

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.021267
ARTICLE
Biological Control of Root-Knot Nematode Meloidogyne incognita in Psoralea corylifolia Plant by Enhancing the Biocontrol Efficacy of Trichoderma harzianum Using Press Mud
1Section of Plant Pathology and Nematology, Department of Botany, Aligarh Muslim University, Aligarh, 202002, India
2Department of Biological and Geological Sciences, Faculty of Education, Ain Shams University, Cairo, 11341, Egypt
3Department of Biology, College of Science and Humanities in Al-Kharj, Prince Sattam Bin Abdulaziz University, Al-Kharj, 11942, Saudi Arabia
*Corresponding Authors: Mohammad Danish. Email: danish.botanica@gmail.com; Heba I. Mohamed. Email: hebaibrahim79@gmail.com; hebaebrahem@edu.asu.edu.eg
Received: 05 January 2022; Accepted: 16 February 2022
Abstract: Meloidogyne incognita is a plant pathogen causing root-knot disease and loss of crop yield. The present study aimed to use Trichoderma harzianum as a biocontrol agent against plant-parasitic nematodes and used press mud, which is a solid waste by-product of sugarcane, as a biocontrol agent and biofertilizer. Therefore, the combined application of T. harzianum and press mud may enhance nematode control and plant growth. Elemental analysis of press mud using scanning electron microscopy (SEM) integrated with an Energy Dispersive X-ray (EDX) analyzer revealed the presence of different elements such as C, O, Mg, Si, P, K, Ca, Cu and Zn. In addition, a greenhouse study was conducted to investigate the combined effects of press mud and T. harzianum on M. incognita reproduction and growth and the biochemical features of Psoralea corylifolia. The results showed that plant length, dry biomass, leaf area, the number of seeds per plant, chlorophyll a, chl b, carotenoid content, nitrate reductase, carbonic anhydrase, and nitrogen content were significantly increased (P ≤ 0.05) in the T2 plants (plants were treated with 100 g of press mud + 50 mL T. harzianum before one week of M. incognita inoculation), over inoculated plants (IC). Antioxidant enzyme activity of ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) in the foliage of P. corylifolia was significantly increased when plants were treated with press mud + T. harzianum. A significant reduction in the number of egg masses, nematode population, and root-knot index (RKI) was found in plants with T2 plants. These results suggest that the combined application of T. harzianum and press mud has the potential to control the M. incognita infection and can be used as an environmentally safe alternative to chemical nematicides and also help in the removal of sugarcane waste that causes environmental pollution.
Keywords: Growth improvement; antioxidant enzymes; nitrate reductase; carbonic anhydrase; root-knot index
Worldwide, traditional medicines are used by many people for therapeutic purposes [1–3]. Psoralea corylifolia Linn., a member of the Fabaceae family, is a critically endangered plant that has long been utilized to cure a variety of clinical symptoms [4]. It is an essential medicinal plant that is used to treat a variety of ailments in traditional medicine [5]. The plant has strong antioxidant properties and, therefore, the seeds of this plant have been used for various kinds of skin diseases such as psoriasis, leukoderma, and leprosy [6]. The plant and seed extracts contain psoralen and isopsoralen, which have antibacterial, anti-tumor, antioxidant, anti-inflammatory, anti-modulatory, and immune activity [7,8]. However, this medicinal plant was frequently attacked by phytopathogens such as nematodes (Meloidogyne incognita), resulting in a significant loss in plant growth and yield productivity [9]. P. corylifolia roots were infected with nematodes and heavily galled, and the soil samples were collected from the rhizosphere of affected plants and contained about 536–845 second juveniles (J2)/200 cm3 of soil. Severe root galling and the presence of a high population density of J2 in the soil around symptomatic plants indicates that the root-knot nematode (RKN) can be a potentially damaging pest of P. corylifolia [10].
The present challenge imposed on us is to devise techniques for increasing crop yield and quality while minimizing pest damage and environmental impact. Plant-parasitic nematodes (PPNs) constitute a major agricultural limitation, causing huge losses of up to 80% of different economically important vegetable crops [11,12]. In order to control these plant parasitic nematodes, chemical nematicides have often been used. However, the continuous and indiscriminate use of these chemical compounds may be harmful to agricultural crops, soil fertility, and ultimately to human health [13]. So, there is an urgent need to find some harmless and eco-friendly solutions. In this context, some beneficial soil fungi are often referred to as plant growth promoting fungi and are used as biocontrol agents for the management of phytonematodes [14].
Trichoderma spp. is a filamentous fungus that has been employed for the biocontrol of nematodes in recent years [15]. Trichoderma spp. has antagonistic effects on nematodes through a wide range of techniques, including (a) direct method, which increases the level of extracellular enzymes like chitinase and protease, which allow the penetration of the fungus into the eggs by directly affecting very abundant structural components of the eggshell, thus reducing the number of eggs capable of hatching and therefore, the number of infective J2 [16] and organic volatile compounds [17], (b) generating of chitinase into the culture or rhizosphere, which may aid in egg hatching suppression [18], (c) conidia of Trichoderma parasitize on nematode cuticle or/and eggshell [18], (d) conidia of Trichoderma enhance root growth and boost inorganic nutrient solubilization [15], (e) Trichoderma conidia produce different types of metabolites (mycotoxins) that inhibit egg-hatching and juveniles [18].
The interaction of numerous factors within the biological control technique represents an effective method for reducing nematode infection and plant damage [19]. Organic compost also plays an effective role in the management of nematodes. The addition of organic compounds to soil has been shown to be beneficial for soil fertility, plant growth, and disease management [20,21]. Different organic matters are used as organic compost, like animal waste, organic fertilizer, litterfall, and organic amendments that have shown nematotoxic properties against nematodes [20]. These organic amendments compete with the phytonematodes through a variety of mechanisms; (i) production of nematode-killing chemicals during decomposition, (ii) improved soil physico-chemical properties, and (iii) increased microbial activity [22].
Press mud is a solid waste and a solid fibrous residue produced during the clarification and filtering of sugarcane juice. It accounts for approximately 2.8%–4.5% (w/w) of the milled sugarcane, which is sustainably used in agriculture worldwide. The sugarcane crop produces a lot of press mud and disposing of this by-product is a big problem. Many times, press mud is burned in brick kilns, resulting in the loss and waste of millions of tonnes of nutrients and, as a result, environmental degradation in India. A common use is for fertilizer, in both the unprocessed and processed form. Composting, microorganism treatment, and mixing with distillery effluents are some of the methods utilized to boost its fertilizer value and enhance soil health [23]. It is rich in inorganic phosphorus (P), potassium (K) and nitrogen (N) [24]. It is an eco-friendly, by-product that protects the crops from different diseases caused by phytopathogens. In this regard, Jonathan et al. [25] have reported that the single/combined application of press mud and neem cake decreases the efficacy of nematode infection and improves the growth of banana.
Very little information is known about the impact of press mud alone or in combination with T. harzianum on the efficacy of the root-knot nematode, M. incognita. As a result, the current study sought to assess the efficacy of combining press mud and T. harzianum in the control of nematode-infected P. corylifolia plants. The plant growth, yield, and biochemical characteristics were measured in response to the combined application of press mud and T. harzianum under the stress of M. incognita. Also, the present study aimed to use solid waste produced during the clarification and filtering of sugarcane juice to reduce the accumulation of pollutants in the environment.
A pot experiment was conducted in the glass house of the Department of Botany at Aligarh Muslim University, Aligarh (27°522 N latitude, 78°512 E longitude, and 187.45 m.a.s.l.), Uttar Pradesh, India.
2.1 Preparation of Nematode Inoculum
Infected roots of eggplant (Solanum melongena L.) with the root-knot nematode (M. incognita) were collected from an eggplant field. Root-knot nematode species M. incognita was identified on the basis of the North Carolina differential host test and perennial pattern morphology. A single egg mass was inoculated on an eggplant to maintain the M. incognita race-1 population. The egg masses were collected from the galled roots using sterilized forceps, transferred in a 20 μm sieve, and kept at room temperature for hatching of eggs following the Baermann funnel technique [26]. In the second stage, juveniles were isolated from the infected plants roots for treatments [27]. The egg masses were extracted from the infected roots using sterile forceps. The egg masses were cleaned in distilled water (DW), put in 15 mesh sieves with an 8 cm diameter cross layer of tissue paper, and allowed to hatch at room temperature on Petri plates with distilled water just deep enough to cover the egg masses.
2.2 Preparation of Pure Culture of Fungal Biocontrol Agents
The culture of T. harzianum was obtained from the Indian Type Culture Collection (ITCC) IARI, New Delhi, India. It was grown and maintained on a potato dextrose agar (PDA) culture medium. The mass production of T. harzianum was done on Richard’s medium. In 250 mL corning flasks, the medium was prepared, filtered through muslin cloth, and sterilized in an autoclave at 15 lb for 15 min. In an aseptic room, the liquid medium was infected with a tiny quantity of fungus and kept on PDA slants with the assistance of an inoculated needle. The inoculated flask was kept in an incubator at 25°C–30°C for about 15 days to allow copious growth of the fungus and it was used throughout the crop experiments.
2.3 Mass Culture Preparation of T. harzianum and Inoculation in Pots
After enmeshing of T. harzianum, (100 spore mL−1) after counting spore density using a haemocytomete (Neubauer-ruled Bright Line counting chambers; Hausser Scientific, Horsham, Pa.) was blended for 2 min at high speed to mix properly into 1000 mL of distilled water in a Waring blender such that 10 mL of suspension contained one gram of mycelium. The fungal suspension of T. harzianum was incorporated into the soil around the root of P. corylifolia by making holes 5–7 cm deep within a radius of 2 cm. After inoculation of the fungus, the holes were plugged with soil.
2.4 Preparation and Characterization of Press Mud
Press mud was obtained from Dwarikesh Sugar Industries Limited Bundki (Bijnor, India). The characterization of press mud was carried out using industry-standard techniques. After oven drying, press mud at 110°C for 2 h and proximate analysis of the powdered sample was performed. The morphological and elemental analysis of press mud was done by using a scanning electron microscope (SEM, JEOL-JSM6100) integrated with an Energy Dispersive X-ray (EDX) analyzer. The X-ray diffraction (XRD) examination was performed at the Department of Physics, AMU Aligarh, India, using copper as the target and nickel as the filter media, with a radiation angle of 1.542 degrees and a goniometer speed of 1/min. The presence of functional groups in the press mud was determined using Fourier Transform Infrared Spectroscopy (FTIR). This was accomplished using the pellet (pressed disk) approach. The chosen spectral range was 4000 to 500 cm−1.
Each experimental unit consisted of a P. corylifolia plant in a pot. Each treatment had five replications (n = 5) in a properly randomized experimental design. The amount of inoculated nematode and fungi were based on the preliminary work in our lab to determine the suitable amounts used in the experiment and also based on literature [28,29]. The treatment counted the number of infective juveniles, biocontrol agents and press mud given to the test plants by making holes of 2.5–5 cm deep near the plant bases carefully without damaging the roots. After inoculation, the holes were covered by soil as soon as possible. The moisture content in the pots was maintained by regular watering. The treatment pattern was as follows:
C = Control un-inoculated
IC = 2,000 J2 of M. incognita.
T1 = 30 mL T. harzianum (T.H) + 50 g press mud (P. M) one week prior to 2,000 J2 inoculation.
T2 = 50 mL T. harzianum (T.H) + 100 g press mud (P. M) one week prior to 2,000 J2 inoculation.
T3 = Simultaneous inoculation of 2,000 J2 inoculation and 30 mL T. harzianum (T.H) + 50 g press mud (P. M).
T4 = Simultaneous inoculation of 2,000 J2 inoculation and 50 mL T. harzianum (T.H) + 100 g press mud (P. M).
T5 = 2,000 J2 inoculation one week prior to 30 mL T. harzianum (T.H) + 50 g press mud (P. M).
T6 = 2,000 J2 inoculation one week prior to 50 mL T. harzianum (T.H) + 100 g press mud (P. M).
The life cycle of the plants was terminated and harvested after 4 months of sowing. Each treated plant was carefully removed from the soil system and cleaned with tap water. A meter scale was used to measure the length of the roots and shoots. Fresh and dry weights were noted, and the number of egg masses and number of galls were observed and counted visually.
2.7.1 Estimation of Photosynthetic Pigments
The amount of chlorophyll a, chlorophyll b, and carotenoid in the leaves of treated and M. incognita infected plants was determined using Maclachlan et al. [30] method.
The formula used was as follows:
V = Total volume of the solution.
W = Weight of the leaves used for extraction of the pigment.
D = Optical Density of sample.
2.7.2 Determination of Enzymatic Activity
The activity of nitrate reductase (NR, 1.6.6.1) in the leaves of treated plants was estimated by Jaworski [31]. One hundred milligrams of young leaves were cut and placed in test tubes containing 0.1 M phosphate buffer (pH 7.4), KNO3, and 5% isopropanol. This combination was kept for 2 h at 25°C. After brooding, 0.2 ml of this solution was transferred to a separate cylinder, and 0.15 mL of 1% sulphanilamide and 0.02% N-(1-naphthyl)-ethylenediamine dihydrochloride (NED-HCl) were blended and left at room temperature for 20 min to achieve the best shading results. The test solution was placed in a cuvette, and the absorbance was measured using a spectrophotometer at 540 nm against a transparent background. A typical bend was plotted using sodium nitrite’s known convergence. After comparing the OD of the sample with the standard curve, the NR activity was expressed in nM NO2 g−1FW h−1.
In the fresh leaves, the carbonic anhydrase activity (CA, 4.2.1.1) was determined by Dwivedi et al. [32]. In a testing tube containing 0.2 M cysteine hydrochloride solution, 100 mg of fresh leaf test were chopped into small pieces. This blend was brooded for 15–20 min at 4°C. 2 mL of phosphate support (pH 6.8), 0.2 M sodium bi carbonate, bromothymol blue, and the methyl red marker were added to each test tube. Before titrating against 0.05 N HCl, each test tube was vigorously shaken. As soon as a red-pink color developed, readings were obtained. A control test was also performed without leaf tissue and titrated against 0.05 N HCl. The CA activity was measured in μM CO2 kg−1 leaf FW S−1.
2.7.3 Determination of Leaf Nitrogen Content
Leaves from each treatment were dried in an oven at 80°C and ground into a fine powder using an electric grinder. A total of 500 mg of leaf powder was digested in a digestion tube containing 2 mL sulfuric acid and 0.5 mL 30% hydrogen peroxide, which was added dropwise. After digestion, the filtrate was completed to a known volume and used to determine nitrogen by using Nessler’s reagent. The optical density (OD) of the solution was measured at 525 nm using a spectrophotometer according to Lindner [33].
2.7.4 Determination of Defense-Related Enzymes Activity
Using a pre-chilled pestle and mortar, 0.5 g of fresh leaf sample was homogenized in 5.0 mL of cold (40°C) extraction buffer. After centrifuging the mixture at 10,000×g for 10 min, the supernatant was collected, and enzyme activity was determined. Peroxidase (POD) (EC 1.11.1.7) activity in the enzyme extract was measured by using the method of Chance et al. [34]. Catalase (CAT) (EC 1.11.1.6) activity was measured by adding a cold sodium phosphate buffer to the enzyme extract. To start the reaction, H2O2 was added to the reaction mixture. The rate of decline in absorbance at 240 nm was measured at 10 s intervals for 1 min [34]. Superoxide dismutase (SOD) (EC1.15.1.1) was assayed by following the method of Giannopolitis et al. [35], and the activity of ascorbate peroxidase (APX) (EC 1.11.1.11) was calculated using Asada et al. [36] method.
2.7.5 Number of Galls, Egg Masses and Root-Knot Index
The number of galls per plant was counted visually, and the size of each gall was recorded using a micrometer to measure its maximum length and breadth (in μm). Infected roots were immersed in phloxin-B solution for at least 20 min. The roots were cleaned thoroughly with tap water, and the red-colored egg masses on infected roots were counted per root system [37]. Disease indexes were measured based on number of gall and number of egg masses per root system (Not per individual plant root). Taylor et al. [38] technique was used to count galls and egg masses on a 0–5 scale after harvest:
0 = 0 galls/egg masses/root system.
1 = 1–2 galls/egg masses/root system.
2 = 3–10 galls/egg masses/root system.
3 = 11–30 galls/egg masses/root system.
4 = 31–100 galls/egg masses/root system.
5 => 100 galls/egg masses/root system.
2.7.6 Nematode Related Parameters
For determination of the nematode population in soil, Cobb’s sieving and the Baermann funnel method were used. Each pot’s soil was carefully mixed, and the juveniles were retrieved because eggs and larva inside the roots are also part of the final population, so they had to be counted. A counting dish was used to count the number of nematodes per root system and per kg of soil. The formula for calculating the reproduction factor (Rf) was:
Pf denotes the final population, whereas Pi denotes the initial population.
The data was analyzed statistically with the program SPSS (Statistics Package for Social Science 26.00) according to Snedecor et al. [39]; five replicas were used on the analysis for variance (ANOVA). The average differences were compared at 5% level of significance by the Duncan’s Multiple Range Test. Correlation was calculated using Stat graphics XVII program Version 17.20 to depict the link between quantitative statistical data.
3.1 Characterization of Press Mud
The SEM is frequently used to investigate the morphological properties and surface characteristics of adsorbent materials. It confirms the shape, surface texture, and porosity of press mud at a qualitative level. Figs. 1A–1C show that SEM image of press mud. The results indicate that the dry press mud is composed of fine particles and shows the loose distribution between them without forming large clustering blocks.

Figure 1: Characterization of press mud; scanning electron microscopic (SEM) examination of press mud representing the surface morphology (panels A, B and C), XRD pattern (D) and FTIR analysis (E). EDX analysis showing the elemental compositions of press mud (F)
The color of press mud was observed as gray or dark gray. The biomass aggregates in the press mud sample were organized into cellulose fibers, and the protein matrix was tightly bonded. The SEM image of sugarcane press mud reveals a fibrous structure with a surface porosity of 20–25 nm. Furthermore, the proximate composition of press mud has been provided in Table 1.

The X-ray diffraction (XRD) pattern of the press mud is shown in Fig. 1D. The XRD spectrum showed two major peaks at 2θ = 20.91° and 26.63° which may be due to the presence of silica in press mud. The other small peaks may be due to the presence of other minerals like Ca, P, K, Zn, Cu.
3.3 Functional Group Analysis of Press Mud Using FTIR
The FTIR analysis of the press mud revealed the existence of several organic functional groups, indicating their respective constituents. The FTIR spectra of the press mud is shown in Fig. 1E. The peak observed at 3400.5 cm−1 may be due to the presence of free OH groups. The stretching vibrations at about 2910 cm−1 indicate the presence of silanol (Si–OH) groups. The C=O stretching frequency is represented by the peak at 1633.56 cm−1. The sharp peak at 1034.01 cm−1 may be due to the C–O stretching vibrations in lactones. The peak at around 549.29 cm−1 is due to Si–H bond stretching.
3.4 Elemental Analysis of Press Mud (EDX)
In order to know the elemental composition of press mud, EDX analysis was performed. The findings revealed that activated press mud has various elemental compositions. Fig. 1F shows the elemental status of press mud (SEM-EDX). The EDX micrographs revealed that the press mud mostly contains silicon (Si) and calcium (Ca) as principal components and oxides of zinc (Zn), magnesium (Mg), copper (Cu) and iron (Fe) in trace amounts.
3.5 Changes in Growth and Yield Attributes
The data in Table 2 shows that all treatments caused a significant increase in morphological criteria and yield as compared to plants inoculated with nematodes. The most pronounced increases were detected in plants treated with 50 or 100 mL of T. harzianum and 50 or 100 g of press mud one week prior to M. incognita followed by plants having a simultaneous inoculation of 2,000 J2 inoculation and 50 or 100 mL of T. harzianum and 50 or 100 g of press mud. The lower values in growth and yield were detected in plants treated with 30 or 50 mL of fungal suspension and 50 or 100 g of press mud at 7 days after M. incognita inoculation. The plants inoculated with 50 mL of T. harzianum and 100 g of press mud one week prior to 2,000 J2 inoculation (T2) caused a significant increase in root length (63.6%), shoot length (93%), root fresh weight (82%), shoot fresh weight (67%), root dry biomass (60%) and shoot dry biomass (79%) over IC (inoculated with 2000 J2 of M. incognita only). Also, the plants inoculated with 30 mL of T. harzianum suspension and 50 g of press mud 7 days after M. incognita inoculation (T5) showed a significant increase and non-significant effect on the growth attributes of plants as compared with inoculated plants (Table 2). For instance, the most pronounced increases in the leaf area (90%) and seed yield (54%) of P. corylifolia plants were detected after inoculation with T. harzianum (50 mL) and press mud (100 g) as compared to plants inoculated with the nematode (Table 2). However, the lowest values were detected in plants treated with 30 mL of fungal suspension and 50 g of press mud at 7 days after M. incognita inoculation.

3.6 Changes in Photosynthetic Pigments
The photosynthetic pigments in the foliage tissues of P. corylifolia plants were significantly decreased (p ≤ 0.05) in plants infected by M. incognita. Plants inoculated with T. harzianum (30 or 50 mL) and press mud (50 or 100 g) press mud before, after nematode infection, and/or simultaneous inoculation reduced the effect of nematodes on photosynthetic pigments compared to control inoculated plants (IC). The higher values in Chl a (60%), Chl b (96%), and carotenoid content (40%) were detected in plants inoculated with T. harzianum (50 mL) and press mud (100 g) before 7 days of infestation with M. incognita over inoculated control (IC) (Table 3).

3.7 Changes in Enzymatic Activity and Leaf Nitrogen Content
In order to assess the impact of fungal biocontrol agents and organic press mud on enzymatic activity, the nitrate reductase (NR) and carbonic anhydrase (CA) in the foliage of M. incognita infected plants were assessed (Table 3). NR, CA, and N content were significantly decreased by about 46.1%, 44.9%, and 41.5%, respectively, in P. corylifolia plants inoculated with nematodes. In addition, all treatments caused a significant increase in the same content as compared with control inoculated plants, while the maximum increase in NR (76%), CA (74%), and leaf N (55%), content was recorded when P. corylifolia plants were detached from soil supplemented with 50 mL of T. harzianum and 100 g of press mud before 7 days of infestation by M. incognita as compared to control inoculated plants.
3.8 Changes in the Activity of Defense-Related Enzymes
The data in Figs. 2A–2D illustrated that inoculation with M. incognita caused a significant boost in APX, CAT, POD and SOD activity in plants as compared to control plants. All treatments caused a significant increase in defense enzymes activity as compared with plants inoculated with nematodes. In addition, treatment with 50 mL of T. harzianum with press mud (100 g) one week prior to nematode inoculation was exhibited as the best treatment, where it enhanced the activity of APX, CAT, POD and SOD by about 150%, 85.2%, 92.3%, and 58.3% respectively over plants inoculated with nematode only.

Figure 2: Combined effect of press mud and T. harzianum on APX (A), CAT (B) POD (C) and SOD activities (D) extracted from fresh foliage of M. incognita inoculated P. corylifolia plant raised under green-house conditions. Each value is a mean (±SE) of five replicates and the different letters on the same bar show significant differences according to Duncan’s test at P ≤ 0.05. C = Control un-inoculated; IC = 2,000 J2 of M. incognita; T1 = 30 mL T. harzianum (T.H) + 50 g press mud (P. M) one week prior to 2,000 J2 inoculation. T2 = 50 mL T. harzianum (T.H) + 100 g press mud (P. M) one week prior to 2,000 J2 inoculation; T3 = Simultaneous inoculation of 2,000 J2 inoculation and 30 mL T. harzianum (T.H) + 50 g press mud (P. M); T4 = Simultaneous inoculation of 2,000 J2 inoculation and 50 mL T. harzianum (T.H) + 100 g press mud (P. M); T5 = 2,000 J2 inoculation one week prior to 30 mL T. harzianum (T.H) + 50 g press mud (P. M); T6 = 2,000 J2 inoculation one week prior to 50 mL T. harzianum (T.H) + 100 g press mud (P. M)
3.9 Changes in the Number of Galls and Number of Egg Masses
In this study, the number of galls, root-knot index, RKI, and reproduction factors (number of egg masses on roots) were significantly increased (P ≤ 0.05) when plants were detached from soil infected only with M. incognita. Besides, inoculation with T. harzianum suspension (50 mL) and press mud (100 g) before nematode infection, exhibited a maximum reduction of 88% and 85% in the number of galls and number of egg masses, respectively (Figs. 3A and 3B). Similarly, gall formation and egg mass production were significantly reduced accordingly by all other treatments. Contrarily, T5 (2,000 J2 inoculation one week prior to 30 mL of T. harzianum and 50 g of press mud) had the lowest reduction in all nematode related parameters.

Figure 3: Combined effect of press mud and T. harzianum on number of galls (A), number of egg masses/plants (B) population of nematodes in roots (C), population of nematodes in soils (D), total nematode population (E), reproduction factor (F) and root knot index (G) of M. incognita inoculated P. corylifolia plants raised under green-house condition. Each value is a mean (±SE) of five replicates and the different letters on the same bar show significant differences according to Duncan’s test at P ≤ 0.05. C = Control un-inoculated; IC = 2,000 J2 of M. incognita; T1 = 30 mL T. harzianum (T.H) + 50 g press mud (P. M) one week prior to 2,000 J2 inoculation. T2 = 50 mL T. harzianum (T.H) + 100 g press mud (P. M) one week prior to 2,000 J2 inoculation; T3 = Simultaneous inoculation of 2,000 J2 inoculation and 30 mL T. harzianum (T.H) + 50 g press mud (P. M); T4 = Simultaneous inoculation of 2,000 J2 inoculation and 50 mL T. harzianum (T.H) + 100 g press mud (P. M); T5 = 2,000 J2 inoculation one week prior to 30 mL T. harzianum (T.H) + 50 g press mud (P. M); T6 = 2,000 J2 inoculation one week prior to 50 mL T. harzianum (T.H) + 100 g press mud (P. M)
3.10 Changes in Nematode Population in Root and Soil System
Nematode development in terms of root and soil population was considerably reduced in all the treatments. The inoculation of T. harzianum suspension (50 mL) and press mud (100 g) before nematode infection had the best effect where it reduced the nematode population and reproduction factor by 80% and 81%, respectively over plants solely infected with nematodes (Figs. 3C–3E). Correlation matrix results are presented between the different parameters studied in Fig. 4.
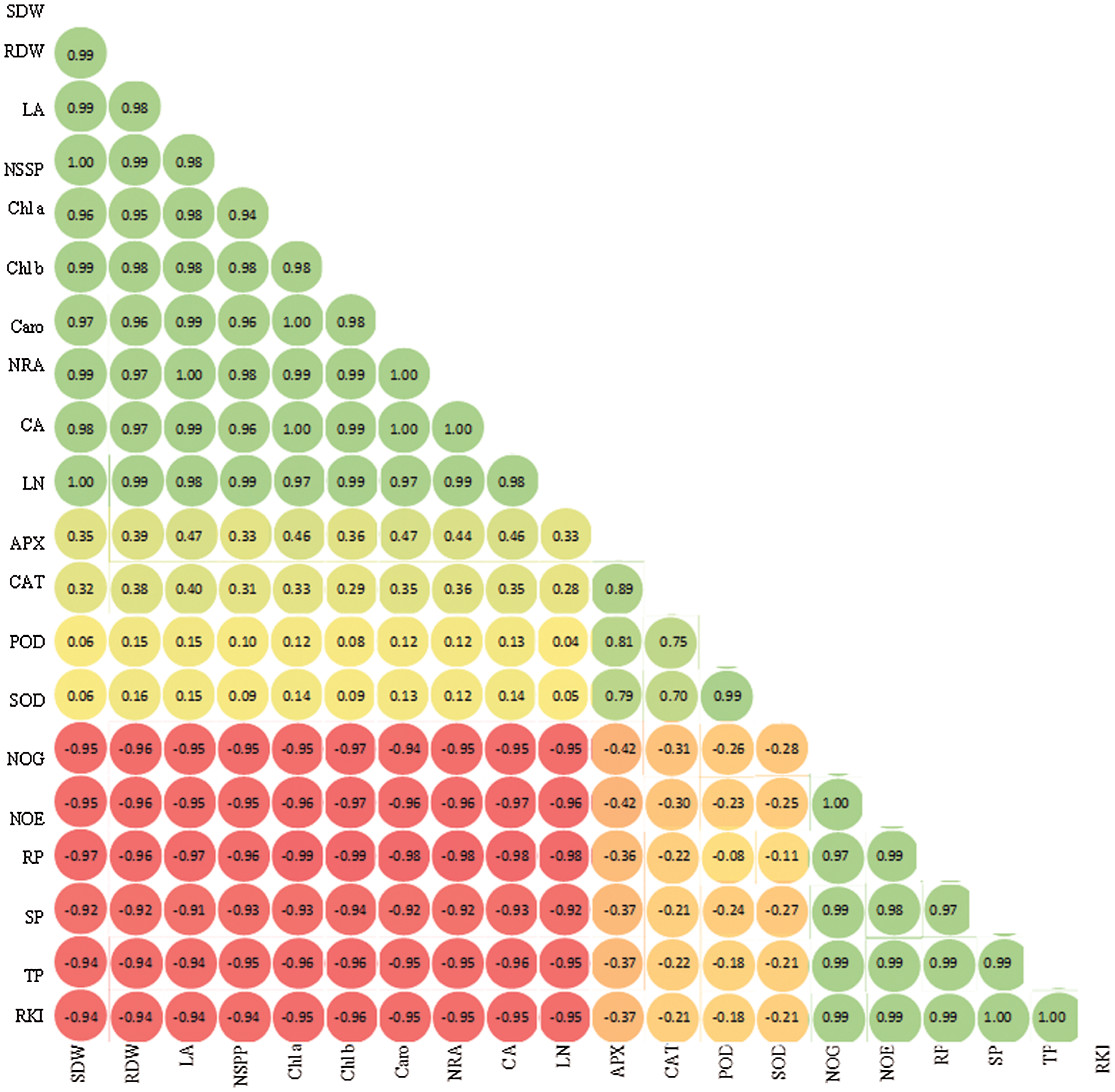
Figure 4: Correlation matrix results between some studied parameters of Psoralea corylifolia at different combined treatments of press mud and T. harzianum before, simultaneously, and after 1 week of M. incognita inoculation. SDW, shoot dry weight; RDW, root dry weight; LA, leaf area; NSPP, Number of seed yield per plant (g); Chl a, chlorophyll a; Chl b, chlorophyll b, NRA, nitrate reductase activity; CA, anhydrase activity; LN, leaf nitrogen concentration; APX, ascorbate peroxidase; CAT, catalase; POD, peroxidase; SOD, superoxide dismutase; NOG, number of galls per root system; and NOE, number of egg masses per root system; RP, root population; SP, soil population; TP, total population; RKI, root-knot index
Naturalists, biologists, environmentalists, and hydrologists are continuously pressuring farmers to use fewer pesticides (nematicides) and synthetic fertilizers. They must, however, preserve crop profitability and crop quality. Plant-parasitic nematode damage is projected to cost the global agriculture industry $100 billion per year. Because pesticides (nematicides) and synthetic fertilizers are expensive, they can contribute to higher crop production costs and, as a result, considerable rises in food prices, especially during low harvest years (in the dry or very rainy). Furthermore, pesticides are usually selective, eliminating only the species that are targeted. They react fast and vigorously [40], but plant protection is limited because their effect is brief and has no other beneficial effects on plant growth or soil quality. As a result, organic fertilizer as a tool for controlling parasitic nematodes and other soil pathogens is advantageous because it is a natural, low-cost material (usually made by growers), from which nutrients and nematicidal substances are gradually released throughout the entire vegetation period [40]. In this context, press mud, a waste product material of sugar industry is used as bio-fertilizers. Here, we had assessed the combined application of T. harzianum (biocontrol agent) and press mud against M. incognita.
The press mud was morphologically characterized using SEM as recently described by Rondina et al. [41]. The XRD spectrum showed two major peaks at 2θ = 20.91° and 26.63°. The FTIR method is a useful tool for identifying the functional groups that distinguish a chemical/compound. The adsorption process also requires an understanding of the chemical structure of adsorbents. By comparing the absorption frequencies of various organic functional groups. The peak at around 549.29 cm−1 is due to Si–H bond stretching [42]. Likewise, Rout et al. [43] investigated a similar pattern when they analyzed the press mud using FTIR spectroscopy.
The data in the present study showed that treatment with press mud and Trichoderma caused a significant boost on morphological criteria and yield as compared to plants inoculated with nematodes. Similar results are recorded by Lakshman et al. [44] who found that treatment with arbuscular mycorrhizal fungi, Press mud and indole acetic acid caused a significant increase in tomato growth, biomass and yield as compared to control plants. It is widely known that press mud nourishes and promotes the growth and yield of plants by assisting in the retention of soil moisture and increasing root multiplication [45]. In agronomic practices, press mud might be used as an organic matter supply, a source of agricultural nutrients, manure, and as a soil ameliorant [46]. Press mud comprises of fiber, crude protein, sugar, crude wax, lipids, ash including oxides of Si, Ca, P, Mg, and K that may play a crucial role in the development of plants [47]. This organic matter is highly soluble, making it easily available for the activity of microbiota and, as a result, easily taken up by the soil [46]. As a valuable source of plant nutrients, press mud may have an impact on the physical, chemical, and biological aspects of a soil [48]. Also, press mud contains a high amount of potassium so that, the involvement of K in nutrient and sugar translocation in plants, as well as turgor pressure in plant cells, may be responsible for the increased plant growth and yield. It also plays a role in cell expansion and meristematic growth [49]. In addition, Trichoderma spp. increased agricultural productivity by improving shoot and root growth [50]. As a result, it is possible that press mud enriches the soil with nutrients that aid T. harzianum development. Enhanced root area allows them to explore larger volumes of soil for nutrients, improve the solubility of insoluble substances, as well as increase the availability of micronutrients, which may also contribute to an increased plant development by Trichoderma spp. [45]. Many researchers have noticed that soil application of Trichoderma spp. to various plants infected with root-knot nematode, resulted in improved growth and biochemical features of plants [45]. Similar to our study, various workers have reported that Trichoderma significantly improved the leaf area and yield of plants [51].
Inoculation with nematodes caused suppression of photosynthetic pigments in leaves of P. corylifolia. Infection with nematodes caused the production of reactive oxygen species (ROS) that could decompose the photosynthetic pigments (i.e., chlorophyll), as well as cause the impairment of the photosynthetic equipment, reduction in electron transport, carbon fixation capacity, and photophosphorylation [52]. On the other hand, T. harzianum and press mud treatment resulted in an increase in photosynthetic pigments (chl a, chl b and carotenoid). Besides, nitrogen (N) is a key component of chlorophyll molecules; nematode inoculation reduces its bioavailability and thus lowers the chlorophyll concentration in the foliage [9]. This might be one of the primary and main reasons that plants infected with phytonematodes including M. incognita often had a lower photosynthetic rate. Our findings showed that T. harzianum along with press mud could possibly increase photosynthetic performance. Likewise, in comparison to plants infected with the nematode (alone), inoculation of tomato roots with T. harzianum UBSTH-501 dramatically increased total chlorophyll content and chitinase activity by increasing the absorption of water, which ultimately resulted in an increase in photosynthetic pigments [53]. The use of a 40% sugarcane press mud treatment increased the chlorophyll content of eggplant, which is likely owed to Fe, Mg, and Mn concentrations in the sugarcane press mud, which are related to chlorophyll synthesis [54]. As a result, the press mud after a 40% treatment includes the optimal levels of nutrients essential for S. melongena to reach its maximum vegetative growth [54].
Infection of plants with nematode caused a significant decrease in NR, CA and N content as compared to control plants. Similar results were recorded by Danish et al. [55] who found that infestation with M. incognita to Trachyspermum ammi (L.) caused a significant decrease in the same contents. On the other hand, treatment with 50 mL of T. harzianum and 100 g of press mud before 7 days of infestation by M. incognita significantly increased NR, CA and N contents. Trichoderma spp. have the ability to invade the root systems of a variety of plants and thus coordinate the host plant’s defense mechanisms [56]. This might have been attributed to an increased water and mineral absorption by the root systems of plants. Similar to our finding, Sofy et al. [50] found that treatment with T. harzianum noticed an increase in nitrogen absorption efficiency of plants. In addition, photosynthetic molecules, comprised of N, Mg, and other essential nutrients are very helpful in the absorption of water and minerals. These minerals are crucial for the metabolic activities and the growth of plants [57]. The increment of nitrogen accumulation by press mud was probably due to mineralization of the organic matter containing proteins and conversion of ammonium-nitrogen into nitrate [13].
Antioxidant enzymes like ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) are often associated with biotic and abiotic factors [58–62]. In contrast, T. harzianum colonization in soil drenched with press mud substantially enhanced the activity of antioxidant enzymes. Trichoderma spp. has been found to boost the activity of plant defense enzymes and the concentration of defense metabolites such as phenolic compounds and flavonoids [61]. In a similar way, Yan et al. [63] observed that pre-soil-inoculation of T. harzianum to tomato plants infected with M. incognita resulted in improved defense-related enzymes and pathogenesis-related (PR) proteins including chitinases, 1,3-glucanase, protease, and amylase.
As a consequence of our findings, T. harzianum mixed with press mud exhibited the greatest nematicidal impact against M. incognita. These findings corroborate those of Olabiyi et al. [64], who found that combining T. harzanium with composted wastes reduced nematode population and gall index in sesame. Additionally, the combined inoculation of the fungal agent Trichoderma vierns with forest debris obtained from oak plants considerably reduced the number of galls in roots which were already infected with M. javanica [65]. It has been hypothesized that reduced galling was due to better proliferation of the fungus T. harzianum in soil drenched with press mud. During planting time, 15 t/ha of cured press mud is applied to help control plant parasitic nematodes. Because it is high in nutrients and organic matter, press mud is an excellent substrate for the growth of nematode antagonistic fungi and bacteria in soil [66]. Trichoderma spp. has been shown to reduce the root gall formation by nematode and worm populations as it has been reported by several workers [67].
The reduction in the nematode population might have been caused by the fungal mycelia colonizing the surface of the roots prior to nematode infestation and by producing various lytic and cell wall degrading enzymes. Trichoderma spp. inhibits the plant-parasitic nematodes in a variety of ways [68] T. harzianum may colonize the roots very fast and effectively decrease the number of feeding sites for RKNs in the rhizosphere [69]. Trichoderma spp. strains have been shown to impact J2 motility, nematode development, egg hatching, nematode reproduction, and disease severity as nematode antagonists [61]. Some biocontrol organisms, such as T. harzianum, colonize soils rich in organic matter, which improves biocontrol activity. Their inhibitory effects against plant nematodes have also been discovered in recent years [70]. Also, Osman et al. [71] observed that T. harzianum had a good effect on root-knot nematode M. incognita management and a moderate improvement in eggplant yield production. They attributed their findings to the fungus’s chitinolytic activity, which induces a chitin layer disintegration in worm eggs. T. harzianum isolates induced systemic resistance in tomato plants against the root-knot nematode, M. javanica, by increasing the accumulation of hydrolytic enzymes that affect nematode invasion [72,73].
The findings of this study indicate that the combination of T. harzianum and press mud is an environmentally friendly and effective treatment for M. incognita root-knot disease. Thus, it might be concluded that the application of soil amendment with press mud and T. harzianum increases plant growth and reduces the nematode population (Fig. 5). Application of 100 g press mud in combination with 50 mL of T. harzianum prior to one week of M. incognita inoculation proved to be the best concentration in comparison to the simultaneous and after inoculation of M. incognita. We discovered that treatment of Trichoderma and press mud prior to one week of M. incognita infestation induced systemic resistance or numerous possible defence mechanisms were responsible for the increase in plant growth and reduction in nematode infestation. The study provides new insights into the enhanced bio-control efficacy of T. harzianum induced by soil drenching with press mud which are considered eco-friendlily methods against the M. incognita infection and also help in the removal of sugarcane wastes that cause pollution to the environment.

Figure 5: Diagram depicts the (1) reducing effects of M. incognita and (2) combined application of T. harzianum and press mud improving the growth, biomass, photosynthetic pigments, antioxidants, and enzymatic activity on P. corylifolia plants
Authorship: The authors confirm contribution to the paper as follows: study conception and design: Y. N., M. D. and H. S.; data collection: Y. N., M. D. and H. S.; analysis and interpretation of results: Y. N., M. D., H. I. M. and H. S.; draft manuscript preparation: Y. N., M. D., H. I. M., H. S., A. E. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgement: The authors are grateful to the (1) Chairperson of the Department of Botany at Aligarh Muslim University (AMU) for providing a laboratory and other essential resources and (2) University Sophisticated Instrument Facility (USIF) of AMU which provided TEM and SEM-EDX analysis.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. El-Beltagi, H. S., Mohamed, H. I., Elmelegy, A. A., Eldesoky, S. E., Safwat, G. (2019). Phytochemical screening, antimicrobial, antioxidant, anticancer activities and nutritional values of cactus (Opuntia Ficus Indicia) pulp and peel. Fresenius Environmental Bulletin, 28(2A), 1534–1551. [Google Scholar]
2. El-Beltagi, H. S., Mohamed, H. I., Abdelazeem, A. S., Youssef, R., Safwat, G. (2019). GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 47(2), 493–505. DOI 10.15835/nbha47211405. [Google Scholar] [CrossRef]
3. Hamed, M. M., Abd El-Mobdy, M. A., Kamel, M. T., Mohamed, H. I., Bayoumi, A. E. (2019). Phytochemical and biological activities of two asteraceae plants Senecio vulgaris and Pluchea dioscoridis L. Pharmacology Online, 2, 101–121. [Google Scholar]
4. Shrestha, S., Jadav, H. R., Bedarkar, P., Patgiri, B. J., Harisha, C. R. et al. (2018). Pharmacognostical evaluation of Psoralea corylifolia Linn. seed. Journal of Ayurveda and Integrative Medicine, 9(3), 209–212. DOI 10.1016/j.jaim.2017.05.005. [Google Scholar] [CrossRef]
5. Chopra, B., Dhingra, A. K., Dhar, K. L. (2013). Psoralea corylifolia L. (Buguchi)—folklore to modern evidence. Fitoterapia, 90, 44–56. DOI 10.1016/j.fitote.2013.06.016. [Google Scholar] [CrossRef]
6. Shenoi, S. D., Prabhu, S. S. (2018). Psychodermatology: An Indian perspective. Clinics in Dermatology, 36(6), 737–742. DOI 10.1016/j.clindermatol.2018.08.013. [Google Scholar] [CrossRef]
7. Agrawal, S. B., Pandey, A. (2019). Pharmaceutical activities and effects of various abiotic stresses/elicitors on bioactive constituents of Psoralea corylifolia L. (Bakuchi). International Journal of Plant and Environment, 5(3), 186–191. DOI 10.18811/ijpen.v5i03.6. [Google Scholar] [CrossRef]
8. Ren, Y., Song, X., Tan, L., Guo, C., Wang, M. et al. (2020). A review of the pharmacological properties of psoralen. Frontiers in Pharmacology, 11, 571535. DOI 10.3389/fphar.2020.571535. [Google Scholar] [CrossRef]
9. Helmi, A., Mohamed, H. I. (2016). Biochemical and ulturasturctural changes of some tomato cultivars to infestation with Aphis gossypii glover (Hemiptera: Aphididae) at Qalyubiya, Egypt. Gesunde Pflanzen, 68, 41–50. DOI 10.1007/s10343-016-0361-9. [Google Scholar] [CrossRef]
10. Ahmad, G., Khan, A., Ansari, S., Khan, A. A., Elhakem, A. et al. (2022). Management of root-knot nematode infection by using fly ash and Trichoderma harzianum in Capsicum annum plants by modulating growth, yield, photosynthetic pigments, biochemical substances, and secondary metabolite profiles. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 50(1), 1259. DOI 10.15835/nbha50112591. [Google Scholar] [CrossRef]
11. Ahmad, G., Khan, A., Khan, A. A., Ali, A., Mohamed, H. I. (2021). Biological control: A novel strategy for the control of the plant parasitic nematodes. Antonie van Leeuwenhoek, 114, 885–912. DOI 10.1007/s10482-021-01577-9. [Google Scholar] [CrossRef]
12. Ahmad, G., Khan, A. A., Mohamed, H. I. (2021). Impact of the low and high concentrations of fly ash amended soil on growth, physiological response and yield of pumpkin (Cucurbita moschata Duch. Ex Poiret L.). Environmental Science and Pollution Research, 28, 17068–17083. DOI 10.1007/s11356-020-12029-8. [Google Scholar] [CrossRef]
13. Mohamed, H. I., Abd–El Hameed, A. G. (2014). Molecular and biochemical markers of some Vicia faba L. genotype in response to storage insect pests infestation. Journal of Plant Interactions, 9(1), 618–626. DOI 10.1080/17429145.2013.879678. [Google Scholar] [CrossRef]
14. Khoja, S., Eltayef, K. M., Baxter, I., Myrta, A., Bull, J. C. et al. (2021). Volatiles of the entomopathogenic fungus, Metarhizium brunneum, attract and kill plant parasitic nematodes. Biological Control, 152, 104472. DOI 10.1016/j.biocontrol.2020. [Google Scholar] [CrossRef]
15. Aly, A. A., Mohamed, H. I., Mansour, M. T. M., Omar, M. R. (2013). Suppression of powdery mildew on flax by foliar application of essential oils. Journal of Phytopathology, 161, 376–381. DOI 10.1111/jph.12080. [Google Scholar] [CrossRef]
16. Abd El-Rahman, S. S., Mohamed, H. I. (2014). Application of benzothiadiazole and Trichoderma harzianum to control faba bean chocolate spot disease and their effect on some physiological and biochemical traits. Acta Physiologia Plantarum, 36(2), 343–354. DOI 10.1007/s11738-013-1416-5. [Google Scholar] [CrossRef]
17. Tchameni, S. N., Cotârleţ, M., Ghinea, I. O., Bedine, M. A. B., Sameza, M. L. et al. (2020). Involvement of lytic enzymes and secondary metabolites produced by Trichoderma spp. in the biological control of Pythium myriotylum. International Microbiology, 23(2), 179–188. DOI 10.1007/s10123-019-00089-x. [Google Scholar] [CrossRef]
18. Fan, H., Yao, M., Wang, H., Zhao, D., Zhu, X. et al. (2020). Isolation and effect of Trichoderma citrino viride Snef1910 for the biological control of root-knot nematode, Meloidogyne incognita. BMC Microbiology, 20(1), 1–11. DOI 10.1186/s12866-020-01984-4. [Google Scholar] [CrossRef]
19. Ansari, S., Ahmad, G., Elhakem, A., Rizvi, R., Tiyagi, S. A. et al. (2021). Effects of organic and inorganic fertilization with bio-inoculants on the sustainable management of plant-parasitic nematodes infesting okra (Abelmoschus esculentus). Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 49(4), 12544. DOI 10.15835/nbha49412544. [Google Scholar] [CrossRef]
20. El-Nagdi, W. M. A. E., El Fattah, A. I. A. (2011). Controlling root-knot nematode, Meloidogyne incognita infecting sugar beet using some plant residues, a biofertilizer, compost and biocides. Journal of Plant Protection Research, 51(2), 1007–113. DOI 10.2478/v10045-011-0019-7. [Google Scholar] [CrossRef]
21. Abu-Shahba, M. S., Mansour, M. M., Mohamed, H. I., Sofy, M. R. (2021). Comparative cultivation and biochemical analysis of iceberg lettuce grown in sand soil and hydroponics with or without microbubbles and macrobubbles. Journal of Soil Science and Plant Nutrition, 21(1), 389–403. DOI 10.1007/s42729-020-00368-x. [Google Scholar] [CrossRef]
22. Aoudia, H., Ntalli, N., Aissani, N., Yahiaoui-Zaidi, R., Caboni, P. (2012). Nematotoxic phenolic compounds from Melia azedarach against Meloidogyne incognita. Journal of Agricultural and Food Chemistry, 60(47), 11675–11680. DOI 10.1021/jf3038874. [Google Scholar] [CrossRef]
23. Tran, G. (2015). Sugarcane press mud. Feedipedia, a programme by INRAE, CIRAD, AFZ and FAO. https://www.feedipedia.org/node/563. [Google Scholar]
24. Prado, R. D. M., Caione, G., Campos, C. N. S. (2013). Filter cake and vinasse as fertilizers contributing to conservation agriculture. Applied and Environmental Soil Science, 2013, 581984. DOI 10.1155/2013/581984. [Google Scholar] [CrossRef]
25. Jonathan, E. I., Cannayane, I., Samiyappan, R. (2004). Field application of biocontrol agents for the management of spiral nematode, Helicotylenchus multicinctus, in banana. Nematologia Mediterranea, 32, 169–173. [Google Scholar]
26. Hussey, R. S., Barker, K. R. A. (1973). Comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Report, 57, 1025–1028. [Google Scholar]
27. Mukhtar, T., Hussain, M. A., Kayani, M. Z. (2017). Yield responses of 12 okra cultivars to southern root-knot nematode (Meloidogyne incognita). Bragantia, 76, 108–112. DOI 10.1590/1678-4499.005. [Google Scholar] [CrossRef]
28. Danish, M., Seikh, H., Robab, M. I. (2018). Study on morphological and biochemical characteristics of babchi (Psoralea corylifolia) infected with the root-knot nematode, Meloidogyne incognita. Journal of Agricultural Science and Technology, 20(3), 633–645. [Google Scholar]
29. Nishat, Y., Danish, M. H. (2022). Management of root-knot nematode, Meloidogyne incognita in Psoralea corylifolia by using press Mud and Glomus mosseae at different time intervals. Research Journal of Agricultural Sciences, 13(1), 053–058. [Google Scholar]
30. Maclachlan, S., Zalik, S. (1963). Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Canadian Journal of Botany, 41(7), 1053–1062. DOI 10.1139/b63-088. [Google Scholar] [CrossRef]
31. Jaworski, E. G. (1971). Nitrate reductase assay in intact plant tissues. Biochemical and Biophysical Research Communications, 43(6), 1274–1279. DOI 10.1016/S0006-291X(71)80010-4. [Google Scholar] [CrossRef]
32. Dwivedi, R. S., Randhawa, N. S. (1974). Evaluation of a rapid test for the hidden hunger of zinc in plants. Plant and Soil, 40(2), 445–451. DOI 10.1007/BF00011531. [Google Scholar] [CrossRef]
33. Lindner, R. C. (1944). Rapid analytical methods for some of the more common inorganic constituents of the plant tissues. Plant Physiology, 19, 76–89. DOI 10.1104/pp.19.1.76. [Google Scholar] [CrossRef]
34. Chance, B., Maehly, A. C. (1955). Assay of catalases and peroxidases. Methods in Enzymology, 2, 764–775. DOI 10.1002/9780470110171.ch14. [Google Scholar] [CrossRef]
35. Giannopolitis, C. N., Ries, S. K. (1977). Superoxide dismutases: I. Occurrence in higher plants. Plant Physiology, 59(2), 309–314. DOI 10.1104/pp.59.2.309. [Google Scholar] [CrossRef]
36. Asada, K., Takahashi, M. (1987). Production and scavenging of active oxygen in chloroplasts. In: Kyle, D. J., Osmond, C. B., Arntzen, C. J. (Eds.Pjotoinhibition, pp. 227–287. Amsterdam, Nederland: Elsevier. [Google Scholar]
37. Holbrook, C. C., Knauft, D. A., Dickson, D. W. (1983). A technique for screening peanut for resistance to Meloidogyne arenaria. Plant Disease, 57, 957–985. DOI 10.1094/PD-67-957. [Google Scholar] [CrossRef]
38. Taylor, A. Z., Sasser, J. N. (1987). Biology, identification and control of root-knot nematode (Meloidogyne spp.), pp. 111. Raleigh: Department of Plant Pathology; North Carolina State University and United States Agency for International Develpoment. [Google Scholar]
39. Snedecor, G. W., Cochran, W. G. (1980). Statistical methods, 7th edition Ames: Iowa State University Press. [Google Scholar]
40. Renčo, M. (2013). Organic amendments of soil as useful tools of plant parasitic nematodes control. Helminthologia, 50(1), 3–14. DOI 10.2478/s11687-013-0101-y. [Google Scholar] [CrossRef]
41. Rondina, D. J. G., Ymbong, D. V., Cadutdut, M. J. M., Nalasa, J. R. S., Paradero, J. B. et al. (2019). Utilization of a novel activated carbon adsorbent from press mud of sugarcane industry for the optimized removal of methyl orange dye in aqueous solution. Applied Water Science, 9(8), 1–12. DOI 10.1007/s13201-019-1063-0. [Google Scholar] [CrossRef]
42. Gupta, N., Tripathi, S., Balomajumder, C. (2011). Characterization of pressmud: A sugar industry waste. Fuel, 90(1), 389–394. DOI 10.1016/j.fuel.2010.08.021. [Google Scholar] [CrossRef]
43. Rout, P. P., Arulmozhiselvan, K. (2019). Investigating the suitability of pressmud and coir pith for use as soilless substrate by SEM, XRF, UV-VIS and FTIR spectroscopy techniques. Nature, 10, 11. DOI 10.35812/CelluloseChemTechnol.2019.53.59. [Google Scholar] [CrossRef]
44. Kavatagi, P. K., Lakshman, H. C. (2014). Effect of arbuscular mycorrhizal fungi, pressmud and growth regulator on Solanum lycopersicum L. International Journal of Pure and Applied Sciences and Technology, 21(2), 11. [Google Scholar]
45. de Oliveira, T. B., Lopes, V. C. P., Barbosa, F. N., Ferro, M., Meirelles, L. A. et al. (2016). Fungal communities in pressmud composting harbour beneficial and detrimental fungi for human welfare. Microbiology, 162(7), 1147–1156. DOI 10.1099/mic.0.000306. [Google Scholar] [CrossRef]
46. Bokhtiar, S. M., Roksana, S., Moslehuddin, A. Z. M. (2016). Soil fertility and productivity of sugarcane influenced by enriched pressmud compost with chemical fertilizers. SAARC Journal of Agriculture, 13(2), 183–197. DOI 10.3329/sja.v13i2.26579. [Google Scholar] [CrossRef]
47. Partha, N., Sivasubramanian, V. (2006). Recovery of chemicals from pressmud-a sugar industry waste. Indian Chemical Engineer, 48(3), 160–163. [Google Scholar]
48. Shah, R. U., Abid, M., Qayyum, M. F., Ullah, R. (2015). Dynamics of chemical changes through production of various composts/vermicompost such as farm manure and sugar industry wastes. International Journal of Recycling of Organic Waste in Agriculture, 4(1), 39–51. DOI 10.1007/s40093-015-0083-5. [Google Scholar] [CrossRef]
49. Kumar, V., Chopra, A. K. (2012). Fertigation effect of distillery effluent on agronomical practices of Trigonella foenum-graecum L. (Fenugreek). Environmental Monitoring and Assessment, 184(3), 1207–1219. DOI 10.3126/on.v8i1.4312. [Google Scholar] [CrossRef]
50. Sofy, M. R., Mohamed, H. I., Dawood, M. F. A., Abu-Elsaoud, A. M., Soliman, M. H. (2021). Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Archives of Agronomy and Soil Science, 1–22, 1949709. DOI 10.1080/03650340.2021. [Google Scholar] [CrossRef]
51. Golzary, H., Panjehkeh, N., Ahmadzadeh, M., Salari, M., Sedaghati-Khoravi, E. (2011). Elucidating the parasitic capabilities of Trichoderma against Meloidogyne javanica on tomato. Insight Plant Disease, 1(1), 12–19. [Google Scholar]
52. Atia, M. A., Abdeldaym, E. A., Abdelsattar, M., Ibrahim, D. S., Saleh, I. et al. (2020). Piriformospora indica promotes cucumber tolerance against root-knot nematode by modulating photosynthesis and innate responsive genes. Saudi Journal of Biological Sciences, 27(1), 279–287. DOI 10.1016/j.sjbs.2019.09.007. [Google Scholar] [CrossRef]
53. Singh, U. B., Singh, S. H., Malviya, D. E., Chaurasia, R. A., Imran, M. O. et al. (2017). Harnessing biocontrol potential of Trichoderma harzianum for control of Meloidogyne incognita in tomato. Indian Phytopathology, 70, 331–335. DOI 10.24838/ip.2017.v70.i3.74239. [Google Scholar] [CrossRef]
54. Kumar, V., Chopra, A. K. (2016). Effects of sugarcane pressmud on agronomical characteristics of hybrid cultivar of eggplant (Solanum melongena L.) under field conditions. International Journal of Recycling of Organic Waste in Agriculture, 5(2), 149–162. DOI 10.1007/s40093-016-0125-7. [Google Scholar] [CrossRef]
55. Danish, M., Altaf, M., Robab, M. I., Shahid, M., Manoharadas, S. et al. (2021). Green synthesized silver nanoparticles mitigate biotic stress induced by Meloidogyne incognita in Trachyspermum ammi (L.) by improving growth, biochemical, and antioxidant enzyme activities. ACS Omega, 6(17), 11389–11403. DOI 10.1021/acsomega.1c00375. [Google Scholar] [CrossRef]
56. Hermosa, R., Viterbo, A., Chet, I., Monte, E. (2012). Plant-beneficial effects of Trichoderma and of its genes. Microbiology, 158(1), 17–25. DOI 10.1099/mic.0.052274-0. [Google Scholar] [CrossRef]
57. Mohamed, H. I., Elsherbiny, E. A., Abdelhamid, M. T. (2016). Physiological and biochemical responses of Vicia faba plants to foliar application with zinc and iron. Gesunde Pflanzen, 68, 201–212. DOI 10.1007/s10343-016-0378-0. [Google Scholar] [CrossRef]
58. Moustafa-Farag, M., Mohamed, H. I., Mahmoud, A., Elkelish, A., Misra, A. N. et al. (2020). Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants, 9(6), 724. DOI 10.3390/plants9060724. [Google Scholar] [CrossRef]
59. Ashry, N. A., Ghonaim, M. M., Mohamed, H. I., Mogazy, A. M. (2018). Physiological and molecular genetic studies on two elicitors for improving the tolerance of six Egyptian soybean cultivars to cotton leaf worm. Plant Physiology and Biochemistry, 130, 224–234. DOI 10.1016/j.plaphy.2018.07.010. [Google Scholar] [CrossRef]
60. Sofy, A. R., Sofy, M. R., Hmed, A. A., Dawoud, R. A., Refaey, E. E. et al. (2021). Molecular characterization of the alfalfa mosaic virus infecting Solanum melongena in Egypt and the control of its deleterious effects with melatonin and salicylic acid. Plants, 10(3), 459. DOI 10.3390/plants10030459. [Google Scholar] [CrossRef]
61. Wann, S. B., Borah, B., Ahmed, R., Gogoi, B., Phukon, P. et al. (2016). Isolation, characterization of nematode-controlling bacteria and fungi from nature. In: Microbial inoculants in sustainable agricultural productivity, pp. 271–295. New Delhi: Springer. [Google Scholar]
62. Ghonaim, M. M., Mohamed, H. I., Omran, A. A. A. (2021). Evaluation of wheat salt stress tolerance using physiological parameters and retrotransposon-based markers. Genetic Resources and Crop Evolution, 68, 227–242. DOI 10.1007/s10722-020-00981-w. [Google Scholar] [CrossRef]
63. Yan, Y., Mao, Q., Wang, Y., Zhao, J., Fu, Y. et al. (2021). Trichoderma harzianum induces resistance to root-knot nematodes by increasing secondary metabolite synthesis and defense-related enzyme activity in Solanum lycopersicum L. Biological Control, 158, 104609. DOI 10.1016/j.biocontrol.2021.104609. [Google Scholar] [CrossRef]
64. Olabiyi, T. I., Gbadamosi, A. R. (2013). The effect of four compost soil amendments based on Trichoderma harzianum on nematode pests of sesame. International Journal of Agronomy and Plant Production, 4, 3859–3863. [Google Scholar]
65. Moradi, R., Moradi, F., Mirehki, K., Abdollahi, M. (2015). Plant debris of oak forest as soil amendment, to improve the biocontrol activity of Pseudomonas fluorescens and Trichoderma vierns against Meloidogyne javanica, in tomato. Journal of Crop Protection, 4(3), 373–384. [Google Scholar]
66. Jayakumar, J., Chandrasekaran, M., Soundararajan, R. P. (2020). Integrated approaches for the management of sugarcane nematodes. Agri Mirror: Future India, 1(4), 52–55. [Google Scholar]
67. Annapurna, M., Bhagawati, B., Kurulkar, U. (2018). Biochemical mechanism of native fungal bioagents in the management of root-knot nematode Meloidogyne incognita on tomato. International Journal of Current Microbiology and Applied Science, 7(11), 380–395. DOI 10.20546/ijcmas.2018.711.047. [Google Scholar] [CrossRef]
68. D’Errico, G., Mormile, P., Malinconico, M., Bolletti Censi, S., Lanzuise, S. et al. (2021). Trichoderma spp. and a carob (Ceratonia siliqua) galactomannan to control the root-knot nematode Meloidogyne incognita on tomato plants. Canadian Journal of Plant Pathology, 43(2), 267–274. DOI 10.1080/07060661.2020.1801844. [Google Scholar] [CrossRef]
69. Mukhtar, T. (2018). Management of root-knot nematode, Meloidogyne incognita, in tomato with two Trichoderma species. Pakistan Journal of Zoology, 50(4), 1589–1592. DOI 10.17582/journal.pjz/2018.50.4.sc15. [Google Scholar] [CrossRef]
70. Abd El-Khair, H., El-Nagdi, W. M., Youssef, M. M., Abd-Elgawad, M. M., Dawood, M. G. (2019). Protective effect of Bacillus subtilis, B. pumilus, and Pseudomonas fluorescens isolates against root knot nematode Meloidogyne incognita on cowpea. Bulletin National Research Centre, 43(1), 1–7. DOI 10.1186/s42269-019-0108-8. [Google Scholar] [CrossRef]
71. Osman, H. A., Ameen, H. H., Mohamed, M., El-Mohamedy, R., Elkelany, U. S. (2018). Field control of Meloidogyne incognita and root rot disease infecting eggplant using nematicide, fertilizers, and microbial agents. Egyptian Journal of Biological Pest Control, 28(1), 1–6. DOI 10.1186/s41938-018-0044-1. [Google Scholar] [CrossRef]
72. Selim, M. E., Mahdy, M. E., Sorial, M. E., Dababat, A. A., Sikora, R. A. (2014). Biological and chemical dependent systemic resistance and their significance for the control of root-knot nematodes. Nematology, 16(8), 917–927. DOI 10.1163/15685411-00002818. [Google Scholar] [CrossRef]
73. Mohamed, H. I., Mohammed, A. H. M. A., Mohamed, N. M., Ashry, N. A., Zaky, L. M. et al. (2021). Comparative effectiveness of potential elicitors of soybean plant resistance against Spodoptera littoralis and their effects on secondary metabolites and antioxidant defense system. Gesunde Pflanzen, 73, 273–285. DOI 10.1007/s10343-021-00546-6. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |