

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.019057
ARTICLE
Effects of Manganese Toxicity on the Growth and Gene Expression at the Seedling Stage of Soybean
1Department of Biotechnology, College of Coastal Agricultural Sciences, Guangdong Ocean University, Zhanjiang, 524088, China
2Department of Resources and Environmental Sciences, College of Chemistry and Environment, Guangdong Ocean University, Zhanjiang, 524088, China
3South China Branch of National Saline-Alkali Tolerant Rice Technology Innovation Center, Zhanjiang, 524088, China
*Corresponding Authors: Ying Liu. Email: liuying85168@126.com; Yingbin Xue. Email: yingbinxue@yeah.net
Received: 31 August 2021; Accepted: 08 October 2021
Abstract: In order to investigate the effects of Manganese (Mn) toxicity stress on the growth and gene expression at the seedling stage of soybean, soybean seedlings were treated with normal Mn concentration (5 μmol/L MnSO4) and excess Mn concentration (100 μmol/L MnSO4) by the method of hydroponic culture in this study. When soybean was subjected to Mn toxicity stress, excessive Mn could affect seedling growth, root development, the number of Mn oxide spots in leaves, and the Mn accumulation content in different parts of soybean. With the increase of exogenous Mn concentration and the prolongation of culture time, the shoot and root biomasses of soybean decreased significantly, but the plant height of soybean had no obvious effects. The total root length, root surface area and root volume of soybean decreased significantly, but the taproot length, taproot tip length and root diameter did not change significantly. The number of Mn spots in the primary leaves (first leaf) was significantly more than that in the old leaves (second leaf) and the youngest leaves (fourth leaf). The Mn concentration in leaves was significantly higher than that in roots, and the Mn concentration in the old leaves was significantly higher than that in youngest leaves with the method of inductively coupled plasma atomic emission spectrometry (ICP-AES). Moreover, the results in the present study suggested that the 10 selected genes were significantly up-regulated or down-regulated by Mn toxicity in the old and young leaves by quantitative real-time PCR (qRT-PCR) analysis. This indicates that these genes might be important in the process of regulation in old and young leaves of the physiological responses and ion transporting to Mn toxicity stress.
Keywords: Manganese toxicity; soybean; gene expression
| Nomenclature | |
| Mn: | Manganese |
| SOD: | Superoxide dismutases |
| POD: | Peroxidase |
| MTP: | Metal tolerance protein |
| ITP: | Ion transport protein |
| NRAMP: | Natural resistance associated macrophage protein |
| ABCT: | ABC transporter |
| YC03-3: | Yuechun 03-3 |
| SPSS: | Statistical product and service solutions |
| ICP-AES: | Inductively coupled plasma atomic emission spectrometry |
| H2O2: | Hydrogen peroxide |
| qRT-PCR: | Quantitative real-time PCR |
| MnSO4: | Manganese sulfate |
Manganese (Mn) is a heavy metal element with a very wide source and distribution. The content of Mn in soil ranges from 450–4000 mg/kg, with an average of about 600 mg/kg [1,2]. Under acidic conditions (pH < 5.5), the Mn in the soil will enter the soil solution in the form of soluble Mn (Mn2+) and then be absorbed and used by plants [3]. Research results show that plants only need 20–40 mg/kg (dry weight) Mn to maintain normal growth and development, but the content of Mn in most plants is as high as 30–500 mg/kg (dry weight), which exceeds the normal Mn requirement of plants [1]. Excessive Mn will produce toxic effects on plants, leading to yellowing, necrosis, leaf crimp, brown spots and other symptoms in plants, which will inhibit plant normal growth and development [4,5]. In addition, the biomass of plants reduced significantly, and the maximum dry weight of aboveground and underground parts decreased respectively under excessive Mn conditions [6]. Mn toxicity is one of the most important factors affecting crop production in acidic soils (pH < 5.5, accounting for 30%–40% of the world’s arable land area), leading to severe crop yield reduction and quality decline [5,7,8]. Moreover, Mn toxicity not only reduces the yield and quality of crops, but also threatens human health [2]. Excessive intake of Mn can cause various kinds of diseases such as Parkinson’s disease, affecting the normal functions of the liver, cardiovascular system and immune system, and having adverse effects on the reproductive system [6,9].
Mn toxicity seriously affects plant growth and development, and its symptoms usually first appear on plant leaves [10]. Brown Mn poisoning spots can be seen on the leaves of cowpea (Vigna unguiculata) and barley (Hordeum vulgare). Also, yellowness and necrosis are observed in the leaves of kidney bean (Phaseolus vulgaris), clover (Trifolium repens), ryegrass (Lolium perenne) and Stylo (Stylosanthes guianensis) too [10,11]. At the same time, photosynthesis will be inhibited under manganese toxic stress and induce the production of excessive reactive oxygen species, which ultimately inhibit plant growth [12]. Studies showed that the photosynthetic rate of wheat (Triticum aestivum) is significantly reduced under Mn toxicity stress [13]. Moreover, the redox reactions of PSI reaction center (P700) and some PSI reaction center polypeptides (PsaA and PsaB) are inhibited in Arabidopsis thaliana [14]. In addition, in the leaves of perennial ryegrass, especially the leaves of sensitive varieties, the quantum yield, electron transport rate and CO2 assimilation rate of PSII all decreased [15]. Furthermore, under Mn toxicity stress, barley (Hordeum vulgare) [16], cucumber (Cucumis sativus) [17], ryegrass [15], polish wheat [18] and rice (Oryza sativa) [7] are all induced oxidative stress response in leaves, which affects the normal growth of plants. When plants absorb excessive Mn, their photosynthetic efficiency and chlorophyll content decrease, young leaves turn yellow, leaf conduction tissue turn necrotic, superoxide dismutase (SOD) activity increase, and veins of old leaves wrinkle and brown spots form, thus seriously affecting plant growth, development and yield [10,19–21].
Soybean (Glycine max) is native to China and is an annual herb of the genus Glycine. As an important economic crop, soybean is an important source of vegetable oil and protein for humans [22]. In acidic soils, Mn toxicity is the second only to aluminum toxicity as an obstacle to crop growth [23]. Soybean is very sensitive to manganese toxicity. When the external soluble manganese (Mn2+) concentration exceeds 50 μM, soybean will suffer serious Mn toxicity [10,24]. However, the study on the effect of Mn toxicity on soybean growth and the mechanism of Mn tolerance are still in its infancy. In this study, the effects of exogenous Mn concentration on soybean growth, uptake of Mn and expression of key genes were studied, which laid a foundation for further study on the mechanism of Mn toxicity tolerance in soybean.
The variety of soybean named Yuechun 03-3 (YC03-3) was used to conduct the experiment at the Root Biology Center of South China Agricultural University [25].
2.2 Acquisition of Cultivated Seedlings and Hydroponic Conditions
Soybean seeds with uniform size were selected, sterilized with 10% NaClO, sown in quartz sand, and germinated in a greenhouse at 25°C. The seedlings were transplanted into 14 L plastic pots for nutrient solution cultivation in the greenhouse. The culture temperature was 25°C/28°C (night/day), the humidity of the greenhouse was 75%, and the illumination intensity was 800 μmol·m−2·s−1. When the first ternately compound leaves were unfolded, seedlings of the same growth were selected and transplanted into 14 L plastic pots for hydroponic culture. The plastic pot was 30 cm wide × 40 cm long ×12 cm high. The components of the hydroponic nutrient solution were 1500 μM KNO3, 400 μM NH4NO3, 25 μM MgCl2, 1200 μM Ca(NO3)2⋅4H2O, 40 μM Fe-EDTA(Na), 500 μM MgSO4⋅7H2O, 300 μM K2SO4, 300 μM (NH4)2SO4, 0.5 μM CuSO4⋅5H2O, 1.5 μM ZnSO4⋅7H2O, 500 μM KH2PO4, 0.16 μM (NH4)5MoO24⋅4H2O, and 2.5 μM NaB4O7⋅10H2O. The nutrient solution was changed every 7 d, and KOH or H2SO4 was used every 2 d to adjust the pH to 5.8 [24,25].
2.3 Treatments with Different Mn Concentrations
After the seedlings were cultivated for 5 d, when the second ternate compound leaves were fully expanded, the soybean seedlings were treated with manganese sulfate (MnSO4), and two Mn concentration gradients were set: 5 (normal Mn concentration) and 100 (Mn toxicity stress) µmol/L. After different times, from the Mn treatment (0, 3, 6, 9, 12 and 15 d), the soybean plants were harvested, and various variables were measured severally.
2.4 Determination of Dry Weight, Fresh Weight and Plant Height
After harvesting the soybean plants, the plant height and the fresh weight of the shoot and root parts were immediately measured. Thereafter, plant materials were transferred to a drying oven at 105°C for 30 min, and then dried until constant weight at 75°C. Finally, their dry weight was determined. Each studied variable has four biological replicates.
The amount of Mn oxidation spots on the leaves was determined by the square method [26], and the number of spots on the middle, back, and front sections of the leaves were measured with a 1 cm2 transparent plastic film. The average value was determined, and the test was repeated 4 times [24].
2.6 Root Morphological Parameters Analysis
Soybean seedling root morphological parameters were analyzed by the WinRHIZO software as described previously [27]. The fresh roots of each treatment were harvested and fully unfurled to acquire a root image on a scanner (Epson, Japan), and then analyzed by the computer image analysis software (WinRhizo Pro, Regent Instruments, Quebec, Canada).
2.7 Determination of Mn Concentrations
The concentrations of Mn in the shoots and roots of the plants were measured by inductively coupled plasma atomic emission spectrometry (ICP-AES) [28]. There were four replicates plants for making each of these measurements.
2.8 Quantitative Real-Time PCR Analysis
Soybean young and old leaves under 5 and 100 µmol/L MnSO4 treatments were harvested for total RNA extraction through TRIzol reagent (Invitrogen, USA). The M-MLV reverse transcriptase (Promega, USA) was used for the synthesis of the first-strand cDNA. The quantitative real-time PCR (qRT-PCR) analysis was conducted by a Step One Plus Real-Time PCR system (ABI, USA). GmEF-1α was used as the housekeeping gene [19]. The relative expression was represented by the ratio of the candidate gene to the housekeeping gene with three biological replicates. According to the results of transcriptome sequencing, the expression of some genes related to physiological responses and ion transport may be affected by Mn toxicity stress [10]. There were ten genes associated with the physiological responses (SOD: Superoxide dismutases; POD: Peroxidase) and ion transport (MTP: Metal tolerance protein; ITP: Ion transport protein; NRAMP: Natural resistance associated macrophage protein; ABCT: ABC transporter). These genes were selected for further qRT-PCR analysis since they were involved which might involve in Mn toxicity response. The primers used for qRT-PCR were shown in Appendix A.
2.9 Statistical Analysis of Data
All data were processed with Microsoft Office Excel 2010. Data analysis was accomplished via the statistical software Statistical Product and Service Solutions (SPSS; 13.0 for MS Windows, USA). When F tests were significant, mean comparisons were made using the Duncan’s multiple comparison test at a significant level of p ≤ 5%.
3.1 Effects of Exogenous Mn Concentration on the Growth of Soybean Seedling
With the increase of exogenous Mn concentration and the extension of culture time, the biomasses of shoots and roots in soybean significantly decreased, while the soybean plant height was not significantly affected (Figs. 1 and 2A–2E). When the culture time was 15 days and the concentration of exogenous Mn was 100 μmol/L, the fresh weight of shoots and roots decreased significantly by 34.50% and 33.33%, respectively (Figs. 2B and 2C). At this time and concentration of Mn, the dry weight of shoots and roots was significantly reduced by 27.73% and 23.44%, respectively (Figs. 2D and 2E). There was no significant difference in plant height between treatments (Fig. 2A).

Figure 1: Soybean plant growth under different Mn treatments. Soybean seedlings were grown under normal conditions for 5 d, and then treated with 5 or 100 μM MnSO4 for 0, 3, 6, 9, 12 and 15 d (bar = 1 cm)
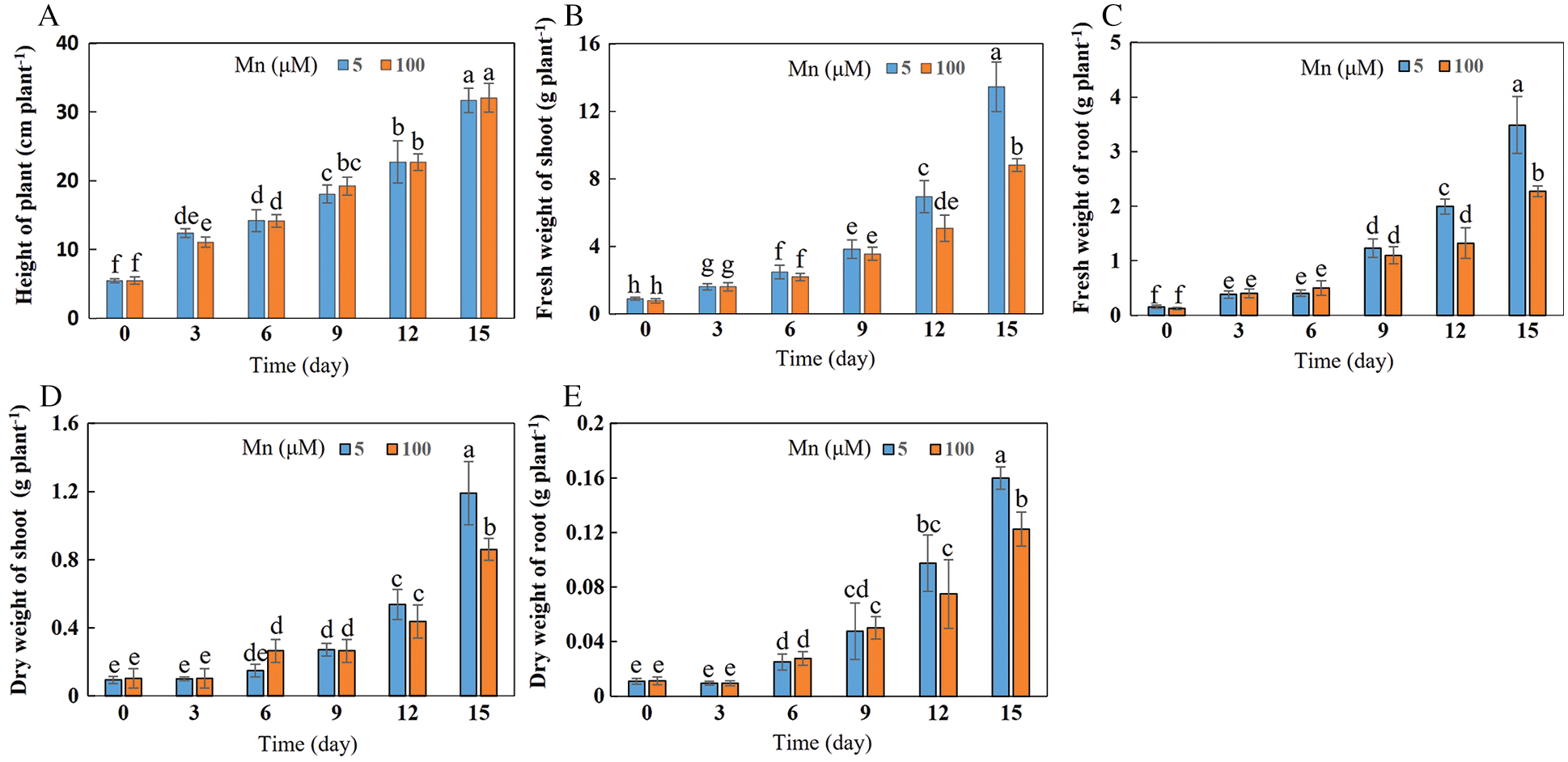
Figure 2: Effects of different Mn concentrations on soybean growth at various times. (A) Plant height; (B) fresh weight of shoots; (C) fresh weight of roots; (D) dry weight of shoots; (E) dry weight of roots. Soybean seedlings were grown under normal conditions for 5 d, and then treated with 5 or 100 μM MnSO4 for 0, 3, 6, 9, 12 and 15 d. Each bar represents the mean of four independent replicates ± 1 standard error. Means followed by different letters are significantly different at the p ≤ 5% level as determined by the Duncan’s multiple range test
3.2 Effects of Exogenous Mn Concentration on Soybean Root Growth and Development
With the increase of exogenous Mn concentration and the extension of culture time, the growth and development of soybean roots were affected to a certain extent (Fig. 3). When the exogenous manganese concentration was 100 μmol/L and the culture time was 15 days, the total root length, root surface area and root volume were significantly decreased by 24.14%, 25.53% and 33.33%, respectively, in comparison with the control Mn (5 μmol/L) concentration (Figs. 3A, 3D and 3F). Nevertheless, the taproot length, taproot tip length, and root diameter did not change significantly (Figs. 3B, 3C, and 3E).
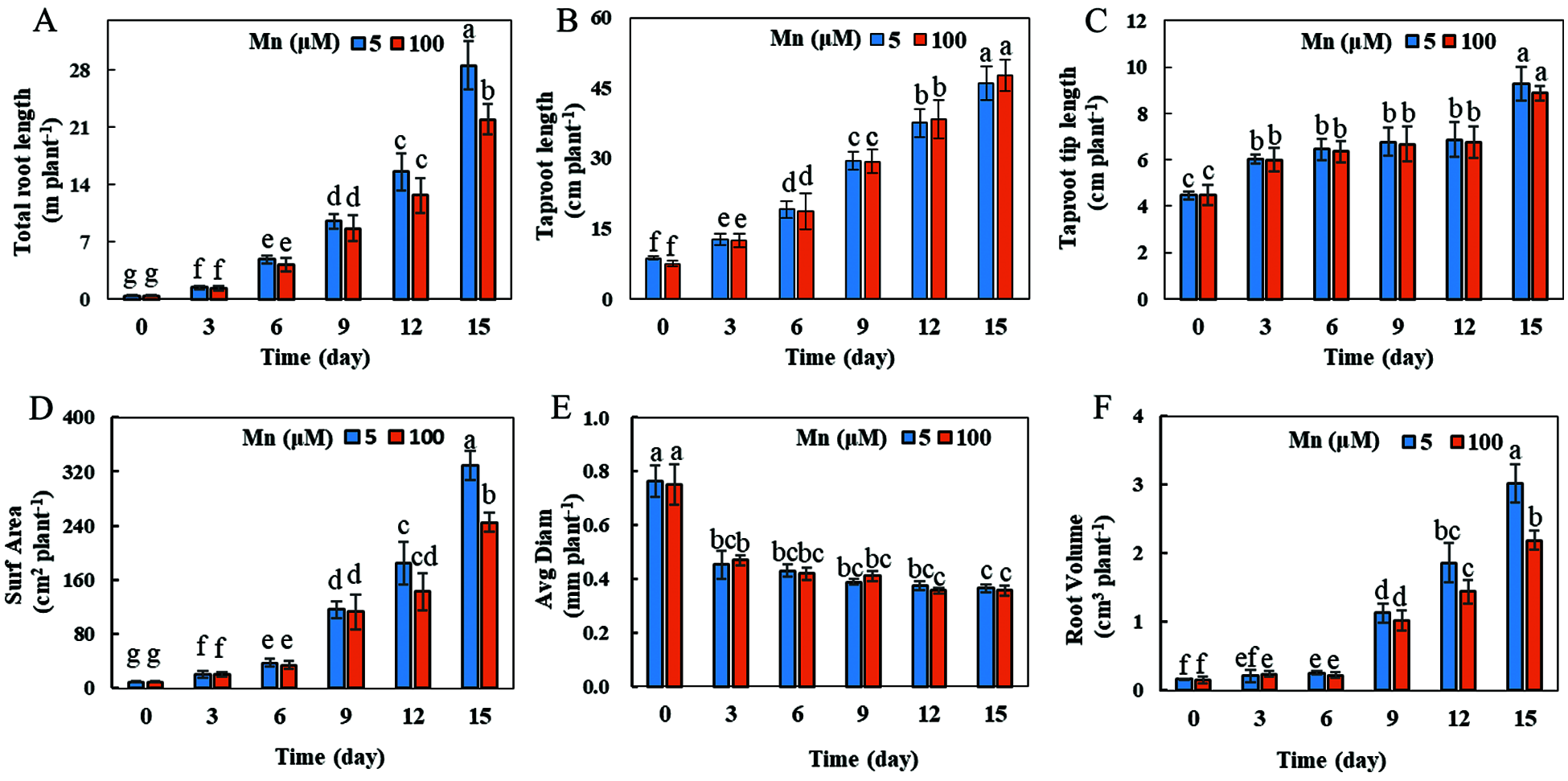
Figure 3: Root growth under different Mn treatments. (A) Total root length; (B) taproot length; (C) taproot tip length; (D) root surface area; (E) average root diameter; (F) root volume. Soybean seedlings were grown under normal conditions for 5 d, and then treated with 5 or 100 μM MnSO4 for 0, 3, 6, 9, 12 and 15 d. Each bar represents the mean of four independent replicates ± 1 standard error. Means followed by different letters are significantly different at the p ≤ 5% level as determined by the Duncan’s multiple range test
3.3 Influence of Exogenous Mn Concentration on Accumulation of Mn Toxicity Spots in Soybean Leaves
With the increase of exogenous Mn concentration and the extension of culture time, the effect on the accumulation of Mn toxicity spots in soybean leaves became more and more obvious (Figs. 4 and 5). Mn toxicity stress caused brown spots in the first leaf of soybean (primary leaf) on the 6th day of hydroponics, and then the symptoms increased with the extension of culture time. When the Mn treatment reached the 9th day, the first leaf (primary leaf) and the second leaf (old leaf) of soybean showed obvious symptoms of Mn spots. After 12 days of Mn treatment, brown spots appeared in the primary leaf, old leaf and third leaf. When the Mn treatment reached the 15th day, Mn spots appeared in the primary leaf, the old leaf, and on the third and fourth leaves (younger leaves). The number of manganese spots in the primary leaf (136.25) was significantly higher than that in the old leaf (88.00) and the youngest leaves (15.25). At the same time, the leaf margins of the primary, old and young leaves showed obvious symptoms of shrinkage (Figs. 4 and 5).
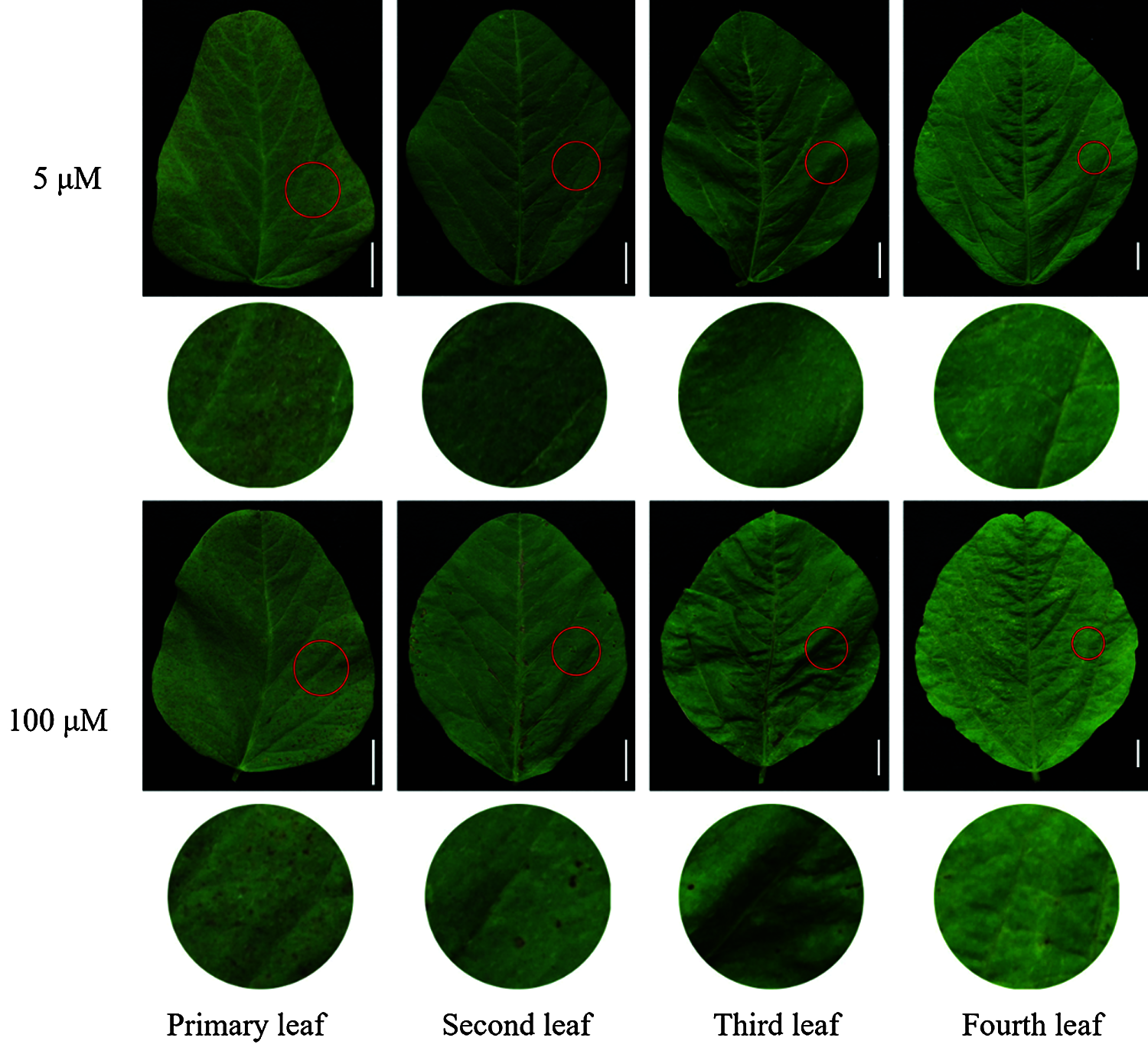
Figure 4: Effects of different Mn concentrations on brown spot accumulation in various types of leaves in soybean. Soybean seedlings were grown under normal conditions for 5 d, and then treated with 5 or 100 μM MnSO4 for 15 d (bar = 1 cm)
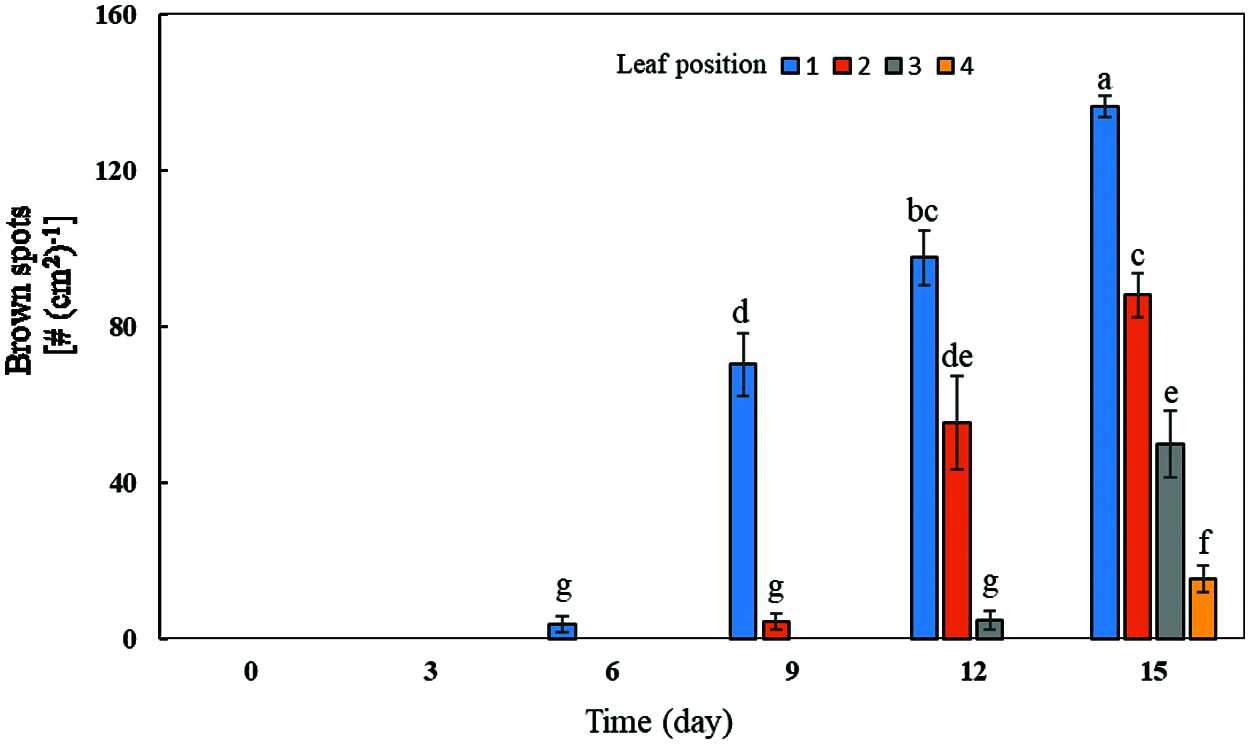
Figure 5: Effects of different Mn concentrations on brown spot accumulation in various types of leaves in soybean. Soybean seedlings were grown under normal conditions for 5 d, and then treated with 5 and 100 μM MnSO4 for 0, 3, 6, 9, 12 and 15 d (bar = 2 cm). 1: Primary leaf; 2: Second leaf (old leaf); 3: Third leaf; 4: Fourth leaf (young leaf). Each bar represents the mean of four independent replicates ± 1 standard error. Means followed by different letters on the columns significantly different at the p ≤ 5% level as determined by the Duncan’s multiple range test
3.4 Effects of Exogenous Mn Levels on Mn Concentrations in Soybean Plants
With the increase of exogenous Mn concentration, the Mn concentrations in soybean plants were significantly affected (Fig. 6). When the culture time was 15 days and the exogenous Mn concentration was 100 μmol/L, the exogenous Mn level significantly affected the Mn concentrations in soybean leaves and roots; the distribution of Mn concentrations was as follows: primary leaf > second leaf > third leaf > young leaf > roots. Compared with the normal Mn treatment group, the Mn concentrations in the primary, second, third, and young leaves and roots increased by 7.41, 9.16, 6.73, 8.38 and 5.74 times, respectively (Fig. 6). Therefore, when soybean was subjected to Mn toxicity stress, the Mn concentration on leaves was significantly higher than that in roots, and the Mn concentration in the old leaf was significantly higher than that in the young leaf.
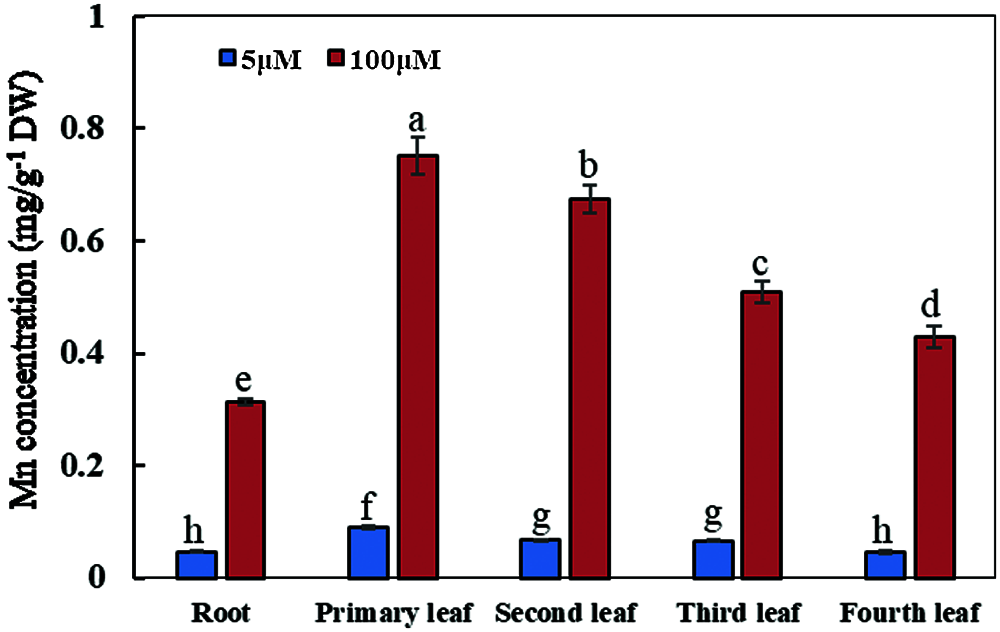
Figure 6: Concentrations of manganese in various types of plant organs. Soybean seedlings were grown under normal conditions for 5 d, and then treated with 5 or 100 μM MnSO4 during 15 days of culture. Each bar represents the mean of four independent replicates ± 1 standard error. Means followed by different letters on the columns are significantly different at the p ≤ 5% level as determined by the Duncan’s multiple range test
3.5 Influence of Exogenous Mn Levels on Several Mn Toxicity Response Genes in Soybean
A total of ten genes may be involved in the Mn toxicity responses measured by qRT-PCR analysis. These genes correlated with the physiological responses (SOD: Superoxide dismutases; POD: Peroxidase) and ion transport (MTP: Metal tolerance protein; ITP: Ion transport protein; NRAMP: Natural resistance associated macrophage protein; ABCT: ABC transporter). They showed a significantly up-regulated or down-regulated expression in old and young leaves exposed to Mn toxicity stress (Fig. 7). GmSOD, GmPOD1, GmPOD2, GmMTP, GmITP, GmNRAMP1b, GmNRAMP2a, GmNRAMP5b and GmABCT showed a significantly up-regulated expression in young leaves subjected to Mn toxicity stress. Only GmNRAMP3a had a significantly down-regulated expression in young leaves (Fig. 7). The genes which showed a significantly up-regulated expression in old leaves were GmPOD1, GmPOD2, GmMTP, GmITP, and GmNramp5b. GmPOD1, GmPOD2, GmMTP, GmITP and GmNRAMP5b were up-regulated in both old and young leaves (Fig. 7).
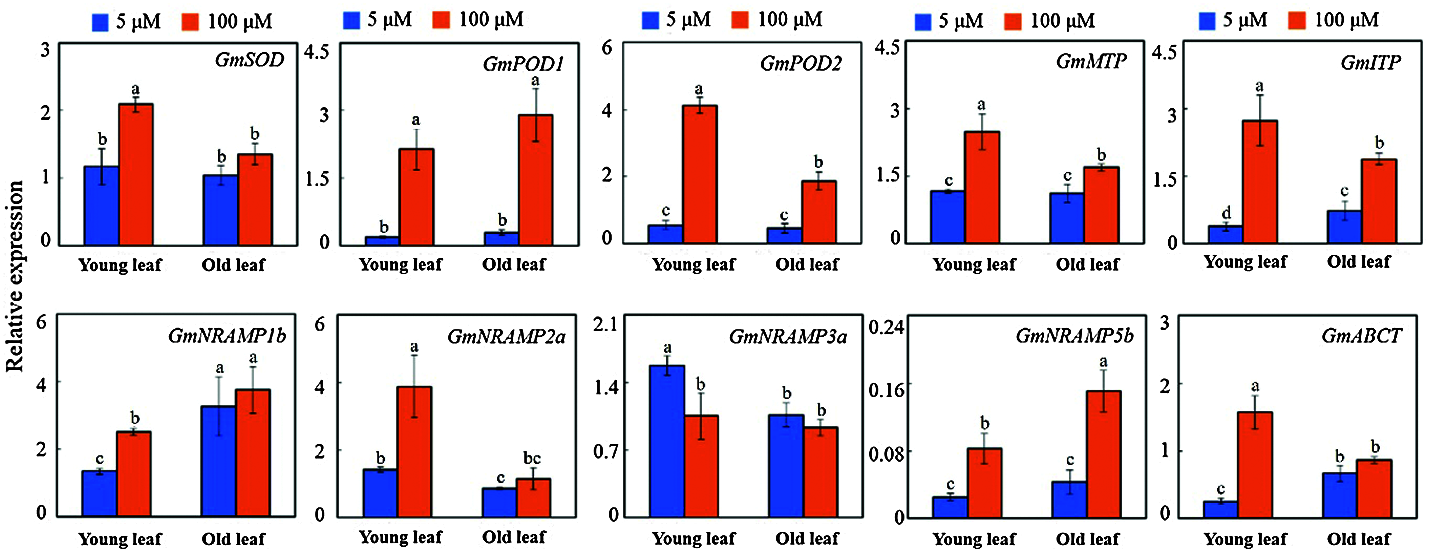
Figure 7: The expression levels of 10 selected genes on the old and young leaves of soybean treated with different concentrations of Mn after testing by qRT-PCR. SOD: Superoxide dismutases; POD: Peroxidase; MTP: Metal tolerance protein; ITP: Ion transport protein; NRAMP: Natural resistance associated macrophage protein; ABCT: ABC transporter
Although Mn is an essential trace element for plant growth, excessive Mn can produce toxic effects on plants, especially in acidic soils; Mn toxicity is considered to be one of the main factors limiting crop production [19,24]. Studies have shown that excessive Mn inhibits the growth of cucumber (Cucumis sativus), leading to a significant decrease in the dry weight of the aboveground and underground parts [17]. The results of this study showed that the biomasses of shoots and roots in soybean were significantly reduced, and root growth was inhibited to a certain extent under the Mn toxicity stress. This may be due to the accumulation of excessive Mn in plants, which can cause serious damage to plant cells [29,30], thus affecting the normal growth and development of plants.
The results of this study demonstrated that Mn toxicity can cause brown Mn spots and wrinkling in soybean leaves. This is similar to previously reported results on rice and pea (Pisum sativum) [31,32]. Mn spots and wrinkles on leaves are one of the main symptoms of manganese poisoning in plants [31,33,34]. The formation of Mn oxidation spots is mainly caused by the accumulation of Mn oxides or oxidized phenols in the outer epidermal cell walls of plants exposed to Mn poisoning conditions [31,33,34]. The results of this study showed that under Mn toxicity stress, significant Mn oxidation spots appeared in the primary, old and young leaves of soybean, indicating that there was also a large amount of Mn oxides or oxidized phenols generated by the soybean leaves.
When soybean was subjected to Mn toxicity stress, the Mn concentration in soybean leaves was significantly higher than that on roots, indicating that the Mn in roots might be actively transported to the leaves to improve the tolerance of soybean to Mn toxicity. At the same time, the concentration of Mn in leaves from various positions was also different, and the concentration of Mn in primary and old leaves was significantly higher than that in young leaves. Translocation or accumulation of excess Mn in aging leaves might be an important mechanism to alleviate the toxic effects of Mn on plant roots and young leaves of soybean. This suggests that there might be some mechanism of Mn distribution in leaves, but the molecular mechanism still remains unclear.
Mn toxicity causes severe oxidative stress. Activation of the plant antioxidant system has been considered as an important mechanism for plant adaptation to Mn toxicity [11]. In our study, GmSOD was up-regulated in young leaves, and GmPOD1/2 were up-regulated in both young and old leaves by Mn toxicity. This suggests that the three genes may contribute to improving the leaf antioxidant system to increase the plant Mn toxicity tolerance.
Studies have shown that the absorption, transport and fixation of Mn in plants may be co-regulated by various metal-transporter proteins [35–37]. For example, OsNRAMP5 and other transporters [e.g., Yellow stripelike (YSL), Cation diffusion facilitator transporter (CAX), Cation diffusion facilitator transporter (CDF) and PIIa-ATPases] may be involved in the Mn uptake and transport processes in rice leaves. However, the mechanism of the interaction between these transporters remains to be further explored [38]. Other studies have shown that metal tolerance proteins (MTP) play an important role in regulating the adaptation of plant leaves to Mn toxicity. In this case, OsMTP8.2 may require the participation of OsMTP8.1 to complete the Mn transport in the process of transferring the excess Mn to leaf vacuoles [39]. Furthermore, research results showed that the calcium-dependent protein kinase (CPKs) family member named CPK4/5/6/11 (which are positioning in the vacuole membrane and known as Mn transporter) interact with the AtMTP8 protein to regulate the Mn steady-state in plants. Moreover, further studies indicated that the serine (numbers 31 and 32) activate of MTP8 can be activated by the CPK4/5/6/11 phosphorylating MTP8, which will cause excess Mn ions in the cytoplasm to be transported into the vacuole, thus enhancing plant tolerance to Mn toxicity [8]. Furthermore, other studies have shown that plants under Mn toxicity stress can respond to this stress by regulating secondary metabolic pathways (such as phenylpropane-like metabolism) and related proteins (such as phenylalanine ammonia-lyase) expression [2]. The results of this study revealed that the 10 selected genes were significantly up-regulated or down-regulated by Mn toxicity stress in the old and young leaves. This suggests that these genes might be important in the process of regulation of the physiological response to Mn toxicity stress and ion transporting in old and young leaves. It is possible that the regulatory mechanism might be complex and require one or more proteins to co-regulate the process of ion transport. However, the specific molecular regulation mechanism remains still unclear, and needs further studies.
The effects of the availability of exogenous Mn were studied on soybean growth and the absorption and distribution of Mn. Growth and development of soybean plants could be inhibited by excessive Mn. Mn tolerance might be related to the accumulation of more Mn in leaves than roots in soybean, the promotion of Mn transport to the shoot, and the existence of different Mn distribution patterns (higher in old than young leaves). This study laid the foundation for further studies on and analysis of the physiological and molecular mechanisms involved in the soybean tolerance to Mn toxicity. Furthermore, this study indicated that the 10 selected genes were significantly up-regulated or down-regulated by Mn toxicity in the old and young leaves. This reveals that these genes might be important in the process of regulation of the physiological responses to Mn toxicity stress and ion transporting in old and young leaves. However, further studies are needed to determine the specific molecular regulation mechanism to Mn toxicity.
Funding Statement: This research was funded by the Guangdong Basic and Applied Basic Research Foundation (2020A1515011570), Nanhai Youth Scholar Project of Guangdong Ocean University (002029001012), Program for Scientific Research Start-Up Funds of Guangdong Ocean University (R17023 and R19031), Foundation of Education Department of Guangdong Province (2019KTSCX059), the Project of Science and Technology of Zhanjiang City (2020B01019), Guangdong Ocean University Graduate Education Innovation Project (Excellent Dissertation Cultivation Project) (040502052101), and the National Natural Science Foundation of China (32002131).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Shao, J. F., Yamaji, N., Shen, R. F., Ma, J. F. (2017). The key to Mn homeostasis in plants: Regulation of Mn transporters. Trends in Plant Science, 22(3), 215–224. [Google Scholar]
2. Liu, P., Huang, R., Hu, X., Jia, Y., Li, J. et al. (2019). Physiological responses and proteomic changes reveal insights into Stylosanthes response to manganese toxicity. BMC Plant Biology, 19(1), 212. [Google Scholar]
3. Blamey, F. P. C., McKenna, B. A., Li, C., Cheng, M., Tang, C. et al. (2018). Manganese distribution and speciation help to explain the effects of silicate and phosphate on manganese toxicity in four crop species. New Phytologist, 217(3), 1146–1160. [Google Scholar]
4. Pan, G., Zhang, H., Liu, P., Xiao, Z., Li, X. et al. (2019). Effects of manganese stress on phenology and biomass allocation in Xanthium strumarium from metalliferous and non-metalliferous sites. Ecotoxicology and Environmental Safety, 172, 308–316. [Google Scholar]
5. Yang, S., Yi, K., Chang, M. M., Ling, G. Z., Zhao, Z. K. et al. (2019). Sequestration of Mn into the cell wall contributes to Mn tolerance in sugarcane (Saccharum officinarum L.). Plant and Soil, 436, 475–487. [Google Scholar]
6. Mora, M., Rosas, A., Ribera, A., Rengel, Z. (2009). Differential tolerance to Mn toxicity in perennial ryegrass genotypes: Involvement of antioxidative enzymes and root exudation of carboxylates. Plant and Soil, 320, 79–89. [Google Scholar]
7. Li, P., Song, A., Li, Z. J., Fan, F. L., Liang, Y. C. (2015). Silicon ameliorates manganese toxicity by regulating both physiological processes and expression of genes associated with photosynthesis in rice (Oryza sativa L.). Plant and Soil, 397, 289–301. [Google Scholar]
8. Zhang, Z., Fu, D., Sun, Z., Ju, C., Miao, C. et al. (2021). Tonoplast-associated calcium signaling regulates manganese homeostasis in Arabidopsis. Molecular Plant, 14(5), 805–819. [Google Scholar]
9. Lucchini, R., Placidi, D., Cagna, G., Fedrighi, C., Oppini, M. et al. (2017). Manganese and developmental neurotoxicity. Advances in Neurobiology, 18, 13–34. [Google Scholar]
10. Liu, Y., Xue, Y., Xie, B., Zhu, S., Lu, X. et al. (2020). Complex gene regulation between young and old soybean leaves in responses to manganese toxicity. Plant Physiology and Biochemistry, 155, 231–242. [Google Scholar]
11. Li, J., Jia, Y., Dong, R., Huang, R., Liu, P. et al. (2019). Advances in the mechanisms of plant tolerance to manganese toxicity. International Journal of Molecular Sciences, 20(20), 5096. [Google Scholar]
12. Fecht-Christoffers, M. M., Führs, H., Braun, H. P., Horst, W. J. (2006). The role of hydrogen peroxide-producing and hydrogen peroxide-consuming peroxidases in the leaf apoplast of cowpea in manganese tolerance. Plant Physiology, 140(4), 1451–1463. [Google Scholar]
13. Sheita, M. M., Taylor, G. J. (1992). The effects of excess manganese on photosynthetic rate and concentration of chlorophyll in Triticum aestivum grown in solution culture. Physiologia Plantarum, 85, 467–475. [Google Scholar]
14. Millaleo, R., Reyes-Díaz, M., Alberdi, M., Ivanov, A. G., Krol, M. et al. (2013). Excess manganese differentially inhibits photosystem I versus II in Arabidopsis thaliana. Journal of Experimental Botany, 64(1), 343–354. [Google Scholar]
15. Ribera, A. E., Reyes-Díaz, M. M., Alberdi, M. R., Alvarez-Cortez, D. A., Rengel, Z. et al. (2013). Photosynthetic impairment caused by manganese toxicity and associated antioxidative responses in perennial ryegrass. Crop and Pasture Science, 64(7), 696–707. [Google Scholar]
16. Demirevska-Kepova, K., Simova-Stoilova, L., Stoyanova, Z., Hölzer, R., Feller, U. (2004). Biochemical changes in barley plants after excess supply of copper and manganese. Environmental and Experimental Botany, 53(2), 253–266. [Google Scholar]
17. Shi, Q., Zhu, Z., Min, X., Qian, Q., Yu, J. (2006). Effect of excess manganese on the antioxidant system in Cucumis sativus L. under two light intensities. Environmental and Experimental Botany, 58, 197–205. [Google Scholar]
18. Sheng, H., Zeng, J., Yan, F., Wang, X., Wang, Y. et al. (2015). Effect of exogenous salicylic acid on manganese toxicity, mineral nutrients translocation and antioxidative system in polish wheat (Triticum polonicum L.). Acta Physiologiae Plantarum, 37, 1–11. [Google Scholar]
19. Chen, Z., Sun, L., Liu, P., Liu, G., Tian, J. et al. (2015). Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiology, 167(1), 176–188. [Google Scholar]
20. Shrestha, A., Dziwornu, A. K., Ueda, Y., Wu, L. B., Mathew, B. et al. (2018). Genome-wide association study to identify candidate loci and genes for Mn toxicity tolerance in rice. PLoS One, 13(2), e0192116. [Google Scholar]
21. Yu, F., Li, Y., Li, F., Li, C., Liu, K. (2019). The effects of EDTA on plant growth and manganese (Mn) accumulation in Polygonum pubescens Blume cultured in unexplored soil, mining soil and tailing soil from the Pingle Mn mine. China Ecotoxicology and Environmental Safety, 173, 235–242. [Google Scholar]
22. Thapa, R., Carrero-Colón, M., Hudson, K. A. (2016). New alleles of to reduce palmitic acid levels in soybean. Crop Science, 56, 1076–1080. [Google Scholar]
23. Foy, C. D., Adams, F. (1984). Physiological effects of hydrogen, aluminum and manganese toxicities in acid soils, in soil acidity and liming. Agronomy Monograph, 12, 57–97. [Google Scholar]
24. Chen, Z., Yan, W., Sun, L., Tian, J., Liao, H. (2016). Proteomic analysis reveals growth inhibition of soybean roots by manganese toxicity is associated with alteration of cell wall structure and lignification. Journal of Proteomics, 143, 151–160. [Google Scholar]
25. Xue, Y. B., Xiao, B. X., Zhu, S. N., Mo, X. H., Liang, C. Y. et al. (2017). GmPHR25, a GmPHR member up-regulated by phosphate starvation, controls phosphate homeostasis in soybean. Journal of Experimental Botany, 68(17), 4951–4967. [Google Scholar]
26. Horst, W. J., Fecht, M., Naumann, A., Wissemeier, A. H., Maier, P. (1999). Physiology of manganese toxicity and tolerance in Vigna unguiculata (L.) Walp. Journal of Plant Nutrition and Soil Science, 162, 263–274. [Google Scholar]
27. Pornaro, C., Macolino, S., Menegon, A., Richardson, M. (2017). Winrhizo technology for measuring morphological traits of bermudagrass stolons. Agronomy Journal, 109(6), 3007–3010. [Google Scholar]
28. Tüzen, M. (2003). Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchemical Journal, 74, 289–297. [Google Scholar]
29. Kochian, L. V., Hoekenga, O. A., Pineros, M. A. (2004). How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology, 55, 459–493. [Google Scholar]
30. Millaleo, R., Reyes-Díaz, M., Ivanov, A. G., Mora, M. L., Alberdi, M. (2010). Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. Journal of Soil Science and Plant Nutrition, 10(4), 470–481. [Google Scholar]
31. Horiguchi, T. (1988). Mechanism of manganese toxicity and tolerance of plants: IV. Effects of silicon on alleviation of manganese toxicity of rice plants. Soil Science and Plant Nutrition, 34(1), 65–73. [Google Scholar]
32. Wissemeier, A. H., Horst, W. J. (1992). Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata (L.) Walp.). Plant and Soil, 143(2), 299–309. [Google Scholar]
33. Santandrea, G., Pandolfini, T., Bennici, A. (2000). A physiological characterization of Mn-tolerant tobacco plants selected by in vitro culture. Plant Science, 150(2), 163–170. [Google Scholar]
34. Fecht-Christoffers, M. M., Maier, P., Horst, W. J. (2003). Apoplastic peroxidases and ascorbate are involved in manganese toxicity and tolerance of Vigna unguiculata. Physiologia Plantarum, 117(2), 237–244. [Google Scholar]
35. Socha, A. L., Guerinot, M. L. (2014). Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Frontiers in Plant Science, 5, 106. [Google Scholar]
36. Pooja, S., Hao, N. H., Samir, K., Christian, L., Kim, S. H. et al. (2021). Efficiency of transporter genes and proteins in hyperaccumulator plants for metals tolerance in wastewater treatment: Sustainable technique for metal detoxification. Environmental Technology & Innovation, 23(9), 101725. DOI 10.1016/J.ETI.2021.101725. [Google Scholar] [CrossRef]
37. Zhang, J., Zhang, M., Song, H, Y., Zhao, J, Q., Shabala, S. et al. (2020). A novel plasma membrane-based NRAMP transporter contributes to Cd and Zn hyperaccumulation in Sedum alfredii Hance. Environmental and Experimental Botany, 176(12), 104121. DOI 10.1016/j.envexpbot.2020.104121. [Google Scholar] [CrossRef]
38. Chen, Z. H., Fujii, Y., Yamaji, N., Masuda, S., Takemoto, Y. et al. (2013). Mn tolerance in rice is mediated by MTP8. 1, a member of the cation diffusion facilitator family. Journal of Experimental Botany, 64, 4375–4387. [Google Scholar]
39. Takemoto, Y., Tsunemitsu, Y., Fujii-Kashino, M., Mitani-Ueno, N., Yamaji, N. et al. (2017). The tonoplast-localized transporter MTP8.2 contributes to manganese detoxification in the shoots and roots of Oryza sativa L. Plant and Cell Physiology, 58, 1573–1582. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |