

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.018996
ARTICLE
Constitutive Activation OsbZIP62 Improves Plant Height and Yield through Regulating the Expression of Agronomic Traits Related Genes in Rice
1Suzhou Chien-Shiung Institute of Technology, Suzhou, 215411, China
2Shanghai Agrobiological Gene Center, Shanghai, 201106, China
3College of Ocean and Bioengineering, Yancheng Institute of Technology, Yancheng, 224051, China
4College of Plant Sciences & Technology, Huazhong Agricultural University, Wuhan, 430070, China
5School of Life Sciences, Hubei University, Wuhan, 430062, China
*Corresponding Author: Kai Xu. Email: kxu@sagc.org.cn
#These two authors contributed equally to this work
Received: 16 September 2021; Accepted: 17 December 2021
Abstract: Plant height is an important morphological trait that affects crop yield. Several genes related to plant height and yield have been reported in rice (Oryza sativa L.), however, the molecular mechanism underlying the regulation of these traits is still not completely understood. VP64 is widely used as a transcriptional activator to investigate the biological function of genes encoding transcription factors. Here, we identified a novel bZIP transcription factor OsbZIP62 that is involved in modulating agronomic traits in rice. Overexpression of OsbZIP62-VP64 (OsbZIP62V) significantly increases the plant height and yield per plant in rice. RNA-seq analysis showed that some plant height and panicle development related genes (i.e., OsEATB, OsDSS1 and OsGA3ox2) were up-regulated in OsbZIP62V overexpressing rice plants. Besides, OsbZIP62 could also bind to the promoters of several putative target genes. These results suggested that OsbZIP62 plays a role as transcriptional regulator in regulating the expression of genes associated with agronomic traits, and OsbZIP62 fused with VP64 would be useful in crop genetic modification with improved plant architecture and yield.
Keywords: Rice; OsbZIP62; panicle length; plant height; transcription factor; yield
Rice is not only an important food crop that provides security for more than 50% of the world’s population, but also the crop with the largest water demand, accounting for about 70% of the total agricultural water consumption [1,2]. In recent years, climate disasters are increasingly frequent, which have a serious impact on crop growth and production, seriously restricting the long-term goal of high and stable yield of crop breeding [3–5]. In order to meet the growing food demand, it is very important to study genetics and molecular mechanism of rice yield to develop new rice varieties with high and stable yields, and ensure the safety of food production [6–8].
The rice yield is mainly determined by three key factors, which are effective panicle number per plant, grain number per panicle and 1000-grain weight. It is necessary to elucidate the molecular mechanisms underlying these traits for developing high yield rice varieties [9–11]. At present, many genes have been reported to be involved in the regulation of rice agronomic traits. For example, the GRAS family protein single tiller gene MOC1, plays an important role in the regulation of rice tiller development [12,13]. The number of tillers and panicles of MOC1 mutants decreased, which regulated the development of tillers and panicles, and then affected the yield of rice [14]. Early heading date 1 (Ehd1) promotes early heading under short sunlight by inducing the expression of FT-like gene, which reduces the number of primary branches, leads to the reduction of grain number per ear, and then affects the field yield of crops [15]. HD1, a gene affecting heading, can promote the expression of Ghd7 under long sunshine, inhibit Ehd1-Hd3a/RFT1 flowering pathway, thus affecting rice yield [16]. Ideal plant architect 1 (IPA1) can disrupt the regulation of OsmiR156 on IPA1, resulting in the reduction of tillers, the increase of grain number per panicle and 1000 grain weight, the stout stem, so as to improve the yield [17].
Plant height is another important agronomic trait of rice that directly affects the yield of this crop. It is also a strategy to increase plant height to increase yield. Genes related to plant height were also reported, such as OsGA3ox2 and OsEATB. OsGA20ox2 is a gene related to GA biosynthesis. Mutation of this site can lead to different degrees of dwarfing in Rice [18]. OsGA3ox2, a gibberellin (GA) biosynthesis related gene, is significantly induced by BR. Its mutation can lead to a decrease in GA accumulation, thus inhibiting cell elongation, resulting in plant growth reduction and dwarfing [19,20]. In the process of internode elongation, OsEATB inhibits ethylene inducing gibberellin response by down regulating the expression of ent-kaurene synthase A, a gene related to gibberellin biosynthesis.
Transcription factors (TFs) play important role in plant development and stress responses [21,22]. Among different types of transcription factors, basic leucine zipper motif (bZIP) transcription factor has been widely studied [23–25]. There are about 89 bZIP transcription factors in rice [25,26]. Studies have shown that several members of the bZIP transcription factors, such as OsbZIP73, OsbZIP75, OsbZIP77 and OsRE1, are involved in the regulation of rice agronomic traits [24,27–29]. Overexpression of OsbZIP75 delays the heading date of rice [28,30]. OsbZIP77 was involved in the regulation of rice heading date through interacting with SAPKs [29,31]. OsRE1 interacts with OsRIP1 to modulate the heading stage of rice by fine regulating the expression of Ehd1 [27].
VP64 is a protein composed of four repeats of an eleven amino acid peptide from the C-terminal of the viral protein 16 (VP16), which exhibits strong activation activity [32]. The transcription activation activities of TFs fused with VP64 can be significantly increased. Therefore, VP64 has been used as a transcriptional activator to investigate the biological function of TFs in rice [33].
Although the functions of several bZIP genes have already been characterized to be involved in modulating agronomic traits in rice, a number of other bZIP genes have not been identified. Previously, we identified a novel bZIP transcription factor, OsbZIP62, which was participated in rice drought tolerance [34]. In this study, we reported that constitutive activation of OsbZIP62 through fused with transactivation domain VP64 significantly improved yield per plant through increasing plant height, panicle length and grain number per panicle in rice. These results showed that OsbZIP62 is a novel regulator in rice agronomic traits and has potential application value in developing novel rice varieties with improved yield.
The full-length cDNA of OsbZIP62 was the seeds of transgenic japonica rice Kitaake with OsbZIP62V were acquired from Fujian Agriculture and Forestry University, and these transgenic rice plants were generated as described previously [33].
2.2 Plant Materials and Growth Conditions
The rice plants (Oryza sativa L. japonica. cv. Nipponbare) used in this study, including the wild-type plants Kitaake and the relevant transgenic plants were in the experimental fields at the Shanghai Agrobiological Gene Center in Shanghai during the natural growing seasons.
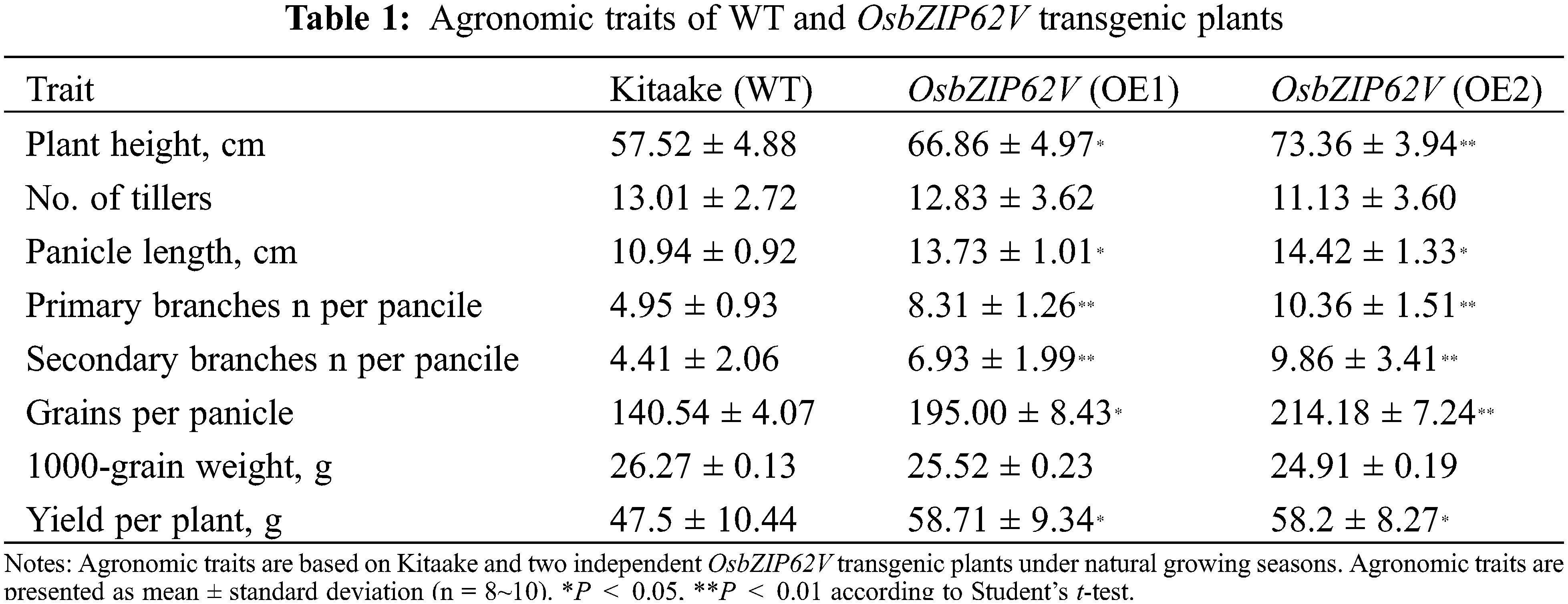
Agronomic traits of WT and OsbZIP62V transgenic rice plants, including plant height, panicle length, primary branches number, secondary branches number, grain number per panicle and 1000-grain weight, were manually measured after plants were harvested. Dry seeds were evaluated for grain width and length via morphology analyzer (http://www.jsjeda.com). Water content of seeds determined by AOAC official method 930.15. Ash content was measured by AOAC Official Method 942.05. Protein content was measured using the Kjeldahl method (984.13). Statistics analysis of the phenotypic data was performed using SPSS version 19.0 (IBM).
2.4 RNA Isolation and Gene Expression Pattern Analysis
Total RNA was extracted from the rice leaves using TRNzol-A+ reagent (Tiangen) according to the manufacturer’s instructions. First-strand cDNA was synthesized via EasyScript One-Step gDNA Removal and cDNA Synthesis Super Mix (Transgen). Real-time quantitative PCR (qPCR) was performed on an optical 96-well plate with a Bio-Rad CFX96 Real-Time PCR Detection System using TransStart Green qPCR SuperMix (Transgen). The PCR procedure was as follows: 94°C for 30 s, followed by 40 cycles at 94°C for 5 s, 55°C for 15 s and 72°C or 10 s. The rice OsAct8 (No. AY212324) was used as an internal control to normalize the target gene expression, and relative expression levels were determined as described previously [35].
The RNA sequencing data of OsbZIP62V transgenic rice plants and wild type plants were obtained previously [34]. We counted the number of differentially expressed genes (DEGs) at different levels of each Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway [36], and then determined the metabolic pathways and signaling pathways in which the DEGs were mainly involved.
2.6 Promoters Sequence Analysis of DEGs
Ten DEGs that are related with rice growth and agronomic traits were chosen for further study. The ~2 kb promoter sequences of these genes were downloaded from the rice genome database and the cis-elements were analyzed through PlantCARE online software.
2.7 Protein Expression and EMSA
OsbZIP62 was ligated to the prokaryotic expression vector pGEX-6P-1, which was expressed by GST fusion label, transformed into E. coli DE3, and was induced by 1 mM IPTG for 18 degrees, and SDS-PAGE was used to detect the expression of GST-OsbZIP62. After induction, the bacteria were collected and GST-OsbZIP62 were purified by Profinia protein purification system. The cis-element of ABRE and G-box were labeled with biotin. EMSA was performed according to the instructions of LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific). The membrane was exposed to a ChemiDoc XRS + imaging system (Bio-Rad).
We selected 10 genes (i.e., LOC_Os01g08220, LOC_Os10g32980, LOC_Os03g04680, LOC_Os11g12740, LOC_Os02g54600, LOC_Os09g16510, LOC_Os09g28440, LOC_Os07g05360, LOC_Os01g41710 and LOC_Os10g32600) that were differentially expressed in OsbZIP62V overexpression transgenic plants. The upstream approximately 0.3 kb long promoters of these potential target genes were cloned from genome DNA of a japonica rice (Nipponbare). In yeast one-hybrid experiment, the promoter sequences of these target genes were digested by SmaI and EcoRI and cloned into yeast expression vector pHIS2.1, and then co-transformed into Y187 yeast cells with the pGAD-T7-OsbZIP62. The interaction between DNA and protein was evaluated by transformation growth analysis on SD/−Leu/−Trp/−His plates, which provided 50 mM of 3-AT.
3.1 Constitutive Expression of OsbZIP62 Enhanced Plant Height of Rice
In the previous studies, we found that OsbZIP62 fused with VP64 (OsbZIP62V) could enhance rice drought tolerance at the seedling stage [34]. To investigate whether OsbZIP62 affects the growth of rice plants, we further charactered and investigated agronomic traits of rice plants overexpressing OsbZIP62V. First of all, we checked the expression level of OsbZIP62V in two independent transgenic lines (OE1, OE2). We found that the expression level of OsbZIP62 in transgenic plants was significantly higher than that of wild type plants (Fig. 1B). Secondly, we measured agronomic traits (i.e., plant height, panicles length and the number of primary/secondary branches) of OsbZIP62V-overexpression rice plants. It was found that OsbZIP62V transgenic plants had slightly fewer tillers, whereas the plant height of OsbZIP62V transgenic plants is noticeably higher than that of the wild type plants, which increased by 16%–27% (Figs. 1C and 2A; Table 1). OsbZIP62V transgenic plants have longer panicles which increased by 25%–31% (Figs. 1D, 1E and 2C; Table 1). Both the numbers of primary and secondary branches of OsbZIP62V transgenic plants increased by more than 50% (Figs. 1F, 2E and 2F; Table 1). The grain numbers per panicle of OsbZIP62V transgenic plants were also significantly higher compared with WT plants, which increased by 38%–52% (Fig. 2D; Table 1). The yield per plant of OsbZIP62V transgenic plants increased by 23–24% (Fig. 2G; Table 1). Finally, we also checked whether OsbZIP62 mutation affected rice phenotype under normal condition. The result showed that there was no significant difference between OsbZIP62 mutant plants and wild type plants (Fig. S1). These data indicated that the constitutive expression of OsbZIP62V markedly improved the plant height and grain yield of transgenic rice plants.
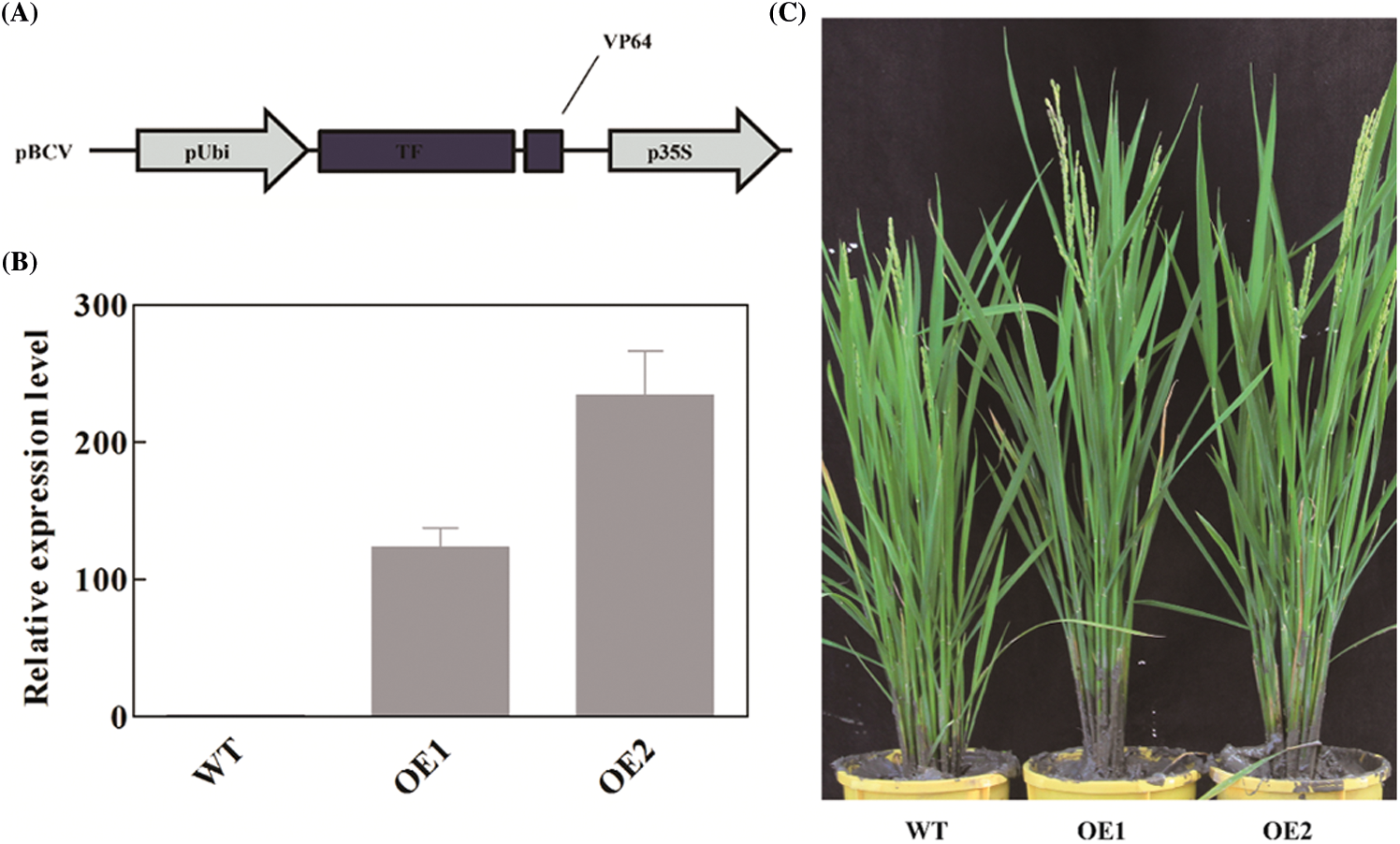

Figure 1: Phenotypic characterization of OsbZIP62V transgenic plants. (A) Structure diagram of OsbZIP62V. (B) Expression levels of OsbZIP62V in Kitaake (WT) and two independent OsbZIP62V transgenic plants. Data represents means ± SE (n = 3). (C) Booting phenotype of OsbZIP62V. (D, E) Panicle morphology of Kitaake (WT) and two independent OsbZIP62V transgenic plants. (F) Primary/ secondary branches per panicle
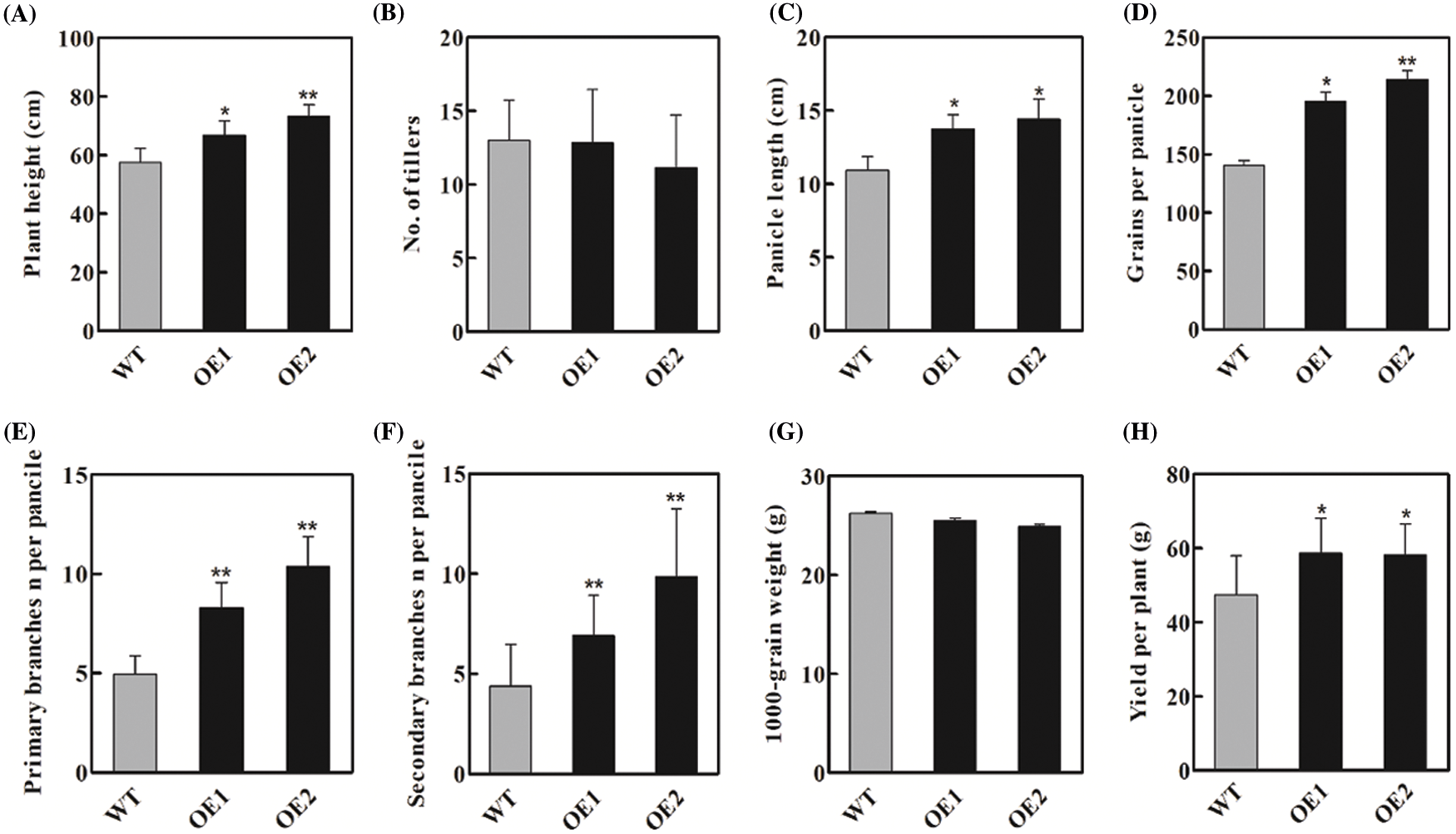
Figure 2: Agronomic traits of WT and OsbZIP62V transgenic plants. (A) Plant height. (B) Number of tillers. (C) Panicle length. (D) Grain number per panicle. (E, F) The number of primary and secondary branches. (G) 1000-grain weight. (H) Yield per plant. Data represents means ± SE (n = 8~10), *P < 0.05, **P < 0.01, Student’s t-test
3.2 OsbZIP62 Plays an Important Role in Grain Shape
Overexpression of OsbZIP62V increased grains number per panicle and yield per plant of transgenic rice plants. It promoted us to investigate whether it also has an effect on grain shape. The result indicated that the grain length of OsbZIP62V transgenic plants was higher, which increased by 8%–13% compared with the wild-type plants (Figs. 3A and 3B). The grain width of OsbZIP62V transgenic plants increased by 5%–11% compared with the wild-type control (Figs. 3C and 3D). Moreover, we also want to know whether OsbZIP62V changes the grain quality of transgenic rice. The results showed that there was no significantly difference between the grain water, ash and protein content of transgenic plants and those of wild-type plants (Table S1). These results indicated that the overexpression of OsbZIP62V affected rice grain shape but did not affect the rice grain quality.
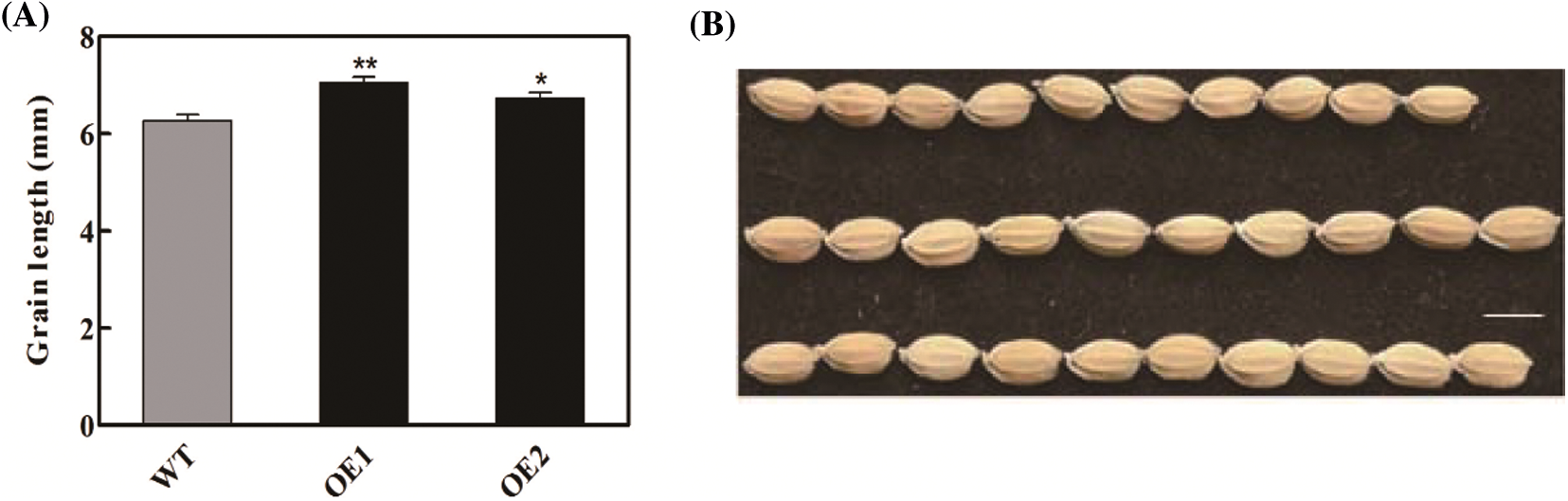
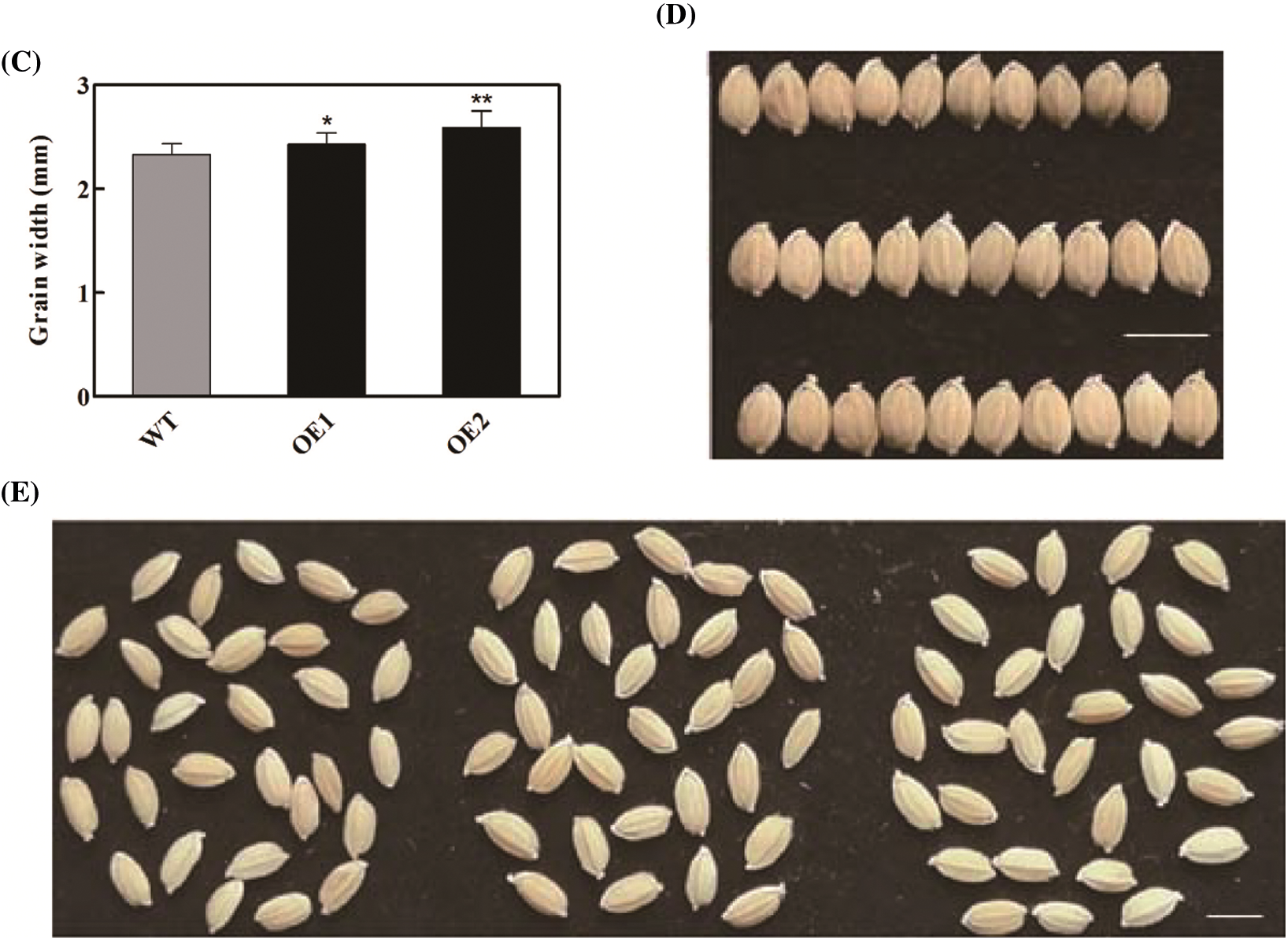
Figure 3: Grain shape was regulated by OsbZIP62. (A, B) Grain length of transgenic plants. (C, D) Grain width of transgenic plants. (E) Grains phenotypes of transgenic plants and wild-type, Bar = 5 mm. Data represents means ± SE, n = 10, *P < 0.05, **P < 0.01, Student’s t-test
3.3 Identification of Putative Target Genes Regulated by OsbZIP62
To elucidate the molecular mechanism of OsbZIP62 on modulating rice agronomic traits and yield traits, we analyzed transcriptome of OsbZIP62V overexpression plants and WT plants through high-throughput sequencing previously [34]. Differentially expressed genes (DEGs) between the transgenic rice and wild plants were analyzed. Many DEGs up-regulated in OsbZIP62V transgenic plants were related to agronomic traits including plant height, panicle length, and yield in rice (Fig. 4A). KEGG metabolic pathway enrichment analysis indicated that the DEGs in OsbZIP62V are significantly enriched in starch and sugar metabolism, hormone signal transduction, photosynthesis and other metabolic pathways (Fig. 4B).
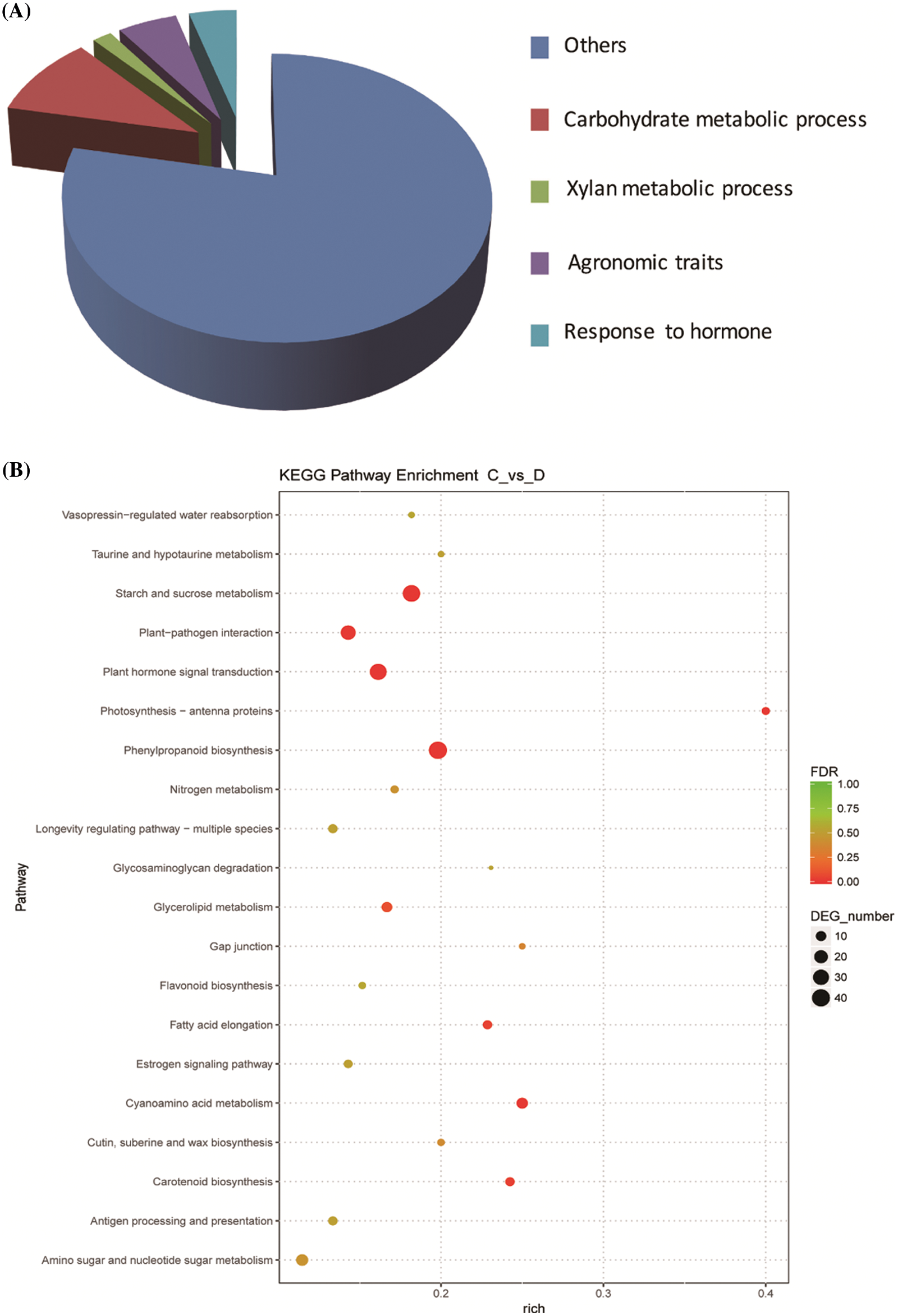
Figure 4: Transcriptome analysis of OsbZIP62V plants and WT plants. (A) Classification of upregulated genes in OsbZIP62V plants. (B) KEGG Pathway functional enrichment of DEGs in the OsbZIP62V vs. WT plants. The x-axis represents the enrichment factor (rich factor). The y-axis shows the pathway names. A larger value of the rich factor indicates a higher enrichment value. The color indicates the P value. Point size indicates DEG number and larger dots refer to higher numbers of DEGs
The expression levels of several DEGs were further checked by qPCR, and the results showed that the expression level of these selected DEGs (i.e., LOC_Os01g08220, LOC_Os10g32980, LOC_Os03g04680, LOC_Os11g12740, LOC_Os02g54600, LOC_Os09g16510, LOC_Os09g28440, LOC_Os07g05360 and LOC_Os01g41710) increased in OsbZIP62V overexpression transgenic lines (Fig. 5).
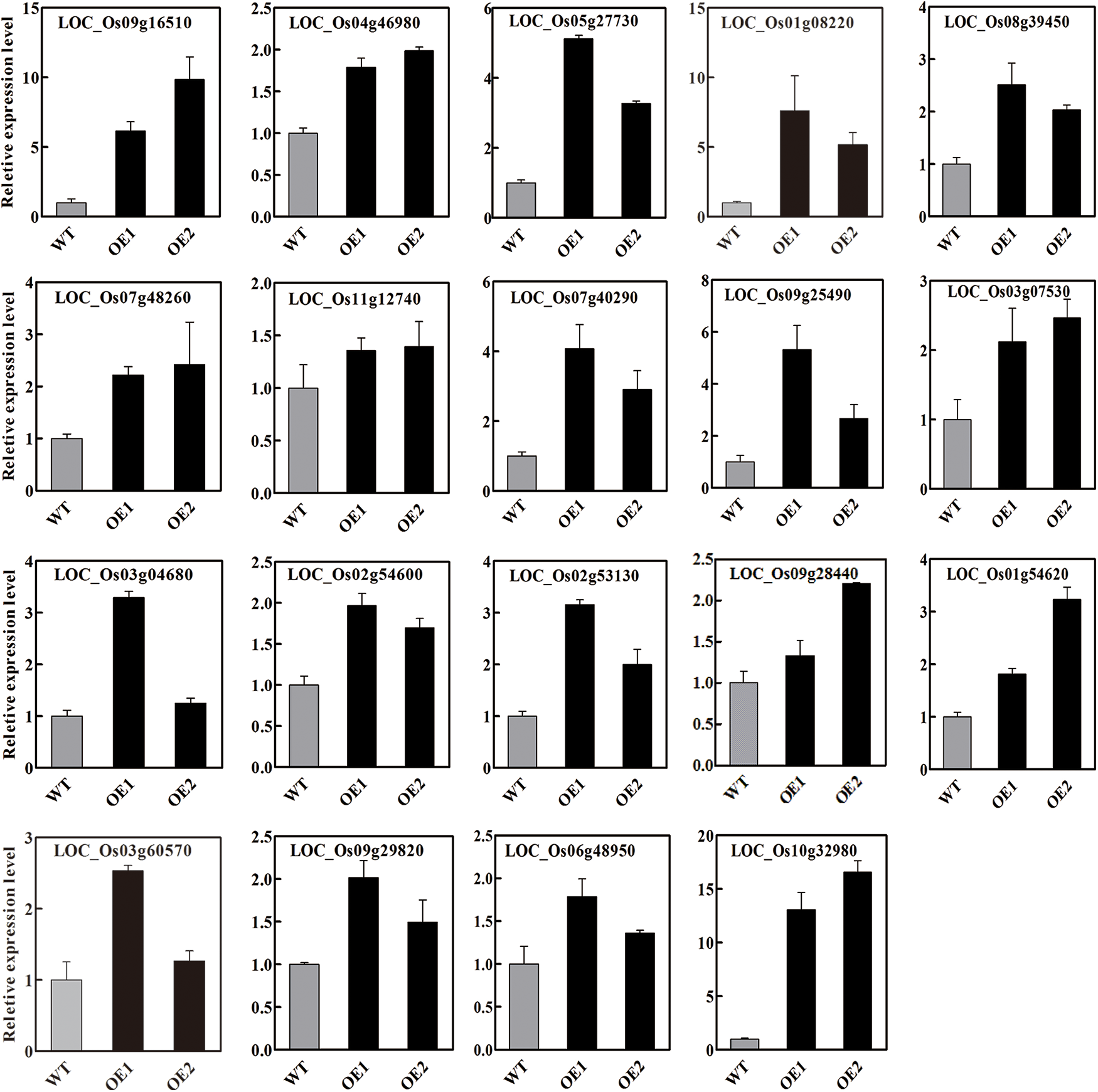
Figure 5: Relative expression level of DEGs involved in agronomic traits in OsbZIP62V and WT plants detected through qPCR. Data represents means ± SE (n = 3)
We also analyzed the expression of DEGs which encode transcription factors and chlorophyll proteins involved in rice growth and development (i.e., LOC_Os01g18240, LOC_Os09g16510 and LOC_Os09g28440 encode a transcription factor; LOC_Os01g41710 and LOC_Os11g12740 encode chlorophyll synthesis protein and polypeptide transporter, respectively) and the results showed the expression of these DEGs were also increased in OsbZIP62V overexpression plants. These results demonstrated that the transcription of these DEGs were affected by OsbZIP62 and these DEGs might be target genes of OsbZIP62.
We also investigated the expression of several important genes affect rice agronomic traits such as Hd1, Ehd1, Ghd7 and DTH8 (Fig. S2). The results showed that the expression of these genes in OsbZIP62V transgenic rice plants were similar with that of WT plants, except that expression of Ehd1 in OsbZIP62V plants were much lower compared with WT plants.
To clarify the mechanism of OsbZIP62V modulating rice agronomic traits, we selected ten DEGs that are related to plant height, rice panicle development and photosynthesis for further study. We analyzed the cis-elements in promoters of these DEGs (i.e., LOC_Os01g08220 (OsGA3ox2), LOC_Os10g32980 (OsCesA7), LOC_Os03g04680 (OsDSS1), LOC_Os11g12740 (OsNPF4.1), LOC_Os02g54600 (OsMKK4), LOC_Os09g16510 (OsWRKY74), LOC_Os10g32600 (Ehd1) and LOC_Os09g28440 (OsEATB)). The results showed that promoters of eight DEGs contain ABREs or G-box cis-elements that may be recognized and bond by bZIP transcription factors (Table 2). We firstly investigated whether OsbZIP62 bind to ABRE and G-box cis-elements through EMSA assay. The results showed that OsbZIP62 protein can bind to the core sequence of ABRE and G-box in vitro (Fig. 6A). We further investigated whether the OsbZIP62 protein could bind to the promoters of these putative target genes through a yeast one-hybrid assay. A pGAD-OsbZIP62 plasmid (containing the OsbZIP62 putative DNA domain fused to the GAL4 active domain) and pHIS-cis reporter construct (~0.3 kb promoters of 10 presumed target genes) were co-transformed into yeast strain Y187 (Fig. 6B). As indicated by the activation of the reporter genes, OsbZIP62 could bind to the promoters of several genes (i.e., LOC_Os01g08220, LOC_Os10g32980, LOC_Os03g04680, LOC_Os11g12740, LOC_Os02g54600, LOC_Os09g16510, LOC_Os07g05360 and LOC_Os01g41710), except for two genes (LOC_Os09g28440 and LOC_Os10g32600) (Fig. 6C). These results indicated that OsbZIP62 exhibits DNA binding activity and might directly regulate the expression of these target genes.
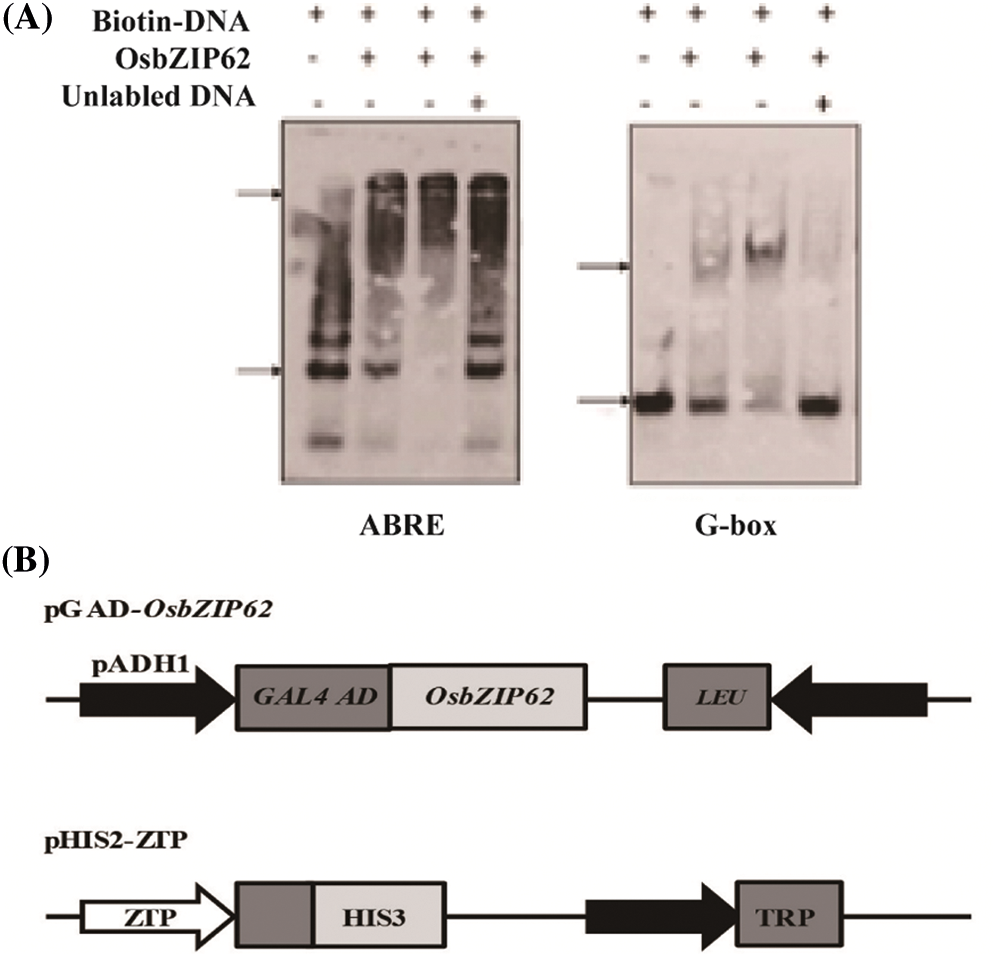
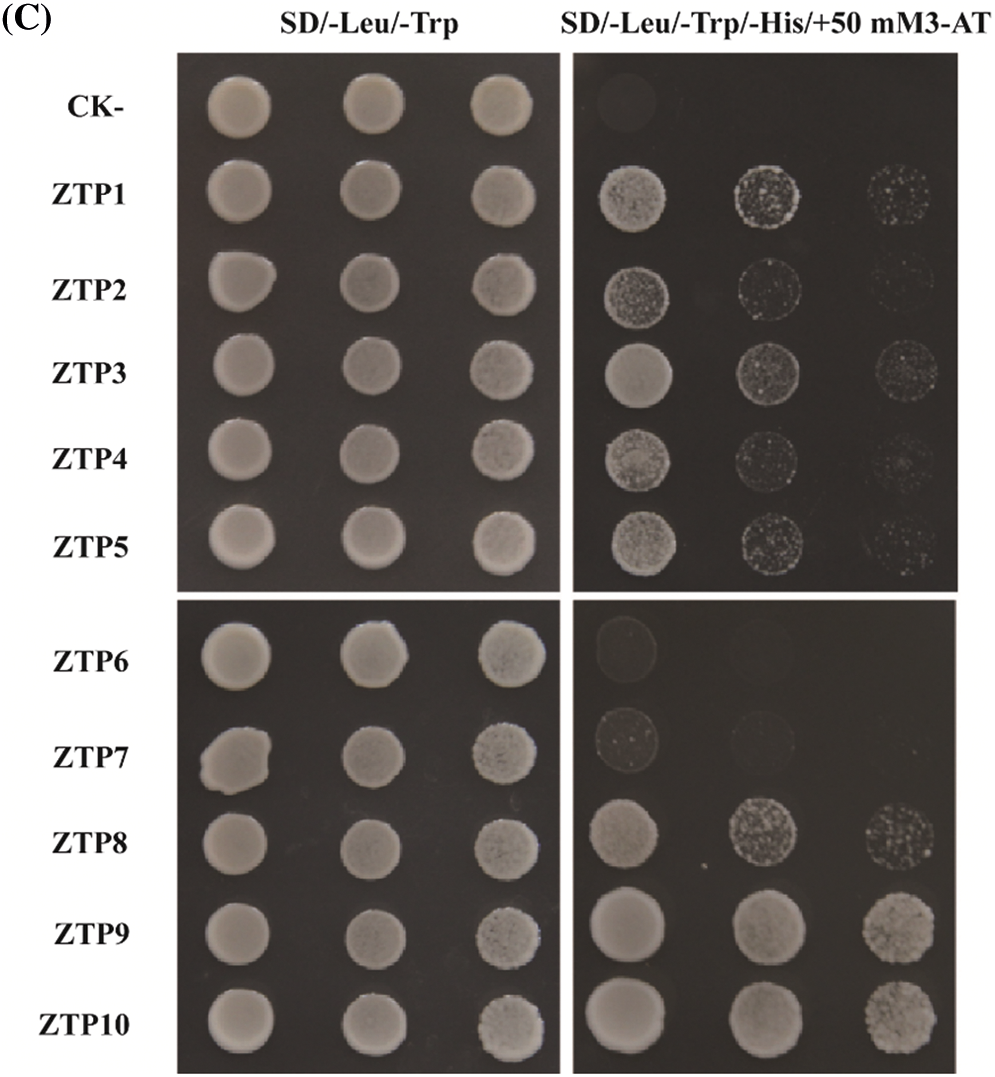
Figure 6: Identification of DNA binding activity of OsbZIP62. (A) EMSA assay of OsbZIP62 and ABRE and G-box. The upper arrow shows the protein-DNA binding complex, while the lower arrow indicates the free DNA. (B) Schematic structure of the yeast one-hybrid expression construct pGAD-OsbZIP62 and the reporter construct pHIS2.1-ZTP (OsbZIP62 putative target gene promoter); (C) Growth performance of transformants on SD/−Leu−/Trp/−His medium containing 50 mM 3-AT. ZTP1-ZTP10 indicates the pGAD-OsbZIP62 plus pHIS2.1-cis (with the promoters of LOC_Os03g04680, LOC_Os07g05360, LOC_Os01g08220, LOC_Os11g12740, LOC_Os09g16510, LOC_Os09g28440, LOC_Os10g32600, LOC_Os10g32980, LOC_Os01g41710 and LOC_Os02g54600 in pHIS2.1, respectively). ck+: positive control, ck–: negative control (pGADT7-rec2-OsbZIP62 plus p53HIS2)
Studies have shown that bZIP transcription factor is involved in plant growth and stress response [23,37–40]. In previous studies, we identified the bZIP family transcription factor OsbZIP62 belonging to the third subfamily of rice bZIP transcription factors [34]. Overexpression of OsbZIP62V improved drought tolerance and antioxidant capacity of rice. However, there are few reports on the relationship between bZIP transcription factors and agronomic traits in rice. Here, we found the overexpression of OsbZIP62V led to increases in several agronomic traits such as plant height, panicle length, primary branch number, secondary branch number and grain number per panicle, which contributed to enhancing the yield of rice (Figs. 1 and 2; Table 1). In addition, we found that overexpression of OsbZIP62V increased the grain length and width, which indicated that the gene had a certain effect on the grain shape (Fig. 3). In contrast, we found that there was no obvious difference between agronomic characters of OsbZIP62 mutants and that of wild type plants (Fig. S1). Therefore, we speculate that OsbZIP62 fused with VP64 can improve agronomic traits and yield, and could be used in rice breeding.
Transcription factors and cis-elements are the core components of plant gene transcription regulatory networks. Identification of direct downstream target genes is an effective strategy to identify the function of OsbZIP62 transcription factor. Not surprisingly, OsbZIP62 altered the expression levels of many genes [34]. Though OsbZIP62 did not affect the expression of several important genes that control rice agronomic traits (Fig. S2), several DEGs were found to be related to rice agronomic traits. For example, Ehd1 expressed lowly in OsbZIP62V plants (Fig. S2). OsbZIP62 was unable to bind to the promoter of Ehd1 (Fig. 6C), it is possible that the expression of Ehd1 was indirectly regulated by OsbZIP62. In contrast, several DEGs (e.g., LOC_Os01g08220 (OsGA3ox2), LOC_Os09g28440 (OsEATB) and LOC_Os03g04680 (OsDSS1)) related to rice agronomic traits were highly expressed in OsbZIP62V plants, and OsbZIP62 might bind to the promoters of these genes (Fig. 6), suggesting that these DEGs might be targets of OsbZIP62.
OsGA3ox2 encodes gibberellin 3β-hydroxylase, and induced expression of OsGA3ox2 led to an increase of GA levels and plant height [19]. OsDSS1 is a member of P450 gene cluster and corresponds to the other reported CYP96B4/SD37 gene. Previous research indicated that dss1 mutants showed dwarfism and reduced grain size, and moderate overexpression SD37 increased rice plant height [41,42]. OsbZIP62 may promote the expression of OsGA3ox2 and OsDSS1, lead to an increase the synthesis of gibberellin, and then improve the plant height. OsEATB is an ethylene responsive transcription factor, which mainly mediates the interaction between ethylene and gibberellin to mediate internode elongation in rice. Overexpression of OsEATB also enhanced the spikelet branching ability [43,44]. In this study, OsbZIP62V increased the number of primary and secondary branches might partially attribute to the enhanced expression of OsEATB. These findings suggest that the increased expression of agronomic trait-related genes by OsbZIP62V contributes to the improvement of plant height and yield of the transgenic rice plants.
In conclusion, we found that OsbZIP62 could regulate the expression of OsGA3ox2, OsEATB, and OsDSS1, and overexpression of OsbZIP62V increased the plant height and grain yield of rice. Combined with the previous studies on the drought resistance function of OsbZIP62 [34], we speculated that overexpression of OsbZIP62V might increase the yield while improving the drought resistance. Our results revealed that fusion of OsbZIP62 with VP64 would have important application value in rice breeding.
Acknowledgement: We thank Professor Chentao Lin (Fujian Agriculture and Forestry University) for providing OsbZIP62-VP64 overexpression rice seeds and Professor Yaoguang Liu (South China Agricultural University) for providing the CRISPR/CAS9 system.
Data Availability: The RNA-seq data supporting the results of this article have been submitted to the GEO at NCBI with the Accession No. GSE122887.
Research Involving Human and Animal Rights: The research does not involve human and/or animal experimentation.
Funding Statement: This research was funded by Funding for Basic Research Projects of Taicang (TC2021JC12), School-Level Research Projects of Yancheng Institute of Technology (xjr2021029), Shanghai Agriculture Applied Technology Development Program (2017-02-08-00-08-F00071) and Natural Science Foundation of Shanghai (18ZR1433300).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Luo, L. J. (2010). Breeding for water-saving and drought-resistance rice (WDR) in China. Journal of Experimental Botany, 61(13), 3509–3517. DOI 10.1093/jxb/erq185. [Google Scholar] [CrossRef]
2. Zhang, Q. (2007). Strategies for developing Green Super Rice. Proceedings of the National Academy of Sciences of the United States of America, 104(42), 16402–16409. DOI 10.1073/pnas.0708013104. [Google Scholar] [CrossRef]
3. Abberton, M., Batley, J., Bentley, A., Bryant, J., Cai, H. et al. (2016). Global agricultural intensification during climate change: A role for genomics. Plant Biotechnology Journal, 14(4), 1095–1098. DOI 10.1111/pbi.12467. [Google Scholar] [CrossRef]
4. Kissoudis, C., van de Wiel, C., Visser, R. G., van der Linden, G. (2016). Future-proof crops: Challenges and strategies for climate resilience improvement. Current Opinion in Plant Biology, 30, 47–56. DOI 10.1016/j.pbi.2016.01.005. [Google Scholar] [CrossRef]
5. Yin, W., Xiao, Y., Niu, M., Meng, W., Li, L. et al. (2020). ARGONAUTE2 enhances grain length and salt tolerance by activating BIG GRAIN3 to modulate cytokinin distribution in rice. Plant Cell, 32(7), 2292–2306. DOI 10.1105/tpc.19.00542. [Google Scholar] [CrossRef]
6. Chen, Y., Dan, Z., Gao, F., Chen, P., Fan, F. et al. (2020). Rice GROWTH-REGULATING FACTOR7 modulates plant architecture through regulating GA and indole-3-acetic acid metabolism. Plant Physiology, 184(1), 393–406. DOI 10.1104/pp.20.00302. [Google Scholar] [CrossRef]
7. Wang, Y., Li, J. (2008). Molecular basis of plant architecture. Annual Review of Plant Biology, 59(1), 253–279. DOI 10.1146/annurev.arplant.59.032607.092902. [Google Scholar] [CrossRef]
8. Xing, Y., Zhang, Q. (2010). Genetic and molecular bases of rice yield. Annual Review of Plant Biology, 61(1), 421–442. DOI 10.1146/annurev-arplant-042809-112209. [Google Scholar] [CrossRef]
9. Wu, B., Hu, W., Xing, Y. Z. (2018). The history and prospect of rice genetic breeding in China. Yi Chuan, 40(10), 841–857. DOI 10.16288/j.yczz.18-213. [Google Scholar] [CrossRef]
10. Xu, C., Wang, Y., Yu, Y., Duan, J., Liao, Z. et al. (2012). Degradation of MONOCULM 1 by APC/C(TAD1) regulates rice tillering. Nature Communications, 3(1), 750. DOI 10.1038/ncomms1743. [Google Scholar] [CrossRef]
11. Zhou, Y., Zhu, J., Li, Z., Yi, C., Liu, J. et al. (2009). Deletion in a quantitative trait gene qPE9-1 associated with panicle erectness improves plant architecture during rice domestication. Genetics, 183(1), 315–324. DOI 10.1534/genetics.109.102681. [Google Scholar] [CrossRef]
12. Li, X., Qian, Q., Fu, Z., Wang, Y., Xiong, G. et al. (2003). Control of tillering in rice. Nature, 422(6932), 618–621. DOI 10.1038/nature01518. [Google Scholar] [CrossRef]
13. Shao, G., Lu, Z., Xiong, J., Wang, B., Jing, Y. et al. (2019). Tiller bud formation regulators MOC1 and MOC3 cooperatively promote tiller bud outgrowth by activating FON1 expression in rice. Molecular Plant, 12(8), 1090–1102. DOI 10.1016/j.molp.2019.04.008. [Google Scholar] [CrossRef]
14. Liao, Z., Yu, H., Duan, J., Yuan, K., Yu, C. et al. (2019). SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nature Communications, 10(1), 2738. DOI 10.1038/s41467-019-10667-2. [Google Scholar] [CrossRef]
15. Endo-Higashi, N., Izawa, T. (2011). Flowering time genes heading date 1 and early heading date 1 together control panicle development in rice. Plant and Cell Physiology, 52(6), 1083–1094. DOI 10.1093/pcp/pcr059. [Google Scholar] [CrossRef]
16. Zong, W., Ren, D., Huang, M., Sun, K., Feng, J. et al. (2020). Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading. New Phytologist, 229(3), 1635–1649. DOI 10.1111/nph.16946. [Google Scholar] [CrossRef]
17. Jiao, Y., Wang, Y., Xue, D., Wang, J., Yan, M. et al. (2010). Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nature Genetics, 42(6), 541–544. DOI 10.1038/ng.591. [Google Scholar] [CrossRef]
18. Qiao, F., Zhao, K. (2011). The influence of RNAi targeting of OsGA20ox2 gene on plant height in rice. Plant Molecular Biology Reporter, 29(4), 952–960. DOI 10.1007/s11105-011-0309-2. [Google Scholar] [CrossRef]
19. Hu, S., Hu, X., Hu, J., Shang, L., Dong, G. et al. (2018). Xiaowei, a new rice germplasm for large-scale indoor research. Molecular Plant, 11(11), 1418–1420. DOI 10.1016/j.molp.2018.08.003. [Google Scholar] [CrossRef]
20. Tong, H., Xiao, Y., Liu, D., Gao, S., Liu, L. et al. (2014). Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell, 26(11), 4376–4393. DOI 10.1105/tpc.114.132092. [Google Scholar] [CrossRef]
21. Riechmann, J. L., Heard, J., Martin, G., Reuber, L., Jiang, C. et al. (2000). Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science, 290(5499), 2105–2110. DOI 10.1126/science.290.5499.2105. [Google Scholar] [CrossRef]
22. Saibo, N. J., Lourenço, T., Oliveira, M. M. (2009). Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Annals of Botany, 103(4), 609–623. DOI 10.1093/aob/mcn227. [Google Scholar] [CrossRef]
23. Jakoby, M., Weisshaar, B., Dröge-Laser, W., Vicente-Carbajosa, J., Tiedemann, J. et al. (2002). bZIP transcription factors in Arabidopsis. Trends in Plant Science, 7(3), 106–111. DOI 10.1016/s1360-1385(01)02223-3. [Google Scholar] [CrossRef]
24. Liu, C., Schläppi, M. R., Mao, B., Wang, W., Wang, A. et al. (2019). The bZIP73 transcription factor controls rice cold tolerance at the reproductive stage. Plant Biotechnology Journal, 17(9), 1834–1849. DOI 10.1111/pbi.13104. [Google Scholar] [CrossRef]
25. Nijhawan, A., Jain, M., Tyagi, A. K., Khurana, J. P. (2008). Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiology, 146(2), 333–350. DOI 10.1104/pp.107.112821. [Google Scholar] [CrossRef]
26. Zhao, Y., Zhao, S., Mao, T., Qu, X., Cao, W. et al. (2011). The plant-specific actin binding protein SCAB1 stabilizes actin filaments and regulates stomatal movement in Arabidopsis. Plant Cell, 23(6), 2314–2330. DOI 10.1105/tpc.111.086546. [Google Scholar] [CrossRef]
27. Chai, J., Zhu, S., Li, C., Wang, C., Cai, M. et al. (2021). OsRE1 interacts with OsRIP1 to regulate rice heading date by finely modulating Ehd1 expression. Plant Biotechnology Journal, 19(2), 300–310. DOI 10.1111/pbi.13462. [Google Scholar] [CrossRef]
28. Ordiz, M. I., Yang, J., Barbazuk, W. B., Beachy, R. N. (2010). Functional analysis of the activation domain of RF2a, a rice transcription factor. Plant Biotechnology Journal, 8(7), 835–844. DOI 10.1111/j.1467-7652.2010.00520.x. [Google Scholar] [CrossRef]
29. Tsuji, H., Nakamura, H., Taoka, K., Shimamoto, K. (2013). Functional diversification of FD transcription factors in rice, components of florigen activation complexes. Plant and Cell Physiology, 54(3), 385–397. DOI 10.1093/pcp/pct005. [Google Scholar] [CrossRef]
30. Dai, S., Petruccelli, S., Ordiz, M. I., Zhang, Z., Chen, S. et al. (2003). Functional analysis of RF2a, a rice transcription factor. Journal of Biological Chemistry, 278(38), 36396–36402. DOI 10.1074/jbc.M304862200. [Google Scholar] [CrossRef]
31. Taoka, K., Ohki, I., Tsuji, H., Furuita, K., Hayashi, K. et al. (2011). 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature, 476(7360), 332–335. DOI 10.1038/nature10272. [Google Scholar] [CrossRef]
32. Yaghmai, R., Cutting, G. R. (2002). Optimized regulation of gene expression using artificial transcription factors. Molecular Therapy, 5(6), 685–694. DOI 10.1006/mthe.2002.0610. [Google Scholar] [CrossRef]
33. Zhao, T., Liu, J., Li, H. Y., Lin, J. Z., Bian, M. D. et al. (2015). Using hybrid transcription factors to study gene function in rice. Science China Life Sciences, 58(11), 1160–1162. DOI 10.1007/s11427-015-4937-x. [Google Scholar] [CrossRef]
34. Yang, S., Xu, K., Chen, S., Li, T., Xia, H. et al. (2019). A stress-responsive bZIP transcription factor OsbZIP62 improves drought and oxidative tolerance in rice. BMC Plant Biology, 19(1), 260. DOI 10.1186/s12870-019-1872-1. [Google Scholar] [CrossRef]
35. Livak, K. J., Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods, 25(4), 402–408. DOI 10.1006/meth.2001.1262. [Google Scholar] [CrossRef]
36. Kanehisa, M., Goto, S., Kawashima, S., Okuno, Y., Hattori, M. (2004). The KEGG resource for deciphering the genome. Nucleic Acids Research, 32, D277–280. DOI 10.1093/nar/gkh063. [Google Scholar] [CrossRef]
37. E, Z. G., Zhang, Y. P., Zhou, J. H., Wang, L. (2014). Mini review roles of the bZIP gene family in rice. Genetics and Molecular Research, 13(2), 3025–3036. DOI 10.4238/2014.April.16.11. [Google Scholar] [CrossRef]
38. Kobayashi, F., Maeta, E., Terashima, A., Takumi, S. (2008). Positive role of a wheat HvABI5 ortholog in abiotic stress response of seedlings. Physiologia Plantarum, 134(1), 74–86. DOI 10.1111/j.1399-3054.2008.01107.x. [Google Scholar] [CrossRef]
39. Liu, A., Zhou, Z., Yi, Y., Chen, G. (2020). Transcriptome analysis reveals the roles of stem nodes in cadmium transport to rice grain. BMC Genomics, 21(1), 127. DOI 10.1186/s12864-020-6474-7. [Google Scholar] [CrossRef]
40. Zhang, Z., Yang, J., Wu, Y. (2015). Transcriptional regulation of Zein gene expression in maize through the additive and synergistic action of opaque2, Prolamine-Box Binding Factor, and O2 heterodimerizing proteins. Plant Cell, 27(4), 1162–1172. DOI 10.1105/tpc.15.00035. [Google Scholar] [CrossRef]
41. Tanaka, W., Ohmori, Y., Ushijima, T., Matsusaka, H., Matsushita, T. et al. (2015). Axillary meristem formation in rice requires the WUSCHEL ortholog TILLERS ABSENT1. Plant Cell, 27(4), 1173–1184. DOI 10.1105/tpc.15.00074. [Google Scholar] [CrossRef]
42. Zhang, J., Liu, X., Li, S., Cheng, Z., Li, C. (2014). The rice semi-dwarf mutant sd37, caused by a mutation in CYP96B4, plays an important role in the fine-tuning of plant growth. PLoS One, 9(2), e88068. DOI 10.1371/journal.pone.0088068. [Google Scholar] [CrossRef]
43. Qi, W., Sun, F., Wang, Q., Chen, M., Huang, Y. et al. (2011). Rice ethylene-response AP2/ERF factor OsEATB restricts internode elongation by down-regulating a gibberellin biosynthetic gene. Plant Physiology, 157(1), 216–228. DOI 10.1104/pp.111.179945. [Google Scholar] [CrossRef]
44. Chen, X., Lu, S., Wang, Y., Zhang, X., Lv, B. et al. (2015). OsNAC2 encoding a NAC transcription factor that affects plant height through mediating the gibberellic acid pathway in rice. Plant Journal, 82(2), 302–314. DOI 10.1111/tpj.12819. [Google Scholar] [CrossRef]

Figure S1: Phenotypic characterization of OsbZIP62 mutant transgenic plants. NIP: Wild type control; C54 and C56 were two osbzip62 knockout mutants
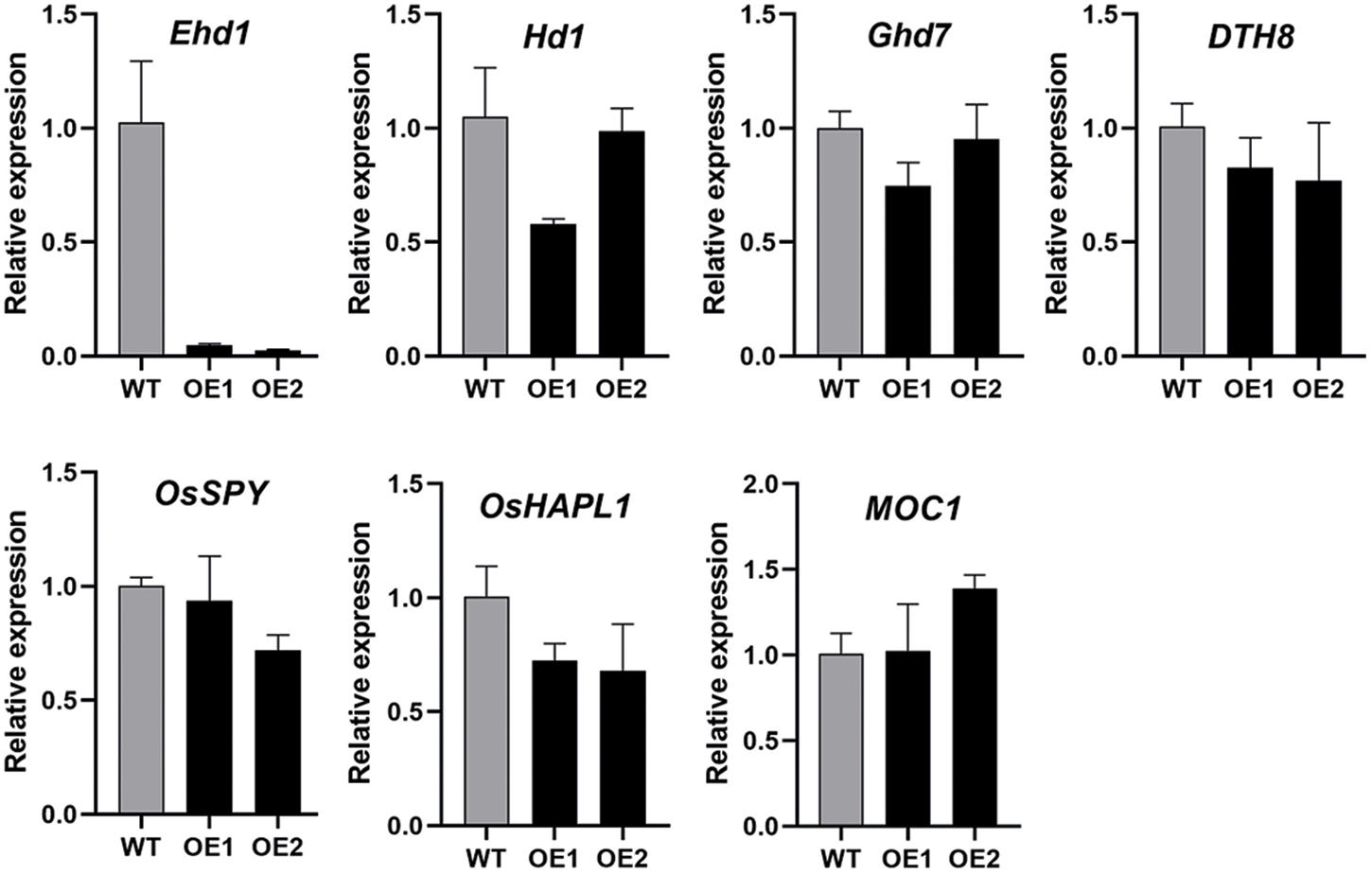
Figure S2: Expression level of important agronomic traits genes in OsbZIP62V transgenic plants detected through qPCR. Data represents means ± SE (n = 3). WT: wild-type. OE1 and OE2: OsbZIP62V overexpression lines
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |