

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020768
ARTICLE
Changes in Florets’ Vertical Direction within Inflorescence Affects Pollinator Behavior, and Fitness in Trifolium repens
College of Life Sciences, Northwest Normal University, Lanzhou, 730070, China
*Corresponding Author: Kun Sun. Email: kunsun@163.com
#These three authors contributed equally in the manuscript
Received: 10 December 2021; Accepted: 09 February 2022
Abstract: Ecological interactions between flowers and pollinators greatly affect the reproductive success. To facilitate these interactions, many flowers are known to display their attractive qualities, such as scent emission, flower rewards and floral vertical direction, in a rhythmic fashion. However, less is known about how plants regulate the relationship between these flower traits to adapt to pollinator visiting behavior and increase reproduction success. Here we investigated the adaptive significance of the flower bending from erect to downward in Trifolium repens. We observed the flowering dynamic characteristics (changes of vertical direction of florets, flowering number, pollen grain numbers, pollen viability and stigma receptivity over time after blossom) and the factors affecting the rate of flower bending in T. repens. Then we altered the vertical direction of florets in inflorescence of different types (upright and downward), and compared the pollinator behaviors and female reproductive success. Our results showed that florets opened sequentially in inflorescence, and then bend downwards slowly after flowering. The bending speed of florets was mainly influenced by pollination, and bending angle increased with the prolongation of flowering time, while the pollen germination rate, stigma receptivity and nectar secretion has a rhythm of “low-high-low” during the whole period with the time going. The visiting frequency of all the four species of pollinators on upward flowers was significantly higher than that of downward flowers, and they especially prefer to visit flowers with a bending angle of 30°–60°, when the flowers was exactly of the highest flower rewards (nectar secretion and number of pollen grains), stigma receptivity and pollen germination rate. The seed set ratio and fruit set ratio of upward flowers were significantly higher than downward flowers, but significantly lower than unmanipulated flowers. Our results indicated that the T. repens could increase female and male fitness by accurate pollination. The most suitable flower angle saves pollinators’ visiting energy and enables them to obtain the highest nectar rewards. This coordination between plants and pollinators maximizes the interests of them, which is a crucial factor in initiating specialized plant-pollinator relationships.
Keywords: Trifolium repens; floral vertical direction; adaptive significances; fitness
Floral traits are considered adaptive because they can improve plant mating success through interacting with abiotic and biotic factors. One of the floral traits interacting with abiotic and biotic agents of selection is floral vertical direction [1–3], which considered to be an inherited trait of some species [4]. Diversification and adaptation of floral vertical direction are usually researched with the expectation that abiotic factors are the mainly selective factors acting on floral vertical direction [2,5–7], because of their influences on mating success [8]. For instance, the downward flowers are considered to have evolved to avoid nectar dilution and pollen damage by rainfall and solar radiation [1,9,10]. In addition, the erect flowers could absorb more heat than downward flowers due to the different air flow convection [10,11], this led to erect flowers improved the flower internal temperature, growth of pollen tube, and seed production in some alpine and arctic species [12]. Therefore, numerous species have evolved adaptive ability to manipulate the microclimate of flower to maintain physiologically optimal conditions [13–15].
Pollinator morphology and behavior are also closely related to the orientation of flowers, as shown by some studies in which parts of the flowers, as lower lips, have been considered as landing organs for pollinators [16], and the function of these organs may be impaired by the change of flower vertical direction. For example, the downward bending reduces the number of flower landing points, while the upward flowers increase the landing points on flower organs [17–19]. Therefore, floral vertical direction determines movement of pollinators within a flower, influence pollinator attraction [3,20], pollination precision [21], pollen transfer [22–24] and foraging behavior [25], and then influences the plant reproduction success, which has led to the exploration of its evolutionary significance and function [26]. However, many previous studies have focused on effect of abiotic factor on flower direction, or the species that produce single flowers [1,2], the effect of floral vertical direction on pollination in more complex inflorescences has rarely been reported.
A series of experimental studies have shown that the flower direction and angle change during flowering, which influence the microenvironment within flowers and pollinator activity, and then affect the success of reproduction [19]. For instance, the change of flower orientation in tropical Convolvulus influences both gynoecium temperature and pollinator activity [2]. Correspondingly, pollinators also promote efficient foraging on different species through a series of behavioral adaptations. Recent studies have showed that the pollinators and plants not only adjust behavioral or floral traits in their organisms, respectively, but also coordinate the precise pollination time [27]. For instance, Nicotiana attenuata flowers regulate their downward or upward orientations in a dynamic way, and synchrony with the activity of their pollinators [28]. These researches indicate that effective cross-pollination requires coordination between plants and their pollinators, as well as involves complex signaling between the environmental cues and circadian clock to adjust the timing within and between each organism to increase plant reproductive fitness.
In this study, we tested the adaptive significance of the changed flower vertical direction in Trifolium repens. The flower of this species was upright in early flowering, but bend slowly after blooming and turn downward in fruiting. To reveal the relationship between flower vertical direction, pollinator preference, flower reward and ability of pollen exportation and pollen reception in T. repens, we observed the flowering dynamic characteristics, tested the factors affecting the flower downward speed, and altered the flower vertical direction. Specifically, we addressed the following questions: (1) what effect does the flower vertical direction have on the behavior and frequency of pollinators? (2) what are the influencing factors of flower bending in T. repens? And (3) what is the adaptive significance of flower bending?
We conducted the study on the campus by Northwest Normal University, Lanzhou (alt. 1550 m, lat. 36°11´19” N, long. 103°74´12” E), Gansu Province, China. The vegetation type of our experimental plot is artificial grassland, which is mainly composed by T. repens. The average annual rainfall is 327 mm and the average annual temperature is 10.3°C. The experiment was conducted from 01 May to 01 June 2019, the flowering peak at the study site.
Trifolium repens is an herbaceous perennial plant in the bean family Fabaceae, commonly found in most grassy areas (gardens and lawns). The plant is capable of both asexual (or vegetative) reproduction through the generation of stolons, and sexual reproduction through seed production and dispersal [29,30]. Each plant can produce about 10 globular racemes inflorescences, and each inflorescence consists of 30–70 florets, which are white and commonly tinged with pink and it began to bend slowly after flowering. Three to four seeds per pod can be produced after pollination [30]. As T. repens is predominantly self-incompatible, cross-pollination is essential for significant seed set [31,32]. T. repens pollen is not easily dispersed by wind and any airborne pollen does not result in effective pollination. Therefore, insect pollinators are normally required to transfer pollen between individual clover plants, the honeybee (Apis mellifera) is the most important pollinator of T. repens [30].
Because the inflorescence types and states required by different experiments are inconsistent, we classify inflorescences according to the number of flowers open or lost at different stages: 1) bud stage, inflorescence before flowering; 2) full-blooming stage, about 1/3 of the flowers have fallen; 3) final flowering stage, more than 2/3 flowers have fallen.
We selected 30 inflorescences (bud stage) from different plants, and observed the changes of flowering number and opening regularity of florets in inflorescence at every 4 h interval until all florets withered. To test the floret duration in different layers of inflorescence, we recorded 30 inflorescence that tagged with a floret on each layer, and measured flower duration as time from opening of the corolla lobes until the flowers withered [33].
390 flower buds were randomly tagged on different inflorescences (full blooming stage), then we collected 30 florets at every 4 h interval after blossom to test for following index on each flower: (1) changes in florets bending angle (n = 30), measured by a protractor. (2) stigma receptivity (n = 30), measured by the benzidine/H2O2 method-place the stigma on the glass slide, drop benzidine/H2O2 solution (1% benzidine: 3% hydrogen peroxide: water = 4:11:22) and observe it under the microscope. The acceptability of the stigma can be judged by the number of bubbles and color of the reaction solution around the stigma. “−” means the stigma is not receptive; “−/+” means that some of the stigmas are receptive; “+” means the stigma is receptive, and more “+” symbols mean the stigma is strongly receptive [34]. (3) pollen germination rate, put all the pollen in a floret into a centrifuge tube containing 10% sucrose solution, incubated under dark conditions at 25°C for 48 h and observed under the microscope. Germination percentage (%) was determined by dividing the number of germinated pollen grains per field of view by the total number of pollen grains per field of view. (4) number of remnant pollen grains in floret, we carefully pinched all the anthers of a flower into 1 ml centrifuge tube, fixed the volume to 1 ml with distilled water, and put them on the shaking table for 48 h. Then we took 20 μl suspension on the slide, counted all pollen under the microscope (repeated 5 times), and finally multiplied by 50 to calculate the total amount of pollen grains in all anthers of a flower. Meanwhile, to detect nectar secretion patterns, we bagged 30 florets in pre-anthesis and measured nectar volume every 4 h until flowers failed. The bags can only be uncovered when measured for eliminate insect interference.
We randomly selected 120 flower buds from 60 inflorescences (full blooming stage) on 30 plants, and estimate the contribution of visitor effects (pressure and pollination) to flower bending through four treatments (n = 30 flowers, respectively): (1) natural condition, exposure to natural pollination environment; (2) bagged, flowers avoiding the influence of visitors through bagged before blossom; (3) artificial pollination, bagged and pollinated with cross-pollen that collected from 1000 m away; (4) artificial pressure, bagged and manually press the flowers with digital push–pull gauge force gauge (KTE HF-5, 0.001–5 N, Shenzhen Enzi Electronics Co., Ltd., China) through simulate pollinator pressure (average pressure of 4 species of pollinators). After each treatment, the flower angle with a clinometer every 2 h until flowers bending completely (perpendicular to horizontal plane). The normality of data was tested using 1-K-S, and then one-way ANOVAs (with Tukey’s multiple contrasts) were used to test the difference in dropping speed between the different treatments.
2.4 Pollinator Observations and Reproductive Success
90 inflorescences (full blooming state) were randomly selected in the different plants, and prepared the following three types of inflorescence (n = 30 flowers, respectively) with different floret vertical direction with adhesive tape: (1) inflorescences with florets turned upright (Up, <90°), (2) inflorescences with florets turned downward (Down, >90°), (3) unmanipulated inflorescences (Unmanipulated) (Fig. 1). Each inflorescence is consistent with the same number of florets and state, all these treatments located the same habitat and grew in the same natural environment. After manipulated, we observed pollinator behaviors (landing position, activity in inflorescence and contact with sexual organs, etc.) and visit frequency on these flowers for 3 sunny days between 08:00–18:00 (total of 30 h) (treat new flowers in every observation day). The seed production was detected after one month of flower exposure. The fruit set ratio was counted as the proportion of seeded flowers in an inflorescence, and the seed set ratio was counted as ovule number/mature seed number.

Figure 1: Inflorescences with different floral vertical direction. A, upright; B, downward; C, unmanipulated
Our results showed that each inflorescence of T. repens includes 71.42 ± 22.26 (Mean ± SD, the same in the following) florets, the florets open sequentially in inflorescence with an average of 10.32 ± 2.54 florets per time, and the inflorescence duration is 8.32 ± 1.28 days (Fig. 2). The florets began to bend at a uniform rate after anthesis (3.15 ± 0.63°/h), but accelerated significantly at 32–40 h (5.10 ± 0.77°/h), bent slowly to 180° under the pressure of the next round of florets after 40 h (Fig. 3). The flower duration is 45.26 ± 5.32 h, and there was no significant difference in flower duration in different layers of inflorescence (Fig. 4).

Figure 2: Flowering dynamic characteristics of Trifolium repens, the sequential opening process of florets in inflorescence

Figure 3: The dynamics of flower angle over time after blossom. Yellow represents period when the flower is upward, and green represents period when the flower is dropping

Figure 4: Left: different layer of inflorescence; Right: floret duration in different layer of inflorescence, different letters on items indicate significant difference at the 0.05 level
The pollen viability, stigma receptivity and nectar secretion of T. repens were relatively low at the beginning of the floret anthesis, while were highest 8–16 h later after anthesis (flower angle was 30°–60°), and then began to decrease continuously as the flowers bend downward (Figs. 5 and 6). These results showed that the pollen germination rate, stigma receptivity and nectar secretion of T. repens showed a “low-high-low” variation tendency during the whole period with the time going. The anthers of T. repens dehisced before anthesis, the number of pollen grains had no significant difference within 8 h after anthesis, and then decreased gradually with the flowering time (Fig. 6).

Figure 5: The dynamics of pollen viability and stigma receptivity over time after blossom. Yellow represents period when the flower is upward, and green represents period when the flower is dropping. “−” means the stigma is not receptive; “−/+” means that some of the stigmas are receptive; “+” means the stigma is receptive, and more “+” symbols mean the stigma is strongly receptive

Figure 6: The dynamics of pollen amounts and nectar volume over time after blossom. Yellow represents period when the flower is upward, and green represents period when the flower is dropping
The rate of flower bending under natural condition is 3.12 ± 0.48°/h, compared with the natural condition flowers, the artificial pollination (3.69 ± 0.33) flowers significantly increased (P < 0.001, df = 3) in rate of flower bending, this indicated that the pollination had a significant influence on flower bending speed. In contrast, there is no significant difference (P = 0.111, df = 3) between the bagged (1.56 ± 0.21°/h) and artificial pressure (1.70 ± 0.22°/h) flowers, but the above treatments were significantly lower (P < 0.001, df = 3) than natural condition flowers (Fig. 7). This indicated that the pollinators pressure had no effect on flower bending.

Figure 7: The bending speed of flower angle under different treatments. Boxplots indicate the bending speed, showing medians, quartiles, interquartile ranges and outliers. Different letters on items indicate significant difference at the 0.05 level
We observed a total of 4 pollinator species during 30 h of observation on T. repens flowers, namely Apis mellifera, A. florea, Bombus religiosus and A. dorsata. The four species of pollinators had the same preference when they were visiting flower, i.e., prefer to land on parallel flowers (bending angle of 90°) to visit flowers with a bending angle of 30°–60° flowers (Figs. 8, 9). When pollinators visit upward flowers, the lower layer of flowers in inflorescence provide a landing platform for them, and this is helpful for them to visit in an upright position (Fig. 8a). However, due to lack of the landing platform, foraging on downward flowers requires pollinators to remain in hanging position or a head-down position (Figs. 8b, 8c). Therefore, the visiting frequency on upward flowers was significantly higher (P < 0.001, df = 5) than downward flowers, and the visiting frequency on these two types of manipulated flowers was significantly lower (P < 0.001, df = 5) than the unmanipulated flowers (Fig. 10).
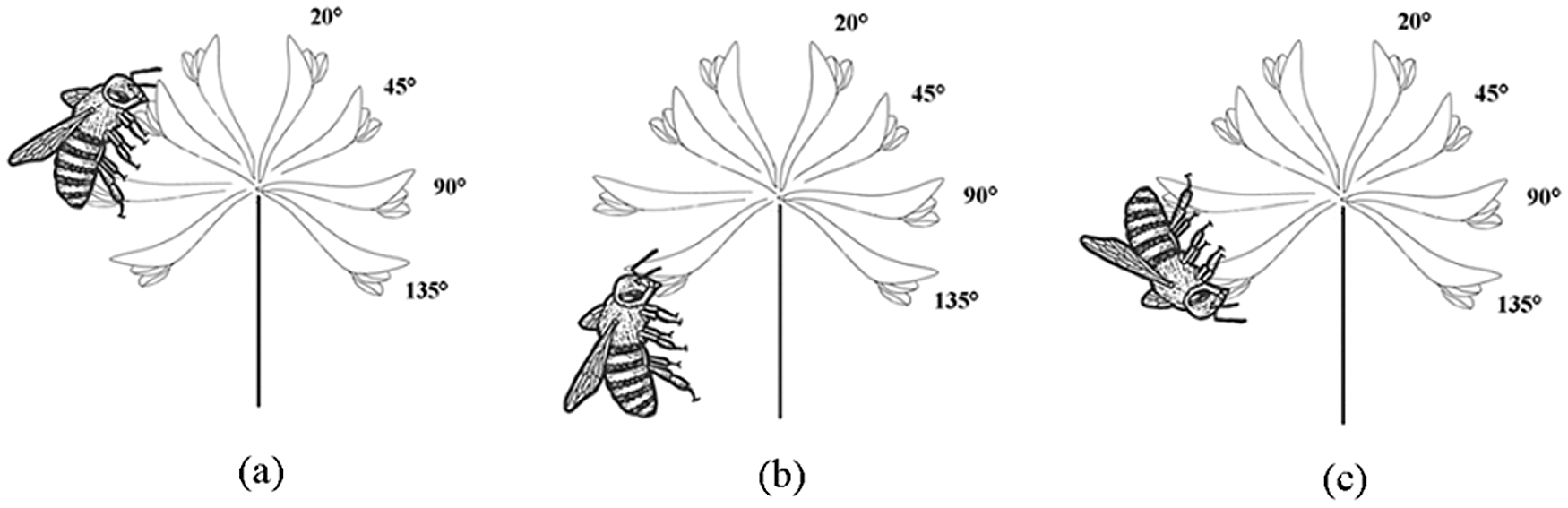
Figure 8: The pollinator visiting and approaching behaviors on T. repens, (a) upright position, (b) hanging position, (c) head-down position

Figure 9: Number of visits on different angle of flowers unmanipulated inflorescence. Color represents the number of visits

Figure 10: Visiting rates of T. repens in different floral vertical direction. The visiting rate expressed as times of flower visit/h/flower
Correspondingly, the seed set ratio and fruit set ratio of upward flowers (0.56 ± 0.06, 0.63 ± 0.06) are significantly higher (P < 0.001, df = 2) than downward flowers (0.05 ± 0.06, 0.23 ± 0.04), but significantly lower (P < 0.001, df = 2) than unmanipulated flowers (0.78 ± 0.04, 0.98 ± 0.05) (Fig. 11).

Figure 11: The seed set ratio and fruit set ratio in different floral vertical direction
Our results showed that, the florets open sequentially in inflorescence, that is, begin to bend slowly after anthesis, and made room for the next round of florets for display. We believe that the enlarged display space will increase the pollination opportunity of florets [35]. Meanwhile, the sequential opening of flowers in the inflorescence prolonged the inflorescence duration, and this will effectively increase the pollination opportunity of all florets in inflorescence [36], and then improve the adaptability of sexual reproduction by this flowering pattern.
Flower bending speed may be related to several environmental factors, such as humidity, temperature, external pressure, and other actions of pollinators [37]. Among them, the main influencing factor is the pollinator, because the flower senescence rate depends on the pollen removal and receipt within a species [38,39]. For instance, Blionis et al. [40] showed that pollinator exclusion improves floral duration in a study on Campanula species. Similarly, in T. repens, hand-pollination significantly increased the rate of flower bending, but was not affected by the pressure generated by the pollinators, suggesting that flower bending is under selective pressure for pollination success. It indicates that the flowers bend rapidly after pollination by pollinators, which may provide some visual signals for pollinators to visit, and improve the display and pollination opportunities of other non-pollinated flowers.
The floral syndrome shaping of entomophilous plants is strongly correlated with pollinators [36]. Slight changes in flower characteristics will affect the landing behavior and frequency of flower visitors [41,42]. Our results showed that all four pollinators of T. repens prefer to visit upward flowers with a bending angle of 30°–60°, but rarely visit downward flowers. We believe that the behavior of pollinators is affected by the following factors. First, the floral vertical direction affects pollinator landing behavior and then influence the pollinator frequency [19]. The lower layer of flowers in inflorescence provide a landing platform for the upper flowers, while the parallel flowers are the best landing platform for pollinators, this makes pollinators easily to visit the flowers with a bending angle of 30°–60°. Pollinators usually fly and forage in an upright position [43–45], the upright position considered to incur less cost in time and energy [44] and it also allows pollinators have a better vision for above than below [44,46]. Moreover, foraging on downward flowers requires bees to remain in hanging position or a head-down position, hence the changes in foraging posture may cause variations in foraging behavior and the fit between pollinators and floral reproductive organs and enlarge the energy consumption of pollinators. Therefore, to save the visiting energy, pollinators prefer to select the most suitable angle of flowers in inflorescence. Second, the upward flowers face towards the sun and may increasing internal temperature of flower, which, in turn, improves pollinator attraction [47]. Finally, the flowers with angles of 30°–60° had the highest pollen grains and nectar secretion, and the downward flowers significantly reduced in this flower rewards, resulting in that it is not as attractive as upward flowers [48,49]. Moreover, corresponding to the preference of pollinators, the flowers with angles of 30°–60° had the highest pollen gemination rate and stigma receptivity. This indicated that plants regulate the rewards for pollinators and the time when they are most suitable for pollination to coordinate the precise timing of pollination, maximizing the interests of insects and plants at the same time.
Corresponding to the frequency of pollinator visits, the seed set ratio and fruit set ratio of upward flowers were significantly higher than those of downward flowers, but were significantly lower than those of unmanipulated flowers. Here, the low seed production of manipulated flowers (downward and upward) may be due to the lower visiting rate of pollinator that the stigma is not fertilized by enough pollen, because changing floral vertical direction may impair the function of floral landing platforms and then decreases the pollinator visiting frequency on manipulated flowers. Another possibility is that the upward flowers do not bend normally after pollination, which may cause the pollen on the stigma to be washed away by rain [8,9], or reduce its vitality through ultraviolet rays [50], and then reduce the seed production [9].
In conclusion, this study focused on the benefits acquired by plant and pollinators which was brought by the change of floral vertical direction in T. repens. Floral vertical direction is a key part of mutualistic plant–insect interactions in pollination systems of T. repens and that a combination of ingenious mechanisms leads plants and pollinators to maximize the interests of them. The evidence provided here demonstrates that the change of floral vertical direction in T. repens exquisitely prepares its flower for pollination by insects and in doing so, exhibits a synchronized adaption between plants and animals.
Data Availability Statement: The data presented in this study are available on request from the corresponding author.
Funding Statement: This research was funded by the National Natural Science Foundation of China, Grant Nos. 31860051 and 31360044.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Huang, S. Q., Takahashi, Y., Dafni, A. (2002). Why does the flower stalk of Pulsatilla cernua (Ranunculaceae) bend during anthesis? American Journal of Botany, 89(10), 1599–1603. DOI 10.3732/ajb.89.10.1599. [Google Scholar] [CrossRef]
2. Patiño, S., Jeffree, C., Grace, J. (2002). The ecological role of orientation in tropical convolvulaceous flowers. Oecologia, 130(3), 373–379. DOI 10.1007/s00442-001-0824-1. [Google Scholar] [CrossRef]
3. Ushimaru, A., Hyodo, F. (2005). Why do bilaterally symmetrical flowers orient vertically? Flower orientation influences pollinator landing behaviour. Evolutionary Ecology Research, 7(1), 151–160. DOI 10.1109/TSE.2006.92. [Google Scholar] [CrossRef]
4. Hodges, S. A., Whittall, J. B., Fulton, M., Yang, J. Y. (2002). Genetics of floral traits influencing reproductive isolation between Aquilegia formosa and Aquilegia pubescens. The American Naturalist, 159(S3), S51–S60. DOI 10.1086/338372. [Google Scholar] [CrossRef]
5. Figueroa-Castro, D. M., Valverde, P. L. (2011). Flower orientation in Pachycereus weberi (cactaceaeEffects on ovule production, seed production and seed weight. Journal of Arid Environments, 75(11), 1214–1217. DOI 10.1016/j.jaridenv.2011.04.027. [Google Scholar] [CrossRef]
6. Tinoco-Ojanguren, C., Molina-Freaner, F. (2000). Flower orientation in Pachycereus pringlei. Canada Journal of Botany, 78(12), 1489–1494. DOI 10.1139/b00-133. [Google Scholar] [CrossRef]
7. Galen, S. C. (1989). Consequences of flower heliotropism for reproduction in an alpine buttercup (Ranunculus adoneus). Oecologia, 78(4), 477–485. DOI 10.1007/BF00378737. [Google Scholar] [CrossRef]
8. Mao, Y. Y., Huang, S. Q. (2009). Pollen resistance to water in 80 angiosperm species: Flower structures protect rain-susceptible pollen. New Phytologist, 183(3), 892–899. DOI 10.1111/j.1469-8137.2009.02925.x. [Google Scholar] [CrossRef]
9. Wang, Y., Meng, L. H., Yang, Y. P., Duan, Y. W. (2010). Change in floral orientation in Anisodus luridus (Solanaceae) protects pollen grains and facilitates development of fertilized ovules. American Journal of Botany, 97(10), 1618–1624. DOI 10.3732/ajb.1000010. [Google Scholar] [CrossRef]
10. Lu, S., Rieger, M., Duemmel, M. J. (1992). Flower orientation influences ovary temperature during frost in peach. Agricultural and Forest Meteorology, 60(3–4), 181–191. DOI 10.1016/0168-1923(92)90037-5. [Google Scholar] [CrossRef]
11. Leibfried, U., Ortjohann, J. (1995). Convective heat loss from upward and downward-facing cavity solar receivers: Measurements and calculations. Journal of Solar Energy Engineering, 117(2), 75–84. DOI 10.1115/1.2870873. [Google Scholar] [CrossRef]
12. Galen, C., Stanton, M. L. (2003). Sunny-side up: Flower heliotropism as a source of parental environmental effects on pollen quality and performance in the snow buttercup, Ranunculus adoneus (Ranunculaceae). American Journal of Botany, 90(5), 724–729. DOI 10.3732/ajb.90.5.724. [Google Scholar] [CrossRef]
13. Corbet, S. A. (1990). Pollination and the weather. Israel Journal of Plant Sciences, 39(1–2), 13–30. DOI 10.1080/0021213X.1990.10677131. [Google Scholar] [CrossRef]
14. van der Kooi, C. J., Kevan, P. G., Koski, M. H. (2019). The thermal ecology of flowers. Annals of Botany, 124(3), 343–353. DOI 10.1093/aob/mcz073. [Google Scholar] [CrossRef]
15. Sun, J. F., Gong, Y. B., Renner, S. S., Huang, S. Q. (2008). Multifunctional bracts in the dove tree Davidia involucrata (Nyssaceae: CornalesRain protection and pollinator attraction. The American Naturalist, 171(1), 119–124. DOI 10.1086/523953. [Google Scholar] [CrossRef]
16. Neal, P. R., Dafni, A., Giurfa, M. (1998). Floral symmetry and its role in plant-pollinator systems: Terminology, distribution, and hypotheses. Annual Review of Ecology and Systematics, 29(1), 345–373. DOI 10.1146/annurev.ecolsys.29.1.345. [Google Scholar] [CrossRef]
17. Inouye, D. W. (1980). The terminology of floral larceny. Ecology, 61(5), 1251–1253. DOI 10.2307/1936841. [Google Scholar] [CrossRef]
18. Maloof, J. E., Inouye, D. W. (2000). Are nectar robbers cheaters or mutualists? Ecology, 81(10), 2651–2661. DOI 10.1890/0012-9658(2000)081[2651:ANRCOM]2.0.CO;2. [Google Scholar] [CrossRef]
19. Irwin, R. E., Maloof, J. E. (2002). Variation in nectar robbing over time, space, and species. Oecologia, 133(4), 525–533. DOI 10.1007/s00442-002-1060-z. [Google Scholar] [CrossRef]
20. Kevan, P. G. (1975). Sun-tracking solar furnaces in high arctic flowers: Significance for pollination and insects. Science, 189(4204), 723–726. DOI 10.1126/science.189.4204.723. [Google Scholar] [CrossRef]
21. Darwin, C. (2011). On the various contrivances by which orchids are fertilized by insects. Chicago: University of Chicago Press. [Google Scholar]
22. Ushimaru, A., Kawase, D., Imamura, A. (2006). Flowers adaptively face down-slope in 10 forest-floor herbs. Functional Ecology, 20(4), 585–591. DOI 10.1111/J.1365-2435.2006.01153.X. [Google Scholar] [CrossRef]
23. Ushimaru, A., Dohzono, I., Takami, Y., Hyodo, F. (2009). Flower orientation enhances pollen transfer in bilaterally symmetrical flowers. Oecologia, 160(4), 667–674. DOI 10.1007/s00442-009-1334-9. [Google Scholar] [CrossRef]
24. Tadey, M., Aizen, M. A. (2001). Why do flowers of a hummingbird-pollinated mistletoe face down? Functional Ecology, 15(6), 782–790. DOI 10.2307/826728. [Google Scholar] [CrossRef]
25. Fenster, C. B., Armbruster, W. S., Dudash, M. R. (2009). Specialization of flowers: Is floral orientation an overlooked first step? The New Phytologist, 183(3), 502–506. DOI 10.1111/j.1469-8137.2009.02852.x. [Google Scholar] [CrossRef]
26. Gómez, J. M., Perfectti, F., Camacho, J. P. M. (2006). Natural selection on Erysimum mediohispanicum flower shape: Insights into the evolution of zygomorphy. The American Naturalist, 168(4), 531–545. DOI 10.1086/507048. [Google Scholar] [CrossRef]
27. Yon, F., Kessler, D., Joo, Y., Cortés Llorca, L., Kim, S. G. et al. (2017). Fitness consequences of altering floral circadian oscillations for Nicotiana attenuata. Journal of Integrative Plant Biology, 59(3), 180–189. DOI 10.1111/jipb.12511. [Google Scholar] [CrossRef]
28. Yon, F., Joo, Y., Cortés Llorca, L., Rothe, E., Baldwin, I. T. et al. (2016). Silencing Nicotiana attenuata LHY and ZTL alters circadian rhythms in flowers. New Phytologist, 209(3), 1058–1066. DOI 10.1111/nph.13681. [Google Scholar] [CrossRef]
29. Harris, W. (1987). Population dynamics and competition. In: Baker, M. J., Williams, W. M. (Eds.) White clover, pp. 203–298. Wallingford, U.K: CAB International. [Google Scholar]
30. Ehmet, N., Wang, Y. P., Zhao, X., Chen, D. W., Hou, Q. Z. (2021). Adaptive response of reproductive characteristics of Trifolium repens on time scale. Flora-Morphology Distribution Functional Ecology of Plants, (2–3), 151923. DOI 10.1016/j.flora.2021.151923. [Google Scholar] [CrossRef]
31. Rodet, G., Vaissière, B. E., Grossa, B. (1998). Status of self-pollen in bee pollination efficiency of white clover (Trifolium repens L.). Oecologia, 114(1), 93–99. DOI 10.1007/s004420050424. [Google Scholar] [CrossRef]
32. Michaelson-Yeates, T. P. T., Marshall, A. H., Williams, I. H., Carreck, N. L., Simpkins, J. R. (1997). The use of isoenzyme markers to determine pollen flow and seed paternity mediated by apis mellifera and bombus spp. in trifolium repens, a self-incompatible plant species. Journal of Apicultural Research, 36(2), 57–62. DOI 10.1080/00218839.1997.11100931. [Google Scholar] [CrossRef]
33. Evanhoe, L., Galloway, L. F. (2002). Floral longevity in Campanula Americana (CampanulaceaeA comparison of morphological and functional gender phases. American Journal of Botaty, 89, 587–591. DOI 10.3732/ajb.89.4.587. [Google Scholar] [CrossRef]
34. Dafni, A., Kevan, P. G., Husband, B. C. (2005). Practical pollination biology. Canada: Enviroquest, Ltd. [Google Scholar]
35. Grindeland, J. M., Sletvold, N., Ims, R. A. (2005). Effects of floral display size and plant density on pollinator visitation rate in a natural population of Digitalis purpurea. Functional Ecology, 19(3), 383–390. DOI 10.1111/j.1365-2435.2005.00988.x. [Google Scholar] [CrossRef]
36. Sánchez, A. M., Albert, M. J., Rodríguez, M., Escudero, A. (2012). Extended flowering in a mediterranean shrub: Seasonal variability in seed quality and quantity. Flora-Morphology, Distribution, Functional Ecology of Plants, 207(11), 821–827. DOI 10.1016/j.flora.2012.09.007. [Google Scholar] [CrossRef]
37. Elzinga, J. A., Atlan, A., Biere, A., Gigord, L., Weis, A. E. et al. (2007). Time after time: Flowering phenology and biotic interactions. Trends in Ecology & Evolution, 22(8), 432–439. DOI 10.1016/j.tree.2007.05.006. [Google Scholar] [CrossRef]
38. Ashman, T. L., Schoen, D. J. (1994). How long should flowers live? Nature, 371(6500), 788–791. DOI 10.1038/371788a0. [Google Scholar] [CrossRef]
39. Blair, A. C., Wolfe, L. M. (2007). The association between floral longevity and pollen removal, pollen receipt, and fruit production in flame azalea (Rhododendron calendulaceum). Botany, 85(4), 414–419. DOI 10.1139/B07-025. [Google Scholar] [CrossRef]
40. Blionis, G. J., Vokou, D. (2001). Pollination ecology of Campanula species on Mt Olympos, Greece. Ecography, 24(3), 287–297. DOI 10.1111/J.1600-0587.2001.tb00201.x. [Google Scholar] [CrossRef]
41. Meléndez-Ackerman, E., Campbell, D. R., Waser, N. M. (1997). Hummingbird behavior and mechanisms of selection on flower color in Ipomopsis. Ecology, 78(8), 2532–2541. DOI 10.1890/0012-9658(1997)078[2532:HBAMOS]2.0.CO;2. [Google Scholar] [CrossRef]
42. Schiestl, F. P., Peakall, R., Mant, J. G., Ibarra, F., Schulz, C. et al. (2003). The chemistry of sexual deception in an orchid-wasp pollination system. Science, 302(5644), 437–438. DOI 10.1126/science.1087835. [Google Scholar] [CrossRef]
43. Sprengel, C. K. (1793). Das entdeckte Geheimniss der Natur im Bau und in der Befruchtung der Blumen. Czech Republic: bei Friendrech Vieveg dem aeltern. [Google Scholar]
44. Waddington, K. D., Heinrich, B. (1979). The foraging movements of bumblebees on vertical “inflorescences”: An experimental analysis. Journal of Comparative Physiology, 134(2), 113–117. DOI 10.1007/BF00610469. [Google Scholar] [CrossRef]
45. Corbet, S. A., Cuthill, I., Fallows, M., Harrison, T., Hartley, G. (1981). Why do nectar-foraging bees and wasps work upwards on inflorescences? Oecologia, 51(1), 79–83. DOI 10.1007/BF00344656. [Google Scholar] [CrossRef]
46. Haynes, J., Mesler, M. (1984). Pollen foraging by bumblebees: Foraging patterns and efficiency on Lupinus polyphyllus. Oecologia, 61(2), 249–253. DOI 10.1007/BF00396768. [Google Scholar] [CrossRef]
47. Comba, L., Corbet, S. A., Hunt, H., Outram, S., Parker, J. S. et al. (2000). The role of genes influencing the corolla in pollination of Antirrhinum majus. Plant Cell and Environment, 23(6), 639–647. DOI 10.1046/J.1365-3040.2000.00580.X. [Google Scholar] [CrossRef]
48. Klinkhamer, P. G., de Jong, T. J. (1990). Effects of plant size, plant density and sex differential nectar reward on pollinator visitation in the protandrous Echium vulgare (Boraginaceae). Oikos, 57(3), 399–405. DOI 10.2307/3565970. [Google Scholar] [CrossRef]
49. Gori, D. F. (1989). Floral color change in Lupinus argenteus (FabaceaeWhy should plants advertise the location of unrewarding flowers to pollinators? Evolution, 43(4), 870–881. DOI 10.1111/j.1558-5647.1989.tb05184.x. [Google Scholar] [CrossRef]
50. Von Hase, A., Cowling, R. M., Ellis, A. G. (2006). Petal movement in cape wildflowers protects pollen from exposure to moisture. Plant Ecology, 184(1), 75–87. DOI 10.1007/s11258-005-9053-8. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |