

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.019572
ARTICLE
Physiological and Biochemical Mechanisms of Exogenous Calcium Chloride on Alleviating Salt Stress in Two Tartary Buckwheat (Fagopyrum tataricum) Varieties Differing in Salinity Tolerance
Key Laboratory of Plant Biotechnology in Universities of Shandong Province, College of Life Science, Qingdao Agricultural University, Qingdao, 266109, China
*Corresponding Author: Tao Zhang. Email: zhangtao9210@sohu.com
Received: 29 September 2021; Accepted: 09 December 2021
Abstract: Salt stress is one of the most serious abiotic stresses limiting plant growth and development. Calcium as an essential nutrient element and important signaling molecule plays an important role in ameliorating the adverse effect of salinity on plants. This study aimed to investigate the impact of exogenous calcium on improving salt tolerance in Tartary buckwheat cultivars, cv. Xinong9920 (salt-tolerant) and cv. Xinong9909 (salt-sensitive). Four-week-old Tartary buckwheat seedlings under 100 mM NaCl stress were treated with and without exogenous calcium chloride (CaCl2), Ca2+ chelator ethylene glycol tetraacetic acid (EGTA) and Ca2+-channel blocker lanthanum chloride (LaCl3) for 10 days. Then, some important physiological and biochemical indexes were determined. The results showed that salt stress significantly reduced seedling growth, decreased photosynthetic pigments, inhibited antioxidants and antioxidant enzyme activities. However, it increased the reactive oxygen species (ROS) levels in the two Tartary buckwheat cultivars. Exogenous 10 mM CaCl2 application on salt-stressed Tartary buckwheat seedlings obviously mitigated the negative effects of NaCl stress and partially restored seedlings growth. Ca2+-treated salt-stressed seedlings diplayed a suppressed accumulation of ROS, increased the contents of total chlorophyll, soluble protein, proline and antioxidants, and elevated the activities of antioxidant enzymes compared with salt stress alone. On the contrary, the addition of 0.5 mM LaCl3 and 5 mM EGTA on salt-stressed Tartary buckwheat seedlings exhibited the opposite effects to those with CaCl2 treatment. These results indicate that exogenous Ca2+ can enhance salt stress tolerance and Ca2+ supplementation may be an effective practice to cultivate Tartary buckwheat in saline soils.
Keywords: Salt stress; calcium; antioxidant enzymes; ROS scavenging; osmoprotection; tartary buckwheat
Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.) originated in southwestern China, and has currently been cultivated all over the world due to its good economic benefits and nutrient values. Tartary buckwheat is an important short-season pseudocereal crop and it produces gluten-free grains which are an important source of complete proteins with a balanced composition of essential amino acids [1]. Furthermore, Tartary buckwheat also contains resistant starch, minerals, proteins, vitamins, especially phenols, which prevent the effects of various chronic human diseases, such as obesity, hypertension, and cardiovascular diseases [2,3]. Therefore, in order to expand the planting area and improve the yield of Tartary buckwheat, it is very important to study the stress resistance of buckwheat [4].
Abiotic stresses, including drought, salt, low or high temperature, and flood, are the main cause of crop productivity decline, which seriously affect the growth and development of crops [5,6]. Among these stresses, salt stress is one of the most important abiotic stress factors restricting plant growth and production [7–9]. Salinity adversely affects plants mainly in three ways including osmotic stress, ionic toxicity, and oxidative damage [10]. The high salt concentration causes the increase of osmotic pressure in the external environment of the root system; therefore, the water potential in the surrounding environment of the root system is lower than that in the cells [11]. Not only can the cells not absorb the water needed, but the free water stored inside them will also flow outward along the water potential gradient, which finally results in cell water shortage and plant physiological drought [12]. Ionic stress mainly refers to ion toxicity and nutrition deficiency. Excessive salt ions destroy the integrity of the plant cell plasma membrane, so that a large number of nutrient elements such as calcium and potassium are extravagated from the cell, while sodium and chlorine are accumulated in it. Ion imbalance and a series of metabolic disorders in cells promote cell senescence and death [13]. In addition, osmotic stress and ionic imbalance will further cause secondary damage to cells, such as excessive production of reactive oxygen species (ROS), including hydrogen peroxide (H2O2), superoxide (O2−.) and hydroxyl radicals (⋅OH), which can cause oxidative damage to plants, such as membrane damage, enzymatic inhibition, cell death, and inhibition of plant growth and development [14–16]. After a long period of adaptation, plants have developed a series of complex defense systems to neutralize and scavenge the generated ROS. Such systems include non-enzymatic antioxidants and enzymatic antioxidants such as ascorbic acid (AsA), glutathione (GSH), ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), and glutathione peroxidase (GPX), which prevent cellular damage from ROS [17–19].
Some studies have shown that the application of different signaling molecules or protective agents on the basal or foliar surface of plants can alleviate the adverse effects of salinity by activating these defense mechanisms [20–24]. Calcium is an essential macronutrient for plants and plays an important role in the regulation of plant cell metabolism and signal transduction [25]. Many findings indicated that exogenous Ca2+ reversed salinity-induced damage in some plant species such as wheat (Triticum aestivum L.) [26], rice (Oryza sativa L.) [23], Cichorium intybus and Cowpea (Vigna unguiculata L.) [27,28]. It has been observed that exogenous application of moderate Ca2+ improved salt tolerance of plants by promoting membrane stability, stabilizing cell wall structures, regulating ion transport, and enhancing antioxidant enzyme activities [23,24,27–30]. Ethylene glycol tetraacetic acid (EGTA) is the specific Ca2+ chelator and it can lead to a decrease of extracellular Ca2+ concentration by chelation, and thus affects the physiological function of Ca2+ [31]. Ca2+ channels also play a crucial role in Ca2+ transfer and regulation, which is involved in responses to abiotic stress. Lanthanum chloride (LaCl3) is the Ca2+ channel blocker and can suppress cytoplasmic Ca2+ elevation via blocking Ca2+ influx [32]. Therefore, LaCl3 can be utilized to analyze the role of Ca2+ signaling in salt stress response. In this research, we aimed to investigate the effect of exogenous Ca2+, Ca2+ chelator EGTA and Ca2+ channel blocker LaCl3 on some physiological and biochemical parameters and revealed the function and mechanism of calcium signaling in two Tartary buckwheat cultivars exposed to salt stress.
2.1 Plant Materials and Treatments
Tartary buckwheat cultivars, cv. Xinong9920 (salt-tolerant) and cv. Xinong9909 (salt-sensitive) were chosen as experimental materials. The healthy and uniform seeds were surface sterilized with 0.01% KMnO4 for 5 min, washed 5 times with distilled water, and then pre-soaked for imbibition in distilled water for 5–6 h. The pre-soaked seeds were sown into plastic pots containing silica sand irrigated with Hoagland nutrient solution, and exposed to natural light and conventional management. Four-week-old seedlings were treated. Five treatments were as follows: untreated materials (CK); 100 mM NaCl treatment (T1); 10 mM CaCl2 + 100 mM NaCl treatment (T2); 0.5 mM LaCl3 + 100 mM NaCl treatment (T3); and 5 mM EGTA + 100 mM NaCl treatment (T4). Ten days after treatments, seedlings were harvested and physiological and biochemical parameters were measured. Each test included five buckwheat seedlings and represented one replicate. All treatments were replicated three times.
2.2 Estimation of Growth Parameters
The growth parameters were evaluated by measuring the length of the shoot and root system, and plant fresh weight (FW). Shoot length was measured from the base of the stem to the tip of the upper, fully expanded leaf, and root length was measured from the base of the root to its tip. Five seedlings under each treatment were weighed to determine FW.
2.3 Measurement of Chlorophyll Content
Chlorophyll a (Chl a), Chlorophyll b (Chl b) and total Chlorophyll a + b (Chl a + b) contents were determined by following the method of Arnon [33]. According to this method, 0.3 g fresh leaves were homogenized in 10 ml of 80% acetone and centrifuged at 10,000× g for 5 min. Then the absorbance of the supernatant was recorded at 645 and 663 nm wavelength using a spectrophotometer.
2.4 Determination of Proline and Soluble Protein Contents
The free proline content in leaf tissues was determined using the method of Bates et al. with some modifications [34]. The fresh leaves (0.1 g) were ground and extracted in 5 ml of 3.0% sulfosalicylic acid solution. Then the homogenate was centrifuged at 3,000× g for 10 min to separate the tissue debris. A total of 2 ml of the supernatant was mixed with 3 ml of 2.5% acid ninhydrin reagent and 2 ml of glacial acetic acid at 100°C for 30 min. After that, the reaction was immediately terminated in an ice bath and the chromophore was extracted with 5 ml of toluene. Finally, the absorbance of the toluene layer was measured at 520 nm. The proline content was quantified by referring to a standard curve for L-proline.
The Bradford protocol was used to quantify soluble protein content in the enzyme extracts [35]. 0.5 g fresh leaves were ground in 5 ml phosphate buffer (0.1 M, pH 7.5) with a pre-chilled mortar and centrifuged at 4,000× g for 10 min at 4°C. 0.1 ml of supernatant was mixed with 0.9 ml of distilled water and 5 ml of Coomassie bright blue. Then, the absorbance at 590 nm was determined using bovine serum albumin V as a standard.
2.5 Determination of MDA, H2O2 and O2−. Generate Rate
The level of lipid peroxidation was measured by estimating the MDA content of leaves following the method of Heath and Packer with slight modifications [36]. 0.1 g fresh plant leaves was homogenized with 5 ml of 10% Trichloroacetic acid (TCA) and centrifuged at 10,000× g for 10 min at 4°C. The 2 ml aliquot of the supernatant was added to 5 ml 20% TCA containing 0.5% of Thiobartituric acid (TBA) and incubated at 100°C for 15 min. After cooling immediately, the reaction mixture was centrifuged again at 10,000× g for 10 min. The absorbance was recorded at 532, 600 and 450 nm.
H2O2 was determined following the method of Lin et al. with some modification [37]. 0.1 g fresh samples were ground with 3 ml ice acetone and centrifuged at 3,000× g for 10 min at 4°C. 1 ml of the supernatant was mixed with 0.1 ml Ti (SO4)2 and 0.2 ml ammonia. After the reaction, the compound was again centrifuged at 3,000× g for 10 min, the supernatant was discarded, and the pellet was washed five times with ice acetone, and dissolved in 1 ml of 2 M H2SO4. The absorbance was measured at 410 nm using a standard curve.
O2−. generation rate was determined according to the method of Elstner et al. [38]. 0.1 g samples were ground in 2 ml phosphate buffer (65 mM, pH 7.8) and centrifuged at 12,000× g for 10 min at 4°C. The 1.5 ml supernatant was added to 0.5 ml 50 mM potassium phosphate buffer (pH 7.8) and 1 ml 1 mM hydroxylamine hydrochloride, and incubated for 20 min at 25°C. Then, 1 ml 17 mM p-aminobenzenesulfonic acid and 1 ml 7 mM α-naphthylamine solution were added to the mixture at 25°C for 30 min. The absorbance was measured at 530 nm, and O2−. generation rate was calculated from a standard curve of NaNO2 reagent.
2.6 Determination of AsA and GSH Contents
0.5 g fresh leaves were homogenized in 5 ml of 5% ice-cold TCA and centrifuged at 3,000× g for 15 min at 4°C. Then the supernatant was collected for analysis of AsA and GSH. AsA content was determined following the method of Huang et al. with some modifications [39]. The 0.5 ml supernatant was added to 0.5 ml NaH2PO4 (pH 7.4) and 0.5 ml H2O and incubated for 30 min at 37°C; then 0.8 ml 10% TCA, 44% H3PO4, 4% bipyridine and 3% FeCl3 were added to the mixture and continue to react for 60 min at 37°C. The absorbance was measured at 525 nm. GSH content was assayed in 200 mM Tris-HCl, 10 mM 5, 5′-Dithiobis-(2-nitrobenzoic acid) (DTNB) and absolute ethanol, and the absorbance was read at 412 nm.
2.7 Antioxidant Enzyme Activity Assays
Fresh leaf samples (0.5 g) were homogenized in 5 ml 0.05 mM phosphate buffer (pH 7.8) using a mortar and pestle on ice and centrifuged at 12,000× g for 15 min at 4°C. The supernatant was used as the crude enzyme extract to determine different enzyme activities.
SOD (EC 1.15.1.1) activity was assayed according to the method of Dhindsa and Matowe with some modification [40]. The 3 ml reaction mixture contained 50 mM phosphate buffer (pH 7.8), 130 mM L-methionine, 0.75 mM NBT, 0.1 mM ethylenediaminetetreacetic acid (EDTA), 0.02 mM riboflflavin and distilled water. Then, 0.02 ml of enzyme extract were added to the reaction mixture. Photoreduction of NBT was measured at 560 nm. One unit was defined as the quantity that causes 50% inhibition of NBT photo-reduction.
POD (EC 1.11.1.7) and CAT (EC 1.11.1.6) activities were measured following the method of Chance and Maehly with some modification [41]. POD activity was measured in a reaction mixture containing 50 mM phosphate buffer (pH 5.5), 50 mM guaiacol, 2% H2O2 and the enzyme extract. The absorbance was determined at 470 nm. CAT activity was assayed in a reaction mixture containing 100 mM phosphate buffer (pH 7.0) and 100 mM H2O. The reaction was started with the addition of the enzyme extract, and CAT activity was evaluated by determining the consumption of H2O2 at 240 nm.
GR activity was assayed in a reaction mixture containing 100 mM phosphate (pH 7.5), 0.5 mM EDTA, 0.75 mM DTNB, 0.1 mM NADPH and oxidized glutathione [42]. The reaction mixture was incubated at 35°C, and the absorbance was measured at 412 nm up to 5 min.
Statistical analysis was performed using SPSS 11.0 software. The obtained data were analyzed by one-way analysis of variance (ANOVA) and the least significant difference (LSD) test was applied to compare differences among the treatments means. Differences were considered significant at P < 0.05.
3.1 Exogenous Ca2+ Alleviated Growth Inhibition in Tartary Buckwheat under Salt Stress
As shown in Fig. 1, 100 mM NaCl treatment significantly inhibited seedling growth and biomass accumulation of both salt-tolerant and salt-sensitive Tartary buckwheat cultivars. Compared with the control, shoot and root length, and FW of salt-stressed Xinong9920 were reduced to 32.19%, 29.47% and 38.33%, respectively, whilst by 39.5%, 33.18% and 47.68%, respectively, in Xinong9909. The inhibition of these growth parameters due to salt stress was recovered after applying 10 mM exogenous CaCl2 by 30.55%, 23.19% and 31.28%, respectively, in Xinong9920. Xinong9909 showed 30.26%, 22.25% and 21.47% accretion, respectively, compared to the seedlings exposed to salt stress alone. The treatments applying Ca2+ chelator EGTA and Ca2+ channel blocker LaCl3 to salt-stressed seedlings greatly reduced those growth parameters on both Tartary buckwheat cultivars, which exhibited the opposite effect to those observed in salt-stressed seedlings with CaCl2 treatment.
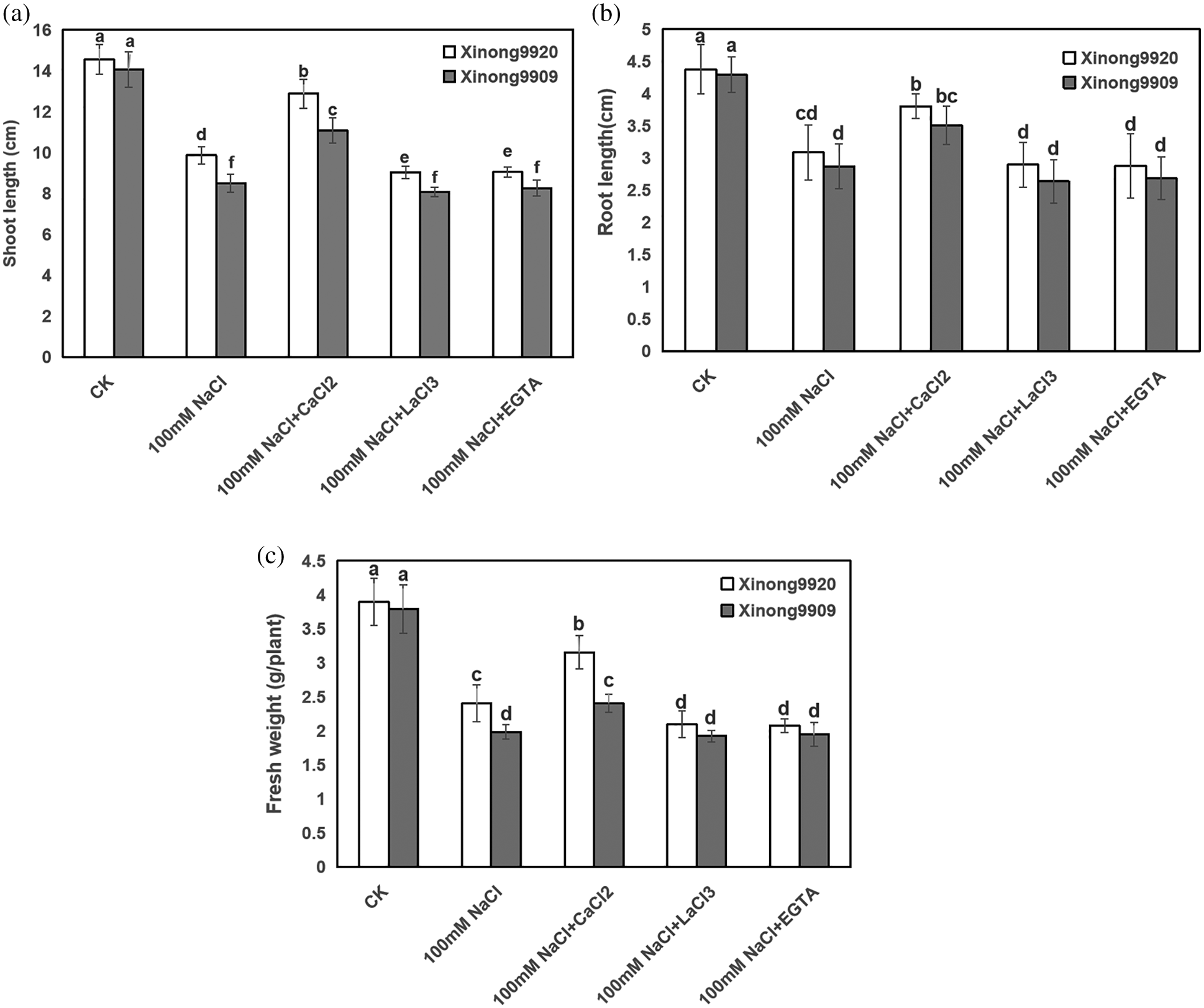
Figure 1: Effects of exogenous Ca2+ on shoot length (a), root length (b), and plant fresh weight (c) of Tartary buckwheat seedlings under salt stress. Each value is the mean ± SD of three replicates. Vertical bars with different letters indicate significant differences according to the LSD test at P < 0.05
3.2 Exogenous Ca2+ Alleviated the Degradation of Chlorophyll Content in Tartary Buckwheat under Salt Stress
Salinity impaired the photosynthetic pigments of Tartary buckwheat seedlings. As shown in Fig. 2, 100 mM NaCl treatment resulted in an obvious decrease of Chl a, Chl b and Chl (a + b) by 42.24%, 46.47% and 43.68%, respectively, in Xinong9920, and 44.9%, 44.34% and 44.71%, respectively, in Xinong9909 compared with control seedlings. Exogenous 10 mM CaCl2 application to both salt-stressed cultivars of Tartary buckwheat seedlings improved Chl a, Chl b and chl (a + b) contents by 53.9%, 60.14% and 55.91%, respectively, in Xinong9920, and 65.96%, 45.67% and 43.95%, respectively, in Xinong9909, in comparison to only salt-stressed seedlings. However, applying 5 mM EGTA and 0.5 mM LaCl3 to salt-stressed seedlings significantly decreased the Chl a, Chl b and Chl (a + b) contents in both cultivars compared with the 10 mM CaCl2 treatment.
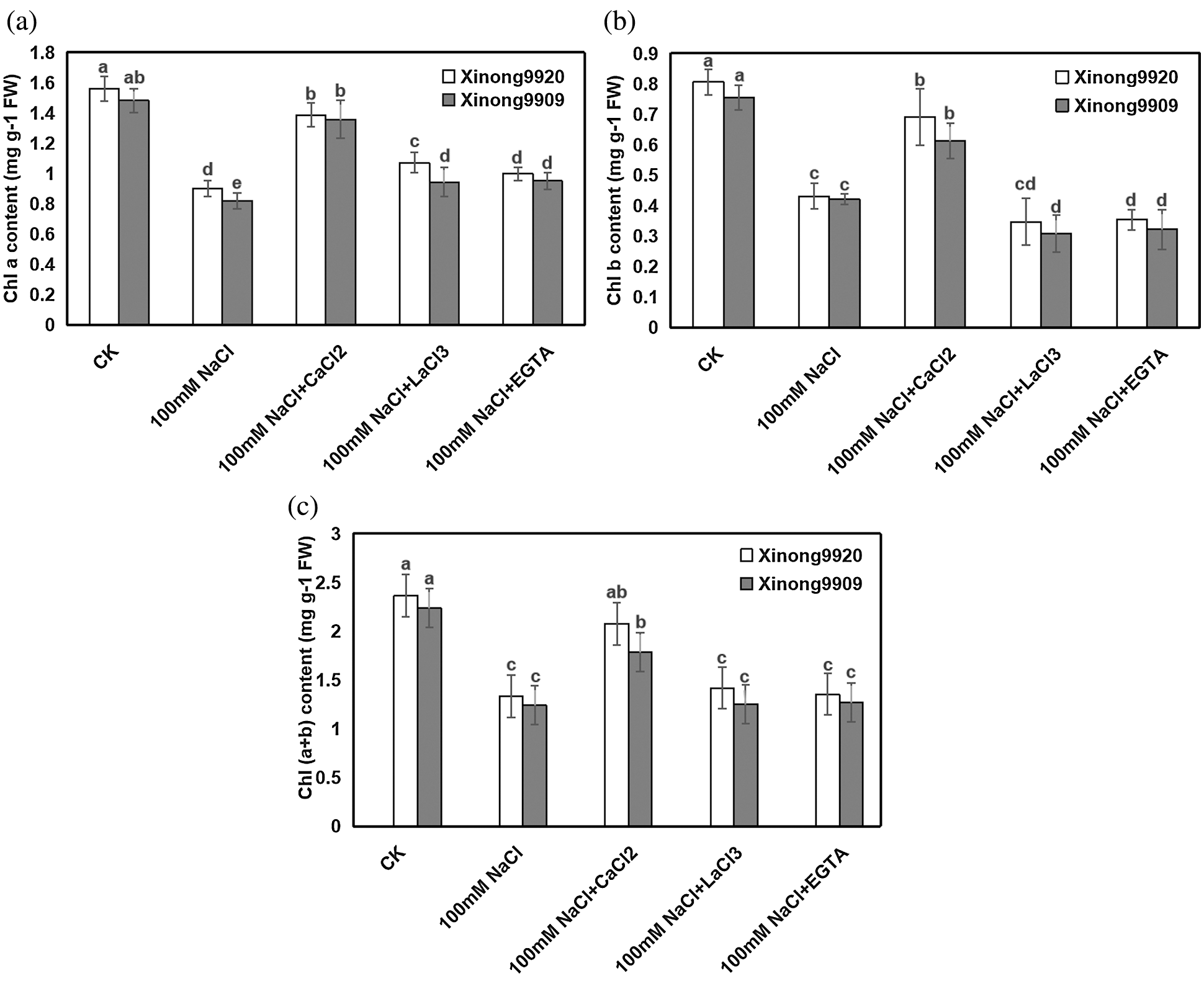
Figure 2: Effects of exogenous Ca2+ on Chla (a), Chlb (b), and Chl (a + b) (c) contents of Tartary buckwheat seedlings under salt stress. Each value is the mean ± SD of three replicates. Vertical bars with different letters indicate significant differences according to the LSD test at P < 0.05
3.3 Exogenous Ca2+ Enhanced Proline and Soluble Protein Contents in Tartary Buckwheat under Salt Stress
Among plant-compatible osmolytes, proline and soluble protein are considered of major importance, as they have been reported to accumulate in many plant species in response to environmental stresses. Our results showed (Fig. 3) that salt stress significantly increased the proline and soluble protein contents by 87.56% and 67.41%, respectively, in salt-tolerant Xinong9920, and 135.13% and 65.98%, respectively, in salt-sensitive Xinong9909 seedlings compared with the control. Exogenous 10 mmol/L CaCl2 further promoted the increase of proline and soluble protein contents in salt-stressed seedlings, which were increased by 44.83% and 31.75%, respectively, in Xinong9920, and 35.98% and 45.57%, respectively, in Xinong9909 compared with NaCl stress alone. The treatments with LaCl3 and EGTA to salt-stressed seedlings greatly reduced the proline content by 23.06% and 26.79%, respectively, in Xinong9920, and by 23.63% and 36.81%, respectively, in Xinong9909, and reduced the soluble protein content by 17.63% and 27.62%, respectively, in Xinong9909 seedlings, and by 29.95% and 35.31%, respectively, in Xinong 9909 compared to the CaCl2 treatment.
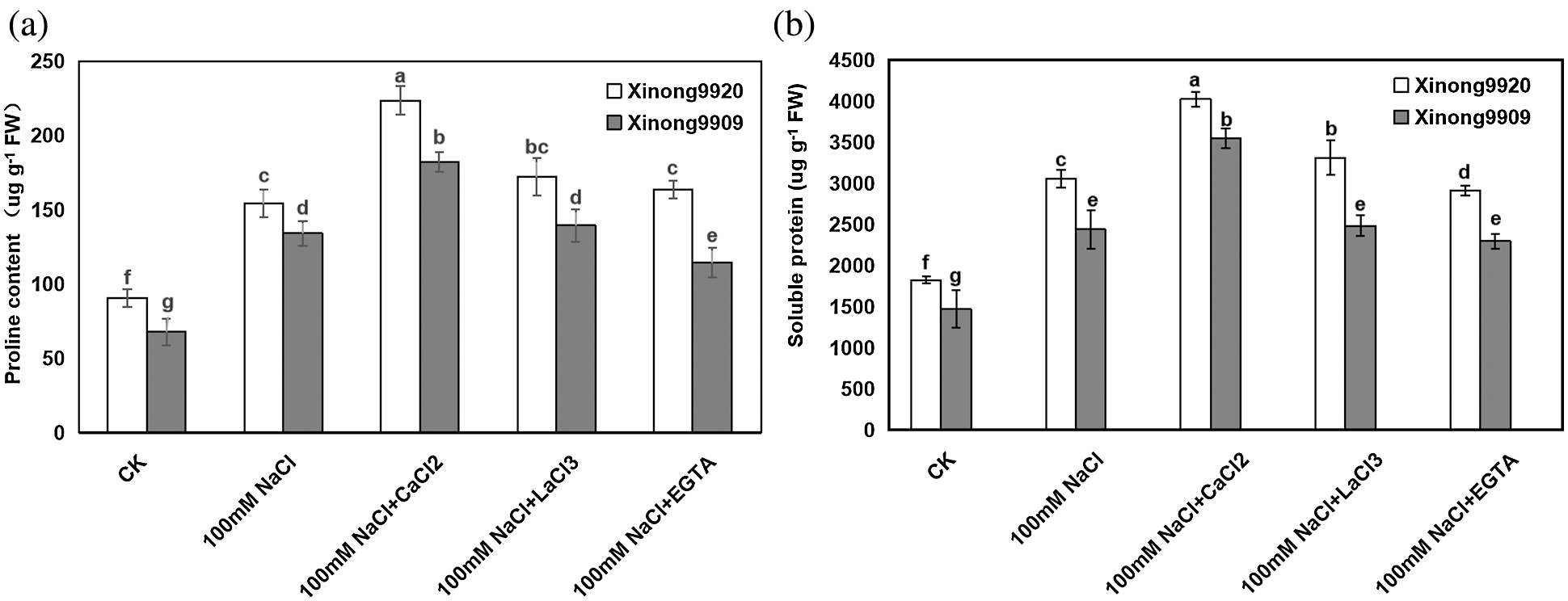
Figure 3: Effects of exogenous Ca2+ on proline (a) and soluble protein (b) contents of Tartary buckwheat seedlings under salt stress. Each value is the mean ± SD of three replicates. Vertical bars with different letters indicate significant differences according to the LSD test at P < 0.05
3.4 Exogenous Ca2+ Decreased MDA Contents and ROS Levels in Tartary Buckwheat under Salt Stress
MDA is one of the main products of membrane lipid peroxidation in plants. The changes in MDA concentration and plasma membrane permeability are important indicators to reflect the degree of membrane lipid peroxidation and plasma membrane damage. As shown in Fig. 4a, under salt stress conditions, the contents of MDA were significantly increased by 107.86% and 122.09%, respectively, in Xinong9920 and Xinong9909 compared with the control seedlings. Applying 10 mmol/L CaCl2 in both salt-stressed Tartary buckwheat seedlings significantly decreased the MDA content by 28.34% and 26.96%, respectively, in Xinong9920 and Xinong9909 compared with the only salt-stressed seedlings.
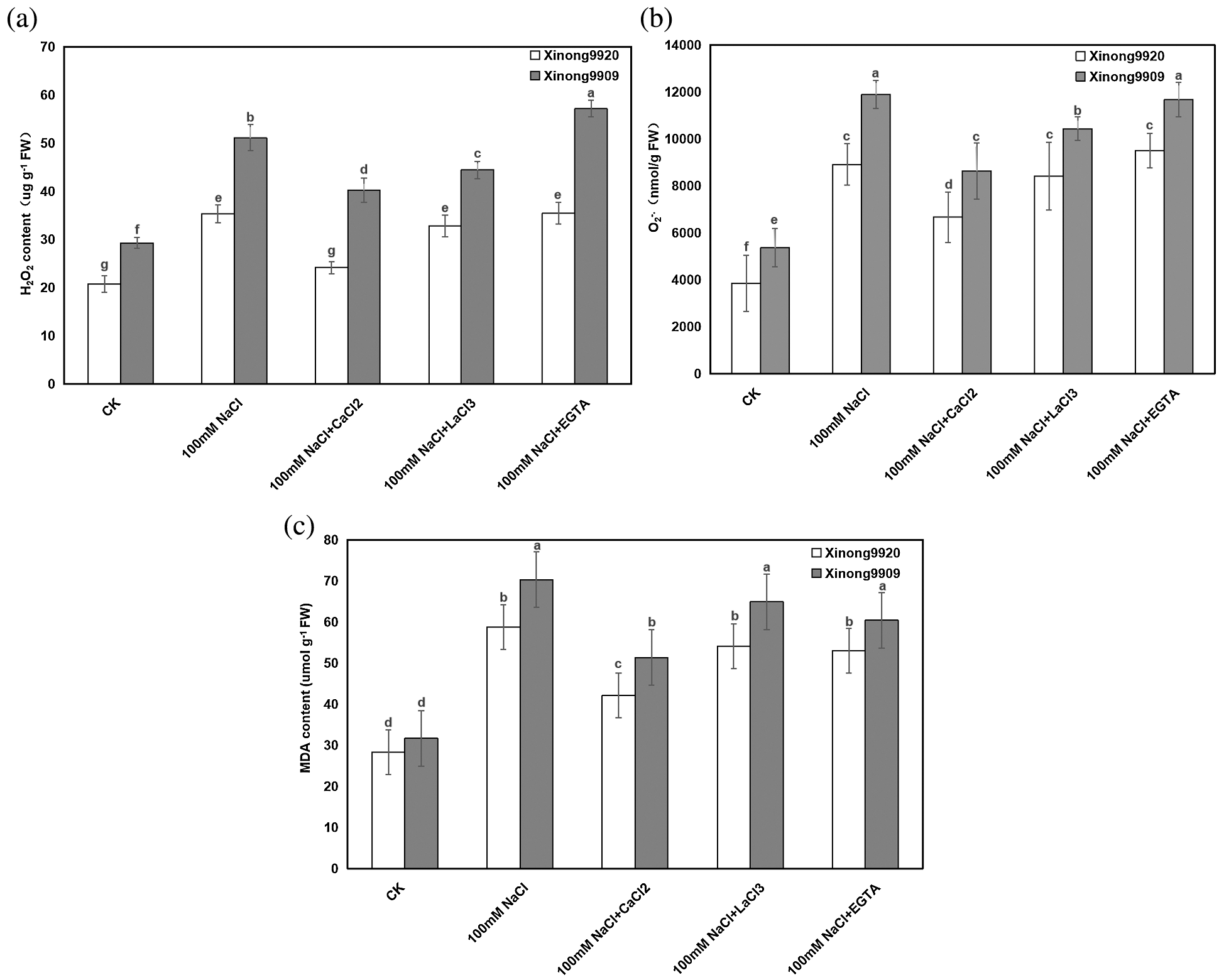
Figure 4: Effects of exogenous Ca2+ on H2O2 (a), O2−. (b) and MDA (c) contents of Tartary buckwheat seedlings under salt stress. Each value is the mean ± SD of three replicates. Vertical bars with different letters indicate significant differences according to the LSD test at P < 0.05
Furthermore, in order to evaluate the effect of Ca2+ on NaCl-induced oxidative stress, H2O2 and O2−. contents were estimated. The results showed (Figs. 4b, 4c) that H2O2 content and O2−. generation rate of salt-stressed Xinong9920 seedlings were significantly increased by 70.02% and 132.08%, respectively, whereas Xinong9909 showed an increase by 74.60% and 121.62%, respectively, compared with those of non-stressed control seedlings. 10 mmol/L CaCl2 treatment on salt-stressed seedlings significantly decreased H2O2 content and O2−. generation rate by 31.58% and 25.21% in Xinong9920, respectively, and by 21.21% and 27.44%, respectively, in Xinong9909, compared to only NaCl-stressed seedlings.
Compared to the Ca2+ treatment, treatments with LaCl3 and EGTA in salt-stress seedlings led to obvious increments in (1) MDA by 28.57% and 26.19%, respectively, in Xinong9920, and by 27.45% and 17.65%, respectively, in Xinong9909, (2) H2O2 content by 33.33% and 45.83% respectively, in Xinong9920, and by 10.00% and 42.50%, respectively, in Xinong9909, and (3) O2−. generation rate by 26.08% and 42.39%, respectively, in Xinong9920, and by 21.01% and 35.30%, respectively, in Xinong9909.
3.5 Exogenous Ca2+ Increased AsA and GSH Contents in Tartary Buckwheat under Salt Stress
As shown in Fig. 5, AsA and GSH contents were higher in Xinong9920 than in Xinong9909 under non-stress conditions. 100 mM NaCl treatment resulted in a significant decrease by 44.81% and 23.86% in Xinong9920 seedlings, respectively, whilst by 53.42% and 29.26%, respectively, in Xinong9909 compared to the control, and the magnitude of decrease was more pronounced in salt-sensitive Xinong9909 than in salt-tolerant Xinong9920. However, 10 mM CaCl2 treatment in NaCl-stressed seedlings overcame the adverse effects of NaCl stress in both cultivars. 10 mM CaCl2 application to the salt-stressed plants significantly increased AsA and GSH contents in the Xinong9920 by 96.77% and 55.21%, respectively, whereas the increase was 128.44% and 25.86% in Xinong9909 plants, respectively, compared to only salt-stressed Tartary buckwheat seedlings. Treatments with LaCl3 and EGTA exhibited opposite effects to those with CaCl2 treatment and significantly decreased AsA content by 40.81% and 44.84%, respectively, in Xinong9920 and by 61.61% and 60.82%, respectively, in Xinong9909, and GSH content by 30.77% and 38.46%, respectively, in Xinong9920 and by 7.69% and 46.15%, respectively in Xinong9909.
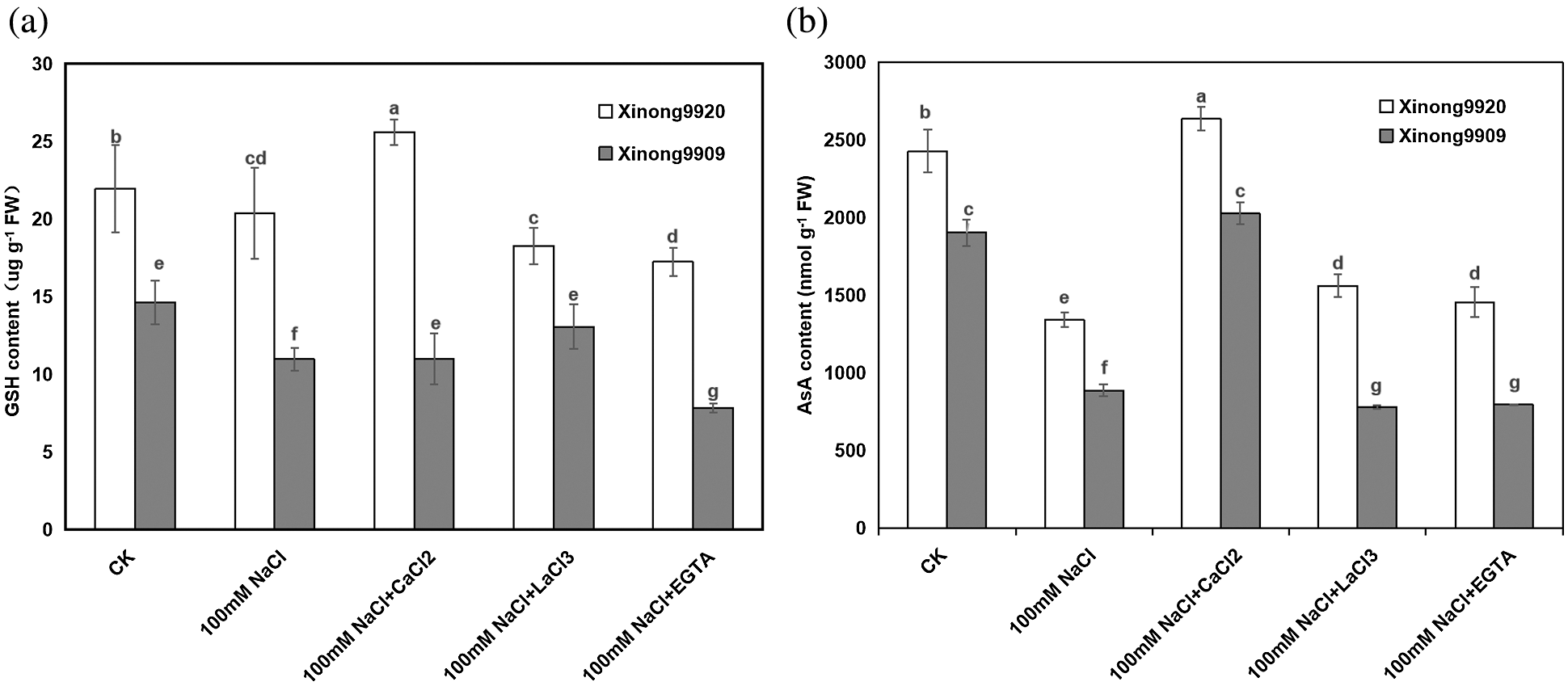
Figure 5: Effects of exogenous Ca2+ on AsA (a) and GSH contents (b) of Tartary buckwheat seedlings under salt stress. Each value is the mean ± SD of three replicates. Vertical bars with different letters indicate significant differences according to the LSD test at P < 0.05
3.6 Exogenous Ca2+ Improves Antioxidant Enzymes Activities in Tartary Buckwheat under Salt Stress
As shown in Fig. 6, 100 mM NaCl treatment significantly increased the SOD activity by 23.40% in salt-tolerant Xinong9920 while in salt-sensitive Xinong9909 it had a slight increase by 5.76% compared to the control seedlings. Exogenous 10 mM CaCl2 supplementation in salt-stressed seedlings further enhanced the SOD activity by 35.68% and 31.49% in Xinong9920 and Xinong9909, respectively, compared to salt stress alone.
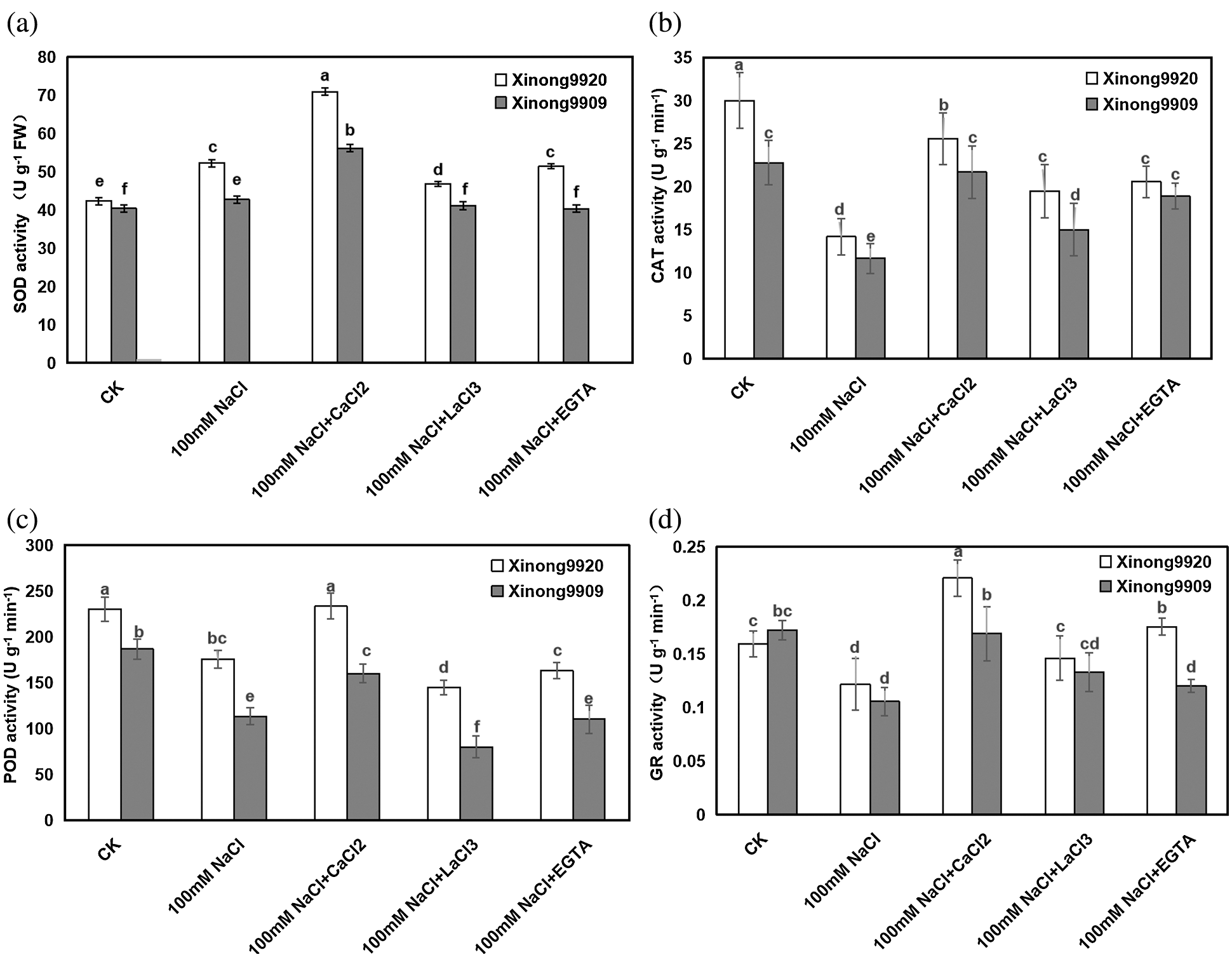
Figure 6: Effects of exogenous Ca2+ on SOD (a), CAT (b), POD (c) and GR (d) activities of Tartary buckwheat seedlings under salt stress. Each value is the mean ± SD of three replicates. Vertical bars with different letters indicate significant differences according to the LSD test at P < 0.05
Different with the SOD, 100 mM NaCl treatment significantly decreased the CAT, POD and GR activities by 52.77%, 23.67% and 23.27% in Xinong9920 seedlings, respectively, and by 48.79%, 39.29%, 38.37% in Xinong9909, respectively, compared to the control seedlings. Exogenous 10 mM CaCl2 application in salt stressed seedlings increased the CAT, POD and GR activities by 23.94%, 32.91% and 81.36% in Xinong9920 seedlings, respectively, and by 85.68%, 41.18%, 60.95% in Xinong9909, respectively, compared with only salt-stressed seedlings. On the contrary, the usage of either LaCl3 or EGTA caused the decrease of SOD by 33.80% and 28.17%, respectively, in Xinong9920, and by 26.79% and 28.57%, respectively, in Xinong9909; the decrease of POD by 37.77% and 30.04%, respectively, in Xinong9920, and by 50.00% and 31.25%, respectively, in Xinong9909; the decrease of CAT by 26.92% and 19.23% respectively, in Xinong9920, and by 33.33% and 13.64%, respectively, in Xinong9909, and the decrease of GR by 31.82% and 18.19%, respectively, in Xinong9920, and by 23.53% and 29.41%, respectively, in Xinong9909, compared with values on the CaCl2 treatment. The only non-significant decrease was on Xinong9909, where salt-stressed seedlings showed similar (P > 0.05) values of CAT under the CaCl2 and EGTA treatments.
Salinity is one of the most brutal abiotic stress factors that can destroy the normal physiological and biochemical processes of plants, thereby adversely affecting plant growth and development [43]. The present study showed that 100 mM NaCl treatment caused the root length, seedling length, and FW of the two Tartary buckwheat cultivars to decrease, but the degree of reduction of the two varieties was different. The growth restriction caused by salt stress may be due to the inhibition of plant cell elongation and imbalance of nutrient homeostasis. A number of studies have shown that exogenous application of CaCl2 has a protective effect on plants under different abiotic stresses [44]. In our study, exogenous 10 mM CaCl2 applying to salt-stressed seedlings significantly enhanced the root length, seedling length, and FW of the two Tartary buckwheat cultivars as compared to salt stress alone, which showed that exogenous application of CaCl2 can partially restore plant growth under salt stress. This result is consistent with previous findings in cowpea [28], tomato [29], wheat [45], Cichorium intybus and rice [27,46]. The growth promotion of Ca2+ might be due to maintaining the balance and stability of the cell membrane and promote cell division and cell elongation which ultimately will lead to promote plant growth [47].
Plant growth is closely related to photosynthesis [48]. In this study, the Chl content of Tartary buckwheat seedlings treated with 100 mM NaCl decreased significantly (Fig. 2). The decrease of Chl content in Tartary buckwheat seedlings under salt stress may be due to the oxidation of chlorophyll and other chloroplast pigments and the instability of pigment-protein complexes [49]. Exogenous 10 mM CaCl2 supplementation prevented the reduction of Chl contents and partially recovered its content on both salt-stressed Tartary buckwheat cultivars (Fig. 2), but the Chl content of Xiong9920 was higher than that of Xinong9909. Similar results were reported in Pisum sativum [50], Festuca arundinacea [51], and Oryza sativus [23,52]. The increment of chlorophyll pigments under salt stress due to application of Ca2+ might be because of limited ROS production (Fig. 4).
In many plants, salt tolerance is achieved by increasing the accumulation of proline and soluble protein, and these organic molecules play an important role in osmotic regulation and protection of membranes and macromolecules [53]. Here, the soluble protein and proline contents of both Tartary buckwheat cultivars increased under salt stressed conditions as compared to controls, but the enhancement of these osmoprotectants was higher in plants supplemented with CaCl2 in comparison to those under the NaCl-induced stress alone (Fig. 3). The elevated proline and soluble protein contents due to salt stress may be an adaptation to compensate the energy for plant growth, development and survival [54]. The addition of CaCl2 to salt stressed plants further increased the proline and soluble protein accumulation, which might be because Ca2+ treatment activates proline and soluble protein biosynthesis [31]. Enhanced synthesis of proline and soluble protein restores photosynthetic efficiency and photoassimilate production, promotes plant growth and reduces salt-induced oxidative stress [53].
Salt stress induced the rapid overproduction of ROS, resulting in the accumulation of cell lipid peroxidation and the destruction of cell membranes, which was characterized by the increase of MDA content [55,56]. Our present study showed that 100 mM NaCl treatment significantly enhanced ROS levels (the accumulation of H2O2 and O2−.) and MDA contents in both Tartary buckwheat cultivars compared to the control, and their accumulation was higher in the sensitive than in the tolerant cultivar. This suggested that oxidative damage in the salt-sensitive cultivar was more severe than that in the salt-tolerant plants. However, supplementation with 10 mM CaCl2 in the NaCl-treated Tartary buckwheat seedlings of both cultivars reduced ROS levels and MDA contents compared with only salt-stressed seedlings (Fig. 4). This indicated that exogenous Ca2+ promoted membranes and protected plant cell membranes from ROS-induced oxidative damage. These results agree with previous studies on rice [23,46], wheat [45] and tomato [29].
In order to dealt with oxidative stress, evolution allowed that plants develop a series of enzymatic and non-enzymatic antioxidants, which work in intimate coordination to eliminate the excess of ROS [57]. SOD is considered the first line of defense against oxidative stress induced by environmental stress, and the increase of SOD activity protected the plant from oxidantive stress damage [58]. In the present study, 100 mM NaCl treatment enhanced SOD activity compared to the control, and 10 mM CaCl2 supplementation on salt-stressed seedlings further enhanced its enzyme activity in both Tartary buckwheat cultivars compared with only salt-stressed plants (Fig. 6a). This is consistent with the results in cucumber [59], bean [60], and wheat [45] under NaCl stress. But SOD catalyzes the dismutation reaction of O2−. into O2 and H2O2. The excessive H2O2 is still toxic and must be converted into H2O in a subsequent reaction to be removed [61]. Some studies showed that the CAT/POD system may work synergistically to remove H2O2 at a maximum rate. In fact, in the present experiment, the NaCl-stressed Tartary buckwheat seedlings exhibited a significantly decreased CAT and POD activities (Figs. 6b, 6c), which agrees with previous findings of Zhang et al. [4], Mishra et al. [62] and Wutipraditkul et al. [63]. The decreased CAT and POD in salt-stressed seedlings might be a cause of excessive accumulation of H2O2, which hampered the Tartary buckwheat seedling growth. However, exogenously applied 10 mM CaCl2 boosted the activities of CAT and POD which scavenged the deleterious H2O2 efficiently. This result is in agreement with previous findings in Brassica juncea and Pennisetum spicatum seedlings [64,65]. GR is the key enzyme in the AsA-GSH cycle, which catalyzes the reaction of reducing GSSG (oxidative state of GSH) to GSH. Previous studies revealed that GR plays critical roles in the stress response [66–68]. In the present study, a decrease in the GR activity was found under NaCl stress in seedlings of both Tartary buckwheat cultivars compared to the control, and the magnitude of the decrease was more obvious in the salt-sensitive Xinong 9909 than in the salt-tolerant Xinong9920 (Fig. 6d). This suggested that the severe oxidative damage imposed by NaCl occurs particularly in the sensitive cultivar. The exogenous application of CaCl2 significantly increased the GR activity in both cultivars of Tartary buckwheat under NaCl stress, and partially reversed the NaCl toxity.
AsA and GSH are the most abundant and powerful non-enzymatic antioxidants in plant tissues. AsA reacts with a series of ROS and directly quenches O2−. and H2O2, which are the basis of its antioxidant activity [69]. Morever, AsA is also responsible for maintaining the reduced form of metal ions, thereby keeping the activity of the antioxidant enzymes [70]. As with AsA, GSH is another antioxidant and plays an important role in ROS detoxification, metabolic conjugation, xenogenetic detoxification and signaling status [71]. Therefore, some studies indicated that the decrease of AsA and GSH contents promotes the production of ROS and oxidative stress in salt-stressed plants. In this study, the AsA and GSH contents sharply decreased due to 100 mM NaCl stress, especially in the salt-sensitive cultivar (Fig. 5). However, Ca2+ supplementation in NaCl-stressed seedlings significantly increased the contents of AsA and GSH compared to only salt-stressed seedlings and alleviated the NaCl toxicity to some extent. This might be because of Ca2+ is taking part in the regeneration of AsA and GHS by upregulating the related enzymes.
Salt stress significantly reduced seedling growth, decreased photosynthetic pigments, and inhibited antioxidants and antioxidant enzyme activities in two Tartary buckwheat seedlings because of excessive ROS production. Application of exogenous CaCl2 alleviated NaCl toxicity through the following mechanisms Supplementation of Ca2+ (1) promoted the synthesis of photosynthetic pigments and the accumulation of osmoprotectants, (2) reduced the content of ROS and the degree of lipid peroxidation, and (3) enhanced the enzymatic and non-enzymatic detoxification systems. Furthermore, the study also found that the exogenous Ca2+-channel blocker LaCl3 and Ca2+ chelator EGTA exhibited the opposite effects to those with CaCl2 treatment in two salt-stressed Tartary buckheat seedlings. This suggests that Ca2+ signaling play an important role in salt stress tolerance in Tartary buckwheat. These results provide the basis for finding the way to regulate the environment and promote the growth of Tartary buckwheat under salt stress conditions.
Author Contribution: T Zhang designed the experiment, analyzed the physiological indexes of Tartary buckwheat and wrote the manuscript. H-B Yang cultivated the plant and treated it. T Zhang and H-B Yang revised and finalized the manuscript.
Funding Statement: This study was supported by the National Nature Science Foundation of China (31101556).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Sinkovic, L., Kokalj, D., Vidrih, R., Meglic, V. (2020). Milling fractions fatty acid composition of common (Fagopyrum esculentum Moench) and tartary (Fagopyrum tataricum (L.) Gaertn) buckwheat. Journal of Stored Products Research, 85, 101551. DOI 10.1016/j.jspr.2019.101551. [Google Scholar] [CrossRef]
2. Huda, M. N., Lu, S., Jahan, T., Ding, M., Jha, R. et al. (2021). Treasure from garden: Bioactive compounds of buckwheat. Food Chemistry, 335, 127653. DOI 10.1016/j.foodchem.2020.127653. [Google Scholar] [CrossRef]
3. Luthar, Z., Golob, A., Germ, M., Vombergar, B., Kreft, I. (2021). Tartary buckwheat in human nutrition. Plants, 10(4), 700. DOI 10.3390/plants10040700. [Google Scholar] [CrossRef]
4. Zhang, J. S., Wang, Y. Q., Song, J. N., Xu, J. P., Yang, H. B. (2020). Effect of aspartic acid on physiological characteristics and gene expression of salt exclusion in Tartary buckwheat under salt stress. Journal of Plant Biochemistry and Biotechnology, 29(1), 94–101. DOI 10.1007/s13562-019-00518-y. [Google Scholar] [CrossRef]
5. Parvaiz, A., Satyawati, S. (2008). Salt stress and phyto-biochemical responses of plants–A review. Plant Soil and Environment, 54, 89–99. DOI 10.17221/2774-PSE. [Google Scholar] [CrossRef]
6. Mittler, R., Blumwald, E. (2010). Genetic engineering for modern agriculture: Challenges and perspectives. Annual Review Plant Biology, 61(1), 443–462. DOI 10.1146/annurev-arplant-042809-112116. [Google Scholar] [CrossRef]
7. Turkan, I., Demiral, T. (2009). Recent developments in understanding salinity tolerance. Environmental Experimental Botany, 1, 2–9. DOI 10.1016/j.envexpbot.2009.05.008. [Google Scholar] [CrossRef]
8. Hasanuzzaman, M., Nahar, K., Fujita, M. (2013). Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmad, P., Azooz, M. M., Prasad, M. N. V. (Eds.Ecophysiology and responses of plants under salt stress, pp. 25–87. New York: Springer. [Google Scholar]
9. Negrao, S., Schmockel, S. M., Tester, M. (2017). Evaluating physiological responses of plants to salinity stress. Annal of Botany, 119(1), 1–11. DOI 10.1093/aob/mcw191. [Google Scholar] [CrossRef]
10. Deinlein, U., Stephan, A. B., Horie, T., Luo, W., Xu, G. et al. (2014). Plant salt-tolerance mechanisms. Trends in Plant Science, 19(6), 371–379. DOI 10.1016/j.tplants.2014.02.001. [Google Scholar] [CrossRef]
11. Yang, Y., Guo, Y. (2018). Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytologist, 217, 523–539. DOI 10.1111/nph.14920. [Google Scholar] [CrossRef]
12. Park, H. J., Kim, W. Y., Yun, D. J. (2016). A new insight of salt stress signaling in plant. Molecules and Cells, 39(6), 447–459. DOI 10.14348/molcells.2016.0083. [Google Scholar] [CrossRef]
13. Shabala, L., Zhang, J., Pottosin, L., Bose, J., Zhu, M. et al. (2016). Cell-type-specifc H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiology, 172, 2445–2458. DOI 10.1104/pp.16.01347. [Google Scholar] [CrossRef]
14. Hasanuzzaman, M., Alam, M. M., Rahman, A., Hasanuzzaman, M., Nahar, K. et al. (2014). Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed Research International, 2014, 1–17. DOI 10.1155/2014/757219. [Google Scholar] [CrossRef]
15. Fallah, F., Nokhasi, F., Ghaheri, M., Kahrizi, D., Beheshti Ale Agha, A. et al. (2017). Effect of salinity on gene expression, morphological and biochemical characteristics of Stevia rebaudiana Bertoni under in vitro conditions. Cellular and Molecular Biology, 63(7), 102–106. DOI 10.14715/cmb/2017.63.7.17. [Google Scholar] [CrossRef]
16. Ma, N. L., Che Lah, W. A., Abd Kadir, N., Mustaqim, M., Rahmat, Z. et al. (2018). Susceptibility and tolerance of rice crop to salt threat: Physiological and metabolic inspections. PLoS One, 13(2), e0192732. DOI 10.1371/journal.pone.0192732. [Google Scholar] [CrossRef]
17. Sofo, A., Scopa, A., Nuzzaci, M., Vitti, A. (2015). Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. International Journal of Molecular Science, 16, 13561–13578. DOI 10.3390/ijms160613561. [Google Scholar] [CrossRef]
18. Mostofa, M. G., Hossain, M. A., Siddiqui, M. N., Fujita, M., Tran, L. S. P. (2016). Phenotypical, physiological and biochemical analyses provide insight into selenium-induced phytotoxicity in rice plants. Chemosphere, 178, 212–223. DOI 10.1016/j.chemosphere.2017.03.046. [Google Scholar] [CrossRef]
19. Saini, P., Gani, M., Kaur, J. J., Godara, L. C., Singh, C. et al. (2018). Reactive oxygen species (ROSA way to stress survival in plants. In: Zargar, S. M., Zargar, M. Y. (Eds.Abiotic stress-mediated sensing and signaling in plants: An omics perspective, pp. 127–153. Singapore: Springer. [Google Scholar]
20. Gautam, S., Singh, P. K. (2009). Salicylic acid-induced salinity tolerance in corn grown under NaCl stress. Acta Physiologiae Plantarum, 31, 1185–1190. DOI 10.1007/s11738-009-0338-8. [Google Scholar] [CrossRef]
21. Hasanuzzaman, M., Oku, H., Nahar, K., Bhuyan, M. H. M. B., Mahmud, J. A. et al. (2018). Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnology Reports, 12, 77–92. DOI 10.1007/s11816-018-0480-0. [Google Scholar] [CrossRef]
22. Tian, X. Y., He, M. R., Wang, Z. L., Zhang, J. W., Song, Y. L. et al. (2015). Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regulation, 77, 343–356. DOI 10.1007/s10725-015-0069-3. [Google Scholar] [CrossRef]
23. Roy, P. R., Tahjib-Ul-Arif, M., Polash, M. A. S., Hossen, M. Z., Hossain, M. A. (2019). Physiological mechanisms of exogenous calcium on alleviating salinity-induced stress in rice (Oryza sativa L.). Physiology and Molecular Biology of Plants, 25(3), 611–624. DOI 10.1007/s12298-019-00654-8. [Google Scholar] [CrossRef]
24. Zhang, D. W., Vu, T. S., Huang, J., Chi, C. Y., Xing, Y. et al. (2019). Effects of calcium on germination and seedling growth in Melilotus offificinalis L. (Fabaceae) under salt stress. Pakistan Journal of Botany, 51, 1–9. DOI 10.30848/PJB2019-1(44). [Google Scholar] [CrossRef]
25. Kader, M. A., Lindberg, S. (2010). Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signaling and Behavior, 5(3), 233–238. DOI 10.4161/psb.5.3.10740. [Google Scholar] [CrossRef]
26. Nemat Alla, M. M., Abogadallah, G. M., Badran, E. G., Nada, R. M., Hassan, N. M. (2014). Supplementary CaCl2 ameliorates wheat tolerance to NaCl. Acta Physiologiae Plantarum, 36, 2103–2112. DOI 10.1007/s11738-014-1587-8. [Google Scholar] [CrossRef]
27. Arshi, A., Ahmad, A., Aref, I. M., Iqbal, M. (2010). Effect of calcium against salinity-induced inhibition in growth, ion accumulation and proline contents in Cichorium intybus L. Journal of Environmental Biology, 31, 939–944. DOI 10.2112/JCOASTRES-D-10-00093.1. [Google Scholar] [CrossRef]
28. Murillo-Amador, B., Jones, H. G., Kaya, C., Aguilar, R. L., García-Hernández, J. L. et al. (2006). Effects of foliar application of calcium nitrate on growth and physiological attributes of cowpea grown under salt stress. Environmental and Experimental Botany, 58, 188–196. DOI 10.1016/j.envexpbot.2005.08.003. [Google Scholar] [CrossRef]
29. Tuna, A. L., Kaya, C., Ashraf, M., Altunlu, H., Yokas, I. et al. (2007). The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environmental Experimantal Botany, 59, 173–178. DOI 10.1016/j.envexpbot.2005.12.007. [Google Scholar] [CrossRef]
30. Khan, M. N., Siddiqui, M. H., Mohammad, F., Naeem, M., Khan, M. M. A. (2010). Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiologiae Plantarum, 32, 121–132. DOI 10.1007/s11738-009-0387-z. [Google Scholar] [CrossRef]
31. Yang, S. L., Lan, S. S., Deng, F. F., Gong, M. (2016). Effect of calcium and calmodulin antagonists on chilling stress-induced proline accumulation in Jatropha curcas L. Journal of Plant Growth Regulation, 35, 815–826. DOI 10.1007/s00344-016-9584-3. [Google Scholar] [CrossRef]
32. Liao, C., Zheng, Y., Guo, Y. (2017). MYB30 transcription factor regulates oxidative and heat stress responses through ANNEXIN-mediated cytosolic calcium signaling in Arabidopsis. New Phytolohist, 216, 163–177. DOI 10.1111/nph.14679. [Google Scholar] [CrossRef]
33. Arnon, D. I. (1949). Copper enzymes in isolated chloroplast: Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1), 1. DOI 10.1104/pp.24.1.1. [Google Scholar] [CrossRef]
34. Bates, L. S., Waldren, R. P., Teare, I. D. (1973). Rapid determination of free proline for water stress studies. Plant Soil, 39, 205–207. DOI 10.1007/BF00018060. [Google Scholar] [CrossRef]
35. Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. DOI 10.1016/0003-2697(76)90527-3. [Google Scholar] [CrossRef]
36. Heath, R. L., Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemsitry and Biophysics, 125, 189–198. DOI 10.1016/0003-9861(68)90654-1. [Google Scholar] [CrossRef]
37. Lin, Z. F., Li, S. X., Lin, G. Z., Guo, J. Y. (1988). Relation between H2O2 accumulation and membrane lipid peroxidation in senescent leaves and chloroplasts. Plant Physiology Journal, 14(1), 16–22. [Google Scholar]
38. Elstner, E. F., Heupel, A. (1976). Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Analytical Biochemistry, 70, 616–620. DOI 10.1016/0003-2697(76)90488-7. [Google Scholar] [CrossRef]
39. Huang, C., He, W., Guo, J., Chang, X., Su, P. et al. (2005). Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. Journal of Experimental Botany, 56(422), 3041–3049. DOI 10.1093/jxb/eri301. [Google Scholar] [CrossRef]
40. Dhindsa, R. S., Matowe, W. (1981). Drought tolerance in 2 mosses correlated with enzymatic defense against lipid peroxidation. Journal of Experimental Botany, 32, 79–92. DOI 10.1093/jxb/32.1.79. [Google Scholar] [CrossRef]
41. Chance, M., Maehly, A. C. (1955). Assay of catalases and peroxidases. Methods in Enzymology, 2, 764–775. DOI 10.1016/S0076-6879(55)02300-8. [Google Scholar] [CrossRef]
42. Smith, G. S., Sirota, E. B., Safinya, C. R., Clark, N. A. (1968). Structure of the Lβ phase in a hydrated phosphatidycholine multimembrane. Physical Review Letters, 60(9), 813–816. DOI 10.1103/PhysRevLett.60.813. [Google Scholar] [CrossRef]
43. Zhao, S. S., Zhang, Q. K., Liu, M. Y., Zhou, H. P., Ma, C. L. et al. (2021). Regulation of plant responses to salt stress. International Journal of Molecular Science, 22(9), 4609. DOI 10.3390/ijms22094609. [Google Scholar] [CrossRef]
44. Seifikalhor, M., Aliniaeifard, S., Shomali, A., Azad, N., Hassani, B. et al. (2019). Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signaling and Behavior, 2019, 14(11), e1665455. DOI 10.1080/15592324.2019.1665455. [Google Scholar] [CrossRef]
45. Al-Whaibi, M. H., Siddiqui, M. H., Basalah, M. O. (2012). Salicylic acid and calcium induced protection of wheat against salinity. Protoplasma, 249(3), 769–778. DOI 10.1007/s00709-011-0322-1. [Google Scholar] [CrossRef]
46. Tahjib-Ul-Arif, M., Roy, P. R., Sohag, A. A. M., Afrin, S., Rady, M. M. et al. (2018). Exogenous calcium supplementation improves salinity tolerance in BRRI Dhan28; a salt-susceptible high-yielding Oryza sativa cultivar. Journal of Crop Science and Biotechnology, 21(4), 383–394. DOI 10.1007/s12892-018-0098-0. [Google Scholar] [CrossRef]
47. Nemat Alla, M. M., Khedr, A. A., Serag, M. M., Abu-Alnaga, A. Z., Nada, R. M. (2012). Regulation of metabolomics in Atriplex halimus growth under salt and drought stress. Plant Growth Regulation, 67, 281–304. DOI 10.1007/s10725-012-9687-1. [Google Scholar] [CrossRef]
48. Bose, J., Munns, R., Shabala, S., Gilliham, M., Pogson, B. et al. (2017). Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. Journal of Experimental Botany, 68, 3129–3143. DOI 10.1093/jxb/erx142. [Google Scholar] [CrossRef]
49. Chaves, M. M., Flexas, J., Pinheiro, C. (2009). Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Annals of Botany, 103, 551–560. DOI 10.1093/aob/mcn125. [Google Scholar] [CrossRef]
50. Ozturk, L., Demir, Y., Unlukara, A., Karatas, I., Kurunc, A. et al. (2012). Effects of long-term salt stress on antioxidant system, chlorophyll and proline contents in pea leaves. Romanian Biotechnology Letters, 17, 7227–7236. DOI 10.1186/1754-6834-5-27. [Google Scholar] [CrossRef]
51. Wang, G., Bi, A., Amombo, E., Li, H., Zhang, L. et al. (2017). Exogenous calcium enhances the photosystem II photochemistry response in salt stressed tall fescue. Frontier in Plant Science, 8, 2032–2043. DOI 10.3389/fpls.2017.02032. [Google Scholar] [CrossRef]
52. Zhang, Z. H., Li, Q., Song, H. X., Ro, X. M., Ismail, A. M. (2012). Responses of different rice (Oryza sativa L.) genotypes to salt stress and relation to carbohydrate metabolism and chlorophyll content. African Journal of Agricultural Research, 7, 19–27. DOI 10.5897/AJAR11.834. [Google Scholar] [CrossRef]
53. Reddy, P. S., Jogeswar, G., Rasineni, G. K., Maheswari, M., Eddy, A. R. et al. (2015). Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum (Sorghum bicolor (L.) Moench). Plant Physiology and Biochemistry, 94, 104–113. DOI 10.1016/j.plaphy.2015.05.014. [Google Scholar] [CrossRef]
54. Ahmad, P., Abdel Latef, A., Hashem, A., Abd Allah, E. F., Gucel, S. et al. (2016). Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Frontier in Plant Science, 7, 347. DOI 10.3389/fpls.2016.00347. [Google Scholar] [CrossRef]
55. Bose, J., Rodrigo-Moreno, A., Shabala, S. (2014). ROS homeostasis in halophytes in the context of salinity stress tolerance. Journal of Experimental Botany, 65, 1241–1257. DOI 10.1093/jxb/ert430. [Google Scholar] [CrossRef]
56. Mansour, M. M. F. (2013). Plasma membrane permeability as an indicator of salt tolerance in plants. Biologia Plantarum, 57(1), 1–10. DOI 10.1007/s10535-012-0144-9. [Google Scholar] [CrossRef]
57. Farooq, M. A., Gill, R. A., Islam, F., Basharat, A., Liu, H. et al. (2016). Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Frontier in Plant Science, 7, 468–468. DOI 10.3389/fpls.2016.00468. [Google Scholar] [CrossRef]
58. Sudhakar, C., Lakshmi, A., Giridarakumar, S. (2001). Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Science, 161, 613–619. DOI 10.1016/S0168-9452(01)00450-2. [Google Scholar] [CrossRef]
59. Shu, S., Yuan, L. Y., Guo, S. R., Sun, J., Yuan, Y. H. (2013). Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplastsin Cucumis sativus L. under salt stress. Plant Physiology and Biochemistry, 63, 209–216. DOI 10.1016/j.plaphy.2012.11.028. [Google Scholar] [CrossRef]
60. Cabot, C., Sibole, J. V., Barcelo, J., Poschenrieder, C. (2009). Sodium-calcium interactions with growth, water, and photosynthetic parameters in salt-treated beans. Journal of Plant Nutrition and Soil Science, 172, 637–643. DOI 10.1002/jpln.200800124. [Google Scholar] [CrossRef]
61. Stepien, P., Klobus, G. (2005). Antioxidant defense in the leaves of C3 and C4 plants under salinity stress. Physiologia Plantarum, 125, 31–40. DOI 10.1111/j.1399-3054.2005.00534.x. [Google Scholar] [CrossRef]
62. Mishra, P., Bhoomika, K., Dubey, R. S. (2013). Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma, 250, 3–19. DOI 10.1007/s00709-011-0365-3. [Google Scholar] [CrossRef]
63. Wutipraditkul, N., Wongwean, P., Buaboocha, T. (2015). Alleviation of salt-induced oxidative stress in rice seedlings by proline and/or glycinebetaine. Biologia Plantarum, 59, 547–553. DOI 10.1007/s10535-015-0523-0. [Google Scholar] [CrossRef]
64. Ahmad, P., Alyemeni, M. N., Ahanger, M. A., Wijaya, L., Alam, P. et al. (2018). Upregulation of antioxidant and glyoxalase systems mitigates NaCl stress in Brassica juncea by supplementation of zinc and calcium. Journal of Plant Interaction, 13(1), 151–162. DOI 10.1080/17429145.2018.1441452. [Google Scholar] [CrossRef]
65. Erinle, K. O., Jiang, Z., Ma, B., Li, J., Chen, Y. et al. (2016). Exogenous calcium induces tolerance toatrazine stress in Pennisetum seedlings and promotes photo-synthetic activity, antioxidant enzymes and psbA gene transcripts. Ecotoxicology and Environmetal Safe, 132, 403–412. DOI 10.1016/j.ecoenv.2016.06.035. [Google Scholar] [CrossRef]
66. Liu, Y. F., Zhang, G. X., Qi, M. F., Li, T. L. (2015). Effect of calcium on photosynthesis, antioxidant system and chlroplast ultrastructure in tomato leaves under low night temperature stress. Journal of Plant Growth Regulation, 34, 263–273. DOI 10.1007/s00344-014-9462-9. [Google Scholar] [CrossRef]
67. Nahar, K., Rahman, M., Hasanuzzaman, M., Mahabub Alam, M., Rahman, A. (2016). Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environmental Science and Pollution Research, 23, 21206–21218. DOI 10.1007/s11356-016-7295-8. [Google Scholar] [CrossRef]
68. Lu, Y., Wang, Q. F., Li, J., Xiong, J., Zhou, L. N. et al. (2019). Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Scientific Reports, 9, 7397. DOI 10.1038/s41598-019-43901-4. [Google Scholar] [CrossRef]
69. Foyer, C. H., Noctor, G. (2005). Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell, 17, 1886–1875. DOI 10.1105/tpc.105.033589. [Google Scholar] [CrossRef]
70. Athar, H. R., Khan, A., Ashraf, M. (2008). Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environmental and Experimental Botany, 63, 224–231. DOI 10.1016/j.envexpbot.2007.10.018. [Google Scholar] [CrossRef]
71. Sumithra, K., Jutur, P. P., Carmel, B. D., Reddy, A. R. (2006). Salinity-induced changes in two cultivars of Vigna radiata: Responses of antioxidative and proline metabolism. Plant Growth Regulation, 50, 11–22. DOI 10.1007/s10725-006-9121-7. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |