 Open Access
Open Access
ARTICLE
Melatonin Promotes Rice Seed Germination under Drought Stress by Regulating Antioxidant Capacity
1 College of Agronomy, Hunan Agricultural University, Changsha, 410128, China
2 The Key Laboratory of Crop Germplasm Innovation and Resource Utilization of Hunan Province, Hunan Agricultural University, Changsha, 410128, China
* Corresponding Authors: Guanghui Chen. Email: ; Yue Wang. Email:
# These authors contributed equally to the work
(This article belongs to the Special Issue: Abiotic and Biotic Stress Tolerance in Crop)
Phyton-International Journal of Experimental Botany 2023, 92(5), 1571-1587. https://doi.org/10.32604/phyton.2023.025481
Received 15 July 2022; Accepted 06 December 2022; Issue published 09 March 2023
Abstract
Drought stress is a serious threat to the germination of plant seeds and the growth of seedlings. Melatonin has been proven to play an important role in alleviating plant stress. However, its effect on seed germination under drought conditions is still poorly understood. Therefore, we studied the effects of melatonin on rice seed germination and physiological characteristics under drought stress. Rice seeds were treated with different concentrations of melatonin (i.e., 0, 20, 100, and 500 μM) and drought stress was simulated with 5% polyethylene glycol 6000 (PEG6000). The results showed that 100 μM melatonin can effectively improve the germination potential, rate and index; the vigor index of rice seeds; and the length of the shoot and root. In addition, that treatment also increased the activity of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT), and reduced the content of malondialdehyde (MDA). The grey relational grade between the shoot MDA content and the melatonin seed-soaking treatment was the highest, which could be useful for evaluating the effect of melatonin on drought tolerance. Two-way analysis of variance showed that the effect of single melatonin treatment on rice seeds was more significant than that of single drought stress and interaction treatment of drought and melatonin (p < 0.05). The subordinate function results showed that 100 μM melatonin significantly improved the germination and physiological indexes of rice seeds and effectively alleviated the adverse effects of drought stress on rice seedlings. The results helped to improve the understanding of the morphological and physiological involvement of melatonin in promoting seed germination and seedling development under drought stress.Keywords
Supplementary Material
Supplementary Material FileAs global temperatures rise, water shortages and drought damage in arid and semi-arid areas have become more and more serious and are affecting the growth and development of plants. Seed germination and seedling growth are sensitive periods at the start of a plant’s life, and drought stress directly affects the field emergence rate of germinating plants, which is related to the success of population establishment.
Drought has become a major limiting factor threatening rice (Oryza sativa L.), one of the world’s most important food crops, in its critical seed germination phase [1]. In China, the problem of seasonal and regional drought and water shortage is very serious, resulting in a reduction of 40 billion kilograms in rice yield [2]. As rising global warming, droughts may become more frequent. It is estimated that approximately 15–20 million hectares of paddy fields may be impacted by drought in major rice-growing countries of worldwide, which may cause up to 40% yield loss [3,4]. High germination ability under drought stress facilitates competition between seedlings and weeds for water and nutrients, and promotes early seedling morphogenesis in direct-seeded rice [5,6]. Therefore, improving the seed germination capacity of rice would enhance productivity under direct-seeded conditions.
Prolonged drought stress leads to the excessive production of reactive oxygen species (ROS) in plants, which leads to oxidative damage and cell death [7]. In response, plants have evolved an efficient antioxidant defense system composed of enzymatic and non-enzymatic components. Enzymatic antioxidants are superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbic acid peroxidase (APX); non enzymatic antioxidants are glutathione, ascorbic acid, as well as polyphenols and vitamins [8].
To improve crop drought resistance, a variety of agronomic strategies have been adopted such as conventional breeding, molecular breeding, improvement of the water supply and irrigation, and improvement of soil [9,10]. It is an important way to protect food security in the future to develop tolerant rice varieties that can be stably produced under drought stress. Conventional breeding strategies aim to find beneficial stress tolerance alleles from traditional local varieties or wild relatives of important crops to improve stress tolerance by crossing and backcrossing. Conventional breeding strategies to improve the drought resistance of rice also has its limitations. Generally, intraspecific hybridization is easy to carry out, but interspecific hybridization is difficult, and it needs more than 5 years of efforts to be successful. With the development of map-based cloning and high-throughput sequencing, more and more drought tolerance genes or quantitative trait loci (QTLs) have been identified and cloned. Molecular breeding can use markers closely linked to drought tolerance genes or developed from actual gene sequences to indirectly select target traits. Drought has been a trait difficult to select through conventional phenotypic identification, so molecular breeding technology is one of the most ideal methods to improve target traits such as molecular marker assisted selection. Identification of drought tolerant genes is a key factor limiting molecular breeding. Improvement of the water supply and irrigation, without a doubt, can significantly reduce drought risk and improve rice productivity. Management strategies to improve water supply and irrigation are either to avoid drought by increasing water input or avoiding drought periods, or to mitigate drought by reducing non-transpirational outflows, so as to leave more water for transpiration. Laborious and cumbersome operation are the key constraints of this strategy. Soil improvement is also an important part of drought management. Drought is directly related to soil nutrients through different growth processes. The uptake of N+, Ca2+, Mg2+ and Na+ in rice is usually controlled by transpiration stream under drought stress. Lower transpiration means lower nutrient uptake so that caused damage to rice growth. Therefore, changing structure, fertility, pH value and oxygen availability of the soil can improve the drought resistance of rice. However, soil improvement is often affected by environmental factors, and it takes many years for the effect to appear. One innovative strategy is to use biological stimulants or plant hormones, which has been found to overcome the effects of drought [11,12].
Melatonin (N-acetyl-5-methoxytryptamine, MT), an indoleamine bioactive molecule, has been shown to enhance tolerance to high and low temperature, drought and salt in maize, wheat, rice, soybean, cotton [13,14]. As little as 300 μM of melatonin increasing SOD activity in wheat variety HG35 thereby alleviating the negative effect of water stress on seed germination [15]. Seed priming with melatonin also improved the germination of waxy maize seeds under chilling stress and protected them from oxidative damage through improving the antioxidant system and starch metabolism [16]. Pretreatment with 50 and 100 μM of melatonin changed the content of endogenous plant hormones and antioxidant enzymes to improve drought resistance in soybean seedlings [17]. Spraying 100 μM of melatonin enhanced the antioxidant enzyme activity of rice seedlings to reduce the effects of drought stress [18]. Exogenous melatonin improved salinity tolerance of rice by blocking the burst of ROS and improving Na+/K+ homeostasis [19]. The foliar application of 100 µM melatonin and soil leaching of 50 µM melatonin alleviate drought damage to maize by maintaining growth, and improving photosynthetic characteristics and antioxidant enzyme activity [20]. Exogenous melatonin could be used to pretreat cucumber seeds to reduce the oxidative damage from salt stress, enhance the gene expression of antioxidants, and reduce the inhibition of salt on seed germination [21]. The germination of cotton seeds can be enhanced by increasing the germination rate, enhancing the activity of antioxidant enzymes, reducing the accumulation of malondialdehyde (MDA) and regulating hormones [22]. Melatonin effectively regulates seminal root length and growth of monocotyledonous rice after germination [23].
While the response mechanism of rice seed germination and seedlings to drought stress have been investigated, information regarding the effects of rice seed priming with melatonin in response to drought stress is limited. Therefore, the effect of melatonin on the germination and physiological characteristics of rice seeds under drought stress was studied by using the rice variety XZX45. XZX45 is widely grown in the rice growing region of southern China and affected by the drought stress. The objectives of this work were (1) to investigate the germination characteristics of rice seeds with melatonin at 0, 20, 100, and 500 μM concentrations under drought stress; (2) to determine the changes in biomass, antioxidant enzyme activity, MDA content and soluble protein content during germination of rice seeds under different melatonin levels; and (3) to evaluate the effective concentration of melatonin in rice. This study provides some novel insights into drought tolerance mechanisms modulated by exogenous melatonin in seed germination.
The rice variety XZX45 was used in the experiment, and was provided by the Rice Research Institute of Hunan Agricultural University. All chemicals used were of analytical grade. The melatonin and polyethylene glycol (PEG6000) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Rice seeds of the same size were selected and sterilized with 5% sodium hypochlorite solution for 40 min, and then rinsed 4 times with sterilized distilled water. After sterilization, they were soaked for 24 h in different concentrations of melatonin: M0 (0 μM, distilled sterile purified water only), M20 (20 μM), M100 (100 μM), and M500 (500 μM). Polyethylene glycol 6000 (PEG6000) was used to simulate drought stress at concentrations of 0 (T0) and 5% (T5).
The germination of rice seeds refers to the method of Cen et al. [24]. One hundred seeds were sown in each germination box, covered with sterile germinating paper, which has a 7 mL PEG6000 solution of different concentrations: 6 repetitions were set for each treatment. All germination boxes were moved to the light incubator after sowing. The light conditions were as follows: fluorescent light with intensity expressed as a PPFD of 150 mmol m−2s−1 for 14 h/day, and 70% relative humidity (RLD-1000E-4, Ningbo Ledian Instrument Manufacturing Co., Ltd., China). During germination the number of germinated rice seeds was observed and recorded every day. Shoot and root observations were taken after 7 d to determine the relevant indicators.
2.3 Measurement Items and Methods
2.3.1 Determination of Relevant Indicators of Seed Germination
Seed germination was tracked daily. When the total stem length was greater than half the seed length and the root length was greater than the seed length, germination was said to have occurred. The germination potential (GP), germination rate (GR), germination index (GI) and vigor index (VI) of seeds 3 and 7 d after germination were measured according to the assay described by Li et al. [15].
2.3.2 Determination of Growth Indices of Seed Roots and Shoots
The biomass tests refer to the method of Zhu et al. [25]. On day 7 after sowing, 20 randomly selected representative and long-standing germinated seeds from each treatment were cleaned with distilled water and then carefully blotted with filter paper to remove residual surface water. They were divided into 4 replicates to determine shoot and root length. Shoot length was measured from the base to the point of growth and the lengths of the shoot and root were determined. Shoot length was the distance from the base to the growth point, and root length was the length of the primary root. Then, the shoot and root of the germinated rice were separated and weighed with a balance. The fresh weight of the aboveground part and root system were recorded, put into a marked bag and placed in an oven at 105°C for 30 min. They were then dried to a constant weight at 65°C, and the dry weight was recorded with a balance.
2.3.3 Physiological and Biochemical Indexes
SOD activity was determined by the nitrogen blue tetrazole method [26]; POD activity by the guaiacol method [27]; CAT activity by UV absorption [28]; MDA content by the thiobarbituric acid chromogenic method [29]; and soluble protein by the Coomassie brilliant blue G-250 staining method [30].
The data of this research were sorted and calculated utilizing the Microsoft Excel 2017 software. The statistical analysis was conducted by the SPSS window version 20.0 data processing system. The figures were drawn by GraphPad Prism 9.3.1. In addition, the difference test at the level of p < 0.05 was carried out through Duncan’s new complex difference method for mean comparison. All data were expressed as mean ± standard error (SE). The index change percentage (α) was calculated as α (%) = ((treatment value – control value)/control value) × 100. In the formula, treatment value is the mean value of the test sample in different melatonin concentrations treatment, and control value is the mean value of the test sample in the control (M0) melatonin concentration treatment. The grey correlation degree refers to the method of Su et al. [31]. The membership function method was used to evaluate the tolerance of rice seeds to melatonin, drought stress and interactive stress [32].
3.1 Germination Characteristics
Under normal treatment (T0) compared with the control (M0), the germination potential, rate, and index and seed vigor index of the M100 concentration increased by 8.87%, 3.19%, 8.57% and 21.32%, respectively (Figs. 1A–1D). Under drought treatment T5, these four criteria were significantly higher in the M20 and M100 concentrations than for M0. The M100 concentration increased by 11.21%, 9.16%, 11.01% and 29.52%, respectively. Compared with the control M0, the germination potential and germination index of M500 treatment significantly increased by 7.75% and 3.13%, respectively, while the seed vigor significantly decreased by 46.82%.
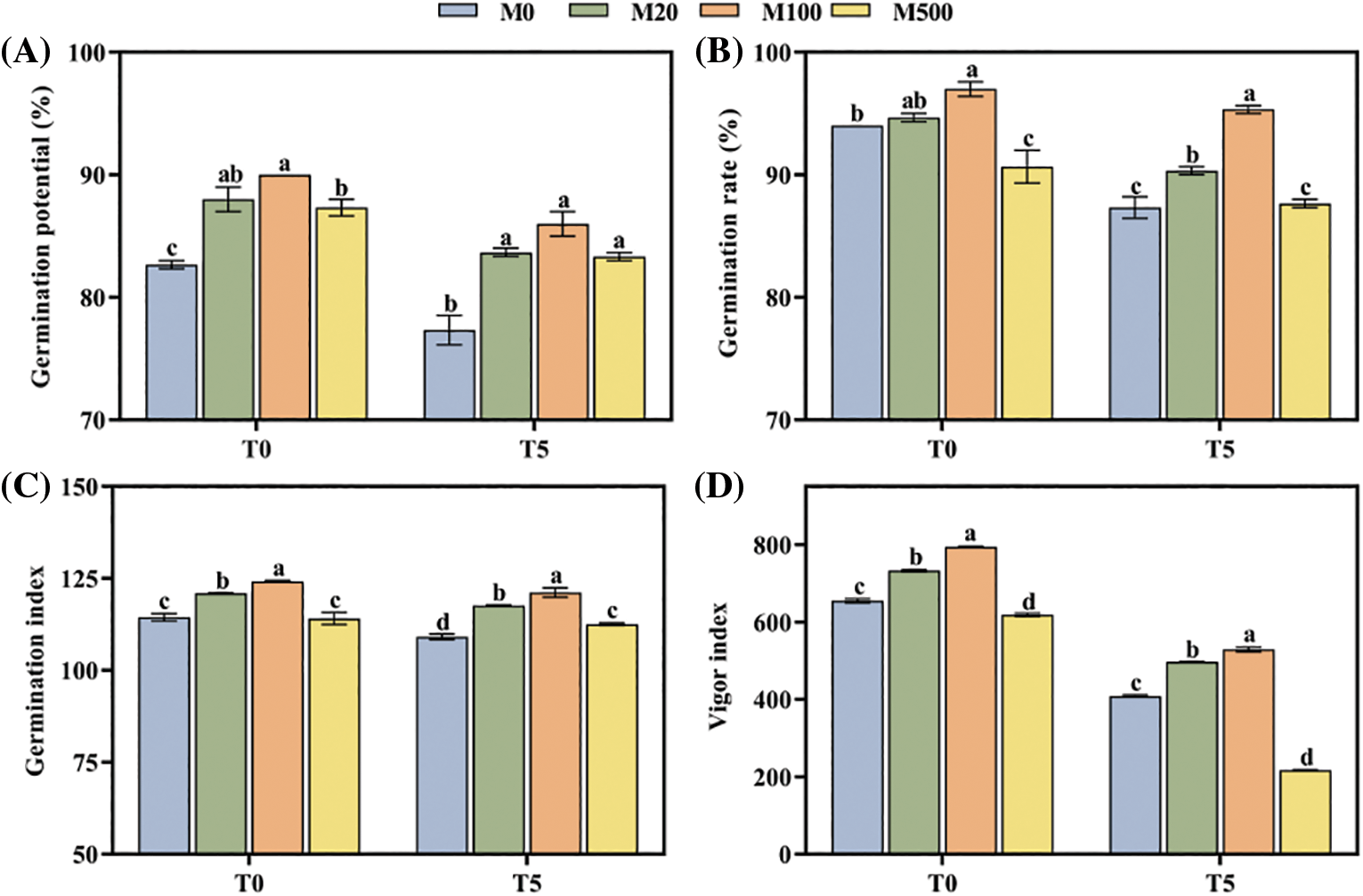
Figure 1: Germination of rice seeds under drought stress. Means ± SEs with different letters denote significant statistical differences (p < 0.05). (A) Germination potential; (B) Germination rate; (C) Germination index; (D) Vigor index
3.2 Biomass of Rice Seed Shoots and Roots
The length, fresh weight, and dry weight of shoot and root were inhibited under drought stress, but seed soaking with an appropriate concentration of melatonin alleviated the damage (Figs. 2A–2F). Under normal treatment (T0), the M100 concentration significantly increased the six shoot and root categories compared to control (M0) by 13.58%, 15.14%, 31.06%, 61.33%, 22.52% and 22.47%, respectively. Under drought treatment (T5), the increases were 15.66%, 29.67%, 15%, 41.83%, 20.16% and 45.59%, respectively.
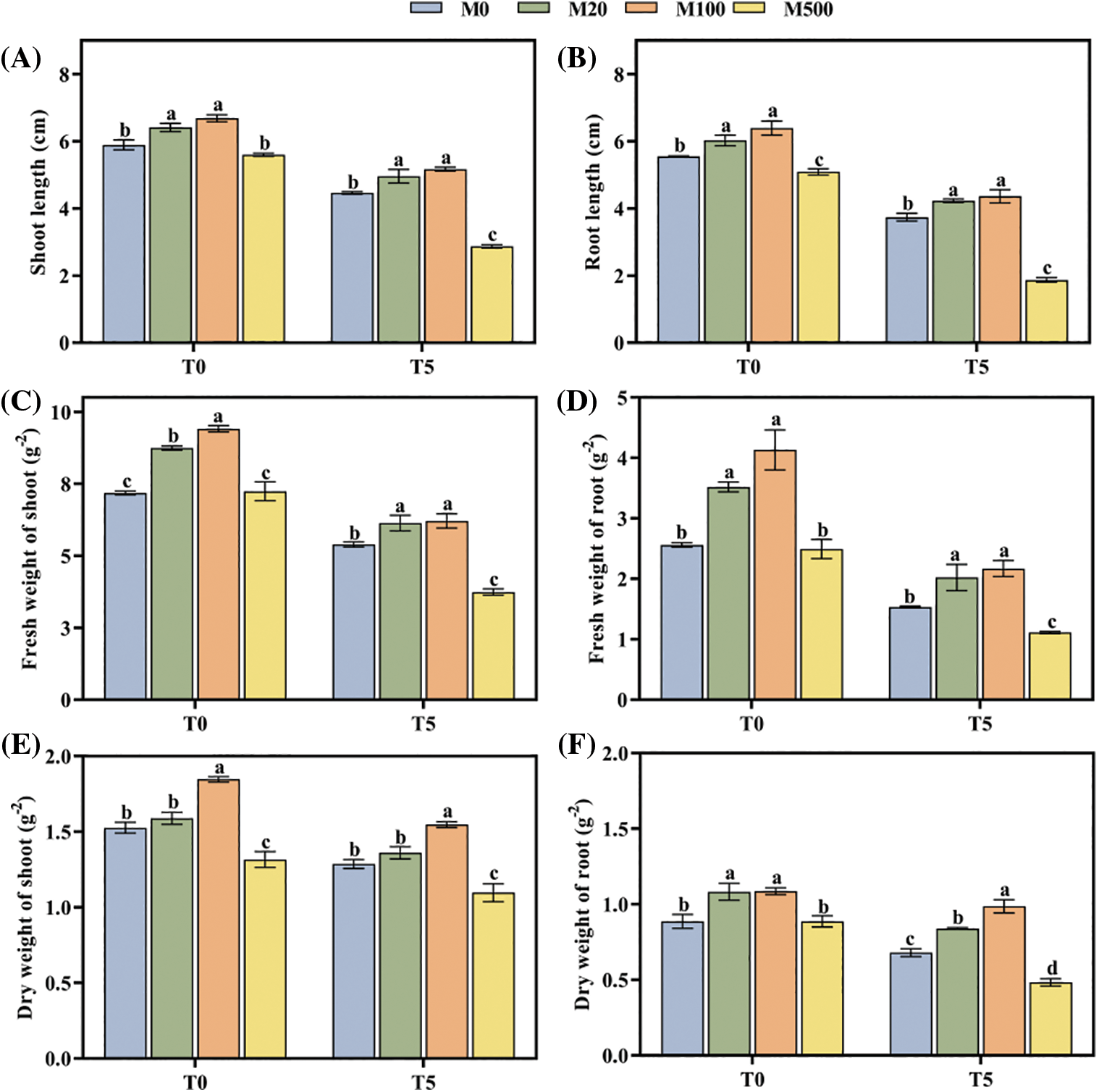
Figure 2: Biomass of rice seeds under drought stress. Means ± SEs with different letters denote significant statistical differences (p < 0.05). (A) Shoot length; (B) Root length; (C) Fresh weight of shoot; (D) Fresh weight of root; (E) Dry weight of shoot; (F) Dry weight of root
3.3 Antioxidant Enzyme Activity in Rice Seed Shoots and Roots
The activities of SOD, POD and CAT of rice seeds under drought stress (T5) were significantly higher than those under normal treatment T0 (Figs. 3A–3G). Under T0, melatonin treatment with M20 and M100 concentration improved SOD, POD and CAT activity of the shoot and root, by 31.87%–52.24%, 19.73%–57.19% and 45.37%–193.32% compared with M0. Under T5, melatonin treatment with M100 concentration increased SOD activity of the shoot, SOD activity of the root, POD activity of the shoot, POD activity of the root, CAT activity of the shoot and CAT activity of the root, by 27.78%, 45.64%, 17.25%, 29.24%, 83.7%, 59.42%, respectively.
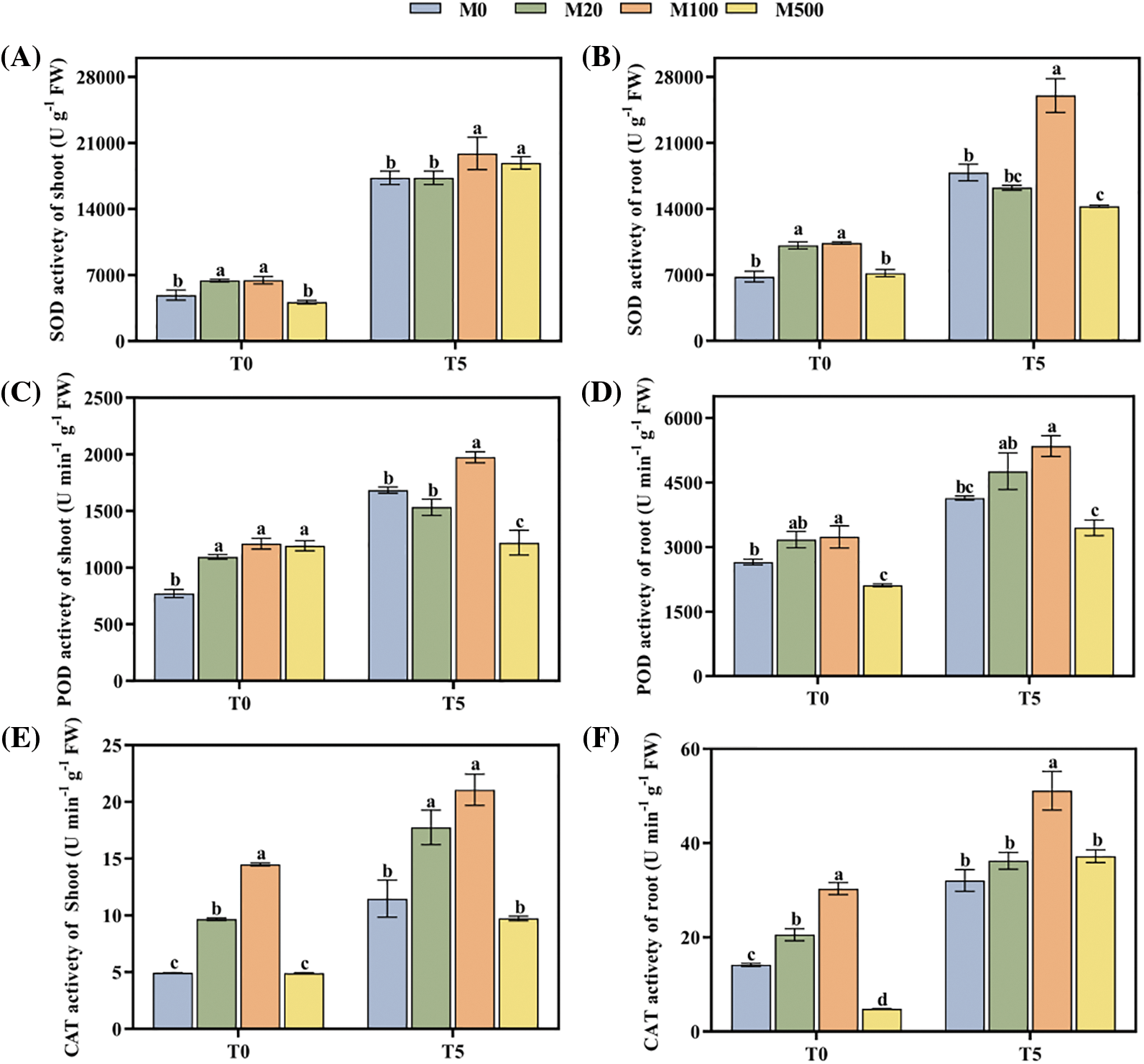
Figure 3: Antioxidant enzyme activity of rice seeds under drought stress. Means ± SEs with different letters denote significant statistical differences (p < 0.05). (A) SOD activity of shoot; (B) SOD activity of root; (C) POD activity of shoot; (D) POD activity of root; (E) CAT activity of shoot; (F) CAT activity of root
3.4 MDA Content in Rice Seed Shoots and Roots
Under normal treatment (T0), compared to control (M0), all the concentrations of melatonin treatment reduced the MDA content of shoot and root. Among them, the M100 concentration was the best, reducing it by 75.16% and 43.46% (Figs. 4A, 4B). Under drought stress treatment T5, all concentrations of melatonin treatment reduced the MDA content of shoot and root by 35.38%–51.6% and 40.02%–61.04%.
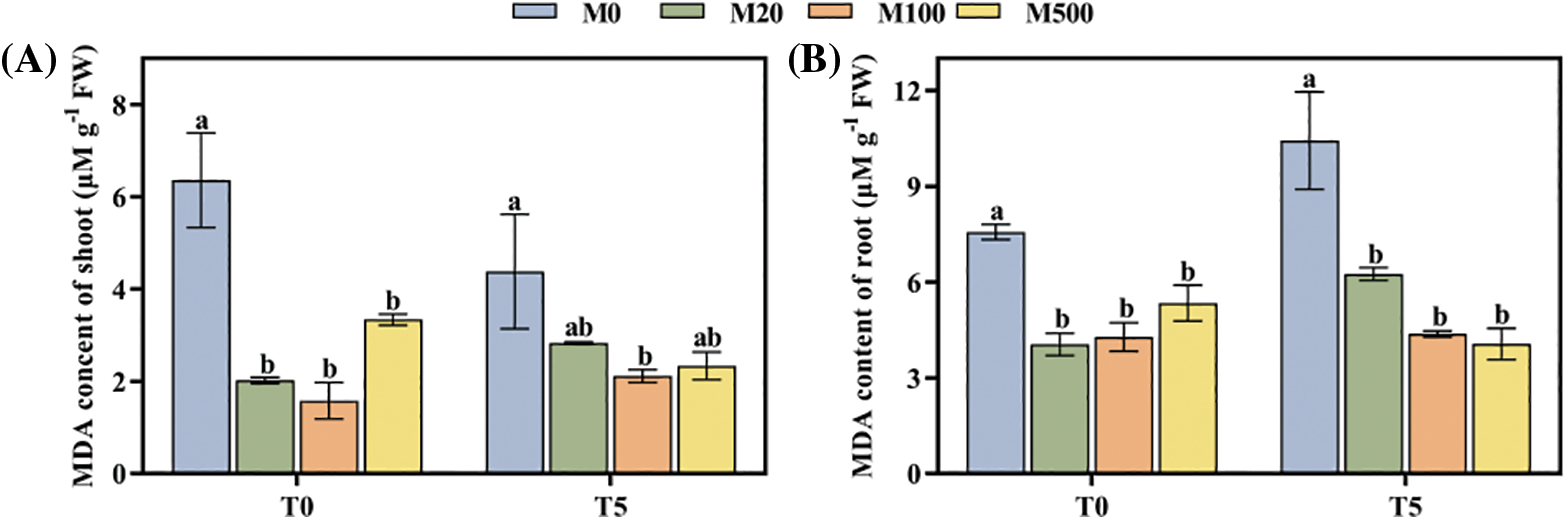
Figure 4: MDA content of rice seeds under drought stress. Means ± SEs with different letters denote significant statistical differences (p < 0.05). (A) MDA content of shoot; (B) MDA content of root
3.5 Soluble Protein Content in Rice Seed Shoots and Roots
Under normal treatment (T0), the M100 concentration increased the soluble protein content of shoots and roots by 17.96% and 17.09% compared with the control M0 (Figs. 5A, 5B). Under drought stress treatment T5, the M20 and M100 concentrations were 8.43%–15.49% and 11.09%–21.38% higher, respectively.
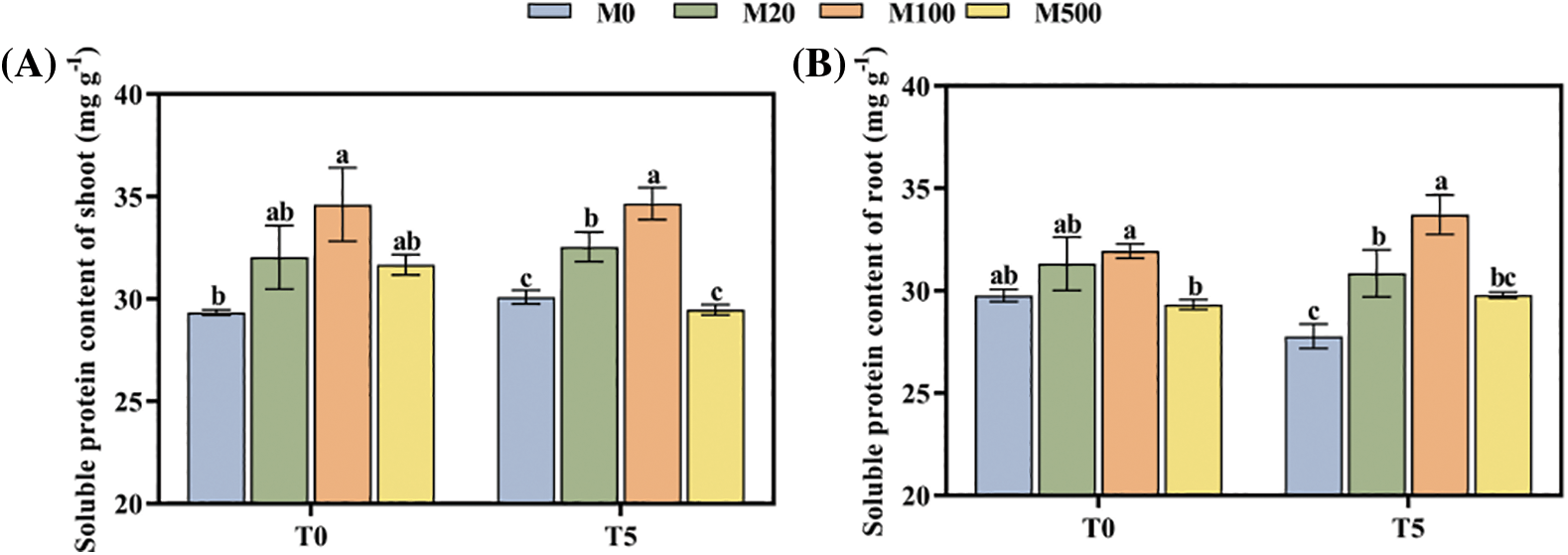
Figure 5: Soluble protein content of rice seeds under drought stress. Means ± SEs with different letters denote significant statistical differences (p < 0.05). (A) Soluble protein content of shoot; (B) Soluble protein content of root
3.6 Grey Relational Grade and Correlation Analysis of Exogenous Melatonin on Rice Seed Germination and Physiological Indicators under Drought Stress
The analysis of the grey relational grade is a quantitative description and comparison of the development and changes of a system, so it is widely applicable in comprehensive evaluation. It can compare multiple trait indexes as a whole, and can more comprehensively and truly reflect the changes of comprehensive factors [24]. The analysis of the grey relational grade between the 20 rice seed germination evaluation items and melatonin seed-soaking concentrations under high drought stress is shown in Fig. 6. The grey correlation coefficient of the MDA content of shoot, the MDA content of root and the SOD activity of shoot were 0.352, 0.33 and 0.308, respectively, which were higher than other evaluation items. It showed that they were closely related to the concentration of melatonin-soaked seeds, and could be used as an indicator to measure the mitigation effect of melatonin on rice seed germination stage under drought stress. In addition, germination potential, germination rate and germination index also clearly indicated the alleviating effect of melatonin on rice seed germination under drought stress. Among these, the grey correlation coefficient between the MDA content of shoot and melatonin concentration was the largest. Therefore, the MDA content of the shoot can best reflect the alleviating effect of melatonin on drought stress.
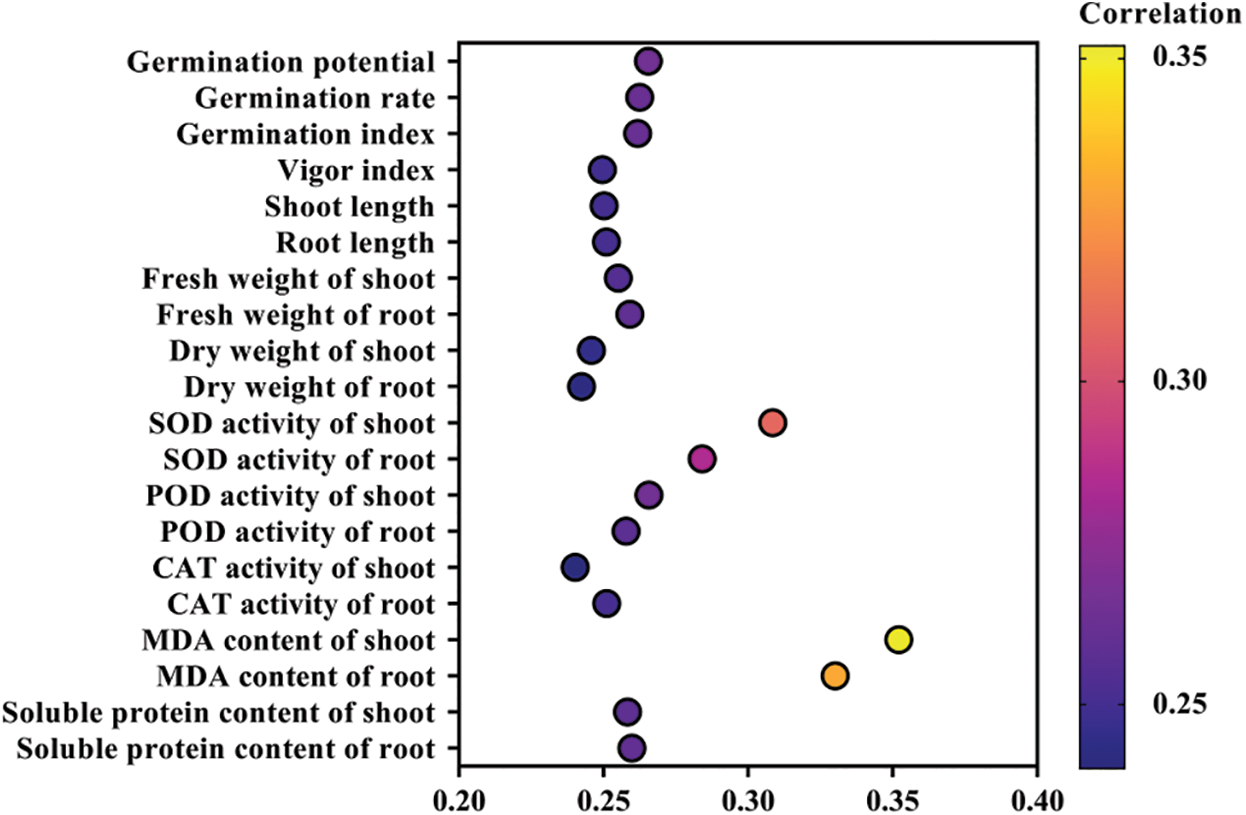
Figure 6: Analysis of the grey relational grade on rice seed germination and physiological indices
Correlation analysis showed that germination potential had a significant positive correlation with the germination rate and index, vigor index, shoot and root length, shoot and root fresh weight, and shoot and root dry weight (p < 0.01). It had a significant negative correlation with MDA content of root (p < 0.01), SOD activity of shoot, and MDA content of shoot (p < 0.05). It did not reach a significant level with POD or CAT activity of shoot, and SOD, POD or CAT activity of root (Fig. 7). The MDA content of shoot negatively correlated with germination potential and index, CAT activity of shoot and root, soluble protein content of shoot and root (p < 0.05).
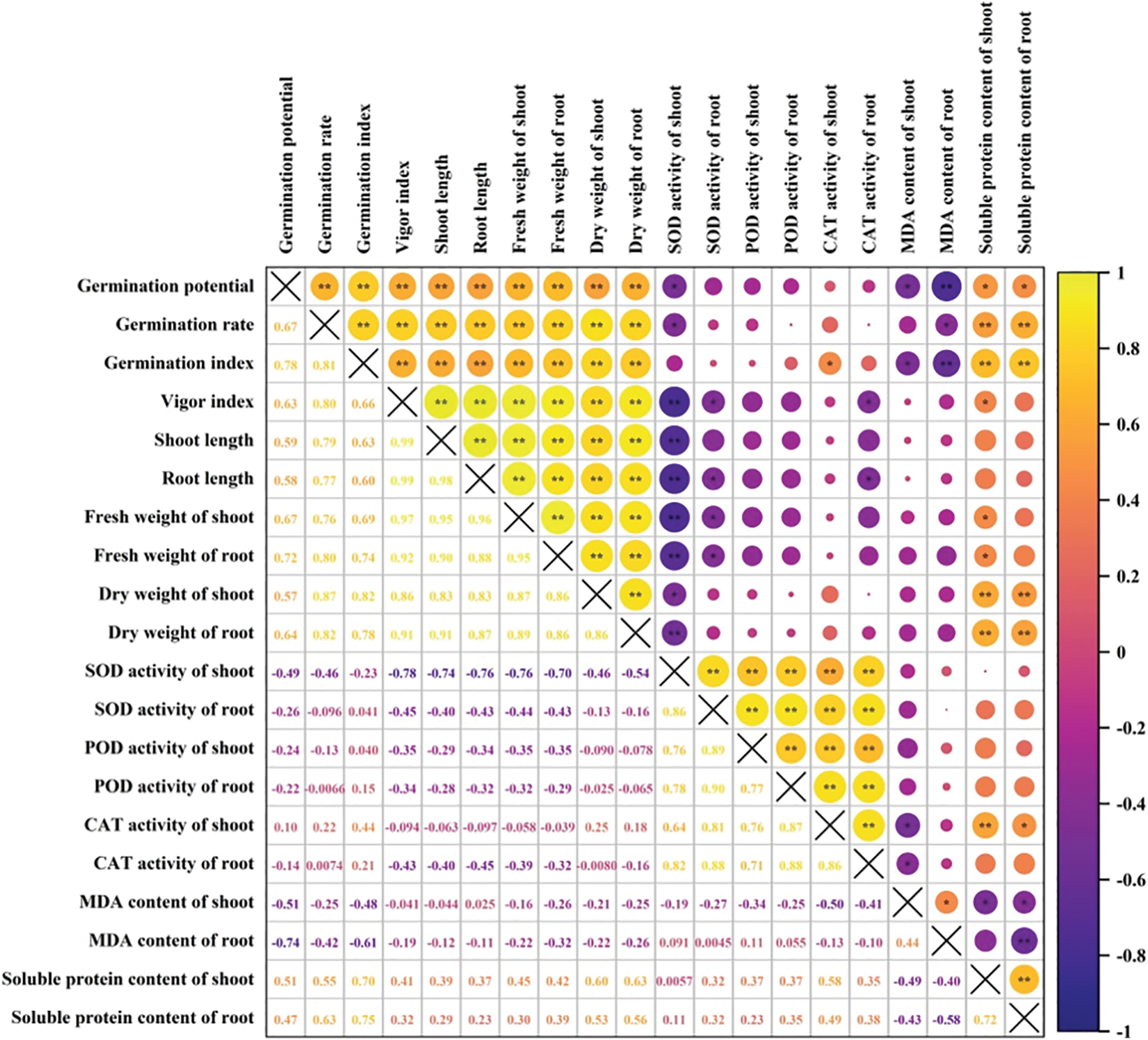
Figure 7: Correlation analysis on rice seed germination and physiological indices. **: significant at the 0.01 probability level; *: significant at the 0.05 probability level
3.7 Principal Component Analysis
Principal component analysis (PCA) was used to evaluate 20 indexes of rice seed germination and physiology under drought stress (Fig. 8). The results showed that the cumulative contribution rate of PCA1 and PCA2 was 79.01%, of which PC1 contributed 49.18% and PCA2 contributed 29.83%. Therefore, it reflected most of the information of melatonin soaked seeds under drought stress. The eigenvalue of PCA1 was 9.84 and the contribution rate was 49.18%. Among them, the indexes with the larger positive eigenvalues were germination rate, germination potential, dry weight and soluble protein content. The indexes with large negative eigenvalues were rice SOD and CAT activity. The eigenvalue of PCA2 was 5.97 and the contribution rate was 29.83%. The indexes with large positive eigenvalues were fresh weight, shoot and root length, and MDA content, while other indexes had negative eigenvalues, which correlated negatively.
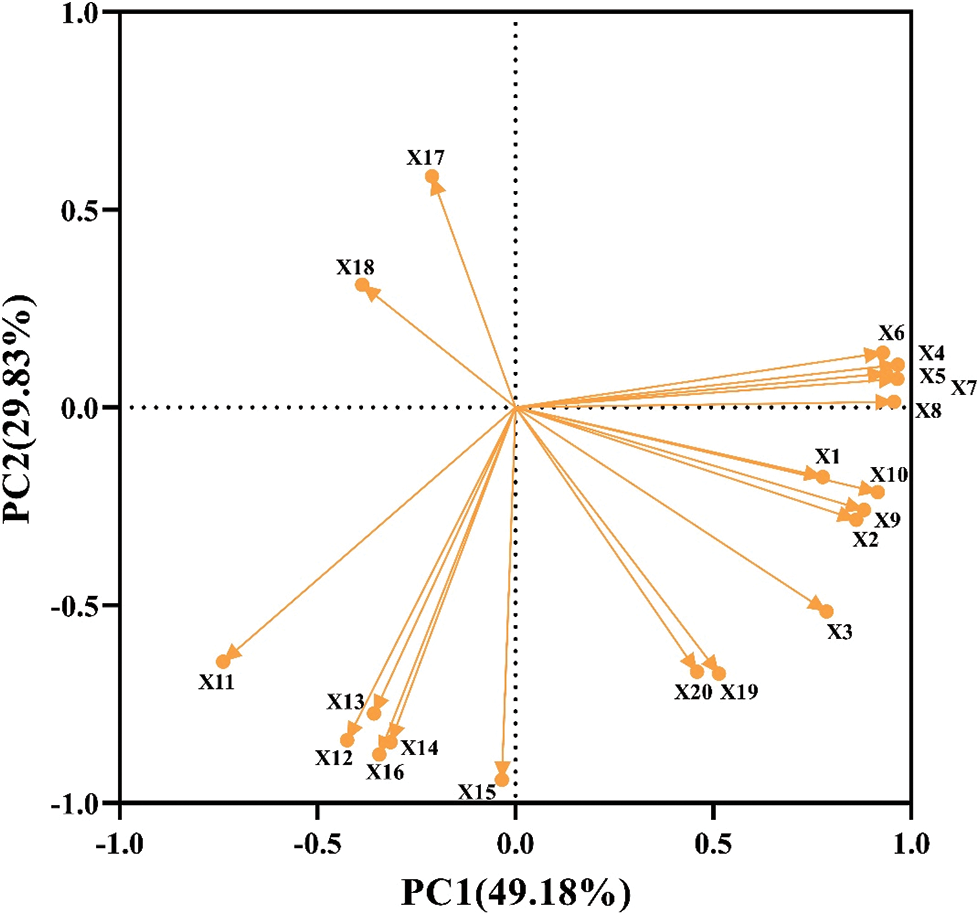
Figure 8: PCA analysis on rice seed germination and physiological indices. X1: Germination potential; X2: Germination rate; X3: Germination index; X4: Vigor index; X5: Shoot length; X6: Root length; X7: Fresh weight of shoot; X8: Fresh weight of root; X9: Dry weight of shoot; X10: Dry weight of root; X11: SOD activity of shoot; X12: SOD activity of root; X13: POD activity of shoot; X14: POD activity of root; X15: CAT activity of shoot; X16: CAT activity of root; X17: MDA content of shoot; X18: MDA content of root; X19: Soluble protein content of shoot; X20: Soluble protein content of root
3.8 Comparison of Differences and Comprehensive Evaluation of Tolerance between Drought Stress and Exogenous Melatonin Interaction
The results of a two-way ANOVA on the effects of drought stress and melatonin interaction on rice seed germination parameters and physiological indexes are shown in Table 1. The germination rate, vigor index, shoot and root length, shoots fresh weight, root dry weight, root SOD activity, shoot POD activity and root CAT activity were significantly different under different treatments (p < 0.01).
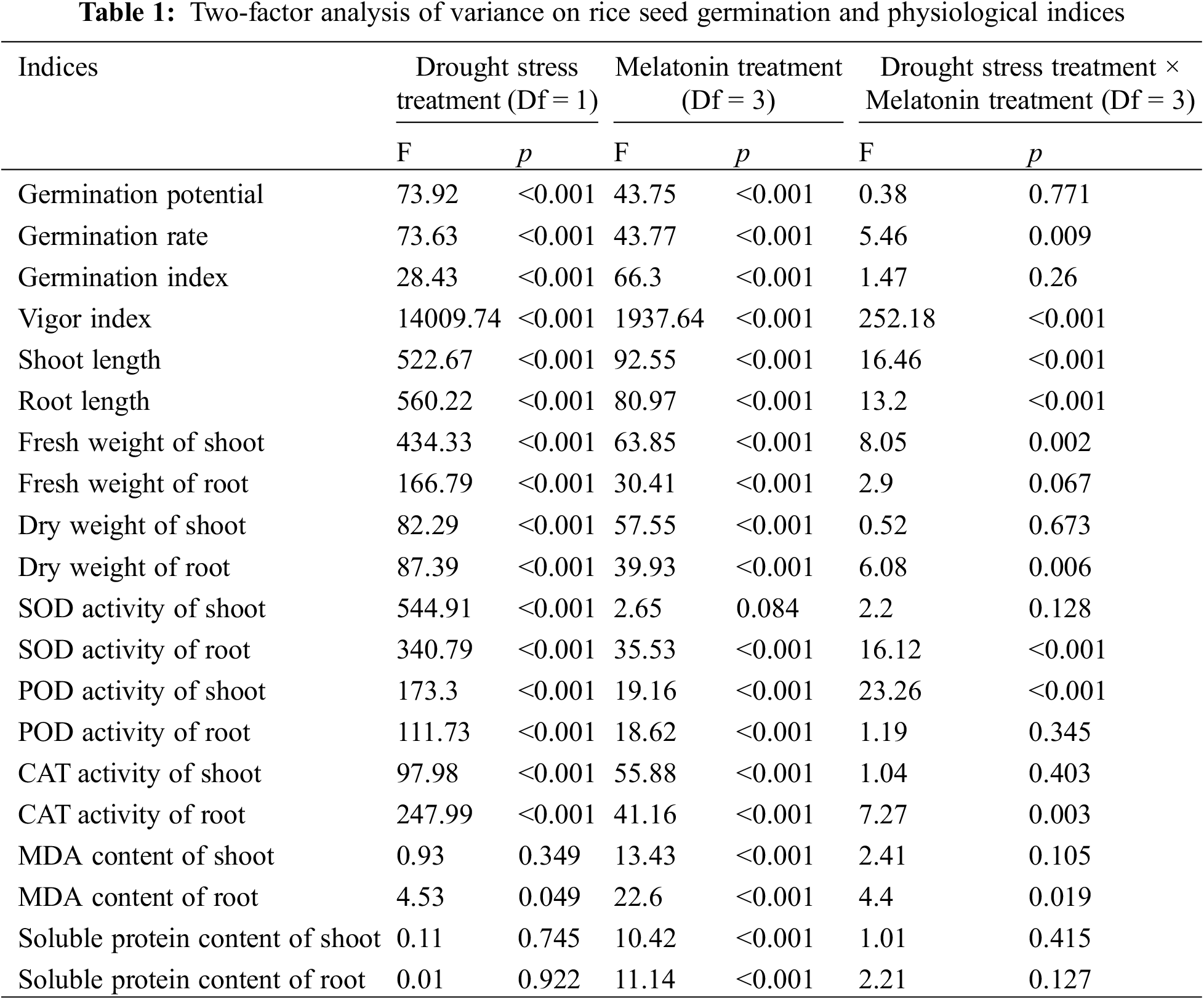
The germination potential, shoot POD activity, root fresh weight, shoot dry weight, root POD activity and shoot CAT activity were different under the single factor treatment of drought stress, exogenous melatonin concentration and the two interactive treatments. Among them, the difference of each index under single-factor treatment was extremely significant (p < 0.01). There was no significant difference in each index under interactive treatment.
The SOD activity of shoot was significantly different under the single factor treatment of drought stress (p < 0.01), but there was no significant difference under the single-factor treatment of exogenous melatonin concentration or the two interactive treatments. The content of MDA in the seed roots was different under the single factor treatment of drought stress and the two interactive treatments (p < 0.05), and extremely significant under the single-factor treatment of exogenous melatonin concentration (p < 0.01). The MDA content, soluble protein content in the shoot, and soluble protein content in the root showed an extremely significant difference under the single-factor treatment of exogenous melatonin concentration (p < 0.01), but there was no significant difference under the single-factor treatment of drought stress or the two interactive treatments.
Figs. 9A–9D show that the response degree of different indexes to drought stress and melatonin concentration was different. To reflect the characteristics of rice seed germination, the subordinate function method was used to evaluate the tolerance to PEG6000 and melatonin under single and interactive treatment. The results showed that under a single drought stress treatment, the subordinate function value of the T5M0 treatment was the largest, and 5% PEG6000 was the critical concentration of drought tolerance. Under a single melatonin treatment, the subordinate function value of T0M100 was the largest. Under the interaction of drought stress and melatonin, the effect of T0M100 treatment was prominent, and 100 μM melatonin was the critical concentration for rice to resist drought stress.
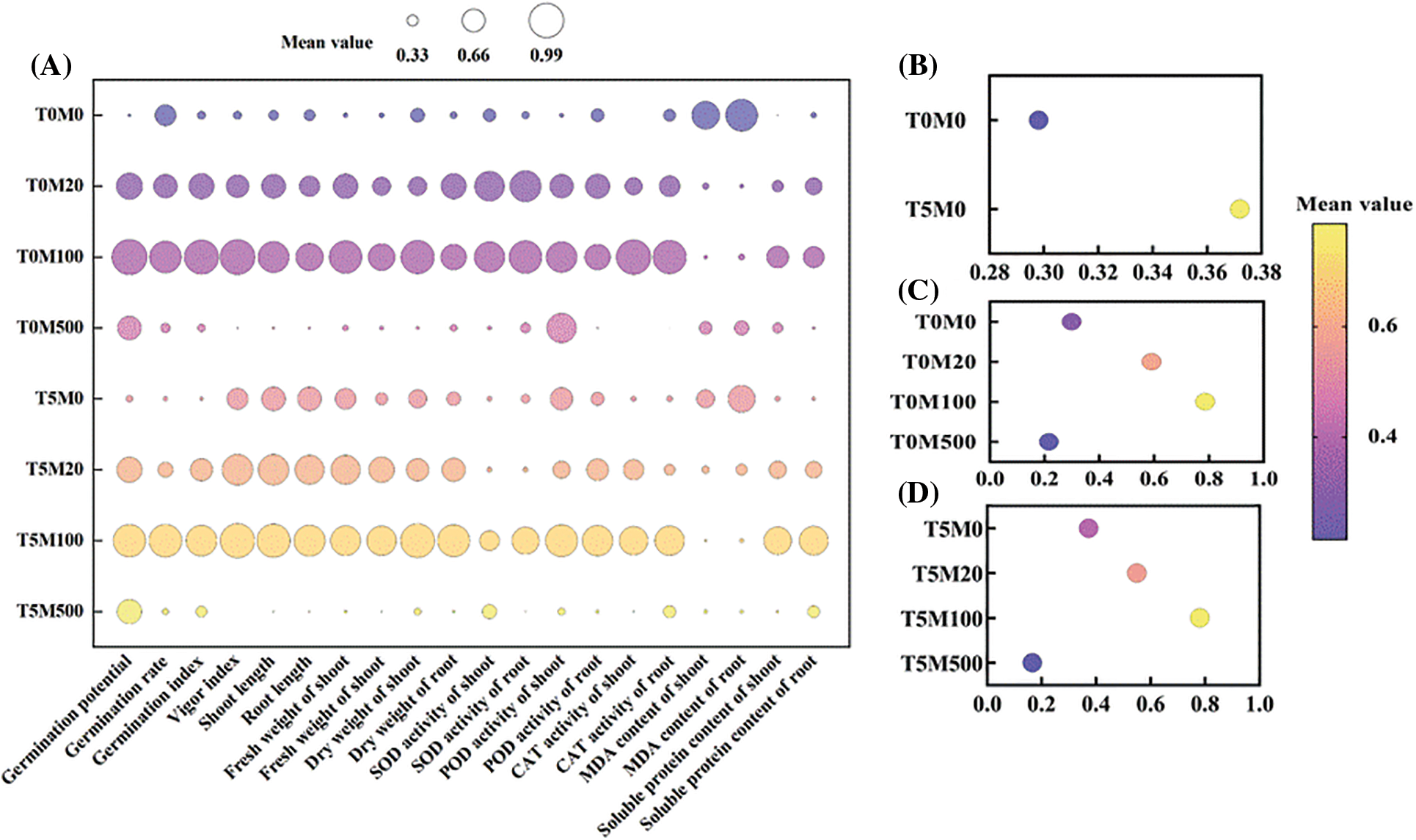
Figure 9: Comprehensive evaluation on rice seed germination and physiological indices
Drought is one of the main abiotic stresses that can significantly damage plant growth and affect seed germination [33,34]. In this study, the application of 5% PEG6000 inhibited the germination and biomass accumulation of rice seeds, which may be closely related to the inhibition of water absorption of seeds by PEG6000. This was similar to the research results of Zhenyi et al. [35]. In addition, the germination vigor was delayed after 5% and 10% PEG6000 treatments but was completely inhibited after 30%. This suggests that germination varied in response to drought stress. Recently, melatonin has been widely used to treat seeds before sowing, improve its resistance to abiotic stress, and promote seed germination [36–38]. In cotton, melatonin (100 µM) presoaked seeds increased the germination rate and potential, free radical length and fresh weight, SOD, POD, α-amylase activity, and decreased H2O2, superoxide anion radical (O2−) and MDA content under drought stress [39]. The results showed that soaking seeds in melatonin alleviated the inhibitory effects of 5% PEG6000 on seed germination and growth. It mainly showed that the speed of seed germination accelerated, and the shoot and, root lengths, and fresh and dry weights all increased. The best concentration to promote rice seed germination under drought stress was 100 μM melatonin. This suggests that melatonin has certain similarities in promoting germination under drought stress.
After sustained drought stress, plant cells produce a large amount of ROS, which destroys physiological and biochemical processes and reduces productivity [40]. On the other hand, plants have evolved an effective antioxidant defense system against this stress, including enzymatic antioxidants such as SOD, POD, CAT and nonenzymatic antioxidants such as glutathione and proline [41]. Previous studies have proven that melatonin triggers this defense system, enhancing the plant’s ability to scavenge harmful ROS, thereby protecting it from the adverse effects of drought-induced oxidative stress [42]. In wheat, melatonin improved the intracellular ROS balance and enhanced the drought tolerance of seedlings by reducing the inhibitory effect of drought stress on photosynthesis [43].
Additionally, melatonin promoted the activity of ABA degrading enzymes and hydrogen peroxide scavenging enzymes under drought stress, and this enhanced activity of the abovementioned enzymes resulted in the reduction of H2O2 in guard cells, indicating the direct involvement of melatonin in scavenging H2O2 [44]. Melatonin reduced the oxidative damage caused by aging of corn seeds by improving the activity of aging seeds, promoting the growth of germ and free radicals, improving the activity of antioxidant enzymes and reducing membrane lipid peroxidation [45]. These conclusions were consistent with the results in the study, which showed that SOD, POD and CAT activities in melatonin-treated rice seeds increased, while the content of MDA decreased. This indicated that under drought stress, melatonin reduced the degree of membrane lipid peroxidation by enhancing the activity of antioxidant enzymes in young roots and shoots, thereby maintaining normal cell metabolism and alleviating drought stress.
The Grey relational grade is a method to measure the degree of correlation between factors [46]. According to the principle of correlation analysis, a sequence with a high degree of correlation is closely related to the reference sequence, and a sequence with a small correlation degree is distant from the reference sequence. The importance of each factor is expressed by the order of its correlation grade. The results showed that among these indicators, MDA content of shoot was the most closely related to melatonin concentration and the first choice for evaluating the effect of melatonin on the alleviation of drought stress. A correlation analysis showed that rice seed germination potential and index positively correlated with length, fresh weight and dry weight of shoot and root (p < 0.01), and negatively correlated with MDA content of shoot (p < 0.05), and MDA content of root (p < 0.01). It indicated that higher MDA content was more detrimental to the germination of rice seeds. Previous studies had also shown that drought stress can cause membrane lipid peroxidation, damage to cell membrane structure and function, delayed germination, and inhibited seedling growth [47]. Rice seed germination is a complex quantitative trait, which is affected by many factors. In rice breeding practice, the role of various influencing factors is of great concern and we should focus on selecting traits according to breeding objectives. The analysis of the grey relational grade can distinguish other factors according to the correlation coefficient. Therefore, in the evaluation of germplasm resources and the breeding of rice varieties, a selection system based on physiological and biochemical characteristics should be established under drought stress, especially based on MDA content of shoot.
To explore the effects of drought treatment and melatonin treatment on the results of this test, the germination and physiological indexes of rice seeds were analyzed by two-way ANOVA. A single melatonin treatment was found to increase the activities of antioxidant enzymes and improve the adaptation of rice to drought environments. The membership function method converted each index into a value of 0–1 through a dimensionless model, put different indexes at the same order of magnitude, enhanced the comparability between different indexes, and quantitatively evaluated each treatment better [48]. The drought tolerance of rice seeds treated with different concentrations of melatonin was evaluated using an integrated subordinate function analysis. It found that the M100 concentration showed a strong tolerance for drought stress, which was consistent with the previous results of physiological indexes. The physiological mechanism of 100 µM melatonin alleviating drought stress is shown in Fig. 10. This study is the first to explore the mechanism by which melatonin alleviates the effect of drought stress on rice seed germination. This may provide a new use for melatonin in regulating drought tolerance in plants. Furthermore, these results provide a theoretical basis for using melatonin to alleviate drought stress in rice. In future research, we will explore the molecular mechanism by which melatonin regulates rice resistance to drought stress.
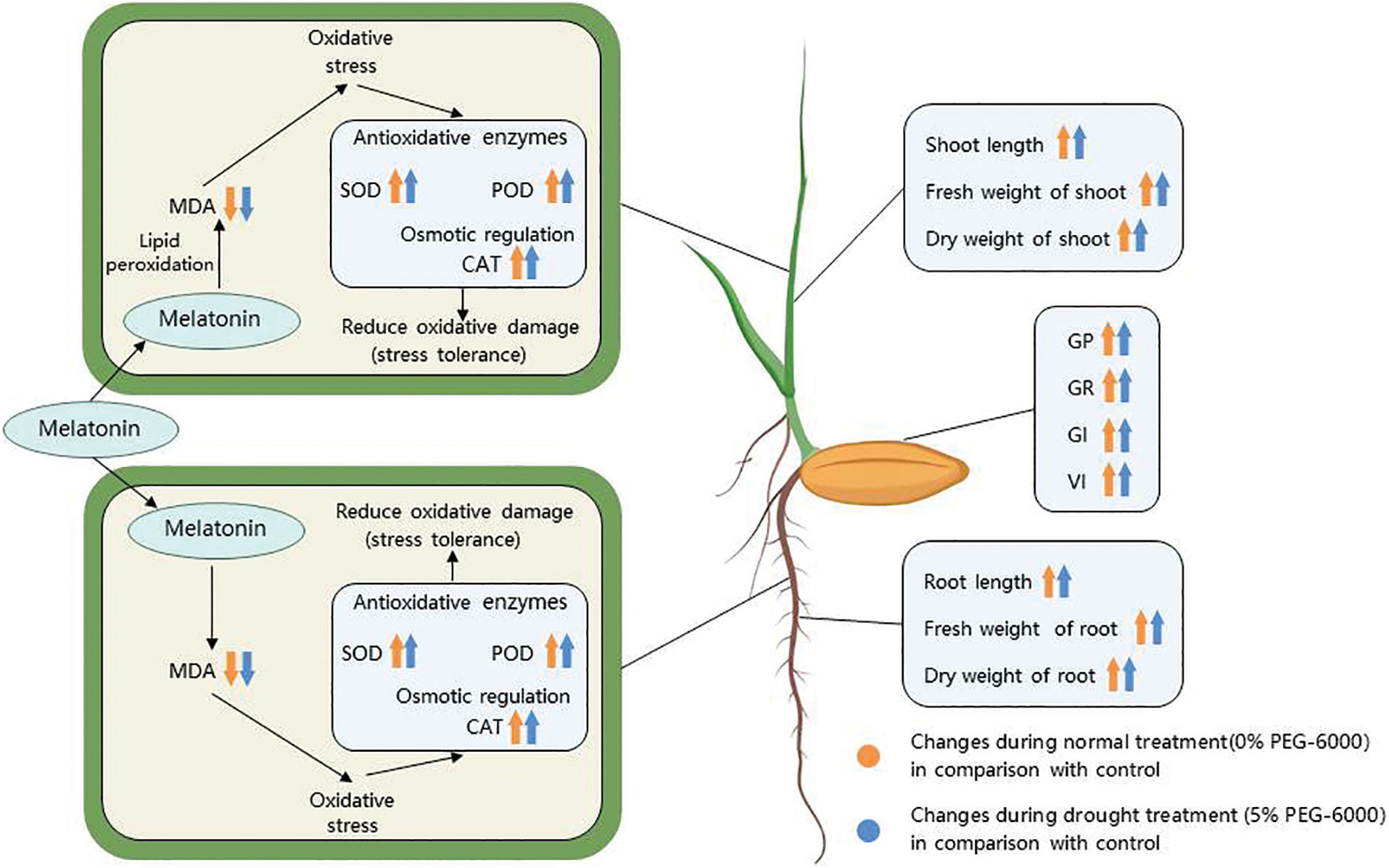
Figure 10: Schematic diagram showing the changes of morphophysiological parameters in response 100 μM melatonin to relieve drought stress in rice seed germination. Symbols such as (↑), (↓), and (|) represent upregulation, downregulation, and no significant changes of various parameters, respectively
Plant hormones are one of the important factors affecting the germination of rice seeds. Based on the interaction between specific plant hormones, genes related to rice seed germination are activated. Therefore, phytohormone pretreatment is an effective method to improve the germination ability of rice seeds. In this study, melatonin seed-soaking effectively improved the germination potential, germination rate, germination index and vigor index, increased the activity of SOD, POD and CAT, and reduced the content of MDA to alleviate the inhibitory effect of drought stress on rice growth. These results indicated that the 100 µM melatonin seed-soaking could be a good option for inducing biostimulation. It can provide a simple and effective method to promote rice seed germination and seedling growth under drought stress.
Acknowledgement: The authors thank Chao Hu, and Xu Mo of Hunan Agricultural University for all their help during the experiment.
Funding Statement: This work was funded by the National Natural Science Foundation of China (31971923; 31301650); the National Key R&D Program of China (2017YFD0301501); the Hunan Provincial Natural Science Foundation of China (2020JJ4360); and the Key Scientific Research Project of Hunan Provincial Education Department of China (19A220); Innovation and Entrepreneurship Training Program for College Students of Hunan Agricultural University (XCX2021038).
Author Contributions: Y.W. and G.C. conceived and supervised the work; L.Z. and X.F. conducted the experiments, analysed data and prepared the figures; N.Y., J.C., H.W., and Q.S. assisted in data analysis; Y.W. and G.C. drafted the manuscript together with L.Z., X.F., N.Y., J.C., H.W., and Q.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Liu, J., Hasanuzzaman, M., Wen, H., Zhang, J., Peng, T. et al. (2019). High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma, 256(5), 1217–1227. [Google Scholar] [PubMed]
2. Yang, G., Wang, L., Wang, H. (2010). Thinking of food security in China based on regional water resources and land cultivation. Transactions of the CSAE, 26(12), 1–5. [Google Scholar]
3. He, H., Wang, Q., Wang, L., Kun, Y., Yang, R. et al. (2021). Photosynthetic physiological response of water-saving and drought-resistant rice to severe drought under wetting-drying alternation irrigation. Physiologia Plantarum, 173(4), 2191–2206. [Google Scholar] [PubMed]
4. Guo, H., Wang, R., Garfin, G., Zhang, A., Lin, D. et al. (2021). Rice drought risk assessment under climate change: Based on physical vulnerability a quantitative assessment method. Science of the Total Environment, 751, 141481. [Google Scholar] [PubMed]
5. Li, J., Xie, Y., Li, X., Wang, J. (2021). Effects of trehalose on seed germination of C4-PEPC transgenic rice for drought. Journal of Nuclear Agricultural Sciences, 35(12), 2879–2892. [Google Scholar]
6. Ali, L. G., Nulit, R., Ibrahim, M. H., Yien, C. Y. S. (2020). Enhancement of germination and early seedling growth of rice (Oryza sativa) var. FARO44 by seed priming under normal and drought stressed conditions. Journal of Plant Nutrition, 43(11), 1579–1593. [Google Scholar]
7. Oladosu, Y., Rafii, M. Y., Samuel, C., Fatai, A., Magaji, U. et al. (2019). Drought resistance in rice from conventional to molecular breeding: A review. International Journal of Molecular Sciences, 20(14), 3519. [Google Scholar] [PubMed]
8. Naeem, M., Naeem, M. S., Ahmad, R., Ahmad, R., Ashraf, M. Y. et al. (2017). Improving drought tolerance in maize by foliar application of boron: Water status, antioxidative defense and photosynthetic capacity. Archives of Agronomy and Soil Science, 64(5), 626–639. [Google Scholar]
9. Ilyas, M., Nisar, M., Khan, N., Hazrat, A., Khan, A. et al. (2020). Drought tolerance strategies in plants: A mechanistic approach. Journal of Plant Growth Regulation, 40, 926–944. [Google Scholar]
10. Serraj, R., McNally, K., Slamet-Loedin, I., Kohli, A., Haefele, S. et al. (2011). Drought resistance improvement in rice: An integrated genetic and resource management strategy. Plant Production Science, 14(1), 1–14. [Google Scholar]
11. Kostopoulou, Z., Therios, I., Roumeliotis, E., Kanellis, A. K., Molassiotis, A. (2015). Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant Physiology and Biochemistry, 86, 155–165. [Google Scholar] [PubMed]
12. Sarropoulou, V., Dimassi-Theriou, K., Therios, I., Koukourikou-Petridou, M. (2012). Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium x Prunus cerasus). Plant Physiology and Biochemistry, 61, 162–168. [Google Scholar] [PubMed]
13. Altaf, M. A., Shahid, R., Ren, M. X., Mora-Poblete, F., Arnao, M. B. et al. (2021). Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiologia Plantarum, 172(2), 820–846. [Google Scholar] [PubMed]
14. Sun, C., Liu, L., Wang, L., Li, B., Jin, C. et al. (2021). Melatonin: A master regulator of plant development and stress responses. Journal of Integrative Plant Biology, 63(1), 126–145. [Google Scholar] [PubMed]
15. Li, D., Batchelor, W. D., Zhang, D., Miao, H., Li, H. et al. (2020). Analysis of melatonin regulation of germination and antioxidant metabolism in different wheat cultivars under polyethylene glycol stress. PLoS One, 15(8), e0237536. [Google Scholar] [PubMed]
16. Cao, Q., Li, G., Cui, Z., Yang, F., Jiang, X. et al. (2019). Seed priming with melatonin improves the seed germination of waxy maize under chilling stress via promoting the antioxidant system and starch metabolism. Scientific Reports, 9(1), 15044. [Google Scholar] [PubMed]
17. Imran, M., Latif Khan, A., Shahzad, R., Aaqil Khan, M., Bilal, S. et al. (2021). Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB PLANTS, 13(4), plab026. [Google Scholar] [PubMed]
18. Silalert, P., Pattanagul, W. (2021). Foliar application of melatonin alleviates the effects of drought stress in rice (Oryza sativa L.) seedlings. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 49(31–13. [Google Scholar]
19. Chen, Y., Li, R., Ge, J., Liu, J., Wang, W. et al. (2021). Exogenous melatonin confers enhanced salinity tolerance in rice by blocking the ROS burst and improving Na+/K+ homeostasis. Environmental and Experimental Botany, 189, 104530. [Google Scholar]
20. Ahmad, S., Kamran, M., Ding, R., Meng, X., Wang, H. et al. (2019). Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ, 7, e7793. [Google Scholar] [PubMed]
21. Zhang, H. J., Zhang, N., Yang, R. C., Wang, L., Sun, Q. Q. et al. (2014). Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA(4) interaction in cucumber (Cucumis sativus L.). Journal of Pineal Research, 57(3), 269–279. [Google Scholar] [PubMed]
22. Xiao, S., Liu, L., Wang, H., Li, D., Bai, Z. et al. (2019). Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS One, 14(6), e0216575. [Google Scholar] [PubMed]
23. Park, S., Back, K. (2012). Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. Journal of Pineal Research, 53(4), 385–389. [Google Scholar] [PubMed]
24. Cen, H., Wang, T., Liu, H., Tian, D., Zhang, Y. (2020). Melatonin application improves salt tolerance of alfalfa (Medicago sativa L.) by enhancing antioxidant capacity. Plants, 9(2), 220. [Google Scholar] [PubMed]
25. Zhu, L., Guan, L., Zhang, J., Wang, Y. (2021). Alleviation effect of exogenous IBA on rice seed germination under salt stress. Fenzi Zhiwu Yuzhong (Molecular Plant Breeding), 19(18), 6126–6134. [Google Scholar]
26. Giannopolitis, C. N., Ries, S. K. (1977). Superoxide dismutases II. purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiology, 59, 315–318. [Google Scholar] [PubMed]
27. Kochba, J., Lavee, S., Spiegelroy, P. (1977). Differences in peroxidase activity and isoenzymes in embryogenic ane nonembryogenic ‘Shamouti’ orange ovular callus lines. Plant and Cell Physiology, 18, 463–467. [Google Scholar]
28. Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126. [Google Scholar] [PubMed]
29. Hodges, D. M., DeLong, J. M., Forney, C. F., Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta, 207, 604–611. [Google Scholar]
30. Read, S. M., Northcote, D. H. (1981). Minimization of variation in the response to different protein of the Coomassie blue G dye-binding assay for protein. Analytica Biochemistry, 116, 53–64. [Google Scholar] [PubMed]
31. Su, D., Mei, L., Lin, H., Luo, H., Zhang, L. et al. (2017). Grey correlation analysis of physiological traits related to drought tolerance in Pennisetum sp. Agricultural Basic Science and Technology, 18(7), 1158–1163. [Google Scholar]
32. Sun, Q., Hu, J. J. (2005). Research technology of plant physiology. Northwest University of Agriculture and Forestry Science and Technology Press, Yangling, China. [Google Scholar]
33. Harb, A., Krishnan, A., Ambavaram, M. M., Pereira, A. (2010). Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiology, 154(3), 1254–1271. [Google Scholar] [PubMed]
34. Wu, L. M., Fang, Y., Yang, H. N., Bai, L. Y. (2019). Effects of drought-stress on seed germination and growth physiology of quinclorac-resistant Echinochloa crusgalli. PLoS One, 14(4), e0214480. [Google Scholar] [PubMed]
35. Zhenyi, W., Xia, P., Zhongjv, M., Yong, G., Xiaohong, D. et al. (2019). Response of Chamecytisus palmensis to drought stress induced by polyethylene glycol during germination. Journal of Plant Nutrition, 42(20), 2814–2823. [Google Scholar]
36. Heshmati, S., Dehaghi, M. A., Farooq, M., Wojtyla, Ł., Maleki, K. et al. (2021). Role of melatonin seed priming on antioxidant enzymes and biochemical responses of Carthamus tinctorius L. under drought stress conditions. Plant Stress, 2, 100023. [Google Scholar]
37. Zhang, M., He, S., Qin, B., Jin, X., Wang, M. et al. (2020). Exogenous melatonin reduces the inhibitory effect of osmotic stress on antioxidant properties and cell ultrastructure at germination stage of soybean. PLoS One, 15(12), e0243537. [Google Scholar] [PubMed]
38. Simlat, M., Szewczyk, A., Ptak, A. (2020). Melatonin promotes seed germination under salinity and enhances the biosynthesis of steviol glycosides in Stevia rebaudiana Bertoni leaves. PLoS One, 15(3), e0230755. [Google Scholar] [PubMed]
39. Bai, Y., Xiao, S., Zhang, Z., Zhang, Y., Sun, H. et al. (2020). Melatonin improves the germination rate of cotton seeds under drought stress by opening pores in the seed coat. PeerJ, 8, e9450. [Google Scholar] [PubMed]
40. Sharma, A., Zheng, B. (2019). Melatonin mediated regulation of drought stress: Physiological and molecular aspects. Plants, 8(7), 190. [Google Scholar]
41. Han, Q. H., Huang, B., Ding, C. B., Zhang, Z. W., Chen, Y. E. et al. (2017). Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Frontiers in Plant Science, 8, 00785. [Google Scholar]
42. Cui, G., Zhao, X., Liu, S., Sun, F., Zhang, C. et al. (2017). Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiology and Biochemistry, 118, 138–149. [Google Scholar] [PubMed]
43. Ye, J., Wang, S., Deng, X., Yin, L., Xiong, B. et al. (2016). Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiologiae Plantarum, 38(2), 48. [Google Scholar]
44. Galano, A., Tan, D. X., Reiter, R. J. (2013). On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. Journal of Pineal Research, 54(3), 245–257. [Google Scholar] [PubMed]
45. Su, X., Xin, L., Li, Z., Zheng, H., Mao, J. et al. (2018). Physiology and transcriptome analyses reveal a protective effect of the radical scavenger melatonin in aging maize seeds. Free Radical Research, 52(10), 1094–1109. [Google Scholar] [PubMed]
46. Wang, Z., Wu, X., Chang, X., Li, R., Jing, R. (2010). Chlorophyll content and chlorophyll fluorescence kinetics parameters of flag leaf and their gray relational grade with yield in wheat. Acta Agronomica Sinica, 36(2), 217–227. [Google Scholar]
47. Mishra, S. S., Panda, D. (2017). Leaf traits and antioxidant defense for drought tolerance during early growth stage in some popular traditional rice landraces from Koraput. India Rice Science, 24(4), 207–217. [Google Scholar]
48. Chen, X., Zhang, Z., Wu, B. (2014). Comprehensive evaluation of salt tolerance and screening for salt tolerant accessions of Naked Oat (Avena nuda L.) at germination stage. Scientia Agricultura Sinica, 47(10), 2038–2046. [Google Scholar]
Appendix
Table S1: Two-factor analysis of variance on rice seed germination and physiological indices
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools