 Open Access
Open Access
ARTICLE
First Report of a Successful Development of Yam Hybrids (Dioscorea alata L.) from Lyophilized and Long-Term Stored Pollens
1 CIRAD, UMR AGAP Institut, Petit Bourg, Guadeloupe, 97170, France
2 UMR AGAP Institut, Univ Montpellier, CIRAD, INRAE, Institut Agro, Montpellier, F-34398, France
3 CIRAD, UMR AGAP Institut, Montpellier, F-34398, France
* Corresponding Author: Komivi Dossa. Email:
Phyton-International Journal of Experimental Botany 2023, 92(10), 2861-2874. https://doi.org/10.32604/phyton.2023.042397
Received 29 May 2023; Accepted 14 July 2023; Issue published 15 September 2023
Abstract
Various biological constraints including erratic and asynchronous flowering between male and female plants hinder successful hybrid development and genetic gains in greater yam breeding programs. Therefore, pollen storage has gained much attention to facilitate artificial pollinations and increase the genetic gains. This 4-year study aimed at developing a practical long-term pollen storage technique for the successful development of yam hybrids. Fresh pollens were collected from two Dioscorea alata males, then lyophilized (two lyophilization treatments were applied), followed by storage at room temperature (24°C–25°C) for 12 months. Moreover, the lyophilized and stored pollens were tested for viability by crossing with four female varieties. Our results showed that lyophilization is effective for achieving viable pollens after 12 months of storage. Treatment 1 (48 h drying) showed higher pollen germination and fertility rates than Treatment 2 (72 h drying). Although we observed a reduction in viability of lyophilized pollens after 12 months of storage, we generated hybrid seedlings with success rates from 12% to 21% compared to 21%–31% when using fresh pollens. Paternity testing based on molecular genotyping confirmed the hybrid status of the obtained seedlings, which grew well in a greenhouse. Lyophilization is a practical approach for a long-term storage of greater yam pollen samples. This protocol will positively impact yam breeding programs particularly in developing countries.Keywords
Supplementary Material
Supplementary Material FileYams are important food security crops and are widely consumed for nutritionally rich tubers, particularly in developing countries [1–4]. Aside from the nutritional composition, including carbohydrates, minerals, and vitamins, yams play a key role in social, cultural, and religious aspects of lifestyle in western Africa [5]. Moreover, yams are rich in some secondary metabolites such as saponins, diterpenoids, and alkaloids [6–8]. Over the years, progress has been made in exploiting the available genetic resources for improving the nutritional quality, and adaptability of yam species. However, the complex genetic architecture of yam species as well as numerous biotic and abiotic factors hinder further breakthroughs, offering new avenues for research and development [9].
Dioscorea alata L., also known as greater yam, is among the most important cultivated yam species from the Dioscorea genus. It is a diecious species widespread across tropical areas of Africa, Asia, Oceania, and South America [10]. Breeding programs significantly contribute to crop improvement, followed by commercial propagation. However, very limited progress has been achieved so far in greater yam breeding programs [9]. The genetic diversity in D. alata germplasms available to breeders is very low [10,11]. Hence, exchange of genetic materials among breeding programs is essential to make substantial genetic gains. However, exchanging yam genetic materials is very complicated because yams are naturally prone to diverse viruses and the sanitation process to achieve virus free vitroplants is a complex and time-consuming task [12,13]. Besides, several biological constraints (polyploidy, erratic flowering, asynchronous flowering, inter-and intra-genotypic incompatibility, long vegetative growth period, low multiplication ratio, and high heterozygosity) impede varietal improvement and genetic gains in D. alata [10,14–19]. These constraints limit the number of compatible fertile parents, resulting in a low number of successful crosses. Dioscorea alata is an erratic flowering dioecious plant. The female flowers mature well before the male flowers, thus making cross-pollination difficult between two plants [19–22]. Moreover, yam pollen is fertile for a very short time (less than 5 h) at ambient temperature [20], complicating the crossing procedure. Therefore, pollen storage has gotten much attention since it enables cross-pollination as early as possible and on a high number of flowers during the short flowering period of female greater yam varieties. Stored yam pollens can be exchanged safely among breeding programs to increase the gene pool and accelerate the genetic gains.
Freeze-preserved pollen is often used to achieve synchronized flowering for a successful crossing program [23–26]. Cryopreservation allows pollen to remain viable over a long period but requires sophisticated equipment [27–29]. The pollen viability under cryopreservation is highly dependent on a stable temperature [28]. Although some studies have demonstrated the viability of cryopreserved pollen in yams [21,24,27], there is an apparent lack of evidence for the successful development of hybrids from long-term cryopreserved pollen. Lyophilization (freeze-drying) is an alternative method for long-term storage, consisting of freezing, sublimation, and adsorption [30]. The pollen, thus dried, can be stored in an ambient atmosphere without keeping it frozen. Previous reports suggested a successful pollen lyophilization for long-term pollen storage in different crops, for instance, sorghum [31], eucalyptus [32], maize [33,34], and pigeon pea [35]. However, lyophilization has never been tested in greater yam for a successful hybrid development. Moreover, considering that yams are mainly produced in the developing world, most laboratories in these regions do not have the facilities to stably preserve the pollen at −80°C for a long period. It is, therefore, necessary to find alternative and practicable pollen conservation methods for yam breeding programs in developing countries.
This study aimed at testing three main hypothesis: (1) the lyophilized D. alata pollens could be stored at room temperature and be viable over a year, (2) the desiccation duration during lyophilization affects pollen viability, (3) a 12-months stored pollens could be used to develop hybrid yam plants.
2.1 Plant Materials, Flower Sampling and Pollen Collection
This study was conducted over 4 years (Table 1) in Guadeloupe at the Centre de coopération Internationale en Recherche Agronomique pour le Développement (CIRAD), research station of Roujol. Two D. alata male varieties (14M and Toufi-Tetea) and four female varieties (74F, Boutou, Ti-violet and CT198) were obtained from the germplasm collection of CIRAD, Guadeloupe. The varieties were selected based on different criteria: different ploidy levels, stable and abundant flowering, high average cross-compatibility rate (ACR > 40%) estimated following details in Mondo et al. [20] and contrasted morphological traits for facilitating hybrid identification. These varieties have been used for pollination in our breeding program since 2002. Varieties 14M (ACR = 41%), Boutou (ACR = 44%) and 74F (ACR = 42%) are diploid, while Toufi-Tetea (ACR = 46%), Ti-violet (ACR = 41%) and CT198 (ACR = 59%) are tetraploid [19].
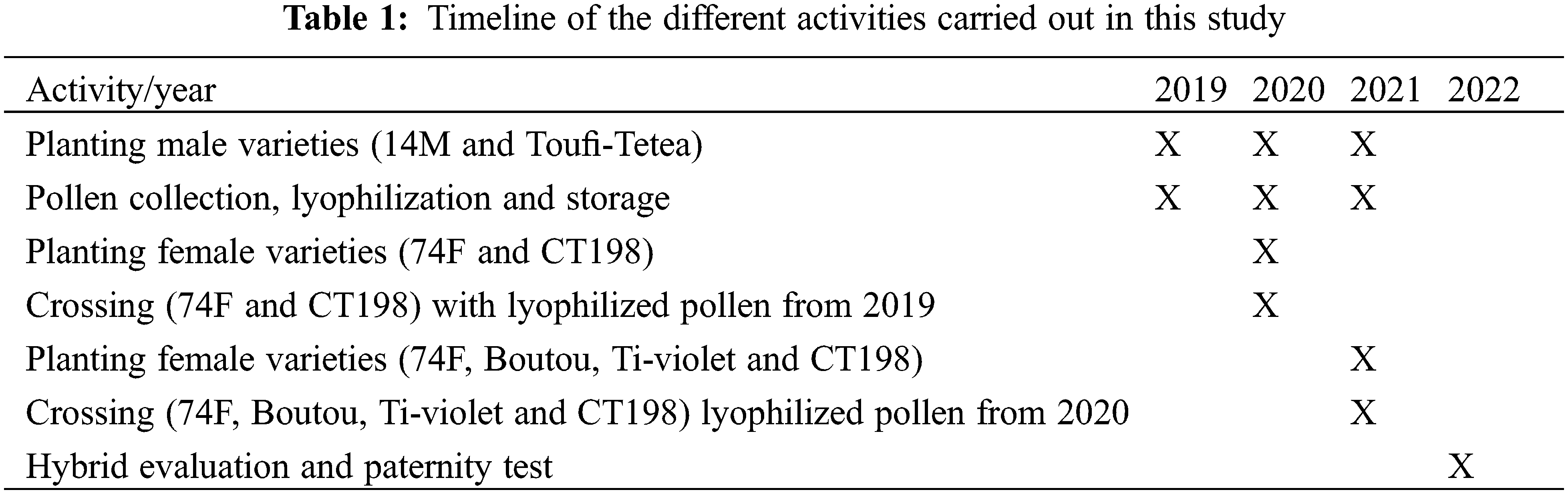
The male varieties were planted over 3 years (2019–2021) in order to collect and store pollen for the pollination of female varieties in consecutive years (Table 1). The female varieties were planted in 2020 (74F and CT198) and 2021 (74F, Boutou, Ti-violet and CT198) at the CIRAD research station of Roujol, in Guadeloupe (16°10′ 56″ N, 61° 35′ 24″ W, 10 m above sea level). The average temperature and relative humidity (RH) from September to December of each year during the study period are: 30/25°C day/night and 82% RH in 2019, 31/25°C day/night and 83.2% RH in 2020, 31/24°C day/night and 82.5% RH in 2021 and 30/25°C day/night and 83% RH in 2022. The flowering of D. alata begins with female varieties in Guadeloupe. The first inflorescences are visible around mid-September. Receptivity of female flowers can be visually checked. When female flowers are fully open and the stigma clearly visible, it means they are ready for pollination. We start pollination in the third day after anthesis, which is within the range of receptivity window reported by Mondo et al. [20]. Usually, around mid-October, the male inflorescences are observed. Full flowering begins towards the end of October. We have noticed that the male tetraploid inflorescences are very close to anthesis from the first hours of the morning around 9 am, while the male diploid flowers do not open until around 11 am. The male flowers were collected from the male varieties by the end October each year when the petals are fully opened (Fig. 1), corresponding to the period of maximum pollen fertility [36]. Inflorescences were cut with a scissor and placed in a plastic bag stored in cooler containing ice. Samples were sent immediately to the laboratory. The fully open spike flowers were taken from the inflorescences and stored in 10 ml Eppendorf tubes. Five filled tubes were stored for each variety.
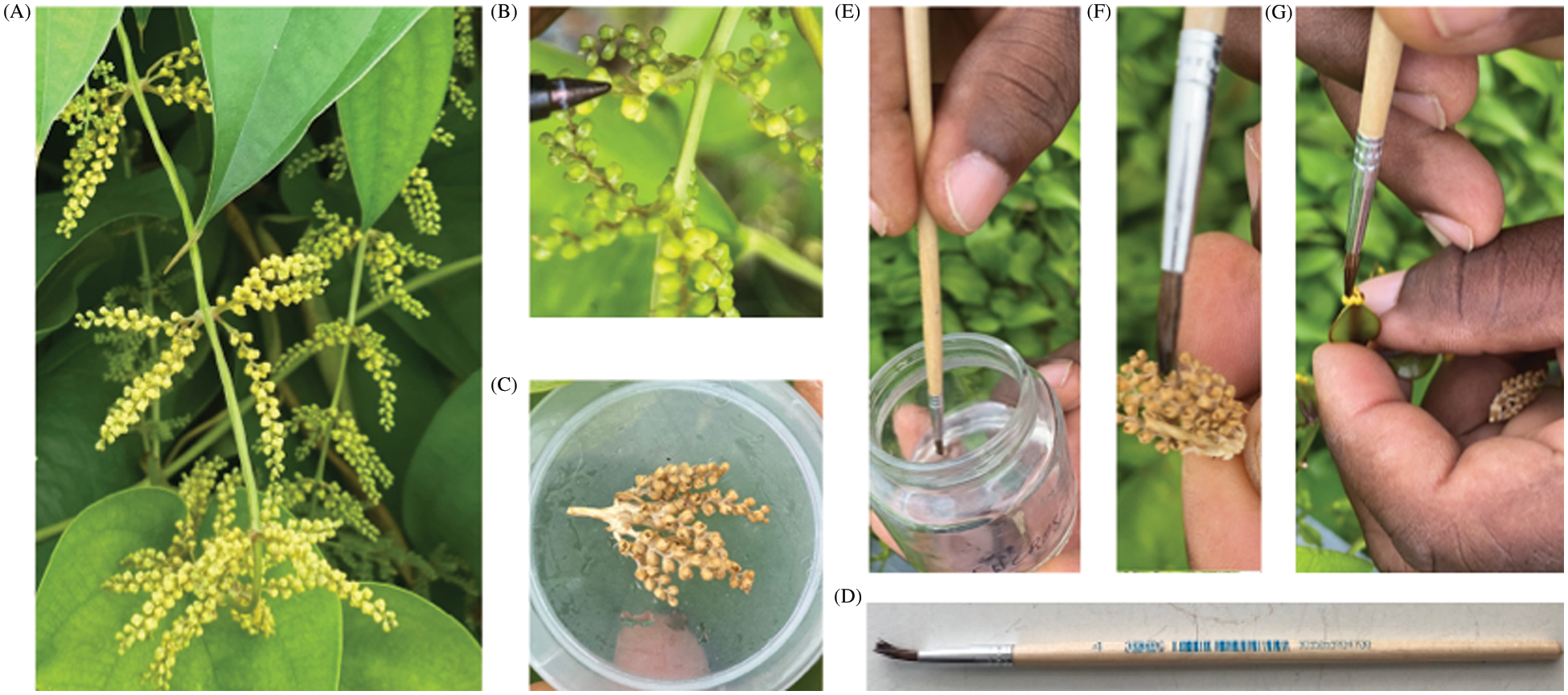
Figure 1: Collection of fresh mature opened male flower samples. (A) Photo of the inflorescences of 14M with mature flowers, (B) Close-up photo of opened male flowers (14M), (C) Photo of lyophilized flower samples (14M), (D) Picture of the flat paintbrush used for artificial pollination, (E) Wetting the paintbrush in a sucrose solution, (F) Brushing the lyophilized flower spike to collect pollen grains, (G) Pollination of the female flower (74F)
2.2 Fertility and Germination Tests of the Fresh Pollens
The fertility rate of the pollen of the flowers collected from each genotype was checked by a colorimetric test [37]. The anthers were removed from the inflorescence and placed on the slide in a drop of alcohol to remove grease and resin and wiped with Joseph paper (Fisherbrand, grade quality 605). Later, the slides were dried at 35°C to fix the pollens. After four alcohol-drying wash cycles, Alexander’s staining solution was added. The pollen staining was observed under a microscope (Ernst Leitz Wetzlar GmbH, Wetzlar, Germany) with a 400 magnification. Three slides were prepared for each genotype and observed for colored pollens.
In addition, an in vitro germination test of the pollen on a synthetic medium was carried out. It consisted in distributing a few drops of the sugar solution containing pollen grains on a Brew-baker and Kwack (BKM) medium composed of 8% of sucrose, 50 mg/L of boric acid (H3BO3), 300 mg/L of Ca(NO3)2·4H2O, 200 mg/L of MgSO4·7H2O, 100 mg/L of KNO3 and 0.7% agar at pH 6.5 [38]. The culture medium was sterilized in an autoclave and poured into three small Petri dishes per genotype.
A minimum of 4 to 5 h of incubation at 25°C on the medium is necessary in order to observe the germination of the pollens. From 24 h, we determined the percentage of germinated pollen grains by observing with a microscope (Ernst Leitz Wetzlar GmbH, Wetzlar, Germany). A pollen grain was considered germinated in vitro if its pollen tube is at least the diameter of the pollen itself [39].
2.3 Pollen Lyophilization and Conservation
Pollen lyophilization was performed using two sets of treatments. Treatment 1 (T1) consisted of 48 h drying, while treatment 2 (T2) consisted of 72 h drying. The freeze dryer Martin Christ, model Alpha 1–4 (Martin Christ Gefriertrocknungsanlagen GmbH, Germany), was used for the lyophilization. Eppendorf tubes containing flower samples were placed in the freeze dryer. The freezing time was kept for 5 h at −35°C. After freeze-drying, the samples were placed in a closed plastic bag and stored in a dark jar containing silica gel at room temperature 24°C–25°C. Freeze-dried pollens were stored for 12 months (Fig. 1C).
2.4 In Vitro Fertility and Germination Test of Lyophilized Pollens
The lyophilized and stored flower samples were monthly checked. Samples were crushed slightly on a slide and placed in a tube containing a sugar solution. After centrifugation, pollen grains were taken and stained with Alexander solution and observed under a microscope, as explained above. Similarly, a germination test, as explained above was carried out. Three replicates per genotype were used.
2.5 Crossing for Obtaining Hybrid Plants from Lyophilized Pollens
Sixteen tuber fragments of each female genotype were planted in an isolated plot [3]. During the flowering period, some immature inflorescences (flowers with green and closed cap) were covered with paper bags. When the female flowers became receptive, lyophilized pollens from the previous year were used to pollinate them (74F × 14M and CT198 × Toufi-Tetea) in 2021 and (74F × 14M, CT198 × Toufi-Tetea, 14M × Boutou and CT198 × Ti-violet) in 2022 (Table 1).
In order to facilitate the cross with the lyophilized flower samples, we set up a new technique. A flat paintbrush was immersed in sucrose solution, then used to wet the female flower (Fig. 1D). A single male flower (entire bud) was detached from the lyophilized flower spikes and placed on top of a female flower (named: single-male-flower approach). We also tried another technique (named: brushing approach) to collect pollen samples by brushing the lyophilized flower spikes using a wet paintbrush (with a sucrose solution) (Figs. 1E and 1F). A colorimetric test [37] showed that the paintbrush was covered by pollen grains (Data not shown). Then, the wet paintbrush covered by pollen grains can be used to brush and pollinate the female flowers (Fig. 1G). Both approaches resulted in successful pollinations.
A total of 100 flowers of each female genotype were crossed using the single-male-flower approach. After crossing, the flowers were protected by the paper bags for 2 weeks, then the fruiting rate was noted. We also used fresh pollen samples for pollination. The fruit seed setting rate and seed with viable embryo were noted 2 months later when fruits are fully dry. The seeds with viable embryos were germinated on a nutrient medium: M20 (Murashige and Skoog medium with vitamins 3 g, Myoinositol 5 g, sucrose 20 g, Gelrite 2.5 g, activated carbon 1 g, for 1000 ml of the medium, the pH was adjusted to 5.8). The obtained seedlings were propagated in vitro and were later acclimatized and planted in pots.
2.6 Genotyping for Paternity Testing
Leaf samples from five hybrids and their parents (74F × 14M) were used for DNA extraction with the Mixed Alkyl Trimethyl Ammonium Bromide method [40]. DNA quality and quantity were checked on agarose gel and Invitrogen Qubit Flex Fluorometer. A total of 19 microsatellite markers (Table S1) were initially tested for polymorphism between the parents. Seven detected polymorphic markers were used for genotyping the five hybrids. M13 tail (CACGACGTTGTAAAACGAC) was added to the primers. The PCR reaction was performed using the QIAGEN kit under the conditions as followed 95°C for 5 min, 10 cycles of 30 s at 95°C, 60°C for 1 min 30 s and 72°C for 30 s, 25 cycles of 30 s at 95°C, Tm °C for 1 min 30 s and 72°C for 30 s, followed by 30 min at 60°C. Migration of the PCR products was conducted on the ABI 3500 xL (Thermo Fisher Scientific Inc., Waltham, MA, USA). Analysis of microsatellite profiles was performed with Genemapper v6.0 (Applied Biosystems™). The software provides DNA sizing and quality allele calls. True hybrids inherit alleles from both parents, allele segregation was analyzed to confirm the hybrid status.
GraphPad Prism v9.0.0121 (GraphPad 159 Software Inc., La Jolla, CA, USA) was used for graph construction and statistical analysis. Statistical differences were performed by independent t-test at p < 0.05.
3.1 Assessment of Fresh Pollen Viability
A systematic approach was followed to evaluate the effect of lyophilization of pollen grains from the two genotypes viz., 14M and Toufi-Tetea on their viability and suitability for crossbreeding (Fig. 2). It is important to ascertain the fertility of pollen grains from the genotypes used for the assay. Hence, fresh pollens were collected from both genotypes and subjected to fertility and germination tests during the first step. The results indicated similar and high pollen fertility (78% and 85% in Toufi-Tetea and 14M, respectively) and pollen germination percent (82% and 86% in 14M and Toufi-Tetea, respectively) in the two genotypes (Fig. 3). Therefore, the selected genotypes are good candidates for pollen lyophilization and long-term storage.
Figure 2: Schematic diagram of hybrid development using lyophilized pollens in Dioscorea alata
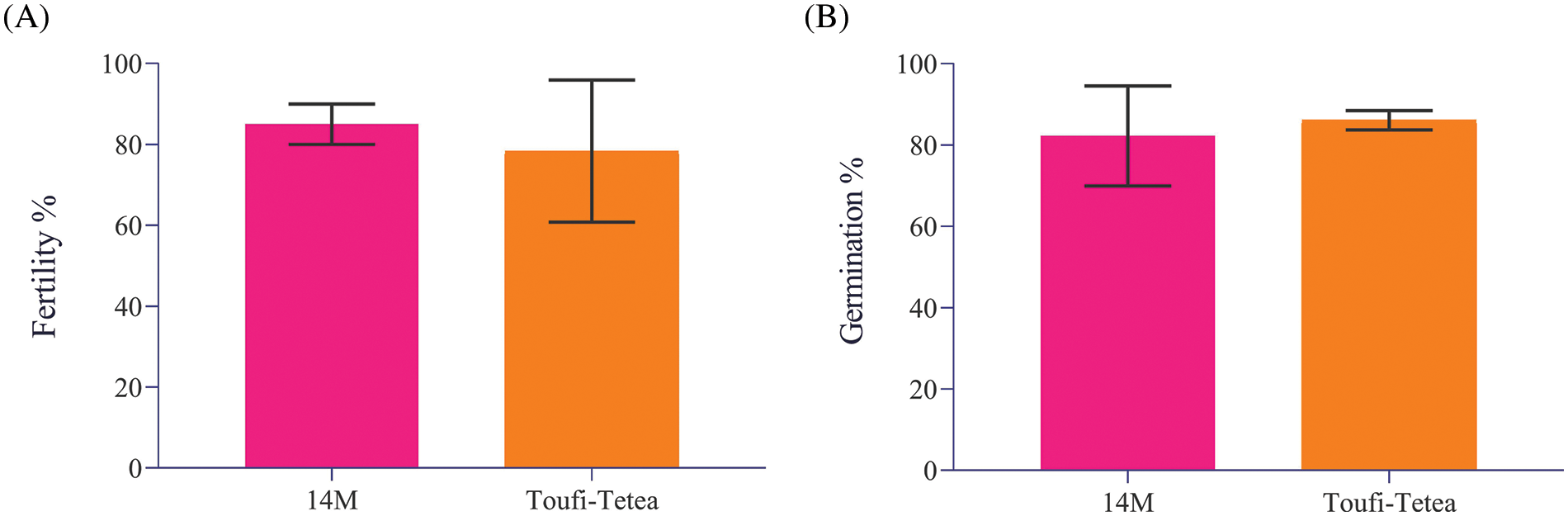
Figure 3: Pollen viability test of two Dioscorea alata male varieties used in the current study. (A) Fertility percent of freshly collected pollens from two D. alata varieties viz., 14M and Toufi-Tetea, (B) Germination percentage of freshly collected pollens from two D. alata varieties viz., 14M and Toufi-Tetea
3.2 Lyophilization, Storage and Their Effects on Pollen Viability
Pollen lyophilization was performed using two treatments (T1 = 48 h drying and T2 = 72 h drying). Both varieties showed a higher germination percent in T1 compared to T2 (Fig. 4). The germination percentage was estimated monthly (from November to July) after the pollen lyophilization, and a continuous decrease in germination percentage was observed. 14M showed a decrease in germination percent from 76% to 25% in T1, while a decrease from 50% to 10% under T2 was observed. Similarly, the germination percentage of lyophilized pollens of Toufi-Tetea decreased from 76% to 25% under T1, while under T2, it decreased from 75% to 20%.
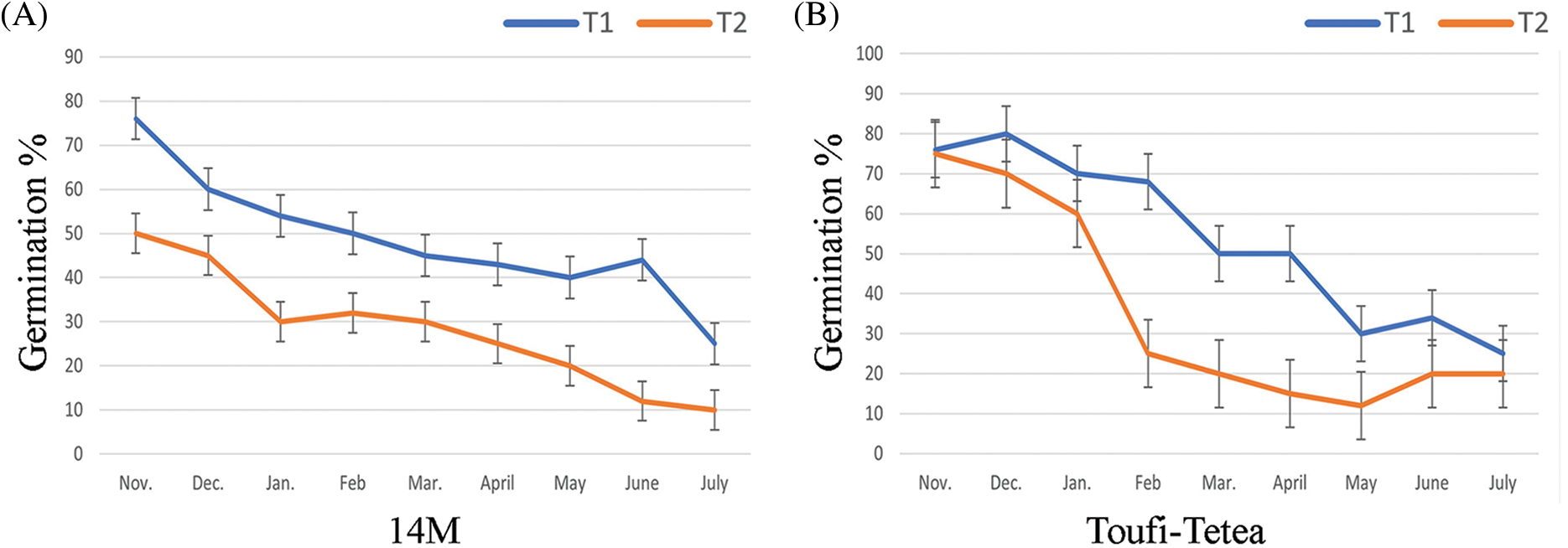
Figure 4: In-vitro germination percentage of pollens after lyophilization in (A) 14M and (B) Toufi-Tetea from November to July. T1 (48 h drying) and T2 (72 h drying) represent the two lyophilization treatments. Data represent the average of two-year experiments
The remaining samples of the lyophilized pollens were further stored for 4 more months (12 months in total). A fertility test was performed to check the pollen viability after 12 months of storage. Interestingly, pollens from both varieties displayed a higher fertility percentage in T1 compared to T2 (Fig. 5). Altogether, our results showed that T1 (48 h drying) is more conducive for the long-term fertility of pollen grains in D. alata.
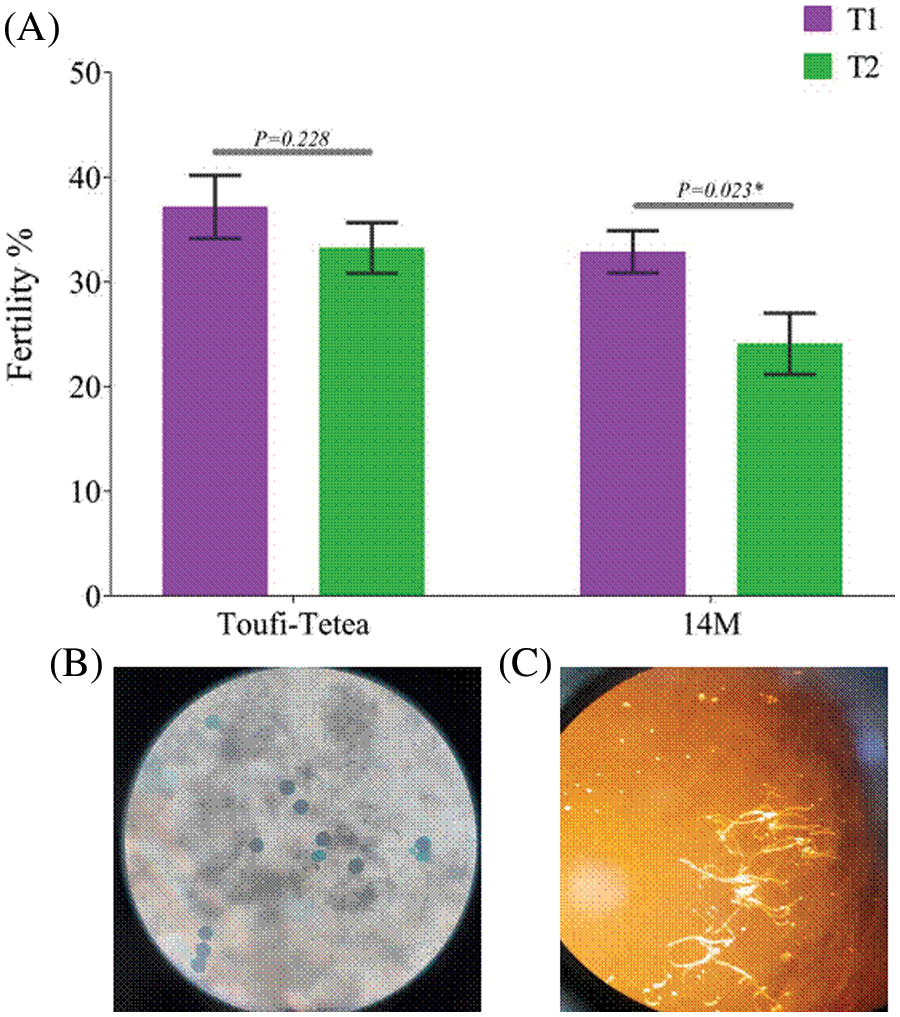
Figure 5: Fertility test of lyophilized pollens after 12 months storage sourced from 14M and Toufi-Tetea. (A) Fertility percentage of lyophilized pollens in 14M and Toufi-Tetea, (B) Photo of colored lyophilized pollen grains from 14M with the Alexander solution, (C) In vitro germination of lyophilized pollen grains from 14M. p < 0.05 was considered significant (*) using a two-tailed t-test
3.3 Development of Hybrid Yam Plants from Lyophilized Pollens
To test whether the lyophilized pollens could be used for developing hybrid yam plants after 12 months storage, we crossed the female plants in the field conditions using pollens from T1. Four varieties, 74F, Boutou, Ti-violet and CT-198, were used as female parents, while lyophilized pollens from 14M and Toufi-Tetea were used for pollination. The cross 74F × 14M, CT-198 × Toufi-Tetea, Boutou × 14M, and Ti-violet × Toufi-Tetea yielded 40%, 50%, 58%, and 56% fruits, respectively (Table 2 and Fig. 6). We also performed crossing using fresh pollens, and the fruit setting rate was higher than lyophilized pollens. The fruits were later evaluated after maturity for viable seeds. Globally, most fruits obtained from lyophilized pollens contain a lot of viable seeds similar to observations with fresh pollens (Table 2).

Figure 6: Development of hybrid plants obtained from crosses with lyophilized and 12-month-stored pollens in Dioscorea alata. (A) Visible swelling ovary of the female flowers 10 days after pollination in 74F × 14M, Photos of seedlings generated from tubers of parents (B) 74F, (C) 14M, and seeds of (D and E) two selected hybrids obtained from lyophilized pollens and grown in a greenhouse. Red arrows show swollen ovary of the female flowers, blue arrows show the pink coloration present on the petiole of 74F, absent on 14M and segregating in the hybrids. The white arrows show the oval leaf shape of 14M present in both hybrids
The obtained viable seeds from the lyophilized pollens were germinated on a nutrient medium (M20), and later seedlings were transplanted in pots. Interestingly, we successfully generated 12–22 hybrid seedlings from the different parent pairs and the seedlings grew normally in a greenhouse (Fig. 6).
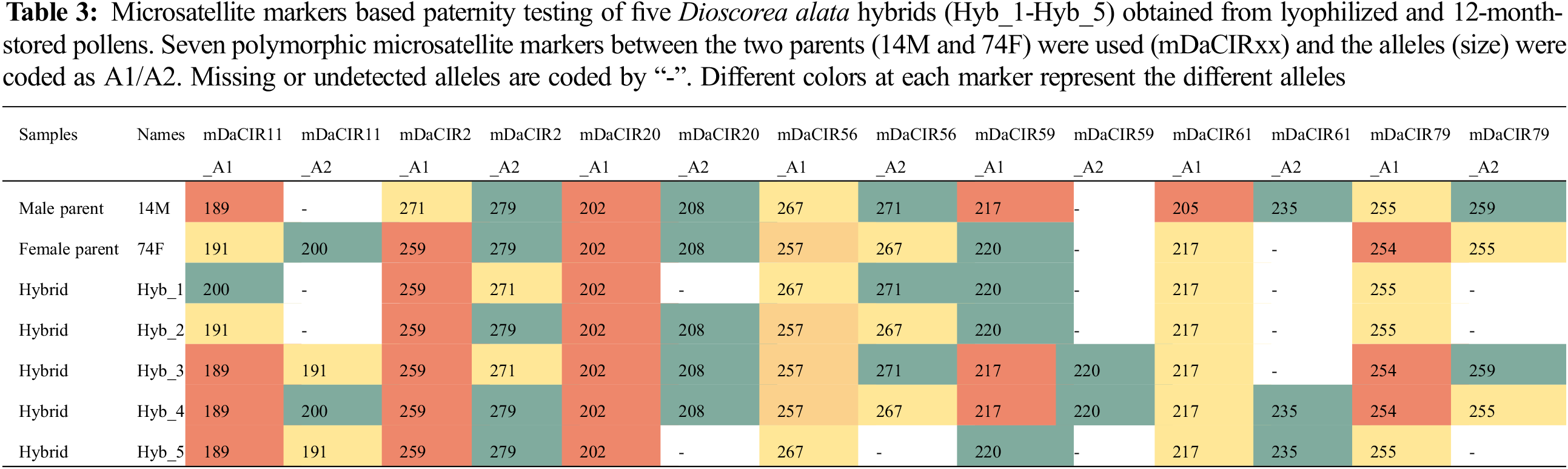
To further confirm the status of the obtained plants, we performed a paternity testing using seven polymorphic microsatellite markers (between the two parents) on five selected hybrids from the cross 74F × 14M. Several missing data were noted due to the quality of the molecular markers or DNA. The genotyping showed a maximum of two alleles for all markers which matches well the ploidy level of the genotypes (Table 3). Overall, the hybrids displayed different allelic combinations from both parents at the different markers (Table 3), confirming that the plants obtained are well from gametes of the two parents.
In brief, our study revealed that long-term conserved lyophilized pollens could be used to develop hybrid plants in D. alata.
Yam is a significant food crop in developing countries feeding over 150 million people [41]. A fast genetic improvement of yams is required in order to satisfy the growing demand of yam tubers owing to the rise of demography coupled with the negative effects of climate change on agriculture. Low tuber yield and nutritional quality as well as the plant susceptibility to biotic (anthracnose and viruses) and abiotic stresses are the major impediments to yam production worldwide [14]. Unfortunately, various biological constraints limit yam improvement [12–16]. It is necessary to overcome these problems to achieve successful breeding programs and accelerate genetic gains. Hereby, we report a practicable protocol for the long-term storage of pollen grains and the first successful development of hybrids of D. alata from lyophilized and long-term stored pollens.
Lyophilization is a valuable technique for long-term pollen preservation and has the key advantage that the lyophilized samples can be stored at room temperature without any sophisticated equipment. Pollen lyophilization has been used in many crops with genetic and biological constraints, such as Eucalyptus [32], maize [33,34], and pigeon pea [35]. However, the success rates vary according to the plant species. Many studies have reported in vitro germination of preserved pollens of yam using different techniques [20,23,24,42–44]. For instance, Mondo et al. [20] achieved 26.4%–59.7% in vitro germination in D. alata. Our study observed a 45% decrease in pollen germination from fresh pollens to 1-year stored pollens. Daniel et al. [44] also observed a sharp decrease in pollen germination of yam species after cryo-preservation. They recommended that wet-cold storage (hermetic cold storage without previous drying) is a superior method for long-term pollen viability compared to air-dried storage and freeze-drying. However, none of these studies used long-term stored pollens for pollination and hybrid development. As previously demonstrated, the decrease in pollen germination can be attributed to the storage conditions and storage time [45,46]. Mathad et al. [47] observed variable germination rates, fruit set, and seed development of cryo-preserved hot pepper pollens under different temperatures. Moreover, our results depicted significant differences between the two treatments used for lyophilization. Treatment 1 with 48 h drying appeared superior to Treatment 2 with 72 h drying. The low efficacy of T2 may be attributed to the reduced moisture content due to longer drying period [44]. Yam pollen is believed to be desiccation-sensitive [20], hence adding cryoprotectants during lyophilization can also be tested in order to improve the viability of the stored pollens.
A key objective of this study was achieved with the development of hybrids using long-term lyophilized pollens. Fruit and seed setting rates in tetraploid and diploid varieties using lyophilized pollens were almost similar but we observed that they were lower than fresh pollens. Likewise, a previous study reported higher fruit and seed setting rates using fresh pollens under field conditions [48]. It is well known that a successful fruit and seed setting is highly dependent on the genotype, environment and requires a precise timing [49]. Nonetheless, our results signify that the normal fruit setting and seed development are achievable using lyophilized pollens in yam breeding. Furthermore, no morphological abnormalities were observed in hybrids developed from lyophilized pollens, in accordance with observations in Eucalyptus [32], maize [33,34], and pigeon pea [35].
Seed setting occurred to a certain extent despite the decrease in pollen viability over time in our study, suggesting that lyophilization is a potential approach for long-term pollen storage to develop hybrids in yam breeding programs. With the availability of lyophilized pollen samples when female flowers become receptive, breeders can overcome the asynchronous flowering issue between male and female D. alata genotypes and make cross on a large quantity of female flowers. We would recommend a large quantity of lyophilized and stored pollens to increase the chances of obtaining enough offspring. Nonetheless, the number of hybrid plants generated from lyophilized pollens in this study was quite similar to those from fresh pollens.
The technique developed in this study will facilitate exchanges of pollen samples and increase the available gene pools among breeding programs in different countries and regions of the world. Pollen samples could be sent and stored at ambient temperature which is very useful for labs in developing countries. In future, more genotypes will be tested for pollen storage using the lyophilization approach with an improved protocol to achieve the maximum viability of stored pollens. We will test whether a reduction of the drying period during lyophilization will permit more viable pollen samples. Moreover, storing lyophilized pollen samples in fridge (~5°C) may also improve the viability. Importantly, the proposed protocol should be tested in another yam growing country to confirm the robustness of the protocol. Although this study was focused on D. alata, we expect that our protocol is applicable to other Dioscorea species since a high similarity of floral biology among yam species has been reported [21,49].
Acknowledgement: Not applicable.
Funding Statement: This research was financially supported by the AfricaYam Project (Grant OPP1052998-Bill and Melinda Gates Foundation).
Author Contributions: Conceptualization: Erick Malédon, Komivi Dossa, Hâna Chair. Experiment: Erick Malédon, Komivi Dossa, Elie Nudol, Christophe Perrot, Marie-claire Gravillon, Ronan Rivallan. Data acquisition, curation and formal analysis: Erick Malédon, Komivi Dossa, Elie Nudol, Christophe Perrot, Marie-Claire Gravillon, Ronan Rivallan, Denis Cornet, Hâna Chair. Funding acquisition: Denis Cornet, Komivi Dossa. Writing ± original draft: Erick Malédon, Komivi Dossa. Writing ± review & editing: Denis Cornet, Hâna Chair. All authors have read and approved the final version of this manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article and its supplementary information file.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/phyton.2023.042397.
References
1. Bakayoko, L., Pokou, D. N., Kouassi, A. B., Agre, P. A., Kouakou, A. M. et al. (2021). Diversity of water yam (Dioscorea alata L.) accessions from côte d’Ivoire based on SNP markers and agronomic traits. Plants, 10(12), 2562. [Google Scholar] [PubMed]
2. Ehounou, A. E., Kouakou, A. M., N’zi, J. C., Dibi, K. B. E., Bakayoko, Y. et al. (2019). Production of hybrid seeds by intraspecific crossing in yam Dioscorea alata L. International Journal of Science and Research, 8(9), 1212–1221. [Google Scholar]
3. Cormier, F., Lawac, F., Maledon, E., Gravillon, M. C., Nudol, E. et al. (2019). A reference high-density genetic map of greater yam (Dioscorea alata L.). Theoretical and Applied Genetics, 132(6), 1733–1744. [Google Scholar] [PubMed]
4. Zou, J., Xu, M., Wen, L., Yang, B. (2020). Structure and physicochemical properties of native starch and resistant starch in Chinese yam (Dioscorea opposita Thunb.). Carbohydrate Polymers, 237, 116188. [Google Scholar] [PubMed]
5. Agre, P. A., Dassou, A. G., Loko, L. E., Idossou, R., Dadonougbo, E. et al. (2021). Diversity of white guinea yam (Dioscorea rotundata Poir.) cultivars from Benin as revealed by agro-morphological traits and SNP markers. Plant Genetic Resources, 19(5), 437–446. [Google Scholar]
6. Sonia, N. (2020). Changes in secondary metabolites during growth and development of milk yam (Ipomoea digitata L.) tubers. Journal of Pharmacognosy and Phytochemistry, 9, 2118–2122. [Google Scholar]
7. Lebot, V., Malapa, R., Abraham, K., Molisalé, T., Van Kien, N. et al. (2018). Secondary metabolites content may clarify the traditional selection process of the greater yam cultivars (Dioscorea alata L.). Genetic Resources and Crop Evolution, 65(6), 1699–1709. [Google Scholar]
8. Vivek, S., Kumar, Y. A. S., Palanisamy, M. (2020). Assessment of secondary metabolites in the yams of Dioscorea oppositifolia L. & Dioscorea pentaphylla L. In: Phytomedicine, pp. 197–202. Boca Raton: CRC Press. [Google Scholar]
9. Friedmann, M., Asfaw, A., Anglin, N., Becerra, L., Bhattacharjee, R. et al. (2018). Genomics-assisted breeding in the CGIAR research program on roots, tubers and bananas (RTB). Agriculture, 8(7), 89. https://doi.org/10.3390/agriculture8070089 [Google Scholar] [CrossRef]
10. Sharif, B. M., Burgarella, C., Cormier, F., Mournet, P., Causse, S. et al. (2020). Genome-wide genotyping elucidates the geographical diversification and dispersal of the polyploid and clonally propagated yam (Dioscorea alata). Annals of Botany, 126(6), 1029–1038. [Google Scholar] [PubMed]
11. Arnau, G., Bhattacharjee, R., Sheela, M. N., Chair, H., Malapa, R. et al. (2017). Understanding the genetic diversity and population structure of yam (Dioscorea alata L.) using microsatellite markers. PLoS One, 12(3), e0174150. [Google Scholar] [PubMed]
12. Umber, M., Filloux, D., Gélabale, S., Gomez, R. M., Marais, A. et al. (2020). Molecular viral diagnosis and sanitation of yam genetic resources: Implications for safe yam germplasm exchange. Viruses, 12(10), 1101. https://doi.org/10.3390/v12101101 [Google Scholar] [PubMed] [CrossRef]
13. Diouf, M. B., Festus, R., Silva, G., Guyader, S., Umber, M. et al. (2022). Viruses of yams (Dioscorea spp.Current gaps in knowledge and future research directions to improve disease management. Viruses, 14(9), 1884. https://doi.org/10.3390/v14091884 [Google Scholar] [PubMed] [CrossRef]
14. Darkwa, K., Olasanmi, B., Asiedu, R., Asfaw, A. (2020). Review of empirical and emerging breeding methods and tools for yam (Dioscorea spp.) improvement: Status and prospects. Plant Breeding, 139(3), 474–497. https://doi.org/10.1111/pbr.12783 [Google Scholar] [CrossRef]
15. Price, E. J., Bhattacharjee, R., Lopez-Montes, A., Fraser, P. D. (2017). Metabolite profiling of yam (Dioscorea spp.) accessions for use in crop improvement programmes. Metabolomics, 13(11), 1–12. [Google Scholar]
16. Abraham, K., Nair, P. G. (1991). Polyploidy and sterility in relation to sex in Dioscorea alata L. (Dioscoreaceae). Genetica, 83(2), 93–97. [Google Scholar]
17. Némorin, A., David, J., Maledon, E., Nudol, E., Dalon, J. et al. (2013). Microsatellite and flow cytometry analysis to help understand the origin of Dioscorea alata polyploids. Annals of Botany, 112(5), 811–819. [Google Scholar]
18. Malapa, R., Arnau, G., Noyer, J. L., Lebot, V. (2005). Genetic diversity of the greater yam (Dioscorea alata L.) and relatedness to D. nummularia Lam. and D. transversa Br. as revealed with AFLP markers. Genetic Resources and Crop Evolution, 52, 919–929. [Google Scholar]
19. Cormier, F., Martin, G., Vignes, H., Lachman, L., Cornet, D. et al. (2021). Genetic control of flowering in greater yam (Dioscorea alata L.). BMC Plant Biology, 21(1), 163. [Google Scholar] [PubMed]
20. Mondo, J. M., Agre, P. A., Asiedu, R., Akoroda, M. O., Asfaw, A. (2021). Optimized protocol for in vitro pollen germination in yam (Dioscorea spp.). Plants, 10(4), 795. [Google Scholar] [PubMed]
21. Mondo, J. M., Agre, P. A., Edemodu, A., Adebola, P., Asiedu, R. et al. (2020). Floral biology and pollination efficiency in yam (Dioscorea spp.). Agriculture, 10(11), 560. [Google Scholar]
22. Ojuederie, O., Balogun, M., Abberton, M. (2016). Mechanism for pollination in African yam bean. African Crop Science Journal, 24(4), 405–416. [Google Scholar]
23. Daniel, I., Ng, N., Tayo, T., Togun, A. (2002). Wet-cold preservation of West African yam (Dioscorea spp.) pollen. The Journal of Agricultural Science, 138(1), 57–62. [Google Scholar]
24. Ng, N. Q., Daniel, I. (2000). Storage of pollens for long-term conservation of yam genetic resources. In: Englemann, F., Tagaki, H. (Eds.Cryopreservation of tropical plant germplasm: Current research progress and application, pp. 136–139. Rome, Italy: JIRCAS/IPGRI. [Google Scholar]
25. Volk, G. M. (2011). Collecting pollen for genetic resources conservation. In: Collecting plant genetic diversity: Technical guidelines, pp. 1–10. [Google Scholar]
26. Hawkes, J., Maxted, N., Ford-Lloyd, B. (2000). Field gene banks, botanic gardens, in vitro, DNA and pollen conservation. In: The ex situ conservation of plant genetic resource, pp. 92–107. Berlin: Springer. [Google Scholar]
27. Towill, L. E., Walters, C. (2000). Cryopreservation of pollen. In: Cryopreservation of tropical plant germplasm, pp. 115–129. [Google Scholar]
28. Panis, B., Lambardi, M. (2005). Status of cryopreservation technologies in plants (crops and forest trees). The Role of Biotechnology, 5, 43–54. [Google Scholar]
29. Reed, B. M. (2001). Implementing cryogenic storage of clonally propagated plants. Cryoletters, 22, 97–104. [Google Scholar] [PubMed]
30. Costantino, H. R., Pikal, M. J. (2004). Lyophilization of biopharmaceuticals, vol. 2, pp. 1–24. Berlin: Springer Science & Business Media. [Google Scholar]
31. Sanchez, R., Smeltzer, D. (1965). Sorghum pollen viability 1. Crop Science, 5(2), 111–113. [Google Scholar]
32. Girijashankar, V. (2010). Effect of Eucalyptus pollen isolation methods on pollen viability, debris content, quantity isolated and pollen density per stigma. Journal of Plant Breeding and Crop Science, 2, 273–279. [Google Scholar]
33. Fearing, P. L., Brown, D., Vlachos, D., Meghji, M., Privalle, L. (1997). Quantitative analysis of CryIA(b) expression in Bt maize plants, tissues, and silage and stability of expression over successive generations. Molecular Breeding, 3(3), 169–176. [Google Scholar]
34. Meissle, M., Zünd, J., Waldburger, M., Romeis, J. (2014). Development of Chrysoperla carnea (Stephens)(Neuroptera: Chrysopidae) on pollen from Bt-transgenic and conventional maize. Scientific Reports, 4(1), 1–9. [Google Scholar]
35. Mallikarjuna, N., Saxena, K. (2002). Production of hybrids between Cajanus acutifolius and C. cajan. Euphytica, 124(1), 107–110. [Google Scholar]
36. Abraham, K., Nair, P. G. (1990). Floral biology and artificial pollination in Dioscorea alata L. Euphytica, 48(1), 45–51. [Google Scholar]
37. de Jesus, L., Silva, R. N. O., da Costa Gomes, M. F., dos Santos Valente, S. E., Gomes, R. L. F. et al. (2018). Efficiency of colorimetric tests to determine pollen viability in peppers. Revista Brasileira de Agropecuária Sustentável, 8(2), 77–82. [Google Scholar]
38. Brewbaker, J. L., Kwack, B. H. (1963). The essential role of calcium ion in pollen germination and pollen tube growth. American Journal of Botany, 50(9), 859–865. [Google Scholar]
39. Te May Ching, K. K. C. (1964). Freeze-drying pine pollen. Plant Physiology, 39(5), 705–709. [Google Scholar]
40. Cummings, B., Wood, T. (1989). A simple and efficient method for isolating genomic DNA from endomycorrhizal spores. Gene Analysis Techniques, 6(5), 89–92. [Google Scholar] [PubMed]
41. Frossard, E., Aighewi, B. A., Aké, S., Barjolle, D., Baumann, P. et al. (2017). The challenge of improving soil fertility in yam cropping systems of West Africa. Frontiers in Plant Science, 8, 1–8. [Google Scholar]
42. Balogun, M. O. (2009). Microtubers in yam germplasm conservation and propagation: The status, the prospects and the constraints. Biotechnology and Molecular Biology Reviews, 4, 1–10. [Google Scholar]
43. Balogun, M., Gueye, B. (2013). Status and prospects of biotechnology applications to conservation, propagation and genetic improvement of yam. In: Ramawal, K. G., Merillon, J. M. (Eds.Bulbous plants: Biotechnology, pp. 92–112. Florida, USA: CRC Press. [Google Scholar]
44. Daniel, I. (2011). Exploring storage protocols for yam (Dioscorea spp.) pollen genebanking. African Journal of Biotechnology, 10(42), 8306–8311. [Google Scholar]
45. Luza, J. G., Polito, V. S. (1985). In vitro germination and storage of English walnut pollen. Scientia Horticulturae, 27(3–4), 303–316. [Google Scholar]
46. Quan, Q. M., Li, Y. X. (2012). A method for mid-term storage of Epimedium pubescens (Berberidaceae) pollen. Pakistan Journal of Botany, 44, 765–768. [Google Scholar]
47. Mathad, R. C., Patil, S., Lokesh, G., Savegowda, B. (2015). Pollen cryo-preservation for hybrid seed production in hot pepper. Seed Research, 43, 53–58. [Google Scholar]
48. Lebot, V., Abraham, K., Kaoh, J., Rogers, C., Molisalé, T. (2019). Development of anthracnose resistant hybrids of the greater yam (Dioscorea alata L.) and interspecific hybrids with D. nummularia Lam. Genetic Resources and Crop Evolution, 66(4), 871–883. [Google Scholar]
49. Mondo, J. M., Agre, P. A., Asiedu, R., Akoroda, M. O., Asfaw, A. (2022). Optimum time for hand pollination in yam (Dioscorea spp.). PLoS One, 17(8), e0269670. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools