 Open Access
Open Access
ARTICLE
Soil Moisture Rather than Soil Nutrient Regulates the Belowground Bud Bank of Rhizomatous Species Psammochloa villosa in Arid Sand Dunes
1
College of Forestry, Henan Agricultural University, Zhengzhou, 450002, China
2
Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang, 110164, China
3
Experimental Center of Desert Forestry, Chinese Academy of Forestry, Inner Mongolia Dengkou Desert Ecosystem National
Observation Research Station, Dengkou, 015200, China
4
Institute of Landscape and Plant Ecology, University of Hohenheim, Stuttgart, 70599, Germany
* Corresponding Authors: Zhiming Zhang. Email: ; Jianqiang Qian. Email:
(This article belongs to the Special Issue: Plant–Environment Interactions)
Phyton-International Journal of Experimental Botany 2023, 92(5), 1301-1309. https://doi.org/10.32604/phyton.2023.027043
Received 10 October 2022; Accepted 18 November 2022; Issue published 09 March 2023
Abstract
In arid and semi-arid sand dune ecosystems, belowground bud bank plays an important role in population regeneration and vegetation restoration. However, the responses of belowground bud bank size and composition to sand burial and its induced changes in soil environmental factors have been rarely studied. In arid sand dunes of Northwestern China, we investigated belowground bud bank size and composition of the typical rhizomatous psammophyte Psammochloa villosa as well as three key soil environmental factors (soil moisture, total carbon and total nitrogen) under different depths of sand burial. Total buds and rhizome buds increased significantly with increasing burial depth, whereas tiller buds first increased and then decreased, with a peak value at the depth of 20–30 cm. Soil moisture increased significantly with sand burial depth, and was positively correlated with the number of all buds and rhizome buds. Soil total carbon concentration first increased and then decreased with sand burial depth, and total nitrogen concentration was significantly lower under deep sand burial than those at shallow depths, and only the number of tiller buds was positively correlated with soil total nitrogen concentration. These results indicate that soil moisture rather than soil nutrient might regulate the belowground bud bank of P. villosa, and that clonal psammophytes could regulate their belowground bud bank in response to sand burial and the most important environmental stress (i.e., soil moisture). These responses, as the key adaptive strategy, may ensure clonal plant population regeneration and vegetation restoration in arid sand dunes.Keywords
In natural ecosystems dominated by perennial species, plant population maintenance and regeneration are closely related to belowground propagule bank (i.e., seed bank and bud bank) [1]. Most clonal plants can reproduce sexually through seed bank and asexually through bud bank [2–4]. Compared with seed bank, bud bank plays more important roles in population regeneration and vegetation recovery after disturbances [5,6]. Additionally, belowground bud bank enables plants to resist frequent disturbances and severe environmental stresses, and is able to accomplish rapid replenishment and renewal of plant population, thereby playing a crucial role in the maintenance and restoration of ecosystem functions [4,7,8].
Belowground bud bank has complex relationships with environmental factors, and the density and composition of belowground bud bank vary with resource availability and disturbances in habitats [8–10]. For instance, soil moisture and nutrients have been confirmed as the critical factors influencing the density and composition of belowground bud bank in wetland ecosystems [11–13]. Soil moisture also significantly influences the bud bank density and composition in grassland ecosystems [5,14]. Along a natural precipitation gradient in Inner Mongolian grasslands, not only the density of belowground bud bank decreases toward the arid and hot ends, but the type of bud bank also changes [10]. Although the relationships between bud bank and environmental factors have been well discussed in grassland and wetland communities, little is known about such relationships in stressful ecosystems such as drylands.
Sand dune ecosystems are characterized by aeolian disturbances and environmental stresses (i.e., sand burial, wind erosion and water stress) [15–17]. The strong aeolian activities in sand dunes often lead to the changes in sand burial depth and corresponding changes in soil properties (mainly soil moisture and nutrients), which pose great threats to individual growth, population maintenance and regeneration, as well as vegetation restoration and rehabilitation. Due to harsh habitat conditions, seeds often fail to germinate or emergent seedlings cannot survive and establish, thus the population regeneration and vegetation restoration in sand dunes rely greatly on belowground bud bank [18–20]. Compared with sexual reproduction, vegetative reproduction has considered to be advantageous since it enables plants to survive severe wind erosion and sand burial by means of rhizome systems [21,22]. A previous study has confirmed that the vegetative reproduction of rhizomatous grasses significantly contributes to population colonization to active sand dunes and plays an important role in the vegetation restoration of sand dunes [23]. As the basis of vegetative reproduction, plants might regulate their bud bank size and composition in adapt to sandy environment [24], and excessive sand burial and wind erosion significantly reduce total bud density and alter bud bank composition [18]. In this sense, exploring how sand burial depth and its resultant soil environmental factors changes affect the size and composition of bud bank of clonal plants in arid dunes is crucial for revealing plant adaptive strategy, assessing sand dune ecosystem stability and predicting plant community dynamics and vegetation restoration progresses.
Therefore, in the present study, the typical rhizomatous grass in arid and semi-arid sand dunes of northwestern China Psammochloa villosa was selected as target clonal species, which possesses a large and extensive belowground rhizome system and has often been selected for sand-fixing and vegetation restoration in sand dunes. A previous study has pointed out that the clonal growth (i.e., number of ramets and rhizomes) rather than clonal morphology (i.e., spacer length and rhizome inter-node length) are responsive to soil water and nutrient changes, and rhizomes function mainly as connections between ramets and as stores of carbohydrate and buds for regeneration [25]. The belowground bud bank is just the base of clonal growth and to some extent determines the number of ramets and rhizomes. Thus, in this study we focused on the belowground bud bank size and composition under different sand burial depth to discuss the adaptive clonal strategy to sandy environments. This study aimed to answer the following two questions: 1) how does the belowground bud bank size and composition of this rhizomatous species change with sand burial depth, 2) what is the key regulating factor of belowground bud bank changing with sand burial depth.
This study was conducted in the sand dunes nearby the Desert Ecosystem National Observation Research Station, Chinese Academy of Forestry, Dengkou, Inner Mongolia, China (40°09′–40°57′N, 106°9′–107°10′E). This region belongs to the temperate continental monsoon climate, with the mean annual temperature of 7.4°C, and the mean annual precipitation is 114 mm, which mainly occurs as rainfall from June to September. The mean annual potential evaporation is 2,372 mm, about 20.8 times of the precipitation. This region belongs to the transition zone between desert and semi-desert in northwestern China. The geomorphology is mainly composed of mobile dunes, semi-mobile dunes and flat sandy land, and soil types mainly consist of aeolian sandy and gray-brown desert soils. The vegetation coverage is low, which is mainly dominated by psammophyte and xerophyte as well as artificial sand-fixing woodlands (i.e., Haloxylon ammodendron). The dominant shrub and herb species include P. villosa, Phragmites australis, Artemisia ordosica, Nitraria tangutorum and A. xerophytica [26].
P. villosa is a rhizomatous perennial grass, mainly distributed in Gansu, Shaanxi, Ningxia, Xinjiang, Qinghai and Inner Mongolia of China, as well as in Mongolia. It is a typical xerophytic and pioneering psammophyte, which often inhabits mobile- and semi-mobile sand dunes. This species can reproduce sexually each year but very few seedlings have been observed, especially in mobile dunes. Its rhizome can grow horizontally in sand profile, and the bud bearing on rhizomes could sprout into offspring ramets under favourable conditions. In the field, it usually forms a multiple-layer network of rhizomes belowground, and by virtue of the large and extensive belowground rhizome systems as well as strong lateral spread and vegetative reproduction ability, P. villosa has been often selected as target species for sand-fixing and vegetation restoration in sand dunes [25].
2.3 Field Investigation and Sampling
During the growing season (May-August) of P. villosa in 2021, six plots (20 m × 20 m) were randomly selected in semi-mobile sand dunes where this species mainly inhabits. According to a previous study and a field survey, the rhizomes of P. villosa are mainly distributed in 10–40 cm sand profile, and very few occur in the shallow sand layer (0–10 cm). Thus we selected three sand burial depths (10–20, 20–30, 30–40 cm) to explore the changes in belowground bud bank size and composition (rhizome bud and tiller bud) with changes in sand burial depth. In six sample plots, at least six clonal fragments (for replicates) with similar size were dug out at each burial depth, and the rhizomes were truncated from the rhizomes’ apical bud backwards for 5 m. Soil samples were taken with cutting rings (8 cm in diameter) at the front, middle and end positions of each rhizome fragment, then taken back to laboratory for subsequent processing. Rhizome fragments were carefully excavated out with a shovel, and record the sand burial depth of each P. villosa rhizome, and the buds were identified to rhizome buds and tiller buds, and counted. Soil samples were dried in an oven at 105°C to constant weight to obtain soil moisture content. Soil samples in sealed bags were taken back to lab and were put in the EA3000 elemental analyzer to measure the soil nutrient concentration (total carbon and total nitrogen).
Prior to data analysis, the mean bud number (total-, rhizome- and tiller buds) of six rhizome fragments at each sand burial depth in each plot was calculated. Data were first tested for normal distribution and homogeneity test of variance, and followed by one-way ANOVA to investigate the differences in the size and composition of the P. villosa bud bank under different sand burial depths. The LSD method was used for subsequent multiple comparisons among the means. Then regression analysis was employed to explore the relationships between belowground bud bank and soil environmental factors (soil moisture, total carbon concentration and total nitrogen concentration). All statistical analyses of the data were completed using SPSS 20.0.
3.1 Belowground Bud Bank Size and Composition under Different Sand Burial Depths
Total bud number under 20–30 and 30–40 cm burial depths were significantly higher than that under 10–20 cm burial depth (p < 0.05, F = 3.413). As regard with the two bud types, the number of rhizome buds under 10–20 and 20–30 cm burial depths were significantly lower than that under 30–40 cm burial depth (p < 0.05, F = 4.716) whereas the number of tiller buds first increased and then decreased and was significantly higher under the burial depth of 20–30 cm (p < 0.05, F = 5.686) (Fig. 1).
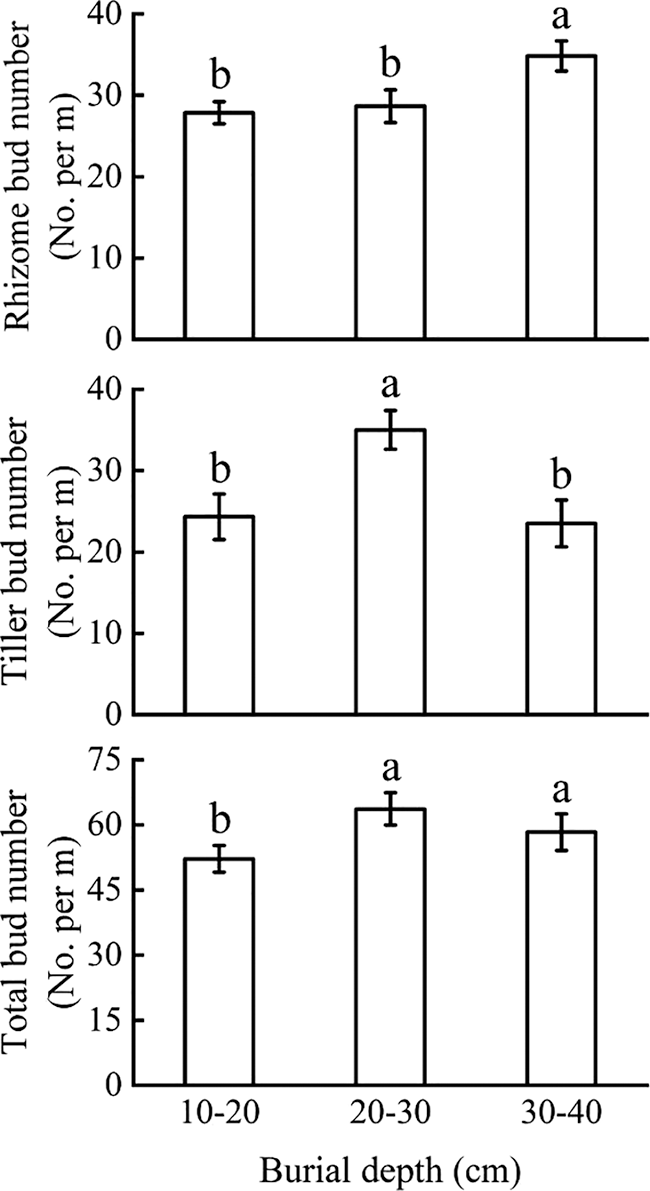
Figure 1: Variation in bud bank size and composition (rhizome buds and tiller buds) (Mean ± SE) under different sand burial depths. Different letters indicate the significant difference in rhizome buds, tiller buds and total buds under different sand burial depth at p < 0.05 level
3.2 Changes in Soil Environmental Factors with Sand Burial Depth
Soil moisture increased significantly with the increasing sand burial depth (p < 0.01, F = 11.361), while soil total carbon concentration first increased and then decreased. The total carbon concentration in 20–30 cm burial depth was significantly higher than those in 10–20 and 30–40 cm burial depths. The total nitrogen concentration in 30–40 cm burial depth was significantly lower than those in the two shallow burial depths (p < 0.01, F = 49.164) (Fig. 2).
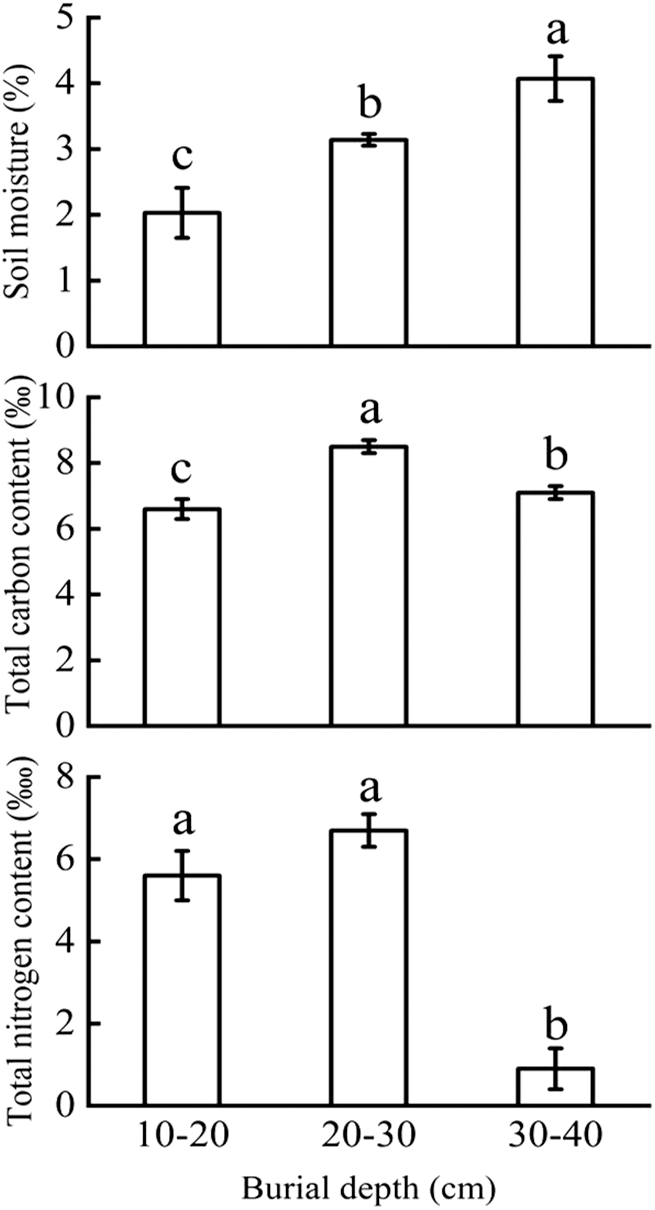
Figure 2: Variation in soil environmental factors (soil moisture, total carbon concentration and total nitrogen concentration) (Mean ± SE) under different sand burial depths. Different letters indicate the significant difference in soil moisture, total carbon concentration and total nitrogen concentration under different sand burial depths at p < 0.05 level
3.3 Relationships between Belowground Bud Bank and Soil Environmental Factors
The number of total buds (df = 17, R2 = 0.24, p < 0.05) and rhizome buds (df = 17, R2 = 0.54, p < 0.01) were positively related with soil moisture while there was no relationship between tiller buds and soil moisture (Fig. 3). There were no significant relationships between bud bank and the other two soil environmental factors (total carbon concentration and total nitrogen concentration) except for the significantly positive relationship between tiller buds and soil total nitrogen concentration (df = 17, R2 = 0.28, p < 0.05) (Fig. 3).
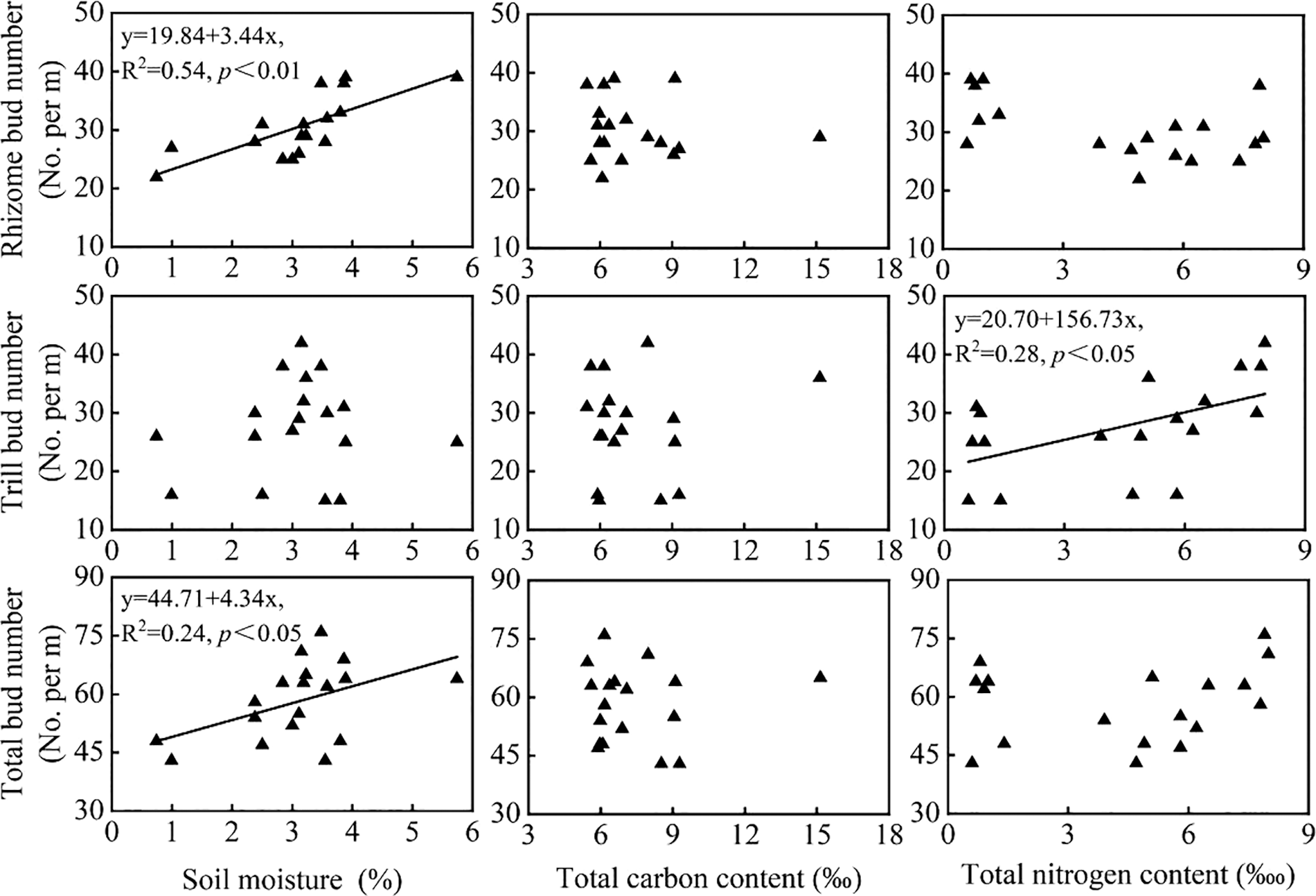
Figure 3: Relationships between soil environmental factors (soil moisture, total carbon concentration and total nitrogen concentration) and bud bank size (total buds) and composition (rhizome buds and tiller buds)
In the present study, for this typical rhizomatous psammophyte in arid sand dunes P. villosa, both total buds and rhizome buds significantly increased with the increasing sand burial depth, which might be attributed to the relatively higher soil moisture under deeper burial depth. Soil moisture in arid and semi-arid regions has been considered as the primary factors limiting plant growth and reproduction [27]. This result was consistent with the positive relationships between belowground bud density and water availability reported in previous studies [5,10]. For instance, belowground bud density increases with average annual precipitation in North American grasslands [5], and from the opposite view it decreases towards the dry, hot end of the climatic gradient in Inner Mongolian grasslands [10]. The positive relationship between total number of buds and soil moisture further confirms that belowground bud bank size is greatly regulated by the changes in soil moisture with sand burial depth, especially in arid sand dunes with severe water stress.
Both for the whole community and specific species, previous studies have found that rhizome buds prefer moist habitats and are more sensitive to soil water availability than tiller buds. For example, along the climatic gradient in the temperate steppe of Northern China, the proportion of rhizome buds is higher at relatively moist sites whereas that of tiller buds fluctuated along the aridity gradient [10]. Similarly with the target species P. villosa in this study, the rhizome buds of dominant species in the temperate steppes of Leymus chinensis (also rhizomatous grass) are more apt to be affected by soil water status compared with tiller buds, and the production of rhizome buds is higher under high soil moisture [14]. Findings of these previous studies and the positive relationship between rhizome buds and soil moisture in this study all support and explain our results that rhizome buds significantly increase with the increasing sand burial depth.
Apart from the changing soil moisture with sand burial depth, the “cost-benefit” theory might also explain the increasing rhizome buds with sand burial depth [28]. Sand burial poses some extent physical barrier for species growth and reproduction [29,30]. Under deep burial depth, clonal species needs more energy and resource for vertical growth and ramet emergence aboveground for future photosynthesis, this process tends to be costly. Under the circumstances, clonal species produce more rhizome buds for horizontal spread, and when wind erosion causes shallow burial, those rhizome buds could sprout into offspring ramets (i.e., P. communis in Liu et al. [31]) and contribute to population regeneration and vegetation restoration in sand dunes.
Despite of major regulation of soil moisture on total buds and rhizome buds, we still need to be aware of that tiller buds and soil nutrient concentration showed similarly trends with sand burial depth, and there was no significant relationship with the number of tiller buds and soil moisture. It manifests that tillers buds are more tolerant to water stress than rhizome buds [10,14], which seem to be mediated by soil nutrient availability. This can be proven by the significantly positive relationship between tiller buds and soil total nitrogen concentration in this study. The tolerance of tiller buds to soil moisture in this water stressful sand dunes might be mainly attributed to their low resource costly since they originated at the shoot bases of clones. In turn, relatively high resource input and cost are needed for supporting the extensive rhizome systems where rhizome buds bearing on. From the perspective of clonal growth form and ecological functions of these two bud types, tiller buds and rhizome buds represent the vertical- and horizontal growth/reproduction potential, respectively [23,31]. Their proportion changes in clonal plants reflect the shift in clonal growth configuration (phalanx or guerilla), which have been considered as an important adaptive strategy of clonal species to resource heterogeneity and disturbances [32,33]. Thus in this study, the higher number of tiller buds under 20–30 cm sand burial depth indicates that clonal species are inclined to produce more tiller buds for their vertical growth to adapt to a medium sand burial, which has been proven that in semi-arid sand dunes, sand burial could facilitate the production of tiller buds [15]. The changes in bud bank composition (tiller buds and rhizome buds) with sand burial depth portend the vertical and horizontal growth/propagation potential, which has also been confirmed by the studies on rhizome species P. communis in semi-arid sand dunes [23,31].
In conclusion, soil moisture greatly determined the bud bank size and composition of the dominant rhizomatous psammophyte P. villosa in arid sand dunes, and rhizomatous species might regulate their bud bank in response to sandy environments. Specifically, the number of total buds and rhizome buds increased with sand burial depth and are greatly regulated by soil moisture (i.e., significantly positive relationship between bud number and soil moisture), whereas tiller buds seem to be positively related to soil total nitrogen concentration as they show similar trend with burial depth. Therefore, our study demonstrated that the adjustment of belowground bud bank size and composition is an important adaptive strategy of clonal species in responses to sandy environments, which ensures plant population maintenance and regeneration and promotes vegetation restoration in arid and semi-arid sand dunes.
Acknowledgement: We thank Chaoqun Ba and Shanshan Zhai for their assistance during the field investigation and sampling. We are also very grateful for the field assistance of colleagues at the Inner Mongolia Dengkou Desert Ecosystem National Observation Research Station.
Funding Statement: This work was financially supported by the National Natural Science Foundation of China (41877542, 41907411).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Zhiming Zhang, Jianqiang Qian; data collection: Yawei Dong, Ziyue Guo, Zhiming Xin, Jin Tao, Jiatai Tian; analysis and interpretation of results: Yawei Dong, Jinlei Zhu; draft manuscript preparation: Yawei Dong, Qun Ma, Jianqiang Qian. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Harper, J. L. (1977). Population biology of plants. New York: Academic Press. [Google Scholar]
2. Yu, F., Dong, M., Krüsi, B. (2004). Clonal integration helps Psammochloa villosa survive sand burial in an inland dune. New Phytologist, 162(3), 697–704. [Google Scholar] [PubMed]
3. Zhang, Y., Zhang, D. (2007). Asexual and sexual reproductive strategies in clonal plants. Frontiers of Biology in China, 2(3), 256–262. [Google Scholar]
4. Klimešová, J., Klimeš, L. (2007). Bud banks and their role in vegetative regeneration–A literature review and proposal for simple classification and assessment. Perspectives in Plant Ecology, Evolution and Systematics, 8(3), 115–129. [Google Scholar]
5. Dalgleish, H. J., Hartnett, D. C. (2006). Below-ground bud banks increase along a precipitation gradient of the North American Great Plains: A test of the meristem limitation hypothesis. New Phytologist, 171(1), 81–89. [Google Scholar] [PubMed]
6. Benson, E. J., Hartnett, D. C. (2006). The role of seed and vegetative reproduction in plant recruitment and demography in tallgrass prairie. Plant Ecology, 187(2), 163–177. [Google Scholar]
7. Dalgleish, H. J., Hartnett, D. C. (2009). The effects of fire frequency and grazing on tallgrass prairie productivity and plant composition are mediated through bud bank demography. Plant Ecology, 201(2), 411–420. https://doi.org/10.1007/s11258-008-9562-3 [Google Scholar] [CrossRef]
8. Ott, J. P., Klimešová, J., Hartnett, D. C. (2019). The ecology and significance of below-ground bud banks in plants. Annals of Botany, 123(7), 1099–1118. https://doi.org/10.1093/aob/mcz051 [Google Scholar] [PubMed] [CrossRef]
9. Qian, J., Wang, Z., Liu, Z., Busso, C. A. (2017). Belowground bud bank responses to grazing intensity in the inner-Mongolia steppe. China Land Degradation & Development, 28(3), 822–832. https://doi.org/10.1002/ldr.2300 [Google Scholar] [CrossRef]
10. Qian, J., Wang, Z., Klimešová, J., Lü, X., Kuang, W. et al. (2017). Differences in below-ground bud bank density and composition along a climatic gradient in the temperate steppe of Northern China. Annals of Botany, 120(5), 755–764. https://doi.org/10.1093/aob/mcx072 [Google Scholar] [PubMed] [CrossRef]
11. Chen, X., Deng, Z., Xie, Y., Li, F., Li, X. (2014). Differential growth and vegetative reproduction of two co-occurring emergent macrophytes along a water table gradient. Pakistan Journal of Botany, 46(3), 881–886. [Google Scholar]
12. Li, L., Lan, Z., Chen, J., Song, Z. (2018). Allocation to clonal and sexual reproduction and its plasticity in Vallisneria spinulosa along a water-depth gradient. Ecosphere, 9(1), e02070. [Google Scholar]
13. Klimeš, L., Klimešová, J. (1999). Root sprouting in Rumex acetosella under different nutrient levels. Plant Ecology, 141(1/2), 33–39. [Google Scholar]
14. Wang, Z., Xu, A., Zhu, T. (2008). Plasticity in bud demography of a rhizomatous clonal plant Leymus chinensis L. in response to soil water status. Journal of Plant Biology, 51(2), 102–107. [Google Scholar]
15. Li, S., Zuidema, P. A., Yu, F., Werger, M. J. A., Dong, M. (2010). Effects of denudation and burial on growth and reproduction of Artemisia ordosica in Mu Us sandland. Ecological Restoration, 25(3), 655–661. [Google Scholar]
16. Samsone, I., Druva-Lūsīte, I., Andersone, U., Ņečajeva, J. (2009). Plasticity of a dune plant Alyssum gmelinii in response to sand burial in natural conditions. Acta Universitatis Latviensis, 753, 125–136. [Google Scholar]
17. Zhang, C., Yu, F., Dong, M. (2002). Effects of sand burial on the survival, growth, and biomass allocation in semi-shrub Hedysarum laeve seedlings. Acta Botanica Sinica, 44(3), 337–343 (in Chinese). [Google Scholar]
18. Ma, Q., Qian, J., Tian, L., Liu, Z. (2019). Responses of belowground bud bank to disturbance and stress in the sand dune ecosystem. Ecological Indicators, 106, 105521. [Google Scholar]
19. Wu, J., Qian, J., Zhou, Q., Yang, W., Liu, Z. (2021). Temporal-spatial changes in the belowground bud bank in interdune lowlands of an active sand dune ecosystem in Northeastern China. Journal of Plant Ecology, 14(1), 170–179. [Google Scholar]
20. Wu, J., Zhou, Q., Yu, F., Liu, Z. (2022). Changes and determinants of belowground bud banks along an interdune lowland sequence. Flora, 289, 152026. [Google Scholar]
21. Marbà, N., Duarte, C. M. (1995). Coupling of seagrass (Cymodocea nodosa) patch dynamics to subaqueous dune migration. Journal of Ecology, 83(3), 381–389. [Google Scholar]
22. Yu, F., Wang, N., He, W., Chu, Y., Dong, M. (2008). Adaptation of rhizome connection in drylands: Increasing tolerance of clones to wind erosion. Annals of Botany, 102(4), 571–577. [Google Scholar] [PubMed]
23. Liu, B., Liu, Z., Wang, L. (2012). The colonization of active sand dunes by rhizomatous plants through vegetative propagation and its role in vegetation restoration. Ecological Engineering, 44, 344–347. [Google Scholar]
24. Wu, J., Chen, X., Xu, L., Qian, J., Liu, Z. (2020). The spatial pattern of the belowground bud bank and its responses to soil water status in the interdune lowlands of active sand dunes of Inner Mongolia. China Restoration Ecology, 29(2), e13223. [Google Scholar]
25. Dong, M., Alaten, B. (1999). Clonal plasticity in response to rhizome severing and heterogeneous resource supply in the rhizomatous grass Psammochloa villosa in an Inner Mongolian dune. China Plant Ecology, 1(2), 53–58. [Google Scholar]
26. Pan, Y., Xiao, H., Xin, Z., Li, J., Miri, A. et al. (2022). Characteristics of energy distribution in a desert ecosystem in Inner Mongolia, Northern China. Frontiers in Environmental Science, 10, 939782. [Google Scholar]
27. Zhao, W., Li, Q., Fang, H. (2007). Effects of sand burial disturbance on seedling growth of Nitraria sphaerocarpa. Plant and Soil, 295(1), 95–102. [Google Scholar]
28. Vesk, P. A., Westoby, M. (2004). Funding the bud bank: A review of the costs of buds. Oikos, 106(1), 200–208. [Google Scholar]
29. Cabaco, S., Santos, R. (2007). Effects of burial and erosion on the seagrass Zostera noltii. Journal of Experimental Marine Biology and Ecology, 340(2), 204–212. [Google Scholar]
30. Luo, W., Zhao, W. (2015). Effects of wind erosion and sand burial on growth and reproduction of a clonal shrub. Flora, 217, 164–169. [Google Scholar]
31. Liu, B., Liu, Z., Wang, L., Wang, Z. (2014). Responses of rhizomatous grass Phragmites communis to wind erosion: Effects on biomass allocation. Plant and Soil, 1(2), 389–398. [Google Scholar]
32. Hutchings, M. J., de Kroon, H.(1994). Foraging in plants: The role of morphological plasticity in resource acquisition. Advances in Ecological Research, 25, 159–238. [Google Scholar]
33. Chen, X., Xie, Y., Deng, Z., Li, F., Hou, Z. (2011). A change from phalanx to guerrilla growth form is an effective strategy to acclimate to sedimentation in a wetland sedge species Carex brevicuspis (Cyperaceae). Flora, 206(4), 347–350. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools