

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.022679
ARTICLE
Allantoin Alleviates Seed Germination Thermoinhibition in Arabidopsis
Shanghai Key Laboratory of Bio-Energy Crops, Research Center for Natural Products, Plant Science Center, School of Life Sciences, Shanghai University, Shanghai, 200444, China
*Corresponding Author: Ping Li. Email: liping80@shu.edu.cn
Received: 21 March 2022; Accepted: 06 April 2022
Abstract: Allantoin as the metabolite of purine catabolism can store and remobilize nitrogen for plant growth and development. However, emerging evidence suggests it also contributes to plant tolerance to stress response through altering abscisic acid (ABA) and reducing reactive oxygen species (ROS) level. 1-CYS PEROXIREDOXIN (PER1) is a seed-specific antioxidant that enhances seed longevity through scavenging ROS over-accumulation. High temperature (HT) suppresses seed germination and induces seed secondary dormancy, called as seed germination thermoinhibition. However, the mechanism that allantoin and PER1 regulate seed germination thermoinhibition remains unknown. In this study, we reported that allantoin treatment enhances seed germination under HT stress. Consistently, the aln mutants displayed higher seed germination, as well as more accumulation of endogenous allantoin, than that of wild-type control. Further biochemical and genetic analyses showed that allantoin reduces ABA content under HT, and allantoin targets PER1 to efficiently scavenge HT-induced ROS accumulation, meanwhile, the function of allantoin requires PER1 during seed gemination thermotolerance. Collectively, our finding proposes a novel function of allantoin in enhancing seed germination tolerance to HT, and uncovers the underlying mechanism by which allantoin regulates seed germination through altering ABA metabolism and PER1-mediated ROS level under HT stress.
Keywords: Seed germination; high temperature; allantoin; PRE1
Plant seed as the main food resource, provides us the energy during human civilization, and the seed production, or crop yield is important for the sustainable agriculture development. Seed germination under favorable condition or dormancy during seed mature to avoid pre-harvest sprouting is also essential for seed quality [1–4]. Seed germination or dormancy is strictly controlled by endogenous phytohormones and environmental factors. Among various phytohormones, gibberellin acid (GA) and abscisic acid (ABA) determine seed germination or dormancy status. GA promotes seed germination, whereas ABA induces seed dormancy [4–8]. Accordingly, a series of mutants deficiency in ABA biosynthesis show less seed dormancy, while mutants with low GA biosynthesis ability show higher seed dormancy. Besides GA/ABA biosynthesis, the component for GA/ABA perception and signal transduction also contribute to seed germination. For example, the transcriptional factors ABI3, ABI4 and ABI5 negatively regulate seed germination [9–14], the DELLAs proteins including GIBBERELLIN INSENSITIVE (GAI), REPRESSOR OF GAL-3 (RGA), RGA-LIKE1 (RGL1), RGL2 and RGL3, repress GA response to delay seed germination. However, GA treatment induces the degradation of DELLAs through E3 ubiquitin ligase SLY1 and GA receptor GID1a/b/c [6,15,16]. What is more, other plant hormones, such as auxin, ethylene and jasmine acid also regulate seed germination by interacting with GA and ABA signal directly or indirectly [17–20]. Besides plant hormones, seed also perceives environmental temperature to ensure germination under favorable condition. And low temperature treatment, such as cold stratification can sufficiently break seed dormancy through PIF1 or SPT [21]. On the contrary, non-dormant seed can be temporarily blocked for germination once exposing to unfavorable high temperature. Such phenome is called as seed germination thermoinhibition [2,22,23]. Expression of many genes involved in ABA, GA and ethylene biosynthesis, metabolism, and response is differentially regulated by high temperature in lettuce [22,24]. Transgenic expressing NCED4 encoding a key regulatory enzyme in ABA biosynthesis results in thermoinhibition, whereas silencing NCED4 results in the loss of thermoinhibition, suggesting that NCED4 is required for thermoinhibition of lettuce seed responding to elevated temperature [24]. Conversely, expressing ETHYLENE RESPONSE FACTOR1 (LsERF1) can promote seed germination by upregulating gibberellin biosynthesis to counter the inhibitory effect of abscisic acid [25]. With the global warming condition, genetic modification of the crop with high temperature tolerance feature shows very important for stable high yield. Therefore, understanding the mechanism underlying seed germination thermotolerance and developing a novel strategy to enhance seed germination tolerance under HT shows more important.
Allantoin as a nitrogen-rich heterocyclic compound is widely existed in plants as an intermediary metabolite of purine catabolism. Allantoin and its acyclic metabolite allantoides can store and transport symbiotically fixed nitrogen in tropic legume plants, and it also serves for nitrogen recycling and remobilization in non-legume plant through sequential degradation of the purine ring and finally releases four molar equivalents of ammonia [26,27]. Accumulated evidence demonstrates that allantoin acts as a major purine metabolite in plant under different stress conditions, such as saline, drought, cold, darkness and pathogen invasion, etc. Knocking out XANTHINE DEHYDROGENASE (XDH) is defective in xanthine oxidation during purine metabolism, also impairs plant stress tolerance and presents early senescence, but such defective phenotype could be reversed by supplementation with allantoin or its precursor uric acid, a product of XDH. Allantoinase converts allantoin into allenoate acid in plants, and the Arabidopsis aln mutant has constitutive high accumulation of allantoin, which enhances seedling tolerance of drought and osmotic stress [26,28]. Exogenous allantoin treatment efficiently scavenges ROS accumulation and increases activity of superoxide dismutase and ascorbate peroxidase under cadmium treatment [29]. 1-CYS PEROXIREDOXIN (PER1) is a seed-specific antioxidant in many plants that uses cysteine residues to scavenge ROS. And ectopic expression of its Nymphaea tetragona (sacred lotus) homolog NnPER1 enhances seed vigor and longevity in Arabidopsis [30,31]. Application with allantoin also activates the critical steps in de-novo ABA biosynthesis and hydrolysis of ABA-glucose conjugate, but does not alter the response to ABA [26]. This evidence suggests the wide role of allantoin, not just as the nitrogen metabolism. However, its function in controlling seed germination is not investigated so far.
As allantoin can alter ABA metabolism and plant’s response to cold stress [32,33], we wonder its function in seed germination under HT stress. Using our previous experimental system, we found that ambient HT induced the quick accumulation of allantoin, and additional allantoin, or aln mutant also alleviated the inhibition effect of HT on seed germination. Furthermore, we found allantoin altered ABA metabolism and reduced ROS accumulation to enhance seed germination. Combination with biochemical and genetic analysis revealed that allantoin activated the expression of PER1 to decrease HT-induced ROS accumulation, ultimately enhanced seed germination tolerance. Thus, our finding identifies a novel function of allantoin in controlling seed germination under HT and uncovers the underlying mechanism that allantoin enhances seed germination thermotolerance through activating PER1-mediated antioxidant system and altering ABA biosynthesis.
2.1 Plant Materials and Growth
Arabidopsis thaliana accession Columbia-0 (Col0) was used as the wild type in this study. The T-DNA inserted seeds of aln-1 (Salk_000325), aln-2 (Salk_146783) and per-1 (Salk_036808) were obtained from ABRC (Arabidopsis Biological Resource Center at the Ohio State University, https://abrc.osu.edu/). To identify homozygous T-DNA insertion lines, PCR-based genotyping of each mutant was performed using a gene-specific primer flanking the insertion in combination with a left-border T-DNA-specific LBa1 primer as described before. Related primers were listed as supplemental Table 1. Surface-sterilized seeds of WT and transgenic plants were sown in 0.1% (w/v) agar plates of standard medium consisting of half-strength Murashige-Skoog (1/2MS) basal salt and 1% (w/v) sucrose. After incubation at 4°C, for 2 d, the plates were placed in a growth cabinet maintained at 22°C under white fluorescent light with a 16 h photoperiod (100 mol photons m–2s) for 10 d, and the seedling was moved into the soil for growing in the green house (100 mol photons m–2s white light, 16 h light/8 h dark) for six to eight weeks for harvested seeds. The seed was harvested at the same time, and was used for the germination percentage testing.
The freshly harvested seed was dried with silica gel for 1–2 months, and seed germination was tested as described method before [23,34]. Briefly, dried seed was imbibed for 3 h after surface sterilizing with 5% (v/v) hypochlorite and 0.02% (v/v) Triton X-100 solution. After 10 min of sterilization, seeds were washed with sterilized water for three times, and sowed on the 1/2MS germination medium supplement with 1% sucrose under constant white light condition (50 μmol m−2 s−1, 22°C) to initiate seed germination. For HT treatment, the plates were placed in the growth cabinet at 32°C to test the effect of high temperature on seed germination. The seed with radical protruded from the seed coat was recorded as the germination. The germinated seeds were observed with stereoscope and the germination percentage was calculated. For each germination assay, at least three biological replicate experiments were performed.
2.3 Allantoin Content Analyses
About 20 mg of germinated seeds by various treatments were ground using liquid nitrogen. Quantification of allantoin was determined by HPLC using an organic acid column and using a mobile phase of 2.5 mM H2SO4. The allantoin standard compound was detected using standard retention time. To prepare the standards, known concentrations of allantoin (Sigma-Aldrich, St. Louis, MO, USA) were used. Both standard allantoin and seed samples were prepared in a solution of ddH2O as solvent. The flow rate was 0.5 mL min−1 and the injection volume was between 10–50 μL. The total run time with this method was 45 min and the compounds were detected at 190 nm using a diode-array detector.
The germinated seeds after different treatments were collected for extracting total RNA. Total RNA was extracted and reversely transcribed to cDNA as described method [23,35]. The relative gene expression was determined by quantitative real-time (qPCR) analysis. RT-qPCR analysis was performed on Roche real-time thermal cycler using SYBR GREEN Real-time PCR master mixture (Roche). Gene-specific primers were listed as Supplemental Table 1. Data represent three biological replicates each consisting of three technical replicates.
The coding region of ALN was obtained by PCR amplification with Primer STAR enzyme (Takara) and cloned into the pRI101-6Flag vector to generate pRI101-ALN-Flag construction. This construct was then introduced into Arabidopsis thaliana Col0 plants by Agrobacterium tumefaciens-mediated transformation [35]. The transgenic seeds were screened on the 1/2MS medium additionally with Kanamycin at 50 mg/L, and several individual lines were obtained by western blotting analysis using anti-Flag antibody.
2.6 Quantification of ROS Level
To test the effect of exogenous allantoin and HT on accumulation of ROS in the germinated seeds, H2O2 levels were determined as reported method [30]. In brief, about 20 mg aliquot of seeds were ground in the liquid nitrogen and extracted in the 250 μL of potassium phosphate buffer (20 mM, pH6.0) containing 5 mM scopoletin (Sigma) and 1 U/mL (final concentration) horseradish peroxidase. H2O2 concentration was measured by the decrease in fluorescence (excitation, 346 nm; emission, 455 nm) of the incubation medium. The total ROS level in the seed was determined using the ROS probe dichlorofluorescein-diacetate (DCFH-DA) as the previous method [30].
3.1 Allantoin Improves Seed Germination under High Temperature Stress
To determine the effect of allantoin on the seed germination under HT, we compared the dose effect of allantoin on seed germination percentage under HT. As shown in Fig. 1A, ambient HT at 32°C obviously suppressed the seed germination for wild type Col line, but additional exogenous allantoin alleviated the inhibition effect of HT on seed germination. And increasing the concentration of allantoin from 1 mg/L to 50 mg/L gradually promoted the seed germination of Col under HT, but such accelerating effect was not obvious for allantoin from over 50 mg/L. Thus, we selected allantoin at 50 mg/L for further study in our experiments. We also compared the positive effect of allantoin on seed germination at different ambient high temperature, and found allantoin treatment obviously increased the seed germination of Col under ambient temperature from 28°C to 32°C (Fig. 1B). Therefore, we propose that allantoin treatment promotes seed germination under HT stress.
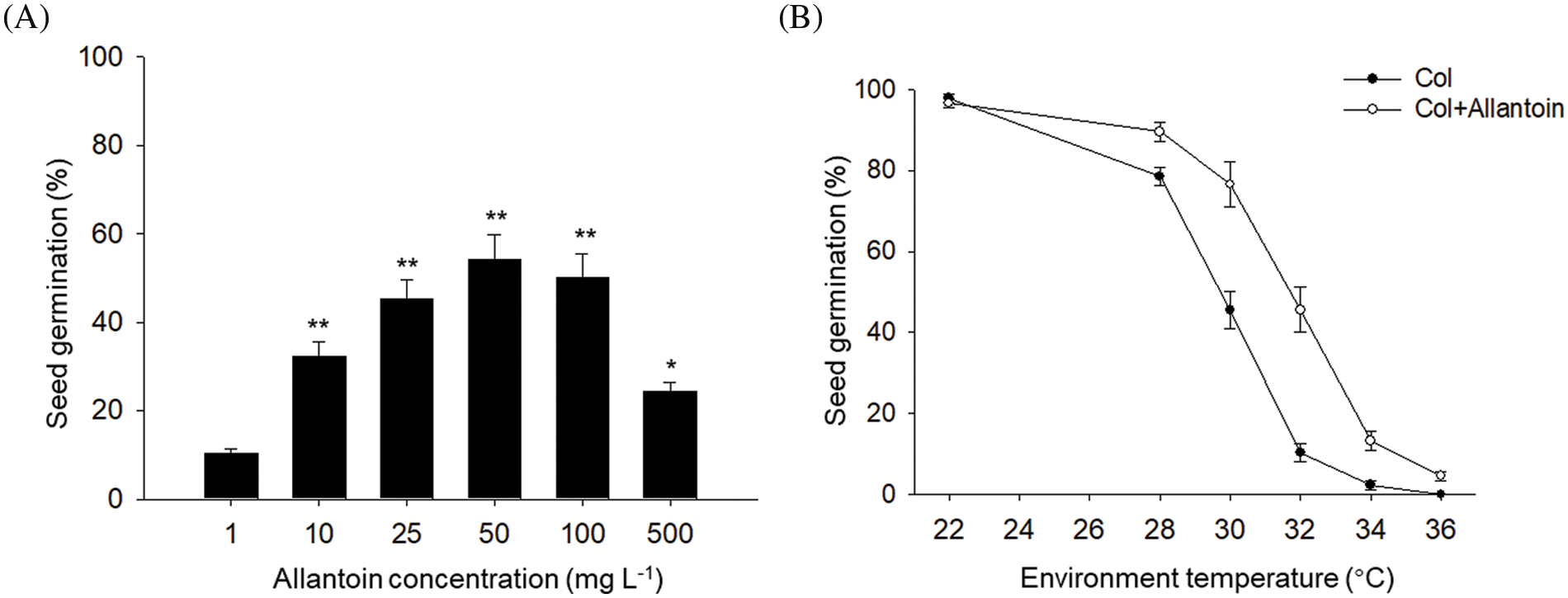
Figure 1: Allantoin treatment increased seed germination under HT stress. (A) Dose effect of allantoin on seed germination under HT stress. The allantoin at indicated concentration was used to treat the imbibed Col seed under HT for 3 d, and seed germination percentage was calculated. (B) The effect of ambient environmental temperature on seed germination. The gradient ambient temperature was used to test the effect of allantoin at 50 mg/L on seed germination. The values are shown as means ± SD of triplicate experiments. Asterisks indicate significant difference between Col at 1 mg/L and other allantoin concentration, as determined by Student’s t test (*P < 0.05, **P < 0.01)
3.2 Genetic Modifications of Allantoin Alter the Seed Germination under HT Stress
In Arabidopsis, allantoin content is controlled by ALN gene. Here we tested the effect of HT on the expression level of ALN. As shown in Fig. 2A, HT treatment at 32°C rapidly induced the expression of ALN during the first 24 h period, and then dropped down during the following 36 h. As the control, the expression ALN did not show obvious change in the germinated seed under 22°C, suggesting that allantoin induced the transiently increase of ALN under HT stress. To further test the function of ALN in controlling seed germination under HT, we obtained the ALN-null line, aln-1 and aln-2, in which the T-DNA fragment was inserted in the twelfth and eleventh exon (Fig. 2B). RT-qPCR analysis showed such T-DNA abolished the functional transcripts of ALN in two aln-null lines (Fig. 2C). Meanwhile, we also generated the transgenic lines by expressing of ALN cDNA fused with Flag under the control of the constitutive 35S promoter. Western blotting analysis showed the strong immunoblotting signal in several individual lines using the anti-Flag (Fig. 2D). We then measured endogenous allantoin content of aln-1, aln-2, and the transgenic ALN-Flag line, and observed the high level of allantoin in aln-1 and aln-2 lines, but relatively lower level of allantoin in several ALN-Flag lines (Fig. 2E), which was consistence with the previous results that ALN was responsible for allantoin catabolism in planta. We then compared the seed germination difference among aln-null mutants and the transgenic lines. As shown in Fig. 2F, we found the aln-1 and aln-2 showed higher germination percentage, while the ALN-Flag line showed lower seed germination rate, compared with Col line under HT stress. Therefore, these genetic data suggest that allantoin enhances seed germination tolerance to HT stress.
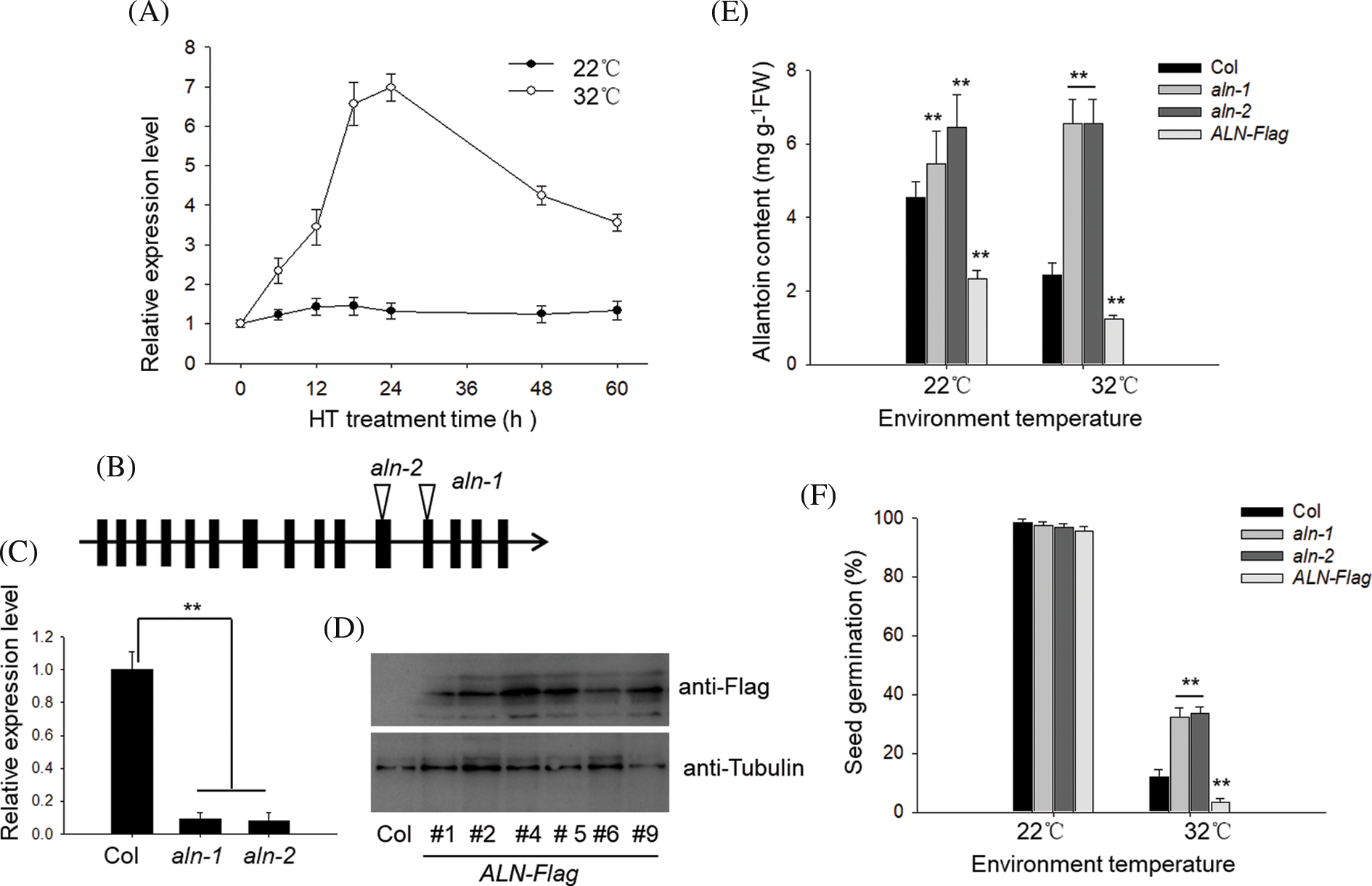
Figure 2: HT induced the biosynthesis of allantoin in the imbibed seeds. (A) The different effects of temperature on the transcriptional expression of ALN in the imbibed seeds. Imbibed seeds of wild type Col were treated with temperature at 22°C or 32°C for the indicated time, and the transcriptional expression of ALN was measured by RT-qPCR. (B&C&D) Confirmation of the aln-null T-DNA insertion mutants and transgenic ALN-Flag lines. (B) The T-DNA location sites were shown, and specific primers were used to confirm the T-DNA insertion both aln-1 and aln-2 mutants. (C) The transcriptional level of ALN was measured by RT-qPCR analysis. (D) The transgenic lines overexpressing ALN-Flag were also measured by anti-Flag antibody. (E) The different content of allantoin in the aln-null mutants and transgenic ALN-Flag line. Seeds were treated with ambient temperature at 22°C or 32°C for 3 d, and the content of allantoin was measured. (F) The effects of HT on the seed germination of aln-null mutants and transgenic ALN-Flag line. Seeds of aln-1, aln-2 and ALN-Flag were incubated under at 22°C or 32°C for 3 d, and seed germination percentage was calculated. The values are shown as means ± SD of triplicate experiments. Asterisks indicate significant difference between Col and aln-1, aln-2 and ALN-Flag lines after different temperature treatments, as determined by Student’s t test (**P < 0.01)
3.3 Allantoin Represses the ABA Biosynthesis and Its Signal Transduction under HT Stress
The endogenous GA and ABA levels determine the seed germination status [5]. Here we also monitored the endogenous bioactive GA4 and ABA levels in aln-1, aln-2, ALN-Flag and Col before or after HT stress. As shown in Fig. 3A, we found that the endogenous GA4 levels in aln-1, aln-2 and ALN-Flag were not significantly changed before or after HT stress, but the level of ABA in ALN-Flag was relatively higher than that in Col line, and the ABA levels in aln-1 and aln-2 was lower than that in Col line (Fig. 3B), suggesting that ALN affects the ABA biosynthesis, rather than GA, in germinated seeds after HT stress. Similarly, we directly treated Col seed with allantoin, and found allantoin treatment also only partially suppressed HT-induced ABA accumulation, but did not alter GA biosynthesis.
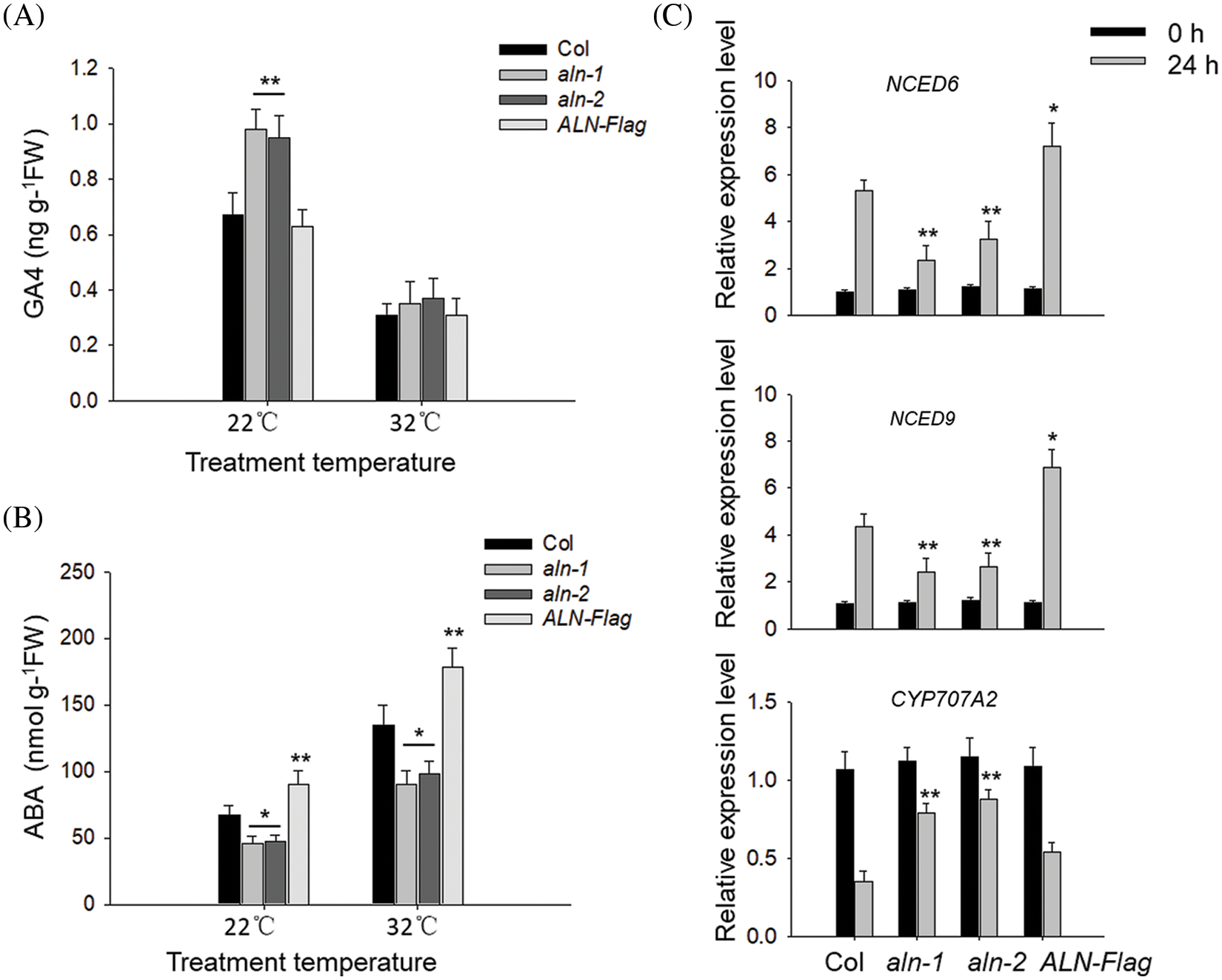
Figure 3: Allantoin regulated the metabolism of GA/ABA in imbibed seeds under HT. (A&B) The different content of GA (A) and ABA (B) in imbibed seeds of Col, aln-1, aln-2 and ALN-Flag. All these seeds were incubated under at 22°C or 32°C for 3 d, and the content of GA and ABA was measured. (C) The expression patterns of NCED6, NCED9 and CYP707A2 in Col, aln-1, aln-2 and ALN-Flag. All these seeds were incubated under at 22°C or 32°C for 24 h, and the expression of these genes was monitored by RT-qPCR analysis. PP2C was used as the internal control. The values are shown as means ± SD of triplicate experiments. Asterisks indicate significant difference between Col and aln-1, aln-2 and ALN-Flag at different temperature treatments by Student’s t test (*P < 0.05; **P < 0.01)
Meanwhile, we also checked the expressing patterns of genes associated with ABA biosynthesis, and found the expression of ABA catabolic gene CYP707A2 could be induced by allantoin treatment under HT stress. And allantoin treatment repressed the HT-induced expression of ABA-biosynthesis genes NCED6 and NCED9. The expression of CYP707A2 in aln-1 and aln-2 was also higher but lower in ALN-Flag after HT treatment (Fig. 3C), suggesting that allantoin regulates ABA level through altering ABA metabolism and biosynthesis related genes.
3.4 Allantoin Scavenges ROS Level to Improve the Seed Germination Tolerance to HT
As allantoin is reported to scavenge ROS to reduce the cadmium toxin [29], it is possible that allantoin suppresses HT-induced ROS overaccumulation to attenuate the HT damage to seed viability. To test such possibility, we measured the content of H2O2 in the imbibed seeds with or without HT or allantoin treatment. Application with allantoin indeed reduced H2O2 level under HT stress (Fig. 4A). Furthermore, we used the ROS specific fluorescence dichlorofluorescein-diacetate (DCFH-DA) to detect the level of ROS, and found HT also induced the accumulation of ROS in the wild type Col line, but such accumulation was lower in the imbibed aln-1 and aln-2 seeds under HT, conversely, HT-induced ROS level was aggravated in the germinated seeds of ALN-Flag (Fig. 4B), hinting that ALN suppresses HT-induced ROS generation.
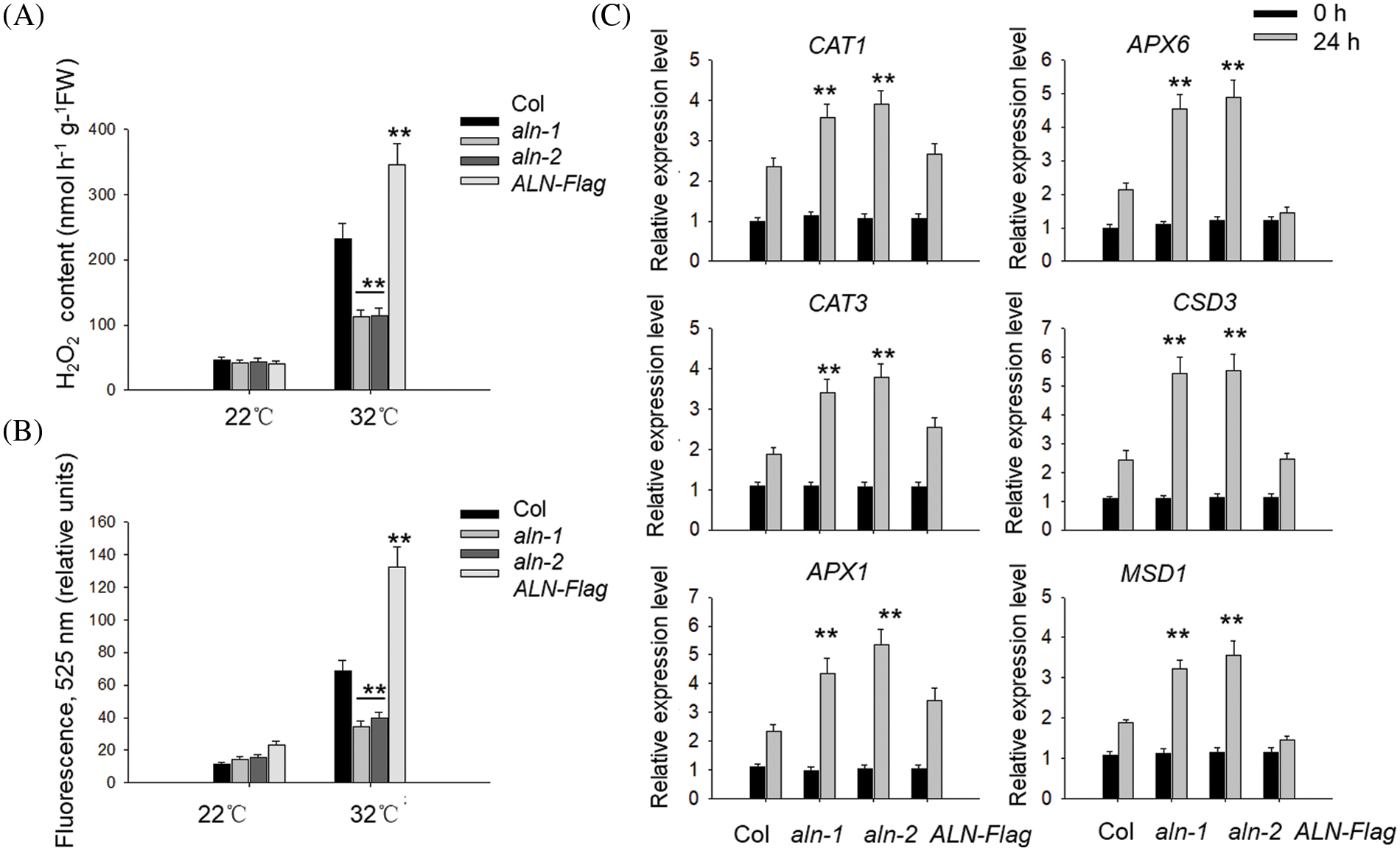
Figure 4: Allantoin treatment alleviated the ROS accumulation under HT. (A&B) The different levels of H2O2 (A) and ROS (B) in the imbibed seeds of Col, aln-1, aln-2 and ALN-Flag. All these seeds were incubated under at 22°C or 32°C for 3 days, and the levels of H2O2 and ROS were monitored. (C) The expression patterns of CAT1, CAT3, APX1, APX6, CSD3 and MSD1 in Col, aln-1, aln-2 and ALN-Flag. All these seeds were incubated under at 22°C or 32°C for 24 h, and the expression of these genes was monitored by RT-qPCR analysis. PP2C was used as the internal control. The values are shown as means ± SD of triplicate experiments. Asterisks indicate significant difference between Col and aln-1, aln-2 and ALN-Flag at different temperature treatment by Student’s t test (**P < 0.01)
Apart from reducing ROS level, exogenous application of allantoin also induced the expression of genes associated with the antioxidant enzyme system, including CAT1, CAT3, APX1, APX6, CSD3 and MSD1. As shown in Fig. 4C, HT treatment partially increased the expressions of these genes in the imbibed seeds of Col, such enhancement effect was also observed in the aln-1 and aln-2 mutants in which the allantoin levels were higher, but overexpressing ALN in the ALN-Flag compromised HT-induced expression of these genes, further supporting the opinion that allantoin signal strengthens the anti-oxidant enzyme system to reduce ROS damage during seed germination under HT.
3.5 Allantoin Induced the Expression of PER1 to Reduce ROS Damage to Seed Vigor
AtPER1 is reported to regulate seed germination or vigor through altering ROS level [25,26]. As allantoin alleviated ROS level to enhance seed germination under HT, we wonder the probable relationship between allantoin signal and AtPER1 during HT. To this end, we first measured the transcriptional level of AtPER1 during HT stress and found that HT induced the rapid expression during the first 24 h and then sustained the high level during the following 48 h, indicating the potential function of AtPER1 during HT stress. As the control, the expression of PER1 in the imbibed seeds under control condition at 22°C did not show a dramatic difference (Fig. 5A). To investigate the function of AtPER1 during HT stress, we obtained the mutant deficiency in AtPER1 (termed as per1). Compared with the wild-type Col line, seed germination of per1 was obviously lower under HT at 28°C or 32°C, probably resulting from the higher accumulation of ROS. To understand the genetic relationship between ALN and PER1, we crossed aln-1 mutant and ALN-Flag with per1 mutant to obtain aln/per1 and ALN-Flag/per1 lines. Though seed germination of aln showed tolerance to HT whereas the seed germination of ALN-Flag showed sensitivity to HT, we found both of aln/per1 and ALN-Flag/per1 showed similarly lower seed germination under HT, similar to per1 line (Fig. 5B). These genetic results indicate that the function of ALN regulate seed germination under HT required PER1, or PER1 is functional epistatic to ALN to control seed germination under HT stress.
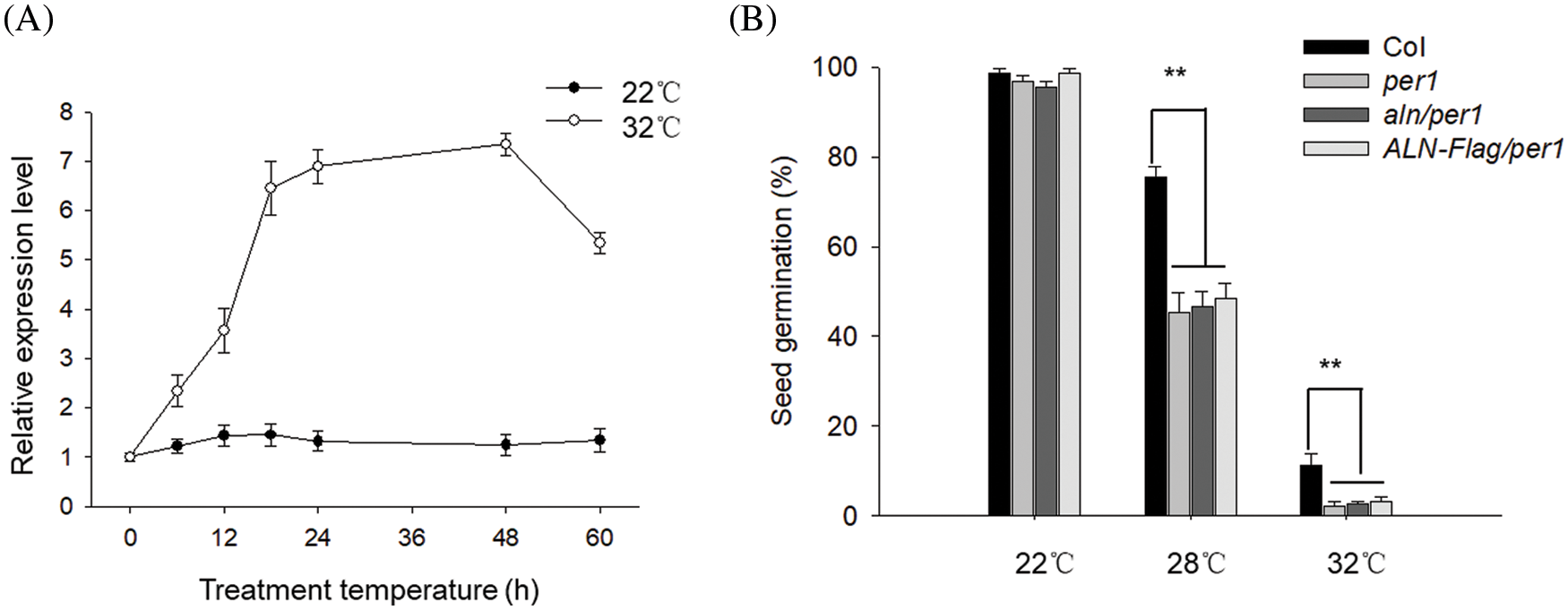
Figure 5: Allantoin alleviated HT-induced damage to seed vigor through PER1. (A) The effect of HT on the expression of PER1 in the imbibed seeds. The transcriptional levels of PER1 were measured by RT-qPCR at the indicated time. PP2C was used as the internal loading control. (B) Genetic analysis of the relationship between ALN and PER1. The Col, per1, aln/per1 and ALN-Flag/per1 seeds were treated with 22°C or 32°C for 3 days, and seed germination percentage was measured. The values are shown as means ± SD of triplicate experiments. Asterisks indicate significant difference by Student’s t test (**P < 0.01)
Allantoin as the deviant from purine catabolism plays multiple function in the response of plant to environmental stress [33], for example, application with allantoin enhances plant tolerance to saline and cadmium stress [29]. Here we tested the potential effect of allantoin in improving seed germination against to HT stress. We have confirmed the novel function of allantoin in enhancing seed germination under HT stress. At first, we directly treated the seed with exogenous allantoin and found application with allantoin indeed obviously enhanced seed germination rate in contrast to the seed without treatment. Allantoin treatment at 50 mg/L was more particularly efficient, and too relatively high concentration of allantoin treatment seems to be partially toxic to seed vigor as the seed germination. We found that HT treatment rapidly induced the accumulation of allantoin, it is possible that HT induced the generation of allantoin to protect seed from HT damage, but long-term HT treatment could impair the sustainable biosynthesis of allantoin, leading to weak seed germination finally. At last, we performed the genetic experiments by inactivating ALN function in aln-1 and aln-2 mutants, or overexpression ALN in the transgenic ALN-Flag line. As ALN is mainly responsible for allantoin degradation in planta, we also found that high allantoin accumulation in aln-1 and aln-2 lines and the lower level of allantoin in ALN-Flag line. In agreement with the endogenous allantoin level, the seeds of aln-1 and aln-2 showed higher seed germination than wild-type Col under HT stress, while the ALN-Flag line displayed lower seed germination under HT stress. Collectively, a series of experiments as above suggest the critical role of allantoin in enhancing seed germination tolerance to HT stress.
The balance of GA/ABA determines the seed germination or dormancy status [5,36,37]. Here we also checked the level of GA and ABA in the aln-null mutants and its transgenic line overexpressing ALN-Flag, and found that allantoin treatment attenuated HT-induced ABA accumulation, as well as repressed ABA anabolic genes NCED6 and NCED9 and increased the expression of ABA catabolic gene CYP707A2, which could explain the promoting effect of allantoin in alleviating HT-induced seed dormancy, but we did not observe the obvious change of allantoin on the GA content under HT treatment. These data coincide with the previous study and suggest that allantoin alter ABA biosynthesis to improve seed germination under HT [26]. Previous study also pointed out that allantoin reduced ROS accumulation after saline stress [29]. Consistently, here we also found that HT-induced the strong accumulation of ROS, mainly H2O2 in seed, and exogenous allantoin treatment obviously reduced ROS generation under HT. In agreement with it, HT-induced ROS level was lower in aln-1 and aln-2 mutants, but higher in ALN-Flag lines, such patterns correlate with the different endogenous H2O2 level in these lines, suggesting that allantoin mainly scavenges HT-induced ROS to enhance seed germination vigor. PER1 is reported to regulate ROS level to enhance seed vigor during seed storage and aging [30,31]. Here we also found HT treatment continuously induced the expression of PER1, suggesting it probably plays the central role for scavenging ROS overaccumulation. Genetic analysis also revealed that the per1 mutant presented lower seed germination than Col under HT, crossing experiments displayed that both of aln/per1 and ALN-Flag/per1 line showed similarly lower seed germination under HT, suggesting that the function of ALN requires PER1 for seed germination under HT.
In summary, we performed the combined physiological, biochemical, and genetic analysis to reveal a new function of allantoin in enhancing seed germination under HT. Allantoin exerts its function through triggering the expression of PER1, subsequently initiating antioxidant enzyme system to scavenge ROS level, and ultimately enhances seed germination under HT. After all, our finding uncovers the underlying mechanism that allantoin elevates seed germination efficiency under HT, and provides the possibility by modifying allantoin as the new plant growth regulator for uniform seed germination during modern agriculture manipulation.
Author Contributions: The authors confirm contribution to the paper as follows: Ping Li designed research and wrote the manuscript. Songbei Ying performed the most experiments and edited the manuscript. Sasa Jing and Leheng Cheng prepared for and identified the all genetic materials. A specific experiment (Quantification of ROS level) was performed by Haiqing Sun. Yuan Tian and Lulu Zhi provided critical analyses of the research. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This work was funded by the National Natural Science Foundation of China (Grant No. 32170562).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Finkelstein, R., Reeves, W., Ariizumi, T., Steber, C. (2008). Molecular aspects of seed dormancy. Annual Review of Plant Biology, 59(1), 387–415. DOI 10.1146/annurev.arplant.59.032607.092740. [Google Scholar] [CrossRef]
2. Buijs, G. (2020). A perspective on secondary seed dormancy in Arabidopsis thaliana. Plants, 9(6), 749. DOI 10.3390/plants9060749. [Google Scholar] [CrossRef]
3. Ali, F., Qanmber, G., Li, F., Wang, Z. (2022). Updated role of ABA in seed maturation, dormancy, and germination. Journal of Advanced Research, 35(10), 199–214. DOI 10.1016/j.jare.2021.03.011. [Google Scholar] [CrossRef]
4. Kozaki, A., Aoyanagi, T. (2022). Molecular aspects of seed development controlled by gibberellins and abscisic acids. International Journal of Molecular Sciences, 23(3), 1876–1891. DOI 10.3390/ijms23031876. [Google Scholar] [CrossRef]
5. Shu, K., Liu, X. D., Xie, Q., He, Z. H. (2016). Two faces of one seed: Hormonal regulation of dormancy and germination. Molecular Plant, 9, 34–45. DOI 10.1016/j.molp.2015.08.010. [Google Scholar] [CrossRef]
6. Vishal, B., Kumar, P. P. (2018). Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Frontiers in Plant Science, 9, 838–853. DOI 10.3389/fpls.2018.00838. [Google Scholar] [CrossRef]
7. Liu, J., Zhang, Y., Jiang, Y., Sun, H., Duan, R. et al. (2022). Formation mechanism and occurrence law of pod shattering in soybean: A review. Phyton-International Journal of Experimental Botany, 91(7), 1327–1340. DOI 10.32604/phyton.2022.019870. [Google Scholar] [CrossRef]
8. Zhang, P., Kong, Z., Liu, J., Liu, Y., Wang, Q. et al. (2022). Transcriptomic analysis of the tolerance response to dehydration and rehydration in wheat seedlings. Phyton-International Journal of Experimental Botany, 91(2), 375–394. DOI 10.32604/phyton.2022.016358. [Google Scholar] [CrossRef]
9. Penfield, S., Li, Y., Gilday, A. D., Graham, S., Graham, I. A. (2006). Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. The Plant Cell, 18(8), 1887–1899. DOI 10.1105/tpc.106.041277. [Google Scholar] [CrossRef]
10. Skubacz, A., Daszkowska-Golec, A., Szarejko, I. (2016). The role and regulation of ABI5 (ABA-insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Frontiers in Plant Science, 7, 1884–1902. DOI 10.3389/fpls.2016.01884. [Google Scholar] [CrossRef]
11. Chandrasekaran, U., Luo, X., Zhou, W., Shu, K. (2020). Multifaceted signaling networks mediated by abscisic acid insensitive 4. Plant Communications, 1(3), 20–29. DOI 10.1016/j.xplc.2020.100040. [Google Scholar] [CrossRef]
12. Chen, B., Fiers, M., Dekkers, B. J. W., Maas, L., van Esse, G. W. et al. (2021). ABA signalling promotes cell totipotency in the shoot apex of germinating embryos. Journal of Experimental Botany, 72(18), 6418–6436. DOI 10.1093/jxb/erab306. [Google Scholar] [CrossRef]
13. Sano, N., Marion-Poll, A. (2021). ABA metabolism and homeostasis in seed dormancy and germination. International Journal of Molecular Sciences, 22(10), 5069–5095. DOI 10.3390/ijms22105069. [Google Scholar] [CrossRef]
14. Yang, M., Han, X., Yang, J., Jiang, Y., Hu, Y. (2021). The Arabidopsis circadian clock protein PRR5 interacts with and stimulates ABI5 to modulate abscisic acid signaling during seed germination. The Plant Cell, 33(9), 3022–3041. DOI 10.1093/plcell/koab168. [Google Scholar] [CrossRef]
15. Debeaujon, I., Koornneef, M. (2000). Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiology, 122(2), 415–424. DOI 10.1104/pp.122.2.415. [Google Scholar] [CrossRef]
16. Piskurewicz, U., Jikumaru, Y., Kinoshita, N., Nambara, E., Kamiya, Y. et al. (2008). The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. The Plant Cell, 20(10), 2729–2745. DOI 10.1105/tpc.108.061515. [Google Scholar] [CrossRef]
17. Steber, C. M., McCourt, P. (2001). A role for brassinosteroids in germination in Arabidopsis. Plant Physiology, 125(2), 763–769. DOI 10.2307/4279701. [Google Scholar] [CrossRef]
18. Chiwocha, S. D., Cutler, A. J., Abrams, S. R., Ambrose, S. J., Yang, J. et al. (2005). The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant Journal, 42(1), 35–48. DOI 10.1111/j.1365-313x.2005.02359.x. [Google Scholar] [CrossRef]
19. Corbineau, F., Xia, Q., Bailly, C., El-Maarouf-Bouteau, H. (2014). Ethylene, a key factor in the regulation of seed dormancy. Frontiers in Plant Science, 5, 539–552. DOI 10.3389/fpls.2014.00539. [Google Scholar] [CrossRef]
20. Shuai, H. W., Meng, Y. J., Luo, X. F., Chen, F., Qi, Y. et al. (2016). The roles of auxin in seed dormancy and germination. Yi Chuan, 38(4), 314–322. DOI 10.16288/j.yczz.15-464. [Google Scholar] [CrossRef]
21. Penfield, S., Josse, E. M., Kannangara, R., Gilday, A. D., Halliday, K. J. et al. (2005). Cold and light control seed germination through the bHLH transcription factor SPATULA. Current Biology, 15(22), 1998–2006. DOI 10.1016/j.cub.2005.11.010. [Google Scholar] [CrossRef]
22. Bertier, L. D., Ron, M., Huo, H., Bradford, K. J., Britt, A. B. et al. (2018). High-resolution analysis of the efficiency, heritability, and editing outcomes of CRISPR/Cas9-induced modifications of NCED4 in lettuce (Lactuca sativa). G3 Genes|Genomes|Genetics, 8(5), 1513–1521. DOI 10.1534/g3.117.300396. [Google Scholar] [CrossRef]
23. Li, P., Zhang, Q. L., He, D. N., Zhou, Y., Ni, H. H. et al. (2020). AGAMOUS-LIKE67 cooperates with the histone mark reader EBS to modulate seed germination under high temperature. Plant Physiology, 184(1), 529–545. DOI 10.1104/pp.20.00056. [Google Scholar] [CrossRef]
24. Huo, H., Dahal, P., Kunusoth, K., McCallum, C. M., Bradford, K. J. (2013). Expression of 9-cis-EPOXYCAROTENOID DIOXYGENASE4 is essential for thermoinhibition of lettuce seed germination but not for seed development or stress tolerance. The Plant Cell, 25(3), 884–900. DOI 10.1105/tpc.112.108902. [Google Scholar] [CrossRef]
25. Yoong, F. Y., O’Brien, L. K., Truco, M. J., Huo, H., Sideman, R. et al. (2016). Genetic variation for thermotolerance in lettuce seed germination is associated with temperature-sensitive regulation of ETHYLENE RESPONSE FACTOR1 (ERF1). Plant Physiology, 170(1), 472–488. DOI 10.1104/pp.15.01251. [Google Scholar] [CrossRef]
26. Watanabe, S., Matsumoto, M., Hakomori, Y., Takagi, H., Shimada, H. et al. (2014). The purine metabolite allantoin enhances abiotic stress tolerance through synergistic activation of abscisic acid metabolism. Plant, Cell & Environment, 37(4), 1022–1036. DOI 10.1111/pce.12218. [Google Scholar] [CrossRef]
27. Takagi, H., Ishiga, Y., Watanabe, S., Konishi, T., Egusa, M. et al. (2016). Allantoin, a stress-related purine metabolite, can activate jasmonate signaling in a MYC2-regulated and abscisic acid-dependent manner. Journal of Experimental Botany, 67(8), 2519–2532. DOI 10.1093/jxb/erw071. [Google Scholar] [CrossRef]
28. Xin, W., Zhang, L., Gao, J., Zhang, W., Yi, J. et al. (2021). Adaptation mechanism of roots to low and high nitrogen revealed by proteomic analysis. Rice, 14(1), 5. DOI 10.1186/s12284-020-00443-y. [Google Scholar] [CrossRef]
29. Nourimand, M., Todd, C. D. (2019). There is a direct link between allantoin concentration and cadmium tolerance in Arabidopsis. Plant Physiology and Biochemistry, 135, 441–449. DOI 10.1016/j.plaphy.2018.11.016. [Google Scholar] [CrossRef]
30. Chen, H., Ruan, J., Chu, P., Fu, W., Liang, Z. et al. (2020). AtPER1 enhances primary seed dormancy and reduces seed germination by suppressing the ABA catabolism and GA biosynthesis in Arabidopsis seeds. The Plant Journal, 101(2), 310–323. DOI 10.1111/tpj.14542. [Google Scholar] [CrossRef]
31. Wang, W. Q., Xu, D. Y., Sui, Y. P., Ding, X. H., Song, X. J. (2022). A multiomic study uncovers a bZIP23-PER1A-mediated detoxification pathway to enhance seed vigor in rice. Proceedings of the National Academy of Sciences of the United States of America, 119(9), e2026355119. DOI 10.1073/pnas.2026355119. [Google Scholar] [CrossRef]
32. Wang, P., Kong, C. H., Sun, B., Xu, X. H. (2012). Distribution and function of allantoin (5-ureidohydantoin) in rice grains. Journal of Agricultural and Food Chemistry, 60(11), 2793–2798. DOI 10.1021/jf2051043. [Google Scholar] [CrossRef]
33. Kaur, H., Chowrasia, S., Gaur, V. S., Mondal, T. K. (2021). Allantoin: Emerging role in plant abiotic stress tolerance. Plant Molecular Biology Reporter, 39(1), 648–661. DOI 10.1007/s11105-021-01280-z. [Google Scholar] [CrossRef]
34. Wei, W. J., Hu, Y. L., Yang, W. J., Li, X. L., Wei, J. L. et al. (2021). S-Nitrosoglutathion reductase activity modulates the thermotolerance of seeds germination by controlling ABI5 stability under high temperature. Phyton-International Journal of Experimental Botany, 90(4), 1075–1087. DOI 10.32604/phyton.2021.016134. [Google Scholar] [CrossRef]
35. Li, X. L., Lu, S. Y., Yang, Y. R., Wei, W. J., Wei, J. L. et al. (2021). The BHLH transcriptional factor PIF4 competes with the R2R3-MYB transcriptional factor MYB75 to fine-tune seeds germination under high glucose stress. Phyton-International Journal of Experimental Botany, 90(5), 1387–1400. DOI 10.32604/phyton.2021.016362. [Google Scholar] [CrossRef]
36. Shu, K., Zhou, W., Chen, F., Luo, X., Yang, W. (2018). Abscisic acid and gibberellins antagonistically mediate plant development and abiotic stress responses. Frontiers in Plant Science, 9, 416. DOI 10.3389/fpls.2018.00416. [Google Scholar] [CrossRef]
37. Deng, G. L., Ying, S. B., Jing, S. S., Zhou, J., Lu, S. Y. et al. (2021). AFP2 coordinates the activity of PIF7 for thermomorphogenesis in Arabidopsis seedlings. Phyton-International Journal of Experimental Botany, 90(4), 1089–1101. DOI 10.32604/phyton.2021.016217. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |