

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020790
ARTICLE
Assessment of Phytochemical Analysis, Nutritional Composition and Antimicrobial Activity of Moringa oleifera
1Department of Botany, The Women University, Multan, 66000, Pakistan
2Department of Forestry and Range Management, Bahauddin Zakariya University, Multan, 60800, Pakistan
3Department of Biotechnology, College of Science, Taif University, Taif, 21944, Saudi Arabia
4Department of Environmental Sciences, University of California, Riverside, 92521, USA
5Department of Forestry, Range, and Wildlife, Ghazi University, Dera Ghazi Khan, 32200, Pakistan
6Department of Agronomy, Ghazi University, Dera Ghazi Khan, 32200, Pakistan
7Biochemistry Department, Faculty of Science, University of Tabuk, Tabuk, 47512, Saudi Arabia
8Biology Department, Faculty of Science, Taif University, Taif, 21944, Saudi Arabia
9Department of Biology, Faculty of Science, University of Tabuk, Tabuk, 71491, Saudi Arabia
10Department of Agronomy, Faculty of Agriculture, University of Kafrelsheikh, Kafr Elsheikh, 33516, Egypt
*Corresponding Authors: Husnain Farooq. Email: hfaro001@ucr.edu; Ayman EL Sabagh. Email: ayman.elsabagh@agr.kfs.edu.eg
Received: 12 December 2021; Accepted: 10 February 2022
Abstract: Moringa oleifera is a miracle plant rich in nutrients, antioxidants, and antibiotic properties. Present study was designed to evaluate various biochemical attributes of leaves and flowers of M. oleifera. Plant parts (leaves, flowers) of M. oleifera, collected from different roadsides of Multan district, Punjab, Pakistan, were used as experimental material. Result indicates that alkaloids, saponin, carbohydrates, fats, and protein had a high value in the aqueous extract of both leaves and flowers of M. oleifera. Whereas phenol content was high in methanolic leaves extract and the phenol contents were high in aqueous extract of flowers. The extract yield of M. oleifera leaves and flowers both showed a higher percentage in aqueous extract (57.5%), followed by methanol extract and lowest in ethyl acetate extract. Flavonoids contents were higher in ethyl acetate extract of leaves (33.67%) and aqueous extract of flowers (53.71%). While crude fiber was high in methanolic extract of leaves (12.40%) and in flowers crude fiber was high in ethyl acetate extract (15.86%). The moisture contents were higher in leaves (8.87%) than flowers (7.3%) and similarly, ash percentage in flowers (52.60%) than leaves (41.84%). Ethyl acetate extracts of M. oleifera leaves show antibacterial activity against Pseudomonas aeruginosa while methanolic extract of M. oleifera flowers shows antibacterial activity against Xanthomonas sp. Maximum growth inhibits show in all extracts of leaves against Aspergillus flavus, F. oxysporum, and P. glabrum except for the concentrated aqueous extract of leaves. While in flowers maximum growth inhibits all extracts against P. glabrum, A. niger, and A. flavus except the diluted ethyl acetate extract. Phytochemicals present in different parts of moringa have significant edible and commercial potential. Moringa extracts exhibited significant antimicrobial activity, therefore have applications in pharmaceuticals.
Keywords: Antifungal; antioxidants; phytochemical; antibacterial; phenolics; plant extract; medicinal properties
The family Morinaceae has 13 known species, including Moringa oleifera [1], which is locally grown in Afghanistan, sub-Himalayas (India, Pakistan, and Bangladesh), Caribbean island, Southeast Asia, Arabia, South America, and Africa. Moringa was dispersed worldwide to tropic and sub-tropic countries [2,3]. Different countries gave the Moringa tree different names, i.e., English Drumstick plant, Mom’s Finest companion; Horseradish plant; Hindi-Shajmah, Mungaara, Segra, Shajna [4]. M. oleifera is termed as ‘horseradish’ plant, ‘drumstick’ plant ‘ben-oil plant’ or ‘cabbage plant’, ‘mom’s finest companion’ or ‘wonder plant [5,6], and widely grown throughout Pakistan [7]. It is a deciduous, fast-growing, and drought-tolerant tree [8,9].
M. oleifera is multipurpose plant, used as a vegetable and possess medicinal potential against many disorders [10,11]. All parts of M. oleifera, from the root to the leaves, are very useful as a fodder spice, food, natural coagulant, fuel, fertilizer [9,11], domestic cleaning agent, biopesticide [12–14] as well as in fine machine lubricating oil, lumber, textile industry, fencing, enhance growth hormones, charcoal, hair-care products, perfumery, water clarification, etc. [15,16]. Extracts, powder and oil of different parts of Moringa plants are commercially available as dietary supplement [1].
Seven times much higher vitamin C is present in moringa as compared to oranges; vitamin A is ten times more than carrots, similarly, much higher Ca and protein than milk, fifteen % higher K as compared to bananas, and M. oleifera contains 25 times much Fe as compared to spinach [17–19].
In Ayurveda, about 300 diseases are claimed to be cured with the moringa leaves and recent studies support the claim [13]. Several phenolic compounds found in Moringa, especially chlorogenic acid, gallic acid, p-coumaric acid, syringic acid and vanillic acid etc. are source of antimicrobial properties of different plant parts [20]. All parts are used traditionally to treat many diseases such as antispasmodic, antiseptic, wound healing, antidiabetic, antimicrobial, cholesterol-lowering, anti-inflammatory, tuberculosis, antioxidant, and anticonvulsant activities [17,21].
Various studies on nutritional, phytochemical, and antibacterial actions in water, ethyl acetate, or alcohol extracts of plant parts were carried out in the world and found a potential medicinal and nutraceutical plant [19,22]. In our present study, different samples from roadside plants in the arid area were used with the assumption that spatial plant distribution not only changes plant growth potential but also their phytochemical properties. Current study was designed to evaluate biochemical attributes of aqueous, methanol and ethyl acetate extracts of M. oleifera parts (leaves and flowers) to determine its antimicrobial, antifungal, and phytochemical activities.
Leaves and flowers of healthy and uninfected M. oleifera plants were collected from different roadsides of Multan district, Punjab, Pakistan (latitude: 30.181 and longitude: 71.492). The area is hot and dry, with sandy to sandy loam soil. The plant leaves (randomly selected from different positions of plant) were collected during January month and flowers were harvested in February and March. The collected parts were washed with water and air-dried at room temperature for three weeks. Subsequently, air-dried samples were ground with an electric grinder until the sample was in coarse powder form. The powder samples were stored in an airtight container and kept at room temperature for further use. All the analysis described were performed at least in triplicate and average values are described.
2.1 Extraction of Moringa oleifera Samples and Estimation of Moisture and Ash Contents
Air dried sample of M. oleifera leaves and flowers (150 g, of each batch), was separated equally into three conical flasks (each containing 50 g air dried sample) and 100 ml ethyl acetate (100 ml) was added in one conical flask. About 100 ml methanol was added to the second flask while in the third bottle 100 ml distilled water was added. These three conical flask mixtures were kept in an incubator shaker to incubate at 60°C at 150 rpm (revolution per minute) for 24 h. After incubation, the mixture was filtered with filter paper (Whatman No. 1) of each flask. The filtrates were divided into three different beakers and covered with fine-pored aluminum foil. The solvent was allowed to evaporate at 65°C in a hot air oven to collect ethyl acetate, methanol, and distilled water extracts.
The dry extract yield depends on the arid mass and is studied by the subsequent equation:
whereas W1 indicates extract weight after solvent evaporation and W2 is dry plant material.
To determine moisture contents ground dry sample (1 g) was taken in a known weighted beaker and kept in an oven at 105°C for 8 h. After cooling down, the sample was weighted to determine the water loss and moisture content using equation below:
where W1 = Weight of air-dried plant sample and W2 = weight of oven-dried plant sample.
Air-dried plant sample (2 g) was incinerated in a Muffle furnace at 600°C for 6 h. Subsequently, samples were left cool down and re-weighted to calculate the percentage of ash using equation below.
2.2.1 Determination of Crude Alkaloids
Harborne method was used to determined crude alkaloids. A sample of 2.5 g was taken in a 250 mL beaker added with 90 ml of ethanol and 10 ml of acetic acid for 4 h after wrapping. After that, the sample was filtered and placed on a water bath to one-fourth of its original volume. Ammonium hydroxide in concentrated form was added dropwise in extract till reached precipitation and allowed to settle down. Precipitations were collected and again filtered after washing with dilute ammonium hydroxide and dried to measure alkaloids [9,23].
where, W1 = Weight of filter paper, W2 = Weight of sample; Wo = weight of the dry flask.
2.2.2 Determination of Saponins
Obadoni and Ochuko method was used to Saponins contents were determined following procedure already described [24]. Briefly, each plant sample (5 g dry powder) was mixed 50 ml of 20% of aqueous ethanol in a conical flask and heated with continuous stirring on a water bath for 4 h at 55°C. Then filtrated and the residue was again re-extracted through 20% of 50 ml ethanol. Again, this mixture was reheated on a water bath at 90°C for reducing the mixture to 10 ml. This mixture was shifted in a separating funnel of 250 ml capacity and added 20 ml diethyl ether. The aqueous layer was retrieved by shaking vigorously. After being thrown away ether layer formed, and the purification processes were repeated. Further, added n-butanol (15 ml) and washed with 10 ml of sodium chloride (5% aqueous sol.) twice. After washing, the sample was heated on a water bath, dried in an oven to calculate saponin as a percentage [24].
2.2.3 Determination of Total Phenolics
A sample extract of 100 μL was taken and mingled with Folin Ciocalteu’s reagent (250 μl) for 5 min at room temperature. Then, 1.5 ml of 20% sodium bicarbonate was added to the extract and incubated for 2 h at room temperature. With a spectrophotometer (Cintra 1010, GBC Scientific Equipment, Melbourne, Australia), the absorbance at 765 nm was measured. By using various gallic acid concentrations, a standard curve was constructed to calculate total phenolic contents (TPC) as equivalents of gallic acid measured in µg/mg of dried extracts [25].
2.3 Determination of Total Flavonoids
Distilled water (4.5 ml) and (NaNO2 (0.03 ml) were added to 250 μl sample extract, and 10% of 0.03 ml of AlCl3 was added and mixture was kept for five minutes at 25°C. After 5 min, that mixture was further treated with 2 ml of 1 M NaOH. 10 ml of distilled water were included to dilute the mixture, and absorbance was calculated at 510 nm. The results were shown as catechin equals (CE) μg/mg of dried extract of leaves or flowers [24].
2.4 Determination of Total Carbohydrate
Association of Official Analytical Chemists (AOAC) Method was used to determine total carbohydrates [26]. Briefly, dried powder of leaf tissues (0.2 g) were homogenized in ethanol extract (5 ml) at 80°C (15 min). Ethanol evaporation was allowed under vacuum at 70°C, subsequently extract was mixed with chloroform and centrifuged (5 min). The absorption was recorded at 630 nm.
2.5 Determination of Crude Fiber
Petroleum ether was added in 2 g of dried sample for removal of fat. Subsequently, the sample was boiled with H2SO4 for 30 min, then filtrated and washed with water. The sample was again boiled with 200 ml sodium hydroxide for 20 min. The sample was filtered and again washed with water and 25 ml ethanol. Residues (W1) were transferred to an ashing dish for drying filtrate for 2 h on 130°C cool (W2), again heated for 30 min at 600°C and cooled, weighted W3 [26].
2.6 Determination of Total Fat
Total fat in the dried samples was extracted with petroleum ether (5–6 h) in the Soxhlet extractor apparatus (Model: H-2 1045, Extraction Unit, Sweden) [26]. Subsequently, the extract was poured into the petri plate and was dried to a constant weight. The fat contents (percent) of the sample were calculated using equation
For determination of protein contents plant dry sample (1 g) was digested using concentrated sulfuric acid and digestion mixture in Kjeldahl apparatus (Model D-40599, Behr Labor Technik, Gmbh-Germany) [26]. The mixture was heated and boiled. After that, this clear or colorless sample was diluted to a volume of 250 ml. Methyl red, indicator, was mixed with the solution and titrated with H2SO4 (0.1 N) to determine nitrogen contents and protein (%) was attained (N% × 6.25).
The Chapman and Pratt (1961) method was used for N digestion, distillation, and quantification. Five grams of dry Moringa leaves and flower were ground, passing through a 2 mm sieve, and digested in sulfuric acid in the presence of a mixture of K2SO4, CuSO4, FeSO4 (10:05:01) using a micro Kjeldahl apparatus to determine N content. Crude protein was calculated by multiplying N content by the factor 6.25.
2.8 Determination of Antimicrobial Activity
Yeast extract (1.25 g), tryptone and sodium chloride (2.5 g) were added in 250 ml of distilled water and maintained the medium pH to 7.2. After maintaining the pH, 3.75 g agar was added in distilled water with continuous stirring and heating until a clear solution was obtained. Now the medium was sterilized in autoclave at 120°C for 20 min. After cooling, the media was poured into Petri plates under sterile conditions or near the flame and kept the plates at room temperature to become hardened. For preparing the bacterial inoculum, 0.25 g of yeast extract, 0.5 g of tryptone, and sodium chloride were dissolved in 50 ml of distilled water. This solution was transferred to 1/3rd in each test tubes and inoculated each test tube with 50 µl of old bacterial culture broth and closed the test tubes. After that, the test tubes were placed on a shaker at 200 rpm, 37°C for 12 h. Antibacterial activity was assessed with the disc diffusion method. Then plates were placed in an incubator for 8–12 h at 37°C and observed the growth inhibition, following the procedure described earlier [27].
Malt extract medium was used to determine antifungal activity. For media preparation, 1.6–1.7% malt extract, 2% agar was taken and NaOH was used to maintain the pH of the medium 5.5. Then media was kept in an autoclave at 0.1 c, 121°C for 20 minutes to sterile it. After cooled down the medium was poured into Petri plates. 50 μl of prepared solution of antibiotics amphotericin B (1%w/v) was added in the medium before pouring into Petri plates to avoid contaminants. For inoculation of the fungal species, the fungal spores were transferred in the middle of the Petri plates with the help of a loop and sealed the plates with parafilm, and kept for 3 days in an incubator at 27°C. After the formation of spores, the pure fungal culture was placed at 4°C. The antifungal activity of the plant extracts was analyzed via the disc diffusion method. Four discs were placed in the plates and after pouring the samples and fungicide and sterile distilled water in the discs the fungal spores were inoculated in the center of the Petri plates. Then plates were incubated at 27°C for 48–72 h [28].
All measurements were performed in triplicate; treatment means, and standard deviations were calculated using Microsoft Excel. Treatment means were statistically analyzed by ANOVA and Duncan’s multiple range tests to identify significant differences among treatment means (P < 0.05) (SAS Institute, 1988).
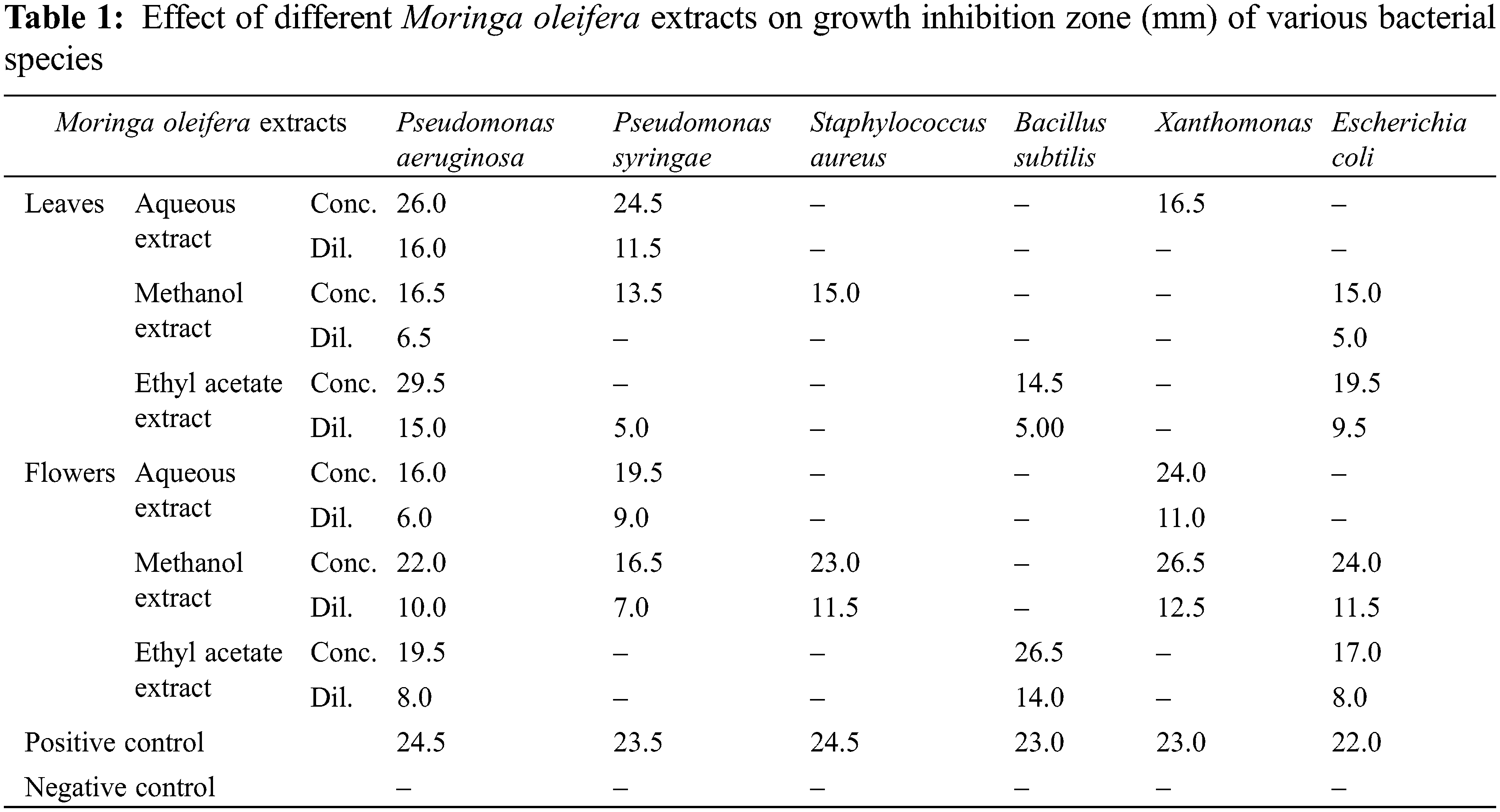
The antimicrobial activity of the aqueous, methanol, and ethyl acetate extracts of M. oleifera leaves and flowers is presented in Table 1. The maximum zone of inhibition measured was found to be observed in Pseudomonas aeruginosa as 29.5 mm in a concentrated sample of ethyl acetate extract of moringa leaves. Whereas aqueous extract of moringa leaves also showed positive potential against P. aeruginosa (concentrated: 26 mm and diluted 16 mm), Pseudomonas syringae (concentrated: 24.5 and diluted 11.5 mm), and Xanthomonas (16.5 mm). Methanol extract of moringa leaves also showed notable activity against P. aeruginosa (16.5 mm), P. syringae (13.5 mm), Staphylococcus aureus (15 mm), and E. coli (15 mm). On the other hand, Methanol extract of moringa flowers possess strong antimicrobial potential against P. aeruginosa (concentrated: 22 mm and diluted 10 mm), P. syringae (concentrated: 16.5 mm and diluted 7 mm), S. aureus (concentrated: 23 mm and diluted 11.5 mm), Xanthomonas (concentrated: 26.5 mm and diluted 12.5 mm) and E. coli (concentrated: 24 mm and diluted 11.5 mm). Ethyl acetate extract inhibited the growth of P. aeruginosa (concentrated: 19.5 mm and diluted 8 mm), Bacillus subtilis (concentrated: 26.5 mm and diluted 14 mm), and E. coli (concentrated: 17 mm and diluted 8 mm).
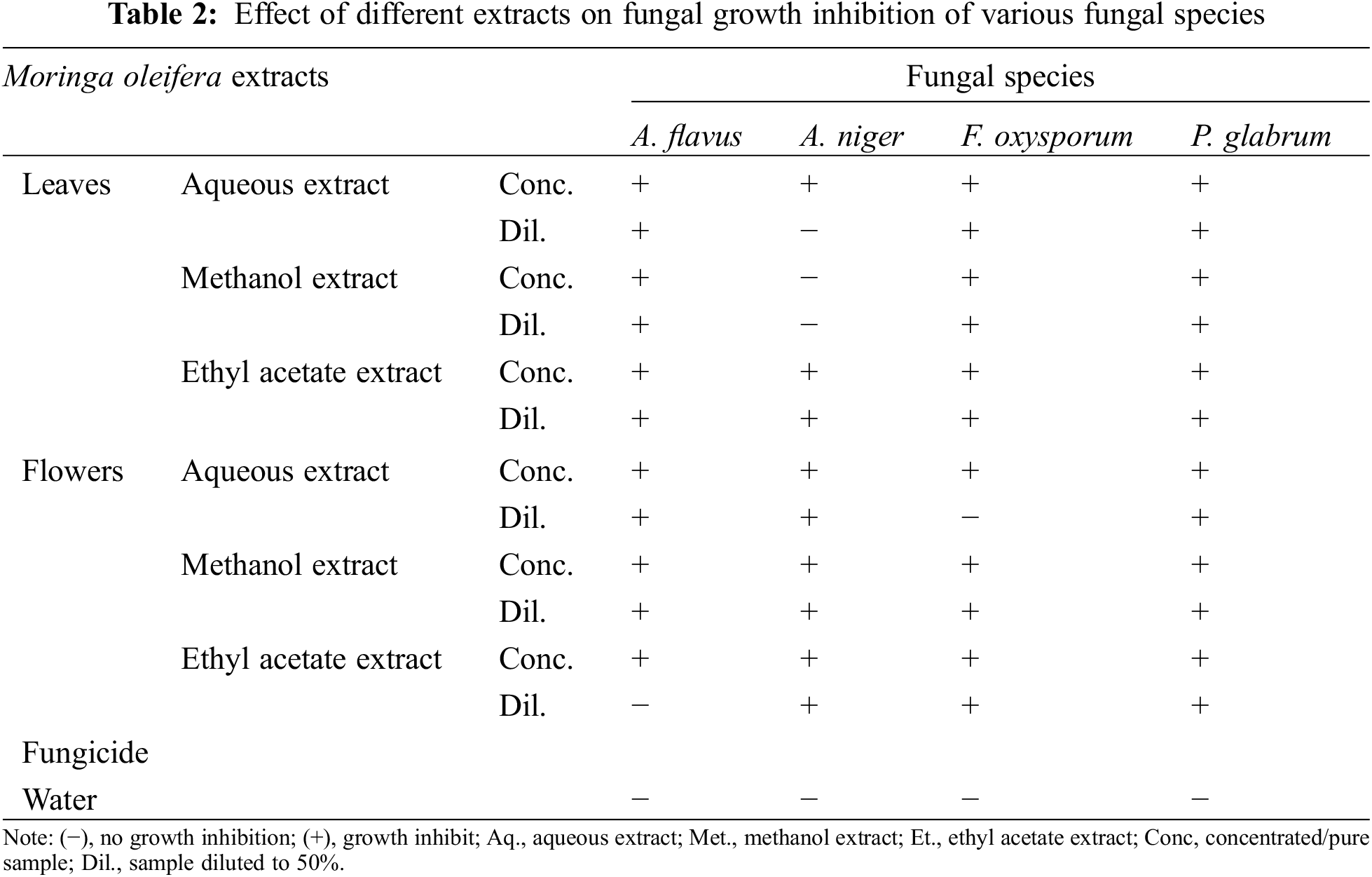
The antifungal activity of M. oleifera leaves and flowers extracts (aqueous, methanol, and ethyl acetate) is presented in Table 2. The results indicated significant antifungal activities of M. olifera leaves and flowers. Maximum growth inhibits show in all extracts of moringa leaves against Aspergillus flavus, F. oxysporum and P. glabrum except for the concentrated aqueous extract of moringa leaves. While in moringa flowers maximum growth inhibits in all extracts against P. glabrum, A. niger, and A. flavus except the diluted ethyl acetate extract.
In our present study, research work has done on aqueous, methanol and ethyl acetate extract of M. oleifera leaves and flowers (Fig. 1) which was not previously studied. Maximum extract yield was obtained using aquous extraction method, however, lowest extract yield was obtained from ethyl acetate extraction. In the present experiment, two parts of M. oleifera, i.e., leaves and flowers showed slight variation in moisture percentage. Similar moisture percentages in various parts of M. oleifera were reported earlier, except flower [29].
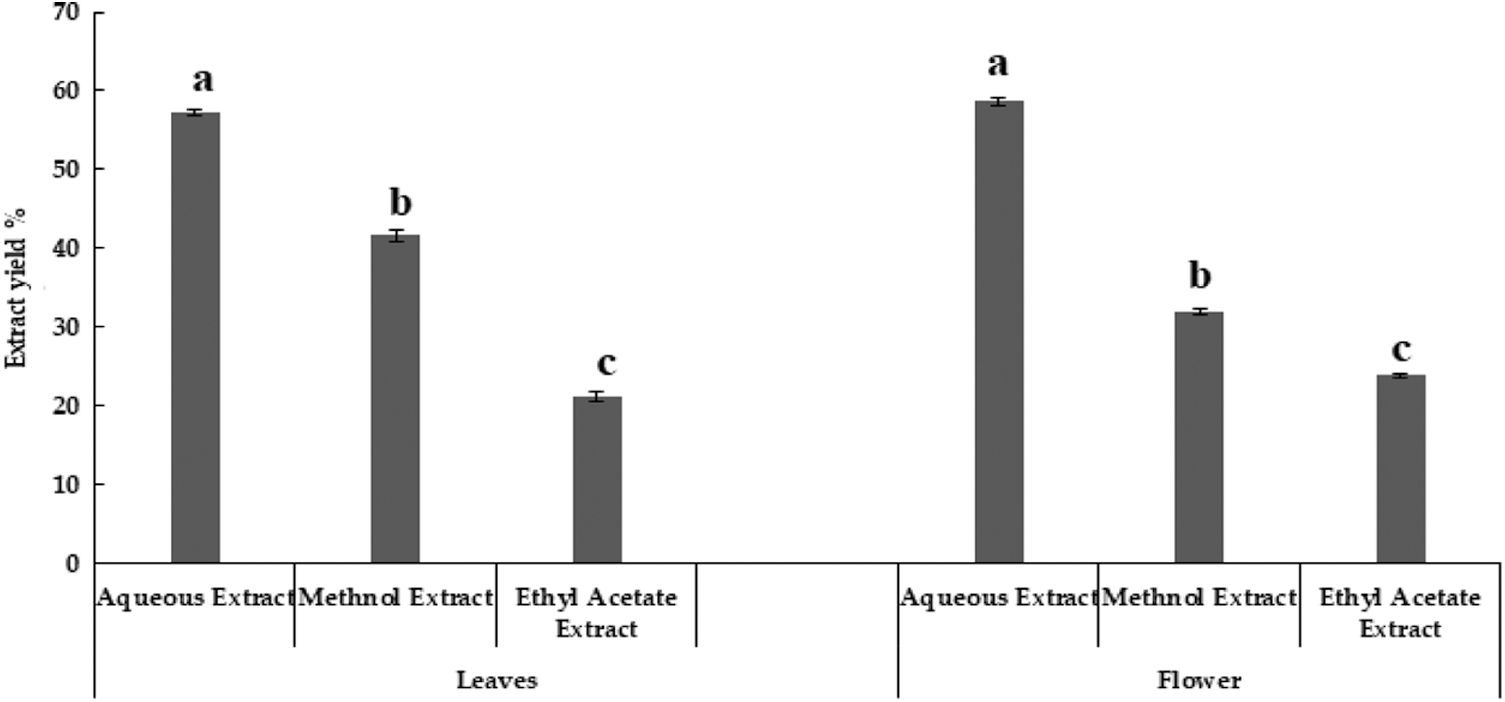
Figure 1: Extract yield of Moringa oleifera leaves and flowers
In the current research, M. oleifera leaves showed slightly higher moisture contents as compared to flowers (Fig. 2). Similar moisture contents in the leaves of Moringa oleifera were reported earlier [30]. The percentage of ash in flowers was higher as compared to leaves of M. oleifera (Fig. 2) Ash contents in leaves of M. oleifera was 35%. Moringa methanolic extracts of leaves have the highest value of phenol [29]. The sequence of phenolic contents in different extracts of M. oleifera leaves was Methanol > ethyl acetate > aqueous, while in flower extract, the sequence of phenolic content was aqueous > methanolic > ethyl acetate (Table 3).
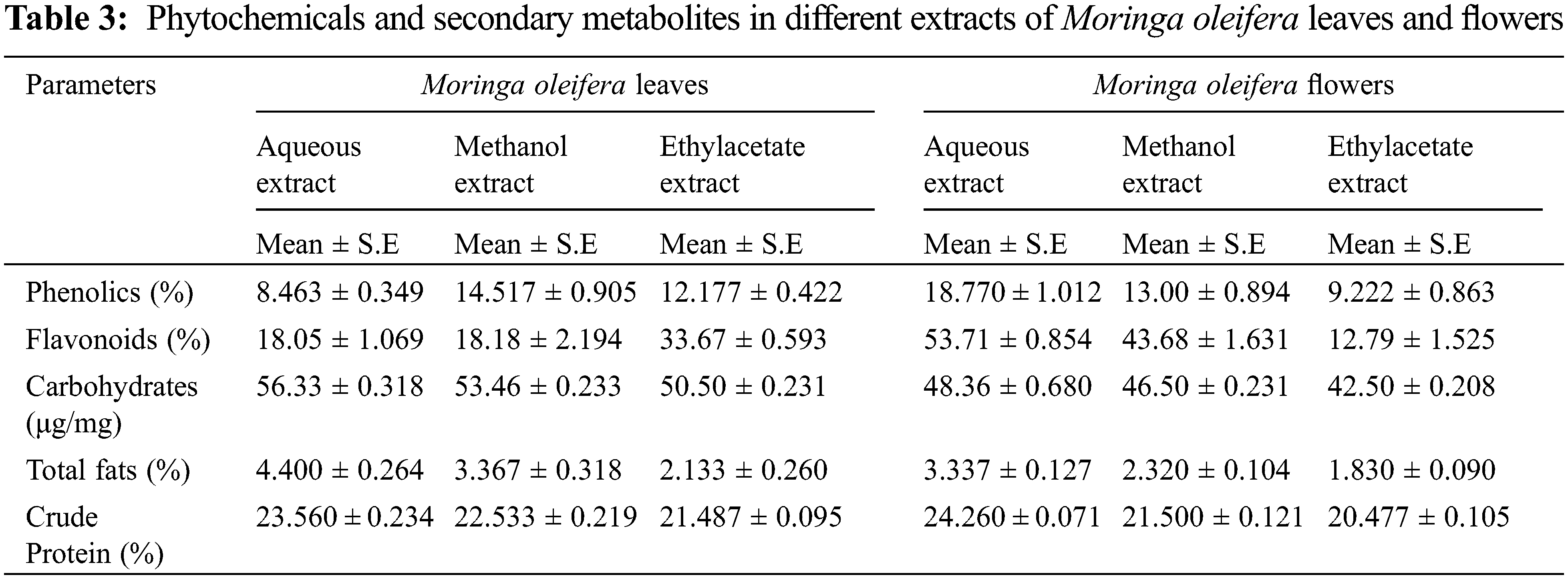
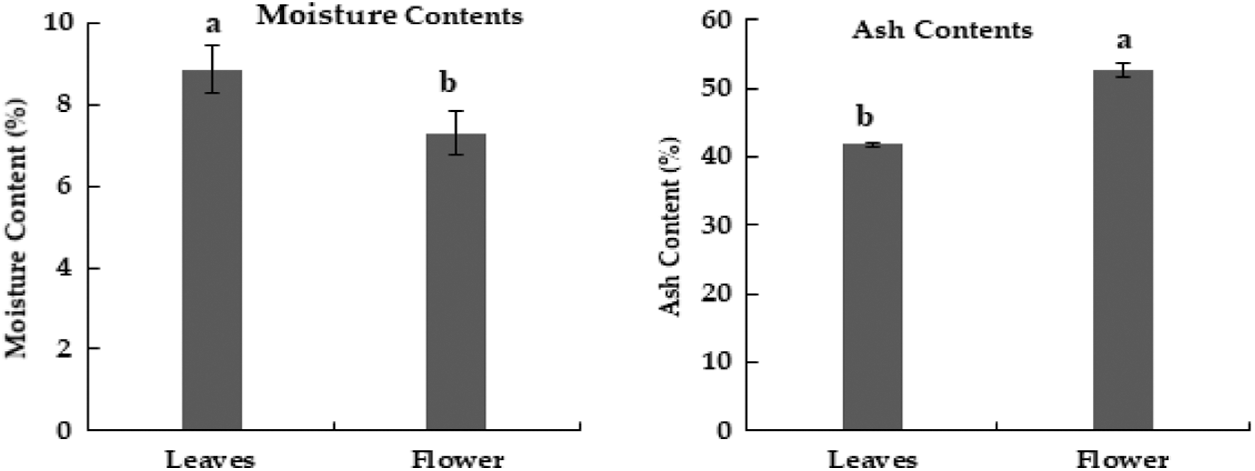
Figure 2: Moisture and ash contents (%) in dried powder of Moringa plant parts
Phenolic content in the leaf of moringa was reported to be higher than in seeds and flowers [31]. Phenolic compounds of M. oleifera leaves and seeds show different results in different extracts [15]. The result shows that M. oleifera methanolic and aqueous extract of leaves had a high value of phenol content than the petroleum benzene and chloroform. The current study revealed that flavonoid content was higher in ethyl acetate extract of leaves of M. oleifera while flowers showed that aqueous extract had a high value of total flavonoids content (Table 1) Maximum value of total flavonoid contents were observed in chloroform extract of M. oleifera [15]. Flower extract of moringa had the highest total phenol content in methanol, however, lower in chloroform extract. While it shows the highest total flavonoid content in aqueous extract of flower [32]. Moreover, the ethanolic extract of M. oleifera leaves contains flavonoids but it was less than the methanolic extract of seed [33].
Various M. oleifera extracts revealed that alkaloids values were high in aqueous extract and lowest in ethyl acetate extract. Likewise, moringa flower extracts were also showed that aqueous extract had a higher value of alkaloids as compared to methanolic and ethyl acetate extracts (Fig. 4). Similarly, M. oleifera leaves had a high value of alkaloids in aqueous extract than other extracts [34]. Methanolic extract of moringa leaves contains higher alkaloids than the hexane extract [33].
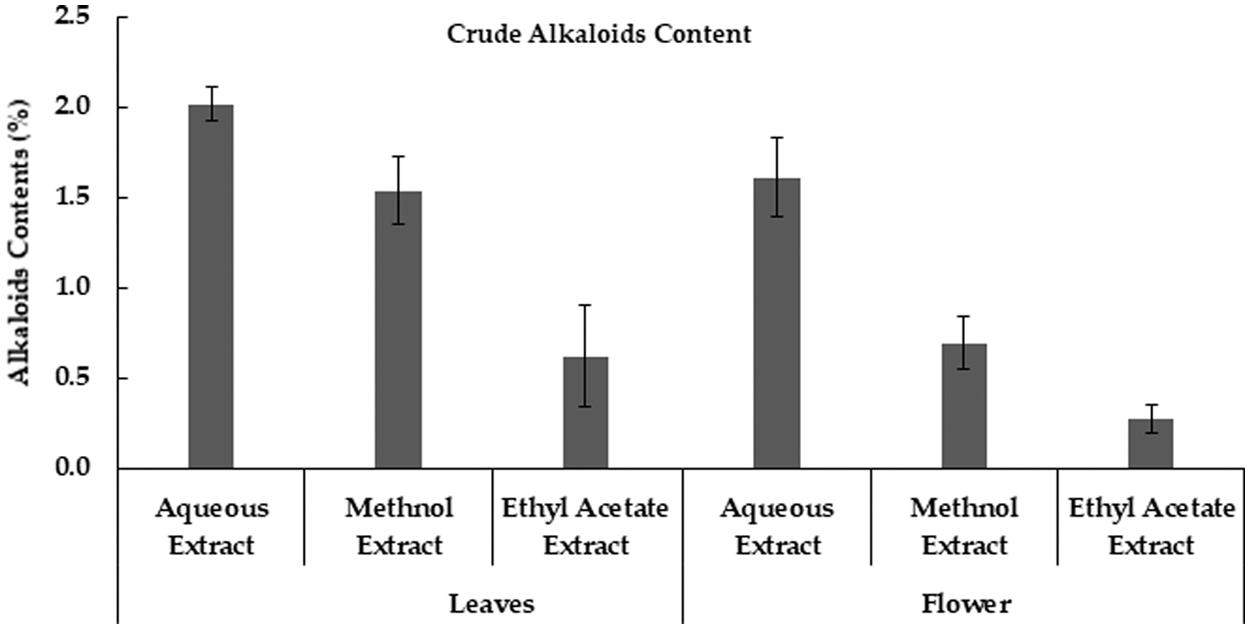
Figure 4: Crude Alkaloids contents in Moringa plant parts obtained using different extraction methods
In current data, aqueous extract of M. oleifera leaves had a high value of saponin content, and low saponin contents were found in ethyl acetate extract. Likewise, the aqueous extract of the moringa flower also has high saponin content as compared to other extracts (Fig. 5). Although saponin was reported to be absent in the aqueous extract of moringa leaves [35], however, another study indicated that saponin was high in methanolic extract of moringa leaves than methanolic extract of seeds [33]. Carbohydrate was maximum in leaves aqueous extracts and the minimum value was noted in ethyl acetate extracts. In different flower extracts of M. oleifera, carbohydrate contents were high in aqueous extract and low carbohydrate contents were found in ethyl acetate extract (Table 1) Carbohydrates contents in moringa leaves varied from 55.97% [36] to 41.2 mg g–1 in dry leaf powder [22].
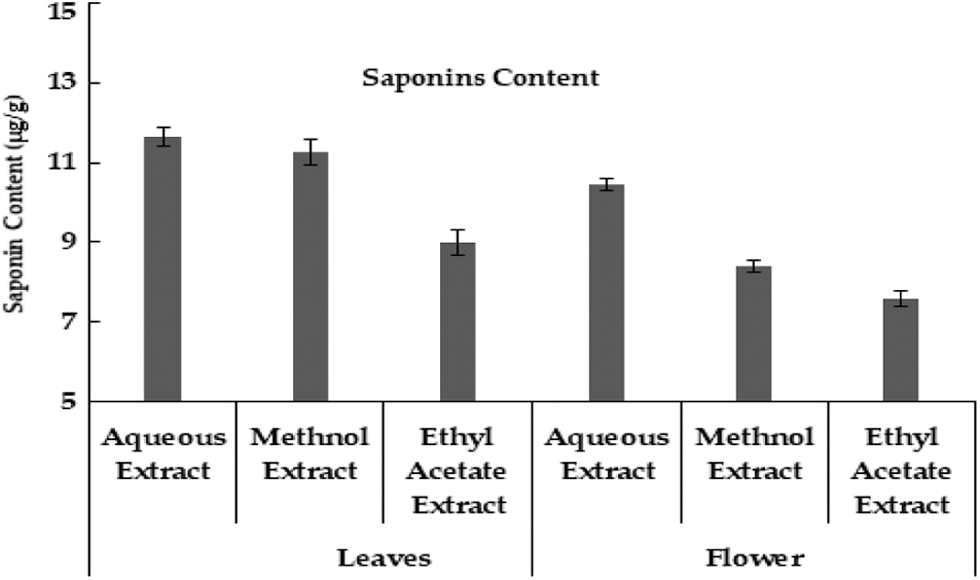
Figure 5: Saponin contents (μg/g) in Moringa plant parts obtained uring different extraction methods
Recent research depicted that different extracts of M. oleifera leaves and flowers had different protein content. Aqueous extract of moringa leaves had high protein content while ethyl acetate extract contains low protein content. Whereas in the Moringa flower, the aqueous extract had high protein content while ethyl acetate extract had low protein content (Table 1). Moringa leaf reported to contains protein, however, is lower than seed. Very limited information is available regarding protein contents in the different extracts of leaves and flowers of M. oleifera [37]. Moreover, protein contains in moringa leaves was 6.8% in dry leaf powder samples [22]. The crude fiber contents in aqueous, methanol and ethyl acetate extract in moringa leaves and flowers were also studied. According to the result, ethyl acetate extract had a high value of crude fiber content and the least in methanol extract of moringa leaves. However, in moringa flowers high content of crude fiber in ethyl acetate extract, followed by methanol extract and lowest in the aqueous extract (Fig. 3). The aqueous extract of M. oleifera leaves has the highest fat content and is low in ethyl acetate extract. Whereas in moringa flower high-fat content in aqueous extract and low in ethyl acetate extract (Table 1). Although, the presence of significant fat contents in moringa leaves is reported [36].
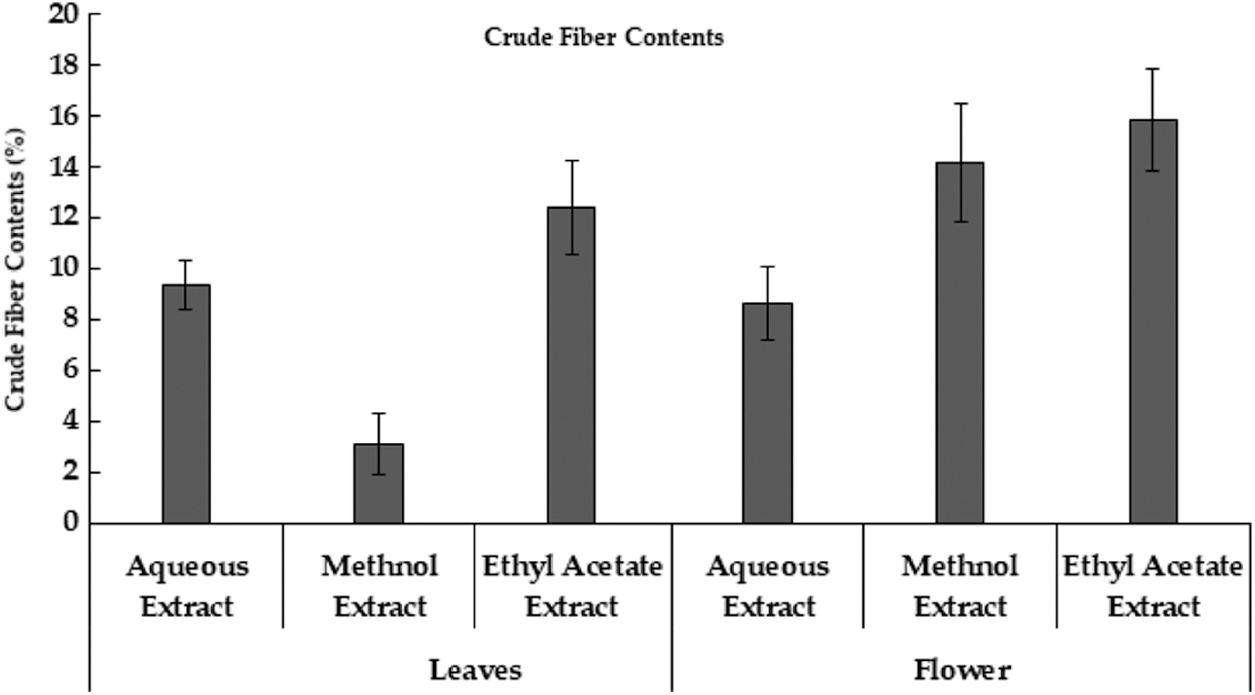
Figure 3: Crude Fiber Contents extracted from Moringa plant parts using different extraction methods
Antibacterial activity of the plant extracts was studied against six different bacterial species P. aeruginosa, P. syringae, B. subtillis, S. aureus, Xanthomonas, and E. coli and compared the results with Amoxycillin. The maximum zone of inhibition measured was found to be observed in P. aeruginosa as 29.5 mm in the concentrated sample of ethyl acetate extract of moringa leaves. The maximum antibacterial activity of methanolic extract of moringa leaves against S. aureus was 23.6 mm [38]. Whereas maximum growth inhibition was found up to 25.5 mm and 20 mm for methanol extracts of moringa leaves and seeds respectively against E. coli, Pseudomonas aeruginosa, Shigella dysenteriae, and Shigella Flexneri [33]. Ethyl acetate extracts of the moringa leaf also showed no inhibition activity against S. aureus, Pseudomonas sp., Klebsiella sp., and E. coli but alcohol extract of leaves produced a little zone of inhibition as 7 to 8 mm against S. aureus [22]. Water, methanol, ethanol, and petroleum ether extracts of M. oleifera significantly vary in different plant parts, i.e., leaves (28 mm), flowers (23 mm), seeds (18 mm), and pulp (15 mm) [39]. All extract of moringa parts showed high antibacterial activity against E. coli compared to S. aureus. Eethanolic extract of moringa leaf indicated a maximum zone of inhibition against E. coli as 22 mm and S. aureus as 25 mm [35].
Antifungal activity of extracts of moringa flowers and leaves was analyzed against four fungal pathogens including A. flavus, A. niger, F. oxysporum, and P. glabrum. moringa leaves extracted in different solvents showed antifungal activity against A. niger, A. fumigatus, and Candida albicans [40]. Generally higher concentration of phytochemicals in the plant extract resulted in better control of fungal species, except A. niger, showing no effect of methanol extract of leaves. Moreover, diluted aqueous extracts [aqueous leaves extract for A. niger; aqueous leaves extract for F. oxysporum); ethanolic flower extract for A. flavus) can be attributed to the lower concentration of certain phytochemicals.
The results indicated significant activity of methanol extract as compared to other extracts including ethanol, ethyl acetate, water, and acetone in A. niger. Aqueous and methanolic extract of leaves inhibited the growth of A. flavus [38]. Ethyl acetate extract of moringa leaves found to be an effective antifungal agent against A. flavus and Trichoderma sp. [39]. The aqueous and ethanol extracts of moringa leaves possess stronger antifungal potential against Saccharomyces cerevisiae, Candida albicans, and Candida tropicalis [40]. Antifungal activity of phytoextracts can be attributed presence of flavonoids [40].
Moringa oleifera possess significant medicinal importance with appreciable quantities of various phytochemicals including phenols, flavonoids, alkaloids and saponin. The extract yield of its leaves and flowers shows a high percentage in aqueous extracts. The moisture content was high in M. oleifera leaves. Variation in the presence of Ash contents, fiber contents, other phytochemicals, and secondary metabolites in both parts of M. oleifera demonstrate promising results. Moringa extracts (leaves and flower) showed appreciable antifungal and antimicrobial activities. Suitable extraction material can increase the concentration of various phytochemicals leading to higher pharmaceutical value.
Acknowledgement: The authors extend their appreciation to Taif University for funding current work by Taif University Researchers Supporting Project No. (TURSP-2020/139), Taif University, Taif, Saudi Arabia.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Leone, A., Spada, A., Battezzati, A., Schiraldi, A., Arstil, J. et al. (2016). Moringa oleifera seeds and oil: Characteristics and uses for human health. International Journal of Molecular Sciences, 17(12), 2141. DOI 10.3390/ijms17122141. [Google Scholar] [CrossRef]
2. Sravanthi, J., Rao, S. G. (2014). Antioxidative studies in Moringa oleifera Lam. Annals of Phytomedicine, 3, 101–105. [Google Scholar]
3. Bhattacharya, A., Tiwari, P., Sahu, P. K., Kumar, S. A. (2018). Review of the phytochemical and pharmacological characteristics of Moringa oleifera. Journal of Pharmaceutical and Bioallied Sciencess, 10(4), 181–191. DOI 10.4103/jpbs.jpbs_126_18. [Google Scholar] [CrossRef]
4. Sawati, A. K., Kumari, C., Ali, A., Garg, P., Thakur, P. et al. (2018). Moringa oleifera-A never die tree: An overview. Asian Journal of Pharmaceutical and Clinical Research, 11(12), 57–65. DOI 10.22159/ajpcr.2018.v11i12.28049. [Google Scholar] [CrossRef]
5. Nwidu, L. L., Elmorsy, E., Aprioku, J. S., Siminialayi, I., Carter, W. G. (2018). In vitro anti-cholinesterase and antioxidant activity of extracts of Moringa oleifera plants from Rivers State, Niger Delta, Nigeria. Medicines, 5(3), 71. DOI 10.3390/medicines5030071. [Google Scholar] [CrossRef]
6. Okorocha, A., Folawiyo, M., Omabe, M., Omabe, K., Uzor, S. et al. (2015). The wonder plant: Moringa oleifera. Journal of Environmental Science, Toxicology and Food Technology, 9(10), 39–47. DOI 10.9790/2402-091023947. [Google Scholar] [CrossRef]
7. Faisal, M. I., Iqbal, S., Basra, S. M. A., Afzal, I., Saddiq, M. S. et al. (2020). Moringa landraces of Pakistan are potential source of premium quality oil. South African Journal of Botany, 129, 397–403. DOI 10.1016/j.sajb.2019.10.002. [Google Scholar] [CrossRef]
8. Devkota, S., Bhusal, K. K. (2020). Moringa oleifera: A miracle multipurpose tree for agroforestry and climate change mitigation from the Himalayas—A review. Cogent Food and Agriculture, 6(1), 1805951. DOI 10.1080/23311932.2020.1805951. [Google Scholar] [CrossRef]
9. Ajantha, A., Kathirvelan, C., Purushothaman, M., Visha, P. (2018). Study on nutrients, mineral and vitamin profile of Moringa oleifera leaf meal. International Journal of Current Microbiolical Applied Sciences, 7(5), 2478–2481. [Google Scholar]
10. Dhakad, A. K., Ikram, M., Sharma, S., Khan, S., Pandey, V. V. et al. (2019). Biological, nutritional, and therapeutic significance of Moringa oleifera Lam. Phytotherapy Research, 33(11), 2870–2903. DOI 10.1002/ptr.6475. [Google Scholar] [CrossRef]
11. Xiao, X., Wang, J., Meng, C., Liang, W., Wang, T. et al. (2020). Moringa oleifera Lam and its therapeutic effects in immune disorders. Frontiers in Pharmacology, 11, 1. DOI 10.3389/fphar.2020.566783. [Google Scholar] [CrossRef]
12. Velázquez-Zavala, M., Peón-Escalante, I. E., Zepeda-Bautista, R., Jiménez-Arellanes, M. A. (2016). Moringa (Moringa oleifera Lam.Potential uses in agriculture, industry and medicine. Revista Chapingo. Serie Horticultura, 22(2), 95–116. [Google Scholar]
13. Kola-Oladiji, K., Fatoki, A., Tewogbade, S., Ojo, O., Ayomide, A. (2014). Consumption pattern and indigenous knowledge of Moringa oleifera among dwellers of rural enclaves around Ibadan Metropolis, Oyo State, Nigeria. Journal of Biology, Agriculture and Healthcare, 4, 2224–3208. [Google Scholar]
14. Neergheen-Bhujun, V. S., Ruhomally, Z. B., Dunneram, Y., Boojhawon, R., Chan Sun, M. (2020). Consumption patterns, determinants and barriers of the underutilised Moringa oleifera Lam in Mauritius. South African Journal of Botany, 129, 91–99. DOI 10.1016/j.sajb.2019.01.027. [Google Scholar] [CrossRef]
15. Vyas, S., Kachhwaha, S., Kothari, S. (2015). Comparative analysis of phenolic contents and total antioxidant capacity of Moringa oleifera Lam. Pharmacognosy Journal, 7(1), 44–51. DOI 10.5530/pj.2015.7.5. [Google Scholar] [CrossRef]
16. Sahay, S., Yadav, U., Srinivasamurthy, S. (2017). Potential of Moringa oleifera as a functional food ingredient: A review. International Journal of Food Science and Nutrition, 2, 31–37. [Google Scholar]
17. Liu, Y., Wang, X. Y., Wei, X. M., Gao, Z. T., Han, J. P. (2018). Values, properties and utility of different parts of Moringa oleifera: An overview. Chinese Herbal Medicines, 10(4), 371–378. DOI 10.1016/j.chmed.2018.09.002. [Google Scholar] [CrossRef]
18. Falowo, A. B., Mukumbo, F. E., Idamokoro, E. M., Lorenzo, J. M., Afolayan, A. J. et al. (2018). Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: A review. Food Research International, 106(4), 317–334. DOI 10.1016/j.foodres.2017.12.079. [Google Scholar] [CrossRef]
19. Gopalakrishnan, L., Doriya, K., Kumar, D. S. (2016). Moringa oleifera: A review on nutritive importance and its medicinal application. Food Science and Human Wellness, 5(2), 49–56. DOI 10.1016/j.fshw.2016.04.001. [Google Scholar] [CrossRef]
20. Prabakaran, M., Kim, S. H., Sasireka, A., Chandrasekaran, M., Chung, I. M. (2018). Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera. Food Bioscience, 26, 23–29. DOI 10.1016/j.fbio.2018.09.003. [Google Scholar] [CrossRef]
21. Tshabalala, T., Ndhlala, A. R., Ncube, B., Abdelgadir, H. A., van Staden, J. (2020). Potential substitution of the root with the leaf in the use of Moringa oleifera for antimicrobial, antidiabetic and antioxidant properties. South African Journal of Botany, 129, 106–112. DOI 10.1016/j.sajb.2019.01.029. [Google Scholar] [CrossRef]
22. Mensah, J., Ikhajiagbe, B., Edema, N., Emokhor, J. (2012). Phytochemical, nutritional and antibacterial properties of dried leaf powder of Moringa oleifera (Lam.) from Edo Central Province, Nigeria. Journal of Natural Products and Plant Resources, 2(1), 107–112. [Google Scholar]
23. Harborne, A. (1998). Phytochemical methods a guide to modern techniques of plant analysis. New Delhi: Springer Science & Business Media. [Google Scholar]
24. Obadoni, B., Ochuko, P. (2002). Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta States of Nigeria. Global Journal of Pure and Applied Sciences, 8(2), 203–208. DOI 10.4314/gjpas.v8i2.16033. [Google Scholar] [CrossRef]
25. McDonald, S., Prenzler, P. D., Antolovich, M., Robards, K. (2001). Phenolic content and antioxidant activity of olive extracts. Food Chemistry, 73(1), 73–84. DOI 10.1016/S0308-8146(00)00288-0. [Google Scholar] [CrossRef]
26. AOAC (1995). Official method of analysis of the Association of Official Analytical Chemist’s 15th edition. Washington, USA: Association of Official Analytical Chemists. [Google Scholar]
27. Balouiri, M., Sadiki, M., Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis, 6(2), 71–79. DOI 10.1016/j.jpha.2015.11.005. [Google Scholar] [CrossRef]
28. Hammer, K. A., Carson, C. F., Riley, T. V. (1999). Antimicrobial activity of essential oils and other plant extracts. Journal of Applied Microbiology, 86(6), 985–990. DOI 10.1046/j.1365-2672.1999.00780.x. [Google Scholar] [CrossRef]
29. Verma, K. S., Rajni, N. (2014). Nutritional assessment of different parts of Moringa oleifera Lamm collected from Central India. Journal of Natural Product and Plant Resources, 4, 81–86. [Google Scholar]
30. Juliani, H. R., Fonseca, Y., Acquaye, D., Malumo, H., Malainy, D. et al. (2009). Nutritional assessment of moringa (Moringa spp.) from Ghana, Senegal and Zambia. In: Juliani, H. R., Ho, C. T. (Eds.African natural plant products: New discoveries and challenges in chemistry and quality, pp. 469–484. Washington, DC: American Chemical Society Publications. [Google Scholar]
31. Nascimento, K. D. O., Reis, I. P., Augusta, I. M. (2017). Total phenolic and antioxidant capacity of flower, leaf and seed of Moringa oleifera. Nutrition Research, 1, 1. [Google Scholar]
32. Gull, I., Javed, A., Aslam, M. S., Mushtaq, R., Athar, M. A. (2016). Use of Moringa oleifera flower pod extract as natural preservative and development of SCAR marker for its DNA based identification. BioMed Research International, 2016(2), 1–12. DOI 10.1155/2016/7584318. [Google Scholar] [CrossRef]
33. Akinyeye, A., Solanke, E., Adebiyi, I. (2014). Phytochemical and antimicrobial evaluation of leaf and seed of Moringa oleifera extracts. International Journal of Research in Medicine and Health Sciences, 4(6), 2307–2083. [Google Scholar]
34. Nweze, N. O., Nwafor, F. I. (2014). Phytochemical, proximate and mineral composition of leaf extracts of Moringa oleifera Lam. from Nsukka, South-Eastern Nigeria. IOSR Journal of Pharmacy and Biological Sciences, 9(1), 99–103. [Google Scholar]
35. Malhotra, S. P. K., Mandal, T. K. (2018). Phytochemical screening and in vitro antibacterial activity of Moringa oleifera (Lam.) leaf extract. Archives of Agriculture and Environmental Science, 3(4), 367–372. DOI 10.26832/24566632.2018.030406. [Google Scholar] [CrossRef]
36. Isitua, C. C., Lozano, M., Jaramillo, C., Dutan, F. (2015). Phytochemical and nutritional properties of dried leaf powder of Moringa oleifera Lam. from machala el oro province of ecuador. Asian Journal of Plant Sciences and Research, 5(2), 8–16. [Google Scholar]
37. Fowoyo, P., Oladoja, E. (2015). Phytochemical screening, nutritional composition and antimicrobial activity of Moringa oleifera seed and leaf extract against selected gastrointestinal pathogens. Journal of Pharmacy and Biological Sciences, 10(6), 116–124. DOI 10.9790/3008-1062116124. [Google Scholar] [CrossRef]
38. Kumar, V., Pandey, N., Mohan, N., Singh, R. P. (2012). Antibacterial & antioxidant activity of different extract of Moringa oleifera leaves—An in vitro study. International Journal of Pharmaceutical Sciences Review and Research, 12(1), 89–94. [Google Scholar]
39. Dodiya, B., Amin, B., Kamlaben, S., Patel, P. (2015). Antibacterial activity and phytochemical screening of different parts of Moringa oleifera against selected gram positive and gram negative bacteria. Journal of Pharmaceutical, Chemical and Biological Sciences, 3(3), 421–425. [Google Scholar]
40. Maqsood, M., Qureshi, R., Arshad, M., Ahmed, M. S., Ikram, M. (2017). Preliminary phytochemical screening, antifungal and cytotoxic activities of leaves extract of Moringa oleifera Lam. from Salt range, Pakistan. Pakistan Journal of Botany, 49(1), 353–359. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |