

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020629
ARTICLE
Rice (Oryza sativa L.) Breeding among Hassawi Landrace and Egyptian Genotypes for Stem Borer (Chilo agamemnon Bles.) Resistance and Related Quantitative Traits
1Department of Agricultural Biotechnology, College of Agriculture and Food Sciences, King Faisal University, Al-Ahsa, 31982, Saudi Arabia
2Rice Research and Training Center, Field Crops Research Institute, Agricultural Research Center, Kafrelsheikh, 33717, Egypt
3Central Laboratories, King Faisal University, Al-Ahsa, 31982, Saudi Arabia
4Department of Virus and Phytoplasma, Plant Pathology Institute, Agricultural Research Center (ARC), Giza, 12619, Egypt
5Institute of Plant Protection, University of Agriculture, Multan, 66000, Pakistan
*Corresponding Author: Jameel M. Al-Khayri. Email: jkhayri@kfu.edu.sa
Received: 03 December 2021; Accepted: 14 February 2022
Abstract: Rice stem borer (Chilo agamemnon Bles.) is a primary insect pest of rice and is a major limiting factor to rice production. Breeding for insect-resistant crop varieties has been an economic way of integrated pest management (IPM) as it offers a viable and ecologically acceptable approach. This study was aimed to evaluate rice genotypes for their resistance against rice stem borer. Seven parental genotypes with twenty one F1 crosses were evaluated for genotypic variation in field experiments. Analysis of variance revealed significant differences for the studied traits in almost all crosses and parents. In addition, the mean squares of parents versus their crosses were significant for stem borer resistance and other associated traits. Moreover, both general combining ability (GCA) and specific combining ability (SCA) variances were highly significant for all characters studied in the F1 generation. Based on GCA, 4 genotypes (Sakha101, Gz6903-3-4-2-1, Gz9577-4-1-1 and Hassawi) exhibited highly significant negative values for stem borer resistance (–0.53, –1.06, –0.18 and –0.49, respectively) indicating they are the best combiners for stem borer resistance. Based on SCA analysis, nine cross combinations showed highly significant negative effects for stem borer resistance. Similarly, the cross Giza178×Hassawi was the best combination with significantly highest value for early maturity. In addition, seven crosses showed highly significant negative SCA for plant height trait. On the other hand, for panicle length, number of primary branches/panicle, panicle weight and 1000-grain weight, seven, four, eight and six crosses showed highly significant positive SCA, respectively. The result further revealed that the non-additive dominance genetic variance was higher than the additive variance for all evaluated traits indicating that non-additive genetic variances have a role in their inheritance. The broad-sense heritability estimates were high for all the studied traits. The stem borer resistance was significantly correlated with panicle weight and 1000-grain weight, which also showed a highly significant correlation with grain yield/plant. Thus these traits can be effectively employed in a breeding program to confer resistance against stem borer infestation in rice. It was further supported by biplot analysis, which clustered these potentially important traits into two quadrants showing their importance in any future breeding program to control stem borer infestation. This study has contributed valuable information for evaluation of genetic diversity in the local rice germplasm and its utilization in futuristic rice genetic improvement programs.
Keywords: Rice stem borer (Chilo agamemnon Bles.); general combining ability; genetic parameters; correlation coefficient; principle component analysis
Rice (Oryza sativa L.) is the primary source of nourishment to nearly half of the global population [1]. Thus, it is critical to improve rice production to ensure sustainability to meet the market demand [2]. Expanding production acreage and monitoring yield losses due to biotic stresses can contribute to meet the global rice demand. Insect pests undoubtedly pose a major challenge to meet the yield potential of various rice cultivars. Of the 52% yield losses due to biotic stresses, insect pests alone inflect 25% reduction in global rice production [3].
Stem borers are principle insect pests of rice crop causing considerable yield losses of about 70% to rice cultivation in some regions [4]. Stem borers ubiquitously infest all types of rice agro-ecological systems and comprise 50 well-known species grouped into three families Pyralidae, Noctuidae (Lepidoptera) and Diopsidae (Diptera) [5]. Rice stem borer (Chilo agamemnon Bles.) belongs to the Lepidoptera group and is a key pest of rice, which have high visual impact on rice plants producing white head panicles with empty grains [6]. It mostly produces destructive effects on terminal shoots by boring holes in the stems through leaf sheath at leaf nodes. During stem borer attack, the young tillering rice plants start to redirect the resources to produce new tillers or to increase the size and number of grains [7]. This causes a delay in grain maturity and ultimately a reduction in biomass, which directly affect the yield and quality of rice grains [8]. Over the past 50 years, a common practice among farmers to reduce the stem borer infestation has been spraying expensive and hazardous chemical insecticides; therefore the situation necessitates the development of environmentally sustainable practices and management strategies to control stem borers in rice [9].
There have been a trending decline on research to find the conventional resistance sources in host plants against insects [10]. Generation of insect-resistant crop cultivars is a viable and eco-friendly integrated pest management (IPM) approach [9]. Because stem borers are polyphagous in nature, it is a challenging task to identify the genes governing host-mediated resistance in rice [11]. Nevertheless, the development of transgenic rice plants transformed with Bacillus thurengenesis (Bt) or new toxins against insect pests has received breeders interest [10]. However, transgenic present additional challenges associated with development, deployment and public concerns [12]. Thus, utilization of native resistance sources against stem borer infestation demands attention, particularly in Asian countries where transgenic crops are not much popular. A primary objective of a rice breeding program is to incorporate insect resistance trait into elite rice cultivars by exploring the insect-resistant native rice germplasm [13]. Resistance, tolerance and vulnerability of the host plant directly influence an effective host plant-insect interaction. Some studies recommended tolerance to stem borer as a criterion for selection of rice varieties [7]. Others consider vulnerability as the selection criterion [5,14].
So far, no resistance genes in rice plants have been reported to exclusively control stem borers infestation [15,16]. Nevertheless, over expression of the mitogen-activated protein kinases 4 (OsMPK4) gene or the endogenous microRNA from the stem borer [16] has been found to positively regulate resistance against rice stem borer; however, it also impairs plant growth [17]. Moreover, few studies are available to control stem borer through conventional breeding due to the lack of identifying resistance sources in cultivated rice, laborious varietal screening protocols and the inherently complex genetics of resistance [9]. The rice parents (Sakha101, Giza175, Gz6903-3-4-2-1 and GZ 9577-4-1-1) were produced at Rice Research & Training Center, Egypt (RRTC) through conventional breeding and have been used as a donor source to transfer the stem borer resistance character in various breeding programs in Egypt [17]. Thus, understanding the genetic background of the resistance in the rice germplasm would contribute to developing effective approaches against stem borer infestation. This would be facilitated by exploiting the knowledge gained from studies related to general combining ability (GCA) among genotypes and specific combining ability (SCA) among their testcrosses, heterosis and heterotic orientation.
Estimation of correlations among morphological and genotypic traits are often useful to determine positive or negative effects of different components on the trait under study. The knowledge of combining ability, type of gene action controlling economic traits and heterosis is useful in fixing the appropriate parent lines, and in designing successful crosses [18]. The half diallel crossing mating design provides reliable information on the general and specific combining ability effects of parents and their cross combinations [19] and has been effectively applied in various previous quantitative genetic investigations in maize [20]. Besides, understating the genetic diversity and variability in agro-morphological traits in the local germplasm is critical to retrieve their genotypic information. Principle component analysis (PCA) is an effective approach to analyze the genetic diversity in a crop population [21]. The biplots generated in PCA contains critical information, which is helpful in explaining the relationship of the experimental groups and their associated variables. It is thus an effective tool to generate reliable information during parental selection for a breeding program [22]. Consequently, the objectives of this study were to investigate GCA and SCA, estimate genetic parameters and inheritance of some quantitative characters in the F1 generation in some rice hybrids, select the suitable combinations for resistance to rice stem borer, in addition, to studying the inheritance of rice stem borer infestation.
This study was carried out during the 2019 and 2020 summer seasons at the Department of Agricultural Biotechnology, College of Agriculture and Food Sciences, King Faisal University, in cooperation with the experimental farm of the Rice Research and Training Center, Sakha, Kafrelsheikh, Egypt to study the magnitude of combining abilities and genetic parameters in some rice genotypes (Table 1).
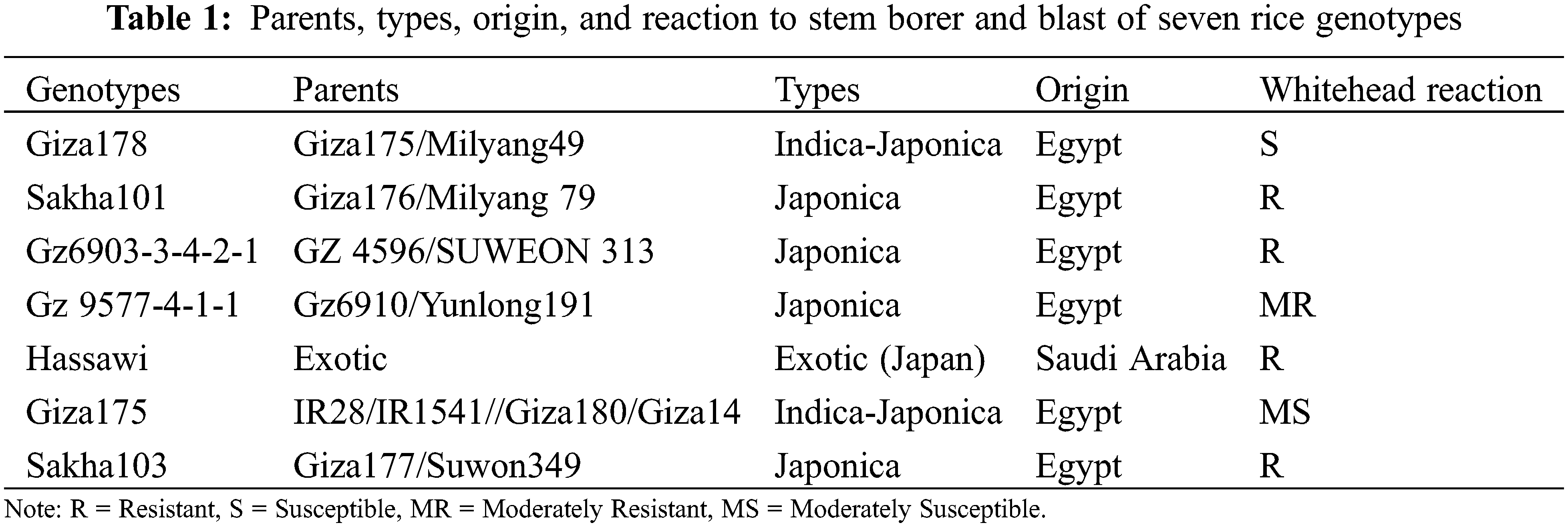
The experimental materials used in this experiment consisted of seven parental genotypes, four of which were resistant (Sakha101, Gz6903-3-4-2-1, Sakha103 and Hassawi), one (Gz9577-4-1-1) was moderately resistant, one was susceptible (Giza178) and one (Giza175) was moderately susceptible, respectively (Table 1, Fig. 1). All these varieties were cultivated varieties except Gz6903-3-4-2-1 and Gz9577-4-1-1, which were promising lines. In addition, crosses were made among those genotypes through half diallel to produce 21 F1 crosses, which were included as experimental materials in this study.
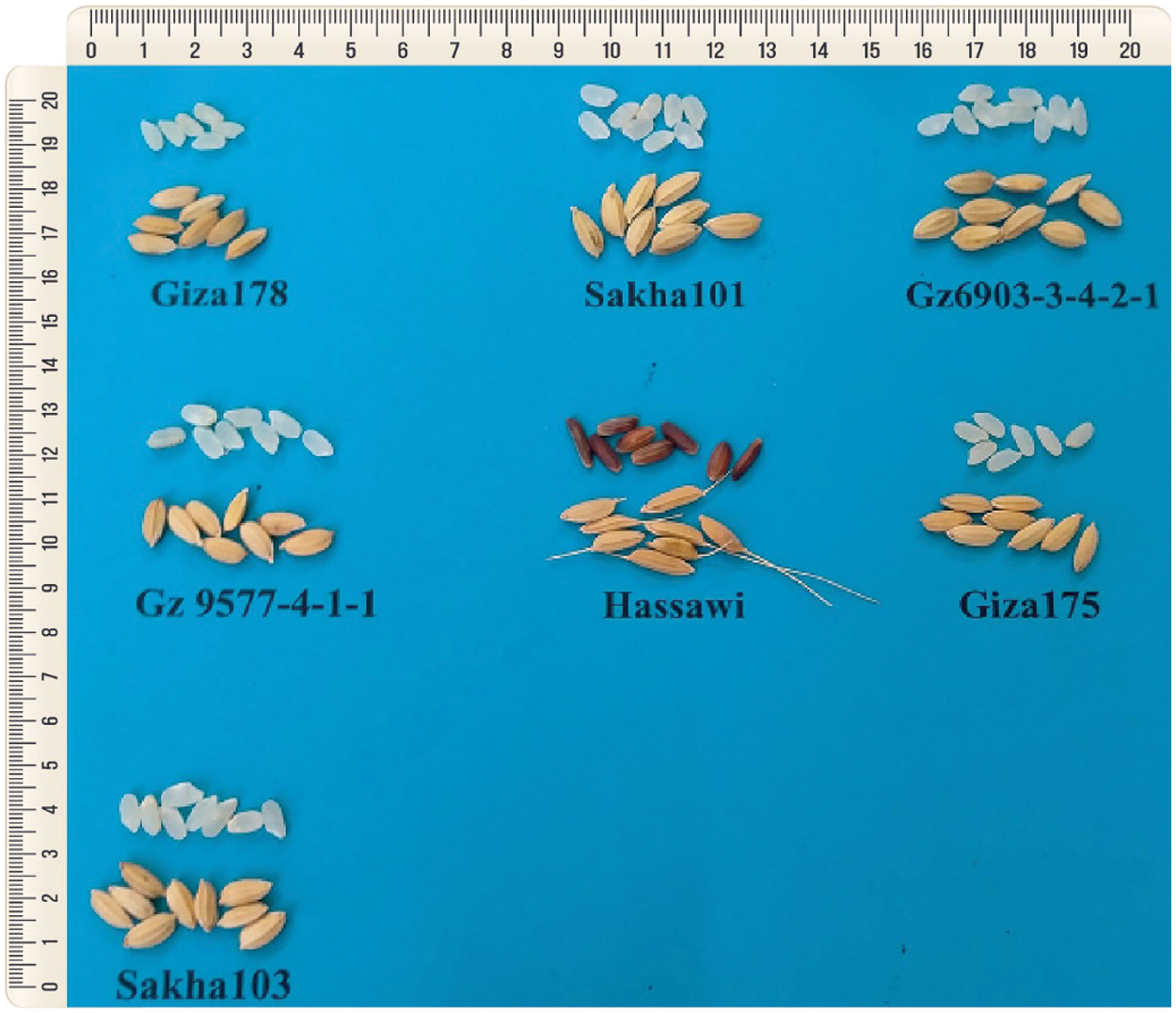
Figure 1: Seeds from seven parental rice genotypes including four cultivated varieties (Giza178, Sakha 101, Giza 175, and Sakha 103) and two promising lines (Gz6903-3-4-2-1 and Gz9577-4-1-1) from Egypt and a Hassawi rice variety cultivated in the Eastern region of Saudi Arabia
In 2019, seven parental genotypes were sown in the summer season in three sowing dates at 15 days intervals to overcome the difference of heading date among the parental varieties. Thirty days after sowing, the seedlings of the parents were transplanted to the experimental field in three rows, of five meters long and 20 × 20 cm apart between plants and rows. A half diallel cross was conducted among the seven parents in the year 2019 to produce 21 crosses. A hot water method for emasculation was utilized for the subsequent hybridization [23]. The parental varieties and the resulting 21 crosses were evaluated and arranged in a randomized complete block design (RCBD) experiment with three replications in the year 2020.
One hundred plants were collected at the maturity stage to calculate the damage of white head percentage. The standard evaluation system of Rice Research and Training Center (RRTC), Sakha, Egypt was followed as resistant (R) = 0%–3%, moderately resistant (MR) = 3%–6%, moderately susceptible (MS) = 6%–9%, susceptible (S) = 9%–12% and highly susceptible (HS) = 12%, respectively. On the other hand, the important agronomic traits such as days to maturity, plant height (cm), 1000-grain weight (g), and number of primary branches ∕panicle were also studied.
The data were statistically analyzed using two-way analysis of variance (ANOVA) for a randomized complete block design as suggested by Panse et al. [24] and the analysis of variance for crossing followed the design of Kempthorne [25]. Whereas, GCA and SCA were analyzed in accordance with the procedure suggested by Kempthorne [25]. Correlation coefficients (r) among all studied traits were computed according to Gomez et al. [26]. The means were separated using least significant difference (LSD) according to the formula suggested by Wynne et al. [27] at P < 0.05 (significant) and P < 0.001 (highly significant). The PCA was performed using XLSTAT v19.1 software (Addinsoft, Paris, France).
3.1 Stem Borer Infestation Percentage
Resistance level of the tested lines is shown in Table 2 and some of the selected parents and their crosses are shown in Fig. 2. Among the parental genotypes, Hassawi produced the least white heads (2.57) and showed highly resistant phenotypes whereas, Giz178 produced the highest white heads (11.36) and can be considered as highly susceptible parent. All other parents were resistant to susceptible with white heads ranging from 3.13 to 7.00, respectively. Among the crosses, Gz9577×Sakha103 was highly resistant with the lowest average white heads (2.57). Whereas, the cross Giza178×Hassawi produced the highest average white heads (5.10) among all the crosses, showing moderate resistance. Nonetheless, twelve crosses were resistant and nine crosses were moderately resistant indicating that the resistance was conferred by dominant genes and inherited in the F1 generation as a dominant trait.
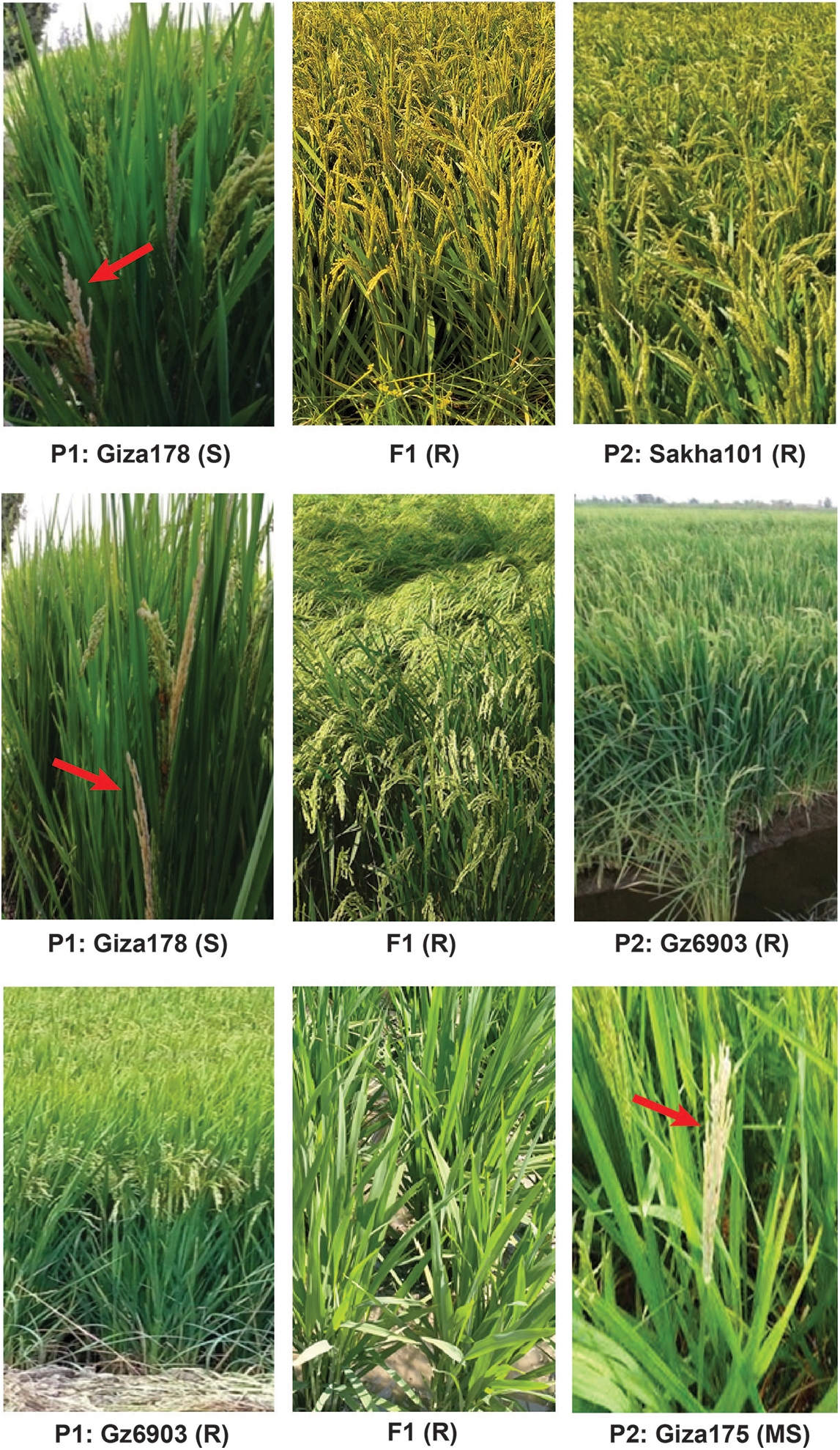
Figure 2: Rice plants showing selected parents and their F1 crosses. The F1 hybrids produced by crossing between Sakha101 (R)×Giza178 (S), Giza178 (S)×Gz6903 (R) and Gz6903 (R)×Giza175 (MS) showed resistance against rice stem borer attack. The susceptible genotypes were showing white heads (indicated with red arrows) while, the resistant genotypes did not produce white head phenotypes
3.2 Mean Performance of Quantitative Traits
The mean performance revealed that Hassawi is a late maturing genotype with 154 days to maturity, while Sakha103 and Gz9577-4-1-1 are early maturing genotypes with 123 and 128 days to maturity, respectively (Table 2). Among the crosses, the Sakha101×Hassawi was a late maturing genotype with 144.33 days to maturity and Gz9577×Sakha103 was an early maturing genotype with 124.67 days to maturity, respectively. The plant height of Hassawi Type-1 was significantly higher (137 cm) than that of all other parents. Among the crosses, the significantly highest plant height (115 cm) was observed for Sakha101×Giza175. The plant heights of Sakha101 × Giza175, Sakha101 × Hassawi, Giza178×Sakha103, Giza178×Giza175, and Giza175 × Sakha103 were also significantly increased ranging 111–115 cm, respectively. The lowest plant height (96.67 cm) was observed for Gz6903×Hassawi. The 1000-grain weight of the parental genotype Sakha101 was the highest (27.30 g) whereas, Giza178 produced the lowest (21.10 g) 1000-grain weight among the parents. The 1000-grain weight of Sakha101×Gz6903 was the highest (29.77g) as compared to other crosses and parental genotypes. Among parents, the parental genotype Giza175 produced the highest number of primary branches/panicle (12.67). Comparatively, the number of primary branches/panicle were higher in crosses as compared to their parents. Among crosses, the cross Gz9577×Hassawi produced the highest number of primary branches/panicle (13), while the lowest number of branches (8.67) was observed in the cross Giza178×GZ6903, respectively. The parental genotype Sakha101 produced the highest (4.08 g) and Giza178 produced the lowest panicle weight (3.10 g), respectively, as compared to other parental genotypes. Among crosses, Sakha101×Hassawi produced the highest panicle weight (5.33 g), while the lowest panicle weight was recorded for Giza178×Giza175 (3.19 g), respectively. Finally, among the parents, Gz9577-4-1-1 produced the highest grain yield/plant (46.49 g). Meanwhile, a significant increase in grain yield/plant was recorded for most of the crosses as compared to the parental genotypes. The highest grain yield/plant (50.70 g) was recorded for Sakha101×Giza175 and lowest (39.37 g) for Giza178×Sakha103 and Hassawi×Giza175, respectively.
3.3 Estimation of Genetic Components
Estimation of genetic components was performed by analysis of variance (ANOVA) to test the difference among parents and their crosses for all studied traits (Table 3). The data revealed that the differences among parents were highly significant for all traits indicating the presence of wide genetic variability among parents (Table 3). Moreover, both the GCA and SCA variances were highly significant for all characters studied in the F1 generation indicating the presence of additive variation and non-additive gene effects in the germplasm. The GCA/SCA ratio in F1 was found to be greater than unity for all the traits indicating that additive and additive×additive types of gene action were of greater importance in the inheritance of these traits.
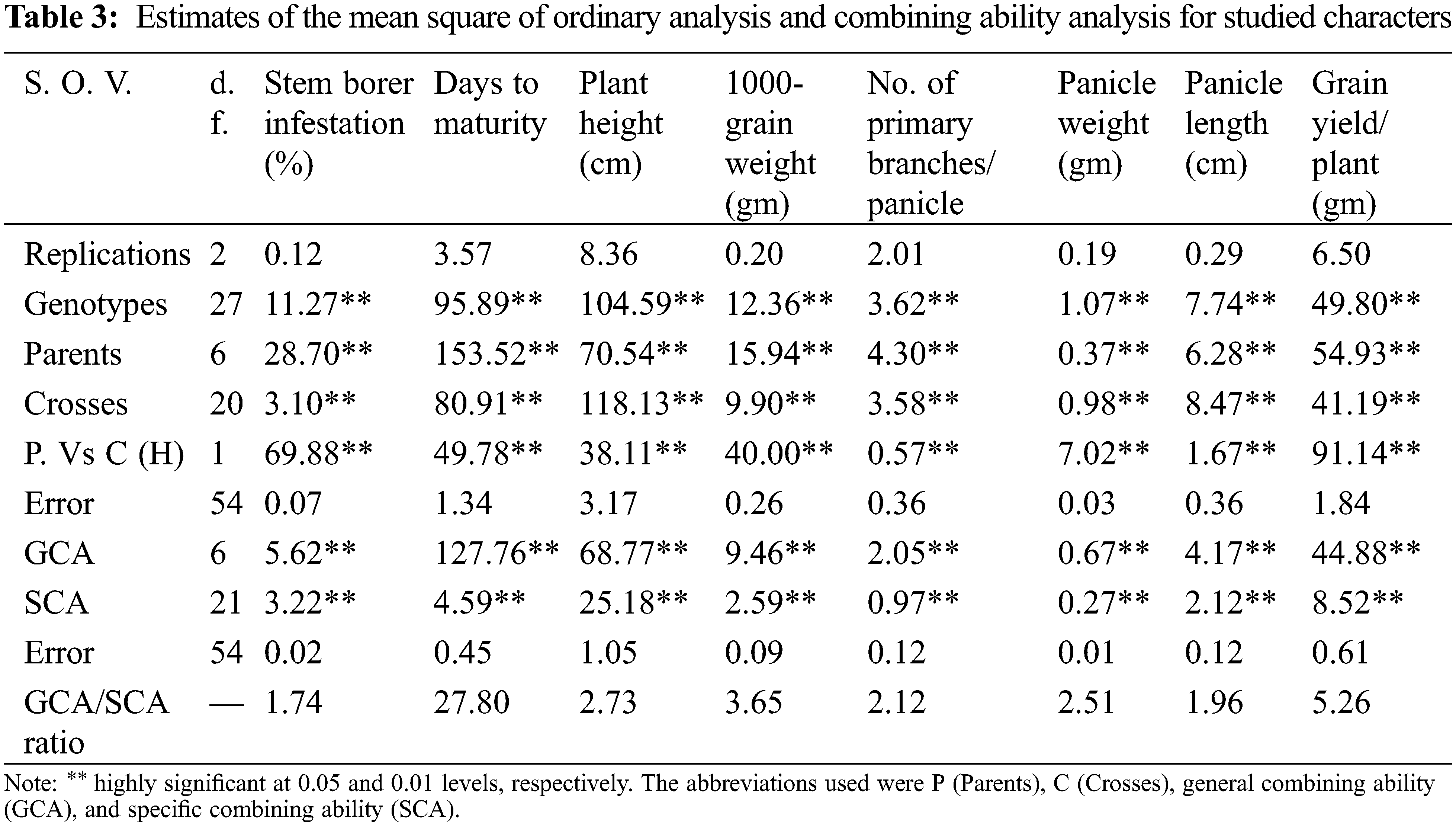
3.4 General Combining Ability Effects
Estimates of GCA effects of parents are presented in Table 4. The results showed that four rice genotypes namely; Gz6903-3-4-2-1, Sakha 101, Hassawi and Gz9577-4-1-1 showed highly significant negative values for stem borer (i.e., –1.06, –0.53, –0.49 and –0.18, respectively). It indicates that these genotypes were the best combiners for stem borer traits. For days to maturity Sakha103, Gz9577-4-1-1 and Giza175 were the best combiners for the early maturity trait with highly significant negative values –5.23, –3.42 and –1.46, respectively. Meanwhile, Sakha101 with highly significant positive value (5.32) represents the best combiner for the late maturity trait. For plant height, the genotypes Gz9577-4-1-1, Gz6903-3-4-2-1 and Sakha101 showed highly significant negative values –3.42, –3.28, and –1.05, respectively.
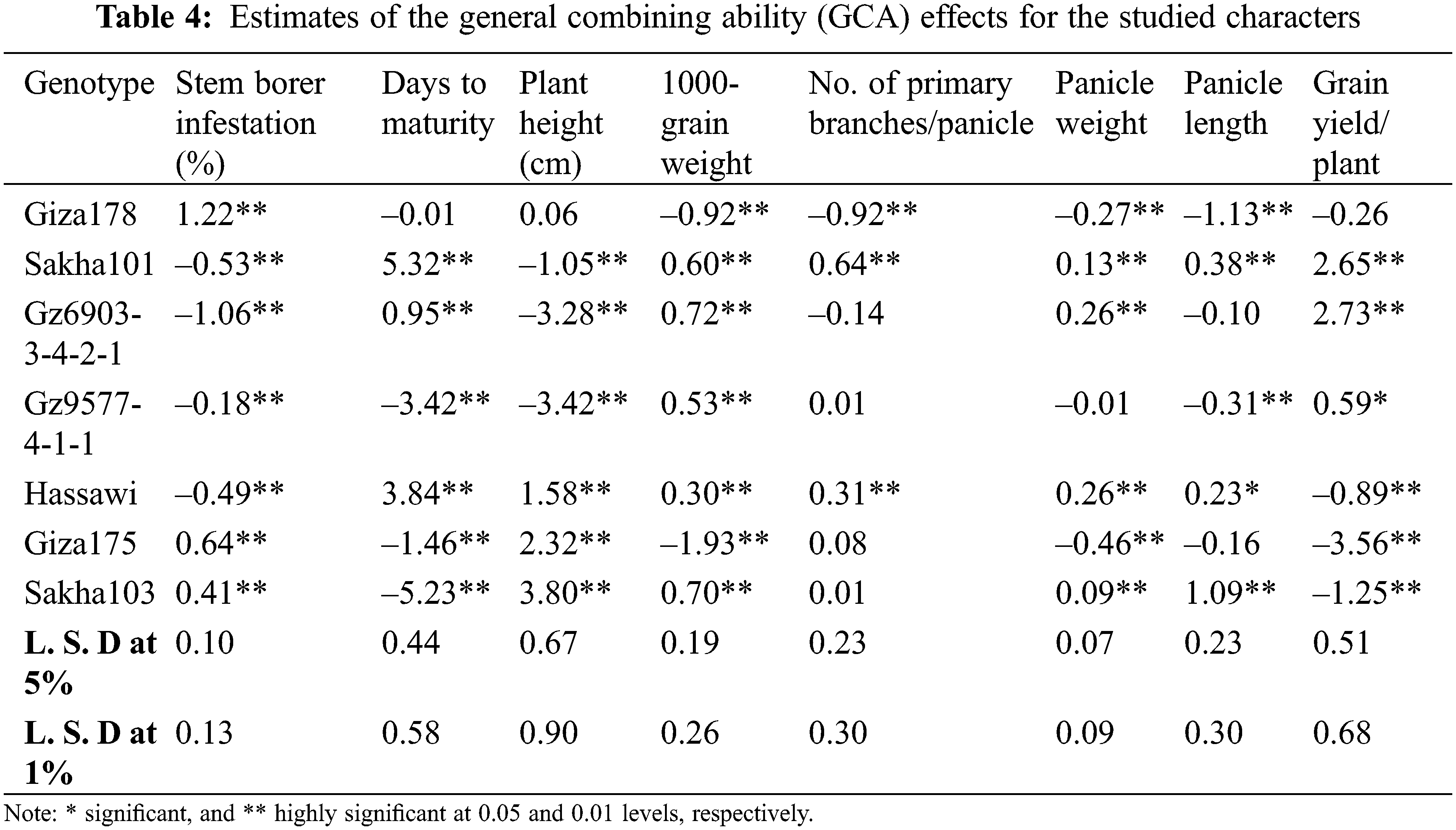
For 1000-grain weight Gz6903-3-4-2-1, Sakha103, Sakha101, Gz9577-4-1-1 and Hassawi showed significant positive GCA effects (0.72, 0.70, 0.60, 0.53 and 0.30, respectively) indicating that these parental genotypes are good combiners for 1000-grain weight trait. For number of primary branches/panicle Sakha101 and Hassawi showed highly significant positive GCA effects as 0.64 and 0.31, proving to be the best general combiners for this trait. Meanwhile, Hassawi, Gz6903-3-4-2-1, Sakha101 and Sakha103 showed highly significant positive GCA values (i.e., 0.26, 0.26, 0.13 and 0.09, respectively) for panicle weight and Hassawi was the best parental line in any breeding program for panicle weight improvement. Whereas, for panicle length three parental genotypes (Sakha103, Sakha101 and Hassawi Type-1) showed highly significant positive GCAs (i.e., 1.09, 0.38 and 0.23, respectively). Thus, Sakha103 represented the best general combiner for panicle length among all parents. Meanwhile, Gz6903-3-4-2-1 and Sakha101 were the best general combiners for grain yield/plant with highly significant positive GCAs (i.e., 2.73 and 2.65, respectively) followed by Gz9577-4-1-1 showing a significant positive GCA (0.59) as compared to all other parental genotypes, respectively (Table 4).
3.5 Specific Combining Ability Effects
Twenty-one rice F1 crosses were evaluated for SCA effects (Table 5). Nine out of the twenty-one cross combinations showed highly significant negative SCA effects for stem borer ranging from −3.44 for Giza178×Giza175 to −0.41 for Gz6903×Sakha103. In addition, five crosses showed highly significant negative SCA and two crosses showed significant negative SCA effects for days to maturity trait (Table 5). The best combination for early maturing trait was the cross Giza178×Hassawi with a highly significant negative value of −3.40. Meanwhile, Sakha101×Sakha103 showed a highly significant positive value 4.14 representing a late maturity genotype. Similarly, for the plant height trait eight crosses showed highly significant negative SCAs while, seven crosses showed highly significant positive SCA effects, respectively. The cross Gz6903×Hassawi showed the highest negative SCA effect (−7.40) and the cross Gz9577×Hassawi showed the highest positive SCA effect (10.43) for plant height.
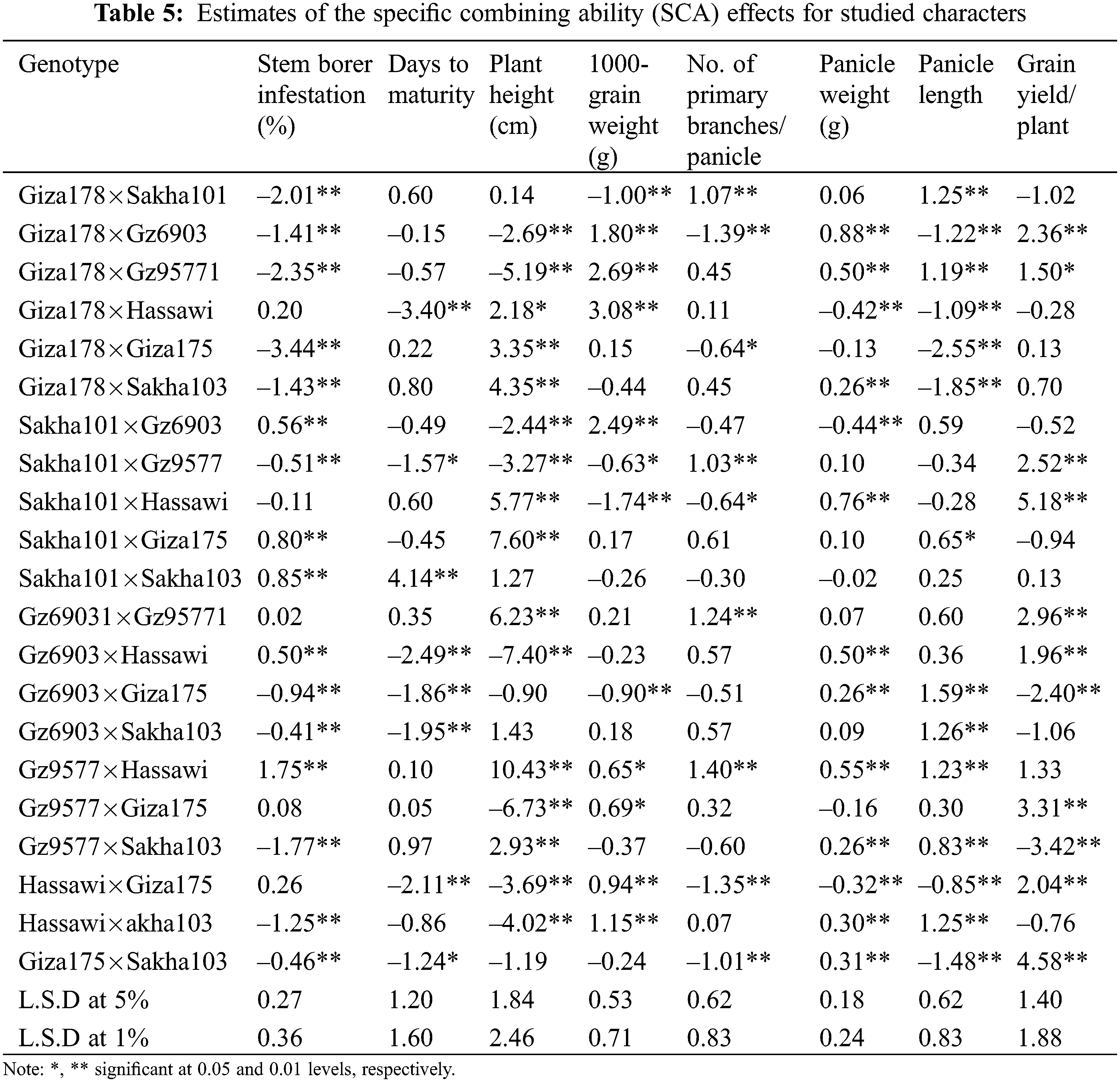
In the cases of panicle length, number of primary branches/panicle, panicle weight and 1000-grain weight, a positive SCA selection is desirable. For 1000-grain weight, six crosses showed highly significant positive SCA ranging 0.94–3.08 with Giza178×Hassawi showing the highest positive SCA (3.08) among all combinations. Four crosses showed highly significant positive SCA effects for the number of primary branches/panicle ranging 1.03–1.40 with the best cross Gz9577×Hassawi showing the highest (1.40) SCA effect. For panicle weight, eight crosses showed highly significant positive SCA effects ranging 0.26–0.88 with Giza178×Gz6903 showing the highest positive SCA (0.88) among all combinations. Meanwhile, seven crosses showed highly significant positive SCA between 0.83–1.59 for the panicle length trait and the cross Gz6903×Giza175 showed the highest positive SCA (1.59) among all combinations. Likewise other yield related traits, eight crosses showed highly significant SCA for grain yield/plant ranging 1.96-5.18 with the cross Sakha101×Hassawi showing the highest SCA (5.18) among all crosses (Table 5).
3.6 Estimates of Genetic Parameters
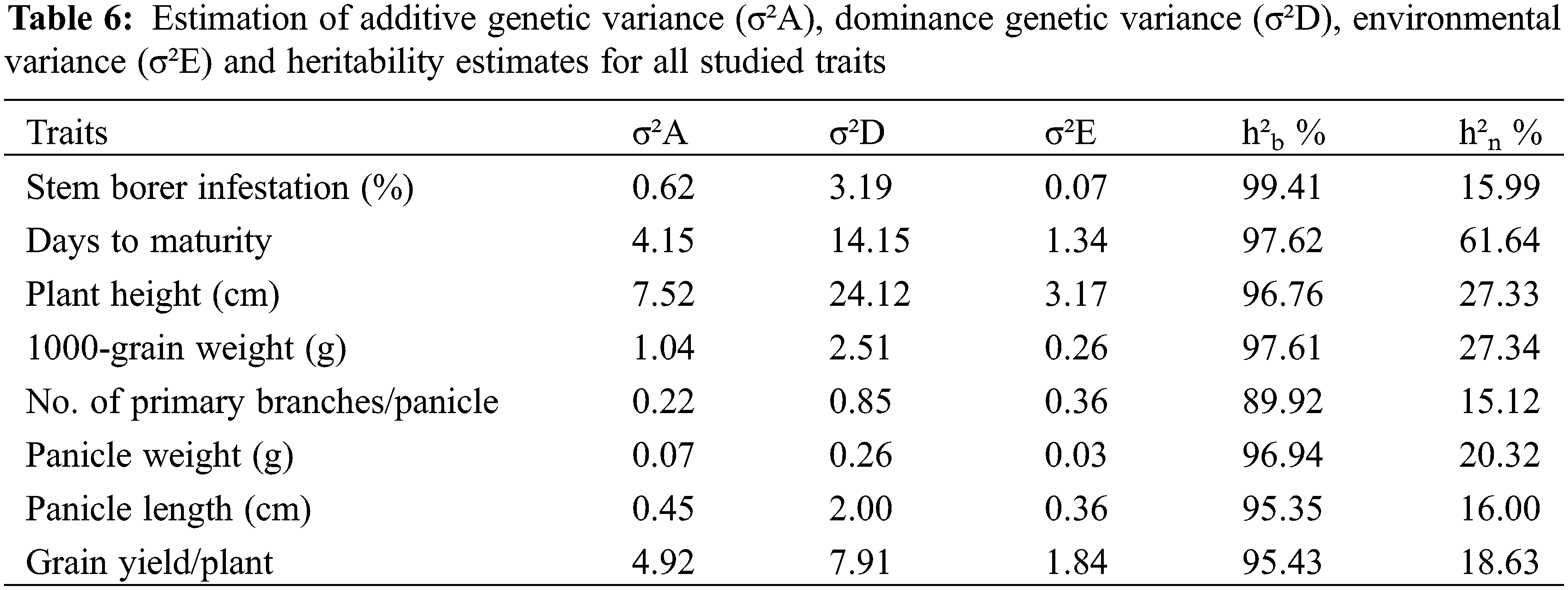
Estimation of genetic components viz. additive, non-additive or dominance genetic variance, as well as broad-sense heritability values are presented in Table 6. The result revealed that the non-additive dominance genetic variance was higher than additive variance for all traits, which indicated a major role of non-additive genetic variances in their inheritance. The broad-sense heritability (h²b %) and narrow-sense heritability (h²n %) values were also estimated for all the studied traits. Our results showed that the estimated values for broad-sense heritability were comparatively higher than the narrow-sense heritability for all the traits. The broad-sense heritability were ranged between 89.92 for primary branches/panicle to 99.41 for stem borer infestation. Thus, the broad-sense heritability estimates were very high for all the studies traits. Whereas, the values for narrow-sense heritability ranged from 15.12 for primary branches/panicle to 61.64 for days to maturity.
3.7 Correlation Coefficients among the Studied Traits
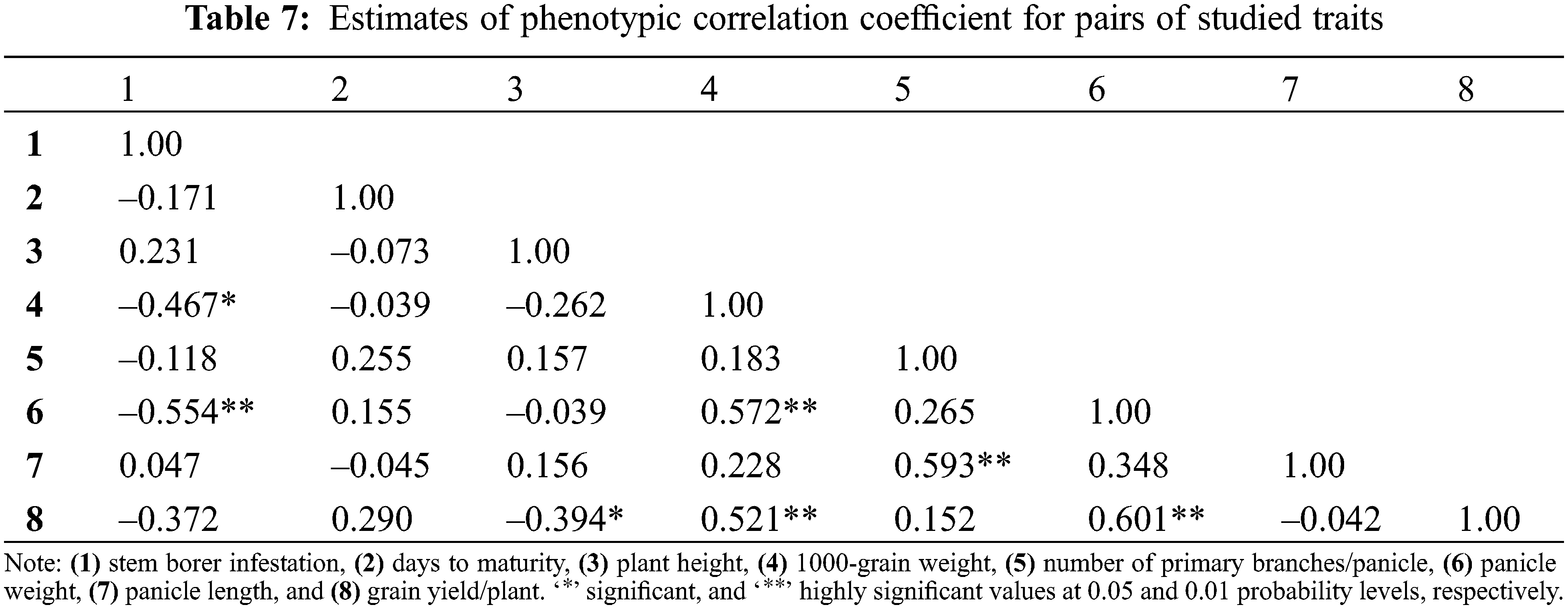
Estimates of phenotypic correlation coefficients between all possible trait combinations (stem borer infestation and quantitative traits) are depicted in Table 7. Stem borer infestation showed highly significant negative correlation with panicle weight (–0.554) and significant negative correlation with 1000-grain weight (–0.467), respectively. Thus, it can be inferred that these three traits can be employed in a breeding program to develop genotypes with the desired resistance against rice stem borer infestation.
At the same time, 1000-grain weight showed highly significant positive correlation with panicle weight (0.572) and grain yield/plant (0.521), respectively. Number of primary branches/panicle showed a positive and highly significant correlation with panicle length (0.593). Similarly, panicle weight and grain yield/plant also showed highly significant positive correlation (0.601).
3.8 Principal Component Analysis
The clustering pattern of parents and their crosses was visualized by plotting a biplot principle component analysis (PCA) (Fig. 3). The compelling results of biplot distribute all accessions based upon the PCA into four quadrants. The genotypes in quadrant-I were characterized by the highest values for days to maturity, grain yield/plant, panicle weight, and no. of primary branches/panicle. The genotypes in quadrant-II recorded the highest values for plant height, and panicle length. Quadrant-III grouped genotypes showing the highest values for 1000-grain weight, while the genotypes in quadrant-IV recorded the highest values for stem borer infestation.
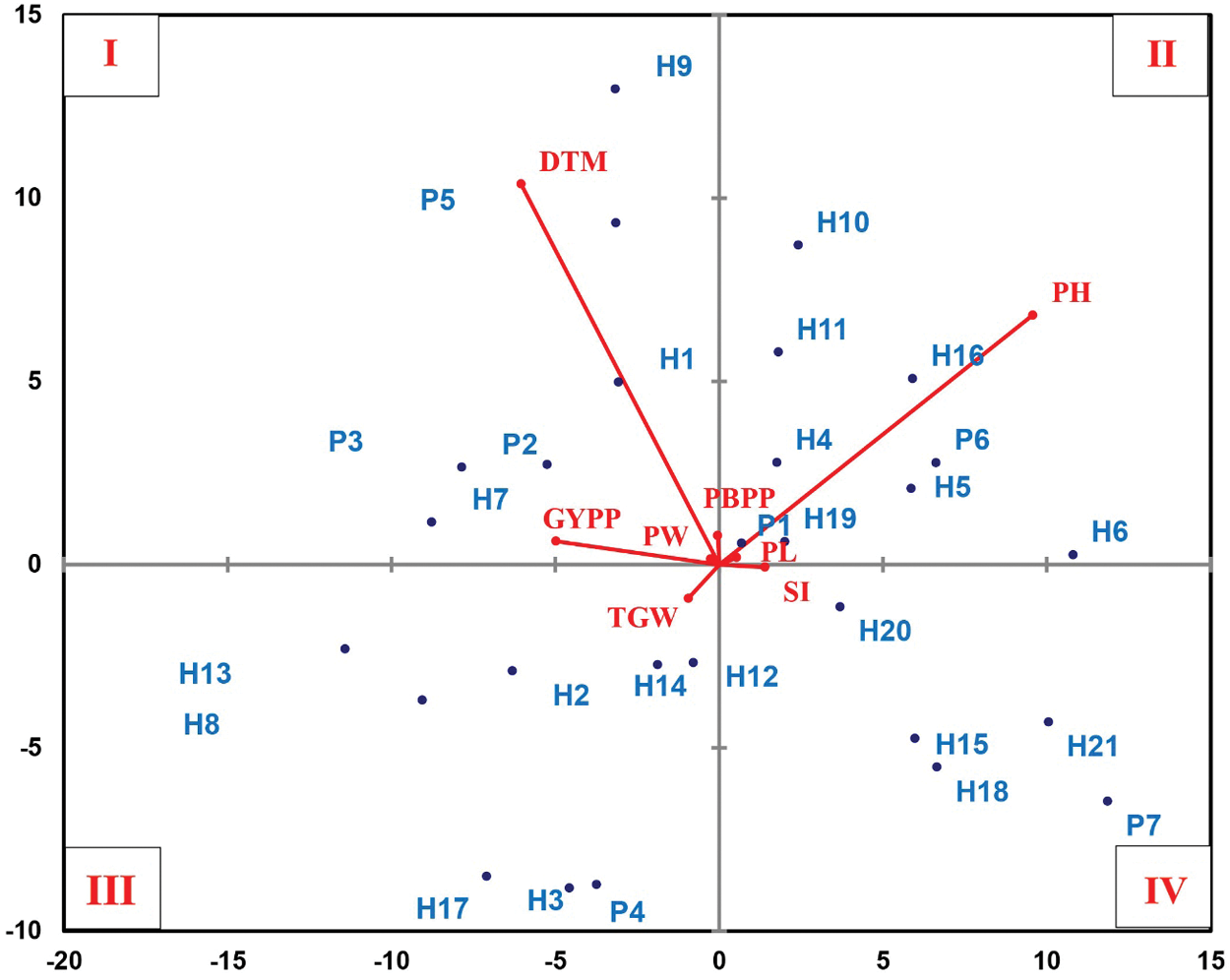
Figure 3: Biplot of the principle component analysis (PCA) for the morpho-physiological, insect resistance-related and yielding traits for all parents and their crosses. The abbreviations used were: stem borer infestation (SI), days to maturity (DTM), plant height (PH), 1000-grain weight (TGW), no. of primary branches/panicle (PBPP), panicle weight (PW), panicle length (PL), and grain yield/plant (GYPP), respectively. PCA was performed using XLSTAT v19.1
Parents: Giza178 (P1), Sakha101 (P2), Gz6903-3-4-2-1 (P3), Gz9577-4-1-1 (P4), Hassawi (P5), Giza175 (P6), Sakha103 (P7).
Crosses: Giza178 × Sakha101 (H1), Giza178 × Gz6903 (H2), Giza178 × Gz95771 (H3), Giza178 × Hassawi (H4), Giza178 × Giza175 (H5), Giza178 × Sakha103 (H6), Sakha101 × Gz6903 (H7), Sakha101 × Gz9577 (H8), Sakha101 × Hassawi (H9), Sakha101 × Giza175 (H10), Sakha101 × Sakha103 (H11), Gz69031 × Gz95771 (H12), Gz6903 × Hassawi (H13), Gz6903 × Giza175 (H14), Gz6903 × Sakha103 (H15), Gz9577 × Hassawi (H16), Gz9577 × Giza175 (H17), Gz9577 × Sakha103 (H18), Hassawi × Giza175 (H19), Hassawi × Sakha103 (H20), and Giza175 × Sakha103 (H21).
The study demonstrated substantial negative effect of stem borer infestation on rice yield related traits. Our results indicated that the Japonica varieties were more resistant to stem borer infestation than Indica-Japonica. These findings are in accordance with the previous findings of El-Malky et al. [28], Hammoud et al. [29] and El-Malky et al. [30]. The best cross combinations were Giza178×Sakha101, Giza178×Gz6903, Giza178×Gz95771, Giza178×Giza175, Sakha101×Gz6903, Sakha101×Hassawi, Gz69031×Gz95771, Gz6903×Hassawi, Gz6903×Giza175, Gz6903×Sakha103 and Gz9577×Sakha103. These crosses can be used in breeding programs to produce new lines resistant to stem borer infestation. Growth of stem borers requires rich diet viz. riboflavin, thiamine, nicotinic acid, pyridoxine, biotin and ascorbic acid [31]. Giza178 is a high yielding cultivar favored for its nutritional value. Therefore, higher rice borer infestation showed that Giza178 cultivar is rich in nutrients necessary for larval growth [32].
Overall, the mean performance of rice hybrids for quantitative traits was better than their parental genotypes. These findings are in accordance with the earlier findings of Hammoud et al. [29], Yang et al. [33] and Rahimi et al. [34]. These plant vigor and yield related parameters could be attributed to roles of small RNAs, non-additive gene expression, and epigenetic involvement along with circadian-associated metabolic cycles in hybrid vigor [35]. Significant negative values are desirable for stem borer infestation, days to maturity and plant height traits, while the positive values are favorable with other studied traits in rice. Estimation of combining abilities is helpful to evaluate the genetic value of suitable parental genotypes prior to their selection in a hybridization program [36]. Four genotypes Gz6903-3-4-2-1, Sakha 101, Hassawi and Gz9577-4-1-1 were the best general combiners for stem borer resistance while, Sakha103, Gz9577-4-1-1 and Giza175 were the best combiners for early maturity. Plant height is an integral part of overall plant yield [37] and selection of semi-dwarf or short statured genotypes may improve the harvest index due to enhanced resistance against rice stem borer. For plant height, Gz9577-4-1-1 was the best combiner and therefore, can be successfully integrated into a breeding program for stem borer resistance. Furthermore, for grain yield/plant Gz6903-3-4-2-1 and Sakha101 were the best combiners with highly significant positive GCA values (Table 4). Our findings for GCA effects are comparable to the findings of Bagheri et al. [38], who have reported that variance values of GCA were less than the values of SCA variances for most of the quantitative traits, which depicts the predominance of non-additive gene action regarding inheritance of these traits.
Our data further showed highly significant differences among parental rice genotypes, which may indicate the presence of wide genetic background. Highly significant GCA and SCA variances of F1 generation (Tables 4 & 5) further supported it. Thus, it showed that additive variation and non-additive gene effect are present in our germplasm. The results concluded that selection procedures based on the accumulation of additive effects would be successful in improving these traits. These results were in agreements with those obtained by Fahmi et al. [39], and Gowayed et al. [40]. It can be attributed to epigenetic mechanisms, which mediate the gene activity, without altering the underlined DNA sequence and can be transferred to the successive generation, thus resulting in phenotypic variations in the offsprings [41,42].
Among crosses, Giza178×Hassawi was the best cross combination for the early maturity trait whereas, Sakha101×Sakha103 was the best combination for the late maturity trait. Depending on the breeder’s orientation, the respective combination for early or late maturity can be selected. Regarding plant height, crosses with significantly positive values could be utilized in rice breeding programs to develop new taller rice varieties. These findings are in accordance with the findings of Hladni et al. [43] who reported that positive SCA values are desirable in plants as they help in acquiring desirable plant height. Similarly, number of primary branches/panicle is a desirable character for breeders and thus, a cross combination with highly significant positive SCA can be used in breeding programs as reported by Wang et al. [44]. Panicle weight and 1000-grain weight are desirable characters and breeder’s first choice to increase the yield of plants infested with insects [39,45,46]. Thus, these can be good parameters to be considered while selecting best genotypes to be used in a breeding program.
The major role of dominance genetic variance was confirmed with the estimates of genetic parameters for all the traits in our study. Thus, initiation of hybrid breeding program can be effectively justified to improve these traits due to presence of non-additive gene action as has been described in other crops [47]. Thus, due to involvement of non-additive or dominance effects, an early selection for all the aforementioned traits cannot be suggested and it should be delayed until later generations to improve their genetic gain [48]. Regarding heritability estimates for all traits under study, the results revealed that the broad-sense heritability was higher than the narrow-sense heritability estimates for all characters. Our results corroborated with El-Malky et al. [28], Hammoud et al. [29], and El-Malky et al. [30]. As a matter of fact, a character or trait influenced by non-additive gene action usually show low genetic advance but high heritability, while for the character under additive gene action, heritability and genetic advance values are always high [49].
The correlation among different traits of genotypes helps to choose components with prior importance to proceed the selection for improved genetic gain in a population. It also determines the strength of relationships among different traits to execute a reliable selection of rice genotypes. The stem borer infestation was in significantly negative correlation with panicle weight and 1000-grain weight. Meanwhile, 1000-grain weight and panicle weight showed a highly significant positive correlation with grain yield/plant (Table 7). Moreover, stem borer infestation also showed a negative correlation with grain yield/plant. Panicle grain yield contributes significantly to the final crop yield [50] and thus a high 1000-grain weight, panicle weight, and grain yield/plant can be good selection indexes for resistance against rice stem borer. Similar results were obtained in previous findings [50–52]. The biplot PCA found interesting information regarding the relationship between rice stem borer resistance and other morpho-physiological and yielding traits (Fig. 3). Quadrant-I gathered late maturing and high yielding rice genotypes showing rice stem borer resistance. Most of the moderately resistant genotypes assembled in quadrant-II were tall and with long panicles. The genotypes in quadrant-III were moderately resistant with a high 1000-grain weight; however, most of them were short-statured. Quadrant-IV accumulated tall-statured and low yielding genotypes showing moderate susceptibility to moderate resistance against stem borer infestation. Usually, number of primary branches/panicle and grain yield/plant are in direct proportion to each other [53]; however, it can also result in a bushy stand, which may result in more insect infestation as Giza178 in quadrant-I. Logically, the accessions appearing in quadrant-I seems potentially suitable for breeding purposes due to many reasons. Most of them are medium statured, late maturing genotypes showing resistance to stem borer infestation with good average yield. The accessions in quadrant-II looks unsuitable for resistance breeding because these genotypes are mostly showing moderate resistance and are tall-statured phenotypes. Usually, tallstatured rice varieties carry short grains and inferior quality characteristics [21].
Breeding for resistance against insects, pests and diseases still remains one of the major objectives in rice breeding programs. Therefore, introduction of new rice varieties resistant to insects is the most economical and effective measure for controlling these pests [54]. Particularly in Asia, the cultivation of resistant rice varieties is an integral part of IPM approaches against insects. However, in last few years there are many constrains such as climate change, high temperatures, and desertification, and the prevalence of diseases are further aggravating the situation. Currently, the management of stem borers mainly depends upon prophylactic treatments and thus, there is a direct need to adopt more holistic approaches for pest management in rice [8]. Growing of resistant cultivars in combination with good management practices can mitigate the negative yield impacts of stem borers in rice growing areas.
The current study highlighted a high level of genetic variability among the parents as well as their cross combinations. It seems that most of the genetic variability was due to the existing genetic variation in all the studied genotypes with little or negligible environmental variations. The results of our study helped to estimate the genetic variability and the correlation among different traits related to stem borer resistance in various rice cultivars. From our study, it may be speculated that low stem borer infestation due to early sowing cannot be considered as a resistance strategy. Our study further revealed that stem borer infestation showed a strong correlation with panicle weight and a 1000-grain weight, and these traits were correlated with grain yield/plant with high significance. These observations were further supported by our PCA analysis. Thus, we may conclude that days to maturity, panicle weight, 1000-grain weight and grain yield/plant can be good parameters to be considered while devising a resistance-breeding strategy against stem borers. These particular traits must be the major focus during the selection of parents to start a varietal improvement program. Furthermore, the selection of resistant rice genotypes should be combined with IPM strategies including the use of pheromone-based traps, biological control agents and crop diversification. The magnitude and nature of yield loss depends upon a complex interaction between the host plant and the insect pest. Thus, overall plant architecture, time of insect attack, type of insect species, soil husbandry and involvement of secondary biotic or abiotic stresses all determine the overall impact of insect infestation on yield losses. Our results may help future breeding programs to confer resistance against stem borer in rice by providing a rich background selection of the potential parental material.
Acknowledgement: The authors fully appreciate the editors and all anonymous reviewers for their constructive suggestions and comments to improve this manuscript.
Funding Statement: The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the Project No. IFT20004.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Kumar, A., Prasad, S., Mishra, V. K., Kumar, R., Singh, J. et al. (2020). Effect of submergence stress on physiological indices and yield of rice (Oryza sativa L.) genotypes. International Journal of Current Microbiology and Applied Sciences, 9(1), 994–999. DOI 10.20546/ijcmas.2020.901.112. [Google Scholar] [CrossRef]
2. Yu, S., Ali, J., Zhang, C., Li, Z., Zhang, Q. (2020). Genomic breeding of green super rice varieties and their deployment in Asia and Africa. Theoretical and Applied Genetics, 133(5), 1427–1442. DOI 10.1007/s00122-019-03516-9. [Google Scholar] [CrossRef]
3. Riegler, M. (2018). Insect threats to food security. Science, 361(6405), 846. DOI 10.1126/science.aau7311. [Google Scholar] [CrossRef]
4. Ogah, E. (2013). Evaluating the impact of new rice for Africa (NERICA) in the management of rice stem borers. Science International, 1(5), 160–166. DOI 10.17311/sciintl.2013.160.166. [Google Scholar] [CrossRef]
5. Horgan, F. G., Romena, A. M., Bernal, C. C., Almazan, M. L. P., Ramal, A. F. (2021). Stem borers revisited: Host resistance, tolerance, and vulnerability determine levels of field damage from a complex of Asian rice stemborers. Crop Protection, 142(2021), 105513. DOI 10.1016/j.cropro.2020.105513. [Google Scholar] [CrossRef]
6. Moghaieb, R. E. A., Khashaba, E. H. K., Abd El Azim, A. M., Ibrahim, S. A. (2021). Genetic diversity studies and screening for rice stem borer (Chilo agamemnon) resistance in six Egyptian rice cultivars (Oryza sativa L.) using DNA based-markers. Genetic Resources and Crop Evolution, 68(7), 2313–2323. DOI 10.1007/s10722-021-01129-0. [Google Scholar] [CrossRef]
7. Horgan, F. G., Crisol-Martínez, E., Almazan, M. L. P., Romena, A., Ramal, A. F. et al. (2016). Susceptibility and tolerance in hybrid and pure-line rice varieties to herbivore attack: Biomass partitioning and resource-based compensation in response to damage. Annals of Applied Biology, 169(2), 200–213. DOI 10.1111/aab.12296. [Google Scholar] [CrossRef]
8. Villegas, J. M., Wilson, B. E., Stout, M. J. (2021). Assessment of tolerance and resistance of inbred rice cultivars to combined infestations of rice water weevil and stemborers. Entomologia Experimentalis et Applicata, 169(7), 629–639. DOI 10.1111/eea.13054. [Google Scholar] [CrossRef]
9. Makkar, G. S., Bentur, J. S. (2017). Breeding for stem borer and gall midge resistance in rice. In: Arora, R., Sandhu, S. (Eds.Breeding insect resistant crops for sustainable agriculture, pp. 323–352. Singapore: Springer. DOI10.1007/978-981-10-6056-4_11. [Google Scholar] [CrossRef]
10. Horgan, F. (2017). Integrated pest management for sustainable rice cultivation: A holistic approach. In: Sasaki, T. (Ed.Achieving sustainable cultivation of rice: Cultivation, pest and disease management, pp. 309–341. Cambridge, UK: Burleigh Dodds. [Google Scholar]
11. Lu, Y., Zhao, Y., Lu, H., Bai, Q., Yang, Y. et al. (2018). Midgut transcriptional variation of Chilo suppressalis larvae induced by feeding on the dead-end trap plant, Vetiveria zizanioides. Frontiers in Physiology, 9(1067), 1–11. DOI 10.3389/fphys.2018.01067. [Google Scholar] [CrossRef]
12. Sosa, B., Fontans-Álvarez, E., Romero, D., da Fonseca, A., Achkar, M. (2019). Analysis of scientific production on glyphosate: An example of politicization of science. Science of the Total Environment, 681(1), 541–550. DOI 10.1016/j.scitotenv.2019.04.379. [Google Scholar] [CrossRef]
13. Zhang, Q. (2007). Strategies for developing green super rice. Proceedings of the National Academy of Sciences of the United States of America, 104(42), 16402–16409. DOI 10.1073/pnas.0708013104. [Google Scholar] [CrossRef]
14. Horgan, F. G., Crisol, E. (2013). Hybrid rice and insect herbivores in Asia. Entomologia Experimentalis et Applicata, 148(1), 1–19. DOI 10.1111/eea.12080. [Google Scholar] [CrossRef]
15. Du, B., Chen, R., Guo, J., He, G. (2020). Current understanding of the genomic, genetic, and molecular control of insect resistance in rice. Molecular Breeding, 40(24), 1–25. DOI 10.1007/s11032-020-1103-3. [Google Scholar] [CrossRef]
16. Zheng, X., Weng, Z., Li, H., Kong, Z., Zhou, Z. et al. (2021). Transgenic rice overexpressing insect endogenous microRNA csu-novel-260 is resistant to striped stem borer under field conditions. Plant Biotechnology Journal, 19(3), 421–423. DOI 10.1111/pbi.13504. [Google Scholar] [CrossRef]
17. Aidy, I. R., Bastawisi, A. O., Sehly, M. R. (2000). Breeding strategy for rice blast resistance in Egypt. In: Tharreau, D., Lebrun, M. H., Talbot, N. J., Notteghem, J. L. (edsAdvances in rice blast research. Developments in plant pathology, vol. 15. Dordrecht: Springer. DOI 10.1007/978-94-015-9430-1_13. [Google Scholar] [CrossRef]
18. Sedeek, S. E. M., El-Shafey, R. A. S., Hammoud, S. A., El-Namaky, R. A. (2012). Gene action and relative importance of some agronomic and biotic stress traits affecting genetic divergence in rice. Journal of Plant Production, 3(12), 2971–2992. DOI 10.21608/jpp.2012.85363. [Google Scholar] [CrossRef]
19. Griffing, B. (1956). Concept of general and specific combining ability in relation to diallel crossing systems. Australian Journal of Biological Sciences, 9(4), 463–493. DOI 10.1071/BI9560463. [Google Scholar] [CrossRef]
20. El-Adl, A. M., Abo Youssef, M. I., El-Diasty, Z. M., Assas, M. S. (2011). Affecting of morphological traits on stem borer resistance in some rice genotypes. Journal of Agricultural Chemistry and Biotechnology, 2(1), 15–21. DOI 10.21608/jacb.2011.56489. [Google Scholar] [CrossRef]
21. Mvuyekure, S., Sibiya, J., Derera, J., Nzungize, J., Nkima, G. (2018). Application of principal components analysis for selection of parental materials in rice breeding. Journal of Genetics & Genomic Sciences, 3(1), 2–7. DOI 10.24966/GGS-2485/100010. [Google Scholar] [CrossRef]
22. Gaballah, M. M., Attia, K. A., Ghoneim, A. M., Khan, N., El-Ezz, A. F. et al. (2022). Assessment of genetic parameters and gene action associated with heterosis for enhancing yield characters in novel hybrid rice parental lines. Plants, 11(3), 266. DOI 10.3390/plants11030266. [Google Scholar] [CrossRef]
23. Butany, W. T. (1961). Mass emasculation in rice. International Rice Community Newsletter, 9, 9–13. [Google Scholar]
24. Panse, V. G., Sukhatme, P. V. (1954). Statistical methods for agricultural workers. Agronomy Journal, 48(7), 323. DOI 10.2134/agronj1956.00021962004800070014x. [Google Scholar] [CrossRef]
25. Kempthorne, O. (1957). An introduction to genetic statistics, pp. 545. Oxford, England: Wiley. DOI10.2307/2310745 [Google Scholar] [CrossRef]
26. Gomez, K. A., Gomez, A. A. (1984). Statistical procedures for agricultural research, pp. 680. New York: John Wiley & Sons. [Google Scholar]
27. Wynne, J. C., Emery, D. A., Rice, P. W. (1970). Combining ability estimates in Arachis hypogaea L. II. Field performance of F1 hybrids. Crop Science, 10(6), 713–715. DOI 10.2135/cropsci1970.0011183X001000060036x. [Google Scholar] [CrossRef]
28. El-Malky, M. M., El-Habashy, M. M., Abdelkhalik, A. F. (2008). Rice germplasm evaluation for agronomic traits and their influence on stem borer (Chilo agamemnon Bles.) resistance. Journal of Agricultural Research, 46(3), 203–213. [Google Scholar]
29. Hammoud, S. A. A., Sedeek, S. E. M., Rewaniy, I. O. A., El-Namaky, R. A. (2012). Genetic behavior of some agronomic traits, blast disease and stem borer resistance in two N-levels. Journal of Agricultural Research (Kafrelsheikh University), 38(1), 83–105. [Google Scholar]
30. El-Malky, M. M. and EL-Zun, H. (2014). Genetic behavior of yield, grain quality, stem borer and storage insect infestation traits for some rice genotypes at different sowing dates. Journal of Plant Production, 5(6), 917–935. DOI 10.21608/JPP.2014.55441. [Google Scholar] [CrossRef]
31. Ishii, S. (1971). Nutritional studies of the rice stem borer, Chilo suppressalis Walker, and its mass rearing. Entomophaga, 16(2), 165–173. DOI 10.1007/BF02371167. [Google Scholar] [CrossRef]
32. Abd Allah, A. A., Badawy, S. A., Zayed, B. A., El-Gohary, A. A. (2010). The role of root system traits in the drought tolerance of rice (Oryza sativa L.). Journal of Plant Production, 1(4), 621–631. DOI 10.21608/jpp.2010.86384. [Google Scholar] [CrossRef]
33. Yang, J., Du, Y., Wu, C., Liu, L., Wang, Z. et al. (2007). Growth and development characteristics of super-high-yielding mid-season japonica rice. Frontiers of Agriculture in China, 1(2), 166–174. DOI 10.1007/s11703-007-0028-5. [Google Scholar] [CrossRef]
34. Rahimi, M., Rabiei, B., Samizadeh, H., Kafi, G. A. (2010). Combining ability and heterosis in rice (Oryza sativa L.) cultivars. Journal of Agricultural Science and Technology A&B, 12, 223–231. [Google Scholar]
35. Chen, Z. J. (2010). Molecular mechanisms of polyploidy and hybrid vigor. Trends in Plant Science, 15(2), 57–71. DOI 10.1016/j.tplants.2009.12.003. [Google Scholar] [CrossRef]
36. Karimizadeh, R., Sharifi, P., Mohammadi, M. (2020). Genetic analysis of morphological traits in wheat hybrids based on the additive-dominance model. Russian Agricultural Sciences, 46(2), 113–120. DOI 10.3103/S1068367420020160. [Google Scholar] [CrossRef]
37. Matusmoto, T., Yamada, K., Yoshizawa, Y., Oh, K. (2016). Comparison of effect of brassinosteroid and gibberellin biosynthesis inhibitors on growth of rice seedlings. Rice Science, 23(1), 51–55. DOI 10.1016/j.rsci.2016.01.006. [Google Scholar] [CrossRef]
38. Bagheri, N., Jelodar, N. B. (2010). Heterosis and combining ability analysis for yield and related-yield traits in hybrid rice. International Journal of Biology, 2(2), 222–231. DOI 10.5539/ijb.v2n2p222. [Google Scholar] [CrossRef]
39. Fahmi, A. I., Eissa Ragaa, A., Nagaty, H. H., El-Malky, M., Sherif, A. I. (2018). Genetic components and correlation coefficient for earliness and grain yield in rice. Vegetos, 31(2), 91–105. DOI 10.5958/2229-4473.2018.00060.5. [Google Scholar] [CrossRef]
40. Gowayed, S., Abd El-Moneim, D., Metwali, E., El-Malky, M. (2020). Combining ability and heterosis studies for some economic traits in rice (Oryza sativa L.). Research Journal of Biotechnology, 15(1), 101–111. [Google Scholar]
41. Berger, S. L., Kouzarides, T., Shiekhattar, R., Shilatifard, A. (2009). An operational definition of epigenetics. Genes & Development, 23(7), 781–783. DOI 10.1101/gad.1787609. [Google Scholar] [CrossRef]
42. Lippman, Z., Martienssen, R. (2004). The role of RNA interference in heterochromatic silencing. Nature, 431(7006), 364–370. DOI 10.1038/nature02875. [Google Scholar] [CrossRef]
43. Hladni, N., Miklič, V., Jocić, S., Kraljević-Balalić, M., Škorić, D. (2014). Mode of inheritance and combining ability for plant height and head diameter in sunflower (Helianthus annuus L.). Genetika, 46(1), 159–168. DOI 10.2298/GENSR1401159H. [Google Scholar] [CrossRef]
44. Wang, Y. H., Cai, Q. H., Xie, H. G., Wu, F. X., Lian, L. et al. (2018). Determination of heterotic groups and heterosis analysis of yield performance in Indica rice. Rice Science, 25(5), 261–269. DOI 10.1016/j.rsci.2018.08.002. [Google Scholar] [CrossRef]
45. El-Malky, M. M., Al-Daej, M. (2018). Studies of genetic parameters and cluster analysis of some quantitative characters through diallel analysis of rice (Oryza sativa L.). Vegetos, 31(1), 1–10. DOI 10.4172/2229-4473.1000377. [Google Scholar] [CrossRef]
46. Upadhyay, M., Jaiswal, H. (2015). Combining ability analysis for yield and earliness in hybrid rice (Oryza sativa L.). Asian Journal of Crop Science, 7(1), 81–86. DOI 10.3923/ajcs.2015.81.86. [Google Scholar] [CrossRef]
47. Varghese, M., Patel, M. (2020). Estimation of combining ability of yield and different agronomic traits in interspecific cotton hybrids. Electronic Journal of Plant Breeding, 11(4), 1015–1020. DOI 10.37992/2020.1104.165. [Google Scholar] [CrossRef]
48. Varona, L., Legarra, A., Toro, M. A., Vitezica, Z. G. (2018). Non-additive effects in genomic selection. Frontiers in Genetics, 9, 78. DOI 10.3389/fgene.2018.00078. [Google Scholar] [CrossRef]
49. Ahmad, F., Mohammad, F., Bashir, M., Khan, H. (2007). Inheritance of important traits in bread wheat over different planting dates using diallel analysis. Sarhad Journal of Agriculture, 23(4), 955–964. [Google Scholar]
50. Oladosu, Y., Rafii, M. Y., Abdullah, N., Abdul Malek, M., Rahim, H. A. et al. (2014). Genetic variability and selection criteria in rice mutant lines as revealed by quantitative traits. Scientific World Journal, 2014(3), 12. DOI 10.1155/2014/190531. [Google Scholar] [CrossRef]
51. Jayasudha, S., Sharma, D. (2010). Genetic parameters of variability, correlation and path-coefficient for grain yield and physiological traits in rice (Oryza sativa L.) under shallow lowland situation. Electronic Journal of Plant Breeding, 1(5), 1332–1338. [Google Scholar]
52. Sabesan, T., Suresh, R., Saravanan, K. (2009). Genetic variability and correlation for yield and grain quality characters of rice grown in coastal saline low land of Tamilnadu. Electronic Journal of Plant Breeding, 1(1), 56–59. [Google Scholar]
53. Dixit, S., Singh, A., Kumar, A. (2014). Rice breeding for high grain yield under drought: A strategic solution to a complex problem. International Journal of Agronomy, 15, 863683. DOI 10.1155/2014/863683. [Google Scholar] [CrossRef]
54. Noorozi, M., Sabertanha, M., Mohammadi, M., Fakheri, B., Sattari, A. (2015). Breeding for stem borer in rice. International Journal of Farming and Allied Sciences, 4(6), 510–513. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |