

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.017365
REVIEW
Salinity Stress in Wheat: Effects, Mechanisms and Management Strategies
1Plant Production Department, College of Food and Agriculture Sciences, King Saud University, Riyadh, 11451, Saudi Arabia
2Department of Crop Sciences, Faculty of Agriculture, Menoufia University, Shibin El-kom, 32514, Egypt
3Department of Agronomy, University of Agriculture, Faisalabad, 38040, Pakistan
4Biology Department, College of Science and Humanity Studies, Prince Sattam Bin Abdulaziz University, Riyadh, 11942, Saudi Arabia
5Research Center on Ecological Sciences, Jiangxi Agricultural University, Nanchang, 330045, China
6Department of Agricultural Sciences and Technology, School of Agriculture and Enterprise Development, Kenyatta University, Nairobi, 00100, Kenya
7Department of Field Crops, Faculty of Agriculture, University of Sutcu Imam, Kahramanmaras, 46040, Turkey
8Department of Agroforestry & Environmental Science, Faculty of Agriculture, Sylhet Agricultural University, Sylhet, 3100, Bangladesh
9Department of Animal Sciences, Cornell University, Ithaca, 14850, USA
*Corresponding Author: Mahmoud F. Seleiman. Email: mseleiman@ksu.edu.sa
#Contributed equally in the manuscript
Received: 05 May 2021; Accepted: 20 August 2021
Abstract: Salinity stress is a major threat to global food production and its intensity is continuously increasing because of anthropogenic activities. Wheat is a staple food and a source of carbohydrates and calories for the majority of people across the globe. However, wheat productivity is adversely affected by salt stress, which is associated with a reduction in germination, growth, altered reproductive behavior and enzymatic activity, disrupted photosynthesis, hormonal imbalance, oxidative stress, and yield reductions. Thus, a better understanding of wheat (plant) behavior to salinity stress has essential implications to devise counter and alleviation measures to cope with salt stress. Different approaches including the selection of suitable cultivars, conventional breeding, and molecular techniques can be used for facing salt stress tolerance. However, these techniques are tedious, costly, and labor-intensive. Management practices are still helpful to improve the wheat performance under salinity stress. Use of arbuscular mycorrhizal fungi, plant growth-promoting rhizobacteria, and exogenous application of phytohormones, seed priming, and nutrient management are important tools to improve wheat performance under salinity stress. In this paper, we discussed the effect of salinity stress on the wheat crop, possible mechanisms to deal with salinity stress, and management options to improve wheat performance under salinity conditions.
Keywords: Breeding techniques; oxidative stress; photosynthesis; phyto-hormones; salinity stress; wheat
Globally, more than 20% of soils are salt-affected and the extent of these soils is continuously increasing owing to anthropogenic activities and climate change [1,2]. Abiotic stresses are considered to be responsible for a 50% reduction in crop production, imposing a serious threat to global food security [3,4]. As a result of the rapid increase in the global population, food production has to be increased by 70% by the end of 2050 [5]. Wheat is the important food crop which ranks first in the global grain production. It is the staple food for more than 36% of the world’s population, and it provides 20% of the calories and 55% of the carbohydrates globally [6,7]. Moreover, wheat is also an important source of micro and macronutrients which are necessary for human health [8–10].
The productivity of wheat crops is negatively affected by salinity stress [11,12]. Wheat crop yield starts to decline at a salinity stress level of 6–8 dS m−1 [13]. According to Food and Agriculture Organization (FAO), 397 million hectares under wheat cultivation are severely affected by salinity stress, which is imposing a serious threat to food security [14]. Salinity stress causes ion toxicity and nutritional imbalance in plants, which disrupts the plant physiological processes, and consequently cause a serious reduction in final yield [15–17]. Initially, salinity stress causes a significant reduction in seed germination, and later it alters growth and reproductive behavior causing serious yield losses [18–20]. Moreover, salt stress disturbs the enzymatic activities, photosynthesis, membrane structure, hormonal balance, water, and nutrient uptake, and induces oxidative stress [21–23].
Salinity stress is a polygenic character, which is regulated by multiple genes. Exclusion of Na+ and retention of K+, maintenance of an optimum K+/Na+ ratio, osmotic adjustment, and enhanced activities of antioxidant system are vital for plants under salinity stress [24,25]. Various techniques including the introduction of desirable genes, selection of suitable genotypes [26–28], screening of genotypes, and conventional breeding techniques have been used across the globe to improve crop performance under salinity stress. However, these techniques are time consuming and costly. Under this scenario, the application of osmoprotectants, seed priming, nutrient management, and hormone application can offer promising results to manage salinity stress [6,29]. Therefore, in this review, we discussed the effect of salinity stress on the wheat crop, resistance mechanisms to salinity stress in wheat, and potential management options to enhance the resilience of wheat under such stress.
2.1 Effects of Salinity Stress on Wheat Germination, Growth and Yield
Germination has a key importance in the plant life cycle and it helps to determine the subsequent growth, development, and yield attributes. Salinity stress reduces seed germination and leads to a serious reduction in the final yield of the wheat crop (Fig. 1). Salt stress reduces osmotic potential, disrupts the normal functioning of enzymes necessary for metabolic activity [30], and reduced the final stand establishment and yield. Salt stress also reduces yield attributes including spikelets number, productive tillers, grain weight, and biomass yield. Plant seedlings are quite sensitive to stress conditions and seedling death also occurs due to salinity stress [31]. Root and shoot parameters are also negatively affected by salinity stress [32,33]. Guo et al. [34] observed a reduction in wheat growth under salinity stress compared to normal conditions Likewise, Zou et al. [35] observed a reduction in root and shoot lengths and their dry weight under salt stress (100 mM NaCl). Salinity stress remarkably decreases the yield of almost all the crops. However, yield reduction percentage may vary on salt-tolerant and sensitive varieties. Asgari et al. [36] examined the reduction in growth attributes of wheat which ultimately reduced wheat production. Chinnusamy et al. [37] observed a 7.1% yield reduction with each unit of increase in salinity up to 6 dSm−1. Afzal et al. [38] noticed a significant reduction in seeds/spike, a thousand seed weight, and economic yield in both salt-sensitive and tolerant varieties of wheat. In conclusion, salinity stress negatively affects the metabolic processes for optimum germination; therefore, it results in a serious reduction in growth and final yield.

Figure 1: Effects of salinity stress on wheat crop. salinity stress reduced germination, photosynthesis, nutrient uptake, and relative water content, and induced ion toxicity and oxidative stress. therefore, it led to a reduction in growth and final yield
2.2 Effect of Salinity Stress on Photosynthesis, Plant Water Relations and, Mineral Uptake
A plant needs optimum photosynthetic activity for its survival, which is greatly influenced by environmental conditions [39]. Photosynthesis is inhibited by the accumulation of ions (Na+ and Cl−) in the chloroplast and a reduction in plant water potential (Fig. 2) due to high salt stress [30]. Guo et al. [34] studied the physiological aspects of wheat under saline conditions; they observed that salinity stress led to stomatal closure, induced less CO2 absorption, and reduced transpiration rate. Furthermore, salinity stress (320 mM NaCl) significantly reduced the photosynthetic pigments in the chloroplast [34,38] which reduced the photosynthetic efficiency and caused a serious reduction in the final productivity.

Figure 2: Possible mechanisms by which salinity stress reduces photosynthesis in wheat crop. Salt stress disturbs the balance between ROS and anti-oxidant species and causes the accretion of ROS which induces oxidative stress in the wheat crop. moreover, salinity stress increases ionic toxicity, reduces leaf growth and imposes early leaf abscission, which reduces the carboxylation and results in a reduction in photosynthesis. additionally, salinity stress also reduces the efficiency of PS-II, stomatal conductance, intercellular CO2 and electron transportation; all of these contributes towards a reduction in photosynthesis
Moisture availability determines the physiological and metabolic processes which occur within the plant body. All the physiological and metabolic alterations in plants mainly depend on moisture availability. Due to high salinity, a plant undergoes osmotic stress that further decreases the water potential of the plant cell. Nassar et al. [40] observed a decreasing trend in relative water contents (RWC) up to 3.5% in a salt-tolerant wheat cultivar, while it was 6.7% in salt-sensitive varieties in comparison to the control. An excess of Na+ and Cl− ions in plants hinders the uptake of essential nutrients from the soil, which alters the plant processes. A reduction in K+, Ca2+, and Zn+2 uptake and an increase in Na+ and Cl− uptake was observed in a salt-affected wheat cultivar [34].
2.3 Salinity-Induced Oxidative Damage
Salinity stress causes stomatal closure and hinder carbon dioxide (CO2) entrance in leaves. This restrains CO2 fixation and enables the chloroplast to stimulate the immense levels of energy, which further develops the reactive oxygen species (ROS) [30,41–46]. These ROS cause damage to major molecules including lipid, protein, and nucleic acids [42,43]. ROS production increased under salinity [44] and induced cellular toxicity in various crop plants [30]. Salt-sensitive wheat cultivars growing under salinity conditions (5.4 and 10.6 dS m−1) had more H2O2 and lipid peroxidation than salt-tolerant cultivars [45]. Zou et al. [35] observed that salt stress (100 mM NaCl) enhanced malondialdehyde (MDA) level up to 35% or 68% after 5 or 10 days of exposure to such stress, respectively, in wheat seedlings.
3 Mechanisms of Salinity Stress in Wheat
Wheat produces alterations at the cellular and organ level to perform best under salt stress (Fig. 3). The resistance mechanisms of salt tolerance in wheat are complex as the plant produces numerous alterations in stomatal conductance, hormonal balance, anti-oxidant defense mechanism, osmotic regulation, and ion exclusion. A comprehensive study of the above-mentioned resistance mechanisms is expressed below.

Figure 3: Responsive mechanisms of wheat crop to salinity stress. APX: ascorbate peroxidase, CAT: catalase, GR: glutathione reductase, POD: per-oxidase, SOD: superoxide dismutase
3.1 Osmoregulation and Osmoprotection
Plants face osmotic stress and implement a well-known strategy (osmoregulation) to lower its adverse effects [46,47]. Plants accumulate various organic compounds (sugars, polyols, amino acids, and quaternary ammonium compounds) that help to reduce the osmotic potential [48]. Osmoregulation is responsible to trigger the defense mechanism against anti-oxidant species for regulating the plant water relationship [49,50]. In nature, osmoprotectants are hydrophilic, and have a low molecular weight, and no net charge [51]. In bean plants, the salt-tolerant cultivars had high proline and amino acids with minimum protein contents as compared to the salt-sensitive varieties [52]. Various concentrations of organic and inorganic solutes result in osmotic adjustment which vary with species and cultivars [53].
Ionic homeostasis is a key process that regulates ion flux to maintain a low Na+ ion concentration and building up a high K+ concentration [44,47]. Regulating intracellular Na+ and K+ ions (homeostasis) is fundamental for the various enzymes’ performance in the cytosol, maintaining the membrane potential as well as cell volume [44]. For equivalence Na+ and K+ concentration in the cytosol, plants rule out the excess salt via primary and secondary active transport [44,54], and accumulate these positively charged ions in the plasma and tonoplast membranes for the sustaining homeostasis during salt stress [54]. Various K+ genes are down and up-regulated by saline stress [55]. For securing the cytosol from the damaging effects of Na+ ions; the extra Na+ is compartmentalized in the vacuole as an efficient mechanism against ion toxicity [47,54]. Cordovilla et al. [55] found a vast diversity for Na+ and K+ within the cytosol among various cultivars of grain crops. Plants use various affinity-based transporters found in the biological membranes for K+ uptake [56], associated with K+/Na+ maintenance [57]. The extra salts are physiologically excluded from the plants as their adaptive trait for salt-resistance. The different effects of salt stress on photosynthetic and physiological attributes of wheat crop are summarized in Table 1.
High sodium concentration in plants interferes with K+ accumulation and stomata regulation [61]. Increasing the Na+ ion concentration in the plant vacuoles via the tonoplast pathway driven by the proton gradient is also considered a crucial strategy against salinity. As a result, plants save their essential organelles like the cytosol from an excess of sodium, thus developing a resistant mechanism against such ion [62]. Plants accumulate Na+ ions in the vacuoles of roots via the tonoplast pathway to lower the sodium transport in the shoot [62]. Optimizing the K+ uptake rate while reducing its omission, plants not only restrict the Na+ entry but also take advantage of sodium exclusion from the cell under saline stress. This mechanism helps to maintain the K+/Na+ ratio in the cytosol [63] and ensures plant survival under salinity conditions.

3.3 Antioxidant Defense System
Reactive oxygen species (ROS), high osmotic stress and ion toxicity develop in plants due to excessive salt accumulation in the root zone of plants [43]. The ROS in plants cause the oxidation of protein degradation and alteration in deoxyribonucleic acid (DNA) sequencing [64]. Plants resistant to salt stress develop an anti-oxidative mechanism by activating various enzymes like the superoxide dismutase (SOD) and catalase (CAT) (Fig. 4) [65,66]. From numerous studies it is clear that the anti-oxidant defense system manages the oxidative damage during abiotic stress in plants [67]. A close association of anti-oxidants and salinity tolerance has been observed in wheat species [68]. Plants indicate the activities of the antioxidant enzymes under salt stress [69]. Plants have water-soluble anti-oxidants that make them strongly redox buffered [70]. Electrons react with oxygen molecules to form hydrogen peroxide (H2O2) as superoxide radicals [43]. Various enzymes are involved in regulating the intracellular H2O2. Among these, the peroxidase (POD) [70], and CAT [71] are crucial ones.

Figure 4: Salt induced oxidative stress and antioxidant defense regulation in wheat crop
To encounter ROS activity, plants activate an antioxidant defense mechanism for their survival. Various antioxidants such as ascorbic acid (AsA), tocopherol, and some phenolics (non-enzymatic) are found in plant, which gives protection from oxidative stress. Athar et al. [72] observed less growth and photosynthetic activity due to high (150 mM NaCl) salinity, with a lower K+/Na+ ratio in the tissue of both sensitive and tolerant wheat cultivars. Conversely, the tolerant wheat varieties produced endogenous AsA and showed an increased CAT activity to counteract the salinity effect. Ascobin, which possesses the quality attribute of both ascorbic acid and citric acid, was found significant for producing wheat yield in a salt-induced environment [73]. In addition to the dose, the application method has a remarkable effect. Athar et al. [74] observed the different effects of AsA when it was used either as a priming agent or applied in a rooting medium or used as a foliar application against salt stress (120 mM NaCl) in wheat. AsA counteracts salinity by activating the SOD, POD, and CAT activities and photosynthesis, and more exclusion of sodium (Na+) ions from leaves in specific cultivar [74].
Generally, five hormones including auxin, gibberellins, cytokinins, ethylene, and abscisic acid affect plants growth and are used externally to alleviate abiotic (salinity) stress. Amongst these hormones indole acetic acid (IAA), gibberellins (GA) and cytokinins (CK) promote plant growth whilst the rest of the hormones are known as growth retardants. Under salinity, auxin promotes wheat germination percentage, shoot dry weight along with maintaining ion homeostasis [75]. Furthermore, auxin priming has been reported to alleviate the salinity up to 15 dSm−1 by increasing wheat assimilation rate and sustaining the balance among various hormones [76]. On the other hand, GA priming strengthens the photosynthetic pigments and enhances plant growth and development by increasing the unit leaf surface area, thus alleviating the severe effects of salt stress in wheat [77]. Cytokinin as a priming agent, enhances wheat grain yield by promoting germination, growth, tiller-number, and a 1000 grain weight under saline conditions [78,79]. Abscisic acid (ABA) priming lowers sodium (Na+) uptake from soil and increased chlorophyll contents [80]. Siddiqui et al. [81] observed the significant role of brassinosteroid on photosynthesis by increasing the assimilating power of wheat and boosting the photosynthetic rate under salt stress. A promising response of wheat to brassinosteroid was also observed in a salt-affected environment [82].
Tetraploid wheat is relatively salt-sensitive as compared to bread wheat [83]. This is because of a lower accumulation of K+ ions in leaves [84] controlled by the chromosome 4D specified with Kna1 loci in bread wheat [85]. Moreover, two loci, Nax1 and Nax2 are concerned for the elimination of high Na+ ions; this was observed in genetic analysis based on populations of durum wheat and Triticum monococcum [86]. The HKT gene is involved in excluding Na+ ions from wheat during salinity. But the mechanism of HKT genes for sodium-ion exclusion under salinity stress has to be further unrevealed. For instance, TaHKT1;5-D alters transcriptional programming in Aegilops tauschii (2n-wheat cultivar) under salt stress [87]. Byrt et al. [88] did not observe any variation in TaHKT1;5-D in hexaploid wheat cv. Bobwhite. Transcription of TaHKT1;5-D was significantly reduced in hexaploid wheat cv. JN177 under salinity [89,90]. This contradictory result arises some fundamental questions on if either the response of TaHKT1;5-D is tissue-specific [88] or based on HKT genes. The sole HKT gene is synchronized by small ribonucleic acid (RNA) and DNA methylation in Arabidopsis [91]. In addition, TaHKT1;5-D mediated salt tolerance in wheat cvs. JN177 and SR3 is also based on DNA methylation [92]. The TaHKT1;5-B1 and TaHKT1;5-B2 have a much lower transcription level than TaHKT1;5-D [88]. Xu et al. [93] noted that epigenetics might be effective in homologous transcription, and there is a need for further investigation on epigenetics of TaHKT1;5-B1 and TaHKT1;5-B2, characterized by a lower expression attributed to the TaHKT1;5-D. Furthermore, salinity tolerance regulated by HKT genes is affected by AtABI4 and OsMYBc [94,95]. Conversely, HKT gene function in complex hexaploid wheat cultivars still needs investigation to identify its function.
The performance of common wheat under salt stress conditions can be increased with the use of some imperative potential traits of wild wheat species and their related cultivars [96]. Likewise, Thinopyrum ponticum (tall wheatgrass) depicts tolerance against abiotic stress [97] and thus, possesses important genes to develop salt-tolerant wheat cultivars. However, a recombinant barrier hampers the production of new hybrids possessing beneficial traits by the combination of both wild and common wheat varieties [98]. Nevertheless, asymmetric somatic hybridization is the best substitute to develop a new cultivar, particularly where the inter-specific cross is not feasible [99]. With the use of this approach, a salt-tolerant wheat cultivar (Shanrong No. 3: SR3) was developed by crossing bread wheat and Thinopyrum ponticum [100]. Biotechnology gets a breakthrough with developing this novel cultivar, which further reveals the salt-tolerant mechanism in wheat. In genetic analysis, the polygenic effect causes homeostasis in ROS for tolerance against salinity. SR3 activates TaCHP (Zn finger transcription factor) with a greater transcription than JN177 [101], which further assists wheat cultivars to enhance POD concentration in leaves for scavenging ROS under salt stress. In another example, TaOPR1-(a gene responsible for the activation of an antioxidant defense mechanism against ROS) limits the MDA to face salinity stress [102]. In somatic hybridization; considerable epigenetic reprogramming occurs commonly known as “Genomic shock” [103]. Scientists need to evaluate the functioning of epigenetic alteration for controlling the expression of genes and they observed differences in transcript abundances of TaFLS1, TaWRSI1, and TaTIP2 between JN177 and SR3. However, it could not be explained by differences in either the promoter or the coding sequences, which were shown to vary concerning the DNA methylation level [104]. A complete investigation is needed to understand whether ROS homeostasis and its deviation among SR3 and JN177 are coupled with DNA methylation for bringing tolerance in wheat cultivars.
4. Salinity Stress Management in Wheat
The development of salt-tolerant cultivars along with appropriate agronomic practices can help to improve crop production under salt stress conditions (Fig. 5). Many opportunities exist regarding genetic diversity in gene banks exist which provides enough support to develop improved salt-tolerant cultivars characterized by a high production over the existing varieties [105,106]. The basic genetic makeup found in the genetic pool of various crop species assists breeders to make progress in developing salt-tolerant cultivars. Moreover, breeders develop salt-tolerant varieties especially for wheat and rice crop [106–108]. When developing salt-tolerant cultivars, the physiology and genetic-based traits also have considerable importance to assure the maximum yield at harvesting.
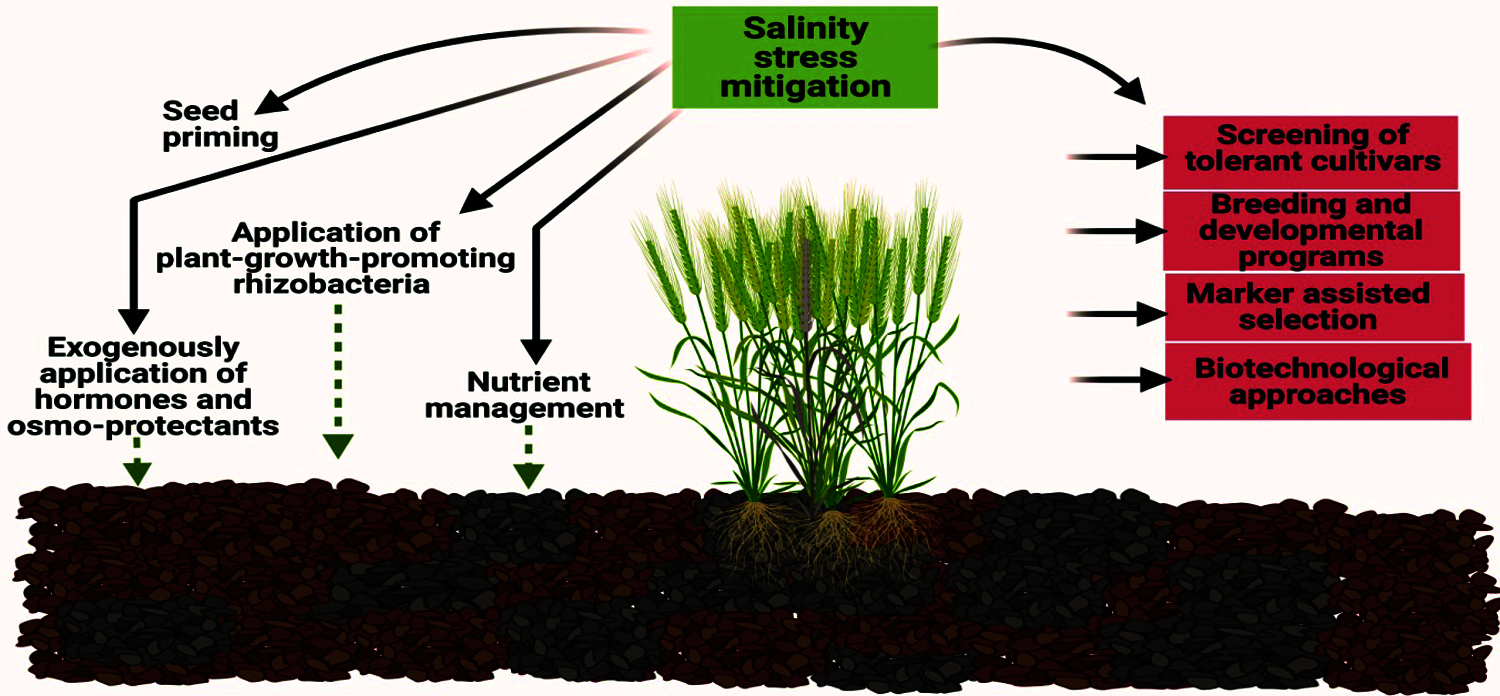
Figure 5: Strategies involved in improving the salinity tolerance in wheat crop
Among all other cereals, wheat is considered a staple food in Asia; however, its yield potential is negatively affected by salinity. In response, breeders make appreciable efforts to develop salt-tolerant cultivars in Asian countries (Pakistan and India) and in Australia. However, the progress is quite slow. In Pakistan, the University of Agriculture, Faisalabad in collaboration with the Saline Agriculture Research Centre (SARC) developed the salt-tolerant lines (LU26S and SARC-1). In addition, the Central Soil Salinity Research Institute (CSSRI) introduced KRL1–4 and KRL-19 in India to cope with salinity threat. All the Indian salt-tolerant cultivars are the progeny of Kharchia 65, which was developed by Indian farmers by selection from the sodic-saline soil of Rajasthan [109]. The Indian salt-tolerant cultivar (KRL1–4) was the cross of Kharchia65 and WL711 and was a promising variety for northern India [110]. KTDH-19 being a doubled haploid wheat line was developed by crossing Kharchia65 with TW161 (a specific line for Na+ exclusion) and gave satisfactory results under the saline condition in Spain [111], India and Pakistan [110]. Its salt tolerant ability along with an earlier maturity of three weeks caused to adopt this line in Asian countries particularly in Pakistan and India [110].
4.2 Transgenic and Biotechnological Approaches for Improving Salinity Tolerance in Wheat
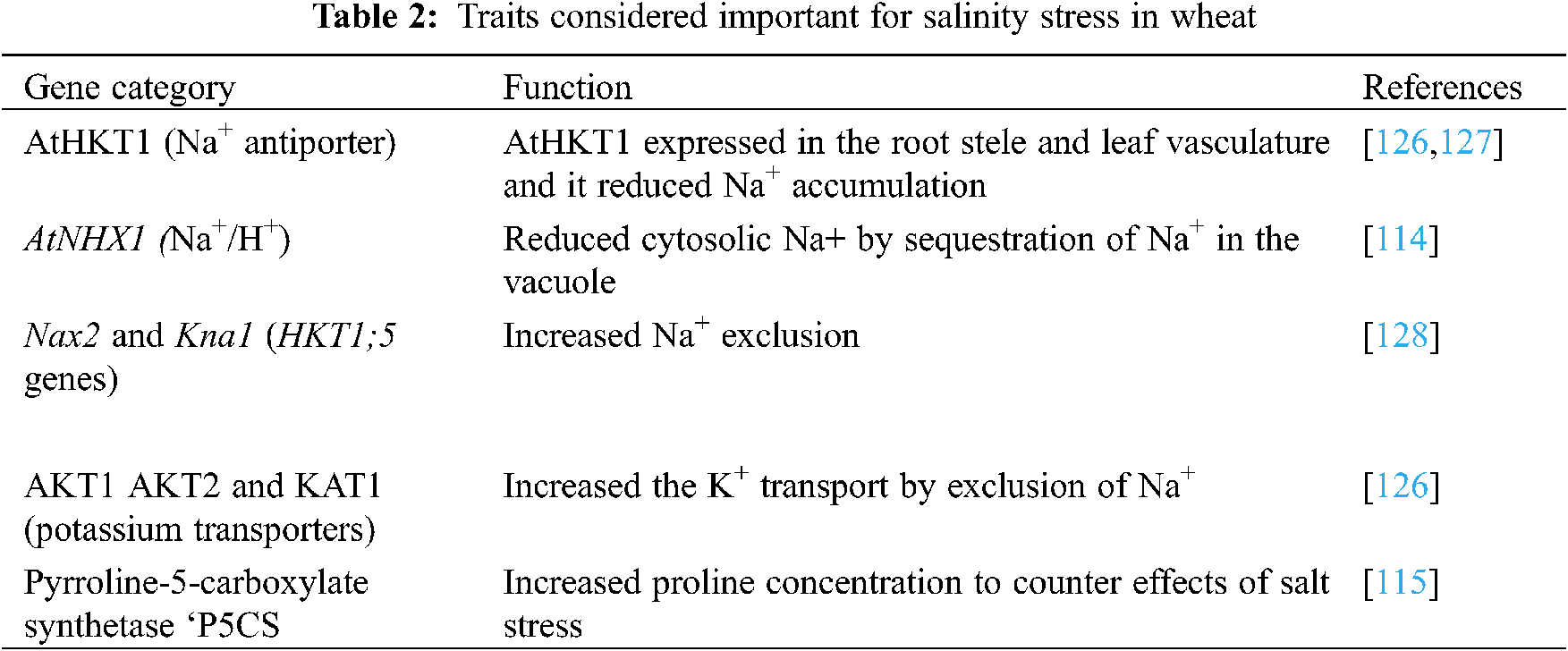
In this technique a desired gene is transferred to a transgenic plant with genetic engineering to obtain the resistance against stress conditions [112,113]. Salinity tolerance is controlled by many minor genes and it is complex to transfer the desired gene for developing the desired trait in a transgenic wheat cultivar (Table 2). Genes are specific in action; the antiporter gene (AtNHXI) controls the overexpression of Na+/H+ ions in the vacuole and improves germination and biomass by lowering the leaf Na+ concentration [114]. Free proline accumulation may increase salt tolerance in plants. An enzyme, the 1-pyrroline-5-carboxylate synthase (P5CS), increased the accumulation of free proline by 2.5 folds higher than the control (non-proline cultivar) to enhance salt tolerance in wheat [115].
With the use of biotechnology, wheat and other crops like rice and tobacco [116] are being equipped with salt-tolerant genes and are producing high yields [117]. Genotypic markers provide phenotypic data to explore hereditary for salt tolerance. The exact phenotypic data is the base of salt-tolerant genes [118]. Recently, advancements in DNA marker and sequencing advances have allowed high-throughput genotyping of numerous individual plant species with moderately minimal effort. Rapid strategies to assess huge amounts of genotypes are critical to completely exploit the immediate improvement of biotechnological systems and to encourage hereditary analysis of complex qualities.
In traditional selection obtaining high yields under saline conditions, the environment has various constraints including innate variables, heterogeneous soils, and climate conditions [118,119]. The physiological attributes along with genes supporting more tolerance against salinity competently discriminate in the natural environment [120]. Assessing grains’ response to salt stress, scientists screened and demonstrated the effective and satisfactory results of their system on different germination media including soil, and sand [121,122], and hydroponic [119]. Particle homeostasis affects the traits involved in genetic analysis with eminent quantitative trait locus (QTLs) described by specific Na+ and K+ contents relieving tolerance against salinity in wheat [123] and rice [124,125].
QTL mapping plays a vital role in examining genetics and producing the complicated plant traits [129]. Our understanding of the complexities of the plant characters is appreciably increased with the use of QTLs for agronomic traits [130]. Marker selection results in an improvement in notable QTLs that have assisted the breeders fundamentally [131] and pyramiding the various apposite forms of genes (alleles) [132]. The basic principle for genes exploring for salt tolerance is based on QTL mapping (biparental), which mainly focuses on the single dividing population incidental from parental genotypes (homozygous) in wheat [133], rice [134], and other grains crops [135]. The HKT group is associated with salinity tolerance in the Knal loci and Nax1 plus Nax2 were encoded with some locus found in common wheat [85]. Some constraints were faced regarding bi-parental QTL mapping that was inefficient for exploring the allelic cultivars in the gene pool and affecting the quality traits [136]. Bi-parental QTL mapping can only exhibit the genes responsible for characterization which normally lies in the range of 10 to 30 cm [137]. Moreover, these chromosomal alleles could demonstrate a few thousand genes [138]. Thus, obviously effective and successful QTLs that are being cloned highlighted with more than one gene [139]. Toconquer the utilization of inherited variability for salt tolerance, further research is mandatory to establish the right mapping sequence for germplasm.
In association mapping interpretation of disequilibrium linkage with phenotypic connection occurs. Differentiation among different loci, which carried the complex genetic information, is also possible and supported with association mapping. Distinctive markers linked with phenotypes and carried gene of interest are used in this technique [140]. Thudi et al. [141] reported the use of association mapping for plant genetics and made underutilization for gene sequencing [142]. Linking disequilibrium and accretion of various genetic makeups determine the use of association mapping [139]. All the possible recombinant events that take place during the crop life span and distribution patterns of small linkage barriers are studied with association mapping [143]. Moreover, their functions based on actual QTLs variation occurs on allele manipulation of the desired character under observation during association mapping of the crop gene pool. Linkage, disequilibrium (LD) informs non-arbitrary association among various alternative forms of genes present on the typical polymorphic locus and thus has a fundamental importance in association mapping. An association mapping group includes vast land regions, areas of adjustment with an upright representation of its evolutionary history typically non-arbitrary because of familial relatedness and distinctive sorts of structure [140]. Association mapping identifies marker quality [144] and requires the proper apparatus to use this technique, which clarifies the complexities [145]. It is necessary to accumulate all the elements associated with mapping and to merge them in the desirable form of a model suitable for markers used within sub-populations [146].
Conventional breeding is time consuming and causes many complexities in polygenic traits. A breeder generally does not phenotypically analyze the crop selecting from the back cross due to varying germ plasmas. Thus, to manage salt stress in tolerant cultivars, breeders use marker-assisted selection for QTL analyses based on polymorphic traits of parental lines [143]. An efficient marker has to be developed to deal with salinity in plants. The genome in major cereals (rice, barley, sorghum, and soybean) has been sequenced, and it has progressed towards a next-generation sequencing (NGS) to modify the genetic maps for obtaining tolerance in plants. Despite these successful models, marker-assisted selection has few constraints at the end of undesirable traits coming from wild donors causing mutants in the genetic programs of cultivars and non-significant results at the farm level. However, NGS is a promising approach in determining molecular markers relatively quicker for obtaining high-density genetic maps. Furthermore, it is necessary to boost salt-resistant QTLs preparation to address salt stress, which can only be achieved with the use of NGS.
4.3 Use of Plant-Growth-Promoting Rhizobacteria (PGPR) to Manage Salinity Stress
Microorganisms are usually colonized in the rhizosphere and they enhance germination, root and shoot length, increase mineral uptake, and yield [147–150], and enhance tolerance against abiotic stress (salinity) [151,152]. Under abiotic stress, many beneficial effects of salt-tolerant rhizobacteria were observed [153,154]. Plant growth is negatively affected by salinity, whereas the function and composition of soil beneficial bacteria also decreased [155]. Saline soils deteriorate the soil structure formed by the soil microbial community near the region of the rhizosphere with a declining quantity and quality of the root exudates [156]. Many strains of PGPR (S. rhizophila e-p10, P. fluorescens SPB2145, P. chlororaphis TSAU 13, Serratia plymuthica RR2–5–10, P. putida TSAU1, P. extremorientalis TSAU20, P. fluorescens PCL1751, and P. aureofaciens TSAU22) induced tolerance against salinity [154,157]. Thus, with the use of these strains, wheat can withstand and perform better with optimum yields under saline stress environments [158,159]. PGPR improve germination rate and growth attributes [160], a thousand-grain weight and grain production [161]. Salt-tolerant rhizobacteria isolated from wheat roots were responsible for increasing root length under saline conditions [162]. Use of PGPR for seed inoculation increased root and shoot length of crops (tomato, pepper, canola, bean, and lettuce) along with increasing their dry biomass, fruiting, and grain yield by enhancing tolerance against salinity [163,164]. Furthermore, PGPR caused osmotic adjustment during saline conditions which also helped to mitigate the adverse effects of salinity stress [165].
4.4 Ameolative Role of AMF against Salinity Stress
Plants under salt-stressed environments undergo hypertonic as well as the hyperosmotic conditions that lead to plant death. Arbuscular mycorrhizal fungi (AMF) are persistent in soil, play a significant role in improving soil health [166], and increases plant growth during salinity [167–169]. AMF enable plants to improve their water and mineral nutrient uptake, increase photosynthesis and accumulation of osmolytes under salt stress [170,171]. Plants with colonized AMF have morphological, physiological, and nutritional changes for normal activities during salinity [172]. In addition, AMF also increase mineral and water uptake [167,173] and water use efficiency (WUE) [170]. However, such development mainly depends upon the type of AMF species that is used for inoculation under saline conditions [174].
Plant exposure to high salinity produces excessive MDA which decreases membrane stability and increases lipid peroxidation [168,175–177]. AMF inoculated plants reduce MDA production with increased anti-oxidant enzymatic activity under salinity stress [178,179]. Studies show that anti-oxidant enzymes were active in AMF colonized plants over un-inoculated plants and showed promising results under salt stress conditions [180–182]. High chlorophyll content was observed in AMF inoculated plants during salt stress that enhanced the photosynthesis rate for carbohydrate production [171,183].
Availability of essential mineral nutrients to plants impedes salinity [184,185]. AMF colonized plants absorb plenty of minerals [186] facilitating a high water uptake [187] and increasing their growth under salt-stressed conditions [188]. Hormones like auxins (IAA, ABA) and gibberellins (GA) improve plant growth. The concentration of these plant growth regulators decreased under salt stress, whilst their concentration was significantly increased on AMF inoculated plants under salinity [178–179,189]. The combined use of PGPR and AMF increased nutrient uptake [190], water absorption, and grain production under saline conditions [191]. The synergistic effect of both PGPR and AMF enables plants to cope with various biotic as well as abiotic stress conditions. Further research is needed for getting the best combination of PGPR and AMF for obtaining a maximum plant production under biotic and abiotic stressed environments.
4.5 Use of Exogenously Applied Hormones and Osmoprotectants to Mitigate Salinity Stress in Wheat
Hormones are chemicals produced in plants and they regulate normal plant functioning, development, and tolerance against various stresses especially under salinity [192]. Moreover, the external use of different synthesized plant hormones (Auxin, CK, ABA, GA, and brassinosteroids) mitigates the effect of various abiotic stresses. Auxin improves germination and shoot dry weight while maintaining ionic homeostasis in salinity; thus, it is well-known as a growth promoter [75]. It alleviates salinity up to 15 dS m−1 with a balanced hormonal concentration in the plant and enhances yield by improving assimilation rate in salt-sensitive and salt-tolerant wheat cultivars [76]. GA3 priming brings improvement in photosynthetic pigments, and leaf and plant growth under salinity stress conditions [193]. ABA primed seeds expresseshigh salinity tolerance with high chlorophyll production and decreased Na+ uptake [80]. High wheat grain yield was observed with the application of brassinosteroid under salt stress [82,194].
Plants produce numerous compatible solutes to survive against ionic, oxidative as well as osmotic stress [195]. These osmolytes include proline, glycine-betaine (GB), β-alanine betaine, dimethylsulfoniopropionate (DMSP), choline, polyols, and sugars [trehalose (Tre), sorbitol, and mannitol] as shown in Table 3. Effective results were observed with the exogenous application of osmoprotectants to alleviate salt and metal stresses on plants [196–198]. Proline, being an osmoprotectant, helps in osmotic adjustment as well as detoxification of ROS, and strengthening the photosystem II structure [199,200]. Various anti-oxidant enzymes (SOD, POD, and CAT) trigger their activities with exogenous applied GB and significantly improve the salinity tolerance of T. aestivum [201]. In another experiment GB application (10 and 30 mM) increased germination and calcium and chlorophyll contents in shoots and leaves, and improved salinity tolerance [202]. Likewise, exogenous proline (60 ppm) up-regulated endogenous hormones (GA, IAA) and down-regulated MDA, and improved salinity tolerance in wheat [203].

Rao et al. [210] suggested that increased production of Pro and GB mitigated the damaging effects of salt stress by activating antioxidant enzymes. The application of trehalose improved the Pro and K+ accumulation, and the ratio of K+/Na+ and stabilized the protein and lipid structure [211,212]. Mannitol lowers lipid peroxidation by amplifying the antioxidant (POD, SOD, APX, CAT, and GR) enzymes, and thus reverses the harmful effects of salinity [213]. Melatonin protects the cell membrane and increases the activities of antioxidants under stress conditions [214]. In conclusion, hormone and osmoprotectant applications improve antioxidants activities, photosynthetic efficiency, membrane stability, and detoxifies ROS, thus leading to substantial improvement under salinity stress.
4.6 Role of Seed Priming under Conditions of Salt Stress
Seed priming is an economical and cheap approach that gives promising results for getting a maximum production under salt stress conditions [215]. Seed priming improves germination rate and seedling establishment in wheat and other crops [32,216–218]. The positive effects of seed priming can be due to the availability of adenosine triphosphate (ATP), enzymatic activation, and de-novo synthesis of some substances that promote germination [219]. Seed priming is a reliable, simple, inexpensive, and low-risk technique [220,221]. Various priming techniques such as hydro-priming (soaking the seeds in water), osmo-priming (soaking the seeds in nutrient, hormone, or chemical solutions), and halo-priming (soaking the seeds in a salt solution) have been developed to increase the speed of germination, seedling establishment and crop production [222]. Seed priming has been shown to effectively increase germination and seeding emergence of many crops in the tropics and subtropics, particularly under salt stress conditions [223].
Increased germination rates and better seedling establishment led to higher levels of salt stress tolerance and crop yields when the seeds were primed [221]. Afzal et al. [224] observed the effect of hydro-priming on salt-sensitive (MH-97) and salt-tolerant (AUQAB-2000) wheat cultivars under salinity (15 dSm−1). They noted seed priming increased tolerance against salinity. Osmopriming of seeds with AsA has been reported to increase endogenous AsA content and CAT activity, which increased salt stress tolerance in wheat [225]. Increased germination rate, early seedling establishment, ABA and Pro accumulation, and plant growth were demonstrated by osmo-priming seeds with 0.05 mM salicylic acid (SA) in wheat [226]. Calcium chloride (CaCl2) used as a priming agent (halopriming) reduced Na+ concentration and increased accumulation of K+ ions that helped to maintain ion homeostasis and salt tolerance in wheat [227]. Moreover, halo-priming also increased the activities of SOD and CAT that detoxified the ROS under salt stress [228]. Phyto hormones are also used as priming substances that induces salinity tolerance. IAA seed priming enhanced amylase activity that triggers the germination process [229] and deteriorates the inhibitory effects of salt stress in wheat production [224]. Photosynthetic pigments (chl a and b) and osmotic adjustment were increased with SA priming (100 mgL−1) in a wheat cultivar during salt stress [230]. The positive response of wheat by lowering Na+ and Cl− ions while accumulating K+ and Ca2+ contents under salt stress was observed with GA (150 mgL−1) priming [76]. Furthermore, it also enabled wheat to increase its germination rate and seedling growth and contributed to a marked increase in its final production.
4.7 Nutrient Management to Improve Salinity Tolerance
Plant nutrients are vital for getting an optimum yield. However, the limited supply of essential nutrients along with a poor soil fertility reduce crop production globally [31]. Sixty percent of soils across the globe are deficient in nutrients; therefore, it is necessary to have satisfactory soil nutrient levels to obtain a maximum yield [231]. Nutrients are essential to alleviate the effects of different abiotic stresses including salinity. Plants use various nutrients including (1) nitrogen (N) and magnesium (Mg) in the photosynthetic process [232], (2) phosphorous (P) for ATP generation, and (3) potassium (K) for stomatal regulation enzyme activation [233,234]. Researchers found a positive response of plants with the use of various mineral nutrients against abiotic stresses; amongst them, tolerance in plants against salinity is acquired with the plenty use of silicon (Si) and potassium (K) [235,236]. Exogenously applied potassium (K) increases salt tolerance in wheat by increasing photosynthetic efficiency, photosynthetic pigments, and antioxidant enzymes activities [237–239]. Foliar application of phosphorous (P) enhances leaf area index and plant biomass, and reduces salinity-induced damages [240]. Nitrogen is an essential part of energy (ATP), and vital for carbon metabolism [241]. Conversely, plants with nitrogen deficiency are susceptible to oxidative damage. Less enzyme activity because of low magnesium (Mg) concentrations occurs in the chloroplast, and it alters the photosynthetic efficiency in salt-affected soils [242,243]. A low Boron concentration improves the antioxidant defense mechanism in wheat and lessens the effect of ROS [233]. ROS production during salt-stress can be minimized with selenium (Se) application [244], and foliar-applied zinc (Zn) enhances grain production in wheat under salt stress conditions [245–250]. All these findings suggest that an adequate nutrient supply is essential for maintaining optimum growth yield and under salinity stress. In Table 4., the salt-induced oxidative stress and antioxidant defense regulations in wheat are presented.

Wheat is the world’s most popular and consumed cereal crop. However, salinity stress is a major threat to global wheat production, food, and nutritional security. Salt stress negatively affects seed germination, plant growth, photosynthesis, ATP production, water relationships, nutrient uptake and yield because of a salt-induced oxidative stress and ionic and hormonal imbalances. Wheat crop shows a wide range of morphological, physiological, and molecular responses under salinity stress. The physiological and molecular mechanisms are very important because they can help the breeders to develop salt tolerance in wheat. These mechanisms against salinity stress are well understood in wheat. However, a better understanding is still needed in many fields, especially in understanding the physiological basis of assimilate partitioning from plant sources to sinks. Additionally, more studies are needed to study the response of roots to salinity stress involving the root-shoot signaling and corresponding impacts on the nutrient and water uptake. Genetic manipulation of salt-tolerant traits is also an important approach to improve salinity tolerance in wheat crops. However, genetic manipulation requires the collaboration of physiologists, breeders, and agronomists to find sustainable ways to improve salinity tolerance in wheat. Moreover, there is a need to assess the wild relatives and accessions of wheat crops having strong salinity tolerance. Modern techniques including molecular markers, genetic engineering, and QTL mapping have contributed to an understanding of the complex salinity traits in wheat. However, there is a large extent for further improvement. As the genotype and environment interactions are still poorly understood, QTL identified under specific conditions cannot perform well in different conditions. Likewise, transgenic plants developed for salinity tolerance may not perform well at the field level. Therefore, research findings need further validation of developed materials at the farmer field scale. The use of plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi can also help to improve wheat responses under salt stress. However, future investigations are needed to find out the mechanisms lying behind the reduction of the effects of salinity stress by these microbes. Additionally, exogenous application of osmo-protectants, phytohormones, seed priming, and nutrient management can also help to (1) improve salt tolerance in wheat. All these efforts would help to alleviate the negative effects of salinity stress on wheat crops, and (2) contribute to an improved wheat productivity and food security.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Munns, R., Tester, M. (2008). Mechanism of salinity tolerance. Annual Review of Plant Biology, 59, 651–681. DOI 10.1146/annurev.arplant.59.032607.092911. [Google Scholar] [CrossRef]
2. Ding, Z., Kheir, A. S., Ali, O. A., Hafez, E., Elshamey, E. A. et al. (2021). A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. Journal of Environmental Management, 277, 111–388. DOI 10.1016/j.jenvman.2020.111388. [Google Scholar] [CrossRef]
3. Acquaah, G. (2007). Principles of plant genetics and breeding. pp. 385. Oxford, UK: Blackwell. [Google Scholar]
4. Seleiman, M. F., Kheir, A. M. S., Al-Dhumri, S., Alghamdi, A. G., Omar, E. S. H. et al. (2019). Exploring optimal tillage improved soil characteristics and productivity of wheat irrigated with different water qualities. Agronomy, 9, 233. DOI 10.3390/agronomy9050233. [Google Scholar] [CrossRef]
5. Hassan, M. U., Chattha, M. U., Khan, I., Chattha, M. B., Barbanti, L. et al. (2020a). Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Bio-System, 155, 211–234. DOI 10.1080/11263504.2020.1727987. [Google Scholar] [CrossRef]
6. Hasanuzzaman, M., Nahar, K., Rahman, A., Anee, T. I., Alam, M. U. et al. (2017). Approaches to enhance salt stress tolerance in wheat. pp. 151–187. UK: IntechOpen Limited 5 Princes Gate Court London. DOI 10.5772/67247. [Google Scholar] [CrossRef]
7. Chattha, M. U., Hassan, M. U., Khan, I., Chattha, M. B., Mahmood, A. et al. (2017a). Biofortification of wheat cultivars to combat zinc deficiency. Frontiers in Plant Science, 8, 281. DOI 10.3389/fpls.2017.00281. [Google Scholar] [CrossRef]
8. Hassan, M. U., Chattha, M. U., Ullah, A., Khan, I., Qadeer, A. et al. (2019). Agronomic biofortification to improve productivity and grain Zn concentration of bread wheat. International Journal of Agriculture and Biology, 21, 615–620. DOI 10.17957/IJAB/15.0936. [Google Scholar] [CrossRef]
9. Hassan, M. U., Aamer, M., Nawaz, M., Rehman, A., Aslam, T. et al. (2021). Agronomic Bio-fortification of wheat to combat zinc deficiency in developing countries. Pakistan Journal of Agriculture Research, 34, 66–78. [Google Scholar]
10. Muhsin, M., Nawaz, M., Khan, I., Chattha, M. B., Khan, S. et al. (2021). Efficacy of seed size to improve field performance of wheat under late sowing conditions. Pakistan Journal of Agriculture Research, 34, 247–254. DOI 10.17582/journal.pjar/2021/34.1.247.253. [Google Scholar] [CrossRef]
11. Al-Ashkar, I., Alderfasi, A., El-Hendawy, S., Al-Suhaibani, N., El-Kafafi, S. et al. (2019). Detecting salt tolerance in doubled haploid wheat lines. Agronomy, 9, 211. DOI 10.3390/agronomy9040211. [Google Scholar] [CrossRef]
12. Al-Ashkar, I., Alderfasi, A., Romdhane, W., Seleiman, M. F., El-Said, R. A. et al. (2020). Morphological and genetic diversity within salt tolerance detection in eighteen wheat genotypes. Plants, 9, 287. DOI 10.3390/plants9030287. [Google Scholar] [CrossRef]
13. Royo, A., Abió, D. (2003). Salt tolerance in durum wheat cultivars. Spanish Journal of Agriculture Research, 1, 27–35. DOI 10.5424/sjar/2003013-32. [Google Scholar] [CrossRef]
14. World Health Organization (2019). Preventing disease through healthy environments: exposure to highly hazardous pesticides: A major public health concern (No. WHO/CED/PHE/EPE/19.4.6). Geneva, Switzerland: World Health Organization. [Google Scholar]
15. Hajihashemi, S., Kiarostami, K., Enteshari, S., Saboora, A. (2009). Effect of paclobutrazol on wheat salt tolerance at pollination stage. Russian Journal of Plant Physiology, 56, 251–257. DOI 10.1134/S1021443709020149. [Google Scholar] [CrossRef]
16. Huang, Y., Bie, Z., He, S., Hua, B., Zhen, A. et al. (2010). Improving cucumber tolerance to major nutrients induced salinity by grafting onto cucurbita ficifolia. Environmental and Experimental Botany, 69, 32–38. DOI 10.1016/j.envexpbot.2010.02.002. [Google Scholar] [CrossRef]
17. Taha, R. S., Seleiman, M. F., Alhammad, B. A., Alkahtani, J., Alwahibi, M. S. et al. (2021). Activated yeast extract enhances growth, anatomical structure, and productivity of Lupinus termis L. plants under actual salinity conditions. Agronomy, 11, 74. DOI 10.3390/agronomy11010074. [Google Scholar] [CrossRef]
18. Hussain, S., Hussain, S., Ali, B., Ren, X., Chen, X. et al. (2021). Recent progress in understanding salinity tolerance in plants: Story of Na+/K+ balance and beyond. Plant Physiology and Biochemistry, 160, 250–256. DOI 10.1016/j.plaphy.2021.01.029. [Google Scholar] [CrossRef]
19. Hafez, E. M., Abou El Hassan, W. H., Gaafar, I. A., Seleiman, M. F. (2015). Effect of gypsum application and irrigation intervals on clay saline-sodic soil characterization, rice water use efficiency, growth, and yield. Journal of Agriculture Science, 7, 208–219. DOI 10.5539/jas.v7n12p208. [Google Scholar] [CrossRef]
20. Seleiman, M. F., Kheir, A. M. (2018). Saline soil properties, quality and productivity of wheat grown with bagasse ash and thiourea in different climatic zones. Chemosphere, 193, 538–546. DOI 10.1016/j.chemosphere.2017.11.053. [Google Scholar] [CrossRef]
21. Taha, R. S., Seleiman, M. F., Shami, A., Alhammad, B. A., Mahdi, A. H. A. (2021). Integrated application of selenium and silicon enhances growth and anatomical structure, antioxidant defense system and yield of wheat grown in salt-stressed soil. Plants, 10, 1040. DOI 10.3390/plants10061040. [Google Scholar] [CrossRef]
22. Ibrahimova, U., Zivcak, M., Gasparovic, K., Rastogi, A., Allakhverdiev, S. I. et al. (2021). Electron and proton transport in wheat exposed to salt stress: Is the increase of the thylakoid membrane proton conductivity responsible for decreasing the photosynthetic activity in sensitive genotypes? Photosynthesis Research, 1–17. DOI 10.1007/s11120-021-00853-z. [Google Scholar] [CrossRef]
23. Seleiman, M. F., Almutairi, K. F., Alotaibi, M., Shami, A., Alhammad, B. A. et al. (2021a). Nano-fertilization as an emerging fertilization technique: Why can modern agriculture benefit from its use? Plants, 10, 2. DOI 10.3390/plants10010002. [Google Scholar] [CrossRef]
24. Shabala, S., Munns, R. (2012). Salinity stress: physiological constraints and adaptive mechanisms. In Shabala S (ed). Plant stress physiology. pp. 59–93. Oxford, London, UK. [Google Scholar]
25. Rahman, A., Nahar, K., Hasanuzzaman, M., Fujita, M. (2016). Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Frontiers in Plant Sciences, 7, 609. DOI 10.3389/fpls.2016.00609. [Google Scholar] [CrossRef]
26. Hassan, M. U., Chattha, M. U., Mahmood, A., Sahi, S. T. (2018a). Performance of sorghum cultivars for biomass quality and biomethane yield grown in semi-arid area of Pakistan. Environmental Science and Pollution Research, 25, 12800–12807. DOI 10.1007/s11356-018-1575-4. [Google Scholar] [CrossRef]
27. Hassan, M. U., Chattha, M. U., Chattha, M. B., Mahmood, A., Sahi, S. T. (2018b). Bio-methane production from sorghum elite lines under the climatic conditions of Pakistan. Maydica, 63, 1–8. [Google Scholar]
28. Chattha, M. U., Hassan, M. U., Khan, I., Chattha, M. B., Aamer, M. et al. (2020). Impact of planting methods on biomass production, chemical composition and methane yield of sorghum cultivars. Pakistan Journal of Agriculture Sciences, 57, 43–51. DOI 10.21162/PAKJAS/20.7112. [Google Scholar] [CrossRef]
29. Erdal, S., Aydın, M., Genisel, M., Taspınar, M. S., Dumlupinar, R. et al. (2010). Effects of salicylic acid on wheat salt sensitivity. African Journal of Biotechnology, 10, 5713–5718. DOI 10.5897/AJB10.1550. [Google Scholar] [CrossRef]
30. Hasanuzzaman, M., Nahar, K., Fujita, M., Ahmad, P., Chandna, R. et al. (2013). Enhancing plant productivity under salt stress: Relevance of poly-omics. In Ahmad P, Azooz M. M., Prasad, M. N. V. (edsSalt Stress in Plants: Omics, Signaling and Responses, pp. 113–156. Berlin, Germany: Springer. DOI 10.1007/978-1-4614-6108-1_6. [Google Scholar] [CrossRef]
31. Saddiq, M. S., Iqbal, S., Hafeez, M. B., Ibrahim, A. M., Raza, A. et al. (2021). Effect of salinity stress on physiological changes in winter and spring wheat. Agronomy, 11(1193), 4. DOI 10.3390/agronomy11061193. [Google Scholar] [CrossRef]
32. Ghiyasi, M., Seyahjani, A. A., Tajbakhsh, M., Amirnia, R., Salehzadeh, H. (2008). Effect of osmopriming with polyethylene glycol (8000) on germination and seedling growth of wheat (Triticum aestivum L.) seeds under salt stress. Journal of Biological Sciences, 3, 1249–1251. [Google Scholar]
33. Jamal, Y., Shafi, M., Bakht, J., Arif, M. (2011). Seed priming improves salinity tolerance of wheat varieties. Pakistan Journal of Botany, 43, 2683–2686. [Google Scholar]
34. Guo, R., Yang, Z., Li, F., Yan, C., Zhong, X. et al. (2015). Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum L.) to salt and alkali stress. BMC Plant Biology, 15, 170. DOI 10.1186/s12870-015-0546-x. [Google Scholar] [CrossRef]
35. Zou, P., Li, K., Liu, S., He, X., Zhang, X. et al. (2016). Effect of sulfated chitooligosaccharides on wheat seedlings (Triticum aestivum L.) under salt stress. Journal of Agriculture and Food Chemistry, 64, 2815–2821. DOI 10.1021/acs.jafc.5b05624. [Google Scholar] [CrossRef]
36. Asgari, H. R., Cornelis, W., Damme, P. V. (2012). Salt stress effect on wheat (Triticum aestivum L.). growth and leaf ion concentrations. International Journal of Plant Production, 6, 195–208. [Google Scholar]
37. Chinnusamy, V., Jagendorf, A., Zhu, J. K. (2005). Understanding and improving salt tolerance in plants. Crop Science, 45, 437–448. DOI 10.2135/cropsci2005.0437. [Google Scholar] [CrossRef]
38. Afzal, I., Rauf, S., Basra, S. M. A., Murtaza, G. (2008). Halopriming improves vigor, metabolism of reserves and ionic contents in wheat seedlings under salt stress. Plant Soil Environment, 54, 382–388. DOI 10.17221/PSE. [Google Scholar] [CrossRef]
39. Badawy, S. A., Zayed, B. A., Bassiouni, S. M. A., Mahdi, A. H. A., Majrashi, A. et al. (2021). Influence of nano silicon and nano selenium on root characters, growth, ion selectivity, yield, and yield components of rice (Oryza sativa L.) under salinity conditions. Plants, 10, 1657. DOI 10.3390/plants10081657. [Google Scholar] [CrossRef]
40. Nassar, R., Kamel, H. A., Ghoniem, A. E., Alarcón, J. J., Sekara, A. et al. (2020). Physiological and anatomical mechanisms in wheat to cope with salt stress induced by seawater. Plants, 9, 237. DOI 10.3390/plants9020237. [Google Scholar] [CrossRef]
41. Wahid, A., Perveen, M., Gelani, S., Basra, S. M. A. (2007). Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. Journal of Plant Physiology, 164, 283–294. DOI 10.1016/j.jplph.2006.01.005. [Google Scholar] [CrossRef]
42. Parida, A. K., Das, A. B. (2005). Salt tolerance and salinity effect on plants: A review. Ecotoxicology and Environmental Safety, 60, 324–349. DOI 10.1016/j.ecoenv.2004.06.010. [Google Scholar] [CrossRef]
43. Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Science, 7, 405–410. DOI 10.1016/S1360-1385(02)02312-9. [Google Scholar] [CrossRef]
44. Hasegawa, P. M., Bressan, R. A., Zhu, J. K., Bohnert, H. J. (2000). Plant cellular and molecular responses to high salinity. Annual Review of Plant Biology, 51, 463–499. DOI 10.1146/annurev.arplant.51.1.463. [Google Scholar] [CrossRef]
45. Hasanuzzaman, M., Hossain, M. A., Fujita, M. (2011). Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnological Reports, 5, 353–365. DOI 10.1007/s11816-011-0189-9. [Google Scholar] [CrossRef]
46. Hasanuzzaman, M., Nahar, K., Fujita, M. (2013b). Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. Ecophysiology and responses of plants under salt stress. pp. 25–87. New York, NY, USA: Springer. DOI 10.1007/978-1-4614-4747-4_2. [Google Scholar] [CrossRef]
47. Farooq, M., Hussain, M., Wakeel, A., Siddique, K. H. M. (2015). Salt stress in maize effects resistance mechanisms and management: A review. Agronomy for Sustainable Development, 35, 461–48. DOI 10.1007/s13593-015-0287-0. [Google Scholar] [CrossRef]
48. Garg, N., Manchanda, N. (2009). Role of arbuscular mycorrhizae in the alleviation of ionic osmotic and oxidative stresses induced by salinity in Cajanus cajan (L.) millsp (pigeonpea). Journal of Agronomy and Crop Science, 195, 110–123. DOI 10.1111/j.1439-037X.2008.00349.x. [Google Scholar] [CrossRef]
49. Bose, J., Rodrigo-Moreno, A., Shabala, S. (2014). ROS homeostasis in halophytes in the context of salinity stress tolerance. Journal of Experimental Botany, 65, 1241–1257. DOI 10.1093/jxb/ert430. [Google Scholar] [CrossRef]
50. Pottosin, I., Velarde-Buendía, A. M., Bose, J., Zepeda-Jazo, I., Shabala, S. et al. (2014). Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: Implications for plant adaptive responses. Journal of Experimental Botany, 65, 1271–1283. DOI 10.1093/jxb/ert423. [Google Scholar] [CrossRef]
51. Phang, T. H., Shao, G., Lam, H. M. (2008). Salt tolerance in soybean. Journal of Integrative Plant Biology, 50, 1196–1212. DOI 10.1111/j.1744-7909.2008.00760.x. [Google Scholar] [CrossRef]
52. El Sayed, H. E. A. (2011). Influence of naCl and Na2SO4 treatments on growth development of broad bean (Vicia faba L.) plant. Journal of Life Science, 5, 513–523. [Google Scholar]
53. Ashraf, M. (2004). Some important physiological selection criteria for salt tolerance in plants. Flora, 199, 361–376. DOI 10.1078/0367-2530-00165. [Google Scholar] [CrossRef]
54. Li, W. Y. F., Wong, F. L., Tsai, S. N., Phang, T. H., Shao, G. et al. (2006). Tonoplast located GmCLC1 andGmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (by)-2 cells. Plant Cell Environment, 29, 1122–1137. DOI 10.1111/j.1365-3040.2005.01487.x. [Google Scholar] [CrossRef]
55. Cordovilla, M. P., Ocana, A., Ligero, F., Lluch, C. (1995). Salinity effects on growth analysis and nutrient composition in four grain legumes. Journal of Plant Nutrition, 18, 1595–1609. DOI 10.1080/01904169509365006. [Google Scholar] [CrossRef]
56. Blumwald, E. (2000). Sodium transport and salt tolerance in plants. Current Opinion in Cell Biology, 2, 431–434. DOI 10.1016/S0955-0674(00)00112-5. [Google Scholar] [CrossRef]
57. Amtmann, A., Sanders, D. (1998). Mechanisms of Na+ uptake by plant cells. Advances in Botanical Research, 29, 76–112. DOI 10.1016/S0065-2296(08)60310-9. [Google Scholar] [CrossRef]
58. Soni, S., Kumar, A., Sehrawat, N., Kumar, A., Kumar, N. et al. (2021). Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi Journal of Biological Sciences, 28, 2510–2517. DOI 10.1016/j.sjbs.2021.01.052. [Google Scholar] [CrossRef]
59. Yadav, T., Kumar, A., Yadav, R. K., Yadav, G., Kumar, R. et al. (2020). Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet-wheat. Saudi Journal of Biological Sciences, 27, 2010–2017. DOI 10.1016/j.sjbs.2020.06.030. [Google Scholar] [CrossRef]
60. Sadak, M. S. (2019). Physiological role of trehalose on enhancing salinity tolerance of wheat plant. Bulletin of the National Research Centre, 43, 1–10. DOI 10.1186/s42269-019-0098-6. [Google Scholar] [CrossRef]
61. Fortmeier, R., Schubert, S. (1995). Salt tolerance of maize (Zea mays L.The role of sodium exclusion. Plant Cell Environment, 18, 1041–1047. DOI 10.1111/j.1365-3040.1995.tb00615.x. [Google Scholar] [CrossRef]
62. Neubert, A. B., Zorb, C. Schubert, S. (2005). Expression of vacuolar Na+/H+ antiporters (ZmNHX) and Na+ exclusion in roots of maize (Zea Mays L.) genotypes with improved salt resistance. in Li C. J. et al. (EdsPlant nutrition for food security human health and environmental protection, pp. 63–89. Beijing, China: Tsinghua University Press. [Google Scholar]
63. Wakeel, A., Farooq, M., Qadir, M., Schubert, S. (2011b). Potassium substitution by sodium in plants. Critical Review in Plant Science, 30, 401–413. DOI 10.1080/07352689.2011.587728. [Google Scholar] [CrossRef]
64. McCord, J. M. (2000). The evolution of free radicals and oxidative stress. American Journal of Medicine, 108, 652–659. DOI 10.1016/S0002-9343(00)00412-5. [Google Scholar] [CrossRef]
65. Garratt, L. C., Janagoudar, B. S., Lowe, K. C., Anthony, P., Power, J. B. et al. (2002). Salinity tolerance and antioxidant status in cotton cultures. Free Radical Biology and Medicine, 33, 502–511. DOI 10.1016/S0891-5849(02)00838-9. [Google Scholar] [CrossRef]
66. Seleiman, M. F., Semida, W. M., Rady, M. M., Mohamed, G. F., Hemida, K. A. et al. (2020). Sequential application of antioxidants rectifies ion imbalance and strengthens antioxidant systems in salt-stressed cucumber. Plants, 9, 1783. DOI 10.3390/plants9121783. [Google Scholar] [CrossRef]
67. Noctor, G., Foyer, C. H. (1998). Ascorbate and glutathione: Keeping active oxygen under control. Annual Review of Plant Physiology, 49, 249–279. DOI 10.1146/annurev.arplant.49.1.249. [Google Scholar] [CrossRef]
68. Meneguzzo, S., Navari-Izzo, F., Izzo, R. (1999). Antioxidative responses of shoots and roots of wheat to increasing NaCI concentrations. Journal of Plant Physiology, 155, 274–280. DOI 10.1016/S0176-1617(99)80019-4. [Google Scholar] [CrossRef]
69. Sreenivasulu, N., Grimm, B., Wobus, U., Weschke, W. (2000). Differential response of antioxidant components to salinity stress in salt-tolerant and salt sensitive seedlings of foxtail millet (Setaria italica). Physiology of Plants, 109, 435–442. DOI 10.1034/j.1399-3054.2000.100410.x. [Google Scholar] [CrossRef]
70. Foyer, C. H., Noctor, G. (2003). Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiology of Plants, 119, 355–364. DOI 10.1034/j.1399-3054.2003.00223.x. [Google Scholar] [CrossRef]
71. Willekens, H., Inze, D., Van Montagu, M., van Camp, W. (1995). Catalases in plants. Molecular Breeding, 1, 207–228. DOI 10.1007/BF02277422. [Google Scholar] [CrossRef]
72. Athar, H. U. R., Khan, A., Ashraf, M. (2007). Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environmental and Experimental Botany, 63, 224–231. DOI 10.1016/j.envexpbot.2007.10.018. [Google Scholar] [CrossRef]
73. Elhamid, E. M. A., Sadak, M. S., Tawfik, M. M. (2014). Alleviation of adverse effects of salt stress in wheat cultivars by foliar treatment with antioxidant 2—Changes in some biochemical aspects, lipid peroxidation, antioxidant enzymes and amino acid contents. Agriculture Science, 5, 1269–1280. DOI 10.4236/as.2014.513135. [Google Scholar] [CrossRef]
74. Athar, H. U. R., Khan, A., Ashraf, M. (2009). Inducing salt tolerance in wheat by exogenously applied ascorbic acid through different modes. Journal of Plant Nutrition, 32, 1–19. DOI 10.1080/01904160903242334. [Google Scholar] [CrossRef]
75. Iqbal, M., Ashraf, M. (2007). Seed treatment with auxins modulates growth and ion partitioning in salt-stressed wheat plants. Journal of Integrative Plant Biology, 49, 1003–1015. DOI 10.1111/j.1672-9072.2007.00488.x. [Google Scholar] [CrossRef]
76. Iqbal, M., Ashraf, M. (2010). Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environmental and Experimental Botany, 86, 76–85. DOI 10.1016/j.envexpbot.2010.06.002. [Google Scholar] [CrossRef]
77. Shaddad, M. A. K., Abd El-Samad, H. M., Mostafa, D. (2013). Role of gibberellic acid (GA3) in improving salt stress tolerance of two wheat cultivars. International Journal Plant Physiology and Biochemistry, 5, 50–57. DOI 10.5897/IJPPB2011.055. [Google Scholar] [CrossRef]
78. Iqbal, M., Ashraf, M., Jamil, A. (2006). Seed enhancement with cytokinins: Changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regulation, 50, 29–39. DOI 10.1007/s10725-006-9123-5. [Google Scholar] [CrossRef]
79. Iqbal, M., Ashraf, M. (2005). Presowing seed treatment with cytokinins and its effect on growth, photosynthetic rate, ionic levels and yield of two wheat cultivars differing in salt tolerance. Journal of Integrative Plant Biology, 47, 1315–1325. DOI 10.1111/j.1744-7909.2005.00163.x. [Google Scholar] [CrossRef]
80. Gurmani, A. R., Bano, A., Najeeb, U., Zhang, J., Khan, S. U. et al. (2013). Exogenously applied silicate and abscisic acid ameliorates the growth of salinity stressed wheat (Triticum aestivum L.) seedlings through Na+ exclusion. Australian Journal of Crop Science, 7, 1123–1130. [Google Scholar]
81. Siddiqui, H., Hayat, S., Bajguz, A. (2018). Regulation of photosynthesis by brassinosteroids in plants. Acta Physiology of Plants, 40, 1–15. DOI 10.1007/s11738-018-2639-2. [Google Scholar] [CrossRef]
82. Eleiwa, M. E., Bafeel, S. O., Ibrahim, S. O. (2011). Influence of brassinosteroids on wheat plant (Triticum aestivum L.) production under salinity stress conditions I—growth parameters and photosynthetic pigments. Australian Journal of Basic Applied Science, 5, 58–65. DOI 10/20113218999. [Google Scholar]
83. Munns, R., Hare, R. A., James, R. A., Rebetzke, G. J. (2000). Genetic variation for improving the salt tolerance of durum wheat. Australian Journal of Agriculture Research, 51, 69–74. DOI 10.1071/AR99057. [Google Scholar] [CrossRef]
84. Gorham, J., Wyn, R. G., Bristol, A. (1990). Partial characterization of the trait for enhanced K+–Na+ discrimination in the D genome of wheat. Planta, 180, 590–597. DOI 10.1007/BF02411458. [Google Scholar] [CrossRef]
85. Dubcovsky, J., Maria, G. S., Epstein, E., Luo, M. C., Dvorak, J. (1996). Mapping of the K+/Na+ discrimination locus kna1 in wheat. Theoretical and Applied Genetics, 92, 448–454. DOI 10.1007/BF00223692. [Google Scholar] [CrossRef]
86. Roy, S. J., Tucker, E. J., Tester, M. (2011). Genetic analysis of abiotic stress tolerance in crops. Current Opinion in Plant Biology, 14, 232–239. DOI 10.1016/j.pbi.2011.03.002. [Google Scholar] [CrossRef]
87. Yang, C., Zhao, L., Zhang, H., Yang, Z., Wang, H. et al. (2014). Evolution of physiological responses to salt stress in hexaploid wheat. Proceedings of Notational Academy of Science, 111, 11882–11887. DOI 10.1073/pnas.1412839111. [Google Scholar] [CrossRef]
88. Byrt, C. S., Xu, B., Krishnan, M., Lightfoot, D. J. (2014). The Na+ transporter, TaHKT1;5-d, limits shoot Na+ accumulation in bread wheat. Plant Journal, 80, 516–26. DOI 10.1111/tpj.12651. [Google Scholar] [CrossRef]
89. Zhao, Y., Dong, W., Zhang, N., Ai, X., Wang, M. et al. (2014). Wheat allene oxide cyclase gene enhances salinity tolerance via jasmonate signaling. Plant Physiology, 164, 1068–1076. DOI 10.1104/pp.113.227595. [Google Scholar] [CrossRef]
90. Wang, M., Yuan, J., Qin, L., Shi, W., Xia, G. et al. (2020). TaCYP81D5, one member in a wheat cytochrome P450 gene cluster, confers salinity tolerance via reactive oxygen species scavenging. Plant Biotechnology Journal, 18, 791–804. DOI 10.1111/pbi.13247. [Google Scholar] [CrossRef]
91. Banerjee, A., Roychoudhury, A., Krishnamoorthi, S. (2016). Emerging techniques to decipher microRNAs (miRNAs) and their regulatory role in conferring abiotic stress tolerance of plants. Plant Biotechnology, 10, 185–205. DOI 10.1007/s11816-016-0401-z. [Google Scholar] [CrossRef]
92. Wang, M., Qin, L., Xie, C., Li, W., Yuan, J. et al. (2014). Induced and constitutive DNA methylation in a salinity-tolerant wheat introgression line. Plant and Cell Physiology, 55, 1354–1365. DOI 10.1093/pcp/pcu059. [Google Scholar] [CrossRef]
93. Xu, B., Waters, S., Byrt, C. S., Plett, D., Tyerman, S. D. et al. (2018). Structural variations in wheat HKT1; 5 underpin differences in Na+ transport capacity. Cell Molecular and Life Science, 75, 1133–1144. DOI 10.1007/s00018-017-2716-5. [Google Scholar] [CrossRef]
94. Shkolnik-Inbar, D., Guy, A., Dudy, B. Z. (2013). ABI4 downregulates expression of the sodium transporter HKT1; 1 in arabidopsis roots and affects salt tolerance. Plant Journal, 73, 993–1005. DOI 10.1111/tpj.12091. [Google Scholar] [CrossRef]
95. Wang, R., Jing, W., Xiao, L., Jin, Y., Shen, L. et al. (2015). The rice high-affinity potassium transporter1; 1 is involved in salt tolerance and regulated by an MYB-type transcription factor. Plant Physiology, 168, 1076–1090. DOI 10.1104/pp.15.00298. [Google Scholar] [CrossRef]
96. van Ginkel, M., Ogbonnaya, F. (2007). Novel genetic diversity from synthetic wheats in breeding cultivars for changing production conditions. Field Crop Research, 104, 86–94. DOI 10.1016/j.fcr.2007.02.005. [Google Scholar] [CrossRef]
97. Colmer, T. D., Munns, R., Flowers, T. J. (2005). Improving salt tolerance of wheat and barley: Future prospects. Australian Journal of Experimental Agriculture, 45, 1425–1443. DOI 10.1071/EA04162. [Google Scholar] [CrossRef]
98. Salse, J., Bolot, S., Throude, M., Jouffe, V., Piegu, B. et al. (2008). Identification and characterization of shared duplications between rice and wheat provide new insight into grass genome evolution. Plant Cell, 20, 11–24. DOI 10.1105/tpc.107.056309. [Google Scholar] [CrossRef]
99. Cui, H., Yu, Z., Deng, J., Gao, X., Sun, Y. et al. (2009). Introgression of bread wheat chromatin into tall wheatgrass via somatic hybridization. Planta, 229, 323–330. DOI 10.1007/s00425-008-0832-z. [Google Scholar] [CrossRef]
100. Xia, G. (2009). Progress of chromosome engineering mediated by asymmetric somatic hybridization. Journal of Genetics and Genomics, 36, 547–556. DOI 10.1016/S1673-8527(08)60146-0. [Google Scholar] [CrossRef]
101. Li, C., Lv, J., Zhao, X., Ai, X., Zhu, X. et al. (2010). TaCHP: A wheat zinc finger protein gene down-regulated by abscisic acid and salinity stress plays a positive role in stress tolerance. Plant Physiology, 154, 211–221. DOI 10.1104/pp.110.161182. [Google Scholar] [CrossRef]
102. Hasanuzzaman, M., Oku, H., Nahar, K., Bhuyan, M. B., Al Mahmud, J. et al. (2018). Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnological Report, 12, 77–92. DOI 10.1007/s11816-018-0480-0. [Google Scholar] [CrossRef]
103. Glombik, M., Bačovský, V., Hobza, R., Kopecký, D. (2020). Competition of parental genomes in plant hybrids. frontiers in . Plant Science, 11, 200. DOI 10.3389/fpls.2020.00200. [Google Scholar] [CrossRef]
104. Wang, M., Xia, G. (2018). The landscape of molecular mechanisms for salt tolerance in wheat. Crop Journal, 6, 42–47. DOI 10.1016/j.cj.2017.09.002. [Google Scholar] [CrossRef]
105. Foolad, M. R. (2004). Recent advances in genetics of salt tolerance in tomato. Plant Cell Tissue and Organ, 76, 101–19. DOI 10.1023/B:TICU.0000007308.47608.88. [Google Scholar] [CrossRef]
106. Ismail, A. M., Heuer, S., Thomson, M. J., Wissuwa, M. (2007). Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Molecular Biology, 65, 547–70. DOI 10.1007/s11103-007-9215-2. [Google Scholar] [CrossRef]
107. Negrao, S., Courtois, B., Ahmadi, N., Abreu, I., Saibo, N. et al. (2011). Recent updates on salinity stress in rice: From physiological to molecular responses. Critical Review in Plant Science, 30, 329–77. DOI 10.1080/07352689.2011.587725. [Google Scholar] [CrossRef]
108. Shahbaz, M., Ashraf, M. (2013). Improving salinity tolerance in cereals. Critical Review in Plant Science, 32, 237–49. DOI 10.1080/07352689.2013.758544. [Google Scholar] [CrossRef]
109. Rana, R. S. (1980). Genetic diversity for salt stress resistance of wheat in India. Rachis, 5, 32–37. QV19870072404. [Google Scholar]
110. Hollington, P. A. (2000). Technological breakthroughs in screening/breeding wheat varieties for salt tolerance. Proceedings of the National Conference ‘Salinity Management in Agriculture, pp. 273–289. CSSRI Karnal, India. [Google Scholar]
111. Hollington, P. A., Royo, A., Miller, T. E., Quarrie, S. A., Mahmood, A. et al. (1994). The use of doubled haploid breeding techniques to develop wheat varieties for saline areas. Proceedings of the 3rd Congress of the European Society of Agronomy, pp. 156–157. Netherlands. [Google Scholar]
112. Flowers, T. J. (2004). Improving crop salt tolerance. Journal of Experimental Botany, 55, 307–319. DOI 10.1093/jxb/erh003. [Google Scholar] [CrossRef]
113. Ashraf, M., Akram, N. A. (2009). Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnology Advances, 27, 744–752. DOI 10.1016/j.biotechadv.2009.05.026. [Google Scholar] [CrossRef]
114. Xue, Z. Y., Zhi, D. Y., Xue, G. P., Zhao, Y. X., Xia, G. M. (2004). Enhanced salt tolerance of transgenic wheat (Triticum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yield in saline soils in the field and a reduced level of leaf Na+. Plant Science, 167, 849–859. DOI 10.1016/j.plantsci.2004.05.034. [Google Scholar] [CrossRef]
115. Sawahel, W. A., Hassan, A. H. (2002). Generation of transgenic wheat plants producing high levels of the osmoprotectant proline. Biotechnology Letters, 24, 721–725. DOI 10.1023/A:1015294319114. [Google Scholar] [CrossRef]
116. Hu, W., Yuan, Q., Wang, Y., Cai, R., Deng, X. et al. (2012). Overexpression of a wheat aquaporin gene, TaAQP8, enhances salt stress tolerance in transgenic tobacco. Plant Cell Physiology, 53, 2127–2141. DOI 10.1093/pcp/pcs154. [Google Scholar] [CrossRef]
117. Bennett, J., Khush, G. S. (2003). Enhancing salt tolerance in crops through molecular breeding: A new strategy. Journal of Crop Production, 7, 11–65. DOI 10.1300/J144v07n01_02. [Google Scholar] [CrossRef]
118. Munns, R., James, R. A., Lauchli, A. (2006). Approaches to increasing the salt tolerance of wheat and other cereals. Journal of Experimental Botany, 57, 1025–1043. DOI 10.1093/jxb/erj100. [Google Scholar] [CrossRef]
119. Chen, Z., Newman, I., Zhou, M., Mendham, N., Zhang, G. et al. (2005). Screening plants for salt tolerance by measuring K+ flux: A case study for barley. Plant Cell Environment, 28, 1230–1246 DOI 10.1111/j.1365-3040.2005.01364.x. [Google Scholar] [CrossRef]
120. Cuin, T. A., Betts, S. A., Chalmandrier, R., Shabala, S. (2008). A root’s ability to retain K+ correlates with salt tolerance in wheat. Journal of Experimental Botany, 59, 2697–2706. DOI 10.1093/jxb/ern128. [Google Scholar] [CrossRef]
121. Munns, R. (2002). Comparative physiology of salt and water stress. Plant Cell Environment, 25, 239–250. DOI 10.1046/j.0016-8025.2001.00808.x. [Google Scholar] [CrossRef]
122. Tavakkoli, E., Rengasamy, P., McDonald, G. K. (2010b). The response of barley to salinity stress differs between hydroponic and soil systems. Functional Plant Biology, 37, 621–633. DOI 10.1071/FP09202. [Google Scholar] [CrossRef]
123. Munns, R., James, R. A., Xu, B., Athman, A., Conn, S. J. (2012). Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Notational Biotechnology, 30, 360–64. DOI 10.1038/nbt.2120. [Google Scholar] [CrossRef]
124. Bonilla, P., Dvorak, J., Mackill, D., Deal, K., Gregorio, G. (2002). RLFP and SSLP mapping of salinity tolerance genes in chromosome 1 of rice (Oryza sativa L.) using recombinant inbred lines. Philippine Agriculture Science, 85, 68–76. [Google Scholar]
125. Ren, Z. H., Gao, J. P., Li, L. G. (2005). A rice quantitative trait locus for salt tolerance encodes a sodium transporter. National Genetics, 37, 1141–1146. DOI 10.1038/ng1643. [Google Scholar] [CrossRef]
126. Maser, P., Eckelman, B., Vaidyanathan, R. (2002). Altered shoot/root Na+ distribution and bifurcating salt sensitivity in arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Letter, 531, 157–161. DOI 10.1016/S0014-5793(02)03488-9. [Google Scholar] [CrossRef]
127. Munns, R., James, R. A. (2003). Screening methods for salinity tolerance: A case study with tetraploid wheat. Plant and Soil, 253, 201–218. DOI 10.1023/A:1024553303144. [Google Scholar] [CrossRef]
128. Byrt, C. S., Platten, J. D., Spielmeyer, W. (2007). HKT 1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiology, 143, 1918–1928. DOI 10.1104/pp.106.093476. [Google Scholar] [CrossRef]
129. Kearsey, M. J., Farquhar, A. G. L. (1998). QTL analysis: Where are we now? Heredity, 80, 137–142. DOI 10.1046/j.1365-2540.1998.00500.x. [Google Scholar] [CrossRef]
130. Salvi, S., Tuberosa, R. (2005). To clone or not to clone plant QTLs: Present and future challenges. Trends in Plant Science, 10, 297–304. DOI 10.1016/j.tplants.2005.04.008. [Google Scholar] [CrossRef]
131. Collard, B. C. Y., Mackill, D. J. (2008). Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Biological Sciences, 363, 557–572. DOI 10.1098/rstb.2007.2170. [Google Scholar] [CrossRef]
132. Ben-Ari, G., Lavi, U. (2012). Marker-assisted selection in plant breeding. Plant biotechnology and agriculture, pp. 163–184. Totowa New Jersey, USA: Academic Press, Springer. DOI 10.1016/b978-0-12-381466-1.00011-0. [Google Scholar] [CrossRef]
133. Genc, Y., Oldach, K., Verbyla, A. P., Lott, G., Hassan, M. et al. (2010). Sodium exclusion QTL associated with improved seedling growth in bread wheat under salinity stress. Theoretical and Applied Genetics, 121, 877–894. DOI 10.1007/s00122-010-1357-y. [Google Scholar] [CrossRef]
134. Lee, S. Y., Ahn, J. H., Cha, Y. S. (2006). Mapping of quantitative trait loci for salt tolerance at the seedling stage in rice. Molecular Biology, 21, 192–196. PMID: 16682812. [Google Scholar]
135. Xue, D. W., Huang, Y. Z., Zhang, X. Q. (2009). Identification of QTLs associated with salinity tolerance at late growth stage in barley. Euphytica, 169, 187–196. DOI 10.1007/s10681-009-9919-2. [Google Scholar] [CrossRef]
136. Mujeeb-Kazi, A., Kazi, A. G., Dundas, I., Rasheed, A., Ogbonnaya, F. et al. (2013). Genetic diversity for wheat improvement as a conduit to food security. Advances in Agronomy, 122, 179–257. DOI 10.1016/B978-0-12-417187-9.00004-8. [Google Scholar] [CrossRef]
137. Bernardo, R. (2008). Molecular markers and selection for complex traits in plants: Learning from the last 20 years. Crop Science, 48, 1649–1664. DOI 10.2135/cropsci2008.03.0131. [Google Scholar] [CrossRef]
138. Ingvarsson, P. K., Hall, D., Tegstrom, C. (2010). Using association mapping to dissect the genetic basis of complex traits in plants. Brief Functional Genomic, 9, 157–165. DOI 10.1093/bfgp/elp048. [Google Scholar] [CrossRef]
139. Mackay, I., Powell, W. (2007). Methods for linkage disequilibrium mapping in crops. Trends in Plant Science, 12, 57–63. DOI 10.1016/j.tplants.2006.12.001. [Google Scholar] [CrossRef]
140. Pritchard, J. K., Stephens, M., Rosenberg, N. A., Donnelly, P. (2000). Association mapping in structured populations. Animal and Human Genetics, 67, 170–181. DOI 10.1086/302959. [Google Scholar] [CrossRef]
141. Thudi, M., Upadhyaya, H. D., Rathore, A., Gaur, P. M., Krishnamurthy, L. et al. (2014). Genetic dissection of drought and heat tolerance in chickpea through genome-wide and candidate gene-based association mapping approaches. PLoS One, 9, e96758. DOI 10.1371/journal.pone.0096758. [Google Scholar] [CrossRef]
142. Zhu, C., Gore, M., Buckler, E. S., Yu, J. (2008). Status and prospects of association mapping in plants. Plant Genome, 1, 5–20. DOI 10.3835/plantgenome2008.02.0089. [Google Scholar] [CrossRef]
143. Nordborg, M., Tavare, S. (2002). Linkage disequilibrium: What history has to tell us. Trends in Genetics, 18, 83–90. DOI 10.1016/S0168-9525(02)02557-X. [Google Scholar] [CrossRef]
144. Zhao, K., Aranzana, M. J., Kim, S. (2007). An arabidopsis example of association mapping in structured samples. PLoS Genetics, 3, e4. DOI 10.1371/journal.pgen.0030004. [Google Scholar] [CrossRef]
145. Patterson, N., Price, A. L., Reich, D. (2006). Population structure and eigenanalysis. PLoS Genetics, 2, 2074–2093. DOI 10.1371/journal.pgen.0020190. [Google Scholar] [CrossRef]
146. Langridge, P., Fleury, D. (2011). Making the most of ‘omics’ for crop breeding. Trends in Biotechnology, 29, 33–40. DOI 10.1016/j.tibtech.2010.09.006. [Google Scholar] [CrossRef]
147. Ashraf, M., Foolad, M. R. (2013). Crop breeding for salt tolerance in the era of molecular markers and marker-assisted selection. Plant Breeding, 132, 10–20. DOI 10.1111/pbr.12000. [Google Scholar] [CrossRef]
148. Chattha, M. U., Maqsood, M., Chattha, M. B., Khan, I., Hassan, M. U. et al. (2017b). Influence of zinc under deficit irrigation on growth and productivity of hybrid maize. Pakistan Journal of Science, 69, 323–327. [Google Scholar]
149. Chattha, M. B., Iqbal, A., Chattha, M. U., Hassan, M. U., Khan, I. et al. (2017c). PGPR Inoculated-seed increases the productivity of forage sorghum under fertilized conditions. Journal of Basic Applied and Science, 13, 150–153. DOI 10.6000/1927-5129.2017.13.25. [Google Scholar] [CrossRef]
150. Chattha, M. B., Chattha, M. U., Hassan, M. U., Khan, I., Nawaz, M. et al. (2017d). Foliar application of growth promoting substances strongly influence the phenology, growth and yield of hybrid maize. International Journal of Biology and Biotechnology, 14, 597–602. [Google Scholar]
151. Arora, N. K., Khare, E., Oh, J. H., Kang, S. C., Maheshwari, D. K. (2008). Diverse mechanisms adopted by fluorescent pseudomonas PGC2 during the inhibition of Rhizoctonia solani and Phytopthora capsisi. World Journal of Microbiology and Biotechnology, 24, 581–585. DOI 10.1007/s11274-007-9505-5. [Google Scholar] [CrossRef]
152. Lugtenberg, B., Kamilova, F. (2009). Plant-growth-promoting-rhizobacteria. Annual Review of Microbiology, 63, 541–556. DOI 10.1146/annurev.micro.62.081307.162918. [Google Scholar] [CrossRef]
153. Adesemoye, A. O., Obini, M., Ugoji, E. O. (2008). Comparison of plant growth-promotion with pseudomonas aeruginosa and bacillus subtilis in three vegetables. Brazilian Journal of Microbiology, 39, 423–426. DOI 10.1590/S1517-83822008000300003. [Google Scholar] [CrossRef]
154. Egamberdieva, D., Kucharova, Z., Davranov, K., Berg, G., Makarova, N. et al. (2011). Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biology and Fertility of Soil, 47, 197–205. DOI 10.1007/s00374-010-0523-3. [Google Scholar] [CrossRef]
155. Ofek, M., Ruppel, S., Waisel, Y. (2006). Effects of salinity on rhizosphere bacterial communities associated with different root types of Vicia faba L. in Ozturk, M., Waisel, Y., Khan, A., Gork, G. (edsBiosaline agriculture and salinity tolerance in plants, pp. 1–21. Basel, Switzerland: Springer. DOI 10.1007/3-7643-7610-4_1. [Google Scholar] [CrossRef]
156. Nelson, D. R., Mele, P. M. (2007). Subtle changes in the rhizosphere microbial community structure in response to increased boron and sodium chloride concentrations. Soil Biology and Biochemistry, 39, 340–351. DOI 10.1016/j.soilbio.2006.08.004. [Google Scholar] [CrossRef]
157. Egamberdieva, D., Kucharova, Z. (2009). Selection for rot colonising bacteria stimulating wheat growth in saline soils. Biology and Fertility of Soil, 45, 563–571. DOI 10.1007/s00374-009-0366-y. [Google Scholar] [CrossRef]
158. Mayak, S., Tirosh, T., Glick, B. R. (2004). Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Science, 166, 525–530. DOI 10.1016/j.plantsci.2003.10.025. [Google Scholar] [CrossRef]
159. Yasmin, F., Othman, R., Saad, M. S., Sijam, K. (2007). Screening for beneficial properties of rhizobacteria isolated from sweet potato rhizosphere. Journal of Biotechnology, 6, 49–52. DOI 10.3923/biotech.2007.49.52. [Google Scholar] [CrossRef]
160. Nabti, E., Sahnoune, M., Ghoul, M., Fischer, D., Hofmann, A. et al. (2010). Restoration of growth of durum wheat (Triticum durum var. waha) under saline conditions due to inoculation with the rhizosphere bacterium azospirillum brasilense NH and extracts of the marine alga ulva lactuca. Journal of Plant Growth Regulatio, 29, 6–22. DOI 10.1007/s00344-009-9107-6. [Google Scholar] [CrossRef]
161. Abbaspoor, A., Zabihi, H. R., Movafegh, S., Asl, M. A. (2009). The efficiency of plant growth promoting rhizobacteria (PGPR) on yield and yield components of two varieties of wheat in salinity condition. American Eurasian Journal of Sustainable Agriculture, 3, 824–828. DOI 10.7324/JABB.2018.60109. [Google Scholar] [CrossRef]
162. Egamberdieva, D., Kamilova, F., Validov, S., Gafurova, L., Kucharova, Z. et al. (2008). High incidence of plant growth stimulating bacteria associated with the rhizosphere of wheat. Environment Microbiology, 10, 1–9. DOI 10.1111/j.1462-2920.2007.01424.x. [Google Scholar] [CrossRef]
163. Barassi, C. A., Ayrault, G., Creus, C. M., Sueldo, R. J., Sobrero, M. T. (2006). Seed inoculation with azospirillum mitigates naCl effects on lettuce. Science Horticulture, 109, 8–14. DOI 10.1016/j.scienta.2006.02.025. [Google Scholar] [CrossRef]
164. Egamberdieva, D., Berg, G., Lindström, K., Räsänen, L. A. (2013a). Alleviation of salt stress of symbiotic Galega officinalis L. (Goat’s Rue) by co-inoculation of rhizobium with root colonising Pseudomonas. Plant Soil, 369, 453–465. DOI 10.1007/s11104-013-1586-3. [Google Scholar] [CrossRef]
165. Creus, C. M., Sueldo, R. J., Barassi, C. A. (2004). Water relations and yield in azospirillum inoculated wheat exposed to drought in the field. Canadian Journal of Botany, 82, 273–281. DOI 10.1139/b03-119. [Google Scholar] [CrossRef]
166. Navarro, J. M., Perez-Tornero, O., Morte, A. (2013). Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the rootstock salt tolerance. Journal of Plant Physiology, 171, 76–85. DOI 10.1016/j.jplph.2013.06.006. [Google Scholar] [CrossRef]
167. Ahanger, M. A., Hashem, A., Abd, E. F., Ahmad, P. (2014a). Arbuscular mycorrhiza in crop improvement under environmental stress. In Ahmad, P., Rasool, S. (edsEmerging technologies and management of crop stress tolerance, pp. 69–95. The Netherlands: Academic Press. [Google Scholar]
168. Alqarawi, A. A., Abd, E. F., Hashem, A. (2014). Alleviation of salt-induced adverse impact via mycorrhizal fungi in ephedra aphylla forssk. Journal of Plant Interaction, 9, 802–810. DOI 10.1080/17429145.2014.949886. [Google Scholar] [CrossRef]
169. Seleiman, M. F., Hardan, A. N. (2021c). Chapter 17: Importance of mycorrhizae in crop productivity. In Awaad H., Abu-Hashim M., Negm A. (edsMitigating environmental stresses for agricultural sustainability in Egypt, pp. 471–484. Basal, Switzerland: Springer Water, Springer International Publishing. [Google Scholar]
170. Sheng, M., Tang, M., Chen, H., Yang, B., Zhang, F. et al. (2008). Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza, 18, 287–296. DOI 10.1007/s00572-008-0180-7. [Google Scholar] [CrossRef]
171. Hajiboland, R., Aliasgharzadeh, N., Laiegh, S. F., Poschenrieder, C. (2010). Colonization with arbuscular mycorrhizal fungi improve salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant and Soil, 331, 313–327. DOI 10.1007/s11104-009-0255-z. [Google Scholar] [CrossRef]
172. Hameed, A., Egamberdieva, D., Abd-Allah, E. F., Hashem, A., Kumar, A. et al. (2014). Salinity stress and arbuscular mycorrhizal symbiosis in plants. In Miransari, M., (ed.) Use of microbes for the alleviation of soil stresses, pp. 139–159. New York, USA: Springer. DOI 10.1007/978-1-4614-9466-9_7. [Google Scholar] [CrossRef]
173. Wu, Q. S., Zou, Y. N., Abd-Allah, E. F. (2014). Mycorrhizal association and ROS in plants. In Ahmad, P., (ed.) Oxidative damage to plants, pp. 453–475. Academic Press, Elsevier. DOI 10.1016/B978-0-12-799963-0.00015-0. [Google Scholar] [CrossRef]
174. Wu, Q. S., Zou, Y. N., Liu, W., Ye, X. F., Zai, H. F. et al. (2010). Alleviation of salt stress in citrus seedlings inoculated with mycorrhiza: Changes in leaf antioxidant defense systems. Plant Soil and Environment, 56, 470–475. DOI 10.17221/PSE. [Google Scholar] [CrossRef]
175. Hashem, A., Abd-Allah, E. F., Alqarawi, A. A., Wirth, S., Egamberdieva, D. (2016a). Arbuscular mycorrhizal fungi alleviate salt stress in lupine (Lupinus termis forsik) through modulation of antioxidant defense systems and physiological traits. Legume Research, 39, 198–207. DOI 10.18805/lr.v39i2.9531. [Google Scholar] [CrossRef]
176. Hashem, A., Abd-Allah, E. F., Alqarawi, A. A., Wirth, S., Egamberdieva, D. (2016b). Comparing symbiotic performance and physiological responses of two soybean cultivars to arbuscular mycorrhizal fungi under salt stress. Saudi Journal of Biological Science, 38–48. DOI 10.33448/rsd-v9i12.11016. [Google Scholar] [CrossRef]
177. Hashem, A., Salwa, A. A., Alqarawi, A. A., Abdullah, E. F., Egamberdieva, D. (2016c). Arbuscular mycorrhizal fungi enhance basil tolerance to salt stress through improved physiological and nutritional status. Pakistan Journal of Botany, 48, 37–45. [Google Scholar]
178. Hashem, A., Abd-Allah, E. F., Alqarawi, A. A., Mona, S. A., Alenazi, M. M. et al. (2015a). Arbuscular mycorrhizal fungi mitigates naCl induced adverse effects on Solanum lycopersicum L. Pakistan Journal of Botany, 47, 327–340. [Google Scholar]
179. Hashem, A., Abd-Allah, E. F., Alqarawi, A. A., Egamberdieva, D. (2015b). Induction of salt stress tolerance in cowpea [Vigna unguiculata (L.) Walp.] by arbuscular mycorrhizal fungi.Legume Research, 38, 579–588. DOI 10.1155/2016/6294098. [Google Scholar] [CrossRef]
180. Latef, A. A. H. A., He, C. X. (2011). Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Science Horticulture, 127, 228–233. DOI 10.1016/j.scienta.2010.09.020. [Google Scholar] [CrossRef]
181. Hashem, A., Abd_Allah, E. F., Alqarawi, A. A., El-Didamony, G., Mona, S. A. et al. (2014). Alleviation of adverse impact of salinity on faba bean (Vicia faba L.) by arbuscular mycorrhizal fungi. Pakistan Journal of Botany, 46, 2003–2013. [Google Scholar]
182. Sarwat, M., Hashem, A., Ahanger, M. A., Abd-Allah, E. F., Alqarawi, A. A. et al. (2016). Mitigation of naCl stress by arbuscular mycorrhizal fungi through the modulation of osmolytes, antioxidants and secondary metabolites in mustard (Brassica juncea L.) plants. Frontiers in Plant Science, 7, 869. DOI 10.3389/fpls.2016.00869. [Google Scholar] [CrossRef]
183. Aroca, R., Ruiz-Lozano, J. M., Zamarreno, A., Paz, J. A., Garcia-Mina, J. M. et al. (2013). Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. Journal of Plant Physiology, 170, 47–55. DOI 10.1016/j.jplph.2012.08.020. [Google Scholar] [CrossRef]
184. Al-Karaki, G. N. (2000b). Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza, 10, 51–54. DOI 10.1007/s005720000055. [Google Scholar] [CrossRef]
185. Kohler, J., Hernandez, J. A., Caravaca, F., Roldan, A. (2009). Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environmental and Experimental Botany, 65, 245–252. DOI 10.1016/j.envexpbot.2008.09.008. [Google Scholar] [CrossRef]
186. Asghari, H. R., Marschner, P., Smith, S. E., Smith, F. A. (2005). Growth response of Atriplex nummularia to inoculation with arbuscular mycorrhizal fungi at different salinity levels. Plant and Soil, 273, 245–256. DOI 10.1007/s11104-004-7942-6. [Google Scholar] [CrossRef]
187. Ruiz-Lozano, J. M., Azcon, R. (1995). Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiology of Plants, 95, 472–478. DOI 10.1111/j.1399-3054.1995.tb00865.x. [Google Scholar] [CrossRef]
188. Jahromi, F., Aroca, R., Porcel, R., Ruiz-Lozano, J. M. (2008). Influence of salinity on the in vitro development of glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microbial Ecology, 55, 45–53. DOI 10.1007/s00248-007-9249-7. [Google Scholar] [CrossRef]
189. Waqas, M., Khan, A. L., Kamran, M., Hamayun, M., Kang, S. M. et al. (2012). Endophytic fungi produce gibberellins and indole acetic acid and promotes host-plant growth during stress. Molecule, 17, 10754. DOI 10.3390/molecules170910754. [Google Scholar] [CrossRef]
190. Shirmardi, M., Savaghebi, G. R., Khavazi, K., Akbarzadeh, A., Farahbakhsh, M. et al. (2010). Effect of microbial inoculants on uptake of nutrient elements in two cultivars of sunflower (Helianthus annuus L.) in saline soils.Notulae Scientia Biologcae, 2, 57–66. DOI 10.15835/nsb234678. [Google Scholar] [CrossRef]
191. Najafi, A., Ardakani, M. R., Rejali, F., Sajedi, N. (2012). Response of winter barley to co-inoculation with azotobacter and mycorrhiza fungi influenced by plant growth promoting rhizobacteria. Annals of Biological Research, 3, 4002–4006. [Google Scholar]
192. Ryu, H., Cho, Y. (2015). Plant hormones in salt stress tolerance. Journal of Plant Biology, 58, 147–155. DOI 10.1007/s12374-015-0103-z. [Google Scholar] [CrossRef]
193. Tabatabei, S. A. (2013). The effect of salicylic acid and gibberellin on enzyme activity and germination characteristics of wheat seeds under salinity stress conditions. International Journal of Agriculture and Crop Science, 6, 236–240. [Google Scholar]
194. Ali, Q., Athar, H., Ashraf, M. (2008). Modulation of growth, photosynthetic capacity and water relations in salt stressed wheat plants by exogenously applied 24-epibrassinolide. Plant Growth and Regulation, 56, 107–116 DOI 10.1007/s10725-008-9290-7. [Google Scholar] [CrossRef]
195. Ahmad, P., Jeleel, C. A., Azooz, M. M., Nabi, G. (2009). Generation of ROS and non-enzymatic antioxidants during abiotic stress in plants. Botanical Research International, 2, 11–20. [Google Scholar]
196. Hasanuzzaman, M., Alam, M. M., Nahar, K., Al-Mahmud, A., Ahamed, K. U. et al. (2014a). Exogenous salicylic acid alleviates salt stress-induced oxidative damage in Brassica napus by enhancing the antioxidant defense and glyoxalase systems. Australian Journal of Crop Science, 8, 631–639. DOI abs/10.3316/informit.292398401589540. [Google Scholar]
197. Aamer, M., Muhammad, U. H., Li, Z., Abid, A., Su, Q. et al. (2018). Foliar application of glycinebetaine (GB) alleviates the cadmium (Cd) toxicity in spinach through reducing Cd uptake and improving the activity of anti-oxidant system. Applied Ecology and Environmental Research, 16, 7575–7583. DOI 10.15666/aeer. [Google Scholar] [CrossRef]
198. Dustgeer, Z., Seleiman, M. F., Imran, K., Chattha, M. U., Alhammad, B. A. et al. (2021). Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 49(1), 12248. DOI 10.15835/nbha49112248. [Google Scholar] [CrossRef]
199. Szabados, L., Savouré, A. (2010). Proline: A multifunctional amino acid. Trends in Plant Science, 15, 89–97. DOI 10.1016/j.tplants.2009.11.009. [Google Scholar] [CrossRef]
200. Hayat, S., Hayat, Q., Alyemeni, M. N., Wani, A. S., Pichtel, J. et al. (2012). Role of proline under changing environments: A review. Plant Signaling Behavior, 7, 1456–1466. DOI 10.4161/psb.21949. [Google Scholar] [CrossRef]
201. Raza, S. H., Athar, H., Ashraf, M. (2006). Influence of exogenously applied glycine-betaine on the photosynthetic efficiency of two differently adapted cultivars under salt stress. Pakistan Journal of Botany, 38, 241–251. [Google Scholar]
202. Akhter, N., Akram, N. A., Shahbaz, M. (2007). Presowing seed treatments with glycinebetaine and mineral nutrients of wheat (Triticum aestivum L.) under saline conditions. Pakistan Journal of Agriculture Science, 44, 236–241. [Google Scholar]
203. Hendawey, M. H. (2015). Biochemical changes associated with induction of salt tolerance in wheat. Global Science Research Journal, 10, 84–99. DOI 10.5829/idosi.gjbb.2015.10.02.1120. [Google Scholar] [CrossRef]
204. Bhaskar, G., Bingru, H. (2014). Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. International Journal of Genomic, 701596, 18. DOI 10.1155/2014/701596. [Google Scholar] [CrossRef]
205. Dorsaf, H., Chedly, A., Habib, A., Muhammad, A., Dorsaf, M. (2018). Effect of salinity on osmotic adjustment, proline accumulation and possible role of ornithine-d-aminotransferase in proline biosynthesis in cakile maritime. Physiology and Molecular Biology of Plants, 24, 1017–1033. DOI 10.1007/s12298-018-0601-9. [Google Scholar] [CrossRef]
206. Mahboob, W., Khan, M. A., Shirazi, M. U. (2016). Induction of salt tolerance in wheat (Triticum aestivum L.) seedlings through exogenous application of proline. Pakistan Journal of Botany, 48, 861–867. [Google Scholar]
207. Tian, F., Wang, W., Liang, C., Wang, X., Wang, G. et al. (2017). Overaccumulation of glycine betaine makes the function of the thylakoid membrane better in wheat under salt stress. Crop Journal, 5, 73–82. DOI 10.1016/j.cj.2016.05.008. [Google Scholar] [CrossRef]
208. Turan, S., Cornish, K., Kumar, S. (2012). Salinity tolerance in plants: Breeding and genetic engineering. Australian Journal of Crop Science, 6, 1337. DOI abs/10.3316/informit.732395810014158. [Google Scholar]
209. Yan, D., Zheng, B. (2016). Effects of soaking seeds in trehalose on physiological characteristics of wheat yangmai-19 under salt stress. Acta Agriculture, 28, 1271–1276. DOI 20163285085. [Google Scholar]
210. Rao, A., Ahmad, S. D., Sabir, S. M., Awan, S., Shah, A. H. et al. (2013). Potential biochemical indicators improve salt tolerance in fifteen cultivars of wheat (Triticum aestivum L.) from Pakistan. International Journal of Science and Engineering and Research, 4, 389–406. [Google Scholar]
211. Duman, F., Aksoy, A., Aydin, Z., Temizgul, R. (2010). Effects of exogenous glycinebetaine and trehalose on cadmium accumulation and biological responses of an aquatic plant (Lemna gibba L.). Water Air Soil Pollution, 217, 545–556. DOI 10.1007/s11270-010-0608-5. [Google Scholar] [CrossRef]
212. Luo, Y., Li, F., Wang, G. P., Yang, X. H., Wang, W. (2010). Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biologia Plantarum, 54, 495–501. DOI 10.1007/s10535-010-0087-y. [Google Scholar] [CrossRef]
213. Seckin, B., Sekmen, A. H., Turkan, I. (2009). An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. Journal of Plant Growth Regulation, 28, 12–20. DOI 10.1007/s00344-008-9068-1. [Google Scholar] [CrossRef]
214. Sadak, M. S. (2016). Mitigation of salinity adverse effects of on wheat by grain priming with melatonin. International Journal of Chemistry and Technology Research, 9, 85–97. [Google Scholar]
215. Bakare, S. O., Ukwungwu, M. N. (2009). On-farm evaluation of seed priming technology in Nigeria. African Journal of Agriculture, 5, 93–97. [Google Scholar]
216. Kaya, M. D., Okcu, G., Atak, M., Cikili, Y., Kolsarici, O. (2006). Seed treatments to overcome salt drought stress during germination in sunflower (Helianthus annuus L.). European Journal of Agronomy, 24, 291–295. DOI 10.1016/j.eja.2005.08.001. [Google Scholar] [CrossRef]
217. Salehzade, H., Shishvan, M. I., Ghiyasi, M., Forouzin, F., Siyahjani, A. A. (2009). Effect of seed priming on germination and seedling growth of wheat (Triticum aestivum L.). Research Journal of Biological Science, 4, 629–631. [Google Scholar]
218. Seleiman, M. F., Al-Suhaibani, N., Ali, N., Akmal, M., Alotaibi, M. et al. (2021b). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants, 10, 259. DOI 10.3390/plants10020259. [Google Scholar] [CrossRef]
219. Hussian, I., Ahmad, R., Farooq, M., Rehman, A., Amin, M. (2015). Seed priming improves the performance of poor quality wheat seed under drought stress. Applied Scientific Reports, 7, 12–18. [Google Scholar]
220. Iqbal, M., Ashraf, M. (2005). Changes in growth, photosynthetic capacity and ionic relations in spring wheat (Triticum aestivum L.) due to pre-sowing seed treatment with polyamines. Plant Growth Regulation, 46, 19–30. DOI 10.1007/s10725-005-5901-8. [Google Scholar] [CrossRef]
221. Tavili, A., Zare, S., Moosavi, S. A., Enayati, A. (2010). Effects of seed priming on germination characteristics of bromus species under salt and drought conditions. American Eurasian Journal of Agriculture and Environmental Science, 10, 163–168. [Google Scholar]
222. Jisha, K. C., Vjayakumari, K., Puthur, J. T. (2013). Seed priming for abiotic stress tolerance: An overview. Acta Physiology of Plants, 35, 1381–1396. DOI 10.1007/s11738-012-1186-5. [Google Scholar] [CrossRef]
223. Yari, L., Abbasian, A., Oskouei, B., Sadeghi, H. (2011). Effect of seed priming on dry matter, seed size and morphological characters in wheat cultivar. Agriculture and Biology Journal of North America, 2, 232–238. DOI 10.5251/abjna.2011.2.2.232.238. [Google Scholar] [CrossRef]
224. Afzal, I., Basra, M. A. S., Iqbal, A. (2005). The effects of seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. Journal of Stress Physiology and Biochemistry, 1, 6–14. [Google Scholar]
225. Al-hakimi, A. M. A., Hamada, A. M. (2001). Counteraction of salinity stress on wheat plants by grain soaking in ascorbic acid, thiamin or sodium salicylate. Biology of Plants, 44, 253–261. DOI 10.1023/A:1010255526903. [Google Scholar] [CrossRef]
226. Shakirova, F. M., Sakhabutdinova, A. R., Bezrukova, M. V., Fatkhutdinova, R. A., Fatkhutdinova, D. R. (2003). Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Science, 164, 317–322. DOI 10.1016/S0168-9452(02)00415-6. [Google Scholar] [CrossRef]
227. Jafar, M. Z., Farooq, M., Cheema, M. A., Afzal, I., Basra, S. M. A. et al. (2012). Improving the performance of wheat by seed priming under saline conditions. Journal of Agronomy and Crop Science, 198, 38–45. DOI 10.1111/j.1439-037X.2011.00485.x. [Google Scholar] [CrossRef]
228. Afzal, I., Basra, S. M. A., Hameed, A., Farooq, M. (2008). Physiological enhancements for alleviation of salt stress in wheat. Pakistan Journal of Botany, 38, 1649–1659. [Google Scholar]
229. Gulnaz, A. J., Iqbal, J., Azam, F. (1999). Seed treatment with growth regulators and crop productivity. II. Response of critical growth stages of wheat (Triticum aestivum L.) under salinity stress. Cereal Research Communication, 19, 419–426. DOI 10.1007/BF03543558. [Google Scholar] [CrossRef]
230. Hamid, M., Ashraf, M. Y., Rehman, K. U., Arashad, M. (2008). Influence of salicylic acid seed priming on growth and some biochemical attributes in wheat grown under saline conditions. Pakistan Journal of Botany, 40, 361–367. [Google Scholar]
231. Cakmak, I. (2002). Plant nutrition research: Priorities to meet human needs for food in sustainable ways. Plant and Soil, 247, 3–24. DOI 10.1023/A:1021194511492. [Google Scholar] [CrossRef]
232. Aslam, M. M., Zeeshan, M., Irum, A., Hassan, M. U., Ali, S. et al. (2015). Influence of seedling age and nitrogen rates on productivity of rice (Oryza sativa L.A review. American Journal of Plant Science, 6, 1361–1369. DOI 10.4236/ajps.2015.69135. [Google Scholar] [CrossRef]
233. Waraich, E. A., Ahmad, R., Ashraf, M. Y., Saifullah, A. M. (2011). Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agronomy Scandavia, 61, 291–304. DOI 10.1080/09064710.2010.491954. [Google Scholar] [CrossRef]
234. Hassan, M. U., Aamer, M., Chattha, M. U., Ullah, M. A., Sulaman, S. et al. (2017). The role of potassium in plants under drought stress: Mini review. Journal of Basic and Applied Science, 13, 268–271. DOI 10.6000/1927-5129.2017.13.44. [Google Scholar] [CrossRef]
235. Munns, R. (2005). Genes and salt tolerance: Bringing them together. New Phytologist, 167, 645–656. DOI 10.1111/j.1469-8137.2005.01487.x. [Google Scholar] [CrossRef]
236. Tahir, M. A., Tariq, A. (2011). Silicon induced growth and yield enhancement in two wheat genotypes differing in salinity tolerance. Communication in Soil Science and Plant Analysis, 42, 395–407. DOI 10.1080/00103624.2011.542219. [Google Scholar] [CrossRef]
237. El-Lethy, S. R., Abdel, M. T., Reda, F. (2013). Effect of potassium application on wheat (Triticum aestivum L.) cultivars grown under salinity stress. World Applied Science Journal, 26, 840–850. DOI 10.5829/idosi.wasj.2013.26.07.13527. [Google Scholar] [CrossRef]
238. Kausar, A., Gull, M. (2014). Effect of potassium sulphate on the growth and uptake of nutrients in wheat (Triticum aestivum L.) under salt stressed conditions. Journal of Agriculture Science, 6, 1–12. DOI 10.5539/jas.v6n8p101. [Google Scholar] [CrossRef]
239. Taha, R. S., Seleiman, M. F., Alotaibi, M., Alhammad, B. A., Rady, M. M. et al. (2020). Exogenous potassium treatments elevate salt tolerance and performances of Glycine max L. by boosting antioxidant defense system under actual saline field conditions. Agronomy, 10, 1741. DOI 10.3390/agronomy10111741. [Google Scholar] [CrossRef]
240. Khan, A., Ahmad, I., Shah, A., Ahmad, F., Ghani, A. et al. (2013). Amelioration of salinity stress in wheat (Triticum aestivum L.) by foliar application of phosphorus. Phyton-International Journal of Experimental Botany, 82, 281–287. DOI 10.32604/phyton.2013.82.281. [Google Scholar] [CrossRef]
241. Huang, Z. A., Jiang, D. A., Yang, Y., Sun, J., Jin, S. H. (2004). Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence and antioxidant enzyme in leaves of rice plants. Photosynthetica, 42, 357–64. DOI 10.1023/B:PHOT.0000046153.08935.4c. [Google Scholar] [CrossRef]
242. Shaul, O. (2002). Magnesium transport and function in plants: The tip of the iceberg. Biometal, 15, 309–323. DOI 10.1023/A:1016091118585. [Google Scholar] [CrossRef]
243. Hermans, C., Verbruggen, N. (2005). Physiological characterization of Mg deficiency in Arabidopsis thaliana. Journal of Experimental Botany, 418, 2153–2161. DOI 10.1093/jxb/eri215. [Google Scholar] [CrossRef]
244. Lobanov, A. V., Hatfield, D. L., Gladyshev, V. N. (2008). Reduced reliance on the trace element selenium during evolution of mammals. Genome Biology, 9, 62. DOI 10.1186/gb-2008-9-3-r62. [Google Scholar] [CrossRef]
245. Yerokun, O. A., Chirwa, M. (2014). Soil and foliar application of zinc to maize and wheat grown on a Zambian Alfisol. African Journal of Agriculture Research, 9, 963–970. DOI 10.5897/AJAR. [Google Scholar] [CrossRef]
246. Chattha, M. U., Hassan, M. U., Khan, I., Chattha, M. B., Ashraf, I. et al. (2017b). Effect of different nitrogen and phosphorus fertilizer levels in combination with nitrogen and phosphorus solubilizing inoculants on the growth and yield of mung bean. Pakistan Journal of Life Social and Science, 15, 31–36. [Google Scholar]
247. Hassan, M., Aamer, M., Chattha, U. M., Haiying, T., Shahzad, B. et al. (2020b). The critical role of zinc in plants facing the drought stress. Agriculture, 10, 396. DOI 10.3390/agriculture10090396. [Google Scholar] [CrossRef]
248. Ngugi, M. M., Gitari, H. I., Muui, C., Gweyi-Onyango, J. P. (2021). Cadmium mobility, uptake and accumulation in spinach, kale and amaranths vegetables as influenced by silicon fertilization. Bioremediation Journal, 192411, 1–14. DOI 10.1080/10889868.2021.1924111. [Google Scholar] [CrossRef]
249. Hassan, M. J., Raza, M. A., Rehman, S. U., Ansar, M., Gitari, H. et al. (2020). Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants, 9, 1575. DOI 10.3390/plants9111575. [Google Scholar] [CrossRef]
250. Nyawade, S., Gitari, H. I., Karanja, N. N., Gachene, C. K., Schulte-Geldermann, E. et al. (2020). Enhancing climate resilience of rain-fed potato through legume intercropping and silicon application. Frontier in Sustainable Food Systems, 4, 566345. DOI 10.3389/fsufs.2020.566345. [Google Scholar] [CrossRef]
251. Mandhania, S., Madan, S., Sawhney, V. (2006). Antioxidant defense mechanism under salt stress in wheat seedlings. Biologia Plantarum, 50, 227–231. DOI 10.1007/s10535-006-0011-7. [Google Scholar] [CrossRef]
252. Ahanger, M. A., Qin, C., Begum, N., Maodong, Q., Dong, X. X. et al. (2019). Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biology, 19, 1–12. DOI 10.1186/s12870-019-2085-3. [Google Scholar] [CrossRef]
253. Dong, Y., Wang, W., Hu, G., Chen, W., Zhuge, Y. et al. (2017). Role of exogenous 24-epibrassinolide in enhancing the salt tolerance of wheat seedlings. Journal of Soil Science and Plant Nutrition, 17, 554–569. DOI 10.4067/S0718-95162017000300001. [Google Scholar] [CrossRef]
254. Zeeshan, M., Lu, M., Sehar, S., Holford, P., Wu, F. (2020). Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy, 10, 127. DOI 10.3390/agronomy10010127. [Google Scholar] [CrossRef]
255. Jabeen, Z., Hussain, N., Irshad, F., Zeng, J., Tahir, A. et al. (2020). Physiological and antioxidant responses of cultivated and wild barley under salt stress. Plant, Soil and Environment, 66, 334–344. DOI 10.17221/PSE. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |