

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020010
ARTICLE
Bioinformatics Analysis of Disease Resistance Gene PR1 and Its Genetic Transformation in Soybeans and Cultivation of Multi-resistant Materials
College of Agronomy, Jilin Agricultural University, Changchun, 1300118, China
*Corresponding Author: Piwu Wang. Email: peiwuw@163.com
Received: 29 October 2021; Accepted: 17 December 2021
Abstract: In agricultural production, a single insect-resistant and disease-resistant variety can no longer meet the demand. In this study, the expression vector pCAMBIA-3301-PR1 containing the disease-resistant gene PR1 was constructed by means of genetic engineering, and the PR1 gene was genetically transformed to contain the PR1 gene through the pollen tube method. In CryAb-8Like transgenic high-generation T7 receptor soybean, a new material that is resistant to insects and diseases is obtained. For T2 transformed plants, routine PCR detection, Southern Blot hybridization, fluorescence quantitative PCR detection, indoor and outdoor pest resistance identification and indoor disease resistance identification were performed. The results showed that there were 9 positive plants in the routine PCR test of T2 generation. In Southern Blot hybridization, both PR1 and CryAb-8Like genes are integrated in soybeans in the form of single copies. Fluorescence quantitative PCR showed that the expression levels of PR1 and CryAb-8Like genes are different in different tissues. The average expression levels of PR1 gene in plant roots, stems, and leaves are 2.88, 1.54, and 5.26, respectively. CryAb-8Like genes are found in roots, stems, and leaves. The average expression levels were 1.36, 1.39, and 4.25, respectively. The insectivorous rate of the CryAb-8Like gene in outdoor plants with positive insect resistance identification was 3.78%. The disc partition method was used indoors for pest resistance identification, and the bud length of transformed plants increased significantly. The average mortality rate of untransformed plants in indoor disease resistance identification was as high as 56.66%, and the average mortality rate of plants transformed with PR1 gene was 10.00%, and disease resistance was significantly improved. Therefore, a new material with resistance to diseases and insects is obtained.
Keywords: Soybean; PR1 gene; CryAb-8Like gene; insect resistance; disease resistance
Soybean (Glycine max L., Merr) is an important oil and food crop in the world [1]. It is one of the main sources of protein and fat for mankind. In China, the protein in soybeans accounts for about 40%, and the fat content accounts for about 20% [2,3]. In recent years, China’s demand for soybeans has increased year by year. In 2017, the total domestic consumption of soybeans reached 1.2 × 1011 Kg. In 2019, the total domestic consumption of soybeans reached 1.964 × 1011 Kg, and soybean consumption accounted for 5.4% of the world [4]. This shows that soybeans play an important role in people’s lives. Diseases and pests of external environmental factors will greatly change the yield of soybeans. In severe cases, it will lead to no harvest of soybeans. Cultivating high-yield and high-quality soybeans is the current goal of crop breeders.
Soybean gray spot disease is also known as brown spot disease. The pathogen is (Cercospora sojina Hara), which is called soybean short fat cell. It belongs to the subphylum Deuteromycota. It is a worldwide disease [5]. The pathogen is Cercosporidium sofinum, which belongs to the subphylum Deuteromycota. The disease type is a fungal disease and spreads all over the world. This pathogen develops in soybean leaves, stems and seeds. The incidence is mainly concentrated in the high temperature and rainy period from July to August [6]. After the onset, the leaves of soybeans will fall off prematurely, which will affect the normal photosynthesis of soybeans, and reduce the yield and quality of soybeans [7]. In recent years, studies have found that the disease has a high incidence in the three northeastern provinces of China, causing a 25%–35% reduction in soybean production, and a serious reduction of more than 50%, which seriously affects soybean yield and quality, causing direct losses of up to 100 million RMB [8].
Pathogenesis related PR1 proteins (PR1s), when plants are invaded by certain pathogenic bacteria or stimulated by some specific compounds, the stress produces related proteins, which are closely related to system resistance and allergic necrosis [9]. In 1980, the PR1 encoding protein gene was first discovered in tobacco [10], and subsequently found in both monocot and dicot plants [11,12]. The PR1 gene encoding protein has been confirmed to be effective in resisting the spread of viruses and blocking fungi. Infringement and resisting the adverse external environment and other functions, the specific mechanism of action is not yet clear, and it has become one of the hot research topics in recent years [13].
Bacillus thuringiensis (Bt) has been discovered more than 100 years ago, and it has played an important role in the biological control of pests [14]. Bacillus thuringiensis can produce two types of endotoxin and exotoxin. Pests ingest the plants containing Bt gene. Pests will die due to starvation, blood necrosis, or neurotoxicity. It has an obvious control effect on Lepidoptera pests (Soybean Heartworm) [14].
This experiment uses biotechnology and genetic engineering principles to construct an overexpression vector for the disease resistance gene PR1 gene, and uses the genetic transformation method to transfer the disease resistance gene PR1 to the high-generation recipient soybean containing the insect resistance gene CryAb-8Like. In the process, through the identification of resistance to offspring, new insect-resistant, disease-resistant and multi-resistant materials are cultivated.
Soybean Jinong 28 and Jinong 38, CryAB-8Like transgenic insect-resistant soybean LND097, ZLHH21, pCAMBIA-3301-PR1 gene expression vector, soybean gray spot and white net cover were used. All the experimental materials were provided by the Biotechnology Center of Jilin Agricultural University.
Plant DNA extraction kit (Kang Wei Reagent Company, Jiangsu, China), High purity plasmid extraction kit (Wegrath Biology Company, Beijing, China), Agarose gel extraction kit (Changchun Weijie Company, Changchun, China), Taq polymerase in PCR, dNTP, Buffering (Fermentas Company, Beijing, China), Southern Hybridization Kit (Roche Company, Shanghai, China), fluorescent quantification kits (Roche Company, Shanghai, China), and Synthetic primer (kumei Company, Changchun, China) were purchased while Other biological reagents were purchased from Beijing Dingguo Company (Beijing, China).
2.3 Identification of Plant Expression Vector pCAMBIA-3301-PR1
The Escherichia coli containing the target disease-resistant gene PR1 was stored in the −80°C refrigerator. The bacterial liquid were activated in the LB solid medium containing kanamycin and incubated overnight at 37°C. A single bacterial colony was taken and , placed it in LB liquid medium containing kanamycin. Then the liquid medium incubated overnight at 37°C. The plasmid containing the PR1 gene sent to Changchun Kume Biotechnology for sequencing. The sequencing analysis identified the expression vector containing the target gene PR1, and the identification results are shown (Fig. 13). The homology reached 100%, indicating that the target gene PR1 is on the overexpression vector.
2.4 Transformation of Recipient Soybean Plants
The method of genetic transformation was carried out by the pollen tube passage method, and the T2 generation plants of transgenic plants are obtained by adding generation planting in the field. The specific operation of the pollen tube passage method is as follows: removed the keel flap, cut the stigma and tied with the rope, respectively. Then the plasmid was introduced in to the plant.
2.5 Routine PCR Identification of Transgenic Plants
In the late three-leaf period of the plant, fresh soybean leaves were taken, and the DNA extraction kit of Kangwei Century Company (Beijing, China) was used to extract soybean plant DNA. Primer 5.0 was used to pair PR1, CryAB-8Like, screening gene Bar, promoter 35S, and terminator Nos. Gene design specific primers (Table 1), plasmid as a positive control group, untransformed plants as a CK control group, and ddH2O as a blank control group. The specific reaction conditions were as shown (Table 2).
2.6 Southern Blot Hybridization Identification of T2 Transgenic Lines
Use DNA genome extraction kit to extract the genome of the T2 generation plant, and store it in the refrigerator at –20°C for subsequent enzyme digestion experiments. The PR1 and CryAB-8Like plasmids were used as the positive control group, and the untransformed plants were used as the CK control group.
The soybean genome was digested with BgLII, and the specific experimental operation method was carried out according to the instructions of Roche’s Southern blot hybridization kit.
2.7 Fluorescence Quantitative PCR Identification of T2 Generation Transgenic Plants
RNA was extracted from the roots, stems, and leaves of T2 transgenic plants, and then reverse-transcribed into cDNA using a fluorescent reverse transcription kit (TakaraBio, RR036A), and designed fluorescent quantitative primers using Primer Quest Tool software (Table 4), Mx3000P fluorescence Quantitative PCR was used to detect the relative expression levels of PR1 gene and CryAB-8Like gene in roots, stems, and leaves, and use the formula 2-ΔΔCT for data analysis.
2.8 Outdoor Pest Resistance Identification of T2 Transgenic Plants
In the outdoor transgenic field, the positive plants genetically transformed with the PR1 gene were planted, and the untransformed soybean Jinong 28CK was used as the control group in the outdoor net shed. During the prevalence of soybean borer in late August, a large number of soybean borer was inserted into the net room. The insect-resistant, disease-resistant and multi-resistant plants and recipient plants containing CryAB-8Like gene and PR1 gene were to be harvested. The plant was threshed and related statistical data analysis was carried out, the soybean insect-eating situation was investigated, and the soybean carbophagous insect-eating situation was photographed.
2.9 Indoor Pest Resistance Identification of T2 Transgenic Plants
50 PR1 gene pest-resistant and disease-resistant soybeans and 50 untransformed soybeans were taken, and conducted the pest resistance identification test. The disc separation method was used for soybean resistance identification [15]. The transformed and untransformed soybeans were soaked in sterilized water for 15 hours for subsequent tests.
Autoclave the glass disc and filter paper. Spread the sterilized filter paper on the surface of the glass dish. Place 10 soybean heartworms on the scoring position in the center of the petri dish. Spread them evenly on both sides of the scoring position on the dish. Soybeans, the transformed soybeans were placed on the left side, and the untransformed soybeans were placed on the right side, and three repeated control experiments were set. After 7 days, record the borer’s condition, bud length and fresh weight (Fig. 1A). According to the actual growth status of soybean seeds, they were divided into insect resistance. In order to ensure the accuracy of the test, four boxes were prepared, and all four boxes were sterilized at high temperatures. The filter paper was spread on the surface of the box and was numbered 1, 2, 3, 4, place 40 transformed CryAB-8Like soybeans in boxes numbered 1, 2, 40 untransformed soybeans, Jinong 28 soybeans in boxes 3 and 4, and 12 soybean heartworms in boxes 1, 3, respectively. Insect resistance identification of transformed CryAB-8Like gene and Jinong 28 resistance identification. Boxes 2 and 4 were used for the identification of CryAB-8Like gene without insect resistance and Jinong 28 for the identification of insect resistance (Fig. 1B).
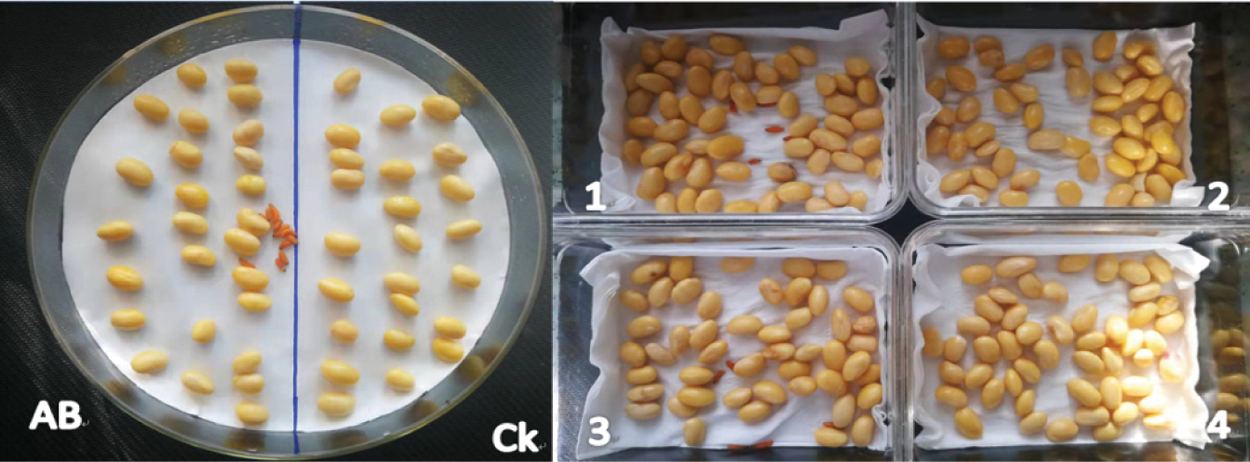
Figure 1: Disc segmentation method of insect resistance identification (A) Comparative experiment (B)
Note: Ab: Bt soybean seeds; CK: untransformed soybean seed; 1, 2: Soybean gene CryAB-8Like transfected; 3, 4: Untransformed soybean Jinong 28; 1, 3: Catch soybean heartworm
2.10 Indoor Disease Resistance Identification of T2 Transgenic Plants
The PR1 genetically modified soybeans were planted and artificially inoculated indoors. The plants containing the PR1 gene and the CK plants of the control group were inoculated with the same bacteria in equal amounts, and the hypocotyls and leaf parts of soybean leaves were scratched. Soybean gray spot was inoculated on the scratched parts of soybean plants at room temperature 25°C At 91% under humid environment, three groups of repeated controls were set up, in which there were two CK plants in each group, and two plants with disease-resistant PR1 gene were inoculated in the late three-leaf stage of soybeans, and soybean gray board disease was inoculated for 3 days and 7 days. Investigation The actual condition of the disease and record the data.
The GmPR1 gene comes from high-throughput sequencing of the differential transcriptome to obtain differentially expressed genes. The over-expression vector pCAMBIA-3301-GmPR1 of this gene was provided by the Biotechnology Center of Jilin Agricultural University.
3.2 Sequence Analysis of Soybean PR1 Gene
Using the NCBI website to perform Blast analysis, the nucleotide sequence of soybean is as much as 100% homologous to GmPR1 (Fig. 2), confirming that this gene is the target gene. The full length of the cDNA is 525 bp, and the ORF sequence is 525 bp in length, which encodes 174 amino acids. The molecular weight of the protein is 18.83 kilodaltons, and the theoretical isoelectric point PI is 4.54.
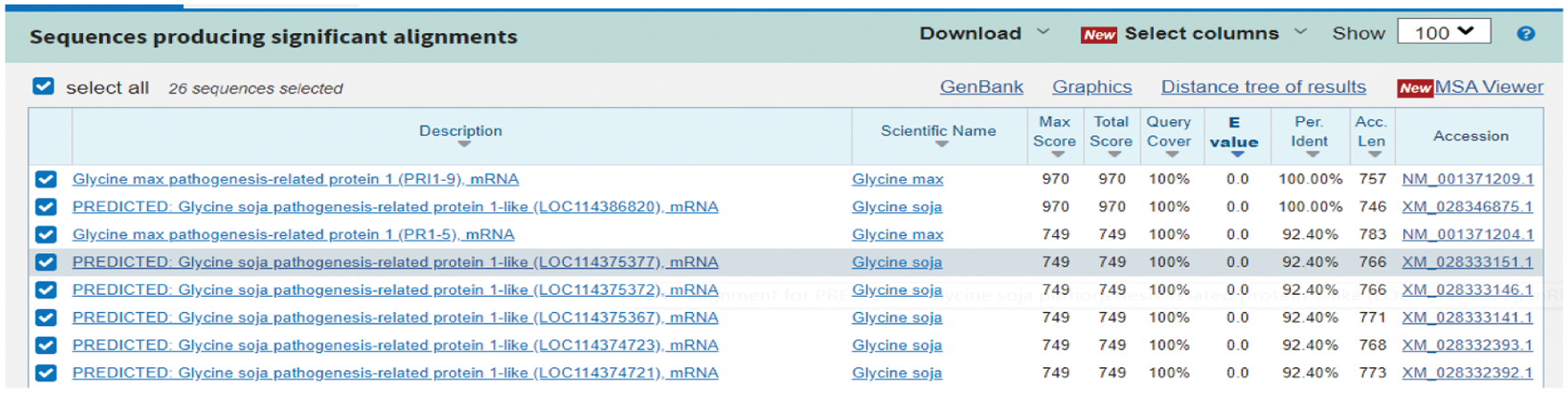
Figure 2: NCBI prediction results of PR1 target gene
3.3 The Signal Peptide Prediction and Subcellular Localization Analysis of Soybean PR1 Encoded Protein
Signalp online analysis was used to predict protein signal peptides (Fig. 3). The protein encoding the PR1 gene is a secreted protein. The maximum signal peptide (Sec/SPI) is 0.9695, and a cleavage site appears at the 27–28 amino acid position, with a probability of 0.8692. The results of PSORT II software on the protein subcellular location analysis of the GmPR1 gene are shown (Fig. 4). The percentage of K-NN prediction 44.4% on the endoplasmic reticulum, 22.2% on the mitochondria and Golgi apparatus were obtained. This result proving that the gene mainly functions on the endoplasmic reticulum. Secondly, it functions on mitochondria and Golgi apparatus, and it can be seen from the side that this gene is a secreted protein.
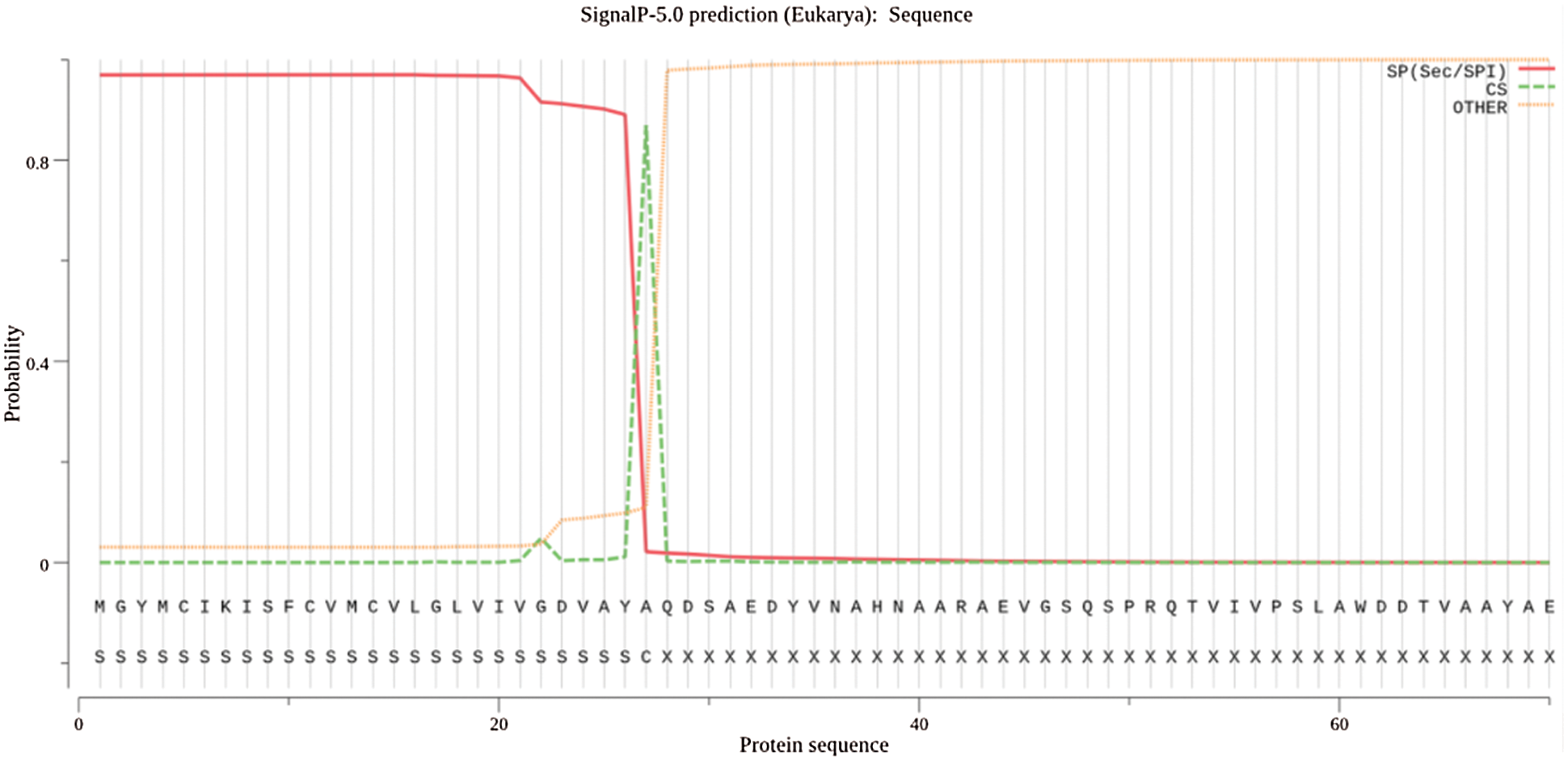
Figure 3: Prediction results of PR1 gene signal peptide

Figure 4: Protein subcellular localization of soybean PR1 gene
3.4 Research on Hydrophilicity and Hydrophobicity of Soybean PR1 Encoded Protein and Analysis of Transmembrane Region
ProtScale online analysis of the hydrophilicity and hydrophobicity of the protein encoded by soybean PR1, Hphob/Kyte & Doolittle is the prediction standard, and the results are as follows (Fig. 5). There are many relatively low peaks, and the amino acids at positions 111–119 have obvious hydrophilic regions. Where the amino acid at position 115 has the smallest value, which is –2.756, where the hydrophilicity is the strongest. However, the amino acid at position 16 has the largest value, which is 3.189, where the amino acid has the strongest hydrophobicity. The hydrophilic amino acids in the entire polypeptide chain account for 72.45%, and the hydrophobic amino acids account for 27.55%. The overall performance is hydrophilic, so the protein is a soluble protein. Using TMHMM Server 2.0 to analyze the transmembrane region of the protein online, the results are shown (Fig. 6). The GmPR1 protein contains a transmembrane structure. It is a transmembrane protein that migrates in the cell.
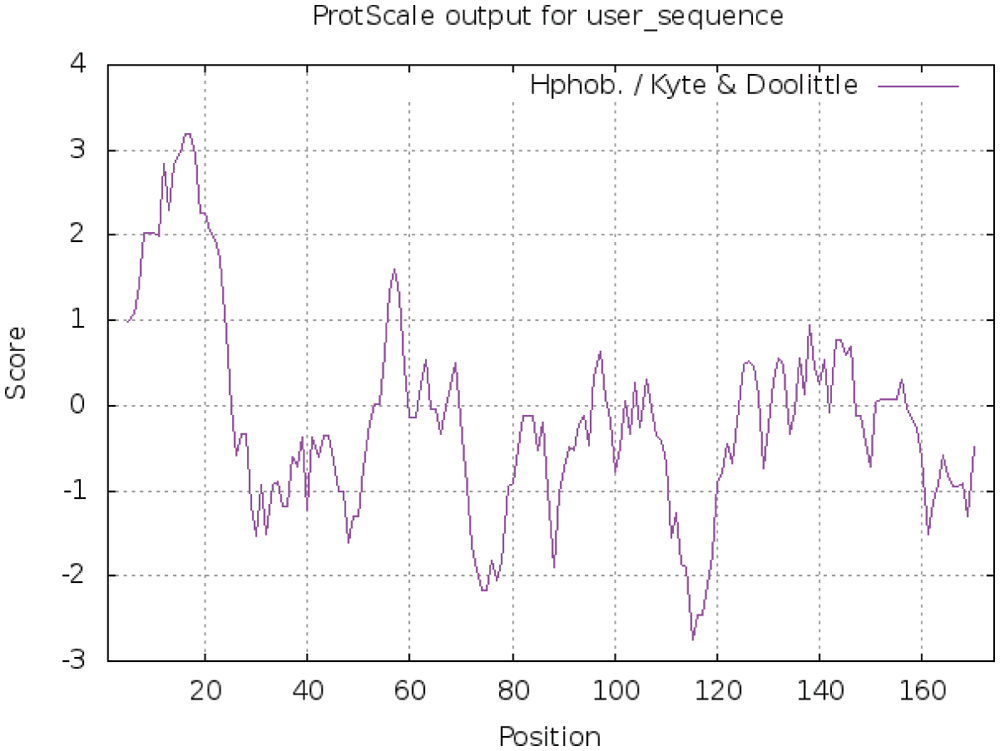
Figure 5: Study on the hydrophilicity and hydrophobicity of PR1 protein
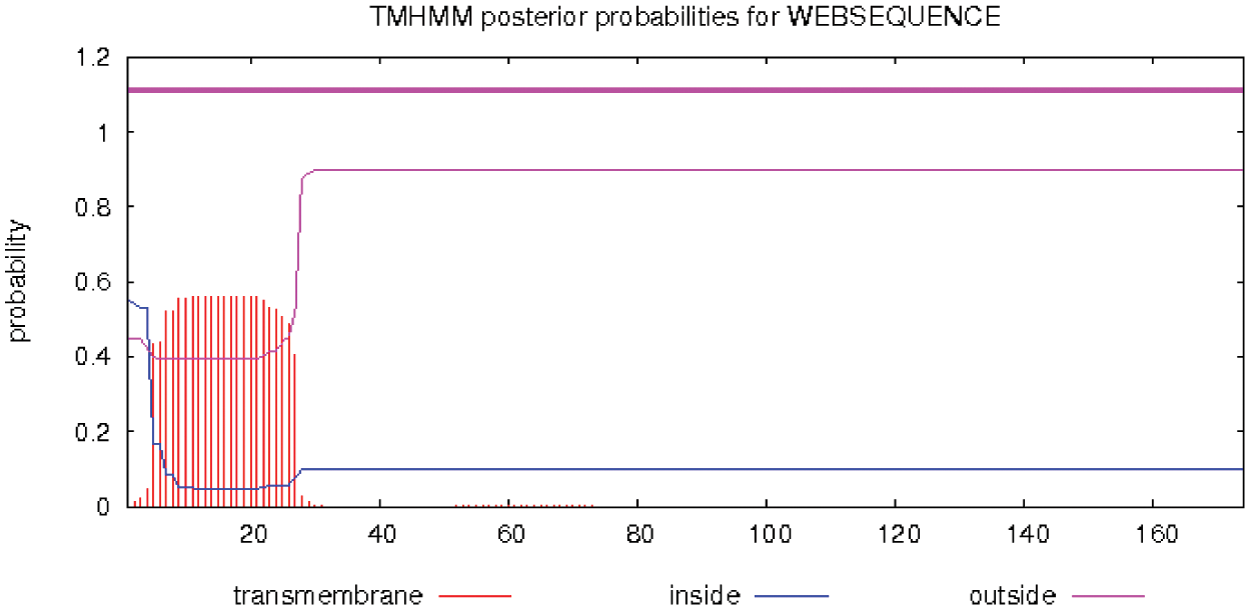
Figure 6: Prediction of transmembrane region of PR1 gene encoding protein
3.5 Prediction of the Phosphorylation Site of Soybean PR1 Protein
NetPhos online analysis for PR1 encoded protein phosphorylation site are shown (Fig. 7). The GmPR1 protein has 19 potential phosphorylation sites, including 10 serine (Ser) phosphorylation sites, 3 threonine (Thr) ) Phosphorylation sites, 6 tyrosine (Tyr) phosphorylation sites.
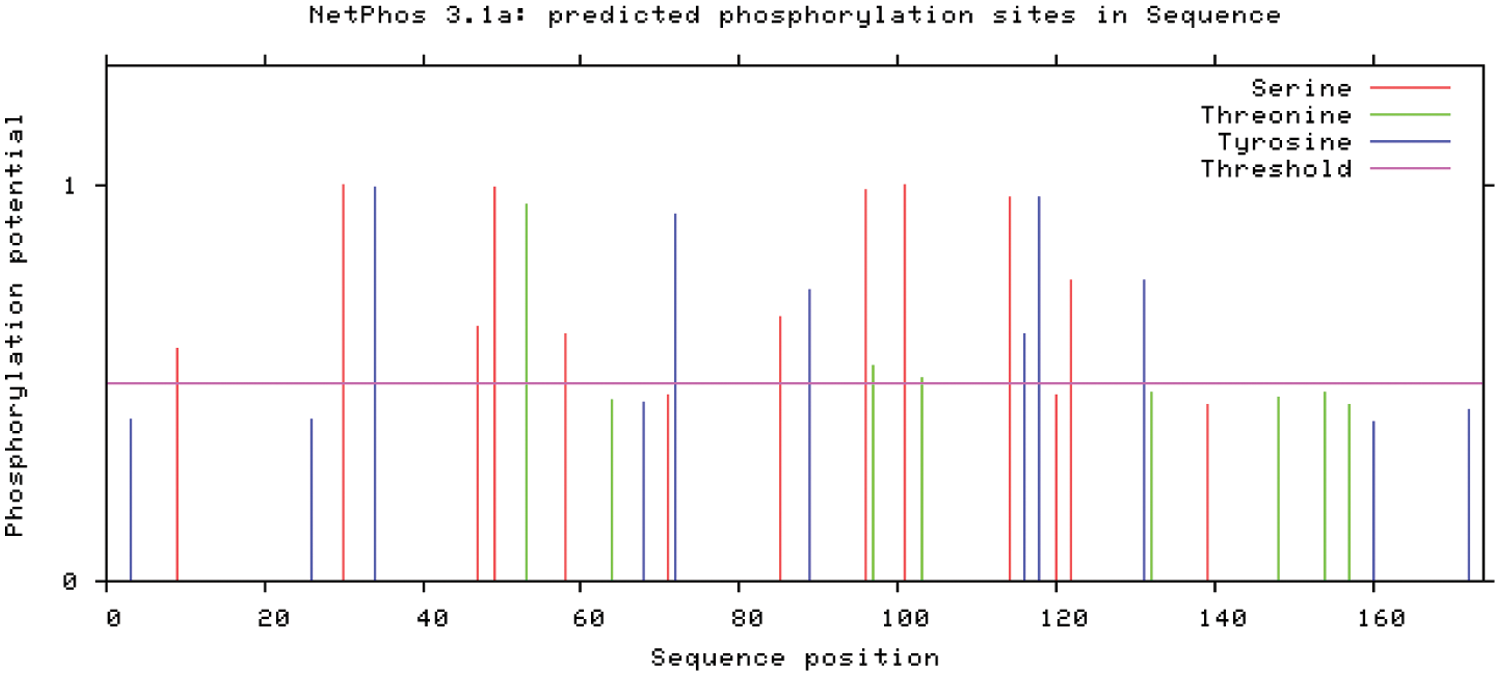
Figure 7: Prediction analysis of phosphorylation of soybean PR1 gene encoding protein
3.6 Analysis of the Results of Soybean PR1 Gene Encoding Protein
SOPMA online was used to analyze the secondary structure of the amino acid sequence of GmPR1, and the results are shown (Fig. 8). GmPR1 protein consists of α-helix (32.76%), extended chain (17.82%), β-turn (5.17%), and random coils (44.25%). The four parts of GmPR1 protein composed of random coils accounted for the highest proportion, followed by α-helical structure, β-turn, and the lowest was β-turn. SWISS-MODEL was used to analyze the tertiary structure of the amino acid sequence of GmPR1 online for predictive analysis. The results show that the tertiary structure and the secondary structure are consistent, and they are composed of four parts (Fig. 9). The GmPR1 encoded protein is in the three-dimensional structure database. The homology to Arabidopsis thaliana is as high as 82.52%.
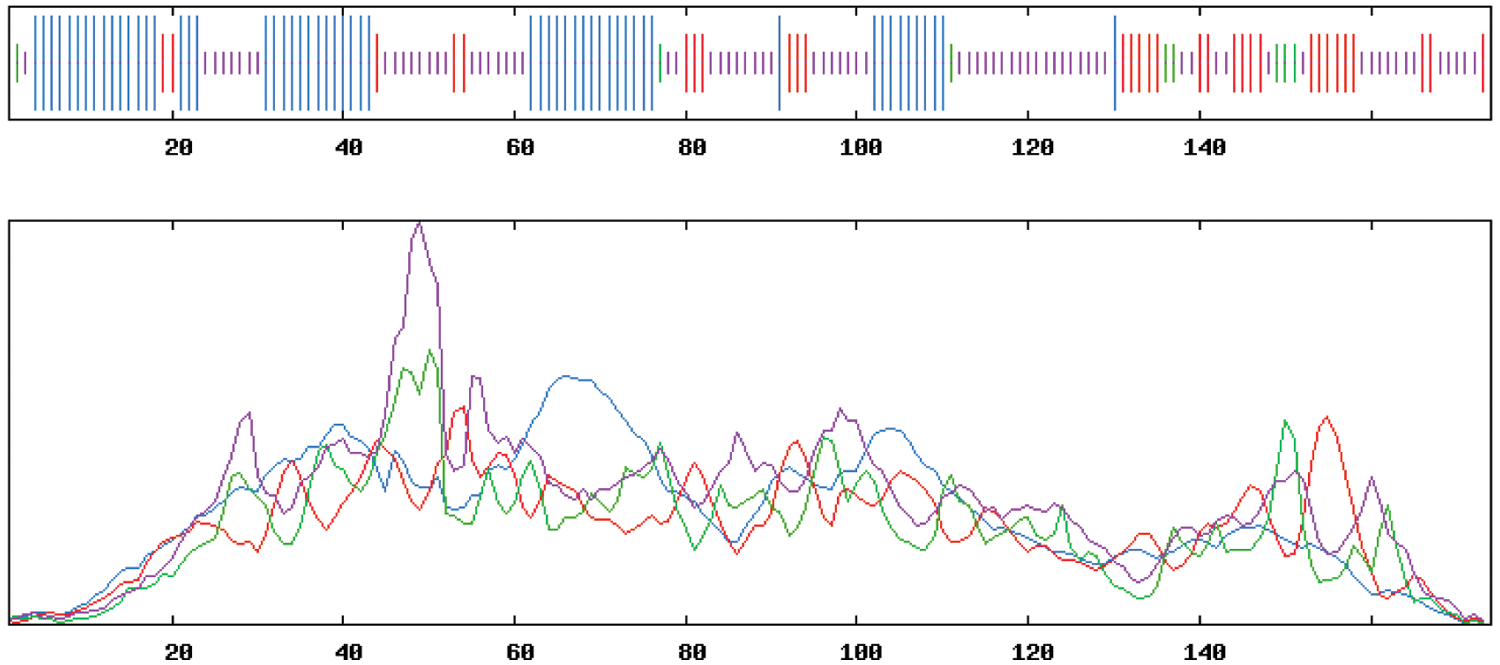
Figure 8: PR1 protein secondary structure prediction
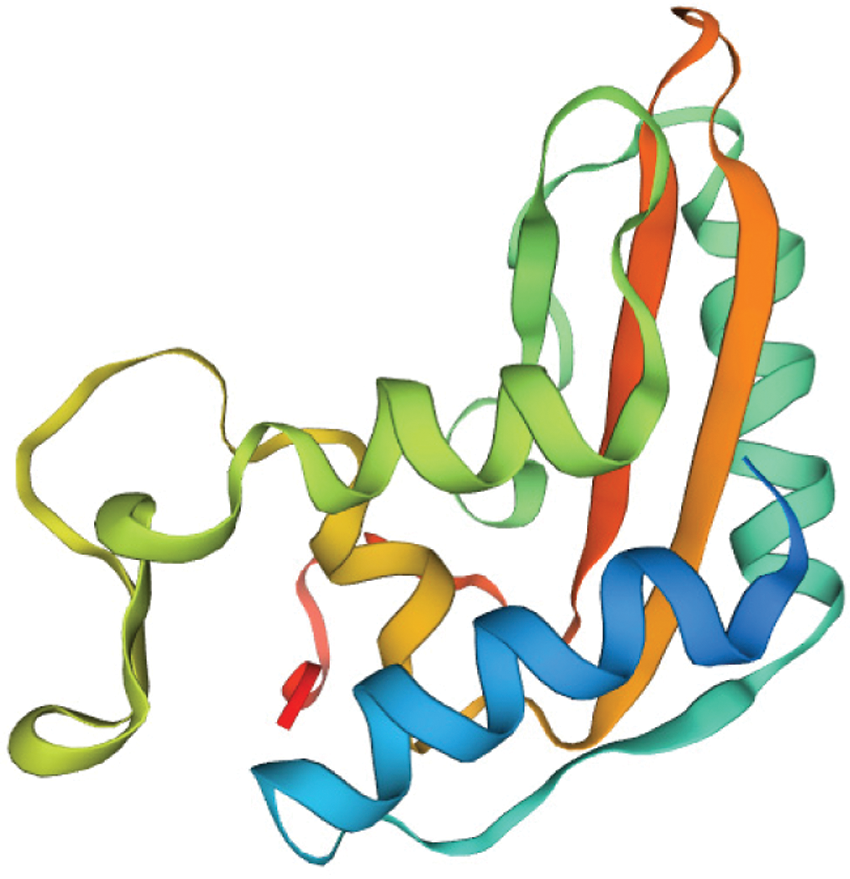
Figure 9: Prediction of the tertiary structure of PR1 protein
3.7 Protein Interaction Analysis of Soybean PR1 Gene
STRING online analysis of GmPR1 gene protein interaction analysis are shown (Fig. 10). PR1 protein is mainly related to defensin-like protein, oxidized allene synthase, chloroplast allene oxide synthase, chitinase class I, peptidase M10A family, NPR1-2 protein, tubulin, etc., interact with each other. Protein interaction analysis showed that GmPR1 protein played an important role and function in response to disease stress.
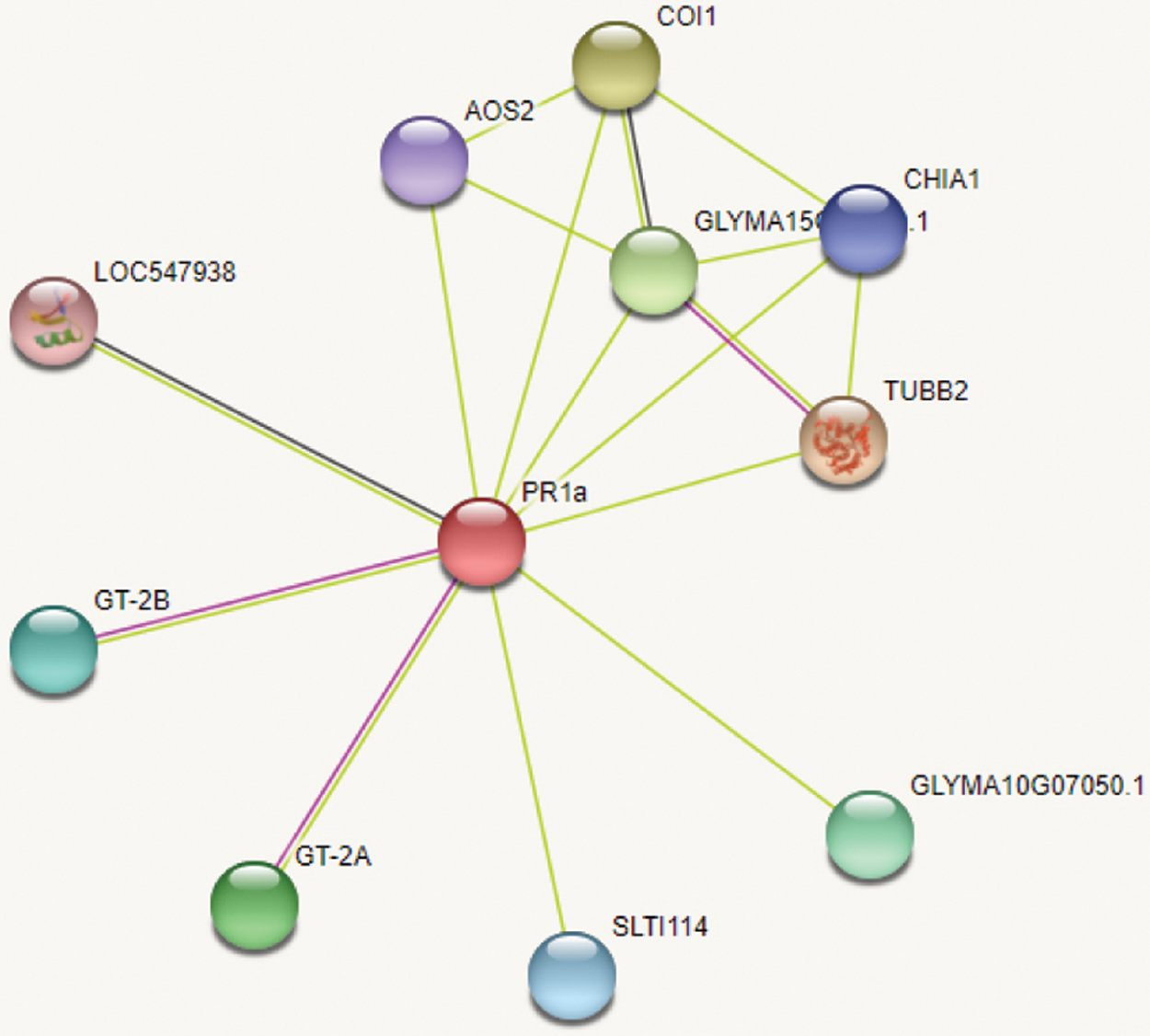
Figure 10: PR1 protein interaction analysis
Note: LOC547938 defensin-like protein; AOS2 oxidized allene synthase, chloroplast allene oxide synthase; SLTI114 peptidase M10A family; CHIA1 chitinase class I; GLYMA15G13320.1 NPR1-2 protein; TUBB2 tubulin.
3.8 Construction of Plant Expression Vector pCAMBIA-3301-PR1
Primer 5.0 software was used to design primer to specifically amplify the nucleotide sequence of the target gene GmPR1 by PCR. Using 1.5% concentration agarose gel electrophoresis at 220 V, 110 mA, the target band is 525bp (Fig. 11) and the size of the expected fragment is consistent. It is preliminarily considered that this fragment is the target gene fragment. In this experiment, the pCAMBIA-3301-PR1 gene expression vector was provided by the Plant Biotechnology Center of Jilin Agricultural University. The vector structure diagram of the disease resistance gene is (Fig. 12) DNA man software and CE design2.0 software were used to design a double digestion. The restriction sites are BglII and BsteII. Seamless cloning technology was used to seamlessly clone the cloned target gene and pCAMBIA-3301 large fragment. Then transformed into Escherichia coli DH5α, LB+Kan (kanamycin) medium plate coating. Sent to the Kumei Biotechnology Company in Changchun City for sequencing. The sequencing results were compared using DNAman homology results as shown (Fig. 13).
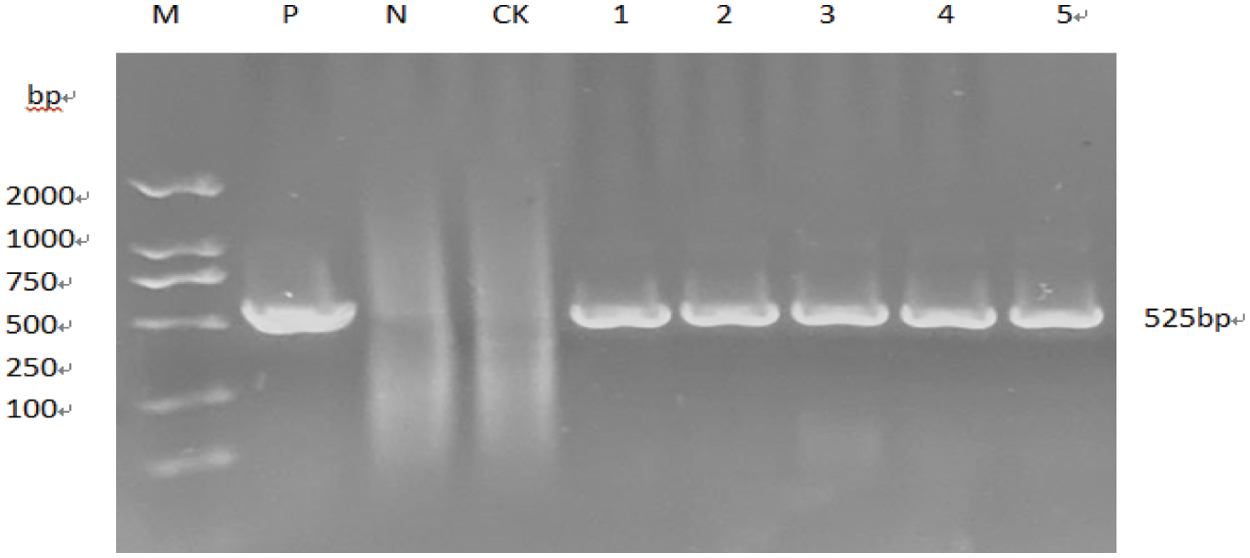
Figure 11: Specific amplified fragment of PR1 gene
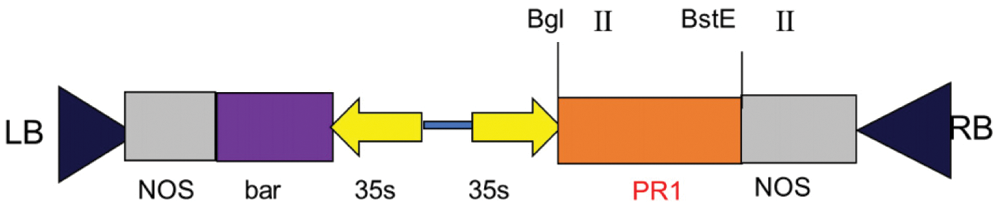
Figure 12: Schematic diagram of pCAMBIA-3301-PR1 vector structure
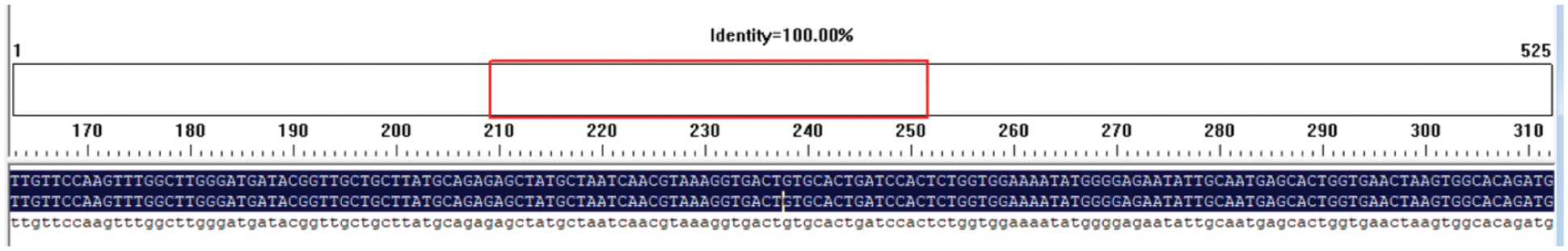
Figure 13: DNAman homology comparison result of PR1 gene
3.9 Transforming Recipient Soybeans
The pollen tube pathway was used to genetically transform 10 T1 positive plants of pCAMBIA-3301-PR1 and 18 T2 positive plants.
3.10 Routine PCR Identification of Transgenic Positive Plants
3.10.1 Identification of T1 Generation Positive Plants
The soybean leaf DNA genome of T1 plant was extracted, and the PR1 gene, Cry-8Like gene, Bar gene, 35S gene, and NOS gene were specifically amplified, and the corresponding fragment sizes were 525, 1987, 552, 500 and 198 bp, respectively (Fig. 14), consistent with the expected results, it can be preliminarily considered that the gene is integrated into the genome of the T1 plant.
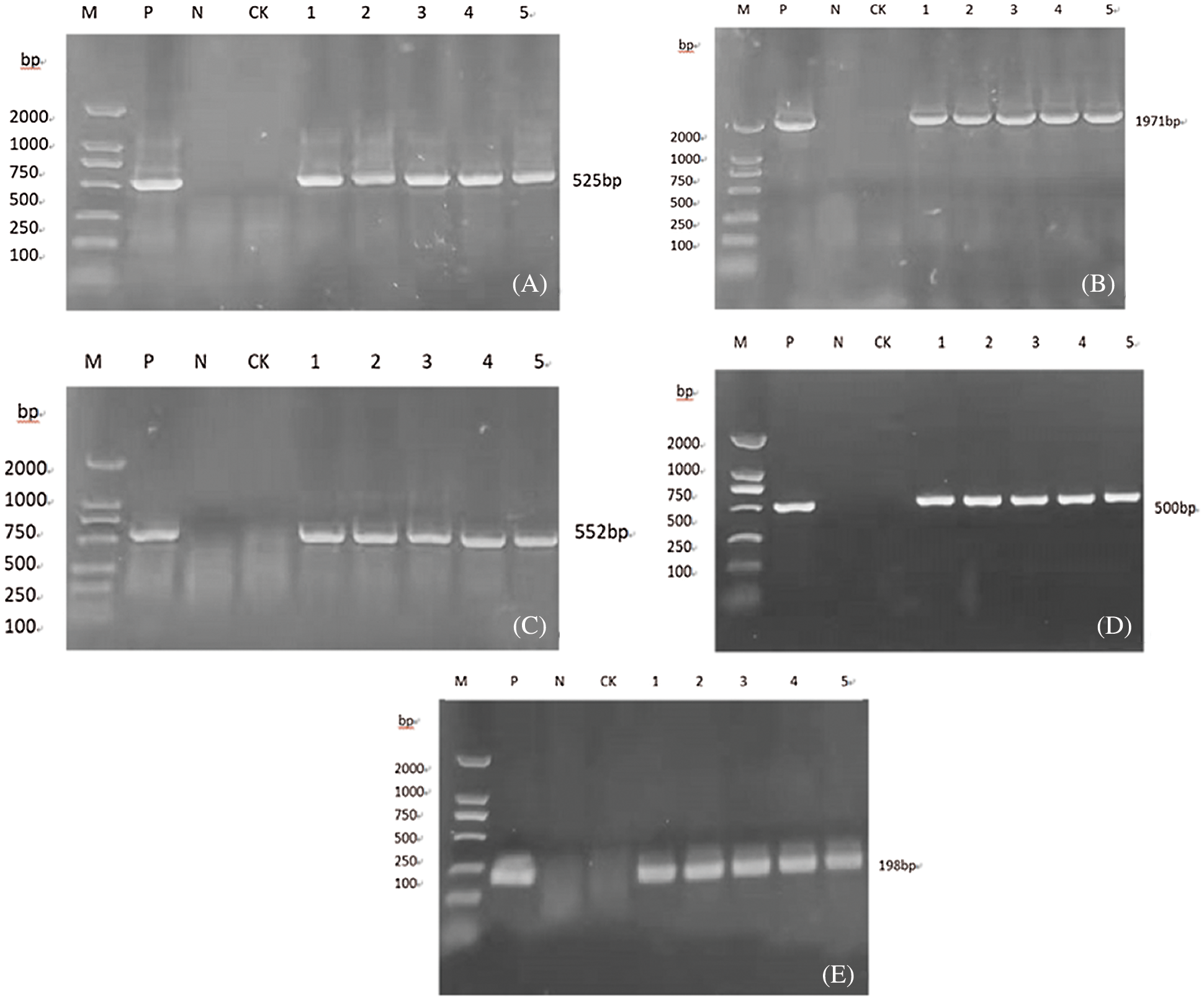
Figure 14: Molecular identification of T1 generation plants
Note: A, B, C, D, E are respectively PR1, Cry8like, bar, 35s, Nos gene detection; M: DL2000 DNA Marker; P: Plasmid (positive control); N: H2O; CK: untransformed plant 1- 5:Transformed plants.
3.10.2 Identification of T2 Generation Positive Plants
The soybean leaf DNA genome of T2 plant was extracted, and the PR1 gene, Cry-8Like gene, Bar gene, 35S gene, and NOS gene were specifically amplified, and the corresponding fragment sizes were 525, 1987, 552, 500 and 198 bp, respectively (Fig. 15), consistent with the expected results, it can be preliminarily considered that the gene is integrated into the genome of the T2 plant.
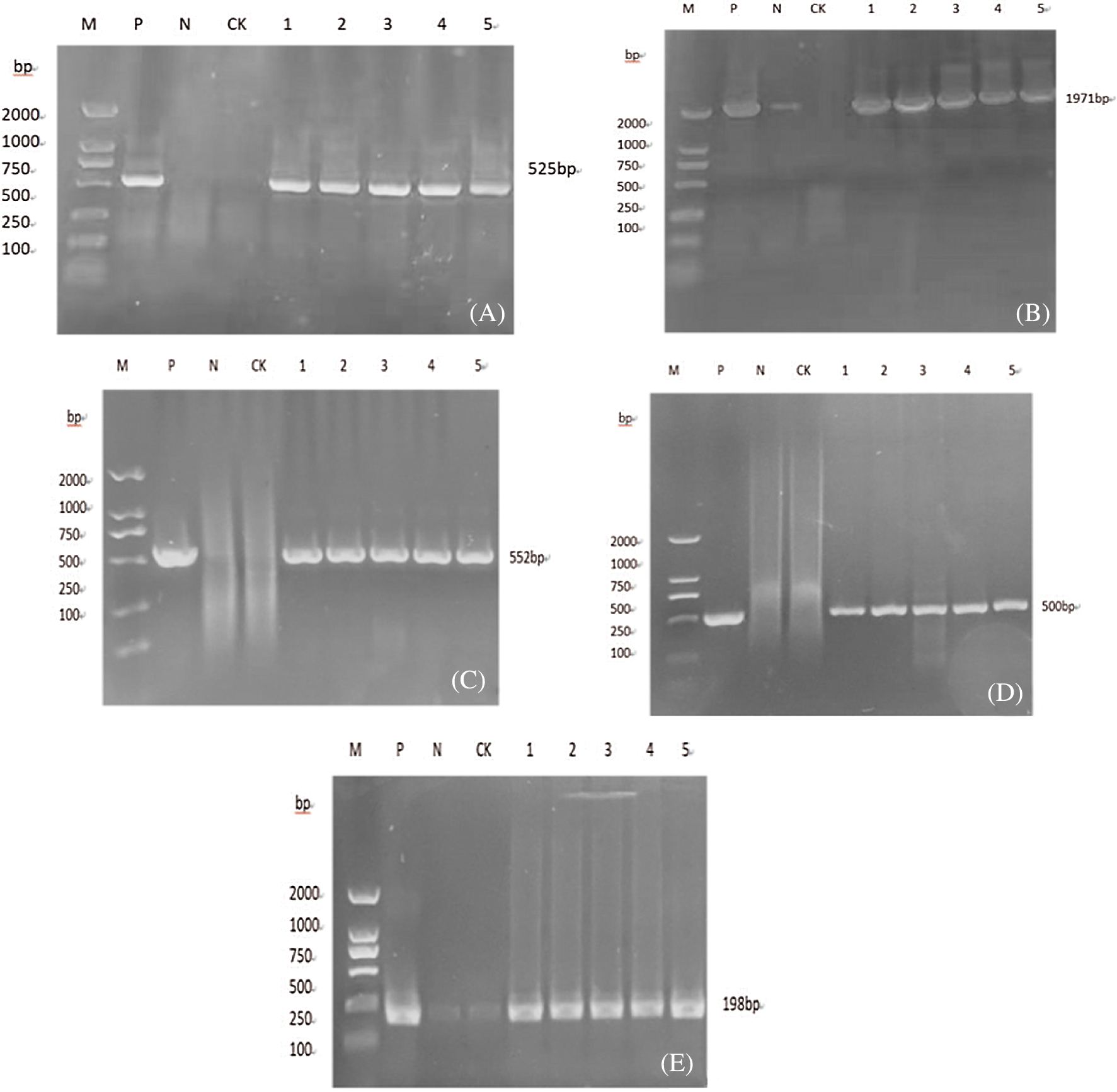
Figure 15: Molecular identification of T2 generation plants
Note: A, B, C, D, E are respectively PR1, Cry8like, bar, 35 s, Nos gene detection; M: DL2000 DNA Marker; P: Plasmid (positive control); N: H2O; CK: untransformed plant; 1-5: Transformed plants.
3.11 Southern Blot Hybridization Identification of Transgenic T2 Plants
Southern Blot hybridization was performed on T2 plants to identify transformed plants and untransformed plants. Hybridization signals appeared in the lanes of transformed plants, and hybridization signal bands appeared in untransformed plants (Fig. 16A) and Cry-8Like gene and (Fig. 16B) genes are integrated in the recipient soybean plant as a single copy, which is consistent with the results of conventional PCR detection. The hybridization bands in the lanes are not used. The position appeared indicates that the integration site of the foreign gene in the soybean genome is inconsistent. The denaturation solution (Nacl, NaOH), neutralization solution (Nacl,Tris.HCl), 20 × SSC (Nacl, sodium citrate, pH = 7.0), mother liquor, washing solution 1, washing solution 2, washing buffer, the molecular probes, and 0.8% agarose gel without nucleic acid dye were prepared to perform the experiment. Finally, the nitrocellulose membrane was hybridized in a hybridization oven and color was developed.
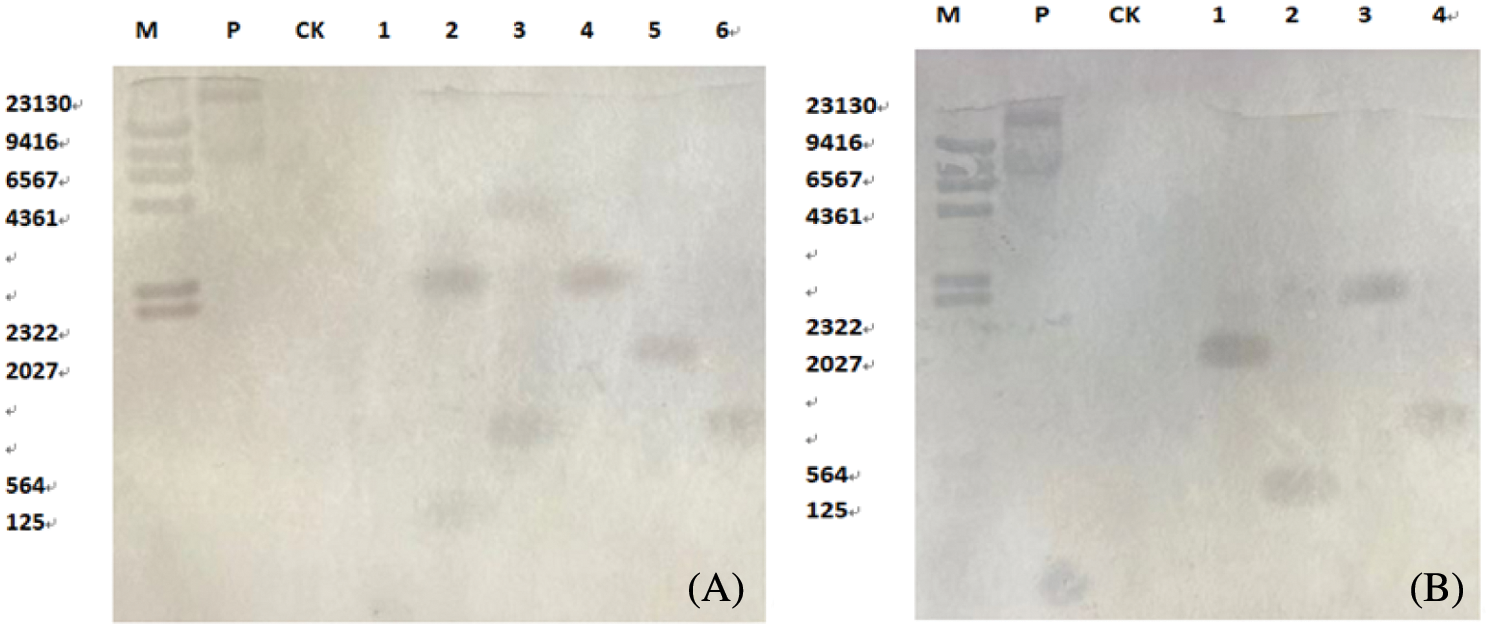
Figure 16: Southern Blot hybridization of T2 transgenic plants
Note: A: Cry8Like; B: PR1; M: Southern dedicated Marker; P: Positive control; CK: untransformed plants; A, B: 1-4/6: transformed plants.
3.12 Fluorescence Quantitative PCR Identification of T2 Generation Plants
For transgenic lines identified by conventional PCR and Southern blot hybridization, the roots, stems, and leaves of the plants were extracted after seed planting, and the cDNA was reverse-transcribed using a fluorescent quantitative reverse transcription kit. Soybean cDNA was used as a specific amplification template. β-actin was used as an internal reference gene in soybean. The relative expression levels of CryAB-8Like gene on roots (Fig. 17A) were 1.47, 0.88, 1.75, respectively. The relative expression levels on stems were 1.34, 1.16, 1.67 and the relative expression levels on leaves were 5.19, 4.12, 3.44, respectively (Fig. 17A). The results indicated that the relative expression of CryAB-8Like gene is the highest in leaves, followed by stems and roots. The relative expression levels of the PR1 gene on the roots (Fig. 17B) were 2.74, 2.65, 3.25, respectively and the relative expression levels on the stems were 2.35, 1.32, and 0.95. The relative expression levels on the leaves were 5.10, 5.65, and 5.05, respectively. The relative expression level of PR1 gene in leaves is 5.65. The relative expression level in the stem is the lowest 0.95. Both PR1 and CryAB-8Like genes are foreign genes. From the above data, it can be shown that the two genes are expressed in different roots, stems, and leaves, and their relative expression levels are not the same.
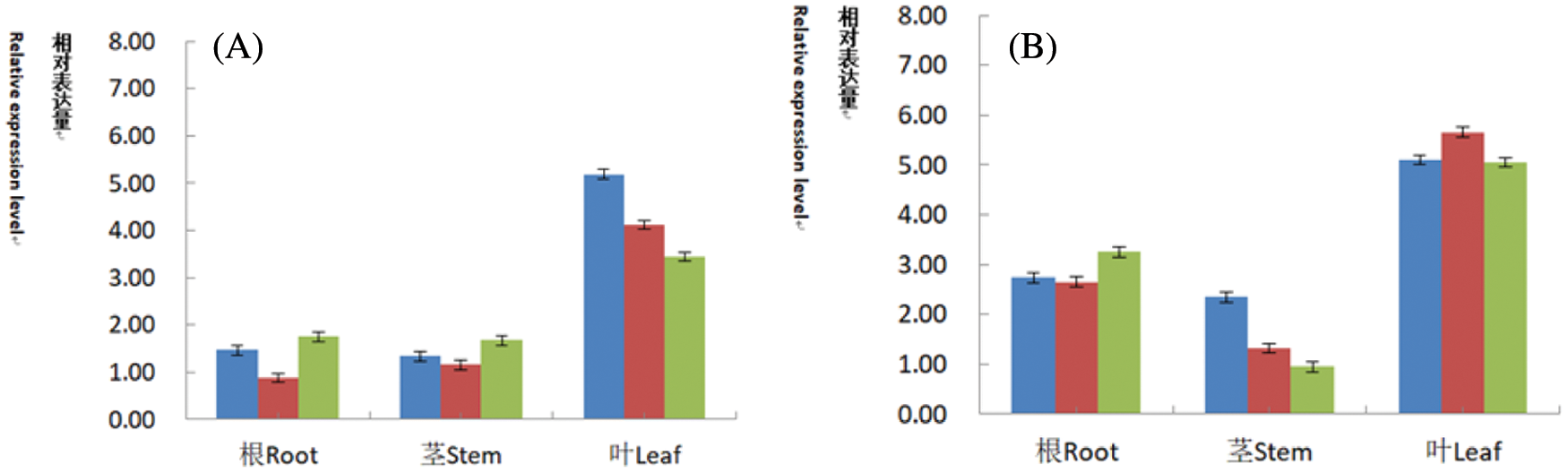
Figure 17: Fluorescence quantitative PCR identification of T2 generation plants
Note: A: Relative expression level of CryAB-8Like gene in roots, stems and leaves; B: Relative expression level of PR1 gene in roots, stems and leaves.
3.13 Outdoor Pest Resistance Identification of T2 Positive Plants
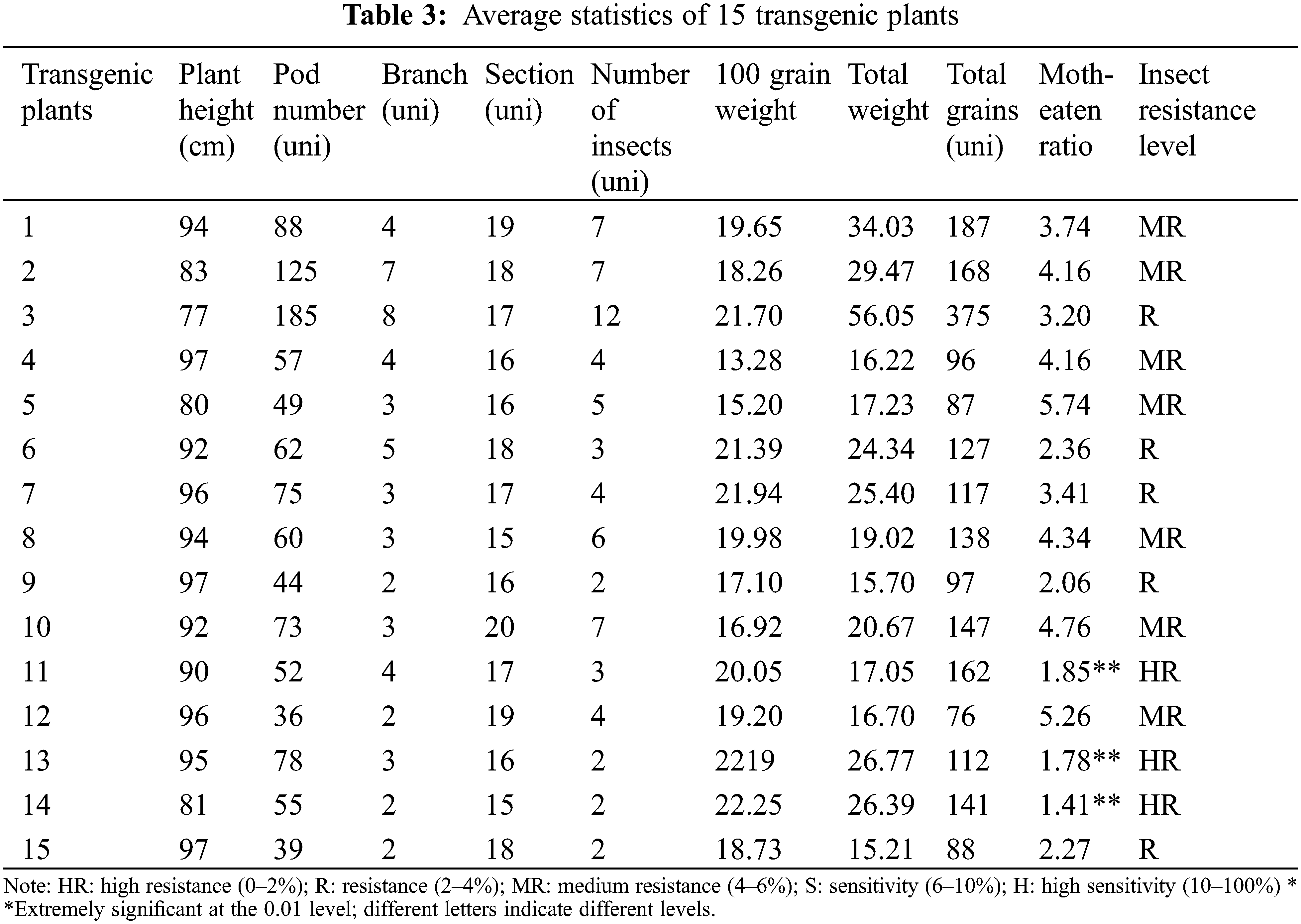
The transgenic plants containing the CryAB-8Like gene and the recipient plants (JN28) were harvested in the transgenic experimental field net laboratory for individual plant test analysis, and single plant threshing for statistical analysis of data (Fig. 18). Among them, 15 transgenic plants were randomly selected for statistical data analysis (Table 3).

Figure 18: Outdoor net room planting situation
The method of identification of resistance to soybean heartworm and its identification criteria refer to the research of Zhang et al. [16]. The comparison of transgenic plants with JN28CK does not occur in agricultural traits such as plant height, branching, number of nodes, and number of pods, except for insect resistance. Comparing JN28 receptors and genetically modified materials, JN28 insectivorous rate was 7.5%, and the average insectivorous rate of plants belonging to the S-sense (6%~10%) CryAB-8Like gene was 3.78% in the grade while R-anti (2%~4%). The insectivory rate of transgenic plants has changed significantly, and the insectivory rate of lines 11, 13 and 14 is extremely significant compared with other transformed lines. The average total weight of soybeans after transformation reached 24.01 g, and the total weight of the acceptor material was 17.55 g. The weight of the transformed material was significantly higher than that of the untransformed acceptor material. Receptor transgenic CryAB-8Like soybeans have obvious insect resistance effects and play an important role in improving soybean yield and quality.
3.14 Indoor Pest Identification
The method used for indoor insect resistance was the disc segmentation method (Table 4). The transformed bud length was 13.4–20.2 cm, with an average of about 17.4 cm. The highest number of borers was 2 heads, the least was 1 head, and the average was 2 heads. The bud length of untransformed plants was 6–12 cm, with an average of about 10.5 cm. The number of borers was 7 at most, 5 at least, and 6 on average. It shows that the transformed grains will release some pheromone during the germination process, which has a certain deworming effect. Under the same circumstances, the growth of transformed plants was better than that of untransformed plants. Some of the untransformed plants even stopped growing, and soybean borer had a certain inhibitory effect on the growth and development of untransformed plants (Fig. 19).

Figure 19: Kernel growth of transformed plants. Note: AB: Transformed seedlings; CK: Untransformed seedlings
3.15 Indoor Disease Resistance Identification
The transformed plants and untransformed plants under the same conditions. After the three-leaf period, a knife was used to lightly scratch the soybean leaves, scratch the hypocotyl part of the neck, and connect the soybean to the leaf wound and the soybean hypocotyl. The result after 3 days (Fig. 20), 3 days after the soybean gray spot disease, the leaves of the recipient plant showed a tendency to turn yellow and gradually wilt, but the positive plants hardly changed. One week after the soybean gray spot disease was inoculated, the recipient plants wilt severely, were broken, and tended to die. However, the transgenic plants are significantly stronger than the recipient plants, and their disease resistance has been improved.

Figure 20: Transformed plants inoculated with soybean gray spot disease
Note: A: transformed plants; B: untransformed plants; C, D: diseased leaves.
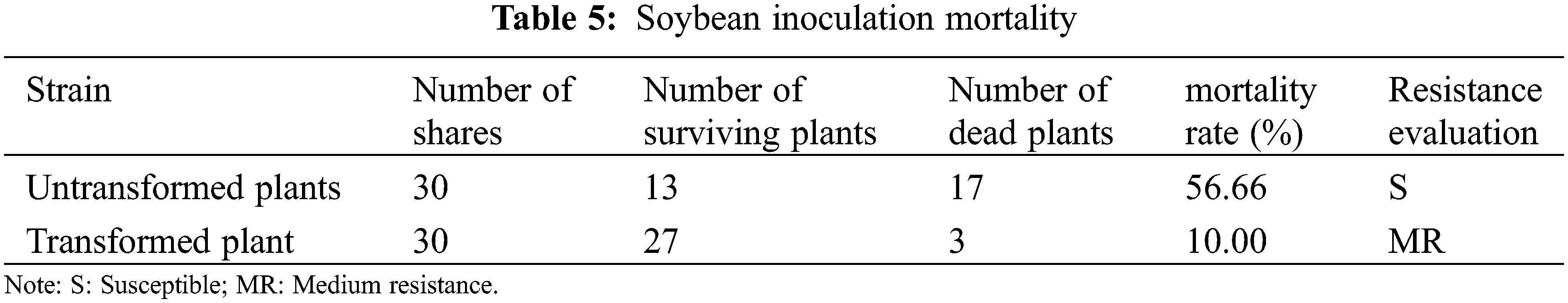
In the survey of the mortality of large gray spot disease in the transformed T2 generation plants, the average mortality rate of untransformed plants was as high as 56.66%, and the average mortality rate of transformed PR1 gene plants was 10.00%, and the mortality rate has changed significantly (Table 5). The disease resistance has been improved, indicating that the insect-resistant materials transformed into PR1 gene plants have obvious disease resistance and improved the disease resistance of soybeans.
Soybean gray spot is a worldwide disease. It occurs most severely in northeastern China. It mainly occurs in the cotyledons, stems, node pods and seeds of soybeans, resulting in decreased photosynthesis and yield of soybean plants. The severity of soybean gray spot disease mainly depends on the number of bacterial sources. Due to the high temperature, long-term rainy weather, little sunshine, and relative air humidity, serious gray spot disease can be caused. The fungal source of cotyledons and node pods are different. The fungal source of leaf parts is the source of overwintering fungi, but the fungal source of node pods comes from the re-infection of diseased leaf spores. It can be seen that the characteristics of soybean gray spot infection are continuous, and the infection process is from work to pod to seed [17]. At present, the prevention of soybean gray spot disease usually adopts conventional cross breeding and molecular assisted breeding. New soybean varieties resistant to gray spot disease are obtained through hybrid breeding, and molecular-assisted breeding which often uses transgenic technology. The disease-related protein gene PR1, which encodes a protein, has been proven to resist the spread of viruses and block fungal damage to resist bad external environments and other functions. Some studies have been reported on soybean [18], rice [19], potato [20] and corn [21]. Bacillus thuringiensis (Bt) Bacillus thuringiensis can produce both endotoxins and exotoxins. When pests eat plants containing Bt genes, the pests will die due to starvation, blood necrosis, or neurotoxicity. It has an obvious control effect on Lepidoptera pests (Soybean Heartworm) [22]. The protein encoded by the CryAb-8Like gene is one of the Bt toxic proteins, and Lin [23] and others have functionally verified that it has a significant anti-insect effect in soybeans.
The pollen tube channel method, when the plant is pollinated, it will form a line that runs through the entire style, and the pollen will enter the ovary along the channel and combine with the ovule to complete fertilization. Pollination within a certain period of time, the pollen tube channel will not be occluded, at this time the foreign DNA fragments along the pollen tube channel deep into the embryo sac [24]. Deng et al. [25] used laser confocal microscopy to clarify that the pathway for foreign DNA to enter the embryo sac is the outer channel of the pollen tube, that is, the foreign DNA enters through the gap between the tissue bundle and the pollen tube, not from the inner cavity of the pollen tube. Factors affecting the efficiency of genetic transformation include the size of inserted DNA fragments, DNA purity, DNA concentration, pH value of DNA vector buffer and different introduction periods. The transformed progeny has a wide variety of traits and rich types, and the various quantitative and quality traits of crops are obvious. Including plant morphology, growth and development, plant physiological and biochemical characteristics, resistance characteristics, yield, quality and many other aspects. At present, the pollen tube passage method is widely used in cotton [26], soybean [27], corn [28], sorghum [29] and many other crops.
There are very few reports about the transfer of foreign genes into resistant transgenic plants through pollen tube passage method to obtain insect-resistant and disease-resistant materials. In this experiment, the plant expression vector pCAMBIA-3301-PR1 containing the Bar marker gene was successfully constructed, and the expression vector was introduced into the recipient soybean containing the insect-resistant gene CryAB-8Like through the pollen tube channel method, and routine PCR identification was performed. Southern blot hybridization of the PR1 gene and CryAB-8Like gene are single copies integrated into the genome of the recipient plant at different sites. In the fluorescence quantitative PCR, the PR1 gene and CryAB-8Like gene are located on the roots, stems, and leaves. Certain expression levels, among which CryAB-8Like gene has the highest expression level on leaves and the lowest expression level on roots. In the outdoor insect resistance test, the insectivory rate of plants containing CryAB-8Like gene was 3.78%, which was classified as R-resistant (2%~4%). The insectivory rate of transgenic plants has changed significantly. Indoor insect resistance test, disc separation method, the transformed plants have obvious insect resistance to soybean coreworm, and their growth is better than untransformed plants, and there is a big difference in bud growth, indicating that the transformed plants have a certain resistance to soybean coreworm. It can be preliminarily judged that the transformed plants have certain insect repellency. In the indoor disease resistance test, 30 PR1 disease-resistant gene plants and 30 untransformed plants were planted respectively. In the survey of the mortality of soybean gray spot disease in the transformed T2 generation plants, the average mortality rate of untransformed plants was as high as 56.66%, and the average mortality rate of transformed PR1 gene plants was 10.00%. The mortality rate has changed significantly, and the transformed plants have certain resistance. Disease effect.
There are many breeding methods to produce insect-resistant and disease-resistant plants using backcross breeding, introduction of germpla sm, and distant hybridization. However, these methods are considered to be time-consuming and labor-intensive. Now a days, the application of biotechnology in crop breeding has solved the challenges related to time and labor. Previous studies only improved a single trait, but few improved two or more traits at the same time. Comparing JN28 receptors and genetically modified materials, JN28 insectivorous rate was 7.5%, and the average insectivorous rate of plants belonging to the S-sense (6%~10%) CryAB-8Like gene was 3.78% in the grade while R-anti (2%~4%). The weight of the transformed material was significantly higher than that of the untransformed acceptor material. The average mortality rate of untransformed plants was as high as 56.66%, and the average mortality rate of PR1 gene transformed plants was 10.00%, and the mortality rate has changed significantly (Table 5).
The total length of the PR1 gene cDNA is 525 bp, and the ORF sequence is 525 bp in length, which encodes 174 amino acids. The molecular weight of the protein is 18.83 kilodaltons, and the theoretical isoelectric point PI is 4.54. Among the protein subcellular locations, 44.4% are located on the endoplasmic reticulum, and 22.2% are located on the endoplasmic reticulum and 22.2% of the mitochondria and the Golgi apparatus. This gene mainly functions on the endoplasmic reticulum, followed by functions on the mitochondria and the Golgi apparatus. It is a secreted protein. The GmPR1 protein contains a transmembrane structure. It is a transmembrane protein that migrates in the cell. Analysis of phosphorylation sites of PR1 encoded protein: GmPR1 protein has 19 potential phosphorylation sites, including 10 serine (Ser) phosphorylation sites, 3 threonine (Thr) phosphorylation sites, and 6 tyrosines (Tyr) phosphorylation site. GmPR1 protein is composed of four parts: α-helix (32.76%), extended chain (17.82%), β-turn (5.17%), and random coils (44.25%). GmPR1 protein has the highest proportion of random coils. GmPR1 encodes a protein. The homology with Arabidopsis thaliana in the three-dimensional structure database is as high as 82.52%. Protein interaction analysis showed that GmPR1 protein played an important role and function in response to disease stress.
Authors Contribution: Huimin Cui is the executive of the experimental design and experimental research of this study; Huimin Cui and Shuo Qu completed the data analysis and the writing of the first draft of the paper; Shuo Qu, Abraham Lamboro and Yaolei Jiao participated in the experimental design, analyzed data, edit the final manuscript; Piwu Wang managed and guided the whole project. All authors have read and agreed to the final text.
Funding Statement: This research was funded by the National Major Special Project for Breeding New Varieties of Genetically Modified Organisms (2016ZX08004-004).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Taha, S. R., Seleiman, M. F., Alotaibi, M., Alhammad, B. A., Rady, M. M. et al. (2020). Exogenous potassium treatments elevate salt tolerance and performances of Glycine max L. by boosting antioxidant defense system under actual saline field conditions. Agronomy, 10(11), 1741. DOI 10.3390/agronomy10111741. [Google Scholar] [CrossRef]
2. Zhang, J., Wang, P., Guan, S., Ma, J., Yao, D. et al. (2010). Breeding of Jinong 27, a new spring soybean variety with high yield, high oil, mid-late maturity and wide adaptability. Seeds, 29(4), 106–107. DOI 10.16590/j.cnki.1001-4705.2010.04.052. [Google Scholar] [CrossRef]
3. Zhao, C., Wang, R., Zhao, X. (2021). Analysis and evaluation of agronomic and quality traits of soybean germplasm resources in foreign countries. Journal of Plant Genetic Resources, 22(3), 665–673. DOI 10.13430/j.cnki.jpgr.20200924002. [Google Scholar] [CrossRef]
4. Quan, H., Gao, W. (2020). The status quo, problems and countermeasures of my country’s soybean international trade. Journal of Tianjin Vocational College of Business, 8(5), 12–17. DOI 10.16130/j.cnki.12-1434/f.2020.05.002. [Google Scholar] [CrossRef]
5. Jiang, H., Zhao, H., Lin, C., Liang, X., Ma, S. et al. (2020). Discovery of excellent allelic variants of soybean varieties resistant to gray spot disease race 7. Chinese Agricultural Science Bulletin, 36(31), 97–102. [Google Scholar]
6. Gao, X., Gu, X., Yang, X., Zhang, M., Yao, L. et al. (2021). Occurrence and control of soybean gray spot. Modern Agricultural Science and Technology, 10, 102–104. DOI 10.3969/j.issn.1007-5739.2021.10.042. [Google Scholar] [CrossRef]
7. Yang, X., Wang, P., Gu, X., Yao, L., Gao, X. et al. (2020). Application of a foliar covering agent in the control of soybean gray spot disease. Acta Agricultural Sciences, 10(11), 11–14. [Google Scholar]
8. Meng, X., Song, Y., Jin, Y., Zhang, L., Wang, P. (2020). Identification of resistance to gray leaf spot of soybean lines transformed into hrpZ_(Psta). Journal of Jilin Agricultural University, 42(5), 502–509. DOI 10.13327/j.jjlau.2020.4273. [Google Scholar] [CrossRef]
9. Qu, S., Jiao, Y., Fu, J., Cui, H., Cai, Q. et al. (2021). Genetic transformation of disease resistance gene PR1 in soybean and cultivation of multi-resistant materials. Molecular Plant Breeding, 16, 12–14. [Google Scholar]
10. Antoniw, J. F., Ritter, C. E., Pierpoint, W. S., van Loon, L. C. (1980). Comparison of three pathogenesis-related Proteins from plants of two cultivars of tobacco infected with TMV. Journal of General Virology, 47(1), 79–87. DOI 10.1099/0022-1317-47-1-79. [Google Scholar] [CrossRef]
11. Liu, Q., Xue, Q. (2006). Computational identification ofnovel PR1-l-type genes in Oryza sativa. Journal of Genefics, 85(3), 193–198. DOI 10.1007/BF02935330. [Google Scholar] [CrossRef]
12. Kim, J. Y., Hwang, B. K. (2000). Pepper gene encoding a basic pathogenesis-related 1 protein is pathogen and ethylene in-ducible. Physiologia Plantarum, 108(1), 51–60. DOI 10.1034/j.1399-3054.2000.108001051.x. [Google Scholar] [CrossRef]
13. Godiard, L., Ragueh, F., Froissard, D., Leguay, J. J., Grosset, J. et al. (2017). Analysis of the synthesis of several pathogenesis-related proteins in tobacco leaves infiltrated with water and with compatible and incompatible isolates of Pseudomonas solanacearum. Molecular Plant-Microbe Interactions, 3(4), 207. DOI 10.1094/MPMI-3-207. [Google Scholar] [CrossRef]
14. Huang, H., Zeng, Y., Wang, L. (2021). Research progress of Bacillus thuringiensis and its parasporal crystal insecticides. Guangdong Chemical Industry, 48(10), 86–87. [Google Scholar]
15. Li, T. T., Han, L. L., Zhao, K. J., Chen, X. H., Zhu, Y. M. (2011). Application of disc segmentation method in the identification of insect resistance in genetically modified soybeans. Crop Journal, 5(4), 20–22. DOI 10.16035/j.issn.1001-7283.2011.04.008. [Google Scholar] [CrossRef]
16. Zhang, L., Jin, Y., Meng, X., Wang, P. (2019). Molecular identification and functional verification of insect-resistant gene Cry1Iem soybean. Molecular Plant Breeding, 17(1), 104–111. DOI 10.13271/j.mpb.017.000104. [Google Scholar] [CrossRef]
17. Chen, G. (2019). The occurrence characteristics of soybean gray spot disease and the research progress of disease resistance genetic breeding. Heilongjiang Science, 10(16), 42–43. [Google Scholar]
18. Jiao, Y., Wang, C., Qu, S., Sun, S., Zhu, T. et al. (2021). Application progress of CRISPR/Cas9 technology in crop genetic breeding. Henan Agricultural Sciences, 50(7), 1–7. DOI 10.15933/j.cnki.1004-3268.2021.07.001. [Google Scholar] [CrossRef]
19. Zhao, C., Lu, G., Du, X., Liu, L., Lin, Y. (2006). Expression dynamics of PR1 and PBZ1 in the pathogenesis of rice sheath blight. Acta Phytopathology, 2006(4), 317–321. DOI 10.13926/j.cnki.apps.2006.04.007. [Google Scholar] [CrossRef]
20. Zhang, Q., Zhao, J., Huang, D., Xiao, X., Xia, W. (2018). Cloning and analysis of potato disease course related protein 1 (PR1) gene and its promoter sequence. Molecular Plant Breeding, 16(7), 2078–2084. DOI 10.13271/j.mpb.016.002078. [Google Scholar] [CrossRef]
21. Wang, J., Liu, L., Zhang, Z., Zhao, M., Pan, G. (2012). Cloning and expression analysis of maize disease course related protein 1 gene. Agriculture and Life Sciences, 38(1), 35–42. [Google Scholar]
22. Song, T., Qian, X., Yao, Y., Yang, J., Xing, G. et al. (2019). Bacillus thuringiensis Cry1Ab and Cry1C homologous recombination and transformation of soybean. Molecular Plant Breeding, 18(8), 2591–2596. DOI 10.13271/j.mpb.018.002591. [Google Scholar] [CrossRef]
23. Lin, N., Wu, N., Feng, G., Yu, M., Rong, J. (2017). Genetic transformation of the insect-resistant gene Cry8-like in soybeans and preliminary identification of insect resistance. Chinese Journal of Oil Crops, 39(2), 221–227. DOI 10.7505/j.issn.1007-9084.2017.02.012. [Google Scholar] [CrossRef]
24. Li, C., Jian, Y. (2000). Application of pollen tube pathway in plant genetic transformation. Journal of Biology, 1, 10–11. [Google Scholar]
25. Deng, D., Guo, S., Yang, Z. (1999). Study on the molecular cytological mechanism of cotton pollen tube pathway transgene. Natural Science Edition, S3, 124–125. [Google Scholar]
26. Yan, S., Zhu, S., Tie, S. (2018). Obtainment and resistance identification of glyphosate-resistant cotton with EPSPS-G6 gene. Genomics and Applied Biology, 37(9), 3944–3949. DOI 10.13417/j.gab.037.003944. [Google Scholar] [CrossRef]
27. He, H., Gao, S., Han, D., Wu, N., Qu, J. (2017). Research on the transformation of insect-resistant gene Cry1Iem into soybean. Journal of Jilin Agricultural University, 39(6), 665–669. DOI 10.13327/j.jjlau.2017.3174. [Google Scholar] [CrossRef]
28. Cao, S., Wang, C., Shi, G., Qi, Y., Wang, W. (2013). Study on the introduction of BcWRKY2 drought resistance gene into maize using pollen tube pathway method. Crop Journal, 2013(1), 32–36. DOI 10.16035/j.issn.1001-7283.2013.01.012. [Google Scholar] [CrossRef]
29. Ou, Q., Cui, W., Wang, W., Ni, J., Wang, H. et al. (2013). Molecular polymerization breeding of new wheat strains created by introducing sorghum DNA into pollen tube channel. Agricultural Research in Arid Areas, 31(2), 6–12 + 22. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |