

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.019870
REVIEW
Formation Mechanism and Occurrence Law of Pod Shattering in Soybean: A Review
1College of Life Sciences, Jilin Agricultural University, Changchun, 130118, China
2College of Agronomy, Jilin Agricultural University, Changchun, 130118, China
*Corresponding Authors: Siyan Liu. Email: siyan_2001@163.com; Shuyan Guan. Email: guanshuyan@jlau.edu.cn
Received: 21 October 2021; Accepted: 20 December 2021
Abstract: Seed shattering refers to the phenomenon in which the pods split along the abdominal and back sutures before the crop is received, so that the seeds are spread. Seed shattering is vital to the reproduction of their offspring in wild plants, but it is also the main cause of crop yield loss reason. Pod-explosion resistance is a complex process of physical and physiological and biochemical reactions. Soybean seed shattering phenomenon is widespread, which severely restricts the development of soybean industry. Seed shattering (pod cracking or fruit dropping) is essential for the reproduction of its offspring in wild plants, but it is also the main cause of crop yield loss. This article analyzes the morphology and structure of pods related to seed shattering from the morphology of pods. On the basis of the regularity of the occurrence of seed shattering and the summary of phenotypic index identification methods, physiologically introduced the regulation mechanism of key enzymes and endogenous hormones on seed shattering. The localization, labeling and cloning of seed shattering genes are introduced in molecular biology. The study focused on reviewing the latest advances in the research on soybean seed shattering characteristics, and discussed with the research results of related crops. Finally, the research and application of soybean seed shattering resistance were prospected for several aspects.
Keywords: Seed shattering; genetic law; identification; mechanism of action
The appearance of fruits represents a major evolutionary innovation of angiosperms. The successful evolution of wild plant species essentially determines their ability to spread offspring [1]. While in the legume, gramaceae and cruciaceae such as Soybean (Glycine max L. Merr), Pea (Pisum sativum L.), Rice (Oryza sativa L.), Arabidopsis (Arabidopsis thaliana), Rape (Brassica campestris), pod shattering plant, etc. [2–6], and seed shattering refers to the fragmentation of the pod shell that breaks the seeds successfully. As early as 1989, Indian scholars Philbrook and Oplinger counted the losses caused by seed shattering in tropical and subtropical soybean growing areas, and found that the field yield loss of easy-shattered pod and medium-shattered pod soybean varieties was 57∼175 kg/ha and 0∼186 kg/ha, respectively [7]. According to the report of Chinese scholar [8], the seed shattering loss of main soybean varieties planted in the Huang-Huai area in my country due to seed shattering is about 112.5 kg/ha. Relevant studies have shown that seed shattering is more common in wild-type and small-grain soybeans [9]. Soybean mechanized harvesting reduces labor costs and can increase seed shattering, but seed shattering is a key factor affecting soybean mechanical harvesting. The pods at the maturity stage are easy to fry pods under the action of any external or internal forces [10]. Reducing seed shattering rate can not only increase crop productivity, but also use combine harvesters, thereby reducing labor costs. Due to the cracking of the pods, the yield of soybeans has decreased significantly, and the deterioration of climatic conditions may cause greater damage to soybean production, severely restricting the development of the soybean industry [11], it can be seen that the occurrence of seed shattering in soybean has brought very serious losses to the soybean industry at home and abroad. Solving the problem seed shattering in soybean is one of the main issues that need to be paid attention to in soybean production and research in my country.
The phenomenon of seed shattering of legumes is widespread. Breeding excellent varieties that are resistant to seed shattering is one of the main goals of people’s breeding. The formation of seed shattering of cultivated crops is an important milestone in domesticated crops [12]. Most soybeans have gradually overcome the problem of seed shattering during the domestication process. However, due to differences in ecological adaptability, the problem of soybean pods has been plagued by breeders. Seed shattering is a complex process of physical, physiological and biochemical reactions that occur together. Changes in any link may inhibit or promote soybean seed shattering [13]. The occurrence of soybean seed shattering is not caused by a single factor. The morphological characteristics of the phenotype, the anatomical structure of the pod suture, the chemical composition of the pod skin, the genetic characteristics of the variety and environmental factors will all affect the occurrence of seed shattering [14]. This article focuses on soybeans and takes into account related crops. First, it summarizes the research on pods from morphology, and then analyzes the rules and main influencing factors of the occurrence of pods. Finally, the latest advances in soybean pods from physiology and molecular biology are analyzed. The summary is intended to provide reference for future studies on the mechanism of seed shattering from the perspective of morphology, physiology and molecular biology. At the same time, it also provides reference for the innovation and variety breeding of seed shattering germplasm.
In the comparative analysis of botanical traits, morphological methods are the oldest but also the most intuitive and accessible, mainly divided into apparent and micromorphology.
In recent years, scholars have studied whether the pods of beans and other seed shattering are affected by pod length, width, thickness, and degree of curvature. Chiapparino et al. [15] overexpression of GmAGL1 gene in Arabidopsis indicated that there may be a relationship between pod length and fried pod. In leguminous crops, pods with a large degree of curvature and sickle-shaped or long pods are not easy to fry. Krisnawati et al. [16] studied the pod thickness related to soybean frying pod. Murgia et al. [17] and other studies found that the number of broken soybean pods per plant is positively correlated with petal weight, and negatively correlated with traits such as hundred-seed weight, pod perimeter, area, width, and height. Among them, the important factor that regulates seed shattering is the width-to-thickness ratio of the pods. Lou et al. [18] found that the seed shattering rate was negatively correlated with the pod’s width-to-thickness ratio. Tsuchiya et al. [19] and Tiwari et al. [20] study the relationship between size and seed shattering rate found that in soybean varieties resistant to seed shattering, the width to thickness ratio of the pods was generally smaller, but whether a smaller width to thickness ratio would inhibit the occurrence of seed shattering has not been confirmed. In peas, there is no significant difference in the width-to-thickness ratio of seed shattering. Therefore, some scholars believe that the occurrence of seed shattering is not directly related to the width-to-thickness ratio of the pods.
The pods are composed of two single sheets of valves, and the central cavity is where the seeds live. Seed shattering originates from the cracks in the abdominal sutures. Christiansen et al. [21] and Summers et al. [22] analyzed the microstructure of the back suture and abdominal suture cross-section of the pod skin during the mature period of soybeans proposed there is a similar structure similar to the cruciferous dehiscence zone (DZ) at the suture line of the abdomen. As the pod matures, the dehiscence zone gradually evolves into a non-ligninized delamination (SL) about two cell layers thick [23]. Degradation of the delamination and separation of parenchyma can improve seed shattering ability [24,25]. In soybeans, the excessive lignification of the fibrous cap cells connecting the two valves in the ventral suture layer of the pod increases the bonding strength of adjacent cells, thereby forming the characteristics of seed shattering of cultivated soybeans. The study of Dong et al. [26] suggested that the thickening of the secondary cell wall of FCC cells is the main factor for the resistance of cultivated soybean varieties to seed shattering. For the degradation of the intercellular layer in the dehiscence zone, the differentiation of the pod wall is essential. The pod wall can be divided into three functional layers, namely exocarp, mesocarp and endocarp [27]. In the process of seed shattering, due to the reduction of cell wall adhesion in the cracking zone, and the tension generated by the mechanical properties of the endocarp and exocarp cells of the pod (the tension produced during the shrinkage process caused by the difference between the endocarp and the exocarp [28]) causing the pods to crack. The key factor that produces the required tension is the geometric arrangement of the cell wall and its histological characteristics (cell wall thickness and composition, lignification, hydration). Suanum et al. [29] found that the composition and characteristics of the cell wall of the pod are related to the fragmentation of cowpea and wild cowpea. In the late stage of maturity, once the valve is fully lignified and the valve attachment becomes weak, the increase in the size of the vacuolated cells in the sidewall of the cracking line will promote the cracking. In other plant species, due to dehydration, the seed shattering is also related to the difference in the expansion of the inner thick-walled cell layer [30]. Murgia et al. [31] proposed that the lignification of the inner valve layer (LFL) contributes to seed shattering, while the study of Bruce et al. [32] showed that the absence of LFL or the presence of a low level of lignification would reduce the stability of the valve, thereby reducing the stability of the valve during drying. In addition, the fracture structure of the pod wall was found to be correlated with the helical moderately, and has visual cracking in some environments.
In summary, the thickness of the secondary cell wall of the lignified FCC cells and the FCC cells in the ventral suture, the structure of the pod wall, and the cause of tension are the main factors that regulate the cracking of the pod. It is believed that subsequent studies will be more detailed analysis for the cytological structure characteristics of soybean pods and some unknown structures and their effects.
3 Occurrence of Seed Shattering and Main Influencing Factors
At present, it is generally believed that the two major factors affecting soybean pod frying are genetics and environment, among which genetic and environmental factors are internal and external, respectively, and the two jointly regulate the occurrence of soybean frying. Now is the era of mechanization, so the design and research of machinery is also closely related to seed shattering.
3.1 Genetic Factors of Seed Shattering
Seed shattering is closely related to its own characteristics, and genetic characteristics are the main factors affecting seed shattering [33]. Research by Li et al. [34–36] showed that different soybean varieties have significant differences in the resistance to seed shattering under the same environmental factors, and there is a certain correlation between the differences in pod structure and water content between different soybean varieties. Nilmani et al. [37] studied the seed shattering rate of different soybean varieties 10 days after the maturity period, and the seed shattering rate was between 0.67% and 67.05%, implying that genetic background has a great influence on soybean seed shattering. For this reason, researchers have studied the genetic mechanism of seed shattering on many model crops such as soybeans, peas and lentils. These studies have shown that the pathways and loci that control seed shattering is widely conserved in species [38], A large number of studies have shown that soybean seed shattering is not determined by a single gene, but a quantitative trait co-regulated by a major gene and multiple minor genes [39].
3.2 Environmental Factors of Seed Shattering
For the initial research of soybean seed shattering, the main focus was on the impact of environmental factors on soybean seed shattering and corresponding measures were proposed to reduce seed shattering so as to avoid the yield loss caused by seed shattering. In 1957, Metcalfe et al. [40] found that mature Lotus japonicus pods showed two phenotypes of cracked pods and non-cracked pods at an environmental humidity of 29.5% and 35%. Relative humidity was first proposed as the main environmental factor affecting legume crop blast pods. Studies have shown that the frying rate of French croissant pods at low temperature and high humidity is significantly lower than that at high temperature and low humidity [41]. Subsequently, Cavines et al., successively proposed that relative humidity is an important environmental factor affecting soybean seed shattering [42,43]; in addition, Zhang et al. [34], not only verified the above views, but also put forward that there is no obvious correlation between the seed shattering rate and the length of sunshine time and the average daily temperature. Yang researched that seed shattering occurred when the moisture content of beans and seed shattering were lower than 25% and 15%, respectively, indicating that the water content of soybeans is also closely related to seed shattering and also proposed that the spatial distribution of pods in identical soybean does not significantly link [44]. In addition, Tsuchiya et al. found that sudden temperature changes can induce soybean pods, which is of great significance for actual production [45]. Travis found that under drought conditions, the twisting force of the pod wall or the structural strength of the cracked area in the pod suture will change, resulting in soybean seed shattering [46].
To sum up, the relative humidity in the air is a direct factor that affects soybean seed shattering, and the rapidly changing environmental factors are the key factors leading to the decrease of the moisture content of the soybean pods. A series of environmental factors such as temperature, light intensity, sunshine hours, wind speed, air pressure, and soil moisture indirectly affect soybean pods by changing air humidity. In order to change the impact of the above reasons on seed shattering, we can harvest them timely, and properly spray water or desiccant to reduce the loss caused by seed shattering, but this will affect the cost and improper operation will cause problems such as mildew of the pods.
3.3 Mechanical Factors of Seed Shattering
Soybean harvesting is a key link in the soybean production process. The use of appropriate harvesting machinery to complete the soybean harvesting operations in a timely manner is an important guarantee for high soybean yield. The mechanical harvest loss of soybeans has always been a serious problem in mechanized soybean harvesting. Langowski et al. [47] conducted a long-term experimental study on the mechanical harvest loss of soybeans. The total mechanical harvest loss rate was 9.8% to 19.3%, and the header loss accounted for 80%, of which shattering loss accounted for 55%. The test results of Deng Si et al. showed that branching and shattering accounted for 94% of the loss. Pods are easy to burst due to the biological characteristics of the crop. Li et al. [48] studied the biological and mechanical characteristics of soybean varieties in the Huanghuaihai area, and provided some parameters for the header and threshing system of the Huanghuaihai soybean combine harvester. Many domestic scholars have made statistics on the start time of soybean frying and found that most varieties start seed shattering 6–12 days after soybeans are fully mature. So timely harvest is also necessary to avoid large soybean production caused by seed shattering. In summary, timely harvesting and modification of machinery based on the biological characteristics of soybeans can avoid seed shattering and reduce yield loss during harvesting.
4 Physiological Study of Seed Shattering
In addition to the pod’s own structure and environmental factors, physiological and biochemical reactions also have an impact on seed shattering. Christiansen et al. [49] conducted an in-depth study on the pod development process of soybean. First, the pod formed a separation zone at the dorsal suture and ventral suture then intercellular layer and cell wall in the separation zone gradually degraded, causing the separation of the separated cells. Eventually a large number of fractures occurred, causing the pods to burst. Certain enzymes in pod cells determine cell degradation and degradation in the dehiscence zone, and corresponding hormones indirectly participate in this process by regulating these enzymes.
4.1 The Impact of Key Enzymes on Seed Shattering
Physiologically, the crush resistance of the pod is essentially related to the amount and composition of carbohydrates in the pod. Carbohydrates, such as cellulose and lignin, are important components of the cell wall of the pod and directly affect the resistance of the pod to mechanical breakage. Cellulose guarantees the toughness of the cell wall, while lignin is an important phenolic compound with a complex structure in plants, which enhances the hydrophobicity and hardness, physical strength and water conductivity of the cell wall. Cellulase can also degrade the cell wall. Agrawal et al. [50] measured the cellulase activity in soybean pods and found that the cellulase activity in the pod germplasm at the mature stage was significantly increased and the high content of cellulose (and hemicellulose) and lignin in the separation layer will hinder the separation of cells in the separation layer and reduce pod damage. Kappara S showed that the seed shattering determined by scoring the seed shattering of cowpea/cowpea has the highest correlation with the pod hemicellulose content, but not the correlation with the pod lignin content [5].
In addition, in the process of seed shattering, β-glucase and polygalacturonic acid endonuclease are the key enzymes that play a role. The activity of glucose hydrolase in the dehiscence zone of the easy-explosive pod germplasm of Brassica is increased and the cell wall is degraded. For the conversion and distribution of β-glucase, this enzyme may be involved in the entire process of layer separation [51]. The endogenous polygalacturonic acid is involved in the degradation of the intercellular layer and is related to the cell separation during the pod shedding process. The pectin is related to the adhesion between the cells. Pectin degradation is found in the plant germplasm that is easy to shatter. Severely, in the process of crop maturation and senescence, increasing the activity of glucose hydrolase and endogenous polygalacturonase can significantly reduce the content of pectin in the cracking zone.
As mentioned above, these enzymes participate in the seed shattering process by separating the cell layer and degrading the cell wall, as well as affecting the adhesion between the cells. However, the activity of these enzymes in the pod, how to express, and the mechanism of action are still unclear, and further research is needed.
4.2 The Effect of Hormones on Seed Shattering
Phytohormones are trace organic substances produced by plants’ own metabolism, also known as plant endogenous hormones, which can regulate plant physiological responses by affecting the activity of enzymes. Among the five endogenous hormones, two are related to β-glucose hydrolase, which can degrade pod cell walls. When the pod is shatter, the content of ethylene in the kernel suddenly increases and a short-term peak appears. At this time, the activity of β-glucose hydrolase increases, indicating that the change of ethylene content indirectly regulates the cracking of the pod. The peak of ethylene content usually occurs earlier than the cracking of the pod suggesting the peak ethylene content may be a signal of pod splitting. Relevant studies have shown that auxin can inhibit the accumulation of β-l, 4-glucohydrolase mRNA in the dehiscence zone to regulate pod dehiscence [52]. Gibberellin plays a role in the establishment of the cell characteristics of the separation layer, but the topical application of cytokinin during fruit development can restore the formation of valve edges and further increase pod dehiscence. Tu [53] found that the synergistic effect of auxin and gibberellin in the cracking zone can increase the activity of vegetable soybean endoglucanase and pectinase, accelerate the decomposition of cellulose and pectin, and form the characteristics of easy-fried pods.
In summary, these findings indicate that several plant hormones are involved in the differentiation of DZ and that their biosynthesis and response are essential. Although the role of hormones in the development of DZ has been extensively investigated, there are relatively few studies on how these hormones coordinate in DZ. Therefore, in the future, it is necessary to in-depth study of the molecular mechanism and interaction of plant hormones behind the DZ differentiation of cracked pods.
5 Soybean Varieties That are Resistant to Seed Shattering
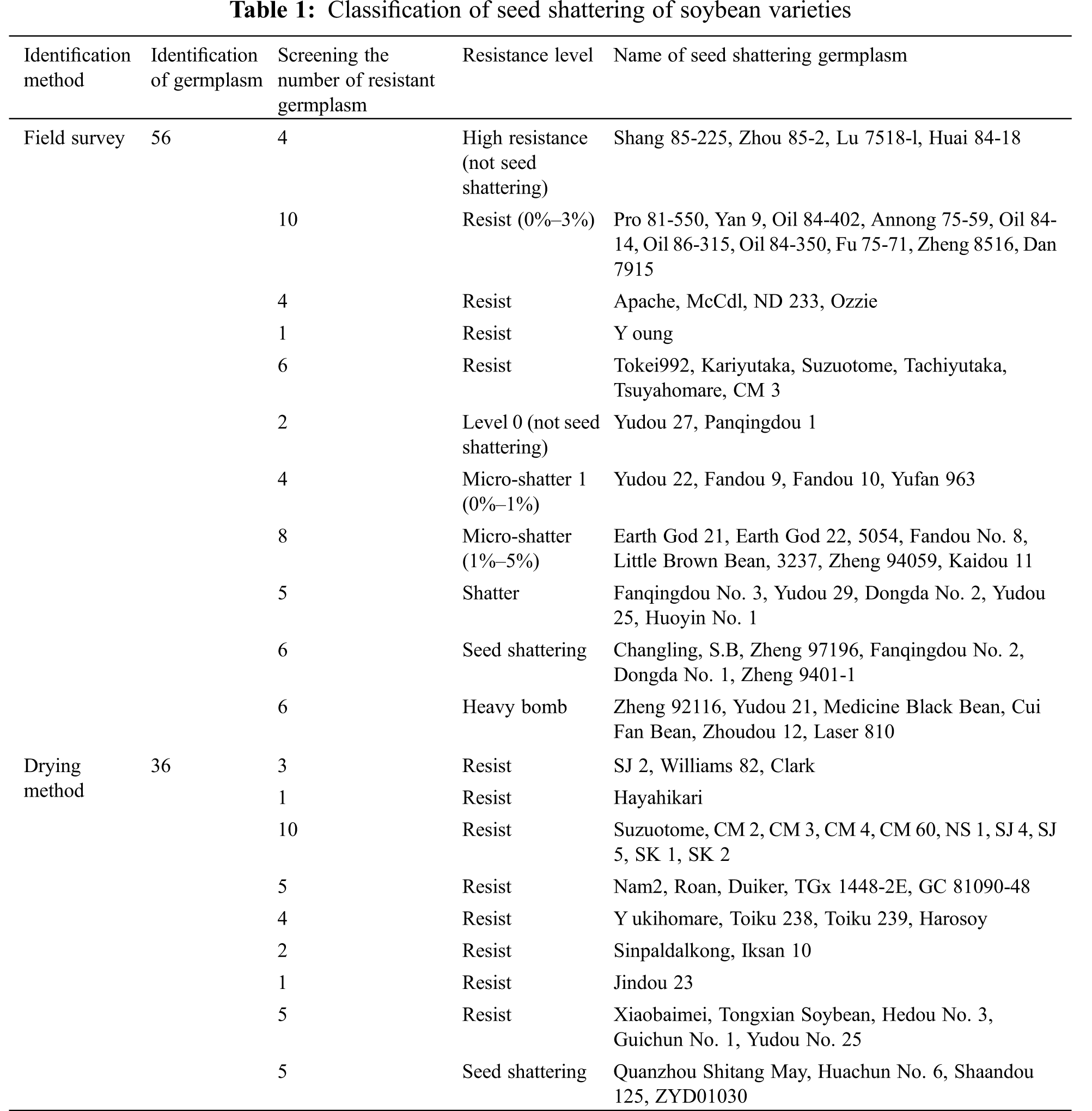
Determining the seed shattering properties of soybean varieties is the basis of application production and scientific research. As shown in Table 1, the field survey method and drying method are mainly used to identify soybean seed shattering germplasm. Compared with the drying method, the field survey method identifies more germplasm. Many of these properties have been used in genetic research on resistance to bombardment. Regarding the breeding of soybean varieties resistant to seed shattering, there are few studies at home and abroad. So far, the domestic publicly reported fried pods include Mengdou No. 7, Longxiaodou No. 1, Heinong 44, Heihe No. 43, Fengshou 27, Heihe No. Nong 58, Gaoyou 1, Xinnong 688, Fengshou 23, Kefeng 34, Jidou 12, etc. [54–58], these anti-pod varieties laid the foundation for soybean variety breeding and genetic background study of pod traits.
6 Molecular Biology Research of Seed Shattering
6.1 Location and Labeling of Seed Shattering
In the past 20 years, some QTLs and genes that control pod dehiscence have been identified, revealing that a major QTL and several minor QTLs co-regulate pod dehiscence. Bailey et al. [59] first used 140 restriction fragment length polymorphism (RFLP) markers to identify the main QTL. Funatsuki et al. [55] detected this major QTL and named it qPDH1. Furthermore, studies have shown that qPDH1 is the main QTL related to pod dehiscence. Funatsuki et al. [60] cloned the gene pdh1 near the QTL “qPDH1” reported on chromosome 16, and promoted pod dehiscence by increasing the twisting of the dry pod wall. In addition, Dong et al. [61] analyzed the changes caused by domestication between cultivated soybeans and wild soybeans and identified the NAC gene SHAT1-5 (Glyma16g02200), proving that this gene activates secondary cell wall biosynthesis and promotes the thickening of fibrous cap cells in pod sutures.
Recently, some new QTLs have been discovered on chromosomes 1, 4, 5, 6, 8, 9, 11, 17, 18, and 20 through genome-wide association analysis (GWAS). 16 QTLs were identified from the high-density linkage maps of two RILs, including the qPDH1 locus, and several new QTLs were identified on chromosomes 2, 6, 11, 13, 14, 16, and 20 [62]. At present, the mechanism of bean pod shatter prevention is not yet fully understood, and the genetic backgrounds of the mapped parents are different, and the genetic effects explained by the mapped QTLs are also different. Therefore, other QTL information from different genetic backgrounds is essential for the development of shatter-resistant soybean varieties.
6.2 Cloning and Function Study of Seed Shattering Gene
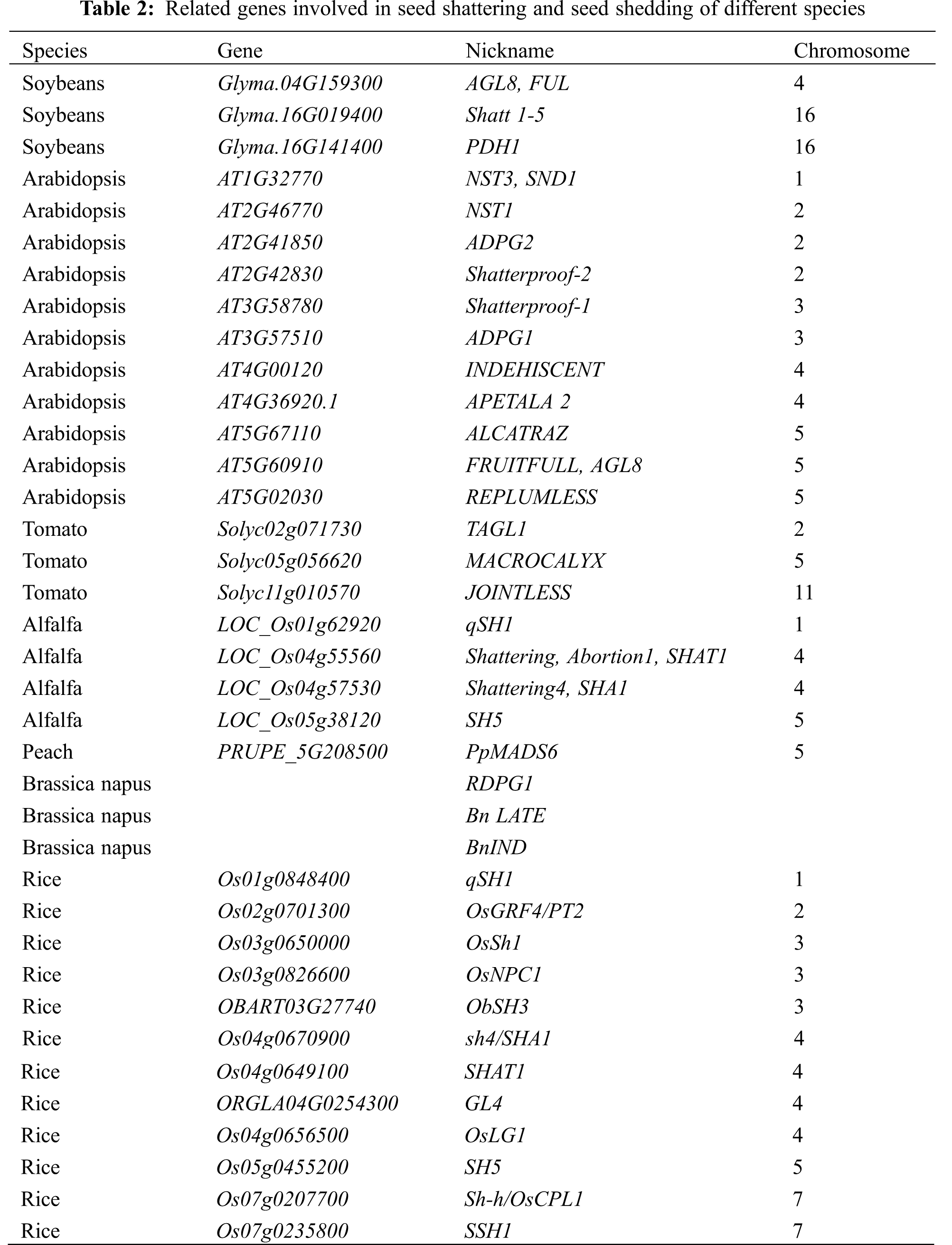
So far, there have been few studies on the cloning and function of the fried pod gene in soybeans. In 2014, Funatsuki et al. [60] obtained the fried pod-resistant gene PDH1 through map-based cloning. Because this gene contains an early stop codon which leads to loss of function so it can reduce the twisting force of the pod skin to reduce the soy pod frying ability. In the same year, Dong et al. [61] found that the 116 kb region of chromosome 16 is related to fried pods, and successfully cloned a seed shattering gene in this region, named SHAT1-5, and proved that SHAT1-5 gene regulates FCC cells, resulting in the thickening of the secondary cell wall and the formation of anti-explosive pods. In 2017, Qing et al. [63] performed a homology comparison of the Arabidopsis FUL gene to obtain the soybean fried pod gene GmAGL8, and found that overexpression of this gene can reduce the rate of soybean and Arabidopsis seed shattering. There are also cloning and functional studies of seed shattering genes in other plants and the most studied is Arabidopsis thaliana. Details are shown in Table 2. Dinneny’s research on the genetic mechanism of Arabidopsis silique seed shattering is shown in the Fig. 1. In summary, seed shattering is a complex process of multi-gene regulation, and more genes related to seed shattering need to be discovered.
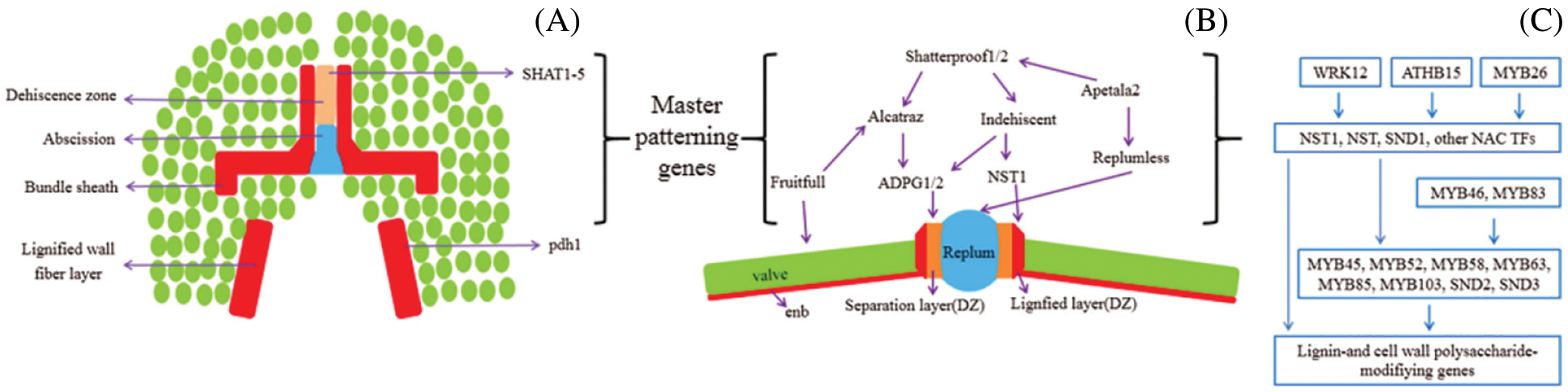
Figure 1: Genetic model of fruit morphology and cell wall development (A) The structure related to cracking in the soybean belly suture, and the mature pod wall fiber layer pulls the suture from both sides. If the cracked zone lacks structural integrity to withstand tension, the cracked zone occurs along the central part of the exfoliated layer and bundle sheath. (B) In Arabidopsis, the pod development in the area around the suture is controlled by several main model genes and their downstream cell wall modification genes. Homologs of Arabidopsis fruit pattern regulators are often used as candidate genes in cracking studies, but so far, there is little experimental evidence to prove their hypnotic role in grain legumes. (C) Detailed view of cell wall modification pathways in Arabidopsis
Studies have shown that the comprehensive phenotypes of legumes resistant to seed shattering are smaller pods, smaller grains, heavier pods, and smaller seed pods. The seed shattering rate is related to 100-seed weight, growth period, and grain yield. There is a negative correlation. The occurrence of seed shattering is likely to be the energy consumption process of the plant. The occurrence of seed shattering is caused by the tension produced by the loss of water in the pod. The moisture content of the pod is related to the tension, and the cellulose and semi-fiber in the pod. The content of lignin and lignin are the decisive factors for seed shattering. In addition, the content of carbon, soluble sugar and sucrose are also related to the occurrence of seed shattering. The dehiscence zone at the suture of the belly of the pod is considered to be an important area that affects the seed shattering, and the degradation of the dehiscence zone is conducive to the formation of seed shattering [64–67]. Cellulase and polygalacturonase are considered to be the key enzymes affecting pod frying in the ventral suture dehiscence zone of soybean pods [50]. Studies have shown that auxin in the dehiscence zone of soybeans can affect the activity of hydrolase. Affect the occurrence of seed shattering [68].
The phenomenon of seed shattering of legumes has been widespread. Breeding high-quality soybean varieties resistant to seed shattering is one of the main goals of people’s breeding. The main factors influencing pods are genetic, environmental and mechanical factors, among which environmental factors cannot be changed. Therefore, it is more important to breed good varieties that are resistant to pods. At present, the research of harvester is also increasing and gradually optimized, but the mechanical design is closely related to the biological characteristics of crops, and the conditions of biological indicators still need to be explored for accurate, not only that, such as the precise impact of the pod identification method on germplasm identification and the impact of environmental factors on the pod research. In the end, genetic factors have the greatest influence and the most complex. Although researchers have studied morphology, physiology, and cloning of related genes for seed shattering, they have learned about the occurrence of seed shattering, the regulatory mechanism of related genes, and the effects of corresponding hormones and enzymes of seed shattering. However, any change in any link may cause seed shattering, so there are still many issues that need to be studied in depth. In the future, research can be carried out in morphology, physiology and molecular biology. Morphology mainly focuses on the structure of the peel, the thickness of the secondary cell wall of FCC cells in the pod wall and ventral sutures, FCC cells and structures that have not yet been discovered and explored. Physiology mainly conducts in-depth research on the key enzymes and endogenous hormones related to seed shattering to clarify the regulatory network. Molecular biology still needs to dig out more genes related to fried pods and perform functional verification to clarify the mechanism of gene action. Frying pods is a complicated process. The exploration from the above three aspects will provide a basis for the study of seed shattering mechanism and provide theoretical support for cultivating new varieties of seed shattering. In molecular biology, some scholars have cloned the main gene that regulates seed shattering, but it is necessary to verify the function of varieties with obvious differences in traits. In addition, it is still necessary to dig out more related seed shattering genes and perform functional verification to clarify the role of the gene. mechanism. In the future research, we will not only explore the physiological mechanism of soybean popping, but also reveal the genetic mechanism of soybean popping from the genetic level, so as to have a more comprehensive and in-depth understanding of the mechanism of soybean popping, and provide theories for the cultivation of new varieties that are resistant to popping. support.
Funding Statement: The research was jointly funded by Jilin Province Education Department Science and Technology Research Project [JJKH20210350KJ], Jilin Province Science and Technology Guidance Program Project [20200402023NC], Jilin Provincial Natural Science Foundation Project [20200201027JC] and Innovation and Entrepreneurship Training Program for College Students in Jilin Province [2021].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Kang, X., Cai, J., Chen, Y., Yan, Y., Yang, S. et al. (2020). Pod-shattering characteristics differences between two groups of soybeans are associated with specific changes in gene expression. Functional & Integrative Genomics, 20(2), 201–210. DOI 10.1007/s10142-019-00702-2. [Google Scholar] [CrossRef]
2. Seo, J. H., Kang, B. K., Dhungana, S. K., Oh, J. H., Choi, M. S. et al. (2020). QTL mapping and candidate gene analysis for pod shattering tolerance in soybean (Glycine max). Plants, 9(9), 1163. DOI 10.3390/plants9091163. [Google Scholar] [CrossRef]
3. Rau, D., Murgia, M. L., Rodriguez, M., Bitocchi, E., Bellucci, E. et al. (2019). Genomic dissection of pod shattering in common bean: Mutations at non-orthologous loci at the basis of convergent phenotypic evolution under domestication of leguminous species. The Plant Journal, 97(4), 693–714. DOI 10.1111/tpj.14155. [Google Scholar] [CrossRef]
4. Parker, T. A., Berny, M. Y., Teran, J. C., Palkovic, A., Jernstedt, J. et al. (2020). Pod indehiscence is a domestication and aridity resilience trait in common bean. New Phytologist, 225(1), 558–570. DOI 10.1111/nph.16164. [Google Scholar] [CrossRef]
5. Kappara, S., Neelamraju, S., Ramanan, R. (2018). Down regulation of a heavy metal transporter gene influences several domestication traits and grain Fe-Zn content in rice. Plant Science, 276, 208–219. DOI 10.1016/j.plantsci.2018.09.003. [Google Scholar] [CrossRef]
6. Łangowski, Ł, Goñi, O., Quille, P., Stephenson, P., Carmody, N. et al. (2019). A plant biostimulant from the seaweed Ascophyllum nodosum (Sealicit) reduces podshatter and yield loss in oilseed rape through modulation of IND expression. Scientific Reports, 9(1), 16644. DOI 10.1038/s41598-019-52958-0. [Google Scholar] [CrossRef]
7. Philbrook, B. D., Oplinger, E. S. (1989). Soybean field losses as influenced by harvest delays. Agronomy Journal, 81(2), 251–258. DOI 10.2134/agronj1989.00021962008100020023x. [Google Scholar] [CrossRef]
8. Zhang, Y. J., Ma, S. F., Gao, Q. Y., Luo, J. Z. (2006). Study on the explosive Pod properties of soybean varieties mainly planted in huanghuai valley. Henan Agricultural Sciences, (6), 56–59. DOI 10.15933/j.cnki.1004-3268. [Google Scholar] [CrossRef]
9. Liu, J., Zhou, R., Wang, W., Wang, H., Qiu, Y. et al. (2020). A copia-like retrotransposon insertion in the upstream region of the SHATTERPROOF1 gene, BnSHP1.A9, is associated with quantitative variation in pod shattering resistance in oilseed rape. Journal of Experimental Botany, 71(18), 5402–5413. DOI 10.1093/jxb/eraa281. [Google Scholar] [CrossRef]
10. Lo, S., Parker, T., Muñoz-Amatriaín, M., Teran, J. C., Jernstedt, J. et al. (2021). Genetic, anatomical, and environmental patterns related to pod shattering resistance in domesticated cowpea [Vigna unguiculata (L.) walp]. Journal of Experimental Botany, 72(18), 6219–6229. DOI 10.1093/jxb/erab259. [Google Scholar] [CrossRef]
11. Mutari, B., Sibiya, J., Nchanji, E. B., Simango, K., Gasura, E. (2021). Farmers’ perceptions of navy bean (Phaseolus vulgaris L.) production constraints, preferred traits and farming systems and their implications on bean breeding: A case study from south east lowveld region of Zimbabwe. Journal of Ethnobiology and Ethnomedicine, 17(1), 13. DOI 10.1186/s13002-021-00442-3. [Google Scholar] [CrossRef]
12. Purugganan, M. D., Fuller, D. Q. (2009). The nature of selection during plant domestication. Nature, 457(7231), 843–848. DOI 10.1038/nature07895. [Google Scholar] [CrossRef]
13. Gulluoglu, L., Arioglu, H., Arslan, M. (2006). Effects of some plant growth regulators and nutrient complexes on pod shattering and yield losses of soybean under hot and dry conditions. Asian Journal of Plant Sciences, 5(2), 368–372. DOI 10.3923/ajps.2006.368.372. [Google Scholar] [CrossRef]
14. Hofhuis, H., Hay, A. (2017). Explosive seed dispersal. New Phytologist, 216(2), 339–342. DOI 10.1111/nph.14541. [Google Scholar] [CrossRef]
15. Chiapparino, E., Lee, D., Donini, P. (2004). Genotyping single nucleotide polymorphisms inbarley by tetra-primer ARMS-PCR. Genome, 47(2), 414–420. DOI 10.1139/g03-130. [Google Scholar] [CrossRef]
16. Krisnawati, A., Adie, M. M. (2017). Variability on morphological characters associated with pod shattering resistance in soybean. Biodiversitas, 18(1), 73–77. DOI 10.13057/biodiv/d180111. [Google Scholar] [CrossRef]
17. Murgia, M. L., Attene, G., Rodriguez, M. (2017). A comprehensive phenotypic investigation of the pod-shattering syndrome in common bean. Frontiers in Plant Science, 8, 251. DOI 10.3389/fpls.2017.00251. [Google Scholar] [CrossRef]
18. Caviness, C. E. (1969). Heritability of pod dehiscence and its association with some agronomic characters in soybeans1. Crop Science, 9(2), 207–209. DOI 10.2135/cropsci1969.0011183X000900020029x. [Google Scholar] [CrossRef]
19. Han, J. Han, D. Guo, Y. Yan, H. Wei, Z. et al. (2019). QTL mapping pod dehiscence resistance in soybean (Glycine max L. Merr.) using specific-locus amplified fragment sequencing. Theoretical and Applied Genetics, 132(8), 2253–2272. DOI 10.1007/s00122-019-03352-x. [Google Scholar] [CrossRef]
20. Tiwari, S. P., Bhatia, V. S. (1995). Characters of pod anatomy associated with resistance to pod-shattering in soybean. Annals of Botany, 76(5), 483–485. DOI 10.1006/anbo.1995.1123. [Google Scholar] [CrossRef]
21. Christiansen, L. C., Degan, F. D., Ulvskov, P., Borkhardt, B. (2002). Examination of the dehiscence zone in soybean pods and isolation of a dehiscenc-related endopolygalacturonase gene. Plant Cell & Environment, 25(4), 479–490. DOI 10.1046/j.1365-3040.2002.00839.x. [Google Scholar] [CrossRef]
22. Summers, J. E., Bruce, D. M., Vancanneyt, G., Redig, P., Werner, C. P. et al. (2003). Pod shatter resistance in the resynthesized Brassica napus line DK142. Journal of Agricultural Science, 140(1), 43–52. DOI 10.1017/S002185960200285X. [Google Scholar] [CrossRef]
23. Tiwari, S., Bhatia, V. S. (1995). Characters of pod anatomy associated with resistance to pod-shattering in soybean. Annals of Botany, 76(5), 483–485. DOI 10.1006/anbo.1995.1123. [Google Scholar] [CrossRef]
24. Takahashi, Y., Kongjaimun, A., Muto, C., Kobayashi, Y., Kumagai, M. et al. (2020). Same locus for non-shattering seed pod in two independently domesticated legumes, Vigna angularis and Vigna unguiculata. Front Genet, 11, 748. DOI 10.3389/fgene.2020.00748. [Google Scholar] [CrossRef]
25. Ferrándiz, C., Liljegren, S. J., Yanofsky, M. F. (2000). Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science, 289(5478), 436–438. DOI 10.1126/science.289.5478.436. [Google Scholar] [CrossRef]
26. Dong, D., Yan, L., Dong, R., Wang, Y., Liu, Z. (2017). Evaluation and analysis of pod dehiscence factors in shatter-susceptible and shatter-resistant common vetch. Crop Science, 57(5), 2770–2776. DOI 10.2135/cropsci2017.03.0191. [Google Scholar] [CrossRef]
27. Meakin, P. J., Roberts, J. A. (1990). Dehiscence of fruit in oilseed rape (Brassica napus L) anatomy of pod dehiscence. Journal of Experimental Botany, 41(8), 995–1002. DOI 10.1093/jxb/41.8.995. [Google Scholar] [CrossRef]
28. Bennett, E. J., Roberts, J. A., Wagstaff, C. (2011). The role of the pod in seed development: strategies for manipulating yield. New Phytologist Foundation, 190(4), 838–853. DOI 10.1111/j.1469-8137. [Google Scholar] [CrossRef]
29. Suanum, W., Somta, P., Kongjaimun, A. (2016). Co-localization of QTLs for pod fiber content and pod shattering in F2, and backcross populations between yard long bean and wild cowpea. Molecular Breeding, 36(6), 80. DOI 10.1007/s11032-016-0505-8. [Google Scholar] [CrossRef]
30. Spence, J., Vercher, Y., Gates, P., Harris, N. (1996). ‘Pod shatter’ in Arabidopsis thalianaBrassica napus and B. juncea. Journal of Microscopy, 181(2), 195–203. DOI 10.1046/j.1365-2818.1996.111391.x. [Google Scholar] [CrossRef]
31. Murgia, M. L., Attene, G., Rodriguez, M., Bitocchi, E., Bellucci, E. et al. (2017). A comprehensive phenotypic investigation of the “pod-shattering syndrome” in common bean. Frontiers in Plant Science, 8, 251. DOI 10.3389/fpls.2017.00251. [Google Scholar] [CrossRef]
32. Bruce, D. M., Farrent, J. W., Morgan, C. L. (2002). PA-Precision agriculture: Determining the oilseed rape pod strength needed to reduce seed loss due to pod shatter. Biosystems Engineering, 81(2), 179–184. DOI 10.1006/bioe. [Google Scholar] [CrossRef]
33. Han, D. Z., Ren, Y. L., Guo, Y., Yan, H. R., Zhang, L., et al. (2015). Occurrence characteristics and molecular genetic basis of pod shattering in soybean. Yi Chuan, 37(6), 553–543. DOI 10.16288/j.yczz.14-456. [Google Scholar] [CrossRef]
34. Zhang, Q., Tu, B., Liu, C. (2018). Pod anatomy, morphology and dehiscing forces in pod dehiscence of soybean (Glycine max (L.) Merrill). Flora, 248, 48–53. DOI 10.1016/j.flora.2018.08.014. [Google Scholar] [CrossRef]
35. Li, C., Zhou, A., Sang, T. (2006). Rice domestication by reducing shattering. Science, 311, 1936–1939. DOI 10.1126/science.1123604. [Google Scholar] [CrossRef]
36. Funatsuki, H., Hajika, M., Yamada, T., Suzuki, M., Hagihara, S. et al. (2012). Mapping and use of QTLs controlling pod dehiscence in soybean. Breeding Science, 61(5), 554–558. DOI 10.1270/jsbbs.61.554. [Google Scholar] [CrossRef]
37. Nilmani, B., Dhirendra, K., Shrivastava, A. N. (2013). Studies on the factors affecting pod shattering in soybean. Indian Journal of Genetics and Plant Breeding, 73(3), 270–277. DOI 10.5958/j.0975-6906.73.3.040. [Google Scholar] [CrossRef]
38. Vittori, V. D., Bitocchi, E., Rodriguez, M., Alseekh, S., Bellucci, E. et al. (2021). Pod indehiscence in common bean is associated with the fine regulation of PvMYB26. Journal of Experimental Botany, 72(5), 1617–1633. DOI 10.1093/jxb/eraa553. [Google Scholar] [CrossRef]
39. Suzuki, M., Fujino, K., Funatsuki, H. (2009). A major soybean QTL, qPDH1, controls pod dehiscence without marked morphological change. Plant Production Science, 12(2), 217–223. DOI 10.1626/pps.12.217. [Google Scholar] [CrossRef]
40. Metcalfe, D. S., Johnson, I. J., Shaw, R. H. (1957). The relation between pod dehiscence, relative humidity and moisture equilibrium in birdsfoot trefoil, Lotus corniculatus1. Agronomy Journal, 49(3), 130–134. DOI 10.2134/agronj1957.00021962004900030006x. [Google Scholar] [CrossRef]
41. Grant, F. W. (1996). Seed pod shattering in the genus lotus (fabaceaeA synthesis of dive. Canadian Journal of Plant Science, 76(3), 447–456. DOI 10.4141/cjps96-079. [Google Scholar] [CrossRef]
42. Maity, A., Lamichaney, A., Joshi, D. C., Bajwa, A., Subramanian, N. et al. (2021). Seed Shattering: A Trait of Evolutionary Importance in Plants. Frontiers in Plant Science, 12, 657773. DOI 10.3389/fpls.2021.657773. [Google Scholar] [CrossRef]
43. Gao, M. Q., Zhu, H. Y. (2013). Fine mapping of a major quantitative trait locus that regulates pod shattering in soybean. Molecular Breeding, 32(2), 485–491. DOI 10.1007/s11032-013-9868-2. [Google Scholar] [CrossRef]
44. Romkaew, J., Umezaki, T., Suzuki, K., Nagaya, Y. (2007). Pod dehiscence in relation to pod position and moisture content in soybean. Plant Production Science, 10(3), 292–296. DOI 10.1626/pps.10.292. [Google Scholar] [CrossRef]
45. Dong, Y., Wang, Y. Z. (2015). Seed shattering: from models to crops. Frontiers in Plant Science, 6, 476. DOI 10.3389/fpls. [Google Scholar] [CrossRef]
46. Travis, A. P., Lo, S., Gepts, P. (2021). Pod shattering in grain legumes: Emerging genetic and environment-related patterns. Plant Cell, 33(2), 179–199. DOI 10.1093/plcell/koaa025. [Google Scholar] [CrossRef]
47. Łangowski, Ł, Goñi, O., Quille, P., Stephenson, P., Carmody, N. et al. (2019). A plant biostimulant from the seaweed Ascophyllum nodosum (Sealicit) reduces podshatter and yield loss in oilseed rape through modulation of IND expression. Scientific Reports, 9(1), 16644. DOI 10.1038/s41598-019-52958-0. [Google Scholar] [CrossRef]
48. Li, L. F., Olsen, K. M. (2016). To have and to hold: Selection for seed and fruit retention during crop domestication. Current Topics in Developmental Biology, 119, 63–109. DOI 10.1016/bs.ctdb.2016.02.002. [Google Scholar] [CrossRef]
49. Christiansen, L. C., Dal, D. F., Ulvskov, P., Borkhardt, B. (2002). Examination of the dehiscence zone in soybean pods and isolation of a dehiscence-related endopolygalacturonase gene. Plant Cell Environment, 25(4), 479–490. DOI 10.1046/j.1365-3040. [Google Scholar] [CrossRef]
50. Agrawal, A. P., Basarkar, P. W., Salimath, P. M. (2002). Role of cell wall-degrading enzymes in pod-shattering process of soybean, Glycine max (L.) Merrill. Current Science, 82(1), 58–60. DOI 10.3390/ijms19020335. [Google Scholar] [CrossRef]
51. Mahmood, U., Fan, Y., Wei, S., Niu, Y., Li, Y. (2021). Comprehensive analysis of polygalacturonase genes offers new insights into their origin and functional evolution in land plants. Genomics, 113(1), 1096–1108. DOI 10.1016/j.ygeno.2020.11.006. [Google Scholar] [CrossRef]
52. Davies, P. J. (1992). Ethylene in plant biology. Saltveit San Diego: Academic Press. [Google Scholar]
53. Tu, B. J. (2020). The formation mechanism of soybean fried pods. University of Chinese Academy of Sciences (Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences). DOI 10.27536/d.cnki.gccdy. [Google Scholar] [CrossRef]
54. Suzuki, M., Fujino, K., Nakamoto, Y., Ishimoto, M., Funatsuki, H. (2010). Fine mapping and development of DNA markers for the qPDH1 locus associated with pod dehiscence in soybean. Molecular Breeding, 25(3), 407–418. DOI 10.1007/s11032-009-9340-5. [Google Scholar] [CrossRef]
55. Funatsuki, H., Ishimoto, M., Tsuji, H., Kawaguchi, K., Hajika, M. et al. (2006). Simple sequence repeat markers linked to a major QTL controlling pod shattering in soybean. Plant Breeding, 125(2), 195–197. DOI 10.1111/j.1439-0523.2006.01199.x. [Google Scholar] [CrossRef]
56. Yamada, T., Funatsuki, H., Hagihara, S., Fujita, S., Tanaka, Y. et al. (2009). A major QTL, qPDH1, is commonly involved in shattering resistance of soybean cultivars. Breed Science, 59(4), 435–440. DOI 10.1270/jsbbs.59.435. [Google Scholar] [CrossRef]
57. Romkaew, J., Umezaki, T. (2006). Pod dehiscence in soybean: Assessing methods and varietal difference. Plant Production Science, 9(4), 373–382. DOI 10.1626/pps.9.373. [Google Scholar] [CrossRef]
58. Kang, S. T., Kwak, M., Kim, H. K., Choung, M. G., Han, W. Y. (2009). Population-specific QTLs and their different epistatic interactions for pod dehiscence in soybean [Glycine max (L.) merr.]. Euphytica, 166(1), 15–24. DOI 10.1007/s10681-008-9810-6. [Google Scholar] [CrossRef]
59. Bailey, M. A., Mian, M. A. R., Carter, T. E., Ashley, D. A., Boerma, H. R. (1997). Pod dehiscence of soybean: Identification of quantitative trait loci. Journal of Heredity, 88(2), 152–154. DOI 10.1093/oxfordjournals.jhered.a023075. [Google Scholar] [CrossRef]
60. Funatsuki, H., Suzuki, M., Hirose, A., Inaba, H., Fujino, K. (2014). Molecular basis of a shattering resistance boosting global dissemination of soybean. Proceedings of the National Academy of Sciences of the USA, 111(50), 17797–17802. DOI 10.1073/pnas.1417282111. [Google Scholar] [CrossRef]
61. Dong, Y., Yang, X., Liu, J., Wang, B. H., Liu, B. L. et al. (2014). Pod shattering resistance associated with domestication is mediated by a NAC gene in soybean. Nature Communications, 5, 3352. DOI 10.1038/ncomms4352. [Google Scholar] [CrossRef]
62. Seo, J. H., Kang, B. K., Dhungana, S. K., Oh, J. H., Choi, M. S. (2020). QTL mapping and candidate gene analysis for pod shattering tolerance in soybean (Glycine max). Plants, 9(9), 1163. DOI 10.3390/plants9091163. [Google Scholar] [CrossRef]
63. Liu, Y. Y., Lu, W. Y., Liu, S., Qin, Y. F., Yang, Y. H. et al. (2017). Preliminary identification of soybean GmAGL8 gene function in Arabidopsis. Journal of Beijing Agricultural College, 35(4), 20–25. DOI 10.13473/j.cnki.issn. [Google Scholar] [CrossRef]
64. Se, B. J. (2020). The formation mechanism of soybean fried pod characteristics. University of Chinese Academy of Sciences (Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences). [Google Scholar]
65. Wang, R., Ripley, V. L., Rakow, G. (2007). Pod shatter resistance evaluation in cultivars and breeding lines of Brassica napus, B. juncea and Sinapis alba. Plant Breeding, 126(6), 588–595. DOI 10.1111/j.1439-0523. [Google Scholar] [CrossRef]
66. Romkaew, J., Nagaya, Y., Goto, M., Suzuki, K., Umezaki, T. (2008). Pod dehiscence in relation to chemical components of pod shell in soybean. Plant Production Science, 11(3), 278–282. DOI 10.1626/pps.11.278. [Google Scholar] [CrossRef]
67. Kuai, J., Sun, Y., Liu, T., Zhang, P., Zhou, M. et al. (2016). Physiological mechanisms behind differences in pod shattering resistance in rapeseed (Brassica napus L.) varieties. PLoS One, 11(6), e0157341. DOI 10.1371/journal.pone.0157341. [Google Scholar] [CrossRef]
68. Tucker, M. L., Sexton, R., Campillo, E. D., Lewis, L. N. (1988). Bean abscission cellulase: Characterization of a cDNA clone and regulation of gene expression by ethylene and auxin. Plant Physiology, 88(4), 1257–1262. DOI 10.1104/pp.88.4.1257. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |