

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020338
ARTICLE
Seed Setting and Its Spatial Characteristics in Tartary Buckwheat (Fagopyrum tataricum)
1Key Laboratory of Coarse Cereal Processing, Ministry of Agriculture and Rural Affairs, College of Food and Biological Engineering, Chengdu University, Chengdu, 610106, China
2French Associates Institute for Agriculture & Biotechnology of Drylands, Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Beersheba, 84990, Israel
3Research Center of Buckwheat Industry Technology, Guizhou Normal University, Guiyang, 550001, China
*Corresponding Author: Gang Zhao. Email: zhaogang@cdu.edu.cn
#This author contributed equally to this work
Received: 17 November 2021; Accepted: 07 January 2022
Abstract: A low seed-setting rate is the main limiting factor influencing Tartary buckwheat production under high-yield cultivation conditions. To investigate the seed setting and its spatial characteristics, two Tartary buckwheat cultivars (high seed-setting rate cultivar Qianku 3; low seed-setting rate cultivar Liuku 3) were compared by a two-year field trial in 2017 and 2018. The results showed that the Tartary buckwheat underwent simultaneous flowering and fruiting. Flowers, generated from branch, were still blooming during the mature stage of grains on stem, which resulting in a greater number of flowers and grains on the branch than those on the stem at the low part of plant. The seed-setting characteristics significantly differed between two cultivars. The high seed-setting rate of Qianku 3 was 26.6% and 33.2% higher than Liuku 3 in 2017 and 2018, respectively. Meanwhile, Qianku 3 showed a higher filled grain number (157.8%) and seed-setting rate (66.4%) on branch than Liuku 3. A network analysis showed that the whole-plant seed-setting rate was positively correlated with grain number, which was closely correlated with flower number at the same position of plant. The path-coefficient analysis revealed that grains number on branches was the most dominant component (Path coefficient (P) = 2.19) of the seed-setting rate, followed by grains number on stem (P = 0.60). The grains number on branches showed the greatest positive direct effect with significant correlation (r = 0.76 and P < 0.01) on the seed-setting rate. Overall, the present study indicated that the grain number of branches may play a vital role in improving the seed-setting rate in Tartary buckwheat.
Keywords: Grain number; path-coefficient analysis; seed-setting rate; tartary buckwheat
Buckwheat (Fagopyrum Mill.) is an important medicinal and edible minor cereal crops planted worldwide owing to its medicinal and nutritive values [1,2]. There are two cultivated species of buckwheat, Tartary buckwheat (Fagopyrum tataricum (L.) Gaertner) and common buckwheat (Fagopyrum esculentum Moench). In generally, the morphological traits of Tartary buckwheat are like common buckwheat but there is some difference. The stem of Tartary buckwheat is more robust, and the plant height of Tartary buckwheat is up to 100~150 cm, while that of common buckwheat is 60~100 cm. More importantly, Tartary buckwheat has greater nutritious and pharmacological values than common buckwheat and other cereal crops, especially in medicinal applications [3–5]. The proteins and vitamins in Tartary buckwheat grains are not only far greater than wheat, rice and maize, but they can also be easily absorbed by the human body. Additionally, Tartary buckwheat contains all the essential amino acids, and the composition is like eggs [6,7]. In recent years, Tartary buckwheat has become more popular because it is rich in flavonoids, which may aid in preventing cancer and cardiovascular disease [1,8–9]. Thus, with the growing commercial interest in Tartary buckwheat, its production requires has caused great attention. In China, especially southwest China, has the largest planting area of Tartary buckwheat, and it plays an important role in the global production [10,11]. However, the yield of Tartary buckwheat is 1200~1500 kg ha–1 in production, because of the inadequate agricultural strategies in production, the low seed-setting rate results in its low and unstable yield of Tartary buckwheat [6].
Crop yield is closely correlated with blooming and fruiting, which is an essential factor among yield components. For most crops, most flowers will develop into fruits. For instance, the seed-setting rates of rice and wheat are more than 80% [12] and 85% [13]. However, Tartary buckwheat had very low seed-setting rate of about 15%–35% [14], because few seeds were developed from numerous flowers produced by Tartary buckwheat plants [6,15]. Peng et al. [16] found that sterile rice mainly aborted at the uninucleate stage. The pollens abortion rate was closely correlated with abnormal tapetum activities, such as the premature disintegration of tapetal cells around anthers or their premature aggregation into a circle. The abnormal growth and burst of pollen tubes may be the key factors causing pollination failure [17–19]. In the grain filling of rice, maize and wheat, enzymes [20,21], water [22], temperature [23,24], spikelet positions [25–27] and the levels of relevant hormones [28–30] play essential roles. In addition to cellular studies, there have been many molecular studies in wheat [31,32]. The low seed-setting rate appears to be influenced by many climatic factors, such as temperature [33], drought stress [34], water [35] and photoperiod [36]. In common buckwheat, Halbrecq et al. [37] found that limited assimilate supplies are not the important factor responsible for the low seed-setting rate. Taylor et al. [38] and Jacquemart et al. [39] stated that common buckwheat fertilization is a limiting process and the lack of fertilization is the main reason for the low seed-setting rate, but not for the factor of flower abortion. However, there are many differences in pollination and fruiting between Tartary and common buckwheat. The studies on the seed-seting rate of Tartary buckwheat are rarely explored. The specific circumstance of its blooming and fruiting remains unknown, especially why the low seed-setting rate became a characteristic of Tartary buckwheat [15]. In addition, there is limited finding on the characteristics and spatial distribution of plant fruiting or the effects and contributions of the grains on each part of plant (including stems and branches, or the upper, middle and lower areas of plant) to the seed-setting rate of the whole plant. Therefore, the objectives of this study were to: (1) investigate the spatial distributions and quantify the grains and flowers throughout the plant; and (2) assess the correlations among seed-setting rate, grain numbers and flower numbers.
2.1 Plant Materials and Site Description
Two Tartary buckwheat cultivars with different seed-setting rates were employed in this experiment [40]: the high seed-setting rate cultivar (Qianku 3, 29.1%) and the low seed-setting rate cultivar (Liuku 3, 23.3%), donated by Weining Institute of Agricultural Sciences and Liupanshui Agricultural Institute of Guizhou, respectively. The field experiment was conducted in 2017 and 2018 during growth stage at the Wufeng Experimental Station of Chengdu University, Sichuan Province, China (104°48′E, 30°60′N, 494 m altitude). The soil at the experimental site was sandy loam that contained 15.2 g kg–1 organic material and 128.0, 23.7 and 116.0 mg kg–1 of available N, P and K, respectively. Before sowing, 45 kg hm–2 urea (N), 120 kg hm–2 phosphorus (P2O5) and 40 kg hm–2 potassium oxide (K2O) were applied and incorporated. An additional 45 kg hm–2 urea (N) was distributed during the seedling stage. Seeds were soaked with 0.1% potassium permanganate before sowing on 05 September 2017 and 07 September 2018 with a hole spacing of 0.3 m × 0.4 m and five seeds per hole. The experiment was arranged as a randomized complete block design with three replications (6 plots). The plot dimensions were 5 m × 10 m. The 17-d and 20-d old seedlings were thinned out, and finally, two seedlings were maintained per hole. The plants were harvested on 25 November 2017 and 28 November 2018. Conventional irrigation, insect and weed controls were utilized as needed.
2.2 Sampling and Measurement Methods
In each experimental plot, 15 consecutive plants were randomly selected. Each panicle generated from branch and stem was placed into a single paper bag, which was also ranked according to the position of panicle from bottom to top of plant. The unfilled and filled grains, identified using water-deposition processing, and sterile flowers were counted for each bag. Thus, the numbers of sterile flowers, unfilled grains and filled grains were determined for each bag and summed for total number of flowers generated from plant. To investigate the spatial distribution, the data of abovementioned measurements was separated into two parts for comparison according to the position where panicle was generated, thus stem and branch. Besides, by regarding the source of panicle, the data was organized according to panicle height on plant which was divided into upper, middle and basal parts with equal length of plant. The seed-setting rate (SR) was calculated as: SR (%) = number of filled grains/total number of flowers × 100.
The statistical analysis was performed using DPS 14.5 (http://www.dpsw.cn), and the differences among treatments were assessed by Duncan’s multiple range test (P < 0.05). R 3.6.0 (http://www.r-project.org/) and Cytoscape 2.7.0 (http://www.cytoscape.org/) were used for network analysis and construction. The average data for the two years were used for network analysis and path-coefficient quantifying the intensity of each direct effect on the variable response because of similar trends.
3.1 The Change of Inflorescence
The Tartary buckwheat reach flower and seed stages simultaneously and maintains many blossoms from the flowering through maturity stages (Fig. 1). At the maturity stage (Fig. 1B), two cultivars have many fresh flowers, filling grains, sterile flowers (Fig. 1C) and unfilled grains (Fig. 1D) on the same spikelet. Thus, the fresh flowers cannot be developed into mature grains till harvest resulting the yield reduction of plant (Fig. 1B).
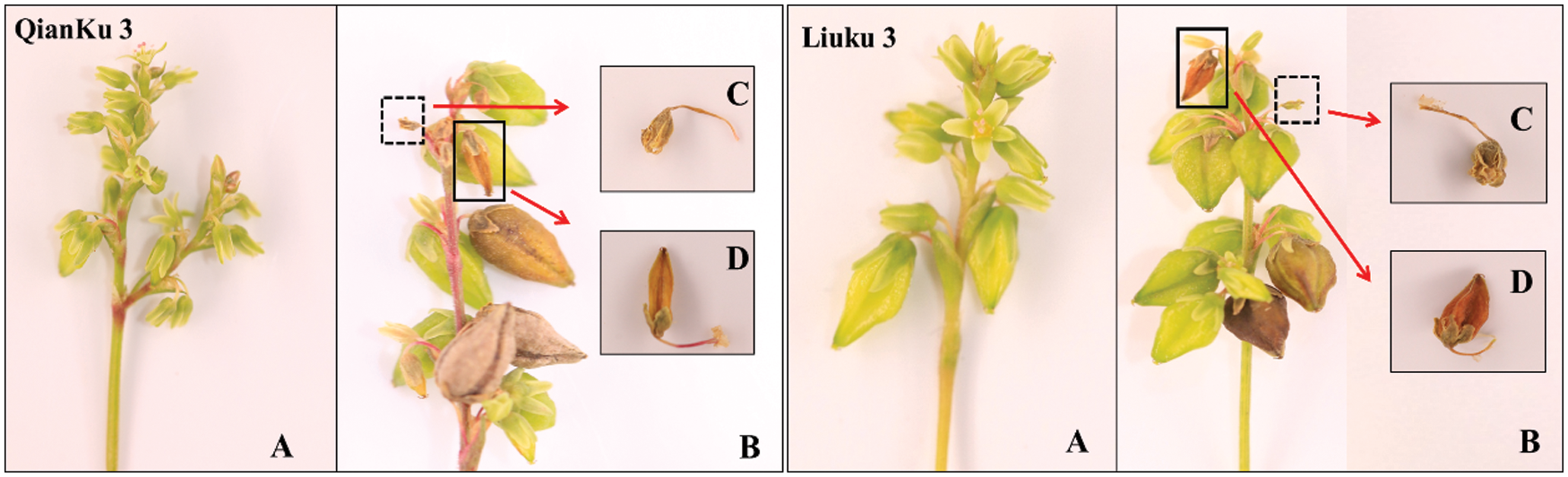
Figure 1: Sterile flowers, unfilled grains and inflorescences of two Tartary buckwheat cultivars. A: spikelet of Tartary buckwheat at the filling stage; B: spikelet of Tartary buckwheat at the mature stage; C: sterile flower; D: unfilled grain
3.2 Flowers, Grains and Seed-Setting Rate
In both growing seasons, significant differences in the number of total flowers, filled grains, unfilled grains and seed-setting rates were observed between two cultivars (Table 1). Qianku 3 generated more flowers, filled and unfilled grains, and higher seed-setting rate than Liuku 3 with average over two years of 945.9, 289.7, 279.3 and 30.6%, respectively. Compared with LiuKu 3, Qianku 3 achieved 26.6% higher seed-setting rate in 2017 and 33.2% higher seed-setting rate in 2018. Significant differences were not observed on sterile flowers between two cultivars in both years.
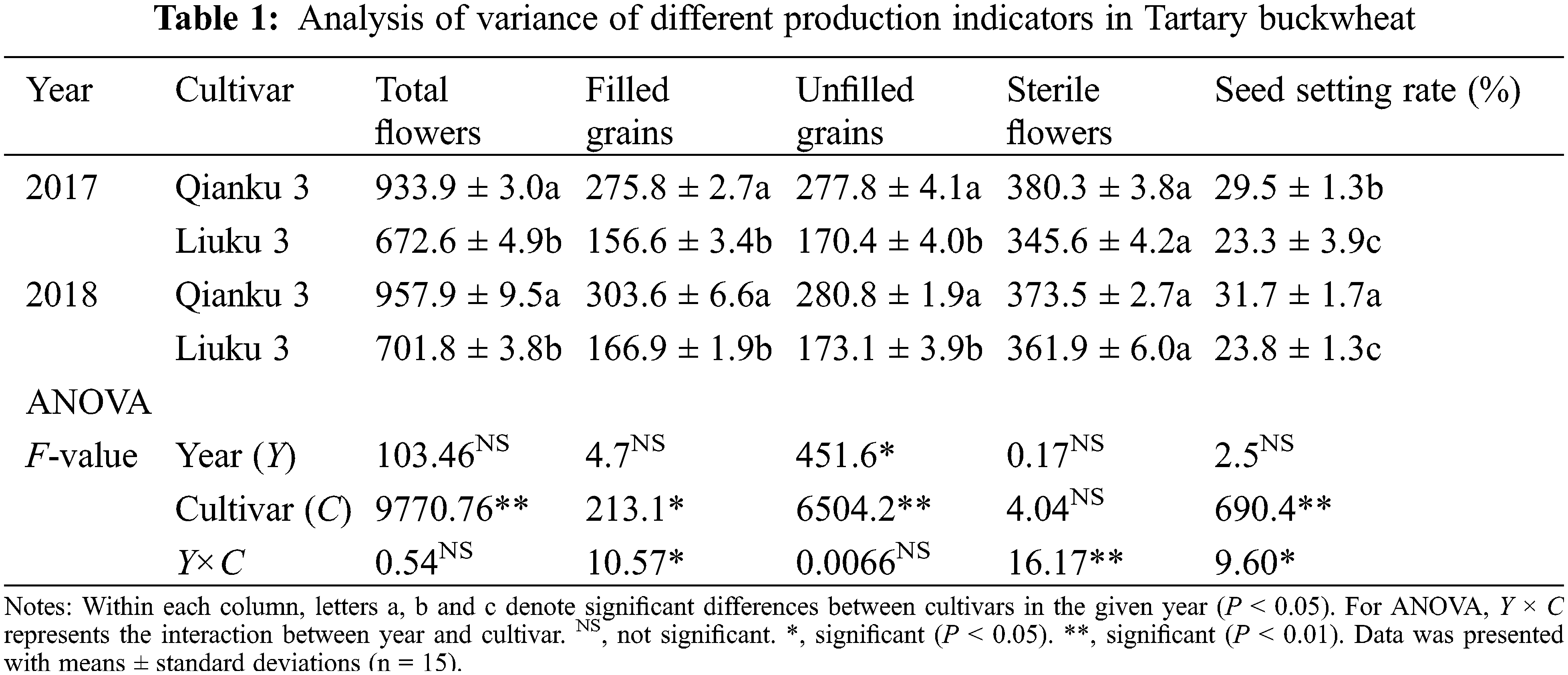
There were different proportions of filled grains, unfilled grains, and sterile flowers in the two cultivars (Fig. 2). As showed in Table 1, Qianku 3 showed lower ratio of sterile flower to total number of flowers than Liuku 3 in 2017 and 2018. Qianku 3 showed no difference between the ratios of filled grains and unfilled grains to total number of flowers in 2017 and 2018. However, the proportion of unfilled grains of Liuku 3 was significantly higher than proportion of filled grains, which was observed in both 2017 and 2018. Although the significant higher proportion of filled grains was observed in QianKu 3 than Liuku 3, the sum proportion of unfilled grains and sterile flowers in LiuKu 3 reached up to 76.7% indicating the great production loss during the reproductive growth period.

Figure 2: The ratio of each indicator (filled grains, unfilled grains, and sterile flowers) to total number of flowers for two Tartary buckwheat cultivars in 2017 and 2018. The letter a, b, and c denote significant differences between indicators of each cultivar at the level of P < 0.05. Vertical lines at the tops of the bars show SE
3.3 The Spatial Distribution of Flower and Grain
The number of filled grains, unfilled grains and sterile flowers differed significantly between main stem and branches in two Tartary buckwheat cultivars in 2017 and in 2018 (Fig. 3). For the cultivar with high seed-setting rate (Qianku 3), the total number of flowers generated from branches including FGB (Filled grains of branches), UGB (Unfilled grains of branches) and SFB (Sterile flowers of branches) is higher than those from main stem in both 2017 and 2018. Besides, the numbers of FGB, UGB and SFB were higher than that from main stem, respectively. Similarly, Liuku 3, the low seed-setting rate cultivar, generated more flowers generated from branches than those on main stem. However, the FGB of Liuku 3 was lower than SFB, UGB in both 2017 and 2018, which showed different trend with that of Qianku 3.
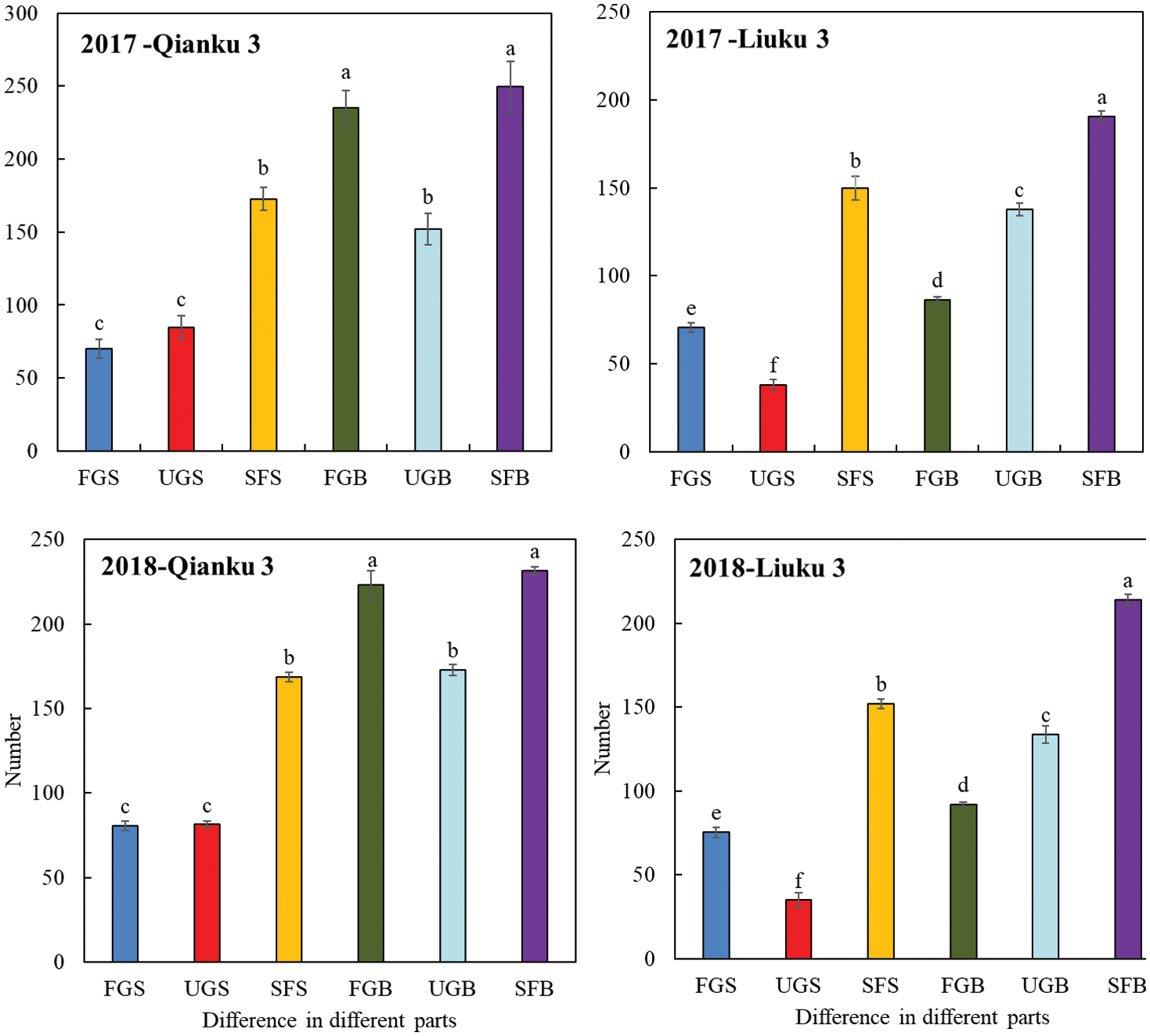
Figure 3: Differences among filled grains, unfilled grains and sterile flowers between main stems and branches in two Tartary buckwheat cultivars. FGS: Filled grains of main stem; UGS: Unfilled grains of main stem; SFS: Sterile flowers of main stem; FGB: Filled grains of branches; UGB: Unfilled grains of branches; SFB: Sterile flowers of branches. The letter above each bar denotes significant differences at the level of P < 0.05. Vertical lines at the tops of the bars show SE
3.4 The Spatial Distributions of Seed-Setting Rate
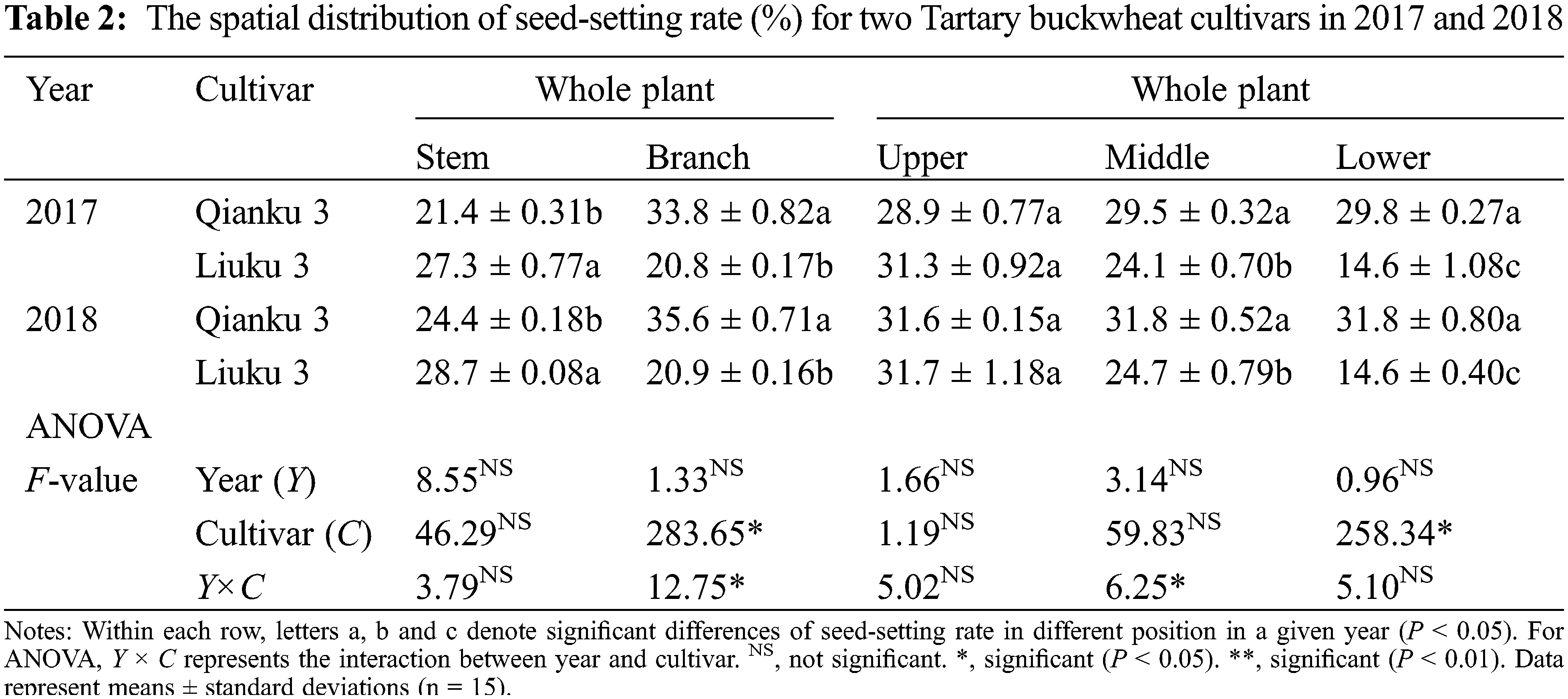
By calculating the seed-setting rate on different parts and the levels of plant, we observed great variations between two cultivars in respect to the spatial distributions of seed-setting rates in different parts of plant (Table 2). Qianku 3 showed significant higher seed-setting rate of the flowers on branch (33.8%) than stem (21.4%). However, the opposite result was found in Liuku 3 that the lower seed-setting rate was observed on branch than on main stem. In addition, the difference of seed-setting rate on branch between two cultivars was greater than that on main stem. Qianku 3 exhibited quite stable seed-setting rates in different levels of plant, showing a range of 28.9%–29.8%, whereas Liuku 3 showed a significant decrease on seed-setting rates from top to bottom parts of plant. The seed-setting rate of lower part of plant was reduced by 16.7% compared with upper part of plant. The similar results were observed in 2018 as for 2017.
Due to consistent changes of results in two years, the mean value of two years for each trait were calculated for performing correlation-based network analysis (Fig. 4). Significant correlations were observed between seed-setting rate of whole plant and four traits, including grains of stems, branches, upper part of plants, and middle part of plants. The grains number of different parts of plant (GS, GB, GMP, GLP, GUP) showed positive correlations with flowers number at the corresponding position of plant (FS, FB, FMP, FLP, FUP), respectively. The GS was significantly and positively correlated with FS, FUP and GUP. Correlation analysis revealed that all traits were significantly correlated with GB, except FS and GS.

Figure 4: Correlation-based network analysis of seed-setting associated traits of Tartary buckwheat. Solely significant correlations were kept (P < 0.05) in network. All the edges between nodes indicate positive correlations; contiguous arrows present the correlations between seed-setting rate and its associated traits; zigzags link the traits derived from the same level of plant. Circles represent grains and flowers of plants at different positions, and hexagons represent seed-setting rates. SR: Seed-setting rate of whole plant; GS: Grains of stems; FS: Flowers of stems; GB: Grains of branches; FB: Flowers of branches; GUP: Grains of upper part of plants; FUP: Flowers of upper part of plants; GMP: Grains of middle part of plants; FMP: Flowers of middle part of plants; GLP: Grains of lower part of plants; FLP: Flowers of lower part of plants
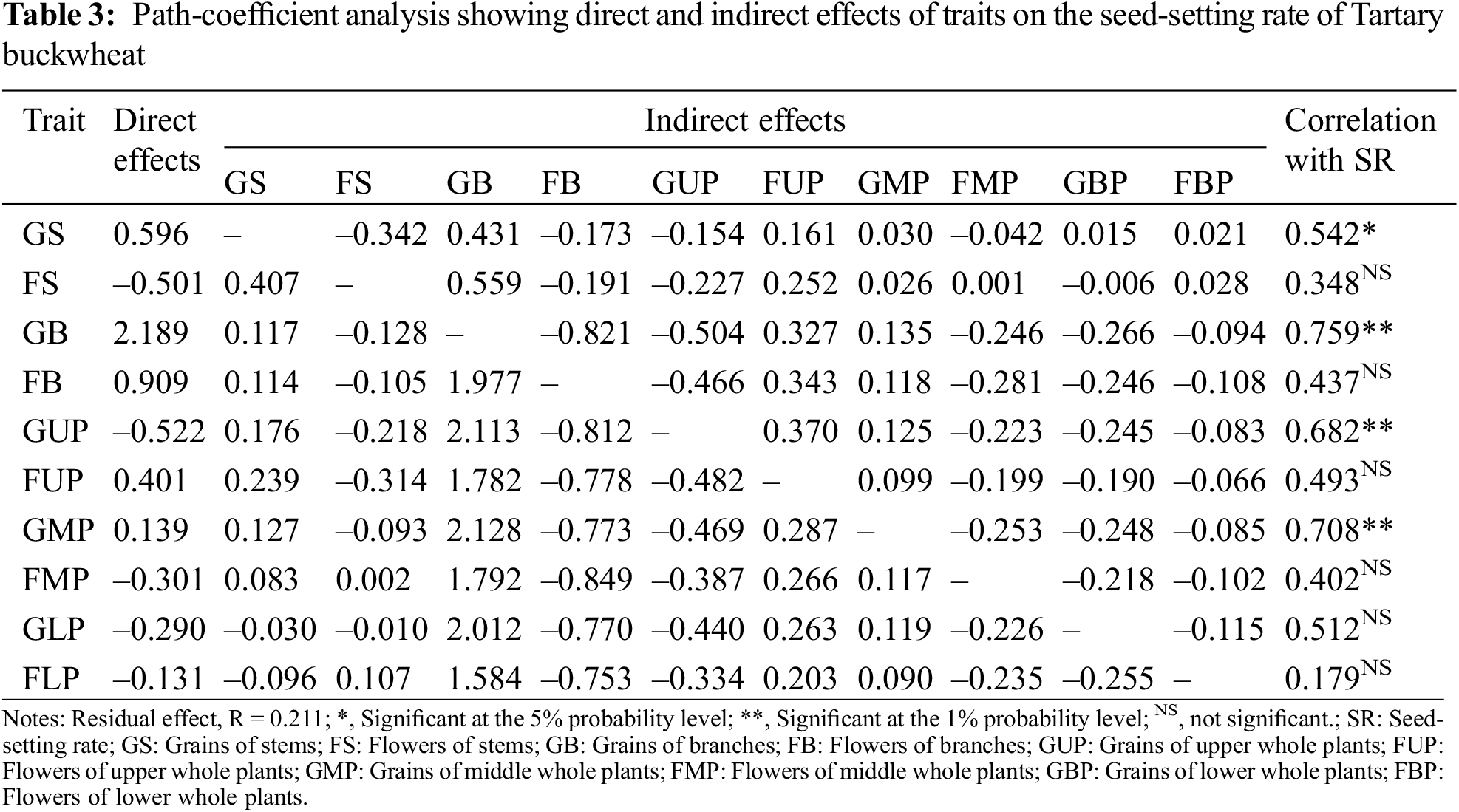
The path-coefficient analysis (Table 3) revealed that grains of branches exhibited a maximum positive direct effect (2.19) on the seed-setting rate, followed by grains of stems (0.60), flowers of upper whole plants (0.40), and grains of middle whole plants (0.14). The direct effects of flowers of stems and branches, grains of upper part of whole plants, flowers of middle part of plants, and both grains and flowers of lower part of plants were negative. The rest of traits had positive indirect effects on the seed-setting rate through grains of branches. The indirect effects of grains on branches through other traits indicated that using grains of branches to directly select high seed-setting rate cultivars will be effective. Grains of branches showed the greatest positive direct effects and correlation (r = 0.76) on seed-setting rate. This strong correlation resulted in a large positive direct effect on the maturation rate.
The residual effect of 0.21 indicated that 78.9% of the contribution of the 10 studied component characteristics to the seed-setting rate was assessed by path-coefficient analysis. The remaining 21.1% was contributed by other factors that still needs to be explored.
The key factor in improving the yield of Tartary buckwheat is to raise seed-setting rate, which depends on increasing the number of grains developed from blossoms (Fig. 1). A single plant can have thousands of flowers, but it may only produce hundreds of mature seeds owing to light, temperature, water and nutrient levels, and the development of its own vegetative organs [41,42]. In addition, the fruiting-rate is closely correlated with the grain-filling characteristic, which are significantly different among the various cultivars. Therefore, the cultivars with many flowers may not have a high seed-setting rate. In this trial, the high seed-setting rate cultivar, Qianku 3, achieved a greater number of total flowers, filled grains, unfilled grains, and sterile flowers than that of Liuku 3 (Table 1). This phenomenon may result from a greater proportion of sterile flowers to total flowers of the cultivar with low seed-setting rate (Fig. 2). Taylor et al. [38] suggested that the main limitation to seed set is the lack of embryo initiation caused by the absence of fertilization. The pollen tube fails to penetrate the micropyle, which may result from the low viability and quality of the pollen [37,38]. The findings reported by Halbrecq et al. [37] suggested that lower part of the inflorescence of high seed-setting cultivar possessed more seeds than the apical part (Table 2). This may be explained that the flowers blossoming earlier may have a greater chance to set seeds than in flowers that blossoms later in the same inflorescence [43].
The seed set of buckwheat plants is globally poor, despite the abundant flowers. Here, the seed-setting rates of both cultivars were less than 32%, which supported the previous results in which the numbers of flowers could surpass 1,000, but the maturation rates of Tartary buckwheat seeds were between 15%–35% [14]. The numbers of total flowers, filled grains, and unfilled grains were different among cultivars. However, there was no significant difference in the numbers of sterile flowers (Table 1). Whether all other cultivars of buckwheat show the similar seed set traits still needs to be explored in future. The maturation rates at different positions showed obvious differences. The more filled grains occurred on branches of Qianku 3 cultivar than on the stem, whereas the maturation rate of seed on branch was not always greater than on stem (Fig. 2, Table 2). It is well documented that rice development was different on different position of plant [26,27]. In this study, the Qianku 3 cultivar had same seed-setting rate at the upper, middle, and lower position of plant, but the seed-setting rate of upper was significantly higher than middle and lower for Liuku 3 (Table 2). It may be related to the activities of key enzymes controlling starch synthesis associated with grain filling at different position of plant [26]. The distribution of flower, grains on other positions (i.e., the secondary branch) in Tartary buckwheat and the contribution of dry matter to flower and grain distribution still requires further study.
A path-coefficient analysis calculated the correlations between grains, flowers and seed-setting rate, considering any cross correlations and dividing the total correlation into direct and indirect effects on different components [44]. In the present study, the maximum positive direct effect (2.19) of grain number of branches on the seed-setting rate suggested that grains of branch is a significant factor in improving seed-setting rate and yield of Tartary buckwheat, which is similar with the findings in soybean [45].
In this experiment, the low seed-setting rates (23.8%–31.7%, Table 1) were observed for both cultivars caused by the great number of sterile flowers and unfilled grains, which might be related to the deficit of available photosynthetic matters for blossoming and grain filling [45]. Moreover, the cultivars with abundant flowers and branches may show an advantage in breeding applications [46]. In production, the cultivation techniques should be applied to optimize the key characteristics of seed-setting, which is increasing the number of filled grains of whole plant to improve the seed-setting and yield of Tartary buckwheat.
Tartary buckwheat undergoes simultaneous flowering and seed setting, which results in many unfilled grains and sterile flowers that cannot form effective grains and negatively influences yield. The seed-setting rate was closely correlated with the position on the plant and the number of filled grains. Especially, the number of filled grains of branches was the most dominant component of the seed-setting rate of Tartary buckwheat. The grain number of branches should be focused on breeding the cultivar with high seed-setting rate and high-yielding Tartary buckwheat.
Funding Statement: We acknowledge the National Natural Science Foundation of China (31771716) and China Agriculture Research System (CARS-07-B-1) to facilitate the research.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Peng, L. X., Huang, Y. F., Liu, Y., Zhang, Z. F., Lu, L. Y. et al. (2014). Evaluation of essential and toxic element concentrations in buckwheat by experimental and chemometric approaches. Journal of Integrative Agriculture, 13, 1691–1698. DOI 10.1016/S2095-3119(13)60724-8. [Google Scholar] [CrossRef]
2. Xiang, D. B., Ma, C. R., Song, Y., Wu, Q., Sun, Y. X. et al. (2019). Post-anthesis photosynthetic properties provide insights into yield potential of tartary buckwheat cultivars. Agronomy, 9, 149. DOI 10.3390/agronomy9030149. [Google Scholar] [CrossRef]
3. Christa, K., Soral-Smietana, M. (2008). Buckwheat grains and buckwheat products-nutritional and prophylactic value of their components—A review. Czech Journal of Food Science, 26, 153–162. DOI 10.1007/978-1-4020-8476-8_13. [Google Scholar] [CrossRef]
4. Zhao, G., Peng, L. X., Wang, S., Hu, Y. B., Zou, L. (2012). HPLC fingerprint—Antioxidant properties study of buckwheat. Journal of Integrative Agriculture, 11, 1111–1118. DOI 10.1016/S2095-3119(12)60104-X. [Google Scholar] [CrossRef]
5. Xiang, D. B., Zhao, G., Wan, Y., Tan, M. L., Song, C. et al. (2016). Effect of planting density on lodging-related morphology, lodging rate, and yield of tartary buckwheat (Fagopyrum tataricum). Plant Production Science, 19, 479–488. DOI 10.1080/1343943X.2016.1188320. [Google Scholar] [CrossRef]
6. Zhao, G., Shan, F. (2009). Chinese tartary buckwheat. China: Science Press. [Google Scholar]
7. Song, C., Xiang, D. B., Yan, L., Song, Y., Zhao, G. et al. (2016). Changes in seed growth, levels and distribution of flavonoids during tartary buckwheat seed development. Plant Production Science, 19, 518–527. DOI 10.1080/1343943X.2016.1207485. [Google Scholar] [CrossRef]
8. Kim, S. H., Cui, C. B., Kang, I. J., Kim, S. Y., Ham, S. S. (2007). Cytotoxic effect of buckwheat (Fagopyrum esculentum Moench) hull against cancer cells. Journal of Medicinal Food, 10, 232–238. DOI 10.1089/jmf.2006.1089. [Google Scholar] [CrossRef]
9. Li, F. H., Yuan, Y., Yang, X. L., Tao, S. Y., Ming, J. (2013). Phenolic profiles and antioxidant activity of buckwheat (Fagopyrum esculentum Möench and Fagopyrum tartaricum L. Gaerth) hulls, brans and flours. Journal of Integrative Agriculture, 12(9), 1684–1693. DOI 10.1016/S2095-3119(13)60371-82012-10-19. [Google Scholar] [CrossRef]
10. Wang, A. H., Xia, M. Z., Cai, G. Z., Zhong, W. (2008). On the center of geographical origin of tartaricum cultivation. Journal of Xichang College, 22, 4–7. [Google Scholar]
11. Xiang, D. B., Song, Y., Wu, Q., Ma, C. R., Zhao, G. (2019). Relationship between stem characteristics and lodging resistance of tartary buckwheat (Fagopyrum tataricum). Plant Production Science, 22, 201–210. DOI 10.1080/1343943X.2019.1577143. [Google Scholar] [CrossRef]
12. Deng, Y. B. (2015). The research on three-line hybrid rice low seed setting rate by using transcriptome data (Master’s Thesis). China: Sichuan Agriculture University. [Google Scholar]
13. Wang, Z. X., Yao, C. Q., Shao, L., Haireguli, A., Su, J. G. et al. (2010). Effect of different pollination methods on setting percentage of wheat and triticale hybrids. Xinjiang Agricultural Science, 47, 569–572. DOI 10.3724/SP.J.1142.2010.40491. [Google Scholar] [CrossRef]
14. Jiang, J. F., Wang, M. H. (1986). A preliminary study on the formal structure of floral organ and flowering biological characteristics in buckwheat. Acta Scientiarum Naturalium Universitatis Intramongolicac, 17, 501–511. [Google Scholar]
15. Douglas, P. T., Ralph, L. O. (2001). Quantitative assessment of some factors limiting seed set in buckwheat. Crop Science, 41(6), 1792–1799. DOI 10.2135/cropsci2001.1792. [Google Scholar] [CrossRef]
16. Peng, H. F., Lu, Y. P., Chen, X. H., Wan, B. H. (2003). Cytological observation of pollen abortion in short day and low temperature induced male sterile rice Yi DS. Hybrid Rice, 18, 65–67. DOI 10.1023/A:1022289509702. [Google Scholar] [CrossRef]
17. Li, S. C., Li, W. B., Huang, B., Cao, X. M., Zhou, X. Y. et al. (2013). Natural variation in PTB1 regulates rice seed setting rate by controlling pollen tube growth. Nature Communication, 4(1), 2793–2805. DOI 10.1038/ncomms3793. [Google Scholar] [CrossRef]
18. Morten, S., Shawn, M. R., Lone, B., Mia, K. J., Michael, G. P. et al. (2004). A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proceedings of the National Academy of Sciences of the United States of America, 101, 9502–9507. DOI 10.1073/pnas.0401542101. [Google Scholar] [CrossRef]
19. Frederic, B., Yuki, H., Mathieu, I., Tetsuya, H. (2008). Double fertilization-caught in the act. Trends in Plant Science, 13, 437–443. DOI 10.1016/j.tplants.2008.05.011. [Google Scholar] [CrossRef]
20. Yang, J. C., Peng, S. B., Gu, S. L., Visperas, R. M., Zhu, Q. S. (2001). Changes in activities of three enzymes associated with starch synthesis in rice grains during grain filling. Acta Agronomica Sinica, 27, 157–164. [Google Scholar]
21. Yang, J. C., Zhang, J. H. (2010). Grain-filling problem in ‘super’ rice. Journal of Experimental Botany, 61, 1–5. DOI 10.1093/jxb/erp348. [Google Scholar] [CrossRef]
22. Yang, D. Q., Dong, W. H., Luo, Y. L., Song, W. T., Cai, T. et al. (2018). Effects of nitrogen application and supplemental irrigation on canopy temperature and photosynthetic characteristics in winter wheat. Journal of Agricultural Science, 156, 13–23. DOI 10.1017/S0021859617000946. [Google Scholar] [CrossRef]
23. Zhang, R. P., Ma, J., Cai, G. Z., Sun, Y. J. (2012). Effects of low temperature stress during flowering stage on flowering and seed setting of rice in Panxi Region, Sichuan Province. Acta Agronomica Sinica, 38(9), 1734–1742. DOI 10.3724/SP.J.1006.2012.01734. [Google Scholar] [CrossRef]
24. Nguyen, C. T., Singh, V., van Oosterom, E. J., Chapman, S. C., Jordan, D. R. et al. (2013). Genetic variability in high temperature effects on seed-set in sorghum. Functional Plant Biology, 40(5), 439–448. DOI 10.1071/FP12264. [Google Scholar] [CrossRef]
25. Xu, Z. J., Chen, W. F., Sun, Z. H., Zhang, S. L., Liu, L. X. et al. (2004). Distribution of rice grain on panicle axis and its relationship with seed setting in Liaoning. Science Agricultura Sinica, 37, 963–967. [Google Scholar]
26. Xu, Y. J., Gu, D. J., Qin, H., Zhang, H., Wang, Z. Q. et al. (2015). Changes in carbohydrate accumulation and activities of enzymes involved in starch synthesis in maize kernels at different positions on an ear during grain filling. Acta Agronomica Sinica, 41(2), 297–307. DOI 10.3724/SP.J.1006.2015.00297. [Google Scholar] [CrossRef]
27. Dong, M. H., Chen, P. F., Xie, Y. L., Qiao, Z. Y., Yang, J. C. (2012). Variations in carbohydrate and protein accumulation among spikelets at different positions within a panicle during rice grain filling. Rice Science, 19(3), 223–232. DOI 10.1016/S1672-6308(12)60044-4. [Google Scholar] [CrossRef]
28. Cao, Z. Q., Yang, J. C. (2014). Research progress in hormonal regulation mechanism in the grain filling of superior and inferior spikelets of rice. China Rice, 20, 12–16. [Google Scholar]
29. Yang, J. C., Wang, Z. Q., Zhu, Q. S., Su, B. L. (1999). Regulation of ABA and GA to the grain filling of rice. Acta Agronomica Sinica, 25, 341–348. [Google Scholar]
30. Yang, J. C., Wang, G. Z., Wang, Z. Q., Liu, L. J., Zhu, Q. S. (2002). Grain-filling characteristics and changes of hormonal content in the grains of dry-cultivated rice during grain-filling. Acta Agronomica Sinica, 28, 615–621. DOI 10.1006/jfls.2001.0409. [Google Scholar] [CrossRef]
31. Singh, S. P., Srivastava, R., Kumar, J. (2014). Male sterility system in wheat and opportunities for hybrid wheat development. Acta Physiologiae Plantarum, 37(1), 1713. DOI 10.1007/s11738-014-1713-7. [Google Scholar] [CrossRef]
32. Ni, F., Qi, J., Hao, Q. Q., Lyu, B., Luo, M. C. et al. (2017). Wheat Ms2 encodes for an orphan protein that confers male sterility in grass species. Nature Communication, 8(1), 15121. DOI 10.1038/ncomms15121. [Google Scholar] [CrossRef]
33. Slawinska, J., Obendorf, R. L. (2001). Buckwheat seed set in planta and during in vitro inflorescence culture: Evaluation of temperature and water deficit stress. Seed Science Research, 11, 223–233. [Google Scholar]
34. Xiang, D. B., Wei, W., Ouyang, J. Y., Le, L. Q., Zhao, G. et al. (2020). Nitrogen alleviates seedling stage drought stress response on growth and yield of tartary buckwheat. International Journal of Agriculture and Biology, 24, 1167–1177. DOI 10.17957/IJAB/15.1546. [Google Scholar] [CrossRef]
35. Cawoy, V., Deblauwe, V., Halbrecq, B., Ledent, J. F., Kinet, J. M. et al. (2006). Morph differences and honeybee morph preference in the distylous species Fagopyrum esculentum moench. International Journal of Plant Science, 167, 853–861. DOI 10.1086/504924. [Google Scholar] [CrossRef]
36. Hara, T., Iwata, H., Okuno, K., Matsui, K., Ohsawa, R. (2011). QTL analysis of photoperiod sensitivity in common buckwheat by using markers for expressed sequence tags and photoperiod-sensitivity candidate genes. Breeding Science, 61, 394–404. DOI 10.1270/jsbbs.61.394. [Google Scholar] [CrossRef]
37. Halbrecq, B., Romedenne, P., Ledent, J. F. (2005). Evolution of flowering, ripening and seed set in buckwheat (Fagopyrum esculentum MoenchQuantitative analysis. European Journal of Agronomy, 23, 209–224. DOI 10.1016/j.eja.2004.11.006. [Google Scholar] [CrossRef]
38. Taylor, D. P., Obendorf, R. L. (2001). Quantitative assessment of some factors limiting seed set in buckwheat. Crop Science, 41, 1792–1799. DOI 10.2135/cropsci2001.1792. [Google Scholar] [CrossRef]
39. Jacquemart, A. L., Cawoy, V., Kinet, J. M., Ledent, J. F., Quinet, M. (2012). Is buckwheat (Fagopyrum esculentum Moench) still a valuable crop today. European Journal of Plant Science and Biotechnology, 6, 1–10. [Google Scholar]
40. Song, Y. (2019). The seed-setting characteristic of different Tartary buckwheat cultivars and its response to source-sink regulation (Master’s Thesis). China: Chengdu University. [Google Scholar]
41. Zheng, C. F., Zhu, H. J., Zhu, Y. J., Guo, T. C., Wang, C. Y. (2016). Responses of floret development and grain setting characteristics of winter wheat to foliar spray boron. Journal of Plant Nutrition and Fertilizers, 22, 550–556. [Google Scholar]
42. Zhu, Y. J., Cui, J. M., Wang, C. Y., Guo, T. C., Xia, G. J. et al. (2002). Effects of nitrogen application at different wheat growth stages on floret development and grain yield of winter wheat. Scientia Agriculture Sinica, 35, 1325–1329. [Google Scholar]
43. Asako, Y., Ujihara, A., Matano, T. (1980). Relation between the position of flowers and their flowering of fruiting in common buckwheat (Fagopyrum esculentum). Hokuriku Crop Science, 15, 27–30. [Google Scholar]
44. Suryavanshi, V. P., Chavan, B. N., Jadhav, K. T., Jadhav, V. T. (2010). Correlation and path analysis of grain yield and yield components in maize. Catalysis Letters, 23, 253–255. [Google Scholar]
45. Carpenter, A. C., Board, J. E. (1997). Branch yield components controlling soybean yield stability across plant populations. Crop Science, 37, 885–891. DOI 10.2135/cropsci1997.0011183X003700030031x. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |