 Open Access
Open Access
ARTICLE
Polyphenolic Compounds and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides L.)
1
Institute of Pharmacy, Faculty of Medicine, University of Tartu, Tartu, 50411, Estonia
2
Chair of Veterinary Biomedicine and Food Hygiene, Institute of Veterinary Medicine and Animal Sciences, Estonian University of
Life Sciences, Tartu, 51006, Estonia
3
Faculty of Pharmacy, Nicolae Testemitanu State Medical and Pharmaceutical University, Chisinau, MD-2001, Republic of Moldova
4
Pharmacognosy Department, The National University of Pharmacy, Kharkiv, 61002, Ukraine
* Corresponding Author: Ain Raal. Email:
Phyton-International Journal of Experimental Botany 2023, 92(11), 2965-2979. https://doi.org/10.32604/phyton.2023.042723
Received 09 June 2023; Accepted 06 August 2023; Issue published 24 October 2023
Abstract
The fruits of the Sea buckthorn (Hippophae rhamnoides L.) are a popular plant food and a valuable health product. Cultivating plants produces a lot of leaves with fine branches as an unnecessary residue, which needs valorization. The aim of the study was to estimate (by HPLC-MS/MS) the qualitative and quantitative content of polyphenolic compounds in different parts of H. rhamnoides (HR), and to determine the level of antioxidant activity of leaves and fruits (by DPPH, ABTS methods and ferrozine test). Up to 19 compounds were identified in different parts of HR. The fruits are rich in flavonoids, including various glycosides of flavonols isorhamnetin, quercetin, and kaempferol. Two isorhamnetin glycosides were not identified in the leaves, while isorhamnetin-3- rhamnosylglactosides and the ellagitannins hippophaenin B, stachyurin and casuarinin were present only in the leaves of the plant. The bark and roots contained considerably more catechins, but minimal flavonols than the fruits and leaves of HR. The total phenolics and flavonols were most abundant in water infusions from leaves of HR (especially quercetin-3-O-glucoside-7-O-rhamnoside), compared to methanolic extracts. No significant differences in the quantitative and qualitative content of the fresh and dried leaves were detected. The highest antioxidant activity by all three methods was detected in the leaves of HR. In addition to the fruits, the leaves may have a perspective as a source of biologically active substances of HR.Keywords
Hippophae rhamnoides L. (family–Elaeagnaceae, common name–Sea buckthorn) is a flowering shrub native to cold temperate regions of Eurasia. Berries, seeds, and leaves of the plant are widely used as a folk medicine to treat hypertension, oedema, inflammation, tissue-regeneration, skin grafts, burns/injury, wounds, and ulcers. Berries are the most prominent feature of the plant [1].
The chemical composition and biological activities of H. rhamnoides (HR) have been studied by many authors [1–37]. Wide variety of secondary metabolites, including phenolics–quercetin, kaempferol, chlorogenic acid, catechins, isorhamnetin, myricetin; carotenoids–lutein, carotene, zeaxanthin, lycopene, tocopherol; tannins, ursolic acid, hippophae cerebroside, cirsiumaldehyde, oleanolic acid, dolichoic acid, 5-hydroxymethyl-2-furancarboxaldehyde, palmitic acid, 19-alpha-hydroxyursolic acid, 1-ohexadecanolenin, octacosanoic acid, linolenic acid (omega-3), linoleic acid (omega-6), palmitoleic acid (omega-7), oleic acid (omega-9), palmitic acid and phytosterols, etc., have been isolated from the plant. About 190 bioactive compounds were identified in its raw materials [1–5]. This wide range of active ingredients present in HR has been used effectively in the cosmetics industry as well as in medicine. The most studied product of HR is its oils [4], dried fruit pulp and berry residues yielding 8%–20%, 20%–25% and 15%–20% of oil, respectively [6]. The valuable substances present in the oil of HR are responsible for healthy and beautiful skin as well as for the proper functioning of the human body [1,7].
The whole plant of HR–berries, roots, leaves, stems and branches–contain various kinds of phenolics, including flavonoids, hydrolysable tannins and phenolic acids [24,25]. The leaves of the plant contain nutrients and bioactive components including various phenolic components-flavonols such as quercetin, kaempferol and isorhamnetin (mostly in glycosidic forms), flavons-3-ols such as various catechins (epicatechin, gallocatechin, epicatechin, epigallocatechin), phenolic acids like gallic, ferulic, and ellagic acids, procyanidins, leucoanthocyanidins [8,9], but also amino acids such as 0.13% methionine, 0.73% lysine, as well as 0.13% cysteine, 20.7% proteins [10]; folic acids, isoprenols, esterified sterols, triterpenols [11], vitamin E and minerals like K, Ca and Mg [8,11]. The fresh leaves of HR are a rich source of chlorophyll (98.8 mg/100 g), which is an indicator of the excellent trait of green vegetables while carotenoids concentration is 26.3 mg/100 g. Hydrolysable ellagitannins and gallotannins of monomeric type like casuarictin, strictinin, casuarinin, isostrictinin, isorhamnetin, quercetin-3-glucoside, quercetin-3-O-galactoside, and kaempferol in leaf extracts of the plant were also identified [9].
The pharmacological studies have demonstrated HR to exhibit antibacterial, anti-sebum, antifungal, anti-psoriasis, anti-atopic dermatitis, and wound healing activities. Besides, sea buckthorn has also been included in various cosmeceuticals for its use in skin-eventone, smoothening, rejuvenation, removal of wrinkles, scars, and pigmentation, and in hair related problems [1].
Various compounds found in HR raw materials (including vitamins, phenolic compounds, and lipids) play an important role in modifying the progression of diseases associated with oxidative stress, i.e., cancer, neurological and cardiovascular diseases [12]. Moreover, the oxidative stress in blood platelets or plasma may promote the development of cardiovascular diseases, neurological diseases, and cancer. Previous experiments showed that the polyphenolic fraction of sea buckthorn fruits, dominated by flavonoids, acts as an antioxidant and antiplatelet aggregation agent [13–15]. Thus, other parts of sea buckthorn that can act as an antioxidant are also interesting, and it is advisable to study their antioxidant activity for complex using.
The authors of the review [17] concluded that the berries, leaves and bark of HR are rich in many bioactive substances valuable for nutritional and health-promoting properties. Leaves of the plant are occasionally used for tea-type infusion because they are rich in nutrients, macro- and microelements. Šne et al. [16] studied total phenolic compounds in HR leaves, shoots, berries, and buds, but not in bark and roots. The leaves from female plants contained the highest total phenolics concentration and antioxidant activity, and less valuable were the shoots from male trees. Michel et al. [37] have been performed the antimicrobial, antioxidant and phytochemical investigations (by HPTLC-method) of HR leaves, stems, roots and seeds. The content of polyphenols in the fruits and leaves of HR has been studied by HPLC-method in several studies [1,6,8,17,18,20,21,23–27,34] but not performed yet in all parts of the plant.
The aim of the study was to estimate the qualitative and quantitative content of main polyphenolic compounds in fruits, leaves, bark and roots of HR. The other focus of this research was determination of the antioxidant activity of leaves and fruits (fresh/dried) extracts of HR employing DPPH, ABTS methods and Ferrozine test for iron chelating capacity.
To the best of our knowledge, this paper is the first to systematically investigate the content of phenolic compounds in fruits, leaves, bark and roots of HR in details using LC-MS/MS-method, and to compare the composition of fresh and dried leaves extracted by different technologies.
Sea buckthorn fruits and leaves (with thin stems) (HR ‘Botanicheskaya’) used in the study were obtained from Mr. Lauri Andressel, a board member of the company Hiiu Astelpaju OÜ, from the company’s Hiiumaa plantation harvested in October 2018. During harvesting, the branches with bunches of fruit were cut from sea buckthorn bushes and frozen. Then the fruits were removed from the frozen branches and the remaining parts crushed, resulting in a mass of shredded leaves and thin branches with individual fruits separated from the plant material (hereinafter: leaves). Fresh fruits and leaves (about a 100 g of each) were stored in a refrigerator (−18°C) until analysis. The leaves were dried at room temperature for 7 days for comparative analyses (samples 1–8).
For comparance, parts of HR (fruits, leaves, bark and roots) were also collected in September 2022 from the forest by the Lilleoru road in Viimsi municipality, Harju county, Estonia. The research material was collected from one bush, from which the branches were cut, the bark was separated with a knife, and the fruits and leaves were removed by hand, while collecting the roots, the finer roots were cut off. The collected plant parts were stored fresh in a freezer at −18°C until the experiments started (samples 9–12).
The Moldavian samples (13–15) were obtained from plants HR, cultivated in the collection of the Scientific Center of Medicinal Plants Cultivation of Nicolae Testemitanu State Medical and Pharmaceutical University in October, 2017. Leaves and fruits were harvested during the fruiting stage. Plant materials (leaves and fruits) were divided into 2 parts. One part was dried at room temperature (25°C) inside the laboratory room, in the dark, weighed, and then extracted, the second part was used fresh.
Samples 1–8 were extracted from plant material collected in 2018 and samples 9–12 in 2022. Samples 1 and 2: A pre-weighed amount of fresh (1) and dried (2) leaves were crushed in a coffee grinder for 10 s and sieved on a 3 mm mesh sieve. The mass remaining on the sieve was further ground in a coffee grinder for 10 s and sieved again on a 3 mm sieve. Methanol was added to the ground mass in a ratio of 1:10, and the sample was allowed to macerate for 24 h and filtered through a paper filter. Sample 3: Fresh fruits were weighed approximately 1 g, the fruits were crushed with a glass rod, but the seeds in the fruits were left intact. Methanol was added in a ratio of 1:10, allowed to macerate for 24 h and filtered through a paper filter. Experiments with dried fruits were not performed, because drying sea buckthorn fruits is very complicated.
Samples 4–7: Approximately 2 g of unground fresh (samples 4 and 5) and dried (samples 6 and 7) leaves, corresponding to one tablespoon, were taken to prepare aqueous extracts. One part of the samples (samples 4 and 6) was poured with 200 ml of boiling water and left to infuse for ten minutes, the other part (samples 5 and 7) was placed on a sieve and poured with 200 ml of boiling water, and filtered through a paper filter. Sample 8: Approximately one tablespoon of fruit was weighed to prepare fruit extract. The fruits were crushed with a glass rod, but the seeds inside the fruit were left intact. 200 ml of boiling water was added to the sample and left to infuse for 10 min. The sample was paper filtered and centrifuged at ~7000 rpm for ten minutes. After filtration, samples 1–8 were centrifuged with a “Centrifuge type MPW-310” at approximately 7000 rpm for 10 min.
Samples 9–12: were extracted as samples 3 (fruits) and 1 (leaves, bark, roots). In addition, before the analyses, the roots, leaves and bark were cut into ~1 cm long pieces with scissors and then ground with a coffee grinder for 10 s. The seeds of the fruits were preserved intact by breaking only the outer skin of the fruits.
The samples 13–15 of fresh or dried leaves and fresh fruits of HR (10–15 g) for study of antioxidant activity were extracted with 60% aqueous ethanol at room temperature. After 30 min of constant shaking, the extracts were filtered through Whatman no. 2 filter paper by vacuum suction, using Buchner funnel. The procedure was repeated 6 times. The combined extracts were evaporated to dryness under reduced pressure at 40°C and stored at −4°C until analysis.
2.3 HPLC-DAD-MS/MS Analysis and Quantification
The compounds were identified and quantified by liquid chromatography-mass spectrometry (LC-MS/MS). Samples 1–8 were analysed using an 1100 series LC/MSD Trap-XCT (ion trap) detector (Agilent Technologies, Palo Alto, USA) with electrospray ionisation in negative mode. The MS and MS/MS chromatograms were obtained in the range of 50–1000 atomic mass units (amu); target mass was set to 400 amu; number of fragment ions, 2; maximum ion collection time, 100 ms; compound stability, 100%; drying gas, nitrogen from the generator; collision gas, helium. HPLC 2D ChemStation software was used for process control and initial processing of the results. Samples 9–12 were analysed on a 1290 Infinity system (Agilent Technologies, Waldbronn, Germany), coupled to an Agilent 6450 Q-ToF mass spectrometer equipped with a Jetstream ESI source working in negative ionisation mode. Data acquisition and initial data processing were conducted using the MassHunter software (Agilent Technologies). The constituents were separated on a reversed-phase column (Zorbax 300SB-C18, 2.1 mm × 150 mm; 5 μm; Agilent Technologies) using a stepwise mobile phase gradient (component A-0.1% formic acid and component B-acetonitrile). For samples 1–8 the flow rate was set to 0.3 ml/min, column temperature was 35°C and sample injection volume 5 μl. For samples 9–12, the flow rate was 0.4 ml/min, column temperature was 40°C and injection volume 1 μl. Substances were identified based on MS/MS fragmentation spectra, retention times, standard compounds, and literature data [38–40].
2.4 Determination of Antioxidant Activity by DPPH Method
The stable 1,1-diphenyl-2-picryl hydrazyl radical (DPPH) was used for the determination of free radical-scavenging activity of the extracts. DPPH is a free radical which at room temperature produces violet colour in ethanol what is reduced in the presence of an antioxidant molecule, to produce a colourless solution. The use of DPPH provides an easy and rapid way to evaluate antioxidants-radical scavengers. Sample stock solutions (1 mg/ml) were diluted to final concentration of 200, 100, 50, 25, 10, 5 and 1 μg/ml in methanol. Different volumes of each extract were added to 0.75 ml of methanolic solution of DPPH (1.5 ml, 20 mg/l). After 15 min at room temperature, the absorbance was recorded at 517 nm. Methanol was used as the blank. DPPH solution (1.5 ml, 20 mg/l) and methanol (0.75 ml) was used as the negative control. The IC50 values denoting the sample concentration required to scavenge 50% of DPPH free radicals were calculated graphically [41,42].
2.5 Determination of Antioxidant Activity by ABTS Assay
The basis of the ABTS/PP assay is the interaction between an antioxidant and the pre-generated ABTS•+ (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) radical cation compared with antioxidant standard (Trolox) [43]. The ABTS•+ was generated by chemical reaction with potassium persulfate (K2S2O8) Firma. For this purpose, 10 ml of ABTS•+ 2 mM was spiked with 0.1 ml of K2S2O8 (70 mM) and allowed to stand in darkness at room temperature for 12–16 h (the time required for formation of the radical). The working solution was prepared by taking a volume of the previous solution (1 ml) and diluting it in 24 ml of methanol until its absorbance at λ = 734 nm was 0.70 ± 0.02. The reaction took place directly in the measuring cuvette. For this purpose, 10 µl of sample or standard were added at 0.99 ml of ABTS•+ solution, at which point the antioxidants present in the sample began to inhibit the radical, producing a reduction in absorbance, with a quantitative relationship between the reduction and the concentration of antioxidants present in the sample [44]. At the same time a Trolox calibration curve was prepared for a concentration range of 2.5–30 µM and the inhibition percentage obtained for the sample was interpolated to calculate the concentration in Trolox equivalents (µM TEAC).
2.6 Metal Chelating Activity by Ferrozine Test
The chelation of ferrous ions by extracts was estimated by method of Ma et al. [22]. Briefly, 50 μl of 2 mM FeCl3 was added to 60 μl of samples (10 mg/ml). The reaction was initiated by the addition of 200 μl of 5 mM ferrozine solution (FerroZine®Iron Reagent, Hach). The mixture was vigorously shaken and left to stand at room temperature for 10 min. The absorbance of the solution was thereafter measured at 562 nm. The percentage inhibition of ferrozine–Fe2+ complex formation was calculated as [(A0- As)/As] × 100, where A0 was the absorbance of the control, and As was the absorbance of the extract/ standard. EDTA was used as a positive control.
Data were expressed as mean of three replicates and standard deviation (SD). Statistical significance (p < 0.05) was evaluated by the Student’s test. All analyses were performed using GraphPad Prism, version 6.01, 2012.
3.1 Identification of Phenolic Compounds in HR
A total of 19 compounds were identified in different parts of HR, including 16 in fruits, 19 in leaves, 15 in bark and 10 in roots (Table 1). HR contains organic acids such as quinic, malic and citric acids in all its parts. In additon, the fruits also contain ascorbic acid. The fruits are particulary rich in flavonoids, including various glycosides of isorhamnetin, quercetin, and kaempferol. The leaves also contain several flavonoids found in the fruits, but two isorhamnetin glycosides were not identified in the leaves. Instead, isorhamnetin-3-rhamnosylgalactoside that was not found in other parts of HR, was identified in leaves. The composition of leaves differs from other parts of the plant due to the presence of catechin and ellagic acid derivatives. Ellagitannins such as hippophaenin B, stachyurin and casuarinin are characteristic compounds found in the leaves, but not in other parts of the plant. The bark of HR is rich in various derivatives of gallic acid and catechin, like procyanidin dimers and trimers. In contrast, roots contain the fewest phenolic components and have minimal phytochemical similarity to the other parts of the plant.

3.2 Total Polyphenols and Flavonols in Extracts of H. rhamnoides
The highest total polyphenols and the lowest total flavonols (1084.5 and 0.8 mg/100 g, respectively) are contained in the methanolic extract of H. rhamnoides roots, followed by the bark (859.5 and 0.8 mg/100 g, Fig. 1). The amounts of polyphenols and flavonols in the samples 9 and 10 was equal, but in the sample 1 flavonols were half as much as other polyphenols (77.6 and 148.9 mg/100 g, respectively). The best results regarding the content of total polyphenols and flavonols are obtained from the aqueous extract of fresh and dried leaves with a maceration time of 10 min (418.5–421.4 and 285.0–287.3 mg/100 g). Both total polyphenols and flavonols are extracted better with water than with methanol. The aqueous extract of fresh leaves contains more polyphenols and flavonols than fresh fruits (1.8 and 1.7 times, respectively). Thus, in addition to the fruits, the antioxidant activity of the leaves of HR is also promising.
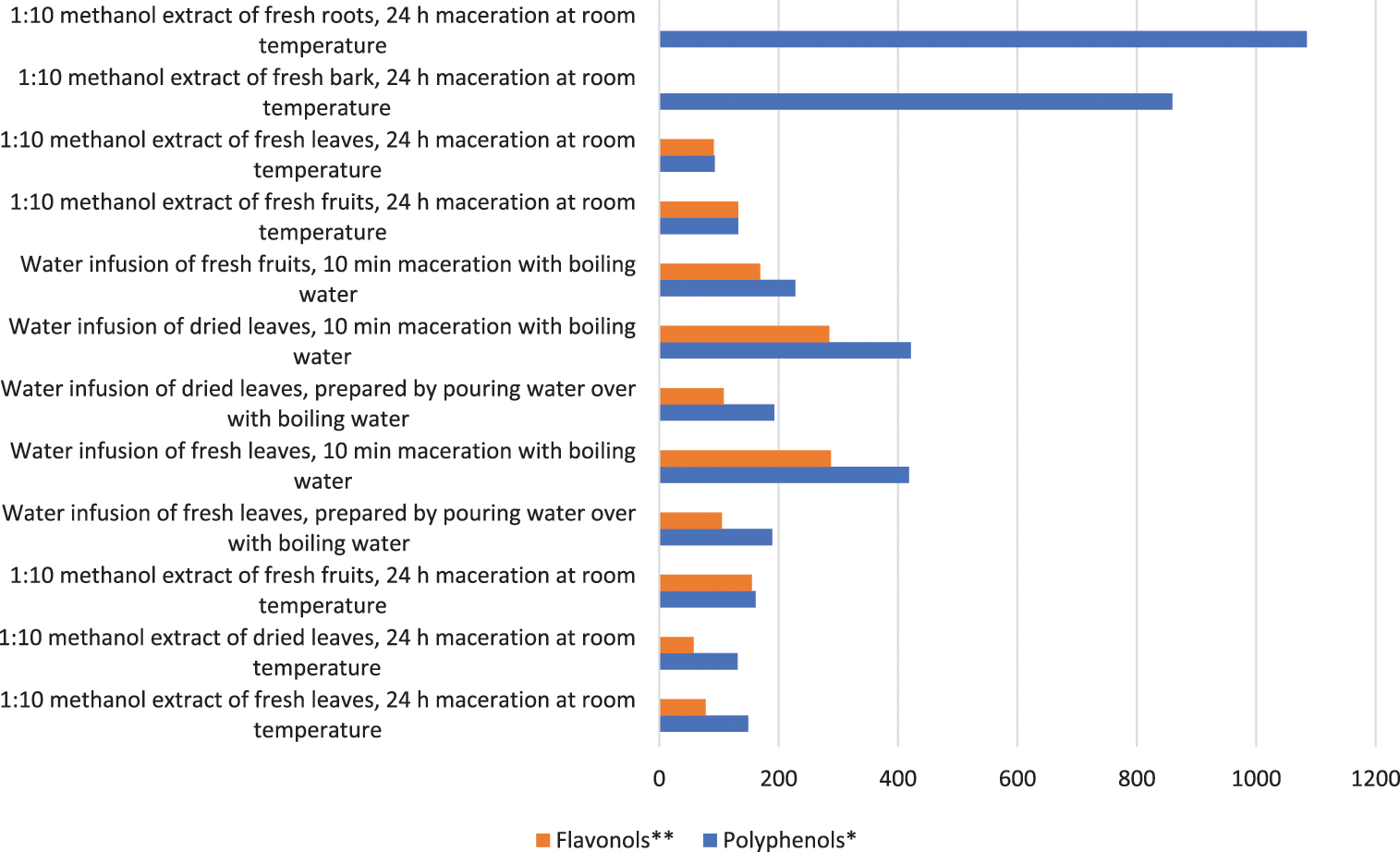
Figure 1: Total polyphenols and flavonols content in samples (mg/100 g DW)
Note: *Calculated as the sum of all compounds in Table 2. **Calculated as the sum of all polyphenols excluding caffeic acid glucoside, dimeric catechin/procyanidin B1 and catechin trimer in Table 2. DW-dry weight.
3.3 Content of Phenolic Compounds Extracted by Different Methods from the Parts of H. rhamnoides
The methanolic extract of dried leaves (sample 2) contained the most catechin dimer −35.6 mg/100 g (Table 2), which concentration was higher (55.7 mg/100 g) in fresh leaves (sample 1). There were also significant amounts of quercetin-3-O-glucoside-7-O-rhamnoside and its isomer (5.7 and 20.3 mg/100 g, respectively), caffeic acid glucoside (26.3 mg/100 g) and catechin trimer (11.6 mg/100 g). In the methanolic extract of fresh leaves (sample 1) catechin dimer was also dominant −55.7 mg/100 g, which exceeds by 1.5 times its content in dried leaves. The dried leaves also contained more quercetin-3-O-glucoside-7-O-rhamnoside and its isomer (8 and 29.5 mg/100 g) than fresh leaves. The largest amount of isorhamnetin rutinoside was found in the fruits −48.5 mg/100 g, almost 10 times more than in the leaves. Also, more quercetin rutinoside (20.5 mg/100 g), isorhamnetin-3-O-glucoside-7-O-rhamnoside (15.9 mg/100 g) and isorhamnetin-glucoside (21.6 mg/100 g) were found in the fruits.
When comparing different water extracts, it becomes clear that the extract obtained by pouring boiling water over the fresh leaves (sample 4) contained the most catechin trimer (36.1 mg/100 g), approximately the same amount was in the water extract standing for 10 min (sample 5 −40.4 mg/100 g, Table 2). It is logical that the sample macerated for 10 min (sample 5) contains 2.3 times more caffeic acid glucoside, 1.5 times more catechin dimer, 7 times more quercetin-3-O-glucoside-7-O-rhamnoside and its isomer, 4.5 times more rutin and 5 times more isorhamnetin rutinoside than the pour-through sample (sample 4). The content of other compounds was approximately the same both in the sample that was macerated in boiling water for 10 min and in the sample prepared by pouring. The methanol and water samples of the fruits (3 and 8) contained significant amounts of isorhamnetin rutinoside (48.5 and 44.4 mg/100 g, respectively), isorhamnetin glucoside (21.6 and 23.3 mg/100 g) and quercetin rutinoside (both about 20 mg/100 g) (Table 2).
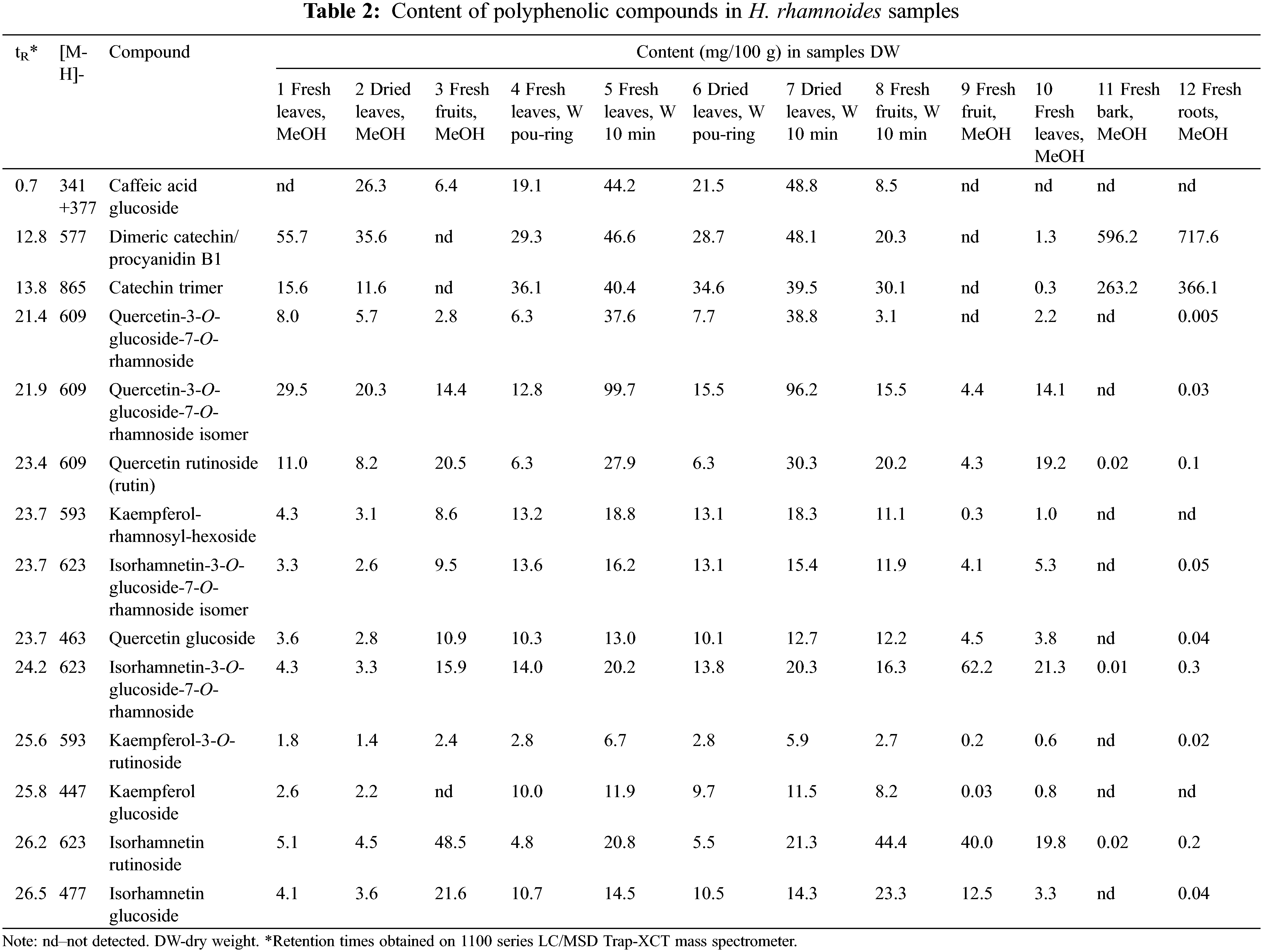
Bark and roots extracts contained less than 1 mg/100 g of identified compounds except dimeric catechin/ procyanidin B1 and catechin trimer (596.2–717.6 and 263.2–366.1 mg/100 g, respectively).
3.4 Antioxidant Activity of Fresh/Dried Leaves and Fruits
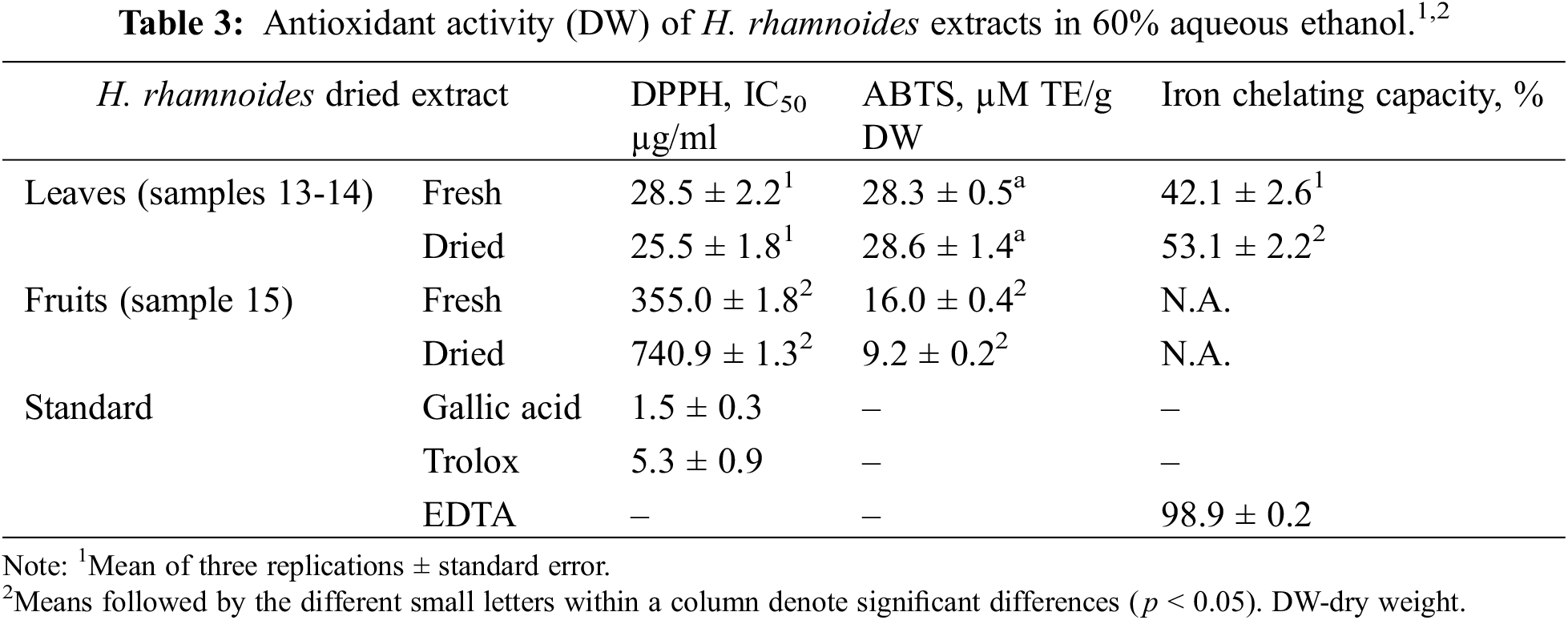
In all assays, HR leaf (fresh/dried) extracts showed significantly (p < 0.05) higher antioxidant (radical scavenging) activity than the fruit extracts (Table 3). Leaf extracts also showed strong iron chelating ability, possibly due to presence of malic and citric acids [45] whereas fruit extracts had no activity (Table 3).
Different ratios of ethanol and water have been used as extractants in solvent extraction process of bioactive substances from HR [28]. Our study showed that the method chosen to prepare the water extract affects the amounts of extracted bioactive substances. Comparing the contents of polyphenols and flavonols in samples 4 and 6 prepared by pouring, it turns out that the contents of both fresh and dried samples were practically equal (Fig. 1). The same result also appears when comparing samples 5 and 7 of dried and fresh leaves that have stood for 10 min. Therefore, it does not matter whether the leaves are used fresh or dried.
In the case of HR fruits, the use of fresh drug is optimal, as their drying under standard conditions is complicated due the high level of oil. Geng et al. [19] analyzed the effects of hot air drying and infrared drying on flavonoid compounds and antioxidant capacity in HR fruits: isorhamnetin and quercetin contents increased in response to both drying methods, while (-)-epigallocatechin and (-)-gallocatechin contents decreased in the fruits. In other similar study [20], they concluded that the pulsed-vacuum–drying of HR fruits had the highest ascorbic acid and total phenolics contents, and is an optimal drying technology for berries.
In this work, the extractability of polyphenolic substances using methanol and water was compared. In the sample 7 macerated with boiling water for 10 min, the concentration of quercetin-3-O-glucoside-7-O-rhamnoside was 6.5 times higher than in the methanol extract of dried leaves (sample 2, Table 2). The amounts of all other compounds in sample 7 are also significantly higher (1.3–4.8 times) than in the extract prepared with methanol. The same tendency is also visible in the extract of fresh leaves prepared with two different solvents (samples 1 and 5), the only exception is the catechin dimer content. Thus, extracting HR leaves with boiling water for 10 min, as is commonly done at home, is optimal. Michel et al. [37] compared the content of hexane, ethyl acetate and water extracts and concluded that the major phenolic compounds were found in water extracts.
In the fruits, the amounts of analysed substances were practically the same in methanol (sample 3) and water extracts (sample 8) (Table 2). However, the water extract also contained 30.1 mg/100 g of catechin trimer, 20.3 mg/100 g of catechin dimer and 8.2 mg/100 g of kaempferol glucoside, which could not be measured in the methanol extract. Therefore, the preparation of water extracts by maceration for 10 min is also justified for fresh fruits.
The technology of reflux extraction of flavonoids from HR leaves with water was studied by Wang. The optimal extraction temperature was 90°C, and the extraction time was 2 h [29]. Various authors [30,31] have optimized the ethanol/water extraction of HR flavonoids, showing ethanol concentration of 60% or 70% as the best choice. On the other hand, the disadvantage of the reflux extraction method is too long extraction time. The comprehensive use of multiple extraction methods compared to ethanol reflux, microwave-assisted extraction, and ultrasound-assisted extraction, increased the extraction rate of flavonoids from HR by 15%, 24%, and 37%, respectively [28]. Guliyev et al. [32] investigated different chromatographic methods to determine the chemical composition of HR, including the use of methanol as an extractant, but they did not determine the chemical composition of the bark and roots. The male leaves of HR are low in caffeine [33], which dissolves easily in water. This may be the reason why tea made from fresh leaves is recommended as a refreshing drink.
If to compare the extractability of polyphenolic compounds when poured with boiling water (samples 4 and 6) and after 10 min of maceration (samples 5 and 7), it is logical that a longer extraction time leads to a significant increase in the concentration of polyphenols. The differences are particularly large in the content of quercetin-3-O-glucoside-7-O-rhamnoside isomers, quercetin-3-O-glucoside-7-O-rhamnoside and isorhamnetin-rutinoside (1.4–7.7: 5.5 times, Table 2). Thus, adequate extraction time (10 min) is necessary for the preparation of aqueous extracts containing the maximum amounts of polyphenolic substances of HR.
The concentration of flavonol glycosides in leaves of HR is higher than in fruits [23]. Isorhamnetin glycosides, followed by quercetin glycosides are typically the most important flavonols of HR fruits and leaves [23]. The fruits are rich in flavonoid glycosides such as isorhamnetin-3-O-rutinoside, isorhamnetin-3-O-glucoside, isorhamnetin-3-glucoside-7-rhamnoside, isorhamnetin-3-neohesperidoside, quercetin-3-rutinoside, quercetin-3-O-glucoside, kaempferol-3-sorphoroside-7-O-rhamnoside, isorhamnertin-3-O-sorphoroside-7-O-rhamnoside, rutin and free isorhamnetin [23,26,27]. According to the Pop et al. [23], isorhamnetin-3-rhamnosylglucoside, isorhamnetin-3-neohesperidoside, isorhamnetin-3-glucoside, quercetin-3-pentoside, kaempferol-3-rutinoside, and quercetin-3-glucoside were predominant in leaves of HR. By Ma et al. [21], isorhamnetin-3-O-glucoside-7-O-rhamnoside and isorhamnetin-3-O-rutinoside were the two major flavonol glycosides in leaves and fruits of HR. Our study revealed that quercetin glycosides are more abundant in leaves, but the level of isorhamnetin glycosides depends on the specific compound (Table 2).
Tannins were present in higher concentrations in HR seeds, roots, flowers, green berries and stems [37]. The bark and root extracts contained the highest amounts of total polyphenols (859.5 and 1084.5 mg/100 g, respectively), but the least of flavonols (0.05 and 0.8 mg/100 g, Fig. 1). The principal polyphenols in bark and roots are catechin di- and trimers. However, these parts of HR contain the identified compounds in such low concentrations that are not of further interest as sources of biologically active substances (Table 2). Ma et al. [21] mentioned that in the leaves of HR ellagitannins are the most abundant polyphenols.
Traditionally, the fruits and leaves of HR, but not the bark and/or roots, have been used in several countries (China, Turkey, Greece, Mongolia, Tajikistan) [34]. In Central and Southeastern Asia every part of HR, including twig, root and bark has been traditionally used as medicine, dietary supplement, fence or firewood [8]. On the other hand, flavonoids are mentioned as the main chemical compounds also in the branches and roots of HR. According to our results (Table 2), the bark and roots contain only minimal amounts of flavonoids. The biological activity of HR roots and bark has been very little studied. For example, root extract has been shown to have antimicrobial effect (63% of inhibition) against Enterococcus durans [1].
Phenolic compounds (flavonoids, tannins and phenolic acids) of HR are considered to be the major constituents possessing antioxidant activity [16]. Li et al. [18] showed strong correlations between total phenolics content, gallic acid and antioxidant capacity. Also, Ciesarová et al. [17] concluded that phenolic compounds are dominantly responsible for antioxidative effect of HR. They mentioned also carotenoids and tocopherols as lipophilic antioxidants. Geng et al. [19] showed that the antioxidant activity of the dried fruits was considerably lower than that of fresh HR berries.
The antioxidant activity of fresh and dried leaves and fruits of HR were investigated in our study by three different methods. All these three methods showed that the antioxidant activity of the leaves is stronger than that of the fruits, which can be explained by the higher concentration of polyphenols in the leaves. This study suggests that HR leaf extracts have great potential for antioxidant activity and may be beneficial for their nutritional and medicinal functions. This is an important problem in the valorisation of plant residues because HR fruits are often collected with the tips of the branches, which are frozen and from which the fruits are later separated.
In our study, the fruits contain more isorhamnetin derivatives than the leaves (Table 2), but the leaves showed stronger activity (Table 3). The antioxidant effect of different parts, particularly leaves of HR, is not only due to polyphenols, but also to the content of vitamins, definite organic acids and polysaccharides [35]. We performed the antioxidant test with 60% aqueous ethanol instead of water, because a previous study [36] showed that ethanol extracts possess a significantly higher antioxidant power than water extracts. More generally, due their free-radical scavenging and anti-inflammatory properties, the popyphenols can be used to treat different conditions associated with metabolic disorders: dyslipidemia, atherosclerosis, obesity, hypertension, elevated blood sugar, accelerating aging, liver intoxication, etc. [8].
The study has some limitations. First, the antioxidant activity of roots and bark was not investigated in this work. It has no practical importance, because the mass collection of these plant parts means the destruction of the bushes. In addition, the organoleptic properties of bark and root water extract are unpleasant. On the other hand, the infusion of the HR leaves has a pleasant taste, somewhat reminiscent of green tea. Michelet al. [37] concluded that root ethanol extracts of HR have higher antioxidant activity (by DPPH and Ferric reducing antioxidant power (FRAP) assays) than extracts from leaves and stems. Thus, the antioxidant activity of roots needs additional study.
Secondly, the phytochemical and antioxidant studies have been performed on plant material collected from different places (Estonia and Moldova, respectively). Therefore, exact correlations between chemical composition and efficacy cannot be calculated. On the other hand, several previous studies [16–18,21] show that polyphenols are more abundant in HR leaves than in fruits and are the most important substances in the plant in terms of antioxidant activity.
Third, the phytochemical studies were performed in plant material collected from two growing sites and antioxidant analyzes only in raw material from one growing place. Probably, the general tendencies in the content and activity of phenolics and flavonols will remain the same, but for more detailed conclusions, the experiments should be repeated with plant parts of HR collected from different places.
A total of 19 phenolic compounds were identified in different parts of H. rhamnoides. Among flavonoids, such compounds as kaempferol glycosides, quercetin glycosides and isorhamnetin glycosides were the most significant ones identified in fruits and leaves. The compositions of bark and roots were very different compared to fruits and leaves as they contain considerably more amounts of polyphenols (mainly catechins but minimal flavonols). The total polyphenols and flavonols contents were the most abundant in water infusions from the leaves of H. rhamnoides. The studied phenolic substances were found in the highest concentration in water extracts that stood for 10 min, while the result was practically the same for fresh and dried raw material and did not depend on the method of preparing the water extract. There were no significant differences in the quantitative or qualitative content of phenolic compounds in the fresh and dried raw materials. Quercetin-3-O-glucoside-7-O-rhamnoside was the most abundant compound in the aqueous extracts. The fresh and dried leaves of H. rhamnoides showed the highest values of antioxidant activity and may have a perspective as sources of biologically active compounds in addition to fruits.
Acknowledgement: Not applicable.
Funding Statement: This work was supported by the European Union in the MSCA4Ukraine project ID Number 1232466.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Ain Raal, Tõnu Püssa, Tatiana Chiru, Nicolae Ciobanu; data collection: Tõnu Püssa, Linda Rusalepp, Kelly Talvistu, Michelle Shusta, Tatiana Chiru, Nicolae Ciobanu; analysis and interpretation of results: Ain Raal, Tõnu Püssa, Linda Rusalepp, Kelly Talvistu, Michelle Shusta, Tatiana Chiru, Nicolae Ciobanu, Oleh Koshovyi; draft manuscript preparation: Ain Raal, Tõnu Püssa, Linda Rusalepp, Tatiana Chiru, Nicolae Ciobanu, Oleh Koshovyi. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Please contact the corresponding author.
Ethics Approval: The study does not include human or animal subjects.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Pundir, S., Garg, P., Dviwedi, A., Ali, A., Kapoor, V. K. et al. (2021). Ethnomedicinal uses, phytochemistry and dermatological effects of Hippophae rhamnoides L.: A review. Journal of Ethnopharmacology, 266, 113434. [Google Scholar] [PubMed]
2. Leskinen, H. M., Suomela, J. P., Yang, B., Kallio, H. P. (2010). Regioisomer compositions of vaccenic and oleic acid containing triacylglycerols in sea buckthorn (Hippophae rhamnoides) pulp oils: Influence of origin and weather conditions. Journal of Agriculture and Food Chemistry, 58(1), 537–545. [Google Scholar]
3. Kallio, H., Yang, B., Peippo, P. (2002). Effects of different origins and harvesting time on vitamin C, tocopherols, and tocotrienols in sea buckthorn (Hippophae rhamnoides) berries. Journal of Agriculture and Food Chemistry, 50(21), 6136–6142. [Google Scholar]
4. Yang, B., Kallio, H. (2002). Composition and physiological effects of sea buckthorn (Hippophae) lipids. Trends in Food Science & Technology, 13(5), 160–167. [Google Scholar]
5. Zheng, R. X., Xu, X. D., Tian, Z., Yang, J. S. (2008). Chemical constituents from the fruits of Hippophae rhamnoides. Natural Products Research, 23, 1451–1456. [Google Scholar]
6. Kumar, R., Phani Kumar, G., Chaurasia, O. P., Singh, S. B. (2011). Phytochemical and pharmacological profile of sea buckthorn oil: A review. Research Journal of Medicinal Plants, 5(5), 491–499. [Google Scholar]
7. Zielinska, A., Nowak, I. (2017). Abundance of active ingredients in sea-buckthorn oil. Lipids in Health and Disease, 16(1), 95–106. [Google Scholar] [PubMed]
8. Suryakumar, G., Gupta, A. (2011). Medicinal and therapeutic potential of sea buckthorn (Hippophae rhamnoides L.). Journal on Ethnopharmacology, 138(2), 268–278. [Google Scholar] [PubMed]
9. Upadhyay, N. K., Yogendra Kumar, M. S., Gupta, A. (2010). Antioxidant, cytoprotective and antibacterial effects of sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chemistry and Toxicology, 48(12), 3443–3448. [Google Scholar]
10. Biswas, A., Bharti, V. K., Acharya, S., Pawar, D. D., Singh, S. B. (2010). Sea buckthorn: Newfeed opportunity for poultry in cold arid Ladakh region of India. World’s Poultry Science Journal, 66(4), 707–714. [Google Scholar]
11. Guan, T. T. Y., Cenkowski, S., Hydamaka, A. (2006). Effect of drying on the nutraceutical quality of sea buckthorn (Hippophae rhamnoides L. ssp. sinensis) leaves. Journal of Food Science, 70(9), E514–E518. [Google Scholar]
12. Olas, B., Żuchowski, J., Lis, B., Skalski, B., Kontek, B. et al. (2018). Comparative chemical composition, antioxidant and anticoagulant properties of phenolic fraction (a rich in non-acylated and acylated flavonoids and non-polar compounds) and non-polar fraction from Elaeagnus rhamnoides (L.) A. Nelson Fruits. Food Chemistry, 247, 39–45. [Google Scholar] [PubMed]
13. Olas, B. (2016). Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chemistry and Toxicology, 97, 199–204. [Google Scholar]
14. Olas, B., Kontek, B., Malinowska, P., Zuchowski, J., Stochmal, A. (2017). Hippophae rhamnoides L. fruits reduce the oxidative stress in human blood platelets and plasma. Oxidative Medicine and Cellular Longevity, 2016, 4692486. [Google Scholar]
15. Olas, B., Kontek, B., Szczesna, M., Grabarczyk, L., Stochmal, A. et al. (2017). Inhibition of blood platelet adhesion by phenolics’ rich fraction of Hippophae rhamnoides L. fruits. Journal of Physiology and Pharmacology, 68(2), 23–29. [Google Scholar]
16. Šne, E., Seglina, D., Galoburda, R., Inta Krasnova, I. (2013). Content of phenolic compounds in various sea buckthorn parts. Proceedings of the Latvian Academy of Sciences. Section B, 67(4/5), 411–415. [Google Scholar]
17. Ciesarová, Z., Murkovic, M., Cejpek, K., Kreps, F., Tobolková, B. et al. (2020). Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Research International, 133, 109170. [Google Scholar]
18. Li, Y., Li, P., Yang, K., He, Q., Wang, Y. et al. (2021). Impact of drying methods on phenolic components and antioxidant activity of sea buckthorn (Hippophae rhamnoides L.) berries from different varieties in China. Molecules, 26(23), 7189. [Google Scholar] [PubMed]
19. Geng, Z., Wang, J., Zhu, L., Yu, X., Zhang, Q. et al. (2023). Metabolomics provide a novel interpretation of the changes in flavonoids during sea buckthorn (Hippophae rhamnoides L.) drying. Food Chemistry, 413, 135598. [Google Scholar] [PubMed]
20. Geng, Z., Zhu, L., Wang, J., Yu, X., Li, M. et al. (2023). Drying sea buckthorn berries (Hippophae rhamnoides L.Effects of different drying methods on drying kinetics, physicochemical properties, and microstructure. Frontiers in Nutrition, 10, 1106009. [Google Scholar] [PubMed]
21. Ma, X., Yang, W., Kallio, H., Yang, B. (2021). Health promoting properties and sensory characteristics of phytochemicals in berries and leaves of sea buckthorn (Hippophaë rhamnoides). Critical Reviews in Food Science and Nutrition, 62(14), 3798–3816. [Google Scholar] [PubMed]
22. Ma, X., Moilanen, J., Laaksonen, O., Yang, W., Tenhu, E. et al. (2019). Phenolic compounds and antioxidant activities of tea-type infusions processed from sea buckthorn (Hippophaäe rhamnoides) leaves. Food Chemistry, 272, 1–11. [Google Scholar] [PubMed]
23. Pop, R. M., Socaciu, C., Pintea, A., Buzoianu, A. D., Sanders, M. G. et al. (2013). UHPLC/PDA-ESI/MS analysis of the main berry and leaf flavonol glycosides from different Carpathian Hippophaäe rhamnoides L. varieties. Phytochemical Analysis, 24(5), 484–492. [Google Scholar] [PubMed]
24. Arimboor, R., Arumughan, C. (2012). HPLC-DAD-MS/MS profiling of antioxidant flavonoid glycosides in sea buckthorn (Hippophae rhamnoides L.) seed. International Journal of Food Science and Nutrition, 63(6), 730–736. [Google Scholar] [PubMed]
25. Fatima, T., Nazir, A., Naseer, B., Hussain, S. Z. (2018). Seabuckthorn (Hippophae rhamnoidesA repository of phytochemicals. International Journal of Pharmaceutical Science and Research, 3, 9–12. [Google Scholar]
26. Guo, R., Guo, X., Li, T., Fu, X., Liu, R. H. (2017). Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of (Hippophaë rhamnoides L.) berries. Food Chemistry, 221, 997–1003. [Google Scholar] [PubMed]
27. Teleszko, M., Wojdylo, A., Rudzinska, M., Oszmianski, J., Golis, T. (2015). Analysis of lipophilic and hydrophilic bioactive compounds content in sea buckthorn (Hippophae rhamnoides L.) berries. Journal of Agriculture and Food Chemistry, 63, 4120–4129. [Google Scholar]
28. He, N., Wang, Q., Huang, H., Chen, J., Wu, G. et al. (2023). A comprehensive review on extraction, structure, detection, bioactivity, and metabolism of flavonoids from sea buckthorn (Hippophae rhamnoides L.). Journal of Food Biochemistry, 2023, 4839124. [Google Scholar]
29. Wang, S. L. (2008). Study on the extraction technology of flavones from leaves of Hippophae rhamnoides L. Food Research and Development, 29, 110–113. [Google Scholar]
30. Li, S. Z., Wu, F., Chen, Y. L., Yang, Y. (2015). The optimization of purification technics of total flavonoids from leaves of Hippophae rhamnoidesL. Journal of Anhui Normal University, 38, 567–571. [Google Scholar]
31. Hui, R. J., Feng, J., Lin, M. H., Feng, B. N. (2017). Optimization of extraction technology for favonoids in leaves and seeds of Hippophae rhamnoides L. by multiindex-orthogonal test. China Pharmacy, 28, 4856–4859. [Google Scholar]
32. Guliyev, V. B., Gul, M., Yildirim, A. (2004). Hippophae rhamnoides L.: Chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. Journal of Chromatography B, 812, 291–307. [Google Scholar]
33. Li, G., Zhang, J., Liu, E., Wang, F. H., Qi, S. et al. (2016). Effects of different sea buckthorn leaf tea processing technologies on nutrient level and fecal microflora in vitro. Journal of Food and Nutrition Research, 55, 205–213. [Google Scholar]
34. Ma, Q. G., He, N. X., Huand, H. L., Fu, X. M., Zhang, Z. L. et al. (2023). Hippophae rhamnoides L.: A comprehensive review on the botany, traditional uses, phytonutrients, health benefits, quality markers, and applications. Journal of Agricultural and Food Chemistry, 71, 4769–4788. [Google Scholar] [PubMed]
35. Wang, K., Xu, Z., Liao, X. (2022). Bioactive compounds, health benefits and functional food products of seabuckthorn: A review. Critical Reviews in Food Science and Nutrition, 62, 6761–6782. [Google Scholar] [PubMed]
36. Šne, E., Ruta Galoburda, R., Dalija Segliņa, D. (2013). Sea buckthorn vegetative parts–A good source of bioactive compounds. Proceedings of the Latvian Academy of Sciences. Section B, 67, 101–108. [Google Scholar]
37. Michel, T., Destandau, E., Le Floch, G., Lucchesi, M. E., Elfakir, C. (2012). Antimicrobial, antioxidant and phytochemical investigations of seabuckthorn (Hippophae rhamnoides L.) leaf, stem, root and seed. Food Chemistry, 131, 754–760. [Google Scholar]
38. Koshovyi, O. N., Vovk, G. V., Akhmedov, E. Y., Komissarenko, A. N. (2015). The study of the chemical composition and pharmacological activity of Salvia officinalis leaves extracts getting by complex processing. Azerbaijan Pharmaceutical and Pharmacotherapy Journal, 15(1), 30–34. [Google Scholar]
39. Raal, A., Jaama, M., Utt, M., Püssa, T., Žvikas, V. et al. (2022). The phytochemical profile and anticancer activity of Anthemis tinctoria and Angelica sylvestris used in Estonian ethnomedicine. Plants, 11, 994. [Google Scholar] [PubMed]
40. Chaika, N., Koshovyi, O., Raal, A., Kireyev, I., Zupanets, A. et al. (2020). Phytochemical profile and pharmacological activity of the dry extract from Arctostaphylos uva-ursi leaves modified with phenylalanine. Sciencerise: Pharmaceutical Science, 6(28), 74–78. [Google Scholar]
41. Brand-Williams, W., Cuvelier, M. E., Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28(1), 25–30. [Google Scholar]
42. Huzio, N., Grytsyk, A., Raal, A., Grytsyk, L., Koshovyi, O. (2022). Phytochemical and pharmacological research in Agrimonia eupatoria L. herb extract with anti-inflammatory and hepatoprotective properties. Plants, 11, 2371. [Google Scholar] [PubMed]
43. Dinis, T. C. P., Madeira, V. M. C., Almeida, M. L. M. (1994). Action of phenolic derivates (acetoaminophen,salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Archives of Biochemistry and Biophysics, 315(1), 161–169. [Google Scholar] [PubMed]
44. Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M. et al. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9–10), 1231–1237. [Google Scholar] [PubMed]
45. Justi, M., Silva, C. A., Rosa, S. D. (2022). Organic acids as complexing agents for iron and their effects on the nutrition and growth of maize and soybean. Archives of Agronomy and Soil Science, 68(10), 1361–1384. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools