

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020913
REVIEW
Opportunities and Challenges of Plant Bioactive Compounds for Food and Agricultural-Related Areas
1Laboratory of Agroindustrial Processes Engineering, Federal University of Santa Maria, Cachoeira do Sul, 96508-010, Brazil
2Department of Teaching, Research and Development, Sul Rio-Grandense Federal Institute, Charqueadas, 96745-000, Brazil
3Department of Food Engineering, Regional Integrated University of Upper Uruguay and Missions, Erechim, 99709-910, Brazil
*Corresponding Author: Marcus V. Tres. Email: marcus.tres@ufsm.br
Received: 19 December 2021; Accepted: 25 January 2022
Abstract: The growing development of biological products highlights the social and environmental responsibility that several industrial companies are facing in recent years. In this context, the advancement of bioprocessing as an alternative for exploring the potential of ecologically based products, especially in biofuels, food, and agro-industrial business, exposes the rational efficiency of the application of renewable sources in different industrial segments. Industries strongly associated with food production concentrate large amounts of wastes rich in bioactive compounds. A range of highly effective technologies has been highly explored to recover large concentrations of prominent compounds present in these materials. The advances in this scenario assurance value addition to these by-products, in addition to highlighting their various technological applications, considering the biorefinery and ecologically based production concepts. Accordingly, this review article described a detailed and systematic approach to the importance of using bioactive compounds and exploring the main sources of these elements. Also, some recent and innovative research that has achieved encouraging results was highlighted. Furthermore, the study included the main extraction technologies that have been investigated as a strategy of prospecting the application of bioactive compounds and optimizing the processes for obtaining natural compounds from plant sources. Finally, future outlooks were presented to contribute to the innovative opportunities and applicability of highly promising technologies and manipulations of bioactive compounds from a range of perspectives.
Keywords: Biological security; renewable biological resources; sustainable production
Bioactive compounds are substances with strong action potential synthesized by the secondary metabolism of plants. These components are extremely important since their production is closely related to plant responses to imposed diversities over time [1]. A diversity of compounds is detected in large amounts in plant materials such as flowers and fruits, vegetables, nut-based, leaves, roots, etc. [2–6]. Additionally, recent studies have shown plant wastes as valuable sources of biocompounds applicable for various purposes [7,8]. These compounds accumulate and large concentrations can be extracted from plant matrices, attending the human requirements and adding value to these materials [9]. This scenario favors a spectrum of opportunities involving the functional application of these components in a range of fields of study [8]. Furthermore, this promising scenario is closely associated with the physicochemical characteristics of the compounds present and their key functional properties.
Plants constitute a range of biocompounds, such as phenolics, flavonoids, anthocyanins, terpenes, and terpenoids, etc. [10–13]. These elements have a high potential for application and are widely explored for their various benefits to human health and the environment. Natural action such as antioxidants, anticancer, anti-inflammatory, and antidiabetic has been investigated and proposes phytochemicals as supplements to the natural requirements of the human body [9,14]. Accordingly, Fig. 1 shows a representation of notable bioactive compounds identified in the shoot and root organs of plants. As previously discussed, these constituents have a high action in combating health disorders and preserving and increasing nutritional properties.
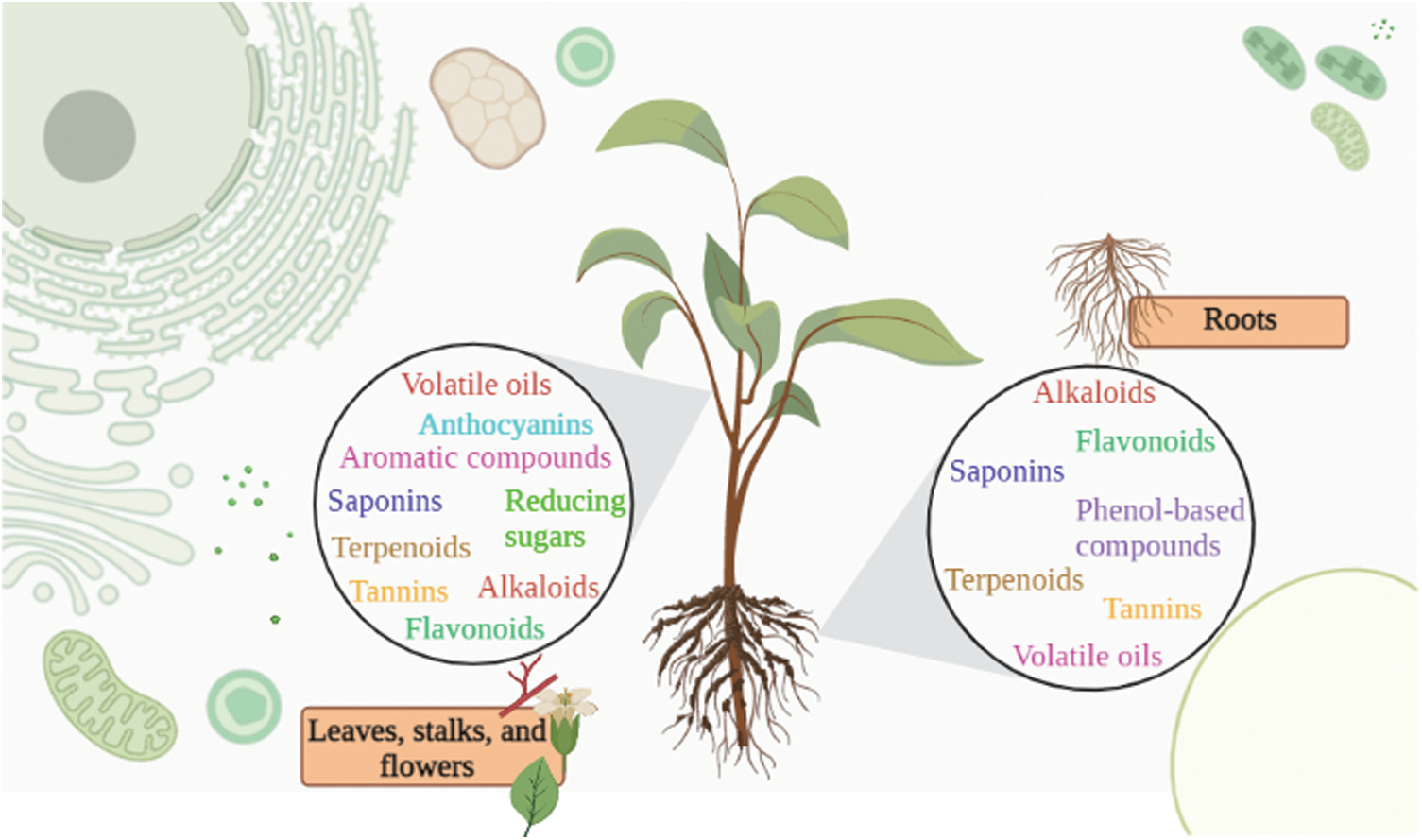
Figure 1: Dominant bioactive compounds present in aerial (leaves, stalks, and flowers) and root organs of plants
Some investigative studies have focused on the action of compounds as mechanisms against the action of viruses causing a range of infections [15]. One of these diseases is the coronavirus, caused by the SARS-CoV-2 virus. One of the main challenges has been to verify the potential of these bioactive compounds as antiviral agents, which has not yet been comprehensively investigated. Bioactive compounds obtained from plants can be used in the form of extracts or essential oils for the prevention or treatment of symptoms caused by infections and diseases. Appropriately, several studies have found the use of bioactive compounds as fundamental components in biotechnology-oriented industries, mainly those directed exclusively to food-related and agricultural purposes. Therefore, investigations have demonstrated the implementation of bioactive compounds as active agents for the preservation and escalation of food quality, increase in the nutritional value of foods, and plant defense, etc. [16–19].
Nonetheless, considering the intensification of population growth and the gradual increase in food demand, several challenges have been considered in the production and maintenance of these elements in the process of exploration of the matrices. One of the main obstacles is the prevalence of healthy eating, which has been an important priority for the population recently. The prevalence of cardiovascular diseases and the emergence of new viral diseases, such as the coronavirus, have stimulated the investigation of plant compounds with potential action against these infections [20]. Also, these challenges have been in constant expansion by the target of research by increasing the shelf-life of the compounds as a strategy to increase their potential [16]. The direct adoption of technologies that increase the production potential of bioactive metabolites, metabolic pathways strategies as precursors in the synthesis of phytocompounds, and in the combination of the performance of different abiotic agents as modelers of morphological and, consequently, the biochemical performance of plants have been significant examples regarding the expansion of promising alternatives for the production of biocompounds [21–23]. Moreover, the evolution of vegetarianism and veganism has been a key element in the development of plant foods, and the increase in consumption of this type of food reports the necessity for innovative strategies that meet new demands [24,25]. Plant bioactive compounds serve as a source of a range of components of considerable health-promoting and notable disease-prevention action, such as proteins, carotenoids, phenolic compounds, etc. [26]. Nevertheless, technological advances that promote large-scale production and generate high interest in the consumer market are demanded. Many of these strategies involve the action of ultrasound equipment, subcritical fluids, supercritical fluids, and enzyme-based extraction [27–30]. These technological advances promote higher concentrations than conventional methods such as Soxhlet extraction, maceration, and reflux methods, mainly due to the action of high pressures, temperatures and consequently, higher penetration of solvents into the plant matrix and prominent extraction of compounds [31]. Accordingly, an economic parameterization is valid, especially when it comes to the association of cost, sensory properties, and nutritional description [24]. Furthermore, a precise configuration of the inputs considered in the extraction procedure, such as temperature, extraction time, solvent characteristics, and pressure is indispensable for process optimization and high productivity [32]. This scenario is evidence of the impacts involving the exploration and systemic consumption of predominantly plant-based compounds and reports the opportunities of these elements in relation to other types of compounds.
Moreover, a precise investigation into the quantitative performance of studies predominantly focused on the application of plant compounds is encouraging and allows us to verify the evolution of the importance and attention given to the subject in recent years. The plant bioactive compounds have been widely investigated as an innovative and sustainable alternative for food and agricultural-related areas. Accordingly, simple research to quantify scientific articles published in the last decade was performed in the Scopus® scientific platform. To conduct the data research, the following keywords were considered: plant/bioactive/compound/food for food and food-related areas (Fig. 2a) and plant/bioactive/compound/agriculture for agriculture and agriculture-related areas (Fig. 2b).
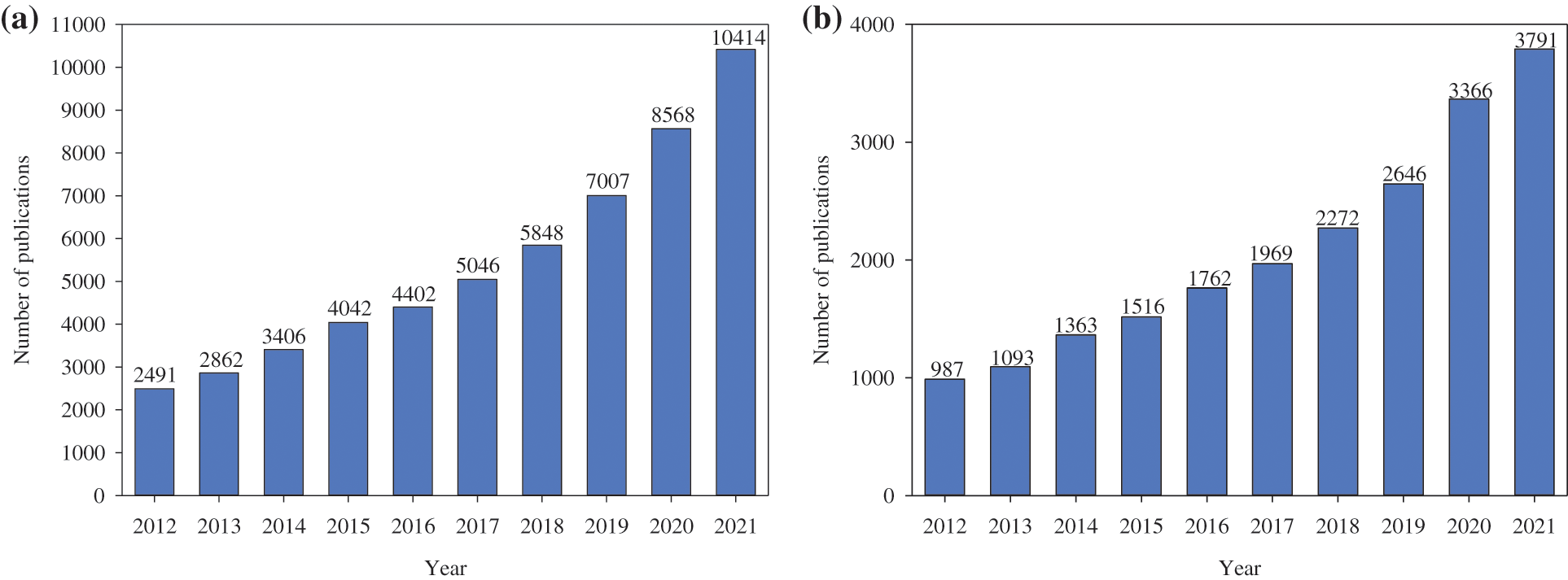
Figure 2: Number of scientific publications on the plant bioactive compounds applied for (a) food and food-related and (b) agriculture and agriculture-related purposes disposed by year from 2012 to 2021 period based on the Scopus® scientific platform according to the following keywords: bioactive/compounds and plant/bioactive/compounds
According to the general research of bioactive compounds, the scientific panorama is widely progressive and promising and presented a significant increase on the subject over the years. Considering the Scopus® database showed that 10414 manuscripts were published in 2021. This scenario is significantly larger than in 2012, where 2491 articles were published. Therefore, the abundance of scientific publications on general bioactive compounds, and particularly the evolution on the subject in the pandemic period 2020 (8568 articles) and 2021 (10414 articles) are significant. This overview indicates the interest of bio-based components in the treatment of comorbidities. Overall, the number of publications grew gradually over the decade, supporting the exploration of these components for application in a range of fields related to distinct fields of study. Moreover, this promising scenario reports the exploration potential for the next few years, since the number of studies on the subject only increased in all the years evaluated in this study.
For plant bioactive compounds applications, the Scopus® database presented about 3791 published manuscripts published in 2021, which represents approximately 36% of the total quantity of scientific studies on bioactive compounds approach published last year. Quantitatively, this number represents approximately 4 times the number of articles published in 2012 (987 articles). This panorama is a role as regards the use of alternative and bio-based strategies from plant sources. Furthermore, as detected for the computation of publications on general bioactive compounds, the scientific expansion on the plant bioactive compounds topic in the pandemic period 2020 (3366 articles) and 2021 (3791 articles) is notable. In general, there was a considerable increase in the application of plant extracts for agricultural principles over the years. As verified for studies based on the application of these compounds, the proposed promising scenario highlights this field as an alternative for exploring the potential of these constituents in the coming years. Additionally, the systemic and gradual adoption of sustainable and biological assumptions has recently been one of the main indications of the development of studies and improvement of methods and technologies that aim at the use of plant bioactive compounds as an interesting strategy.
Furthermore, a global network map of country bibliographic coupling considering the previous Scopus® database research was applied. The VOSviewer visualization provided an exceptional panorama to promote networking information among worldwide collaborators. This collaboration set is formed by clusters, which indicate the specific periodicity of co-occurring items characterizing each country, indicating the co-occurred items into colored clusters (countries with the same color configure a cluster). Moreover, the dimensions of each circle describe the quantification of documents of the specific country, and the thickness of lines that connect the countries represent the proportion aspects of collaboration. The largest set of connected items consisted of 115 countries in 14 clusters. Based on the analysis, the largest cluster comprised 15 countries. Accordingly, Fig. 3 presents the synergic global network of countries that published at least one document from 1974 to 2022 (115 countries). Adequate perception of the link strength comprises the collaboration between India and Morroco, with a total link strength of 2301, representing one line. In contrast, the line between El Salvador and Spain had a link strength of 5. Indian researchers were predominant considering this study. These researchers presented significant collaborations with Saudi Arabia, Spain, the United States, Malaysia, Pakistan, Brazil, South Korea, Turkey and Italy.
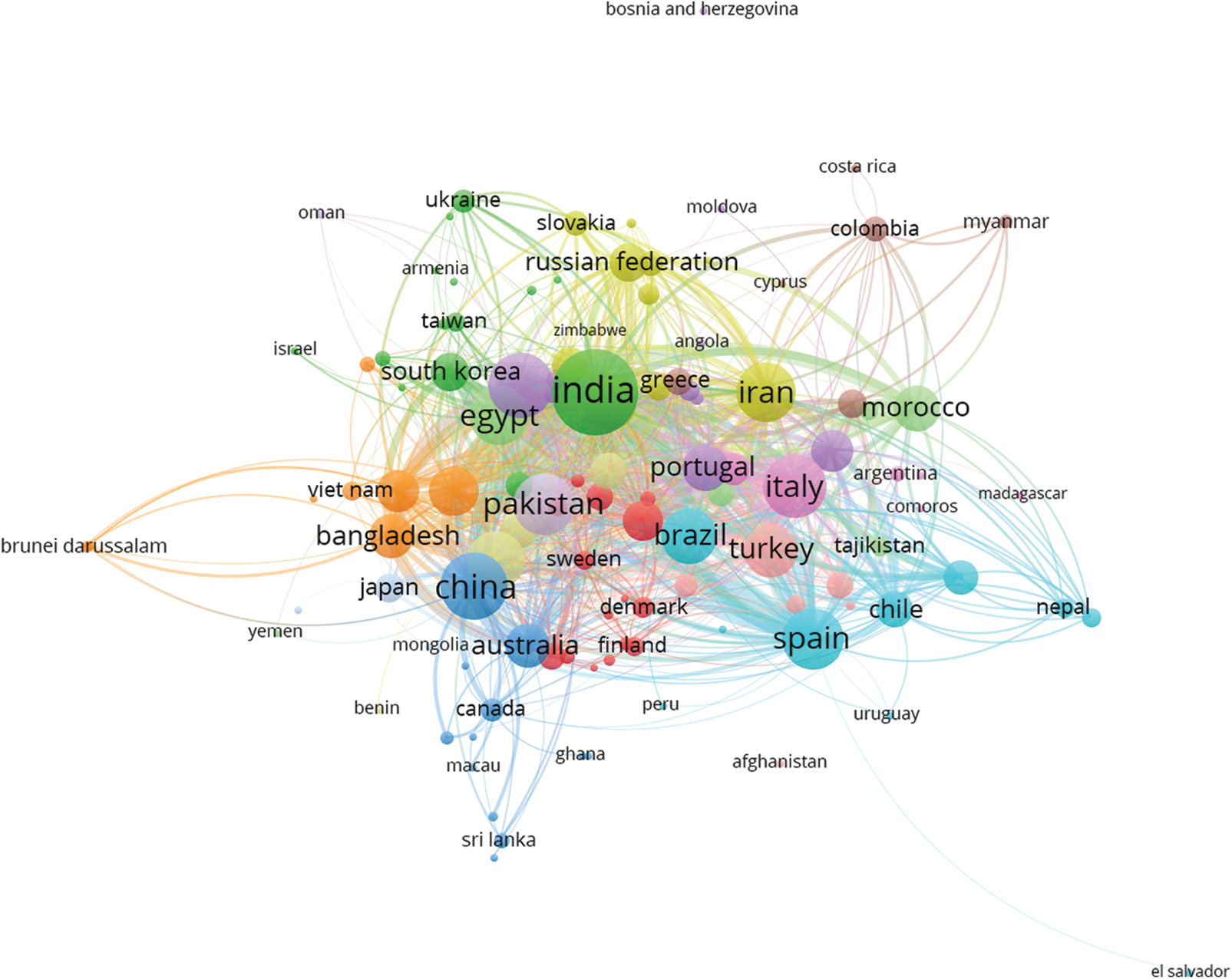
Figure 3: VOSviewer global networks map of country bibliographic coupling considering a minimum of one published document, from 1974 to 2022, based on the Scopus® scientific platform according to the following keywords: plant/bioactive/compounds. 115 countries were considered in 14 clusters; the largest cluster comprised 15 countries
For the parameterization of the scientific studies on the plant bioactive compounds, the network visualization can be attributed to the keywords of each document. The keywords identify the main instruments of a bibliographic research and provide answers and the solution of research problems. As verified for the characterization of the global network mapping, this study delineated the frequency co-occurrence bibliographic map on the set of keywords based on the Scopus® scientific platform, considering 10 as a minimum number of occurrences of a keyword, from 1974 to 2022. Accordingly, 704 keywords were considered for current research. These items formed 6 clusters, where the largest set of items consisted of 264 keywords. The main keywords observed were “non-human”, “plant extract”, “article”, “antioxidant” and “bioactive compounds” (Fig. 4).
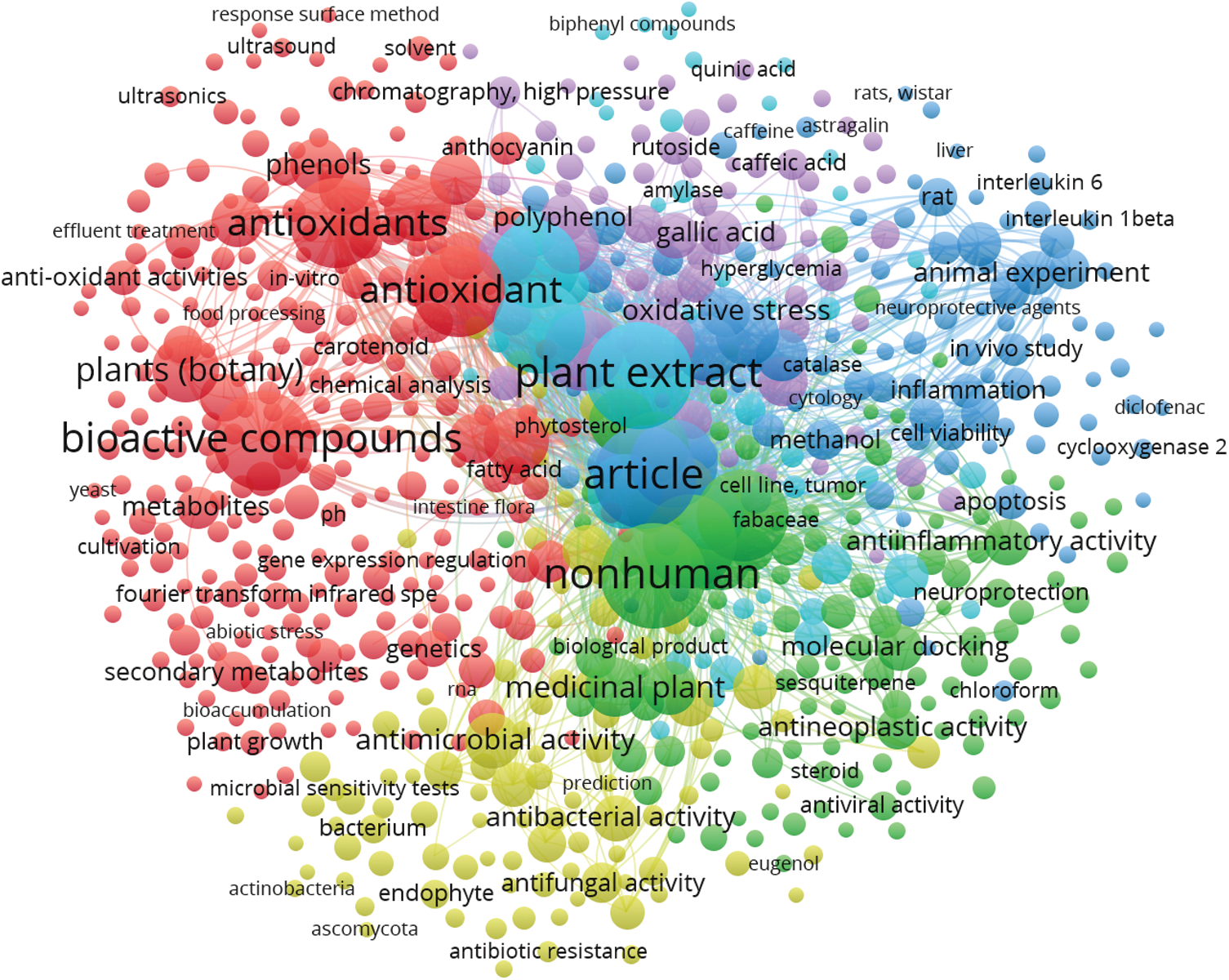
Figure 4: VOSviewer frequency of co-occurrence keywords considering 10 as a minimum number of occurrences of a keyword, from 1974 to 2022, based on the Scopus® scientific platform according to the following keywords: plant/bioactive/compounds. 704 keywords were considered in 6 clusters; the largest cluster comprised 264 items
Appropriately, the purpose of this review was to perform a detailed and systematic review of the importance of plant-based bioactive compounds and explore the main sources of these elements for different fields of application. Furthermore, the main applications, emerging technologies and strategies aimed at adding value to plant-based materials and future perspectives are presented.
Bioactive compounds are the secondary metabolites widely detected in plant tissues and are composed of vitamins, carbohydrates, alkaloids, saponins, polyphenols, gluconates, proteins and total phenolic compounds. Most of these compounds are generally thermolabile and the antioxidant activity of fruit and vegetable extracts is due to their presence [33]. Among the main classifications, flavonoids include anthocyanins, flavanols, flavones and flavanones and compounds that are not flavonoids include tannins, stilbenes and phenolic acids [34,35]. Besides, the scope of employment of bioactive compounds encompasses numerous applications, from medicine and pharmaceutical purposes to weed and pest management (Fig. 5).
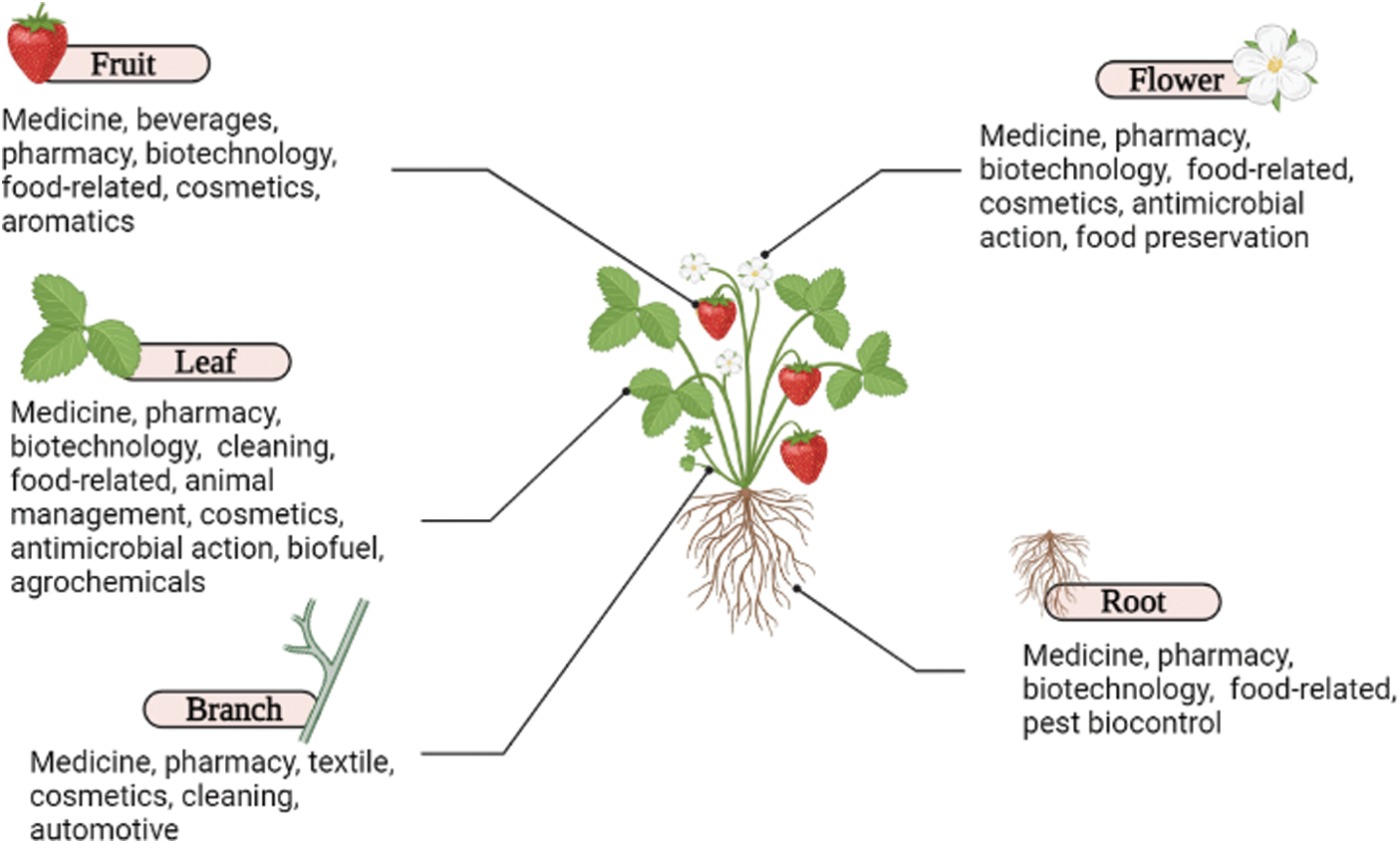
Figure 5: Predominant industrial, agricultural and food-related applications of the diversity of bioactive compounds present in different plant organs
Additionally, antioxidant compounds are orderly categorized as primary and secondary. Primary antioxidants perform as free radical scavengers, delay or even inhibit the initiation step and can interrupt the self-oxidation propagation step. Secondary antioxidants, also called preventatives, offer their antioxidant activity through different mechanisms to decrease the rate of oxidation reactions. Secondary antioxidants generally increase the antioxidant activity of primary antioxidants and, in addition, do not convert free radicals into stable molecules. They act as pro-oxidant metal ion chelators or catalysts, in addition to providing H+ to primary antioxidants, decomposing hydroperoxide to non-radical species, deactivating singlet oxygen, consuming ultraviolet radiation [36]. In general, for protection against oxidative stress to occur, the natural antioxidant protects cells from oxidative stress by obstructing the generation of Reactive Oxygen Species (ROS) [37].
Regarding phenolic compounds, several compounds with biological properties are found in fruits, vegetables and their residues. However, it is believed that the bioactive effect results from the various components responsible for the beneficial biological properties rather than a single chemical class or compound. Furthermore, all possible applications of bioactive compounds from different plant sources have not yet been fully reported. The use of fruits, fruit, or vegetable pulp for scientific research is very popular [38–40]. Recently, there has been spreading attention in transforming waste into value-added products. The extraction of biocompounds directly detected in fruit peels and other residues has been explored and the results are favorable for its use. Other residues are being used as substrates for the production of enzyme-producing microorganisms or other compounds of interest [41–45].
3 Technologies for Extraction of Plant Bioactive Compounds
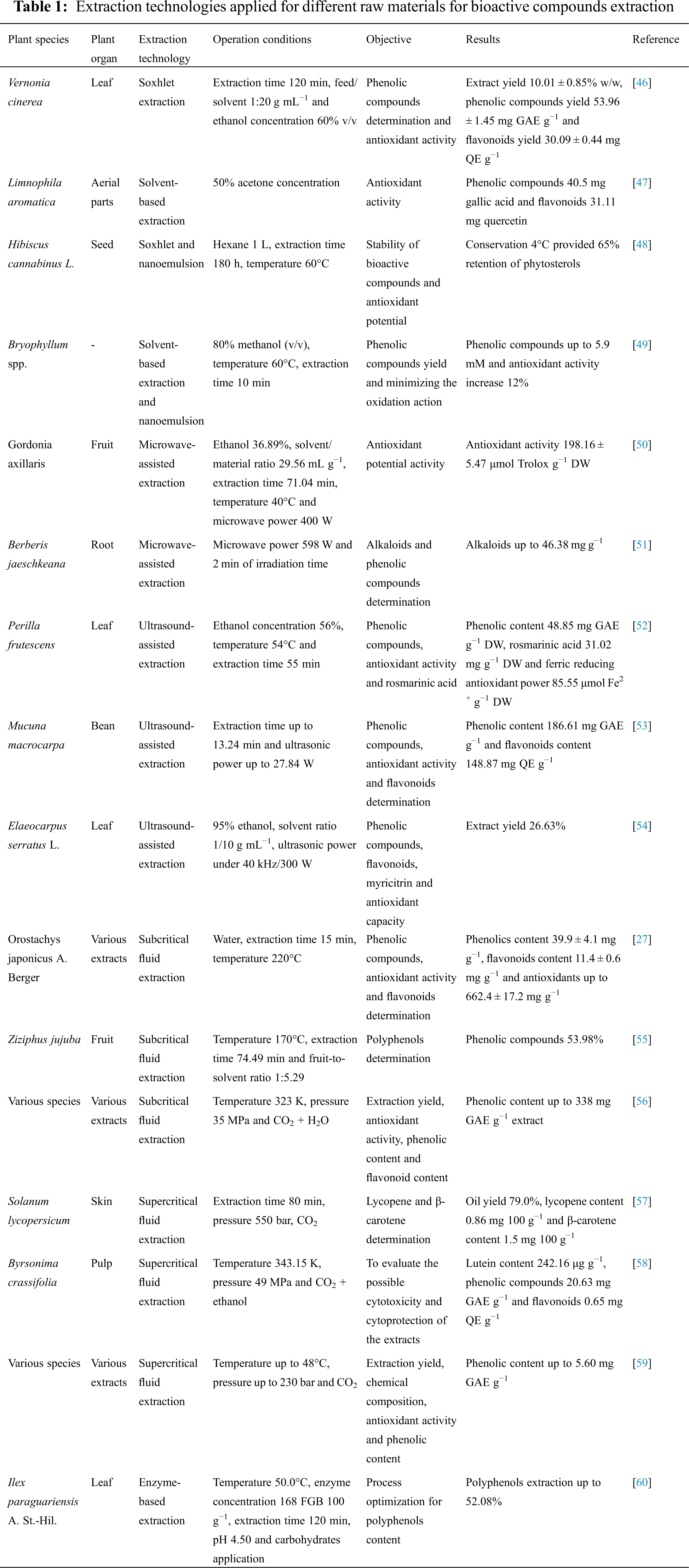
A significant diversity of extraction technologies applied to obtain bioactive compounds from plant matrices is observed in the scientific literature, with their best process conditions and limitations. Conventional methods, such as Soxhlet and the application of chemical solvents are widely investigated over the years. Nonetheless, innovative technologies have emerged as effective strategies to enhance the biocompounds access in plants. Emulsification, microwave and ultrasound-assisted, subcritical and supercritical fluids and enzyme-based extraction are largely employed as trending techniques on the subject.
Properly, the extraction technology and/or integration of technologies are a fundamental component of the compounding process. The search for improved technologies and higher extraction efficiency considers a series of requirements that enable a viable cost/benefit and that do not generate problems of environmental contamination and risks to human health. Accordingly, the intensive use of chemical solvents, difficulties in handling equipment and/or unskilled labor, the high energy demand, the inclination of corrosion or damage to the extraction apparatus, the significant degradation of the matrix to be extracted, has been reported as pertinent concerns when considering the technological process of extraction. Furthermore, recent studies that have aimed to improve concentrations of extracted compounds based on the application of technologies have been considered in the current literature approach. Some of these studies showed highly satisfactory results regarding the feasibility of application in plant matrices for the extraction of compounds of interest perpetuate adding value to a range of plant products and by-products (Table 1).
4 Potential Activity and Applications
Different technologies have been the theme of studies for bioactive compounds recovery from several types of food and food wastes. In the same way, the variety of uses for bioactive compounds also has gained the attention of works involving pharmaceutical and medicinal applications, food, animal feeding, bioenergy generation as well as improvement of nutritional properties of the compounds. Fig. 6 illustrates the potential employments of bioactive compounds addressed in recent studies in different areas. Some of these studies, reporting interesting data, are described in this topic.
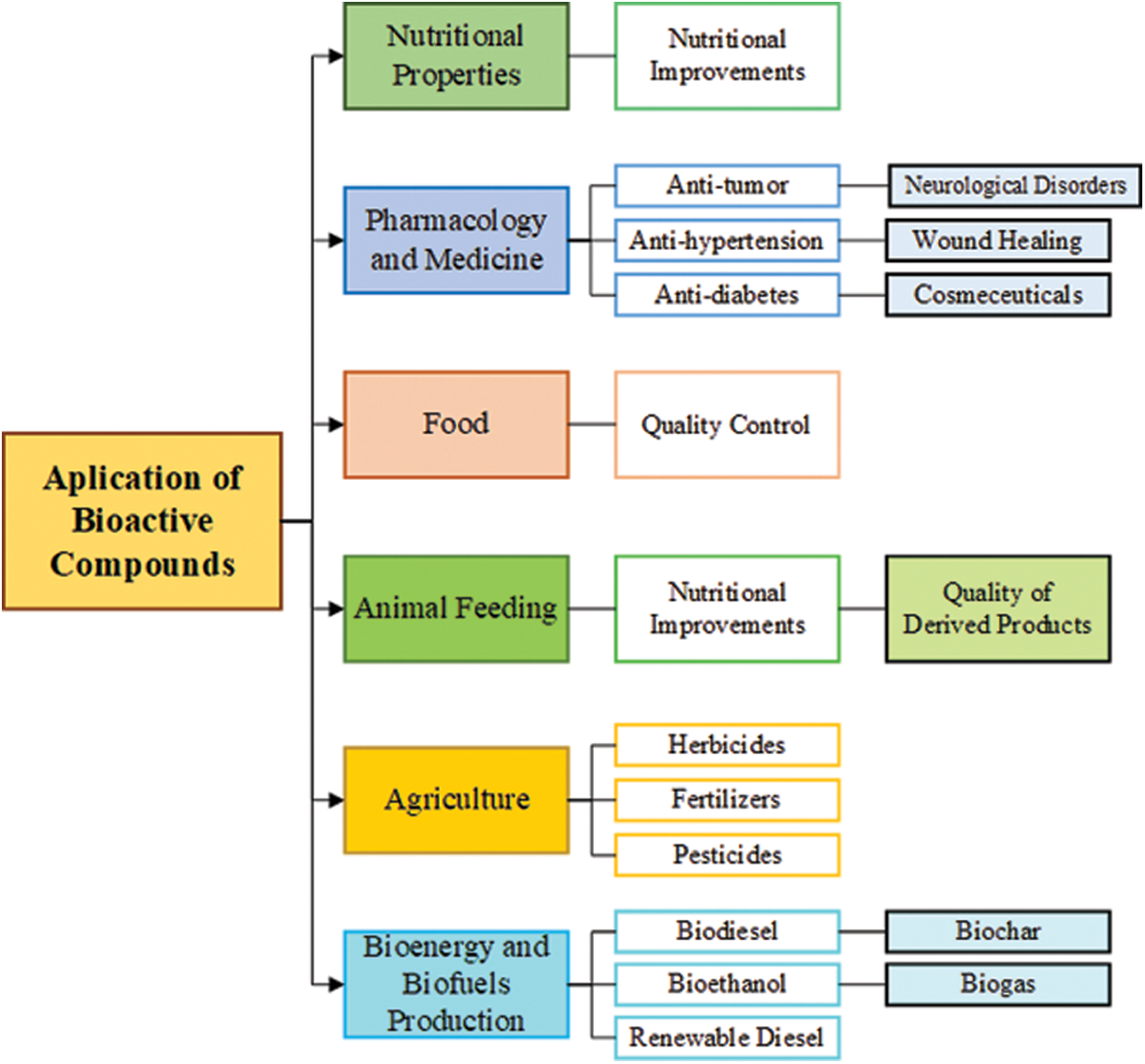
Figure 6: Scheme of the areas with the main applications involved in studies recently published about bioactive compounds
The demand for food with functional characteristics has been increasing in the last years once consumers have been looking for products that have the ability to contribute to improvements in health [61]. Functional foods offer benefits to health when appropriately consumed additionally to their essential nutritional properties [61,62]. Numerous functional foods are rich in bioactive compounds and several studies published have focused on discovering alternative sources of these compounds as well as improving their nutritional properties.
The effects of the application of passion fruit and buriti pulps on probiotic and starter lactic acid bacteria growth on fermented milk was evaluated [63]. Fruit pulp can contribute to enhancing the nutritional properties of fermented milk since it contains high amounts of bioactive compounds, such as phenolic compounds, tannins, flavonoids, among others [64]. According to results reported by these authors, buriti pulp presented higher amounts of biocompounds when compared to passion fruit. The addition of buriti pulp increased the total phenolic compounds in the fermented milk from 33.4% to 141.6%. Moreover, buriti pulp was able to increase the antioxidant activity of the fermented milk, indicating the potential of this material to be used as a functional food.
Moreover, the recovery and concentration of bioactive compounds from jambolan fruit extract by ultra and nanofiltration were investigated for potential nutritional and medical applications [65]. Jambolan–Syzygium cumini (L.)–is a fruit native from India and commonly found in Brazil. The authors used membranes to concentrate the extracts and, consequently, improve the nutritional properties of the bioactive compounds. Concentration factors higher than four times were achieved for anthocyanins, gallic acid and catechin after nanofiltration extraction. The study indicated that membrane technologies are interesting strategies faced to thermal alternatives usually applied to concentrate bioactive compounds since is common for these conventional techniques to be associated with negative effects such as color degradation and loss of nutritional values of the compounds. A similar panorama was verified for cardoon capitula [66]. Cardoon edible parts present high concentrations of free sugars, carbohydrates, oleic and linoleic acids and minerals, such as K, Ca, Mg and Fe. Moreover, even though predominantly applied in the field of bioenergy, the cardoon biocompounds have been explored as components of human food due to their high nutritional value and chemical compounds.
Researches focusing on improving the nutritional properties of foods and other feedstocks rich in bioactive compounds go further, such as extracts from moringa shrubs and trees–a native plant from many tropical and arid countries [67], wheat bran [68], date fruit [69], blueberry and blackcurrant powder [70], Peruvian Andean maize [71], edible seaweeds [72], edible flowers [73], among others. All these feedstocks are rich in bioactive compounds that include carbohydrates, phenolic compounds, oils and fatty acids, proteins and functional peptides with the potential for food products.
Bioactive compounds that present antioxidant activity have the capacity to reduce the oxidative stress that could be involved in health problems such as cancer, obesity, cardiovascular risks, diabetes and neurological disorders [74]. For this reason, several types of research developed in recent years have focused on developing processes for compounds extraction from food and agricultural residues, testing their application aiming to cure diseases and seeking health benefits. Currently, some studies report several types of research that address the extraction of bioactive compounds from fish wastes (skin, bones, frame protein and internal organs) and their application as antioxidative, antihypertensive, antitumor and anticoagulant components [75]. Furthermore, the implications of mango peel ethanolic extracts on colon cancer cell lines was explored [76]. The material characterization indicated the relevance of phenolic compounds that could act as anti-cancer agents. The researches go further, with studies reporting the extraction of bioactive compounds from vine shoots wastes [77], coffee silverskin [78], pomegranate (Punica granatum) peel [79], water chestnut (Trapa natans) peel [80], coconut (Coco nucifera L.) [81], and a plethora of others food/agro-industrial wastes, all focused on pharmacology, medicine and aesthetic applications.
In this context, a study suggested a cost-effective process to extracting antioxidant compounds from Yerba mate (Ilex paraguariensis St. Hil.) waste, a tea-beverage largely consumed in South America [82]. The results reported showed that the content of antioxidant phenolic was higher than most of those usually reported for agro-industrial residues. Using ethanol (50%vol) in the extraction process, a liquid-to-solid ratio of 20 mL g−1 and 180 min, total phenolic content of 63.1 mg of gallic acid equivalents per gram and total flavonoid content of 148.5 mg rutin equivalents were obtained. Additionally, the antioxidant activity of 111.2 mg Trolox equivalents g−1 was achieved.
An investigation on the extraction of bioactive compounds from camu-camu (Myrciaria dubia) seeds, a common fruit from the Amazon region was developed to explore the pharmacological potential of these components [83]. The authors reported that the fruit seed extract (rich in phenolic compounds–mainly vescalagin and castalagin) presented in vitro antioxidant activity, antihypertensive, antimicrobial, antihemolytic, antihyperglycemic and anti-inflammatory effects. Furthermore, the extract demonstrated cytotoxic and antiproliferative potentials against cancer cells.
In another research, the co-culture of breast cancer cell lines (MCF7 and MDA-MB-231) with bromelains extracted from different tissues of the pineapple (fruit pulp, peel, fruit stalk, young stem and mature stem) was evaluated [84]. Treatments for breast cancer have side effects and, according to the authors, the most effective drugs are highly toxic. For this reason, the authors suggested a safe alternative medication, aiming to search for a cure for breast cancer. The results reported that the growth of MCF7 and MDA-MB-231 cells could be inhibited by bromelains, where the fruit stalk bioactive compounds showed promising impediments to the growth of the cancer cells.
Application of bioactive compounds obtained from agro-industrial residues in the food sector and animal feeding involves studies focused on the optimization of different methods for extracting components that improve the organoleptic characteristics of food, as well as to improve the diet of animals aiming to increase the nutritional quality of products generated by them.
Researches recently developed investigated the use of bioactive compounds as materials capable of, at the same time, storing and monitoring the food quality. An example is the manufacture of “smart biodegradable films” compounded of derived food and food wastes (anthocyanins, curcumin, betalains, carotenoids, etc.) with biopolymeric matrices to fabricate biomaterials for monitoring the spoilage and quality of meat products, seafood, milk and others [85]. These biofilms are sensitive to alterations in pH, temperature, CO2, ammonia emission and bacterial activities on foods. Moreover, biodegradable films from cassava starch and glycerol considering different natural extracts of green tea and basil were developed [86]. With the presence of phenolic compounds, the biofilm presented significant antioxidant activity and exhibited color changes according to variations in pH. The same idea was applied to biofilms produced from red pitaya peel for fish meat monitoring [87], eggplant for spoilage indicator of milk [88], and films based on κ-carrageenan incorporated with curcumin for quality monitoring of pork meat [89]. The development of these smart biofilms, in addition to meeting environmental demands arising from society’s concern with the growing of the incorrect disposal of plastics, also meets issues related to consume of food that has its quality monitored throughout the storage period until the time that precedes its consumption.
In another interesting research, the authors evaluated the extraction of bioactive compounds from Brazilian olive leaves using microwave-assisted extraction and the potential of the extract as a food additive with antibacterial and antioxidant activities [90]. Under 100°C, pH 6 and 2 min, it was verified the antioxidant activity of 92.87% and total phenolics 103.87 mg GAE g−1. Additionally, the extracts obtained presented antibacterial activity against Escherichia coli. Despite the interesting results obtained and although the use of microwaves helps the extraction process, intensifying the mass transfer of the operation in general, the 1,000 W of equipment power associated with a temperature of 100°C raises questions about the energy demand of the process and its viability to be implemented in a scale superior to the laboratory.
In relation to animal feeding, bioactive compounds existing on agro-industrial wastes can offer nutritional and functional properties to animal health. Improvements in the antioxidant system, ruminal fermentation, muscle composition and animal performance have been verified due to the reasonable intake of phenolic compounds added to the diet of ruminants [91–94]. Furthermore, the ingestion of bioactive compounds can enhance the quality of derived food products from these animals. Also, the use of artichoke bracts and whole plant silage in the dairy goat diet provided slightly improvement in the mineral and lipid profiles of the milk due to the higher polyunsaturated fatty acids and conjugated linoleic acid contents presents in these compounds [95]. Moreover, a study evaluated the effect of dietary supplementation with dried apple, chokeberry, black currant, strawberry and carrot pomace on meat quality in fattening pigs [96]. The highest oxidative stability and vitamin E content were achieved after supplementation with black currant, a material rich in phenolic compounds, anthocyanins and α-tocopherol.
Besides, it has been reported that a diet rich in phenolic compounds can decrease the methane and nitrogen emissions in ruminants, reducing emissions that have serious impacts [97]. On the other side, it should be highlighted that the exceeding ingestion of food wastes rich in phenolic compounds can reduce nutrient digestibility and the voluntary feed intake in some cases [98].
Regular application of chemical pesticides and herbicides to agricultural management has led to several toxic effects on human health, environment and food as well as facilitated the emergence of weed and pests resistance [99]. These recent concerns have encouraged researches focusing on isolation, quantification and characterization of new bioactive compounds with effectiveness and low toxicity to application in the agricultural sector [100]. Nonetheless, studies show that many plants secrete specific toxins with antimicrobial action [101–103]. One of the main examples is the allelochemicals produced by plants that affect weeds and do not damage crops, due to the difference in sensitivity in the target enzymes or the occurrence of particular receptors in weeds that react directly to the action of secreted biocompounds [104].
Correspondingly, a study investigated the allelopathic capacity of flowers of Lantana camara (Sage-plant), extracted via the methanolic pathway, counter to weeds viz. Rumex dentatus (Knotweed), Avena fatua (Wild oat), Chenopodium album (Goosefoot), Phalaris minor (Canary-grass) and Euphorbia helioscopia (Sun-spurge) [105]. The examination of the bioassays for three methanolic fractions of the Combiflash from Lantana camara was performed at 50 wt%, 75 wt% and 100 wt% of concentration employing parameters of germination percentage, plumule inhibition and radicle size. The second fraction of Combiflash powerfully repressed all weeds with insignificant effects on Triticum aestivum (wheat). The easy dispersion of L. camara seeds results in high reproduction of the species, forming a plant stand that promotes a high release of allelochemicals that act as competitors with the other species. Furthermore, L. camara allelochemicals have been obtained from different parts of the plant and have high concentrations of terpenes, flavonoids, furanonaphoquinones, etc., promoting efficiency in inhibiting a diversity of plant species such as Oryza sativa L. and Brassica juncea L. [106].
Herein, a scientific report examined the herbicidal activity of residues from Brucea javanica (L.) Merr. and its active compounds for weed management [107]. For this proposal, the authors accomplished biotests against the gramineous weed–Eleusine indica (L.) Gaertn.–and the broad-leaved weed–Bidens pilosa L. where it was confirmed that ethyl acetate and n-butanol extracts, containing 4-(9H-b-carbolin-1-yl)-4-oxobut-2-enoic acid methyl ester, bruceines, carisphthalate, pityriacitrin, protocatechuic acid, and vanillic acid, had effective biological activities on seed germination and seedling growth of Eleusine indica at 5 mg⋅mL−1. The results are promising: samples where 31.25 μg mL−1 of bruceine were applied could inhibit completely the root growth of the Eleusine indica; samples, where 125 μg mL−1 of bruceine were used, showed a complete inhibition of the seed germination and shoot elongation of the Eleusine indica. Moreover, in relation to Bidens pilosa, complete inhibition of the shoot growth was observed when 125 μg mL−1 of bruceine was employed.
In terms of pesticides, a study was conducted extensive research exploring twenty-five new matrine-type alkaloid analogs from non-food bioactive compounds–semi-synthesized from matrine isolated from roots of Sophora flavescens Aiton (Fabaceae)–and tested to pesticide applications [107]. According to the data presented, all compounds extracted exhibited pronounced insecticidal and acaricidal activities against M. separata and T. cinnabarinus [do not abbreviate the genus (i.e., M. and T.) if it cited for the first time]. Extracts from the roots and stems of S. flavescens showed a range of alkaloids (matrine, sofocarpine, sophocarpine, and cytisine) of significant insecticidal function [108]. Moreover, the introduction of molecules of the species with groups of 1-pyrrolidinecarbodithioate and diethylcarbamodithioate promoted the increase of the pesticide potential, showing that the combination engineering of biomolecules involving plant extracts is a new strategy to enhance the pesticide activity of these components [109].
Nowadays, the concept of bioenergy through the production of renewable fuels and the use of biomass to generate energy has proved to be an interesting alternative aiming to reduce society’s energy dependence on petrochemical fuels [110–112]. Biofuels such as bioethanol, biodiesel and biogas, and biomass have emerged not only as an alternative energy source but also in relation to environmental issues.
Based on the current scientific approach, there are several examples of the employment of agro-industrial and food wastes, rich in bioactive compounds, with this proposal. A study was conducted to obtain fatty acid methyl esters (FAME) from citrus wax, an agro-industrial waste originating from the purification process of citrus oils [113]. Results demonstrated that citrus wax is an important source of a range of biocompounds, including flavonoids, saponins, carbohydrates, phenolics, and fatty acid esters. Employing extraction with ethanol, the authors obtained value-added products and a “washed wax” that served as feedstock to the FAME synthesis. Using a transesterification reaction in acid media, a mixture of methyl esters was obtained. Based on these results and considering that around 56 tons of citrus wax are generated annually, the authors concluded that the process would allow the production of up to 1.7 tons of biodiesel.
Spent grounds coffee, a material rich in lipids, carbohydrates, carbonaceous, and nitrogen is the focus of studies aiming to synthesize biodiesel, renewable diesel, bioethanol, biochar, and biogas [114–116]. Also, several bioactive compounds can be obtained: polyhydroxyalkanoates, biosorbent, activated carbon, polyol, polyurethane foam, carotenoid, and phenolic antioxidants [117,118]. In terms of biofuel production, a performance of a large-scale in-situ transesterification using spent coffee grounds as raw material to prepare biodiesel (with 4 kg of material) provided 81.8% of FAME yield, achieved after 180 min of reaction [119].
In a similar approach, orange peel waste was used to produce bioethanol besides lactic acid and acetic acid [120]. The management of this material is quite complex, where most of this residue produced from orange juice manufacturing is dumped on landfills causing impacts on the environment [121]. In this scenario, employing an ensiling anaerobic process, these authors obtained the production of several value-added chemical compounds. During this process, anaerobic fermentation occurs reconsidering the necessity for any inoculation or operation. The yields achieved by this research are promising: 120 g kg−1 of total solids to the bioethanol, 55 g kg−1 of total solids to the lactic acid, and 26 g kg−1 of total solids to the acetic acid have been produced.
Furthermore, marigold (Calendula officinalis) wastes–leaves and stem–were explored to produce biogas and bioactive materials (phenolic compounds–vanillin and vanillic acid) [122]. After pretreatment, black liquor was obtained and submitted to a high-pressure oxidation process, with yields of 5.09 wt% and 1.16 wt% for vanillin and vanillic acid, respectively. The remaining solid fraction was fermented through anaerobic digestion, achieving biogas productivity of 1,621.3 mL g−1 of volatile solids.
Another example of the application of agro-industrial/food wastes, rich in bioactive compounds, in biofuels production, includes the preparation of bioethanol from mango peel and pulp, a material with high content of fermentable sugars [123]. Also, an investigation on the bioethanol production from the banana peel, a residue rich in carbohydrates that through fermentation can synthesize the biofuel [124]. Finally, the conduction of bioethanol synthesis (and bromeliad extraction) from pineapple residues, a biomass rich in bioactive compounds (antioxidants), beyond simple and complex sugars that can be used in the fermentation process [125].
Furthermore, agro-industrial/food wastes can be used for the synthesis of catalysts and nanocomposites. Corn, rice, and areca nut husk are some of the examples of raw materials considered residues that can be used in the biofuel production chain [126–129]. For example, Rice Husk (RH) and Rice Husk Ash (RHA) can be used to synthesize silica-based materials into zeolites and MCM-type structures due to the silicon (Si) contained in these co-products. Pedott et al. [130] demonstrated the synthesis of MCM-48 material using ionic liquid as a structure-directing agent employing Si obtained from RH and RHA. Moreover, MCM-type structures can be used in the immobilization of enzymes for use as catalysts in hydrolysis and esterification reactions [131].
5 Conclusions and Future Outlooks
A significant diversity of studies involving the exploration of plant bioactive compounds has indicated an extremely promising scenario regarding the use of these components for a range of applications. The growing number of scientific publications on this particular subject reflects the global interest in adopting sustainable proposals and adding value to products and by-products with potential use in different industries. In general, plant bioactive compounds have strong antioxidants, anticancer, antimicrobial, etc.
Appropriately, recent studies showed the discovery of a range of bioactive compounds of interest in foods. These substances vary widely morphologically, in distribution and concentrations, and in the characterization of chemical properties. These compounds are largely found in cereals, legumes, fruits, vegetables, trees, nuts, etc. Considering the growing human concern with a balanced diet rich in nutrients and highly beneficial compounds, recent studies evidence a continuous exploration of plant extracts as promoters of the inhibition of health disorders and providers of nutritional properties and of minimizing disease risks. Furthermore, the diversity of molecules present in these extracts has also been explored as an antimicrobial action and control strategy. This scenario has been observed in the application of medicine and agriculture targets, which demand the conduction of more studies that explore the mechanisms of action and preservation of phytochemicals extracted from plants.
Finally, extraordinary progress has been reported in the position of bioactive compounds as tools to promote human health and efficient management in agricultural-based discussions. Under a large population growth and a significant detailing of registered compounds, the importance of developing studies aimed at the narratives proposed in this review is evident, and which ensure the effectiveness of essentially natural products such as the spread trending highlight in the coming years.
Acknowledgement: This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES), the National Council of Technological and Scientific Development (CNPq), and the Research Support Foundation of the State of Rio Grande do Sul (FAPERGS).
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Vivaldo, G., Masi, E., Taiti, C., Caldarelli, G., Mancuso, S. (2017). The network of plants volatile organic compounds. Scientific Reports, 7, 1–18. DOI 10.1038/s41598-017-10975-x. [Google Scholar] [CrossRef]
2. Demoliner, F., de Carvalho, L. T., de Liz, G. R., Prudêncio, E. S., Ramos, J. C. et al. (2020). Improving the nutritional and phytochemical compounds of a plant-based milk of sapucaia nut cake using block freeze concentration. International Journal of Food Science & Technology, 55(8), 3031–3042. DOI 10.1111/ijfs.14568. [Google Scholar] [CrossRef]
3. Tian, B., Pei, Y., Huang, W., Ding, J., Siemann, E. (2021). Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive plant. The ISME Journal, 15, 1919–1930. DOI 10.1038/s41396-021-00894-1. [Google Scholar] [CrossRef]
4. Kasmi, S., Hamdi, A., Atmani-Kilani, D., Debbache-Benaida, N., Jaramillo-Carmona, S. et al. (2021). Characterization of phenolic compounds isolated from the Fraxinus angustifolia plant and several associated bioactivities. Journal of Herbal Medicine, 29. DOI 10.1016/j.hermed.2021.100485. [Google Scholar] [CrossRef]
5. Ketsuwan, N., Leelarungrayub, J., Kothan, S., Singhatong, S. (2017). Antioxidant compounds and activities of the stem, flower, and leaf extracts of the anti-smoking Thai medicinal plant: Vernonia cinerea less. Drug Design, Development and Therapy, 13(11), 383–391. DOI 10.2147/DDDT.S126882. [Google Scholar] [CrossRef]
6. Botella, M. Á., Hernández, V., Mestre, T., Hellín, P., García-Legaz, M. F. et al. (2021). Bioactive compounds of tomato fruit in response to salinity, heat and their combination. Agriculture, 11(6), 1–12. DOI 10.3390/agriculture11060534. [Google Scholar] [CrossRef]
7. Shirahigue, L. D., Ceccato-Antonini, S. R. (2020). Agro-industrial wastes as sources of bioactive compounds for food and fermentation industries. Ciência Rural, 50(4). DOI 10.1590/0103-8478cr20190857. [Google Scholar] [CrossRef]
8. Chiocchio, I., Mandrone, M., Tomasi, P., Marincich, L., Poli, F. (2021). Plant secondary metabolites: An opportunity for circular economy. Molecules, 26(2), 495. DOI 10.3390/molecules26020495. [Google Scholar] [CrossRef]
9. Altemimi, A., Lakhssassi, N., Baharlouei, A., Watson, D. G., Lightfoot, D. A. (2017). Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants, 6(4), 42. DOI 10.3390/plants6040042. [Google Scholar] [CrossRef]
10. Boncan, D. A. T., Tsang, S. S. K., Li, C., Lee, I. H. T., Lam, H. M. et al. (2020). Terpenes and terpenoids in plants: Interactions with environment and insects. International Journal of Molecular Sciences, 21(19), 1–19. DOI 10.3390/ijms21197382. [Google Scholar] [CrossRef]
11. Yonekura-Sakakibara, K., Higashi, Y., Nakabayashi, R. (2019). The origin and evolution of plant flavonoid metabolism. Frontiers in Plant Science, 10, 1–16. DOI 10.3389/fpls.2019.00943. [Google Scholar] [CrossRef]
12. Cosme, P., Rodríguez, A. B., Espino, J., Garrido, M. (2020). Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants, 9(12), 1–20. DOI 10.3390/antiox9121263. [Google Scholar] [CrossRef]
13. Cappellini, F., Marinelli, A., Toccaceli, M., Tonelli, C., Petroni, K. (2021). Anthocyanins: From mechanisms of regulation in plants to health benefits in foods. Frontiers in Plant Science, 12. DOI 10.3389/fpls.2021.748049. [Google Scholar] [CrossRef]
14. Lourenço, S. C., Moldão-Martins, M., Alves, V. D. (2019). Antioxidants of natural plant origins: From sources to food industry applications. Molecules, 24(22), 14–16. DOI 10.3390/molecules24224132. [Google Scholar] [CrossRef]
15. Jamali, N., Soureshjani, E. H., Mobini, G. R., Samare-Najaf, M., Clark, C. C. T. et al. (2021). Medicinal plant compounds as promising inhibitors of coronavirus (COVID-19) main protease: An in silico study. Journal of Biomolecular Structure and Dynamics, 1–12. DOI 10.1080/07391102.2021.1906749. [Google Scholar] [CrossRef]
16. Brilli, F., Loreto, F., Baccelli, I. (2019). Exploiting plant volatile organic compounds (VOCS) in agriculture to improve sustainable defense strategies and productivity of crops. Frontiers in Plant Science, 10, 1–8. DOI 10.3389/fpls.2019.00264. [Google Scholar] [CrossRef]
17. Ahmadi, F., Samadi, A., Rahimi, A. (2020). Improving growth properties and phytochemical compounds of Echinacea purpurea (L.) medicinal plant using novel nitrogen slow release fertilizer under greenhouse conditions. Scientific Reports, 10, 1–11. DOI 10.1038/s41598-020-70949-4. [Google Scholar] [CrossRef]
18. Ahmed, M., Peiwen, Q., Gu, Z., Liu, Y., Sikandar, A. et al. (2020). Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.). Scientific Reports, 10, 1–10. DOI 10.1038/s41598-019-57092-5. [Google Scholar] [CrossRef]
19. Oleszek, M., Kowalska, I., Oleszek, W. (2019). Phytochemicals in bioenergy crops. Netherlands: Springer. [Google Scholar]
20. Remali, J., Aizat, W. M. (2021). A review on plant bioactive compounds and their modes of action against coronavirus infection. Frontiers in Pharmacology, 11, 1–13. DOI 10.3389/fphar.2020.589044. [Google Scholar] [CrossRef]
21. Kumar, K., Srivastav, S., Sharanagat, V. S. (2021). Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrasonics Sonochemistry, 70, 105325. DOI 10.1016/j.ultsonch.2020.105325. [Google Scholar] [CrossRef]
22. Yoon, H. I., Kim, H. Y., Kim, J., Son, J. E. (2021). Quantitative analysis of UV-B radiation interception and bioactive compound contents in kale by leaf position according to growth progress. Frontiers in Plant Science, 12. DOI 10.3389/fpls.2021.667456. [Google Scholar] [CrossRef]
23. Yang, L., Yang, C., Li, C., Zhao, Q., Liu, L. et al. (2016). Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Science Bulletin, 61(1), 3–17. DOI 10.1007/s11434-015-0929-2. [Google Scholar] [CrossRef]
24. Rubio, N. R., Xiang, N., Kaplan, D. L. (2020). Plant-based and cell-based approaches to meat production. Nature Communications, 11, 1–11. DOI 10.1038/s41467-020-20061-y. [Google Scholar] [CrossRef]
25. Alcorta, A., Porta, A., Tárrega, A., Alvarez, M. D., Pilar Vaquero, M. (2021). Foods for plant-based diets: Challenges and innovations. Foods, 10(2), 1–23. DOI 10.3390/foods10020293. [Google Scholar] [CrossRef]
26. Samtiya, M., Aluko, R. E., Dhewa, T., Moreno-Rojas, J. M. (2021). Potential health benefits of plant food-derived bioactive components: An overview. Foods, 10(4), 1–25. DOI 10.3390/foods10040839. [Google Scholar] [CrossRef]
27. Ko, M. J., Nam, H. H., Chung, M. S. (2020). Subcritical water extraction of bioactive compounds from Orostachys japonicus A. berger (Crassulaceae). Scientific Reports, 10, 1–10. DOI 10.1038/s41598-020-67508-2. [Google Scholar] [CrossRef]
28. Hassim, N., Markom, M., Rosli, M. I., Harun, S. (2021). Scale-up approach for supercritical fluid extraction with ethanol–water modified carbon dioxide on Phyllanthus niruri for safe enriched herbal extracts. Scientific Reports, 11, 1–19. DOI 10.1038/s41598-021-95222-0. [Google Scholar] [CrossRef]
29. Ying, X., Agyei, D., Udenigwe, C., Adhikari, B., Wang, B. (2021). Manufacturing of plant-based bioactive peptides using enzymatic methods to meet health and sustainability targets of the sustainable development goals. Frontiers in Sustainable Food Systems, 5, 1–22. DOI 10.3389/fsufs.2021.769028. [Google Scholar] [CrossRef]
30. Noore, S., Rastogi, N. K., O’Donnell, C., Tiwari, B. (2021). Novel bioactive extraction and nano-encapsulation. Encyclopedia, 1(3), 632–664. DOI 10.3390/encyclopedia1030052. [Google Scholar] [CrossRef]
31. Awad, A. M., Kumar, P., Ismail-Fitry, M. R., Jusoh, S., Ab Aziz, M. F. et al. (2021). Green extraction of bioactive compounds from plant biomass and their application in meat as natural antioxidant. Antioxidants, 10(9), 1–39. DOI 10.3390/antiox10091465. [Google Scholar] [CrossRef]
32. Kumar, M., Dahuja, A., Tiwari, S., Punia, S., Tak, Y. et al. (2021). Recent trends in extraction of plant bioactives using green technologies: A review. Food Chemistry, 353, 129431. DOI 10.1016/j.foodchem.2021.129431. [Google Scholar] [CrossRef]
33. Shinwari, K. J., Rao, P. S. (2018). Stability of bioactive compounds in fruit jam and jelly during processing and storage: A review. Trends in Food Science & Technology, 75, 181–193. DOI 10.1016/j.tifs.2018.02.002. [Google Scholar] [CrossRef]
34. Montoro, P., Serreli, G., Gil, K. A., D’Urso, G., Kowalczyk, A. et al. (2020). Evaluation of bioactive compounds and antioxidant capacity of edible feijoa (Acca sellowiana (O. berg) burret) flower extracts. Journal of Food Science and Technology, 57, 2051–2060. DOI 10.1007/s13197-020-04239-2. [Google Scholar] [CrossRef]
35. Peanparkdee, M., Iwamoto, S. (2020). Encapsulation for improving in vitro gastrointestinal digestion of plant polyphenols and their applications in food products. Food Reviews International, 1–19. DOI 10.1080/87559129.2020.1733595. [Google Scholar] [CrossRef]
36. Lorenzo, J. M., Pateiro, M., Domínguez, R., Barba, F. J., Putnik, P. et al. (2018). Berries extracts as natural antioxidants in meat products: A review. Food Research International, 106, 1095–1104. DOI 10.1016/j.foodres.2017.12.005. [Google Scholar] [CrossRef]
37. Chen, W., Feng, L., Shen, Y., Su, H., Li, Y. et al. (2013). Myricitrin inhibits acrylamide-mediated cytotoxicity in human Caco-2 cells by preventing oxidative stress. BioMed Research International, 2013, 1–7. DOI 10.1155/2013/724183. [Google Scholar] [CrossRef]
38. Aguirre Calvo, T. R., Santagapita, P. R. (2017). Encapsulation of a free-solvent extract of lycopene in alginate-Ca(II) beads containing sugars and biopolymers. Chemical and Biological Technologies in Agriculture, 4(16), 4–11. DOI 10.1186/s40538-017-0099-3. [Google Scholar] [CrossRef]
39. Cruz, P. N., Pereira, T. C. S., Guindani, C., Oliveira, D. A., Rossi, M. J. et al. (2017). Antioxidant and antibacterial potential of butia (Butia catarinensis) seed extracts obtained by supercritical fluid extraction. Journal of Supercritial Fluids, 119, 229–237. DOI 10.1016/j.supflu.2016.09.022. [Google Scholar] [CrossRef]
40. da Silva, F. D., Itoda, C., Rosa, C. I. L. F., Vital, A. C. P., Yamamoto, L. N. et al. (2018). Effects of blackberries (Rupus sp.; cv. Xavante) processing on its physicochemical properties, phenolic contents and antioxidant activity. Journal of Food Science and Technology, 55(11), 4642–4649. DOI 10.1007/s13197-018-3405-6. [Google Scholar] [CrossRef]
41. de Carvalho Silva, J., de França, P. R. L., de Melo, A. H. F., Neves-Petersen, M. T., Converti, A. et al. (2019). Optimized production of Aspergillus aculeatus URM4953 polygalacturonases for pectin hydrolysis in hog plum (Spondias mombin L.) juice. Process Biochemistry, 79, 18–27. DOI 10.1016/j.procbio.2018.12.014. [Google Scholar] [CrossRef]
42. de Castro, R. J. S., Ohara, A., Nishide, T. G., Albernaz, J. R. M., Soares, M. H. et al. (2015). A new approach for proteases production by Aspergillus niger based on the kinetic and thermodynamic parameters of the enzymes obtained. Biocatalysis and Agricultural Biotechnology, 4(2), 199–207. DOI 10.1016/j.bcab.2014.12.001. [Google Scholar] [CrossRef]
43. Scaglia, B., D’Incecco, P., Squillace, P., Dell’Orto, M., de Nisi, P. et al. (2020). Development of a tomato pomace biorefinery based on a CO2-supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. Journal of Cleaner Production, 243, 118650. DOI 10.1016/j.jclepro.2019.118650. [Google Scholar] [CrossRef]
44. Jalgaonkar, K., Mahawar, M. K., Bibwe, B., Kannaujia, P. (2020). Postharvest profile, processing and waste utilization of dragon fruit (Hylocereus Spp.A review. Food Reviews International, 1–27. DOI 10.1080/87559129.2020.1742152. [Google Scholar] [CrossRef]
45. Sari, S. N., Melati, A. (2019). Facile preparation of carbon nanofiber from banana peel waste. Materials Today: Proceedings, 13, 165–168. DOI 10.1016/j.matpr.2019.03.208. [Google Scholar] [CrossRef]
46. Alara, O. R., Abdurahman, N. H., Ukaegbu, C. I. (2018). Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. Journal of Applied Research on Medical and Aromatic Plants, 11, 12–17. DOI 10.1016/j.jarmap.2018.07.003. [Google Scholar] [CrossRef]
47. Do, Q. D., Angkawijaya, A. E., Tran-Nguyen, P. L., Huynh, L. H., Soetaredjo, F. E. et al. (2014). Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. Journal of Food and Drug Analysis, 22(3), 296–302. DOI 10.1016/j.jfda.2013.11.001. [Google Scholar] [CrossRef]
48. Cheong, A. M., Tan, C. P., Nyam, K. L. (2018). Stability of bioactive compounds and antioxidant activities of kenaf seed Oil-in-water nanoemulsions under different storage temperatures. Journal of Food Science, 83(10), 2457–2465. DOI 10.1111/1750-3841.14332. [Google Scholar] [CrossRef]
49. García-Pérez, P., Losada-Barreiro, S., Bravo-Díaz, C., Gallego, P. P. (2020). Exploring the use of Bryophyllum as natural source of bioactive compounds with antioxidant activity to prevent lipid oxidation of fish oil-in-water emulsions. Plants, 9(8), 1–18. DOI 10.3390/plants9081012. [Google Scholar] [CrossRef]
50. Li, Y., Li, S., Lin, S. J., Zhang, J. J., Zhao, C. N. et al. (2017). Bin microwave-assisted extraction of natural antioxidants from the exotic Gordonia axillaris fruit: Optimization and identification of phenolic compounds. Molecules, 22(9), 1481. DOI 10.3390/molecules22091481. [Google Scholar] [CrossRef]
51. Belwal, T., Pandey, A., Bhatt, I. D., Rawal, R. S. (2020). Optimized microwave assisted extraction (MAE) of alkaloids and polyphenols from Berberis roots using multiple-component analysis. Scientific Reports, 10, 1–10. DOI 10.1038/s41598-020-57585-8. [Google Scholar] [CrossRef]
52. Li, H. Z., Zhang, Z. J., Xue, J., Cui, L. X., Hou, T. Y. et al. (2016). Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants and rosmarinic acid from Perilla leaves using response surface methodology. Food Science and Technology, 36(4), 686–693. DOI 10.1590/1678-457X.13516. [Google Scholar] [CrossRef]
53. Aware, C. B., Patil, R. R., Vyavahare, G. D., Gurme, S. T., Jadhav, J. P. (2019). Ultrasound-assisted aqueous extraction of phenolic, flavonoid compounds and antioxidant activity of Mucuna macrocarpa beans: Response surface methodology optimization. Journal of the American Nutrition Association, 38(4), 364–372. DOI 10.1080/07315724.2018.1524315. [Google Scholar] [CrossRef]
54. Chen, Y. H., Yang, C. Y. (2020). Ultrasound-assisted extraction of bioactive compounds and antioxidant capacity for the valorization of Elaeocarpus serratus L. leaves. Processes, 8(10), 1–11. DOI 10.3390/pr8101218. [Google Scholar] [CrossRef]
55. Niazmand, R., Shahidi Noghabi, M., Niazmand, A. (2021). Optimization of subcritical water extraction of phenolic compounds from Ziziphus jujuba using response surface methodology: Evaluation of thermal stability and antioxidant activity. Chemical and Biological Technologies in Agriculture, 8(6), 1–13. DOI 10.1186/s40538-020-00203-6. [Google Scholar] [CrossRef]
56. Veggi, P. C., Cavalcanti, R. N., Meireles, M. A. A. (2014). Production of phenolic-rich extracts from Brazilian plants using supercritical and subcritical fluid extraction: Experimental data and economic evaluation. Journal of Food Engineering, 131, 96–109. DOI 10.1016/j.jfoodeng.2014.01.027. [Google Scholar] [CrossRef]
57. Pellicanò, T. M., Sicari, V., Loizzo, M. R., Leporini, M., Falco, T. et al. (2020). Optimizing the supercritical fluid extraction process of bioactive compounds from processed tomato skin by-products. Food Science and Technology, 40(3), 692–697. DOI 10.1590/fst.16619. [Google Scholar] [CrossRef]
58. Pires, F. C. S., Oliveira, J. C. D., Menezes, E. G. O., Ferreira, M. C. R., Siqueira, L. M. M. et al. (2021). Bioactive compounds and evaluation of antioxidant, cytotoxic and cytoprotective effects of murici pulp extracts (Byrsonima crassifolia) obtained by supercritical extraction in hepG2 cells treated with H2O2. Foods, 10(4), 737. DOI 10.3390/foods10040737. [Google Scholar] [CrossRef]
59. Čižmek, L., Kralj, M. B., Čož-rakovac, R., Mazur, D., Ul’yanovskii, N. et al. (2021). Supercritical carbon dioxide extraction of four medicinal Mediterranean plants: Investigation of chemical composition and antioxidant activity. Molecules, 26(18), 1–18. DOI 10.3390/molecules26185697. [Google Scholar] [CrossRef]
60. Heemann, A. C. W., Heemann, R., Kalegari, P., Spier, M. R., Santin, E. (2019). Enzyme-assisted extraction of polyphenols from green yerba mate. Brazilian Journal of Food Technology, 22, 1–10. DOI 10.1590/1981-6723.22217. [Google Scholar] [CrossRef]
61. Gul, K., Singh, A. K., Jabeen, R. (2016). Nutraceuticals and functional foods: The foods for the future world. Critical Reviews in Food Science and Nutrition, 56(16), 2617–2627. DOI 10.1080/10408398.2014.903384. [Google Scholar] [CrossRef]
62. Sandner, G., König, A., Wallner, M., Weghuber, J. (2020). Functional foods-dietary or herbal products on obesity: Application of selected bioactive compounds to target lipid metabolism. Current Opinions in Food Science, 34, 9–20. DOI 10.1016/j.cofs.2020.09.011. [Google Scholar] [CrossRef]
63. Borgonovi, T. F., Casarotti, S. N., Penna, A. L. B. (2021). Lacticaseibacillus casei SJRP38 and buriti pulp increased bioactive compounds and probiotic potential of fermented milk. LWT, 143(2), 111124. DOI 10.1016/j.lwt.2021.111124. [Google Scholar] [CrossRef]
64. Turgut, T., Cakmakci, S. (2018). Probiotic strawberry yogurts: Microbiological, chemical and sensory properties. Probiotics and Antimicrobial Proteins, 10(1), 64–70. DOI 10.1007/s12602-017-9278-6. [Google Scholar] [CrossRef]
65. Koop, B. L., Knapp, M. A., Di Luccio, M., Pinto, V. Z., Tormen, L. et al. (2021). Bioactive compounds from jambolan (Syzygium cumini (L.)) extract concentrated by ultra-and nanofiltration: A potential natural antioxidant for food. Plant Foods for Human Nutrition, 76(1), 90–97. DOI 10.1007/s11130-021-00878-8. [Google Scholar] [CrossRef]
66. Petropoulos, S. A., Pereira, C., Tzortzakis, N., Barros, L., Ferreira, I. C. F. R. (2018). Nutritional value and bioactive compounds characterization of plant parts from Cynara cardunculus L. (Asteraceae) Cultivated in Central Greece. Frontiers in Plant Science, 9, 1–12. DOI 10.3389/fpls.2018.00459. [Google Scholar] [CrossRef]
67. Saucedo-Pompa, S., Torres-Castillo, J. A., Castro-López, C., Rojas, R., Sánchez-Alejo, E. J. et al. (2018). Moringa plants: Bioactive compounds and promising applications in food products. Food Research International, 111, 438–450. DOI 10.1016/j.foodres.2018.05.062. [Google Scholar] [CrossRef]
68. Mao, M., Wang, P., Shi, K., Lu, Z., Bie, X. et al. (2020). Effect of solid state fermentation by Enterococcus faecalis M2 on antioxidant and nutritional properties of wheat bran. Journal of Cereal Science, 94, 102997. DOI 10.1016/j.jcs.2020.102997. [Google Scholar] [CrossRef]
69. Maqsood, S., Adiamo, O., Ahmad, M., Mudgil, P. (2020). Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chemistry, 308, 125522. DOI 10.1016/j.foodchem.2019.125522. [Google Scholar] [CrossRef]
70. Hui, X., Wu, G., Han, D., Gong, X., Stipkovits, L. et al. (2021). Bioactive compounds from blueberry and blackcurrant powder alter the physicochemical and hypoglycaemic properties of oat bran paste. LWT, 111167. DOI 10.1016/j.lwt.2021.111167. [Google Scholar] [CrossRef]
71. Salvador-Reyes, R., Clerici, M. T. P. S. (2020). Peruvian Andean maize: General characteristics, nutritional properties, bioactive compounds, and culinary uses. Food Research International, 130, 108934. DOI 10.1016/j.foodres.2019.108934. [Google Scholar] [CrossRef]
72. Gullón, B., Gagaoua, M., Barba, F. J., Gullón, P., Zhang, W. et al. (2020). Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends in Food Science & Technology, 100, 1–18. DOI 10.1016/j.tifs.2020.03.039. [Google Scholar] [CrossRef]
73. Zhao, L., Fan, H., Zhang, M., Chitrakar, B., Bhandari, B. et al. (2019). Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Research International, 126, 108660. DOI 10.1016/j.foodres.2019.108660. [Google Scholar] [CrossRef]
74. Correia, R. T. P., Borges, K. C., Medeiros, M. F., Genovese, M. I. (2012). Bioactive compounds and phenolic-linked functionality of powdered tropical fruit residues. Food Science and Technology International, 18(6), 539–547. DOI 10.1177/1082013211433077. [Google Scholar] [CrossRef]
75. Atef, M., Mahdi Ojagh, S. (2017). Health benefits and food applications of bioactive compounds from fish byproducts: A review. Journal of Functional Foods, 35, 673–681. DOI 10.1016/j.jff.2017.06.034. [Google Scholar] [CrossRef]
76. Lauricella, M., Lo Galbo, V., Cernigliaro, C., Maggio, A., Palumbo Piccionello, A. et al. (2019). The anti-cancer effect of Mangifera indica L. peel extract is associated to γH2AX-mediated apoptosis in colon cancer cells. Antioxidants, 8(10), 422. DOI 10.3390/antiox8100422. [Google Scholar] [CrossRef]
77. Moreira, M. M., Barroso, M. F., Porto, J. V., Ramalhosa, M. J., Švarc-Gajić, J. et al. (2018). Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Science of the Total Environment, 634, 831–842. DOI 10.1016/j.scitotenv.2018.04.035. [Google Scholar] [CrossRef]
78. Ballesteros, L. F., Teixeira, J. A., Mussatto, S. I. (2014). Selection of the solvent and extraction conditions for maximum recovery of antioxidant phenolic compounds from coffee silverskin. Food and Bioprocess Technology, 7(5), 1322–1332. DOI 10.1007/s11947-013-1115-7. [Google Scholar] [CrossRef]
79. Malik, L. A., Ad’hiah, A. H., Aziz, G. M. (2019). Phytochemical content and the potential of Punica granatum peel extracts as radical scavengers and dipeptidyl peptidase-4 inhibitors. Journal of Biotechnology. Research Center, 13. DOI 10.24126/jobrc.2019.13.1.561. [Google Scholar] [CrossRef]
80. Ahmad, N., Sharma, A. K., Sharma, S., Khan, I., Sharma, D. K. et al. (2019). Biosynthesized composites of Au-Ag nanoparticles using trapa peel extract induced ROS-mediated p53 independent apoptosis in cancer cells. Drug and Chemical Toxicology, 42(1), 43–53. DOI 10.1080/01480545.2018.1463241. [Google Scholar] [CrossRef]
81. Nevin, K. G., Rajamohan, T. (2010). Effect of topical application of virgin coconut oil on skin components and antioxidant status during dermal wound healing in young rats. Skin Pharmacology and Physiology, 23(6), 290–297. DOI 10.1159/000313516. [Google Scholar] [CrossRef]
82. Gullón, B., Eibes, G., Moreira, M. T., Herrera, R., Labidi, J. et al. (2018). Yerba mate waste: A sustainable resource of antioxidant compounds. Industrial Crops and Products, 113, 398–405. DOI 10.1016/j.indcrop.2018.01.064. [Google Scholar] [CrossRef]
83. Fidelis, M., do Carmo, M. A. V., da Cruz, T. M., Azevedo, L., Myoda, T. et al. (2020). Camu-camu seed (Myrciaria dubia)–from side stream to anantioxidant, antihyperglycemic, antiproliferative, antimicrobial, antihemolytic, anti-inflammatory, and antihypertensive ingredient. Food Chemistry, 310, 125909. DOI 10.1016/j.foodchem.2019.125909. [Google Scholar] [CrossRef]
84. Shu, H. Y., Ma, F. N., Li, K. M., Sun, W., Xu, G. Y. et al. (2020). Growth of breast cancer cells inhibited by bromelains extracted from the different tissues of pineapple. Folia Biologica, 68(3), 81–88. [Google Scholar]
85. Bhargava, N., Sharanagat, V. S., Mor, R. S., Kumar, K. (2020). Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends in Food Science & Technology, 105, 385–401. DOI 10.1016/j.tifs.2020.09.015. [Google Scholar] [CrossRef]
86. Medina-Jaramillo, C., Ochoa-Yepes, O., Bernal, C., Famá, L. (2017). Active and smart biodegradable packaging based on starch and natural extracts. Carbohydrate Polymers, 176, 187–194. DOI 10.1016/j.carbpol.2017.08.079. [Google Scholar] [CrossRef]
87. Ardiyansyah, A. M. W., Wahyono, A., Fatoni, M., Poerwanto, B., Suryaningsih, W. (2018). The potency of betacyanins extract from a peel of dragon fruits as a source of colourimetric indicator to develop intelligent packaging for fish freshness monitoring. IOP Conference Series: Earth and Environmental Science, 207, 12038. DOI 10.1088/1755-1315/207/1/012038. [Google Scholar] [CrossRef]
88. Yong, H., Wang, X., Zhang, X., Liu, Y., Qin, Y. et al. (2019). Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocolloids, 94, 93–104. DOI 10.1016/j.foodhyd.2019.03.012. [Google Scholar] [CrossRef]
89. Liu, J., Wang, H., Wang, P., Guo, M., Jiang, S. et al. (2018). Films based on κ-carrageenan incorporated with curcumin for freshness monitoring. Food Hydrocolloids, 83, 134–142. DOI 10.1016/j.foodhyd.2018.05.012. [Google Scholar] [CrossRef]
90. Martiny, T. R., Raghavan, V., de Moraes, C. C., da Rosa, G. S., Dotto, G. L. (2021). Optimization of green extraction for the recovery of bioactive compounds from Brazilian olive crops and evaluation of its potential as a natural preservative. Journal of Environmental Chemical Engineering, 9(2), 105130. DOI 10.1016/j.jece.2021.105130. [Google Scholar] [CrossRef]
91. de la Luz Cadiz-Gurrea, M., del Carmen Villegas-Aguilar, M., Leyva-Jiménez, F. J., Pimentel-Moral, S., Fernandez-Ochoa, A. et al. (2020). Revalorization of bioactive compounds from tropical fruit by-products and industrial applications by means of sustainable approaches. Food Research International, 138, 109786. DOI 10.1016/j.foodres.2020.109786. [Google Scholar] [CrossRef]
92. Sharifi, M., Bashtani, M., Naserian, A. A., Farhangfar, H. (2015). The effect of feeding low quality date palm (Phoenix dactylifera L.) on the performance, antioxidant status and ruminal fermentation of mid-lactating saanen dairy goats. Small Ruminant Research, 130, 95–100. DOI 10.1016/j.smallrumres.2015.07.031. [Google Scholar] [CrossRef]
93. Hernández-López, S. H., Rodríguez-Carpena, J. G., Lemus-Flores, C., Grageola-Nuñez, F., Estévez, M. (2016). Avocado waste for finishing pigs: Impact on muscle composition and oxidative stability during chilled storage. Meat Science, 116, 186–192. DOI 10.1016/j.meatsci.2016.02.018. [Google Scholar] [CrossRef]
94. Joseph, R., Saminathan, K., Deepthi, M. P., Kathireswari, P. (2020). Comparative analysis on bioactive compounds presents in dung material of Bos Taurus and Bos indicus. Materials Today: Proceedings. DOI 10.1016/j.matpr.2020.06.457. [Google Scholar] [CrossRef]
95. Monllor, P., Romero, G., Sendra, E., Atzori, A. S., Díaz, J. R. (2020). Short-term effect of the inclusion of silage artichoke by-products in diets of dairy goats on milk quality. Animals, 10(2), 339. DOI 10.3390/ani10020339. [Google Scholar] [CrossRef]
96. Pieszka, M., Szczurek, P., Bederska-Łojewska, D., Migdał, W., Pieszka, M. et al. (2017). The effect of dietary supplementation with dried fruit and vegetable pomaces on production parameters and meat quality in fattening pigs. Meat Science, 126, 1–10. DOI 10.1016/j.meatsci.2016.11.016. [Google Scholar] [CrossRef]
97. Correddu, F., Lunesu, M. F., Buffa, G., Atzori, A. S., Nudda, A. et al. (2020). Can agro-industrial by-products rich in polyphenols be advantageously used in the feeding and nutrition of dairy small ruminants?. Animals, 10. DOI 10.3390/ani10010131. [Google Scholar] [CrossRef]
98. Correddu, F., Fancello, F., Chessa, L., Atzori, A. S., Pulina, G. et al. (2019). Effects of supplementation with exhausted myrtle berries on rumen function of dairy sheep. Small Ruminant Research, 170, 51–61. DOI 10.1016/j.smallrumres.2018.11.003. [Google Scholar] [CrossRef]
99. Lawrance, S., Varghese, S., Varghese, E. M., Asok, A. K., Jisha, M. S. (2019). Quinoline derivatives producing Pseudomonas aeruginosa H6 as an efficient bioherbicide for weed management. Biocatalysis and Agricultural Biotechnology, 18, 101096. DOI 10.1016/j.bcab.2019.101096. [Google Scholar] [CrossRef]
100. Todero, I., Confortin, T. C., Luft, L., Seibel, J., Kuhn, R. C. et al. (2020). Concentration of exopolysaccharides produced by Fusarium fujikuroi and application of bioproduct as an effective bioherbicide. Environmental Technology, 41(21), 2742–2749. DOI 10.1080/09593330.2019.1580775. [Google Scholar] [CrossRef]
101. Tibugari, H., Marumahoko, P., Mandumbu, R., Mangosho, E., Manyeruke, N. et al. (2020). Allelopathic sorghum aqueous extracts reduce biomass of hairy beggarticks. Cogent Biology, 6(1), 1810382. DOI 10.1080/23312025.2020.1810382. [Google Scholar] [CrossRef]
102. Mekky, M. S., Hassanien, A. M. A., Kamel, E. M., Ismail, A. E. A. (2019). Allelopathic effect of Ocimum basilicum L. extracts on weeds and some crops and its possible use as new crude bio-herbicide. Annals of Agricultural Sciences, 64(2), 211–221. DOI 10.1016/j.aoas.2019.12.005. [Google Scholar] [CrossRef]
103. Hussain, I., Singh, N. B., Singh, A., Singh, H. (2017). Allelopathic potential of sesame plant leachate against Cyperus rotundus L. Annals of Agricultural Sciences, 15(1), 141–147. DOI 10.1016/j.aasci.2016.10.003. [Google Scholar] [CrossRef]
104. Hasan, M., Ahmad-Hamdani, M. S., Rosli, A. M., Hamdan, H. (2021). Bioherbicides: An eco-friendly tool for sustainable weed management. Plants, 10(6), 1–21. DOI 10.3390/plants10061212. [Google Scholar] [CrossRef]
105. Anwar, T., Qureshi, H., Mahnashi, M. H., Kabir, F., Parveen, N. et al. (2021). Bioherbicidal ability and weed management of allelopathic methyl esters from Lantana camara. Saudi Journal of Biological Sciences, 28(8), 4365–4374. DOI 10.1016/j.sjbs.2021.04.026. [Google Scholar] [CrossRef]
106. Veraplakorn, V. (2018). Allelopathic hormesis and slow release of lantana (Lantana camara L.) callus extract. Agriculture and Natural Resources, 52(4), 335–340. DOI 10.1016/j.anres.2018.10.004. [Google Scholar] [CrossRef]
107. Huang, Y., Chen, L., He, B., Liu, S. Y., Zeng, D. Q. et al. (2021). Herbicidal activity and bioactive components of Brucea javanica (L.) Merr. residue. Arabian Journal of Chemistry, 14(7), 103228. DOI 10.1016/j.arabjc.2021.103228. [Google Scholar] [CrossRef]
108. Xiong, X., Yao, M., Fu, L., Ma, Z., Qing, Z. X. (2016). The botanical pesticide derived from Sophora flavescens for controlling insect pests can also improve growth and development of tomato plants. Industrial Crops and Products, 92, 13–18. DOI 10.1016/j.indcrop.2016.07.043. [Google Scholar] [CrossRef]
109. Paredes-Sánchez, F. A., Rivera, G., Bocanegra-García, V., Martínez-Padrón, H. Y., Berrones-Morales, M. et al. (2021). Advances in control strategies against spodoptera frugiperda. A review. Molecules, 26(18), 5587. DOI 10.3390/molecules26185587. [Google Scholar] [CrossRef]
110. Vedovatto, F., Bonatto, C., Bazoti, S. F., Venturin, B., Alves Jr., S. L. et al. (2021). Production of biofuels from soybean straw and hull hydrolysates obtained by subcritical water hydrolysis. Bioresource Technology, 328, 124837. DOI 10.1016/j.biortech.2021.124837. [Google Scholar] [CrossRef]
111. Dos Santos, M. S., Zabot, G. L., Mazutti, M. A., Ugalde, G. A., Rezzadori, K. et al. (2020). Optimization of subcritical water hydrolysis of pecan wastes biomasses in a semi-continuous mode. Bioresource Technology, 306, 123129. DOI 10.1016/j.biortech.2020.123129. [Google Scholar] [CrossRef]
112. Wancura, J. H. C., Fantinel, A. L., Ugalde, G. A., Donato, F. F., de Oliveira, J. V. et al. (2020). Semi-continuous production of biodiesel on pilot scale via enzymatic hydroesterification of waste material: Process and economics considerations. Journal of Cleaner Production, 285. DOI 10.1016/j.jclepro.2020.124838. [Google Scholar] [CrossRef]
113. Cruz, A. G., Mtz-Enríquez, A. I., Díaz-Jiménez, L., Ramos-González, R., Valdés, J. A. A. et al. (2020). Production of fatty acid methyl esters and bioactive compounds from citrus wax. Waste Management, 102, 48–55. DOI 10.1016/j.wasman.2019.10.021. [Google Scholar] [CrossRef]
114. Karmee, S. K. (2018). A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Management, 72, 240–254. DOI 10.1016/j.wasman.2017.10.042. [Google Scholar] [CrossRef]
115. Vardon, D. R., Moser, B. R., Zheng, W., Witkin, K., Evangelista, R. L. et al. (2013). Complete utilization of spent coffee grounds to produce biodiesel, Bio-oil, and biochar. ACS Sustainable Chemistry & Engineering, 1(10), 1286–1294. DOI 10.1021/sc400145w. [Google Scholar] [CrossRef]
116. Banerjee, A., Singh, V., Solanki, K., Mukherjee, J., Gupta, M. N. (2013). Combi-protein coated microcrystals of lipases for production of biodiesel from oil from spent coffee grounds. Sustainable Chemical Processes, 1(14), 1–9. DOI 10.1186/2043-7129-1-14. [Google Scholar] [CrossRef]
117. Obruca, S., Petrik, S., Benesova, P., Svoboda, Z., Eremka, L. et al. (2014). Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Applied Microbiology and Biotechnology, 98(13), 5883–5890. DOI 10.1007/s00253-014-5653-3. [Google Scholar] [CrossRef]
118. Anastopoulos, I., Karamesouti, M., Mitropoulos, A. C., Kyzas, G. Z. (2017). A review for coffee adsorbents. Journal of Molecular Liquids, 229, 555–565. DOI 10.1016/j.molliq.2016.12.096. [Google Scholar] [CrossRef]
119. Tuntiwiwattanapun, N., Monono, E., Wiesenborn, D., Tongcumpou, C. (2017). In-situ transesterification process for biodiesel production using spent coffee grounds from the instant coffee industry. Industrial Crops and Products, 102, 23–31. DOI 10.1016/j.indcrop.2017.03.019. [Google Scholar] [CrossRef]
120. Fazzino, F., Mauriello, F., Paone, E., Sidari, R., Calabrò, P. S. (2020). Integral valorization of orange peel waste through optimized ensiling: Lactic acid and bioethanol production. Chemosphere, 271. DOI 10.1016/j.chemosphere.2021.129602. [Google Scholar] [CrossRef]
121. Ricci, A., Diaz, A. B., Caro, I., Bernini, V., Galaverna, G. et al. (2019). Orange peels: From by-product to resource through lactic acid fermentation. Journal of the Science of Food and Agriculture, 99(15), 6761–6767. DOI 10.1002/jsfa.9958. [Google Scholar] [CrossRef]
122. Poveda-Giraldo, J. A., Alzate, C. A. C. (2021). A biorefinery for the valorization of marigold (Calendula officinalis) residues to produce biogas and phenolic compounds. Food and Bioproduct Process, 125, 91–104. DOI 10.1016/j.fbp.2020.10.015. [Google Scholar] [CrossRef]
123. Fernando, S. E. L., Peréz-Sariñana Bianca, Y., Sergio, S. T., Eapen, D., Sebastian, P. J. (2014). Evaluation of agro-industrial wastes to produce bioethanol: Case study-mango (Mangifera indica L.). Energy Procedia, 57, 860–866. DOI 10.1016/j.egypro.2014.10.295. [Google Scholar] [CrossRef]
124. Prakash, H., Chauhan, P. S., General, T., Sharma, A. K. (2018). Development of eco-friendly process for the production of bioethanol from banana peel using inhouse developed cocktail of thermo-alkali-stable depolymerizing enzymes. Bioprocess and Biosystems Engineering, 41(7), 1003–1016. DOI 10.1007/s00449-018-1930-3. [Google Scholar] [CrossRef]
125. Seguí, L., Fito Maupoey, P. (2018). An integrated approach for pineapple waste valorisation. Bioethanol production and bromelain extraction from pineapple residues. Journal of Cleaner Production, 172, 1224–1231. DOI 10.1016/j.jclepro.2017.10.284. [Google Scholar] [CrossRef]
126. Chen, M., Zhang, S., Su, Y., Niu, X., Zhu, S. et al. (2022). Catalytic co-pyrolysis of food waste digestate and corn husk with CaO catalyst for upgrading bio-oil. Renewable Energy, 186, 105–114. DOI 10.1016/J.RENENE.2021.12.139. [Google Scholar] [CrossRef]
127. Li, Z., Zhong, Z., Yang, Q., Ben, H., Seufitelli, G. V. S. et al. (2022). Parametric study of catalytic hydropyrolysis of rice husk over a hierarchical micro-mesoporous composite catalyst for production of light alkanes, alkenes, and liquid aromatic hydrocarbons. Fuel (Part C), 310, 122457. DOI 10.1016/J.FUEL.2021.122457. [Google Scholar] [CrossRef]
128. Dien, L. X., Chinh, H. D., Nga, N. K., Luque, R., Osman, S. M. et al. (2022). Facile synthesis of CO3O4@SiO2/Carbon nanocomposite catalysts from rice husk for Low-temperature CO oxidation. Molecular Catalysis, 518, 112053. DOI 10.1016/J.MCAT.2021.112053. [Google Scholar] [CrossRef]
129. Vinu, V., Binitha, N. N. (2020). Lithium silicate based catalysts prepared using arecanut husk ash for biodiesel production from used cooking oil. Matererials Today: Procedings, 25, 241–245. DOI 10.1016/J.MATPR.2020.01.210. [Google Scholar] [CrossRef]
130. de Aguilar Pedott, V., Bordin, I., Oro, C. E. D., Macieski, R. J., Petkowicz, D. I. et al. (2021). Synthesis of Nb/MCM-48 material using rice husk ash as silica source with different SI/NB molar ratios. Revista Perspectiva, 45(171), 85–95. DOI 10.31512/persp.v.45.n.171.2021.159.p.85-95. [Google Scholar] [CrossRef]
131. Bordin, I., de Aguilar Pedott, V., Oro, C. E. D., Junges, A., Dallago, R. M. et al. (2021). Nb-MCM-type mesoporous material synthesis using ionic solid as structure-directing agent for in situ lipase immobilization. Applied Biochemistry and Biotechnology, 193(4), 1072–1085. DOI 10.1007/s12010-020-03484-7. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |