reduction in disease incidence was noted in Metarhizium spp.-treated plants at 15 DAI relative to R. solani-inoculated controls. Furthermore, the suppressive effects of Metarhizium sp. against plant pathogens have been documented in other studies. For example, Metarhizium sp. inhibited Cochliobolus heterostrophus in maize, and Fusarium solani f. sp. phaseoli was suppressed in haricot beans through soil application of this species [108,109]. These differential effects may be attributed to distinct colonization patterns, with Metarhizium spp. primarily inhabiting the rhizosphere and root tissues, and Beauveria spp. exhibiting a broader host range, colonizing roots, stems, and leaves [27,76,110,111]. The biocontrol efficacy of B. bassiana and Metarhizium spp. is attributed to multiple mechanisms, including antagonistic interactions and direct suppression of plant pathogens via mycoparasitism, competition, and antibiosis, as well as indirect effects through endophytic colonization and induction of systemic resistance [30,31,34,37].
Application of B. bassiana strain B2 resulted in effective management of rice sheath blight, associated with increased accumulation of defense enzymes, including polyphenol oxidase, peroxidase, chitinase, and lipoxygenase [112]. Reference [113] identified biocontrol mechanisms underlying B. bassiana strain TS12 management of R. solani-induced tomato damping-off, involving induced systemic resistance through increased accumulation of defense enzymes such as chitinases, peroxidases, and phenyl ammonia lyase and phenolic compounds [113]. Metarhizium spp. treatment reduced H2O2 and MDA levels by 5.21% and 14.96%, respectively, in pathogen-inoculated plants by enhancing antioxidant enzyme activities (ascorbate peroxidase, glutathione S-transferase, peroxidase, and catalase) [107]. Furthermore, Metarhizium spp. increased secondary metabolites, proline, carbohydrates, and soluble sugars in okra, promoting improved osmotic adjustment against diseases. Increased antioxidant enzyme activity mitigates oxidative damage and restores photosynthetic imbalance caused by pathogen-induced lesions [114]. Reference [27] reported increased peroxidase activity four weeks post-planting following treatment with Metarhizium sp. and B. bassiana, suggesting that soil and rhizosphere colonization of potatoes enhanced root peroxidase activity. Activation of plant-protective enzymes (chitinase, polyphenol oxidase, and peroxidase) has been documented in plant-entomopathogenic fungi interactions [60,115]. Independent endophytic colonization with entomopathogenic fungi can induce plant resistance to phytopathogens and increase protective compound activity [27,38].
Acknowledgement: The authors are grateful to the review editor and the anonymous reviewers for their helpful comments and suggestions to improve the clarity of the research paper.
Funding Statement: We did not receive financial support; we used our facilities available.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Abdelhak Rhouma, Lobna Hajji-Hedfi; data collection: Abdelhak Rhouma, Lobna Hajji-Hedfi, Samar Dali, Omaima Bargougui, Amira Khlif; analysis and interpretation of results: Abdelhak Rhouma, Lobna Hajji-Hedfi, Nahla Alsayd Bouqellah; draft manuscript preparation: Abdelhak Rhouma, Lobna Hajji-Hedfi, Nahla Alsayd Bouqellah, Pravin Babasaheb Khaire, Laith Khalil Tawfeeq Al-Ani. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the authors and/or corresponding authors upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Gu D, Andreev K, Dupre ME. Major trends in population growth around the world. China CDC Wkly. 2021;3:604–13. doi:10.46234/ccdcw2021.160. [Google Scholar] [PubMed] [CrossRef]
2. Mok WK, Tan YX, Chen WN. Technology innovations for food security in Singapore: a case study of future food systems for an increasingly natural resource-scarce world. Trends Food Sci Technol. 2020;102:155–68. doi:10.1016/j.tifs.2020.06.013. [Google Scholar] [PubMed] [CrossRef]
3. SFSNW. State of Food Security and Nutrition in the World (SFSNW) is an annual flagship report jointly prepared by FAO, IFAD, UNICEF, WFP, and WHO. Rome, Italy: Building Climate Resilience for Food Security and Nutrition; 2018. p. 1–202. [Google Scholar]
4. FAO. Coping with water scarcity in agriculture: a global framework for action in a changing climate. Rome, Italy: FAO; 2016. p. 1–12. [Google Scholar]
5. Srivastava P, Giri N, Mandal D. 137 Cs technology for soil erosion and soil carbon distribution. Curr Sci. 2019;116:888–9. [Google Scholar]
6. Dolan F, Lamontagne J, Calvin K, Snyder A, Narayan KB, Di Vittorio AV, et al. Modeling the economic and environmental impacts of land scarcity under deep uncertainty. Earth’s Fut. 2022;10:e2021EF002466. doi:10.1029/2021EF002466. [Google Scholar] [CrossRef]
7. Roberts TL, Johnston AE. Phosphorus use efficiency and management in agriculture. Resour Conserv Recycl. 2015;105:275–81. doi:10.1016/j.resconrec.2015.09.013. [Google Scholar] [CrossRef]
8. Al Rawashdeh R. World peak potash: an analytical study. Resour Policy. 2020;69:101834. doi:10.1016/j.resourpol.2020.101834. [Google Scholar] [CrossRef]
9. Hajji-Hedfi L, Rhouma A, Hajlaoui H, Hajlaoui F, Rebouh NY. Understanding the influence of applying two culture filtrates to control gray mold disease (Botrytis cinerea) in tomato. Agronomy. 2023;13(7):1774. doi:10.3390/agronomy13071774. [Google Scholar] [CrossRef]
10. Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, Johnston M, et al. Solutions for a cultivated planet. Nature. 2011;478:337–42. doi:10.1038/nature10452. [Google Scholar] [PubMed] [CrossRef]
11. Garnett T, Appleby MC, Balmford A, Bateman IJ, Benton TG, Bloomer P, et al. Sustainable intensification in agriculture: premises and policies. Science. 2013;341:33–44. doi:10.1126/science.1234485. [Google Scholar] [CrossRef]
12. Smith P, Gregory PJ. Climate change and sustainable food production. Proc Nutr Soc. 2013;72:21–8. doi:10.1017/S0029665112002832. [Google Scholar] [PubMed] [CrossRef]
13. Samba N, Nunomura O, Lu N, Johkan M, Nakano A, Tsukagoshi S. Cucumber (Cucumis sativus L.) growth and productivity under solar radiation-based quantitative nutrient management in hydroponic system. Agronomy. 2024;14(2):296. doi:10.3390/agronomy14020296. [Google Scholar] [CrossRef]
14. Mahmoud AF, Abdalla OA. Biological control of fungi associated with damping-off and root rot disease of cucumber (Cucumis sativus L.). Arch Phytopathol Plant Prot. 2021;54:870–85. doi:10.1080/03235408.2020.1860412. [Google Scholar] [CrossRef]
15. Ristaino JB, Johnston SA. Ecologically based approaches to management of Phytophthora blight on bell pepper. Plant Dis. 1999;83:1080–9. doi:10.1094/PDIS.1999.83.12.1080. [Google Scholar] [PubMed] [CrossRef]
16. Morsy SM, Drgham EA, Mohamed GM. Effect of garlic and onion extracts or their intercropping on suppressing damping-off and powdery mildew diseases and growth characteristics of cucumber. Egypt J Phytopathol. 2009;37:35–46. [Google Scholar]
17. Simsek Ersahin Y, Haktanir K, Yanar Y. Vermicompost suppresses Rhizoctonia solani Kühn in cucumber seedlings. J Plant Dis Prot. 2009;116:182–8. doi:10.1007/BF03356308. [Google Scholar] [CrossRef]
18. El-Komy MH, Al-Qahtani RM, Widyawan A, Molan Y, Almasrahi A. First report of Fusarium root and stem rot caused by Fusarium oxysporum f. sp. radicis-cucumerinum on greenhouse cucumbers in Saudi Arabia. Plant Dis. 2021;105:3758. doi:10.1094/PDIS-01-21-0122-PDN. [Google Scholar] [PubMed] [CrossRef]
19. Heflish AA, Abdelkhalek A, Al-Askar AA, Behiry SI. Protective and curative effects of Trichoderma asperelloides Ta41 on tomato root rot caused by Rhizoctonia solani Rs33. Agronomy. 2021;11(6):1162. doi:10.3390/agronomy11061162. [Google Scholar] [CrossRef]
20. Wallon T, Sauvageau A, Van der Heyden H. Detection and quantification of Rhizoctonia solani and Rhizoctonia solani AG1-IB causing the bottom rot of lettuce in tissues and soils by multiplex QPCR. Plants. 2021;10(1):57. doi:10.3390/plants10010057. [Google Scholar] [PubMed] [CrossRef]
21. Almaghasla MI, El-Ganainy SM, Ismail AM. Biological activity of four Trichoderma species confers protection against Rhizoctonia solani, the causal agent of cucumber damping-off and root rot diseases. Sustainability. 2023;15(9):7250. doi:10.3390/su15097250. [Google Scholar] [CrossRef]
22. Williamson-Benavides BA, Dhingra A. Understanding root rot disease in agricultural crops. Horticulturae. 2021;7(2):33. doi:10.3390/horticulturae7020033. [Google Scholar] [CrossRef]
23. Li S, Peng X, Wang Y, Hua K, Xing F, Zheng Y, et al. The effector AGLIP1 in Rhizoctonia solani AG1 IA triggers cell death in plants and promotes disease development through inhibiting PAMP-triggered immunity in Arabidopsis thaliana. Front Microbiol. 2019;10:2228. doi:10.3389/fmicb.2019.02228. [Google Scholar] [PubMed] [CrossRef]
24. Zachow C, Grosch R, Berg G. Impact of biotic and a-biotic parameters on structure and function of microbial communities living on sclerotia of the soil-borne pathogenic fungus Rhizoctonia solani. Appl Soil Ecol. 2011;48:193–200. doi:10.1016/j.apsoil.2011.03.006. [Google Scholar] [PubMed] [CrossRef]
25. Rhouma A, Mehaoua MS, Mougou I, Rhouma H, Shah KK, Bedjaoui H. Combining melon varieties with chemical fungicides for integrated powdery mildew control in Tunisia. Eur J Plant Pathol. 2023;165:189–201. doi:10.1007/s10658-022-02599-3. [Google Scholar] [CrossRef]
26. Branine M, Bazzicalupo A, Branco S. Biology and applications of endophytic insect-pathogenic fungi. PLoS Pathog. 2019;15(7):e1007831. doi:10.1371/journal.ppat.1007831. [Google Scholar] [PubMed] [CrossRef]
27. Tomilova OG, Shaldyaeva EM, Kryukova NA, Pilipova YV, Schmidt NS, Danilov VP, et al. Entomopathogenic fungi decrease Rhizoctonia disease in potato in field conditions. PeerJ. 2020;8:e9895. doi:10.7717/peerj.9895. [Google Scholar] [PubMed] [CrossRef]
28. Moonjely S, Barelli L, Bidochka MJ. Insect pathogenic fungi as endophytes. Adv Genet. 2016;94:107–35. doi:10.1016/bs.adgen.2015.12.004. [Google Scholar] [PubMed] [CrossRef]
29. Jaber LR, Enkerli J. Fungal entomopathogens as endophytes: can they promote plant growth? Biocontrol Sci Techn. 2017;27:28–41. doi:10.1080/09583157.2016.1243227. [Google Scholar] [CrossRef]
30. Jaber LR, Ownley BH. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol Control. 2018;116:36–45. doi:10.1016/j.biocontrol.2017.01.018. [Google Scholar] [CrossRef]
31. Vega FE. The use of fungal entomopathogens as endophytes in biological control: a review. Mycologia. 2018;110(1):4–30. doi:10.1080/00275514.2017.1418578. [Google Scholar] [PubMed] [CrossRef]
32. Bamisile BS, Dash CK, Akutse KS, Keppanan R, Wang L. Fungal endophytes: beyond herbivore management. Front Microbiol. 2018;9:544. doi:10.3389/fmicb.2018.00544. [Google Scholar] [PubMed] [CrossRef]
33. Garrido-Jurado I, Resquín-Romero G, Amarilla SP, Ríos-Moreno A, Carrasco L, Quesada Moraga E. Transient endophytic colonization of melon plants by entomopathogenic fungi foliar applications for the control of Bemisia tabaci Gennadius (Hemiptera: aleyrodidae). J Pest Sci. 2017;90(1):319–30. doi:10.1016/j.jip.2016.03.003. [Google Scholar] [PubMed] [CrossRef]
34. Ownley BH, Griffin MR, Klingeman WE, Gwinn KD, Moulton JK, Pereira RM. Beauveria bassiana: endophytic colonization and plant disease control. J Invertebr Pathol. 2008;98(3):267–70. doi:10.1016/j.jip.2008.01.010. [Google Scholar] [PubMed] [CrossRef]
35. Collemare J, Griffiths S, Yuichiro L, Mansoor KJ, Battaglia E, Russell JC. Secondary metabolism and biotrophic lifestyle in the tomato pathogen Cladosporium fulvum. PLoS One. 2014;9:e85877. doi:10.1371/journal.pone.0085877. [Google Scholar] [PubMed] [CrossRef]
36. Ríos-Moreno A, Garrido-Jurado I, Resquín-Romero G, Arroyo-Manzanares N, Arce L, Quesada-Moraga E. Destruxin A production by Metarhizium brunneum strains during transient endophytic colonisation of Solanum tuberosum. Biocontrol Sci Techn. 2016;26(11):1574–85. doi:10.1080/09583157.2016.1223274. [Google Scholar] [CrossRef]
37. Ownley BH, Gwinn KD, Vega FE. Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. BioControl. 2010;55:113–28. doi:10.1007/s10526-009-9241-x. [Google Scholar] [CrossRef]
38. Maksimov IV, Sorokan AV, Nafikova AR, Benkovskaya GV. On principal ability and action mechanisms of joint use of Bacillus subtilis 26D and Beauveria bassiana Ufa-2 preparation for potato protection against Phytophthora infestans and Leptinotarsa decemlineata. Micol Fitopatol. 2015;49(5):317–24. [Google Scholar]
39. El-Mageed TAA, El-Mageed SAA, El-Saadony MT, Abdelaziz S, Abdou NM. Plant growth-promoting rhizobacteria improve growth, morph-physiological responses, water productivity, and yield of rice plants under full and deficit drip irrigation. Rice. 2022;15:16. doi:10.1186/s12284-022-00564-6. [Google Scholar] [PubMed] [CrossRef]
40. Bhattacharyya C, Banerjee S, Acharya U, Mitra A, Mallick I, Haldar A. Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci Rep. 2020;10:15536. doi:10.1038/s41598-020-72439-z. [Google Scholar] [PubMed] [CrossRef]
41. Namasivayam E, Mariappan K, Jiji A, Kumar M, Richard L. Production of extracellular pectinase by Bacillus Cereus isolated from market solid waste. J Bioanal Biomed. 2011;3:3. doi:10.4172/1948-593X.1000046. [Google Scholar] [CrossRef]
42. Naik PR, Raman G, Narayanan KB, Sakthivel N. Assessment of genetic and functional diversity of phosphate solubilizing Fluorescent Pseudomonads isolated from rhizospheric soil. BMC Microbiol. 2008;8:230. doi:10.1186/1471-2180-8-230. [Google Scholar] [PubMed] [CrossRef]
43. Méndez-Santiago EW, Gómez-Rodríguez O, Sánchez-Cruz R, Folch-Mallol JL, Hernández-Velázquez VM, Villar-Luna E, et al. Serratia sp., an endophyte of Mimosa Pudica nodules with nematicidal, antifungal activity and growth-promoting characteristics. Arch Microbiol. 2021;203:549–59. doi:10.1007/s00203-020-02051-2. [Google Scholar] [PubMed] [CrossRef]
44. Faramarzi MA, Fazeli M, Yazdi MT, Adrangi S, Al-Ahmadi KJ, Tasharrofi N, et al. Optimization of cultural conditions for production of chitinase by a soil isolate of Massilia Timonae. Biotechnology. 2009;8:93–9. doi:10.3923/biotech.2009.93.99. [Google Scholar] [CrossRef]
45. Hankin L, Anagnostakis SL. The use of solid media for detection of enzyme production by fungi. Mycologia. 1975;67:597–607. doi:10.1080/00275514.1975.12019782. [Google Scholar] [CrossRef]
46. Carling DE, Pope EJ, Brainard KA, Carter DA. Characterization of mycorrhizal isolates of Rhizoctonia solani from an orchid, including AG-12, a new anastomosis group. Phytopathol. 2007;89:942–6. doi:10.1094/PHYTO.1999.89.10.942. [Google Scholar] [PubMed] [CrossRef]
47. Kator L, Ogo-Oluwa AT, Kemi AB. Isolation and identification of seed borne fungi of common bean (Phaseolus vulgaris L.) from selected markets in Makurdi. Int J Agric Sci. 2016;2(5):75–8. doi:10.11648/j.ijaas.20160205.11. [Google Scholar] [CrossRef]
48. Metwally RA, Abdelhameed RE, Soliman SA. Potential use of beneficial fungal microorganisms and C-phycocyanin extract for enhancing seed germination, seedling growth and biochemical traits of Solanum lycopersicum L. BMC Microbiol. 2022;22:108. doi:10.1186/s12866-022-02509-x. [Google Scholar] [PubMed] [CrossRef]
49. Matrood AAA, Rhouma A. Evaluating eco-friendly botanicals as alternatives to synthetic fungicides against the causal agent of early blight of Solanum melongena. J Plant Dis Prot. 2021;128(6):1517–30. doi:10.1007/s41348-021-00530-2. [Google Scholar] [CrossRef]
50. Hmouni A, Hajlaoui MR, Mlaiki A. Résistance de Botrytis cinerea aux benzimidazoles et aux dicarboximides dans les cultures abritées de tomate en Tunisie. OEPP/EPPO Bull. 1996;26:697–705. doi:10.1111/j.1365-2338.1996.tb01513.x. [Google Scholar] [CrossRef]
51. Hajji-Hedfi L, Rhouma A, Al-Judaibi AA, Hajlaoui H, Hajlaoui F, Abdel Azeem AM. Valorization of Capsicum annuum seed extract as an antifungal against Botrytis cinerea. Waste Biomass Valor. 2024;15:2559–73. doi:10.1007/s12649-023-02322-1. [Google Scholar] [CrossRef]
52. Okon OG, Rhouma A, Ismaila U, Matrood AAA, Hajji-Hedfi L. Biological control of fruit rot of postharvest orange (Citrus aurantium) by aqueous plant extracts. Indian J Agr Sci. 2023;93(11):1243–7. doi:10.56093/ijas.v93i11.141146. [Google Scholar] [CrossRef]
53. Bhuvaneshwari V, Amsaveni R, Kalaiselvi M, Rajeshwari R, Paul PK. Induced resistance by neem extracts in plants. Int J Biosci Nanosci. 2015;2(12):221–4. [Google Scholar]
54. Velazhahan R, Vidhyasekaran P. Role of phenolic compounds, peroxidase and polyphenol-oxidase in resistance of groundnut to rust. Acta Phytopathol Entomol Hung. 1994;29:23–9. [Google Scholar]
55. Xing Z, Wang Y, Feng Z, Tan Q. Effect of different packaging films on postharvest quality and selected enzyme activities of Hypsizygus marmoreus mushrooms. J Agric Food Chem. 2008;56:11838–44. doi:10.1021/jf8024387. [Google Scholar] [PubMed] [CrossRef]
56. Zhou XR, Xiao YJ, Meng XH, Liu BJ. Full inhibition of Whangkeumbae pear polyphenol oxidase enzymatic browning reaction by L-cysteine. Food Chem. 2018;266:1–8. doi:10.1016/j.foodchem.2018.05.086. [Google Scholar] [PubMed] [CrossRef]
57. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58. [Google Scholar]
58. Schields R, Burnett W. Determination of protein bound carbohydrate in serum by a modified anthrone method. Anal Chem. 1960;32:885–6. [Google Scholar]
59. Chaudhary PJ, Raghunandan BL, Patel HK, Mehta PV, Patel NB, Sonth B. Plant growth-promoting potential of entomopathogenic fungus Metarhizium pinghaense AAUBC-M26 under elevated salt stress in tomato. Agronomy. 2023;13(6):1577. doi:10.3390/agronomy13061577. [Google Scholar] [CrossRef]
60. Rhouma A, Hajji-Hedfi L, El Amine Kouadri M, Chihani-Hammas N, Babasaheb Khaire P. Investigating plant growth promoting and antifungal potential of Metarhizium spp. against Fusarium wilt in tomato. Nova Hedwigia. 2024;2:1–24. doi:10.1127/nova_hedwigia/2024/0958. [Google Scholar] [CrossRef]
61. Ghazi MV, Matrood AAA, Rhouma A, Hajji-Hedfi L. Efficacy of Beauveria bassiana and Trichoderma viride against Bemisia tabaci (Hemiptera: aleyrodidae) on tomato plants. J Biol Control. 2024;38(2):179–85. doi:10.18311/jbc/2024/36616. [Google Scholar] [CrossRef]
62. Agbessenou A, Akutse KS, Yusuf AA, Ekesi S, Subramanian S, Khamis FM. Endophytic fungi protect tomato and nightshade plants against Tuta absoluta (Lepidoptera: gelechiidae) through a hidden friendship and cryptic battle. Sci Rep. 2020;10(1):22195. doi:10.1038/s41598-020-78898-8. [Google Scholar] [PubMed] [CrossRef]
63. Deb L, Dutta P, Mandal MK, Singh SB. Antimicrobial traits of Beauveria bassiana against Rhizoctonia solani, the causal agent of sheath blight of rice under field conditions. Plant Dis. 2023;107(6):1739–56. doi:10.1094/PDIS-04-22-0806-RE. [Google Scholar] [PubMed] [CrossRef]
64. Aljarah NS. The activity of Metarhizium sp. to control Pythium aphanidermatum causal agent of cucumber damping off under greenhouse conditions, 2017;6(8):1098–1102. doi:10.21275/ART20176132. [Google Scholar] [CrossRef]
65. Freimoser FM, Hu G, St Leger RJ. Variation in gene expression patterns as the insect pathogen Metarhizium anisopliae adapts to different host cuticles or nutrient deprivation in vitro. Microbiology. 2005;151(2):361–71. doi:10.1099/mic.0.27560-0. [Google Scholar] [PubMed] [CrossRef]
66. Schrank A, Vainstein MH. Metarhizium anisopliae enzymes and toxins. Toxicon. 2010;56(7):1267–74. doi:10.1016/j.toxicon.2010.03.008. [Google Scholar] [PubMed] [CrossRef]
67. Bai N, Remadevi O, Sasidharan T, Balachander M, Dharmarajan P. Cuticle degrading enzyme production by some isolates of the entomopathogenic fungus, Metarhizium anisopliae (metsch.). J Bio-Sci. 2014;20:25–32. doi:10.3329/jbs.v20i0.17648. [Google Scholar] [CrossRef]
68. Bakker AW, Schippers B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp.-mediated plant growth-stimulation. Soil Biol Biochem. 1987;19(4):451–7. doi:10.1016/0038-0717(87)90037-X. [Google Scholar] [CrossRef]
69. Olanrewaju OS, Glick BR, Babalola OO. Mechanisms of action of plant growth promoting bacteria. World J Microbiol Biotechnol. 2017;33(11):197. doi:10.1007/s11274-017-2364-9. [Google Scholar] [PubMed] [CrossRef]
70. Abd El-Rahman AF, Shaheen HA, Abd El-Aziz RM, Ibrahim DSS. Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt J Biol Pest Control. 2019;29(1):41. doi:10.1186/s41938-019-0143-7. [Google Scholar] [CrossRef]
71. Sehrawat A, Sindhu SS, Glick BR. Hydrogen cyanide production by soil bacteria: biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere. 2022;32(1):15–38. doi:10.1016/S1002-0160(21)60058-9. [Google Scholar] [CrossRef]
72. Rai M, Rathod D, Agarkar G, Dar M, Brestic M, Pastore GM, et al. Fungal growth promotor endophytes: a pragmatic approach towards sustainable food and agriculture. Symbiosis. 2014;62(2):63–79. doi:10.1007/s13199-014-0273-3. [Google Scholar] [CrossRef]
73. Pham MT, Huang CM, Kirschner R. The plant growth-promoting potential of the mesophilic wood-rot mushroom Pleurotus pulmonarius. J Appl Microbiol. 2019;127(4):1157–71. doi:10.1111/jam.14375. [Google Scholar] [PubMed] [CrossRef]
74. Mesquita E, Hu S, Lima TB, Golo PS, Bidochka MJ. Utilization of Metarhizium as an insect biocontrol agent and a plant bioinoculant with special reference to Brazil. Front Fungal Biol. 2023;4:1276287. doi:10.3389/ffunb.2023.1276287. [Google Scholar] [PubMed] [CrossRef]
75. Fu SF, Sun PF, Lu HY, Wei JY, Xiao HS, Fang WT, et al. Plant growth-promoting traits of yeasts isolated from the phyllosphere and rhizosphere of Drosera spatulata lab. Fungal Biol. 2016;120(3):433–48. doi:10.1016/j.funbio.2015.12.006. [Google Scholar] [PubMed] [CrossRef]
76. Barelli L, Moreira CC, Bidochka MJ. Initial stages of endophytic colonization by Metarhizium involves rhizoplane colonization. Microbiology. 2018;164:1531–40. doi:10.1099/mic.0.000729. [Google Scholar] [PubMed] [CrossRef]
77. Russo ML, Vianna MF, Scorsetti AC, Ferreri N, de Abajo JM, Troncozo MI, et al. Entomopathogenic fungi as dual control agents against two phytopathogens and the lepidopteran pest rachiplusia nu in soybean (Glycine max (L.) Merr). J Fungi. 2024;10(2):93. doi:10.3390/jof10020093. [Google Scholar] [PubMed] [CrossRef]
78. Jaber JR. Grapevine leaf tissue colonization by the fungal entomopathogen Beauveria bassiana sl and its effect against downy mildew. BioControl. 2015;60:103–12. doi:10.1007/s10526-014-9618-3. [Google Scholar] [CrossRef]
79. Culebro-Ricaldi JM, Ruíz-Valdiviezo VM, Rodríguez-Mendiola MA, Ávila-Miranda MA, Miceli FG, Cruz-Rodríguez RI, et al. Antifungal properties of Beauveria bassiana strains against Fusarium oxysporum f. sp. lycopersici race 3 in tomato crop. J Environ Biol. 2017;38:821. doi:10.22438/jeb/38/5/MRN-412. [Google Scholar] [CrossRef]
80. Jaber L, Alananbeh KM. Fungal entomopathogens as endophytes reduce several species of Fusarium causing crown and root rot in sweet pepper (Capsicum annuum L.). Biol Control. 2018;126:117–26. doi:10.1016/j.biocontrol.2018.08.007. [Google Scholar] [CrossRef]
81. Kang BR, Han JH, Kim JJ, Kim YC. Dual biocontrol potential of the entomopathogenic fungus, Isaria javanica, for both aphids and plant fungal pathogens. Mycobiology. 2018;46:440–7. doi:10.1080/12298093.2018.1538073. [Google Scholar] [PubMed] [CrossRef]
82. Barra-Bucarei L, France IA, Gerding GM, Silva AG, Carrasco-Fernández J, Castro JF, et al. Antifungal activity of Beauveria bassiana endophyte against Botrytis cinerea in two solanaceae crops. Microorganisms. 2019;8(1):65. doi:10.3390/microorganisms8010065. [Google Scholar] [PubMed] [CrossRef]
83. Aguilera-Sammaritano J, Caballero J, Deymié M, Rosa M, Vazquez F, Pappano D, et al. Dual effects of entomopathogenic fungi on control of the pest Lobesia botrana and the pathogenic fungus Eutypella microtheca on grapevine. Biol Res. 2021;54:44. doi:10.1186/s40659-021-00367-x. [Google Scholar] [PubMed] [CrossRef]
84. Sinno M, Ranesi M, Di Lelio I, Iacomino G, Becchimanzi A, Barra E, et al. Selection of endophytic Beauveria bassiana as a dual biocontrol agent of tomato pathogens and pests. Pathogens. 2021;10:1242. doi:10.3390/pathogens10101242. [Google Scholar] [PubMed] [CrossRef]
85. Grabka R, d’Entremont TW, Adams SJ, Walker AK, Tanney JB, Abbasi PA, et al. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants. 2022;11:384. doi:10.3390/plants11030384. [Google Scholar] [PubMed] [CrossRef]
86. Pozo MJ, Baek JM, Garcia JM, Kenerley CM. Functional analysis of tvsp 1, a serineprotease-encoding gene in the biocontrol agent Trichoderma virens. Fungal Genet Biol. 2003;41(3):336–48. doi:10.1016/j.fgb.2003.11.002. [Google Scholar] [PubMed] [CrossRef]
87. Espinoza EO, Rodríguez FV, Sánchezl PD, Arteaga LESH, Sánchez JM, Tobías HMR, et al. Inoculation with entomopathogenic fungi reduces seed contamination, improves seed germination and growth of chilli seedlings. Afr J Agric Res. 2019;14(32):1463–71. doi:10.5897/AJAR2019.14061. [Google Scholar] [CrossRef]
88. Russo ML, Pelizza SA, Vianna MF, Allegrucci N, Cabello MN, Toledo AV, et al. Effect of endophytic entomopathogenic fungi on soybean Glycine max (L.) Merr. growth and yield. J King Saud Univ Sci. 2019a;31:728–36. doi:10.1016/j.jksus.2018.04.008. [Google Scholar] [CrossRef]
89. Trizelia T. The effect of seed treatment duration with entomopathogenic fungi Beauveria Bassiana on seed germination and seedling growth of chili. Indones J Crop Sci. 2020;3(1):25–9. doi:10.25077/jijcs.3.1.25-29.2020. [Google Scholar] [CrossRef]
90. Jaber LR, Enkerli J. Effect of seed treatment duration on growth and colonization of Vicia faba by endophytic Beauveria bassiana and Metarhizium brunneum. Biol Control. 2016;103:187–95. doi:10.1016/j.biocontrol.2016.09.008. [Google Scholar] [CrossRef]
91. Raya-Dıaz S, Sanchez-Rodrıguez AR, Segura-Fernandez JM, del Campillo MC, Quesada-Moraga E. Entomopathogenic fungi-based mechanisms for improved Fe nutrition in sorghum plants grown on calcareous substrates. PLoS One. 2017;12(10):e0185903. doi:10.1371/journal.pone.0185903. [Google Scholar] [PubMed] [CrossRef]
92. Russo ML, Scorsetti AC, Vianna MF, Cabello M, Ferreri N, Pelizza S. Endophytic effects of Beauveria bassiana on corn (Zea mays) and its herbivore, Rachiplusia nu (Lepidoptera: noctuidae). Insects. 2019b;10(4):110. doi:10.3390/insects10040110. [Google Scholar] [PubMed] [CrossRef]
93. Liu Y, Yang Y, Wang B. Entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae play roles of maize (Zea mays) growth promoter. Sci Rep. 2022;12:15706. doi:10.1038/s41598-022-19899-7. [Google Scholar] [PubMed] [CrossRef]
94. Felten J, Kohler A, Morin E, Bhalerao RP, Palme K, Martin F, et al. The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through transport and signaling. Plant Physiol. 2009;151(4):1991–2005. doi:10.1104/pp.109.147231. [Google Scholar] [PubMed] [CrossRef]
95. Wu L, Lv Y, Meng Z, Chen J, Guo SX. The promoting role of an isolate of dark-septate fungus on its host plant Saussure a involucrate Kar. Et Kir Mycorrhiza. 2010;20:127–35. doi:10.1007/s00572-009-0268-8. [Google Scholar] [PubMed] [CrossRef]
96. Ahmad I, Jimenez-Gasco MDM, Barbercheck ME. Water stress and black cutworm feeding modulate plant response in maize colonized by Metarhizium robertsii. Pathogens. 2024;13(7):544. doi:10.3390/pathogens13070544. [Google Scholar] [PubMed] [CrossRef]
97. Shoresh M, Harman GE, Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol. 2010;48:21–43. doi:10.1146/annurev-phyto-073009-114450. [Google Scholar] [PubMed] [CrossRef]
98. Lazim AH, Matrood AAA. The effectiveness of the Fungus Beauveria bassiana in reducing the infection of okra plant (Abelmosechuse seulentus) with fungal root diseases. J Al-Muthanna Agric Sci. 2021;8(4):83. doi:10.52113/mjas04/8.4/8. [Google Scholar] [CrossRef]
99. Sarven MS, Hao Q, Deng J, Yang F, Wang G, Xiao Y, et al. Biological control of tomato gray mold caused by Botrytis Cinerea with the entomopathogenic fungus Metarhizium anisopliae. Pathogens. 2020;9(3):213. doi:10.3390/pathogens9030213. [Google Scholar] [PubMed] [CrossRef]
100. Crespo R, Pedrini N, Juarez MP, Dal Bello GM. Volatile organic compounds released by the entomopathogenic fungus Beauveria bassiana. Microbiol Res. 2008;163:148–51. doi:10.1016/j.micres.2006.03.013. [Google Scholar] [PubMed] [CrossRef]
101. Xu Y, Orozco R, Kithsiri WEM, Espinosa-Artiles P, Leslie GAA, Patricia SS, et al. Biosynthesis of the cyclooligomer depsipeptide bassianolide, an insecticidal virulence factor of Beauveria bassiana. Fungal Genet Biol. 2009;46:353–64. doi:10.1016/j.fgb.2009.03.001. [Google Scholar] [PubMed] [CrossRef]
102. Watjen W, Debbab A, Hohlfeld A, Chovolou Y, Proksch P. The mycotoxin beauvericin induces apoptotic cell death in H4IIE hepatoma cells accompanied by an inhibition of NF-κB-activity and modulation of MAP-kinases. Toxicol Lett. 2014;231:9–16. doi:10.1016/j.toxlet.2014.08.021. [Google Scholar] [PubMed] [CrossRef]
103. Mallebrera B, Juan-Garcia A, Font G, Ruiz MJ. Mechanisms of beauvericin toxicity and antioxidant cellular defense. Toxicol Lett. 2016;246:28–34. doi:10.1016/j.toxlet.2016.01.013. [Google Scholar] [PubMed] [CrossRef]
104. Bamisile BS, SenyoAkutse K, Dash CK, Qasim M, Ramos Aguila LC, Ashraf HJ, et al. Effects of seedling age on colonization patterns of Citrus limon plants by endophytic Beauveria bassiana and Metarhizium anisopliae and their influence on seedlings growth. J Fungi. 2020;6(1):29. doi:10.3390/jof6010029. [Google Scholar] [CrossRef]
105. Bishop DG. Assessing the growth promoting characteristics, and effectiveness of selected bacteria and the entomopathogenic fungus, Beauveria bassiana, in the control of Rhizoctonia solani on tomato (MS Thesis). University of Tennessee: Knoxville, TN, USA; 1999. [Google Scholar]
106. Seth D. Effects of inoculum, cultivar and the biological control fungus Beauveria bassiana on damping-off by Rhizoctonia solani on tomato (MS Thesis). University of Tennessee: Knoxville, TN, USA; 2001. [Google Scholar]
107. Mimma AA, Akter T, Haque MA, Baki Bhuiyan MA, Chowdhury MZH, Sultana S, et al. Effect of Metarhizium anisopliae (MetA1) on growth enhancement and antioxidative defense mechanism against Rhizoctonia root rot in okra. Heliyon. 2023;9:e18978. doi:10.1016/j.heliyon.2023.e18978. [Google Scholar] [PubMed] [CrossRef]
108. Sasan RK, Bidochka MJ. Antagonism of the endophytic insect pathogenic fungus Metarhizium robertsii against the bean plant pathogen Fusarium solani f. sp. phaseoli. Can J Plant Pathol. 2013;35:288–93. doi:10.1080/07060661.2013.823114. [Google Scholar] [CrossRef]
109. Ahmad I, del Jimenez-Gasco M, Luthe DS, Barbercheck ME. Endophytic Metarhizium robertsii suppresses the phytopathogen, Cochliobolus heterostrophus and modulates maize defenses. PLoS One. 2022;17(9):e0272944. doi:10.1371/journal.pone.0272944. [Google Scholar] [PubMed] [CrossRef]
110. Behie SW, Jones SJ, Bidochka MJ. Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecol. 2015;13:112–9. doi:10.1016/j.funeco.2014.08.001. [Google Scholar] [CrossRef]
111. Steinwender MB, Enkerli J, Widmer F, Eilenberg J, Kristensen HL, Bidochka MJ. Root isolations of Metarhizium spp. from crops reflect diversity in the soil and indicate no plant specificity. J Invertebr Pathol. 2015;132:142–8. doi:10.1016/j.jip.2015.09.007. [Google Scholar] [PubMed] [CrossRef]
112. Karthiba L, Saveetha K, Suresh S, Raguchander T, Saravanakumar D, Samiyappan R. PGPR and entomopathogenic fungus bioformulation for the synchronous management of leaf folder pest and sheath blight disease of rice. Pest Manage Sci. 2010;66:555–64. doi:10.1002/ps.1907. [Google Scholar] [PubMed] [CrossRef]
113. Azadi N, Shirzad A, Mohammadi H. A study of some biocontrol mechanisms of Beauveria bassiana against Rhizoctonia disease on tomato. Acta Biol Szeged. 2016;60:119–27. [Google Scholar]
114. Ramzan M, Sana S, Javaid N, Shah AA, Ejaz S, Malik WN, et al. Mitigation of bacterial spot disease induced biotic stress in Capsicum annuum L. cultivars via antioxidant enzymes and isoforms. Sci Rep. 2021;11:9445. doi:10.1038/s41598-021-88797-1. [Google Scholar] [PubMed] [CrossRef]
115. Prabhukarthikeyan SR, Keerthana U, Archana S, Raguchander T. Induced resistance in tomato plants to Helicoverpa armigera by mixed formulation of Bacillus subtilis and Beauveria bassiana. Res J Biotechnol. 2017;12(10):53–9. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools