

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020329
ARTICLE
Seasonal Changes in Soil Respiration with An Elevation Gradient in Abies nephrolepis (Trautv.) Maxim. Forests in North China
1Xinzhou Teachers University, Xinzhou, 034000, China
2School of Geography Science, Taiyuan Normal University, Taiyuan, 030619, China
*Corresponding Author: Zhijie Tian. Email: tianzhijie12@mails.ucas.ac.cn
Received: 17 November 2021; Accepted: 24 December 2021
Abstract: Soil respiration (Rs) plays an important role in regulating carbon cycle of terrestrial ecosystems and presents temporal and spatial heterogeneity. Abies nephrolepis is a tree species that prefers the cold and wet environment and is mainly distributed in Northeast Asia and East Asia. The Rs variations of Abies nephrolepis forests communities are generally environmental-sensitive and can effectively reflect the adaptive responses of forest ecosystems to climate change. In this study, the growing-seasonal variations of Rs, soil temperature, soil water content and soil properties of Abies nephrolepis forests were analyzed along an altitude gradient (2000, 2100, 2200 and 2300 m) over two years on Wutai Mountain in North China. As the main results showed, soil respiration keeps the same change trend as soil temperature and reached peaks in July at 2000 m in 2019 and 2020. During 26th July to 25th October in 2019 and 27th May to 23rd October in 2020, on the whole, the soil temperature independently explained 76.2% of Rs variations while the soil water content independently explained 26.8%. Soil temperature and soil water content jointly explained 81.8% of Rs variations. Soil properties explained 61.8% and 69.6% of Rs variation in 2019 and 2020, respectively. Soil organic carbon content and soil enzyme activity had the significant (P < 0.01) negative and positive relationships, respectively, with Rs variation. With altitudes evaluated from 2000 to 2300 m, soil respiration temperature sensitivity (Q10) and the soil organic carbon content increased by 12.4% and 10.4%, respectively, while invertase activity, cellulase activity and urease activity dropped by 41.2%, 29.45% and 38.19%, respectively. The results demonstrate that (1) soil temperature is the major factor affecting Rs variations in Abies nephrolepis forests; (2) weakened microbial carbon metabolism in high-altitude areas results in the accumulation of soil organic carbon; (3) with a higher Q10, forest ecosystems in high-altitude areas might be more easily affected by climate change; (4) climate warming might accelerate the consumption of soil organic carbon sink in forest ecosystems, especially in high-altitude areas.
Keywords: Soil respiration; Abies nephrolepis; altitude; soil respiration temperature sensitivity; soil organic carbon
Soil respiration (Rs) is the process of biological metabolism and chemical oxidation of carbon-containing substances in the soil [1,2]. As the second largest carbon flux (about 90 Pg C·yr–1) of terrestrial ecosystems, Rs reflects the impact of environmental changes on vegetation ecosystems and plays an important role in reflecting global climate change [3,4]. Forest ecosystems are the largest carbon sink with more than 75% organic carbon storage in terrestrial ecosystems [5]. With ongoing global warming, the risk of forest ecosystems changing from carbon sinks to carbon sources might continue to increase [6]. Thus, the dynamic changes in Rs in forest ecosystems have become one of the most urgent research objects for the global long-term monitoring of CO2 fluxes [7]. Many high altitude areas have always been ecologically fragile areas and are easily affected by climate changes and environmental factors [8,9]. An increase in Rs in these areas can effectively accelerate soil organic carbon consumption and affect stability of vegetation communities in forest ecosystems.
Rs is affected by many biotic and abiotic factors and shows significant temporal and spatial differences [10,11]. Elevation is an important topographical factor affecting Rs in mid- and high-latitude areas [12]. Due to the changes in hydrothermal conditions, soil properties, biological metabolisms and vegetation communities, the soil CO2 flux and carbon cycle in forest ecosystems obviously differ with altitude [13,14]. Soil temperature and the water content are the two mainly important factors affecting Rs by changing the growth of plant roots, soil microbial metabolism, and other oxidation and decomposition processes of soil organic matter [15]. Studies have shown that there is an obvious positive correlation between Rs and soil temperature, while the influence of soil moisture on Rs is more complex [16]. Only when soil moisture becomes the main factor restricting plant growth under drought conditions will it have a significant positive correlation with Rs [17,18]. Moreover, soil properties also have important effects on Rs. Soil organic carbon and nitrogen have been found to affect Rs by regulating plant root growth and biological metabolism [19]. Soil C:N can effectively indicate the soil dissolved organic carbon concentration [20]. Soil microorganisms have an important influence on soil respiration, and the carbon metabolism process can be better reflected by the activity of soil enzymes [21]. Thus, the Rs variation of forest ecosystems with altitude is a comprehensive progress. Rs characteristics of special plant communities in ecologically fragile areas or border areas is more important for us to predict the development of terrestrial ecosystems with climate change [22].
Abies nephrolepis (Trautv.) Maxim. is a genus of evergreen coniferous trees that favors cold and humid environment. It has a limited distribution in Northeast Asia and East Asia, mainly in the Russian Far East and North China [23,24]. Wutai Mountain is an ecologically fragile region with the highest elevation (3061 m) in North China and significant zonal characteristics associated with elevation and vegetation, which provides suitable fertility conditions for Abies nephrolepis. It is also the southernmost natural distribution of the Abies nephrolepis population in Asia. Thus, the Rs characteristics of the Abies nephrolepis community in Wutai Mountain play an important ecological role in indicating the responses of vegetation sensitivity and ecosystem stability to climate change in high-altitude areas at mid-latitudes.
In this study, four altitudes with typical Abies nephrolepis communities in Wutai Mountain were conducted for two years. Seasonal and interannual variations in Rs, soil temperature, and the soil water content were determined and then used to build correlation models. Soil properties were also measured to analyze their relationships with Rs changes. This study aims to explain (1) how the Rs, soil temperature and soil water content change with different altitudes and seasons; (2) how the environmental factors such as soil temperature, soil water content and soil properties affect Rs variation; (3) how the soil carbon metabolism responses to the change of climate and altitude gradient. We hypothesize that (1) soil temperature has a major effect on Rs variation, while soil water content and soil properties are also partly in regulating soil CO2 flux; (2) along an altitude gradient, soil respiration temperature sensitivity increases with decreasing Rs.
The experiment was conducted at a long-term positioning experimental station in the Nature Reserve of Abies nephrolepis in Shanxi Province, China (Fig. 1). It has a humid and semi-humid climate in a warm temperate zone, with an average precipitation of 760 mm mainly occurring in July, August and September. It is dry and windy in spring, warm and humid in summer, cool and rainy in autumn, with a cold and long winter. The temperature varies greatly along the altitude. The annual average temperature is 4°C, and temperatures change from 30°C to –30°C, with 100 frost-free days. The soil types are mainly brown soil or cinnamon soil. The forest community is mainly composed of cold-temperate coniferous species such as Abies nephrolepis (Trautv.) Maxim., Picea wilsonii Mast., Larix principis-rupprechtii Mayr., Sorbus pohuashanensis (Hance) Hedl., etc.
Figure 1: Study site locations
Abies nephrolepis is mostly distributed in the altitude range of 1900~2500 m in the Nature Reserve. It has survived here for more than 40 years and the population density is approximately 1100 plants·hm–2. Along the increased altitudes, the diversity of plant communities decreased. For example, the total relative abundance of Larix principis-rupprechtii and Sorbus pohuashanensis populations decreased from 30% at 2000 m to 10% at 2300 m, while the relative abundance of Abies nephrolepis population increased from 20% at 2000 m to 60% at 2300 m. Thus, four sites with typical Abies nephrolepis communities were set up along altitudes of 2000 m (113°29′51″E, 39°04′23″N), 2100 m (113°29′50″E, 39°04′10″N), 2200 m (113°29′50″E, 39°04′04″N) and 2300 m (113°29′50″E, 39°03′52″N). Three experimental plots sized 20 m × 20 m were set up at each altitude.
2.2 Determination of Soil Respiration Rate, Soil Temperature and Soil Moisture
At each site, four polyvinyl chloride (PVC) collars sized 10 cm (height) × 20 cm (diameter) were randomly installed in each plot for Rs determination. Collars were put into the soil organic layer and exposed to the ground at a 3–4 cm height. All the living plants and plant residues above the ground in the PVC collars were cut 24 h before the determination.
Rs (μmol·m–2·s–1) was measured from 09:00 to 11:00 am on a relatively fixed day with clear weather every month from 26th July 2019 to 3rd October 2020 (except the months from December 2019 to April 2020 during the winter freezing period) by the Soil CO2 Flux Automatic Measurement System (Li-8100, USA). Soil temperature probes (6000-09TC, USA) and soil moisture probes (8100-204 Delta-Theta, USA) were used to measure the soil temperature and water content at a depth of 5 cm at the same time. The measurement was repeated three times for each PVC collar.
2.3 Soil Sample Collection and Testing
Four soil samples at the top 0–15 cm in each plot (near the PVC collar, at approximately 50 cm) were collected on 26th July 2019 and 28th July 2020. Plant roots, rocks and other impurities were removed from fresh soil samples, which were then air-dried, crushed and passed through a 2 mm sieve to determine the physical and chemical properties of the soil. Soil pH was determined by extraction with a soil-water ratio of 1:2.5 and was measured by a potentiometric method. Soil organic carbon (SOC) and total nitrogen (TN) contents were measured by a Total Organic Carbon Analyzer (Multi C/N 2100, Germany). Soil invertase activity and cellulase activity were determined by 3,5-dinitrosalicylic acid colormetry to detect glucose (mg) per gram dry soil in 24 h. Soil urease activity was determined by sodium phenate-sodium hypochlorite colormetry to measure the release of NH3-N (mg) per gram dry soil in 24 h.
2.4 Data Processing and Analysis
The relationship between Rs and soil temperature (T, °C) and soil respiration temperature sensitivity (Q10) was fitted with exponential function models as shown in Eq. (1). The relationship between Rs and the soil water content (W, %) was fitted with a quadratic model as shown in Eq. (2). The relationship among ln (Rs) and T and W was fitted with a binary quadratic model as shown in Eq. (3).
In the above equations, a, b, c, d, f, g, h, j, and k are all fitting parameters.
The differences in Rs, soil temperature, the soil water content and soil properties among altitudes and years were analyzed using one-way analysis of variance (ANOVA) with Tukey’s test in SPSS 20.0. The association analysis between Rs and soil properties was conducted by redundancy analysis (RDA) using R. Figures were generated using Origin 9.0, and tables were generated using Office 2016.
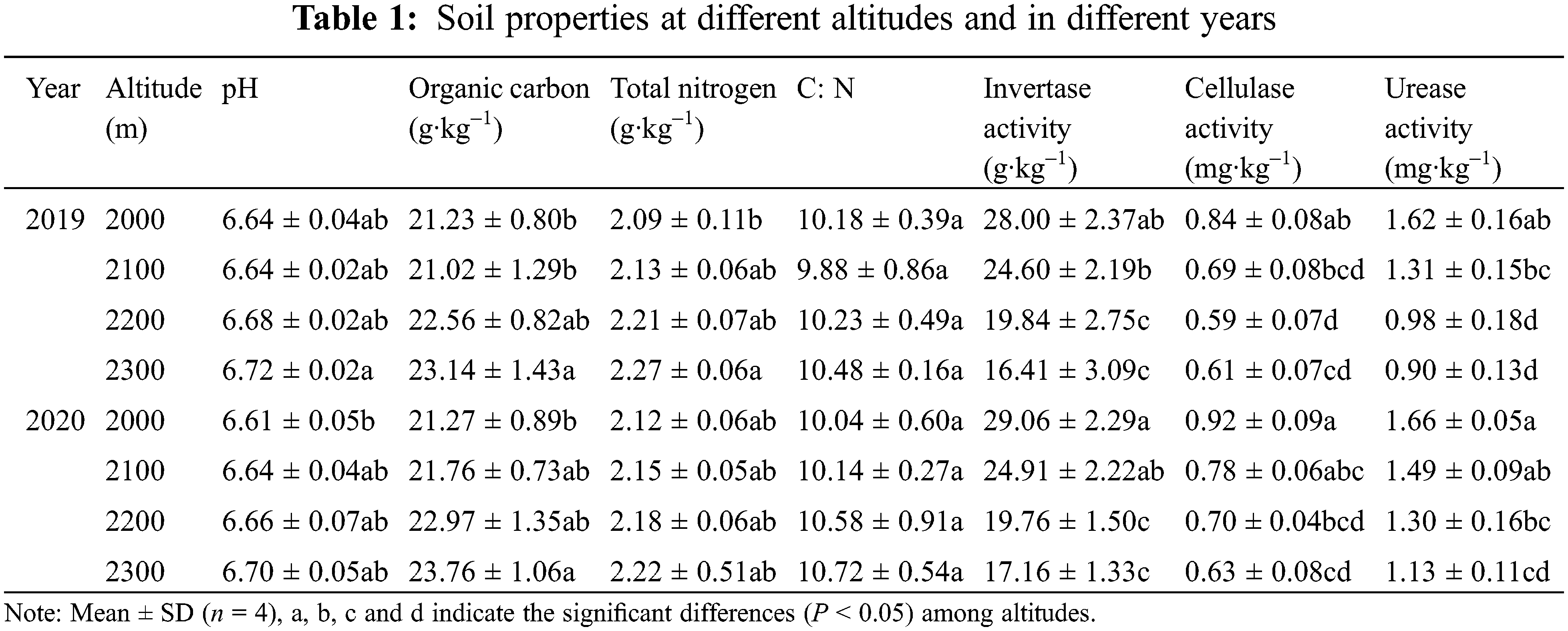
3.1 Soil Properties at Different Altitudes and in Different Years
From the results in Table 1, the forest soil of the Abies nephrolepis community was neutral to acidic and had different properties with increasing altitude. Soil SOC and TN showed increasing trends with increasing altitude from 2000 to 2300 m, especially SOC, with significant (P < 0.05) increases of 9.00% and 11.71% in 2019 and 2020, respectively. The TN and C:N changed around 2.20 g kg–1 and 10 across four altitudes and two years. Contrary to the above soil properties, soil enzymes significantly (P < 0.05) decreased from 2000 to 2300 m. Invertase activity, cellulase activity and urease activity decreased by 41.39%, 27.38% and 44.44%, respectively, in 2019 and by 40.95%, 31.52% and 31.93%, respectively, in 2020. Moreover, the soil properties mentioned above in 2020 were slightly higher than those in 2019.
3.2 Soil Temperature, Soil Water Content and Rs Dynamics
In the plant growing season, soil temperature, the soil water content and Rs showed obvious seasonal changes (Fig. 2). Across the two years, Rs generally tracked soil temperature and reached a peak in July and then decreased to the lowest value in October (Figs. 2a and 2b). Based on the means of the four altitudes from July to October, soil temperature changed from 13.39°C (2019, Fig. 2a) to 8.82°C (2020, Fig. 2b), and Rs changed from 2.96 μmol·m–2·s–1 (2019, Fig. 2e) to 2.50 μmol·m–2·s–1 (2020, Fig. 2f). Within the determined months, the soil water content was mostly maintained at higher than 60% in 2019 and reached the highest value of 78.22% in October (Fig. 2c). In 2020, the soil water content increased to a peak (74.71%) in August and dropped to a minimum (43.68%) in October (Fig. 2d). The interannual differences in the soil temperature and soil water content were caused by the changes in climate and hydrothermal conditions over the two years.
With raising altitude, soil temperature and Rs showed decreased, but the soil water content increased over the two years. Compared with the value from 2000 to 2300 m at July (highest Rs around the year), there is a relative decrease in Rs of 27.65% and 42.26% in 2019 and 2020, respectively. While there is a relative increase in soil water content of 21.12% and 7.27% in 2019 and 2020, respectively.
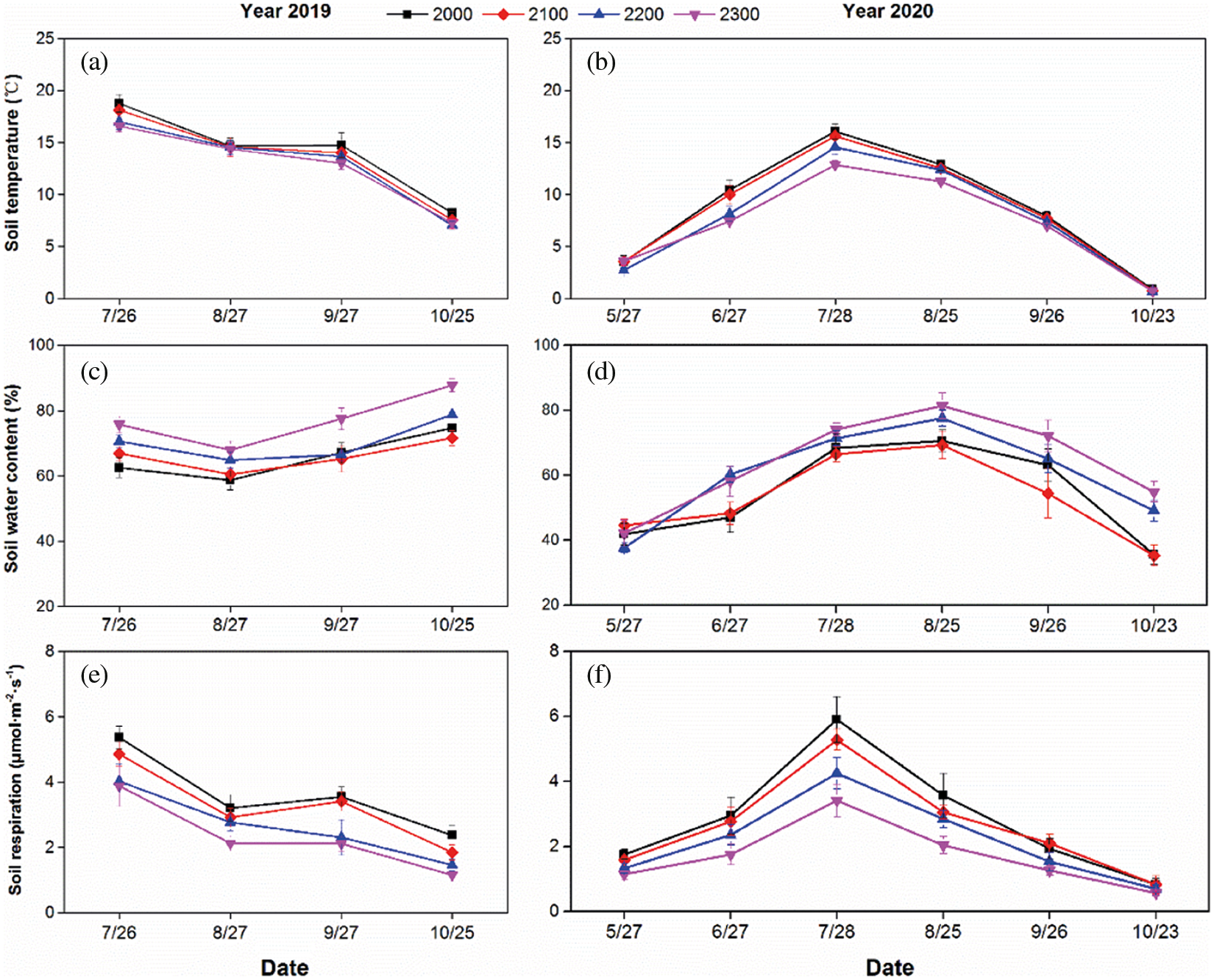
Figure 2: Monthly dynamics of soil temperature (a, b), soil water content (c, d) and Rs (e, f) respectively in 2019 and 2020. Data are shown as means ± SD (n = 12)
3.3 Relationships among Soil Temperature, Soil Water Content and Rs
There was a significant exponential correlation (P < 0.001) between Rs and soil temperature across all altitudes (Fig. 3). Soil temperature explained 72.5% to 82.1% of the seasonal variation in Rs at the four altitudes. The correlation (R2) between Rs and soil temperature were similar with the average of 0.818 at 2000 m and 2100 m (Figs. 3a and 3b), but obviously declined to 0.774 and 0.725 at 2200 m and 2300 m, respectively (Figs. 3c and 3d). In contrast, soil respiration temperature sensitivity (Q10) increased from the mean value of 2.40 at 2000 m and 2100 m (Figs. 3a and 3b) to 2.48 and 2.71 at 2200 m and 2300 m, respectively.
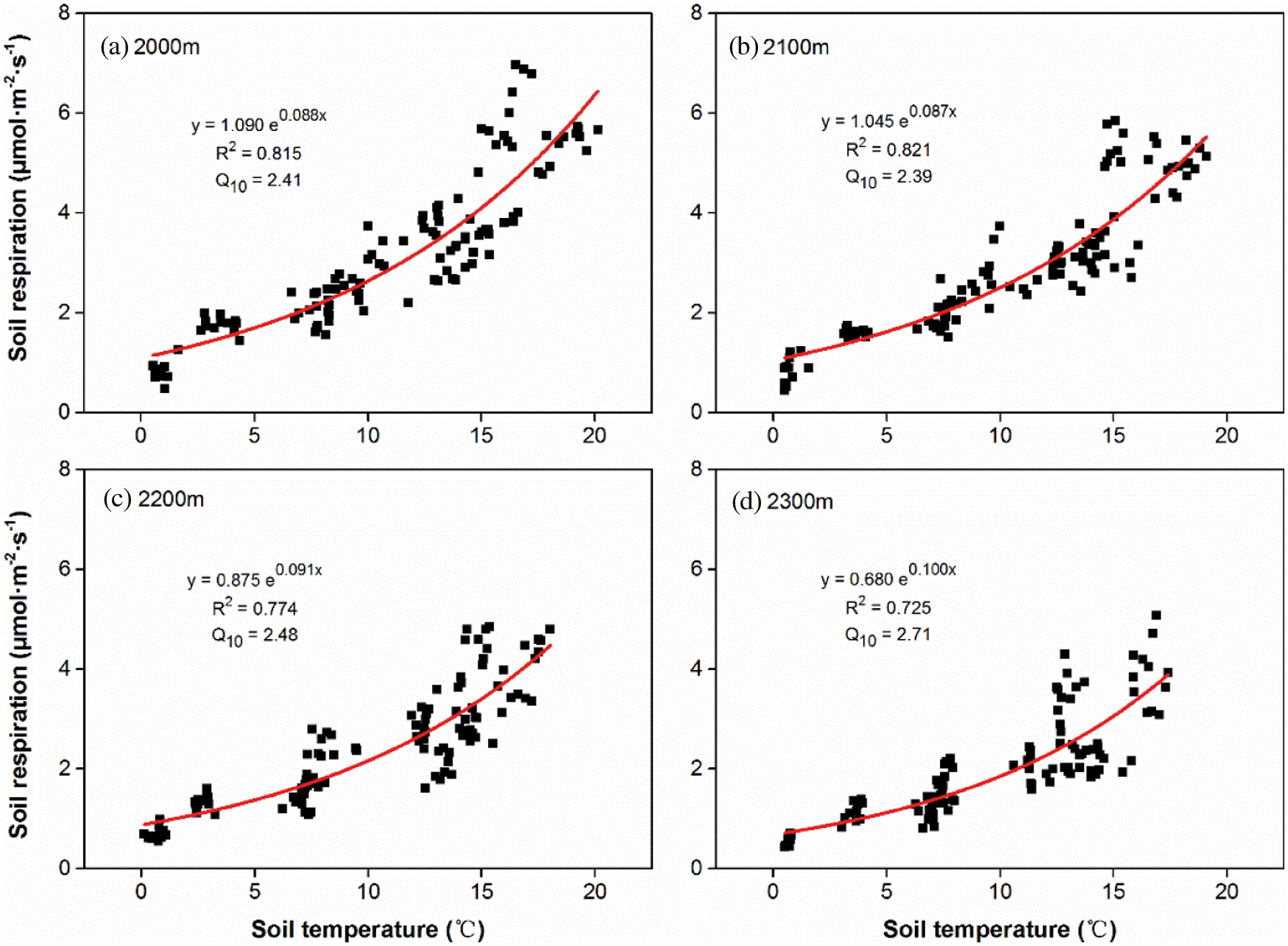
Figure 3: Relationships between soil temperature and Rs, respectively at the altitude of 2000 m (a), 2100 m (b), 2200 m (c) and 2300 m (d). Solid curves indicate the fitting trends (n = 120) of Rs variation with soil temperature
Quadratic functions (P < 0.001) were fitted between Rs and the soil water content (Fig. 4), which showed that Rs first increased and then decreased with increasing soil water content. The soil water content explained 21.3% to 42.5% of the seasonal variation in Rs among the four altitudes. R2 showed the values of 0.402 and 0.425 respectively at 2000 m and 2100 m (Figs. 4a and 4b), and decreased to 0.260 and 0.213 respectively at 2200 m and 2300 m (Figs. 4c and 4d). With increasing altitude, R2 decreased from 0.402 at 2000 m (Fig. 4a) to 0.213 at 2300 m (Fig. 4d); however, a value of 0.425 was measured at 2100 m (Fig. 4b). By contrast, the corresponding soil water content of the peak Rs showed increasing trends with altitude gradients, i.e., 63.52% and 65.66% at 2000 m and 2100 m, respectively, compared with 75.61% and 72.57% at 2200 and 2300 m, respectively.
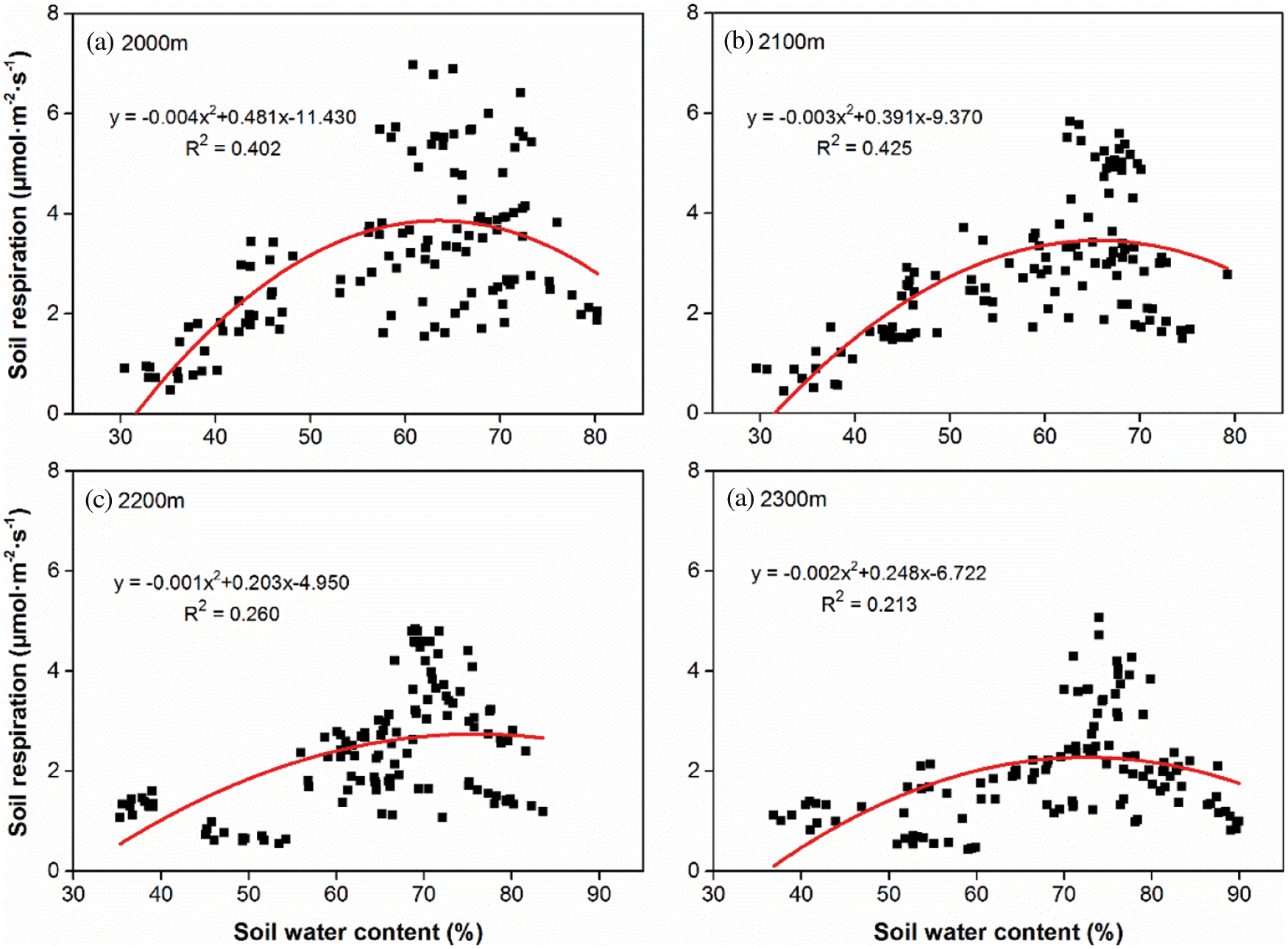
Figure 4: Relationships between soil water content and Rs, respectively at the altitude of 2000 m (a), 2100 m (b), 2200 m (c) and 2300 m (d). Solid curves indicate the fitting trends (n = 120) of Rs variation with soil water content
The fittings results showed significant (P < 0.001) correlations among soil temperature, soil water content and Rs (Fig. 5); R2 changed from 0.824 (Fig. 5c) to 0.862 (Fig. 5b) at four altitudes. These values were higher than the individual correlations between Rs and the soil temperature (0.725~0.821) and were much higher than the individual correlations between Rs and the soil water content (0.213~0.425). The relationships among soil temperature, soil water content and Rs were higher at the altitude of 2000 m (0.856) and 2100 m (0.862) than that in the altitude of 2200 m (0.824) and 2300 m (0.828).
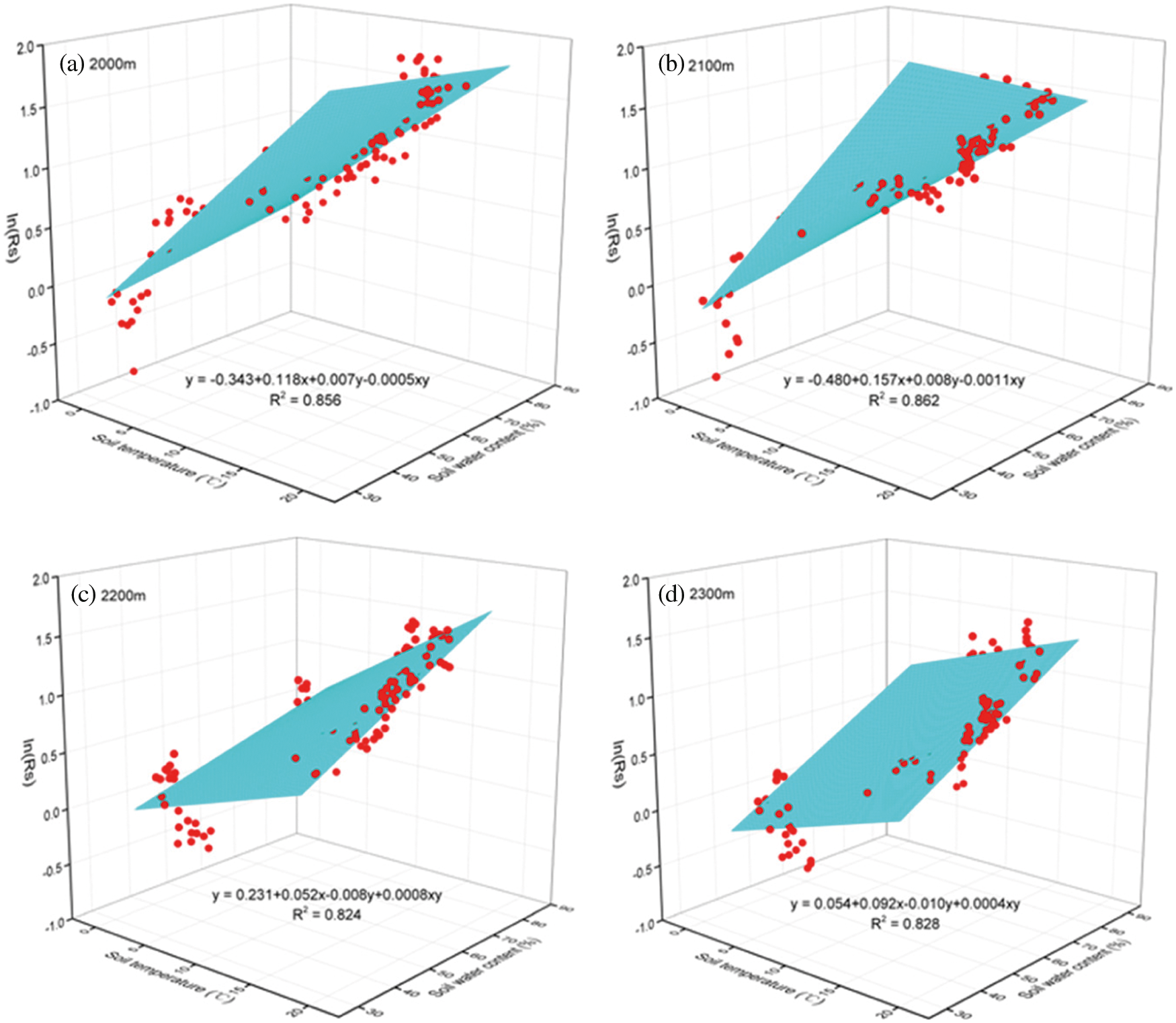
Figure 5: Relationships among soil temperature, soil water content and Rs, respectively at the altitude of 2000 m (a), 2100 m (b), 2200 m (c) and 2300 m (d). Curved surfaces indicate the fitting trends (n = 120) of Rs variation with soil temperature and soil water content
3.4 Relationships between Rs and Soil Properties
According to relationship analysis, Rs was significantly positively correlated (P < 0.01) with invertase, cellulase and urease activities but negatively correlated with soil pH, SOC, TN and C:N (Fig. 6). These properties jointly explained 61.76% (Fig. 6a) and 69.95% (Fig. 6b) of the Rs variation in 2019 and 2020, respectively, and the correlations changed in different seasons and different years. In 2019, Rs had a more obvious correlation with soil properties especially in July and September, whereas in 2020, the highest correlation occurred in July. In addition, SOC had stronger negative effects (P < 0.01) on Rs variation than pH, TN and C:N (Figs. 6a and 6b). Invertase activity had stronger positive (P < 0.01) effects on Rs variation than urease activity and cellulase activity. Rs variation was mainly affected by soil chemical properties at 2200 and 2300 m, but was affected by soil enzyme activity at 2000 and 2100 m.
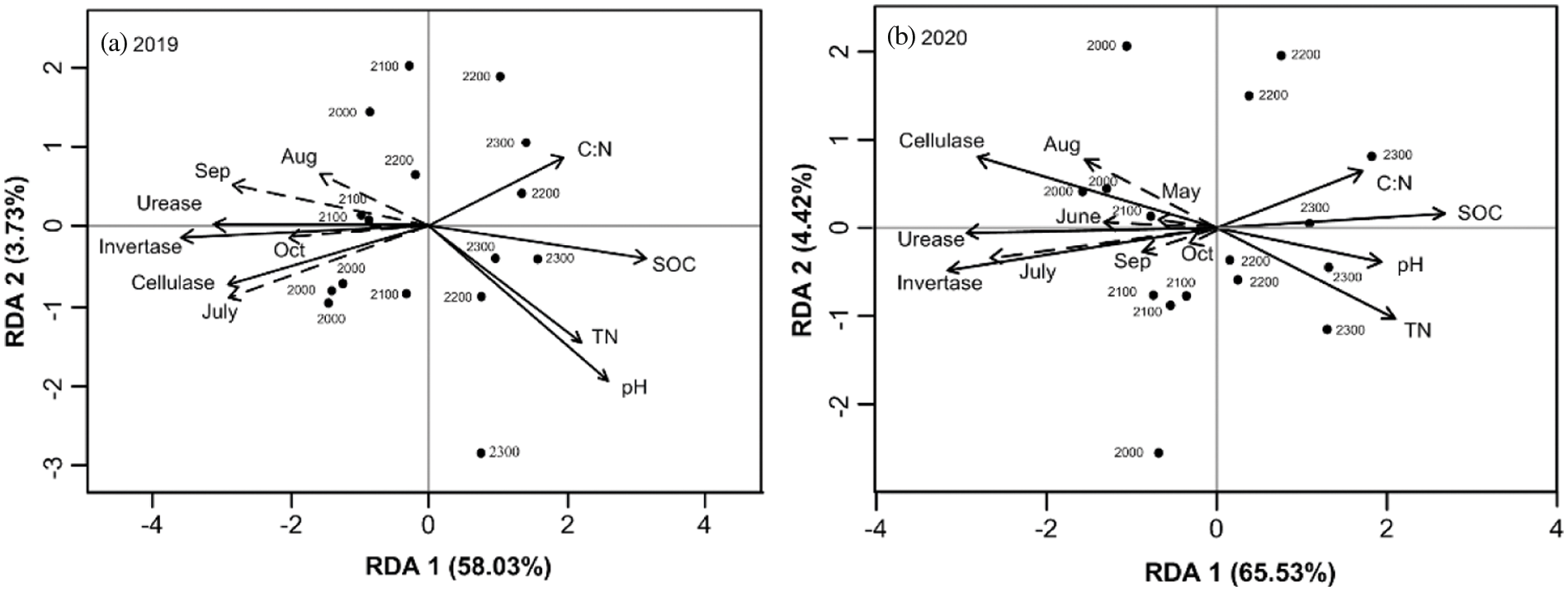
Figure 6: Redundancy analysis (RDA) of Rs and soil properties in 2019 (a) and 2020 (b). Dashed lines indicate Rs in different months, while solid lines indicate soil properties. Abbreviations: SOC: soil organic carbon; TN: soil total nitrogen
4.1 Soil Temperature Effects on Rs with Altitude
Rs changes in a seasonal and an interannual scale and has a significant positive correlation with soil temperature [25,26]. In this study, Rs of Abies nephrolepis forest in Wutai Mountain showed a significant exponential correlation (P < 0.001) with soil temperature and reached the peak in July in both years because of the highest soil temperature. Soil temperature explained 76.2% of the growing season change in Rs across the four altitudes, which is consistent with previous studies [27]. Rs had a mean of 2.58 μmol·m–2·s–1 throughout all measuring seasons, which was higher than the annual average forest Rs of 2.21 μmol·m–2·s–1 in China and 2.24 μmol·m–2·s–1 around the world [28]. This is mainly due to the excluded Rs during the non-growing season in our study.
Global warming profoundly affects the distribution, structure and function of the terrestrial ecosystem [29]. Temperature sensitivity of Rs (Q10) is a key parameter in reflecting the feedback relationship between the terrestrial C cycle and climate change, especially in vegetation ecosystems located in the border zone of ecologically fragile areas [30,31]. It has been found that the higher the Q10, the greater impact of climate change on Rs [32,33]. As an infrequent species preferring cold and wet conditions, the Abies nephrolepis community only survives in limited areas with shady slopes and high altitudes on Wutai Mountain. As the altitude increased from 2000 to 2300 m, we found the increased Q10 with a range from 2.39 to 2.71, which is in the mid-lower range of the China forest ecosystems (1.09~5.53) and global forest ecosystems (0.98~8.90) [34]. This result is consistent with previous studies showing that Q10 is higher in high latitude and high altitude areas, indicating that Rs at high altitudes is more susceptible to temperature changes [35]. Thus, soil carbon emissions in high latitude and high altitude areas are more sensitive to temperature variation [36]. Moreover, the correlation (R2) between Rs and soil temperature showed a decreasing trend from 0.815 to 0.725 at 2000 and 2300 m, respectively. This means that there are other factors commonly influencing Rs variation with changing altitudes.
4.2 Soil Water Content Effects on Rs with Altitude
Soil water content directly affects the growth metabolism of plant roots and microorganisms. Indirectly, it changes oxygen diffusion and organic decomposition [37]. In contrast, the soil water content is also regulated by soil temperature, soil properties, soil life events and other factors [38,39]. In the dry season, Rs remains low value because of the less rainfall, closed plant stomata, and weakened microbial activity. Under this condition, increasing moisture can be effectively improved Rs [40]. On the other hand, excessive soil water content seriously restrains Rs due to the shortage of oxygen in soil and the weakened processes of oxidation and decomposition [41]. In most forest ecosystems, the soil water content generally ranges from approximately 50%~80% and explains approximately 5%~20% of Rs variation [16,42]. In this study, Rs showed an obvious opposite trend with changes in the soil water content from August to October over the two years. In 2019, a longer rainy season and heavier rainfall jointly contributed to a high soil water content that exceeded 60% and even a continuous increase to 90%. In 2020, in contrast, the lack of rain and rapidly dropping air temperatures led to a decline in the soil water content. Compared with the results in 2020, the average higher soil water content might have partly contributed to the Rs increase in 2019, especially in September and October.
Across seasons and altitudes, there was a significant (P < 0.001) parabolic correlation between Rs and the soil water content, with the peak Rs occurring at 65.3% soil water content. This indicates that when the optimum range is exceeded, the soil water content will restrict soil respiration instead, especially in mid- and high-altitude areas [41]. Moreover, the soil water content explained only 26.8% of the growing-season change in Rs overall, which means that the soil water content had much smaller effects on Rs than soil temperature [43]. Contrary to the decreased soil temperature, the soil water content increased with elevated altitudes. At the same time, the soil water content at peak Rs also showed increasing trends from 63.52% to 75.61% at 2000 m and 2200 m, respectively, while the correlation (R2) between Rs and the soil water content weakened. These results suggest that the soil water content only makes little roles in regulating Rs variation. Moreover, the climate cools and moistens with the altitude gradient, which is more suitable for the Abies nephrolepis community. However, the Abies nephrolepis community might suffer more negative effects once the climate changes to one that is warm and dry.
4.3 Comprehensive Effects of Soil Temperature and the Soil Water Content on Rs with Altitude
Soil temperature and soil water content have a complex effect on the seasonal changes in Rs, especially in the ecologically fragile areas [44,45]. Scottdenton et al. found that soil temperature in high-altitude forest communities mainly affected inter-season Rs, while the soil water content mainly affected inter-annual Rs [46]. Liu et al. found that throughfall reduction trends to restrain effects of soil warming to Rs in a transitional oak forest [47]. Smith et al. found that Rs is about 20% higher at edge than at the interior in temperate forests [48]. Previous studies also showed that soil temperature and the soil water content accounted for 70~97% of the Rs variation [35,49]. In this study, we consistently constructed a binary quadratic model and found that soil temperature and the soil water content jointly explained 81.8% of the changes in Rs across altitudes and years, which is higher than the individual explanations based on soil temperature (76.2%) or the soil water content (26.8%). This proved that the combined effect of soil temperature and the soil water content is more accurate and reasonable to study soil respiration, and soil temperature is the major factor affecting Rs variation [50]. In addition, the correlation (R2) among Rs, soil temperature and the soil water content declined with increasing altitude. This may indicate that there may be other factors impacting Rs variation in the Abies nephrolepis community.
4.4 Soil Properties Effects on Rs with Altitude
In addition to soil temperature and the soil water content, soil properties are also closely related to Rs variation and have some effects with changing altitudes [51,52]. The RDA results and correlation analyses showed that seven soil properties in this study jointly explained 61.76% and 69.95% of the Rs variation in 2019 and 2020, respectively. Among the four soil chemical properties studied in this experiment, SOC had the closest correlation with Rs variation. Peng et al. has found an increase in Rs with improved SOC, root biomass, or microbial activity [53]. Moreover, both lower soil C:N and lower pH can promote soil microbial metabolism and soil enzyme activity and increase Rs [12]. In this study, SOC (P < 0.01), pH (P < 0.05), TN (P < 0.05) and C/N had negative relationships with Rs variations. This indicates that altitude may have changed the strategies of microbial carbon metabolism in some way. At low altitudes, higher soil temperature contributes to faster carbon decomposition and higher consumption but lower substrate use efficiency of SOC [54]. In contrast, soil SOC at high altitudes is characterized by slower decomposition and less consumption but better substrate use efficiency under cooler conditions [55]. Previous studies also showed that Q10 increases with the increases of SOC, pH and TN [56–58]. Thus, Rs is more sensitive to the changes of soil nutrient properties at higher altitude areas, which is consistent with RDA result about the close relationships between Rs and SOC, pH, and C:N at 2200 m and 2300 m in this study. As a predictor of soil microbial activity, higher SOC is generally corresponding to the higher soil enzyme activity [59]. However, in this study, soil enzyme activity has a significantly (P < 0.01) positive relationship and declined with increasing altitude. This suggests that soil microbial activity may contribute greatly to Rs and is mainly regulated by soil temperature [38,60]. Thus, microbial activity is also an effective index in reflecting Rs response of ecologically sensitive forest ecosystems to climate change [61]. According to results mentioned above, soil CO2 flux in forest ecosystems may be more sensitive to climate change at higher altitude areas, while microbial decomposition of soil carbon sink may be promoted by climate warming especially at lower altitude areas [62,63]. Plant root development, soil microbial metabolism and nutrient cycling processes should be further studied to explain the mechanism of soil carbon flux in forest ecosystems.
In this study, Rs, soil temperature, soil water content, and soil properties of Abies nephrolepis forests at four altitudes were studied over two years on Wutai Mountain, North China. The results showed obvious temporal and spatial variations in Rs, which provided a view on the responses of soil carbon emission of forest communities to changes in climates and soil properties at ecologically fragile region. In addition, this study highlighted that soil temperature had a much greater impact on Rs variation than the soil water content, especially in the high-altitude areas. At the same time, soil organic carbon and soil enzyme activity were also important factors in regulating Rs variation. This demonstrated that climate change can significantly affect microbial carbon decomposition and soil CO2 flux. Thus, forests in high-altitude areas might be more sensitive to climate change. The future long-term research should focus on the microbial effects to soil property and soil carbon metabolism, especially to the forest ecosystems with climate change sensitivity in cold and high-altitude areas.
Acknowledgement: We thank all the reviewers for their helpful remarks; Hongwei Qiu for suggestions and comments on the experimental design and result analysis; Xiaoying Liu, Zhenhua Wang, Huimin Yan, Zhizhong Gao and Xinchao Yang for assistance in the experiment execution; Xinping Zhao and Yongping Gao for experimental plot construction.
Funding Statement: This research was funded by the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi, China (2019L0826).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. van Hees, P. A., Jones, D. L., Finlay, R., Godbold, D. L., Lundström, U. S. (2005). The carbon we do not see-the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: A review. Soil Biology and Biochemistry, 37(1), 1–13. DOI 10.1016/j.soilbio.2004.06.010. [Google Scholar] [CrossRef]
2. Yang, K., Yang, Y., Xu, Z., Wu, Q. (2018). Soil respiration in a subtropical forest of Southwestern China: Components, patterns and controls. PLoS One, 13(9), e0204341. DOI 10.1371/journal.pone.0204341. [Google Scholar] [CrossRef]
3. Kuzyakov, Y. V., Larionova, A. A. (2006). Contribution of rhizomicrobial and root respiration to the CO2 emission from soil (A Review). Eurasian Soil Science, 39(7), 753–764. DOI 10.1134/S106422930607009X. [Google Scholar] [CrossRef]
4. Deng, Q., Zhang, D., Han, X., Chu, G., Zhang, Q. et al. (2018). Changing rainfall frequency rather than drought rapidly alters annual soil respiration in a tropical forest. Soil Biology and Biochemistry, 121(7), 8–15. DOI 10.1016/j.soilbio.2018.02.023. [Google Scholar] [CrossRef]
5. Pan, Y., Birdsey, R. A., Fang, J., Houghton, R., Kauppi, P. E. et al. (2011). A large and persistent carbon sink in the world’s forests. Science, 333(6045), 988–993. DOI 10.1126/science.1201609. [Google Scholar] [CrossRef]
6. Bispo, A., Andersen, L., Angers, D. A., Bernoux, M., Brossard, M. et al. (2017). Accounting for carbon stocks in soils and measuring GHGs emission fluxes from soils: Do we have the necessary standards? Frontiers in Environmental Science, 5, 41. DOI 10.3389/fenvs.2017.00041. [Google Scholar] [CrossRef]
7. Bond-Lamberty, B. (2018). New techniques and data for understanding the global soil respiration flux. Earth’s Future, 6(9), 1176–1180. DOI 10.1029/2018EF000866. [Google Scholar] [CrossRef]
8. Ohmura, A. (2012). Enhanced temperature variability in high-altitude climate change. Theoretical and Applied Climatology, 110(4), 499–508. DOI 10.1007/s00704-012-0687-x. [Google Scholar] [CrossRef]
9. Dahri, Z. H., Ludwig, F., Moors, E., Ahmad, S., Ahmad, B. et al. (2021). Climate change and hydrological regime of the high-altitude Indus basin under extreme climate scenarios. Science of the Total Environment, 768, 144467. DOI 10.1016/j.scitotenv.2020.144467. [Google Scholar] [CrossRef]
10. Li, W., Bai, Z., Jin, C., Zhang, X., Guan, D. et al. (2017). The influence of tree species on small scale spatial heterogeneity of soil respiration in a temperate mixed forest. Science of the Total Environment, 590(G4), 242–248. DOI 10.1016/j.scitotenv.2017.02.229. [Google Scholar] [CrossRef]
11. Tian, Q., Wang, D., Tang, Y., Li, Y., Wang, M. et al. (2019). Topographic controls on the variability of soil respiration in a humid subtropical forest. Biogeochemistry, 145(1), 177–192. DOI 10.1007/s10533-019-00598-x. [Google Scholar] [CrossRef]
12. Lu, S. B., Xu, Y., Fu, X. P., Xiao, H., Ding, W. et al. (2019). Patterns and drivers of soil respiration and vegetation at different altitudes in southern China. Applied Ecology and Environmental Research, 17(2), 3097–3106. DOI 10.15666/aeer/1702_30973106. [Google Scholar] [CrossRef]
13. Buchmann, N. (2000). Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biology and Biochemistry, 32(11–12), 1625–1635. DOI 10.1016/S0038-0717(00)00077-8. [Google Scholar] [CrossRef]
14. Unger, M., Leuschner, C., Homeier, J. (2010). Variability of indices of macronutrient availability in soils at different spatial scales along an elevation transect in tropical moist forests (NE Ecuador). Plant and Soil, 336(1), 443–458. DOI 10.1007/s11104-010-0494-z. [Google Scholar] [CrossRef]
15. Kim, D. G., Vargas, R., Bond-Lamberty, B., Turetsky, M. R. (2012). Effects of soil rewetting and thawing on soil gas fluxes: A review of current literature and suggestions for future research. Biogeosciences, 9(7), 2459–2483. DOI 10.5194/bg-9-2459-2012. [Google Scholar] [CrossRef]
16. Mo, W., Lee, M. S., Uchida, M., Inatomi, M., Saigusa, N. et al. (2005). Seasonal and annual variations in soil respiration in a cool-temperate deciduous broad-leaved forest in Japan. Agricultural and Forest Meteorology, 134(1–4), 81–94. DOI 10.1016/j.agrformet.2005.08.015. [Google Scholar] [CrossRef]
17. Goodrick, I., Connor, S., Bird, M. I., Nelson, P. N. (2016). Emission of CO2 from tropical riparian forest soil is controlled by soil temperature, soil water content and depth to water table. Soil Research, 54(3), 311–320. DOI 10.1071/SR15040. [Google Scholar] [CrossRef]
18. Kim, G. S., Joo, S. J., Lee, C. S. (2020). Seasonal variation of soil respiration in the Mongolian oak (Quercus mongolica Fisch. Ex Ledeb.) forests at the cool temperate zone in Korea. Forests, 11(9), 984. DOI 10.3390/f11090984. [Google Scholar] [CrossRef]
19. Chen, S., Zou, J., Hu, Z., Chen, H., Lu, Y. (2014). Global annual soil respiration in relation to climate, soil properties and vegetation characteristics: Summary of available data. Agricultural and Forest Meteorology, 198(6), 335–346. DOI 10.1016/j.agrformet.2014.08.020. [Google Scholar] [CrossRef]
20. Aitkenhead, J. A., McDowell, W. H. (2000). Soil C:N ratio as a predictor of annual riverine DOC flux at local and global scales. Global Biogeochemical Cycles, 14(1), 127–138. DOI 10.1029/1999GB900083. [Google Scholar] [CrossRef]
21. Merino, C., Godoy, R., Matus, F. (2016). Soil enzymes and biological activity at different levels of organic matter stability. Journal of Soil Science and Plant Nutrition, 16(1), 14–30. DOI 10.4067/S0718-95162016005000002. [Google Scholar] [CrossRef]
22. Shi, W. Y., Tateno, R., Zhang, J. G., Wang, Y. L., Yamanaka, N. et al. (2011). Response of soil respiration to precipitation during the dry season in two typical forest stands in the forest-grassland transition zone of the Loess Plateau. Agricultural and Forest Meteorology, 151(7), 854–863. DOI 10.1016/j.agrformet.2011.02.003. [Google Scholar] [CrossRef]
23. Woo, L. S., Hoon, Y. B., Don, H. S., Ho, S. J., Joo, L. J. (2008). Genetic variation in natural populations of Abies nephrolepis Max. in South Korea. Annals of Forest Science, 65(3), 1. DOI 10.1051/forest:2008006. [Google Scholar] [CrossRef]
24. Wang, J., Zhang, C., Xia, F., Zhao, X., Wu, L. et al. (2011). Biomass structure and allometry of Abies nephrolepis (Maxim) in Northeast China. Silva Fennica, 45(2), 211–226. [Google Scholar]
25. Yu, L., Wang, H., Wang, Y., Zhang, Z., Chen, L. et al. (2020). Temporal variation in soil respiration and its sensitivity to temperature along a hydrological gradient in an alpine wetland of the Tibetan Plateau. Agricultural and Forest Meteorology, 282(4), 107854. DOI 10.1016/j.agrformet.2019.107854. [Google Scholar] [CrossRef]
26. Masyagina, O. V., Evgrafova, S. Y., Menyailo, O. V., Mori, S., Koike, T. et al. (2021). Age-dependent changes in soil respiration and associated parameters in Siberian permafrost larch stands affected by wildfire. Forests, 12(1), 107. DOI 10.3390/f12010107. [Google Scholar] [CrossRef]
27. Mayer, M., Sandén, H., Rewald, B., Godbold, D. L., Katzensteiner, K. (2017). Increase in heterotrophic soil respiration by temperature drives decline in soil organic carbon stocks after forest windthrow in a mountainous ecosystem. Functional Ecology, 31(5), 1163–1172. DOI 10.1111/1365-2435.12805. [Google Scholar] [CrossRef]
28. Chen, S., Huang, Y., Zou, J., Shen, Q., Hu, Z. et al. (2010). Modeling interannual variability of global soil respiration from climate and soil properties. Agricultural and Forest Meteorology, 150(4), 590–605. DOI 10.1016/j.agrformet.2010.02.004. [Google Scholar] [CrossRef]
29. Steinbauer, M. J., Grytnes, J. A., Jurasinski, G., Kulonen, A., Lenoir, J. et al. (2018). Accelerated increase in plant species richness on mountain summits is linked to warming. Nature, 556(7700), 231–234. DOI 10.1038/s41586-018-0005-6. [Google Scholar] [CrossRef]
30. Bond-Lamberty, B., Bailey, V. L., Chen, M., Gough, C. M., Vargas, R. (2018). Globally rising soil heterotrophic respiration over recent decades. Nature, 560(7716), 80–83. DOI 10.1038/s41586-018-0358-x. [Google Scholar] [CrossRef]
31. Li, J., Pendall, E., Dijkstra, F. A., Nie, M. (2020). Root effects on the temperature sensitivity of soil respiration depend on climatic condition and ecosystem type. Soil and Tillage Research, 199, 104574. DOI 10.1016/j.still.2020.104574. [Google Scholar] [CrossRef]
32. Yan, T., Song, H., Wang, Z., Teramoto, M., Wang, J. et al. (2019). Temperature sensitivity of soil respiration across multiple time scales in a temperate plantation forest. Science of the Total Environment, 688(2), 479–485. DOI 10.1016/j.scitotenv.2019.06.318. [Google Scholar] [CrossRef]
33. Chen, S., Wang, J., Zhang, T., Hu, Z. (2020). Climatic, soil, and vegetation controls of the temperature sensitivity (Q10) of soil respiration across terrestrial biomes. Global Ecology and Conservation, 22(3), e00955. DOI 10.1016/j.gecco.2020.e00955. [Google Scholar] [CrossRef]
34. Sun, H., Zhou, G., Xu, Z., Wang, Y., Liu, X. et al. (2020). Temperature sensitivity increases with decreasing soil carbon quality in forest ecosystems across northeast China. Climatic Change, 160(3), 1–12. DOI 10.1007/s10584-019-02650-z. [Google Scholar] [CrossRef]
35. Ma, M., Zang, Z., Xie, Z., Chen, Q., Xu, W. et al. (2019). Soil respiration of four forests along elevation gradient in northern subtropical China. Ecology and Evolution, 9(22), 12846–12857. DOI 10.1002/ece3.5762. [Google Scholar] [CrossRef]
36. Zimmermann, M., Davies, K., Zimmermann, V. P., Bird, M. I. (2015). Impact of temperature and moisture on heterotrophic soil respiration along a moist tropical forest gradient in Australia. Soil Research, 53(3), 286–297. DOI 10.1071/SR14217. [Google Scholar] [CrossRef]
37. Yan, L., Chen, S., Huang, J., Lin, G. (2010). Differential responses of auto-and heterotrophic soil respiration to water and nitrogen addition in a semiarid temperate steppe. Global Change Biology, 16(8), 2345–2357. DOI 10.1111/j.1365-2486.2009.02091.x. [Google Scholar] [CrossRef]
38. Niklinska, M., Klimek, B. (2007). Effect of temperature on the respiration rate of forest soil organic layer along an elevation gradient in the Polish Carpathians. Biology and Fertility of Soils, 43(5), 511–518. DOI 10.1007/s00374-006-0129-y. [Google Scholar] [CrossRef]
39. Meisner, A., Bååth, E., Rousk, J. (2013). Microbial growth responses upon rewetting soil dried for four days or one year. Soil Biology and Biochemistry, 66, 188–192. DOI 10.1016/j.soilbio.2013.07.014. [Google Scholar] [CrossRef]
40. Rubio, V. E., Detto, M. (2017). Spatiotemporal variability of soil respiration in a seasonal tropical forest. Ecology and Evolution, 7(17), 7104–7116. DOI 10.1002/ece3.3267. [Google Scholar] [CrossRef]
41. Huang, Y. H., Hung, C. Y., Lin, I. R., Kume, T., Menyailo, O. V. et al. (2017). Soil respiration patterns and rates at three Taiwanese forest plantations: Dependence on elevation, temperature, precipitation, and litterfall. Botanical Studies, 58(1), 1–12. DOI 10.1186/s40529-017-0205-7. [Google Scholar] [CrossRef]
42. Jassal, R. S., Black, T. A., Novak, M. D., Gaumont-Guay, D. A. V. I. D., Nesic, Z. (2008). Effect of soil water stress on soil respiration and its temperature sensitivity in an 18-year-old temperate Douglas-fir stand. Global Change Biology, 14(6), 1305–1318. DOI 10.1111/j.1365-2486.2008.01573.x. [Google Scholar] [CrossRef]
43. Han, M., Jin, G. (2018). Seasonal variations of Q10 soil respiration and its components in the temperate forest ecosystems, Northeastern China. European Journal of Soil Biology, 85, 36–42. DOI 10.1016/j.ejsobi.2018.01.001. [Google Scholar] [CrossRef]
44. Shi, B., Gao, W., Cai, H., Jin, G. (2016). Spatial variation of soil respiration is linked to the forest structure and soil parameters in an old-growth mixed broadleaved-Korean pine forest in Northeastern China. Plant and Soil, 400(1), 263–274. DOI 10.1007/s11104-015-2730-z. [Google Scholar] [CrossRef]
45. Li, G., Kim, S., Han, S. H., Chang, H., Son, Y. (2017). Effect of soil moisture on the response of soil respiration to open-field experimental warming and precipitation manipulation. Forests, 8(3), 56. DOI 10.3390/f8030056. [Google Scholar] [CrossRef]
46. Scott-Denton, L. E., Sparks, K. L., Monson, R. K. (2003). Spatial and temporal controls of soil respiration rate in a high-elevation, subalpine forest. Soil Biology and Biochemistry, 35(4), 525–534. DOI 10.1016/S0038-0717(03)00007-5. [Google Scholar] [CrossRef]
47. Liu, Y., Liu, S., Wan, S., Wang, J., Luan, J. et al. (2016). Differential responses of soil respiration to soil warming and experimental throughfall reduction in a transitional oak forest in Central China. Agricultural and Forest Meteorology, 226(5), 186–198. DOI 10.1016/j.agrformet.2016.06.003. [Google Scholar] [CrossRef]
48. Smith, I. A., Hutyra, L. R., Reinmann, A. B., Thompson, J. R., Allen, D. W. (2019). Evidence for edge enhancements of soil respiration in temperate forests. Geophysical Research Letters, 46(8), 4278–4287. DOI 10.1029/2019GL082459. [Google Scholar] [CrossRef]
49. Rey, A., Pegoraro, E., Tedeschi, V., de Parri, I., Jarvis, P. G. et al. (2002). Annual variation in soil respiration and its components in a coppice oak forest in Central Italy. Global Change Biology, 8(9), 851–866. DOI 10.1046/j.1365-2486.2002.00521.x. [Google Scholar] [CrossRef]
50. Hu, T., Sun, L., Hu, H., Weise, D. R., Guo, F. (2017). Soil respiration of the Dahurian Larch (Larix gmelinii) forest and the response to fire disturbance in Da Xing’an Mountains. China Scientific Reports, 7(1), 1–10. DOI 10.1038/s41598-017-03325-4. [Google Scholar] [CrossRef]
51. Chen, D., Yu, M., González, G., Zou, X., Gao, Q. (2017). Climate impacts on soil carbon processes along an elevation gradient in the tropical Luquillo experimental forest. Forests, 8(3), 90. DOI 10.3390/f8030090. [Google Scholar] [CrossRef]
52. Wang, Y., Liu, S., Wang, J., Chang, S. X., Luan, J. et al. (2020). Microbe-mediated attenuation of soil respiration in response to soil warming in a temperate oak forest. Science of the Total Environment, 711(7), 134563. DOI 10.1016/j.scitotenv.2019.134563. [Google Scholar] [CrossRef]
53. Peng, Y., Song, S. Y., Li, Z. Y., Li, S., Chen, G. T. et al. (2020). Influences of nitrogen addition and aboveground litter-input manipulations on soil respiration and biochemical properties in a subtropical forest. Soil Biology and Biochemistry, 142, 107694. DOI 10.1016/j.soilbio.2019.107694. [Google Scholar] [CrossRef]
54. Li, X., Xie, J., Zhang, Q., Lyu, M., Xiong, X. et al. (2020). Substrate availability and soil microbes drive temperature sensitivity of soil organic carbon mineralization to warming along an elevation gradient in subtropical Asia. Geoderma, 364(3), 114198. DOI 10.1016/j.geoderma.2020.114198. [Google Scholar] [CrossRef]
55. Massaccesi, L., Feudis, M. D., Leccese, A., Agnelli, A. (2020). Altitude and vegetation affect soil organic carbon, basal respiration and microbial biomass in Apennine forest soils. Forests, 11(6), 710. DOI 10.3390/f11060710. [Google Scholar] [CrossRef]
56. Zhou, T., Shi, P. J., Hui, D. F., Luo, Y. Q. (2009). Global pattern of temperature sensitivity of soil heterotrophic respiration (Q10) and its implications for carbon-climate feedback. Journal of Geophysical Research, 114(G2), 271–274. DOI 10.1029/2008JG000850. [Google Scholar] [CrossRef]
57. Liu, Y., He, N. P., Zhu, J. X., Li, X., Yu, G. R. et al. (2017). Regional variation in the temperature sensitivity of soil organic matter decomposition in China’s forests and grasslands. Global Change Biology, 23(8), 3393–3402. DOI 10.1111/gcb.13613. [Google Scholar] [CrossRef]
58. Zhao, J. X., Li, R. C., Li, X., Tian, L. X. (2017). Environmental controls on soil respiration in alpine meadow along a large altitudinal gradient on the central Tibetan Plateau. Catena, 159, 84–92. DOI 10.1016/j.catena.2017.08.007. [Google Scholar] [CrossRef]
59. Wallenius, K., Rita, H., Mikkonen, A., Lappi, K., Lindström, K. et al. (2011). Effects of land use on the level: Variation and spatial structure of soil enzyme activities and bacterial communities. Soil Biology and Biochemistry, 43(7), 1464–1473. DOI 10.1016/j.soilbio.2011.03.018. [Google Scholar] [CrossRef]
60. Xu, Z., Yu, G., Zhang, X., Ge, J., He, N. et al. (2015). The variations in soil microbial communities, enzyme activities and their relationships with soil organic matter decomposition along the northern slope of Changbai Mountain. Applied Soil Ecology, 86, 19–29. DOI 10.1016/j.apsoil.2014.09.015. [Google Scholar] [CrossRef]
61. Karhu, K., Auffret, M. D., Dungait, J. A., Hopkins, D. W., Prosser, J. I. et al. (2014). Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature, 513(7516), 81–84. DOI 10.1038/nature13604. [Google Scholar] [CrossRef]
62. Li, Y., Zhou, G., Huang, W., Liu, J., Fang, X. (2016). Potential effects of warming on soil respiration and carbon sequestration in a subtropical forest. Plant and Soil, 409(1), 247–257. DOI 10.1007/s11104-016-2966-2. [Google Scholar] [CrossRef]
63. Chen, J., Luo, Y., García-Palacios, P., Cao, J., Dacal, M. et al. (2018). Differential responses of carbon-degrading enzyme activities to warming: Implications for soil respiration. Global Change Biology, 24(10), 4816–4826. DOI 10.1111/gcb.14394. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |