 Open Access
Open Access
ARTICLE
Aggressiveness Assessment of Two Fusarium spp. on Durum Wheat Grain Coleoptiles under Controlled Conditions
1 Centre de Recherche en Aménagement du Territoire (CRAT), Campus Zouaghi Slimane, Route de Ain el Bey, Constantine, 25000, Algérie
2 Ecole Nationale Supérieure Agronomique (ENSA), Alger, 16200, Algérie
3 Department of Geographical Sciences, University of Maryland, College Park, MD 20742, USA
4 Plant Production Department, College of Food and Agriculture Sciences, King Saud University, Riyadh, 11451, Saudi Arabia
5 Centre de Recherche en Agropastoralisme (CRAPast), Djelfa, 17000, Algérie
* Corresponding Author: Salah Hadjout. Email:
Phyton-International Journal of Experimental Botany 2024, 93(11), 2983-2992. https://doi.org/10.32604/phyton.2024.056982
Received 04 August 2024; Accepted 21 October 2024; Issue published 30 November 2024
Abstract
Fusarium head blight (FHB) is a disease caused by several Fusarium species, notably, F. culmorum and F. graminearum. These pathogens adversely affect the technological and sanitary qualities of cereal grains, particularly durum wheat. Under favorable environmental conditions and in susceptible varieties, these Fusarium species can significantly reduce both the quantity and quality of crops. This study evaluated the pathogenicity of the two Fusarium species (FC2006 and FG2008) in the growth of durum wheat coleoptiles. The plant material included four commercially grown parental varieties (G9, G10, G11, G12) and eight breeding lines (G1, G2, G3, G4, G5, G6, G7, G8). In vitro tests revealed that both Fusarium species significantly reduced the coleoptile growth across the studied varieties and lines (p ≤ 0.001). The control test had an average coleoptile length of 37.87 mm. In contrast, seeds inoculated with FC2006 had an average length of 0.62 mm, and those inoculated with FG2008 had only 0.064 mm. Although there was a slight difference in aggressiveness between the two species, it was not statistically significant (p > 0.05). Some variability was also noted in the responses of the durum wheat varieties and lines. The G8 genotype showed remarkable behavior in both isolates, with an average length of 1.83 mm for FC2006 and 0.4 mm for FG2008. The other genotypes showed total inhibition of coleoptile growth (0 mm). These findings highlight the importance of conducting further research on the defense mechanisms of durum wheat against Fusarium and assessing the local varieties’ pathogenicity to better explore the interactions between these pathogens and durum wheat genotypes under in vitro conditions.Keywords
Cereal crops are cultivated over an estimated 3.3 million hectares annually, with 1.5 million hectares of durum wheat and 600.000 hectares of soft wheat. The total cereal production in Algeria is approximately 4 million tons, with bread wheat contributing only 1% of the overall harvest [1]. Durum wheat (Triticum turgidum var. durum) is the most widely grown cereal crop in the Mediterranean basin, and ranks as the tenth most cultivated species globally [2]. This cereal is a main crop and staple food in certain Mediterranean regions, serving as an essential raw material for finished products such as pasta, couscous, bulgur, and various types of bread, consumed worldwide [3]. In Algeria, wheat has long been the primary dietary staple for consumers [4], and globally, it is the third most harvested crop and the most consumed grain [5].
However, wheat production is affected by severe abiotic factors, including drought, irregular rainfall, frost, extreme temperatures, and increased atmospheric CO2 levels, all of which can drastically reduce yield and grain quality [6,7]. Additionally, plant diseases caused by pathogenic fungi are prevalent and can lead to significant losses in yield, as well as a decline in grain quality [8,9]. Fusarium head blight (FHB) is one of the most destructive fungal diseases affecting durum wheat, causing yield reductions of up to 61%. FHB is characterized by flower abortion, reduction in grain number and weight, and deterioration of grain quality due to the accumulation of harmful mycotoxins in the grain [10–12]. Consequently, FHB is a major threat to cereal production worldwide with significant economic implications [10].
The Fusarium genus is recognized as one of the most pathogenic and aggressive fungal groups, comprising multiple species that infect a wide range of cultivated plants and cereals, many of which are essential for both human and animal nutrition, some of them causing FHB [10]. Key targets of Fusarium infection include leaves, roots, stems, heads, and crop residues [13,14]. In recent years, several studies have been conducted in Algeria to identify specific Fusarium species involved in FHB in wheat [15–17]. Using morphological and molecular techniques, these studies have confirmed that F. culmorum is the dominant species in durum wheat crops affected by FHB in the region [15,17,18]. Additionally, F. graminearum and F. pseudograminearum, have been identified, with F. cerealis being reported for the first time in Algeria [17]. Globally, F. graminearum and F. culmorum are the two most prevalent species responsible for root, stem, and ear rots, as well as seedling blight in small-grain cereals, including durum wheat [17,19,20].
The epidemiological cycle of Fusarium begins with the survival of the inoculum in the soil or on plant debris, where it persists as a saprophytic mycelium or thick-walled resting spores. When crops are sown in contaminated soils, diseases such as foot rot and seedling blight, can develop. During flowering, fungal infections can spread to wheat heads through conidia dispersed by rain splash or ascospores carried by the wind [21]. Fusarium-contaminated seeds further Cereal crops are cultivated over an estimated 3.3 million hectares annually, with 1.5 million hectares of durum wheat and 600.000 hectares of soft wheat. The total cereal production in Algeria is approximately 4 million tons, with bread wheat contributing only 1% of the overall harvest [1]. Durum wheat (Triticum turgidum var. durum) is the most widely grown cereal crop in the Mediterranean basin, and ranks as the tenth most cultivated species globally [2]. This cereal is a main crop and staple food in certain Mediterranean regions, serving as an essential raw material for finished products such as pasta, couscous, bulgur, and various types of bread, consumed worldwide [3]. In Algeria, wheat has long been the primary dietary staple for consumers [4], and globally, it is the third most harvested crop and the most consumed grain [5].
However, wheat production is affected by severe abiotic factors, including drought, irregular rainfall, frost, extreme temperatures, and increased atmospheric CO2 levels, all of which can drastically reduce yield and grain quality [6,7]. Additionally, plant diseases caused by pathogenic fungi are prevalent and can lead to significant losses in yield, as well as a decline in grain quality [8,9]. Fusarium head blight (FHB) is one of the most destructive fungal diseases affecting durum wheat, causing yield reductions of up to 61%. FHB is characterized by flower abortion, reduction in grain number and weight, and deterioration of grain quality due to the accumulation of harmful mycotoxins in the grain [10–12]. Consequently, FHB is a major threat to cereal production worldwide with significant economic implications [10].
The Fusarium genus is recognized as one of the most pathogenic and aggressive fungal groups, comprising multiple species that infect a wide range of cultivated plants and cereals, many of which are essential for both human and animal nutrition, some of them causing FHB [10]. Key targets of Fusarium infection include leaves, roots, stems, heads, and crop residues [13,14]. In recent years, several studies have been conducted in Algeria to identify specific Fusarium species involved in FHB in wheat [15–17]. Using morphological and molecular techniques, these studies have confirmed that F. culmorum is the dominant species in durum wheat crops affected by FHB in the region [15,17,18]. Additionally, F. graminearum and F. pseudograminearum, have been identified, with F. cerealis being reported for the first time in Algeria [17]. Globally, F. graminearum and F. culmorum are the two most prevalent species responsible for root, stem, and ear rots, as well as seedling blight in small-grain cereals, including durum wheat [17–20].
The epidemiological cycle of Fusarium begins with the survival of the inoculum in the soil or on plant debris, where it persists as a saprophytic mycelium or thick-walled resting spores. When crops are sown in contaminated soils, diseases such as foot rot and seedling blight can develop. During flowering, fungal infections can spread to wheat heads through conidia dispersed by rain splash or ascospores carried by the wind [21]. The use of Fusarium-contaminated seeds and further exacerbates the disease cycle by providing the primary inoculum for the development of FHB and foot rot [22]. The presence of Fusarium in seed lots can significantly reduce the germination capacity, resulting in lower emergence rates and seedling blight after germination [23]. Even seeds with healthy, viable embryos may fail to germinate or produce normal seedlings owing to fungal contamination during the germination process [21]. Previous studies have demonstrated that high Fusarium aggressiveness is associated with a marked reduction in germination rates, elevated Area Under Disease Progression Curve (AUDPC) values, and a significant decrease in coleoptile length [24].
The present study aimed to evaluate the pathogenicity of Fusarium culmorum and Fusarium graminearum isolates on durum wheat coleoptile growth while identifying response variations between different varieties and lines. Objectives include measuring the impact of isolates on coleoptile length, comparing the aggressiveness of the two species on the tested genotypes, and analyzing the variability of genotype responses to identify those with increased tolerance or susceptibility. Finally, this research aims to provide useful data for future studies on the management of fusarium head blight under controlled conditions.
In this study, we used four parental durum wheat varieties: Ardente, Waha, Simeto, and Vitron (Table 1). Additionally, we included eight breeding lines derived from F15 seeds, which were produced through diallel crosses involving five parental varieties: Ardente, Waha, Simeto, Vitron, and Saadi [25]. The seeds of these lines, used as experimental material, were obtained in June 2011 from plots managed by the Higher National School of Agronomy (ENSA), within the Plant Production Department.
The pathogenic fungal material that was subjected to in vitro test consisted of two Fusarium isolates, FG2008 and FC2006, belonging to F. graminearum and F. culmorum species, respectively. These isolates were identified using a combination of morphological observations of mycelia growth on Potato Dextrose Agar (PDA) and microscopic examination of conidia, following the method described by Leslie et al. [26]. Species identification was further confirmed by PCR, using species-specific primers [15,17].
Both isolates were sourced from the mycological collection of the ENSA in El-Harrach, Algiers. The FG2008 isolate was obtained from soft wheat spikelets collected at the ITGC experimental station (Technical Institute of Field Crops) in Algiers, in May 2008. The FC2006 isolate was derived from soft wheat spikelets harvested at the ENSA experimental station in El-Harrach, 2006. Both isolates were obtained from wheat ears displaying typical symptoms of FHB, such as pink to orange discoloration, indicative of significant spore mass formation.
2.3 In Vitro Pathogenicity Test on Durum Wheat Grains
The pathogenicity of the fungal isolates was evaluated according to a modified version of the protocol established by Mesterhazy [27] using PDA medium. Conidia, represented by mycelium post-conidial germination, were used to prepare the inocula. Conidial concentration and purity were controlled to ensure a consistent amount of inoculum for both species [9]. Mycelial mass was quantified using the method outlined by Brennan et al. [28].
To produce mycelia, 50 mL of Potato Dextrose Broth (PDB) was prepared. For each isolate, four 5-mm mycelial portions were obtained from 7-day-old cultures on PDA medium. These explants were added to PDB and incubated at 20°C on an orbital shaker at 250 rpm for seven days. After incubation, mycelia were harvested by centrifugation at 5000 × G for 10 min, homogenized, and diluted to a concentration of 13.3 mg/mL in a solution containing 0.2% Tween 20.
Petri dishes (85 mm in diameter) containing the PDA medium were prepared. A sterilized filter paper disk with the same diameter was placed on each PDA medium. Homogenized mycelia, diluted in 0.2% Tween 20, were applied evenly at a rate of 8 mL per dish. A second sterile filter paper disk was placed on the top of the inoculum. For each sample, 10 durum wheat grains were disinfected using 2% sodium hypochlorite for 10 min, rinsed thrice with sterile distilled water, and dried between two sterile blotting papers. These pre-disinfected grains were placed in Petri dishes. Plates containing grains inoculated with each Fusarium isolate were incubated in the dark at 25°C using a randomized design, with three replicates per isolate. Control plates were subjected to identical conditions, except that sterile distilled water was used instead of mycelial inoculum.
The growth of durum wheat coleoptiles was measured on the fourth day of incubation, and the results were expressed as differences in growth compared to the non-inoculated control.
Statistical analysis of the results was performed using Statgraphics software version 15.1.0. Multiple comparisons of means were conducted using the Least Significant Difference (LSD) test at a 5% significance level to determine homogeneous groups.
To assess the impact of the two Fusarium isolates, FC2006 from Fusarium culmorum and FG2008 from Fusarium graminearum, on the growth of coleoptiles in 12 durum wheat genotypes, we developed an in vitro evaluation method under controlled laboratory conditions. This experimental approach enabled the precise measurement of the effects of the two isolates on coleoptile growth across different durum wheat varieties and breeding lines. This method provided a clear basis for comparing the differences in coleoptile growth after artificial inoculation with the two Fusarium species.
Coleoptile length was measured in millimeters after a 4-day incubation period at 25°C in the dark (Fig. 1). To determine the level of aggressiveness of each Fusarium isolate on the different genotypes, coleoptile growth was compared with that of the uninoculated controls. The degree of reduction in coleoptile length was used to quantify the severity of the infection caused by the isolates.

Figure 1: Inhibition of seed germination by both Fusarium isolates after 4 days of incubation at 25°C and in darkness, illustrated with some representative images
As presented in Fig. 1, coleoptile length was significantly reduced in grains inoculated with either of the Fusarium isolates compared to uninoculated controls. This reduction in growth highlights the inhibitory effect of the isolates on durum wheat coleoptiles, consistent with the known pathogenic characteristics of Fusarium. The observed inhibition is indicative of diseases, such as grain rot, which are common in infected plants and can lead to substantial economic losses in cereal production.
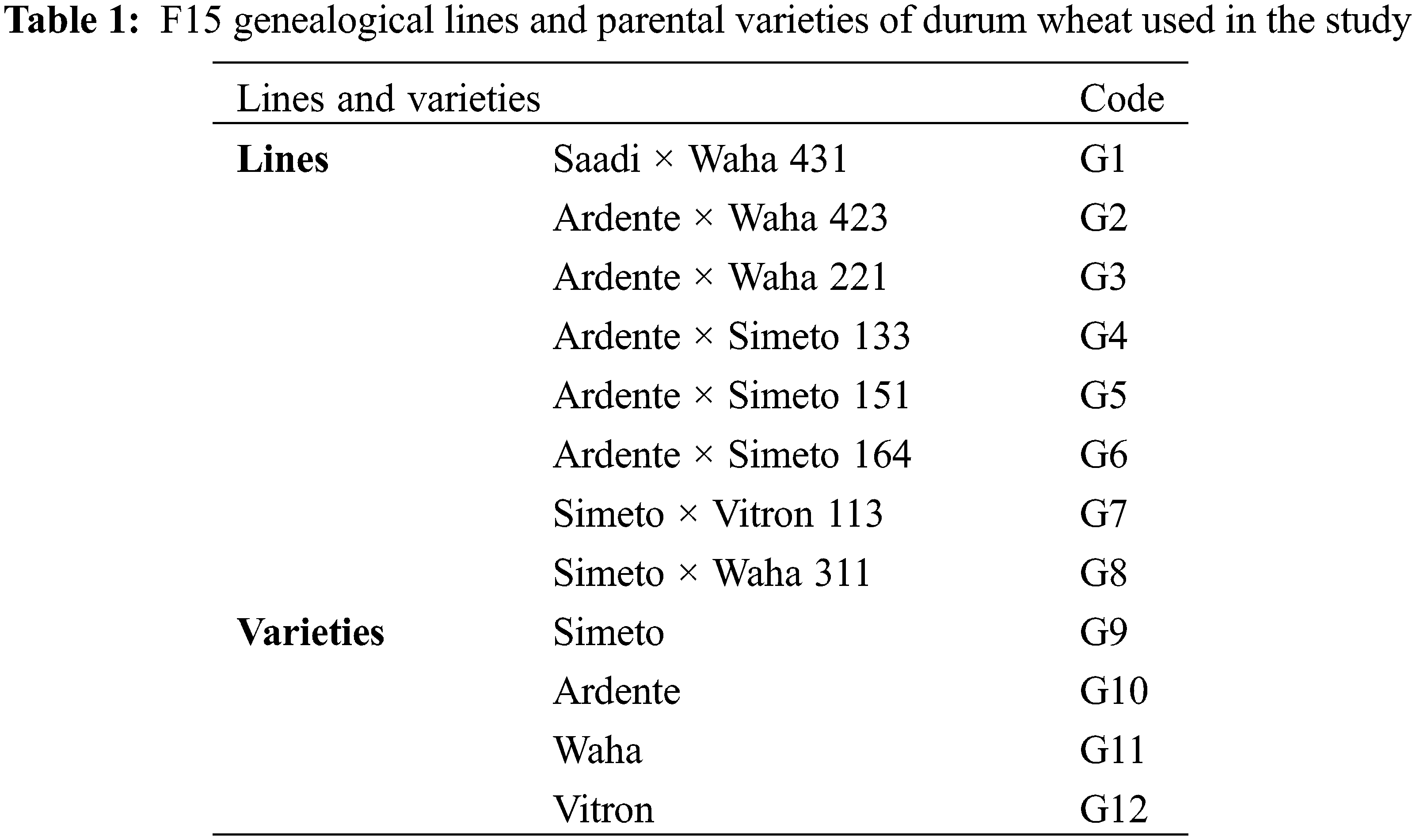
Statistical analysis of variance revealed that the effects of genotypes, treatments, and the interaction between genotypes and treatments on coleoptile length were highly significant (p ≤ 0.001). To further compare the mean coleoptile lengths across the three treatments (controls, genotypes inoculated with FC2006, and genotypes inoculated with FG2008), we conducted an LSD test. The test identified three homogeneous groups, with the control group having the longest average coleoptile length of 37.87 mm. By contrast, seeds inoculated with the FC2006 isolate had an average coleoptile length of 0.62 mm, while those inoculated with FG2008 exhibited the shortest average coleoptile length of 0.064 mm (Fig. 2).
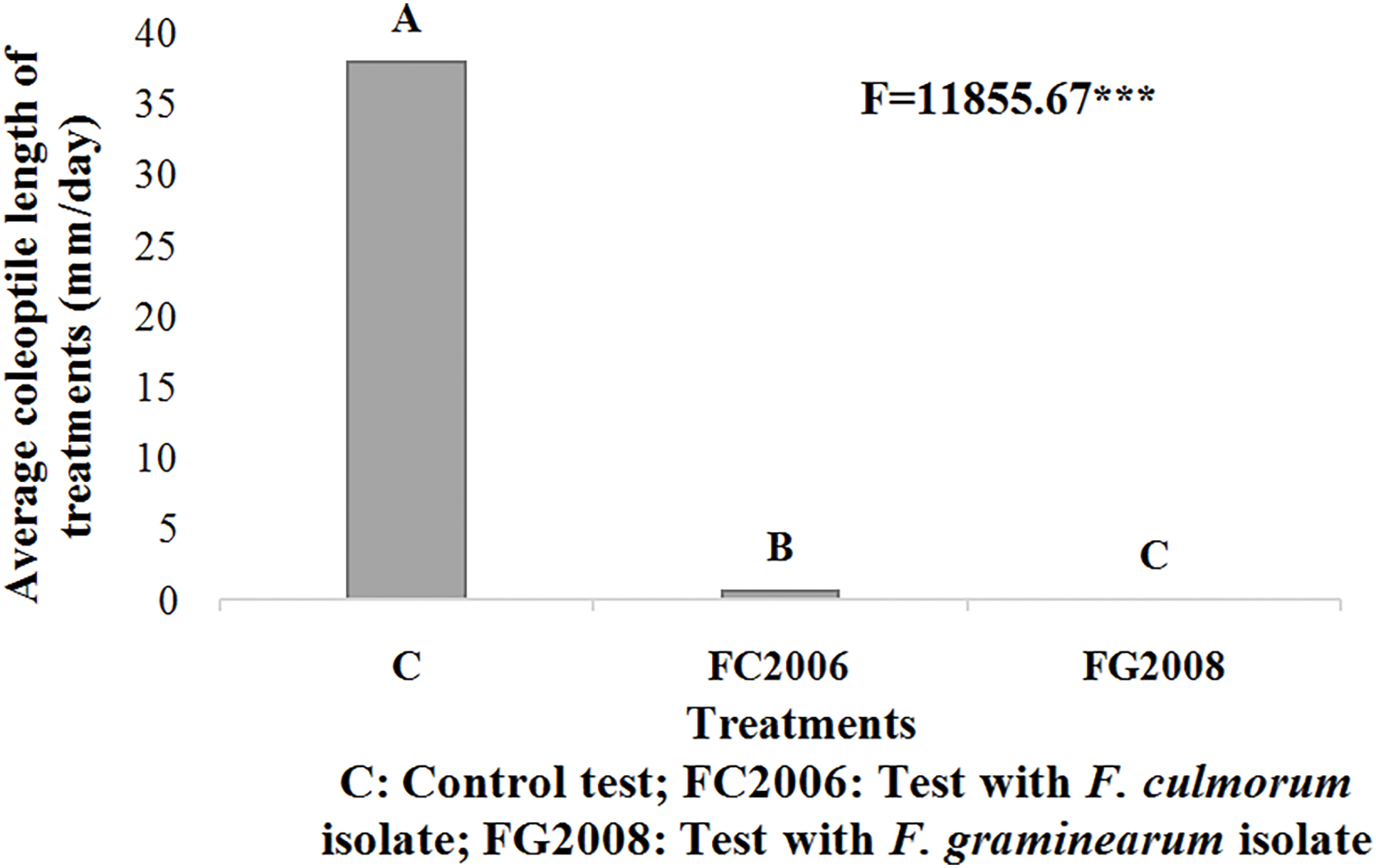
Figure 2: Mean coleoptile length of the three treatments after 4 days of incubation at 25°C and in the dark
The interaction between genotypes and treatments, analyzed using the LSD test, distinguished 10 homogeneous groups (Fig. 3). The results indicated that the coleoptile growth in all genotypes inoculated with Fusarium isolates differed significantly from that of the uninoculated controls (p ≤ 0.001). In the control group, genotype G4 exhibited the longest coleoptile length at 44.50 mm, whereas G1 recorded the shortest length at 32.17 mm after 4 days of incubation.
Figure 3: Effect of three treatments on the average coleoptile length after 4 days of incubation at 25°C and in darkness
Among the genotypes inoculated with FC2006, genotype G8 displayed the longest average coleoptile length of 1.83 mm, while genotypes G1, G5, G7, and G11 showed complete inhibition of coleoptile growth, with an average length of 0 mm. Similarly, for the genotypes inoculated with FG2008, genotype G8 again had the highest average coleoptile growth, measuring 0.4 mm, while the other genotypes (G1, G2, G3, G4, G5, G6, G7, G9, G10, and G11) all exhibited total inhibition of coleoptile growth with an average length of 0 mm (Fig. 3). Interestingly, these results revealed that several genotypes exhibited complete inhibition of coleoptile growth following inoculation with both FC2006 and FG2008 isolates.
Coleoptiles are conical structures that emerge from seeds and develop into leaves when exposed to light and moisture, making their length a critical indicator for early growth, wheat breeding, and plant development [29,30]. In this study, inoculation of durum wheat grains with Fusarium isolates was aimed at evaluating the aggressiveness of the fungi in inhibiting coleoptile growth in different durum wheat varieties andbreeding lines. The observed growth inhibition confirms that both F. culmorum and F. graminearum fungi have detrimental effects on seed germination and subsequent coleoptile development. Notably, a slight difference in aggressiveness was detected between the two isolates, with F. graminearum (FG2008) exhibiting slightly higher aggressiveness than F. culmorum (FC2006).
Our findings align with those of previous research, which identified F. culmorum and F. graminearum as among the most aggressive pathogens affecting the coleoptile growth of wheat and barley [28,31]. Reduced germination rates and shorter coleoptile lengths are commonly used as key indicators of fungal aggressiveness, particularly in F. graminearum [24]. Brennan et al. [28] similarly reportedreport that both F. culmorum and F. graminearum exhibit optimal pathogenicity at temperatures between 20°C and 25°C, reducing coleoptile growth by over 89.3% compared to uninoculated controls. Their study further concluded that F. graminearum is a more aggressive species, reducing coleoptile growth by as much as 96% at 25°C, which is consistent with the findings of the present study.
Additional studies have reported that F. culmorum can reduce coleoptile length by up to 91.32%, whereas F. graminearum can cause a 78.32% reduction in growth [31]. Furthermore, in vitro seed inoculation test revealed that F. culmorum isolates can be highly aggressive, with some strains completely inhibiting coleoptile growth [16]. This extreme sensitivity to the initial infection was especially pronounced in durum wheat genotypes, where F. culmorum caused a significant reduction in germination rates. For example, the “Hogar” durum wheat variety experienced a 52.17% reduction in germination after just three days of inoculation [32].
Therefore, both F. culmorum and F. graminearum are regarded as highly aggressive pathogens, although their relative degrees of aggressiveness may vary depending on several factors. These include the type of host plant, environmental conditions, interactions with other microorganisms, and the genetic characteristics of the specific fungal isolates.
Other studies reported other Fusarium species (F. equiseti, F. chlamydosporum, F. poae, Microdochium nivale, F. verticillioides and F. solani) having negative effect on seed germination and coleoptile length in durum wheat, but with coleoptile length reduction variable depending on the fungal species and isolates [12,28,33,34].
This study examined the effects of two Fusarium isolates, F. culmorum (FC2006) and F. graminearum (FG2008), on coleoptile growth in different durum wheat genotypes. The results demonstrated that both isolates significantly inhibited coleoptile development across all genotypes tested, with wheat coleoptiles exposed to the fungi exhibiting markedly reduced growth compared to the controls. Moreover, there was a slight but notable difference in aggressiveness between the two isolates, with F. graminearum showing a marginally stronger inhibitory effect on coleoptile growth than F. culmorum.
These findings offer valuable insights for farmers and breeders in selecting wheat varieties that are more resistant to Fusarium infections. It is possible to mitigate the effect of Fusarium on crop yields by identifying varieties with increased resistance to these aggressive fungal species, ultimately enhancing the sustainability of wheat production in regions vulnerable to these pathogens.
Acknowledgement: The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R390), King Saud University, Riyadh, Saudi Arabia, and to la Direction Générale de la Recherche Scientifique et du Développement Technologique (DGRSDT), Algeria.
Funding Statement: This Research was funded by la Direction Générale de la Recherche Scientifique et du Développement Technologique (DGRSDT), Algeria, and the Researchers Supporting Project number (RSP2024R390), King Saud University, Riyadh, Saudi Arabia.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Salah Hadjout, Fathi Abdellatif Belhouadjeb, Houcine Bougrine, Abdeldjalil Belkendil; data collection: Salah Hadjout, Mohamed Zouidi, Amer Zeghmar; analysis and interpretation of results: Salah Hadjout, Mohamed Zouidi, Walid Ouaret; draft manuscript preparation: Salah Hadjout, Mohamed Zouidi, Fathi Abdellatif Belhouadjeb, Walid Soufan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Besaoud O, Pellissier JP, Rolland JP, Khechimi W. Rapport de synthèse sur l’agriculture en Algérie (Ph.D. Thesis). CIHEAM-IAMM de Montepellier: France; 2019. [Google Scholar]
2. Chaouachi L, Marín-Sanz M, Barro F, Karmous C. Genetic diversity of durum wheat (Triticum turgidum ssp. durum) to mitigate abiotic stress: drought, heat, and their combination. PLoS One. 2024;19:e0301018. doi:10.1371/journal.pone.0301018 [Google Scholar] [PubMed] [CrossRef]
3. Blanco A. Structure and trends of worldwide research on durum wheat by bibliographic mapping. Inter J Plant Biol. 2024;15(1):132–60. doi:10.3390/ijpb15010012. [Google Scholar] [CrossRef]
4. Bekkis S, Benmehaia MA, Kaci A. Les enjeux de la dépendance de la filière de blé en Algérie: analyse par asymétries de réponses de l’offre dans la chaîne de valeur. N Medit, A Medit J Eco, Agri and Env. 2022;21(1):133–47. doi:10.30682/nm2201h. [Google Scholar] [CrossRef]
5. Nedjah I. Changements physiologiques chez des plantes de blé dur (Triticum durum Desf.) exposées à une pollution par un métal lourd (plomb) (Ph.D. Thesis). Université Badji Mokhtar: Algerie; 2015. [Google Scholar]
6. Kalaycı M, Küçüközdemir Ü. Impact of abiotic stresses on wheat growth and adaptation. In: Advances in wheat breeding: Towards climate resilience and nutrient security. Springer; 2024. p. 183–313. doi:10.1007/978-981-99-9478-6_4. [Google Scholar] [CrossRef]
7. Nyaupane S, Poudel MR, Panthi B, Dhakal A, Paudel H, Bhandari R. Drought stress effect, tolerance, and management in wheat–a review. Cog Food Agri. 2024;10(1):2296094. doi:10.1080/23311932.2023.2296094. [Google Scholar] [CrossRef]
8. Haile JK, N’Diaye A, Walkowiak S, Nilsen KT, Clarke JM, Kutcher HR, et al. Fusarium head blight in durum wheat: recent status, breeding directions, and future research prospects. Phytopath. 2019;109(10):1664–75. doi:10.1094/PHYTO-03-19-0095-RVW [Google Scholar] [PubMed] [CrossRef]
9. Hadjout S, Zouidi M. Morphological and cultural characterization of two Fusarium isolates causing wheat fusarium head blight in Algeria. Not Scien Biol. 2022;14:11318. doi:10.55779/nsb14411318. [Google Scholar] [CrossRef]
10. Shikur Gebremariam E, Sharma-Poudyal D, Paulitz TC, Erginbas-Orakci G, Karakaya AZİZ, Dababat AA. Identity and pathogenicity of Fusarium species associated with crown rot on wheat (Triticum spp.) in Turkey. Eur J Plant Patho. 2018;150(2):387–99. doi:10.1007/s10658-017-1285-7. [Google Scholar] [CrossRef]
11. Beccari G, Senatore MT, Tini F, Sulyok M, Covarelli L. Fungal community, Fusarium head blight complex and secondary metabolites associated with malting barley grains harvested in Umbria, central Italy. Inter J Food Microb. 2018;273:33–42. doi:10.1016/j.ijfoodmicro.2018.03.005 [Google Scholar] [PubMed] [CrossRef]
12. Bencheikh A, Rouag N, Mamache W, Belabed I. First report of Fusarium equiseti causing crown rot and damping-off on durum wheat in Algeria. Arch Phytopath Plant Protect. 2020;53:915–31. doi:10.1080/03235408.2020.1804303. [Google Scholar] [CrossRef]
13. Karlsson I, Persson P, Friberg H. Fusarium head blight from a microbiome perspective. Front Microb. 2021;12:628373. doi:10.3389/fmicb.2021.628373 [Google Scholar] [PubMed] [CrossRef]
14. Askun T. Introductory chapter: Fusarium: pathogenicity, infections, diseases, mycotoxins, and management. In: Fusarium–plant diseases, pathogen diversity, genetic diversity, resistance and molecular markers. UK: IntechOpen; 2018. doi:10.5772/intechopen.76507. [Google Scholar] [CrossRef]
15. Touati-Hattab S, Barreau C, Verdal-Bonnin MN, Chereau S, Richard-Forget F, Hadjout S, et al. Pathogenicity and trichothecenes production of Fusarium culmorum strains causing head blight on wheat and evaluation of resistance of the varieties cultivated in Algeria. Eur J Plant Pathol. 2016;145:797–814. doi:10.1007/s10658-016-0869. [Google Scholar] [CrossRef]
16. Abdallah-Nekache N, Laraba I, Ducos C, Barreau C, Bouznad Z, Boureghda H. Occurrence of fusarium head blight and fusarium crown rot in Algerian wheat: identification of associated species and assessment of aggressiveness. Euro J Plant Pathol. 2019;154(3):499–512. doi:10.1007/s10658-019-01673-7. [Google Scholar] [CrossRef]
17. Hadjout S, Chéreau S, Mekliche L, Marchegay G, Ducos C, Boureghda H, et al. Molecular identification of some Fusarium isolates and their chemotypes involved in fusarium head blight on durum wheat in Algeria. Arch Phytopatho Plant Prot. 2022b;55(4):499–513. doi:10.1080/03235408.2022.2034363. [Google Scholar] [CrossRef]
18. Laraba I, Boureghda H, Abdallah N, Bouaicha O, Obanor F, Moretti A, et al. Population genetic structure and mycotoxin potential of the wheat crown rot and head blight pathogen Fusarium culmorum in Algeria. Fun Gen Biol. 2017;103:34–41. doi:10.1016/j.fgb.2017.04.001 [Google Scholar] [PubMed] [CrossRef]
19. Nicol JM, Elekçioğlu IH, Bolat N, Rivoal R. The global importance of the cereal cyst nematode (Heterodera spp.) on wheat and international approaches to its control. Com Agri Appl Biol Sci. 2007;72:677–86. [Google Scholar]
20. Tunali B, Obanor F, Erginbaş G, Westecott RA, Nicol J, Chakraborty S. Fitness of three Fusarium pathogens of wheat. FEMS Microb Ecol. 2012;81:596–609. doi:10.1111/j.1574-6941.2012.01388.x [Google Scholar] [PubMed] [CrossRef]
21. Tekle S, Skinnes H, Bjørnstad Å. The germination problem of oat seed lots affected by Fusarium head blight. Eur J Plant Patho. 2013;135:147–158. doi:10.1007/s10658-012-0074-6. [Google Scholar] [CrossRef]
22. Parry DW, Jenkinson P, McLeod L. Fusarium ear blight (scab) in small grain cereals—a review. Plant Patho. 1995;44:207–38. doi:10.1111/j.1365-3059.1995.tb02773.x. [Google Scholar] [CrossRef]
23. Jones RK. Seedling blight development and control in spring wheat damaged by Fusarium graminearum group 2. Plant Dis. 1999;83(11):1013–8. doi:10.1094/PDIS.1999.83.11.1013 [Google Scholar] [PubMed] [CrossRef]
24. Purahong W, Alkadri D, Nipoti P, Pisi A, Lemmens M, Prodi A. Validation of a modified Petri-dish test to quantify aggressiveness of Fusarium graminearum in durum wheat. Eur J Plant Patho. 2012;132:381–91. doi:10.1007/s10658-011-9883-2. [Google Scholar] [CrossRef]
25. Mekliche A, Dahlia F, Hanifi-Mekliche L. Agromorphological diversity and stability of durum wheat lines (Triticum durum Desf.) in Algeria. Acta Agro Hung. 2013;61:149–59. doi:10.1556/AAgr.61.2013.2.6. [Google Scholar] [CrossRef]
26. Leslie JF, Summerell BA. Morphological and molecular identification of fusarium head blight isolates from wheat in the north of Iran. Aust J Crop Sci. 2006;6:1356–61. [Google Scholar]
27. Mesterhazy A. Breeding wheat for resistance to Fusarium graminearum and Fusarium culmorum. Zeit für Pflanzen. 1983;91:295–311. [Google Scholar]
28. Brennan JM, Fagan B, Van Maanen A, Cooke BM, Doohan FM. Studies on in vitro growth and pathogenicity of European Fusarium fungi. Eur J Plant Patho. 2003;109:577–87. doi:10.1023/A:1024712415326. [Google Scholar] [CrossRef]
29. Pucciariello C. Molecular mechanisms supporting rice germination and coleoptile elongation under low oxygen. Plants. 2020;9:1037. doi:10.3390/plants9081037 [Google Scholar] [PubMed] [CrossRef]
30. Wei N, Zhang S, Liu Y, Wang J, Wu B, Zhao J, et al. Genome-wide association study of coleoptile length with Shanxi wheat. Front Plant Sci. 2022;13:188. doi:10.3389/fpls.2022.1016551 [Google Scholar] [PubMed] [CrossRef]
31. Hudec K. Pathogenicity of fungi associated with wheat and barley seedling emergence and fungicide efficacy of seed treatment. Biologia. 2007;62(3):287–91. doi:10.2478/s11756-007-0050-3. [Google Scholar] [CrossRef]
32. Bouanaka H, Bellil I, Khelifi D. Multiple methods for varietal resistance assessment of durum wheat cultivars against Fusarium culmorum the causal agent of fusarium head blight and crown rot in Algeria. Physio Mol Plant Patha. 2021;115(6):101683. doi:10.1016/j.pmpp.2021.101683. [Google Scholar] [CrossRef]
33. Bencheikh A, Rouag N, Boutalbi W, Belabed I. First report of Fusarium chlamydosporum causing crown rot and dumping off on durum wheat in Algeria. J Nov Res Plant Protect. 2018;9:309–24. [Google Scholar]
34. Sakr N. Intra- and inter-species variability of the aggressiveness in four fusarium head blight species on durum wheat plants detected in an in vitro Petri-dish assay. Arch Phytopat Plant Protect. 2018;51:814–23. doi:10.1080/03235408.2018.1495390. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools