 Open Access
Open Access
REVIEW
Effect of Dandelion (Taraxacum mongolicum Hand.-Mazz.) Intercropping with Different Plant Spacing on Blight and Growth of Pepper (Capsicum annuum L.)
1
College of Horticulture and Landscape Architecture, Northeast Agricultural University, Harbin, 150030, China
2
Key Laboratory of Biology and Genetic Improvement of Horticulture Crops (Northeast Region), Ministry of Agriculture and Rural
Affairs, Harbin, China
3
State Key Laboratory of Improvement and Utilization of Saline-Alkali Soils (Inland Saline-Alkali Land of Northeast China),
Ministry of Agriculture and Rural Affairs of China, Jilin Agricultural University, Jilin, China
* Corresponding Authors: Xihong Yu. Email: ; Yao Cheng. Email:
# Equivalent contributing authors
(This article belongs to the Special Issue: Advances in Molecular Genetics and Physiology towards a Better Understanding of Agricultural Crop Plants)
Phyton-International Journal of Experimental Botany 2023, 92(8), 2227-2244. https://doi.org/10.32604/phyton.2023.027392
Received 27 October 2022; Accepted 06 May 2023; Issue published 25 June 2023
Abstract
Intercropping of crops that can secrete bacteriostatic active substances can not only inhibit the occurrence of disease but also have an important effect on plant growth. However, the effects of dandelion intercropping on pepper blight control and pepper growth remain unclear. In this study, the control effect of dandelion on pepper blight was studied by inoculating the pepper leaves with Phytophthora infestans, and it also discusses the correlation of the occurrence of pepper epidemic disease with the pepper canopy environment, soil environment, pepper photosynthesis, and yield index. The results showed that best plant distance for dandelion intercropping was 20 cm (P20), and the control effect reached 43.31%. As compared to the CK, SOD enzyme, POD enzyme, and PAL enzyme were significantly up-regulated during the growth of pepper; chlorophyll content in pepper leaves was significantly increased; photosynthetic characteristics were significantly increased; stem diameter and yield of crop pepper were effectively improved; and the quality of the pepper product was better, but intercropping dandelion resulted in a significant decrease of nutrients in the soil environment of pepper, so a reasonable intercropping distance was needed. The correlation analysis shows that the incidence of pepper blight (A) was significantly positively correlated with soil temperature (Q), intercellular carbon dioxide (L), and canopy air temperature (O). The incidence of capsicum blight (A) was significantly negatively correlated with chlorophyll content (F), net photosynthetic rate (K), stomatal conductance (M), ww rate (N), soil sucrase activity (W), vitamin C (AB), and leaf PAL enzyme (J). Finally, it was deduced that intercropping dandelion could effectively control the occurrence of pepper blight while also demonstrating a complex interaction with the pepper growing environment.Keywords
Supplementary Material
Supplementary Material FilePepper (Capsicum frutescence L.) is one of the most widely cultivated vegetables in the world. Pepper is loved by people for its unique flavor and rich nutrition [1–3]. Phytophthora capsici is a destructive soil-borne disease caused by Phytophthora capsici Leonian [4–6]. The disease is easily spread in production and difficult to control, resulting in significant losses to pepper production, generally resulting in a yield reduction of 20%–30%, serious to more than 50%, or even out of production [7]. The pathogen of Phytophthora capsici is Phytophthora capsica, and as a soil-borne disease, it can spread not only in soil but also through air, watering, wind, rain, and plant remains that break out in a short time [8].
Chemical control is one of the most effective means to control pepper blight, but with the increase in service life, the drug resistance of pathogens increases, which leads to a decrease in the field control effect [9]. At the same time, the use of chemical fungicides also has a serious impact on the environment and food safety [10]. Therefore, it is of great significance to use cultivation or physical means to control pepper blight. The application of intercropping to control soil-borne diseases has become a research hotspot in recent years. Through proper intercropping, a variety of crops can make better use of water, fertilizer, light, air, and other environmental conditions, improve intercropping population stability, improve field conditions, improve land utilization, and control diseases and insect pests [11,12]. The use of intercropping crops that can secrete bacteriostatic substances can effectively inhibit the occurrence of diseases. For example, the volatiles, extracts, and root exudates of garlic have antibacterial activity, which can interfere with and inhibit the infection of Phytophthora capsici [13,14].
Dandelion (Taraxacum mongolicum), a perennial herb of the genus Compositae [15], is also known as being rich in nutrition and having certain health care functions. Dandelion is a leafy vegetable [16], which is homologous to medicine and food and has high nutritional value [17]. The term “medicine and food homology” refers to the fact that “medicine and food have the same origin, the same root.” Medicine and food-homologous plants can not only be used as medicine to treat diseases but also be eaten for a long time to strengthen the body [18]. Dandelion extract has also been reported to have protective properties for plants. For example, dandelion root extract can improve the seed germination rate of rape [19]. It also has strong antibacterial activity against Fusarium wilt in cucumber [20]. Exogenous spraying of dandelion extract can improve the physiological and biochemical characteristics of cabbage and improve product quality [21].
Plant extracts have traditionally been used as a source of natural antimicrobial compounds. Dandelion roots contain high amounts of 9-hydroxyoctadecatrienoic acid and 9-hydroxyoctadecadienoic acid, and at the same time, phenolic compounds such as vanillin, acylaldehyde, and p-methoxyphenacetaldehyde with enhanced antibacterial activity were also identified. It was found that dandelion extract had a strong inhibitory effect on the microbial growth of Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, and Bacillus cereus [22,23]. Secondary metabolites, or PIEs, were extracted from the roots, leaves, and flowers of dandelion, including triterpenoid acetate (TritAc) and sesquiterpenoid lactone taraxic acid-D-glucopyranoglucopyranoester (TA-G). It has a strong repellent effect against cucumber beetle larvae (Diabrotica balteata) [24].
At present, there is a lack of studies to check the effect of intercropping dandelion on capsicum blight resistance, for evaluating the effects of photosynthetic characteristics and growth indexes of crops. In this study, the control effect of different intercropping spacings of dandelion on pepper blight and the influence of growth factors (canopy environment, soil environment, photosynthesis, and yield index) on capsicum were studied. We provide a reference for the effective production of pepper in order to obtain effective cultivation for pepper disease prevention and growth.
The pepper variety “Qingjiao No.1” was purchased from Beidahuang Kinfeng Seed Industry Co., Ltd. (China). Dandelion seeds were provided by Green Funong Traditional Chinese Medicine Seed Industry, and the SD33 strain of Phytophthora capsici was provided by the Key Laboratory of Vegetable Disease and Insect Biology, School of Plant Protection, Shandong Agricultural University, China.
Pepper seedlings were raised routinely in a greenhouse on May 17, 2019. Pepper plants with the same growth were selected and planted in the greenhouse with the high border double-row method on July 06. As shown in Figs. 1A and 1B, the border width was 120 cm, the border length was 480 cm, the plant spacing was 30 cm, the row spacing between two rows on the border was 40 cm, and the planting density was 3600 plants/667 m2.
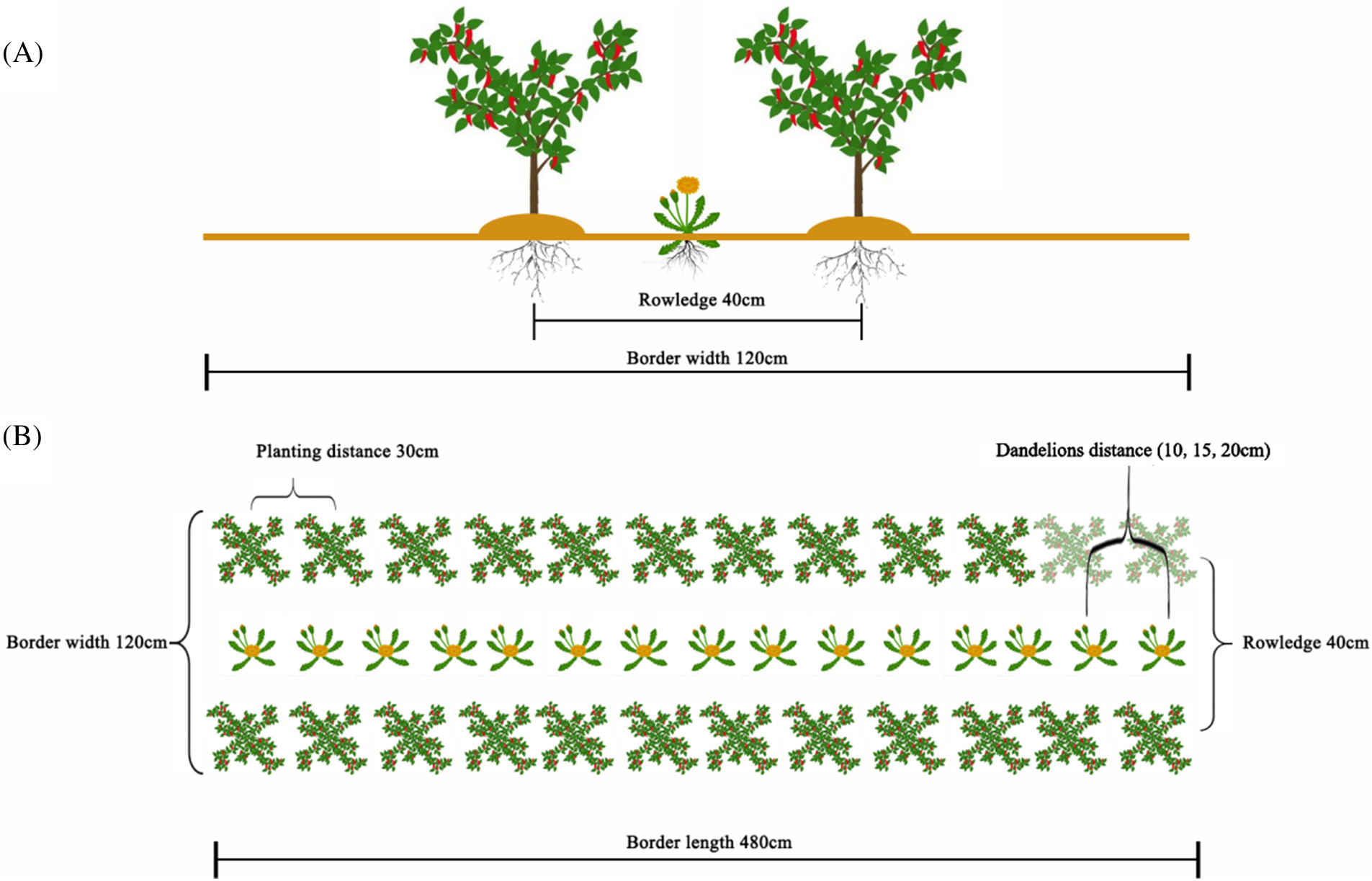
Figure 1: Diagram of dandelion intercropping at different plant spacings. (A) side view, (B) top view
Dandelion was sown between two rows of pepper to be planted in the greenhouse on July 06, 2019. The width of the dandelion planting row was 10 cm. When the seedlings grew to 3–4 leaves, the dandelion was fixed at the spacings of 10 cm (P10), 15 cm (P15), and 20 cm (P20), and the single cropping pepper was used as a control. The field layout is shown in Fig. 1, e.g., random block design, three times repeated, each plot with two beds, an area of 12 square meters.
After 15 days of colonization, capsicum was inoculated with Phytophthora capsicum by cutting the stem, and the inoculation method was based on the method of Yi et al. [25]. After inoculation, 10 pepper plants were randomly selected from each group of treatments with different plant spacings of dandelion to investigate the final disease index and incidence of pepper blight (36D) and calculate the control effect. The growth factors of pepper were investigated at 0, 12, 24, and 36 days after inoculation, including plant growth and yield, leaf enzyme activity, photosynthesis, canopy environment, soil environment, and quality indexes.
2.3 Determination of Related Indicators
To investigate the final disease index and incidence of pepper blight (36D) and calculate the control effect. The disease index was determined by referring to the whole plant field investigation method [26]. The incidence was divided into grades 0, 1, 3, 5, 7, and 9; Grade 0 was disease-free. Grade 1: only leaves and fruit have diseased spots above ground. Grade 3: brown rot spots on stems and branches above ground. Grade 5: brown rot spots at the stem base. Grade 7: brown rot spots on stems, branches, and stems on the ground. Grade 9: plant death (Supplementary Fig. S1).
Disease index = ∑[number of diseased plants × number of diseased grade]/[total number of investigated plants × number of highest diseased grade] × 100. Determination of disease rate: incidence rate (%) = number of diseased plants/total number of investigated plants × 100%. Control effect measurement: control effect (%) = (control disease index − treatment disease index)/control disease index × 100%.
2.3.2 Growth and Biomass Parameter Analysis
Plant height, strain amplitude was measured with a ruler (stem base to the growing point height was prevailed and plant with leaf maximum degree was subjected). Stem diameter was measured with a vernier caliper (thick stems were subjected to the root to the first split in the middle). Fruit began to mature in August and September, and the yield per plant was calculated using an electronic balance (per mu yield = yield per plant * 3600 plants).
2.3.3 Determination of Antioxidant Enzyme Activity and Photosynthetic Characteristics
Leaf disease-related enzyme activities were measured, including superoxide dismutase (SOD), peroxidase (POD), polyphenol oxidase (PPO), and phenylalanine ammonia lyase (PAL). In each group, plant leaves were randomly obtained from 10 listed pepper plants for mixing and then thoroughly ground after quick-freezing with liquid nitrogen and storing in the refrigerator at −80°C. The related enzyme activities were measured after weighing 10 g of tissue. SOD activity was measured by the NBT reduction method, POD activity by the guaiacol method, PPO activity by the catechol method, and PAL activity by the L-phenylalanine method [27].
Photosynthetic characteristics were measured, including net photosynthetic rate, stomatal conductance, transpiration rate, intercellular carbon dioxide concentration, and leaf chlorophyll. From 9:00 to 11:00 in the morning, the fifth functional leaf from top to bottom of pepper plants was selected, and the portable photosynthetic rate instrument LI-6400 (Beijing Licotai Technology Co., Ltd., China) was used to measure the results. Chlorophyll content was determined by the acetone method [28].
2.3.4 Determination of Spatial and Soil Environmental Indices
The canopy temperature and air relative humidity were measured by the TM820M hygrograph (the measurement position was less than 15 cm in the pepper canopy), and the soil temperature and soil relative humidity were measured by the TA8670 soil tester when the soil depth was 10 cm. Pepper population soil samples were obtained with a 45 cm * 50 cm ring knife soil collector of 100 cubic centimeters. Three soil samples were randomly obtained under each group of treatments and stored in a 4°C refrigerator. The contents of soil nitrate nitrogen, soil available phosphorus, soil available potassium, soil urease activity, soil phosphatase activity, and soil sucrase activity were measured in 10.0 g of soil. The content of soil nitrate nitrogen was measured by ultraviolet spectrophotometry [29], the contents of soil available phosphorus and soil available potassium were measured by a soil nutrient tachometer. Soil urease activity was measured by the colorimetric method [30], soil phosphatase activity was measured by the phenyldisodium phosphate colorimetric method [31], and soil sucrase activity was measured by the colorimetric method [32].
2.3.5 Determination of Quality Characteristics of Pepper
The pepper quality indices included soluble sugar, soluble protein, and vitamin C, which were harvested from August to September at the fruit ripening stage. Some fruits were randomly obtained from the 10 labeled pepper plants in each group for mixing, and then fully ground with liquid nitrogen after quick freezing and stored in the refrigerator at −80°C. After weighing 10 g of tissue, related quality indices were determined. Soluble sugar content was determined by anthrone colorimetry, soluble protein content was determined by the Coomassie bright blue G-250 method, and vitamin C (VC) content was determined by 2, 6-dichlorophenol indophenol titration [33].
SPSS (v25.0. IBM Corporation, USA) software was used to analyze One-way ANOVA, Two-way ANOVA and Duncan test multiple comparisons between mean values were obtained by performing the Pearson correlation coefficient was used to analyze the correlation, and all statistical analyses were performed at a 95% confidence level. The data were plotted using GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, USA).
3.1 Effects of Intertreatment and Sampling Time on Physiological Indexes of Pepper by Two-Factor Analysis of Variance
After inoculation of capsicum mildew at the seedling stage, the physiological indices of plant growth at different periods were determined under different intercropping distance treatments. According to the two-factor analysis of variance (see Supplementary Table S1), the main effect of the time variable on each physiological index is significant. The main effect of treatment variables except plant height and the atmospheric relative humidity index was not obvious, and the main effect of other indicators was obvious. The main interaction effect of time variables and treatment variables was not obvious except for plant height, stem diameter, and atmospheric relative humidity, but the interaction effect of other indexes was obvious. Two-factor analysis showed that during the growth of pepper, each index changed obviously at the sampling point. In particular, plant height and atmospheric relative humidity were almost entirely affected by the time variable; the main effects of treatment and Time * Treatment were not significant, indicating that they were not affected by intercropping dandelion treatment. Plant height, crown diameter and atmospheric relative humidity were not significantly affected by the interaction of time and treatment, but time or treatment had a significant impact on a single factor (see Supplementary Tables S2 and S3). The leaf contents, leaf SOD enzyme, leaf POD enzyme, leaf PPO enzyme, leaf PAL enzyme, net photosynthetic rate, intercellular CO2 concentration, stomatal conductance, transpiration rate, canopy air temperature, soil temperature, soil relative humidity. Soil nitrate nitrogen content, Soil available phosphorus content, Soil available potassium content, Soil urease activity, Soil sucrase activity, Soil phosphatase activity was influenced by the main effect of sampling time variable and inter-range distance treatment variable, as well as the interaction effect of Time * Treatment (see Supplementary Table S4).
3.2 Intercropping Dandelion Reduces the Pepper Blight Occurrence
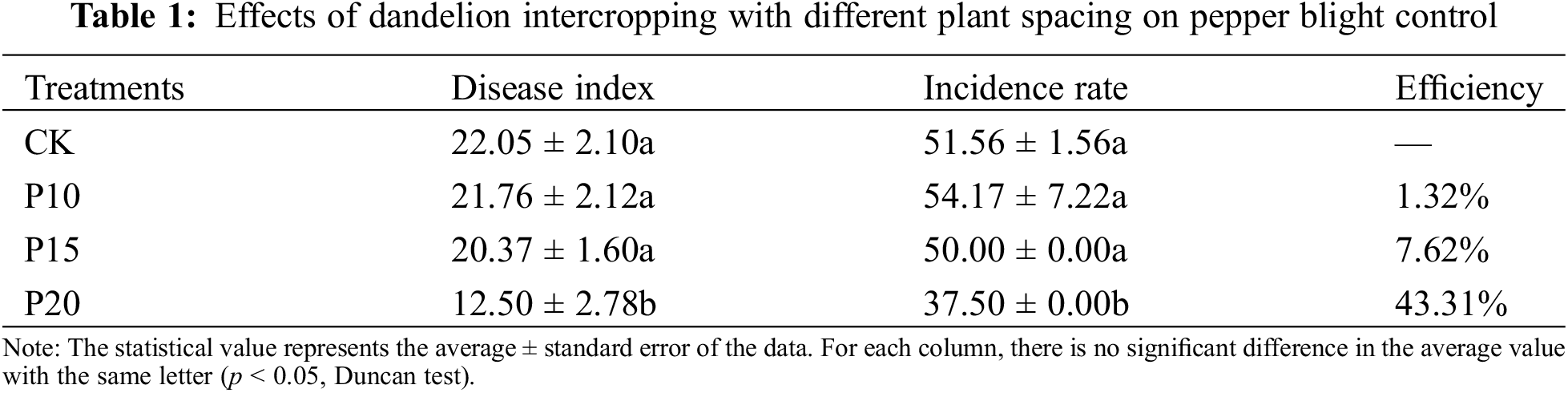
The intercropping mode of dandelions and peppers could inhibit the occurrence of pepper blight to a certain degree. Intercropping dandelions with plant spacing of 20 cm (P20) obtained the best effect on inhibiting pepper blight, and the disease index and incidence of pepper blight decreased significantly compared with pepper monoculture, with the control effect reaching 43.31% (Table 1). Intercropping dandelions with plant spacings of 10 cm and 15 cm (P10, P15) achieved little effect on pepper blight in comparison with pepper monoculture.
3.3 Intercropping Dandelion Affects the Growth of Pepper
The effect of intercropping dandelions on the growth of peppers is shown in Fig. 2. As compared with pepper monoculture, the plant height of peppers was not obviously affected by intercropping dandelions (Fig. 2a). During 0 and 12 days, the crown diameter of peppers showed no significant difference between intercropping dandelions and pepper monoculture, while with the pepper growing, the plant width increased rapidly compared with other treatments (Fig. 2b). When compared to other treatments and pepper monoculture, the stem diameter of intercropping dandelions with planting spacing of 20 cm increased rapidly. The stem diameter of pepper increased after intercropping dandelions with different plant spacing (Fig. 2c). When compared to pepper monoculture, intercropping dandelions with planting spacing of 15 cm and 20 cm increased pepper yield significantly, while intercropping dandelions with planting spacing of 10 cm had no discernible effect on pepper yield (Fig. 2d). These findings indicated that intercropping dandelions with a planting spacing of 20 cm contributed to pepper growth and yield accumulation, and to some extent, this can promote pepper blight resistance.
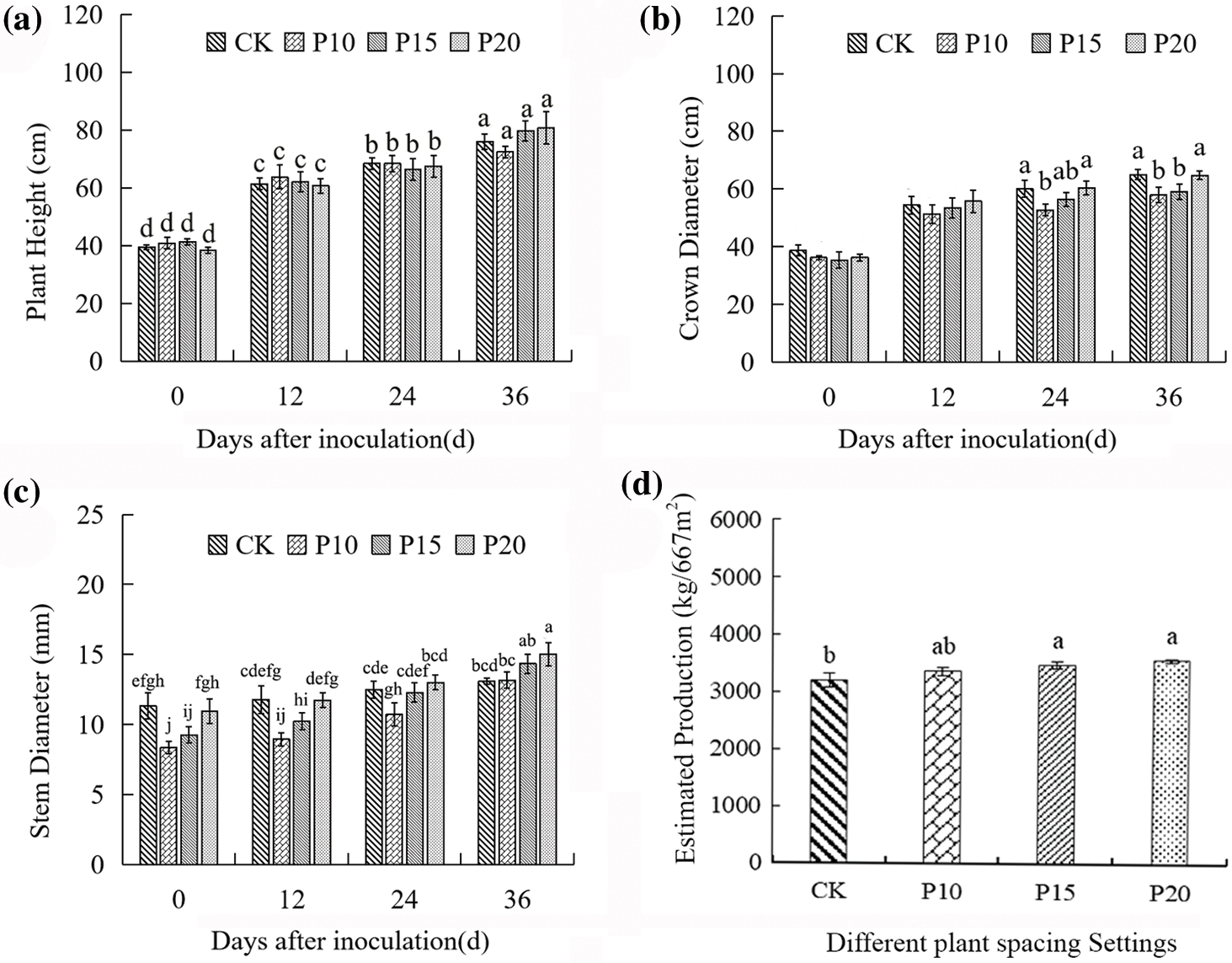
Figure 2: Effects of dandelion interplanting with different plant spacing on pepper growth Note: For each column, there is no significant difference in the average value with the same letter (p < 0.05, Duncan test)
3.4 Intercropping Dandelions Affected Enzymes Activity in Pepper Leaves
As shown in Fig. 3, intercropping the dandelion influenced the enzyme activity in pepper leaves compared with pepper monoculture. The activity of disease-resistance enzymes in leaves was influenced by infection time and intercropping. Leaf disease-resistance-related enzyme activities were significantly up-regulated in CK group and treatment group at 12 days, and then slowly decreased. Compared with CK group, intercropping dandelion at 20 cm could significantly increase SOD, POD, PPO and PAL enzyme activities in leaves. However, SOD, POD, PPO and PAL activities of intercropped dandelion leaves in 10 cm and 15 cm were not significantly up-regulated, but down-regulated. These findings indicated that intercropping dandelions with a planting spacing of 20 cm increased protected enzyme activity in pepper leaves, which may contribute to pepper blight resistance.
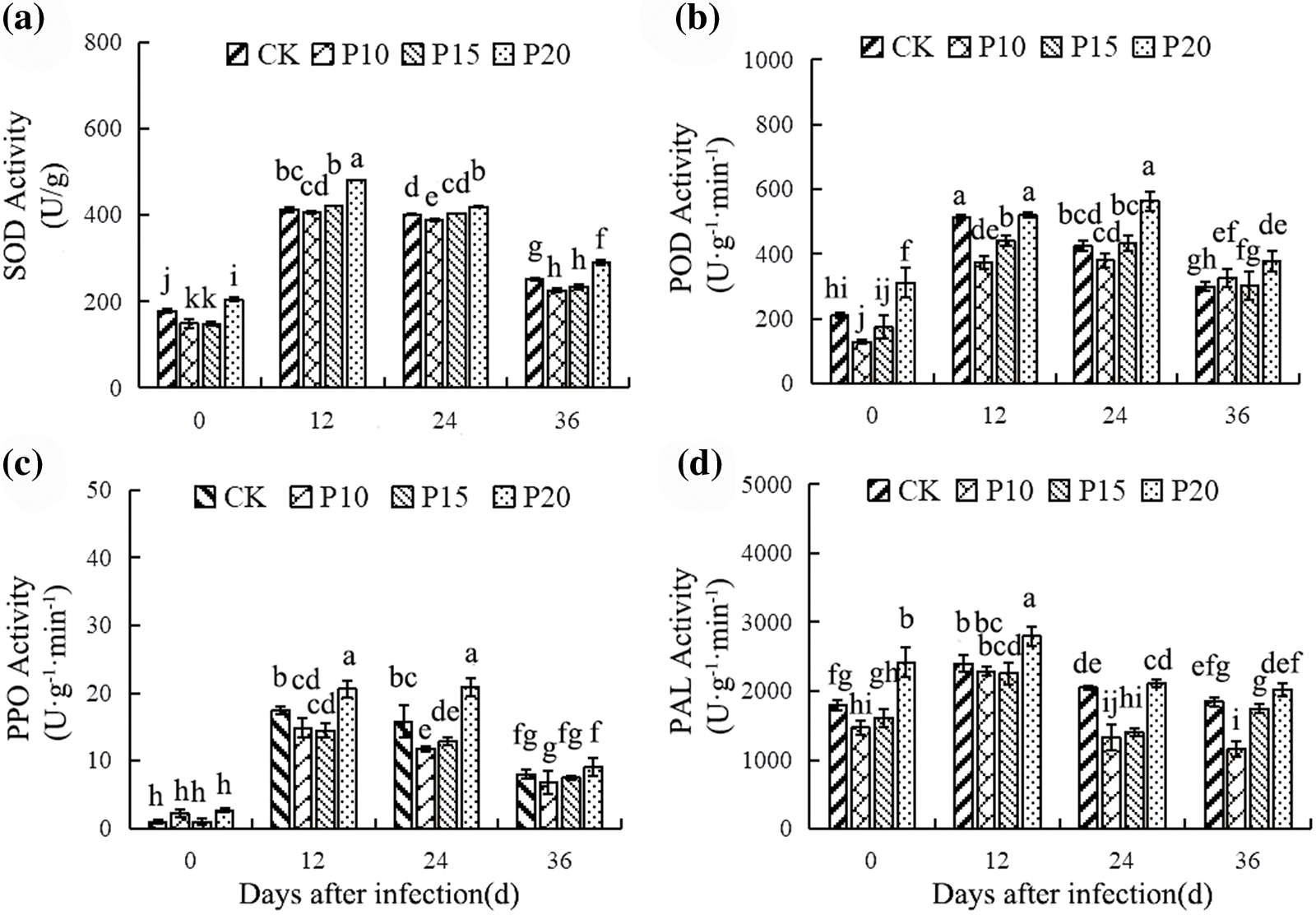
Figure 3: Effects of dandelion interplanting with different plant spacing on enzyme activities in pepper leaves At each date, columns followed by different letters were statistically significantly different (p < 0.05, Duncan test)
3.5 Intercropping Dandelion Affected Photosynthetic Characteristics of Pepper
The intercropping of dandelions with different plant spacing had an effect on the photosynthetic characteristics of pepper. As shown in Fig. 4, the net photosynthetic rate, stomatal conductance and transpiration rate increased rapidly, but the intercellular CO2 concentration did not change strongly along with peppers growing. Intercropping dandelions elevated the net photosynthetic rate, stomatal conductance and transpiration rate significantly, and showed obvious difference compared with peppers monoculture. Additionally, under the treatment of intercropping dandelions with 20 cm planting spacing, the net photosynthetic rate, stomatal conductance and transpiration rate were higher, while the intercellular CO2 concentration decreased significantly compared with other treatment and peppers monoculture. It is worth noting that intercropping dandelions with 10 cm and 15 cm planting spacing reduced the intercellular CO2 concentration apparently, compared with peppers monoculture.
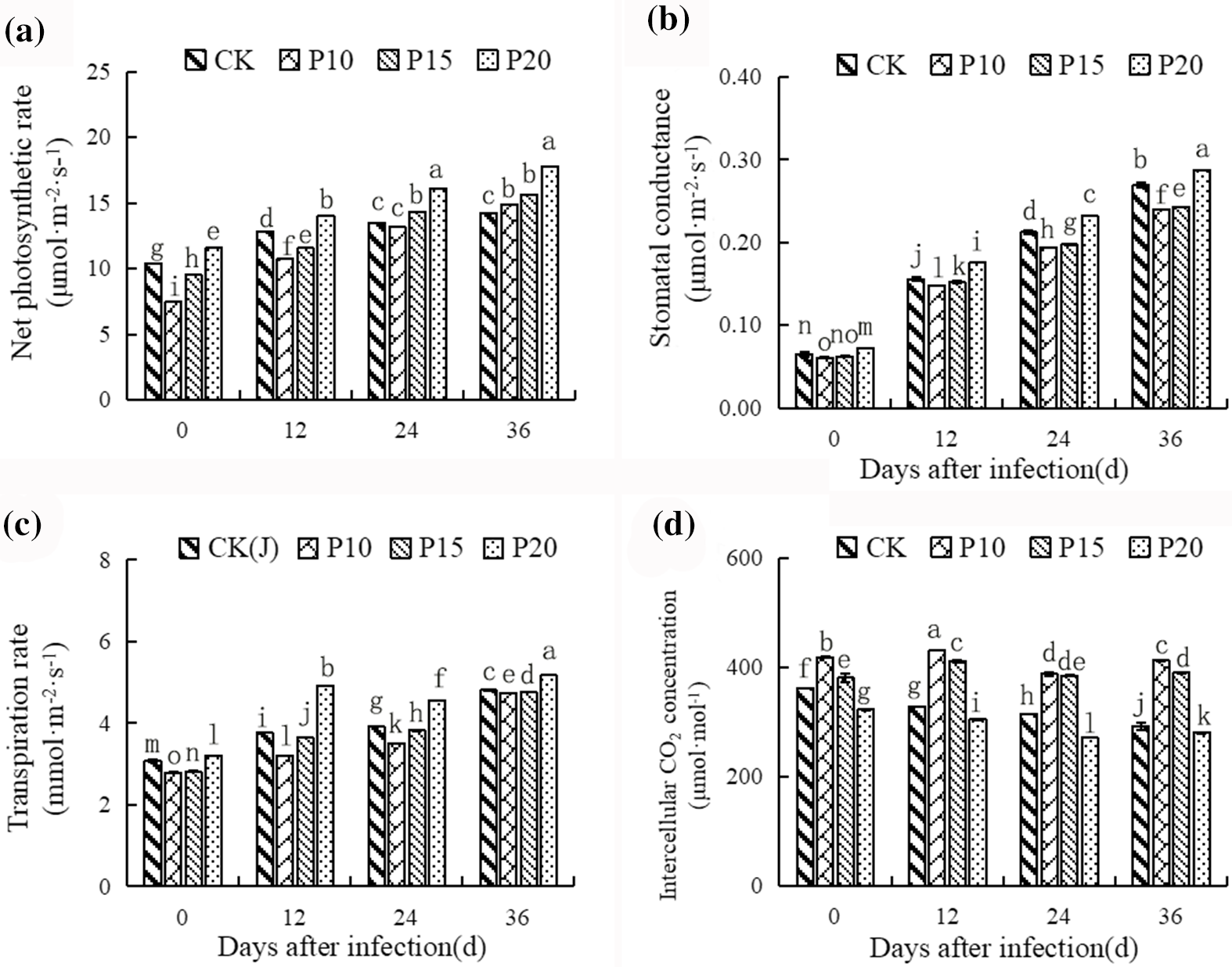
Figure 4: Effects of dandelion intercropping with different plant spacing on photosynthetic characteristics of Pepper. At each date, columns followed by different letters were statistically significantly different (p < 0.05, Duncan test)
Chlorophyll is one of the important factors affecting photosynthesis, and dandelion intercropping can significantly affect the chlorophyll content of pepper leaves. It can be seen from Fig. 5 that the content of chlorophyll increases with the growth of pepper. On the 12th day, the chlorophyll content of P20 treatment was significantly higher than that of the control, while that of P10 treatment was significantly lower than that of the control, on the 24th day, the chlorophyll content of P20 treatment and control was significantly higher than that of P10 and P15 treatments, on the 36th day, the chlorophyll content of P20 treatment was significantly higher than that of the other two treatments and control.
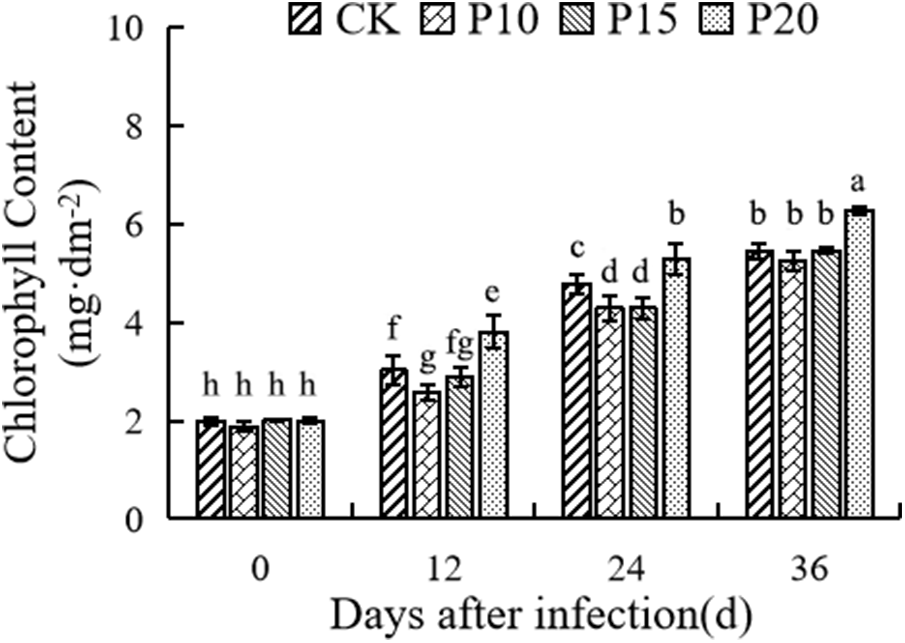
Figure 5: Effects of dandelion interplanting with different plant spacing on photosynthetic characteristics (chlorophyll content) of pepper. For each column, there is no significant difference in the average value with the same letter (p < 0.05, Duncan test)
3.6 Intercropping Dandelion Affected the Canopy Environment of Pepper
With the growth of crops, the intercropping of dandelion with different plant spacing had a certain effect on the canopy temperature of pepper. As shown in Fig. 6a, there was no significant difference in canopy temperature between 6 days and 12 days after inoculation, but the canopy temperature of pepper under dandelion intercropping began to change at 18 days, which showed that the canopy temperature of P15 and P20 treatments was significantly lower than that of the control, and that of P20 treatments was significantly lower than that of P10 and P15 treatments at 24 days. As can be seen from Fig. 6b, the soil temperature decreased gradually with the growth of the plant and increased at 24 days. From the 6th to 24th day after inoculation, the soil temperature decreased with the increase of the distance between dandelion plants, especially on the 24th day, the difference among the three treatments was significant, in which P10 was slightly higher than that of the control, but the difference between them was not significant, but P20 was significantly lower than that of the control.
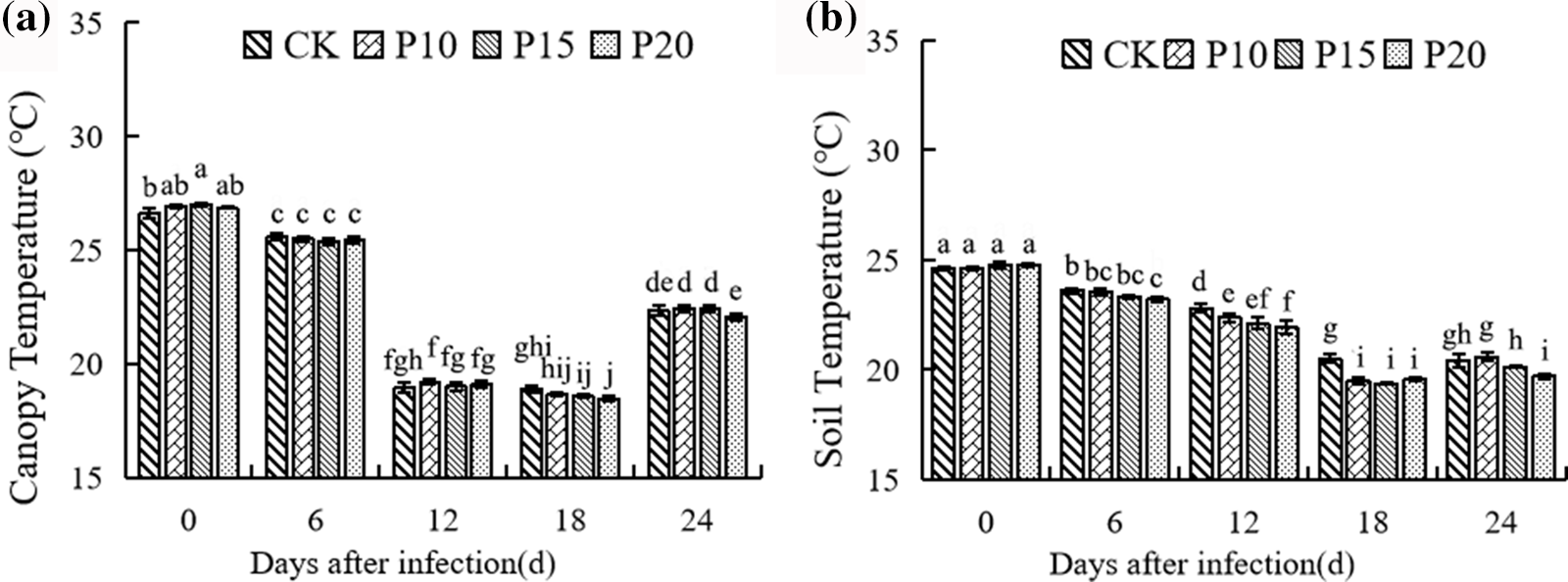
Figure 6: Effects of different plant spacing on population temperature of Dandelion. For each column, there is no significant difference in the average value with the same letter (p < 0.05, Duncan test)
It can be seen from Fig. 7a that dandelion intercropping had little effect on the air relative humidity of the population, except that the P15 treatment was significantly lower than the control at 24 days, and there was no significant difference between each treatment and the control. For soil relative humidity, as shown in Fig. 7b, the larger the plant spacing, the smaller the soil relative humidity. On the day of inoculation (0 days), the soil relative humidity of dandelion treatments with different plant spacing was significantly higher than that of pepper monoculture, and from 6 days to 24 days after inoculation, the soil relative humidity of P20 treatment was significantly lower than that of P10 treatment, but there was no significant difference between P10 treatment and control except P10 treatment for 18 days and 24 days.
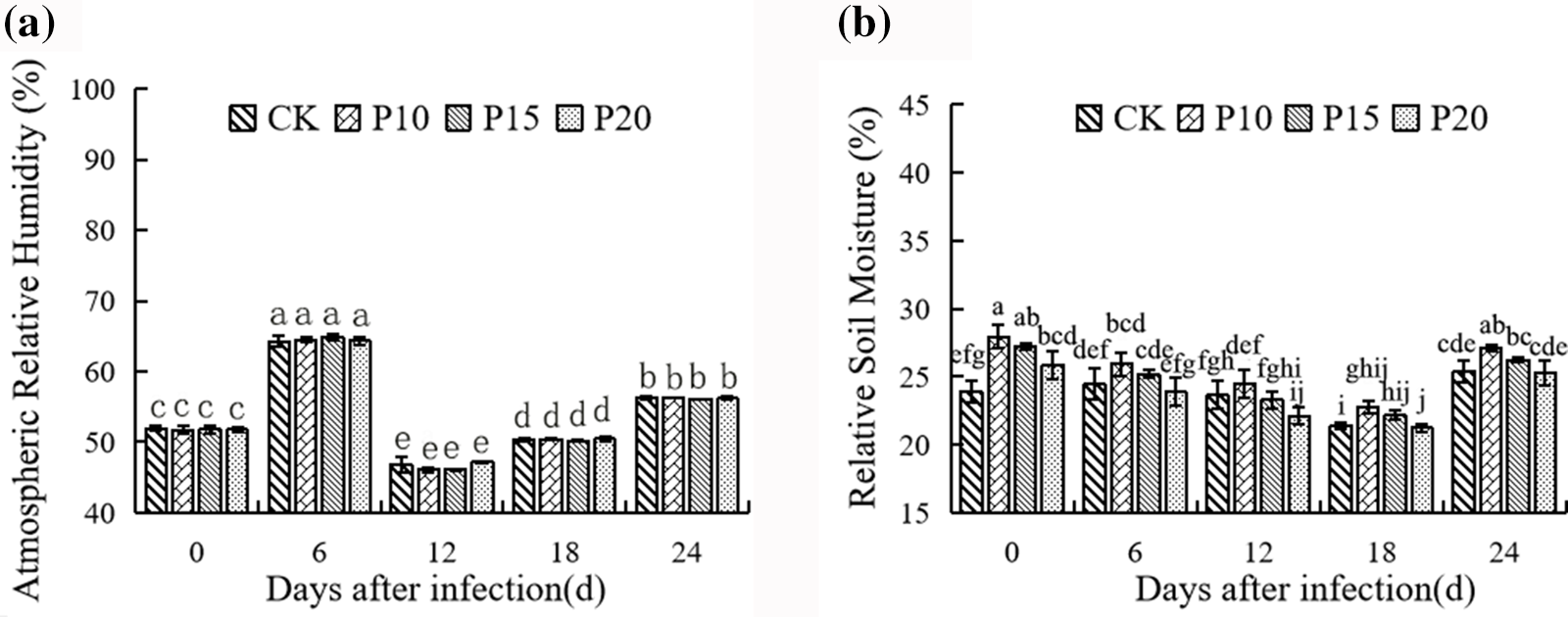
Figure 7: Effects of different plant spacing on population humidity of Dandelion. For each column, there is no significant difference in the average value with the same letter (p < 0.05, Duncan test)
3.7 Intercropping Dandelion Affects Soil Environment of Pepper
Intercropping dandelion could affect the soil environment of peppers. Compared with the pepper monoculture system, the contents of nitrate nitrogen and available phosphorus under the system of dandelion intercropping pepper decreased obviously in soil, however, the available potassium was not existed difference obviously in the pepper and dandelion intercropping and monoculture system (Fig. 8). In addition, the planting spacing of dandelion in the intercropping system could affect the accumulation of soil nutrients, with the planting spacing of the dandelion increasing, the contents of nitrate nitrogen and available phosphorus increased. While the available potassium showed various trends, the 10 cm planting spacing of dandelions accumulated higher available potassium under the 12d and 24d compared with other treatments. These results demonstrated that intercropping dandelions could elevate the nutrient use efficiency in peppers.
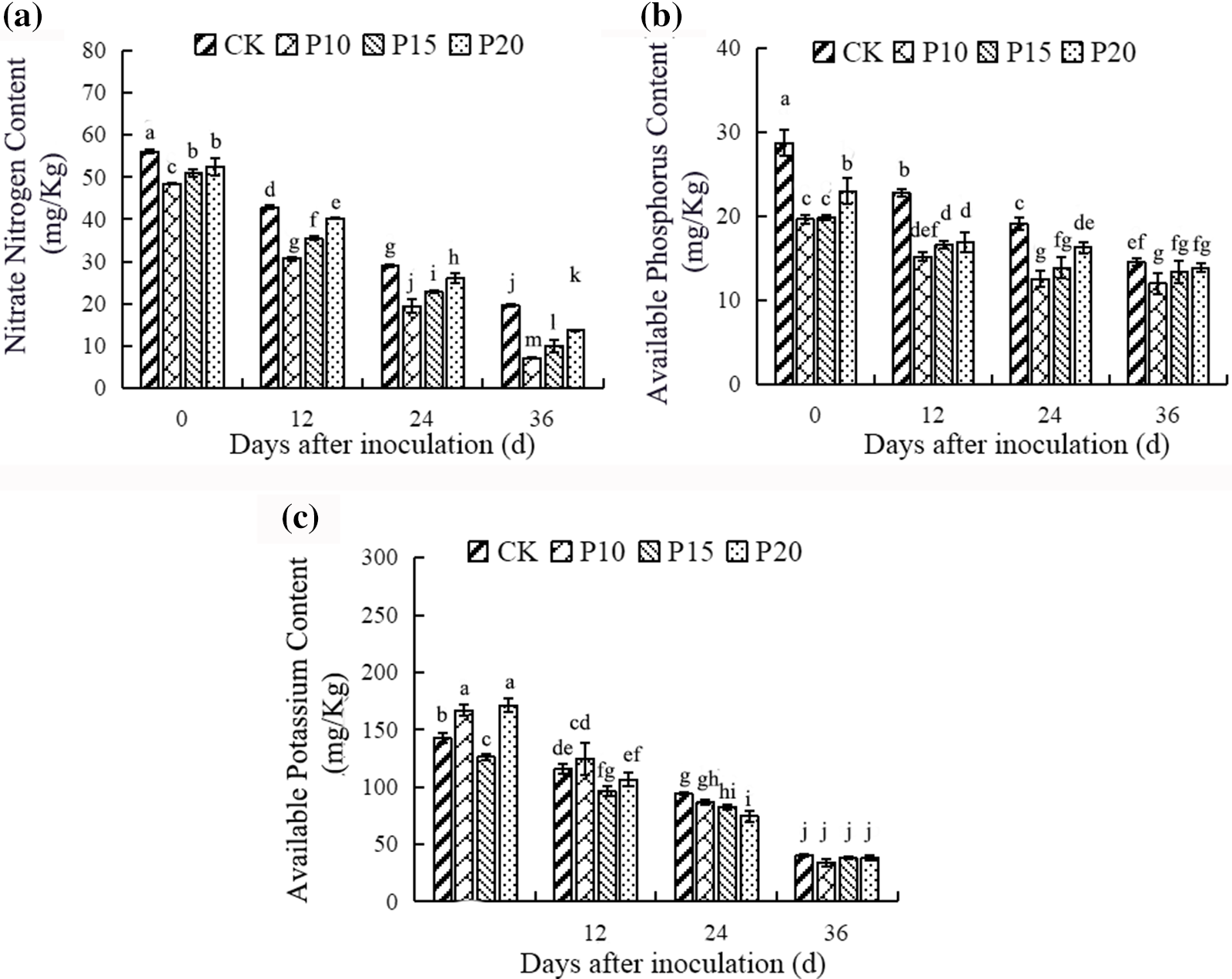
Figure 8: Effects of different plant spacing on soil nutrients of Dandelion. For each column, there is no significant difference in the average value with the same letter (p < 0.05, Duncan test)
In addition, the soil enzyme activities were affected in the pepper and dandelion intercropping and monoculture system. As shown in Fig. 9, intercropping dandelions reduced the soil urease activity, the soil urease activity of all treatments was significantly lower than that of the control at 0 d, 24 d, and 36 d. Contrasted to the soil urease activity, intercropping dandelions inhibited the soil sucrose activity of peppers in a certain degree in comparison with peppers monoculture, and moreover, the planting spacing of dandelions affected the soil sucrose activity of peppers, compared with the planting spacing 10 cm and 15 cm of dandelions, the soil sucrose activity of peppers was elevated significantly when the dandelion was planted with 20 cm planting spacing. The soil phosphatase activity of peppers intercropping dandelions with 10 cm planting spacing was elevated in discernible during 0 d and 12 d compared with 15 cm and 20 cm planting spacing, while during 24 d and 36 d, the soil phosphatase activity of peppers was higher obviously with the dandelion planting 15 cm and 20 cm compared with other treatments and peppers monoculture. Consequently, intercropping dandelions negatively regulated activities of the soil urease and sucrose, while having no obvious impacts on the soil phosphatase activity of peppers.
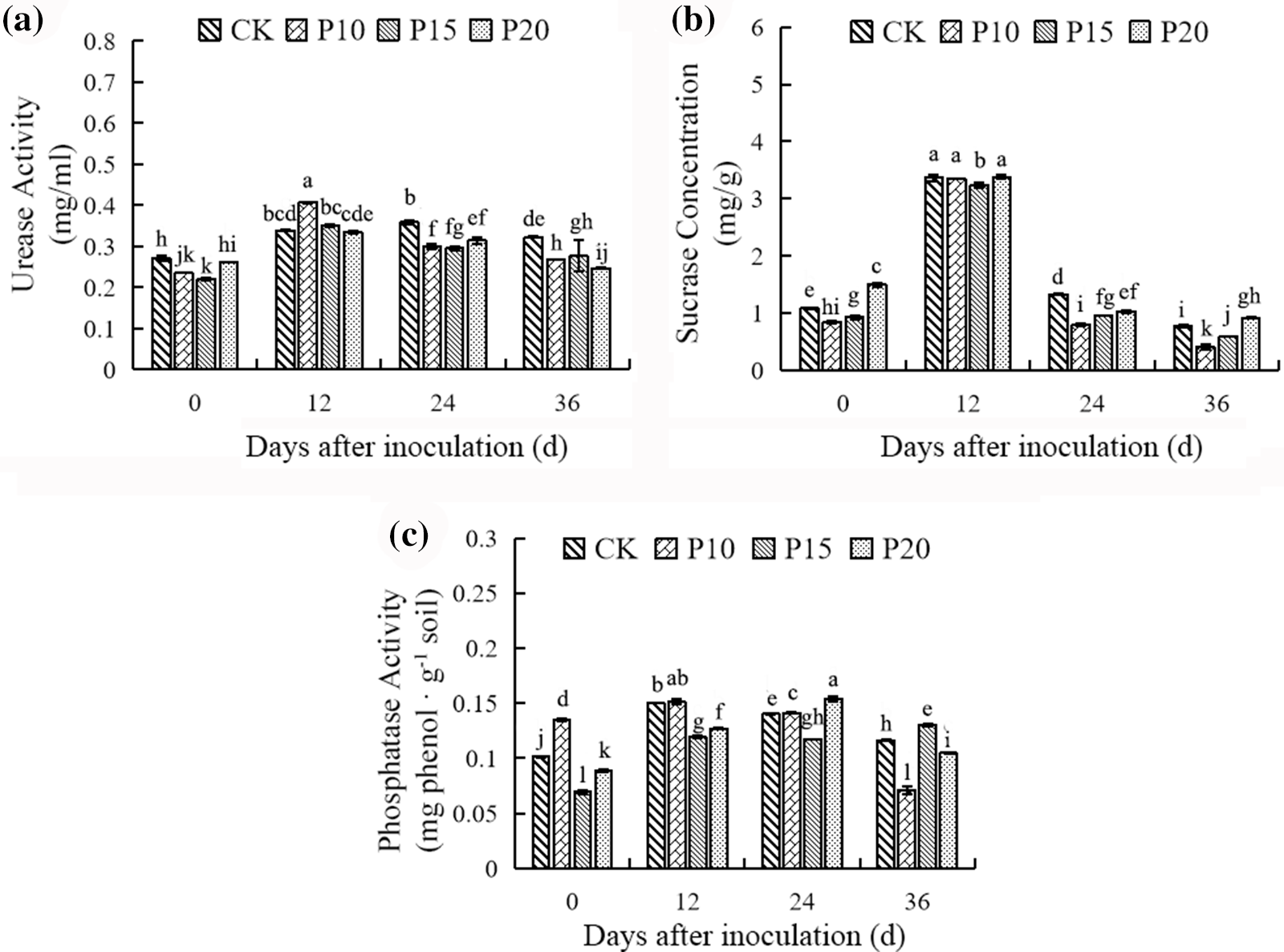
Figure 9: Effects of different plant spacing on soil enzyme activities of Dandelion. For each column, there is no significant difference in the average value with the same letter (p < 0.05, Duncan test)
3.8 Intercropping Dandelion Affects the Quality of Pepper
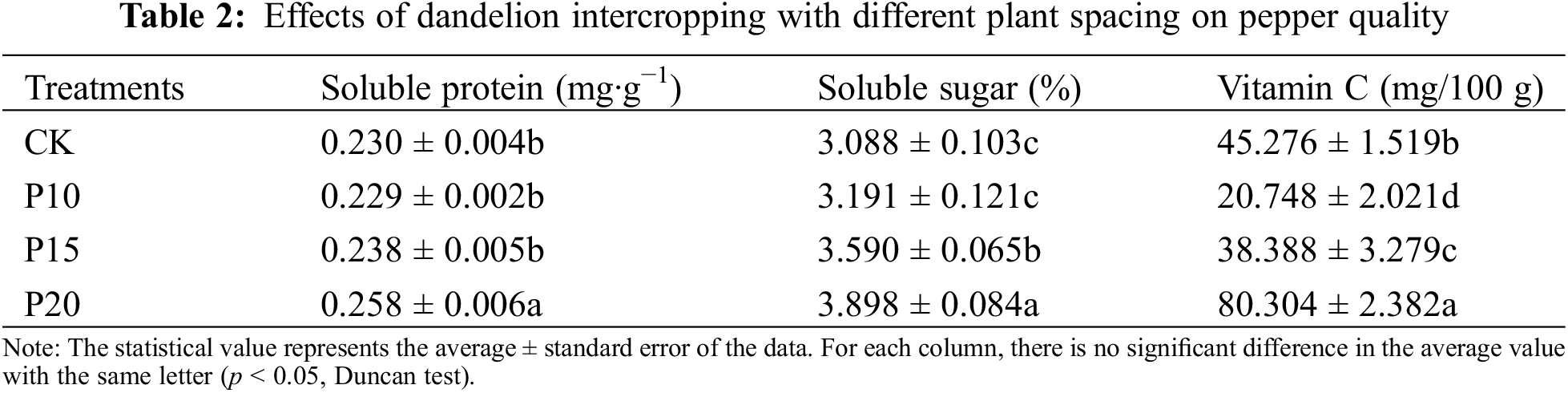
Intercropping dandelions affected the accumulation of qualities in pepper leaves. The soluble protein, soluble sugar and vitamin C was accumulated more higher obviously with intercropping dandelions with planting spacing 30 cm, compared with other treatments and peppers monoculture, see Table 2. The contents of soluble sugar, soluble protein and vitamin C in dandelion treatment with large plant distance (P20) were significantly higher than those in other treatments, and the quality was the best, while there was no significant difference in soluble protein content between P10 and P15 treatments and the control. The soluble sugar content and vitamin C content increased with the increase of plant distance, and the vitamin C content of P10 and P15 treatments with smaller plant distance was significantly lower than that of the control, while the soluble sugar content of P10 treatment was slightly higher than that of the control. But there was no significant difference between them.
3.9 Analysis of Factors Affecting the Incidence of Capsicum Blight
The correlation analysis (Fig. 10) showed that the incidence of pepper blight (A) was positively correlated with intercellular carbon dioxide (L), canopy temperature (O), air relative humidity (P), soil temperature (Q), soil relative humidity (R), soil urease activity (V) and soluble protein content (Z). And control effect (B), plant height (C), plant width (D), stem diameter (E), chlorophyll content (F), leaf SOD enzyme (G), leaf POD enzyme (H), leaf PPO enzyme (I), leaf PAL enzyme (J), net photosynthetic rate (K), stomatal conductance (M), transpiration rate (N), soil nitrate nitrogen content (S), soil available phosphorus content (T), soil available potassium content (U), Soil sucrase activity (W), soil phosphatase activity (X), pepper yield (Y), soluble sugar content (AA) and vitamin C (AB) were negatively correlated.
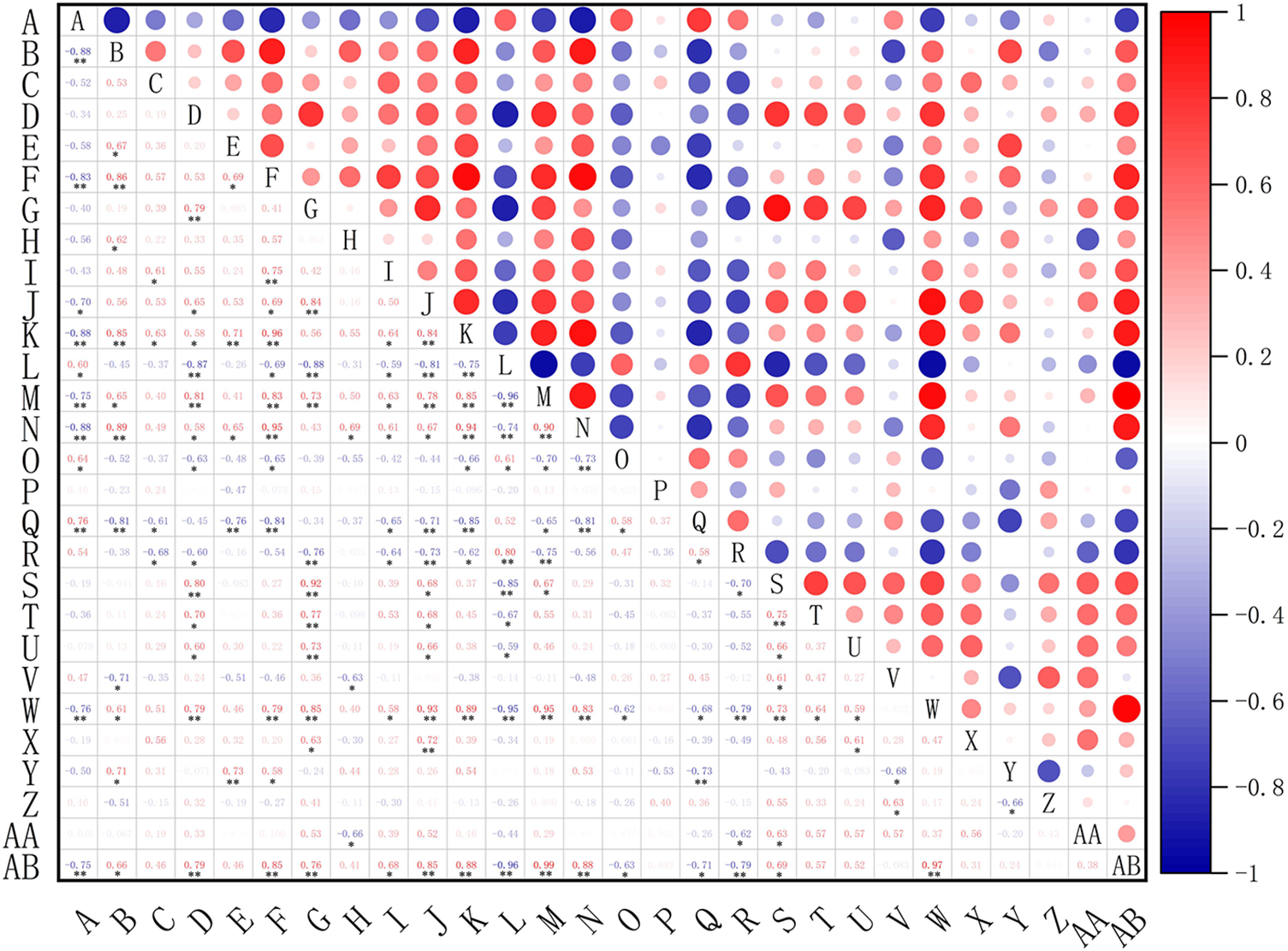
Figure 10: Correlation analysis of incidence of pepper blight. A, Incidence, B, Control effect, C, Plant height, D, Plant width, E, Stem diameter, F, Chlorophyll content, G, Leaf SOD enzyme, H, Leaf POD enzyme, I, Leaf PPO enzyme, J, Leaf PAL enzyme, K, Net photosynthetic rate, L, Intercellular CO2 concentration, M, Stomatal conductance, N, Transpiration rate, O, Canopy air temperature, P, Air relative humidity, Q, Soil temperature, R, Soil relative humidity. S, Soil nitrate nitrogen content, T, Soil available phosphorus content, U, Soil available potassium content, V, Soil urease activity, W, Soil sucrase activity, X, Soil phosphatase activity, Y, Pepper yield, Z, Soluble protein content, AA, Soluble sugar content, AB, Vitamin C. * and * * represent 5% and 1% significant levels, respectively
Among them, the correlation coefficient between pepper blight incidence (A) and soil temperature (Q), intercellular carbon dioxide (L) and canopy air temperature (O) were 0.763, 0.604 and 0.643, respectively. The correlation coefficients between incidence (A) and control effect (B), chlorophyll content (F), net photosynthetic rate (K), stomatal conductance (M), transpiration rate (N) and soil sucrase activity (W) were −0.883, −0.827, −0.877, −0.751, −0.883 and −0.756, respectively. The correlation with vitamin C (AB) reached −0.747, reaching a very significant negative correlation level, and the correlation coefficient with leaf PAL enzyme (J) reached −0.699, reaching a significant negative correlation level. Therefore, in the cultivation of pepper crops, it is possible to appropriately increase the chlorophyll content (F), net photosynthetic rate (K), stomatal conductance (M), transpiration rate (N), soil sucrase activity (W), vitamin C (AB), leaf PAL enzyme and other factors. Properly reduce the influencing factors of soil temperature (Q), intercellular carbon dioxide (L), canopy air temperature (O), so as to reduce the incidence of pepper blight.
In this study, we also found that the correlation coefficient between fruit yield (Y) and control effect (B) was 0.709, and that with stem diameter (E) was 0.728, and the correlation coefficient with chlorophyll content (F) was 0.582, which reached a significant positive correlation level, and that with soil temperature (Q) was −0.729, which reached an extremely significant negative correlation. The correlation coefficient between soil urease activity and soil urease activity (V) was −0.677, which was a significantly negative correlation. Therefore, improving the control effect (B) and stem diameter (E) influencing factors, reducing chlorophyll content (F) and soil temperature (Q) can increase the fruit yield of pepper per plant (Y).
The intercropping model is beneficial to improve the diversity of farmland ecosystems, change the population environment in the intercropping system, and then reduce the incidence of diseases and insect pests [34]. The results showed that in the intercropping of dandelion and pepper, different plant distances of dandelion had different control effects on pepper blight, and the treatment with a dandelion plant distance of 20 cm (P20) had the best control effect, which could significantly reduce the disease index and incidence of pepper blight, while the treatments with a plant distance of 10 cm (P10) and 15 cm (P15) had a lower control effect on pepper blight. The reason for the low control effect under P10 and P15 treatments may be that the dandelion root system is strong, and dense planting causes it to compete for nutrients in the soil, inhibit the growth of pepper, reduce the resistance of pepper itself, and then reduce the ability of dandelion to prevent and control pepper blight.
In the intercropping model, too high or too low density affects plant growth and yield; too high planting density leads to fierce competition among intercropping species (for nutrients, light, etc.); too low planting density, such as in soybeans with nitrogen fixation functions, will lead to no significant difference in the yield of main plant materials [35,36]. In this experiment, the yield of the P20 and P15 treatments increased significantly, but the yield of the P10 treatment with the smallest plant distance was not significantly different from that of pepper monoculture (CK). It shows that properly increasing the plant distance of dandelion can effectively prevent and control pepper blight in the intercropping of dandelion and pepper. In agricultural production, different cultivation measures and crop interaction principles can reduce the occurrence of plant diseases and insect pests. For example, coumaric acid secreted by wheat roots can significantly inhibit the germination, sporulation, and growth of watermelon Fusarium wilt spores, and then effectively reduce the occurrence of Fusarium wilt in watermelon [37]. However, what kind of bacteriostatic substance is secreted by dandelion root, how this bacteriostatic substance works, and so on need to be further studied.
Intercropping can create a suitable canopy and soil environment to improve stress conditions and promote plant growth. In this experiment, at the initial stage of intercropping between dandelion and pepper, it had little effect on the change in air canopy temperature and air relative humidity. With the formation of a dandelion and pepper intercropping population, dandelion leaves gradually cover the soil; soil temperature decreases with the decrease of dandelion intercropping distance; the shielding effect of dandelion leaves on the surface increases; and the exposed soil absorbs less solar radiation, thus reducing the soil temperature. Shading reduced the evaporation of soil water into the air, and the water absorption capacity of roots also had a significant effect on soil moisture. The P10 treatment had poor plant growth and weak root water absorption capacity, so the soil’s relative humidity was higher than that of other treatments. Dandelion leaves shielded soil temperature on day 24 after inoculation; soil temperature of the intercropping treatment decreased significantly on day 36; and soil temperature of the intercropping treatment decreased significantly on day 48 as intercropping time was extended. There was no significant difference in soil temperature between the intercropping treatment and the control. This may be due to the lack of light for 36 days and the tendency for the decrease of soil temperature to be consistent due to the influence of ventilation, but also because the shielding effect of dandelion leaves has a certain limitation on soil temperature and there is little difference in soil temperature in the absence of light. However, there is no significant difference in air relative humidity and soil relative humidity due to greenhouse ventilation. Different plant spacing for dandelion intercropping has a certain prevention and control effect on pepper blight, so it can be concluded that when the temperature in the environment decreases more and the humidity does not change much, it is more conducive to the prevention and control of pepper blight. Especially when the intercropping distance of dandelion was 20 cm (P20), the temperature was lower than the optimum temperature for the growth of Phytophthora capsici strains, and the disease index and incidence were significantly lower than those of P10 and P15. This shows that the proper increase in the distance between dandelion intercropping plants and the creation of a suitable temperature and humidity environment are more conducive to the prevention and control of pepper blight.
The intercropping population structure formed by each intercropping method is different, which has an effect on plant photosynthesis. In this experiment, dandelion intercropping will form different population structures: pepper functional leaves are in a high position, dandelion leaves are in a low position, and this structure can effectively improve light energy utilization efficiency. Under the treatment of dandelion intercropping distance of 20 cm (P20), the photosynthetic parameters at different stages were significantly higher than those of other treatments and controls, and the chlorophyll content was also significantly increased. In this experiment, it was found that the photosynthetic rate and chlorophyll content of P10 and P15 treatments with small intercropping distances were lower than those of the control. This may be due to the high intercropping density and the nutrient competition between dandelion and pepper, which inhibited the growth of pepper, and the disease index and incidence of pepper blight treated with P10 and P15 were not significantly different from those of the control. This shows that the occurrence of the disease may seriously affect the photosynthesis of pepper. For example, the chlorophyll content in cucumber will gradually increase with plant growth. When cucumber is damaged by downy mildew, the decrease in chlorophyll content in damaged leaves leads to a decrease in photosynthesis [38]. The chlorophyll content of the plant after inoculation was determined in this experiment, but the change law of chlorophyll content in the early stages of inoculation needs to be studied further.
Yield is an important index to measure whether the intercropping method is reasonable [39]. Because the pepper variety used in this experiment was sweet, the content and yield of soluble sugar, soluble protein, and vitamin C were determined. In this experiment, the yield of dandelion intercropping with different plant spacing was increased, especially the quality of the P20 treatment with large plant spacing, which was also significantly improved, while the content of soluble sugar and soluble protein in the P10 treatment was not significantly different from that of the control. The content of vitamin C decreased significantly. This demonstrates that increasing the plant distance between dandelion intercropping increases yield and improves fruit quality. This is different from the results of soybean as an intercropping plant, although there are many factors affecting intercropping yield, intercropping crop types, environmental changes, disease severity, and plant growth can affect the yield to a certain extent [40–42], compared with the nitrogen fixation of soybean, dandelion may play a greater role in providing some substances resistant to pepper blight in the middle of this study, and the intercropping density of dandelion should not be too high, otherwise, it will compete with the main crop pepper and affect the yield and quality of pepper.
Understanding the epidemic trend of pepper blight disease is the key to disease prediction and control measures. In this study, the correlation between the occurrence of blight and canopy environment, soil environment, photosynthesis, and yield index under different intercropping patterns was studied. The results showed that the incidence of pepper blight (A) was positively correlated with soil temperature (Q), intercellular carbon dioxide (L), and canopy air temperature (O). According to the previous research, the high temperature and humidity environment of the protected ground is conducive to the growth of Phytophthora capsici. The incidence of pepper blight (A) was significantly negatively correlated with the control effect (B), chlorophyll content (F), net photosynthetic rate (K), stomatal conductance (M), transpiration rate (N), soil sucrase activity (W), and vitamin C (AB), and significantly negatively correlated with the PAL enzyme (J) in leaves. Chlorophyll content reflects the ability of plants to accumulate organic matter and resist external environmental stress. It is an important pigment for plants to carry out photosynthesis, participates in various processes of light energy utilization, and affects leaf net photosynthetic rate, stomatal conductance, transpiration rate, and intercellular CO2 concentration [43]. The enhancement of photosynthesis is beneficial to the growth of plants, and the resistance of plants to diseases is improved. Some studies have found that when plants are damaged by pathogens, the chlorophyll content of damaged leaves decreases [38], which is consistent with our results. We also found that there was a potential relationship between chlorophyll and net photosynthetic rate, stomatal conductance, transpiration rate, and intercellular concentration. Chlorophyll content (F) was significantly positively correlated with net photosynthetic rate (K), stomatal conductance (M), and transpiration rate (N) and negatively correlated with intercellular CO2 concentration (N). All the evidence shows that the correlation study can provide reliable guidance for reducing the incidence of pepper blight. In the cultivation of pepper crops, the incidence of pepper blight can be reduced by properly increasing chlorophyll content (F), net photosynthetic rate (K), stomatal conductance (M), transpiration rate (N), soil sucrase activity (W), vitamin C (AB), leaf PAL enzyme, and reducing soil temperature (Q), intercellular carbon dioxide (L), and canopy air temperature (O).
In conclusion, intercropping dandelion could significantly increase the control effect of pepper on pepper blight. Moreover, intercropping dandelion reliably improved the growth of young pepper plants. Studies show that when dandelions are intercropped at different distances, It can directly affect the physiological indexes of pepper and the surrounding environment (air and soil), thus affecting the occurrence of pepper blight.
Acknowledgement: All authors are grateful to the Key Laboratory of Biology and Genetic Improvement of Horticulture Crops (Northeast Region), Ministry of Agriculture and Rural Affairs, for kindly providing the SD33 strain of Phytophthora capsici and other relevant experiment materials used in this study.
Funding Statement: This work was supported by the Young Talent Project of Northeast Agricultural University (20QC03) and Disciplinary Team Project of Northeast Agricultural University.
Author Contributions: P.X. and H.L. conducted the entire experiment, data curation, formal analysis. M.J. and M.W. assisted in the formal analysis and data curation. Y.T.C., X.Z., N.C. and Y.S. assisted in the formal analysis, scientific writing, reviewing, and editing of manuscript. X.T. assisted in the theoretical guidance. X.J. provided the experimental resources and useful practical guidance. X.Y. and Y.C. supervised the research project, reviewed & edited the manuscript.
Conflicts of Interest: We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
1. Shi, N., Hu, C. H. (2017). The present situation of pepper cultivation and breeding trend in China. Anhui Agricultural Bulletin, 23, 61–62. https://doi.org/10.3969/j.issn.1007-7731.2017.22.025 [Google Scholar] [CrossRef]
2. Rosa-Martínez, E., García-Martínez, M. D., Adalid-Martínez, A. M., Pereira-Dias, L., Casanova, C. et al. (2021). Fruit composition profile of pepper, tomato and eggplant varieties grown under uniform conditions. Food Research International, 147(2), 110531. https://doi.org/10.1016/j.foodres.2021.110531 [Google Scholar] [PubMed] [CrossRef]
3. Batiha, E. S., Alqahtani, A. M., Ojo, O. A., Shahee, H. M., Hetta, H. F. (2020). Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. International Journal of Molecular Sciences, 21(15), 5179. https://doi.org/10.3390/ijms21155179 [Google Scholar] [PubMed] [CrossRef]
4. Chen, L. F., Xu, J. Y. (2001). Agricultural plant pathology in China, pp. 320–322. Beijing, China: China Agriculture Press. [Google Scholar]
5. Leonian, L. H. (1922). Stem and fruit blight of peppers caused by Phytophtora capsica sp. nov. Phytopathology, 12(4), 401–408. https://doi.org/10.1086/333130 [Google Scholar] [CrossRef]
6. Barchenger, D. W., Lamour, K. H., Bosland, P. W. (2018). Challenges and strategies for breeding resistance in Capsicum annuum to the multifarious pathogen, Phytophthora capsici. Frontiers in Plant Science, 9, 628. https://doi.org/10.3389/fpls.2018.00628 [Google Scholar] [PubMed] [CrossRef]
7. Sang, M. K., Kim, J. D., Kim, B. S., Kim, K. D. (2011). Root treatment with rhizobacteria antagonistic to Phytophthora blight affects anthracnose occurrence, ripening, and yield of pepper fruit in the plastic house and field. Phytopathology, 101(6), 666–678. https://doi.org/10.1094/PHYTO-08-10-0224 [Google Scholar] [PubMed] [CrossRef]
8. Lamour, K. H., Stam, R., Jupe, J., Huitema, E. (2012). The oomycete broad-host-range pathogen Phytophthora capsici. Molecular Plant Pathology, 13(4), 329–337. https://doi.org/10.1111/j.1364-3703.2011.00754.x [Google Scholar] [PubMed] [CrossRef]
9. Bellini, A., Pugliese, M., Guarnaccia, V., Meloni, G. R., Gullino, L. M. (2021). Calcium oxide, potassium phosphite and a Trichoderma enriched compost water suspension protect Capsicum annuum against Phytophthora capsici by priming the immune system. Pest Management Science, 77(7), 3484–3490. https://doi.org/10.1002/ps.6401 [Google Scholar] [PubMed] [CrossRef]
10. Casida, J. E., Durkin, K. A. (2017). Pesticide chemical research in toxicology, lessons from nature. Chemical Research in Toxicology, 30(1), 94–104. https://doi.org/10.1021/acs.chemrestox.6b00303 [Google Scholar] [PubMed] [CrossRef]
11. Chen, P., Song, C., Liu, X. M., Zhou, L., Yang, H. et al. (2019). Yield advantage and nitrogen fate in an additive maize-soybean relay intercropping system. Science of the Total Environment, 657, 987–999. https://doi.org/10.1016/j.scitotenv.2018.11.376 [Google Scholar] [PubMed] [CrossRef]
12. Fan, Y., Wang, Z., Liao, D., Raza, M. A., Wang, B. et al. (2020). Uptake and utilization of nitrogen, phosphorus and potassium as related to yield advantage in maize-soybean intercropping under different row configurations. Scientific Reports, 10(1), 9504. https://doi.org/10.1038/s41598-020-66459-y [Google Scholar] [PubMed] [CrossRef]
13. Liu, Y. X., Liao, J. J., Zhu, S. S. (2016). Chemical and ecological mechanism of garlic/pepper intercropping to control pepper blight. Proceedings of the Annual Meeting of Chinese Society for Plant Pathology, Nanjing, China. [Google Scholar]
14. Hayat, S., Cheng, Z., Ahmad, H., Ali, M., Chen, X. et al. (2016). Garlic, from remedy to stimulant, evaluation of antifungal potential reveals diversity in phytoalexin allicin content among garlic cultivars, allicin containing aqueous garlic extracts trigger antioxidants in cucumber. Frontiers in Plant Science, 7, 1235. https://doi.org/10.3389/fpls.2016.01235 [Google Scholar] [PubMed] [CrossRef]
15. Vijverberg, K., Welten, M., Kraaij, M., van Heuven, B. J., Smets, E. et al. (2021). Sepal identity of the pappus and floral organ development in the common dandelion (Taraxacum officinale, Asteraceae). Plants, 10(8), 1682. https://doi.org/10.3390/plants10081682 [Google Scholar] [PubMed] [CrossRef]
16. Meng, Q. F., Feng, X. C. (2011). Development and further utilization of Dandelion. Forestry Exploration and Design, 87 (in Chinese). [Google Scholar]
17. Olas, B. (2022). New perspectives on the effect of dandelion, its food products and other preparations on the cardiovascular system and its diseases. Nutrients, 14(7), 1350. https://doi.org/10.3390/nu14071350 [Google Scholar] [PubMed] [CrossRef]
18. Xue, L., Zhou, Y. Z., Gao, L., Qin, X. M., Du, G. H. et al. (2017). A review of recent literature on anti-aging activity of medicinal and edible traditional Chinese herbs. Food Science, 38, 302–309, (in Chinese). [Google Scholar]
19. Szparaga, A., Kocira, S. (2018). Generalized logistic functions in modelling emergence of Brassica napus L. PLoS One, 13(8), e0201980. https://doi.org/10.1371/journal.pone.0201980 [Google Scholar] [PubMed] [CrossRef]
20. Meng, Y. J. (2013). Study on the inhibitory effect and antibacterial mechanism of dandelion extract on Fusarium oxysporum wilt of cucumber (Master Thesis). Shanxi Agricultural University, China. [Google Scholar]
21. Godlewska, K., Pacyga, P., Michalak, I., Biesiada, A., Szumny, A. et al. (2021). Effect of botanical extracts on the growth and nutritional quality of field-grown white head cabbage (Brassica oleracea var. capitata). Molecules, 26(7), 1992. https://doi.org/10.3390/molecules26071992 [Google Scholar] [PubMed] [CrossRef]
22. Sharifi-Rad, M., Roberts, T. H., Matthews, K. R., Bezerra, C. F., Morais-Braga, M. et al. (2018). Ethnobotany of the genus Taraxacum—Phytochemicals and antimicrobial activity. Phytotherapy Research, 32(11), 2131–2145. https://doi.org/10.1002/ptr.6157 [Google Scholar] [PubMed] [CrossRef]
23. Kenny, O., Brunton, N. P., Walsh, D., Hewage, C. M., McLoughlin, P. et al. (2015). Characterisation of antimicrobial extracts from dandelion root (Taraxacum officinale) using LC-SPE-NMR. Phytotherapy Research, 29(4), 526–532. https://doi.org/10.1002/ptr.5276 [Google Scholar] [PubMed] [CrossRef]
24. Huber, M., Triebwasser-Freese, D., Reichelt, M., Heiling, S., Paetz, C. et al. (2015). Identification, quantification, spatiotemporal distribution and genetic variation of major latex secondary metabolites in the common dandelion (Taraxacum officinale agg.). Phytochemistry, 115, 89–98. https://doi.org/10.1016/j.phytochem.2015.01.003 [Google Scholar] [PubMed] [CrossRef]
25. Yi, T. Y., Zhang, B. X., Xie, B. Y., Gao, B. D., Ma, X. W. (2003). Comparison of three inoculation methods for capsicum blight. China Vegetables, (2), 16–18. https://doi.org/10.3969/j.issn.1000-6346.2003.02.006 [Google Scholar] [CrossRef]
26. Jiang, X. M., Zhang, Q., Chen, Y. T., Bai, G. L., Wang, J. et al. (2020). Effects of intercropping cucumber on Phytophthora blight and growth and development of pepper. Journal of Northeast Agricultural University, 51, 18–25+35. [Google Scholar]
27. Yingsanga, P., Srilaong, V., Kanlayanarat, S., Noichinda, S., McGlassonc, W. B. (2008). Relationship between browning and related enzymes (PAL PPO and POD) in rambutan fruit (Nephelium Lappaceum Linn.) cvs. Rongrien and See-Chompoo. Postharvest Biology and Technology, 50(2–3), 164–168. https://doi.org/10.1016/j.postharvbio.2008.05.004 [Google Scholar] [CrossRef]
28. Shu, Z., Zhang, X. S., Chen, J., Chen, G. S., Xu, D. Q. (2010). Simplification of the determination of chlorophyll content. Plant Physiology Communications, 46, 399–402. https://doi.org/10.13592/j.cnki.ppj.2010.04.001 [Google Scholar] [CrossRef]
29. Sun, B., Song, G., Zhang, Y. M., Chen, A. L., Fu, J. et al. (2016). Determination of nitrate in soil-Ultraviolet spectrophotometry, GB/T 32737-2016. Domestic-National Standard-State Administration of Market Supervision and Administration CN-GB, 2016-08-29 (in Chinese). [Google Scholar]
30. Feng, X., Duan, J. P., Pu, X. P., Zou, Y., Li, C. R. (2008). Comparison of two methods for determining soil urease activity. Grassland and Turf, (2), 70–72. https://doi.org/10.13817/j.cnki.cyycp.2008.02.004 [Google Scholar] [CrossRef]
31. Shi, C. F., Wang, Z. Y., Leng, X. Y., Liu, J. M., Cao, J. M. (2016). Improvement of determination method of soil phosphatase activity. Experimental Technology and Management, 33, 48–49+54. https://doi.org/10.16791/j.cnki.sjg.2016.07.012 [Google Scholar] [CrossRef]
32. Guan, S. Y., Zhang, D., Zhang, Z. (1986). Soil enzyme and its research methods, pp. 274–297. Beijing, China: Chinese Agricultural Press. [Google Scholar]
33. Li, H. S. (2000). Guidance of plant physiological and biochemical experiments. Beijing, China: Beijing Education Press. [Google Scholar]
34. Staudacher, K., Schallhart, N., Thalinger, B., Wallinger, C., Juen, A. et al. (2013). Plant diversity affects behavior of generalist root herbivores, reduces crop damage, and enhances crop yield. Ecological Applications, 23(5), 1135–1145. https://doi.org/10.1890/13-0018.1 [Google Scholar] [PubMed] [CrossRef]
35. Zhang, Y., Li, L., Guo, Y. P., Shao, Y. (2018). Effects of different planting densities on root rot and crop growth of broad bean under intercropping mode. Anhui Agricultural Bulletin, 24(18), 60–61. https://doi.org/10.16377/j.cnki.issn1007-7731.2018.18.028 [Google Scholar] [CrossRef]
36. Kim, J., Song, Y., Dong, W. K., Fiaz, M., Chan, H. K. (2018). Evaluating different interrow distance between corn and soybean for optimum growth, production and nutritive value of intercropped forages. Journal of Animal Science and Technology, 60(1), 1. https://doi.org/10.1186/s40781-017-0158-0 [Google Scholar] [PubMed] [CrossRef]
37. Lv, H., Cao, H., Nawaz, M. A., Sohail, H., Huang, Y. et al. (2018). Wheat intercropping enhances the resistance of watermelon to fusarium wilt. Frontiers in Plant Science, 9, 696. https://doi.org/10.3389/fpls.2018.00696 [Google Scholar] [PubMed] [CrossRef]
38. Meng, Q., Zhou, X., Pang, B., Sun, X., Feng, Y. (2014). Effects of cucumber downy mildew infection on physiological and biochemical indexes of cucumber leaves. Acta Agriculturae Boreali-Occidentalis Sinica, 23(6), 141–146. [Google Scholar]
39. Raza, M. A., Cui, L., Qin, R., Yang, F., Yang, W. (2020). Strip-width determines competitive strengths and grain yields of intercrop species in relay intercropping system. Scientific Reports, 10(1), 21910. https://doi.org/10.1038/s41598-020-78719-y [Google Scholar] [PubMed] [CrossRef]
40. Ocimati, W., Were, E., Groot, J., Tittonell, P., Nakato, G. V. et al. (2018). Risks posed by intercrops and weeds as alternative hosts to Xanthomonas campestris pv. musacearum in banana fields. Frontiers in Plant Science, 9, 1471. https://doi.org/10.3389/fpls.2018.01471 [Google Scholar] [PubMed] [CrossRef]
41. He, X., Xie, H., Gao, D., Khashi, U., Rahman, M. et al. (2021). Biochar and intercropping with potato-onion enhanced the growth and yield advantages of tomato by regulating the soil properties, nutrient uptake, and soil microbial community. Frontiers in Microbiology, 12, 695447. https://doi.org/10.3389/fmicb.2021.695447 [Google Scholar] [PubMed] [CrossRef]
42. Luo, C., Ma, L., Zhu, J., Guo, Z., Dong, K. et al. (2021). Effects of nitrogen and intercropping on the occurrence of wheat powdery mildew and stripe rust and the relationship with crop yield. Frontiers in Plant Science, 12, 637393. https://doi.org/10.3389/fpls.2021.637393 [Google Scholar] [PubMed] [CrossRef]
43. Nasar, J., Shao, Z., Arshad, A., Jones, F. G., Liu, S. et al. (2020). The effect of maize-alfalfa intercropping on the physiological characteristics, nitrogen uptake and yield of maize. Plant Biology, 22(6), 1140–1149. https://doi.org/10.1111/plb.13157 [Google Scholar] [PubMed] [CrossRef]
Supplementary Materials
Supplementary Figure S1: Classification of pepper blight disease
Supplementary Table S1: Two-factor analysis of variance (ANOVA)
Supplementary Table S2: Single factor analysis of plant height and crown diameter
Supplementary Table S3: Single factor analysis table of Atmospheric relative humidity
Supplementary Table S4: Interactive analysis variance analysis
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools