

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020578
ARTICLE
Antifungal Activity of Crude Extracts of Tectona grandis L.f. against Wood Decay Fungi
1Facultad de Ingeniería en Tecnología de la Madera, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, 58030, México
2Instituto de Investigaciones Químico-Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, 58030, México
*Corresponding Author: Crisanto Velázquez-Becerra. Email: cvelazquez@umich.mx
Received: 01 December 2021; Accepted: 08 February 2022
Abstract: Wood is mainly made up of cellulose, hemicelluloses, lignin polymers and other organic and inorganic substances, making it susceptible to deteriorate by various biological agents. Tectona grandis L.f. (Teak) is a timber species with high resistance to biological deterioration, valued for its durability, beauty, and mechanical resistance. The purpose of this work was to evaluate the antifungal activity of crude extracts from teak on various fungi that cause wood deterioration. For this, Teak heartwood was obtained, then fragmented and pulverized until obtaining a flour which was used for compounds extraction using the Soxhlet technique coupled to a rotary evaporator through solvents of increasing polarity (hexane, dichloromethane, tetrahydrofuran, and acetone). The extracts obtained were tested against fungal organisms collected in the field, and the LC50 was determined using teak crude extracts on Artemia salina as a biological model. The results obtained showed that a high flour yield was obtained with hexane (0.951 g), followed by tetrahydrofuran (0.446 g), dichloromethane (0.348 g), and acetone (0.152 g). By using nine fungal organisms that predominantly correspond to the genus Aspergillus, the extractable compounds were tested, inhibiting 25% of mycelial growth with tetrahydrofuran (T. versicolor), and 40.9% with dichloromethane (G. trabeum). Likewise, the biological model of A. salina showed an LC50 of 84.9 μg/mL with hexane, 43.3 μg/mL with dichloromethane, 59.6 μg/mL with tetrahydrofuran, and 54.7 μg/mL with acetone. For this reason, it is concluded that Teak wood contains many extractable compounds in relation to its weight, besides having antimicrobial activity when extracted through polar compounds such as dichloromethane and tetrahydrofuran.
Keywords: Tectona grandis; wood deterioration; naphthoquinone; antifungal activity; wood protection
Nomenclature
| LC50 | Lethal Concentration 50 |
| T-Hex | Hexane |
| T-Dic | Dichloromethane |
| T-Thf | Tetrahydrofuran |
| T-Ace | Acetone |
Wood is a material that has historically been used by man in house construction, tool making, handicrafts, musical instruments and much more. Because of the organic nature of this material, wood is susceptible to deterioration by various biological agents, among which is a varied group of chromogenic and rotting fungi, bacteria, full-cycle xylophagous insects (anobiid, lyctids and cerambycids), social insects (termites) and marine xylophages [1,2].
Wood is mainly made up of cellulose molecules (C6H10O5)n, hemicelluloses (xylan, D-glycans, β-glucans, and xyloglycans) and lignin polymers, besides other organic and inorganic substances like secondary compounds like fats, waxes, resin acids, terpenoids, steroids, flavonoids, tannins, and water. These compounds in the heartwood area cause the resistance to biological deterioration, e.g., the arboreal species Tectona grandis L.f., (Teak) is a timber species capable of persisting, dominating, and regenerating against biological deterioration; it is one of the most valuable and best valued tropical woods and it has been planted extensively to produce wood for construction, fine furniture, and general carpentry [3,4]. It is a precious wood because of its durability, appreciated color, mechanical resistance, and endurance to water deterioration [5]. Teak heartwood durability is given by the presence of chemical compounds like tectoquinone (2-Methylanthraquinone) and naphthoquinone (Lawsone, Vitamin K1, Plumbagine, Juglone, Javanicin), the latter group of compounds showing greater resistance to decomposition against rot fungi [6]. Among the most dangerous fungal organisms that damage wood, we find the so-called white rot (Trametes versicolor, Phanaerochaete chrysosporium and Pleurotus ostreatus), brown rot (Serpula lacrymans, Coniophora puteana, Antrodia vaillantii, Lentinus lepideus and Gloumeophyllum trabeumeophyllum), and soft rot fungi (Chaetomium globesum, Monodictys putredinis, Hypocrea muroiana, Cryphonectria parasitica and Fusarium oxysporum) [7,8], responsible for the loss of physical, chemical and mechanical properties in wood [9]. Each type of rot fungi affects differently the chemical components of wood, i.e., they can degrade and use wood as food (cellulose and hemicellulose) by catabolizing the structural components through enzymes such as oxidases and peroxidases (EC 1.11.1.7) or laccases (EC 1.10.3.2) [10,11].
Wood susceptible to biological deterioration must be artificially preserved. The industry for the chemical preservation of wood developed at the beginning of the 19th century and has been of great technological, social, historical, and economic importance, using extremely effective mixtures of mineral salts with organic molecules. Mixtures such as Copper/Chromium/Arsenical salts (CCA); Copper/Azoles/Organics (CA); Coppers/Azoles/Organics/Boron (CAB); Pentachlorophenol (PCF); Arsenic/Copper/Ammonia (ACA); Chromium/Zinc/Chlorine (CZC), among others, to control the growth and proliferation of bacteria, fungi, and insects in wood [12,13]. Those compounds are highly effective to inhibit and eliminate microbial growth since lignocellulosic materials treated with CCA and exposed to extreme environmental conditions (high humidity and temperature) can maintain a useful life for decades. However, to increase the half-life of timber products at a minimum cost, an irrational amount of pesticides has been applied to wood, contributing to the alarming deterioration of the ecosystem, a sociocultural practice that is now an emerging and a multifactorial environmental problem whose solution is complex [14]. In the twentieth century, some control measures for the release of pesticides were implemented in developed countries, which were later copied and adapted in underdeveloped countries. One of them was the “Food Quality Protection Act” issued by the US government in 1996. In it, it was proposed to drastically restrict the use of conventional insecticides. At the same time, the definition of pesticide proposed by the World Health Organization (WHO) was modified and included biopesticides such as deterrents with a confounding effect as oviposition inhibitor, antinutritional and repellent, as well as biological control agents [15]. Besides, protection methods with natural components are the technological alternative to replace the conventional and harmful preservatives for low-durability wood. The use of this eco-friendly technology is slow and less popular due to the practicality, effectiveness, and low cost of conventional preservatives.
Among the alternative preservation methods, there are technological flaws that must be overcome, and there is the challenge that needs to be solved rapidly due to the alarming environmental deterioration and the change in government policies. Some of these methods include the differences obtained under controlled conditions and the yield in the field, difficulties in efficiency related to exposure, environmental conditions, and legislation conflicts worldwide [16]. However, different natural compounds have justified their efficiency as wood preservatives. Historically, plant extracts were the molecular basis for the chemical synthesis of pyrethroids nicotinoids. Another successful example of insecticides is derived from the Azadirachta indica (neem) or Enterolobium cyclocarpum (guanacaste) trees where their essential oils or compounds contained in the heartwood, such as azadiracthin can work as insecticides [17]. Likewise, microbial agents for biological control are useful and efficient, and comprise a paradigm in the control of pests, like Bacillus thuringensis, encouraging researchers to know the style and the life cycle of bacteria and other microorganism to use them in wood preservation. Also, novel synthetic molecules and natural products with biological activity such as pest controllers are surfacing, as a partial solution for the control of deleterious organisms that damage wood [14,18], so, with these measures adopted, a new academic, biotechnological, and commercial opportunity is provided to design preservatives for wood with low durability. The purpose of this work was to evaluate the antifungal activity of crude extracts from T. grandis on the growth of various fungi that cause wood deterioration.
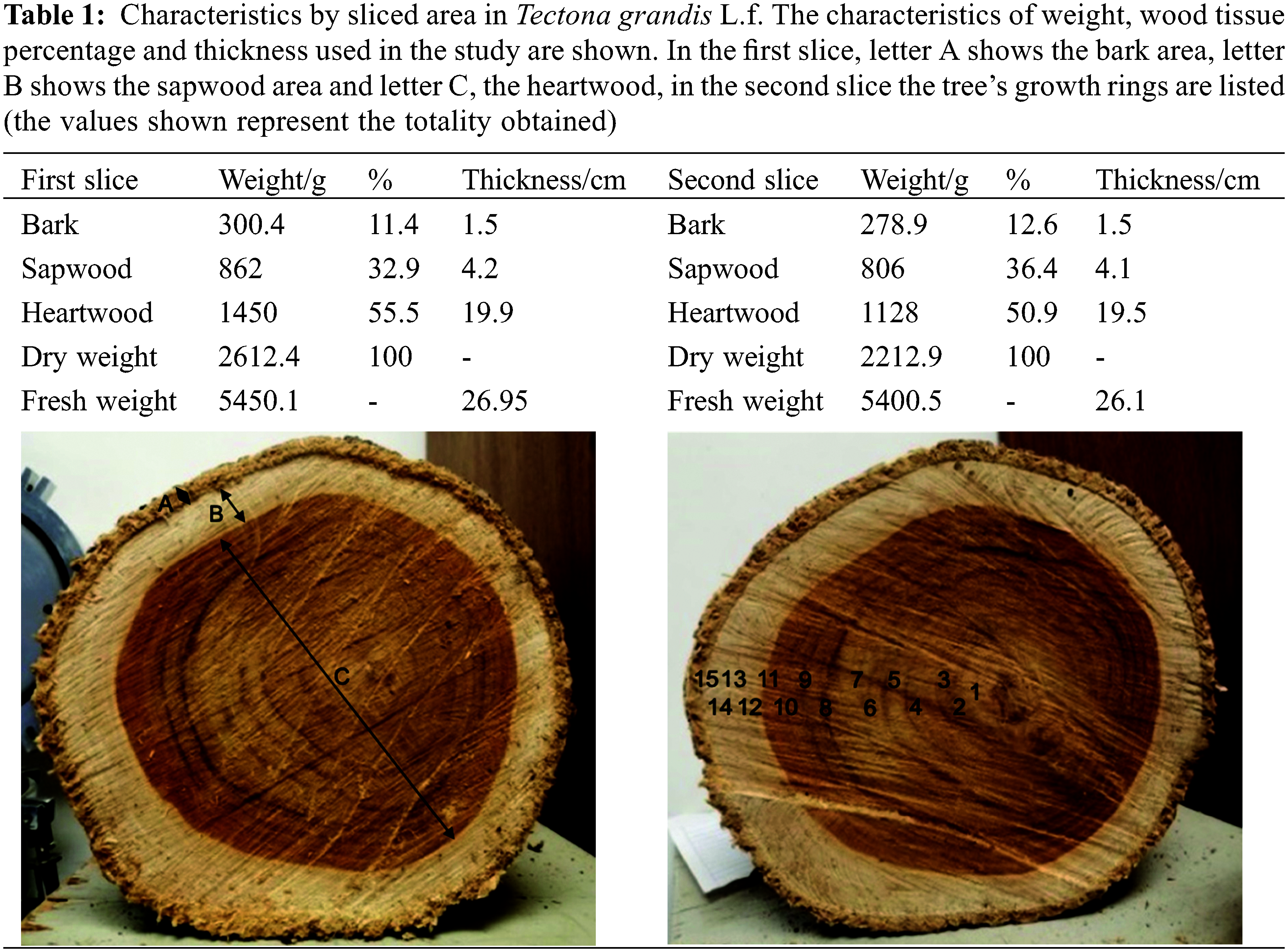
2.1 Obtaining the Wood of Tectona Grandis
The wood was obtained from the “Rancho Guadalupe” forest plantation, located in the municipality of Nuevo Urecho, Michoacán (19°12′10.4"N 101°52′22.1"W/19.202874, −101.872814), and the selected tree had an approximate age of 14 years. To obtain the samples, two consecutive cuts were made in a transversal way, at a height of 1.5 m from the base of the trunk (Table 1). Subsequently, the samples were processed by removing the bark from the samples and carefully separating the sapwood and heartwood of both slices, fragmenting them with the help of a hammer (TRUPERTM model MA-7, Mexico) and chisel (TRUPERTM model FT-1/2, Mexico) until obtaining splinters of approximately 5 × 1 cm. Later, with the help of a mill (MICRON-K2OF) the splinters were pulverized, obtaining heartwood flour. The flour obtained was sieved with a TYLER equipment (model RX-29, USA), through 20 and 40 mesh sizes (850 and 425 microns, respectively). The samples obtained were placed in a desiccator until use.
2.2 Obtaining Raw Extracts by the Soxhlet Technique
Using 12 g of wood flour (Heartwood) obtained previously, compounds were extracted using the Soxhlet technique coupled to a rotary evaporator (BUCHI brand, Switzerland) [19]. The samples were placed in an extraction thimble (brand Whatman GE Healthcare) with the following working conditions: 4 h at reflux with solvents by ascending polarity, hexane (CAS 110-54-3), dichloromethane (CAS 75-09-2), tetrahydrofuran (CAS 109-99-9) and acetone (CAS 67-64-1) (Performed three times independently for each solvent). The extraction of compounds was derived from the heartwood. Subsequently, the extracts were stored in glass containers and placed in a desiccator for 24 h.
2.3 Collection and Identification of Wood Decay Fungus
The fungal organisms Trametes versicolor CTB-863A and Gloeophyllum trabeum BAM Ebw 109 were donated by the Department of Wood, Cellulose and Paper of the University of Guadalajara Jalisco, Mexico (DMCP). The rest of the fungal organisms were collected in the field, which consisted of visiting the town of “Los Azufres, Ciudad Hidalgo Michoacán, Mexico” (Latitude: 19.7833 Longitude: −100.65) during the month of August 2019. On the site, and with the help of a camping knife, the carpophores from dead tissue of felled wood and standing trees were carefully extracted (Fig. 1). Separately, the samples were placed in sterile plastic bags and were transferred to the Wood Microbiology Laboratory of the Michoacan University of San Nicolás de Hidalgo, to immediately continue with the isolation protocol of the organisms [20].

Figure 1: Fungal organisms in the original substrate under natural conditions are shown and correspond to the genus Aspergillus (UM-2, UM-2 (7), UM-G1, UM-A4, UM-4 (9) and UM-A5). The remaining isolates correspond to Macrophomina (UM-E2) and Schizophyllum (UM-D1). The isolated UM-C3 was not identified
For the isolation of the collected organisms (with code UM-), a laminar flow hood (Novatech Model CFLH-120E, Mexico) was used, where the samples were washed with sterilized water for 5 min. The samples were culture using Sabouraud Dextrose Agar (SDA, BD Bioxon) as a culture medium in a Petri dish system, prepared in an autoclave (AESA model CV300, Mexico) (121°C/15 lb pressure for 15 min) according to the manufacturer’s specifications, then, the plates inoculated with the fungus were incubated (ECOSHEL model 9052, Mexico) in the dark at 28°C for 5 days. Subcultures were made from the plates with bacterial growth until pure cultures were obtained.
2.4 Molecular Identification of UM-Xylophagous Isolates
From pure cultures, the DNA extraction of the UM-isolates was carried out with the method described by Raeder et al. [21] with some modifications, first, 100 mg of mycelium were weighed into a 1.5 ml tube (Eppendorf, USA) and 0.4 g of glass beads, 200 μl of lysis buffer (0.2 M Tris-HCl pH 7.5, 0.5 M NaCl, 0.01 M EDTA, 1% SDS), and 200 μl phenol:chloroform:isoamyl (25:24:1) were added. The contents of the tube were vortexed (Scientific Industries Inc., USA) for 6 min, and then, 300 μl of phenol: chloroform: isoamyl and 300 μl of lysis buffer were added. The samples were vortexed again for 30 s. Samples were centrifuged (Eppendorf, USA) at 14,000 rpm for 30 s, and the first phase was transferred to a clean tube and 300 μl of chloroform:isoamyl (24:1) were added by mixing with a vortex for 10 s. The samples were centrifuged (Thermo Scientific model 75002478, USA) at 14,000 rpm for 30 s and the aqueous phase was transferred to a clean tube and 2.5 volumes of isopropanol were added. The samples were kept in a freezer at −20°C (Torrey Model CHTC-115D, Mexico) for 30 min, and then, samples were centrifuged again at 14,000 rpm for 5 min. The pellet was washed with 500 μl of 70% ethanol and centrifuged for 3 min at 14,000 rpm. The pellet obtained was left in the hood until it was dried. Subsequently, it was resuspended in 30 μl of sterile deionized water. Finally, the nucleic acid pellet was purified using the PureLink Plant DNA Purification Kit (Invitrogen, USA), according to the product specifications. DNA was visualized on 1% agarose gel (Sigma-Aldrich, USA) and then the optical density quantification was carried out at 260 nm with an UV-VIS spectrophotometer (Thermo Scientific, Genesys, USA), using 3 μl of DNA and 997 μl of sterile deionized water for the readings. The DNA concentration was calculated according to the formula used by Barbas III, etc., DNA concentration = A260 × 50 × 1000/3.
For the identification of the fungal isolates, the amplification of the 18S gene was carried out using the oligonucleotides NS1 and NS6. The PCR reaction was carried out using a BioRad-iCycler kit (USA) with a final volume of 20 μl, obtained as follows: 3 to 8 ng of DNA, 0.5 μM NS1, 0.5 μM NS6, and 12 μl PCR Master Mix (Promega Corp., USA). The samples were centrifuged and heated at 94°C for 2 min, then, 35 PCR cycles were carried out under the following temperatures: 94°C for 1 min, 55°C for 1 min and 72°C for 1 min; finally, an additional extension cycle was given at 72°C for 3 min (BioRad model 580BR, USA). The amplification products were visualized on a 1% agarose gel. Once the amplification of the 18S gene was confirmed, the purification of the PCR product was carried out with a Wizard SV Gel and PCR Clean-Up System (Promega Corp., USA), according to the manufacturer’s specifications. This material was sequenced with the Sanger technique in automated capillary and fluorescence technology systems (langebio.cinvestav.mx/). The sequences obtained were compared with other sequences deposited in the GenBank database, and compared using the basic alignment search tool (BLAST) (blast.ncbi.nlm.nih.gov/Blast.cgi) to find the closest homologous sequences.
2.5 Antifungal Activity Assays
The fungal organisms donated by the DMCP and those collected in the field were used in the Petri dish inhibition assays. SDA was used as culture medium prepared according to the manufacturer’s specifications. The treatments used are shown in Table 3. Once the SDA culture medium was solidified, filter paper (1 cm2 previously sterilized in an autoclave at 121°C/15 lb pressure for 15 min) was placed at the edge of the Petri dish, on the surface of the medium, then 1.2 mg/mL of crude extract were added. The other end of the Petri dish was inoculated by taking a sample of the mycelium of the fungal isolates, and incubated for 5 days at 28°C [22]. After the incubation, the fungus growth on the culture medium was quantified and the inhibition percentage of the extracts was determined as follows:
Percentage of inhibition = [(major diameter of the control – major diameter of the treatment)/major diameter of the control] × 100
2.6 Toxicity Test of Crude Extracts of Heartwood of Tectona Grandis
2.6.1 Growth of Brine Shrimp (Artemia salina L.)
This test was carried out in the Plant Nutrition Laboratory of the Center for Innovation and Food Development of Michoacan. Two and a half liters of salty water (3% Halita “Instant Ocean” sea salt, especially for aquariums) were prepared in a fish tank (4-L capacity*), coupled with a thermostat*. The water was kept at 25°C and exposed to a light source (approximately 2000 lux, standard aquarium equipment*) on the surface of the water and constant aeration, at oxygen levels higher than 2 mg/L [23]. The A. salina cysts were allowed to hatch until reaching the nauplius phase after 72 h the Artemia culture.
2.6.2 Determination of LC50 on Brine Shrimp Model
The crude extracts from the heartwood, obtained through hexane, dichloromethane, tetrahydrofuran, and acetone were tested as follows. A stock solution of 3 mg/mL of extract dissolved in dimethyl sulfoxide (DMSO) (CAS 67-68-5) was prepared. The larvae were placed in standard 96-well plates (Costar), 6 larvae per well, and for each extract, the concentrations 7.5, 15, 22.5, 30, 60, 90 and 120 μg/ml were used, in addition, salty water (192 μl) with DMSO (8 μl) was used as control. The lethal concentration 50 (LC50) was determined through the loss or death of the Artemia salina nauplii, quantified every 12 h for a maximum period of 24 h, the nauplii were observed with a stereoscopic magnifying glass (Celestron S20 Labs brand, USA) through a 4X objective. Using Excel Microsoft, a linear regression was performed to calculate the LC50 [24].
The experiments were analyzed with the software STATISTICA v10.0 (TIBCO Statistica, Palo Alto, CA) using an ANOVA and subsequent Tukey test (P < 0.05).
3.1 Characteristics of the Woody Tissue of Tectona grandis
The woody tissue of the T. grandis tree showed a high moisture content, with 52.1 and 59.2 percent for slices 1 and 2. Also, it is important to mention that the heartwood showed the highest portion with up to 55.5% and 50.5% (slices 1 and 2, respectively), followed by sapwood and bark area (Table 1).
3.2 Heartwood Compound Extraction
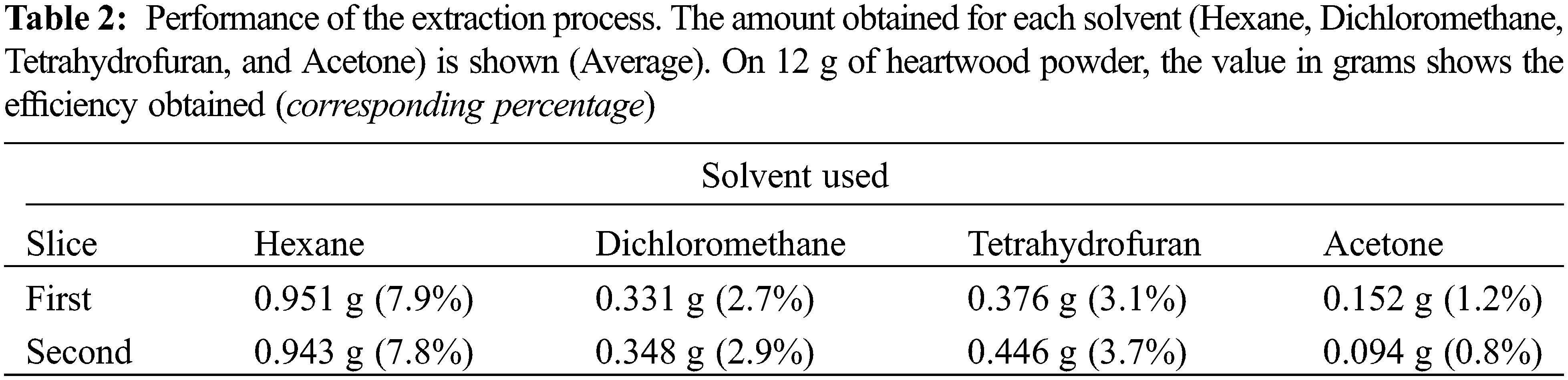
As previously described, when using 12 g of heartwood flour for each extraction, similar yields were obtained for each sample, that is, using the hexane solvent, 0.951 and 0.943 g were obtained using slices 1 and 2, for dichloromethane, 0.331 and 0.348 g, for tetrahydrofuran, 0.376 and 0.446 g, and for acetone, 0.152 and 0.094 g, which corresponds to yields between 0.8% and 7.9%, depending on the solvent used (Table 2).
3.3 Fungal Organisms Collected in the Field
A total of 9 fungal organisms were collected, which were mainly found colonizing dead or felled wood but still fresh (Fig. 1).
3.4 Assays of Fungal Inhibition by Extracts of Tectona Grandis
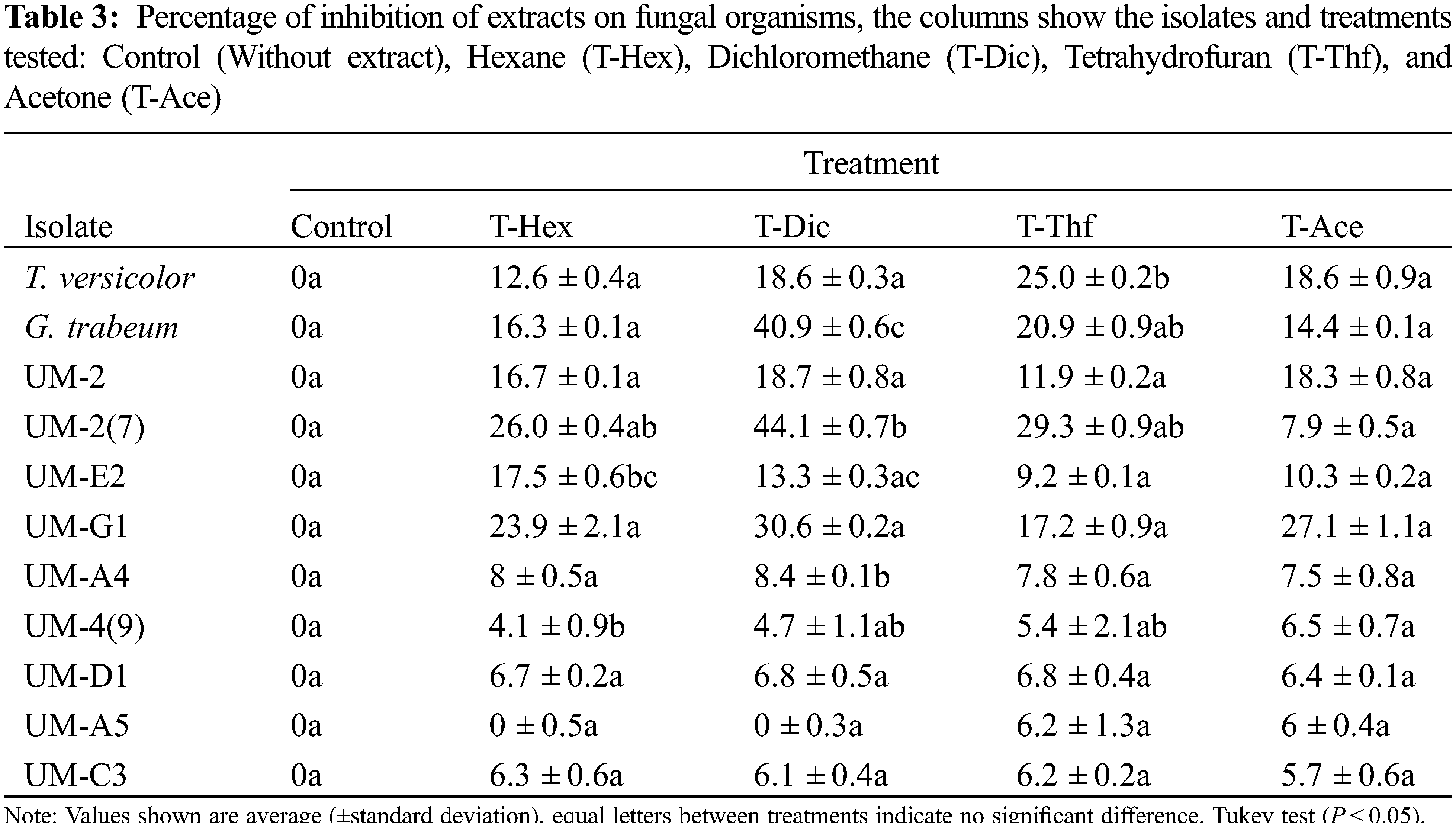
The inhibition tests were carried out on the 9 isolates collected in the field, as well as on the organisms donated by DMCP. It was found that T. versicolor was inhibited by 25% because of the exposition to the compounds extracted with tetrahydrofuran (T-Thf). On the other hand, G. trabeum also showed strong inhibition of 40.9% and 20.9% with compounds extracted with dichloromethane (T-Dic) and tetrahydrofuran (T-Thf), respectively. Also, it is important to mention that several of the fungal isolates showed susceptibility to the extractable compounds tested, e.g., the UM-2 isolate (7) showed an inhibition of up to 44.1% (T-Dic) in relation to the control treatment, the isolate UM-E2 showed an inhibition of 17.5% (T-Hex), UM-A4 exhibited an inhibition of 8.4% (T-Dec) and UM-4 (9) an inhibition of 4.1% regarding mycelial growth. On the other hand, the rest of the isolates tested (UM-2, UM-G1, UM-D1, UM-A5 and UM-C3) did not show any significant inhibition of mycelial growth, however, a clear trend towards repression, since all values show inhibition of mycelial growth in different percentages (Table 3).
3.5 Taxonomic Identity of the Fungal Isolates
The various fungal isolates found corresponded mainly to organisms of the genus Aspergillus (UM-2, UM-2 (7), UM-G1, UM-A4, UM-4 (9) and UM-A5). The remaining isolates correspond to Macrophomina (UM-E2) and Schizophyllum (UM-D1) (the isolated UM-C3 was not identified).
Of the organisms identified, the literature denotes that they correspond to fungi that deteriorate wood, e.g., Aspergillus niger is a soft-rot fungus, responsible for cavity formation (soft-rot) and erosion detected in archaeological wood especially from terrestrial environments [25]. Macrophomina has been reported as a fungal trunk pathogen in various Prunus species [26–28]. Schizophyllum has been identified as an organism that produces dark pigmented lines in bleached wood, and in some cases produces white rot decay (Table 4) [29–31].

3.6 Toxicological Evaluation of Crude Extracts of Tectona grandis L.f. Using Brine Shrimp (Artemia salina L.) as a Model (LC50)
The extracts obtained through the various solvents were shown to exert a toxic effect on the A. salina model. Specifically, the hexane extract (T-Hex) showed that at a concentration of 84.9 μg/mL, the LC50 is achieved, for its part, the LC50 with dichloromethane (T-Dic) extracts were found with 43.3 μg/mL, tetrahydrofuran (T-Thf) with 59.6 μg/mL and acetone (T-Ace) with 54.7 μg/mL (Fig. 2).
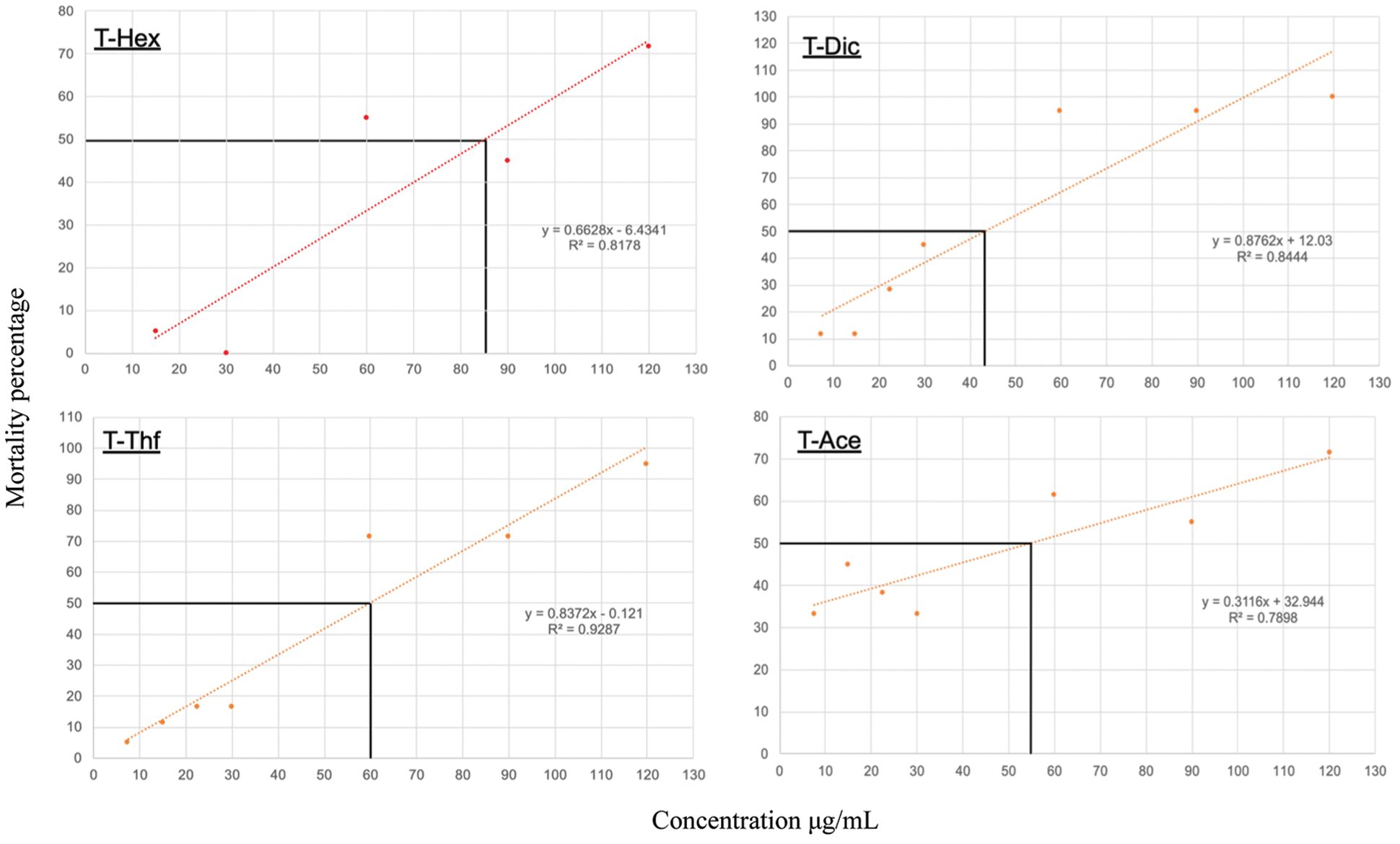
Figure 2: Toxicological evaluation of crude extracts of Tectona grandis L.f. using Brine Shrimp (Artemia salina L.) as a model (Lethal Concentration 50 LC50). Heartwood raw extracts: hexane (T-Hex), dichloromethane (T-Dic), tetrahydrofuran (T-Thf) and acetone (T-Ace)
Tectona grandis (Teak) wood is one of the most valuable and known tropical timber species and is highly valued for its use in ship building, outdoor equipment, furniture, and general carpentry. It is prized mostly for its natural durability and high dimensional stability in association with pleasant aesthetics. Also, it is important to say that the age of the tree (Teak) from which the wood is obtained is related to the concentration of secondary metabolites in the woody tissue such as heartwood, since there are many compounds like alkane, olefin, benzene, alcohols, ketones, aldehyde, esters, acid anhydride, phenols, quinone, and others, some responsible for antimicrobial activity. Teak wood over 12 years of age is considered mature wood, and the samples used in the present work exhibited an age of at least 15 years (tree), which, according to works of Miranda et al., [32,33]. they reported that they contained a significant amount of extractable compounds. Also, it is important to say that the general chemical composition and presence of extractable compounds with antimicrobial activity in teak wood is appropriately documented in the literature, which is why this document does not explore this aspect, but a lack of information regarding the effect of extractables on the great variety of fungal organisms related to damage or wood rotting, this is where this work finds its value.
Under field conditions, fungal organisms exhibit a nutritional and growth behavior different from that presented in the laboratory, which is why their reproduction is not viable in many occasions. In this work, nine fungal organisms were collected and all grew under a Petri dish system in an adequate way, achieving their isolation and reproduction successfully, revealing that they corresponded to organisms related to damage or wood rotting, for example, fungi belonging to the genus Aspergillus (A. niger) is a soft-rot (type I) fungus, responsible for cavity formation and erosion (type II) detected in archaeological wood especially from terrestrial environments [25,31]. Macrophomina spp., is the most damaging seed and soil borne pathogen, infecting about 500 plant species in more than 100 families throughout the world [27,34,35]. Schizophyllum commune is wood-decay fungus that has a world-wide distribution and this basidiomycete had been shown to produce indole as well as dark pigmented lines in bleached wood [29,36–38]. However, due to the experimental strategy used to identify fungal organisms, it is still pending to find the species to which they belong, since it is necessary to use oligonucleotides with a greater amplitude of base pairs, in addition to enriching the information in databases available online.
However, when testing the fungal organisms against the different crude extracts, it was found that six showed statistically significant differences, though, the remaining organisms tested exhibited an interesting inhibition of mycelial growth, being the crude extracts from dichloromethane those that presented an increased biological activity. In the literature there are several reports showing extractable compounds derived from organic solvents, waxes and other non-polar compounds that are soluble in dichloromethane, making up for the remaining bark extractives, representing 16% of the total extractives. But, due to the polar nature of compounds such as Tectoquinones and Naphthoquinones, solvents such as dichloromethane and tetrahydrofuran have a high retention capacity, which is why crude extracts show antimicrobial activity, since, according to various reports, it is known that dichloromethane and tetrahydrofuran have a retention capacity of molecules like Lapachol, Isolapachol, Deoxylapachol, Betulinic Acid, Pachybasin, Juglone, Plumbagin, Lawsone, 1-Hydroxy-2-methylanthraquinone, Dehydrotectol, Xyloidone, 2-methylquinazolin-4-ol, and 1–4 naphthoquinone [39–46] molecules related to antifungal and insecticidal activity.
Some studies on the antifungal properties of teak hardwood suggest that they may result from the synergistic effect of various extractive compounds (anthraquinines and tectoquinones), however, it is still unclear whether one mechanism of action is more prevalent than the others because quinones are organic compounds with chemical properties that allow them to interact with biological targets, this through the formation of covalent bonds, acting as an electron transfer agent in oxide-reduction reactions [47]. The biological activity of quinones has been related to their oxidation-reduction and acid-base properties [48]. Also, quinones are oxidized to semiquinone, to form a radical intermediate that when reacting with molecular oxygen can generate superoxide anion, promoting hydrogen peroxide and reactive oxygen species (ROS) causing oxidative stress in the body, manifesting in severe cell damage of nucleic acids, proteins, and membranes [49]. On the other hand, it is known that naphthoquinones alter mitochondrial function and inhibit respiration by interfering with the cytochrome bc1 complex, besides promoting the generation of ROS. The stimulation of oxidative stress is caused by an imbalance between the formation of ROS, such as superoxide anion, hydrogen peroxide, hydroxyl radical, in addition to the ability of the biological system to react against these agents, which can even cause cell death. ROS can act with macromolecules generating lipid peroxidation and membrane disintegration, loss of enzymatic activity, DNA degradation and mutagenesis [50,51].
It has been reported that antifungal activity of methanol crude extract of T. grandis was investigated against Alternaria cajani, Curvularia lunata, Fusarium sp., Bipolaris sp. and Helminthosporium sp. at different concentrations. The best antifungal activity was observed with the T. grandis extracts at concentration of 5000 μg/ml with other plant extracts, showing four types of phenolic acids, tannic acid, gallic acid, ferulic acid and caffeic acid that are present in varying amount. A new naphthoquinone derivative was isolated from the heartwood of the teak stem and the chemical structure of this compound, 4’,5’-dihydroxy-epiisocatalponol. A high negative correlation was found between its concentration and the mass losses of the wood samples after exposure to the brown rot Antrodia sp., demonstrating that 4’, 5’-dihydroxyepiisocatalponol acted as a fungicide against T. versicolor at 58 mg/ml [16]. Haupt et al. [52] studied decay resistance of teak wood from Panama, and identified tectoquinone as a bioactive compound inhibiting the growth of C. puteana. Thulasidas et al. [6] reported high resistance of teak heartwood against brown-rot fungi (Polypomus palustris and G. trabeum) and white-rot fungi (P. sanguineus, T. hirsuta and T. versicolor), specifying naphthoquinone as the most important active compound. Anda et al. [53] showed high natural resistance of teak wood from Mexico to white-rot fungi (P. chrysosporium) and brown-rot fungi (G. trabeum). They identified tectoquinone, deoxylapachol, isolapachol and dehydrotectol as the components responsible for wood durability [54].
It is difficult to identify the degree of toxicity of the extracts obtained, but when comparing with other substances tested in the biological model of A. salina, we can understand their biological activity. Authors such as Camara et al. [55] shown the toxic profile of lawsone (2-hydroxy- [1,4] naphthoquinone) and a series of naphthoquinone derivatives was evaluated against the Artemia salina, and o the derivatives tested, nine fell below the threshold of 100 μg/mL setting the basis for potential molluscicidal activity by the World Health Organization. As a general rule, derivatives with non-polar substituents presented the highest molluscicidal activities. These substances showed significant toxicity in A. salina lethality bioassay. Also, other works have indicated that the isolated compounds are good candidates for anticancer and antitumor research, unlike conventional wood preservatives based on heavy metals and inorganic compounds, their toxicity is known and differs from these new alternatives, they are also known to be carcinogenic, contrary to extracts of plant origin, where research suggest to prevent microbial degradation, but also as agents involved in reducing problems such as cancer, tumors, diabetes, antioxidant, and insecticide [35,56].
The extractions carried out in Teak heartwood showed a high yield, especially when obtained through hexane, however, the extractions with dichloromethane showed the highest inhibitory capacity against the organisms tested. Also, the results showed that the extractable compounds obtained act on different genera of fungi (Trametes, Gloeophyllum, Aspergillus, Macrophomina, Schizophyllum), which broadens the knowledge about the scope and use of this type of compounds for the protection of lignocellulosic materials.
Funding Statement: Thanks to CONACYT for the scholarship granted (2019-000002-01NACF-13536).
Conflicts of Interest: The authors declare that no competing interests exist.
1. Ibach, R. E., Rowell, R. M. (2013). Biological properties of wood. Handbook of Wood Chemistry and Wood Composites, 2, 99–126. [Google Scholar]
2. Muthukrishnan, R., Remadevi, O. K. (2017). Powder post beetle menace in wooden handicraft industries and their management, pp. 277–285. Springer, Singapore. DOI 10.1007/978-981-10-3115-1_26. [Google Scholar] [CrossRef]
3. Muñoz, F., Tenorio, C., Moya, R., Navarro-Mora, A. (2022). CLT fabricated with Gmelina arborea and Tectona grandis wood from fast-growth forest plantations: Physical and mechanical properties. Journal of Renewable Materials, 10(1), 1–17. DOI 10.32604/jrm.2022.017392. [Google Scholar] [CrossRef]
4. Rivera-Tenorio, M., Moya, R., Navarro-Mora, Á. (2020). Wooden trusses using metal plate connections and fabricated with Gmelina arborea, Tectona grandis and Cupressus lusitanica timber from forest plantations. Journal of the Indian Academy of Wood Science, 17(2), 183–94. DOI 10.1007/S13196-020-00271-Z. [Google Scholar] [CrossRef]
5. Shukla, S. R., Sharma, S. K. (2021). Estimation of density, moisture content and strength properties of Tectona grandis wood using near infrared spectroscopy. Maderas: Ciencia y Tecnología, 23, 1–12. DOI 10.4067/S0718-221X2021000100418. [Google Scholar] [CrossRef]
6. Thulasidas, P. K., Bhat, K. M. (2007). Chemical extractive compounds determining the brown-rot decay resistance of teak wood. Holz Roh Werkst, 65(2), 121–124. DOI 10.1007/s00107-006-0127-7. [Google Scholar] [CrossRef]
7. Krah, F. S., Bässler, C., Heibl, C., Soghigian, J., Schaefer, H. et al. (2018). Evolutionary dynamics of host specialization in wood-decay fungi. BMC Evolutionary Biology, 18(1), 1–13. DOI 10.1186/S12862-018-1229-7. [Google Scholar] [CrossRef]
8. Marcot, B. G. (2017). A review of the role of fungi in wood decay of forest ecosystems, vol. 31, pp. 1–31. Res. Note. PNW-RN-575. Portland, OR: US Department of Agriculture, Forest Service, Pacific Northwest Research Station. [Google Scholar]
9. Días-Rivera, E., Montejo-Mayo, W., Martínez-Pacheco, M., Munro-Rojas, A., Ambriz-Parra, E. et al. (2021). Chemical-mechanical damage caused by the brown-rot fungus Gloeophyllum trabeum (Pers.) murrill on Pinus pseudostrobus lindl. wood. Revista Chapingo Serie Ciencias Forestales y del Ambiente, 27(2), 199–214. DOI 10.5154/R.RCHSCFA.2020.05.033. [Google Scholar] [CrossRef]
10. Hiscox, J., O’leary, J., Boddy, L. (2018). Fungus wars: Basidiomycete battles in wood decay. Studies in Mycology, 89, 117–124. DOI 10.1016/J.SIMYCO.2018.02.003. [Google Scholar] [CrossRef]
11. Kahl, T., Arnstadt, T., Baber, K., Bässler, C., Bauhus, J. et al. (2017). Wood decay rates of 13 temperate tree species in relation to wood properties, enzyme activities and organismic diversities. Forest Ecology and Management, 391, 86–95. DOI 10.1016/J.FORECO.2017.02.012. [Google Scholar] [CrossRef]
12. García-Ortiz, V. R., Benítez-Rocha, G., Martínez-Pacheco, M., Velázquez-Becerra, C. (2018). Preservadores maderables y exudados microbianos con actividad antagonista contra agentes biológicos deletéreos. Revista Mexicana de Fitopatología, 36(1), 56–78. DOI 10.18781/R.MEX.FIT.1704-2. [Google Scholar] [CrossRef]
13. Gérardin, P. (2016). New alternatives for wood preservation based on thermal and chemical modification of wood–A review. Annals of Forest Science, 73(3), 559–570. DOI 10.1007/S13595-015-0531-4. [Google Scholar] [CrossRef]
14. González-Laredo, R. F., Rosales-Castro, M., Rocha-Guzmán, N. E., Gallegos-Infante, J. A., Moreno-Jiménez, M. R. et al. (2015). Wood preservation using natural products. Madera y Bosques, 21, 63–76. DOI 10.21829/MYB.2015.210427. [Google Scholar] [CrossRef]
15. Lebow, S. T. (2010). Wood preservation. General Technical Report FPL-GTR-190. Madison, WI: US Department of Agriculture, Forest Service, Forest Products Laboratory. https://www.fpl.fs.fed.us/documnts/fplgtr/fplgtr190/chapter_15.pdf. [Google Scholar]
16. Singh, T., Singh, A. P. (2012). A review on natural products as wood protectant. Wood Science and Technology, 46(5), 851–870. DOI 10.1007/S00226-011-0448-5. [Google Scholar] [CrossRef]
17. Raya-González, D., Martínez-Muñoz, R. E., Ron-Echeverría, O. A., Flores-García, A., Macías-Rodríguez, L. I. et al. (2013). Dissuasive effect of an extract aqueous from Enterolobium cyclocarpum (Jacq) griseb on the dry wood termite Incisitermes marginipennis (Isoptera: Kalotermitidae) (Latreille). Emirates Journal of Food and Agriculture, 25, 524–530. DOI 10.9755/EJFA.V25I7.15987. [Google Scholar] [CrossRef]
18. Ramírez-López, C. B., García-Sánchez, E., Martínez-Muñoz, R. E., Del Río, R. E., Martínez-Pacheco, M. M. (2016). Chemical composition of the essential oil from Ageratina jocotepecana and its repellent effect on dry wood termite Incisitermes marginipennis. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, 15(1), 53–60. [Google Scholar]
19. Schwanninger, M., Hinterstoisser, B. (2002). Comparison of the classical wood extraction method using a soxhlet apparatus with an advanced extraction method. Holz Roh Werkst, 60(5), 343–346. DOI 10.1007/s00107-002-0312-2. [Google Scholar] [CrossRef]
20. García-Ortiz, V., Hernández-Soberano, C., Martínez-Pacheco, M., Ambriz-Parra, E., Velázquez-Becerra, C. (2019). Protective effect on wood by metabolic extracts from plant growth-promoting rhizobacteria against decay fungi. Revista Argentina de Microbiología, 52(2), 164–165. DOI 10.1016/j.ram.2019.04.003. [Google Scholar] [CrossRef]
21. Raeder, U., Broda, P. (1985). Rapid preparation of DNA from filamentous fungi. Letters in Applied Microbiology, 1(1), 17–20. DOI 10.1111/j.1472-765X.1985.tb01479.x. [Google Scholar] [CrossRef]
22. Rutiaga-Quiñones, J. G. (2001). Chemische und biologische untersuchungen zum verhalten dauerhafter holzarten und ihrer extrakte gegenüber holzabbauenden pilzen. Buchverl Gräfelfing. [Google Scholar]
23. Vanhaecke, P., Persoone, G., Claus, C., Sorgeloos, P. (1981). Proposal for a short-term toxicity test with Artemia nauplii. Ecotoxicology and Environmental Safety, 5(3), 382–387. DOI 10.1016/0147-6513(81)90012-9. [Google Scholar] [CrossRef]
24. Hamidi, M. R., Jovanova, B., Panovska, T. K. (2014). Toxicological evaluation of the plant products using brine shrimp (Artemia salina L.) model. Macedonian Pharmaceutical Bulletin, 60(1), 9–18. DOI 10.33320/MacedPharmBull. [Google Scholar] [CrossRef]
25. Goffredo, G. B., Citterio, B., Biavasco, F., Stazi, F., Barcelli, S. et al. (2017). Nanotechnology on wood: The effect of photocatalytic nanocoatings against Aspergillus niger. Journal of Cultural Heritage, 27, 125–136. DOI 10.1016/J.CULHER.2017.04.006. [Google Scholar] [CrossRef]
26. Akçay, Ç., Birinci, E., Birinci, C., Kolaylı, S. (2020). Durability of wood treated with propolis. BioResources, 15(1), 1547–1562. DOI 10.1080/14756366.2016.1186023. [Google Scholar] [CrossRef]
27. Ansar, M. (2017). Variability in sensitivity among different host origin-Macrophomina phaseolina isolates to azoxystrobin fungicide. International Journal of Plant Protection, 10(1), 26–33. DOI 10.15740/HAS/IJPP/10.1/26-33. [Google Scholar] [CrossRef]
28. Gramaje, D., Agustí-Brisach, C., Pérez-Sierra, A., Moralejo, E., Olmo, D. et al. (2012). Fungal trunk pathogens associated with wood decay of almond trees on mallorca (Spain). Molecular Phylogeny and Evolution of Fungi, 28(1), 1–13. DOI 10.3767/003158512X626155. [Google Scholar] [CrossRef]
29. Krause, K., Jung, E. M., Lindner, J., Hardiman, I., Poetschner, J. et al. (2020). Response of the wood-decay fungus Schizophyllum commune to co-occurring microorganisms. PLoS One, 15(4), e0232145. DOI 10.1371/JOURNAL.PONE.0232145. [Google Scholar] [CrossRef]
30. Padhiar, A., Albert, S. (2012). Anatomical studies on decaying wood of Mangifera indica by two white rot fungi Schizophyllum commune and Flavadon flavus. Journal of the Indian Academy of Wood Science, 9(2), 143–153. DOI 10.1007/S13196-012-0079-Y. [Google Scholar] [CrossRef]
31. Zhao, Y. N., He, S. H., Nakasone, K. K., Chen, C. C., Liu, S. L. et al. (2021). Global phylogeny and taxonomy of the wood-decaying fungal genus Phlebiopsis (Polyporales, Basidiomycota). Frontiers in Microbiology, 12, 1–20. DOI 10.3389/FMICB.2021.622460. [Google Scholar] [CrossRef]
32. Miranda, I., Sousa, V., Pereira, H. (2011). Wood properties of teak (Tectona grandis) from a mature unmanaged stand in East Timor. Journal of Wood Science, 57(3), 171–178. DOI 10.1007/S10086-010-1164-8. [Google Scholar] [CrossRef]
33. Qiu, H., Liu, R., Long, L. (2019). Analysis of chemical composition of extractives by acetone and the chromatic aberration of teak (Tectona Grandis L.F.) from China. Molecules, 24(10), 2–10. DOI 10.3390/molecules24101989. [Google Scholar] [CrossRef]
34. Kwaśniewska-Sip, P., Bartkowiak, M., Cofta, G., Nowak, P. B. (2019). Resistance of Scots pine (Pinus sylvestris L.) after treatment with caffeine and thermal modification against Aspergillus niger. BioResources, 14(1), 1890–1898. DOI 10.15376/BIORES.13.3.6555-6564. [Google Scholar] [CrossRef]
35. Aminin, D., Polonik, S. (2020). 1, 4-naphthoquinones: Some biological properties and application. Chemical and Pharmaceutical Bulletin, 68(1), 46–57. DOI 10.1248/CPB.C19-00911. [Google Scholar] [CrossRef]
36. Bucher, V. V. C., Hyde, K. D., Pointing, S. B., Reddy, C. A. (2004). Production of wood decay enzymes, mass loss and lignin solubilization in wood by marine ascomycetes and their anamorphs. Fungal Divers, 15, 1–14. [Google Scholar]
37. Campi, M., Maubet, Y., Armoa, J., Sandoval, P. (2017). Donkia pulcherrima (Polyporales, Phanerochaetaceae) una especie hidnoide poco conocida, nueva cita para el Paraguay. Steviana, 9(1), 25–30. [Google Scholar]
38. Żółciak, A., Sierota, Z., Małecka, M. (2012). Characterization of some Phlebiopsis gigantea isolates with respect to enzymatic activity and decay of Norway spruce wood. Biocontrol Science and Technology, 22(7), 777–790. DOI 10.1080/09583157.2012.691156. [Google Scholar] [CrossRef]
39. Dulo, B., Phan, K., Githaiga, J., Raes, K., de Meester, S. (2021). Natural quinone dyes: A review on structure, extraction techniques, analysis and application potential. Waste and Biomass Valorization, 12, 6339–6374. DOI 10.1007/S12649-021-01443-9. [Google Scholar] [CrossRef]
40. Duval, J., Pecher, V., Poujol, M., Lesellier, E. (2016). Research advances for the extraction, analysis and uses of anthraquinones: A review. Industrial Crops and Products, 94, 812–833. DOI 10.1007/S12649-021-01443-9. [Google Scholar] [CrossRef]
41. Kaur, P., Gupta, R. C., Dey, A., Pandey, D. K. (2019). Simultaneous quantification of oleanolic acid, ursolic acid, betulinic acid and lupeol in different populations of five Swertia species by using HPTLC-densitometry: Comparison of different extraction methods and solvent selection. Industrial Crops and Products, 130, 537–546. DOI 10.1016/J.INDCROP.2018.12.089. [Google Scholar] [CrossRef]
42. Lukmandaru, G. (2015). Quinone contents in teak heartwood isolated by cold extraction. Jurnal Ilmu dan Teknologi Kayu Tropis, 13(1), 28–38. DOI 10.51850/JITKT.V13I1.57. [Google Scholar] [CrossRef]
43. Qadariyah, L., Azizah, N., Syafa’atullah, A. Q., Bhuana, D. S., Mahfud, M. (2019). The extraction of natural dyes from henna leaves (Lawsonia inermis L.) by ultrasound-assisted method. IOP Conference Series. Materials Science and Engineering, 543(1), 1–7. DOI 10.1088/1757-899X/543/1/012082. [Google Scholar] [CrossRef]
44. Santos, M. C., Gonçalves, É. C. (2016). Effect of different extracting solvents on antioxidant activity and phenolic compounds of a fruit and vegetable residue flour. Scientia Agropecuaria, 7(1), 7–14. DOI 10.17268/SCI.AGROPECU.2016.01.01. [Google Scholar] [CrossRef]
45. Sumthong, P., Romero-González, R. R., Verpoorte, R. (2008). Identification of anti-wood rot compounds in teak (Tectona grandis L.f.) sawdust extract. Journal of Wood Chemistry and Technology, 28(4), 247–260. DOI 10.1080/02773810802452592. [Google Scholar] [CrossRef]
46. Viana, L. M., Freitas, M. R., Rodrigues, S. V., Baumann, W. (2003). Extraction of lapachol from Tabebuia avellanedae wood with supercritical CO2: An alternative to soxhlet extraction? Brazilian Journal of Chemical Engineering, 20, 317–325. DOI 10.1590/S0104-66322003000300011. [Google Scholar] [CrossRef]
47. Monks, T. J., Jones, D. C. (2002). The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinone-thioethers. Current Drug Metabolism, 3(4), 425–438. DOI 10.2174/1389200023337388. [Google Scholar] [CrossRef]
48. El-Najjar, N., Gali-Muhtasib, H., Ketola, R. A., Vuorela, P., Urtti, A. et al. (2011). The chemical and biological activities of quinones: Overview and implications in analytical detection. Phytochemistry Reviews, 10(3), 353–370. DOI 10.1007/s11101-011-9209-1. [Google Scholar] [CrossRef]
49. Snezhkina, A. V., Kudryavtseva, A. V., Kardymon, O. L., Savvateeva, M. V., Melnikova, N. V. et al. (2019). ROS generation and antioxidant defense systems in normal and malignant cells. Oxidative Medicine and Cellular Longevity, 2019, 1–17. DOI 10.1155/2019/6175804. [Google Scholar] [CrossRef]
50. Feng, Z., Sedeeq, M., Daniel, A., Corban, M., Woolley, K. L. et al. (2020). Comparative in vitro toxicology of novel cytoprotective short-chain naphthoquinones. Pharmaceuticals, 13(8), 1–20. DOI 10.3390/PH13080184. [Google Scholar] [CrossRef]
51. Xavier, M. R., Santos, M. M. S., Queiroz, M. G., de Lima, S. M. S., Goes, A. J. S. et al. (2020). Lawsone, a 2-hydroxy-1, 4-naphthoquinone from Lawsonia inermis (hennaproduces mitochondrial dysfunctions and triggers mitophagy in Saccharomyces cerevisiae. Molecular Biology Reports, 47(2), 1173–1185. DOI 10.1007/S11033-019-05218-3. [Google Scholar] [CrossRef]
52. Haupt, M., Leithoff, H., Meier, D., Puls, J., Richter, H. G. (2003). Heartwood extractives and natural durability of plantation-grown teakwood (Tectona grandis L.)–A case study. European Journal of Wood and Wood Products, 61(6), 473–474. DOI 10.1007/S00107-003-0428-Z. [Google Scholar] [CrossRef]
53. Anda, R. R., Koch, G., Richter, H. G., Talavera, F. J. F., Guzmán, J. A. S. et al. (2019). Formation of heartwood, chemical composition of extractives and natural durability of plantation-grown teak wood from Mexico. Holzforschung, 73(6), 547–557. DOI 10.1515/HF-2018-0109. [Google Scholar] [CrossRef]
54. Broda, M. (2020). Natural compounds for wood protection against fungi–A review. Molecules, 25(15), 3538. DOI 10.3390/MOLECULES25153538. [Google Scholar] [CrossRef]
55. Camara, C. A., Silva, T., Da-Silva, T. G., Martins, R. M., Barbosa, T. P. et al. (2008). Molluscicidal activity of 2-hydroxy-[1, 4] naphthoquinone and derivatives. Annals of the Brazilian Academy of Sciences, 80, 329–334. DOI 10.1590/S0001-37652008000200011. [Google Scholar] [CrossRef]
56. Zhang, X., Li, X., Li, Z., Wu, X., Wu, Y. et al. (2018). An NAD (P) H: Quinone oxidoreductase 1 responsive and self-immolative prodrug of 5-fluorouracil for safe and effective cancer therapy. Organic Letters, 20(12), 3635–3638. DOI 10.1021/ACS.ORGLETT.8B01409. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |