

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020062
ARTICLE
Optimization of Callus Induction Conditions from Immature Embryos of Maize under Stress
1College of Life Sciences, Jilin Agricultural University, Changchun, 130118, China
2Jilin Academy of Agricultural Sciences, Changchun, 130118, China
3Joint Laboratory of International Cooperation in Modem Agricultural Technology of Ministry of Education, Jilin Agricultural University, Changchun, 130118, China
*Corresponding Authors: Shuyan Guan. Email: guanshuyan@jlau.edu.cn; Yiyong Ma. Email: m18404319202_1@126.com
#These authors contributed equally to this work
Received: 02 November 2021; Accepted: 05 January 2022
Abstract: The embryos of maize (Zea mays L.) inbred lines GS02, GS07, GS08, GS11 and GS15 were used as receptor materials to optimize the receptor system from the aspects of genotype, medium components and stress (PEG6000, mannitol, salt and low phosphorus). The results showed that GS07 had the highest induction rate (95.2%). Orthogonal test analysis showed that the best combination of medium components in induction was A2B3C1D3(2), namely, the concentration of 2, 4-dichlorophenoxy acetic acid (2,4-D) was 4 mg·mL-1, the concentration of L-Proline (L-Pro) was 0.8 mg·mL-1, and the concentration of silver nitrate (AgNO3) was 10 mg·mL-1 (or 5 mg·mL). Interestingly, we found that the optimal medium supplemented with 30 g·L-1 PEG6000 or 80 g·L-1 mannitol was suitable for antioxidant enzyme activity and malondialdehyde (MDA) content in GS07 callus. Exogenous 10 mmol·L-1 Ca2+ in the medium components with 100 mmol·L-1 sodium chloride (NaCl) could improve the activity of antioxidant enzymes in GS07 callus. Callus of GS07 could divide normally and grow well in medium components with 27 mg·L-1 KH2PO4. This study enhanced the adaptability of maize callus to stress and optimized the culture conditions.
Keywords: Maize; immature embryo; adversity stress; medium optimization
Maize is a kind of widely used crop, which can be used as food, feed and industrial raw material, and is of great significance to human survival and development. Maize is a relatively rich genetic background crop. Many excellent agronomic traits are distributed among different strains [1]. Traditional breeding usually costs the breeders decades, and transgenic breeding offers the possibility to shorten the breeding years of new varieties of maize, but transgenic breeding depends on genetic transformation technology and excellent receptor system of maize [2]. Maize immature embryos and embryogenic callus are important receptor materials for the maize genetic transformation system [3]. Therefore, the screening of recipient materials is a key step in transgenic breeding.
After the emergence of plant tissue culture technology, a plant tissue culture upsurge, and the study of maize tissue culture became popular. In 1954, the study of maize callus began, and the endosperm of triploid was used for the callus culture of maize for the first time [4]. So far, the embryonic callus has been successfully induced from maize tissues and organs such as immature embryos [5], young ears [6], root tips [7], leaves [8], mature embryos [9], anthers [10], suspended cells and protoplasts [11,12]. These studies showed that embryogenic callus could be induced from most explants of maize; however, different explants have different abilities to induce embryogenic callus. In these explants, immature embryos have a good ability of induction, subgeneration and regeneration [13,14]. Therefore, maize immature embryos were used as the research object in this study. With the wide application of plant tissue culture technology in maize genetic transformation, screening and establishing of a maize somatic cell regeneration system with high induction rate and high efficiency can lay a foundation for genetic transformation. However, how to induce callus with good quality and plant regeneration ability is an important factor to improve maize genetic transformation. Callus can be divided into three types: Type I callus is milky white with compact a structure; Type II callus is light yellow, loosely structured, fragile, with an obvious granular shape, and can be continued for generations; Type III callus is white and transparent, easy to brown [15].
In the study of the relationship between water stress and active oxygen metabolism, Bewley et al. [16] first proposed the free radical hypothesis on plant drought damage: On the one hand, a large number of free radicals are produced in plant cells, and the balance of free radical metabolism is destroyed; One of the toxic effects of excess free radicals is to induce or aggravate membrane lipid peroxidation, which damages the cell membrane system and leads to plant cell death in serious cases. Drought-tolerant plants, on the other hand, can be divided into enzyme-induced and non-enzyme-induced defense systems to protect cells from oxidative damage. Free radicals are normal metabolites of the body. Although they are continuously produced in the process of cell metabolism, there is a defense system in the body; thus, under normal circumstances, its metabolism maintains a balanced state. Enzymatic systems include superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), etc. [17]. Recent studies indicate that the activities of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) are closely related to drought tolerance of plants, and can be used as physiological indicators of drought resistance of plants [18]. SOD is the main protective enzyme of the membrane lipid peroxidation defense system. It catalyzes disproportionation of reactive oxygen species and produces non-toxic molecular oxygen and water, so as to avoid plant injury. Higher SOD activity is the physiological basis of plant resistance to stress [19]. POD and CAT can remove H2O2 accumulated in the plant physiological system under drought stress. Malondialdehyde (MDA) is one of the main products of membrane lipid peroxidation, which is cytotoxic and can cause cell membrane dysfunction. Moreover, it can destroy many functional molecules, so the increase of MDA content is the direct cause of plant cell damage [20]. Salt damage can affect a range of physiological activities of plants, leading to plant death in severe cases [21]. Calcium is an essential nutrient element in plants. It plays an important role in maintaining the stability of cell membrane and cell wall, regulating inorganic ion transport, and various enzyme activities, and so on. It is also the second messenger coupling extracellular signals and intracellular physiological reactions [22]. Ca2+ plays a decisive role in maintaining the cell membrane by tightly binding phosphate and phosphate esters on the surface of the cell membrane, as well as hydroxyl groups in the embedded proteins, thereby increasing the integrity and stability of the cell membrane [23]. Although there are many studies on the physiological response of maize to stress at home and abroad, most of them focus on seeds or seedlings as experimental materials, and there are few reports on the adaptability of maize callus to stress.
In this study, immature embryos and callus of maize were used as experimental materials to study the effects of stress on the activity of antioxidant enzymes and the degree of membrane lipid peroxidation in maize callus, so as to optimize the culture conditions of maize callus and provide theoretical guidance and technical support for maize genetic transformation receptor materials.
The tested materials were maize inbred lines GS02, GS07, GS08, GS11, GS15. The seeds of each inbred line were sown in the experimental field of the Key Laboratory of Crop Molecular Breeding of Jilin Agricultural University. After 10–13 days of self-pollination, immature embryos with a length of 1.5–2 mm were selected for tissue culture experiment.
2.2.1 Exfoliation of Immature Embryo of Maize
After artificial pollination, the pollinated female corn ears aged 10–13 days were selected to strip the immature corn embryos, sterilized with 70% alcohol for 5–15 min on a super clean working table (clean bench, HS-1300-U, Sujing Group Suzhou Antai Air Technology Co., Ltd., China), soaked in 8% NaClO for 10–15 min, washed with sterile water for 3–5 times, and the endosperm of 1/3 of the upper corn kernel was cut with a blade. Under aseptic conditions, 1–2 mm of immature embryos shield slices were selected and inoculated upward, respectively, on a N6 induction medium to induce callus. In the dark culture at 26°C, the germ and rhizoid were cut off in time 1–2 weeks later, the initial callus of Type I was generated, which were non-embryonic calluses with compact structure, hard, wrinkled surface, milky white or white transparent, and difficult to subculture. Then, subculture was performed every two weeks to induce Type II callus with a granular and crisp material, milky white or light yellow and rapid growth.
2.2.2 Effects of Immature Embryos of Different Genotypes on Embryogenic Callus Induction Rate
In this experiment, immature embryos of 5 different genotypes GS02, GS07, GS08, GS11 and GS15 were cultured on a N6 induction medium for about 2 weeks for observation, and most of them were Type I calluses. The induction rate of each genotype and embryogenic callus growth were calculated when most calluses were transformed into stable Type II calluses after 2–3 subgenerations [24]. Inbred lines with the strongest induction ability were selected as the main research object.
2.2.3 Optimization of Induction Medium
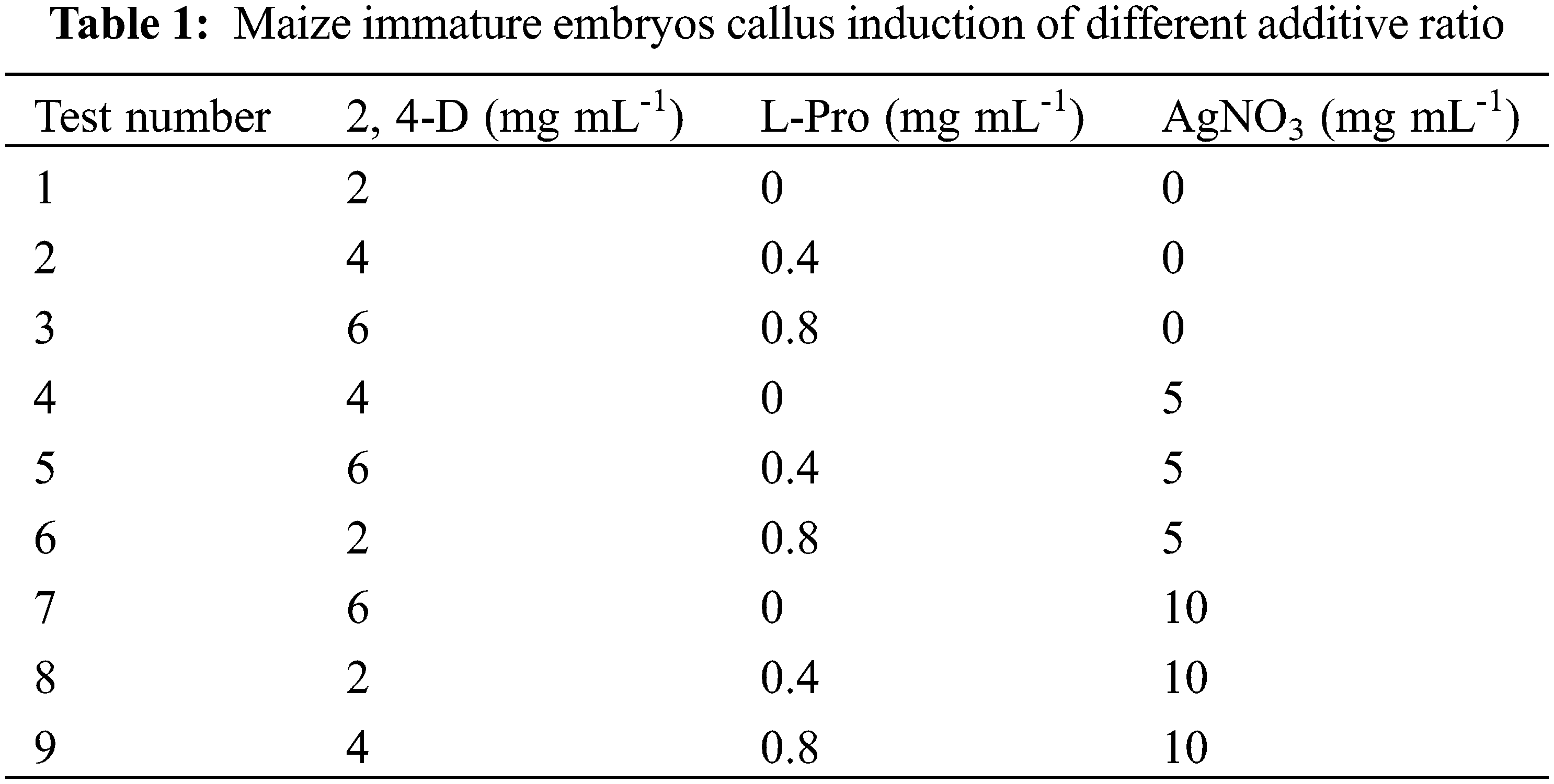
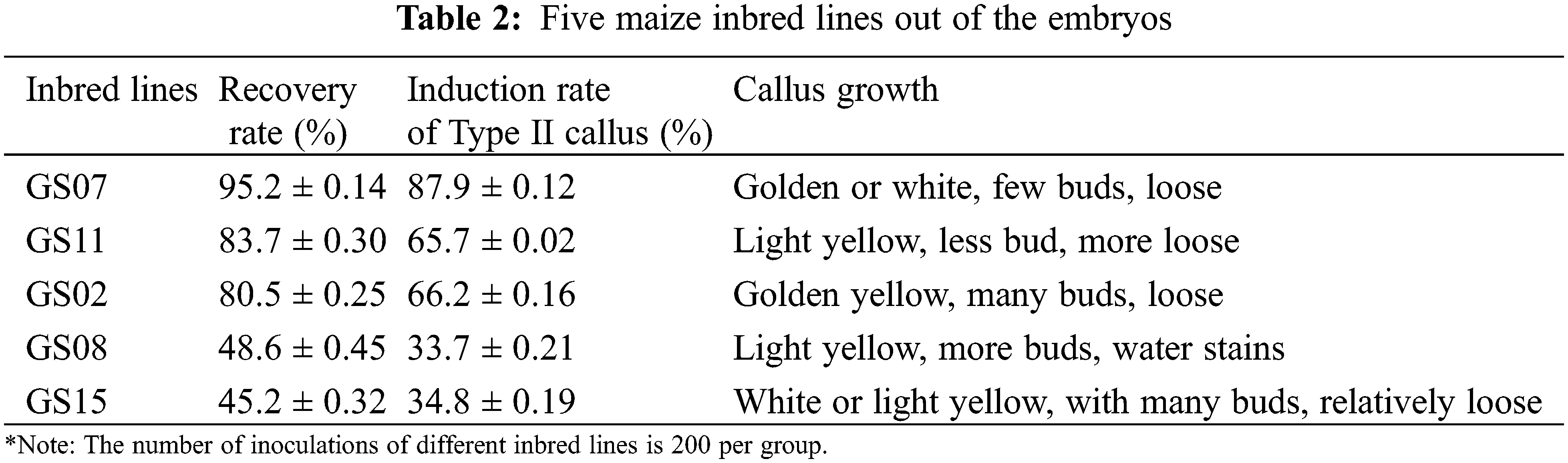
Immature embryos of 1.5–2 mm inbred line GS07 were inoculated into a N6 induction medium (Table 1) and dark culture was performed at 26°C. Ten tissue culture vials were inoculated, and 5 vials were repeated in each group. By orthogonal experiment, nine groups of data were processed by orthogonal table L9(34) with three factors and three levels. The results were observed after 20 days of induction, and the browning rate and average value of calluses were calculated [24].
2.2.4 Stress Treatment of Embryogenic Callus of Maize
GS07 embryogenic callus was subcultured on a modified N6 medium for 20 days and grew well. Calluses with consistent growth were selected. 200 calluses (20 pieces per petri dish, 10 petri dishes in total) were treated in each group, and three replicates were set up. A modified N6 liquid medium with a concentration gradient of PEG-6000 (0, 10, 20, 30 g·L-1) or mannitol (0, 20, 40, 60, 80, 100 g·L-1) was used to shake the medium for 6 h,12 h, 24 h, 36 h (25°C, 150 r·min-1). NaCl (0, 25, 50, 100, 150 mmol·L-1) or low phosphorus (0, 9, 18, 27, 36, 50 mg·L-1) were added to the modified N6 liquid medium, shaking in a shake-table for 24 h (25°C, 150 r·min-1), and then placed on filter paper to dry. Liquid nitrogen was stored in an ultra-low temperature refrigerator [Haier medical cryogenic storage box, DW-88L388(J), Qingdao Haier Special Electric Appliance Co., Ltd., China] at –80°C for testing.
2.2.5 Determination of Antioxidant Physiological Indexes of Embryogenic Callus of Maize under Stress
The activity of SOD was determined by nitroblue tetrazolium (NBT) [25]; POD activity was determined by guaiacol colorimetry [25]. Catalase (CAT) activity was determined by ultraviolet spectrophotometry [26]. The con of malondialdehyde (MDA) was determined by the thiobarbituric acid method [27]. Each index was repeated 3 times and the average value was taken.
Each experiment was repeated three times, and significance analysis was completed by multivariate analysis of variance using SPSS 24 software. P < 0.05 was a significant difference, P < 0.01 was an extremely significant difference. GraphPad Prism 8.0.2 was used for mapping.
3.1 Effects of Immature Embryos of Different Genotypes on Embryogenic Callus Induction Rate
The immature embryos of GS02, GS07, GS08, GS11 and GS15 were inoculated on a N6 medium for induction. The embryogenic callus of Type II was yellow or milky white, and showed a dry and rice grain structure, but the number of callus was different. Fig. 1 shows the callus growth of five inbred lines after one subgeneration. According to the results of repeated experiments, the induction rate of the 5 genotypes in Table 1 was GS07 > GS02 > GS11 > GS08 > GS15. GS07 had the highest callus extraction rate, up to 95.2%. GS11 and GS02 showed a similar rate of 83.7% and 80.5%, respectively. GS08 and GS15 showed a rate of less than 50% (Table 2).
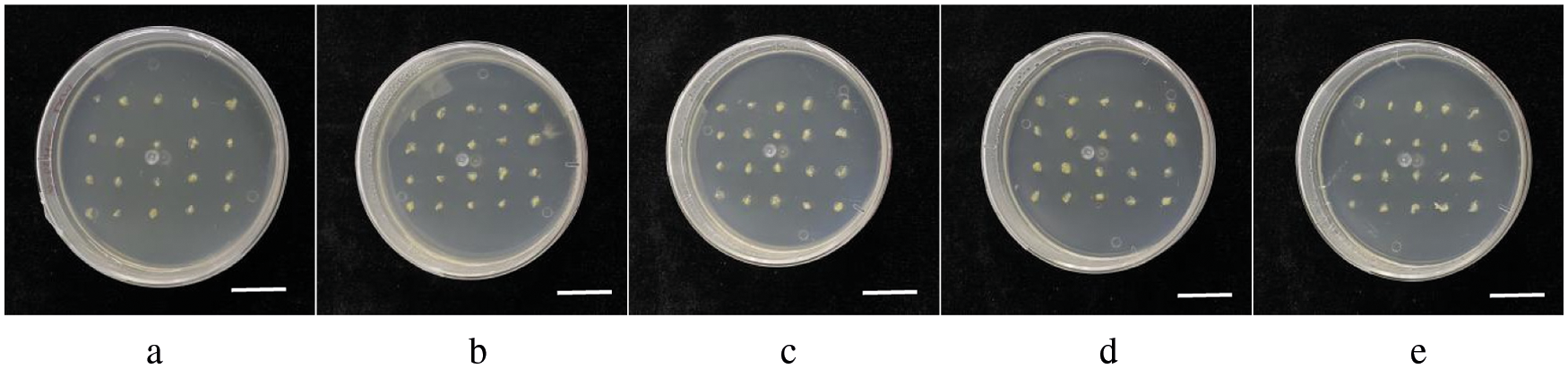
Figure 1: Growth status of immature embryos from five maize inbred lines after one successive generation. a: inbred line GS02; b: inbred line GS07; c: inbred line GS08; d: inbred line GS11; e: inbred line GS15. Scale bar = 2 cm
3.2 Optimization of Induction Medium
The purpose of this study was to further study the effect of the relative strength and the relationship between the factors on callus induction rate of maize. A L9(34) orthogonal experiment was used to design the different induction combinations. The results showed that the callus induction rate varied with the three different factors. The range R directly reflected the influence of various factors on callus induction rate of maize. The larger the R value, the greater its degree of influence [24]. According to the R value, the influence of various factors on callus induction rate of maize in this study was determined in descending order: 2, 4-D concentration (A) > L-Pro concentration (B) > AgNO3 concentration(D). The optimal level combination was determined as A2B3C1D3(2) according to the average callus induction rate of all factors and levels. That is, 2, 4-D concentration was 4 mg·mL-1, L-Pro concentration was 0.8 mg·mL-1, AgNO3 concentration was 10 mg/mL (or 5 mg/mL) (Table 3).
3.3 Optimization of Embryogenic Calluses of Maize under Stress
3.3.1 Effects of PEG6000 Stress on the Antioxidant Enzyme Activity and MDA Con in Maize Calluses
PEG6000 is a macromolecular penetrant that can quickly deprive water and cause osmotic stress to plants [28]. In each group of stress time, SOD, POD, CAT activities and MDA content under different concentration of PEG6000 stress showed significant difference. Under PEG6000 stress with the same concentration, SOD, POD, CAT activities and MDA content have significant differences in a certain range with the increase of stress time, but the differences are relatively small. When the concentration of PEG6000 was low, the SOD activity increased continuously with the stress time, but under the treatment of 30 g·L-1 PEG6000, the activity showed a downward trend, and the change was significant (Fig. 2A). It indicates that the PEG stress process is fast. POD activity decreased with the increase of stress concentration and the extension of stress time. 30 g·L-1 PEG6000 for 6 h was significantly higher than that of the control (P < 0.05) (Fig. 2B). With the increase of stress time, CAT activity varied greatly: After low concentration stress (10 g·L-1), it showed a trend of rising first and then decreasing; After 30 g·L-1 stress treatment, the enzyme activity decreased below the control level at 36 h (Fig. 2C). MDA is one of the main products of lipid peroxidation in plant cells, and its content can indicate the degree of membrane lipid peroxidation. MDA content increased with the increase of stress concentration and stress time, compared with the control, MDA content increased significantly during short stress time, when the stress time was longer, the increase rate decreased, but the overall level still increased. The peak value of MDA content appeared at 30 g·L-1 PEG6000 concentration and 36 h stress, and the difference was significant compared with the control (P < 0.05) (Fig. 2D). The results indicated that under osmotic stress, the callus of maize was affected by membrane lipid peroxidation, and the slower growth rate may be related to the enhanced activity of antioxidant enzymes. The above experiments showed that the PEG6000 concentration of 30 g·L-1 was the critical value of callus enzyme activity and membrane lipid peroxidation. Therefore, the addition of 30 g·L-1 PEG6000 in the culture medium was beneficial to the growth of the embryogenic callus of maize.
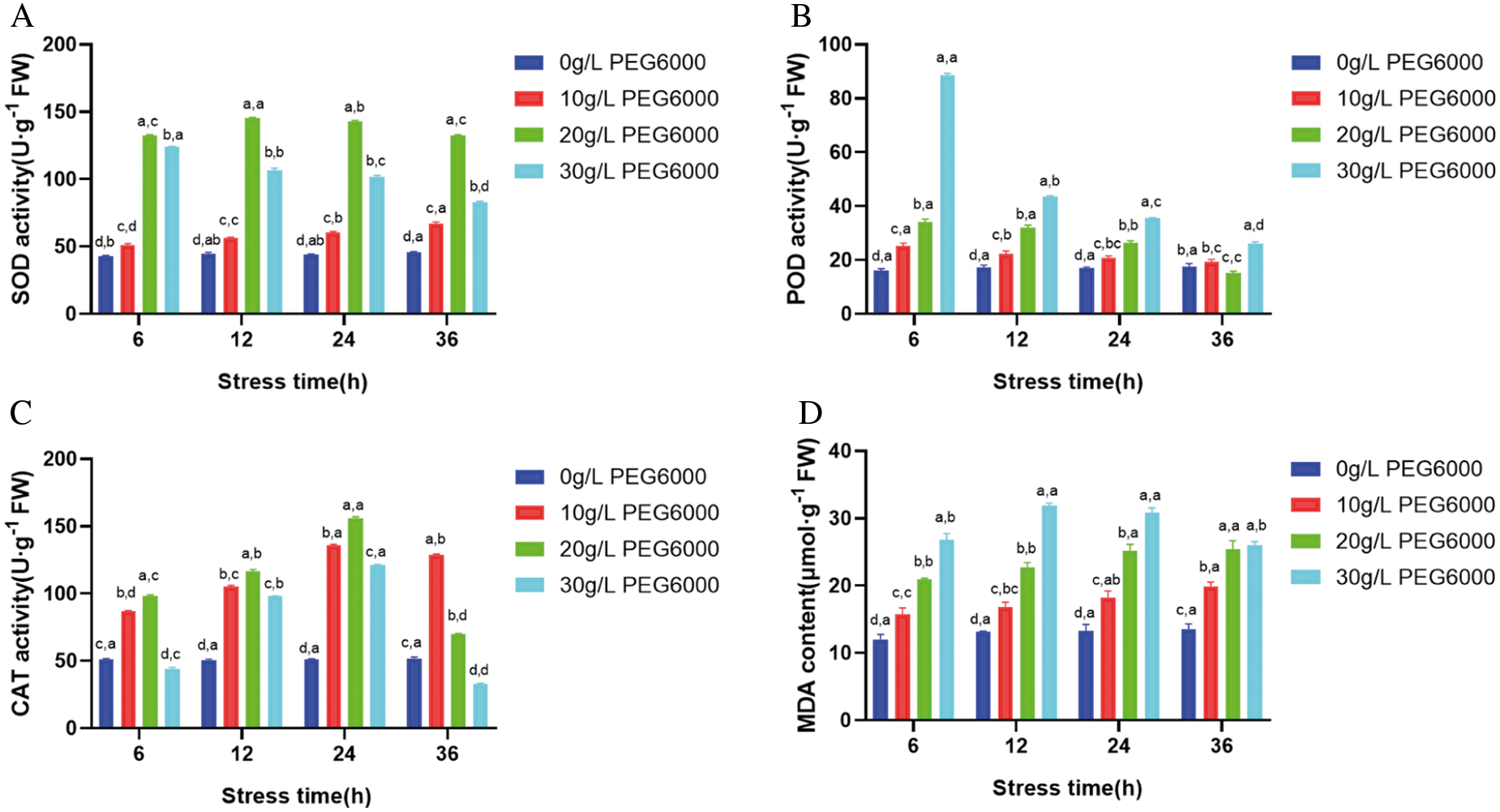
Figure 2: Effects of PEG6000 stress on antioxidant enzyme activity and MDA concentration in GS07 callus. A: Changes of SOD enzyme activity; B: Changes of POD enzyme activity; C: Changes of CAT enzyme activity; D: Change of MDA concentration. Data are the mean of n = 3. Vertical bars represent 1 SD. Within each bar, different letters before the comma indicate significant differences (P < 0.05) stress treatments. Different letters after the comma indicate significant differences (P < 0.05) time after stress exposure
3.3.2 Effects of Mannitol Stress on Antioxidant Enzyme Activity and MDA Content in Maize Callus
The mechanism of mannitol is to decrease cell turgor pressure, increase the osmotic potential negative value of the medium and cause water stress. In each group of stress time, SOD, POD, CAT activities and MDA content under different concentration of mannitol stress showed significant difference. However, under the stress of mannitol with the same concentration, SOD, POD, CAT activities and MDA content were significantly different in a certain range with the increase of stress time. With the increasing concentration of mannitol (20–80 g·L-1), SOD activity in maize embryogenic callus increased and remained at a high level. It indicated that the SOD activity in cells was induced to increase under stress treatment, and it began to decline slowly until the concentration was up to 100 g·L-1, which might be caused by the fact that the high concentration treatment exceeded the physiological tolerance and response limit of cells and destroyed the normal physiological activities of cells (Fig. 3A). POD activity increased slowly under low concentration mannitol stress, and increased first and then decreased under moderate concentration mannitol stress. POD activity peaked at 100 g·L-1 mannitol treatment for 6 h, and then decreased sharply (Fig. 3B). The variation trend of CAT activity was complicated, except that the activity increased continuously under 20 g·L-1 mannitol stress and the concentration decreased continuously under 40 g·L-1 mannitol stress, the other concentrations increased first and then decreased, and the concentration of 100 g·L-1 mannitol for 6h was even lower than that of the control (Fig. 3C). MDA content increased with the increase of stress concentration and stress time, and reached the peak at 36 h (Fig. 3D). In conclusion, 80 g·L-1 mannitol concentration is the most suitable for the enzymatic activity and lipid peroxidation of maize GS07 callus, which is beneficial to the growth of maize embryogenic callus.
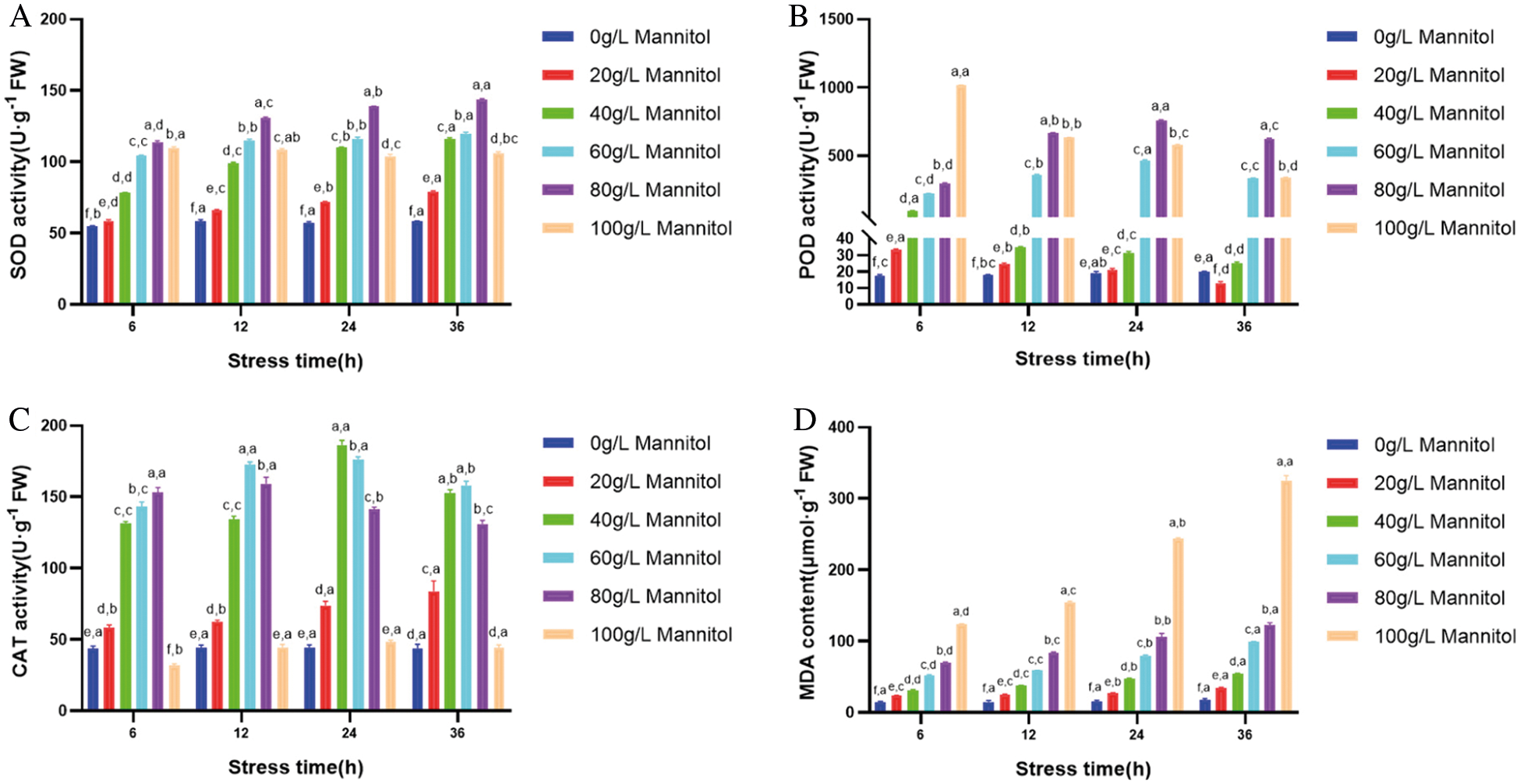
Figure 3: Effects of mannitol stress on antioxidant enzyme activity and MDA content in callus of GS07. A: Changes of SOD enzyme activity; B: Changes of POD enzyme activity; C: Changes of CAT enzyme activity; D: Change of MDA content. Data are the mean of n = 3. Vertical bars represent 1 SD. Within each bar, different letters before the comma indicate significant differences (P < 0.05) stress treatments. Different letters after the comma indicate significant differences (P < 0.05) time after stress exposure
3.3.3 Alleviating Effect of Exogenous Ca2+ on Maize Callus under Salt Stress
Under salt stress, when NaCl concentration ≥100 mmol·L-1, SOD activity was significantly increased (P < 0.05), indicating that the callus alleviated the damage caused by salt stress by increasing SOD activity. When NaCl concentration was 25 mmol·L-1, SOD activity increased with the increase of Ca2+ concentration, and the highest activity increased by 25.58% compared with that without Ca2+. When NaCl concentration was 50 and 100 mmol·L-1, SOD activity was the highest when Ca2+ concentration was 10 mmol·L-1, and SOD activity increased by 27.49% and 32.28% with the increase of Ca2+ concentration, respectively, compared with that without Ca2+, and then showed a downward trend. When the NaCl concentration is 150 mmol·L-1 and the Ca2+ concentration is 5 mmol·L-1, the SOD activity is the highest, which is 26.86% higher than when Ca2+ is not applied (Fig. 4A).
Under salt stress, POD activity in callus of maize increased with increasing salt stress, and the difference was significant (P < 0.05). When exogenous Ca2+ was applied, POD activity increased with the increase of Ca2+ concentration when NaCl concentration was 25 mmol·L-1, and when Ca2+ concentration was ≥10 mmol·L-1, POD activity increased significantly compared with that of the non-Ca2+ group (P < 0.05). When NaCl concentration ≥100 mmol·L-1, POD activity first increased and then decreased with the increase of Ca2+ concentration. When NaCl concentration was 100 mmol·L-1, POD activity reached the maximum value when exogenous Ca2+ concentration was 10 mmol·L-1, which increased by 49.4% compared with that without Ca2+. When NaCl concentration reached 150 mmol·L-1, it reached the maximum value at Ca2+ concentration of 5 mmol·L-1, increasing by 26.69% (Fig. 4B). The higher POD activity of callus under salt stress, the stronger physiological metabolism, antioxidant activity and better adaptability to stress conditions.
Under salt stress, CAT activity increased first and then decreased with the increase of NaCl concentration, and reached the highest level under NaCl concentration of 100 mmol·L-1. With the addition of exogenous Ca2+, CAT activity increased at first and then decreased at different NaCl concentrations. When NaCl concentration ≤100 mmol·L-1, CAT activity was the highest at Ca2+ concentration of 10 mmol·L-1, which was 9.88% and 14.63% higher than that without exogenous Ca2+, respectively. When salt concentration reached 150 mmol·L-1, CAT activity was the highest at Ca2+ concentration of 5 mmol·L-1, which increased by 9.84% and then decreased (Fig. 4C). Therefore, CAT activity can be activated under low salt stress, while CAT activity can be inhibited under high salt stress. This may be due to the accumulation of H2O2 in plants under low salt concentration stress, and the increase of CAT activity is a kind of self-defense response of the body, which can improve the ability of plants to remove H2O2, so as to reduce the damage of excessive H2O2 to cells. When the salt stress concentration continued to increase, exceeding the cell defense ability, CAT activity also decreased accordingly.
After salt stress, the MDA mass molality of maize callus increased with the increase of salt concentration. When NaCl concentration was 150 mmol·L-1, MDA content was significantly higher than that of other treatments (P < 0.05). Under the same NaCl concentration under salt stress, MDA content showed a continuous downward trend with the increase of exogenous Ca2+ concentration. Compared with no exogenous Ca2+, MDA content decreased to the lowest at Ca2+ concentration of 15 mmol·L-1 under 50 mmol·L-1 NaCl stress, and decreased by 48.40%. Under NaCl stress of other concentrations, MDA content reached the lowest at Ca2+ concentration of 10 mmol·L-1, which decreased by 42.89%, 20.28% and 12.32% (Fig. 4D), respectively. These results indicated that the callus adapted to the salt stress environment by increasing the reactive oxygen species in the body, and there was a certain degree of membrane lipid peroxidation, and the injury degree of cells increased with the increase of salt concentration.
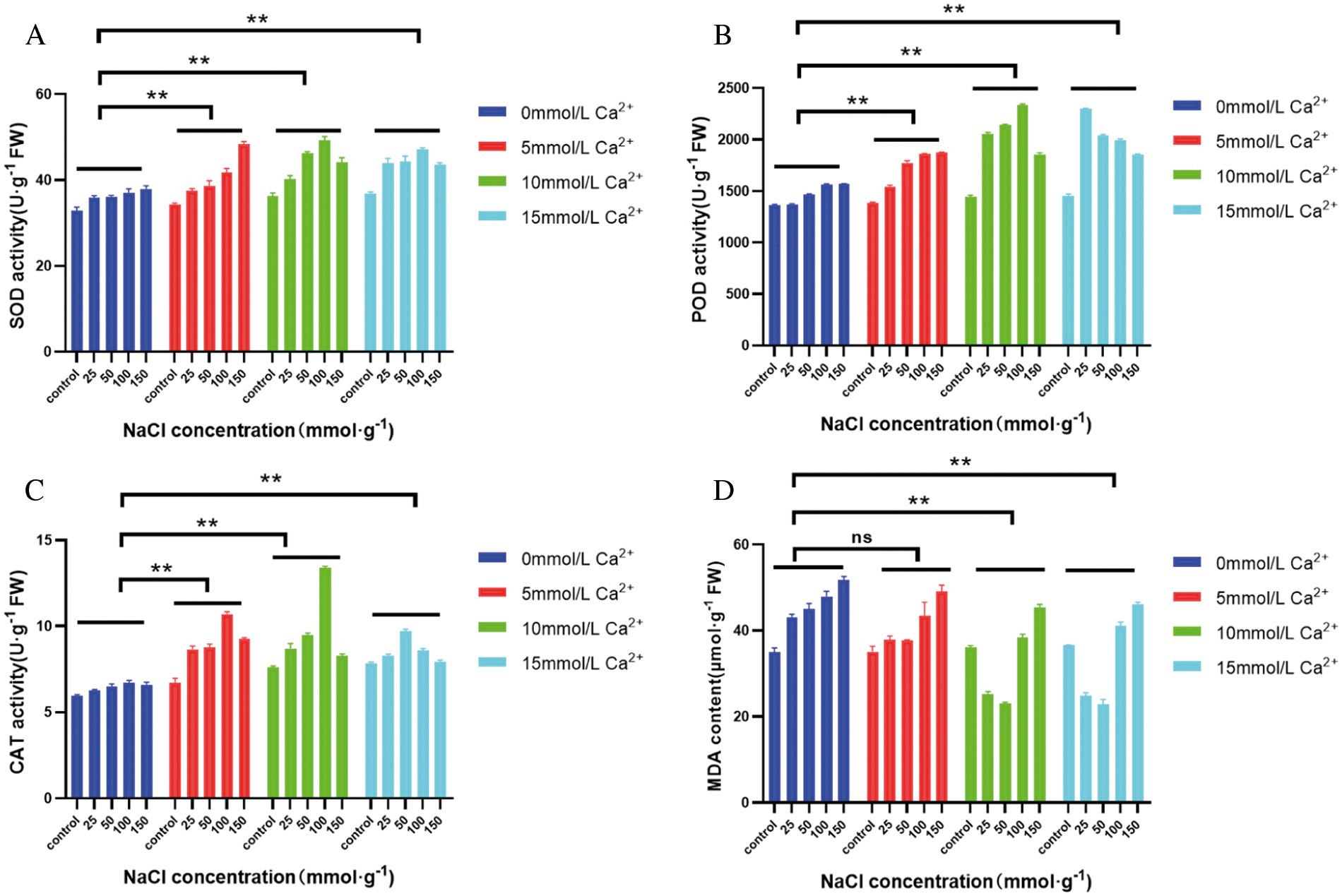
Figure 4: Effect of exogenous Ca2+ on enzyme activity and membrane lipid oxidation degree of GS07 maize callus under salt stress. A: Changes of POD enzyme activity; B: Changes of SOD enzyme activity; C: Change of MDA content; D: Changes of CAT enzyme activity. Data are expressed as the mean of triplicate values and error represent the SD. Non-significant (ns), P < 0.05 (**)
3.3.4 Effects of Low Phosphorus Stress on Callus Growth Status of Maize
Phosphorus is an important component of cell structure, cell division and genetic material. In tissue culture, phosphorus is supplied with the mass element KH2PO4. In modified N6 medium without phosphorus supply, callus was found to prolifize and changes of CAT enzyme activity browning appeared after one week, and most of them were Type I callus. However, the time of half and full death of callus was 16 d and 25 d, respectively. Under low-phosphorus nutrition, the callus divided slowly, and the browning phenomenon was obvious, and the tissue was soft and watery, with the formation of bud. Concentrations above 27 mg·L-1 KH2PO4 have no significant effect on the growth of callus, and callus division of 50 mg·L-1 KH2PO4 is still slow (Fig. 5). Therefore, after a large number of repeated experiments, we found that GS07 callus grew well under 27 mg·L-1 KH2PO4.
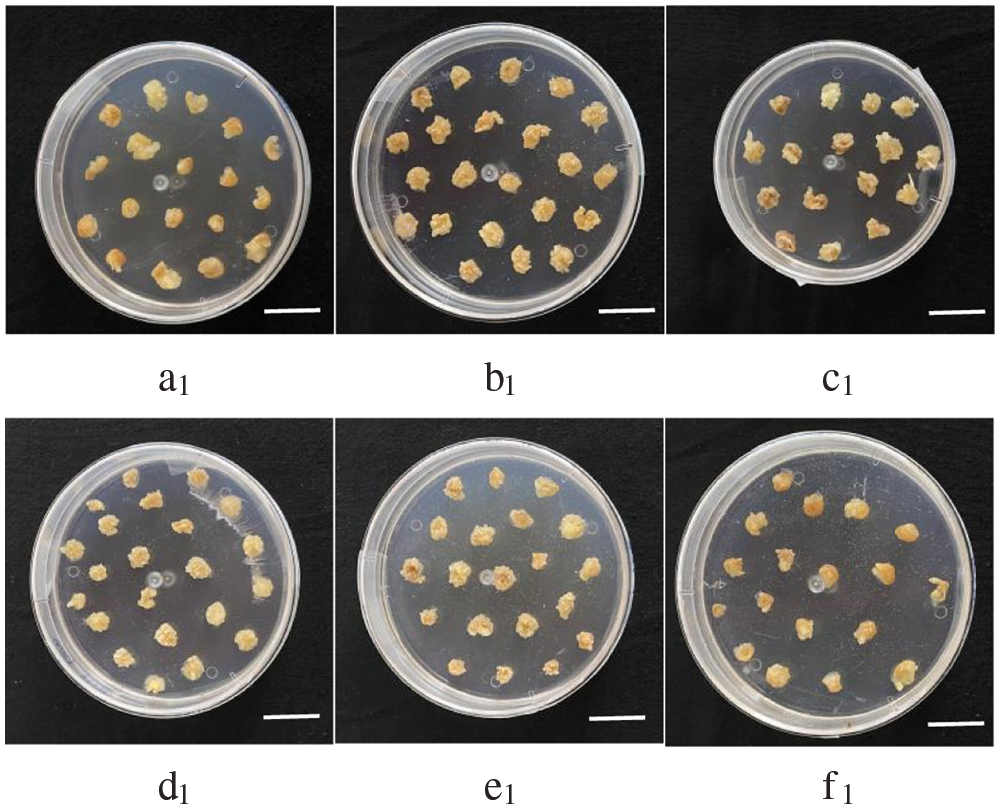
Figure 5: Effects of low phosphorus stress on callus growth status of maize GS07. a1–c1: At 0,9,18 mg·L-1 KH2PO4, the callus hardly divided, and most of them were Type I callus, divided slowly, browning was obvious, and there were buds; d1: Under 27 mg·L-1 KH2PO4, the callus could divide normally with golden color, and most of them were Type II callus; e1–f1: At 36, 50 mg·L-1 KH2PO4, the callus division was slow and browning was observed. Scale bar = 2 cm
Maize callus is induced by many factors, including genotype, hormone content, temperature, humidity, etc. Genotype is an important factor in callus induction of maize. Suitable maize explants of genotype can produce callus more easily under suitable conditions. In this study, callus induction from Immature embryo of maize inbred line GS07 was the most successful. The best combination of additives is A2 (2, 4-D concentration is 4 mg·mL-1), B3 (L-Pro concentration is 0.8 mg·mL-1) C1 and D3(2) (AgNO3 concentration is 10 mg·mL-1 or 5 mg/mL). This combination of additives laid a foundation for the induction of high-quality embryogenic callus. In order to optimize the optimum conditions for callus growth of maize, we subjected them to osmotic stress, salt stress and low phosphorus stress, and measured the activity of antioxidant enzymes and the degree of membrane lipid peroxidation. All aerobic organisms depend on oxygen for energy and life, and the growth of callus must involve reactive oxygen species. SOD, POD and CAT are important antioxidant enzymes in plants, which can remove reactive oxygen species caused by external damage in plants [29]. Under adversity conditions, when the amount of active oxygen exceeds the damage threshold, it directly triggers or aggravates the peroxidation of membrane lipids, which leads to an increase in the content of the final product MDA and a decrease in membrane fluidity, which makes membrane material transport, information transmission, and metabolic regulation and selection The normal function of permeability is impaired, and it can cause cell death in severe cases [30,31].
Adversity stress often destroys the physiological and biochemical states of plants, but appropriate stress is beneficial to the growth and development of plants. Cai et al. [32] identified the salt tolerance of maize varieties through changes in antioxidant enzyme activity and changes in related gene expression. PEG dissolved in water immediately produces high osmotic pressure, which causes the accumulation of intracellular reactive oxygen species and stimulates the protective ability of the SOD enzyme system in plant cells. Therefore, PEG stress results in a rapid increase of SOD activity in plant cells, and with the sharp increase of free radicals, the antioxidant enzyme system is damaged, resulting in a significant decrease in SOD activity. This study found that under the stress of different concentrations of PEG6000, the activity of antioxidant enzymes (POD, CAT, SOD) in maize callus changed to different degrees, all of which showed an upward trend, and the MDA content continued to increase until the concentration reached 30 g·L-1. At that time, various physiological and biochemical indicators began to turn. Under different concentrations of mannitol stress, we found that the antioxidant enzyme activity and MDA content are the best values when the mannitol concentration is 80 g·L-1, which is more conducive to the growth of callus; under salt stress, each physiological indexes reached the optimal value at 100 mmol·L-1 NaCl, but the MDA content was high. We reduced the level of membrane lipid peroxidation by adding 10 mmol·L-1 Ca2+. Therefore, the appropriate concentration of exogenous Ca2+ can effectively alleviate the damage of maize callus caused by salt stress, but the excessive application of Ca2+ has no obvious relieving effect, and even shows an inhibitory effect. However, the alleviating effect of exogenous calcium is also limited, that is, the appropriate concentration of Ca2+ can promote plant growth and alleviate the salt damage effect, but the too high concentration of exogenous Ca2+ can reduce the alleviating ability, and even inhibit plant growth. The effects of high concentrations of Ca2+ on plants need to be further studied. Under low phosphorus stress, we found that the callus of GS07 grew well at 27 mg·L-1 KH2PO4, callus can also grow in a non-phosphorus medium, but most of them are Type I callus, the browning phenomenon is significant, and the splitting phenomenon is not significant. As a major nutrient element, phosphorus is not only a component of nucleic acids, lipids and proteins in plants, but also participates in many basic life activities of plants, such as energy transfer and metabolic regulation. Although soil total phosphorus content is high, PO42- in the soil is easily absorbed and fixed by various cations and minerals or converted into organophosphorus, which cannot be absorbed. Therefore, it is of great significance to use plant genetic engineering to breed new varieties with efficient absorption of phosphorus. The purpose of this experiment was to optimize the growth conditions of maize callus, genotypes were screened, induction medium was optimized and physiological and biochemical indexes of callus under different stress were analyzed. Combined with the growth status of callus, the callus culture conditions of maize could be optimized, which laid a good foundation for perfecting the maize genetic transformation system.
During embryogenic callus induction, induction rate and regeneration ability were controlled by genetic factors of embryogenic callus. Different genotypes showed different results. The induction rate of immature embryos of maize inbred line GS07 is higher, up to 95.2%. According to the callus induction rate of each factor and each level, the optimal level of induction medium for each factor was A2 (2, 4-D concentration is 4 mg·mL-1), B3 (L-Pro concentration is 0.8 mg·mL-1) C1 and D3(2) (AgNO3 concentration is 10 mg·mL-1 or 5 mg/mL). In order to optimize the embryogenic callus of maize under stress, we found that when 30 g·L-1 PEG6000 or 80 g·L-1 mannitol was added to the culture medium, the enzymatic activity and membrane lipid peroxidation of corn GS07 callus were the most suitable, which was helpful to the growth of corn embryogenic callus. Adding 10 mmol·L-1 Ca2+ to 100 mmol·L-1 NaCl increased the activity of antioxidant enzymes in maize GS07 callus, and it reduces the degree of membrane lipid oxidation. The corn GS07 at 27 mg·L-1 KH2PO4 is mostly Type II callus, which can divide normally, the browning phenomenon is not significant, and the growth state is well.
Funding Statement: This work was supported by the Science and Technology Project of Jilin Provincial Department of Education [JJKH20210351KJ, JJKH20210346KJ]; Jilin Province Science and Technology Development Plan Project [20200402023NC]. Furthermore, the funding body has no role in designing of study, collection and interpretation of data or writing up the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Sahoo, S., Adhikari, S., Joshi, A., Singh, N. K. (2021). Use of wild progenitor teosinte in maize (Zea mays subsp. mays) improvement: Present status and future prospects. Tropical Plant Biology, 14(2), 156–179. DOI 10.1007/s12042-021-09288-1. [Google Scholar] [CrossRef]
2. Du, D. X., Jin, R. C., Guo, J. J., Zhang, F. D. (2019). Infection of embryonic callus with agrobacterium enables high-speed transformation of maize. International Journal of Molecular Sciences, 20(2), 279. DOI 10.3390/ijms20020279. [Google Scholar] [CrossRef]
3. Zhang, S., Yan, S., An, P., Cao, Q., Wang, C. et al. (2021). Embryogenic callus induction from immature zygotic embryos and genetic transformation of Larix kaempferi 3x Larix gmelinii 9. PLoS One, 16(10), e0258654. DOI 10.1371/journal.pone.0258654. [Google Scholar] [CrossRef]
4. Straus, J., Larue, C. D. (1954). Maize endosperm tissue grown in vitro. I. culture requirements. American Journal of Botany, 41(8), 687–693. DOI 10.2307/2438296. [Google Scholar] [CrossRef]
5. Ge, F., Hu, H., Huang, X., Zhang, Y., Wang, Y. et al. (2017). Metabolomic and proteomic analysis of maize embryonic callus induced from immature embryo. Scientific Reports, 7(1), 1004. DOI 10.1038/s41598-017-01280-8. [Google Scholar] [CrossRef]
6. Long, Y., Yang, Y., Ge, F., Pan, G., Shen, Y. (2020). Establishment of a maize callus regeneration system from haploid shoot tips. Plant Cell, Tissue and Organ Culture, 141(3), 583–592. DOI 10.1007/s11240-020-01817-2. [Google Scholar] [CrossRef]
7. Wang, C. C., Ma, H. Z., Zhu, W. W., Zhang, J. D., Zhao, X. Y. et al. (2021). Seedling-derived leaf and root tip as alternative explants for callus induction and plant regeneration in maize. Physiologia Plantarum, 172(3), 1570–1581. DOI 10.1111/ppl.13347. [Google Scholar] [CrossRef]
8. Ahmadabadi, M., Ruf, S., Bock, R. (2007). A leaf-based regeneration and transformation system for maize (Zea mays L.). Transgenic Research, 16(4), 437–448. DOI 10.1007/s11248-006-9046-y. [Google Scholar] [CrossRef]
9. Huang, X. Q., Wei, Z. M. (2004). High-frequency plant regeneration through callus initiation from mature embryos of maize (Zea mays L.). Plant Cell Reports, 22(11), 793–800. DOI 10.1007/s00299-003-0748-9. [Google Scholar] [CrossRef]
10. Ma, H. C., Liang, G. H., Wassom, C. E. (1991). Effects of growth regulators and genotypes on callus and embryoid induction from maize anther culture. Plant Breeding, 106(1), 47–52. DOI 10.1111/j.1439-0523.1991.tb00478.x. [Google Scholar] [CrossRef]
11. Oswald, T. H., Nicholson, R. L., Bauman, L. F. (1977). Cell suspension and callus culture from somatic tissue of maize. Physiologia Plantarum, 41(1), 45–50. DOI 10.1111/j.1399-3054.1977.tb01520.x. [Google Scholar] [CrossRef]
12. Kamo, K. K., Chang, K. L., Hodges, M. (1987). Embryogenic callus formation from maize protoplasts. Planta, 172(2), 245–251. DOI 10.2307/23379100. [Google Scholar] [CrossRef]
13. Fan, W., Zhen, H., Wang, G. (2021). Proteomic analysis of ubiquitinated proteins in maize immature kernels. Journal of Proteomics, 243(1), 104261. DOI 10.1016/j.jprot.2021.104261. [Google Scholar] [CrossRef]
14. Duncan, D. R., Williams, M. E., Zehr, B. E., Widholm, J. M. (1985). The production of callus capable of plant regeneration from immature embryos of numerous Zea mays genotypes. Planta, 165(3), 322–332. DOI 10.1007/BF00392228. [Google Scholar] [CrossRef]
15. Carvalho, C., Bohorova, N., Bordallo, P. N., Abreu, L. L., Valicente, F. H. et al. (1997). Type II callus production and plant regeneration in tropical maize genotypes. Plant Cell Reports, 17(1), 73–76. DOI 10.1007/s002990050355. [Google Scholar] [CrossRef]
16. Bewley, J. D., Pacey, J. (1978). Desiccation-induced ultrastructural changes in drought-sensitive and drought-tolerant plants 1. Dry Biological Systems, 53–73. DOI 10.1016/B978-0-12-198080-1.50007-7. [Google Scholar] [CrossRef]
17. Du, H., Huang, Y., Qu, M., Li, Y., Hu, X. et al. (2020). A maize ZmAT6 gene confers aluminum tolerance via reactive oxygen species scavenging. Frontiers in Plant Science, 11, 1016. DOI 10.3389/fpls.2020.01016. [Google Scholar] [CrossRef]
18. Zhou, L., Tian, X., Cui, B., Hussain, A. (2021). Physiological and biochemical responses of invasive species Cenchrus pauciflorus benth to drought stress. Sustainability, 13(11), 5976. DOI 10.3390/su13115976. [Google Scholar] [CrossRef]
19. Liu, J., Wang, J., Lee, S., Wen, R. (2018). Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS One, 13(9), e0203612. DOI 10.1371/journal.pone.0203612. [Google Scholar] [CrossRef]
20. Liu, M., Li, J., Niu, J., Wang, R., Song, J. et al. (2016). Interaction of drought and 5-aminolevulinic acid on growth and drought resistance of leymus chinensis seedlings. Acta EcologicaSinica, 36(3), 180–188. DOI 10.1016/j.chnaes.2016.04.004. [Google Scholar] [CrossRef]
21. Wang, Y., Gu, W., Meng, Y., Xie, T., Li, L. et al. (2017). γ-aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Scientific Reports, 7, 43609. DOI 10.1038/srep43609. [Google Scholar] [CrossRef]
22. Liu, W., Yuan, X., Zhang, Y., Xuan, Y., Yan, Y. (2014). Effects of salt stress and exogenous Ca2+ on Na+ compartmentalization, ion pump activities of tonoplast and plasma membrane in Nitraria tangutorum Bobr. leaves. Acta Physiologiae Plantarum, 36(8), 2183–2193. DOI 10.1007/s11738-014-1595-8. [Google Scholar] [CrossRef]
23. Zhang, X. Y., Ma, M. J., Ye, B., Liu, L., Ji, S. J. (2021). Calcium ion improves cold resistance of green peppers (Capsicum annuum L.) by regulating the activity of protective enzymes and membrane lipid composition. Scientia Horticulturae, 277(12), 109789. DOI 10.1016/j.scienta.2020.109789. [Google Scholar] [CrossRef]
24. Jiao, P., Ma, R., Qi, Z., Jiang, Z., Ma, Y. (2019). Optimization of callus induction conditions from immature embryos in maize and plant regeneration. Phyton-International Journal of Experimental Botany, 88(3), 121–130. DOI 10.32604/phyton.2020.07980. [Google Scholar] [CrossRef]
25. Miteva, E., Hristova, D., Nenova, V., Maneva, S. (2005). Arsenic as a factor affecting virus infection in tomato plants: Changes in plant growth, peroxidase activity and chloroplast pigments. Scientia Horticulturae, 105(3), 343–358. DOI 10.1016/j.scienta.2005.01.026. [Google Scholar] [CrossRef]
26. Jia, Y., Xiao, W., Ye, Y., Wang, X., Liu, X. et al. (2020). Response of photosynthetic performance to drought duration and re-watering in maize. Agronomy, 10(4), 533. DOI 10.3390/agronomy10040533. [Google Scholar] [CrossRef]
27. Ren, T., Chen, N., Mahari, W. A. W., Xu, C., Feng, H. et al. (2021). Biochar for cadmium pollution mitigation and stress resistance in tobacco growth. Environmental Research, 192, 110273. DOI 10.1016/j.envres.2020.110273. [Google Scholar] [CrossRef]
28. Liu, W., Zhao, B. G., Chao, Q., Wa Ng, B., Li, X. (2020). The maize AP2/EREBP transcription factor ZmEREB160 enhances drought tolerance in Arabidopsis. Tropical Plant Biology, 13(9), 251–261. DOI 10.1007/s12042-020-09259-y. [Google Scholar] [CrossRef]
29. Liang, W., Ma, X., Wan, P., Liu, L. (2018). Plant salt-tolerance mechanism: A review. Biochemical Biophysical Research Communications, 495(1), 286–291. DOI 10.1016/j.bbrc.2017.11.043. [Google Scholar] [CrossRef]
30. Peng, Y., Li, T., Jiang, H., Gu, Y., Chen, Q. et al. (2020). Postharvest biochemical characteristics and ultrastructure of Coprinus comatus. PeerJ, 8, e8508. DOI 10.7717/peerj.8508. [Google Scholar] [CrossRef]
31. Zhang, S. Z., Hua, B. Z., Fan, Z. (2008). Induction of the activities of antioxidative enzymes and the levels of malondialdehyde in cucumber seedlings as a consequence of Bemisia tabaci (Hemiptera: Aleyrodidae) infestation. Arthropod-Plant Interactions, 2(4), 209–213. DOI 10.1007/s11829-008-9044-5. [Google Scholar] [CrossRef]
32. Cai, Z., Feng, K., Li, X., Yan, H., Zhang, Z. et al. (2019). Pre-breeding: The role of antioxidant enzymes on maize in salt stress tolerance. Acta Physiologiae Plantarum, 41(6), 102. DOI 10.1007/s11738-019-2880-3. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |