

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.021734
REVIEW
Antagonistic Potential of Bacterial Species against Fungal Plant Pathogens (FPP) and Their Role in Plant Growth Promotion (PGP): A Review
1Department of Plant Protection, Sivas University of Science and Technology, Sivas, 58140, Turkey
2Department of Plant Pathology, College of Agriculture, University of Sargodha, Sargodha, 40100, Pakistan
3College of Bioscience, University of Birmingham, Birmingham, B152TT, UK
4Department of Plant Pathology, PMAS University of Arid Agriculture, Rawalpindi, 43490, Pakistan
5Department of Soil Science, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan, 60800, Pakistan
6Citrus Research Institute, Sargodha, 40100, Pakistan
7Department of Agriculture and Agribusiness Management, University of Karachi, 75270, Karachi, Pakistan
8Pest warning & Quality Control of Pesticides, Sahiwal, Punjab, 40210, Pakistan
9Institute of Soil and Environmental Sciences, University of Agriculture, Faisalabad, 38000, Pakistan
*Corresponding Author: Yasir Iftikhar. Email: yasir.iftikhar@uos.edu.pk
Received: 01 February 2022; Accepted: 09 March 2022
Abstract: Since the 19th century to date, the fungal pathogens have been involved in causing devastating diseases in plants. All types of fungal pathogens have been observed in important agricultural crops that lead to significant pre and postharvest losses. The application of synthetic fungicide against the fungal plant pathogens (FPP) is a traditional management practice but at the same time these fungicides kill other beneficial microbes, insects, animal, and humans and are harmful to environment. The antagonistic microorganism such as bacteria are being used as an alternate strategy to control the FPP. These antagonistic species are cost-effective and eco-friendly in nature. These biocontrol bacteria have a broad mechanism against fungal pathogens present in the phyllosphere and rhizosphere of the plant. The antagonistic bacteria have different strategies against the FPP, by producing siderophore, biofilm, volatile organic compounds (VOCs), through parasitism, antibiosis, competition for limited resources and induce systemic resistance (ISR) in the host plant by activating the immune systems. The commercial bio-products synthesized by the major bacterial species Pseudomonas syringae, Burkholderia cepacia, Streptomyces griseoviridis, Pseudomonas fluorescens and Bacillus subtilis are used to control Fusarium, Pythium, Rhizoctonia, Penicillium, Alternaria, and Geotrichum. The commercial bio-formulations of bacteria act as both antifungal and plant growth regulators. The Plant growth-promoting rhizobacteria (PGPR) played a significant role in improving plant health by nitrogen-fixing, phosphorus solubilization, phytohormones production, minimizing soil metal contamination, and by ACC deaminase antifungal activities. Different articles are available on the specific antifungal activity of bacteria in plant diseases. Therefore, this review article has summarized the information on biocontrol activity of bacteria against the FPP and the role of PGPR in plant growth promotion. This review also provided a complete picture of scattered information regarding antifungal activities of bacteria and the role of PGPR.
Keywords: Fungi; plant pathogens; synthetic fungicide; antagonism; bio-products; PGPR
Bacterial species associated with the phyllo-sphere and rhizosphere soil of plants may have valuable effects on plant health by providing essential plant beneficial nutrients and Plant Growth Promoters (PGR) or by producing induction host resistance, siderophore, and antibiotics against plant pathogenic species of bacteria and fungi, respectively [1–3]. PGPR colonize the plant root to nourish the plant health. PGPR increase the nutrient supply to the plants and act as antagonist against plant pathogenic fungi at the same time [4]. The Rhizobacteria also act as shield against abiotic stress in pulses [5]. Basically, bacteria are unicellular prokaryotic microorganisms with achlorophyllous properties and having an ambient environment. Bacteria as an antagonistic played a significant role by killing and suppressing the growth of other pathogenic harmful microorganisms especially fungi that cause diseases on important agricultural crops [6,7]. The fungal pathogens cause severe infection on fruit, vegetable, and other cereal crops worldwide. The major fungal pathogens attacking fruits and vegetables are Fusarium, Botrytis, Penicillium, Rhizoctonia, Pythium, Ascochyta, Alternaria, Leptosphaeria, Blumeria graminis, Melampsora lini, and Sclerotinia [8–10]. Biological control of these fungal pathogens is an alternative to synthetic fungicide. A broad range of microorganisms such as bacteria, fungi, nematodes are being used as biocontrol agents worldwide. These biocontrol agents not only retard the growth of pathogenic target microorganisms but also improve plant health by providing nutrients through mutualistic associations. Furthermore, the application of these biocontrol agents (BCAs) is cost-effective, eco-friendly, with some more features such as these do not contaminate the water, plant product, and soil [11]. The BCAs, have different modes of action such as antibiosis, parasitism, competition, and induction of host plant resistance to control the target pathogens [3,12]. This review highlights the bacteria as biocontrol against different plant pathogenic fungi. Both the gram-negative and gram-positive bacteria are significantly used as a BCAs [13]. These biocontrol bacteria produced Siderophore and formed the biofilm against the many-targeted fungal pathogens of fruit crops [14]. Bacterial species used as antagonists belong to genera Bacillus, Pseudomonas, Rhizobium, Stenotrophomonas, Pantoea, and Paenibacillus. The antagonistic bacteria Bacillus amyloliquefaciens is being used to control the fungal pathogen Botryosphaeria dothidea, causative agent of apple ring rot [15]. The biocontrol bacteria retard the growth of fungal pathogens both in-vivo and in-vitro by producing antifungal activities. The antifungal activities of Pseudomonas synxantha against stone fruit fungal pathogens Monilinia fructigena and M. fructicola cause brown rot [16]. These bacteria also compete with target fungal pathogens for nutrients and space on fruit surfaces. Bio-commercial products prepared from bacterial species are being used to control many fungal and bacterial pathogens. A commercially available product Avogreen® was synthesized by Bacillus subtilis against a major Avocado plant disease Cercospora spot in South Africa [17]. Three bacterial bio-commercial products named Bio-Save® (10LP, 11LP, Blight-Ban® A506) were also synthesized by using Pseudomonas spp. against plant pathogenic fungal and bacterial species. Bio-Save 10LP® and 11LP® prepared from P. syringae strains ESC-10 and ESC-11 applied against pathogenic fungal storage pathogens of pome, potato, and citrus fruits, but the product Blight-Ban A506 used against bacterial diseases of apple and pear tree [18]. P. syringae strain ESC-10 and 11 was effective to control the Helminthosporium solani (Silver scurf of potato) and similarly, Fusarium spp. (Dry rot of potato) was controlled by the bio-commercial products Bio-Save 10LP® and Bio-Save 11 LP® [19]. Plant growth-promoting rhizobacteria (PGPR) are very prominent and important bacteria present in the rhizosphere. These bacteria have a vital role in plant growth promotion and to enhance soil fertility. These bacteria are also beneficial in heavy metal stress, salinity, drought and nutrient deficiency [4]. These PGPR improve plant health to combat plant diseases.
2 Control of FPP by Using Antagonistic Bacterial Species
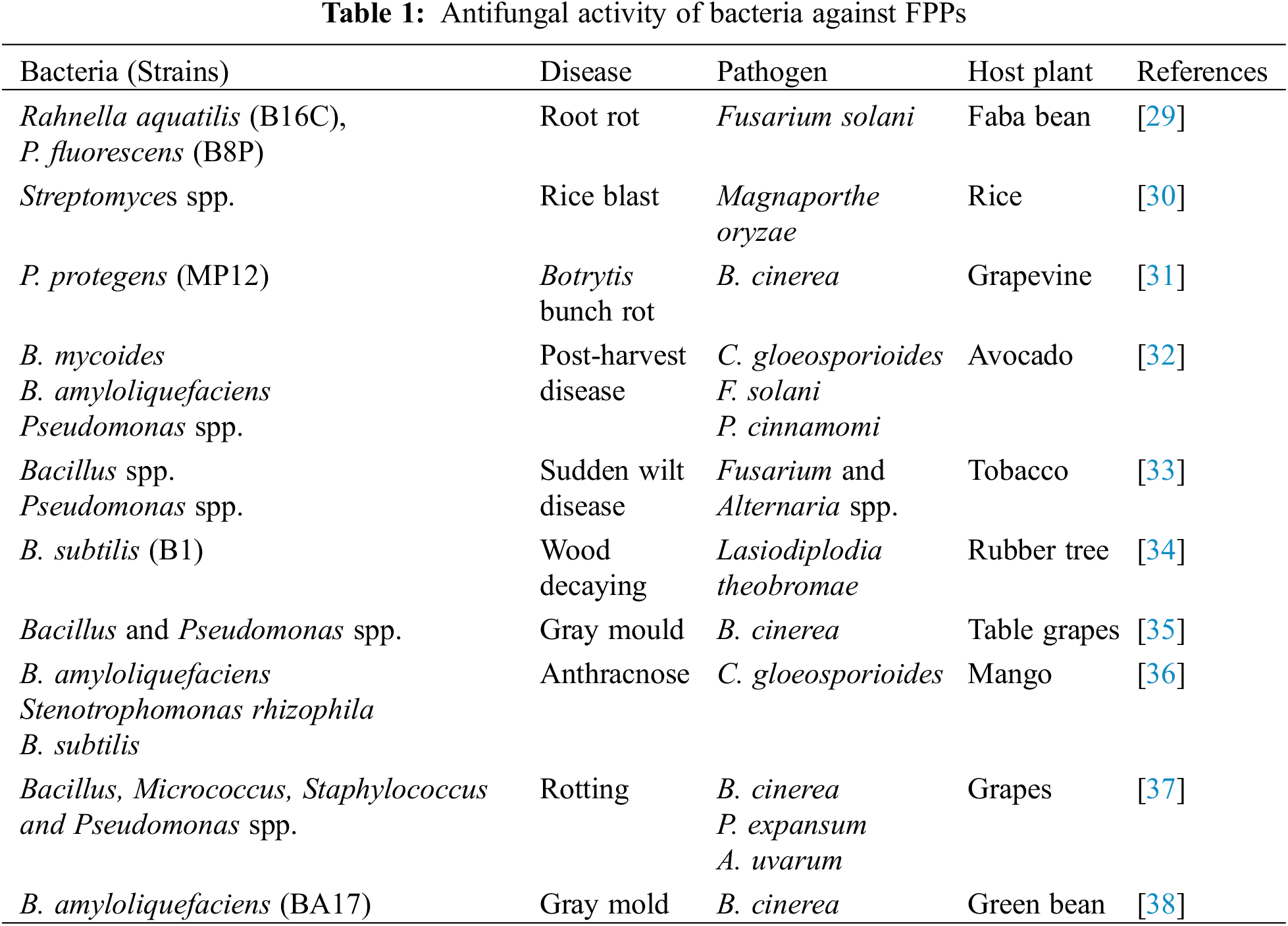
The demand of the population for food is increasing day by day with the increase in population of the world. Every day, the scientist, researchers tried to develop new varieties for the production of high yield but after a few years, each variety becomes susceptible against various pathogens. However, various pathogenic microbes (fungi, fungi-like organisms, prokaryotes, nematodes, viruses, and virus-like pathogens) contaminate the food and are responsible for decreasing the value for human consumption [20,21]. At the early and later stages of the crop, the soil-borne fungal pathogens attack and kill the seedlings and plants. Different species of Fusarium, Rhizoctonia, Pythium, Phytophthora, Rhizoctonia [22,23] Botrytis, Penicillium, Alternaria, Aspergillus, Ascochyta, and Colletotrichum are examples of soil borne pathogens at different stages of host plants [24–26]. Biological control of these pathogenic microbes is necessary to control the pathogens because the biocontrol agents are cost-effective and eco-friendly [27,28]. Different bacterial species act as antagonists against FPPs (Table 1).
3 Biocontrol Potential of Lactic Acid Bacteria against the FPP
Lactic acid bacteria (LAB) are gram-positive bacteria with some salient features such as non-sporulation, fastidious, rod shape, and catalase-negative. Due to safe food bio-preservation status, the LAB was used as a biocontrol agent against many fungal and bacterial pathogens [39]. In food industries, LAB produced anti-microbial compounds (e.g., anti-fungal and anti-bacterial) for the preservation of food during packaging and storing. Various types of antifungal compounds [Proteinaceous, Lactic acid, 4-hydroxyphenyl acetic acid, Benzoic acid, Pitocin TV35b, Sodium acetate, cyclo (Phe-OH-Pro, Mevalonolactone, Phenyl-lactate, Phenolic compound, Propionic acid, Diketopiperazines, and 2-hydroxy-4-methylpentanoic acid)] are produced by these bacteria to kill fungal pathogens. The antifungal activities of two Lactic acid bacteria named Plantarum UFG 121 and Plantarum UFG 108 have been observed against the fungal pathogens Aspergillus flavus, A. niger, P. chrysogenum, P. roqueforti, and F. culmorum [40]. The LAB inhibited the growth of these fungal pathogens by producing different organic compounds. The antifungal compounds produced by LAB were isolated and reported from different sources such as; Lactobacillus Plantarum was reported from grass silage [41], isolated from a wax gourd [42], and Pediococcus pentosaceus from maize [43]. The LAB strain 58 and 13 retarded the spore germination on fruit surface by P. expansum [44]. The LAB produced antifungal metabolites include cyclic dipeptides, biosurfactants, phenyl-lactic, and reuterin. The L. plantarum strain (LR14) inhibited the hyphal and spore germination of four fungi; A. niger, Mucor racemosus, Rhizopus stolonifer, and P. chrysanthemum by using antifungal peptides [45]. Post-harvest application by coating the grapes with Lactococcus. plantarum controlled the fruit decay by B. cinerea in storage [46]. PFL9 and PFR77 isolates of LAB inhibited the spore germination and delayed the infection caused by Alternaria alternata, Orynespora cassiicola, and Phomopsis varsoniana on pomegranate fruit [20]. The four LAB named L. plantarum, L. paracasei, L. hilgardii and L. lactis retarded the growth of F. oxysporum and promoted the plant growth in tomato plant [47]. In-vitro, these LABs reduced the disease incidence from 55–76% indicating that LAB is a good biocontrol agent against soil-borne pathogenic fungi [48].
4 Mechanism of Action Used by Bacteria against Fungal Pathogenic Species
Bacteria follow two major antagonistic modes of action (direct and indirect) against pathogenic fungi. During the direct mode of action, the bacteria parasitize the targeted pathogen through parasitism while during the indirect method the bacteria activate the systemic resistance of the host plant and also produce antibiotic substances to suppress the growth of opposite pathogens [13]. There are more antagonistic modes of action (Siderophore production, competition for nutrients and space, production of volatile organic compounds, biofilm formation on the fruit surface, and quorum sensing) applied by the bacteria to suppress the growth of FPP (Fig. 1).
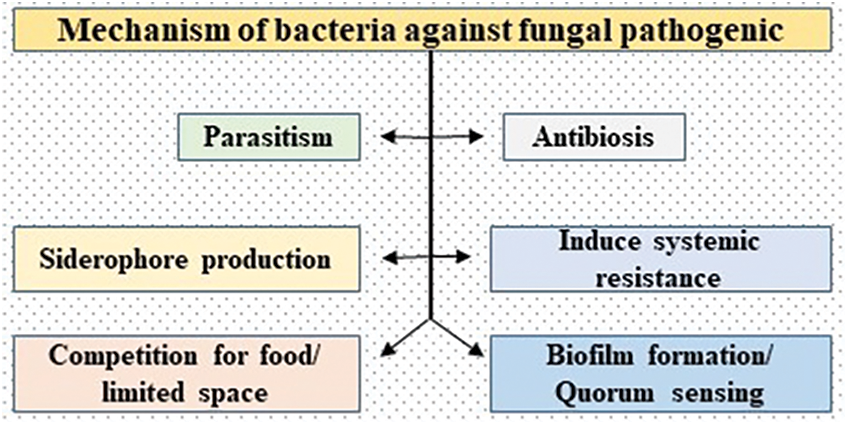
Figure 1: Mechanism of bacteria against fungal plant pathogens
Micronutrients are essential elements for the development of any microorganism. Among all the micronutrients the iron (Fe2+) is very important for the growth of microorganisms. Bacteria utilize iron for energy purposes and body development. Iron is oxidized from Fe2+ to Fe3+ in the presence of water and oxygen [2]. These complex molecules secluded during siderophore production, by using these molecules bacteria with functional categories, are: 1) Catolate phenate 2) Pyridoxines 3) Carboxylates and 4) Hydroxamates. The produced siderophore has the ability to inhibit the hyphal and mycelial growth of target pathogens in a medium plate and repel the pathogenic fungi from the host plant surface [49]. Siderophore production by Pseudomonas aeruginosa FP6 was compared in two mediums (Succinate medium and King’s B medium) [50]. The siderophore production is high in succinate medium as compared to King’s B medium. This siderophore production strain was also tested against two fungal pathogens of the chili plant Colletotrichum gloeosporioides and Rhizoctonia solani under controlled conditions. Siderophore production by Pseudomonas strains SPs9 inhibited the growth of F. oxysporum causing wilt in chili plant [51]. P. protegens MP12 had the ability to produce siderophore with antifungal activity against the fungal pathogen of grapevine B. cinerea [31].
4.2 Competition for Food and Limited Space
Biocontrol bacteria have the ability to (1) colonize in scratched fruit to ingest the food (Carbon source) for their existence, and (2) restrict the carbohydrates tendency for the target fungus by decreasing its germination rate and consequently reduce its invasion capability on the host surfaces [52]. An in-vitro study showed that in the presence of a biocontrol agent the fungal pathogen slowed down the utilization of sucrose, glucose, and fructose [53]. Other bacterial species belonging to the genera Pseudomonas, Bacillus, and Pantoea also acted as a biocontrol agent against infectious fungi due to their colonization of all food resources under controlled conditions. In-vitro and in-vivo research was conducted to measure the incidence and severity of fungal pathogens on the bell pepper plant [54]. The biocontrol bacteria (Arthrobacter Pseudomonas spp.) was used to inhibit the growth of the fungal pathogens B. cinerea (cause of gray mold) and A. alternate (cause of Alternia rot on bell pepper). The biocontrol bacteria compete for nutrients with fungal pathogens on the fruit surface.
The biocontrol bacterial species secrete chitinases, glucanases, and protease enzymes for the degradation of the fungi cell wall [3]. In a simple way, the cell wall degradation is impossible because of chitin present an insoluble form and designed by N-Acetyl glucosamine which is interlinked with β1-4 that provide support to the cell wall. In addition, β1-3 glucan is one of the essential components in which several other components were covalently associated and gave mechanical stability as well as integrity to the fungal cell wall. As antagonistic agents, the bacteria need diverse antifungal enzymes to degrade the cell wall of pathogenic fungi [55]. Glucan is an antifungal enzyme which is secreted by a few antagonistic bacterial species to hydrolyze the glucans present in the fungal cell wall by two noteworthy mechanisms. In first, the bacteria secrete exo-1,3 glucanases that had the capability to hydrolyze the concerned glucans by successive integration of glucose particles with some non-reducer residues. Secondly, the bacteria secrete endo-1,3 glucanases [56,57]. To breakdown the chitin of the fungal cell wall, the antagonistic bacteria secreted chitinases for the hydrolyzation of chitin. Non-splitting N-Acetyl glucosamines were found with β1,4 linkages by following these two mechanisms: 1) NAG residues successively segmented by exo-chitinase, and 2) aleatory sites concerned with polymer chain activated by the endo-chitinase. The species of Pseudomonas and Bacillus were found to be a good biocontrol agent by secreting antifungal enzymes against fungal pathogens. The antifungal activities of different Bacillus strains (EPP21, EPP32, EPP49, EPP65, EPP74, and EPP100) have been recorded against the fungal pathogens R. solani, S. rolfsii, and F. solani of the pearl millet plant [58]. These antagonistic bacterial strains inhibited the growth of fungal targeted pathogens and also improved the plant health and provided systemic resistance by producing IAA. Bacillus safensis produced two antifungal compounds named iturin A2 and iturin A6 against the rice blast pathogen Magnaporthe oryzae. In dual culture plate, the iturin A2 inhibited the spore and hyphal germination of the mention fungal pathogen [59]. B. subtilis strain (DZSY21) produced antifungal volatile compounds (2-Methylbutyric acid and isopentyl acetate) in-vitro that inhibited the mycelial growth and sporulation of the fungus Curvularia lunata causing brown leaf spot on maize [60].
4.4 Biofilm Formation and Quorum Sensing
Biofilm formation is an effective antimicrobial property of bacteria on fruit surface against pathogenic fungi. This biocontrol mechanism of bacterial species assists its adherence, colonization as well as multiplication on fruit lesions against target pathogens [61]. Bacteria use it as a biocontrol agent forming micro-colonies around the lesion of fruits and protect them from harmful pathogens. The existence of these micro-colonies is measured by using the quorum sensing with concerned regulators (farnesol, phenethyl alcohol, and tyrosol) [62,63]. Through this quorum-sensing, the bacterial species interlink with each other and adapt themselves according to their environment during protection [64]. However, the biocontrol mechanism of this biofilm formation by the bacteria is not fully understood. The biocontrol of F. oxysporum through the bacteria B. amyloliquefaciens strain W19 with OF (organic fertilizer) reduced the effect of the target fungal pathogen and formed a thick biofilm formation on the soil against the pathogen. The biofilm is formed by lipopeptide. On the fruit surface, the formation of bacteria micro-colonies also competed for food and space. The species of the genus Bacillus as compared to other genera (Micrococcus, Brevibacillus, Pseudomonas, and Curtobacterium) had more antifungal antagonistic potential on grapes fruit surfaces against the fungal pathogens B. cinerea, P. expansum, and A. uvarum [37]. Surfactin, an antifungal compound, produced by Pseudomonas and Bacillus, also formed biofilm against many fungal and other pathogenic microbes on the fruits and soil surfaces [65]. Under controlled conditions, B. velezensis (QST713) triggered the surfactin and fengycin related genes (surFF and fenA) to control the fungal pathogen [66].
4.5 Induced Systemic Resistance
Induction of host plant resistance can also be generated by antagonistic bacterial species against FPP through activating the different chemical and biochemical reactions (such as a change in protein profiling structure, change in tissue structure, and change in PR genes) inside the host plant [67]. Induce systemic resistance (ISR) produced by biocontrol bacteria activate the immune systems of the host plant that were triggered through the production of ROS (Reactive Oxygen Species) and by closing the stomata [68]. An experiment conducted to control the black rot disease of grapes, caused by Aspergillus spp. through an ISR in grapes fruit [69]. The application of B. subtilis OTPB1 induced systemic resistance against A. solani and P. infactent in tomato plants. Production of IAA and gibberellic acid by biocontrol bacteria promoted root and shoot growth of tomato plants, and at the same time controlled the associated pathogens [70]. Bacillus species produced ISR in cotton plants against charcoal root pathogens. The application of two Bacillus species, B. subtilis IAGS174 and B. megatherium, combined with Benzothiadiazide activated defense biochemical reactions related to enzymes and phenolic compounds production [71]. Endophyte bacteria Bacillus, Stenotrophomonas, and Lysinibacillus determined ISR in tomato plants against collar rot diseases caused by Sclerotium rolfsii. These bacterial species protected the plant cell wall by providing strengthening through lignification, lipid peroxidation, and suberization [72].
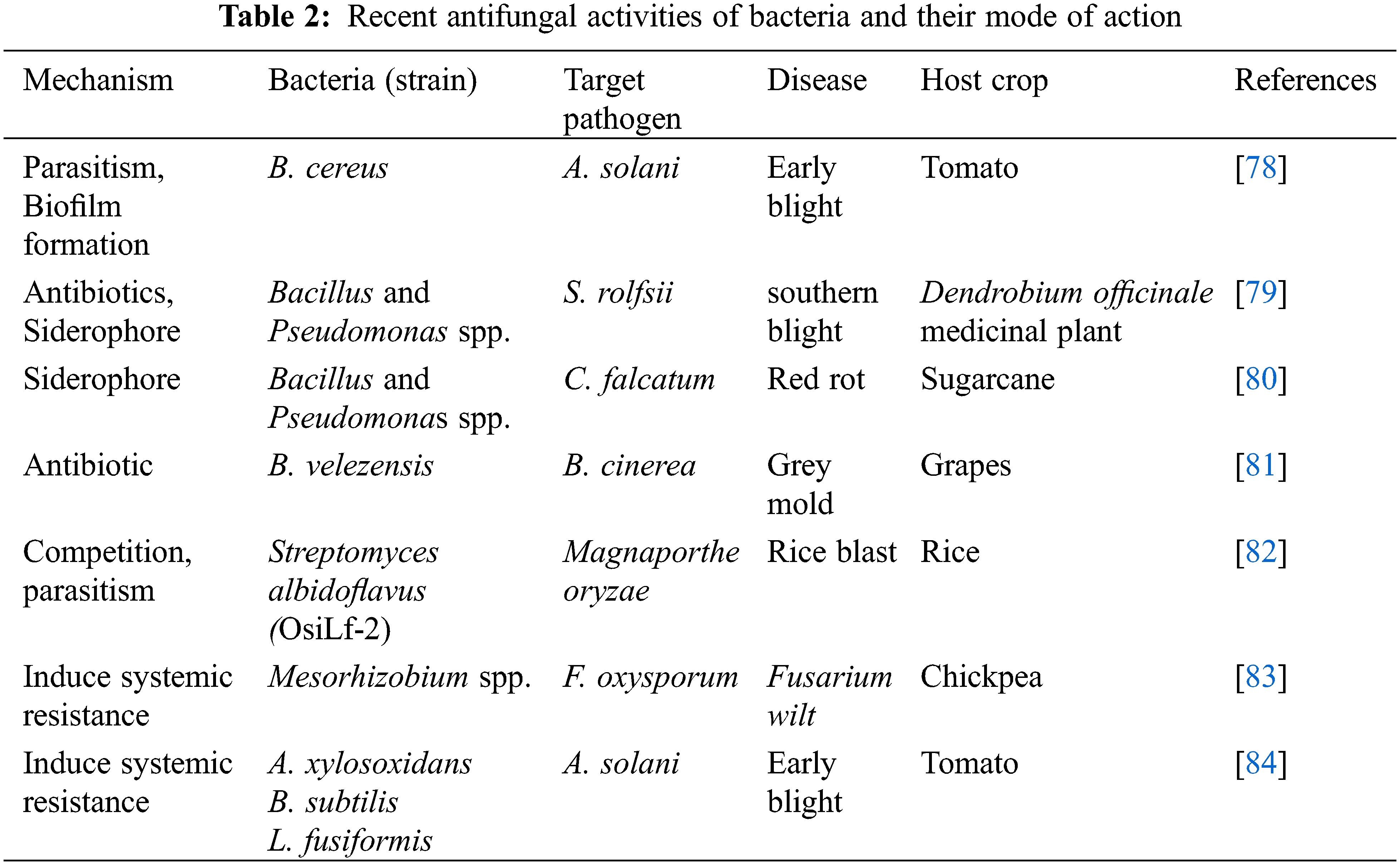
Antimicrobial metabolites of bacterial species, also known as secondary metabolites, are low molecular weight and have been proved lethal against other plant pathogenic microbes [73]. Three main antibiotics, iturin, trichothecene and pyyrrolnitrin have been emitted by the bacteria B. subtilis, Myrothecium roridum, and Pseudomonas cepacia, respectively, to control fungal diseases [74]. Antibiotics proved to be effective at low concentrations associated with the chemical groups alcohols, esters, aldehydes, terpenes, ketones, sulfur compounds, and lactones. Due to their volatile ability in the environment, they travelled unrestricted distances in solid and in liquid media as well as gas complexes, having a great advantageous effect as BCA. Currently, researchers are paying attention to produce commercial products related to volatile metabolism. Bio fumigation of fruits was possible via microorganisms able to emit VOCs in a locked and protected chamber that was verified as an alternative good source to control some important phytopathogens [75]. The genus Bacillus as BCA, produced some secondary metabolites against some major fungal pathogens [76]. The two species that secrete volatile compounds are suggested to be good BCA, which includes Brevibacillus breves, that emits fengycin and iturin A, and B. subtilis, that emits gramicidin. Both bacterial species were suggested to be good BCA which prevented the growth of FPPs [77]. Some antifungal activities of bacteria have been documented in Table 2.
5 Success Stories of Bacterial Bio-Products against FPPs
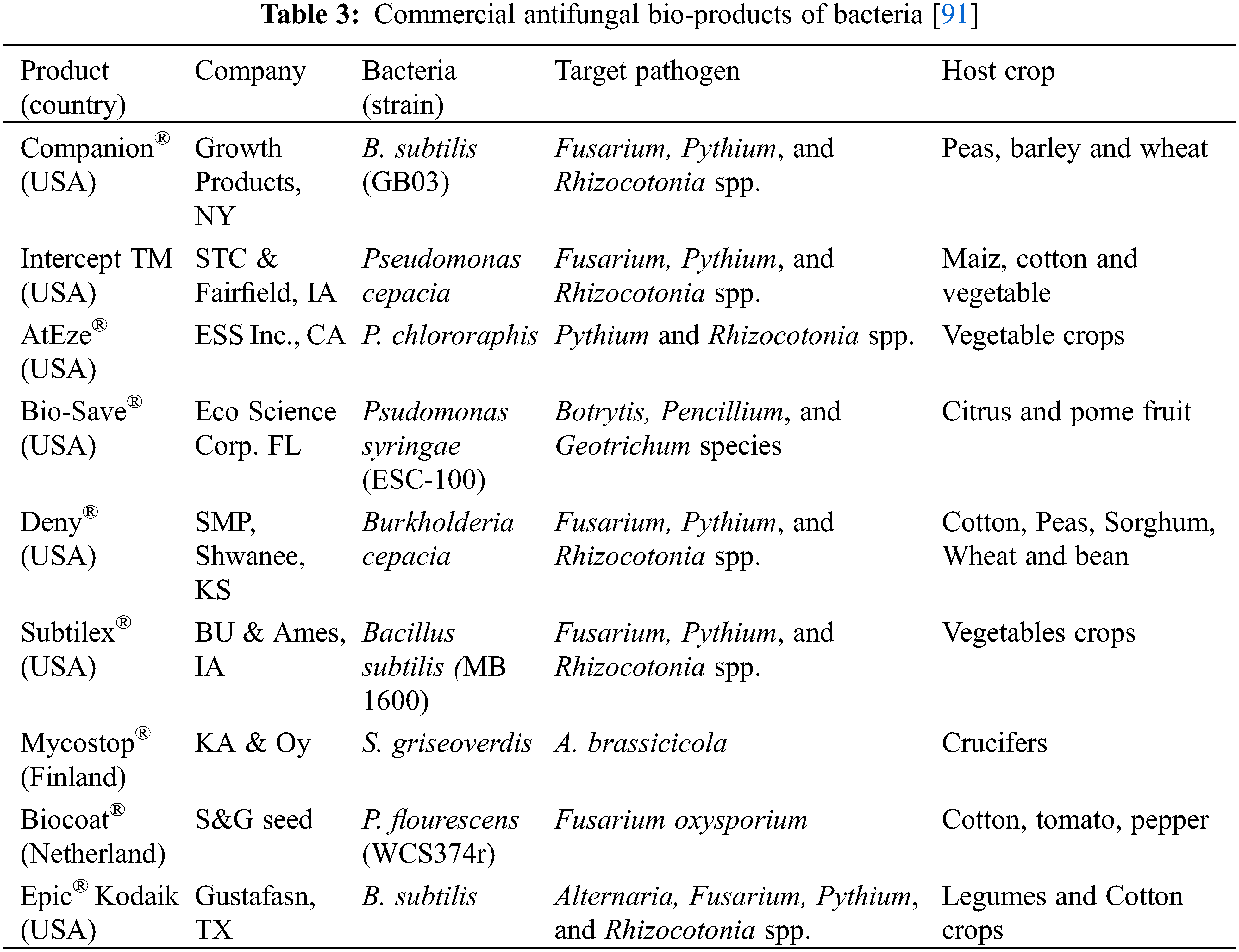
To date, numbers of bio commercial products, prepared from antagonistic bacteria, are available in the market to protect the economically important crops from plant pathogenic fungi [85]. Commercially available bio-products can be applied in the field pre-harvest and post-harvest. During soil application of these bacterial commercial products, a few disadvantages have been noticed such as not all the products are suitable for all types of soils. Soil pH and moisture also affect the product solubility whereas, clay contents retard the adherences of products. Other bacteria, already present in the soil, were a hindrance to the progression of the biocontrol agent. Soil application of these biocontrol agents enhanced the competition between fungal and bacterial pathogens. Antagonistic bacteria utilized more the nutrients, minerals, competed for carbon sources, and colonized efficiently around the roots side which gave protection against pathogenic microbes [86]. Pre-harvest application of biocontrol agents in the phyllospheric part of plants also reduced the fungal infection by suppressing them. Premature fruit application of bacterial biocontrol agents restricted the many fungal pathogens such as Botrytis, Penicillium, Colletotrichum, Aspergillus, and Fusarium species [87]. An experiment was conducted to control the two pathogenic fungi P. italicum and P. digitatum by application of a commercial pre-harvest bacterial product [45]. The pre-harvest application successfully suppressed the growth of fungal pathogens as compared to the control. The pre-harvest application of bacterial products also reduced the fungal infections in mango, banana, and citrus fruits [88]. Different bacterial bio-products were suitable to control the post-harvest fungal pathogens and are commercially available in the market to date. The application of these products as single or combined with other products produced effective results in controlling the postharvest fungal pathogens [89]. Bacterial species belonging to the genera Pseudomonas, Bacillus, Enterobacter, and Burkholderia are being used to prepare commercial bio-products. The commercial products had broad-spectrum mechanisms to control the targeted pathogens by producing different volatile metabolites, production of antifungal enzymes and by activating systemic resistance in host plants [35,90]. Commercial biocontrol agents such as B. thuringiensis, Pantoea agglomerans, Serratia plymuthica, B. subtilis, and Enterobacter cloacae have already expressed their antifungal activities against different post-harvest fungal pathogens. Some of the commercially available bio-products have been tabulated as success stories in Table 3.
6 Role of Plant Growth Promoting Rhizobacteria (PGPR)
A huge diversity of soil-borne microorganisms associated with plant roots provide nutrients to plants, while in response to the nutrient supply, plants reversely provide shelter for the survival of these microbes [92]. This mutualistic mechanism is supported by both of these species; without this mechanism the survival of both species would not be possible [93]. Finally, the bacterial species, present in the rhizosphere and associated with roots of different crops, provide nutrients to the plants by colonization around the roots and the root surface, converting N2 into Ammonia (NH3), and then to nitrite (NO2−) and nitrate (NO3−). These beneficial bacteria secrete some hormones that promote plant growth, and also act as biocontrol agents against plant pathogenic microbes [94]. The rhizospheric beneficial bacteria have been categorized into two groups 1) PGPRs and, 2) Endophyte bacteria. PGPR species belong to multiple genera such as Agrobacterium, Arthrobacter, Azotobacter, Azospirilum, Bacillus, Burkholderia, Caulobacter, Chromobacterium, and Erwinia which exist in the rhizospheric soil, and they perform multiple functions to promote plant growth [95]. The role of PGPR is shown in Fig. 2. In this figure, the PGPRs were categorized on functional bases such as (a) Biofertilizers (enhance the availability of nutrients in the soil) (b) Biopesticides (secretion of specific lytic enzymes to control plant pathogens) (c) Phytostimulators (produce phytohormones to enhance plant growth) and, (d) Rhizomediators (balanced the accessible pollutants with metal solubilizations). The members of Endophytic bacteria belong to the genera Allorrhizobium, Azorhizobium, Bradyrhizobiom, Mesorhizobium, and Rhizobium [96]. The mechanism of plant growth-promoting by PGPRs is still unknown; however, the researchers continue their efforts since the last 30 years to identify the mechanisms.
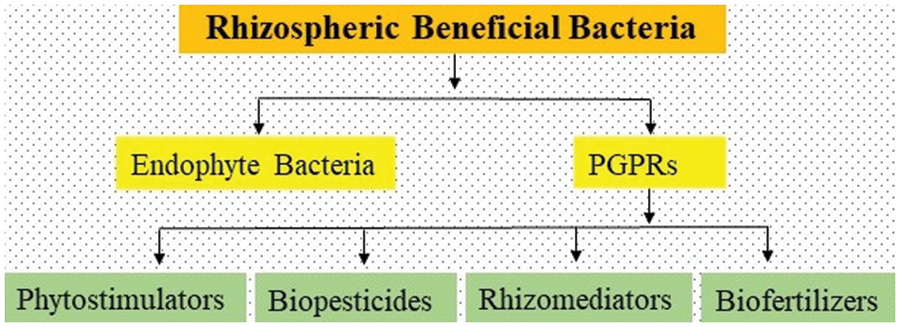
Figure 2: Role of plant growth promoting bacteria and their antifungal activities
6.1 Nitrogen Fixation by PGPRs
Two types of bacteria have been reported to convert atmospheric nitrogen into nitrite and nitrate, and then the plant species utilize the atmospheric nitrogen. The first types of bacteria were interlinked with plant roots symbiotically with the resulting formation of nodules [97]. The second type of bacteria were free-living bacteria. These free-living bacteria fully participated in NF (nitrogen fixation), e.g., Azotobacter, Paenibacillus, Bacillus, Azospirilum, Burkholderia, and Herbaspirillum. The free-living bacteria converted 20–30 kg/ha of nitrogen per year [98]. Mixed application of the NF bacteria A. brasilense, Sinorhizobium americanum, and Rhizobium phaseoli in maize crop increased crop yield with increases in root and shoot lengths [99]. Cedecea davisae RS3 as a PGRP also increased crop yield of chickpea by nitrogen fixation and with production of IAA [100].
6.2 Solubilization of Phosphorus (P)
Phosphorus (P) has a vital role in the development of living cells and plant tissues [101]. The plant takes up P in the forms of mono-phosphate and dibasic phosphate [102]. The beneficial bacterial species (PGPR) produced some specific enzymes (phytase and phosphatase), which are used in the solubilization of P into the phosphate form; this process is also known as mineralization [103]. PGPRs synthesize and release some specific acids that act like chelators to control Ca cations; these cations have the ability to convert soluble phosphate into an insoluble form. Therefore, it is concluded that the PGPRs are recognized as good and primary mediators of phosphate solubilization [104].
The phytohormones (auxins, cytokinins, gibberellins, abscisic acid, and ethylene) produced by PGPRs have a chief role in the development of important agricultural crops. These phytohormones are used by the plant for the development of body parts such as excessive cell division and cell enlargement, and extension of roots and shoots [105,106]. During the production of these types of hormones, the PGPRs develop a symbiotic association with plant roots. The phytohormones, auxin, synthesize the IAA that promote root and plant growth by improving the branch size, number, and weight. For the production of IAA, the PGPRs utilize the L-tryptophan from plant roots. However, the proper mechanism of IAA production by this process is still unknown. The gibberellic acid produced by the bacterial species A. diazotrophicus, Rhizobium meliloti, Herbaspirillum seropedicae, Bacillus spp., and Azospirilum spp. is used for the development of flowering, seed germination, fruit set, and stem elongation. Cytokinins are synthesized by important bacterial species such as Pseudomonas, Bacillus, Azospirilum, Xanthomonas, Klebsiella, and Proteus [95].
6.4 Role of ACC (1-aminocyclopropane-1-carboxylic acid) Activity of Bacteria
The PGPRs play an important role to compensate for this situation by their ACC deaminase production ability [95]. The bacteria synthesize an enzyme (ACC synthetase) and this enzyme convert the S-adenosylmethionine (SAM) to 1-aminocyclopropane-1-carboxylic acid (ACC), resulting in the production of indole acetic acid which is helpful for plant development [107]. This ACC deaminase is also used for the conversion of nitrogen into ammonia in the rhizospheric soil of the plant. The ACC deaminase antifungal activity of rhizospheric bacteria was used to control the fungal pathogen Fusarium culmorum, the causal agent of the seedling blight of wheat [108]. The ACC activity of P. fluorescence promoted the excessive modulation in an alpha crop [109].
6.5 Minimize the Rhizospheric Metal Contamination
Excessive application of synthetic chemicals/fertilizers in soil has a bad impact on both soil and plant health [110]. The beneficial microorganisms available in the rhizosphere become weaker and are suppressed due to more applications of trace metals like cobalt, magnesium, zinc, iron, chromium, selenium, and copper [111]. The PGPRs not only improve plant health, but also maintain the toxicity effects of metals [112]. The biofertilizer of the bacteria Paenibacillus sp. reduced the effect of cadmium in cotton plants by improving plant health through an increase in height, weight, chlorophyll contents, and root length [113]. The biofertilizer application of E. aerogenes reduced the cadmium contamination in rice plants and increased root and shoot lengths with more chlorophyll production [114].
6.6 Production of Volatile Metabolites or Volatile Organic Compounds
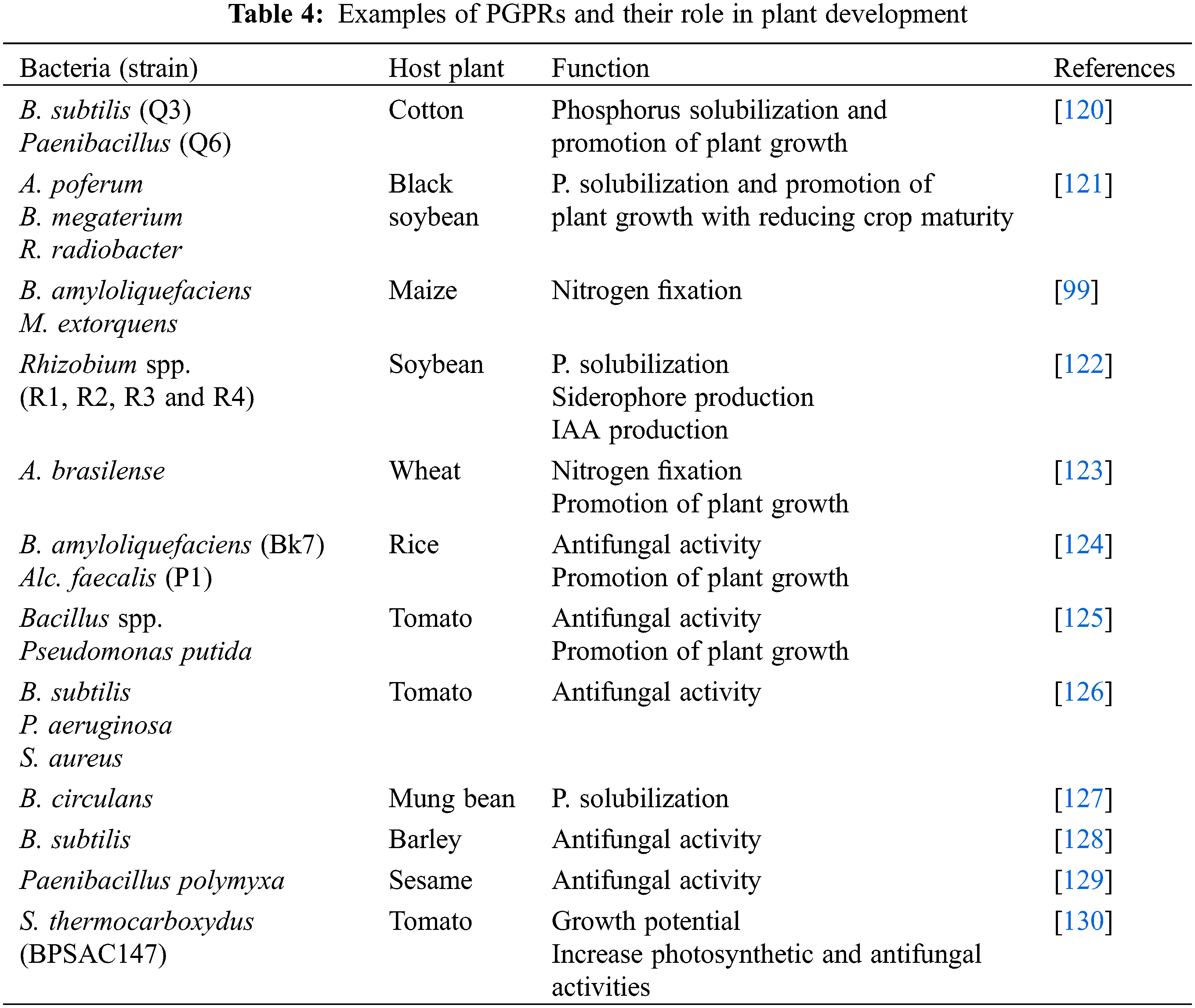
The volatile organic compounds (VOC) emitted by the PGRPs, are used to control phytopathogens and at the same time, these bacteria promote plant growth by ISR [115]. The bacterial species belong to different genera producing VOCs like acetoin and 2, 3-Butanediol, produced by members of the genus Bacillus [116]. The species of the genus Bacillus, Stenotrophomonas, Pseudomonas, Serratia, and Arthrobacter mostly suppressed the growth of FPPs by producing antifungal VOCs. These VOCs produce resistance in the host plant against a variety of pathogenic microbes and abiotic stresses by the activation of both ISR and SAR [117]. A number of PGPRs are soil borne bacterial species and they synthesize and emit VOCs in the soil such as tetradecane, dodecane, cyclohexane, benzene, 1-(N-phenylcarbamyl)-2-morpholinocyclohexene, methyl, dotriacontane, decane, 2-(benzyloxy) ethanamine, benzene(1-methylnonadecyl), 11-decyldocosane, 2,6,10-trimethyl, and 1-chlorooctadecane [118,119]. The different PGPRs and their roles in plant growth are presented in Table 4.
7 Conclusion and Future Prospective
By using bacteria as an effective biocontrol agent we can improve our soil fertility, plant health and crop production. These multitalented biocontrol agents improved seed health by coating it before sowing, and afterwards the soil was made more beneficial for cropping. A few previous studies reported by many researchers showed that the mixing of biocontrol agents with artificially prepared pesticides improved plant health and stimulated many genes in host plants against infectious plant pathogens. The application of biocontrol agents is cost-effective and ecofriendly. We should need to understand the biochemistry of biopesticides and increase them by better ways. Bacterial biopesticide chemistry is still not fully understood and explains the need of future research.
Compliance with Ethical Standards: The authors declare that the review is in compliance with ethical standards of the journal.
Research Involving Human Participants and/or Animals: The authors declare that the manuscript does not contain research involving Human Participants and/or Animals.
Authorship: All authors have equally contributed in gathering literature and in writing and formatting the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Parewa, H. P., Meena, V. S., Jain, L. K., Choudhary, A. (2018). Sustainable crop production and soil health management through plant growth-promoting rhizobacteria. In: Role of rhizospheric microbes in soil, pp. 299–329. Singapore: Springer. [Google Scholar]
2. Golonka, R., San Yeoh, B., Vijay-Kumar, M. (2019). The iron tug-of-war between bacterial siderophores and innate immunity. Journal of Innate Immunity, 11(3), 249–262. DOI 10.1159/000494627. [Google Scholar] [CrossRef]
3. Carmona-Hernandez, S., Reyes-Pérez, J. J., Chiquito-Contreras, R. G., Rincon-Enriquez, G., Cerdan-Cabrera, C. R. et al. (2019). Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy, 9(3), 121. DOI 10.3390/agronomy9030121. [Google Scholar] [CrossRef]
4. Umer, M., Mubeen, M., Iftikhar, Y., Shad, M. A., Usman, H. M. et al. (2021). Role of Rhizobacteria on plants growth and biological control of plant diseases: A review. Plant Protection, 5(1), 59–73. DOI 10.33804/pp.005.01.3565. [Google Scholar] [CrossRef]
5. Zafar-ul-Hye, M., Akbar, M. N., Iftikhar, Y., Abbas, M., Zahid, A. et al. (2021). Rhizobacteria inoculation and caffeic acid alleviated drought stress in lentil plants. Sustainability, 13(17), 9603. DOI 10.3390/su13179603. [Google Scholar] [CrossRef]
6. Abedon, S. T. (2015). Ecology of anti-biofilm agents II: Bacteriophage exploitation and biocontrol of biofilm bacteria. Pharmaceuticals, 8(3), 559–589. DOI 10.3390/ph8030559. [Google Scholar] [CrossRef]
7. Markelova, N. Y. (2010). Predacious bacteria, Bdellovibrio with potential for biocontrol. International Journal of Hygiene and Environmental Health, 213(6), 428–431. DOI 10.1016/j.ijheh.2010.08.004. [Google Scholar] [CrossRef]
8. Dean, R., van Kan, J. A., Pretorius, Z. A., Hammond-Kosack, K. E., di Pietro, A. et al. (2012). The top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology, 13(4), 414–430. DOI 10.1111/j.1364-3703.2011.00783.x. [Google Scholar] [CrossRef]
9. Mbengue, M., Navaud, O., Peyraud, R., Barascud, M., Badet, T. et al. (2016). Emerging trends in molecular interactions between plants and the broad host range fungal pathogens Botrytis cinerea and Sclerotinia sclerotiorum. Frontiers in Plant Science, 7, 422. DOI 10.3389/fpls.2016.00422. [Google Scholar] [CrossRef]
10. Vilanova, L., Vall-Llaura, N., Torres, R., Usall, J., Teixidó, N. et al. (2017). Penicillium expansum (compatible) and Penicillium digitatum (non-host) pathogen infection differentially alter ethylene biosynthesis in apple fruit. Plant Physiology and Biochemistry, 120, 132–143. DOI 10.1016/j.plaphy.2017.09.024. [Google Scholar] [CrossRef]
11. Torres, M. J., Brandan, C. P., Petroselli, G., Erra-Balsells, R., Audisio, M. C. (2016). Antagonistic effects of Bacillus subtilis subsp. subtilis and B. amyloliquefaciens against Macrophomina phaseolina: SEM study of fungal changes and UV-MALDI-TOF MS analysis of their bioactive compounds. Microbiological Research, 182, 31–39. DOI 10.1016/j.micres.2015.09.005. [Google Scholar] [CrossRef]
12. Haidar, R., Fermaud, M., Calvo-Garrido, C., Roudet, J., Deschamps, A. (2016). Modes of action for biological control of Botrytis cinerea by antagonistic bacteria. Phytopathologia Mediterranea, 301–302. DOI 10.14601/Phytopathol_Mediterr-18079. [Google Scholar] [CrossRef]
13. Mansoori, M., Heydari, A., Hassanzadeh, N., Rezaee, S., Naraghi, L. (2013). Evaluation of Pseudomonas and Bacillus bacterial antagonists for biological control of cotton Verticillium wilt disease. Journal of Plant Protection Research, 53(2), 154–157. [Google Scholar]
14. Beneduzi, A., Ambrosini, A., Passaglia, L. M. (2012). Plant growth-promoting rhizobacteria (PGPRTheir potential as antagonists and biocontrol agents. Genetics and Molecular Biology, 35, 1044–1051. [Google Scholar]
15. Chen, X., Zhang, Y., Fu, X., Li, Y., Wang, Q. (2016). Isolation and characterization of Bacillus amyloliquefaciens PG12 for the biological control of apple ring rot. Postharvest Biology and Technology, 115, 113–121. DOI 10.1016/j.postharvbio.2015.12.021. [Google Scholar] [CrossRef]
16. Aiello, D., Restuccia, C., Stefani, E., Vitale, A., Cirvilleri, G. (2019). Postharvest biocontrol ability of Pseudomonas synxantha against Monilinia fructicola and Monilinia fructigena on stone fruit. Postharvest Biology and Technology, 149, 83–89. DOI 10.1016/j.postharvbio.2018.11.020. [Google Scholar] [CrossRef]
17. Demoz, B. T., Korsten, L. (2006). Bacillus subtilis attachment, colonization, and survival on avocado flowers and its mode of action on stem-end rot pathogens. Biological Control, 37(1), 68–74. DOI 10.1016/j.biocontrol.2005.11.010. [Google Scholar] [CrossRef]
18. Stockwell, V. O., Stack, J. P. (2007). Using Pseudomonas spp. for integrated biological control. Phytopathology, 97(2), 244–249. DOI 10.1094/PHYTO-97-2-0244. [Google Scholar] [CrossRef]
19. Al-Mughrabi, K. I., Vikram, A., Peters, R. D., Howard, R. J., Grant, L. et al. (2013). Efficacy of Pseudomonas syringae in the management of potato tuber diseases in storage. Biological Control, 64(3), 315–322. DOI 10.1016/j.biocontrol.2012.11.011. [Google Scholar] [CrossRef]
20. Gajbhiye, M. H., Kapadnis, B. P. (2016). Antifungal-activity-producing lactic acid bacteria as biocontrol agents in plants. Biocontrol Science and Technology, 26(11), 1451–1470. DOI 10.1080/09583157.2016.1213793. [Google Scholar] [CrossRef]
21. Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A., Dufresne, A. (2015). The importance of the microbiome of the plant holobiont. New Phytologist, 206(4), 1196–1206. DOI 10.1111/nph.13312. [Google Scholar] [CrossRef]
22. Huang, D., Tian, W., Feng, J., Zhu, S. (2020). Interaction between nitric oxide and storage temperature on sphingolipid metabolism of postharvest peach fruit. Plant Physiology and Biochemistry, 151, 60–68. DOI 10.1016/j.plaphy.2020.03.012. [Google Scholar] [CrossRef]
23. Huang, S., Gong, B., Wei, F., Ma, H. (2017). Pre-harvest 1-methylcyclopropene application affects post-harvest physiology and storage life of the cut rose cv. Carola. Horticulture, Environment, and Biotechnology, 58(2), 144–151. DOI 10.1007/s13580-017-0081-9. [Google Scholar] [CrossRef]
24. Moradi, M., Nejad, F. J., Bonjar, G. H. S., Fani, S. R., Mimand, B. M. et al. (2018). Efficacy of Bacillus subtilis native strains for biocontrol of Phytophthora crown and root rot of pistachio in Iran. Tropical Plant Pathology, 43(4), 306–313. DOI 10.1007/s40858-018-0226-0. [Google Scholar] [CrossRef]
25. Aktaruzzaman, M., Afroz, T., Lee, Y. G., Kim, B. S. (2018). Post-harvest anthracnose of papaya caused by Colletotrichum truncatum in Korea. European Journal of Plant Pathology, 150(1), 259–265. DOI 10.1007/s10658-017-1265-y. [Google Scholar] [CrossRef]
26. Li, H., Chen, Y., Zhang, Z. Q., Li, B. Q., Qin, G. Z. et al. (2018). Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Quality and Safety, 2(3), 111–119. DOI 10.1093/fqsafe/fyy016. [Google Scholar] [CrossRef]
27. Yahaya, S. M., Kamalu, A. B., Ali, M. U., Lawan, M., Ajingi, Y. S. et al. (2019). A study of pathogenic fungi causing post harvest losses of pineapple sold at Wudil and Yan Lemo markets of Kano State. Journal of Experimental Agriculture International, 38(2), 1–6. DOI 10.9734/jeai/2019/v38i230297. [Google Scholar] [CrossRef]
28. Andreote, F. D., Silva, M. D. C. P. (2017). Microbial communities associated with plants: Learning from nature to apply it in agriculture. Current Opinion in Microbiology, 37, 29–34. DOI 10.1016/j.mib.2017.03.011. [Google Scholar] [CrossRef]
29. Siegel-Hertz, K., Edel-Hermann, V., Chapelle, E., Terrat, S., Raaijmakers, J. M. et al. (2018). Comparative microbiome analysis of a Fusarium wilt suppressive soil and a Fusarium wilt conducive soil from the Chateaurenard region. Frontiers in Microbiology, 9, 568. DOI 10.3389/fmicb.2018.00568. [Google Scholar] [CrossRef]
30. Bahroun, A., Jousset, A., Mhamdi, R., Mrabet, M., Mhadhbi, H. (2018). Anti-fungal activity of bacterial endophytes associated with legumes against Fusarium solani: Assessment of fungi soil suppressiveness and plant protection induction. Applied Soil Ecology, 124, 131–140. DOI 10.1016/j.apsoil.2017.10.025. [Google Scholar] [CrossRef]
31. Law, J. W. F., Ser, H. L., Khan, T. M., Chuah, L. H., Pusparajah, P. et al. (2017). The potential of Streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Frontiers in Microbiology, 8, 3. DOI 10.3389/fmicb.2017.00003. [Google Scholar] [CrossRef]
32. Andreolli, M., Zapparoli, G., Angelini, E., Lucchetta, G., Lampis, S. et al. (2019). Pseudomonas protegens MP12: A plant growth-promoting endophytic bacterium with broad-spectrum antifungal activity against grapevine phytopathogens. Microbiological Research, 219, 123–131. DOI 10.1016/j.micres.2018.11.003. [Google Scholar] [CrossRef]
33. Guevara-Avendaño, E., Bejarano-Bolívar, A. A., Kiel-Martínez, A. L., Ramírez-Vázquez, M., Méndez-Bravo, A. et al. (2019). Avocado rhizobacteria emit volatile organic compounds with antifungal activity against Fusarium solani, Fusarium sp. associated with Kuroshio shot hole borer, and Colletotrichum gloeosporioides. Microbiological Research, 219, 74–83. DOI 10.1016/j.micres.2018.11.009. [Google Scholar] [CrossRef]
34. Santhanam, R., Menezes, R. C., Grabe, V., Li, D., Baldwin, I. T. et al. (2019). A suite of complementary biocontrol traits allows a native consortium of root-associated bacteria to protect their host plant from a fungal sudden-wilt disease. Molecular Ecology, 28(5), 1154–1169. DOI 10.1111/mec.15012. [Google Scholar] [CrossRef]
35. Sajitha, K. L., Dev, S. A., Florence, E. M. (2018). Biocontrol potential of Bacillus subtilis B1 against sapstain fungus in rubber wood. European Journal of Plant Pathology, 150(1), 237–244. DOI 10.1007/s10658-017-1272-z. [Google Scholar] [CrossRef]
36. Kasfi, K., Taheri, P., Jafarpour, B., Tarighi, S. (2018). Identification of epiphytic yeasts and bacteria with potential for biocontrol of grey mold disease on table grapes caused by Botrytis cinerea. Spanish Journal of Agricultural Research, 16(1), 23. DOI 10.5424/sjar/2018161-11378. [Google Scholar] [CrossRef]
37. Hernandez Montiel, L. G., Zulueta Rodriguez, R., Angulo, C., Rueda Puente, E. O., Quiñonez Aguilar, E. E. et al. (2017). Marine yeasts and bacteria as biological control agents against anthracnose on mango. Journal of Phytopathology, 165(11–12), 833–840. DOI 10.1111/jph.12623. [Google Scholar] [CrossRef]
38. Lorenzini, M., Zapparoli, G. (2020). Epiphytic bacteria from withered grapes and their antagonistic effects on grape-rotting fungi. International Journal of Food Microbiology, 319, 108505. DOI 10.1016/j.ijfoodmicro.2019.108505. [Google Scholar] [CrossRef]
39. Li, Y., Cai, Y., Liang, Y., Ji, P., Xu, L. (2020). Assessment of antifungal activities of a biocontrol bacterium BA17 for managing postharvest gray mold of green bean caused by Botrytis cinerea. Postharvest Biology and Technology, 161, 111086. DOI 10.1016/j.postharvbio.2019.111086. [Google Scholar] [CrossRef]
40. Cheong, E. Y., Sandhu, A., Jayabalan, J., Le, T. T. K., Nhiep, N. T. et al. (2014). Isolation of lactic acid bacteria with antifungal activity against the common cheese spoilage mould Penicillium commune and their potential as biopreservatives in cheese. Food Control, 46, 91–97. DOI 10.1016/j.foodcont.2014.05.011. [Google Scholar] [CrossRef]
41. Russo, P., Arena, M. P., Fiocco, D., Capozzi, V., Drider, D. et al. (2017). Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. International Journal of Food Microbiology, 247, 48–54. DOI 10.1016/j.ijfoodmicro.2016.04.027. [Google Scholar] [CrossRef]
42. Prema, P., Smila, D., Palavesam, A., Immanuel, G. (2010). Production and characterization of an antifungal compound (3-phenyllactic acid) produced by Lactobacillus plantarum strain. Food and Bioprocess Technology, 3(3), 379–386. DOI 10.1007/s11947-008-0127-1. [Google Scholar] [CrossRef]
43. Lan, W. T., Chen, Y. S., Wu, H. C., Yanagida, F. (2012). Bio-protective potential of lactic acid bacteria isolated from fermented wax gourd. Folia Microbiologica, 57(2), 99–105. DOI 10.1007/s12223-012-0101-1. [Google Scholar] [CrossRef]
44. Dalie, D. K. D., Deschamps, A. M., Atanasova-Penichon, V., Richard-Forget, F. (2010). Potential of Pediococcus pentosaceus (L006) isolated from maize leaf to suppress fumonisin-producing fungal growth. Journal of Food Protection, 73(6), 1129–1137. DOI 10.4315/0362-028X-73.6.1129. [Google Scholar] [CrossRef]
45. Matei, S., Matei, A., Matei, G. M., Cornea, C. P. (2015). Utilization of lactic acid bacteria and extracellular compounds in biological control of fungal species. Research Journal of Agricultural Science, 47(3), 122–132. [Google Scholar]
46. Hacquard, S., Spaepen, S., Garrido-Oter, R., Schulze-Lefert, P. (2017). Interplay between innate immunity and the plant microbiota. Annual Review of Phytopathology, 55, 565–589. DOI 10.1146/annurev-phyto-080516-035623. [Google Scholar] [CrossRef]
47. Marín, A., Plotto, A., Atarés, L., Chiralt, A. (2019). Lactic acid bacteria incorporated into edible coatings to control fungal growth and maintain postharvest quality of grapes. Horticultural Science, 54(2), 337–343. DOI 10.21273/HORTSCI13661-18. [Google Scholar] [CrossRef]
48. López-Seijas, J., García-Fraga, B., da Silva, A. F.,Sieiro, C. (2020). Wine lactic acid bacteria with antimicrobial activity as potential biocontrol agents against Fusarium oxysporum f. sp. lycopersici. Agronomy, 10(1), 31. DOI 10.3390/agronomy10010031. [Google Scholar] [CrossRef]
49. Zebboudj, N., Yezli, W., Hamini-Kadar, N., Kihal, M., Henni, J. E. (2014). Antifungal activity of lactic acid bacteria against Fusarium oxysporum f. sp. albedinis isolated from diseased date palm in South Algeria. International Journal of Biosciences, 5(9), 99–106. DOI 10.12692/ijb/5.9.99-106. [Google Scholar] [CrossRef]
50. Carroll, C. S., Moore, M. M. (2018). Ironing out siderophore biosynthesis: A review of non-ribosomal peptide synthetase (NRPS)-independent siderophore synthetases. Critical Reviews in Biochemistry and Molecular Biology, 53(4), 356–381. DOI 10.1080/10409238.2018.1476449. [Google Scholar] [CrossRef]
51. Sasirekha, B., Srividya, S. (2016). Siderophore production by Pseudomonas aeruginosa FP6, a biocontrol strain for Rhizoctonia solani and Colletotrichum gloeosporioides causing diseases in chilli. Agriculture and Natural Resources, 50(4), 250–256. DOI 10.1016/j.anres.2016.02.003. [Google Scholar] [CrossRef]
52. Arya, N., Rana, A., Rajwar, A., Sahgal, M., Sharma, A. K. (2018). Biocontrol efficacy of siderophore producing indigenous Pseudomonas strains against Fusarium wilt in tomato. National Academy Science Letters, 41(3), 133–136. DOI 10.1007/s40009-018-0630-5. [Google Scholar] [CrossRef]
53. Hernandez-Montiel, L. G., Gutierrez-Perez, E. D., Murillo-Amador, B., Vero, S., Chiquito-Contreras, R. G. et al. (2018). Mechanisms employed by Debaryomyces hansenii in biological control of anthracnose disease on papaya fruit. Postharvest Biology and Technology, 139, 31–37. DOI 10.1016/j.postharvbio.2018.01.015. [Google Scholar] [CrossRef]
54. Yu, S. M., Lee, Y. H. (2015). Genes involved in nutrient competition by Pseudomonas putida JBC17 to suppress green mold in postharvest satsuma mandarin. Journal of Basic Microbiology, 55(7), 898–906. DOI 0.1002/jobm.201400792. [Google Scholar]
55. Luo, M., Purdy, H., Avis, T. J. (2019). Compost bacteria provide antifungal activity against grey mold and Alternaria rot on bell pepper fruit. Botany, 97(3), 221–230. DOI 10.1139/cjb-2018-0180. [Google Scholar] [CrossRef]
56. Safdarpour, F., Khodakaramian, G. (2019). Assessment of antagonistic and plant growth promoting activities of tomato endophytic bacteria in challenging with Verticillium dahliae under in-vitro and in-vivo conditions. Biological Journal of Microorganism, 7(28), 77–90. [Google Scholar]
57. Spadaro, D., Droby, S. (2016). Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends in Food Science & Technology, 47, 39–49. DOI 10.1016/j.tifs.2015.11.003. [Google Scholar] [CrossRef]
58. Stoykov, Y. M., Pavlov, A. I., Krastanov, A. I. (2015). Chitinase biotechnology: Production, purification, and application. Engineering in Life Sciences, 15(1), 30–38. DOI 10.1002/elsc.201400173. [Google Scholar] [CrossRef]
59. Kushwaha, P., Kashyap, P. L., Srivastava, A. K., Tiwari, R. K. (2020). Plant growth promoting and antifungal activity in endophytic Bacillus strains from pearl millet (Pennisetum glaucum). Brazilian Journal of Microbiology, 51(1), 229–241. DOI 10.1007/s42770-019-00172-5. [Google Scholar] [CrossRef]
60. Rong, S., Xu, H., Li, L., Chen, R., Gao, X. et al. (2020). Antifungal activity of endophytic Bacillus safensis B21 and its potential application as a biopesticide to control rice blast. Pesticide Biochemistry and Physiology, 162, 69–77. DOI 10.1016/j.pestbp.2019.09.003. [Google Scholar] [CrossRef]
61. Xie, S., Liu, J., Gu, S., Chen, X., Jiang, H. et al. (2020). Antifungal activity of volatile compounds produced by endophytic Bacillus subtilis DZSY21 against Curvularia lunata. Annals of Microbiology, 70(1), 1–10. DOI 10.1186/s13213-020-01553-0. [Google Scholar] [CrossRef]
62. Shafi, J., Tian, H., Ji, M. (2017). Bacillus species as versatile weapons for plant pathogens: A review. Biotechnology & Biotechnological Equipment, 31(3), 446–459. DOI 10.1080/13102818.2017.1286950. [Google Scholar] [CrossRef]
63. Kalia, V. C. (2013). Quorum sensing inhibitors: An overview. Biotechnology Advances, 31(2), 224–245. DOI 10.1016/j.biotechadv.2012.10.004. [Google Scholar] [CrossRef]
64. Conway, B. A. D., Venu, V., Speert, D. P. (2002). Biofilm formation and acyl homoserine lactone production in the Burkholderia cepacia complex. Journal of Bacteriology, 184(20), 5678–5685. DOI 10.1128/JB.184.20.5678-5685.2002. [Google Scholar] [CrossRef]
65. Camele, I., Elshafie, H. S., Caputo, L., Sakr, S. H., de Feo, V. (2019). Bacillus mojavensis: Biofilm formation and biochemical investigation of its bioactive metabolites. Journal of Biological Research-Bollettino della Società Italiana di Biologia Sperimentale, 92(139–45. DOI 10.4081/jbr.2019.8296. [Google Scholar] [CrossRef]
66. Jasim, B., Sreelakshmi, S., Mathew, J., Radhakrishnan, E. K. (2016). Identification of endophytic Bacillus mojavensis with highly specialized broad spectrum antibacterial activity. 3 Biotech, 6(2), 1–10. DOI 10.1007/s13205-016-0508-5. [Google Scholar] [CrossRef]
67. Pandin, C., Darsonval, M., Mayeur, C., Le Coq, D., Aymerich, S. et al. (2019). Biofilm formation and synthesis of antimicrobial compounds by the biocontrol agent Bacillus velezensis QST713 in an Agaricus bisporus compost micromodel. Applied and Environmental Microbiology, 85(12), e00327–19. DOI 10.1128/AEM.00327-19. [Google Scholar]
68. Aziz, A., Verhagen, B., Magnin-Robert, M., Couderchet, M., Clément, C. et al. (2016). Effectiveness of beneficial bacteria to promote systemic resistance of grapevine to gray mold as related to phytoalexin production in vineyards. Plant and Soil, 405(1), 141–153. DOI 10.1007/s11104-015-2783-z. [Google Scholar] [CrossRef]
69. Guo, H., Nolan, T. M., Song, G., Liu, S., Xie, Z. et al. (2018). FERONIA receptor kinase contributes to plant immunity by suppressing jasmonic acid signaling in Arabidopsis thaliana. Current Biology, 28(20), 3316–3324. DOI 10.1016/j.cub.2018.07.078. [Google Scholar] [CrossRef]
70. El-Shanshoury, A. E. R. R., Bazaid, S. A., El-Halmouch, Y., Waheed, M., Ghafar, E. (2013). Control the postharvest infection by Aspergillus spp. to Taify table grape using grape epiphytic bacteria. Life Science Journal, 10(1), 1821–1836. [Google Scholar]
71. Chowdappa, P., Kumar, S. M., Lakshmi, M. J., Upreti, K. K. (2013). Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biological Control, 65(1), 109–117. DOI 10.1016/j.biocontrol.2012.11.009. [Google Scholar] [CrossRef]
72. Adrees, H., Haider, M. S., Anjum, T., Akram, W. (2019). Inducing systemic resistance in cotton plants against charcoal root rot pathogen using indigenous rhizospheric bacterial strains and chemical elicitors. Crop Protection, 115, 75–83. DOI 10.1016/j.cropro.2018.09.011. [Google Scholar] [CrossRef]
73. Sahu, P. K., Singh, S., Gupta, A., Singh, U. B., Brahmaprakash, G. P. et al. (2019). Antagonistic potential of bacterial endophytes and induction of systemic resistance against collar rot pathogen Sclerotium rolfsii in tomato. Biological Control, 137, 104014. DOI 10.1016/j.biocontrol.2019.104014. [Google Scholar] [CrossRef]
74. Seyedsayamdost, M. R. (2019). Toward a global picture of bacterial secondary metabolism. Journal of Industrial Microbiology and Biotechnology, 46(3–4), 301–311. DOI 10.1007/s10295-019-02136-y. [Google Scholar] [CrossRef]
75. Ciancio, A., Pieterse, C. M., Mercado-Blanco, J. (2019). Harnessing useful rhizosphere microorganisms for pathogen and pest biocontrol, vol. 2. Lausanne: Frontiers Media SA. [Google Scholar]
76. Hanschen, F. S., Winkelmann, T. (2020). Biofumigation for fighting replant disease—A review. Agronomy, 10(3), 425. DOI 10.3390/agronomy10030425. [Google Scholar] [CrossRef]
77. Latorre, J. D., Hernandez-Velasco, X., Wolfenden, R. E., Vicente, J. L., Wolfenden, A. D. et al. (2016). Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Frontiers in Veterinary Science, 3, 95. DOI 10.3389/fvets.2016.00095. [Google Scholar] [CrossRef]
78. Guan, Q., Huang, S., Jin, Y., Campagne, R., Alezra, V. et al. (2019). Recent advances in the exploration of therapeutic analogues of gramicidin S, an old but still potent antimicrobial peptide. Journal of Medicinal Chemistry, 62(17), 7603–7617. DOI 10.1021/acs.jmedchem.9b00156. [Google Scholar] [CrossRef]
79. Karthika, S., Midhun, S. J., Jisha, M. S. (2020). A potential antifungal and growth-promoting bacterium Bacillus sp. KTMA4 from tomato rhizosphere. Microbial Pathogenesis, 142, 104049. DOI 10.1016/j.micpath.2020.104049. [Google Scholar] [CrossRef]
80. Shen, Y. C., Korkor, N. L., Xiao, R., Pu, Q., Hu, M. et al. (2020). Antagonistic activity of combined bacteria strains against southern blight pathogen of Dendrobium officinale. Biological Control, 151, 104291. DOI 10.1016/j.biocontrol.2020.104291. [Google Scholar] [CrossRef]
81. Shastri, B., Kumar, R., Lal, R. J. (2020). Isolation, characterization and identification of indigenous endophytic bacteria exhibiting PGP and antifungal traits from the internal tissue of sugarcane crop. Sugar Tech, 22(4), 563–573. DOI 10.1007/s12355-020-00824-z. [Google Scholar] [CrossRef]
82. Calvo, H., Mendiara, I., Arias, E., Gracia, A. P., Blanco, D. et al. (2020). Antifungal activity of the volatile organic compounds produced by Bacillus velezensis strains against postharvest fungal pathogens. Postharvest Biology and Technology, 166, 111208. DOI 10.1016/j.postharvbio.2020.111208. [Google Scholar] [CrossRef]
83. Gao, Y., Zeng, X. D., Ren, B., Zeng, J. R., Xu, T. et al. (2020). Antagonistic activity against rice blast disease and elicitation of host-defence response capability of an endophytic Streptomyces albidoflavus OsiLf-2. Plant Pathology, 69(2), 259–271. DOI 10.1111/ppa.13118. [Google Scholar] [CrossRef]
84. Kumari, S., Khanna, V. (2020). Induction of systemic resistance in chickpea (Cicer arietinum L.) against Fusarium oxysporum f. sp. ciceris by antagonistic rhizobacteria in assistance with native mesorhizobium. Current Microbiology, 77(1), 85–98. DOI 10.1007/s00284-019-01805-6. [Google Scholar] [CrossRef]
85. Attia, M. S., El-Sayyad, G. S., Abd Elkodous, M., El-Batal, A. I. (2020). The effective antagonistic potential of plant growth-promoting rhizobacteria against Alternaria solani-causing early blight disease in tomato plant. Scientia Horticulturae, 266, 109289. DOI 10.1016/j.scienta.2020.109289. [Google Scholar] [CrossRef]
86. Granada, D., López-Lujan, L., Ramírez-Restrepo, S., Morales, J., Peláez-Jaramillo, C. et al. (2020). Bacterial extracts and bioformulates as a promising control of fruit body rot and root rot in avocado cv. Hass. Journal of Integrative Agriculture, 19(3), 748–758. DOI 10.1016/S2095-3119(19)62720-6. [Google Scholar] [CrossRef]
87. Abbey, J. A., Percival, D., Abbey, L., Asiedu, S. K., Prithiviraj, B. et al. (2019). Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)-prospects and challenges. Biocontrol Science and Technology, 29(3), 207–228. DOI 10.1080/09583157.2018.1548574. [Google Scholar] [CrossRef]
88. Udhayakumar, R., Rajamohan, K., Sanjaygandhi, S., Vengadeshkumar, L. (2019). Effect of pre harvest application of bioformulations on the anthracnose disease incidence and biometrics of mango. Journal of Biopesticides, 12(1), 61–71. [Google Scholar]
89. Parveen, S., Wani, A. H., Bhat, M. Y., Koka, J. A. (2016). Biological control of postharvest fungal rots of rosaceous fruits using microbial antagonists and plant extracts—A review. Czech Mycology, 68(141–66. [Google Scholar]
90. Wang, X., Glawe, D. A., Kramer, E., Weller, D., Okubara, P. A. (2018). Biological control of Botrytis cinerea: Interactions with native vineyard yeasts from Washington State. Phytopathology, 108(6), 691–701. DOI 10.1094/PHYTO-09-17-0306-R. [Google Scholar] [CrossRef]
91. Wallace, R. L., Hirkala, D. L., Nelson, L. M. (2019). Pseudomonas fluorescens and low doses of chemicals inhibit postharvest decay of apples in commercial storage. Canadian Journal of Plant Pathology, 41(3), 355–365. DOI 10.1080/07060661.2019.1605539. [Google Scholar] [CrossRef]
92. Bhattacharyya, P. N., Jha, D. K. (2012). Plant growth-promoting rhizobacteria (PGPREmergence in agriculture. World Journal of Microbiology and Biotechnology, 28(4), 1327–1350. DOI 10.1007/s11274-011-0979-9. [Google Scholar] [CrossRef]
93. Harish, S., Parthasarathy, S., Durgadevi, D., Anandhi, K., Raguchander, T. (2019). Plant growth promoting Rhizobacteria: Harnessing its potential for sustainable plant disease management. In: Plant growth promoting rhizobacteria for agricultural sustainability, pp. 151–187. Singapore: Springer. [Google Scholar]
94. Etesami, H., Adl, S. M. (2020). Plant growth-promoting rhizobacteria (PGPR) and their action mechanisms in availability of nutrients to plants. In: Phyto-microbiome in stress regulation, pp. 147–203. Singapore: Springer. [Google Scholar]
95. Kumar, A., Kumar, R., Kumari, M., Goldar, S. (2020). Enhancement of plant growth by using PGPR for a sustainable agriculture: A review. International Journal of Current Microbiology and Applied Sciences, 9(2), 152–165. DOI 10.20546/ijcmas.2020.902.019. [Google Scholar] [CrossRef]
96. Prasad, M., Srinivasan, R., Chaudhary, M., Mahawer, S. K., Jat, L. K. (2020). Endophytic bacteria: Role in sustainable agriculture. In: Microbial endophytes, pp. 37–60. Woodhead Publishing. [Google Scholar]
97. Oberson, A., Frossard, E., Bühlmann, C., Mayer, J., Mäder, P. et al. (2013). Nitrogen fixation and transfer in grass-clover leys under organic and conventional cropping systems. Plant and Soil, 371(1), 237–255. DOI 10.1007/s11104-013-1666-4. [Google Scholar] [CrossRef]
98. Peoples, M. B., Herridge, D. F., Ladha, J. K. (1995). Biological nitrogen fixation: An efficient source of nitrogen for sustainable agricultural production? In Management of biological nitrogen fixation for the development of more productive and sustainable agricultural systems, pp. 3–28. Dordrecht: Springer. [Google Scholar]
99. Gómez-Godínez, L. J., Fernandez-Valverde, S. L., Romero, J. C. M., Martínez-Romero, E. (2019). Metatranscriptomics and nitrogen fixation from the rhizoplane of maize plantlets inoculated with a group of PGPRs. Systematic and Applied Microbiology, 42(4), 517–525. DOI 10.1016/j.syapm.2019.05.003. [Google Scholar] [CrossRef]
100. Mazumdar, D., Saha, S. P., Ghosh, S. (2019). Isolation, screening and application of a potent PGPR for enhancing growth of Chickpea as affected by nitrogen level. International Journal of Vegetable Science, 26(4), 333–350. DOI 10.1080/19315260.2019.1632401. [Google Scholar] [CrossRef]
101. Saia, S., Aissa, E., Luziatelli, F., Ruzzi, M., Colla, G. et al. (2020). Growth-promoting bacteria and arbuscular mycorrhizal fungi differentially benefit tomato and corn depending upon the supplied form of phosphorus. Mycorrhiza, 30(1), 133–147. DOI 10.1007/s00572-019-00927-w. [Google Scholar] [CrossRef]
102. Jha, C. K., Saraf, M. (2015). Plant growth promoting Rhizobacteria (PGPRA review. Journal of Agricultural Research, 5, 108–119. [Google Scholar]
103. Pii, Y., Mimmo, T., Tomasi, N., Terzano, R., Cesco, S. et al. (2015). Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biology and Fertility of Soils, 51(4), 403–415. DOI 10.1007/s00374-015-0996-1. [Google Scholar] [CrossRef]
104. Gusain, P., Bhandari, B. (2019). Rhizosphere associated PGPR functioning. Journal of Pharmacognosy and Phytochemistry, 8, 1181–1191. [Google Scholar]
105. Banerjee, A., Roychoudhury, A. (2019). The regulatory signaling of gibberellin metabolism and its crosstalk with phytohormones in response to plant abiotic stresses. In: Plant signaling molecule, pp. 333–339. Woodhead Publishing. DOI 10.1016/B978-0-12-816451-8.00020-4. [Google Scholar] [CrossRef]
106. Mihaljević, S., Milanović, J., Oklestkova, J., Novák, O. (2020). The role of phytohormones in plant-viroid interactions. In: Applied plant biotechnology for improving resistance to biotic stress, pp. 321–342. Elsevier. [Google Scholar]
107. Zerrouk, I. Z., Rahmoune, B., Khelifi, L., Mounir, K., Baluska, F. et al. (2019). Algerian Sahara PGPR confers maize root tolerance to salt and aluminum toxicity via ACC deaminase and IAA. Acta Physiologiae Plantarum, 41(6), 91. DOI 10.1007/s11738-019-2881-2. [Google Scholar] [CrossRef]
108. Imriz, G., Özdemir, F., Karaca, M. S., Taş, M. N., Topal, I. et al. (2020). Biological control potential of rhizosphere bacteria with ACC-deaminase activity against Fusarium culmorum in wheat. Zemdirbyste-Agriculture, 107(2105–112. DOI 10.13080/z-a.2020.107.014. [Google Scholar] [CrossRef]
109. Nascimento, F. X., Tavares, M. J., Franck, J., Ali, S., Glick, B. R. et al. (2019). ACC deaminase plays a major role in Pseudomonas fluorescens YsS6 ability to promote the nodulation of Alpha-and Betaproteobacteria rhizobial strains. Archives of Microbiology, 201(6), 817–822. DOI 10.1007/s00203-019-01649-5. [Google Scholar] [CrossRef]
110. Upadhayay, J., Rana, M., Juyal, V., Bisht, S. S., Joshi, R. (2020). Impact of pesticide exposure and associated health effects. In: Pesticides in crop production: Physiological and biochemical zoology, pp. 69–88. [Google Scholar]
111. Tripathi, S., Srivastava, P., Devi, R. S., Bhadouria, R. (2020). Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. Agrochemicals Detection, Treatment and Remediation, 25–54. DOI 0.1016/B978-0-08-103017-2.00002-7. [Google Scholar]
112. Muhammad, H., Wei, T., Cao, G., Yu, S., Ren, X. et al. (2021). Study of soil microorganisms modified wheat straw and biochar for reducing cadmium leaching potential and bioavailability. Chemosphere, 273, 129644. DOI 10.1016/j.chemosphere.2021.129644. [Google Scholar] [CrossRef]
113. Kumari, M., Thakur, I. S. (2018). Biochemical and proteomic characterization of Paenibacillus sp. ISTP10 for its role in plant growth promotion and in rhizostabilization of cadmium. Bioresource Technology, 3, 59–66. DOI 10.1016/j.biteb.2018.06.001. [Google Scholar] [CrossRef]
114. Pramanik, K., Mitra, S., Sarkar, A., Maiti, T. K. (2018). Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. Journal of Hazardous Materials, 351, 317–329. DOI 10.1016/j.jhazmat.2018.03.009. [Google Scholar] [CrossRef]
115. Syed-Ab-Rahman, S. F., Carvalhais, L. C., Chua, E. T., Chung, F. Y., Moyle, P. M. et al. (2019). Soil bacterial diffusible and volatile organic compounds inhibit Phytophthora capsici and promote plant growth. Science of the Total Environment, 692, 267–280. DOI 10.1016/j.scitotenv.2019.07.061. [Google Scholar] [CrossRef]
116. Santoro, M. V., Bogino, P. C., Nocelli, N., Cappellari, L. D. R., Giordano, W. F. et al. (2016). Analysis of plant growth-promoting effects of fluorescent Pseudomonas strains isolated from Mentha piperita rhizosphere and effects of their volatile organic compounds on essential oil composition. Frontiers in Microbiology, 7, 1085. DOI 10.3389/fmicb.2016.01085. [Google Scholar] [CrossRef]
117. Gouda, S., Kerry, R. G., Das, G., Paramithiotis, S., Shin, H. S. et al. (2018). Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiological Research, 206, 131–140. DOI 10.1016/j.micres.2017.08.016. [Google Scholar] [CrossRef]
118. Kanchiswamy, C. N., Malnoy, M., Maffei, M. E. (2015). Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Frontiers in Plant Science, 6, 151. DOI 10.3389/fpls.2015.00151. [Google Scholar] [CrossRef]
119. Myo, E. M., Liu, B., Ma, J., Shi, L., Jiang, M. et al. (2019). Evaluation of Bacillus velezensis NKG-2 for bio-control activities against fungal diseases and potential plant growth promotion. Biological Control, 134, 23–31. DOI 10.1016/j.biocontrol.2019.03.017. [Google Scholar] [CrossRef]
120. Ahmad, M., Ahmad, I., Hilger, T. H., Nadeem, S. M., Akhtar, M. F. et al. (2018). Preliminary study on phosphate solubilizing Bacillus subtilis strain Q3 and Paenibacillus sp. strain Q6 for improving cotton growth under alkaline conditions. PeerJ, 6, e5122. DOI 10.7717/peerj.5122. [Google Scholar] [CrossRef]
121. Tyagi, V., Durgapal, A. (2019). Synergetic effect of PGPR on growth, yield and nutrients of black soybean. Annals of Plant and Soil Research, 21, 326–332. [Google Scholar]
122. Igiehon, N. O., Babalola, O. O., Aremu, B. R. (2019). Genomic insights into plant growth promoting rhizobia capable of enhancing soybean germination under drought stress. BMC Microbiology, 19, 159. DOI 10.1186/s12866-019-1536-1. [Google Scholar] [CrossRef]
123. Karimi, N., Zarea, M. J., Mehnaz, S. (2018). Endophytic Azospirillum for enhancement of growth and yield of wheat. Journal of Environmental Sustainability, 1, 149–158. DOI 10.1007/s42398-018-0014-2. [Google Scholar] [CrossRef]
124. Kakar, K. U., Nawaz, Z., Cui, Z., Almoneafy, A. A., Ullah, R. et al. (2018). Rhizosphere-associated Alcaligenes and Bacillus strains that induce resistance against blast and sheath blight diseases, enhance plant growth and improve mineral content in rice. Journal of Applied Microbiology, 124(3), 779–796. DOI 0.1111/jam.13678. [Google Scholar]
125. He, Y., Pantigoso, H. A., Wu, Z., Vivanco, J. M. (2019). Co-inoculation of Bacillus spp. and Pseudomonas putida at different development stages acts as a biostimulant to promote growth, yield and nutrient uptake of tomato. Journal of Applied Microbiology, 127, 196–207. DOI 10.1111/jam.14273. [Google Scholar] [CrossRef]
126. Chanthini, K. M. P., Senthil-Nathan, S., Soranam, R., Thanigaivel, A., Karthi, S. et al. (2018). Bacterial compounds, as biocontrol agent against early blight (Alternaria solani) and tobacco cut worm (Spodoptera litura Fab.) of tomato (Lycopersicon esculentum Mill.). Archives of Phytopathology and Plant Protection, 51(13–14), 729–753. DOI 10.1080/03235408.2018.1496525. [Google Scholar] [CrossRef]
127. Otieno, N., Lally, R. D., Kiwanuka, S., Lloyd, A., Ryan, D. et al. (2015). Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Frontiers in Microbiology, 6, 745. DOI 10.3389/fmicb.2015.00745. [Google Scholar] [CrossRef]
128. Prathap, M., Ranjitha Kumari, B. (2015). A critical review on plant growth promoting rhizobacteria. Journal of Plant Pathology & Microbiology, 6, 1–4. [Google Scholar]
129. Ngumbi, E., Kloepper, J. (2016). Bacterial-mediated drought tolerance: Current and future prospects. Applied Soil Ecology, 105, 109–125. DOI 10.1016/j.apsoil.2016.04.009. [Google Scholar] [CrossRef]
130. Passari, A. K., Upadhyaya, K., Singh, G., Abdel-Azeem, A. M., Thankappan, S. et al. (2019). Enhancement of disease resistance, growth potential, and photosynthesis in tomato (Solanum lycopersicum) by inoculation with an endophytic actinobacterium, Streptomyces thermocarboxydus strain BPSAC147. PLoS One, 14(7), e0219014. DOI 10.1371/journal.pone.0219014. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |