 Open Access
Open Access
REVIEW
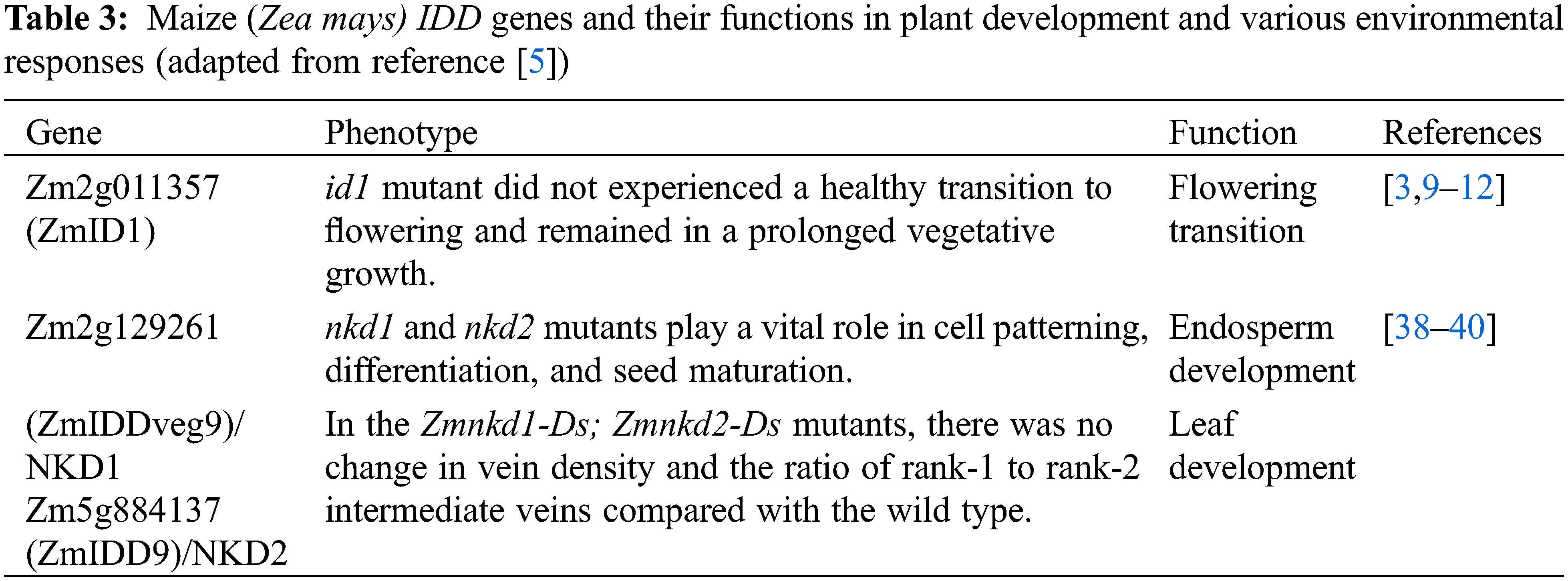
The IDD Transcription Factors: Their Functions in Plant Development and Environmental Response
1 School of Life Sciences, Qilu Normal University, Jinan, 250200, China
2 School of Life Sciences, Shandong Normal University, Jinan, 250014, China
* Corresponding Authors: Jing Liu. Email: ; Dayong Cui. Email:
Phyton-International Journal of Experimental Botany 2024, 93(1), 63-79. https://doi.org/10.32604/phyton.2023.045940
Received 12 September 2023; Accepted 21 November 2023; Issue published 26 January 2024
Abstract
INDETERMINATE-DOMAIN proteins (IDDs) are a plant-specific transcription factor family characterized by a conserved ID domain with four zinc finger motifs. Previous studies have demonstrated that IDDs coordinate a diversity of physiological processes and functions in plant growth and development, including floral transition, plant architecture, seed and root development, and hormone signaling. In this review, we especially summarized the latest knowledge on the functions and working models of IDD members in Arabidopsis, rice, and maize, particularly focusing on their role in the regulatory network of biotic and abiotic environmental responses, such as gravity, temperature, water, and pathogens. Understanding these mechanisms underlying the function of IDD proteins in these processes is important for improving crop yields by manipulating their activity. Overall, the review offers valuable insights into the functions and mechanisms of IDD proteins in plants, providing a foundation for further research and potential applications in agriculture.Keywords
Transcription factors (TFs) have played an important role in plant development and response to various environmental changes. Transcription factors recognize and bind to specific DNA sequences (cis-acting elements), thus activating or inhibiting the expression of target genes [1]. Cys2His2 (C2H2) zinc-finger structure transcription factors are one of the largest transcription factor families [2]. The plant-specific INDETERMINATE DOMAIN (IDD) family belongs to the subfamily of C2H2 transcription factors and has been identified by its DNA-binding domain, also named the INDETERMINATE (ID) domain [3]. The ID domain includes C2H2 and C2HC zinc-finger domains and is highly conserved at the N-terminal of proteins [4,5] (Fig. 1). Recently, many functions of IDD genes have been reported, especially in Arabidopsis thaliana, but also in the Zea mays (maize) and Oryza sativa (rice) [5–8] (Fig. 2). In this paper, we reviewed the recent advances in the biological functions and mechanism of IDD gene, especially the roles of IDD in plant development and various environmental response.
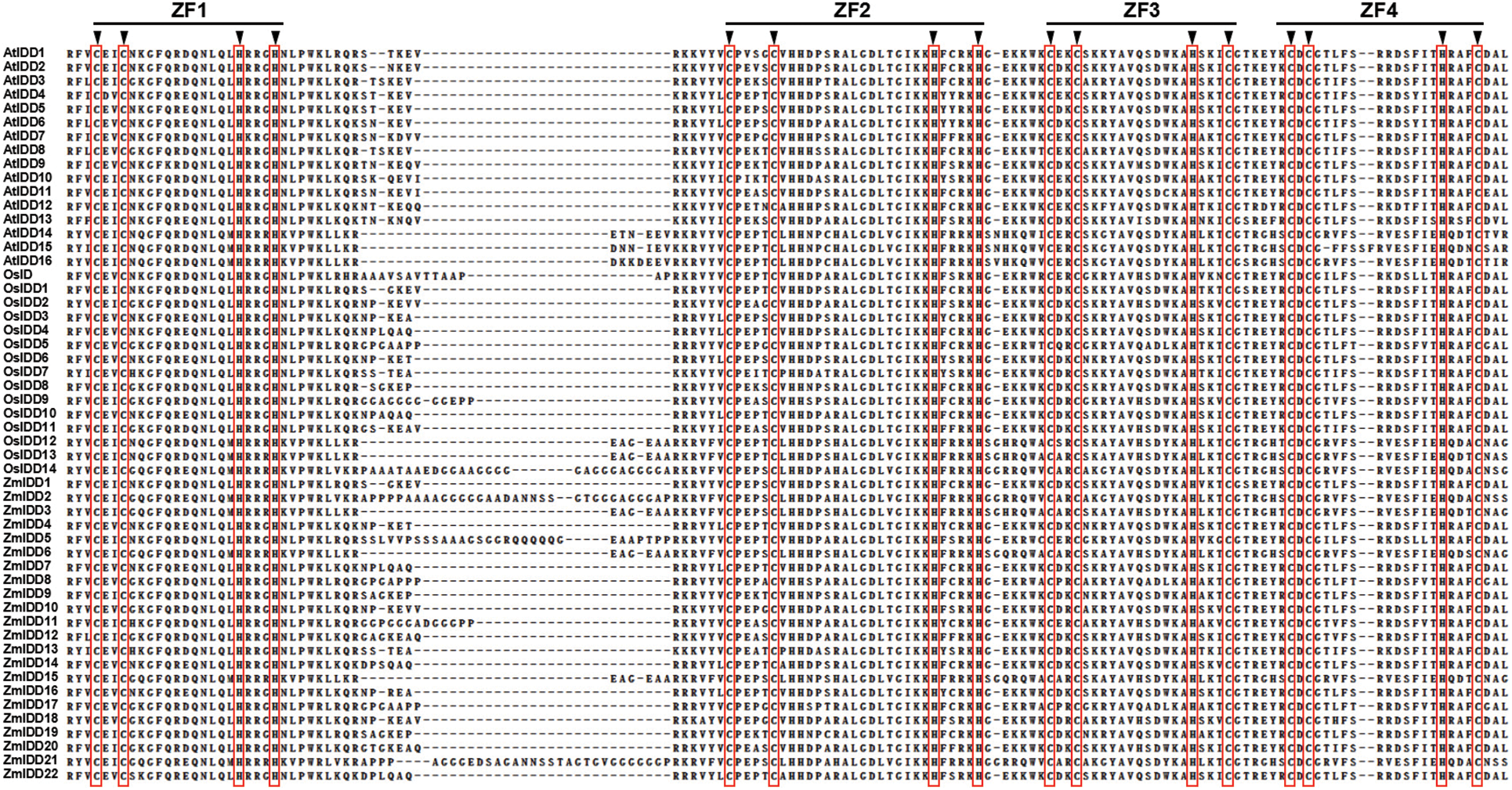
Figure 1: Alignment of INDETERMINATE DOMAIN (IDD) domains conserved amino acid sequence in different species (adapted from reference [5]), including Arabidopsis thaliana (AtIDD), Oryza sativa (OsIDD), and Zea mays (ZmIDD). ZF1-ZF4 represents the four C2H2-type zinc finger motifs. The arrowheads indicate the conserved cysteine and histidine residues
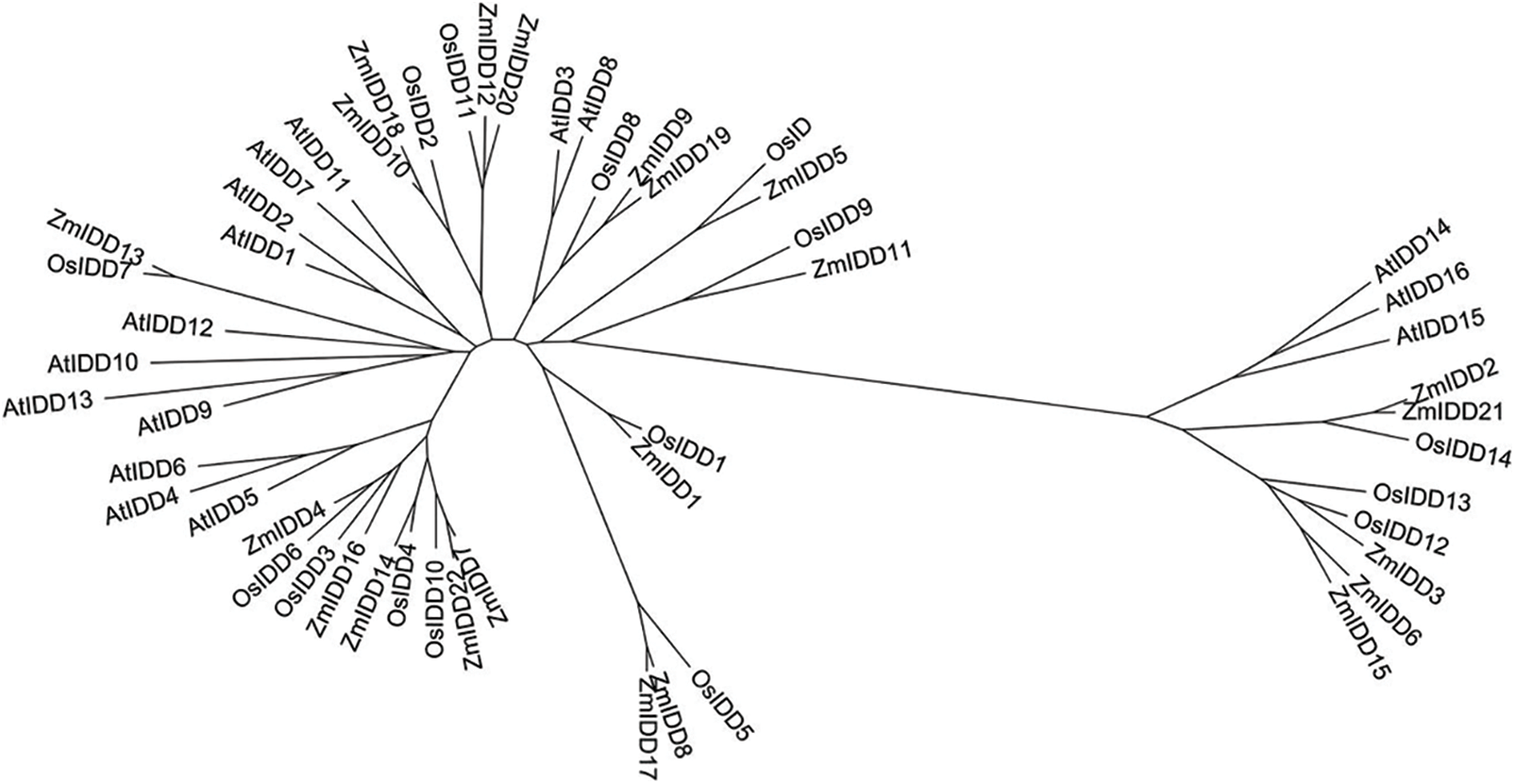
Figure 2: Phylogenetic tree of full-length sequences AtIDD, OsIDD, and ZmIDD proteins. The phylogenetic tree was drawn using the MEGA version 5.1 software
2 Functions in Plant Development
The transition from the vegetative stage to the reproductive stage is a key development change in the plant life cycle. The IDD family proteins are highly conserved in angiosperms [4]. Since the first IDD family gene (ZmID1) was isolated from maize and identified as a causal gene for late-flowering [3,9], the functions of this family have been reported on flowering time regulation first. The production of a mobile florigenic (F) signal was proposed to move to the shoot apex, which is controlled by ZmID1 [10–12]. Further studies showed that Zea mays CENTRORADIALIS 8 (ZCN8) acts as the mobile signal to function downstream of ZmID1 [13]. It is unclear how ZmID1 regulates the transcription of the ZCN8 gene. ZCN8 is unlikely to be the direct target of ZmID1 because there are no obvious ID1 binding sites in ZCN8 promoter regions [9]. Thus, ZmID1 probably regulates the expression of other transcription factors to activate the transcription of ZCN8 (Fig. 3).
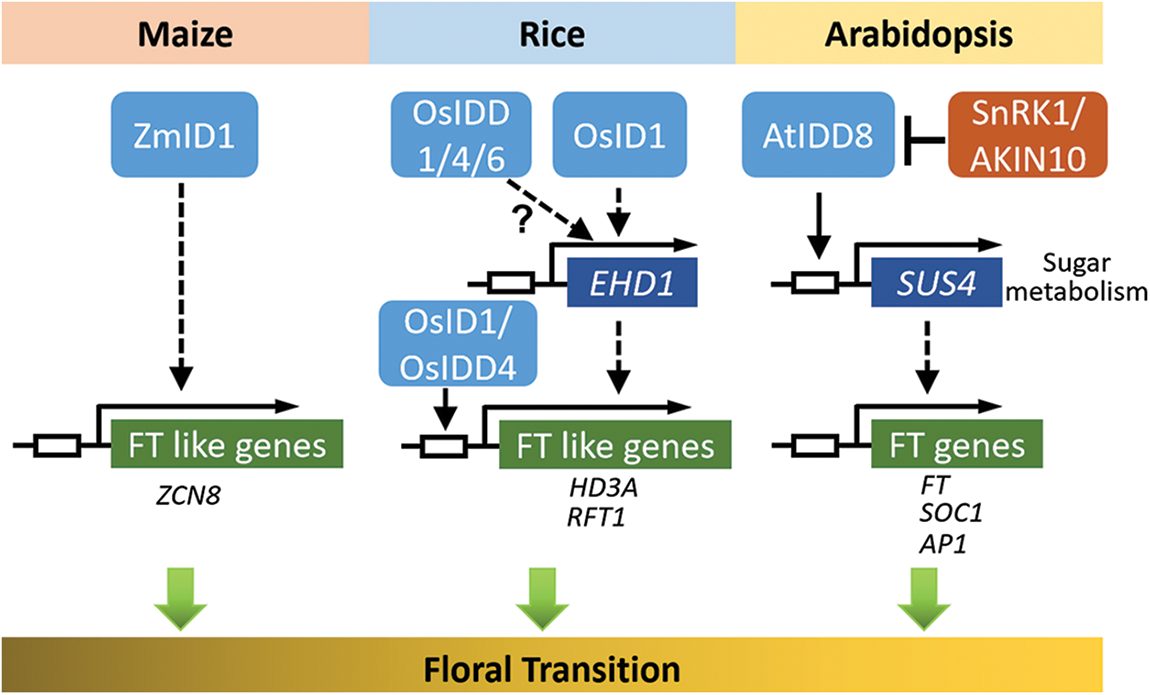
Figure 3: Schematic diagram of IDD members that may have participated in the floral transition of maize, rice, and Arabidopsis thaliana (adapted from reference [5]). The white box represents the region of the promoter. OsID1 and OsIDD4 could specially bind to the consensus motif TTTGTC in the promoter regions of Hd3a or RFT1. AtIDD8 binds directly to the SUS4 gene promoter containing the conserved CTTTTGTCC motif. The arrows and T-shaped lines represent positive and negative regulation, respectively. Solid arrows indicate direct activation and dashed arrows indicate indirect activation
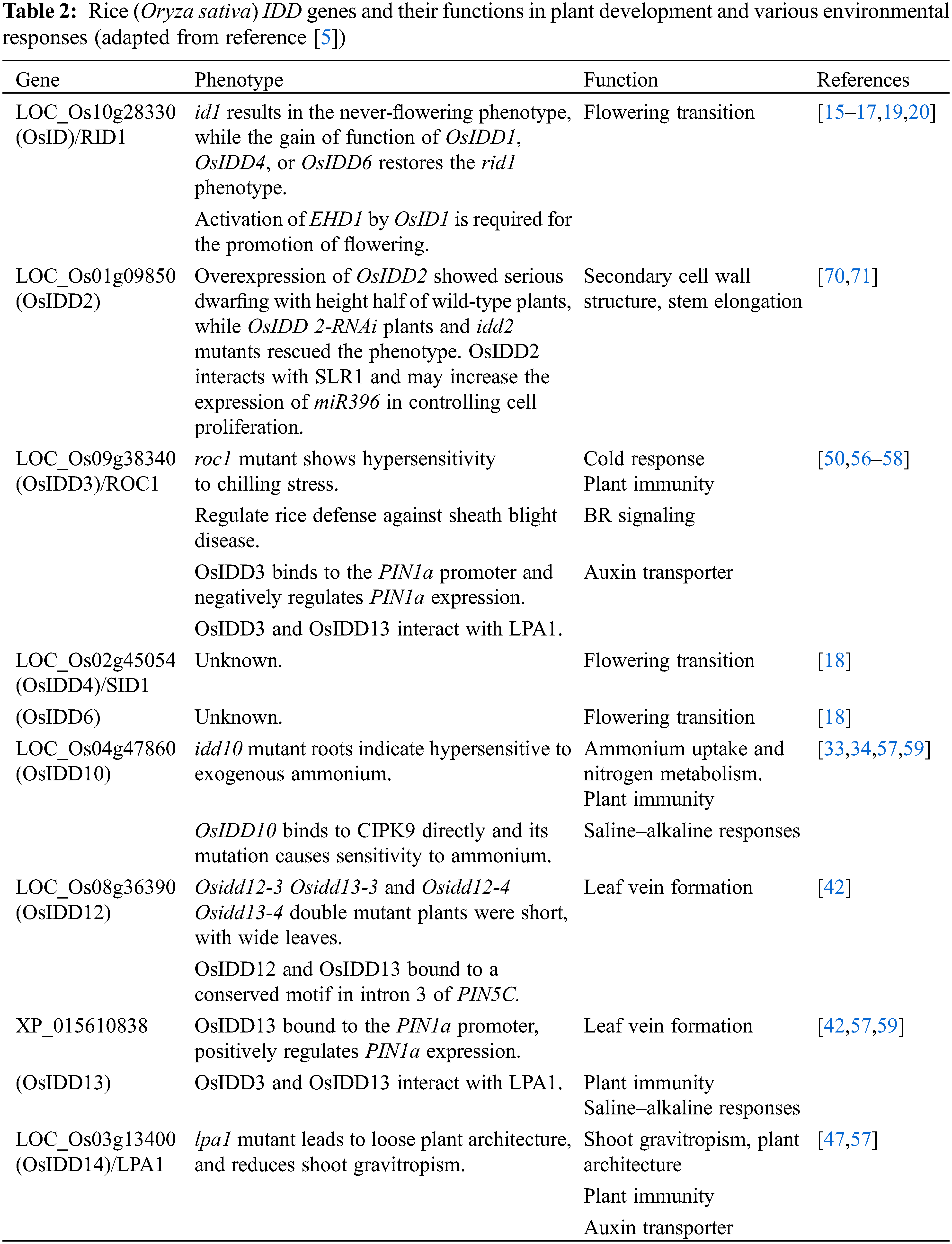
The IDD transcription factor is also characterized by the regulation of flowering time in rice. Previous studies on rice mutants or natural variants have reported two important ways to participate in the regulation of rice heading date, called the HD1 (HEADING DATE 1)-HD3A (HEADING DATE 3A)/RFT1 (RICE FLOWERING LOCUS T1) and EHD1 (EARLY HEADING DATE 1)-HD3A/RFT1 pathways [14]. INDETERMINATE 1 (OsID1)/EARLY HEADING DATE (EHD2)/RICE INDETERMINATE1 (RID1), a ZmID1 ortholog, is related to the flowering regulation of rice [15–19]. The OsID1/EHD2/RID1 make a role in the main switch of transition from vegetation to reproduction and may start the activation of florigen genes (including HD3A and RFT1) by directly binding to their promoters, and then the floral signals are collected to promote floral transition [15–18]. In addition, id1 results in a non-flowering phenotype, which is restored by the functional gain of OsIDD1 or OsIDD6. Thus, it is likely that the functions of OsIDD1, OsIDD4, and OsIDD6 are redundant, and that over-expression of any of these genes could replace OsID1 to initiate the flowering transition in the absence of OsID1 [18]. However, EHD1 is slightly reduced in idd1 plants, but almost completely repressed in the id1 mutant, indicating that the EHD1-mediated flowering pathway may differ between id1 and idd1 mutants. In addition, OsID1 partly accelerates flowering through negative regulation of rice OsERF#136 (a repressor of rice flowering), which acts as the repressor of rice flowering and mainly inhibits flowering by the EHD1-HD3A/RFT1 pathway [20] (Fig. 3).
Sugar metabolism is likely to be involved in flowering, and IDD proteins are core members of this pathway. Transcriptome and metabolic spectrum of maize id1 mutant leaves showed that in the pre-flowering stage, transcription of genes encoding polysaccharide metabolizing enzymes increased significantly, and the sucrose output level was low [21]. Sufficient sucrose and starch in the mutant of id1 revealed that ZmID1 guided the utilization of carbohydrates in source leaves rather than storage, thus promoting the output of carbohydrates to the shoot apex during flowering [21]. In rice, the overexpression of OsIDD1, OsIDD4/SID1, and OsIDD6 rescue the late flowering phenotype of OsID1, indicating that the IDDs might have some functional redundancy in sugar metabolism and floral transition [18]. Similarly, IDD members in Arabidopsis thaliana also act as transcriptional factors of floral transition by controlling sucrose signal transduction. It has been found that AtIDD8/NUTCRACKER (NUC) can promote the photoperiodic flowering time of plants by binding to the promoter region of downstream SUCROSE SYNTHASE (SUS) gene directly and up-regulating the gene [22]. Furthermore, SUCROSE NONFERMENTING-1-RELATED PROTEIN KINASE 1 (SnRK1)/AKIN10 interacts with AtIDD8 in the nucleus and phosphorylates AtIDD8 mainly on two serine (Ser) residues. AKIN10-mediated phosphorylation does not influence the DNA-binding properties and subcellular localization of AtIDD8, however, the AtIDD8 activation of transcriptiona was decreased after phosphorylation. In addition, AKIN10 has the function of antagonizing AtIDD8 to control flowering time, which is consistent with the late flowering phenotype of AKIN10 overexpressed plants and the idd8-3 mutants. In this signal regulation, AKIN10 signals are integrated into the regulatory network mediated by AtIDD8 directly, which regulates flowering time according to the fluctuation of sugar metabolism, further supporting the regulation of flowering metabolism [23]. To summarize, these findings suggest that IDD genes may be involved in the regulation of flowering time through direct or indirect connections with sugar metabolism (Fig. 3).
An interesting functional analogy is the Arabidopsis IDD proteins in root development. Numerous studies reveal that both epidermal cell and ground tissue characters are formed by IDD proteins. Four IDD proteins, AtIDD3/MAGPIE (MGP), AtIDD8/NUTCRACKER (NUC), AtIDD9/BALDIBIS (BIB), and AtIDD10/JACKDAW (JKD) have overlapping roles in the specification of the cortical cell layer [24–26]. AtIDD3 and AtIDD10 regulate root tissue boundaries and asymmetric cell division through mediating SHORT-ROOT (SHR) and SCARECROW (SCR) activity in a transcriptional and protein interaction network [24,27,28]. Moreover, AtIDD10 activates transient expression of the LUC reporter gene in protoplasts, and its binding sequence is upstream of the start codon ATG of SCR and AtIDD3. These results suggest that AtIDD10 acts with SHR, SCR, and AtIDD3 to directly regulate the expression of SCR and AtIDD3 [25]. AtIDD10 and its close homolog AtIDD9 modulate SHR movement by enhancing its nuclear retention and cooperating with AtIDD3 and AtIDD8 to activate the formative divisions that pattern the ground tissue into the cortex and endodermis. The normal cell division patterns are operated partly by transcriptional inhibition of CYCLIND6 (CYCD6) [26,29]. Studies showed that AtIDD10 and AtIDD9 restrict CYCD6 gene expression to the cortex-endois initial/daughter (CEI/CEID) [26]. AtIDD6/BLUEJAY (BLJ) and AtIDD4/IMPERIAL EAGLE (IME), regulate the ground tissue after embryogenesis. Their functions were as the determinants of CEI, which act as effectors of asymmetric cell divisions of the CEID when SHR is activated [30]. In vivo, FRET-FLIM results indicate SCR promoted AtIDD10-SHR interaction, and SHR boosted AtIDD10-SCR association, suggesting that SHR, SCR, and AtIDD10 form a ternary complex [31]. Besides SHR and SCR, another transcription factor, SCHIZORIZA (SCZ) was reported to regulate AtIDD10-mediated ground tissue patterning and vasculature formation before emergence at the step of dome-shape primordial [30,32].
Ammonium and nitrate nitrogen are the main sources of nitrogen in the roots of plants, and recent studies have also shown that some IDD members can regulate root growth and development by affecting nitrogen homeostasis. It was reported that OsIDD10 is involved in regulating ammonium absorption and nitrogen metabolism of roots, which activates the transcription of Ammonium transporter 1;2 (AMT1;2) and Glutamate dehydrogenase 2 (GDH2) by binding to the promoter region of AMT1;2 and the intron of GDH2. Moreover, OsIDD10 has made significant contributions to the activation of genes participated in N-linked metabolic and cellular responses, for example, genes encoding nitrite reductase, trehalose-6-phosphate (T6P) synthase, and glutamine synthetase 2 [33]. In addition, studies have found that OsIDD10 can directly activate the transcription of Calcineurin B-like protein (CBL)-interacting protein kinase 9 (CIPK9) and CIPK14, and the expression of CIPK9 and CIPK14 was sensitive to exogenous NH4+. At the same time, analysis of the phenotypes of idd10 mutant and CIPK9 OX plants indicated that the overexpressed plant was able to rescue the root growth defects in idd10 that relied on NH4+.
This suggests that CIPK9 is an NH4+-dependent regulator involved in root growth and seems to act downstream of OsIDD10 [34]. In Arabidopsis thaliana, AtIDD8-overexpression promoted the primary root growth in both normal and nitrogen-deficient situations. There are AtIDD8-binding sites in the promoter regions of the N-responsive and root-related genes TGACG SEQUENCE-SPECIFIC BINDING PROTEIN 1 (TGA1) and NITRATE TRANSPORTER 2.4 (NRT2.4), and AtIDD8 can activate and up-regulate their expression under nitrogen deficiency conditions, thereby increasing the number and length of lateral roots [35].
Seed maturation and germination are known to be essential for the production of viable seeds. Heterotopic expression of the Arabidopsis AtIDD1/ENHYDROUS (ENY) gene leads to abnormal seed maturation, and the function of AtIDD2/GAI-ASSOCIATED FACTOR1 (GAF1) in GA homeostasis regulation reveals a role of IDDs in Arabidopsis seed development, such as IDDs have been reported to determining aleurone layers [36,37]. In addition, studies in maize have shown that maize ID transcription factors ZmIDDveg9 (NKD1) and ZmIDD9 (NKD2) are both core regulators of gene expression during endosperm development of maize seeds and can participate in aleurone cell fate regulation and cell differentiation [38–40].
We know that cell proliferation and expansion can lead to leaf growth and formation and that the establishment of leaf polarity is a necessary condition for normal leaf morphogenesis and effective photosynthesis. The Arabidopsis genes HD-ZIP III and KANADI are typical regulators of leaf abaxial/adaxial patterns, and they play opposite regulatory roles in leaf polarity. Both AtIDD4 and AtIDD11/WARBLER promoters have binding sites for HD-ZIP III protein REVOLUTA (REV). In addition, the transcripts of four IDDs (AtIDD4, AtIDD5, AtIDD10, and AtIDD14) of the 12 family members measured were downregulated by KAN1, and ChIP-seq results showed that 7 of the Arabidopsis IDD gene promoters contained REV binding sites. This suggests that promoter regions of these IDD genes are potential targets for REV action [41]. Recently, SHR, IDD, and PIN (PIN-FORMED) family members were reported to play a role in vascular development and ground cell proliferation in rice leaves. Additionally, it was revealed that OsIDD12 and OsIDD13 directly interact with the auxin transporter gene OsPIN5c [42].
3 Responses to Diverse Environmental Conditions
The functions of multiple IDD members in plant development have been well-characterized. Increasing shreds of evidence indicate that IDDs also have an impact on a diverse range of responses to biological and abiotic environmental conditions, such as gravity, temperature, water, and pathogens.
3.1 Responses to Abiotic Environmental Factors
Geotropism is a vital factor in plant development, which influences the growth direction of plant organs on the gravity vector [43]. It has been reported that Arabidopsis AtIDD15/SHOOT GRAVITROPISM5 (SGR5) is involved in the gravity perception of the stem. Analysis of the phenotype of the SGR5 mutant revealed that the deposition rate of starch granules in the mutant was slower than the WT due to the decrease in the total starch accumulation. Moreover, the stem circumnutation movement of SGR5 was severely weakened, which was manifested by decreased amplitude and periodicity [44,45]. In short, loss of SGR5 activity affects the accumulation of starch in stem tissues, resulting in reduced sensitivity to gravity and diminished circulation movement in Arabidopsis. Furthermore, our results also indicate that SGR5 belongs to the IDD subfamily classified by AtIDD14/AtIDD15/AtIDD16 in Arabidopsis, which can co-regulate auxin biosynthesis and transport genes, such as AtPIN1 and YUCCA5. It can also regulate the gravitropic responses and the orientation change of branches and siliques [46]. Similarly, rice OsIDD14/Loose Plant Architecture 1 (LPA1), a homologous gene of Arabidopsis SGR5, modulates the sedimentation rate of amyloplasts, tiller, and leaf angles by regulating the adaxial growth of tiller node and lamina joint, thus regulating shoot gravitropism [47]. Taken together, these studies suggest that IDD family transcription factors may coordinate the gravisensing and morphogenesis of aerial organs by acting as intermediates in starch metabolism and hormone signaling.
Several studies have found that the IDD family is also involved in high and low-temperature responses. For example, the role of Arabidopsis AtIDD14 and SGR5 in temperature change provides an unexpected gene regulatory mechanism in which the two isoforms produced by alternative splicing play different roles. Interestingly, the AtIDD14α protein gathers under normal temperatures, while the AtIDD14β protein is at low temperatures. Under low temperatures, AtIDD14β can physically interact with AtIDD14α protein, and inhibit their interaction with downstream target genes (for example Qua-Quine Starch, QQS), thus causing starch degradation to decrease. In short, the self-regulatory circuit of IDD is specifically involved in regulating starch metabolism under cold conditions [48]. There are two splicing variants of the SGR5 gene in Arabidopsis thaliana, a full-size SGR5α and another a truncated SGR5β form that lacks functional ZF motifs [49], and this alternative splicing may be accelerated at high temperatures, leading to high levels of collection of the SGR5β protein. The truncated form of SGR5β may inhibit the function of SGR5α by forming non-functional complex heterodimers. Moreover, SGR5-overexpressing SGR5β plants also showed a reduced response to inflorescence stem geotropism, similar to the sgr5-1 phenotype in Arabidopsis [49]. In rice, the transcriptional regulator of CBF1 was isolated using the promoter of Dehydration-responsive element-binding protein1s (DREB1s)/C-repeat binding factors (CBFs)/CBF1, cold-induced gene, by yeast one-hybrid assay. The results showed that OsIDD3/ROC1 (Regulator of CBF1) can directly bind to CBF1 promoter. Meantime, idd3 mutants showed a cold-sensitive phenotype and can inhibit the induction of cold-mediated genes CBF1 and CBF3, showing that OsIDD3 is a positive factor involved in cold stress response [50]. Recently, our study found that in drought conditions, idd14-1D, a gain-of-function mutant, showed reduced water loss rate of leaves and enhanced drought resistance, while a loss-of-function mutant idd14-1 showed improved water loss rate of leaves and decreased drought tolerance. The expression of IDD14 also affects the sensitivity to ABA and ABA-mediated stomatal closure. At the same time, we further illustrated that IDD14 can directly interact with ABRE-binding factor 1-4 (ABF1-4) to promote its transcriptional activity, thereby improving drought resistance. Taken together, we suggest that the Arabidopsis IDD14 transcription factor, as a component of the ABA signaling pathway, is involved in positively regulating the drought-stress responses [51]. Overexpression of the IDD16 gene decreased the stomatal density of the abaxial leaf in Arabidopsis, and ChIP analysis suggested that IDD16 directly combined with the promoter region of the stomatal development gene SPCH. Moreover, water use efficiency (WUE) and drought tolerance of Arabidopsis overexpressing IDD16 were significantly increased while leaf transpiration was reduced. In summary, AtIDD16 can directly regulate the transcription of the SPCH gene as a negative regulator, thereby affecting the initiation of stomatal development and resulting in decreased stomatal density, while Arabidopsis thaliana with overexpression of IDD16 shows enhanced drought stress tolerance and WUE [52].
3.2 Responses to Biotic Environmental Factors
The plant immune system is the basis of plant survival, and numerous pieces of evidence support those plants have two immune systems, namely pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) [53]. Research have shown that AtIDD4 mutations increase resistance to the hemibiotrophic pathogen Pseudomonas syringae, and AtIDD4 may be an inhibitor of the underlying immune response and PTI. Comparative transcriptome studies of idd4 and IDD4ox plants, consistent with the whole genome AtIDD4 DNA binding sites studies, identified a target gene responsible for biodefense processes, namely AtIDD4, which interacts with MAP kinase MPK6 and is phosphorylated by the latter at two conserved sites. DNA binding studies of AtIDD4 and AtIDD4 phosphate site mutants treated with FLAGELLIN22 (flg22) show that AtIDD4 has enhanced binding affinity with ID1-containing motif promoters and transcriptional regulation. Additionally, the AtIDD4 chimeric inhibitor (idd4SRDX, SRDX, the chimeric repressor gene-silencing technology) expressed in WT increased basal resistance after hemibiotrophic infection, especially after infection with Botrytis cinerea. Moreover, high levels of the immune hormones SA and jasmonic acid (JA) in idd4SRDX plants suggest that AtIDD4 and other members may form the center of plant immunity, which mediates the defense response and regulation of hormonal pathways [54,55].
As far as we know, the rice sheath blight disease (ShB) seriously affected rice production. It was found that ABI3/VP1-like 1 (RAVL1) participated in the negative regulation of the anti-ShB defense mechanism in rice, while OsIDD3 was positively regulated by RAVL1, and RAVL1 directly bound to the OsIDD3 promoter region. There was no significant difference in the response of OsIDD3 mutants to ShB, while OsIDD3 overexpression plants were more sensitive to ShB [56]. It was found that OsIDD14/LPA1 was almost not expressed in leaves, but the infection of Rhizoctonia solani could significantly induce the expression of OsIDD14 in leaves, and the susceptibility of lpa1 to R. solani was higher than that of wild type and related plants. OsIDD14 overexpression significantly improved rice resistance to sheath blight disease (ShB) via activating PIN-FORMED 1a (PIN1a). In addition, the expression of OsIDD3, OsIDD5, OsIDD10, and OsIDD13 could be changed by infection with R. solani, and OsIDD14 could interact with OsIDD3 and OsIDD13. OsIDD13 RNAi plants were susceptible to ShB, while plants that overexpress OsIDD13 were less susceptible to ShB. OsIDD3 and OsIDD13 regulate the transcription of PIN1a negatively and positively via binding to the PIN1a promoter, respectively. Moreover, OsIDD3, OsIDD13, and OsIDD14 form transcription factor complexes that regulate the expression of the PIN1a gene [57,58]. Taken together, these analyses demonstrated that OsIDD3, OsIDD13, and OsIDD14/LPA1 constitute transcriptional regulatory complexes that may influence rice defense against ShB by regulating PIN1a and PIN1b.
Studies have shown that the absorption of NH4+ ions can promote the resistance of rice to saline-alkaline stress and ShB. OsIDD10, which encodes a core TF for NH4+ signaling, causes roots to be sensitive to NH4+ under light conditions but not under dark conditions. OsIDD10 interacted with brassinazole-resistant 1 (BZR1) to activate AMT1;2. When the rice was inoculated with R. solani, phytochrome B (PhyB) and OsIDD10 negatively regulated the rice resistance to ShB, while AMT1 and BZR1 were positively regulated. In addition, PhyB has a negative function, and OsIDD10 and AMT1 have a positive regulatory effect on the rice resistance to saline-alkaline stress. Taken together, these findings suggested that PhyB-OsIDD10-AMT1;2 signaling pathway operates the saline-alkaline reaction, while PhyB-BZR1-AMT1; 2 pathway controls ShB resistance [59].
4 Functions in Hormone Signal Transduction Pathway
DELLA proteins, such as GIBBERELLIC ACID INSENSITIVE (GAI), REPRESSOR OF GA1-3 1 (RGA1), are transcription factors of the GRAS family in Arabidopsis thaliana, which regulate gene expression in response to GA signals. Increasing evidence indicates that IDD family members can act as DNA-binding transcription factors directly or as cofactors of DELLAs indirectly. For example, RGA1 interacts and activates transcription of GA-positive regulator SCARECROW-LIKE3 (SCL3) by interacting with any of the five proteins AtIDD3, AtIDD4, AtIDD5, AtIDD9, and AtIDD10 [60]. More research has revealed that DELLAs and SCL3 regulators play a role as co-regulators, and IDD transcription factors bound to DNA regulate downstream gene expression by balancing SCL3 and DELLA protein levels. Therefore, IDDs family TFs are participated in GA feedback regulation as DNA-binding scaffolds [30,60,61] (Fig. 4a). Additionally, AtIDD2/GAF1 (GAI-ASSOCIATED FACTOR1) and AtIDD1/ENY interact with GAI to regulate the GA20ox2 gene [37,61–64]. AtIDD2 can also interact with the transcriptional co-suppressor TOPLESS (TPL), for example without the DELLA proteins, AtIDD2-TPL forms complexes that inhibit the transcription of target genes. Recent studies indicated that the GRAS domain of DELLA protein has activation activity, while the GRAS domain of SCL3 has transcriptional repression activity. It was also found that SCL3 represses the activation of AtIDD2-DELLA complex by inhibiting activity rather than by competitively inhibiting AtIDD2-DELLA interaction. In addition, AtIDD2 was found to enhance the repression activity of SCL3 in a manner independent of TPL. In short, SCL3 can form ternary complexes with AtIDD2 and DELLA proteins [65]. Above all, these results provide an important reference for the interpretation of the IDD-DELLA-regulated GA signaling pathway.
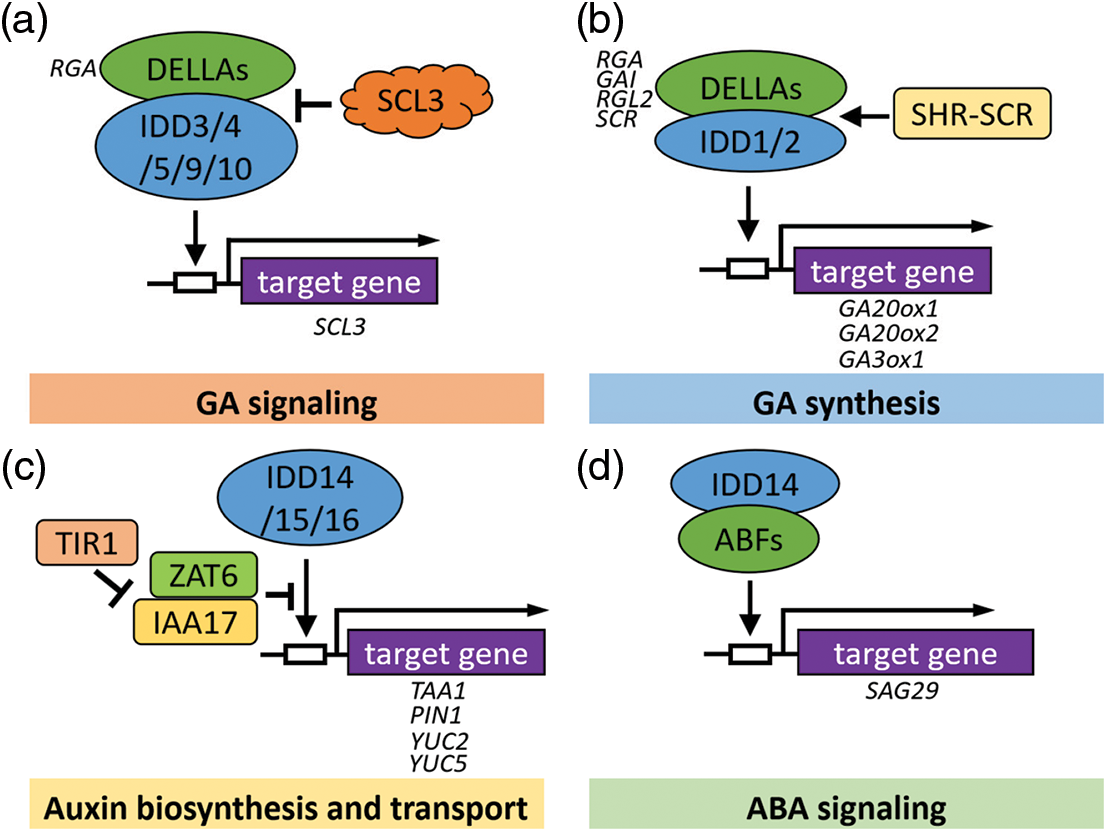
Figure 4: Models of IDDs that might be involved in the hormone signal transduction pathway in Arabidopsis. (a) IDDs participate in the GA signaling pathway. IDD3 protein binds to DNA sequences containing AGACAA as a core motif. (b) IDDs play a role in the GA synthesis process. IDD1 and IDD2/GAF1 proteins bind to DNA sequences containing TTTTGTC or TTTTGT. (c) IDDs coordinate auxin biosynthesis and transport. IDD15 protein binds to DNA sequences containing the TACAAT motif in the promoter. IDD16 could bind to a specific 11 bp DNA consensus motif, TTTGTCG/CT/CT/aT/aT. (d) IDDs mediate the ABA signaling pathway. The white box represents the region of the promoter. The arrows and T-shaped lines represent positive and negative regulation, respectively. Solid arrows indicate direct activation
In addition, it also indicated that AtIDD1 interacted directly with DELLA proteins, which confirmed that AtIDD1 was a component in the hormone signaling pathway during seed maturation [36]. It has recently been summarized that, not only RGA, SHR-SCR also can act as a co-activator of AtIDD2, promoting the expression of SCR, SCL3, and AtGA3ox1, and that these complexes may regulate and coordinate the expression of genes related to root formation [65,66]. Interestingly, in Arabidopsis, AtIDD2 also participates in the GA-dependent flowering pathway by modulating the expression of FT and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1). Under the action of GAs, AtIDD2 forms a transcription suppressor complex and activates the transcription of FT and SOC1 by inhibiting the expression of four flowering suppressor genes, EARLY FLOWERING3 (ELF3), SHORT VEGETATIVE PHASE (SVP), TEMPRANILLO1 (TEM1), and TEM2 [64]. Collectively, AtIDD1 and AtIDD2 are involved in seed and root development, and flowering in a GA-dependent manner (Figs. 4a, 4b).
The morphogenesis of plant lateral organs and the establishment of plant structure mainly depend on the spatial accumulation of auxin in the organs, which is determined by the local biosynthesis and polar transportation of auxin. Our previous study showed that the AtIDD14, AtIDD15, and AtIDD16 of the Arabidopsis IDD transcription factor family can activate the gene expression of downstream TRYPTOPHAN AMINOTRANSFERASE of ARABIDOPSIS1 (TAA1), PINFORMED1 (PIN1), and YUCCA5 (YUC5) (YUC5) via directly binds to their promoter regions, thereby promoting the auxin biosynthesis and transportation [46]. Additionally, an investigation showed that the zinc finger of Arabidopsis thaliana 6 (ZAT6) represses the transcription of IDD15 on the YUC2 promoter, while ZAT6 repressed the interaction of TRANSPORT INHIBITOR RESPONSE 1 (TIR1) and INDOLE-3-ACETIC ACID 17 (IAA17) through competitively binding to IAA17. Currently, AtIDD15 and IAA17 interacting with ZAT6 have been found in vivo, providing a new perspective to elucidate the ZAT6-mediated auxin signaling pathway [67] (Fig. 4c).
In rice, OsIDD3 expression is widely in different tissues and stages and is transcribed by exogenous auxin. Furthermore, OsIDD3 OX is sensitive to polar transporter inhibitor N-1-naphthylphalamic acid (NPA) and auxin. OsIDD3 directly inhibits PIN1b expression through binding to the promoter. After inoculation with R. solani, PIN1b RNAi are more susceptible to ShB infection than WT plants [57,58]. In conclusion, these analyses indicate that OsIDD3 influences resistance to ShB in rice by regulating the auxin transporter PIN genes. Additionally, compared with WT, the transcription of brassinosteroid-related genes (D2, D11, and BRI1) decreased in OsIDD3 repressors, but increased in OsIDD3 overexpressors. In BRI1 mutant d61-1, OsIDD3 overexpression resulted in decreased OsIDD3 activity. Compared with OsIDD3 overexpression plants and WT plants, OsIDD3 was less sensitive to ShB, suggesting that OsIDD3 negatively regulates the defense mechanism of rice against ShB via activating the BR pathway [56].
Recently, we demonstrated that Arabidopsis IDD14 interacts with ABF1, ABF2, ABF3, and ABF4 directly, and activates their transcriptional activities, resulting in enhanced drought resistance. We compared the expression levels of three ABA signaling marker genes, SAG29, RAB18, and AIL1, in WT and IDD14 mutants treated or not with ABA. We found that transcription levels of these ABA-response marker genes were further increased in gain-of-function mutant idd14-1D and suppressed in loss-of-function mutant idd14-1. These findings indicate that the Arabidopsis IDD14 transcription factor, as a component of the ABA signaling pathway, is related to the ABA pathway and participates in the positive regulation of drought-stress responses [51] (Fig. 4d). Moreover, researchers took advantage of the SRDX to broaden our understanding on the roles of Arabidopsis AtIDD4 and IDD members in plant immunity. Results showed that the growth of idd4SRDX lines was impaired and displayed a strong autoimmune phenotype. Through hormone analyses, the results showed that SA and JA accumulate in plants, indicating that IDDs may play role in regulating the metabolism of these hormones [54] (Tables 1–3).
In plants, transcription factors appear to be susceptible to being influenced as a result of environmental factors and events [1]. IDD proteins are TFs that play a vital role in modulating various developmental processes in plants. These proteins are identified by the presence of a conserved DNA-binding domain known as the IDD domain [3,5]. The majority of these transcription factors have been studied in Arabidopsis, including seed and root development (Table 1) [24–30,36,37,62,63]. However, some functions of IDDs have been reported in other plants, such as rice and maize (Tables 2, 3). They are involved in root development, flowering, sugar homeostasis, starch metabolism, drought/hot/cold-stress signaling, plant immunity, GA signaling and biosynthesis, plant architecture, shoot gravitropism, auxin biosynthesis and transport, and ammonium uptake (Tables 1–3, Figs. 3, 4). In fact, IDDs are reported in almost all aspects of plant development and growth. Studies have indicated that IDD proteins are participated in the development of the integument, which is the outermost layer of cells that protect the plant from external stresses. Mutations in IDD genes can lead to abnormal development of the integument, resulting in reduced seed production and quality [36–39]. In particular, IDD proteins also play a critical role in abiotic stress tolerance in plants. They regulate the expression of stress-responsive genes and help plants adapt to adverse environmental factors such as drought, salinity, and extreme temperatures [49–52,59]. Recent studies have identified several molecular mechanisms that regulate the activity of IDD proteins, including post-translational modifications, protein-protein interactions, and epigenetic regulation [7,39,66,72]. Understanding these mechanisms is essential for developing strategies to manipulate IDD protein function and improve crop yields. In conclusion, IDD proteins have a crucial impact on plant development and stress responses, making them potential targets for crop improvement. Further research is needed to fully elucidate the functions and mechanisms of IDD proteins in plants and develop strategies to enhance their activity for agricultural applications.
Acknowledgement: We are thankful to Kumar, M., Le, D. T., Hwang, S., Seo, P. J., Kim, H. U. [5] and all anonymous reviewers for their valuable input, which greatly improved the quality of this manuscript.
Funding Statement: This work was supported by the National Natural Science Foundation of China (31800225 and 32370363) and by the Natural Science Foundation of Shandong Province (ZR2020MC027 and ZR2021QC213).
Author Contributions: The authors confirm their contribution to the paper as follows: J. Liu and D. Cui wrote the manuscript; D. Shu, Z. Tan, M. Ma, H. Yang, N. Guo and S. Li finalized the manuscript. All authors read and approved the final manuscript.
Availability of Data and Materials: Data sharing does not apply to this article as no new data were created or analyzed in this study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Shiu SH, Shih MC, Li WH. Transcription factor families have much higher expansion rates in plants than in animals. Plant Physiol. 2005;139(1):18–26. [Google Scholar] [PubMed]
2. Englbrecht CC, Schoof H, Böhm S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genom. 2004;5(1):39. [Google Scholar]
3. Colasanti J, Yuan Z, Sundaresan V. The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell. 1998;93(4):593–603. [Google Scholar] [PubMed]
4. Locascio A, Marqués MC, García-Martínez G, Corratgé-Faillie C, Andrés-Colás N, Rubio L, et al. BCL2-ASSOCIATED ATHANOGENE4 regulates the KAT1 potassium channel and controls stomatal movement. Plant Physiol. 2019;181(3):1277–94. [Google Scholar] [PubMed]
5. Kumar M, Le DT, Hwang S, Seo PJ, Kim HU. Role of the INDETERMINATE DOMAIN genes in plants. IJMS. 2019;20(9):2286. [Google Scholar] [PubMed]
6. Coelho CP, Huang P, Lee DY, Brutnell TP. Making roots, shoots, and seeds: IDD gene family diversification in plants. Trend Plant Sci. 2018;23(1):66–78. [Google Scholar]
7. Zhang T, Tan M, Geng L, Li J, Xiang Y, Zhang B, et al. New insight into comprehensive analysis of INDETERMINATE DOMAIN (IDD) gene family in rice. Plant Physiol Biochem. 2020;154:547–56. [Google Scholar] [PubMed]
8. Feng X, Yu Q, Zeng J, He X, Ma W, Ge L, et al. Comprehensive analysis of the INDETERMINATE DOMAIN (IDD) gene family and their response to abiotic stress in Zea mays. IJMS. 2023;24:6185. [Google Scholar] [PubMed]
9. Kozaki A, Hake S, Colasanti J. The maize ID1 flowering time regulator is a zinc finger protein with novel DNA binding properties. Nucleic Acids Res. 2004;32(5):1710–20. [Google Scholar] [PubMed]
10. Wong AY, Colasanti J. Maize floral regulator protein INDETERMINATE1 is localized to developing leaves and is not altered by light or the sink/source transition. J Exp Bot. 2007;58(3):403–14. [Google Scholar] [PubMed]
11. Colasanti J, Tremblay R, Wong AY, Coneva V, Kozaki A, Mable BK, et al. The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family in higher plants. BMC Genom. 2006;7:158. [Google Scholar]
12. Coneva V, Zhu T, Colasanti J. Expression differences between normal and indeterminate1 maize suggest downstream targets of ID1, a floral transition regulator in maize. J Exp Bot. 2007;58(13):3679–93. [Google Scholar] [PubMed]
13. Meng X, Muszynski MG, Danilevskaya ON. The FT-like ZCN8 gene functions as a floral activator and is involved in photoperiod sensitivity in maize. Plant Cell. 2011;23(3):942–60. [Google Scholar] [PubMed]
14. Zhou S, Zhu S, Cui S, Hou H, Wu H, Hao B, et al. Transcriptional and post-transcriptional regulation of heading date in rice. New Phytol. 2021;230(3):943–56. [Google Scholar] [PubMed]
15. Park SJ, Kim SL, Lee S, Je BI, Piao HL, Park SH, et al. Rice Indeterminate 1 (OsId1) is necessary for the expression of Ehd1 (Early heading date 1) regardless of photoperiod. Plant J. 2008;56(6):1018–29. [Google Scholar] [PubMed]
16. Matsubara K, Yamanouchi U, Wang ZX, Minobe Y, Izawa T, Yano M, et al. Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up-regulating Ehd1. Plant Physiol. 2008;148(3):1425–35. [Google Scholar] [PubMed]
17. Wu C, You C, Li C, Long T, Chen G, Byrne ME, et al. RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc Natl Acad Sci USA. 2008;105(35):12915–20. [Google Scholar] [PubMed]
18. Deng L, Li L, Zhang S, Shen J, Li S, Hu S, et al. Suppressor of rid1 (SID1) shares common targets with RID1 on florigen genes to initiate floral transition in rice. PLoS Genet. 2017;13(2):e1006642. [Google Scholar] [PubMed]
19. Hu S, Dong G, Xu J, Su Y, Shi Z, Ye W, et al. A point mutation in the zinc finger motif of RID1/EHD2/ OsID1 protein leads to outstanding yield-related traits in japonica rice variety Wuyunjing 7. Rice. 2013;6(1):24. [Google Scholar] [PubMed]
20. Zhang S, Deng L, Zhao L, Wu C. Genome-wide binding analysis of transcription factor Rice Indeterminate 1 reveals a complex network controlling rice floral transition. J Integr Plant Biol. 2022;64(9):1690–705. [Google Scholar] [PubMed]
21. Coneva V, Guevara D, Rothstein SJ, Colasanti J. Transcript and metabolite signature of maize source leaves suggests a link between transitory starch to sucrose balance and the autonomous floral transition. J Exp Bot. 2012;63(14):5079–92. [Google Scholar] [PubMed]
22. Seo PJ, Ryu J, Kang SK, Park CM. Modulation of sugar metabolism by an INDETERMINATE DOMAIN transcription factor contributes to photoperiodic flowering in Arabidopsis. Plant J. 2011;65(3):418–29. [Google Scholar] [PubMed]
23. Jeong EY, Seo PJ, Woo JC, Park CM. AKIN10 delays flowering by inactivating IDD8 transcription factor through protein phosphorylation in Arabidopsis. BMC Plant Biol. 2015;15:110. [Google Scholar] [PubMed]
24. Welch D, Hassan H, Blilou I, Immink R, Heidstra R, Scheres B. Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT- ROOT action. Gene Dev. 2007;21(17):2196–204. [Google Scholar] [PubMed]
25. Ogasawara H, Kaimi R, Colasanti J, Kozaki A. Activity of transcription factor JACKDAW is essential for SHR/SCR-dependent activation of SCARECROW and MAGPIE and is modulated by reciprocal interactions with MAGPIE, SCARECROW and SHORT ROOT. Plant Mol Biol. 2011;77(4–5):489–99. [Google Scholar] [PubMed]
26. Long Y, Smet W, Cruz-Ramírez A, Castelijns B, de Jonge W, Mähönen, AP, et al. Arabidopsis BIRD zinc finger proteins jointly stabilize tissue boundaries by confining the cell fate regulator SHORT-ROOT and contributing to fate specification. Plant Cell. 2015;27(4):1185–99. [Google Scholar] [PubMed]
27. Heidstra R, Welch D, Scheres B. Mosaic analyses using marked activation and deletion clones dissect Arabidopsis SCARECROW action in asymmetric cell division. Gene Dev. 2004;18(16):1964–9. [Google Scholar] [PubMed]
28. Hassan H, Scheres B, Blilou I. JACKDAW controls epidermal patterning in the Arabidopsis root meristem through a non-cell-autonomous mechanism. Dev. 2010;137(9):1523–9. [Google Scholar]
29. Sozzani R, Cui H, Moreno-Risueno MA, Busch W, van Norman JM, Vernoux T, et al. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature. 2010;466(7302):128–32. [Google Scholar] [PubMed]
30. Moreno-Risueno MA, Sozzani R, Yardgmcg GG, Petricka JJ, Vernoux T, Blilou I, et al. Transcriptional control of tissue formation throughout root development. Sci. 2015;350(6259):426–30. [Google Scholar]
31. Long Y, Stahl Y, Weidtkamp-Peters S, Postma M, Zhou W, Goedhart J, et al. In vivo FRET-FLIM reveals cell- type-specific protein interactions in Arabidopsis roots. Nature. 2017;548(7665):97–102. [Google Scholar] [PubMed]
32. Bustillo-Avendaño E, Ibáñez S, Sanz O, Sousa Barros JA, Gude I, Perianez-Rodriguez J, et al. Regulation of hormonal control, cell reprogramming, and patterning during De Novo root organogenesis. Plant Physiol. 2018;176(2):1709–27. [Google Scholar]
33. Xuan YH, Priatama RA, Huang J, Je BI, Liu JM, Park SJ, et al. Indeterminate domain 10 regulates ammonium-mediated gene expression in rice roots. New Phytol. 2013;197(3):791–804. [Google Scholar] [PubMed]
34. Xuan YH, Kumar V, Han X, Kim SH, Jeong JH, Kim CM, et al. CBL-INTERACTING PROTEIN KINASE 9 regulates ammonium-dependent root growth downstream of IDD10 in rice (Oryza sativa). Ann. Bot. 2019;124(6):947–60. [Google Scholar] [PubMed]
35. Ling J, Huang X, Jia Y, Li W, Zhang X. The overexpression of NUC promotes development and increases resistance to nitrogen deficiency in Arabidopsis thaliana. IJMS. 2021;22(21):11413. [Google Scholar] [PubMed]
36. Feurtado JA, Huang D, Wicki-Stordeur L, Hemstock LE, Potentier MS, Tsang EW, et al. The Arabidopsis C2H2 zinc finger INDETERMINATE DOMAIN1/ENHYDROUS promotes the transition to germination by regulating light and hormonal signaling during seed maturation. Plant Cell. 2011;23(5):1772–94. [Google Scholar] [PubMed]
37. Fukazawa J, Teramura H, Murakoshi S, Nasuno K, Nishida N, Ito T, et al. DELLAs function as coactivators of GAI-ASSOCIATED FACTOR1 in regulation of gibberellin homeostasis and signaling in Arabidopsis. Plant Cell. 2014;26(7):2920–38. [Google Scholar] [PubMed]
38. Yi G, Neelakandan AK, Gontarek BC, Vollbrecht E, Becraft PW. The naked endosperm genes encode duplicate INDETERMINATE domain transcription factors required for maize endosperm cell patterning and differentiation. Plant Physiol. 2015;167(2):443–56. [Google Scholar] [PubMed]
39. Gontarek BC, Neelakandan AK, Wu H, Becraft PW. NKD transcription factors are central regulators of maize endosperm development. Plant Cell. 2016;28(12):2916–36. [Google Scholar] [PubMed]
40. Hughes T, Sedelnikova O, Thomas M, Langdale J. Mutations in NAKED-ENDOSPERM IDD genes reveal functional interactions with SCARECROW during leaf patterning in C4 grasses. PLoS Genet. 2023;19(4):e1010715. [Google Scholar] [PubMed]
41. Reinhart BJ, Liu T, Newell NR, Magnani E, Huang T, Kerstetter R, et al. Establishing a framework for the Ad/abaxial regulatory network of Arabidopsis: ascertaining targets of class III homeodomain leucine zipper and KANADI regulation. Plant Cell. 2013;25(9):3228–49. [Google Scholar] [PubMed]
42. Liu Q, Teng S, Deng C, Wu S, Li H, Wang Y, et al. SHORT ROOT and INDETERMINATE DOMAIN family members govern PIN-FORMED expression to regulate minor vein differentiation in rice. Plant Cell. 2023;35(8):2848–70. [Google Scholar] [PubMed]
43. Tasaka M, Kato T, Fukaki H. The endodermis and shoot gravitropism. Trend Plant Sci. 1999;4(3):103–7. [Google Scholar]
44. Morita MT, Sakaguchi K, Kiyose S, Taira K, Kato T, Nakamura M, et al. A C2H2-type zinc finger protein, SGR5, is involved in early events of gravitropism in Arabidopsis inflorescence stems. Plant J. 2006;47(4):619–28. [Google Scholar] [PubMed]
45. Tanimoto M, Tremblay R, Colasanti J. Altered gravitropic response, amyloplast sedimentation and circumnutation in the Arabidopsis shoot gravitropism 5 mutant are associated with reduced starch levels. Plant Mol Biol. 2008;67(1–2):57–69. [Google Scholar] [PubMed]
46. Cui D, Zhao J, Jing Y, Fan M, Liu J, Wang Z, et al. The Arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. PLoS Genet. 2013;9(9):e1003759. [Google Scholar] [PubMed]
47. Wu X, Tang D, Li M, Wang K, Cheng Z. Loose plant Architecture1, an INDETERMINATE DOMAIN protein involved in shoot gravitropism, regulates plant architecture in rice. Plant Physiol. 2013;161(1):317–29. [Google Scholar] [PubMed]
48. Seo PJ, Kim MJ, Ryu JY, Jeong EY, Park CM. Two splice variants of the IDD14 transcription factor competitively form nonfunctional heterodimers which may regulate starch metabolism. Nat Commun. 2011;2:303. [Google Scholar] [PubMed]
49. Kim JY, Ryu JY, Baek K, Park CM. High temperature attenuates the gravitropism of inflorescence stems by inducing SHOOT GRAVITROPISM 5 alternative splicing in Arabidopsis. New Phytol. 2016;209(1):265–79. [Google Scholar] [PubMed]
50. Dou M, Cheng S, Zhao B, Xuan Y, Shao M. The indeterminate domain protein ROC1 regulates chilling tolerance via activation of DREB1B/CBF1 in rice. IJMS. 2016;17(3):233. [Google Scholar] [PubMed]
51. Liu J, Shu D, Tan Z, Ma M, Guo N, Gao S, et al. The Arabidopsis IDD14 transcription factor interacts with bZIP-type ABFs/AREBs and cooperatively regulates ABA-mediated drought tolerance. New Phytol. 2022;236(3):929–42. [Google Scholar] [PubMed]
52. Qi SL, Lin QF, Feng XJ, Han HL, Liu J, Zhang L, et al. IDD16 negatively regulates stomatal initiation via trans-repression of SPCH in Arabidopsis. Plant Biotechnol J. 2019;17(7):1446–57. [Google Scholar] [PubMed]
53. Yuan M, Jiang Z, Bi G, Nomura K, Liu M, Wang Y, et al. Pattern-recognition receptors are required for NLR- mediated plant immunity. Nature. 2021;592(7852):105–9. [Google Scholar] [PubMed]
54. Völz R, Kim SK, Mi J, Mariappan KG, Siodmak A, Al-Babili S, et al. A chimeric IDD4 repressor constitutively induces immunity in Arabidopsis via the modulation of salicylic acid and jasmonic acid homeostasis. Plant & Cell Physiol. 2019;60(7):1536–55. [Google Scholar]
55. Völz R, Kim SK, Mi J, Rawat AA, Veluchamy A, Mariappan KG, et al. INDETERMINATE-DOMAIN 4 (IDD4) coordinates immune responses with plant-growth in Arabidopsis thaliana. PLoS Pathog. 2019;15(1):e1007499. [Google Scholar]
56. Sun Q, Yang S, Guo XF, Wang ST, Jia XT. RAVL1 activates IDD3 to negatively regulate rice resistance to sheath blight disease. Rice Sci. 2021;28(2):146–55. [Google Scholar]
57. Sun Q, Li DD, Chu J, Yuan DP, Li S, Zhong LJ, et al. Indeterminate domain proteins regulate rice defense to sheath blight disease. Rice. 2020;13(1):15. [Google Scholar] [PubMed]
58. Wang ST, Guo XF, Yao TS, Xuan YH. Indeterminate domain 3 negatively regulates plant erectness and the resistance of rice to sheath blight by controlling PIN-FORMED gene expressions. Plant Signal Behav. 2020;15(11):1809847. [Google Scholar] [PubMed]
59. Jung JH, Li Z, Chen H, Yang S, Li D, Priatama RA, et al. Mutation of phytochrome B promotes resistance to sheath blight and saline-alkaline stress via increasing ammonium uptake in rice. Plant J. 2023;113(2):277–90. [Google Scholar] [PubMed]
60. Yoshida H, Hirano K, Sato T, Mitsuda N, Nomoto M, Maeo K, et al. DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proc Natl Acad Sci USA. 2014;111(21):7861–6. [Google Scholar] [PubMed]
61. Fukazawa J, Miyamoto C, Ando H, Mori K, Takahashi Y. DELLA-GAF1 complex is involved in tissue-specific expression and gibberellin feedback regulation of GA20ox1 in Arabidopsis. Plant Mol Biol. 2021;107(3):147–58. [Google Scholar] [PubMed]
62. Fukazawa J, Ito T, Kamiya Y, Yamaguchi S, Takahashi Y. Binding of GID1 to DELLAs promotes dissociation of GAF1 from DELLA in GA dependent manner. Plant Signal Behav. 2015;10(10):e1052923. [Google Scholar] [PubMed]
63. Fukazawa J, Mori M, Watanabe S, Miyamoto C, Ito T, Takahashi Y. DELLA-GAF1 complex is a main component in gibberellin feedback regulation of GA20 oxidase 2. Plant Physiol. 2017;175(3):1395–406. [Google Scholar] [PubMed]
64. Fukazawa J, Ohashi Y, Takahashi R, Nakai K, Takahashi Y. DELLA degradation by gibberellin promotes flowering via GAF1-TPR-dependent repression of floral repressors in Arabidopsis. Plant Cell. 2021;33(7):2258–72. [Google Scholar] [PubMed]
65. Ito T, Fukazawa J. SCARECROW-LIKE3 regulates the transcription of gibberellin-related genes by acting as a transcriptional co-repressor of GAI-ASSOCIATED FACTOR1. Plant Mol Biol. 2021;105(4–5):463–82. [Google Scholar] [PubMed]
66. Aoyanagi T, Ikeya S, Kobayashi A, Kozaki A. Gene regulation via the combination of transcription factors in the INDETERMINATE DOMAIN and GRAS families. Genes. 2020;11(6):613. [Google Scholar] [PubMed]
67. Shi H, Zhang S, Lin D, Wei Y, Yan Y, Liu G, et al. Zinc finger of Arabidopsis thaliana 6 is involved in melatonin-mediated auxin signaling through interacting INDETERMINATE DOMAIN15 and INDOLE-3- ACETIC ACID 17. J Pineal Res. 2018;65(2):e12494. [Google Scholar] [PubMed]
68. Ingkasuwan P, Netrphan S, Prasitwattanaseree S, Tanticharoen M, Bhumiratana S, Meechai A, et al. Inferring transcriptional gene regulation network of starch metabolism in Arabidopsis thaliana leaves using graphical Gaussian model. BMC Syst. Biol. 2012;6:100. [Google Scholar] [PubMed]
69. Asaoka M, Sakamoto S, Gunji S, Mitsuda N, Tsukaya H, Sawa S, et al. Contribution of vasculature to stem integrity in Arabidopsis thaliana. Dev. 2023;150(3):dev201156. [Google Scholar]
70. Huang P, Yoshida H, Yano K, Kinoshita S, Kawai K, Koketsu E, et al. OsIDD2, a zinc finger and INDETERMINATE DOMAIN protein, regulates secondary cell wall formation. J Integr Plant Biol. 2018;60(2):130–43. [Google Scholar] [PubMed]
71. Lu Y, Feng Z, Meng Y, Bian L, Xie H, Mysore KS, et al. SLENDER RICE1 and Oryza sativa INDETERMINATE DOMAIN2 regulating OsmiR396 are involved in stem elongation. Plant Physiol. 2020;182(4):2213–27. [Google Scholar] [PubMed]
72. Völz R, Rayapuram N, Hirt H. Phosphorylation regulates the activity of INDETERMINATE-DOMAIN (IDD/BIRD) proteins in response to diverse environmental conditions. Plant Signal Behav. 2019;14(10):e1642037. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF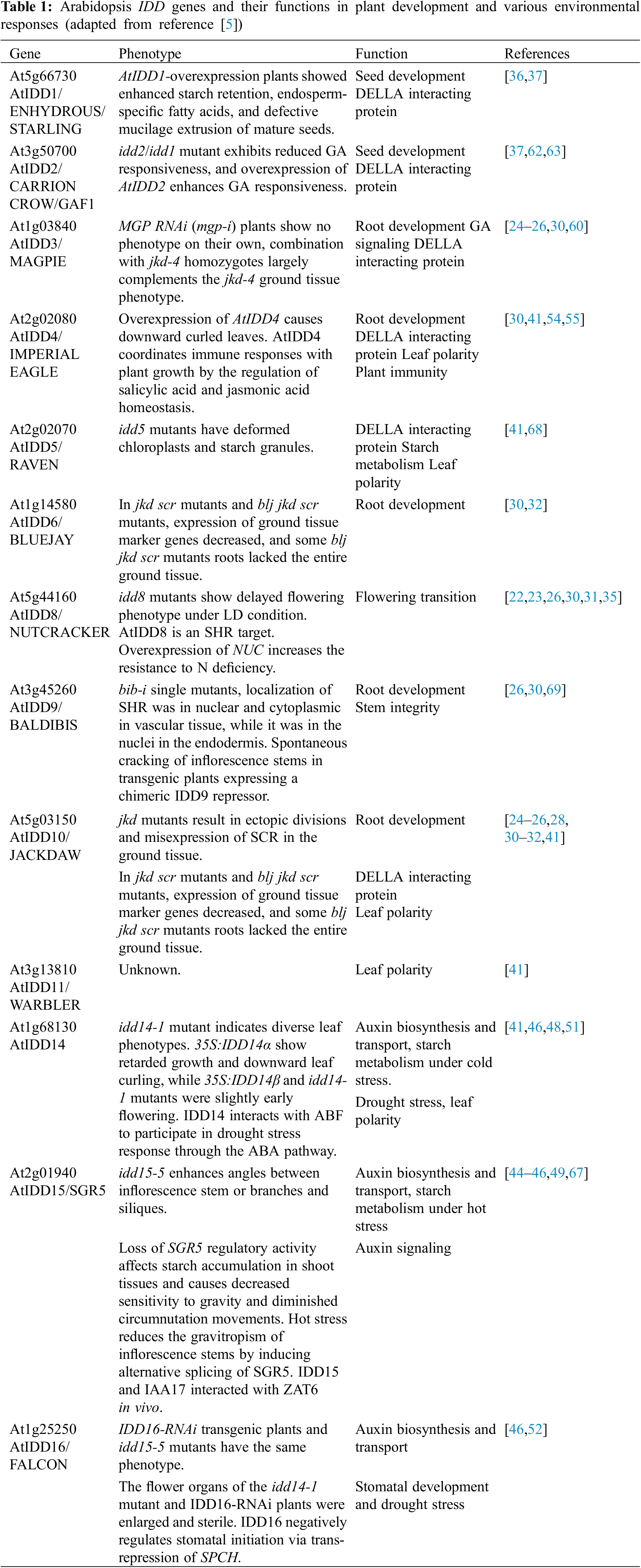
 Downloads
Downloads
 Citation Tools
Citation Tools