 Open Access
Open Access
ARTICLE
Toxic and Antifeedant Effects of Different Pesticidal Plant Extracts against Beet Armyworm (Spodoptera exigua)
1
College of Life Science, Fujian Agriculture and Forestry University, Fuzhou, 350002, China
2
Department of Entomology, University of Agriculture, Faisalabad, 38000, Pakistan
3
Department of Plant Protection, College of Agricultural Engineering Science, University of Baghdad, Baghdad, 10071, Iraq
4
Department of Forestry and Range Management, University of Agriculture, Faisalabad, 38000, Pakistan
5
College of Plant Protection Fujian Agriculture and Forestry University, Fuzhou, 350002, China
6
College of Plant Protection, Gansu Agricultural University, Lanzhou, 730070, China
7
Department of Plant Sciences, Quaid-i-Azam University, Islamabad, 45320, Pakistan
8
Department of Biology, College of Science, Taif University, Taif, 21944, Saudi Arabia
9
Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, 11671, Saudi Arabia
* Corresponding Authors: Rashad Rasool Khan. Email: ; Khadiga Alharbi. Email:
(This article belongs to the Special Issue: Agricultural Intensification, Climate Change, and Food Security)
Phyton-International Journal of Experimental Botany 2023, 92(4), 1161-1172. https://doi.org/10.32604/phyton.2023.026513
Received 09 September 2022; Accepted 25 October 2022; Issue published 06 January 2023
Abstract
The beet armyworm (BAW), Spodoptera exigua (Lepidoptera: Noctuidae) is a highly destructive pest of vegetables and field crops. Management of beet armyworm primarily relies on synthetic pesticides, which is threatening the beneficial community and environment. Most importantly, the BAW developed resistance to synthetic pesticides with making it difficult to manage. Therefore, alternative and environment-friendly pest management tactics are urgently required. The use of pesticidal plant extracts provides an effective way for a sustainable pest management program. To evaluate the use of pesticidal plant extracts against BAW, we selected six plant species (Lantana camara, Aloe vera, Azadirachta indica, Cymbopogon citratus, Nicotiana tabacum , and Ocimum basilicum) for initial screening experiment. Four out of six plant species such as A. indica, N. tabacum, C. citratus and O. basilicum showed promising mortality of more than 50%. Therefore, we selected these four plant extracts for the subsequent experiments. Through contact bioassay, A. indica showed high mortality 66.63%, followed by the N. tabacum 53.33%, at 10% w/v concentration. Similarly, N. tabacum showed the highest mortality rate, 66% at 10% w/v concentration, followed by the A. indica 46% through feeding bioassay. Furthermore, the feeding deterrence assay showed that C. citratus had a high antifeedant index (−50) followed by A. indica (−39), and N. tabacum (−28). In living plant assay, the N. tabacum extract showed a low mean damage score 3.6 on living cotton plant followed by C. citratus 4.5 and A. indica 5.5. Hence, extracts of three plant species provided promising results against the BAW, which can minimize the use of synthetic chemicals, particularly for small landholding farmers. Further studies are also required to evaluate the effects of these plant extract against BAW on cotton plants under field conditions to optimize the further use.Keywords
Agricultural researchers and producers have always struggled with insect pest management. Controlling insect pest infestations will become more difficult as insecticide resistance grows and more toxic pesticides are phased out [1]. Farmers will be challenged to produce high-quality, pest-free crops on a budget without jeopardizing the environment or endangering worker safety [2,3]. In response to this struggle, more research is being done into alternative control methods that are both cost-effective and environmentally friendly.
The beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) (BAW) is a polyphagous insect pest that has been established all over the world, including the Indian sub-continent [4]. In Pakistan, BAW is one of the major pests of horticultural and field crops (cotton, maize), especially on vegetables (tomato, cotton, cauliflower, spinach) and cereal (chickpea, lentils) crops, respectively [5]. This insect is more likely to persist throughout the year due to the availability of a wide range of hosts [6]. Its larvae feed mainly on foliage and fruit [7]. Young larvae graze and skeletonize leaves. Larvae become solitary as they mature and chew irregular crevices in the foliage. BAW is a heavy foliage feeder, leading to a 100% loss of cereal crops [8].
Huge amounts of synthetic insecticides are being used to bring down the infestation of BAW in Pakistan [9]. The BAW has developed resistance to the widespread use of these synthetic insecticides, especially the organophosphates, carbamates and pyrethroids on which most Pakistani farmers rely [5,10,11]. As sub-continent farmers especially, Pakistan has a long history of using plants with pesticidal properties as an integrated approach to developing more eco-friendly and effective control of different crop pests [12]. Therefore, pesticidal plants would be an alternative and eco-friendly approach to managing the BAW.
Plants are a rich source of bioactive chemical compounds, making botanical insecticides an appealing alternative to synthetic chemical insecticides [13]. Aside from their insecticidal potential, they are reported to be less harmful to the environment and human health than synthetic pesticides [13]. Plant chemicals with pesticidal properties are target-specific, biodegradable to non-toxic products, and potentially suitable for use in an integrated pest management (IPM) program [14]. Many recent studies evaluated the use of botanical biopesticides in small-scale farming, which provides comparable yields to commercial synthetic pesticides by avoiding severe environmental damage. The leaf extract of Phyllanthus acidus showed 100% larvicidal effect Artemia salina after 24 h of spraying [15]. Similarly, the leaves extract of Lippia javanica and Nicotiana tabacum have shown promising results in controlling the fall S. frugiperda [16].
Keeping the above facts in mind, we conducted a study to evaluate some of the more encouraging pesticidal plant species to develop new, effective, and agro-ecologically sustainable methods for controlling BAW based on previously available knowledge of their abundance, phytochemistry, and safe use. The ultimate goal of the study presented here was to assess the potential effects of pesticidal plants on larval stages, including direct toxicity and feeding deterrence. Finally, the most promising plant extracts were applied to cotton plants to evaluate the foliar damage caused by BAW larvae under cropping conditions to see whether these plant extracts reduced the foliar damage.
2.1 Experimental Site, Collection and Rearing of Beet Armyworm
The current study was performed at the Eco-Toxicological Laboratory, Department of Entomology, University of Agriculture Faisalabad, Punjab, Pakistan. The fourth and fifth instar larvae of BAW were collected from different host plants in different districts of Punjab province in Pakistan. The BAW larvae were initially reared on young cotton leaves collected from the cotton field to develop a large population. However, the larvae were reared on a semi-synthetic diet after establishing a large population. The diet contained cotton leaf powder (300 g), methyl-4-hydroxybenzoate (3 g), flour (300 g), ascorbic acid (4.7 g), corn oil (12 mL), sorbic acid (1.5 g), vitamin mixture (10 mL), streptomycin (1.5 g), agar (17 g), yeast extract (48 g). The agar and yeast were mixed with 750 mL boiling water, and added all other components [5]. The diet was placed in plastic cups at room temperature for one hour to become a solid paste.
The newly born larvae were placed in a 450 mL plastic cup and fed on young cotton leaves until the 2nd instar. The cotton leaves were replaced with new ones daily. After the 2nd instar, the larvae were transferred into a prepared diet until the pupal stage. The pupae were collected and transferred to the adult mating cage. The adults were fed on a 10% honey solution. The honey-soaked cotton wool was placed in mating cages. Additionally, the filter paper was placed into the cage for egg collection. The filter paper, along with eggs, was collected every day and placed into the hatching box.
2.2 Collection of Plant Materials and Extract Preparation
We initially selected six plant species: L. camara, A.vera, A. indica, C. citratus, N. tabacum and O. basilicum. These plant species were selected based on previous studies about the phytochemical and efficacy. The leaves of all plant species were collected from the different known locations of Punjab province in Pakistan. These collected leaves were dried and ground into the powder. The powder form of these leaves was kept at cool places until further use. For extract preparation, we used 100 g of fine powder of each plant in 1 L water and kept it at room temperature for 24 h. Subsequently, the prepared extract was filtered and used for bioassay. The two control treatments (positive and negative) were used during this experiment. Positive control included the field-recommended dose of chlorpyrifos, and negative control had the water.
Furthermore, we selected four (A. indica, C. citratus, N. tabacum, and O. basilicum) out of six plant species for subsequent experiments. The extract was prepared by dissolving the 150 g powder into 1 L methanol and kept at room temperature for 24 h to improve the extract efficacy. To prepare the five concentrations (0.1%, 1%, 5%, 10% w/v), we weighed and resolubilized the dried residues of extract in acetone. The two control treatments (positive and negative) were used during this experiment. Positive control included the field-recommended dose of synthetic insecticide chlorpyrifos, and negative control included the acetone.
Contact bioassay is a topical application of plant extracts directly to the body of BAW larvae. We applied 15 μL of each plant extract directly to the bodies of 2nd instar BAW with the help of a 100 μL pipette. The treated larvae were then placed into the Petri plates containing the prepared semi-synthetic diet. Two control treatments were used during this bioassay method. The field-recommended dose of chlorpyrifos 40% EC was used as positive control, while the distilled water was used as a negative control. A completely randomized design with eight treatments and three replications was used. Each replication contained five 2nd instar larvae of BAW. Finally, the dead larvae were counted after seven days of treatment and calculated the mortality percentage.
Feeding bioassay is also known as a leaf-dip bioassay method. Cotton leaf disks with 5 cm diameter were prepared, dipped into different plant extracts for a few seconds and then allowed to dry on tissue paper for 1 h. Each treated leaf disk was placed separately in Petri dishes, and five 2nd instar larvae were released on treated leaves. Eight treatments with three replicates of five larvae were used for each concentration. The field-recommended dose of chlorpyrifos 40% EC was used as a positive control, while the distilled water was used as a negative control. Similarly, the dead larvae were counted after seven days of treatment and calculated the mortality percentage.
2.3.3 Feeding Deterrence Assay
A feeding deterrence assay was performed to evaluate the antifeedant activity of four plant extracts against the BAW larvae by leaf disc no-choice method [17]. Fresh cotton leaves were used to prepare the leaf discs of 3-cm diameter. The prepared leaf discs were dipped into 10% w/v solution of four plant extracts. The leaf disc treated with water was used as a control treatment. The treated leaves were placed into the Petri dishes. The leaf discs were weighed using the analytical balance before the treatment application. Five 2nd instar larvae of BAW were released in each Petri plate. Three replications of each treatment were used. The leaf consumption by BAW larvae after in control and treated leaf discs was evaluated. The remaining uneaten leaf area in control and treated discs were weighed after 72 h. The percentage of the antifeedant index was calculated using the following formula [18]:
where C represents the leaf weight in control leaf discs and T represents the leaf weight in treated leaf discs.
A living plant assay was used to evaluate the foliar damage of BAW on living cotton plants. The non-BT cotton variety FH-1000 was planted in pots. Five cotton seeds were planted in each pot and later were thinned into two after successful germination. The recommended dose of fertilizers (120:60:60, N:P:K) was applied through basal and top dressing. Standard agronomic practices, including hand weeding and watering, were used in all cotton plants. Cotton plants were infested with five larvae after 30 days of plant emergence. To avoid harsh conditions, larvae were placed on the cotton plant early in the morning. After the artificial infestation, cotton plants were covered with a cage to avoid the free movement of larvae. This experiment was carried out with a randomized complete block design with six treatments, including positive control (chlorpyrifos) and negative control (water). Six replications for each treatment were used in this experiment. The plant extract with 10% w/v concentration was applied uniformly to all plants through a handheld plastic sprayer. The first treatment applications were made after 48 h of larval infestation, and subsequent application was used after seven days. The damage data was collected after seven days of treatment applications upto 9 weeks. The foliar damage of each plant was recorded through the Williams’ 0–9 scale for the whole plant damage method [19]. According to this scale: 0 = no visible damage, 1 = Pin hole damage, 2 = both shot hole and pinhole lesions, 3 = small 1cm holes on few leaves, 4 = many leaves with 1–3 cm elongated holes, 5 = Large, elongated lesions or tiny pieces chewed on several leaves, 6 = Large, elongated lesions and large pieces chewed on several leaves, 7 = On around half of the leaves, there are numerous holes of all sizes and forms. 8 = the 70% leaf eaten, 9 = almost completely destroyed the leaves [20].
A randomized complete block design was used during the experiments. Two-way ANOVA was used to analyze the effects of treatments and interactions with bioassay methods and concentrations. Treatments and interactions mean were compared through Tukey’s (HSD) test at the 95% confidence interval. All statistical analyses were performed using the GRAPHPAD 8.02 (GraphPad Software, La Jolla, San Diego, CA, USA). The graphs were also prepared with GRAPHPAD 8.02.
3.1 Initial Toxicity Screening of Plant Extracts
Six plant extracts were used for contact and feeding bioassays during initial screening experiments. Water extracts (10% w/v) were prepared and used against BAW, showing different mortality ranges (Fig. 1). L. camara exhibited the lowest mortality percentage (6.33%) in contact bioassay, geared up to 20% in feeding bioassay. Similarly, A. vera plant extract showed low mortality in contact bioassay (13.34%) compared to feeding bioassay (26.67%).
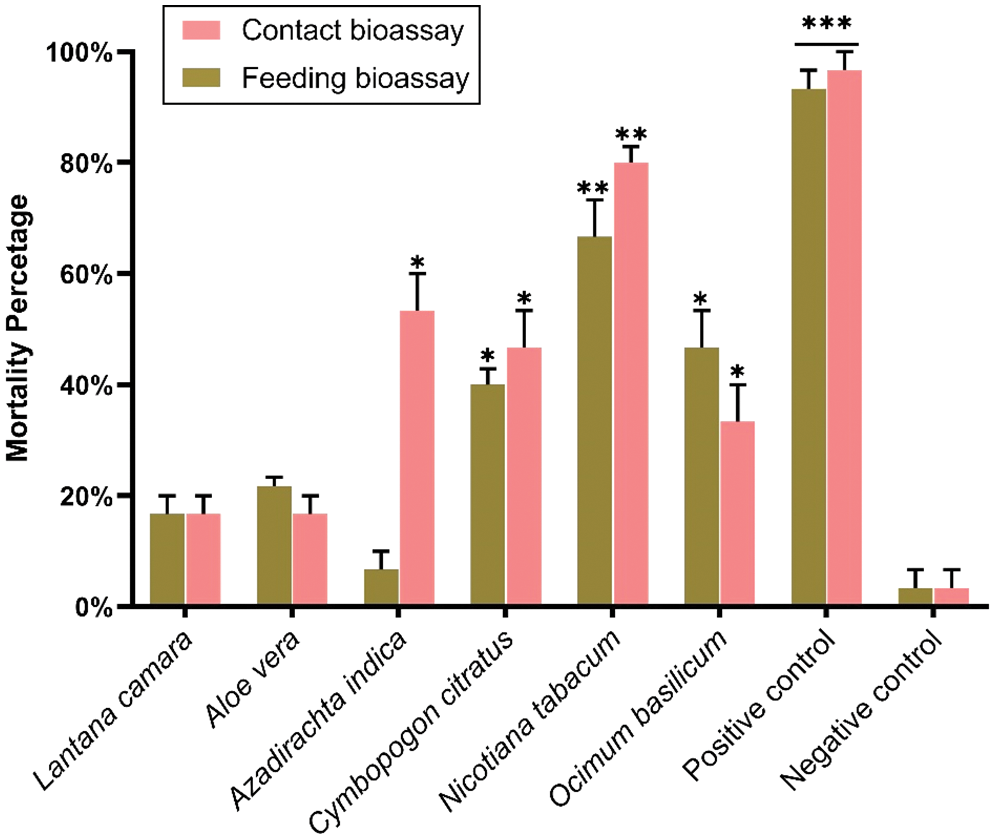
Figure 1: Mortality percentage of BAW larvae after application of different plant extracts through contact and feeding bioassays. A significant difference between the treatments with untreated control is highlighted with *. *** indicates the highly significant (p < 0.001), ** indicates the significant difference at p < 0.005, * indicates the significant difference at p < 0.05, and ns represents the non-significant difference between treatments and control
The most effective plant extracts against BAW larvae were N. tabacum, with a high mortality rate of 60%–73.34% in both (contact and feeding) bioassays, followed by A. indica 6.53%–53.33% (feeding and contact bioassay), C. citratus 40%–46%, and O. basilicum 26.66%–40%. The highest mortality percentage was observed in N. tabacum, 73.34%. Overall, these results showed that four plant species are most effective against the BAW larvae and demonstrated the effectiveness of different bioassay methods, which is confirmed by statistical analysis. Based on the initial screening results, we selected four (N. tabacum, A. indica, C. citratus and O. basilicum) out of six plant species for subsequent experiments.
3.2 Toxicity of Four Selected Plant Extracts against Baw through Two Different Bioassay Methods
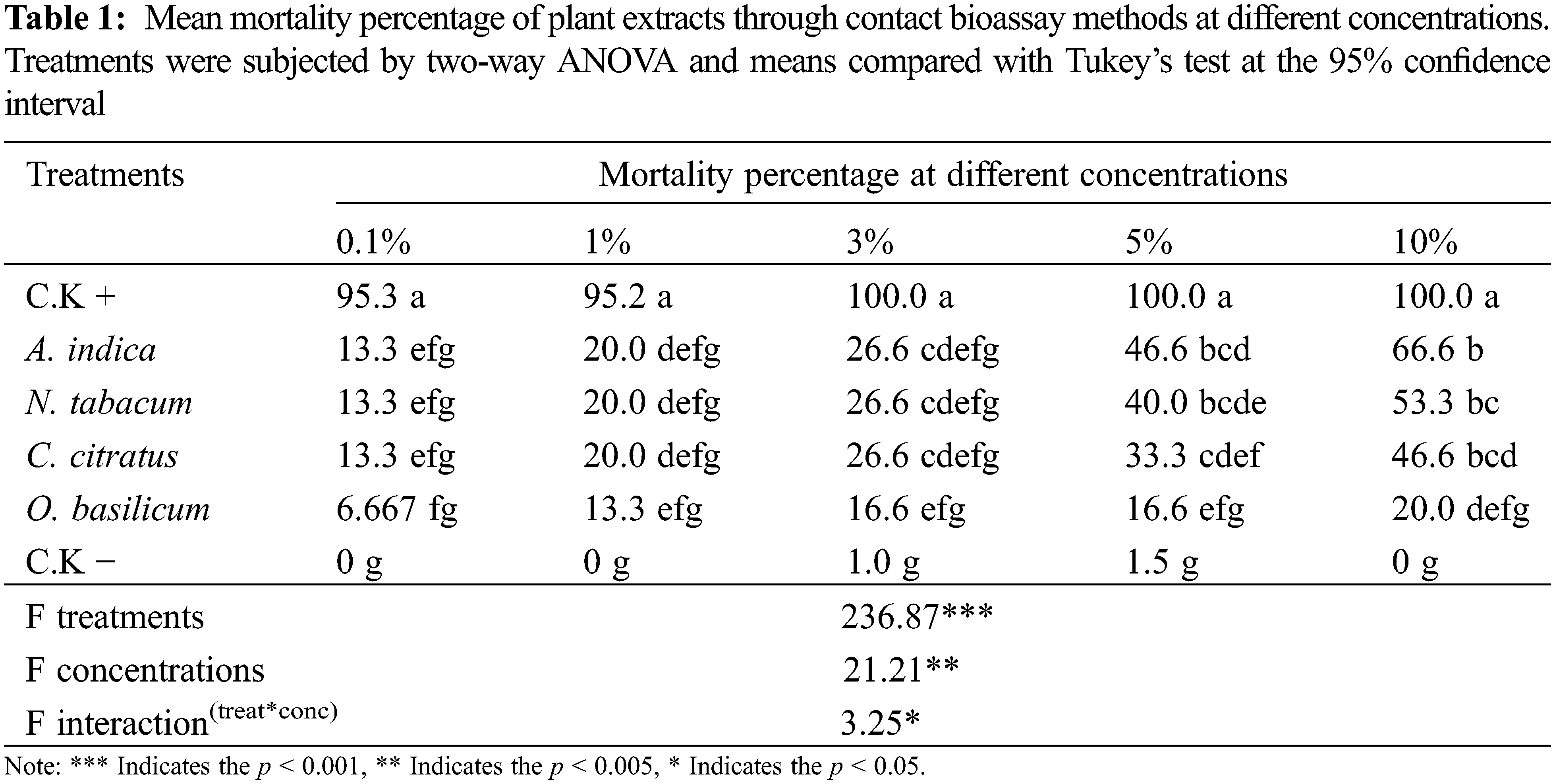
Different concentrations of four selected plant extracts were topically applied to BAW larvae, with considerable mortality percentages, except the O. basilicum (Table 1). High mortality percentage of BAW larvae was observed at 10% w/v concentrations rather than the other concentrations. At 10% (w/v) concentration, A. indica showed high mortality (66.63%), followed by the N. tabacum (53.33%), C. citratus (46,67%), and displayed a highly significant difference from untreated control (p < 0.05). O. basilicum showed a low mortality percentage at all concentrations, which was not significantly different from the negative control. The positive control treatment (chlorpyrifos) showed nearly 100% mortality which is superior to all plant extracts.
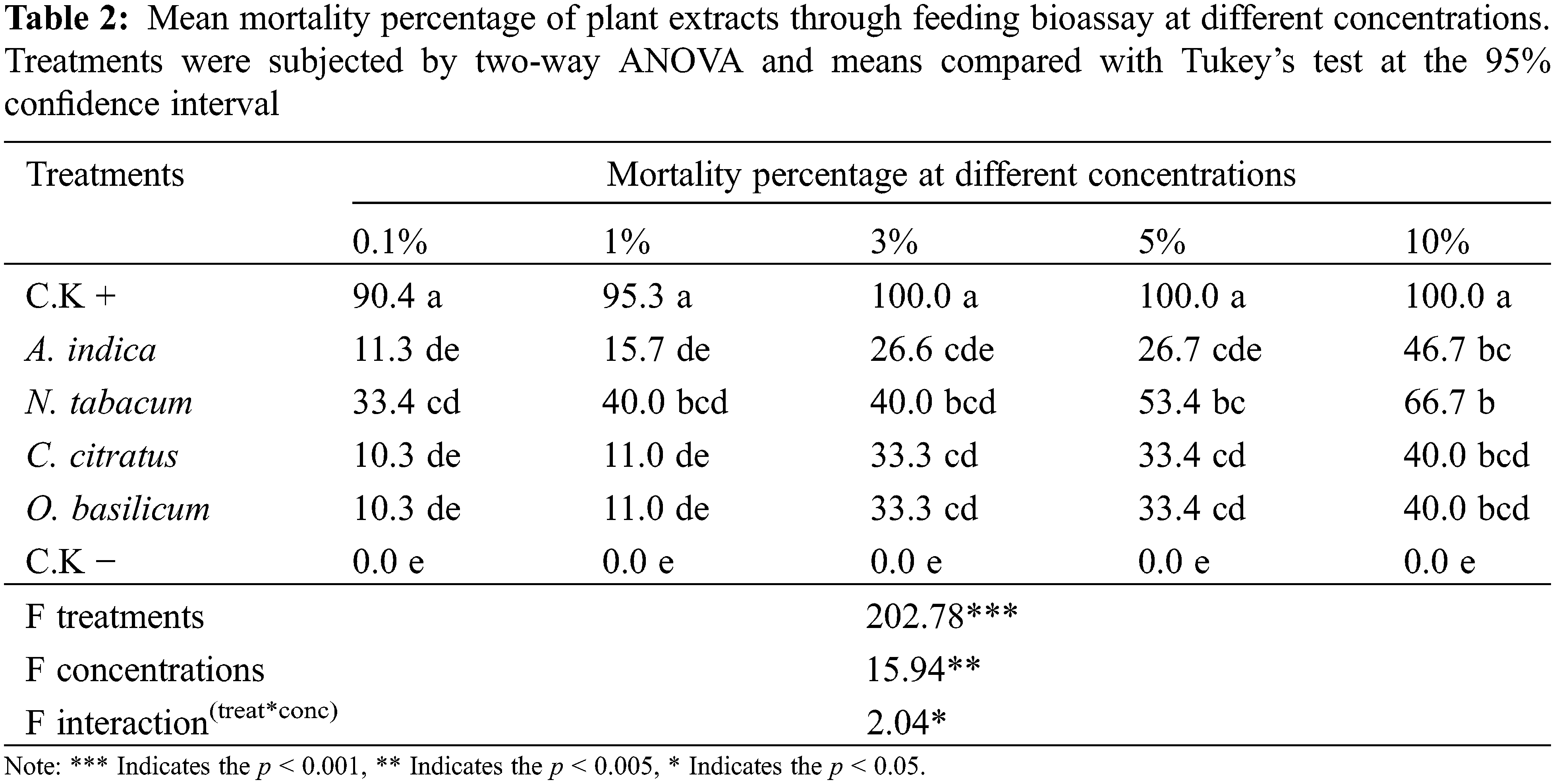
For feeding bioassay, the cotton leaves were treated with different plant extracts and offered to BAW larvae which showed a significant mortality rate compared to untreated control (Table 2). N. tabacum showed the highest mortality (66%) at 10% w/v concentration, followed by the A. indica (46%) and C. citratus (40%), with a high significant difference from untreated control. The lowest mortality percentage (35%) was observed in O. basilicum. Although the mortality percentage in O. basilicum treated larvae showed a significant difference from untreated but was comparatively lower than other treatments. Some plant extracts showed better toxicity in one bioassay method, such as A. indica (66.67%) through contact bioassay.
3.3 Antifeedant Activity of Selected Plant Extracts
Four plant extracts were used to determine the antifeedant activity against the BAW larvae. The results of this trial indicated that all four plant extracts exhibited some degree of feeding deterrence when sprayed with 10% w/v concentrations (Fig. 2). C. citratus showed a high antifeedant index (−50) followed by A. indica (−39), N. tabacum (−28). The lowest antifeedant index was observed in O. basilicum (−9). Overall results showed that C. citratus and A. indica plant extracts had the best antifeedant effects against the BAW larvae compared to other plant extracts.
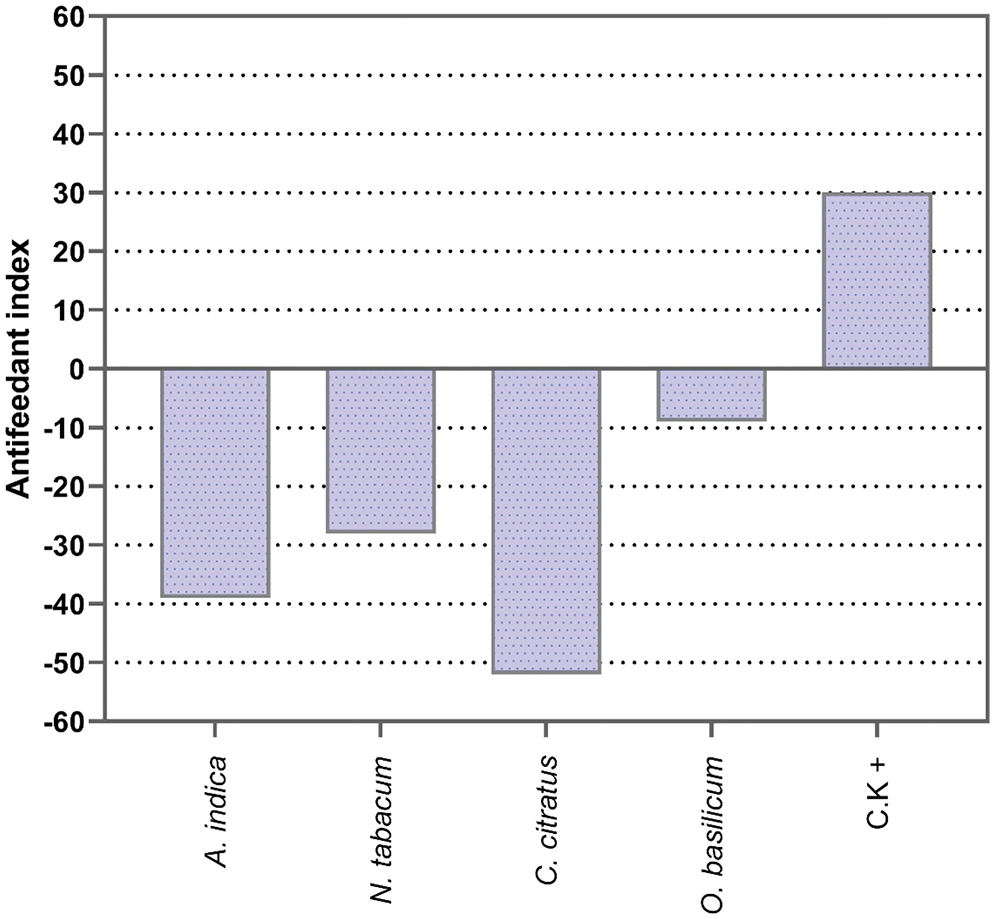
Figure 2: Antifeedant effects of four different pesticidal plant extract against the BAW larvae after 72 h of exposure. Bars represent the percentage of feeding deterrence
3.4 Damage Count of BAW Larvae on Living Cotton Plant
The four most active pesticidal plant extracts were applied to living cotton plants and BAW larvae were allowed to feed on treated plants. The ability of damage caused by the BAW larvae on cotton plants was observed, which showed a significant difference at p < 0.05 among the treatments compared to control treatments. The lowest mean damage score was recorded in the positive control (chlorpyrifos) treated plants, and the highest mean damage score was recorded in negative control (water) treated plants. Among the pesticidal plant extracts, N. tabacum showed a low mean damage score of 3.6, followed by C. citratus 4.5 and A. indica 5.7 (Fig. 3). A high mean damage score was observed in O. basilicum (6.5) and negative control (7.5). The lowest foliar damage score of 1.3 was observed in chlorpyrifos-treated plants.
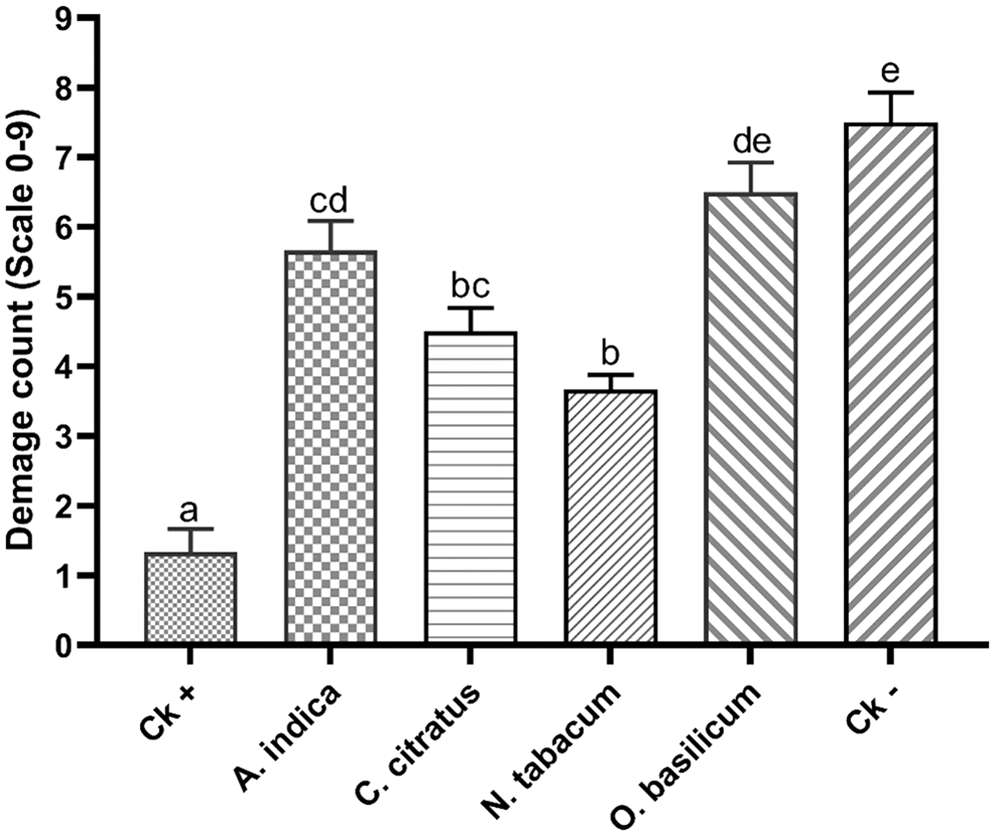
Figure 3: Beet armyworm damage score on cotton after the application of different treatments. The bars indicate the mean ± SEM of different treatments. Different letters highlight the significant differences among the treatments at p < 0.05, and the treatments share similar letters are not significantly different from each
The extensive use of synthetic pesticides to manage pest infestation in developing counties has some negative impact on human health and the ecosystem [21]. The use of pesticidal plant extracts is an alternative and biocontrol option for sustainable pest management programmes for developing countries, and our results showed that the pesticidal plant extracts can effectively control and reduce the damage caused by BAW larvae which would be an eco-friendly and sustainable pest management approach for smallholder farmers [22].
The L. camara and A. vera showed comparatively lower mortality percentages during the initial toxicity screening trial than other treatments. These results are surprising because previous studies showed that L. camara had high mortality percentage against a wide range of insect species [23–26]. In the current experiment, we used the same conditions and extraction methods for L. camara as previously described [23,26]. However, the mortality percentage in our case is lower than the previous studies. Similarly, A. vera plant extract also showed low mortality in contact bioassay compared to feeding bioassay. These results differ slightly from previous studies in which the A. vera plant extract showed considerable mortality at 10% w/v concentration against housefly and maize storage pest (Sitotroga cerealella and Sitophilus oryzae) [27–29]. A. vera plant extract was also used to manage the aphid population under field conditions [30].
The most effective plant extracts against BAW larvae were N. tabacum, with a high mortality rate in both contact and feeding bioassays, followed by A. indica, C. citratus, and O. basilicum. In previous studies, N. tabacum, A. indica, C. citratus and O. basilicum showed at least 50% larval mortality with one application method, but in our case, it showed different mortality percentages with respect to different application methods [31–34]. Overall, these results showed that four plant species are most effective against the BAW larvae and demonstrated the effectiveness of different bioassay methods.
Further, we tested different concentrations of four selected pesticidal plant extract against BAW with two bioassay methods. High mortality percentage was observed in N. tabacum at (10% w/v) through both bioassay methods. Overall, N. tabacum and A. indica extracts showed a more significant mortality percentage of BAW larvae. These results are similar to previous studies in which the N. tabacum and A. indica showed high mortality against different insect species, including lepidopteran insects and also provided the eco-friendly control of harmful pest species [35–39]. A. indica showed high mortality percentage at (10% w/v) concentration through the topical bioassay method. These results are consistent with previous studies in which the A. indica showed better mortality through topical application [40–42]. Furthermore, some differences were observed in mortality rates in plant extracts prepared with water and methanol. The mortality rate between extracts prepared in methanol and water was almost similar except the O. basilicum, which exhibited a lower mortality rate in methanol extract than water extract. The reason behind the low mortality rate is unclear; however, the difference in mortality rate might be due to different extraction efficiency in different solvents (water and methanol). The variations in mortality rate and lack of an obvious dosage impact between methanol and water trials might be caused by differences in feeding rates of BAW by feeding deterrent behaviour [16].
The antifeedant activity of four selected plant extracts was evaluated in which C. citratus and A. indica plant extracts had the best antifeedant effects against the BAW larvae compared to other plant extracts. Although, these two plant extracts caused relatively low mortality rates against BAW. But, these two plants may have some feeding deterrent compounds which help to minimize the damage to cotton plants. In accordance with our results, the previous studies also showed antifeedant effects of different plant extracts and essential oils against different insect species. Six plant extracts, including A. indica and N. tabacum were tested against Demotispa neivai, which exhibited strong antecedent effects [43]. The extracts from C. citratus showed high antifeedant effects against Dinoderus porcellus [44], Crocidolomia binotalis [45], S. litura [46], Tribolium castaneum [47,48], and S. oryzae [48]. The antifeedant effects of A. indica against various insects, especially for lepidopteran pests, are also well demonstrated in previous studies [49–54].
Furthermore, the mean damage score was calculated on living cotton plants treated with four most active plant extracts. The lowest mean damage score was observed in N. tabacum and C. citratus-treated cotton plants. The reduction in foliar damage of cotton crops might be attained due to the antifeedant, toxicity, and repellent effects of these four pesticidal plants, consistent with previous studies. The foliar damage reduction caused by the antifeedant, toxicity, and repellent effects of specific synthetic and biopesticides against different crop pests has been demonstrated in previous studies [18,33,45,46,55,56]. Synthetic pesticides reduce the plant damage caused by BAW more than pesticidal plant extracts, as shown in chlorpyrifos-treated plants. However, some additional studies showed that almost equal damage and the mortality rate was observed when pesticidal plants extract compared with synthetic chemicals [22,57–59]. Further, additional studies must be performed to know if these pesticidal plant extracts can maintain a similar yield to synthetic pesticides under field conditions.
Our study suggests that the pesticidal plant species are providing eco-friendly sustainable pest management of BAW. Out of all tested species, three pesticidal plant extracts (N. tabacum, A. indica and C. citratus) showed a significant mortality rate and antifeedant effects against BAW larvae. Furthermore, these plant extracts also showed low foliar damage caused by BAW in living cotton plants. However, the O. basilicum extract failed to show the expected results, although it somehow showed mortality rate and antifeedant activity.
Author’s Contributions: Study, conception and design: M.A. and R.R.K.; methodology, M.A. and M.H.U.R.; software, U.K., I.U.H. and A.B.; validation, M.A. and R.R.K.; formal analysis, M.A. and A.H.; writing original draft preparation, M.A. and M.H.U.R.; writing review and editing, R.R.K., A.N. and K.A.; funding acquisition, R.R.K., A.N. and K.A. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement:: This research was funded by Princess Nourah bint Abdulrahman University, Researchers Supporting Project No. (PNURSP2023R188), Riyadh, Saudi Arabia.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Aktar, M. W., Sengupta, D., Chowdhury, A. (2009). Impact of pesticides use in agriculture: Their benefits and hazards. Interdisciplinary Toxicology, 2(1), 1–12.
2. Sastry, P. (2017). A review on adverse effects of herbicides and pesticides on environment. International Journal of Environmental Chemistry, 3(2), 26–30.
3. Asad, M., Ahmed, S., Khan, R. R., Ali, A., Raza, M. F. et al. (2021). Effects of soil application of two different fipronil formulations on some soil-dwelling non-target arthropods. International Journal of Tropical Insect Science, 41(3), 663–669.
4. Li, H., Jiang, W., Zhang, Z., Xing, Y., Li, F. (2013). Transcriptome analysis and screening for potential target genes for RNAi-mediated pest control of the beet armyworm, Spodoptera exigua. PLoS One, 8(6), e65931.
5. Ahmad, M., Farid, A., Saeed, M. (2018). Resistance to new insecticides and their synergism in Spodoptera exigua (Lepidoptera: Noctuidae) from Pakistan. Crop Protection, 107(5), 79–86.
6. Anwar, T. H., Qureshi, N., Parveen, S., Mahmood, M. Z., Haider, S. et al. (2023). Herbicidal effectiveness of wild poisonous plant Rhazya stricta using different media by the sandwich method. Pakistan Journal of Botany, 55(2) DOI 10.30848/PJB2023-2(10).
7. Metayi, M. H., Abd El-Naby, S. S., El-Habal, N. A., Fahmy, H. H., Abdou, M. S. et al. (2022). Omani Frankincense nanoemulsion formulation efficacy and its latent effects on biological aspects of the spiny bollworm Earias insulana (Boisd.). Frontiers in Physiology, 13, 1001136.
8. Al-Zaban, M. I., Alhag, S. K., Dablool, A. S., Ahmed, A. E., Alghamdi, S. et al. (2022). Manufactured nano-objects confer viral protection against cucurbit chlorotic yellows virus (CCYV) infecting Nicotiana benthamiana. Microorganisms, 10(9), 1837.
9. Hafeez, M., Qasim, M., Ali, S., Yousaf, H. K., Waqas, M. et al. (2020). Expression and functional analysis of P450 gene induced tolerance/resistance to lambda-cyhalothrin in quercetin fed larvae of beet armyworm Spodoptera exigua (Hübner). Saudi Journal of Biological Sciences, 27(1), 77–87.
10. Ahmad, M., Arif, M. I. (2010). Resistance of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) to endosulfan, organophosphorus and pyrethroid insecticides in Pakistan. Crop Protection, 29(12), 1428–1433.
11. Ahmad, M., Gull, S. (2017). Susceptibility of armyworm Spodoptera litura (Lepidoptera: Noctuidae) to novel insecticides in Pakistan. The Canadian Entomologist, 149(5), 649–661.
12. Damalas, C. A. (2011). Potential uses of turmeric (‘Curcuma longa’) products as alternative means of pest management in crop production. Plant Omics, 4(3), 136–141.
13. Campos, E. V., Proença, P. L., Oliveira, J. L., Bakshi, M., Abhilash, P. et al. (2019). Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecological Indicators, 105(10), 483–495.
14. Guleria, S., Tiku, A. K. (2009). Botanicals in pest management: Current status and future perspectives. In: Peshin, R., Dhawan, A. K. (Eds.Integrated pest management: Innovation-development process, pp. 317–329. Dordrecht: Springer.
15. Prakash, N. U., Bhuvaneswari, S., Divyasri, D., Kurien, N. A., Uma, P. et al. (2013). Studies on the phytochemistry and bioactivity of leaves of few common trees in Chennai, Tamilnadu. India International Journal of Pharmacy and Pharmaceutical Sciences, 5(3), 88–91.
16. Phambala, K., Tembo, Y., Kabambe, V. H., Stevenson, P. C., Belmain, S. R. (2020). Bioactivity of common pesticidal plants on fall armyworm larvae (Spodoptera frugiperda). Plants, 9(1), 112.
17. Isman, M. B., Koul, O., Luczynski, A., Kaminski, J. (1990). Insecticidal and antifeedant bioactivities of neem oils and their relationship to azadirachtin content. Journal of Agricultural and Food Chemistry, 38(6), 1406–1411.
18. Jeyasankar, A. (2012). Antifeedant, insecticidal and growth inhibitory activities of selected plant oils on black cutworm, Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae). Asian Asian Pacific Journal of Tropical Disease, 2(1), S347–S351.
19. Williams, W. P., Buckley, P. M., Davis, F. M. (1989). Combining ability for resistance in corn to fall armyworm and Southwestern corn borer. Crop Science, 29(4), 913–915.
20. Toepfer, S., Fallet, P., Kajuga, J., Bazagwira, D., Mukundwa, I. P. et al. (2021). Streamlining leaf damage rating scales for the fall armyworm on maize. Journal of Pest Science, 94(4), 1075–1089.
21. Sola, P., Mvumi, B., Ogendo, J., Mponda, O., Kamanula, J. et al. (2014). Botanical pesticide production, trade and regulatory mechanisms in Sub-Saharan Africa: Making a case for plant-based pesticidal products. Food Security, 6(3), 369–384.
22. Tembo, Y., Mkindi, A. G., Mkenda, P. A., Mpumi, N., Mwanauta, R. et al. (2018). Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Frontiers in Plant Science, 9, 1425.
23. Rajashekar, Y., Ravindra, K., Bakthavatsalam, N. (2014). Leaves of Lantana camara Linn. (Verbenaceae) as a potential insecticide for the management of three species of stored grain insect pests. Journal of Food Science, 51(11), 3494–3499.
24. Dua, V., Pandey, A., Dash, A. (2010). Adulticidal activity of essential oil of Lantana camara leaves against mosquitoes. Indian Journal of Medical Research, 131(3), 434.
25. Ogendo, J., Belmain, S., Deng, A., Walker, D. (2003). Comparison of toxic and repellent effects of Lantana camara L. with Tephrosia vogelii hook and a synthetic pesticide against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) in stored maize grain. International Journal of Tropical Insect Science, 23(2), 127–135.
26. Mkindi, A., Mpumi, N., Tembo, Y., Stevenson, P. C., Ndakidemi, P. A. et al. (2017). Invasive weeds with pesticidal properties as potential new crops. Industrial Crops and Products, 110(12), 113–122.
27. Mahmood, S. I. J. (2018). Evaluation of Aloe vera extract efficiency to control Sitotroga cerealella (Lepidoptera: Gelechiidae). Plant Archives, 18(2), 1366–1368.
28. Mallavadhani, U., Prasad, B. R., Soujanya, P. L., Rao, M. B., Ratanlal, M. (2016). Aloe vera (L.) Burm. f.: A highly useful Indian traditional plant for the management of maize storage pest, Sitophilus oryzae L. (Coleoptera: Curculionidae). Journal of Biopesticides, 9(2), 157–166.
29. Jesikha, M. (2012). Evaluation of larvicidal efficacy of Aloe Vera extract against Musca domestica. Journal of Environmental Science and Toxicology, 2(2), 1–3.
30. Sarwar, M. (2013). The inhibitory properties of organic pest control agents against aphid (Aphididae: Homoptera) on canola Brassica napus L. (Brassicaceae) under field environment. International Journal of Scientific Research in Environmental Sciences, 1(8), 195–201.
31. Sakadzo, N., Makaza, K., Chikata, L. (2020). Biopesticidal properties of aqueous crude extracts of tobacco (Nicotiana Tabacum L.) against fall armyworm (Spodoptera Frugiperda JE Smith) on maize foliage (Zea Mays L.) diets. Agricultural Science, 2(1), 47.
32. Trdan, S., Cirar, A., Bergant, K., Andjus, L., Kač, M. et al. (2007). Effect of temperature on efficacy of three natural substances to Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Acta Agriculturae Scandinavica, 57(4), 293–296.
33. Sisay, B., Tefera, T., Wakgari, M., Ayalew, G., Mendesil, E. (2019). The efficacy of selected synthetic insecticides and botanicals against fall armyworm, Spodoptera frugiperda, in maize. Insects, 10(2), 45.
34. Siazemo, M. K. (2020). An evaluation of the efficacy of botanical pesticides for fall armyworm control in maize production. Open Access Library Journal, 7(9), 103177.
35. Noureldeen, A., Kumar, U., Asad, M., Darwish, H., Alharthi, S. et al. (2022). Aphicidal activity of five plant extracts applied singly or in combination with entomopathogenic bacteria, Xenorhabdus budapestensis against rose aphid, Macrosiphum rosae (Hemiptera: Aphididae). Journal of King Saud University-Science, 34(8), 102306.
36. Sagheer, M., Ali, K., Rashid, A., Sagheer, U., Alvi, A. (2013). Repellent and toxicological impact of acetone extracts of Nicotiana tabacum, Pegnum hermala, Saussurea costus and Salsola baryosma against red flour beetle, Tribolium castaneum (Herbst). Pakistan Journal of Zoology, 45(6), 1735–1739.
37. Pavela, R., Kazda, J., Herda, G. (2009). Effectiveness of Neem (Azadirachta indica) insecticides against Brassica pod midge (Dasineura brassicae Winn.). Journal of Pest Science, 82(3), 235–240.
38. Karkanis, A. C., Athanassiou, C. G. (2021). Natural insecticides from native plants of the Mediterranean basin and their activity for the control of major insect pests in vegetable crops: Shifting from the past to the future. Journal of Pest Science, 94(2), 187–202.
39. Pérez-Guerrero, S., Mateus, C. (2019). Field evaluation of commercial plant extracts against Drosophila suzukii (Diptera: Drosophlidae) in raspberry. International Journal of Pest Management, 65(1), 53–58.
40. Alim, M. A., Song, J., Lim, U. T., Choi, J. J., Hossain, M. A. (2017). Bioassay of plant extracts against Aleurodicus dispersus (Hemiptera: Aleyrodidae). Florida Entomologist, 100(2), 350–357.
41. Nisbet, A. (2000). Azadirachtin from the neem tree Azadirachta indica: Its action against insects. Anais da Sociedade Entomológica do Brasil, 29(4), 615–632.
42. Pereira, R. C., Barbosa, W. F., Lima, M. A. P., Vieira, J. O. L., Guedes, R. N. C. et al. (2020). Toxicity of botanical extracts and their main constituents on the bees Partamona helleri and Apis mellifera. Ecotoxicology, 29(3), 246–257.
43. Martínez, L. C., Plata-Rueda, A., Zanuncio, J. C., Serrao, J. E. (2015). Bioactivity of six plant extracts on adults of Demotispa neivai (Coleoptera: Chrysomelidae). Journal of Insect Science, 15(1), 34.
44. Loko, Y. L. E., Medegan Fagla, S., Kassa, P., Ahouansou, C. A., Toffa, J. et al. (2021). Bioactivity of essential oils of Cymbopogon citratus (DC) Stapf and Cymbopogon nardus (L.) W. Watson from benin against Dinoderus porcellus Lesne (Coleoptera: Bostrichidae) infesting yam chips. International Journal of Tropical Insect Science, 41(1), 511–524.
45. Facknath, S., Kawol, D. (1993). Antifeedant and insecticidal effects of some plant extracts on the cabbage webworm, Crocidolomia binotalis. International Journal of Tropical Insect Science, 14(5–6), 571–574.
46. Arivoli, S., Tennyson, S. (2013). Antifeedant activity, developmental indices and morphogenetic variations of plant extracts against Spodoptera litura (Fab) (Lepidoptera: Noctuidae). Journal of Entomology and Zoology Studies, 1(4), 87–96.
47. Olivero-Verbel, J., Nerio, L. S., Stashenko, E. E. (2010). Bioactivity against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia. Pest Management Science, 66(6), 664–668.
48. Stefanazzi, N., Stadler, T., Ferrero, A. (2011). Composition and toxic, repellent and feeding deterrent activity of essential oils against the stored-grain pests Tribolium castaneum (Coleoptera: Tenebrionidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Pest Management Science, 67(6), 639–646.
49. Martinez, S., van Emden, H. (1999). Sublethal concentrations of azadirachtin affect food intake, conversion efficiency and feeding behaviour of Spodoptera littoralis (Lepidoptera: Noctuidae). Bulletin of Entomological Research, 89(1), 65–71.
50. Martinez, S. S., van Emden, H. F. (2001). Growth disruption, abnormalities and mortality of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) caused by azadirachtin. Neotropical Entomology, 30(1), 113–125.
51. Rioba, N. B., Stevenson, P. C. (2020). Opportunities and scope for botanical extracts and products for the management of fall armyworm (Spodoptera frugiperda) for smallholders in Africa. Plants, 9(2), 207.
52. Farder-Gomes, C. F., Saravanan, M., Martínez, L. C., Plata-Rueda, A., Zanuncio, J. C. et al. (2021). Azadirachtin-based biopesticide affects the respiration and digestion in Anticarsia gemmatalis caterpillars. Toxin Reviews, 41(2), 466–475.
53. Jucelio Peter, D., Luiza Rodrigues, R., Simone Mundstock, J., Samuel, T. (2019). Effect of Azadirachta indica (Sapindales: Meliaceae) oil on Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae and adults. Florida Entomologist, 102(2), 408–412.
54. Khorshidi, M., Hejazi, M. J., Iranipour, S. (2017). Effect of azadirachtin, chlorantraniliprole and some insect growth regulators on vegetable leafminer, liriomyza sativae (Blanchard) (Diptera: Agromyzidae). Journal of Crop Protection, 6(1), 115–123.
55. Silva, M. S., Broglio, S. M. F., Trindade, R. C. P., Ferrreira, E. S., Gomes, I. B. et al. (2015). Toxicity and application of neem in fall armyworm. Comunicata Scientiae, 6, 359–364.
56. Iamba, K., Malapa, S. (2020). Efficacy of selected plant extracts against diamondback moth (Plutella xylostella L.) on round cabbage in situ. Journal of Entomology and Zoology Studies, 8(1), 1240–1247.
57. Kanwal, A., Ahmad, M., Khurshid, I., Khan, M. P., Khan, S. et al. (2020). Comparison of efficacy of synthetic pesticides with botanical extracts under field condition on cabbage white butterfly (Pieris brassicae). Advances in Entomology, 9(1), 44–48.
58. Ahmad, M. (2020). Comparative efficacy of synthetic pesticides with botanical extracts under field condition on cabbage white butterfly (Pieris brassicae Linnaeus) (Lepidoptera: Pieridae). Agricultural Science Journal, 2(2), 79–83.
59. Dougoud, J., Toepfer, S., Bateman, M., Jenner, W. H. (2019). Efficacy of homemade botanical insecticides based on traditional knowledge. A review. Agronomy for Sustainable Development, 39(4), 1–22.
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools