

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020041
ARTICLE
Effects of Auxin at Different Concentrations on the Growth, Root Morphology and Cadmium Uptake of Maize (Zea mays L.)
College of Resources and Environment, Yunnan Agricultural University, Kunming, 650201, China
*Corresponding Author: Yongmei He. Email: heyongmei06@126.com
Received: 31 October 2021; Accepted: 17 January 2022
Abstract: Indoleacetic acid (IAA) is an important regulator that plays a crucial role in plant growth and responses to abiotic stresses. In the present study, a sand cultivation experiment was carried out to investigate the effects of IAA at different concentrations (0, 0.01, 0.1, 0.5, 1, and 2.5 mmol/L) on maize growth, root morphology, mineral elements (Ca, Mg) and Cd uptake under 20 mg/kg Cd stress. The results showed that 0.01 mmol/L is the optimal IAA concentration for enhancing the Cd tolerance of maize. Compared with the control treatment, 0.01 mmol/L IAA promoted maize growth, with significant increases in the height, shoot and root biomass by 34.6%, 25.0% and 16.3%; altered the root morphology, with increases in root length, root tip number, and root tip density by 8.9%, 31.4% and 20.7%, respectively; and enhanced the mineral element uptake of maize, resulting in significant increases in the Ca content in shoots and roots by 640.6% and 1036.4% and in the Mg content in shoots by 205.8%, respectively. In addition, 0.01 mmol/L IAA decreased the Cd content and uptake in the shoots by 51.9% and 39.6%, respectively. Furthermore, the Cd content and uptake exhibited a significant negative correlation with Ca content in roots and a significantly positive correlation with root morphology, and the Cd content in shoots was significantly and negatively correlated with root tip number. Thus, 0.01 mmol/L IAA was effective in enhancing the Cd tolerance and plant growth of maize.
Keywords: Abiotic stress; mineral nutrient; plant growth; phytohormone; root trait
Cadmium (Cd) is an unnecessary element for plants. After being absorbed by the soil, Cd is transported and taken up by plants [1]. When Cd uptake occurs to a certain extent, it will inhibit plant growth, damage the photosynthetic system, interfere with the metabolism of mineral elements, induce oxidative damage, accelerate plant senescence, and even cause plant death [2,3]. In plants that live in Cd stress environments for a long time, a series of adaptive mechanisms can form, including changes in plant root morphology, nutrient absorption, reactive oxygen levels, hormone homeostasis, and gene expression [4].
Auxin is an important plant growth regulator that can promote cell division and elongation and the differentiation and formation of new organs, change the morphology of plant roots, and regulate the absorption of nutrients by plants. It plays an important role in plant Cd tolerance [5,6]. Under Cd stress, 40 mg/L IAA can significantly increase the total root length, root surface area and root tip number of Solanum nigrum [7], and 10−9 mol/L IBA can significantly increase the content of nutrient elements such as nitrogen, phosphorus, potassium, calcium, sulfur, magnesium, iron, manganese, zinc, copper and other elements [8]. While auxin affects plant root development and nutrient absorption, auxin affects the absorption, transfer and uptake of Cd by plants. Zhang et al. [9] reported that 2 and 10 μmol/L IAA can inhibit the absorption and uptake of Cd and reduce the Cd content in the roots, stems, mature leaves and tender leaves of tea seedlings (Camellia sinensis). Ran et al. [7] reported that 20 mg/L IAA significantly increased the Cd content of the Cd hyperaccumulator Solanum nigrum and the nonhyperaccumulator Solanum melongena plants, and 40 mg/L IAA significantly increased the Cd content of Solanum nigrum but significantly decreased the Cd content of Solanum melongena. The effects of IAA on the Cd uptake characteristics of plants are related to the concentration of IAA and plant species.
Maize has a large biomass, is easy to harvest, and is a widely planted crop worldwide [10]. Maize has a certain ability to absorb, transfer and uptake Cd, is a good material studying plant Cd resistance mechanism. In recent years, there have been studies that IAA can change the transport pathway or as a signal substance to enhance the stress resistance of maize [11], but has less related studies of maize root morphology and nutrient elements. Most of maize Cd resistance mechanism was carried out at a single IAA concentration. But the influence mechanism of IAA on plant growth, physiology and Cd tolerance is related to the concentration of IAA and plant species. Therefore, it is necessary to study the effects of different concentrations IAA on the growth and Cd uptake of maize. We used low-Cd maize cultivar maize Huidan No. 4 as the material to study the effects of IAA on plant height, biomass, root morphology, root traits, mineral element content and Cd uptake characteristics of maize under 20 mg/kg Cd stress, to provide basic data and theoretical support in IAA alleviate the toxicity of Cd to maize. We hypothesize that IAA can inhibit the absorption and uptake of Cd in maize by promoting the development of maize roots and the absorption of mineral elements to enhance maize Cd tolerance and promote maize growth.
The test material was a native low-Cd maize cultivar (Huidan No. 4) from Yunnan Province, China. The culture substrate was quartz sand (the main component of which is SiO2), with a particle size of 0.5–1 mm, white powder. IAA (3-indoleacetic acid, C10H9NO2) is a white crystal that is poorly soluble in water. IAA was purchased from Beijing Coollab Technology Co., Ltd. (China) (Coolaber RT Purity > 99%). The culture vessel was an uncovered cylindrical sealed tank with a diameter of 12 cm and glass bottles with a diameter of 6.5 cm and a height of 25 cm.
First, the seeds of Huidan No. 4 were surface-sterilized by immersion in 75% ethanol for 10 min and in 10% sodium hypochlorite for 10 min, followed by rinsing with water until colorless. The seeds were germinated in a petri dish (150 mm) at 25°C for 3 days. After germination to about 1 cm, two seedlings with uniform growth were transplanted to a glass bottle, which 0.4 kg quartz sand and 40 ml of 50% Hoagland nutrient solution were added. Seedlings were cultured for 14 days in a greenhouse (average temperature 25°C, average humidity 56%).
Second, after 14 days, two maize seedlings with the same growth were transplanted to a sealable tank. To each sealed tank, 1.475 kg of quartz sand and 100 ml of 50% Hoagland nutrient solution were added. The nutrient solution contained 20 mg/kg Cd and the corresponding concentration of IAA (0, 0.01, 0.1, 0.5, 1, 2.5 mmol/L IAA), Cd was added in the form of CdCl2·2.5H2O, 4 replicates per treatment. Seedlings were grown for 30 days and harvested in greenhouse.
2.3 Determination of Plant Height and Biomass
After the maize seedlings were harvested, the plant height was measured with a measuring tape, and then the seedlings were divided into shoots and roots. The samples were soaked in ethylenediaminetetraacetic acid solution (EDTA) for 15 min. The shoots and roots soaked in EDTA were rinsed with tap water and deionized water 3 times and then dried in an oven at 105°C for 30 min and then at 75°C. The dried shoots and roots were weighed to a constant weight to obtain the shoot and root biomass [12].
2.4 Determination of Root Morphology
The root was rinsed with tap water and then with distilled water 2–3 times, and the water was absorbed with filter paper. The root morphology was scanned with an Epson Perfection V700 scanner (Seiko Epson, Japan), the scanned image was saved, and the root length, root surface area, root volume, root average diameter, root tip number, and branch number were analyzed with Win RHIZO PRO STD4800 (Regent, Canada) software [13].
According to the root length, root surface area, root tip number and branch number recorded by WinRHIZO and the root biomass, the specific root length (SRL), specific surface area (SRA), root tip density (RTD) and root branching intensity (RBI) of the root system were calculated. The calculation formula was as follows: specific root length (m/g) = root length (m)/biomass (g), specific surface area (cm2/g) = root surface area (cm2)/biomass (g), root tip density (No./cm) = root tip number(number)/root length (cm), branch density (No./cm) = branch number(number)/root length (cm) [14].
2.5 Determination of Plant Cd, Ca and Mg Contents
Plant samples were dried, ground and passed through a 100-mesh sieve. A 0.5-g sample was transferred to polyethylene digestion irrigation, concentrated HNO3 and H2O2 (5:3) were added to the sample, and the sample was digested in a pressure digestion tank and measured by flame atomic absorption spectrophotometry (AAS ICE 3000 Series, Thermo Scientific, Franklin, USA) [15,16].
2.6 Determination of Biological Transport Factors and Translocation Factors
Plant Cd uptake characteristics are expressed by the biological transfer factor (BTF) and the transfer factor (TF) [17] with the following formula: Cd uptake (µg/plant) = Cd content (mg/kg) × plant biomass (g/plant); biological transport factor (BTF) = shoot Cd content (mg/kg) × shoot biomass (g)/root Cd content (mg/kg) × root biomass (g); transfer factor (TF) =shoot Cd content (mg/kg)/root Cd content (mg/kg).
The test data were the average of 4 replicates, and all data were sorted and analyzed in Excel. The least significant difference method (LSD) has a relatively simple method, in all possible pairwise comparison of mean of multiple treatments. LSD overcomes some drawbacks of the T-test method. Therefore, the LSD method was used to mean analysis [18]. Analysis of variance, significance test (LSD method, significance level P = 0.05) and correlation analysis (Pearson method, significance level P = 0.05) were performed by IBM SPSS Statistics 21 software. Origin (Version 9.0 Pro, Origin Lab Company, Massachusetts, USA) was used for charting.
3.1 The Effect of IAA on Maize Growth
Under 20 mg/kg Cd stress, 0.01 mmol/L IAA resulted in a significant increase in maize plant height and shoot and root biomass, which increased by 34.6%, 25.0%, and 16.3%, respectively. IAA (0.1 mmol/L) resulted in a significant increase in plant height by 21.4% but resulted in a significant decrease in root biomass by 20.4%. IAA (0.5 mmol/L) resulted in significant decreases in plant height and shoot and root biomass, which were decreased by 24.3%, 13.9%, and 18.4%, respectively. IAA at 1 and 2.5 mmol/L resulted a significant decrease in root biomass of 26.5% and 12.2%, respectively. Under Cd stress, 0.01 mmol/L IAA significantly promoted maize growth (Table 1).
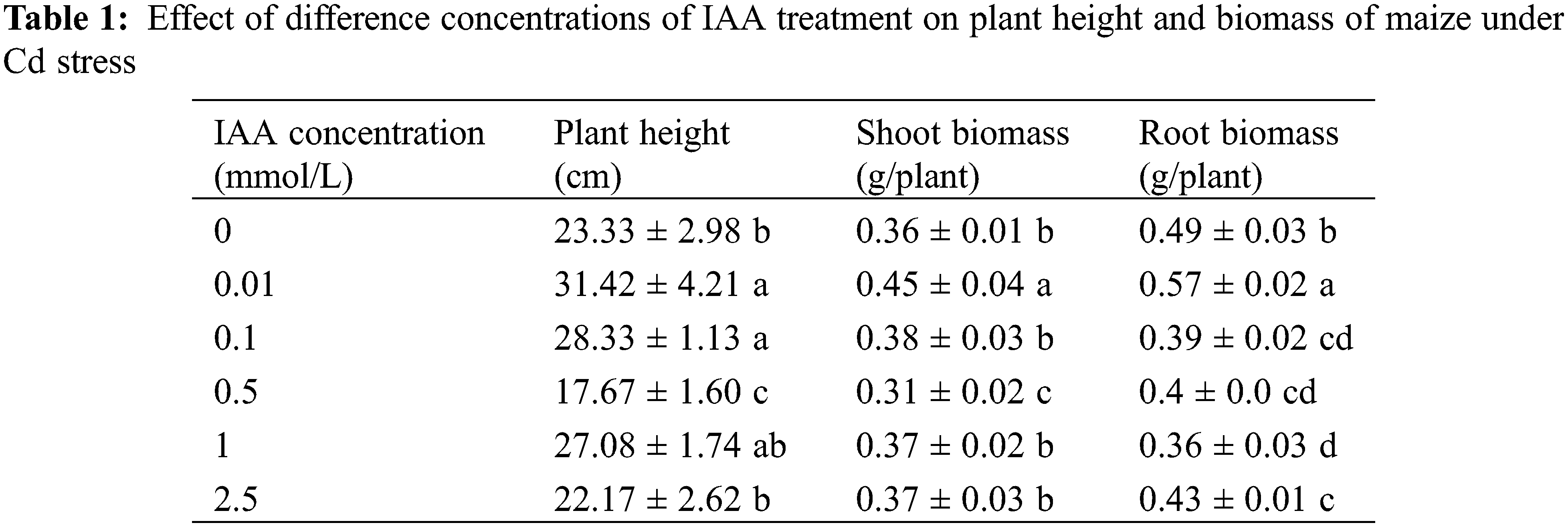
All values represent the means ± standard deviations; different lowercase letters within a column indicate significant differences based on one-way analysis of variance in SPSS 21 followed by the least significant differences at the 5% confidence level.
3.2 The Effects of IAA on Root Morphology and Traits of Maize
Under 20 mg/kg Cd stress, 0.01 mmol/L IAA resulted in a significant increase in root length of maize by 8.9%. IAA at 0.1 and 2.5 mmol/L resulted in significant decreases in root length of 14.4% and 12.8%, respectively. IAA at 0.01, 0.1, 0.5, 1, and 2.5 mmol/L resulted in significant decreases in root surface area and root volume; the root surface area was decreased by 11.1%, 20.2%, 12.5%, 16.9% and 17.8%, the root volume was decreased by 11.1%, 20.2%, 11.1%, 16.9%, 17.8%, and 0.5 mmol/L AA resulted in a significant decrease in the average root diameter by 20.0%. IAA (0.01 mmol/L) resulted in a significant increase in the number of root tips by 31.4%. IAA (1 mmol/L) resulted in a significant decrease in the number of root tips by 12.9%. There was no significant effect on the number of branches treated with IAA (Fig. 1).
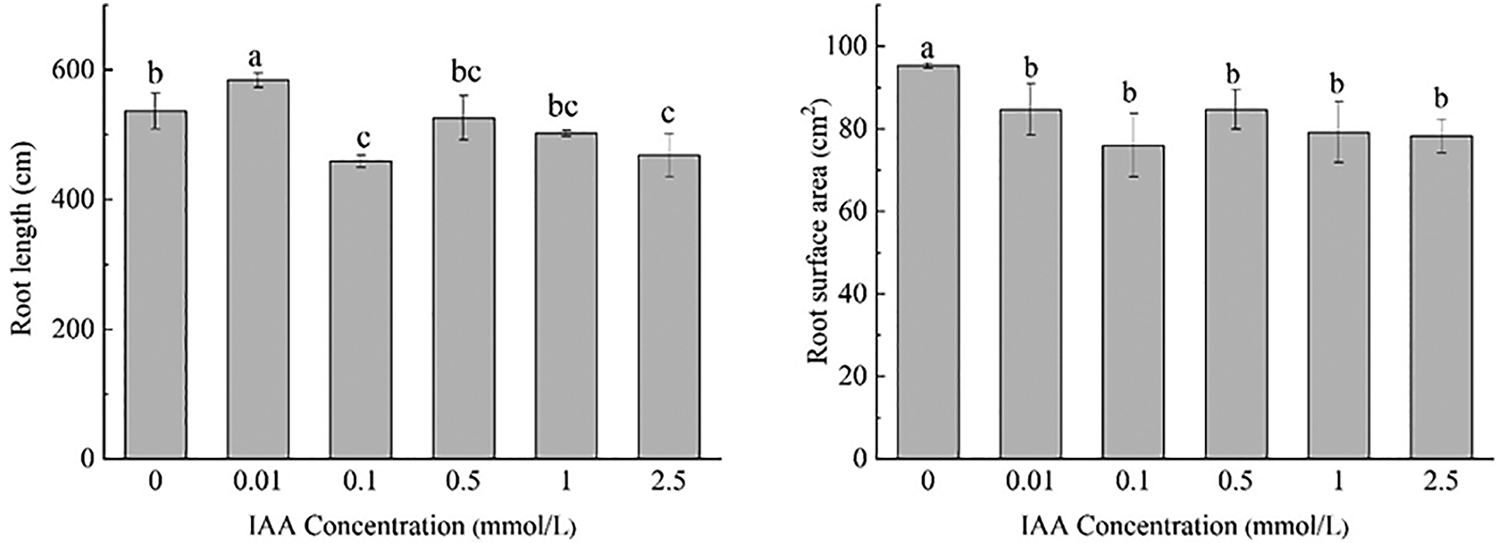
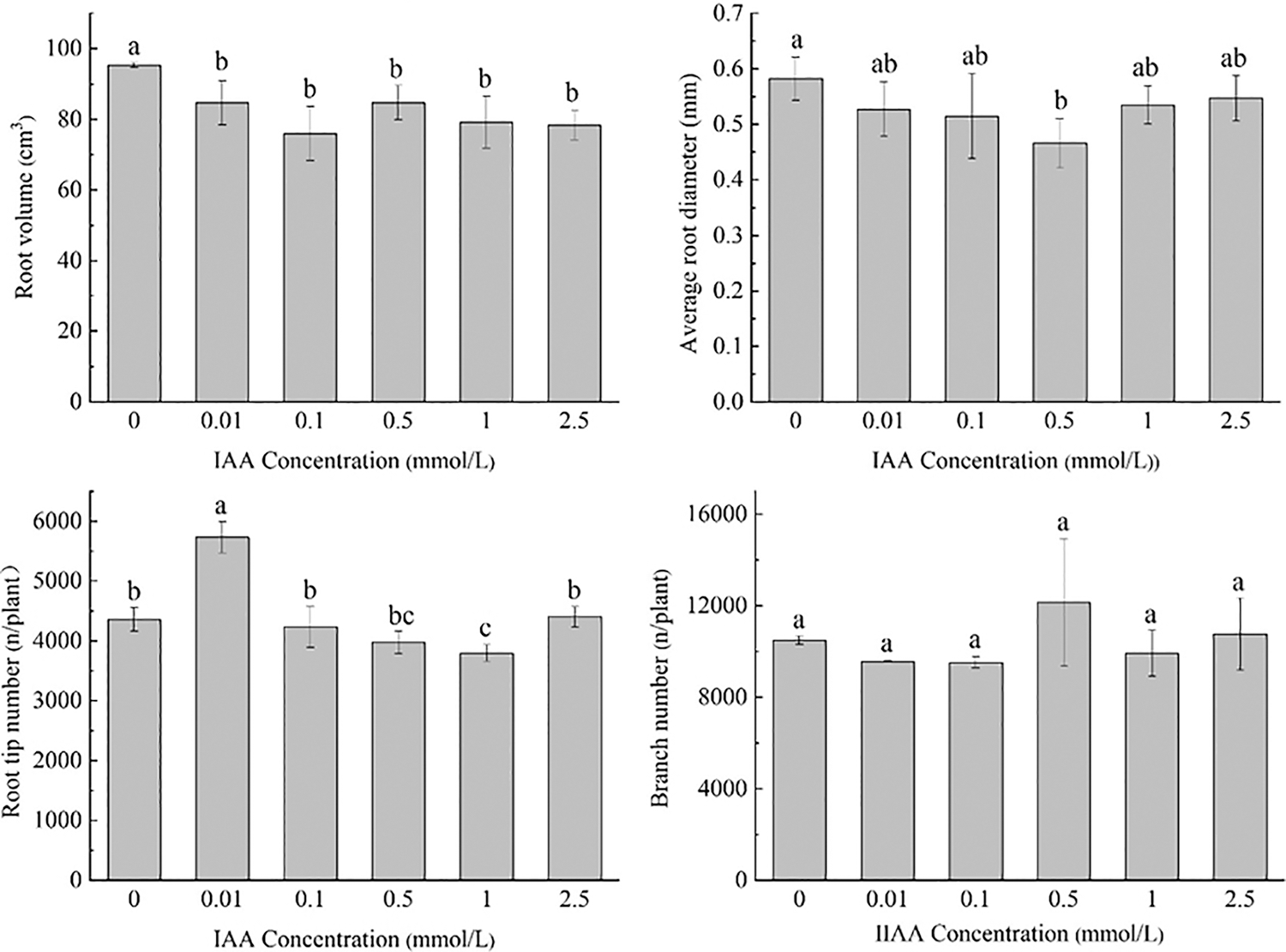
Figure 1: Effect of difference concentrations of IAA treatment on root morphology of maize under Cd stress; different little letters refer to P < 0.05 according to LSD test
Under 20 mg/kg Cd stress, 0.5 and 1 mmol/L IAA resulted in significant increases in the specific root length of maize of 19.2% and 28.4%, respectively. IAA (0.01 mmol/L) resulted in a significant decrease in the specific surface area by 24.7%. IAA at 0.01, 0.1, and 2.5 mmol/L resulted in a significant increase in root tip density, which was increased by 20.7%, 13.7%, and 16.2%, respectively. IAA (2.5 mmol/L) resulted in a significant increase in root branch density by 16.8% (Fig. 2). In general, under Cd stress, exogenous application of IAA has significant effects on maize root morphology and traits.
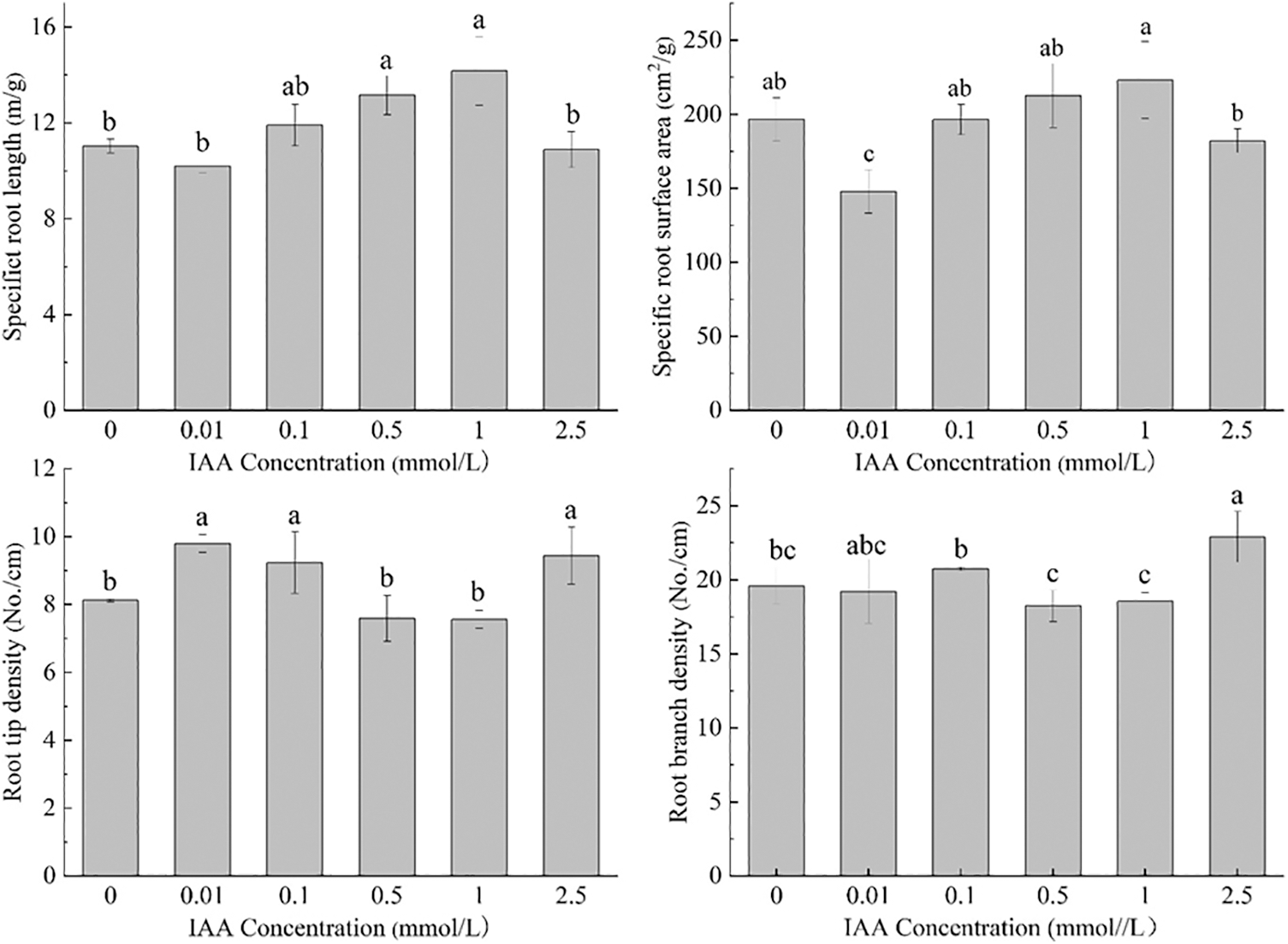
Figure 2: Effect of difference concentrations of IAA treatment on root traits of maize under Cd stress; different little letters refer to P < 0.05 according to LSD test
3.3 The Effects of IAA on the Contents of Ca and Mg in Maize
Under 20 mg/kg Cd stress, 0.01, 0.1, 0.5, 1, and 2.5 mmol/L IAA resulted in significant increases in the Ca content of maize of 640.6%, 241.8%, 371.2%, and 387.5%. In addition, the root Ca content increased by 1036.4%, 1136.3%, 1265.4%, 902.1%, and 668.1%, respectively, and 0.01, 0.1, 0.5, 1, and 2.5 mmol/L IAA resulted in significant increases in shoot Mg content, of 205.8%, 126.2%, 162.9%, 156.5%, and 155.5%, respectively. IAA had no significant effect on the root Mg content. In general, under Cd stress, applying IAA can promote the absorption of Ca and Mg in maize (Fig. 3).
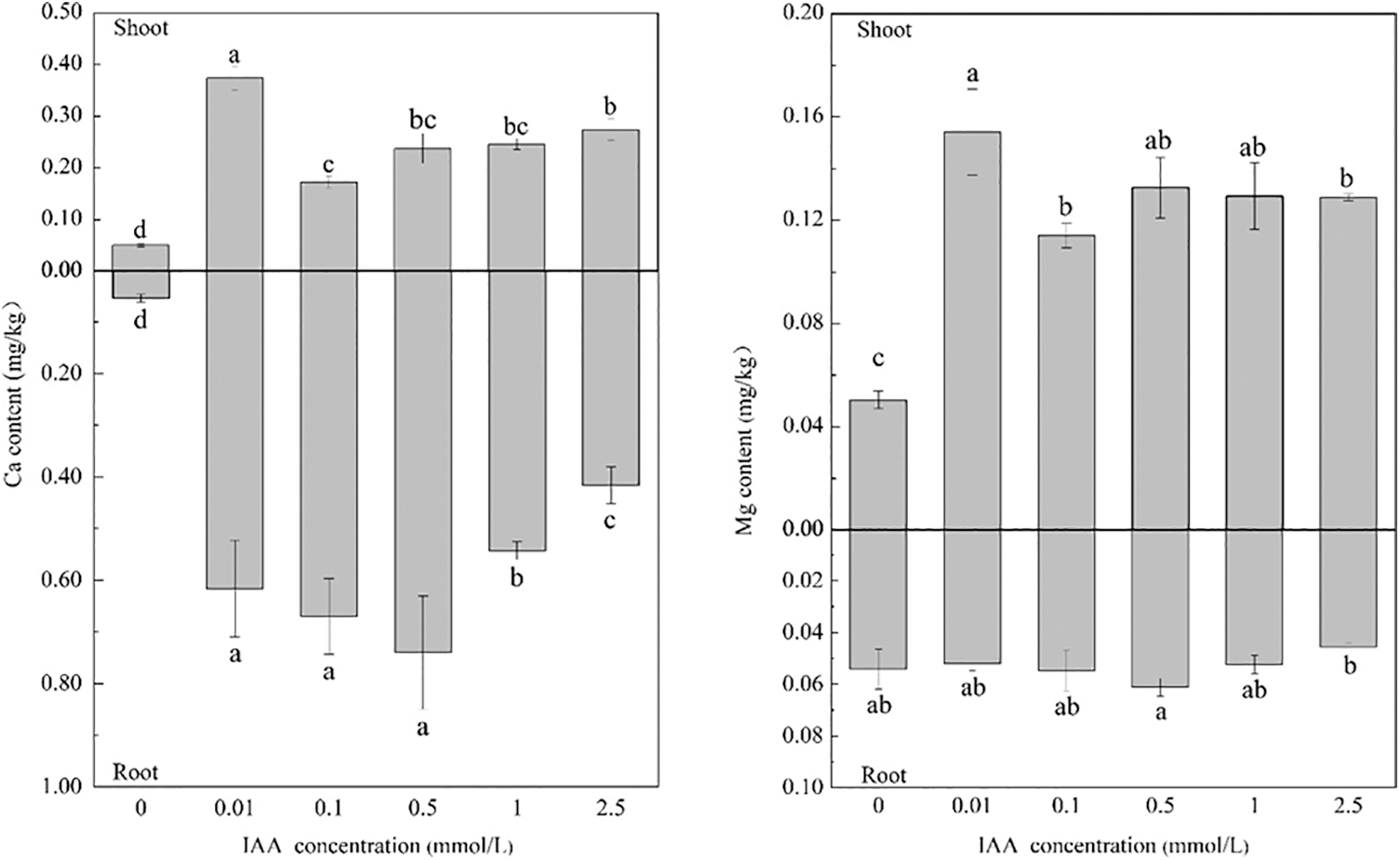
Figure 3: Effect of difference concentrations of IAA treatment on Ca and Mg content of maize under Cd stress; different little letters refer to P < 0.05 according to LSD test
3.4 The Effects of IAA on Maize Cd Content and Uptake
Under 20 mg/kg Cd stress, 0.01 mmol/L IAA resulted in significant decreases in the Cd content, and the uptake of maize shoots decreased by 51.9% and 39.55%, respectively. IAA at 0.5 and 2.5 mmol/L resulted in significant increases in Cd content and shoot uptake. The Cd content increased by 141.5% and 36.3%, respectively, and the uptake increased by 108% and 41.9%, respectively. IAA at 0.1, 0.5, 1 and 2.5 mmol/L resulted in significant decreases in Cd content and uptake of maize roots; the Cd content decreased by 58.0%, 30.0%, 32.0%, and 47.4%, respectively, and the uptake decreased by 67.2%, 42.2%, 49.9%, and 53.4%, respectively. It can be seen that 0.01 mmol/L IAA can significantly reduce the Cd content and uptake of maize shoots (Fig. 4).
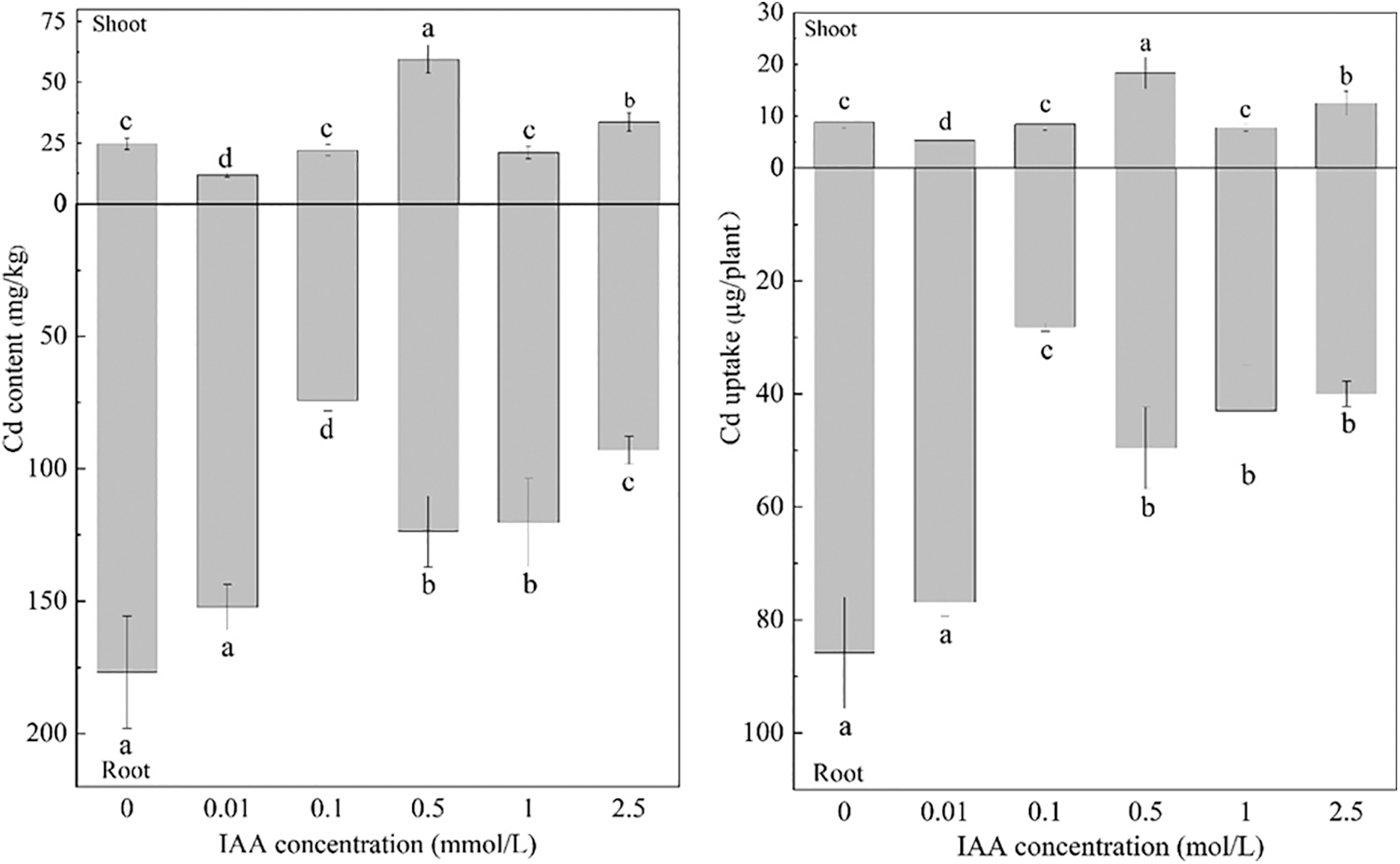
Figure 4: Effect of difference concentrations of IAA treatment on Cd content and uptake of maize; different little letters refer to P < 0.05 according to LSD test., Cd: cadmium
3.5 The Effects of IAA on the Biological Transport Factor and Transfer Factor of Cd in Maize
Under 20 mg/kg Cd stress, 0.1, 0.5, 1 and 2.5 mmol/L IAA resulted in significant increases in the biological transport factor of maize Cd of 113.8%, 244.5%, 27.3% and 159.2%, respectively. IAA (0.1, 0.5 and 2.5 mmol/L) resulted in significant increases in transfer factor of 189.3%, 258.3% and 204.7%, respectively. In general, the application of IAA can promote maize Cd from root transfer shoots (Table 2).
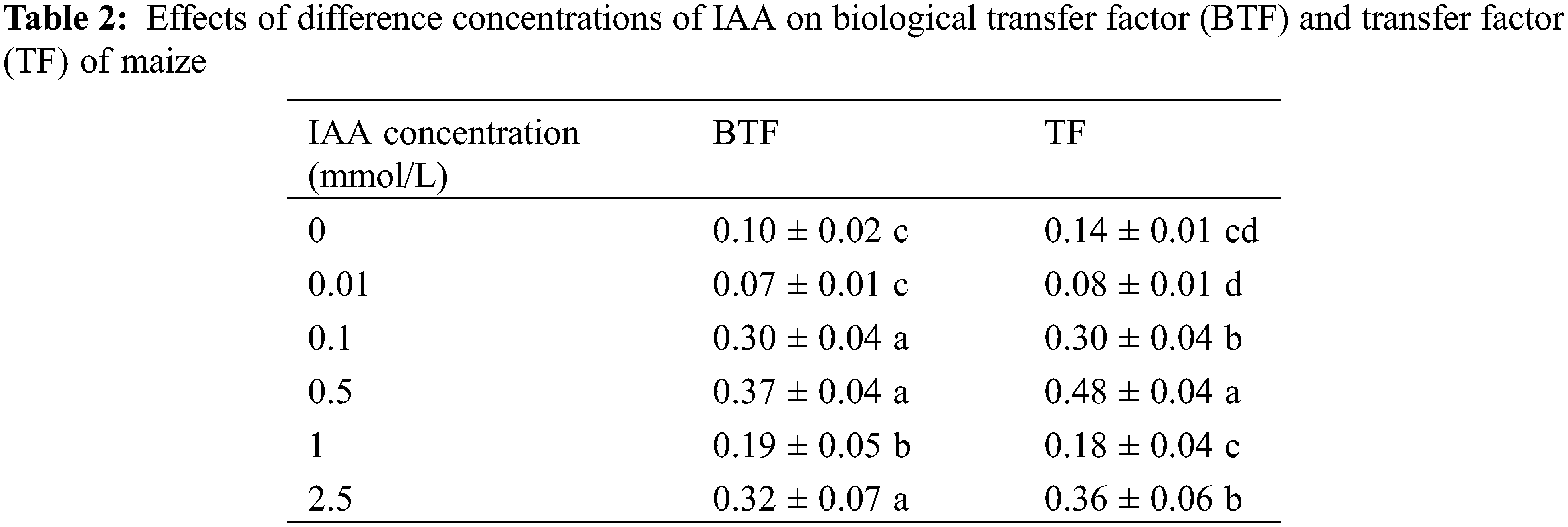
Different lowercase letters within a column indicate significant differences based on one-way analysis of variance in SPSS 21 followed by the least significant differences at the 5% confidence level.
Correlation analysis indicated that the Cd content of the shoots was significantly negatively correlated with the number of root tips. The Cd content of the roots was extremely negatively correlated with the IAA concentration, extremely positively correlated with root length and root surface area, and positively correlated with root volume. The Cd uptake of roots was extremely negatively correlated with IAA concentration, extremely positively correlated with root length and root surface area, and significantly positively correlated with root volume, root tip number and branch number (Table S1). In addition, the root Ca content was significantly negatively correlated with the Cd content (r = –0.490, P < 0.05) and the uptake amount (r = –0.528, P < 0.05).
Growth status is an effective indicator of plant resistance. Cd can inhibit plant seed germination, photosynthesis, respiration, nutrient absorption, and other processes, cause plant metabolism disorders, slow growth, and reduce biomass [19,20]. IAA can alleviate the inhibitory effect of Cd on plant growth. Khan et al. found that under 100 µmol/L Cd stress, 5 µmol/L IAA can significantly increase the fresh weight of tomato (Lycopersicum esculantum L.) shoots [21]. In this experiment, 0.01 mmol/L IAA resulted in significant increases in maize plant height and shoot biomass. This may be because IAA regulates the plant’s antioxidant system, reduces the uptake of malondialdehyde, and enhances plant photosynthesis to alleviate the damage caused by heavy metals to plants, thereby promoting plant growth [7,22]. IAA (0.5 mmol/L) resulted in significant decreases in the plant height and shoot biomass of maize seedlings. This may be due to the high concentration of IAA, which inhibited plant growth. Other studies have shown that an IAA concentration that is too high will destroy the membrane structure of plant cells, and the toxicity of IAA modulators is greater than that of naturally synthesized IAA at the same concentration [23].
The root is the first organ in which plants perceive soil abiotic stress. When roots are subjected to environmental stress, it usually manifests as a decrease in root biomass and a change in root morphology [24,25]. For example, under Cd stress, the root length, root surface area, root volume and total root tip number of peanuts (Arachis hypogaea L.) were significantly decreased, but the average root diameter was significantly increased [26]. Studies have found that IAA can activate the entire pericycle to form a lateral root primordium, split the lateral root primordium, and release pectin lyase and other substances to modify the cell wall of its surrounding cortex or epidermal cells so that the lateral roots can break through the cortex and epidermal cells for exposure, which plays important roles in plant root development and the response to environmental stress [27,28]. Under Pb stress, 10 and 100 mmol/L IAA can significantly increase the root length, root surface area and root biomass of Picris divaricata Vant [29]. IAA (0.1 nmol/L) can significantly increase sunflower (Helianthus annuus L.) root volume, root surface area, and average root diameter [30]. In this experiment, 0.01 mmol/L IAA resulted in significant increases in maize root biomass, root length, root tip number, and root tip density. This may be because IAA promoted cell division and differentiation, thereby promoting the growth of plant roots [31]. However, the regulatory effect of IAA on roots is closely related to the concentration of IAA. Under Cr stress, 10 µmol/L IAA can significantly increase pea (Pisum sativum L.) root biomass, but 100 µmol/L IAA significantly reduces root biomass [32]. In this experiment, 0.1, 0.5, 1, and 2.5 mmol/L IAA resulted in significant reductions in maize root biomass, root surface area and root volume. This may be due to the high concentration of IAA, which inhibited root development. The decrease in root biomass may be indirectly caused by the decreases in root surface area and root volume.
Both Ca and Mg are trace elements that are necessary for plant growth and are the same divalent ions as Cd, Ca and Mg have ionic radii similar to those of Cd, and the ions will compete for absorption and transport sites [33]. Under heavy metal stress, Ca or Mg can enhance cell antioxidant enzyme activity, affect root secretion of low molecular weight organic acids, and help root cells maintain a normal osmotic system [34–36]. Exogenous Ca or Mg can attenuate the inhibitory effect of Cd on plant growth [37]. Recent studies have shown that exogenous Ca mitigates Cd toxicity in plants by alleviation of growth inhibition, regulation of metal uptake and translocation, improvement of photosynthesis, mitigation of oxidative damages and the control of signal transduction in the plants [38]. Kikuchi et al. [39] reported that MgO suppression of transfer of Cd into the edible part of rice plants (Oryza sativa L. cv. Kinuhikari) from the contaminated soils. But there are few studies on the interaction mechanism between plant exogenous Mg and Cd, most studies are about the effect of magnesium deficiency on plant Cd accumulation. Wu et al. [37] review that deficient Mg facilitates Cd accumulation is beneficial for Cd phytoextraction plants. Studies have found that IAA is directly involved in the transportation of nutrient elements. For example, changes in plant endogenous IAA content will affect the absorption of nitrogen by plant roots [40]. In this experiment, IAA treatment significantly increased the plant Ca and shoot Mg contents. The increase in Ca content may be related to the regulation of Ca2+-ATPase by IAA [41]. Dindas et al. [42] performed measurements with scanning Ca2+-selective extracellular electrodes in Arabidopsis thaliana root hairs, to test whether IAA triggers a calcium influx. The study suggested that IAA provoked a transient influx of Ca2+, IAA triggers elevation of cytosolic calcium in root hairs. IAA triggers cytoplasmic Ca2+ transients, which require SCFTIR1/AFB-based auxin signaling, and transcriptional analyses revealed that Ca2+-dependent, feedback control circuit within a functional signaling pathway consisting of AUX1 mutants, SCFTIR1/AFB receptor and CNGC14 Ca2+ channel. But the mechanism by which IAA increases the Mg content of maize shoots still needs to be studied.
In this study, 0.01 mmol/L IAA resulted in significant decreases in the Cd content and uptake of maize shoots, while 0.5 and 2.5 mmol/L IAA resulted in significant increases in the Cd content and uptake of maize shoots. Correlation analysis indicated that the Cd content of the shoots was significantly negatively correlated with the number of root tips. This may be because under 0.01 mmol/L IAA, the number of root tips increased significantly, which promoted maize growth, diluted the Cd in the plant, increased the content of hemicellulose 1, and prevented the transfer of Cd from roots to shoots [43]. While 0.5 and 2.5 mmol/L IAA significantly inhibited the growth of maize, the increase in Cd content may be due to the enhancement of transpiration or stomatal conductance of plants by IAA, which promotes the Cd absorption of roots and the transfer from roots to shoots [44]. This study also found that 0.1, 0.5, 1, and 2.5 mmol/L IAA resulted in significant decreases in the Cd content and uptake of maize roots. The root Cd content was significantly or extremely negatively correlated with the IAA concentration and root Ca content and was significantly or extremely positively correlated with root length, root surface area, and root volume. Root Cd uptake was significantly or extremely negatively correlated with IAA concentration, and root Ca content was significantly or extremely negatively correlated with root length, root surface area, root volume, root tip number, and branch number. This shows that IAA inhibits root development and promotes the absorption of the nutrient element Ca, which is one of the main reasons why IAA reduces the absorption and uptake of Cd in maize roots.
Biological transport factors and transfer factors are indicators to measure the ability of plants to transport heavy metals from roots to shoots after absorbing heavy metals [45,46]. In this study, 0.1, 0.5, 1, and 2.5 mmol/L IAA resulted in a significant increase in the biological transport factor, and 0.1, 0.5, and 2.5 mmol/L IAA resulted in a significant increase in the transfer factor. However, the Cd biological transport factor and the transfer factor of maize under the six IAA concentrations were less than 0.5, indicating that the ability of maize to transport from roots to shoots was weak. This may be related to the low-uptake maize variety Huidan No. 4 used as the experimental material in this experiment.
Under 20 mg/kg Cd stress, 0.01 mmol/L IAA changed maize root morphology and promoted mineral element uptake in maize, decreased the Cd content and uptake in shoots, and promoted maize growth. In conclusion, the application of optimal concentrations can help maize adapt to cadmium stress. Therefore, this study may help to better understand the role of phytohormones in promoting Cd tolerance in host plants.
Funding Statement: This work was financially supported by the National Natural Science Foundation of China (No. 41877130), the Key Project of Yunnan Agricultural Foundation (2017FG001-014), and the Reserve Talents Fund for Young and Middle-Aged Academic and Technological Leaders in Yunnan Province (Nos. 2018HB043 and 202005AC160038).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Seregin, I. V., Kozhevnikova, A. D. (2008). Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel and strontium. Russian Journal of Plant Physiology, 55(1), 1–22. DOI 10.1134/S1021443708010019. [Google Scholar] [CrossRef]
2. Rizwan, M., Ali, S., Rehman, M. Z. U., Maqbool, A. (2019). A critical review on the effects of zinc at toxic levels of cadmium in plants. Environmental Science and Pollution Research, 26(7), 6279–6289. DOI 10.1007/s11356-019-04174-6. [Google Scholar] [CrossRef]
3. Zeng, P., Guo, Z., Xiao, X., Peng, C., Liu, L. et al. (2019). Physiological stress responses, mineral element uptake and phytoremediation potential of Morus alba L. in cadmium-contaminated soil. Ecotoxicology and Environmental Safety, 189(15), 109973. DOI 10.1016/j.ecoenv.2019.109973. [Google Scholar] [CrossRef]
4. Hu, Y. F., Zhou, G. Y., Na, X. F., Yang, L. J., Nan, W. B. et al. (2013). Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. Journal of Plant Physiology, 170(11), 965–975. DOI 10.1016/j.jplph.2013.02.008. [Google Scholar] [CrossRef]
5. Zhao, F. Y., Hu, F., Zhang, S. Y., Wang, K., Zhang, C. R. et al. (2013). MAPKs regulate root growth by influencing auxin signaling and cell cycle-related gene expression in cadmium-stressed rice. Environmental Science and Pollution Research, 20(8), 5449–5460. DOI 10.1007/s11356-013-1559-3. [Google Scholar] [CrossRef]
6. San-Francisco, S., Houdusse, F., Zamarreñ0, A. M., Garnica, M., Casanova, E. et al. (2005). Effects of IAA and IAA precursors on the development, mineral nutrition, IAA content and free polyamine content of pepper plants cultivated in hydroponic conditions. Scientia Horticulturae, 106(1), 38–52. DOI 10.1016/j.scienta.2005.03.006. [Google Scholar] [CrossRef]
7. Ran, J., Zheng, W., Wang, H., Wang, H., Li, Q. (2020). Indole-3-acetic acid promotes cadmium (Cd) accumulation in a Cd hyperaccumulator and a non-hyperaccumulator by different physiological responses. Ecotoxicology and Environmental Safety, 191, 110213. DOI 10.1016/j.ecoenv.2020.110213. [Google Scholar] [CrossRef]
8. Šípošová, K., Labancová, E., Kučerová, D., Kollárová, K., Vivodová, Z. (2021). Effects of exogenous application of indole-3-butyric acid on maize plants under cadmium stress. Research Square. DOI 10.21203/rs.3.rs-416113/v1. [Google Scholar] [CrossRef]
9. Zhang, C., He, Q., Wang, M., Gao, X., Shen, C. (2019). Exogenous indole acetic acid alleviates Cd toxicity in tea (Camellia sinensis). Ecotoxicology and Environmental Safety, 190, 110090. DOI 10.1016/j.ecoenv.2019.110090. [Google Scholar] [CrossRef]
10. Redjala, T., Zelko, I., Sterckeman, T., Legué, V., Lux, A. (2011). Relationship between root structure and root cadmium uptake in maize. Environmental and Experimental Botany, 71(2), 241–248. DOI 10.1016/j.envexpbot.2010.12.010. [Google Scholar] [CrossRef]
11. Wei, L., Huang, Y., Huang, L., Huang, Q., Li, Y. et al. (2021). Combined biochar and soda residues increases maize yields and decreases grain Cd/Pb in a highly Cd/Pb-polluted acid Udults soil. Agriculture, Ecosystems and Environment, 306(3), 107198. DOI 10.1016/j.agee.2020.107198. [Google Scholar] [CrossRef]
12. He, Y. M., Yang, Z. X., Li, M. R., Jiang, M., Zhan, F. D. et al. (2017). Effects of a dark septate endophyte (DSE) on growth, cadmium content, and physiology in maize under cadmium stress. Environmental Science Pollution Research, 24(2), 18494–18504. DOI 10.1007/s11356-017-9459-6. [Google Scholar] [CrossRef]
13. Yu, Z. H., Zhao, X. L., Su, L., Yan, K., Li, B. et al. (2021). Effect of an arbuscular mycorrhizal fungus on maize growth and cadmium migration in a sand column. Ecotoxicology and Environmental Safety, 225, 112782. DOI 10.1016/j.ecoenv.2021.112782. [Google Scholar] [CrossRef]
14. He, Y. M., Fan, X. M., Zhang, G. Q., Li, B., Li, T. G. et al. (2020). Effects of arbuscular mycorrhizal fungi and dark septate endophytes on maize performance and root traits under a high cadmium stress. South African Journal of Botany, 134, 415–423. DOI 10.1016/j.sajb.2019.09.018. [Google Scholar] [CrossRef]
15. Bao, S. D. (2000). Soil and agricultural chemistry analysis (3rd ed.). Beijing, China: Chinese Agriculture Publication. [Google Scholar]
16. Li, X. F., Zhang, Z. L. (2016). Experimental guidance of plant physiology (5th ed.). Beijing, China: Higher Education Press. [Google Scholar]
17. Wang, H., Chen, W., Sinumvayabo, N., Li, Y., Zhang, Y. (2020). Phosphorus deficiency induces root proliferation and Cd absorption but inhibits Cd tolerance and Cd translocation in roots of Populus × euramericana. Ecotoxicology and Environmental Safety, 204(2), 111148. DOI 10.1016/j.ecoenv.2020.111148. [Google Scholar] [CrossRef]
18. Li, C. X., Shao, Y., Jiang, L. N. (2008). Biostatistics (5th ed.). Beijing, China: Science Press. [Google Scholar]
19. Chen, J., Duan, B., Xu, G., Korpelainen, H., Niinemets, ü et al. (2016). Sexual competition affects biomass partitioning, carbon-nutrient balance, Cd allocation and ultrastructure of Populus cathayana females and males exposed to Cd stress. Tree Physiology, 36(11), 1353–1368. DOI 10.1093/treephys/tpw054. [Google Scholar] [CrossRef]
20. Khanna, K., Jamwal, V. L., Gandhi, S. G., Ohri, P., Bhardwaj, R. (2019). Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Scientific Reports, 9(1), 1–14. DOI 10.1038/s41598-019-41899-3. [Google Scholar] [CrossRef]
21. Khan, M. Y., Prakash, V., Yadav, V., Chauhan, D. K., Prasad, S. M. et al. (2019). Regulation of cadmium toxicity in roots of tomato by indole acetic acid with special emphasis on reactive oxygen species production and their scavenging. Plant Physiology Biochemistry, 142(14), 193–201. DOI 10.1016/j.plaphy.2019.05.006. [Google Scholar] [CrossRef]
22. Bashri, G., Prasad, S. M. (2015). Indole acetic acid modulates changes in growth, chlorophyll a fluorescence and antioxidant potential of Trigonella foenum-graecum L. grown under cadmium stress. Acta Physiologiae Plantarum, 37(3), 49. DOI 10.1007/s11738-014-1745-z. [Google Scholar] [CrossRef]
23. Hąc-Wydro, K., Sroka, A., Jabłońska, K. (2016). The impact of auxins used in assisted phytoextraction of metals from the contaminated environment on the alterations caused by lead (II) ions in the organization of model lipid membranes. Colloids and Surfaces B: Biointerfaces, 143, 124–130. DOI 10.1016/j.colsurfb.2016.03.018. [Google Scholar] [CrossRef]
24. Schiefelbein, J. W., Benfey, P. N. (1991). The development of plant roots: New approaches to underground problems. Plant Cell, 3(11), 1147–1154. DOI 10.1105/tpc.3.11.1147. [Google Scholar] [CrossRef]
25. He, J. Y., Zhu, C., Ren, Y. F., Jiang, D. A., Sun, Z. X. (2007). Root morphology and cadmium uptake kinetics of the cadmium-sensitive rice mutant. Biologia Plantarum, 51(4), 791–794. DOI 10.1007/s10535-007-0162-1. [Google Scholar] [CrossRef]
26. Ostonen, I., Püttsepp, Ü., Biel, C., Alberton, O., Bakker, M. R. et al. (2007). Specific root length as an indicator of environmental change. Plant Biosystems, 141(3), 426–442. DOI 10.1080/11263500701626069. [Google Scholar] [CrossRef]
27. Du, Y., Scheres, B. (2018). Lateral root formation and the multiple roles of auxin. Journal of Experimental Botany, 69(2), 155–167. DOI 10.1093/jxb/erx223. [Google Scholar] [CrossRef]
28. Péret, B., Rybel, B. D., Casimiro, I., Benková, E., Bennett, M. J. (2009). Arabidopsis lateral root development: An emerging story. Trends in Plant Science, 14(7), 399–408. DOI 10.1016/j.tplants.2009.05.002. [Google Scholar] [CrossRef]
29. Du, R. J., He, E. K., Tang, Y. T., Hu, P. J., Ying, R. R. et al. (2011). How phytohormone IAA and chelator EDTA effect lead uptake by Pb/Zn hyperaccumulator Picris divaricate. International Journal of Phytoremediation, 13(10), 1024–1036. DOI 10.1080/15226514.2010.549862. [Google Scholar] [CrossRef]
30. Faessler, E., Evangelou, R. W., Robinson, R. H., Schulin, R. (2010). Effects of indole-3-acetic acid (IAA) on sunflower growth and heavy metal uptake in combination with ethylene diamine disuccinic acid (EDDS). Chemosphere, 80(8), 901–907. DOI 10.1016/j.chemosphere.2010.04.077. [Google Scholar] [CrossRef]
31. Fu, X. D., Harberd, N. P. (2003). Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature, 421(6924), 740–743. DOI 10.1038/nature01387. [Google Scholar] [CrossRef]
32. Gangwar, S., Singh, V. P. (2011). Indole acetic acid differently changes growth and nitrogen metabolism in Pisum sativum L. seedlings under chromium (VI) phytotoxicity: Implication of oxidative stress. Scientia Horticulturae, 129(2), 321–328. DOI 10.1016/j.scienta.2011.03.026. [Google Scholar] [CrossRef]
33. Khaliq, M. A., James, B., Yan, H. C., Saqib, H., Wang, G. (2018). Uptake, translocation, and accumulation of Cd and its interaction with mineral nutrients (Fe, Zn, Ni, Ca, Mg) in upland rice. Chemosphere, 215, 916–924. DOI 10.1016/j.chemosphere.2018.10.077. [Google Scholar] [CrossRef]
34. Lu, L., Tian, S., Zhang, M., Zhang, J., Yang, X. et al. (2010). The role of Ca pathway in Cd uptake and translocation by the hyperaccumulator Sedum alfredii. Journal of Hazardous Materials, 183(1), 22–28. DOI 10.1016/j.jhazmat.2010.06.036. [Google Scholar] [CrossRef]
35. Magdziak, Z., Kozlowska, M., Kaczmarek, Z., Mleczek, M., Chadzinikolau, T. et al. (2011). Influence of Ca/Mg ratio on phytoextraction properties of Salix viminalis. II. Secretion of low molecular weight organic acids to the rhizosphere. Ecotoxicology and Environmental Safety, 74(1), 33–40. DOI 10.1016/j.ecoenv.2010.09.003. [Google Scholar] [CrossRef]
36. Drzewiecka, K., Mleczek, M., Gąsecka, M., Magdziak, Z., Chadzinikolau, T. (2014). Copper phytoextraction with Salix purpurea×viminalis under various Ca/Mg ratios, Effect on organic acid, phenolics and salicylic acid contents. Acta Physiologiae Plantarum, 36(4), 903–913. DOI 10.1007/s11738-013-1469-5. [Google Scholar] [CrossRef]
37. Wu, J., Li, R., Lu, Y., Bai, Z. (2021). Sustainable management of cadmium-contaminated soils as affected by exogenous application of nutrients: A review. Journal of Environmental Management, 295, 113081. DOI 10.1016/j.jenvman.2021.113081. [Google Scholar] [CrossRef]
38. Huang, D., Gong, X., Liu, Y., Zeng, G., Lai, C. et al. (2017). Effects of calcium at toxic concentrations of cadmium in plants. Planta, 245(5), 863–873. DOI 10.1007/s00425-017-2664-1. [Google Scholar] [CrossRef]
39. Kikuchi, T., Okazaki, M., Kimura, S. D., Motobayashi, T., Baasansuren, J. et al. (2008). Suppressive effects of magnesium oxide materials on cadmium uptake and accumulation into rice grains: II: Suppression of cadmium uptake and accumulation into rice grains due to application of magnesium oxide materials. Hazard Mater, 154(1–3), 294–299. DOI 10.1016/j.jhazmat.2007.10.025. [Google Scholar] [CrossRef]
40. Ma, W., Li, J., Qu, B., Xue, H., Zhao, X. et al. (2014). Auxin biosynthetic gene TAR2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. Plant Journal, 78(1), 70–79. DOI 10.1111/tpj.12448. [Google Scholar] [CrossRef]
41. Navarro-Avino, J. P., Bennett, A. B. (2005). Role of a Ca2+-ATPase induced by ABA and IAA in the generation of specific Ca2+ signals. Biochemical and Biophysical Research Communications, 329(1), 406–415. DOI 10.1016/j.bbrc.2005.01.142. [Google Scholar] [CrossRef]
42. Dindas, J., Scherzer, S., Roelfsema, M. R. G., Meyer, K. V., Müller, H. M. et al. (2018). AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling. Nature Communications, 9(1), 1174. DOI 10.1038/s41467-018-03582-5. [Google Scholar] [CrossRef]
43. Xiao, F. Z., Zhi, W. W., Fang, D., Gui, J. L., Yuan, Z. S. et al. (2013). Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. Journal of Hazardous Materials, 263(239), 398–403. DOI 10.1016/j.jhazmat.2013.09.018. [Google Scholar] [CrossRef]
44. Chaoui, A., Jarrar, B., Ferjani, E. E. (2004). Effects of cadmium and copper on peroxidase, NADH oxidase and IAA oxidase activities in cell wall, soluble and microsomal membrane fractions of pea roots. Journal of Plant Physiology, 161(11), 1225–1234. DOI 10.1016/j.jplph.2004.02.002. [Google Scholar] [CrossRef]
45. Sun, Y., Zhou, Q., Diao, C. (2008). Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. Bioresource Technology, 99(5), 1103–1110. DOI 10.1016/j.biortech.2007.02.035. [Google Scholar] [CrossRef]
46. Dhananjay, K., Sushil, K. B., Sangeeta, A. (2018). Bioaccumulation and biochemical responses of Vetiveria zizanioides grown under cadmium and copper stresses. Environmental Sustainability, 1(2), 133–139. DOI 10.1007/s42398-018-0009-z. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |