

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.018688
REVIEW
Recent Developments to Mitigate Selenium Deficiency in Agricultural Eco-Systems
1Institute of Environment and Ecology School of the Environment and Safety Engineering, Jiangsu University, Zhenjiang, 21201, China
2College of Agriculture, Fujian Agriculture and Forestry University, Fuzhou, 350002, China
3Chinese Academy of Agricultural Sciences, Nanning, 530007, China
4Laboratory of Bioclimatology, Department of Ecology and Environmental Protection, Poznan University of Life Sciences, Poznań, 60-649, Poland
5Academy of Biology and Biotechnology, Southern Federal University, Rostov-on-Don, 344006, Russia
6Department of Agronomy, University of Agriculture, Faisalabad, 38040, Pakistan
7College of Food Science and Biotechnology, Key Laboratory of Fruits and Vegetables Postharvest and Processing Technology Research of Zhejiang Province, Zhejiang Gongshang University, Hangzhou, 310018, China
8Department of Soil and Environmental Science, University of Agriculture, Faisalabad, 38000, Pakistan
9Department of Agronomy, Faculty of Agriculture, University of Poonch Rawalakot, Rawalakot, 12350, Pakistan
10Department of Entomology, Faculty of Agriculture, University of Poonch Rawalakot, Rawalakot, 12350, Pakistan
11Department of Agronomy, Hajee Mohammad Danesh Science and Technology University, Dinajpur, 5200, Bangladesh
12Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, 22233, Saudi Arabia
13Princess Dr. Najla Bint Saud Al-Saud Center for Excellence Research in Biotechnology, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
14Department of Biosystem Engineering, Faculty of Agriculture, Siirt University, Siirt, 56220, Turkey
15Faculty of Agriculture, Department of Field Crops, Siirt University, Siirt, 56100, Turkey
16Department of Agronomy, Faculty of Agriculture, Kafrelsheikh University, Kafrelsheikh, 33156, Egypt
17Seed Science and Technology, University of Agriculture Faisalabad, Faisalabad, 38040, Pakistan
*Corresponding Authors: Khalid Rehman Hakeem. Email: kur.hakeem@gmail.com; Ayman EL Sabagh. Email: ayman.elsabagh@agr.kfs.edu.eg
Received: 10 August 2021; Accepted: 05 November 2021
Abstract: Under changing climate, trace elements like selenium (Se) have emerged as vital constituent of agro-ecosystems enabling crop plants to off-set the adverse effects of suboptimal growth conditions. The available form of selenium is important for boosting its bioavailability to crop plants having varied agro-botanical traits and root architectural systems. As compared to selenite, the selenate has a weaker soil bonding, higher absorption in the soil solution which results in a comparatively absorption by plant roots. Various factors including dry climate, high pH, optimal ambient air temperature, less accumulation of water, and low concentration of organic matter in the soil tend to boost the selenate ratio in the soil. The use of selenium pelleted seeds has emerged as an interesting and viable alternative to alleviate selenium deficiency in agricultural eco-systems. Similarly, the co-inoculation of a mixture of Selenobacteria and Arbuscular mycorrhizal fungi represents an evolving promising strategy for the bio-fortification of wheat plants to produce selenium-rich flour to supplement human dietary needs. Furthermore, in-depth research is required to assure the effectiveness of biological fertilization procedures in field conditions as well as to explore and increase our understanding pertaining to the underlying main mechanisms and channels of selenium absorption in plants. The focus of this review is to synthesize the recent developments on Se dynamics in soil-plant systems and emerging promising strategies to optimize its levels for crop plants. Recent developments regarding the use of micro-organisms as a biotechnological mean to enhance plant nutrition and crop quality have been objectively elaborated. The study becomes even more pertinent for arid and semi-arid agro-ecosystems owing to the potential role of selenium in providing stress tolerance to crop plants. Moreover, this review synthesizes and summarizes the recent developments on climate change and bioavailability, and the protective role of selenium in crop plants.
Keywords: Abiotic stresses; bioavailability; selenate; selenite; climate change; sustainable agriculture
Selenium (Se) is one of the rare elements on earth with an average concentration of around 0.05 mg · kg−1 in igneous rocks, which is lesser than that for other nutrients [1–3]. The Se resources are limited in the globe because there is no ore from which Se can be mined as the main product [4]. The Se has been regarded as an essential element for various ruminants and mammals, however, its consumption by these animals remains largely insufficient owing to a variety of factors especially the low Se concentration present in forage crops and other feedstuffs. Additionally, Se has not been recognized as an essential element in plants, but it tends to stimulate the plant metabolism, leading to an enhanced synthesis of molecules such as catalase, ascorbate peroxidase, and glutathione peroxidase along with several non-enzymatic antioxidants including glucosinolates, vitamins, phenolic compounds, and chlorophylls [5]. Selenium has one of the narrowest ranges between dietary deficiency (<40 μg · day−1) and toxicity levels (>400 μg · day−1). Geology has profoundly controlled the concentration of Se in the soil, where we grow crops and animals that form the human food chain. Due to different ecological conditions, the Se status differs significantly across the world depending on the population, animals, and crops. Conversely, health outcomes depend not only on the total amount of Se in rocks and soil, but also on the amount of Se absorbed/accumulated by plant roots and that bioavailable to animals [5]. The soil Se status can be estimated through plant Se concentrations. It has been reported earlier that plants can accumulate almost a thousand µg · g of Se [6]. Plants can accumulate less than 1 μg · g of Se under optimal and normal soil conditions. Plants grown in normal soils accumulate a lot of sulfur (S), and Se accumulation is higher than that of S [7]. Although anthropogenic Se is the main source of Se content in the soil, its major part could not be used biologically. Some microorganisms (fungi and bacteria) reduce the bioavailable form of Se to insoluble forms, while others convert it (Se) to volatile organic forms; both of these forms are not available for plant absorption. Some bacteria can oxidize colloidal Se, making it biologically available to crop plants [8]. The Se bioavailability in the soil environment has been predicted to be critical for agricultural productivity under changing climatic scenarios. Efforts are needed for the quantification of plant-available Se in the soil, and the amount, and form of absorption. It is recommended to assess suspicious areas for soil and crop analysis to show that there will not be a non-obvious Se deficiency in the animals [9].
It is interesting to note that Se enters the food chain through plants, while its concentration in plants is directly regulated by the Se concentration in the soil solution. The bioavailability and absorption of Se by plants depend largely on the redox balance in the soil, environmental factors and the plant species. The pertinence of Se for agricultural ecosystems is clear from the fact that the global atlas illustrates the variability of Se distribution globally [2]. Se accumulation in the plant is a worldwide concern. In various regions, the Se content is not enough to meet the nutritional demands of animals. In these low-Se areas, people have tried to increase its concentration in plant and animal tissues [8]. It has been suggested that Se may play an important role in plant growth, development, and productivity, but it has not been confirmed. In soil, the plant utilization rate of Se6+ is usually many times higher than that of Se4+. Additionally, soil physical and chemical characteristics such as texture, pH, organic matter content, and the presence of numerous ions (SO24− and PO34−) also significantly affect the absorption of Se by plants [10]. Techniques for increasing the tissue concentration of Se include applying Se to the soil, seeds, and plant leaves. Regardless of the method used, the addition of Se6+ will result in a higher concentration of plant Se than the addition of Se4+. Due to the low Se content in the soil or the difficulty of absorbing soil Se by plants (or a combination of these two factors), crops are deficient in Se [8,11,12]. Due to the expansion of modern agricultural firms in formerly poor countries, especially in Asia and Latin America, many of these countries may now face problems alike Europe because of the high bioavailability of soil Se [13]. The cultivated land is usually lower than before. Due to population and economic growth, this problem may become more common soon. Therefore, this review synthesizes and summarizes the recent literature on the bioavailability of Se under climate change and its protective role in crop plants.
The form of Se is important which directly affects the Se bioavailability for various crop species. It is interesting to note that selenate has weaker bonds with soil particles compared with selenite which promotes its higher absorption by the plant roots [14]. In addition, various other factors such as high pH, low concentration of soil organic matter, high ambient air temperature, dry climate, and reduced water uptake could multiply the selenate to selenite ratio in the soil solution. However, selenite is the main form of inorganic Se in soils of high organic matter content (e.g., Nordic countries with low soil temperature causes the degradation of soil organic matter to be much slower than in tropical countries), and it is likely to be in waterlogged soils under rice transplantations [15]. In New Zealand, insufficient Se absorption and Se concentration in the volcanic rock bedrock can well explain the underlying reasons for Se scarcity in crop plants. Selenite ions could not only be highly adsorbed to the ferric oxide/hydroxide minerals but could also be strongly adsorbed to ureidomethane formed through weathering of the volcanic glass [2]. After manufacturers removed arsenic from superphosphate fertilizers, the Se status in animals and plants declined in New Zealand [16]. The process used to remove arsenic also removes Se, and the color of the fertilizer changes from pink (due to the fine distribution of elemental Se) to white or gray [17]. In the short term, sheep began to show Se deficiency symptoms and died, followed by bulldozer burial in thousands of mass graves.
Low Se contents in the soil solution are possibly due to the co-precipitation of phosphate and selenite ions, sometimes needing the addition of Se fertilizers [18]. Another concern is the mechanism involved in selenite absorption by plant roots and mycorrhizas, and there might be either specific membrane transporters for selenite or selenite ions as well as other more abundant anions such as phosphates or these anions may share a common membrane transport protein [19]. If the latter is the case, it must be expected that phosphate tends to act as a competitive inhibitor of selenite ion migration to plant roots, even if there is no interaction between the elements binding to minerals (adsorption or co-precipitation). This may also be true in the case of interactions. In soil, the mechanism of active transport of selenite ions to other types of organisms may also cause the same problem to get into various planktonic algae [20]. Not only the level of Se but also its bioavailability in soil has to be high enough (Fig. 1). It is important to avoid the use of phosphorus-rich and Se-poor commercial fertilizers that could damage the bioavailability of soil Se to crop plants through extensive roots networks [21]. This will contribute to the development of natural Se-fortified soils for growing Se-rich foods (cereals grains, fruits, vegetables, etc.).
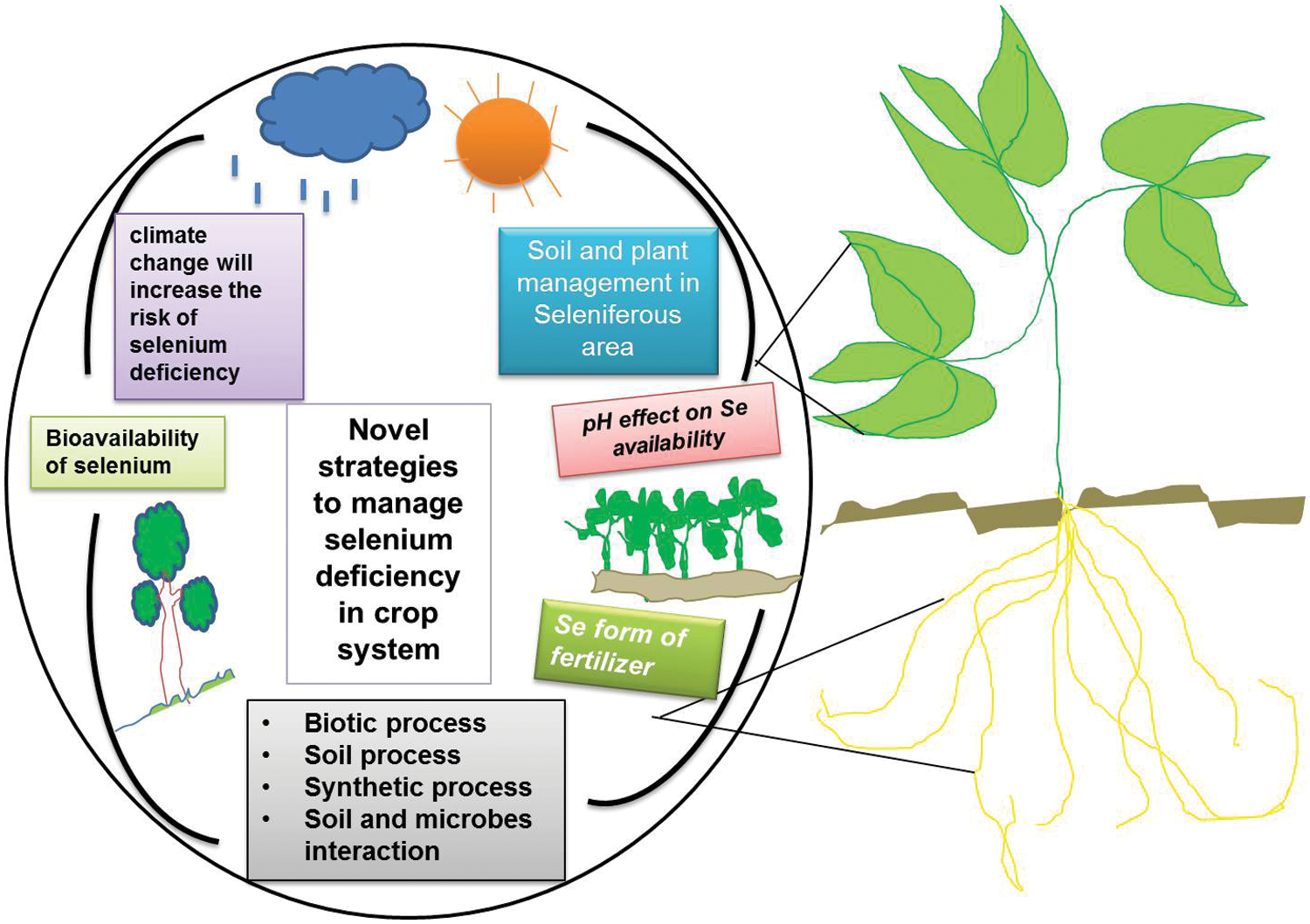
Figure 1: Abiotic and biotic processes affecting Se availability in soil-crop interacting systems
2.2 Se Accumulation and Plant Species
The absorption and accumulation of Se in either form depend on the species as well as the agro-botanical traits, especially the root architecture of crop plants. The main Se accumulators, such as Astragalus spp. and Stanleya pinnata may contain up to 10–15 g of Se per kg of DW. Previous studies found that the accumulation capacity of Se changed up to 15 times in Brassica crop species [22]. Some mushrooms may accumulate Se. Some Se accumulator forest mushrooms are antagonic with the effects of toxic metals such as Cd. This is important in Southern Norway forests which are exposed to heavy metal toxicity, such as Cd, because of the frequent acid rains [23]. In addition, some areas of Norway and Sweden have the bedrock Cambrian bituminous shale (alum shale) rich in Cd, which contributes significantly to heavy metal toxicity A Se-rich soybean variety has also been found [20].
The Se content varied in the different plant parts of Astragalus bisulcatus and the mature leaves contained the highest Se content [24]. Selenium excessively accumulated in the trichomes which were approximately 47% and 53% in γ-glutamyl-Se-methylselenocystine (GGSeMeSeCys) and selenomethylselenocystine (Se-MeSeCys) (organic), respectively. Young leaves contained 70% of Se-MeSeCys and 30% of inorganic Se content in the form of selenite (SeO32−) and selenate (SeO42−). In the young leaves of Stanleya pinnata, high Se concentration was observed in the periphery of the epidermal cells and near the leaf edges where selenocystathionine and Se-MeSeCys were 12 and 88%, respectively [25]. Higher peripheral Se concentration could act as a basic line of defense in plants. Other applications of Se-enriched sprouted seeds can be considered, such as those of bean sprouts, alfalfa-lucerne, etc., which can be mixed with various foods for Se fortification [26,27]. It has been studied the germination of the seeds in wheat, alfalfa (Medicago sativa), and sunflower (Helianthus annuus), and the absorption rate of inorganic selenium [28].
It is necessary to formulate biologically viable strategies to manage the rare Se resources in the world by reducing the waste of this fragile resource. Se is essential for living organisms, i.e., human and animal health, and the quality of animal products; it can also potentially improve plant growth and development along with the quality of agricultural products. Large-scale bio-augmentation programs that add Se to commercial fertilizers may be a very wasteful method that cannot be applied to most areas of our planet because a large amount of Se used in making them will be lost for future use [29]. Careful consideration of plant bio-enhancement is needed to avoid wastage of Se. Countries should evaluate these supplementary methods based on Se resource planning and sustainability. The food industry can directly add Se compounds to food products (process intensification). Adding Se to food can aid in avoiding oxidation by heating treatments. Selenium bio-fortification is the addition of Se-enriched sprouts to Se-rich environments [30]. Special attention needs to be paid to the development of supplement diets in developing countries, as the number of processed and fortified foods in these countries is limited which has contributed to food and nutritional insecurity [2].
Selenium in the soil exists in several inorganic forms including elemental Se (Se), selenide (Se2), selenite (SeO32−), selenate (SeO42−) and many organic forms such as Se-met. The SeO32− and SeO42− dominate in cultivated soils, while other forms are scant in ploughed soils. In addition, other factors such as soil reaction, aeration, hydrological conditions, and soil redox potential also affect Se concentration and its bioavailability to crop plants [31]. Depending on the dynamics of these factors, Se tends to undergo transformations that are regulated by redox processes. However, the availability of the Se primarily depends on the pH and redox potential as shown in Fig. 1. Previous research demonstrated that enhanced Se content in wheat grain was observed by co-inoculation of Selenobacteria and Arbuscular mycorrhizal fungi [32]. Li et al. [33] reported that Arbuscular mycorrhizal fungi increased the bioavailability of Se in soil and enhanced the uptake Se in wheat (Triticum aestivum L.) plants. Furthermore, inoculation with Selenobacteria and Arbuscular mycorrhizal fungi resulted in an enhanced Se content in lettuce plants, and improved tolerance against drought stress [34]. Inoculation of arbuscular mycorrhizal fungi (AMF), foliar application of sodium selenate, and the combination AMF + Se on garlic and onion regarding bulb yields, and biochemical characteristics and mineral composition were assessed, and reported that application of AMF + Se resulted in the highest yields, monosaccharides, and Se content in both garlic and onion bulbs, increased the ascorbic acid and flavonoids in onion, and flavonoids in garlic, increased macronutrients (P, K, Ca and Mg) and microelements (B, Cu, Fe, Mn, Si, Zn) in bulbs, as well as Se with or without AMF, promoted the accumulation of B and Si in onion, and Mo and Zn in garlic bulbs [35]. Overall, the combined use of arbuscular mycorrhizal fungus and selenium fertilizer also shaped the microbial community structure and enhanced the organic Se accumulation in crop plants [36].
3 Climate Change and Selenium Availability
During the last decade, climate change and global warming effects have become more pronounced on soil fertility status and nutrient availability for crop plants [1]. Being a trace element, Se has been recognized as very important for human health, while a narrow dietary range to maintain normal biological activity is required. It has been estimated that insufficient Se intake has affected almost one billion people worldwide [37]. The availability of dietary Se is controlled mainly by the interaction between soil and plants, but the exact mechanism that controls its widespread soil distribution is undecided [38]. Jones et al. [39] used data mining techniques to model the distribution of Se at the global level during 1980–1999, and identified climate-soil interaction as the main controlling factor. In addition, using moderate climate change predictions, it has been anticipated that soil Se losses in the future (2080–2099) might go up to 8.4% as recorded in 58% of the modeled area [1]. The predicted farmland loss is even higher, with 66% of the farmland expected to lose 8.7% of Se annually. These intensive losses may multiply the severity of Se deficiency worldwide. Therefore, it is a major goal to identify a broad mechanism to enhance soil-Se retention and availability to crop plants. However, for the time being, the data used may be inadequate to evaluate and determine the impact of many small to regional-scale factors (local sources, specific soils, types of rocks, etc.) that affect soil-Se retention. To evaluate the small-scale soil Se distribution or test local correlation hypotheses, a suitable scale model is needed [40]. Some of the impacts of climate change on global food security are foreseeable including a noticeable decline of food products obtained from C3 crops due to a limited water supply. Nevertheless, their general reduction in the soil nutrient profile, especially Se concentration, under climate change has remained unforeseen. Variations in other factors (such as specific sources of Se, soil properties, weathering of soil and rocks, etc.) may have additional effects on the soil-Se content, but these factors have not been analyzed because of their impact on soil pH, soil clay content, and the future of space forecast. There is currently no available information on the contribution of man-made and natural sources of Se [41]. These sources may also have an impact on the soil-Se concentration. For example, considering the changes in industrial SOx and NOx emissions, the pH of the soil may increase. Considering the inverse relationship between soil Se and soil pH, an increase in pH may lead to a further decrease in soil Se concentrations. Therefore, as more data becomes available, updated soil Se predictions may be changed [42].
Among many other adverse consequences of climate change, the profound reduction in the concentration of the essential trace elements including Se in the soil may pose a challenge to the sustainability of modern farming systems. As a result, the Se fraction in crops may also decrease which may lead to an increase in the risk of Se deficiency in various parts of the globe. This is demonstrated by a study that used data mining to model the global distribution of Se [43]. The Se, as an essential micronutrient is obtained from dietary sources such as grains, while Se contents in food principally depend on their concentration in the soil. Previous studies have shown that the low Se contents are directly associated with high soil pH and oxygen utilization, as well as low clay and soil organic carbon content. It is evident in regional studies that in Europe, Se-poor soils are particularly present in Germany, Denmark, Scotland, Finland, and some Balkan countries [44]. Two main factors affecting soil Se content are precipitation and drought index (potential evaporation: precipitation ratio) [45]. Among negatively affecting factors of Se concentration, precipitation remains the top factor as it causes the leaching of Se from the soil. Simultaneously, precipitation has a positive effect on the Se concentration, as the oxygen content in moist soil is lower than that in dry soil. As a result, it serves to lower the solubility of Se which leads to lower mobility of this micronutrient [46]. Moreover, frequent precipitation leads to a drop in the soil pH, which helps in promoting the binding of negatively charged Se to soil particles. Areas with high Se content are usually present in regions that receive low to moderate precipitation and have a high clay content, while low Se content is found in arid areas where soils have a high pH and low clay content [39].
4 pH Effects on Se Availability
Among many other factors, soil pH has been found to influence Se concentration in the soil solution and its availability to crop plants. It usually exists in the form of water-soluble selenate in well-aerated alkaline soils [47]. Some soils also contain rotten selenomethionine from selenium plants, and some plants have the potential to absorb this methionine [48]. The effect of Se and sodium selenide (Na2SeO4) from various organic matters (4 mg Se/kg) was studied on Trifolium alexandrinum (Berseem) and Lactuca sativa (lettuce) under controlled conditions of greenhouse in both alkaline (pH = 8.0) and acidic (pH = 6.3) soils. Previous research findings indicated a higher Se content in T. alexandrinum that was grown in acidic soil, while up to a 45% reduction in biomass of lettuce was observed under the combined application of Se and Na2SeO4. It depicted a negative correlation between Se and organic matter (OM) of the soil whereby higher OM content caused significant reductions of Se bioavailability and absorption by crop plants. Additionally, results indicated that T. alexandrinum planted on acid soil had a higher Se content. It has also been observed that the combined application of Se in acid soil significantly reduces the plant biomass of lettuce (approximately by 45%). While a decreased Se content was observed in the plants grown on alkaline soil, it indicated the negative correlation between pH and Se content due to the decrease in a number of binding sites (pH-dependent) with the increase of pH resulting in the accumulation of selenates (a usable form of Se). Soil organic matter (SOM) was seemed to be compensating the effect of pH on the Se absorption [49].
However, the SOM in alkaline soil increased by 4.5%–8.5%, which resulted in a decrease of Se utilization, indicating that high contents of organic matter can limit the absorption of Se by plants because of the soil pH. Therefore, based on earlier findings, increasing soil pH does not seem to be an appropriate regulating method to increase Se-utilization in soils rich with organic matter. Treatment of berseem and lettuce with sodium selenate resulted in a lower Se-content in lettuce than in berseem; the original Se content of both alkaline and acidic soils did not seem to have any effect on both plant species [50]. Since other soil characteristics (i.e., competitive anions, microbial activity and redox potential) could affect the effectiveness of Se, further research is required to confirm the Se absorption mechanism and strategies of Se fertilization under field conditions [51]. The utilization rate of Se in vegetation is affected by the following factors, i.e., climate variables, soil physiology, soil weathering and pH of soil. Mobile Se is known as the form of water moving under aerobic soil conditions that can be used for plant absorption [52]. Selenate is a readily available form of Se and has been absorbed by plants. The Se absorption leads to reduce the plant growth in order to: SeO42− > H2SeO3 > SeO32− > Se [53,54].
5 Selenium Metabolism in Plants
Although Se is not an essential element for plants to complete their life cycle, it might be beneficial for triggering growth and ensuring the survival of plants under sub-optimal and harsh soil, and environmental conditions [55]. Excessive Se concentration tends to impart toxic effects which damage plant tissues and cause disruption of plant vital physiological mechanisms. The ability to chelate Se in vacuoles, synthesize non-toxic Se metabolites or volatilize Se compounds determines the highest concentration of Se in tissues and the ability of plants to colonize selenized soils [45]. The difference in Se metabolism determines the maximum Se concentration in plant tissues, which is very important for the delivery of Se to the diet of herbivores and the evolution of plant species that colonize Se soils [56]. It has been observed that boron is easily absorbed by crop plants from the soil solution due to its weaker bond with soil particles through electrostatic attraction. The excessive absorption and mobilization of selenate have been observed in many plants due to its resemblance with S and sulfates, and the use of the S-assimilation pathway [57]. Selenic acid is first activated by the ATP sulfurylase-adenosine 5′-phosphate selenate (APSE), and then reduced to selenite by the adenosine 5′-phosphosulfate reductase in reducing glutathione during the reduction of peptides; selenium is also reduced to selenide (II) [56]. The metabolic process and final products of Se metabolism depend on the accumulation capacity of plants [58]. In non-Se enriched plants, five steps lead to the formation of dimethyl selenide (DMSE), while in Se enriched plants, the final product is dimethyl diselenide (DMDSe) [59]. The five steps of Se metabolism (Fig. 2) include reduction of selenate to selenite, reduction of selenite to selenide; conversion of selenide into selenocysteine; conversion of selenocysteine into selenomethionine, and lastly conversion of methionine to dimethyl selenide [60–66]. The conversion of selenite to selenide and volatile substances (such as dimethyl selenide) in plants may be the detoxification mechanism of toxic Se species [56].
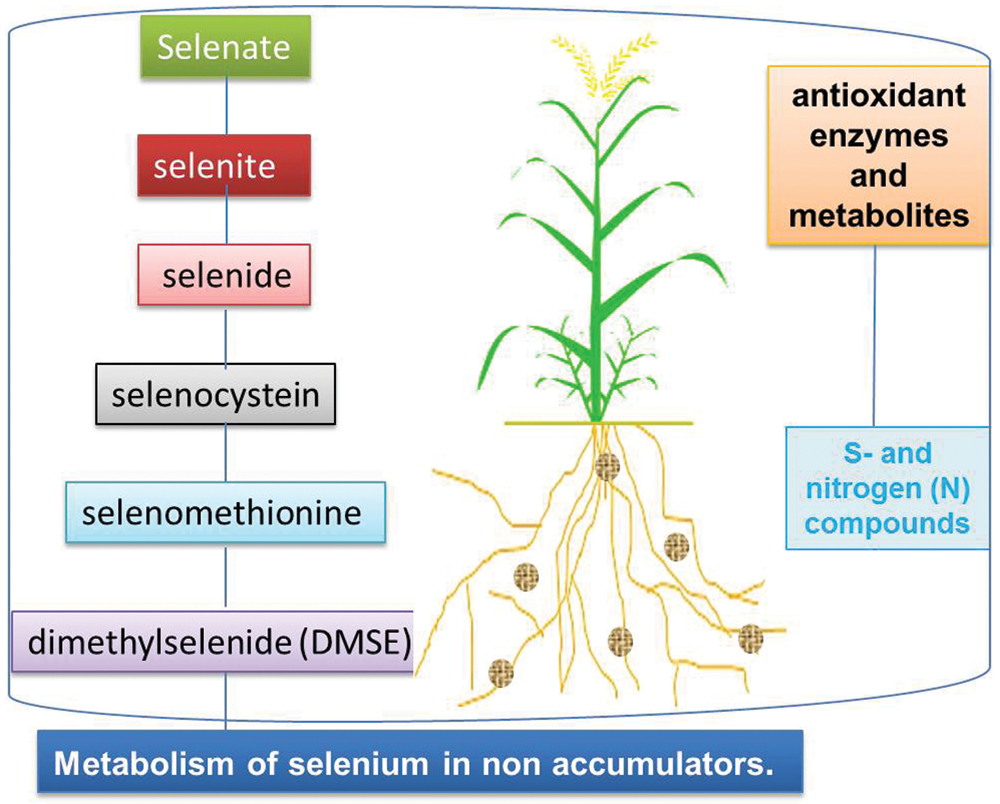
Figure 2: Impact of Se on metabolic processes on plants
6 Selenium Nanoparticles: Characterization and Application
There is a fine line between optimum limit/or deficiency and excess of Se in the living system which may cause toxicity. It is known that the Se nanoparticles prepared from biological material are less toxic than bulk Se nanoparticles prepared from chemicals. The biomolecules present in the extract act both as reducing agents and stabilizers of Se nanoparticles. Bacteria, algae, dry fruits and plant extracts are used to produce nanoparticles. Green synthesis of selenium nanoparticles from selenious acid was achieved from dried extracts of raisin (Vitis vinifera) [66]. Like other biological materials, raisin also contains sugar, flavonoids and phenols in addition to minerals such as K, Ca and Fe [67]. A change from colorless to the deeply brick-red color indicated the formation of nanoparticles. The formation of Se-nanoballs was examined at different intervals of time. It took nearly 6 min to start the conversion of Se ion to Se nanoparticles which were indicated by a decrease in the Se ion concentration in the solution. The nature of nanoparticles was analyzed by transmission electron microscopy (TEM) images. It showed that the diameter of nanoballs ranged between 3 and 18 nm. They were found to be encapsulated with a thin polymorphic layer. The formation of Se nanoparticles was confirmed from energy-dispersive x-ray spectroscopy. The Se nanoballs were identified from their characteristic absorption peaks at 1.37, 11.22 and 12.49 KeV [68]. The morphology of Se nanoparticles can be analyzed by X-ray diffraction (XRD) analysis. The broad diffraction peak suggests the presence of the amorphous nature of Se nanoparticles [69]. Their particle size [70] is in the order of 12 nm.
Stable Se nanoparticles in colloidal form were prepared from Terminalia arjuna extract in the aqueous medium. They were characterized by UV–vis, energy dispersive X-ray analysis (EDAX) and TEM analysis. The maximum absorbance in the colloidal solution was recorded at 390 nm. Its IR spectrum showed peaks corresponding to O-H, NH, C = O and C-O stretches suggesting the presence of hydroxyl, amino, ketonic and carbonyl functional groups in the extract which act both as stabilizer and capping agents for the Se nanoparticles [71]. The Se nanoparticles synthesized from fenugreek seed extract in aquous medium at room temperature are between 50–150 nm. They have been found to be active against human breast cancer cells [72]. Biological Se nanoparticles were successfully used to suppress Triticum aestivum L. crown rot and root rot diseases induced by Fusarium species, and to improve yield under drought and heat stress [73].
7 Conclusion and Future Prospects
Recently, a number of researches have established that dietary intake of micronutrients in sufficient quantity is essential for the physical and physiological well-being of humans. Similarly, Se insufficiency in food can pose serious threats not only to human life but also to animals by causing numerous diseases including different types of cancers. It has been estimated that over 15% of the world’s population is deficient in Se which gives rise to the need for Se bio-fortification in staple grains as well as leafy vegetables. Since wheat is the most used edible food, and thus it has become the need of time to develop and promote Se-bio-fortification strategies that are not only biologically viable but also economically feasible. These strategies have also to be safe in terms of Se-enrichment in the safer limits for humans and ruminants. They are highly desirable for semi-arid and arid regions having soils poor in Se contents, and need special attention regarding Se-supplementation. This is because it will not only increase the dietary content of Se but also increase crop production through enhancement of the drought resistance capability of plants. Regarding future research needs, it becomes pertinent to determine the appropriate doses of Se for staple crops that will generate productive outcomes for humans, and plants and humans along with agricultural soils. Since available data from previous research findings, a computational model may be designed for predicting the need and efficiency of Se for various food crops of economic and nutritional significance. An interesting challenge posed to researchers for Se bio-fortification is to determine how much variability exists among crop genotypes regarding safe limits of Se tolerance. Future studies need to explore this aspect to formulate strategies for Se enrichment in food crops.
Acknowledgement: The authors thank Muhammad Zaryab Khalid and Sunny Ahmar for critically reviewing and editing the manuscript.
Funding Statement: This manuscript received no external funding.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Jones, G. D., Droz, B., Greve, P., Gottschalk, P., Poffet, D. et al. (2017). Selenium deficiency risk predicted to increase under future climate change. Proceedings of the National Academy of Sciences of the United States of America, 114(11), 2848–2853. DOI 10.1073/pnas.1611576114. [Google Scholar] [CrossRef]
2. Haug, A., Graham, R. D., Christophersen, O. A., Lyons, G. H. (2009). How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microbial Ecology in Health and Disease, 19(4), 209–228. DOI 10.1080/08910600701698986. [Google Scholar] [CrossRef]
3. Ramkissoon, C., Degryse, F., da Silva, R. C., Baird, R., Young, S. D. et al. (2019). Improving the efficacy of selenium fertilizers for wheat biofortification. Scientific Reports, 9(1), 3292. DOI 10.1038/s41598-019-55914-0. [Google Scholar] [CrossRef]
4. Oliveira, C. S., Piccoli, B. C., Aschner, M., Rocha, J. B. T. (2017). Chemical speciation of selenium and mercury as determinant of their neurotoxicity. Neurotoxicity of Metals, 18, 53–83. DOI 10.1007/978-3-319-60189-2. [Google Scholar] [CrossRef]
5. Fordyce, F. M. (2013). Selenium deficiency and toxicity in the environment. In: Selinus, O. (Ed.Essentials of Medical geology, pp. 375–416. Dordrecht: Springer. [Google Scholar]
6. Girling, C. A. (1984). Selenium in agriculture and the environment. Agriculture, Ecosystems, & Environment, 11(1), 37–65. DOI 10.1016/0167-8809(84)90047-1. [Google Scholar] [CrossRef]
7. Kaur, N., Sharma, S., Kaur, S., Nayyar, H. (2014). Selenium in agriculture: A nutrient or contaminant for crops? Archives of Agronomy and Soil Science, 60(12), 1593–1624. DOI 10.1080/03650340.2014.918258. [Google Scholar] [CrossRef]
8. Mikkelsen, R. L., Page, A. L., Bingham, F. T. (1989). Factors affecting selenium accumulation by agricultural crops. Selenium in Agriculture and the Environment, 23, 65–94. DOI 10.2136/sssaspecpub23.c4. [Google Scholar] [CrossRef]
9. El-Ramady, H. R., Domokos-Szabolcsy, É., Shalaby, T. A., Prokisch, J., Fári, M. (2015). Selenium in agriculture: Water, air, soil, plants, food, animals and nanoselenium. In: Lichtfouse, E., Schwarzbauer, J. Robert, D. (Eds.CO2 sequestration, biofuels and depollution. pp. 153–232. Berlin, Germany: Springer. [Google Scholar]
10. Al-Ani, L. K. T., Franzino, T., Aguilar-Marcelino, L., el Zahar Haichar, F., Furtado, E. L. et al. (2020). The role of microbial signals in plant growth and development: Current status and future prospects. In: New and future developments in microbial biotechnology and bioengineering, pp. 225–242.Elsevier. [Google Scholar]
11. Wang, J., Wang, Z., Mao, H., Zhao, H., Huang, D. (2013). Increasing Se concentration in maize grain with soil-or foliar-applied selenite on the Loess Plateau in China. Field Crops Research, 150, 83–90. DOI 10.1016/j.fcr.2013.06.010. [Google Scholar] [CrossRef]
12. Lyons, G. H., Stangoulis, J. C., Graham, R. D. (2004). Exploiting micronutrient interaction to optimize biofortification programs: The case for inclusion of selenium and iodine in the HarvestPlus program. Nutrition Reviews, 62(6), 247–252. DOI 10.1301/nr2004.jun247-252. [Google Scholar] [CrossRef]
13. Galić, L., Vinković, T., Ravnjak, B., Lončarić, Z. (2021). Agronomic biofortification of significant cereal crops with selenium–A review. Agronomy, 11(5), 1015. DOI 10.3390/agronomy11051015. [Google Scholar] [CrossRef]
14. Ali, F., Peng, Q., Wang, D., Cui, Z., Huang, J. et al. (2017). Effects of selenite and selenate application on distribution and transformation of selenium fractions in soil and its bioavailability for wheat (Triticum aestivum L.). Environmental Science and Pollution Research, 24(9), 8315–8325. DOI 10.1007/s11356-017-8512-9. [Google Scholar] [CrossRef]
15. Dinh, Q. T., Li, Z., Tran, T. A. T., Wang, D., Liang, D. (2017). Role of organic acids on the bioavailability of selenium in soil: A review. Chemosphere, 184, 618–635. DOI 10.1016/j.chemosphere.2017.06.034. [Google Scholar] [CrossRef]
16. Allen, R. O., Steinnes, E. (1980). Contribution from long-range atmospheric transport to the heavy metal pollution of surface soil. International Conference on the Ecological Impact of Acid Precipitation, Sandefjord (Norway). SNSF Project. [Google Scholar]
17. Gupta, M., Gupta, S. (2017). An overview of selenium uptake, metabolism, and toxicity in plants. Frontiers in Plant Science, 7, 160. DOI 10.3389/fpls.2016.02074. [Google Scholar] [CrossRef]
18. Hopper, J. L., Parker, D. R. (1999). Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant and Soil, 210(2), 199–207. DOI 10.1023/A:1004639906245. [Google Scholar] [CrossRef]
19. Paulraj, S., Kumar, M. S. (2016). Selenium Bioavailability through Microbes. In: Singh, U., Praharaj, C. S., Singh, S. S., Singh, N. P. (Eds.Biofortification of food crops, pp. 303–316. New Delhi: Springer. [Google Scholar]
20. di Tullo, P., Pannier, F., Thiry, Y., Le Hécho, I., Bueno, M. (2016). Field study of time-dependent selenium partitioning in soils using isotopically enriched stable selenite tracer. Science of the Total Environment, 562, 280–288. DOI 10.1016/j.scitotenv.2016.03.207. [Google Scholar] [CrossRef]
21. Power, A. G. (2010). Ecosystem services and agriculture: Tradeoffs and synergies. Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1554), 2959–2971. DOI 10.1098/rstb.2010.0143. [Google Scholar] [CrossRef]
22. Hladun, K. R., Parker, D. R., Trumble, J. T. (2011). Selenium accumulation in the floral tissues of two Brassicaceae species and its impact on floral traits and plant performance. Environmental and Experimental Botany, 74, 90–97. DOI 10.1016/j.envexpbot.2011.05.003. [Google Scholar] [CrossRef]
23. Yasin, M., El-Mehdawi, A. F., Jahn, C. E., Anwar, A., Turner, M. F. et al. (2015). Seleniferous soils as a source for production of selenium-enriched foods and potential of bacteria to enhance plant selenium uptake. Plant and Soil, 386(1), 385–394. DOI 10.1007/s11104-014-2270-y. [Google Scholar] [CrossRef]
24. Freeman, J. L., Zhang, L. H., Marcus, M. A., Fakra, S., McGrath, S. P. et al. (2006). Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalusbisulcatus and Stanleyapinnata. Plant Physiology, 142(1), 124–134. DOI 10.1104/pp.106.081158. [Google Scholar] [CrossRef]
25. Wuilloud, R. G., Berton, P. (2013). Selenium speciation in the environment. In: Bakirdere, S. (Ed.Speciation studies in soil, sediment and environmental samples, pp. 43.Boca Raton: CRC Press. [Google Scholar]
26. El-Ramady, H. R., Domokos-Szabolcsy, É., Shalaby, T. A., Prokisch, J., Fári, M. (2015). Selenium in agriculture: Water, air, soil, plants, food, animals and nanoselenium. In: Lichtfouse, E., Schwarzbauer, J., Robert, D. (Eds.CO2 sequestration, biofuels and depollution, pp. 153–232. Berlin: Springer. [Google Scholar]
27. Lintschinger, J., Fuchs, N., Moser, J., Kuehnelt, D., Goessler, W. (2000). Selenium-enriched sprouts. A raw material for fortified cereal-based diets. Journal of Agricultural and Food Chemistry, 48(11), 5362–5368. DOI 10.1021/jf000509d. [Google Scholar] [CrossRef]
28. Puccinelli, M., Malorgio, F., Rosellini, I., Pezzarossa, B. (2019). Production of selenium biofortified microgreens from selenium enriched seeds of basil. Journal of the Science of Food and Agriculture, 99(12), 5601–5605. DOI 10.1002/jsfa.9826. [Google Scholar] [CrossRef]
29. Ahmad, Z., Anjum, S., Skalicky, M., Waraich, E. A., Tariq, R. M. S. et al. (2021). Selenium alleviates the adverse effect of drought in oilseed crops camelina (Camelina sativa L.) and canola (Brassica napus L.). Molecules, 26(6), 1699. DOI 10.3390/molecules26061699. [Google Scholar] [CrossRef]
30. Schrauzer, G. N. (2000). Selenomethionine: A review of its nutritional significance, metabolism and toxicity. Journal of Nutrition, 130(7), 1653–1656. DOI 10.1093/jn/130.7.1653. [Google Scholar] [CrossRef]
31. Ježek, P., Škarpa, P., Lošák, T., Hlušek, J., Jůzl, M. (2012). Selenium-an important antioxidant in crops biofortification. In: El-Missiry, M. A. (Ed.Antioxident enzyme, pp. 343–368. Rijeka, IntechOpen. [Google Scholar]
32. Durán, P., Acuña, J. J., Jorquera, M. A., Azcón, R., Borie, F. et al. (2013). Enhanced selenium content in wheat grain by co-inoculation of selenobacteria and arbuscular mycorrhizal fungi: A preliminary study as a potential Se biofortification strategy. Journal of Cereal Science, 57(3), 275–280. DOI 10.1016/j.jcs.2012.11.012. [Google Scholar] [CrossRef]
33. Li, J., Awasthi, M. K., Xing, W., Liu, R., Bao, H. et al. (2020). Arbuscular mycorrhizal fungi increase the bioavailability and wheat (Triticum aestivum L.) uptake of selenium in soil. Industrial Crops and Products, 150, 112383. DOI 10.1016/j.indcrop.2020.112383. [Google Scholar] [CrossRef]
34. Durán, P., Acuña, J. J., Armada, E., López-Castillo, O. M., Cornejo, P. et al. (2016). Inoculation with selenobacteria and arbuscular mycorrhizal fungi to enhance selenium content in lettuce plants and improve tolerance against drought stress. Journal of Soil Science and Plant Nutrition, 16(1), 211–225. DOI 10.4067/S0718-95162016005000017. [Google Scholar] [CrossRef]
35. Golubkina, N., Amagova, Z., Matsadze, V., Zamana, S., Tallarita, A. et al. (2020). Effects of arbuscular mycorrhizal fungi on yield, biochemical characteristics, and elemental composition of garlic and onion under selenium supply. Plants, 9(1), 84. DOI 10.3390/plants9010084. [Google Scholar] [CrossRef]
36. Chen, X., Zhang, Z., Gu, M., Li, H., Shohag, M. J. I. et al. (2020). Combined use of arbuscular mycorrhizal fungus and selenium fertilizer shapes microbial community structure and enhances organic selenium accumulation in rice grain. Science of the Total Environment, 748, 141166. DOI 10.1016/j.scitotenv.2020.141166. [Google Scholar] [CrossRef]
37. El-Ramady, H., Abdalla, N., Alshaal, T., Domokos-Szabolcsy, E., Elhawat, N. et al. (2015). Selenium in soils under climate change, implication for human health. Environmental Chemistry Letters, 13(1), 1–19. DOI 10.1007/s10311-014-0480-4. [Google Scholar] [CrossRef]
38. Priya, M. D. L., Geetha, A. (2011). Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biological Trace Element Research, 142(2), 148–158. DOI 10.1007/s12011-010-8766-2. [Google Scholar] [CrossRef]
39. Jones, G. D., Droz, B., Greve, P., Gottschalk, P., Poffet, D. et al. (2017). Selenium deficiency risk predicted to increase under future climate change. Proceedings of the National Academy of Sciences of the United States of America, 114(11), 2848–2853. DOI 10.1073/pnas.1611576114. [Google Scholar] [CrossRef]
40. Bañuelos, G. S., Lin, Z. Q., Broadley, M. (2017). Selenium biofortification. In: Pilon-Smits, E. A. H., Winkel, L. H. E., Lin, Z. Q. (Eds.Selenium in plants: Molecular, physiological, ecological and evolutionary aspects, pp. 231–255. Cham: Springer International Publishing. [Google Scholar]
41. Finley, J. W. (2005). Selenium accumulation in plant foods. Nutrition Reviews, 63(6), 196–202. DOI 10.1111/j.1753-4887.2005.tb00137.x. [Google Scholar] [CrossRef]
42. Pilon-Smits, E. A. H., LeDuc, D. L. (2009). Phytoremediation of selenium using transgenic plants. Current Opinion in Biotechnology, 20(2), 207–212. DOI 10.1016/j.copbio.2009.02.001. [Google Scholar] [CrossRef]
43. Gupta, U. C., Gupta, S. C. (2008). Selenium in soils and crops, its deficiencies in livestock and humans: Implications for management. Communications in Soil Science and Plant Analysis, 31(11–14), 1791–1807. DOI 10.1080/00103620009370538. [Google Scholar] [CrossRef]
44. Fang, Y., Wang, L., Xin, Z., Zhao, L., An, X. et al. (2008). Effect of foliar application of zinc, selenium, and iron fertilizers on nutrients concentration and yield of rice grain in China. Journal of Agricultural and Food Chemistry, 56(6), 2079–2084. DOI 10.1021/jf800150z. [Google Scholar] [CrossRef]
45. Saha, U., Fayiga, A., Sonon, L. (2017). Selenium in the soil-plant environment: A review. International Journal of Applied Agricultural Sciences, 3(1), 1–18. DOI 10.11648/j.ijaas.20170301.11. [Google Scholar] [CrossRef]
46. Sager, M. (2006). Selenium in agriculture, food, and nutrition. Pure and Applied Chemistry, 78(1), 111–133. DOI 10.1351/pac200678010111. [Google Scholar] [CrossRef]
47. Eich-Greatorex, S., Sogn, T. A., Øgaard, A. F., Aasen, I. (2007). Plant availability of inorganic and organic selenium fertiliser as influenced by soil organic matter content and pH. Nutrient Cycling in Agroecosystems, 79(3), 221–231. [Google Scholar]
48. White, P. J. (2016). Selenium accumulation by plants. Annals of Botany, 117(2), 217–235. [Google Scholar]
49. Tsioubri, M., Gasparatos, D., Economou-Eliopoulos, M. (2020). Selenium uptake by lettuce (Lactuca sativa L.) and berseem (Trifolium alexandrinum L.) as affected by the application of sodium selenate, soil acidity and organic matter content. Plants, 9(5), 605. [Google Scholar]
50. Mayland, H. F., James, L. F., Panter, K. E., Sonderegger, J. L. (1989). Selenium in seleniferous environments. Selenium in Agriculture and the Environment, 23, 15–50. [Google Scholar]
51. McCauley, A., Jones, C., Jacobsen, J. (2009). Soil pH and organic matter. Nutrient Management Module, 8(2), 1–12. [Google Scholar]
52. El-Ramady, H., Abdalla, N., Alshaal, T., El-Henawy, A., Salah, E. D. F. et al. (2015). Selenium and its role in higher plants. In: Lichtfouse, E., Schwarzbauer, J., Robert, D. (Eds.Pollutants in buildings, water and living organisms, pp. 235–296. Cham: Springer. [Google Scholar]
53. Singh, M., Singh, N. (1979). The effect of forms of selenium on the accumulation of selenium, sulfur, and forms of nitrogen and phosphorus in forage cowpea (Vigna sinensis). Soil Science, 127(5), 264–269. [Google Scholar]
54. Zhang, P., Ganje, T. J., Page, A. L., Chang, A. C. (1988). Growth and uptake of selenium by swiss chard in acid and neutral soils. Journal of Environmental Quality, 17(2), 314–316. [Google Scholar]
55. Wen, D. (2021). Selenium in horticultural crops. Scientia Horticulturae, 289, 110441. [Google Scholar]
56. Chauhan, R., Awasthi, S., Srivastava, S., Dwivedi, S., Pilon-Smits, E. A. H. et al. (2019). Understanding selenium metabolism in plants and its role as a beneficial element. Critical Reviews in Environmental Science and Technology, 49(21), 1937–1958. [Google Scholar]
57. Pilon-Smits, E. A. H., Quinn, C. F. (2010). Selenium metabolism in plants. In: Hell, R., Mendel, R. R. (Eds.Cell biology of metals and nutrients, pp. 225–241. Berlin: Springer. [Google Scholar]
58. Sors, T. G., Ellis, D. R., Salt, D. E. (2005). Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynthesis Research, 86(3), 373–389. [Google Scholar]
59. Germ, M., Stibilj, V., Kreft, I. (2007). Metabolic importance of selenium for plants. European Journal of Plant Science and Biotechnology, 1(1), 91–97. [Google Scholar]
60. Farooq, M. U., Tang, Z., Zheng, T., Asghar, M. A., Zeng, R. et al. (2019). Cross-talk between cadmium and selenium at elevated cadmium stress determines the fate of selenium uptake in rice. Biomolecules, 9(6), 247. [Google Scholar]
61. Rahim, F. P., Rocio, C. G., Adalberto, B. M., Lidia Rosaura, S. C., Maginot, N. H. (2020). Agronomic biofortification with selenium in tomato crops (Solanum lycopersicon L. Mill). Agriculture, 10(10), 486. DOI 10.3390/agriculture10100486. [Google Scholar] [CrossRef]
62. Márquez, G. V., Moreno, M. Á., Mendoza, B. A., Macías, M. J. (2020). Ionic selenium and nanoselenium as biofortifiers and stimulators of plant metabolism. Agronomy, 10(9), 1399. DOI 10.3390/agronomy10091399. [Google Scholar] [CrossRef]
63. Golubkina, N., Gomez, L. D., Kekina, H., Cozzolino, E., Simister, R. et al. (2020). Joint selenium-iodine supply and arbuscular mycorrhizal fungi inoculation affect yield and quality of chickpea seeds and residual biomass. Plants, 9(7), 804. DOI 10.3390/plants9070804. [Google Scholar] [CrossRef]
64. Siddiqui, S. A., Blinov, A. V., Serov, A. V., Gvozdenko, A. A., Kravtsov, A. A. et al. (2021). Effect of selenium nanoparticles on germination of Hordeum vulgare barley seeds. Coatings, 11(7), 862. DOI 10.3390/coatings11070862. [Google Scholar] [CrossRef]
65. Galić, L., Vinković, T., Ravnjak, B., Lončarić, Z. (2021). Agronomic biofortification of significant cereal crops with selenium—A review. Agronomy, 11, 1015. DOI 10.3390/agronomy11051015. [Google Scholar] [CrossRef]
66. Sharma, G., Sharma, A. R., Bhavesh, R., Park, J., Ganbold, B. et al. (2014). Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules, 19(3), 2761–2770. [Google Scholar]
67. Fesharaki, P. J., Nazari, P., Shakibaie, M., Rezaie, S., Banoee, M. et al. (2010). Biosynthesis of selenium nanoparticles using Klebsiella pneumoniae and their recovery by a simple sterilization process. Brazilian Journal of Microbiology, 41, 461–466. [Google Scholar]
68. Dhanjal, S., Cameotra, S. S. (2010). Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microbial Cell Factories, 9(1), 1–11. [Google Scholar]
69. Ingole, A. R., Thakare, S. R., Khati, N. T., Wankhade, A. V., Burghate, D. K. (2010). Green synthesis of selenium nanoparticles under ambient condition. Chalcogenide Letters, 7(7), 485–489. [Google Scholar]
70. Klug, H. P., Alexander, L. E. (1967). X-ray diffraction procedures for polycrystalline and amorphous materials. New York, USA: Wiley. [Google Scholar]
71. Prasad, K. S., Selvaraj, K. (2014). Biogenic synthesis of selenium nanoparticles and their effect on As(III)-induced toxicity on human lymphocytes. Biological Trace Element Research, 157(3), 275–283. [Google Scholar]
72. Ramamurthy, C. H., Sampath, K. S., Arunkumar, P., Kumar, M. S., Sujatha, V. et al. (2013). Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess and Biosystems Engineering, 36(8), 1131–1139. [Google Scholar]
73. El-Saadony, M. T., Saad, A. M., Najjar, A. A., Alzahrani, S. O., Alkhatib, F. M. et al. (2021). The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi Journal of Biological Sciences, 28, 4461–4471. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |