 Open Access
Open Access
ARTICLE
Anatomical and Molecular Identification of Ornamental Plant Ficus L. Species
1
Department of Botany, Faculty of Science, University of Sabratha, Sabratha, 999116, Libya
2
Department of Botany, Faculty of Science, Cairo University, Giza, 12111, Egypt
3
Department of Botany and Microbiology, Faculty of Science (Boys Branch), Al-Azhar University, Cairo, 11884, Egypt
4
Department of Agricultural Botany, Faculty of Agriculture, Al-Azhar University, Cairo, 11884, Egypt
5
Department of Horticulture, Faculty of Agriculture, Ain Shams University, Cairo, 11566, Egypt
6
Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, 11671, Saudi Arabia
7
Department of Science and Technology, College of Ranyah, Taif University, Taif, 21944, Saudi Arabia
8
Department of Biology, College of Science, Taif University, Taif, 21944, Saudi Arabia
9
Department of Biology, College of Science, University of Jeddah, Jeddah, 21959, Saudi Arabia
10 Department of Plant Production, (Genetic Branch), Faculty of Environmental Agricultural Sciences, Arish University, El-Arish,
45511, Egypt
11 Department of Botany and Microbiology, Faculty of Science, Suez Canal University, Ismailia, 41522, Egypt
* Corresponding Author: Fatmah A. Safhi. Email:
(This article belongs to the Special Issue: Plant–Environment Interactions)
Phyton-International Journal of Experimental Botany 2023, 92(5), 1329-1347. https://doi.org/10.32604/phyton.2023.026888
Received 30 September 2022; Accepted 21 November 2022; Issue published 09 March 2023
Abstract
This present study includes twelve species that represent the Ficus genus, namely; aspera, carica, tinctoria subsp. gibbosa, hirta, hispida, neriifolia, palmata, pumila, racemosa, septica, sur, and sycomorus, belonging to the Moraceae family. The species samples were collected from various locations in Egypt. The study focused on the anatomical and molecular characteristics of mature foliage leaves. Since the identification and classification of taxa are highly dependent on the anatomical features of leaves, the anatomical characteristics were recorded in the form of a comparison between the examined plants in the data matrix. This study aims to contribute to the identification of the studied species based on the anatomical details of the matured leaves. Anatomical characterization includes the variations in upper and lower epidermal layers that are covered by a thin or thick cuticle; the number of palisade and spongy layers; crystals; secretory elements; lithocysts; the midrib zone has parenchyma associated with mechanical tissue, vascular system, and investigation of trichomes; on the other hand, in the current study, the phylogenetic analysis was conducted by using the ITS and 5.8 S sequences. From the analysis of all the available data, it could be stated that there is an overall agreement with the anatomical character dendrogram.Keywords
Ficus species are rich sources of naturally occurring antioxidants, of which phenolic compounds and flavanoids play a vital role in preventing innumerable health disorders related to oxidative stress including cardiovascular diseases, neurodegenerative diseases, and cancer [1]. Many researchers have successfully determined the antioxidant activity and total phenolic content (TPC) of crude extracts from F. carica leaves, Furthermore, the TPC and antioxidant activity of F. carica Linn latex from 18 cultivars were investigated, the latex of these three F. carica L. cultivars could be a potential source of natural antioxidants and polyphenols [2]. Ficus species contain a high polyphenol content and ideal ferric reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) values, which could be an excellent source of antioxidants. Moreover, the polysaccharide has the function of improving immunity, which indicates that it may be beneficial for treating diseases [3]. Chemical constituent and bioactivity investigation showed that the stems and leaves of F. tikoua mainly contain antifungal isoflavonoids [4], antioxidant lignans and phenolic compounds [5].
The genus Ficus belongs to the family Moraceae. This family includes figs, banyan, breadfruit, jackfruit, and mulberry. More than 845 species are included in the genus Ficus, of which the common fig (F. carica L.) and sycamore (F. sycomorus L.) have been widely recorded since pharaonic times in Egypt for their edible fruit and valuable wood [6]. Ficus is a pantropical genus native to the tropics, with a few species extending into the semi-warm temperate zone. It includes trees, shrubs, vines, epiphytes, hemi-epiphytes, and vines occupying various ecological niches. Based on morphological characteristics and distributional patterns, the genus Ficus has recently been classified into six subgenera, 19 sections, and 27 Subsections [7]. In addition, Pederneiras (2015) revised nomenclatural, which leads to the displacement of three subgeneric names (F. subg. Terega Raf., F. subg. Sycomorus Raf., F. subg. Spherosuke Raf.), and four sectional names (F. sect. Cordifoliae G. Don, F. sect. Pogonotrophe (Miq.) Miq., F. sect. Platyphyllae Mildbr. & Burret, F. sect. Urostigma (Endl.) Griseb.) that have priority over other long-used sub-generic and sectional names [8].
In this study, Ficus is represented by twelve species belonging to four subgenera distributed into seven sections. Most Egyptian species fall under the section Sycomorus of the subgenus Sycomorus. Taxonomically, the genus Ficus has been considered a problematic taxon. Infraspecific classification of the genus is based on morphological traits of leaves, flowers, and fruits. Syconium contains a minute flower found inside a fleshy receptacle. The syconium represented a challenging structure of the genus [9,10].
Anatomy represents another tool for the infraspecific delimitation of the genus Ficus [11–15]. Moreover, leaf indumentum should be a helpful tool for the same purpose [16–21]. Recently, DNA barcoding has been used extensively to resolve the classification of many proplomatic taxa [22–24]. DNA barcoding facilitates fast species identification based on standardized and short DNA sequences from organelle genomes or inter-spacer ribosomal genes [25–27]. Li et al. proposed a combination of several loci as Ficus barcodes, including rbcL, matK, psbK-psbI, trnH-psbA, atpF-atpH, and ITS [28–30].
The objectives of this contribution were to investigate the leaf anatomical traits and molecular characteristics of the 12 cultivated using previously published ITS sequences. Ficus taxa from Egypt.
Fresh samples for each species were collected from three botanical gardens in Giza and Aswan Governorates. All samples were taxonomically revised to confirm the identification using the available literature [9,10,31–33]. An extra confirmatory identification check using the valuable materials of the herbaria of Cairo University Herbarium (CAI) and The Agriculture Museum in Cairo (CAIM) was performed. All collected samples were then kept in the booth mentioned herbaria above (Table 1).

Twelve leaf samples were prepared for anatomical investigation according to the method proposed by Nassar and El-Sahhar [34]. A square centimeter of the leaf was removed, and it was dehydrated in a succession of solutions with ethyl alcohol concentrations ranging from 50% to 100%. The samples were then embedded in paraffin wax [the melting point of paraffin wax range is 58°C–62°C using xylol as a solvent. Sections were cut at a thickness of 15 microns using a rotary microtome and then mounted on slides using egg albumin as an adhesive agent. The slides were subjected to a declining sequence of ethyl alcohol solutions ranging from 100% to 50% ethyl alcohol concentrations.
A double stain composed of safranin and light green was used. Canada balsam was used as a mounting medium. Sections were investigated using a light microscope, Serico XSZ-107BN, at the Department of Botany and Microbiology, Faculty of Science, Cairo University, Egypt. Photomicrographs were taken using a photomicroscope; Optika fitted with a premiere MA88-900 digital camera in the Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Egypt.
All terms used to describe the leaf anatomical traits were after [12,17,19,20]. Forty anatomical traits of the leaf were scored and coded to build up a numerical data matrix. Statistical analysis using PC-ORD (Software, Version 5) was conducted to compare the investigated taxa. To build a data matrix for numerical analysis, a total of 40 comparative anatomical features for the analyzed taxa were scored and coded. Finally, artificial keys were generated using the statistical programme DELTA [35].
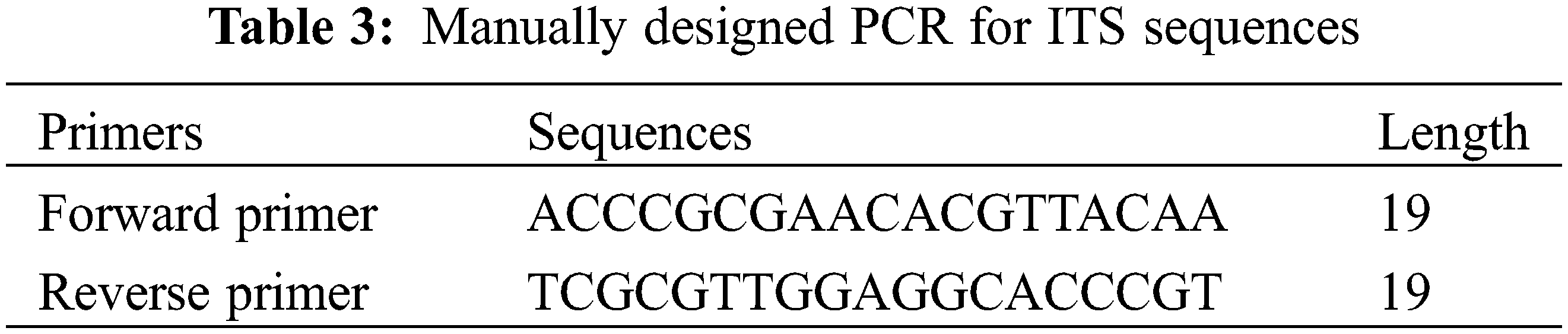
To investigate the molecular characteristics of Egyptian Ficus species. We retrieved the ITS and 5.8 S sequences from NCBI (Table 2). Next, we used 19 bp upstream and downstream of the sequence of F. neriifolia as a potential primer for in-silico PCR (Table 3). The Primer-Blast module of NCBI extracted the amplicons from the target sequences. Finally, we used the ClustalW module on Mega7 [36] to execute Multiple Sequence Alignment (MSA) for the amplicons and construct a phylogenetic tree based on a maximum likelihood algorithm. While the maximum likelihood tree shows the most likelihood topology for the taxonomical tree and branches, it also shows a general collinearity agreement with the hierarchical morphological tree.
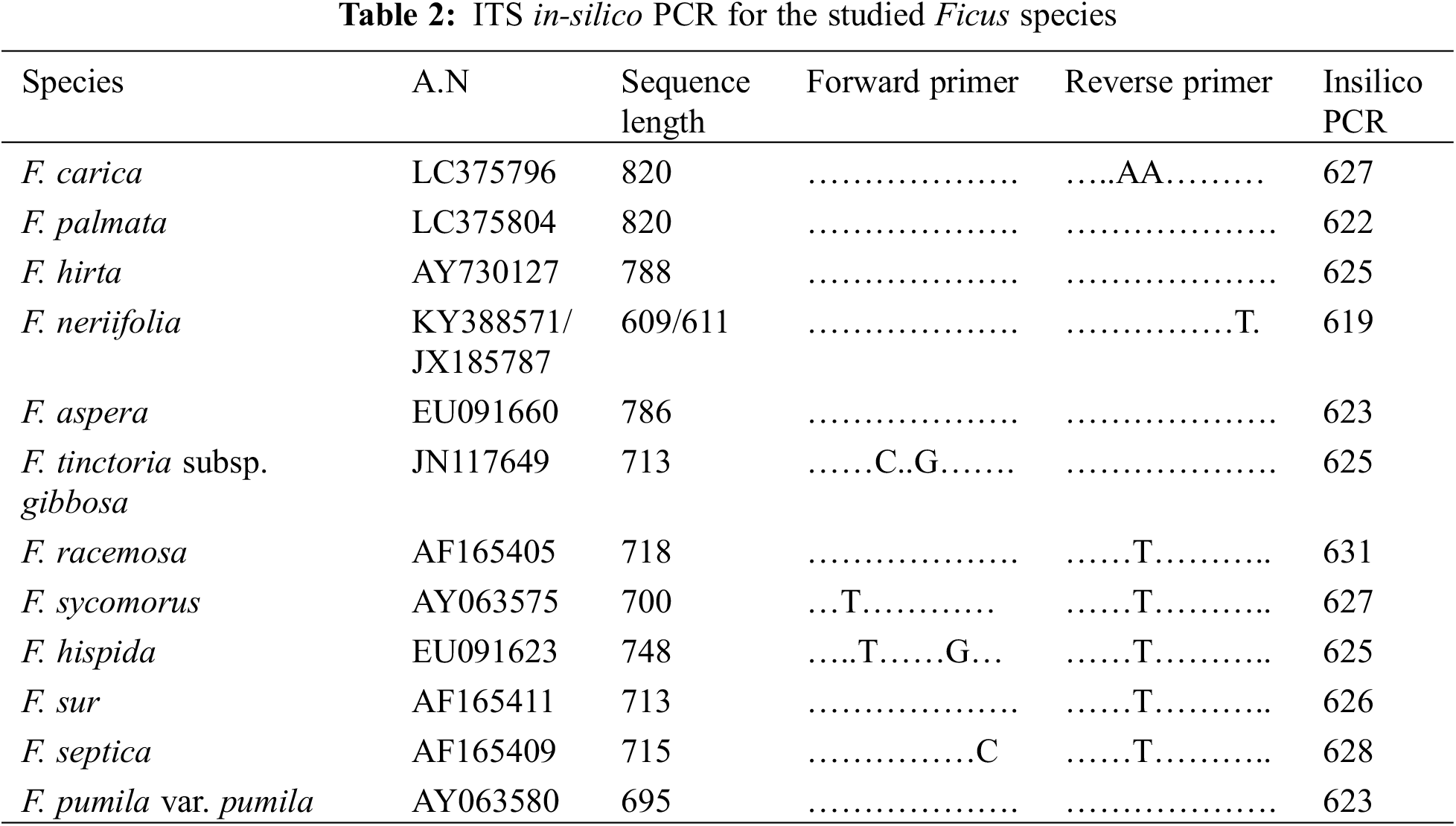
Hairy leaf surfaces were recorded in ten Ficus species. The leaves of F. neriifolia and F. septica were glabrous. The following types of trichomes were recognized on both abaxial and adaxial leaf surfaces (Table 4 and Fig. 1).
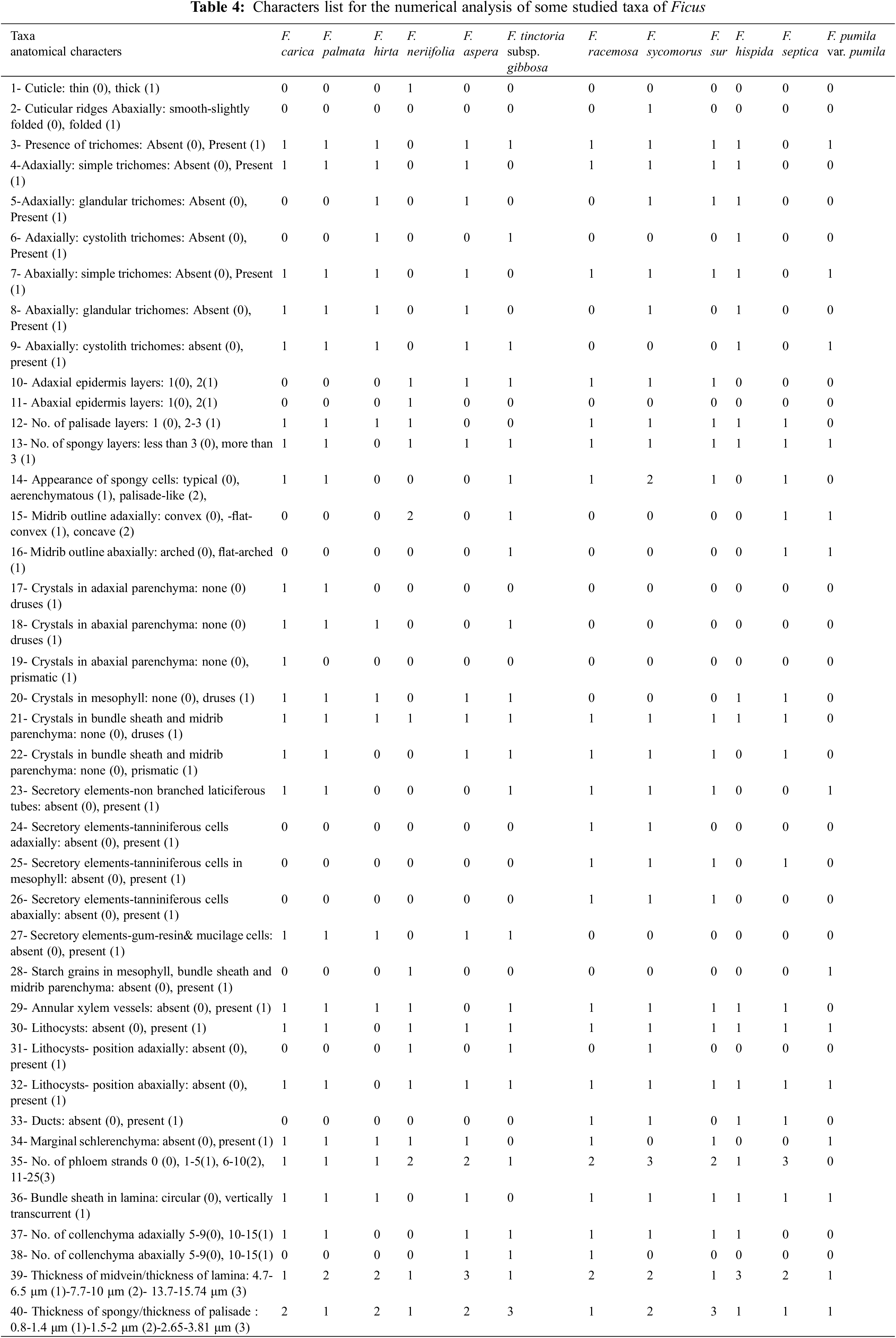
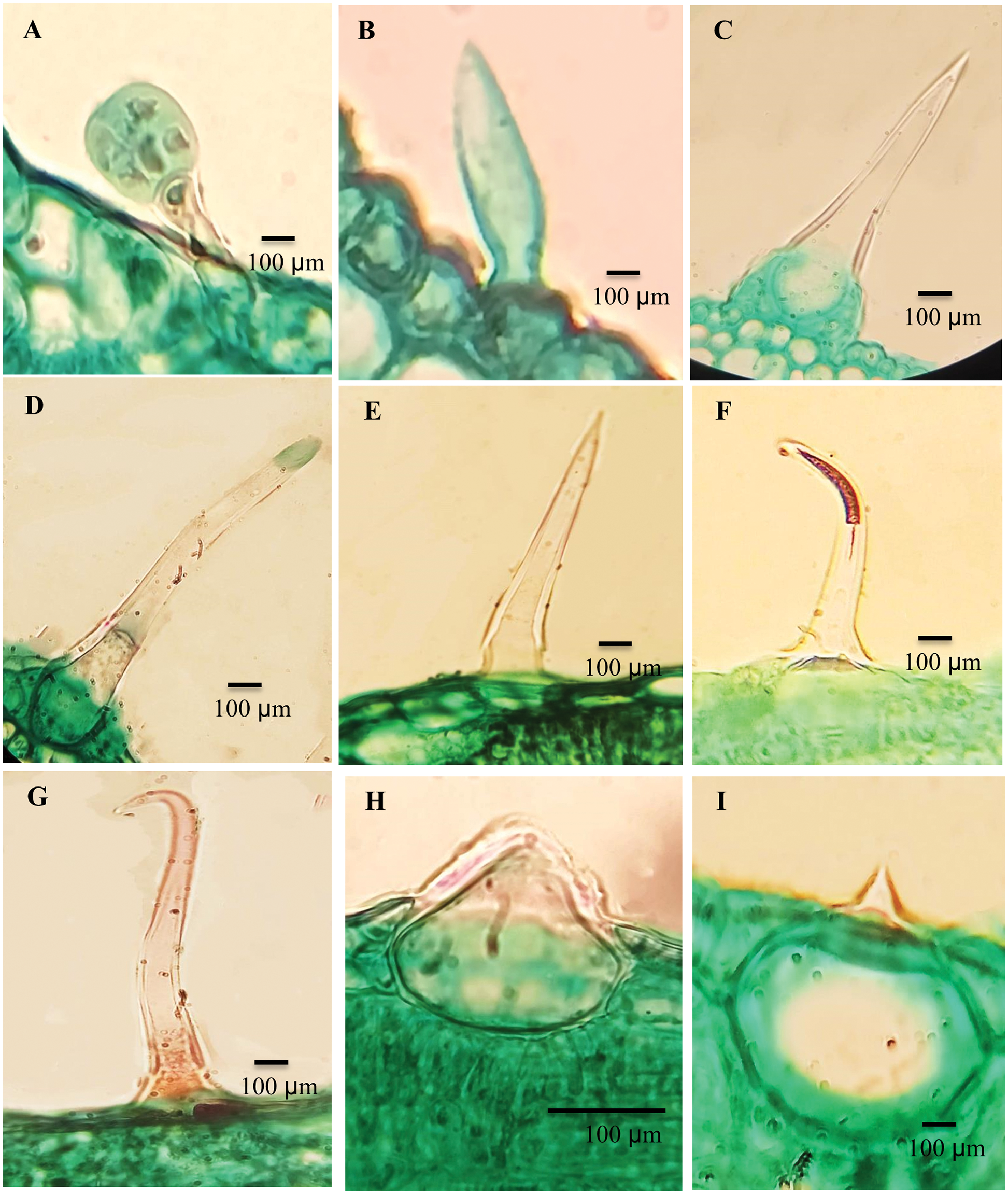
Figure 1: Trichomes of the studied taxa Ficus. A. F. hispida, B. F. sycomorus, C. F. carica, D. F. hispida, E. F. carica, F. F. hirta, G. F. sycomorus, H. F. carica and, I. F. tinctoria subsp. gibbosa
The stalks are composed of 1–2 cells with spherical heads: F. sur, F. hirta, F. carica; F. aspera; F. palmata and F. hispida (Fig. 1A).
They are categorized into four groups:
a) Unicellular trichome: F. sycomorus (Fig. 1B).
b) Unicellular trichomes with prominent bases surrounded by raised epidermal cells: F. hirta; F. carica (Fig. 1C) and curved end in F. palmata, and F. hispida (Fig. 1D).
c) Multicellular, slender bristle trichomes, terminating gradually into the tapered end, F. racemosa, F. sur, F. palmata, F. carica, F. aspera and F. hispida (Fig. 1E), while in F. hirta, the trichome was found to be slightly curved (Fig. 1F).
d) The adaxial leaf surfaces have trichomes that are mainly described as long clothing hairs: F. carica, and F. sycomorus (Fig. 1G).
This type of trichome is composed of a rounded, sometimes swollen base sunken into the mesophyll layer of the leaf, with very short mucronate at the apex. Cystolith was found in F. hirta, F. hispida, and F. carica (Fig. 1H). Cytoliths were recorded for all studied taxa, with a large base sunken in mesophyll and a sharp mucronate apex more on the abaxial surface than adaxial; F. palmata, F. aspera, F. pumila and F. tinctoria subsp. gibbosa (Fig. 1I).
Table 4 and Figs. 2 and 3 summarize the anatomical features of the leaf that permit effective discrimination between the studied taxa.
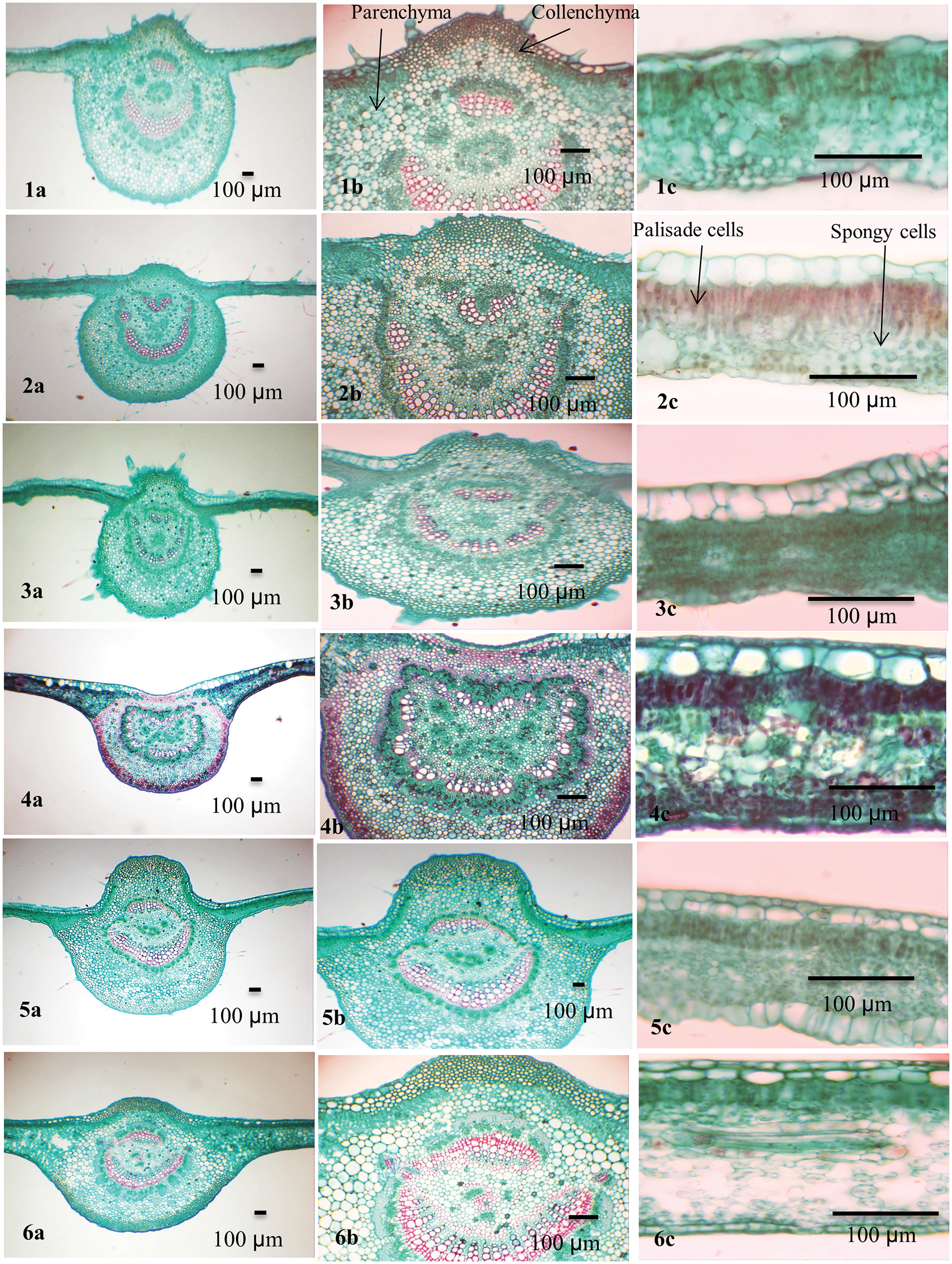
Figure 2: Blade anatomy of the studied taxa of Ficus. 1. F. carica, 2. F. palmata, 3. F. hirta, 4. F. neriifolia, 5. F. aspera, 6. F. tinctoria subsp. gibbosa
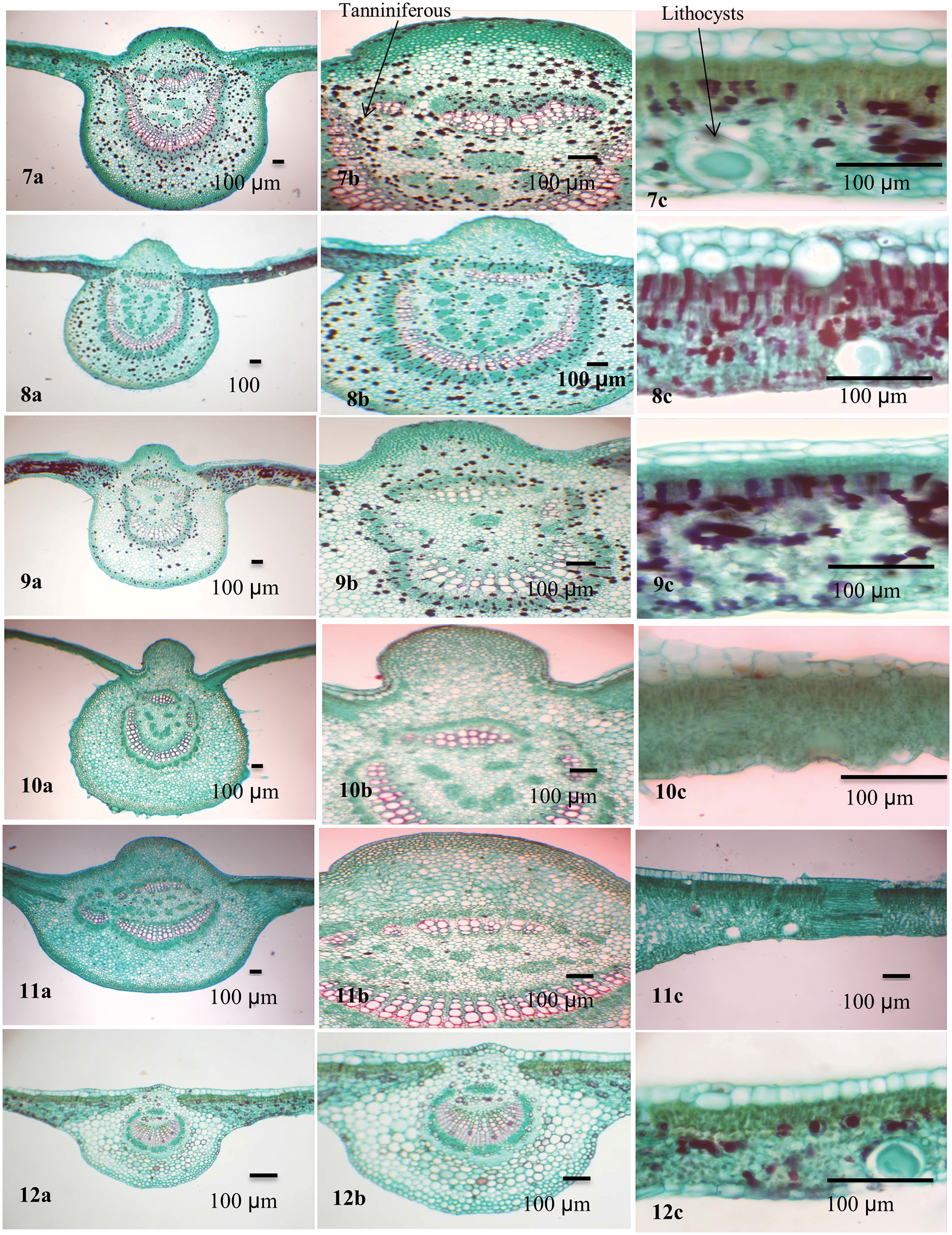
Figure 3: Blade anatomy of the studied taxa of Ficus. 7. F. racemosa, 8. F. sycomorus, 9. F. sur, 10. F. hispida, 11. F. septica, 12. F. pumila var. pumila
In the transverse section (TS), the Ficus leaf is dorsiventral and consists of a central midrib and two wings. The epidermis is covered by a thin cuticle in eleven taxa while it was found in F. neriifolia. Cuticular ridges are found on the abaxial surface of the leaf. The cuticle layer was folded in F. sycomorus and slightly folded in the other Ficus species under investigation.
The convex midrib is found in most studied taxa. It was flat-convex in F. tinctoria subsp. gibbosa, F. septica, and F. pumila var. pumila, and concave in F. neriifolia only. In F. tinctoria subsp. gibbosa, F. septica, and F. pumila var. pumila, the midrib is abaxially outlined and flat-arched.
Collenchymal tissue circulates the central vascular bundle. It is composed of 5–9 layers in F. hirta, F. neriifolia, F. septica, and F. pumila var. pumila; 10–15 layered in F. hispida; F. sycomorus; F. carica, F. tinctoria subsp. gibbosa; F. racemosa, F. aspera and F. palmata; and 10–15 layers in F. aspera, F. tinctoria subsp. gibbosa, and F. racemosa, F. carica, F. palmata, F. hirta, F. hispida, F. septica, and F. pumila var. pumila. Two layers of hypodermis were recorded in F. neriifolia, F. aspera, F. tinctoria subsp. gibbosa, F. racemosa and F. sycomorus, while it is composed of a single layer in F. hispida, F. sycomorus, F. carica and F. septica. Sclerenchyma tissue is found as marginal strands in F. tinctoria subsp. gibbosa, F. sycomorus, F. hispida, and F. septica.
The bundle sheath extends to both abaxial and adaxial epidermal layers (vertically transcurrent) in most of the taxa while closed, not extending to adaxial and abaxial epidermal layers (circular) as in F. neriifolia and F. tinctoria subsp. gibbosa. Annular xylem vessels are present in all studied taxa except F. aspera and F. pumila var. pumila. Lithocysts occur in both the abaxial and adaxial epidermis and are recorded in most studied taxa but are absent in F. hirta only, and they mostly appear on the abaxial epidermis of the lamina.
The mesophyll was dorsiventral with predominantly one to three layers of adaxial palisade cells. Spongy tissue consists of two layers in F. hirta; other taxa under investigation have multiple layers of spongy tissue. Parenchyma cells are found in the spongy layer. The parenchyma has various forms and might help in distinguishing among species: typical, palisade-like aerenchyma: F. hirta, F. neriifolia, F. aspera, F. hispida, and F. pumila var. pumila; typical aerenchymatous: F. carica, F. palmata, F. tinctoria subsp. gibbosa, F. racemosa, F. sur, and F. septica, whereas they were palisade-like in F. sycomorus only.
Two types of crystals are recorded in the leaves of the studied taxa: druses and prismatic crystals. Druse crystals are commonly present in the adaxial and abaxial parenchyma in F. carica and F. palmata. While present abaxially only in F. hirta and F. tinctoria subsp. gibbosa. Druse crystals are also recorded in mesophyll in F. carica, F. palmata, F. hirta, F. aspera, F. tinctoria subsp. gibbosa, F. hispida, and F. septica. Prismatic crystals occur in the abaxial parenchyma in F. carica only. Both druses and prismatic crystals are mainly found in the bundle sheath and midrib parenchyma in all studied taxa. Secretory element -tanniniferous cells are present adaxially and abaxially in F. racemosa and F. sycomorus, abaxially only in F. sur and absent in the rest. It is found in the mesophyll in F. racemosa, F. sycomorus, F. sur, and F. septica. Secretory element-nonbranched laticiferous tubes are present in F. carica, F. palmata, F. tinctoria subsp. gibbosa, F. racemosa, F. sycomorus, F. sur, and F. pumila var. pumila. Secretory elements-tanniniferous secretory elements-gum-resin and mucilage cells are present in F. carica, F. palmata, F. hirta, F. aspera, and F. tinctoria subsp. gibbosa. Starch grains are recorded in the mesophyll, bundle sheath and midrib parenchyma in F. neriifolia and F. pumila.
3.3 Computer Based Generating Key
In the present study, 12 taxa were used as the Operational Taxonomic Units (OTU). A total of 37 anatomical leaf aspects were examined and recorded. The construction of the key was carried out by using DELTA (Description Language for Taxonomy).

Table 4 shows 40 leaf anatomical traits of 12 Ficus species. These traits are used for numerical analysis. In Fig. 4, the anatomical data showed that the studied taxa belonged to two main clusters in the dendrogram produced using PCORD. Cluster 1 consisted of three Ficus taxa, which were further split into two subgroups; group No. 3 included two taxa, F. neriifolia and F. septica, while group No. 4 had one taxon, F. pumila var. pumila. Cluster 2 was divided into two groups. Group No. 5 was divided into two subgroups. Subgroup 7 included F. carica, F. palmata, F. hirta, F. hispida, and F. aspera, and was separated into a distinct subgroup. Subgroup 8 consisted of the studied taxa of F. racemosa, F. sycomorus, and F. sur, whereas the second group 6 contained F. tinctoria subsp. gibbosa.
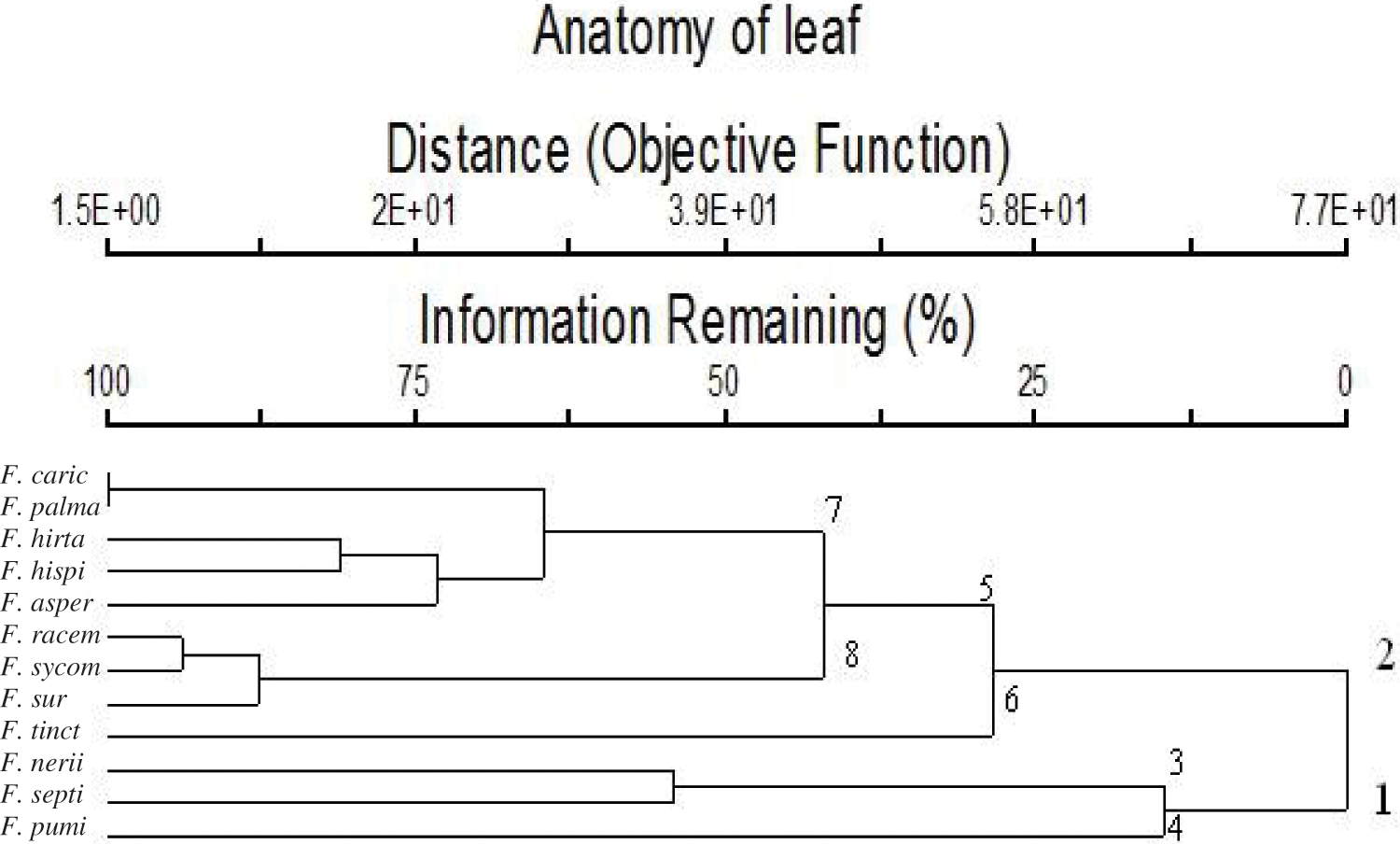
Figure 4: Dendrogram showing the interrelationships between 12 taxa of Ficus based on 40 leaf anatomical characters by using the PCORD program
Dendrogram cluster based on data from leaf anatomy was illustrated in Fig. 4, this revealed that studied taxa classified into two main groups. The first group included three taxa (F. pumi, F. septi, and F. neni). The second group included two sub-group; first sub-group contained F. tinct and the second sub-group contained other eight studied taxa. The second sub-group divided into two sub-subgroups; first sub-subgroup included (F. surr, F. sycom, and F. racem). the second sub-subgroup included the other five studied taxa.
On the other hand, the molecular characteristics data showed that the studied taxa belonged to two main clusters in the dendrogram produced using the Clustal W module on Mega7 to execute MSA. Cluster (A) consisted of two taxa, F. carica and F. palmata. Cluster (B) was divided into two groups. Group No. (C) was divided into two subgroups. Subgroup (E) includes F. aspera, F. tinctoria subsp. gibbosa and F. hirta. Subgroup (F) consisted of the studied taxa F. neriifolia and F. pumila var. pumila, whereas the second group (D) contains F. hispida, F. septica, F. racemosa, F. sur and F. sycomorus (Fig. 5).
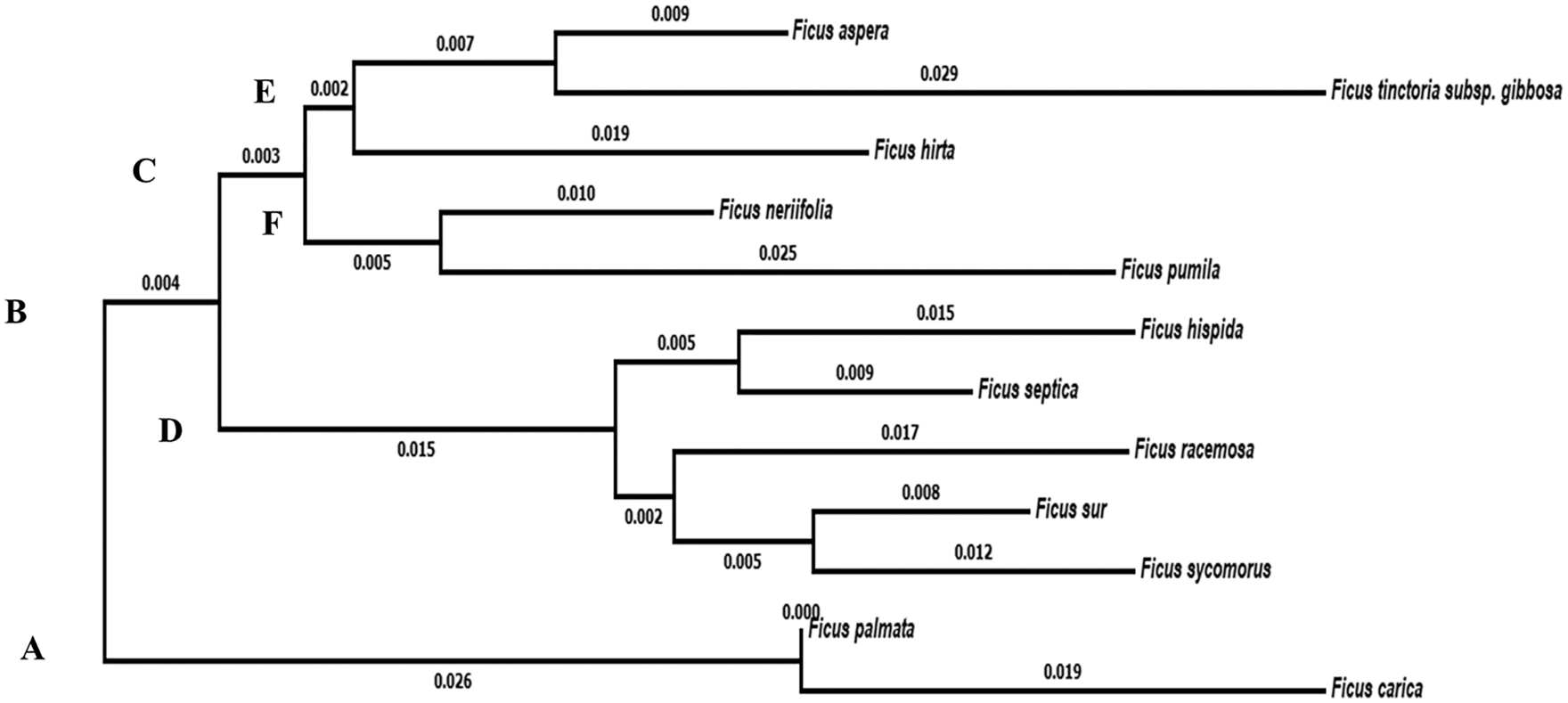
Figure 5: Dendrogram showing the interrelationships between the twelve taxa of Ficus based on ITS sequence
In many branches of biology, species delimitation is crucial. Despite its significance, there is no consensus on the criteria for defining species because different biological disciplines have different points of view. Traditional anatomical and molecular diagnostic characters are the two main categories frequently used to differentiate between species. Since it has been studied for more than a century, traditional morphological taxonomy is a very sophisticated method of identifying species. There still seem to be some differences within the method and disagreements about its efficacy among those who study it, despite a century of continuous improvement. The use of molecular markers as tools for determining the boundaries of species has significantly increased. In principle, all three plant genomes (nuclear, mitochondrial, and chloroplastic) can be used to delimit species. Nuclear and chloroplastic genomes are most frequently utilized in plants. The fundamental idea behind using molecular markers to identify species is that the “species tree” should be deduced from a “gene tree”. In other words, one or more DNA pieces can be used to infer the evolutionary history of a phylum.
The twelve Ficus taxa showed substantial leaf anatomical variations. These variations would help in the infraspecific classification of the taxa under investigation. The identification and classification of taxa are highly dependent on the anatomical traits of leaves, including cuticle, trichome, crystals, secretory elements, lithocysts, number of layers of palisade and spongy tissues. In the majority of taxa under investigation, the epidermis was covered by a thin or thick cuticle; the midrib has parenchyma associated with mechanical tissue.
Sonibare et al. examined the leaf anatomy of 25 different Ficus species from Nigeria and concluded that foliar anatomical traits might be helpful in the infraspecific classification of the genus Ficus [15]. According to Berg & Corner’s study on Ficus leaf anatomy, the microscopic features of leaves can be used to distinguish subgenera, series, and subseries, but at the species level, more data are needed [7]. However, Van Greuning and Grobbelaar observed that the anatomical characteristics of the leaves of Ficus are significant for taxonomy even at the species level [17]. Corner classified leaves using a combination of morphological and anatomical traits, such as the position of the lithocysts [37,38]. Chew also combined leaf anatomy, such as lithocysts and hypodermis, with morphology to identify Ficus subgenera, sections, and series [39]. Lithocyst and epidermal features were used by Berg and Corner and Berg et al. [7,40].
Calcium oxalate crystals have been used in the taxonomic classification of plants; the appearance and position of calcium oxalate or calcium carbonate crystals (such as cystolith, a crystal associated with the cell wall) can be distinctive and helpful [22,23,41]. In the lower epidermis of the leaf of F. deltoidea var. motleyana, a cystolith, was found in a cell known as a lithocyst [41].
According to Berg and Corner, in section of subgen. Ficus, cystoliths can only be found on the lower side of the leaf lamina [7]. This is contrary to research by Awang et al. [42]. Lithocysts were recorded in the current study on the abaxial side the leaves of F. palmata and F. carica. A bundle sheath outline is considered a valuable taxonomical trait [17]. Circular vascular bundles were recorded in F. hispida, F. pumila var. pumila and F. sycomorus.
The current research showed that the epidermal cells were simple and covered by a thin cuticle layer or thick cuticle layer, druses and sandy crystals, hypodermis and cystolith (calcium carbonate), and a secretory cell ridge with papillae, which distinguishes them from the hypodermis. The results were consistent with cystolith appearance in several mulberry family species (Moraceae), such as those recorded in F. elastica [43].
In the Moraceae family, the spongy parenchyma has taxonomic importance. Three different types of spongy parenchyma, including typical spongy parenchyma, palisade-like parenchyma, and aerenchymatous spongy parenchyma tissue, may be recognized in this study, which agrees with Van-Greuning et al. In their study of leaves from four mulberry cultivars’ leaves, they observed that the mesophyll had 8–10 layers and significant air spaces [44].
The trichomes were recorded in three types simple, glandular, and cystolith. All the trichomes on the adaxial and abaxial epidermises of leaves in both studied taxa, except for F. neriifolia and F. septica, are glabrous. Sonibare et al. revealed that trichomes are distributed differently in upper and lower leaves and that the taxonomic value of the trichomes is a critical-essential diagnostic for classifying Ficus species from others [15]. According to Mamoucha et al. [45] and Sosnovsky [20], F. carica only has glandular trichomes on the lower surface, but Giordano et al., discovered them on both the upper and lower surfaces [20,21,45], but in this study, the glandular trichomes in F. carica are present on the lower surface, agreeing with Mamoucha et al. [45] and Sosnovsky [20].
As one of the most promising DNA barcoding locations, ITS is frequently utilized to shed light on the taxonomical relationships of plants [27,46]. Even though the ITS was very useful for phylogenetic analyses, it was quite challenging to resolve Ficus connections. Unfortunately, the sequences were considerably divergent from those of other taxa, and the overlapping indels made the alignment process and phylogenetic construction difficult [47]. Fortunately, the alignment in our experiment was simple, and the ML phylogenetic tree produced exhibits collinearity with the morphological taxonomical approach.
We conclude that anatomical characteristics, notably the blade, are important for recognizing and differentiating the species under consideration here. The selected Ficus species’ differences and affinities have been fairly described by the anatomical characterization used in this study. Additionally, it is crucial to distinguish between taxa based on the presence or absence of crystals, starch grains, lithocysts, bundle sheaths in the lamina, and trichomes. Based on the features examined in this article, a key is offered for the identification of the investigated taxa. The outcome of the numerical analysis demonstrates that anatomical traits are crucial in discriminating between species that belong to the same genus. Additionally, the anatomical character dendrogram and the phylogenetic analysis of the ITS and 5.8 S sequences indicated general concordance. The traits assessed in this study were consistent with previous classifications of the genus Ficus.
Acknowledgement: The authors wish to thank Mohamed Helmy (Food and Biotechnology Innovation, Agency for Science, Technology, and Research, Singapore) and Mohamed Awad (Faculty of Agriculture, Al-Azhar University, Cairo, Egypt) for supporting this work. The authors also acknowledge Omar A. Hewedy (University of Guelph, Canada) for the English revision. We want to extend our thanks to Princess Nourah bint Abdulrahman University Researchers Supporting Project No. (PNURSP2023R318), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding Statement: Princess Nourah bint Abdulrahman University Researchers Supporting Project No. (PNURSP2023R318), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author Contributions: Conceptualization, A.B., R.H., O.R.; methodology, A.B., R.H., O.R., H.E., A.A.; software, A.B., R.H., O.R., H.E., A.A., A.T.; validation, A.T., A.El., F.S., S.A., A.E., A.El.B.; formal analysis, A.B., R.H., O.R., H.E., A.A., A.T., A.El.; investigation, A.B., R.H., O.R., H.E. A.A., A.T., A.El., F.S., S.A., A.E., A.El.B.; resources, A.B., R.H., O.R,. H.E., A.A., A.T., A.El., F.S., S.A.; data curation, A.B., R.H., O.R., H.E., A.A., A.T., A.E., A.El.B.; writing—original draft preparation, A.B., R.H., O.R., H.E., A.A., A.T., A.E., A.El.B.; writing—review and editing, A.B., R.H., O.R., H.E., A.A., A.T., A.El., F.S., S.A., A.E., A.El.B.; visualization, A.El., F.S., S.A., A.E., A.El.B.; supervision, A.T., A.El., F.S., S.A., A.E., A.El.B.; project administration, R.H., O.R., A.T., A.El., F.S., S.A., A.E., A.El.B.; funding acquisition, A.A., A.T., A.El., F.S., S.A. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Sirisha, N., Sreenivasulu, M., Sangeeta, K., Madhusudhana, C. (2010). Antioxidant properties of ficus species–A review. International Journal of PharmTech Research, 2(4), 2174–2182. [Google Scholar]
2. Shahinuzzaman, M., Yaakob, Z., Anuar, F. H., Akhtar, P., Kadir, N. H. A. et al. (2020). In vitro antioxidant activity of Ficus carica L. latex from 18 different cultivars. Scientific Reports, 10(1), 10852. https://doi.org/10.1038/s41598-020-67765-1 [Google Scholar] [PubMed] [CrossRef]
3. Gong, Y., Luo, W., Chen, H., Ren, B., Hu, W. et al. (2022). Systematical ingredient investigations of Ficus tikoua Bur. fruit and immunoregulatory and antioxidant effects of different fractions. Molecules, 27(20), 6880. https://doi.org/10.3390/molecules27206880 [Google Scholar] [PubMed] [CrossRef]
4. Wei, S. P., Luan, J. Y., Lu, L. N., Wu, W. J., Ji, Z. Q. (2011). A new benzofuran glucoside from Ficus tikoua Bur. International Journal of Molecular Sciences, 12(8), 4946–4952. https://doi.org/10.3390/ijms12084946 [Google Scholar] [PubMed] [CrossRef]
5. Wei, S., Wu, W., Ji, Z. (2012). New antifungal pyranoisoflavone from Ficus tikoua Bur. International Journal of Molecular Sciences, 13(6), 7375–7382. https://doi.org/10.3390/ijms13067375 [Google Scholar] [PubMed] [CrossRef]
6. Roskov, Y., Ower, G., Orrell, T., Nicolson, D., Bailly, N. et al. (2022). Species 2000 & ITIS catalogue of life, 25th March 2019. Leiden, The Netherlands: Species 2000. www.catalogueoflife.org/col. [Google Scholar]
7. Berg, C. C., Corner, E. J. H. (2005). Flora malesiana. Series I, Spermatophyta moraceae (Ficus). Noordhoff: Groningen. [Google Scholar]
8. Pederneiras, L. C., Carauta, J. P. P., Neto, S. R., Vidal de Mansano, F. (2015). An overview of the infrageneric nomenclature of Ficus (Moraceae). TAXON, 64(3), 589–594. https://doi.org/10.12705/643.12 [Google Scholar] [CrossRef]
9. Berg, C. C., Hijman, M. E. E. (1989). Flora of Tropical East Africa-Moraceae. In: Polhill, R. M. (Ed.Flora of tropical East Africa/prepared at the royal botanic gardens, kew with assistance from the East African Herbarium. Rotterdam, The Netherland. [Google Scholar]
10. Soliman, A. T., Hamdy, R. S., Mahdy, R. A. (2021). Numerical taxonomy of genus Ficus L. 1753 (Moraceaewith addition new record species to Egypt. Bulletin of the Iraq Natural History Museum, 16(4), 429–467. https://doi.org/10.26842/binhm.7.2021.16.4.0429 [Google Scholar] [CrossRef]
11. Renner, O. (1906). Beiträge zur anatomie und systematik der artocarpeen und conocephaleen insbesondere der gattung Ficus. Leipzig: Wilhelm Engelmann. [Google Scholar]
12. Metcalfe, C. R., Chalk, L. (1950). Moraceae. In: Anatomy of dicotyledons, vol. 2, pp. 1259–1271. Oxford: Clarendon Press. [Google Scholar]
13. Philpott, J. (1953). A blade tissue study of leaves of forty-seven species of Ficus. Botanical Gazette, 115(1), 15–35. https://doi.org/10.1086/335794 [Google Scholar] [CrossRef]
14. Mello Filho, L. E., Neves, L. J., Isaias, R. M. S. (1990). Anatomia foliar de Ficus Benghalensis L. Bradea, 5(30), 324–333. [Google Scholar]
15. Sonibare, M. A., Jayeola, A. A., Egunyomi, A. (2006). Comparative leaf anatomy of Ficus Linn. species (Moraceae) from Nigeria. Journal of Applied Sciences, 6(15), 3016–3025. https://doi.org/10.3923/jas.2006.3016.3025 [Google Scholar] [CrossRef]
16. Gangadhara, M., Inamdar, J. A. (1977). Trichomes and stomata, and their taxonomic significance in theurticales. Plant Systematics and Evolution, 127(12), 121–137. https://doi.org/10.1007/BF00984146 [Google Scholar] [CrossRef]
17. van Greuning, J. V., Robbertse, P. J., Grobbelaar, N. (1984). The taxonomic value of leaf anatomy in the genus Ficus. South African Journal of Botany, 3(5), 297–305. [Google Scholar]
18. Azizian, D. (2002). Morphology and distribution of trichomes in some genera (Morus, Ficus, Broussonetia and Maclura) of moraceae in Iran. The Iranian Journal of Botany, 9, 195–202. [Google Scholar]
19. Chantarasuwan, B., Baas, P., van Heuven, B. J., Baider, C., van Welzen, P. C. (2014). Leaf anatomy of Ficus subsection U Rostigma (MoraceaeLeaf anatomy of Ficus subsection Urostigma. Botanical Journal of the Linnean Society, 175(2), 259–281. https://doi.org/10.1111/boj.12165 [Google Scholar] [CrossRef]
20. Sosnovsky, Y. (2015). Microscopical investigation of the leaf architecture in greenhouse-cultivated Ficus (Moraceae). Plant Systematics and Evolution, 301(6), 1669–1692. https://doi.org/10.1007/s00606-014-1184-8 [Google Scholar] [CrossRef]
21. Giordano, C., Maleci, L., Agati, G., Petruccelli, R. (2020). Ficus carica L. leaf anatomy: Trichomes and solid inclusions. Annals of Applied Biology, 176(1), 47–54. https://doi.org/10.1111/aab.12557 [Google Scholar] [CrossRef]
22. Safhi, F. A., Alshamrani, S. M., Fiteha, Y. G. (2022). Abd El-Moneim, D.2022 DNA barcoding of endangered and rarely occurring plants in Faifa mountains (Jazan, Saudi Arabia). Agriculture, 12, 1931. https://doi.org/10.3390/agriculture12111931 [Google Scholar] [CrossRef]
23. EL-Banhawy, A., ElKordy, A., Farag, R., Abd Elbar, O., Faried, A. et al. (2021). Taxonomic Significance of the leaf geometric and micrometric attributes in the discrimination of some cultivars of Mangifera Indica L.(Anacardiaceae). Egyptian Journal of Botany, 61(1), 255–269. https://doi.org/10.21608/ejbo.2020.40870.1550 [Google Scholar] [CrossRef]
24. El-Banhawy, A., Uluer, D. A., Fayed, A., Mohamed, M., Faried, A. (2020). DNA barcoding and phylogenetic placement of the genus Euphorbia L. (Euphorbiaceae) in Egypt. Biology and Life Sciences Forum, 4, 58. https://doi.org/10.3390/IECPS2020-08620 [Google Scholar] [CrossRef]
25. Safhi, F. A., ALshamrani, S. M., Jalal, A. S., El-Moneim, D. A., Alyamani, A. A. et al. (2022). Genetic characterization of some Saudi Arabia’s accessions from commiphora gileadensis using physio-biochemical parameters, molecular markers, DNA barcoding analysis and relative gene expression. Genes, 13, 2099. https://doi.org/10.3390/genes13112099 [Google Scholar] [PubMed] [CrossRef]
26. Savolainen, V., Cowan, R. S., Vogler, A. P., Roderick, G. K., Lane, R. (2005). Towards writing the encyclopaedia of life: An introduction to DNA barcoding. Philosophical Transactions of the Royal Society B: Biological Sciences, 360(1462), 1805–1811. https://doi.org/10.1098/rstb.2005.1730 [Google Scholar] [PubMed] [CrossRef]
27. Kress, W. J., Wurdack, K. J., Zimmer, E. A., Weigt, L. A., Janzen, D. H. (2005). Use of DNA barcodes to identify flowering plants. PNAS, 102(23), 8369–8374. https://doi.org/10.1073/pnas.0503123102 [Google Scholar] [PubMed] [CrossRef]
28. Li, H. Q., Chen, J. Y., Wang, S., Xiong, S. Z. (2012). Evaluation of six candidate DNA barcoding loci in Ficus (Moraceae) of China. Molecular Ecology Resources, 12(5), 783–790. https://doi.org/10.1111/j.1755-0998.2012.03147.x [Google Scholar] [PubMed] [CrossRef]
29. Olivar, J. E. C., Brillantes, R. Y., Rubite, R. R., Alejandro, G. J. D. (2014). Evaluation of three candidate DNA barcoding loci in selected Ficus L.(Moraceae). International Journal of Scientific and Technology Research, 3(9), 43–48. [Google Scholar]
30. Castro, C., Hernandez, A., Alvarado, L., Flores, D. (2015). DNA barcodes in fig cultivars (Ficus carica L.) using ITS regions of ribosomal DNA, the PsbA-TrnH spacer and the MatK coding sequence. American Journal of Plant Sciences, 6(1), 95–102. https://doi.org/10.4236/ajps.2015.61011 [Google Scholar] [CrossRef]
31. Corner, E. J. H. (1977). Moraceae. In: Dassanayake, M. D. (Ed.A revised handbook to the flora of ceylon, vol. 1, pp. 111–165. New Delhi: Amerind Publishing Co. Pvt. Ltd. [Google Scholar]
32. Ghafoor, A. (1985). Moraceae. In: Nasir, E., Ali, S. I. (Eds.Flora of Pakistan, vol. 171, pp. 1–54. Shamim Printing Press. [Google Scholar]
33. Berg, C. C. (1991). Moraceae. In: Launert, E., Pope, G. V. (Eds.Flora Zambesiaca: Ulmaceae-Ceratophyllaceae (excluding Myricaceae), vol. 9, pp. 13–76. London, UK: Royal Botanic Gardens for Flora Zambesiaca Managing Committee. [Google Scholar]
34. Nassar, M. A., El-Sahhar, K. F. (1998). Botanical preparations and microscopy (Microtechnique). Dokki, Giza, Egypt: Academic Bookshop. [Google Scholar]
35. Dallwitz, M. J. (2016). Overview of the DELTA System. https://www.delta-intkey.com/www/overview.htm. [Google Scholar]
36. Kumar, S., Stecher, G., Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874. https://doi.org/10.1093/molbev/msw054 [Google Scholar] [PubMed] [CrossRef]
37. Corner, E. J. H. (1959). Taxonomic note on Ficus L., Asia and Australasia. I. subgen. urostigma (Gasp.) Miq. Gard Bull Singapore, 17, 368–415. [Google Scholar]
38. Corner, E. J. H. (1981). Moraceae. In: Dassanayake, M. D. (Ed.). A revised handbook to the flora of ceylon, vol. 3, pp. 213–292. New Delhi, India: Amerind Publishing Co. Pvt. Ltd. [Google Scholar]
39. Chew, W. L. (1989). Moraceae. In: George, A. S. (Ed.). Flora of Australia. Hamamelidales to Casuarinales, vol. 3, pp. 15–68. Canberra: Australian Government Publishing Services. [Google Scholar]
40. Berg, C. C., Pattharahirantricin, N., Chantarasuwan, B. (2011). Cecropiaceae & Moraceae. In: Santisuk, T., Larsen, K. (Eds.Flora of Thailand, vol. 10, pp. 475–675. Bangkok: Forest Herbarium. [Google Scholar]
41. Esau, K. (1977). Anatomy of seed plants, 2nd edition. New York: Wiley. [Google Scholar]
42. Awang, N. A., Hasan, S. M. Z., Shafie, M. S. (2011). Evaluation on morphological variability of mas cotek (Ficus Deltoidea Jack) collected in Malaysia. Proceedings of the Proceedings of Universiti Malaysia Terengganu 10th International Annual Symposium, pp. 11–13. Kuala Terengganu, Malaysia. [Google Scholar]
43. Cutler, D. F., Botha, C. E. J., Stevenson, D. W. (2008). Plant anatomy: An applied approach. Malden, USA: Blackwell Pub. [Google Scholar]
44. Kumar, V., Kodandaramaiah, J., Rajan, M. V. (2012). Leaf and anatomical traits in relation to physiological characteristics in mulberry (Morus Sp.) cultivars. Turkish Journal of Botany, 36(6), 683–689. https://doi.org/10.3906/bot-1003-48 [Google Scholar] [CrossRef]
45. Mamoucha, S., Fokialakis, N., Christodoulakis, N. (2016). Leaf structure and histochemistry of Ficus carica (Moraceaethe fig tree. Flora-Morphology, Distribution, Functional Ecology of Plants, 218(1), 24–34. https://doi.org/10.1016/j.flora.2015.11.003 [Google Scholar] [CrossRef]
46. Yao, H., Song, J., Liu, C., Luo, K., Han, J. et al. (2010). Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS One, 5, e13102. [Google Scholar] [PubMed]
47. Weiblen, G. D. (2000). Phylogenetic relationships of functionally dioecious FICUS (Moraceae) based on ribosomal DNA sequences and morphology. American Journal of Botany, 87(9), 1342–1357. https://doi.org/10.2307/2656726 [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools