

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.018933
ARTICLE
Effects of Salt-Alkaline Stress on Carbohydrate Metabolism in Rice Seedlings
1Jilin Agricultural University, Changchun, 130118, China
2Key Laboratory of Germplasm Innovation and Physiological Ecology of Grain Crops in Cold Region, Ministry of Education, Harbin, 150030, China
*Corresponding Authors: Yanqiu Geng. Email: ccgyq@163.com; Liying Guo. Email: guoliying0621@163.com
Received: 25 August 2021; Accepted: 02 November 2021
Abstract: The aim of this study was to investigate carbohydrate metabolism in rice seedlings subjected to salt-alkaline stress. Two relatively salt-alkaline tolerant (Changbai 9) and sensitive (Jinongda 138) rice cultivars, grown hydroponically, were subjected to salt-alkaline stress via 50 mM of salt-alkaline solution. The carbohydrate content and the activities of metabolism-related enzymes in the leaves and roots were investigated. The results showed that the contents of sucrose, fructose, and glucose in the leaves and roots increased under salt-alkaline stress. Starch content increased in the leaves but decreased in the roots under salt-alkaline stress. The activities of sucrose-phosphate synthase, sucrose synthase, amylase, and ADP-glucose pyrophosphorylase increased whereas the activities of neutral invertase and acid invertase decreased in the leaves under salt-alkaline stress. The activities of sucrose-phosphate synthase, sucrose synthase, amylase, neutral invertase, and acid invertase increased in the roots under salt-alkaline stress. In conclusion, salt-alkaline stress caused the accumulation of photosynthetic assimilates in the leaves and decreased assimilation export to the roots.
Keywords: Rice; salt-alkaline stress; starch metabolism; sucrose metabolism
The main salt components of salt-alkaline soil are Na2CO3 and NaHCO3, and because the pH of the soil is mostly above 8.5, the strong alkalinity contributes to poor physical properties [1,2]. The salt-alkaline soil is mainly found in the western region of Jilin and Heilongjiang Province, and comprises an area of up to 3 million hectares [3]. In recent decades, with the interference of human factors and the deterioration of the ecological environment, the area of salt-alkaline land has further increased and the problem of salt-alkaline land has become increasingly serious. Salt-alkaline soils have huge development potential as a reserve resource for potential cultivated land. Planting rice is one of the most effective ways to control, improve and utilize salt-alkaline land [4]; the practice can truly achieve economic benefits and sustainable development within the ecological environment. However, salt-alkaline stress has many adverse effects on plants; it causes growth inhibition, reduced transpiration, reduced net photosynthetic rate, imbalance of mineral nutrition, and metabolic disorders [5–7].
Photosynthesis is the basis of plant growth and development, and sucrose and starch are the main products of photosynthesis [8]. The activity of sucrose-phosphate synthase (SPS) plays an important role in the synthesis of sucrose in plants, and is a key enzyme for catalyzing the synthesis of sucrose [9]. The degradation of sucrose is mainly catalyzed by the activity of sucrose synthase (SS) and invertase [10,11]. ADP-glucose pyrophosphorylase (ADP-GPPase) and amylase play important roles in starch synthesis and degradation, respectively [12,13]. Under stress conditions, the accumulation of carbohydrates (such as soluble sugars) plays an important role in osmotic regulation, energy storage, signal transduction and scavenging of reactive oxygen species [14]. As the end product of photosynthetic carbon assimilation, sugar is considered to be an important signal molecule for measuring the degree of stress in plants and the ability of plants to adapt to adversity [15,16]. Therefore, the impact of salt-alkaline adversity on plant carbon metabolism is mainly manifested in the process of carbohydrate metabolism. The soluble sugar content in plant tissues increased under salt-alkaline stress and gradually increased with the increasing of salt concentration [17,18]. However, some studies have noted that the influence of salt-alkaline stress on soluble sugar content, and the change in the trend of soluble sugar content with stress intensity and stress time, varies with species and plant parts [19,20]. Further obvious differences were observed even among different varieties of the same species. Increasing the concentration of soluble sugar is beneficial for reducing the osmotic potential of plant cells and improving their water absorption capacity. However, at the same time, it might also reduce the assimilation rate of CO2, thereby inhibiting the photosynthesis of plants through feedback mechanisms [21–23]. Consequently, the change in soluble sugar under salt-alkaline stress is complex and controversial. In addition, salt-alkaline stress can seriously affect the distribution of assimilation products between the source and sink, leading to the accumulation of photosynthetic products, such as soluble sugar and starch in the source leaves, resulting in photosynthetic feedback inhibition [24,25]. At the same time, salt-alkaline stress blocks the transport of carbohydrates from the source leaves to the underground parts, thereby affecting the growth of the root system. Research on the effects of salt-alkaline stress on carbohydrate metabolism in rice is scattered, and there is a lack of systematic reports. Therefore, this study aims to explore the damage mechanism of salt-alkaline stress on rice, and improve the regulation network of plant salt-alkaline tolerance, to lay a theoretical foundation for ensuring a high and stable yield of rice in salt-alkaline areas.
Two rice cultivars “Changbai 9” (salt-alkaline tolerant) and “Jinongda 138” (salt-alkaline sensitive) were used in this study. The seeds were sterilized, soaked, germinated, and cultured in an artificial climate chamber (During the day 27.0 ± 1.5°C, at night 21.0 ± 1.5°C, the period is 16/8 h, and the effective light radiation is 250 μmol m–2s–1) with distilled water. At the two-leaf heart stage, uniform and robust seedlings were selected and transferred to 3500 mL plastic buckets filled with 1/2 nutrient solution. A uniform foam board for drilling holes (each plate with 21 holes) was placed on the plastic bucket (height of 17.2 cm and inner diameter of 20.1 cm). Two seedlings were planted in each hole and 42 seedlings were planted in each bucket. The nutrient solution used refers to the conventional nutrient solution of the International Rice Institute: its formula is: 0.715 mM NH4NO3, 0.16 mM NaH2PO4, 0.323 mM K2SO4, 0.5 mM CaCl2, 0.83 mM MgSO4, 0.036 mM Fe-EDTA, 0.1 mM Na2SiO3, 4.55 μM MnCl2, 0.077 μM ZnSO4, 0.078 μM CuSO4, 9.25 μM H3BO3, 0.236 μM H2MoO4. The nutrient solution was renewed every 3 days, and the pH of the nutrient solution was regulated to 5.2 with a portable pH meter.
Based on the main salt types and pH values of salt-alkaline soil in Northeast China [26], four kinds of salt compounds (NaCl, Na2SO4, Na2CO3, and NaHCO3) were selected in a molar ratio of 1:9:1:9 and mixed. The salt-alkaline concentration in the treatment was 50 mM and the pH of the mixed salt solution was 8.9. At the three-leaf heart stage, salt-alkaline treatment was initiated. Two treatments were set up: these were the control (CK) and the saline-alkali treatment (50 mM). After 5, 10 and 15 days of salt-alkaline treatment, the leaves and roots of the rice were harvested, immediately frozen in liquid N2 and stored at −80°C until analysis. All experiments were performed with three technical replications, each containing 10–15 seedlings.
2.2 Measurement of Carbohydrate Content
The soluble sugar content was determined according to the method described by Maness [27], and the starch content was determined according to the method described by Yang et al. [28].
2.3 Measurement of Enzyme Activity Related to Carbohydrate Metabolism
The enzyme solution was prepared according to the method described by Hubbard et al. [29]. The activity of sucrose-phosphate synthase was determined according to the method of Dong et al. [30]. The activity of sucrose synthase (the direction of sucrose decomposition) was determined using the SS assay kit manufactured by Genmed Scientifics Inc., USA, following the manufacturer’s instructions. The soluble protein content was measured following the method of Bradford [31] and the enzyme activity was presented as a unit of protein. The extraction and determination of acid invertases (AI) and neutral invertases (NI) were carried out according to the methods of Miron et al. [32] and Rosales et al. [33], respectively.
The extraction and determination of β-amylase were performed according to the methods described by Duffus et al. [34] and Bhatia et al. [35]. ADP-glucose pyrophosphorylase (ADP-GPPase) was extracted as described by Rosa et al. [36], and its activity was determined using an ADP-GPPase assay kit (GenMed Scientifics Inc., USA).
Statistical analysis of the data was performed using IBM SPSS software (version 22.0: IBM Corporation, Armonk, NY, USA), and Statistical significance was determined by the t-test. All data were represented as an average of the three replicates and their standard deviation (S.D.). Figures were produced using OriginPro 2020b (OriginLab Corp., Northampton, MA, USA) software.
3.1 Effects of Salt-Alkaline Stress on Glucose, Fructose, and Sucrose Contents in the Roots and Leaves of Rice
In the leaves of both rice cultivars, the contents of glucose, fructose, and sucrose were significantly increased under salt-alkaline stress (Table 1). Compared to the CK treatment, the hexose/sucrose ratios of Jinongda 138 and Changbai 9 decreased by 5.3%–27.1% and 22.8%–30.0%, respectively, after 5, 10, and 15 days of salt-alkaline treatment. In the leaves of both rice cultivars, the content of starch was significantly increased, and the accumulation of starch reached a maximum on the 15th day under salt-alkaline stress (Table 1). Compared to the CK treatment, the sucrose/starch ratios of Jinongda 138 and Changbai 9 increased by 3.3%–17.8% and 29.6%–56.8%, respectively, after 5, 10, and 15 days of salt-alkaline treatment.
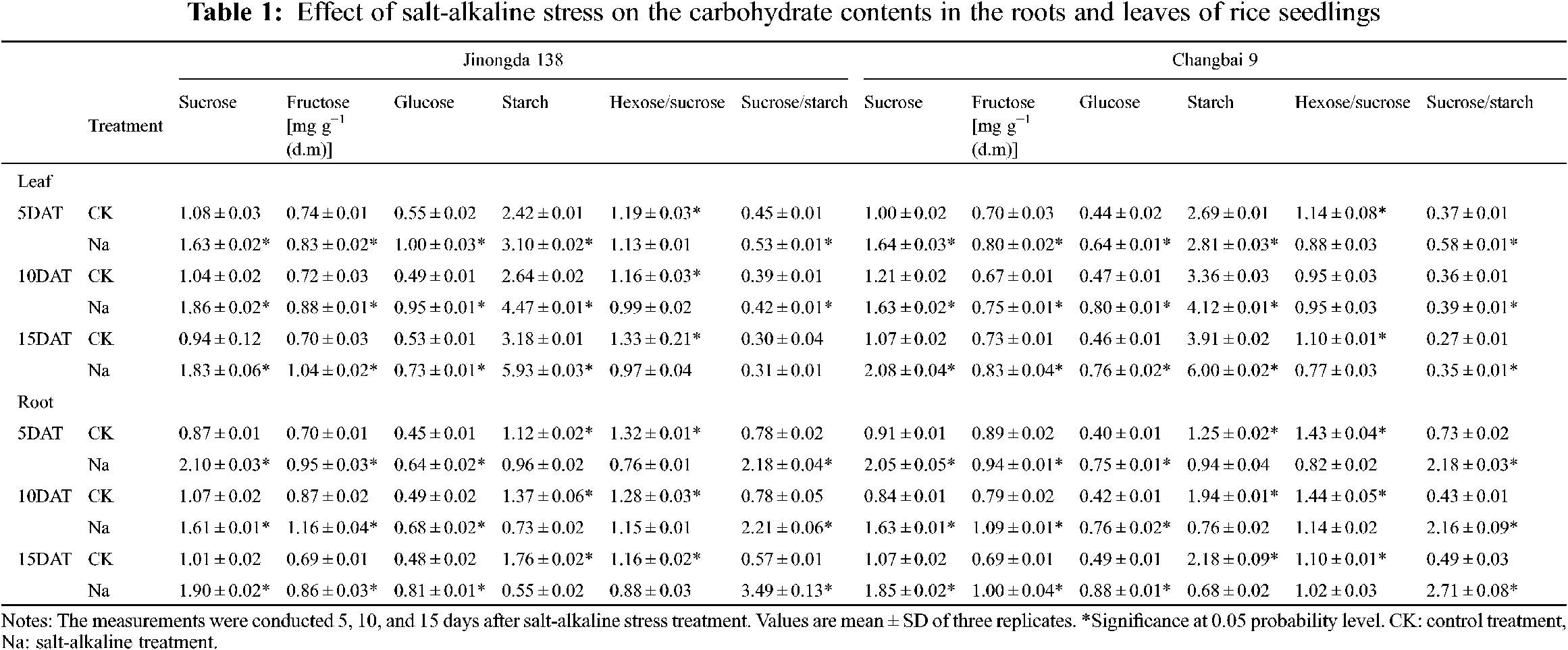
In the roots of both rice cultivars, the contents of glucose, fructose, and sucrose were significantly increased under salt-alkaline stress (Table 1). Compared to the CK treatment, the hexose/sucrose ratios of Jinongda 138 and Changbai 9 decreased by 24.1%–42.4% and 7.3%–42.7%, respectively, after 5, 10, and 15 days of salt-alkaline treatment. Furthermore, the starch content significantly decreased in the rice roots of both rice cultivars under salt-alkaline stress (Table 1). Compared to the CK treatment, the sucrose/starch ratios of Jinongda 138 and Changbai 9 increased by 179.5%–512.3% and 198.6%–453.1%, respectively, after 5, 10, and 15 days of salt-alkaline treatment.
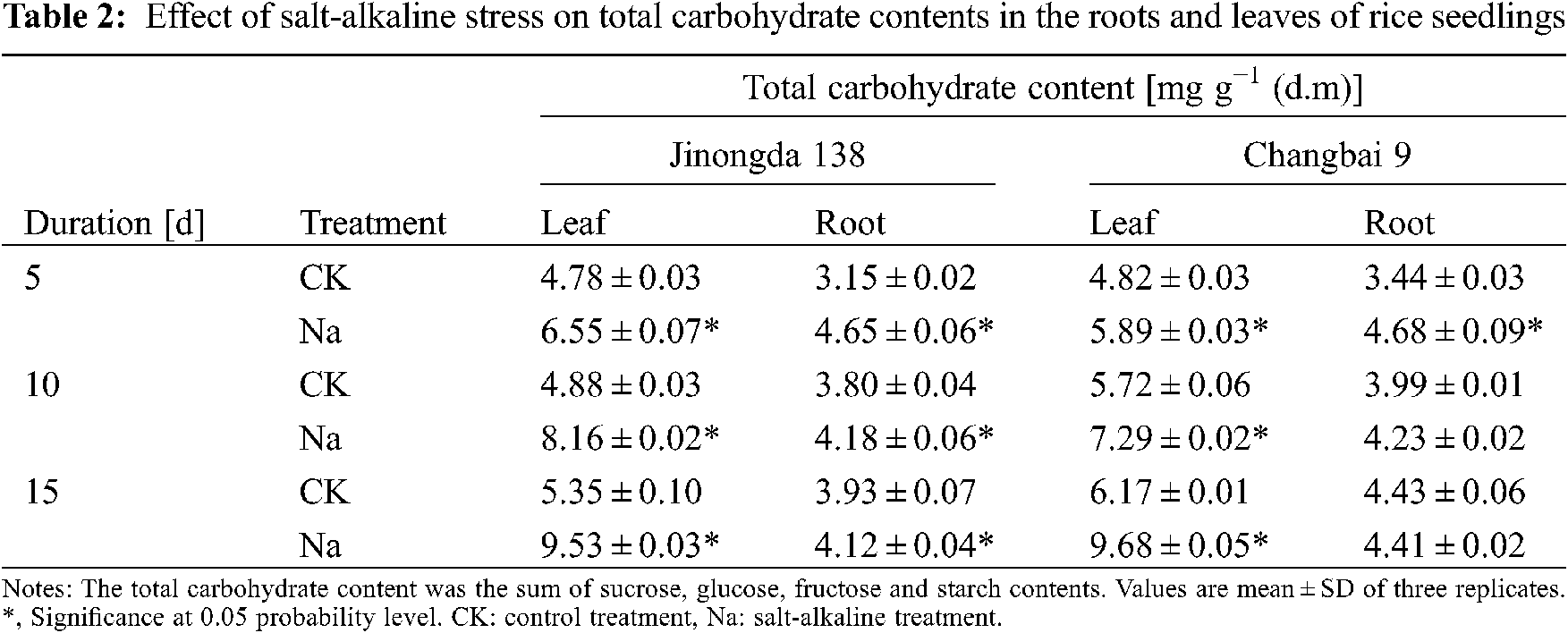
3.2 Effects of Salt-Alkaline Stress on the Total Carbohydrates in the Roots and Leaves of Rice
The total contents of carbohydrates (glucose, fructose, sucrose, and starch) increased in the leaves of both rice cultivars under salt-alkaline stress (Table 2). The total carbohydrate content in the roots of Changbai 9 increased on the 5th and 10th day of salt-alkaline treatment, but it was decreased on the 15th day of salt-alkaline treatment.
3.3 Effects of Salt-Alkaline Stress on SPS Activity in the Roots and Leaves of Rice
Compared with the control, the SPS activity in the roots and leaves of both rice cultivars increased under salt-alkaline stress (Figs. 1D, 1H and 2D, 2H). The SPS activity of rice leaves and roots was significantly positively correlated with the sucrose content under salt-alkaline stress (Fig. 3).
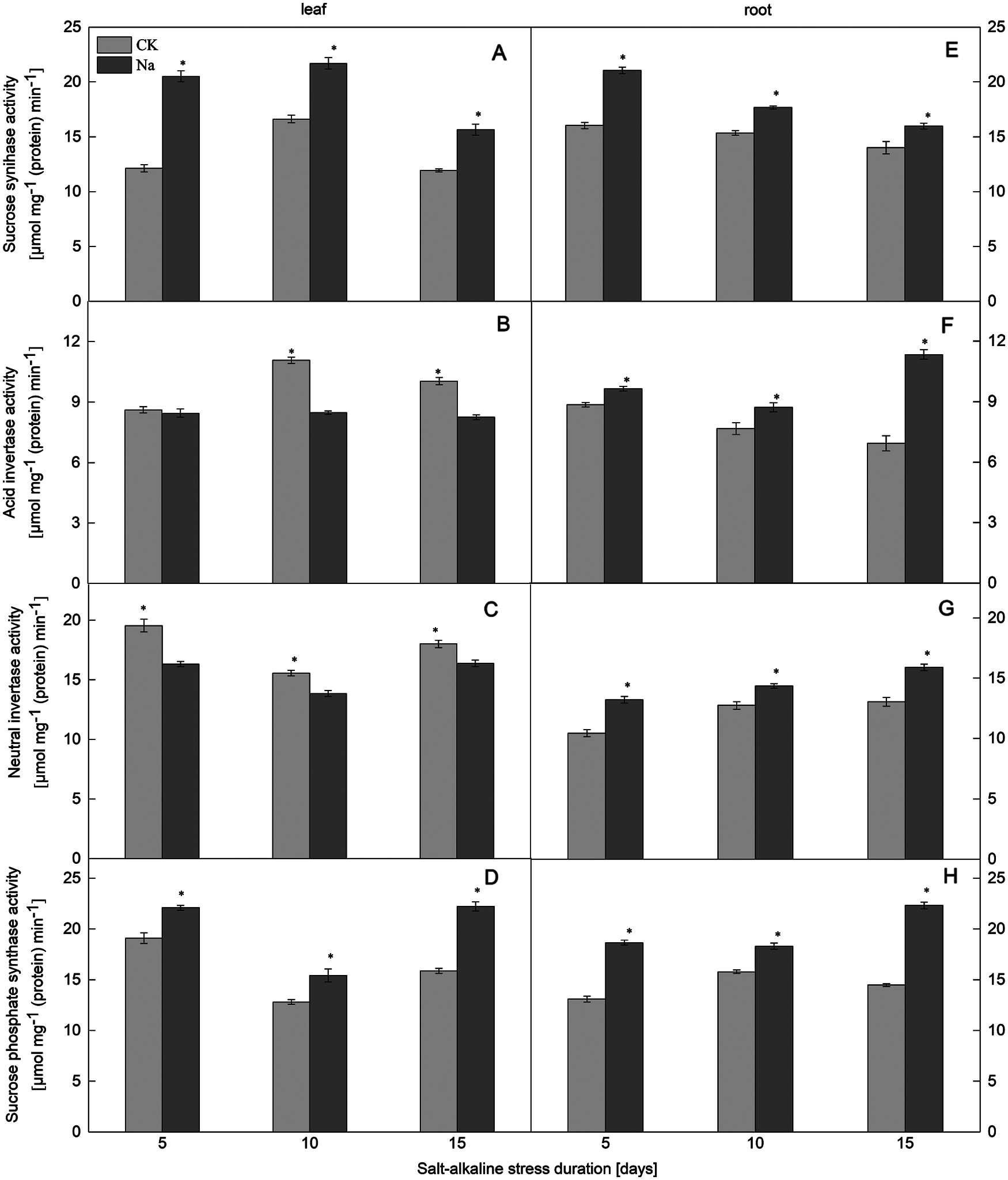
Figure 1: Effect of salt-alkaline stress on the activities of enzymes involved in sucrose synthesis and catabolism in ‘Jinongda 138’. (A–D) leaf; (E–H) root; (A and E) sucrose synthase (SS); (B and F) acid invertase (AI); (C and G) neutral invertase (NI); (D and H) sucrose-phosphate synthase (SPS). Values are mean ± SD of three replicates. *Significance at 0.05 probability level. CK: control treatment, Na: salt-alkaline treatment
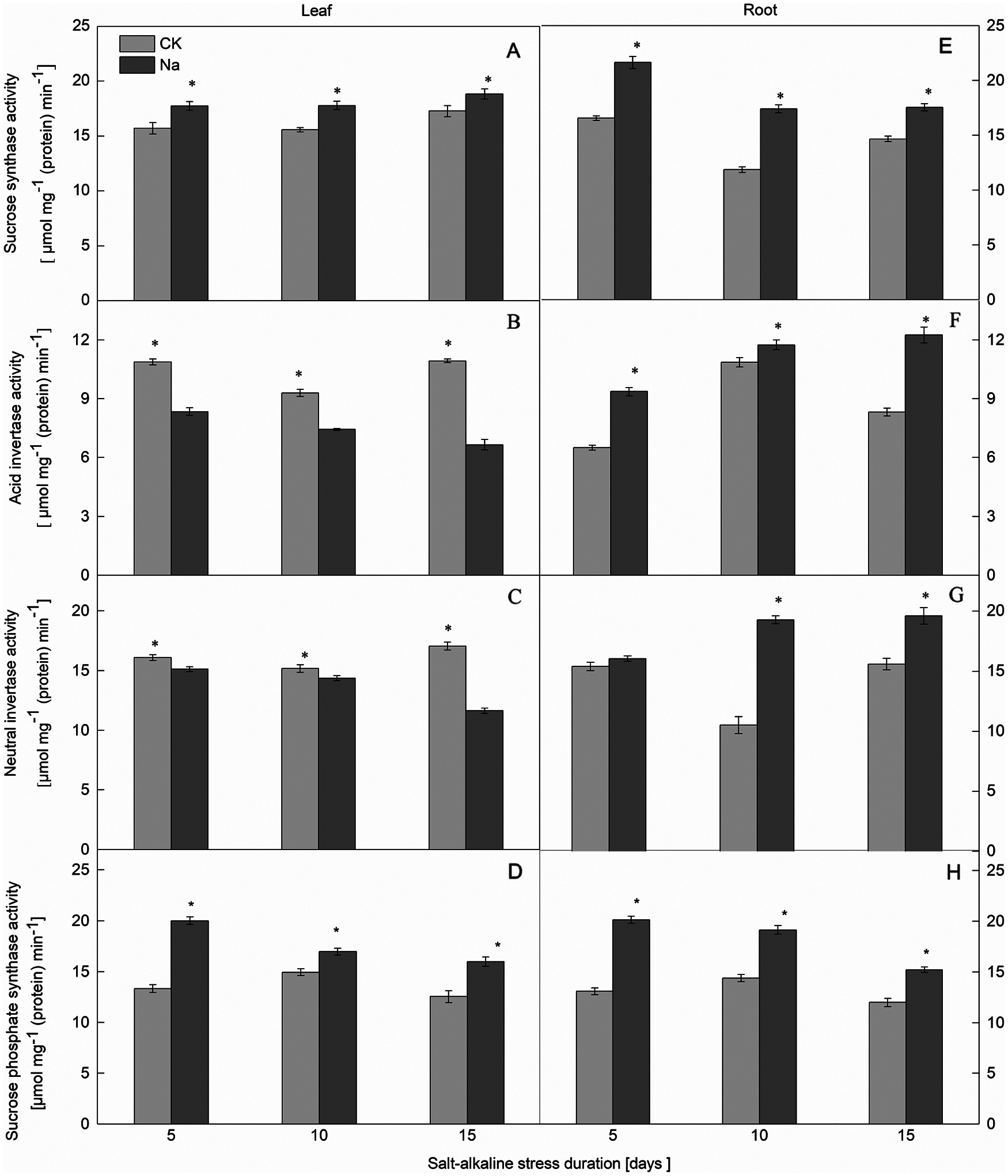
Figure 2: Effect of salt-alkaline stress on the activities of enzymes involved in sucrose synthesis and catabolism in ‘Changbai 9’. (A–D) leaf; (E–H) root; (A and E) sucrose synthase (SS); (B and F) acid invertase (AI); (C and G) neutral invertase (NI); (D and H) sucrose-phosphate synthase (SPS). Values are mean ± SD of three replicates. *Significance at 0.05 probability level. CK: control treatment, Na: salt-alkaline treatment
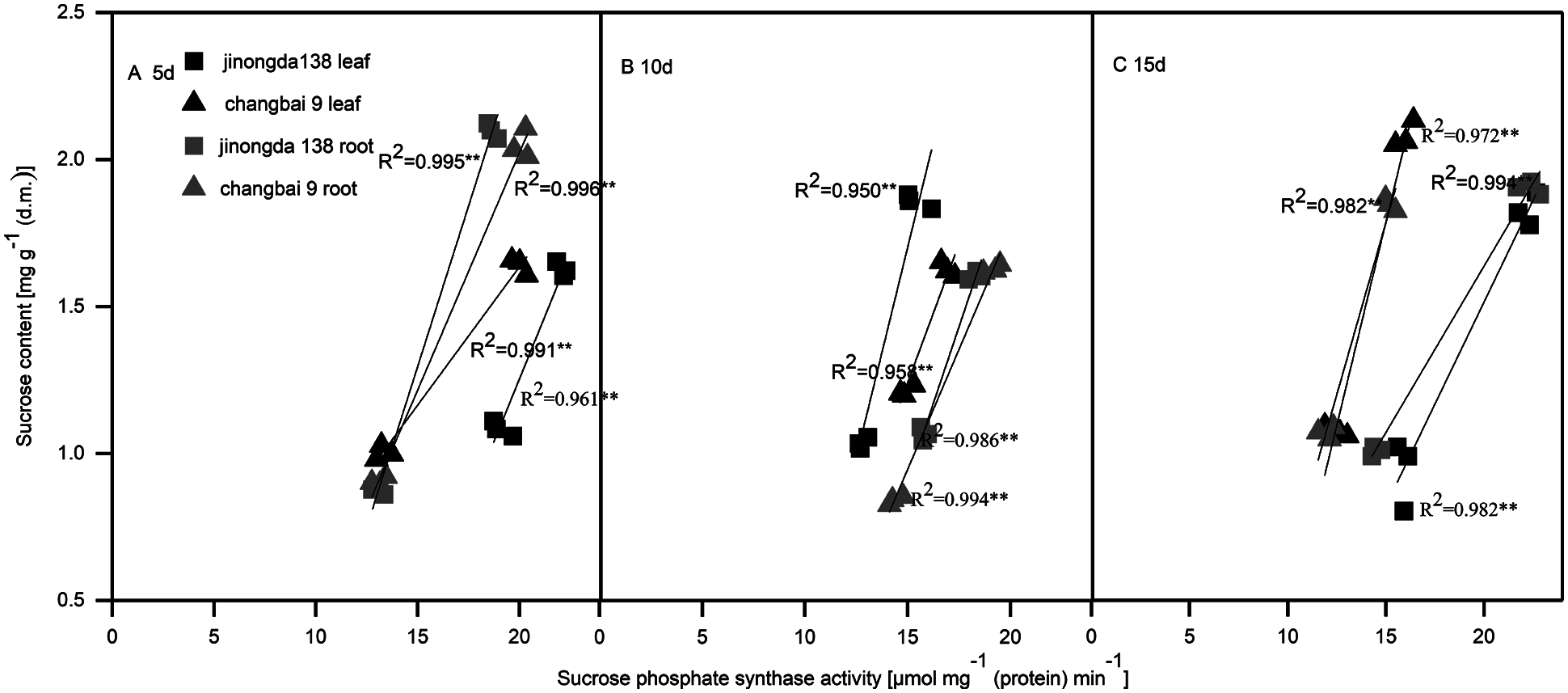
Figure 3: Correlation between sucrose content and sucrose phosphate synthase activity in the leaves and roots of rice seedlings after salt-alkaline treatment for 5 d (A), 10 d (B) and 15 d (C). **Significance at 0.01 probability level
3.4 Effects of Salt-Alkaline Stress on the Activities of SS, AI, and NI in the Roots and Leaves of Rice
The SS activity in the roots and leaves of both rice cultivars increased under salt-alkaline stress (Figs. 1A, 1E and 2A, 2E). Compared to the CK treatment, the activities of AI and NI in the leaves of both rice cultivars decreased after 5, 10, and 15 days of salt-alkaline treatment. On the 5th day of salt-alkaline treatment, there was no significant difference in the activity of AI of Jinongda 138 (Figs. 1B, 1C and 2B, 2C). In the roots of both rice cultivars, compared to the CK treatment, after 5, 10, and 15 days of salt-alkaline treatment, the activities of AI and NI increased. On the 5th day of salt-alkaline treatment, there was no significant difference in the activity of NI of Changbai 9 (Figs. 1F, 1G and 2F, 2G).
3.5 Effect of Salt-Alkaline Stress on the Activities of β-Amylase in the Roots and Leaves of Rice
Compared to the CK treatment, the amylase activity in the leaves of both rice cultivars increased under salt-alkaline stress (Figs. 4B, 4D). There was no significant difference in the amylase activity of Jinongda 138 on the 10th day between the CK and salt-alkaline stress treatments. Compared to the CK treatment, the amylase activity in the roots of both rice cultivars increased under salt-alkaline stress (Figs. 4A, 4C). However, there was no significant difference in the amylase activity of Changbai 9 on the 5th day between the CK and salt-alkaline stress treatments.
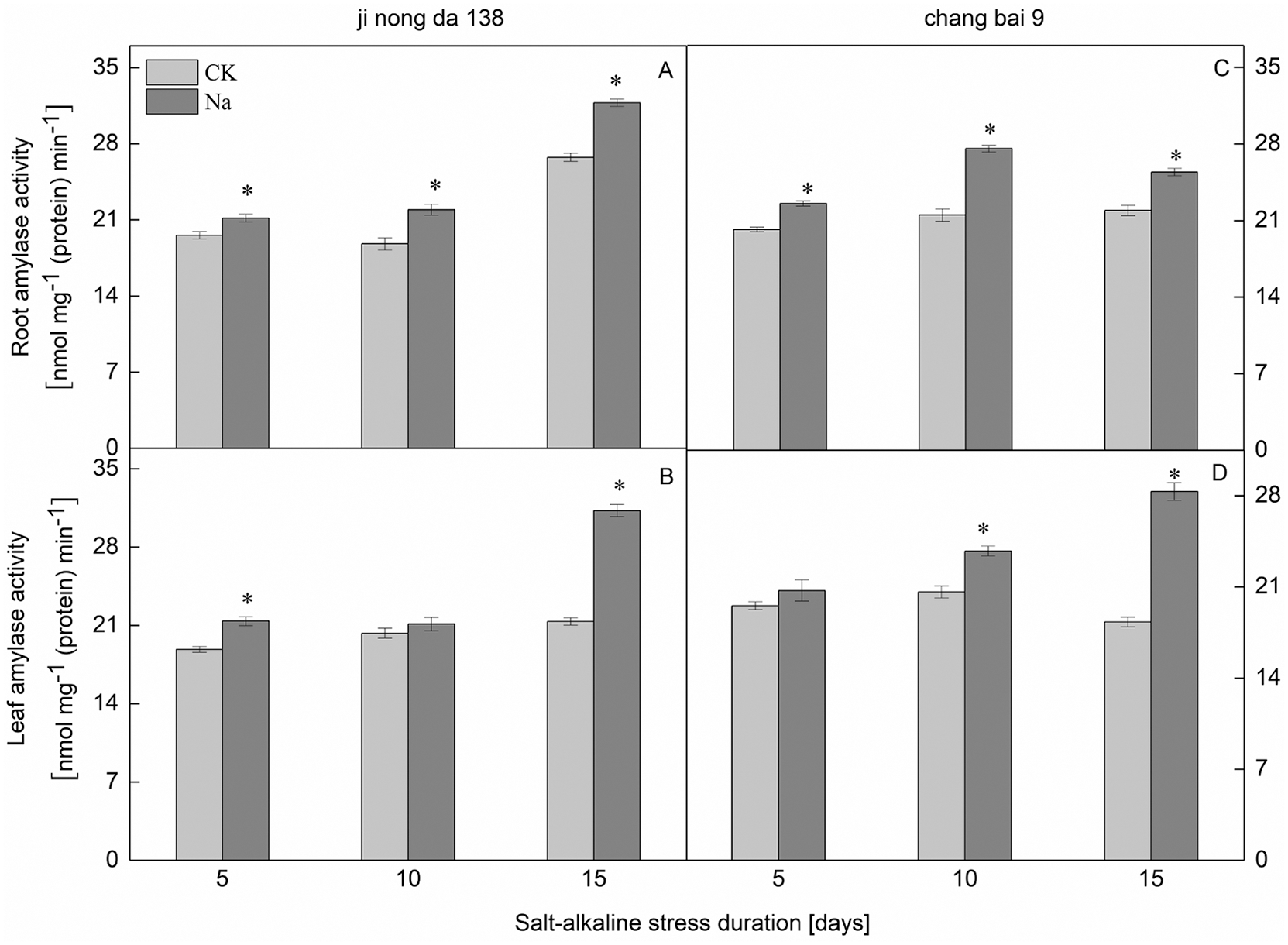
Figure 4: Effect of salt-alkaline stress on the activities of β-amylase in the roots and leaves of rice. (A and C) root; (B and D) leaf. Values are mean ± SD of three replicates. *Significance at 0.05 probability level. CK: control treatment, Na: salt-alkaline treatment
3.6 Effect of Salt-Alkaline Stress on the Activities of ADP-GPPase in the Leaves of Rice
Compared to the CK treatment, the ADP-GPPase activity in the leaves of both rice cultivars increased under salt-alkaline stress (Fig. 5). Notably, the difference between treatments was significant.
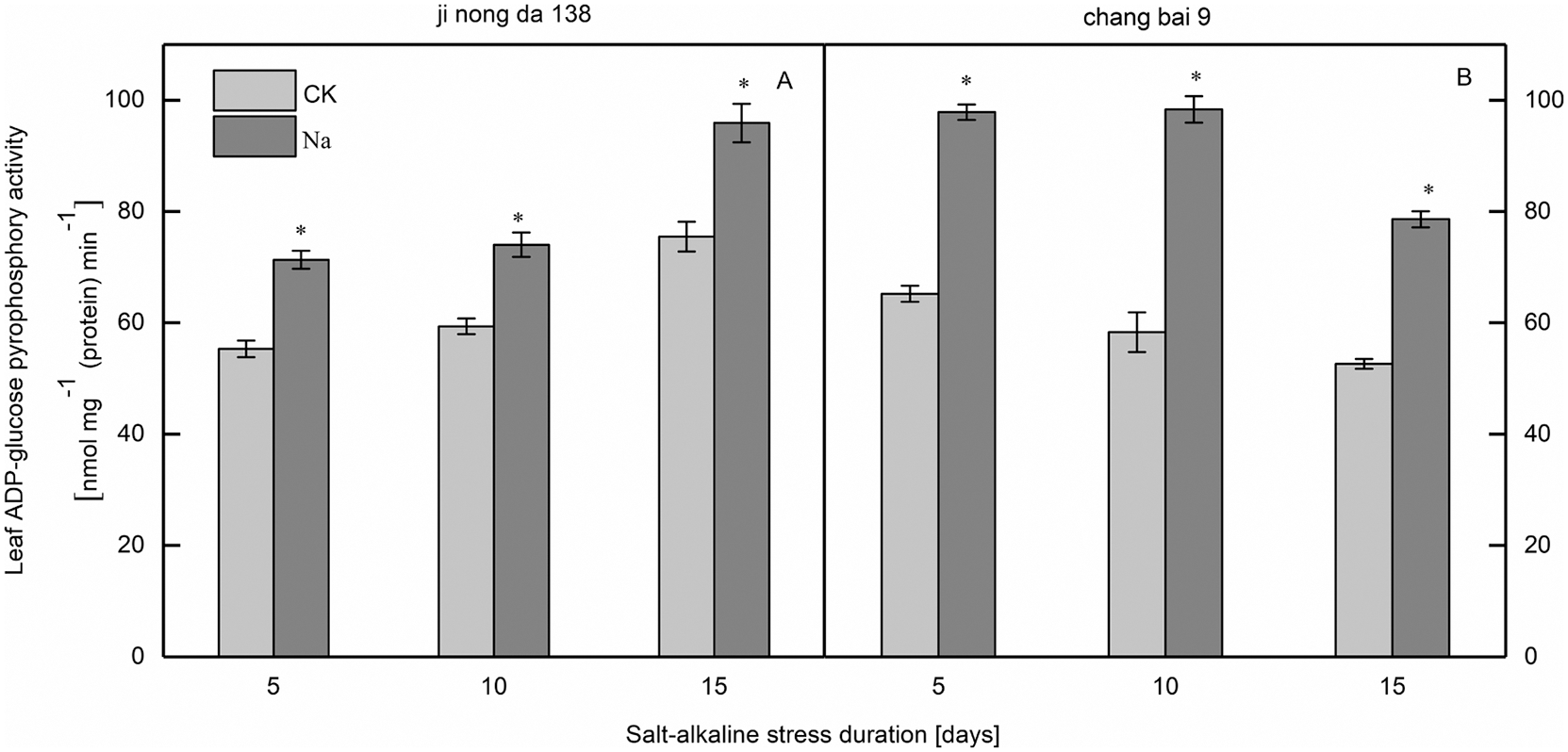
Figure 5: Effect of salt-alkaline stress on the activities of ADP-glucose pyrophosphorylase in rice leaves. ADP-GPPase, ADP-glucose pyrophosphorylase. Values are mean ± SD of three replicates. *Significance at 0.05 probability. CK: control treatment, Na: salt-alkaline treatment
Carbohydrates are the material basis of plant metabolism and they play an important role in maintaining the normal physiological activities of plants [37]. The physiological activity status of plants and the response of plants to environmental changes can be reflected by changes in the content of non-structural carbohydrates. The accumulation of carbohydrates (such as soluble sugars) plays an important role in osmotic regulation, energy storage, signal transduction and scavenging of reactive oxygen species under stress conditions [14].
Sucrose is one of the main products of plant photosynthesis. The transformation of sucrose decomposition into hexose for the physiological metabolism of leaves is conducive to maintaining the normal growth and development of plants and helping plants to cope with abiotic stress [38–40]. In terms of regulating the relationship between source and sink, sucrose can be used as a signal molecule to regulate gene expression and improve the ability of plants to resist stress [41,42]. Therefore, to ensure the normal growth of plants, it is very important to maintain a dynamic balance between sucrose synthesis, transportation, distribution and use in plants. Previous studies have shown that drought stress significantly increased the contents of sucrose and fructose, as well as the activities of SPS and SS [43]. In this study, the salt-alkaline stress caused an increase in the contents of glucose, fructose, and sucrose in rice leaves and a decrease in the hexose/sucrose ratio (Table 1). This may be related to the increased activity of the enzyme SPS that catalyzes the synthesis of sucrose under salt-alkaline stress (Figs. 1D and 2D), and the decreased activity of the enzymes AI and NI that catalyze the decomposition of sucrose (Figs. 1B, 1C and 2B, 2C). In addition, the activity of SS increases while the activity of AI and NI decreases (Figs. 1A–1C and 2A–2C) but the content of glucose and fructose increases (Table 1) in the leaves of rice under salt-alkaline stress. This indicated that SS might be the main enzyme that catalyzes the hydrolysis of sucrose in the leaves of rice under salt-alkaline stress. Because the content of sucrose in rice leaves did not decrease under salt-alkaline stress, it indicated that the synthesis of sucrose in rice leaves was greater than its decomposition under salt-alkaline stress. The significant positive correlation between SPS activity and sucrose content also confirmed this conclusion. The production, transportation, and distribution of carbohydrates in plants are complex physiological processes that result from the coordination of “source-flow-sink”. In this study, the accumulation of sucrose in rice leaves under salt-alkaline stress was not only related to the synthesis and degradation of sucrose, but also its transport. Nevertheless, the effect of salt-alkaline stress on carbohydrate transport in rice needs to be further studied. Soluble carbohydrates, as a “bridge metabolite” between source and sink, could buffer the damage to plants caused by stress through osmotic adjustment. In this study, salt-alkaline stress increased the accumulation of soluble sugars in the leaves (Table 1), which was consistent with the results of Munir et al. [18]. These soluble sugars could participate in the osmotic adjustment of plants, which facilitates water absorption under stress conditions. The decomposition of sugar accumulated in plants is beneficial for resisting adversity stress under conditions of adversity [44].
Starch is the main storage form of carbohydrates in plants, and can be quickly broken down into soluble sugars to provide energy for plants [45,46]. The balance of starch metabolism is affected by abiotic stresses, such as water and temperature [47,48]. To adapt to changes in the environment, the starch in plants will show the corresponding reactions of synthesis and degradation. Under drought stress, high starch content translates to plant resistance to stress and recovery of growth [49]. ADP-GPPase and amylase are two very important enzymes involved in starch synthesis and decomposition [35,50]. Starch degradation can provide sufficient carbon sources, energy sources and carbon-derived metabolites for plants under stress. In this study, under salt-alkaline stress, the starch content (Table 1), ADP-GPPase activity (Figs. 4B, 4D), and β-amylase activity (Figs. 5A, 5B) were increased in rice leaves. Previous studies have shown that water stress can significantly increase β-amylase activity [51]. Starch and sucrose can be interconverted through a series of enzymatic reactions. The increase in the activity of starch-degrading enzymes in plants promotes the decomposition of starch into more soluble sugars, which translates into the accumulation of sucrose in leaves. Theoretically, an increase in amylase activity would promote the hydrolysis of starch, leading to a reduction in starch content within the leaf. However, in this study, the starch content in the rice leaves did not decrease but instead increased under salt-alkaline stress. This might be because starch synthesis was greater than its decomposition under salt-alkaline stress. The accumulation of sugar is related to the salt-alkaline tolerance mechanism of many species. In this study, compared with salt-alkaline-tolerant (Changbai 9) variety, the soluble sugars and starch of salt-alkaline-sensitive (Jinongda 138) variety accumulate relatively more in rice leaves under salt-alkaline stress (Table 1). Under stress conditions, soluble sugars and starch tend to accumulate in large amounts in plants, resulting in the feedback inhibition of photosynthesis, which seriously affects the normal progress of plant photosynthesis, thereby retarding plant growth [52,53]. The large accumulation of carbohydrates in salt-alkaline-sensitive varieties may be one of the reasons for their growth restriction under salt-alkaline stress environment.
To improve the buffering capacity of plants under adverse conditions, the transport of non-structural carbohydrates from source organs to sink organs is essential [54,55]. The accumulation of sucrose in the roots is beneficial for improving their osmotic regulation ability and water absorption capacity. In this study, the contents of glucose, fructose, and sucrose were increased and the ratio of hexose/sucrose decreased in the roots under salt-alkaline stress (Table 1); this might be attributed to the synthesis of sucrose being greater than its decomposition in the roots. Furthermore, the increase in fructose and glucose contents might be attributed to an increase in the activities of SS, AI, and NI in the roots (Figs. 1E–1G and 2E–2G). In this study, the starch content of roots under salt-alkaline stress significantly decreased. However, the starch content of salt-alkaline-tolerant (Changbai 9) in the root system is higher than that of salt-alkaline-sensitive (Jinongda 138) variety under salt-alkaline stress (Table 1). In plants, increasing the starch content in the root is highly beneficial for improving plant resistance to stress and it can provide plants with more energy stores to maintain normal plant growth under conditions of adversity [30]. The salt-alkaline-tolerant varieties maintain a higher starch content in the root system to facilitate their growth and development. The reduction in root starch content may be because the activity of amylase in the roots of rice was significantly increased under salt-alkaline stress and the accumulation of carbohydrates in rice leaves affected the transport of assimilates to the underground parts. This might have then caused the root system to consume its own starch to provide energy for the normal physiological metabolism of the plant.
Salt-alkaline stress was found to affect the activity of carbohydrate metabolism-related enzymes in the leaves and roots, resulting in the disorder of carbohydrate metabolism in the leaves and roots. Salt-alkaline stress caused the accumulation of photosynthetic assimilates in the leaves and decreased assimilation export to the roots. Consequently, our results may help understand the damage mechanism of salt-alkaline stress in rice.
Funding Statement: This research was funded by Key Scientific and Technological Research Projects of Jilin Province, China (20210509032RQ), Key Laboratory of Straw Biology and Utilization (Jilin Agricultural University) (klos2020-001), Ministry of Education, The Open Project of Key Laboratory of Cold Region Grain Crop Germplasm Innovation and Physiological Ecology of Ministry of Education (CXSTOP2021003).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Chi, C. M., Zhao, C. W., Sun, X. J., Wang, Z. C. (2012). Reclamation of saline-sodic soil properties and improvement of rice (Oriza sativa L.) growth and yield using desulfurized gypsum in the west of Songnen Plain, Northeast China. Geoderma, 187–188, 24–30. DOI 10.1016/j.geoderma.2012.04.005. [Google Scholar] [CrossRef]
2. Luo, S. S., Tian, L., Chang, C. L., Wang, S. J., Zhang, J. F. et al. (2018). Grass and maize vegetation systems restore saline-sodic soils in the songnen plain of Northeast China. Land Degradation and Development, 29(4), 1107–1119. DOI 10.1002/ldr.2895. [Google Scholar] [CrossRef]
3. Yang, F., An, F. H., Ma, H. Y., Wang, Z. C., Zhou, X. et al. (2016). Variations on soil salinity and sodicity and its driving factors analysis under microtopography in different hydrological conditions. Water, 8(6), 227. DOI 10.3390/w8060227. [Google Scholar] [CrossRef]
4. Liu, M. A., Liang, Z. W., Yang, F., Ma, H. Y., Huang, L. H. et al. (2010). Impacts of sand amendment on rice (Oryza sativa L.) growth and yield in saline-sodic soils of North-East China. Journal of Food Agriculture and Environment, 8(2), 412–418. DOI 10.1016/S0092-8674(00)80467-5. [Google Scholar] [CrossRef]
5. Tuna, A. L., Kaya, C., Ashraf, M., Altunlu, H., Yokas, I. et al. (2007). The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environmental and Experimental Botany, 59(2), 173–178. DOI 10.1016/j.envexpbot.2005.12.007. [Google Scholar] [CrossRef]
6. Cardarelli, M., Rouphael, Y., Rea, E., Colla, G. (2010). Mitigation of alkaline stress by arbuscular mycorrhiza in zucchini plants grown under mineral and organic fertilization. Journal of Plant Nutrition and Soil Science, 173(5), 778–787. DOI 10.1002/jpln.200900378. [Google Scholar] [CrossRef]
7. Hu, L. P., Xiang, L. X., Li, S. T., Zou, Z. R., Hu, X. H. (2016). Beneficial role of spermidine in chlorophyll metabolism and D1 protein content in tomato seedlings under salinity-alkalinity stress. Physiologia Plantarum, 156(4), 468–477. DOI 10.1111/ppl.12398. [Google Scholar] [CrossRef]
8. Goldschmidt, E. E., Huber, S. C. (1992). Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiology, 99(4), 1443–1448. DOI 10.1104/pp.99.4.1443. [Google Scholar] [CrossRef]
9. Huber, S. C., Huber, J. L. (1996). Role and regulation of sucrose-phosphate synthase in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology, 47, 431–444. DOI 10.1146/annurev.arplant.47.1.431. [Google Scholar] [CrossRef]
10. Ranwala, A. P., Miller, W. B. (1998). Sucrose-cleaving enzymes and carbohydrate pools in Lilium longiflorum floral organs. Physiologia Plantarum, 103(4), 541–550. DOI 10.1034/j.1399-3054.1998.1030413.x. [Google Scholar] [CrossRef]
11. Fukuda, A., Yoshinaga, S., Nagata, K., Shiratsuchi, H. (2008). Rice cultivars with higher sucrose synthase activity develop longer coleoptiles under submerged conditions. Plant Production Science, 11(1), 67–75. DOI 10.1626/pps.11.67. [Google Scholar] [CrossRef]
12. Cardemil, L., Varner, J. E. (1984). Starch degradation metabolism towards sucrose synthesis in germinating araucaria araucana seeds. Plant Physiology, 76(4), 1047–1054. DOI 10.1104/pp.76.4.1047. [Google Scholar] [CrossRef]
13. Bowsher, C. G., Scrase-Field, E. F. A. L., Esposito, S., Emes, M. J., Tetlow, I. J. (2007). Characterization of ADP-glucose transport across the cereal endosperm amyloplast envelope. Journal of Experimental Botany, 58(6), 1321–1332. DOI 10.1093/jxb/erl297. [Google Scholar] [CrossRef]
14. Parvaiz, A., Satyawati, S. (2008). Salt stress and phyto-biochemical responses off plants–A review. Plant Soil and Environment, 54(3), 89–99. DOI 10.17221/2774-PSE. [Google Scholar] [CrossRef]
15. Zhang, W. J., Wang, J. Q., Huang, Z. L., Mi, L., Xu, K. F. et al. (2019). Effects of low temperature at booting stage on sucrose metabolism and endogenous hormone contents in winter wheat spikelet. Frontiers in Plant Science, 10, 498. DOI 10.3389/fpls.2019.00498. [Google Scholar] [CrossRef]
16. Zhen, F. X., Zhou, J. J., Mahmood, A., Wang, W., Chang, X. N. et al. (2020). Quantifying the effects of short-term heat stress at booting stage on nonstructural carbohydrates remobilization in rice. The Crop Journal, 8(2), 194–212. DOI 10.1016/j.cj.2019.07.002. [Google Scholar] [CrossRef]
17. Li, R., Shi, F., Fukuda, K. (2010). Interactive effects of various salt and alkali stresses on growth, organic solutes, and cation accumulation in a halophyte Spartina alterniflora (Poaceae). Environmental and Experimental Botany, 68(1), 66–74. DOI 10.1016/j.envexpbot.2009.10.004. [Google Scholar] [CrossRef]
18. Munir, A., Shehzad, M. T., Qadir, A. A., Murtaza, G., Khalid, H. I. (2019). Use of potassium fertilization to ameliorate the adverse effects of saline-sodic stress condition (ECw: SARw levels) in rice (Oryza Sativa L.). Communications in Soil Science and Plant Analysis, 50, 1975–1985. DOI 10.1080/00103624.2019.1648657. [Google Scholar] [CrossRef]
19. Wang, X. P., Geng, S. J., Ri, Y. J., Cao, D. H., Liu, J. et al. (2011). Physiological responses and adaptive strategies of tomato plants to salt and alkali stresses. Scientia Horticulturae, 130(1), 248–255. DOI 10.1016/j.scienta.2011.07.006. [Google Scholar] [CrossRef]
20. Saeedipour, S. (2014). The combined effects of salinity and foliar spray of different hormones on some biological aspects, dry matter accumulation and yield in two varieties of indica rice differing in their level of salt tolerance. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 84(3), 721–733. DOI 10.1007/s40011-013-0292-5. [Google Scholar] [CrossRef]
21. Roitsch, T. (1999). Source-sink regulation by sugar and stress. Current Opinion in Plant Biology, 2(3), 198–206. DOI 10.1016/S1369-5266(99)80036-3. [Google Scholar] [CrossRef]
22. Munns, R., Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651–681. DOI 10.1146/annurev.arplant.59.032607.092911. [Google Scholar] [CrossRef]
23. Dionisio-Sese, M. L., Tobita, S. (2000). Effects of salinity on sodium content and photosynthetic responses of rice seedlings differing in salt tolerance. Journal of Plant Physiology, 157(1), 54–58. DOI 10.1016/s0176-1617(00)80135-2. [Google Scholar] [CrossRef]
24. Khelil, A., Menu, T., Ricard, B. (2007). Adaptive response to salt involving carbohydrate metabolism in leaves of a salt-sensitive tomato cultivar. Plant Physiology and Biochemistry, 45(8), 551–559. DOI 10.1016/j.plaphy.2007.05.003. [Google Scholar] [CrossRef]
25. Hu, T., Hu, L. X., Zhang, X. Z., Zhang, P. P., Zhao, Z. J. et al. (2013). Differential responses of CO2 assimilation, carbohydrate allocation and gene expression to NaCl stress in perennial ryegrass with different salt tolerance. PLoS One, 8(6), e66090. DOI 10.1371/journal.pone.0066090. [Google Scholar] [CrossRef]
26. Peng, Y. L., Gao, Z. W., Gao, Y., Liu, G. F., Sheng, L. X. et al. (2008). Eco-physiological characteristics of alfalfa seedlings in response to various mixed salt-alkaline stresses. Journal of Integrative Plant Biology, 50(1), 29–39. DOI 10.1111/j.1744-7909.2007.00607.x. [Google Scholar] [CrossRef]
27. Maness, N. (2010). Extraction and analysis of soluble carbohydrates. In: Sunkar, R. (edsPlant stress tolerance. Methods in molecular biology, vol. 639. USA: Humana Press. DOI 10.1007/978-1-60761-702-0_22. [Google Scholar] [CrossRef]
28. Yang, J., Zhang, J., Wang, Z., Zhu, Q. (2001). Activities of starch hydrolytic enzymes and sucrose-phosphate synthase in the stems of rice subjected to water stress during grain filling. Journal of Experimental Botany, 52(364), 2169–2179. DOI 10.1093/jexbot/52.364.2169. [Google Scholar] [CrossRef]
29. Hubbard, N. L., Huber, S. C., Pharr, D. M. (1989). Sucrose phosphate synthase and acid invertase as determinants of sucrose concentration in developing muskmelon (Cucumis melo L.) fruits. Plant Physiology, 91(4), 1527–1534. DOI 10.1104/pp.91.4.1527. [Google Scholar] [CrossRef]
30. Dong, C. J., Wang, X. L., Shang, Q. M. (2011). Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Scientia Horticulturae, 129(4), 629–636. DOI 10.1016/j.scienta.2011.05.005. [Google Scholar] [CrossRef]
31. Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. DOI 10.1016/0003-2697(76)90527-3. [Google Scholar] [CrossRef]
32. Miron, D., Schaffer, A. A. (1991). Sucrose phosphate synthase, sucrose synthase, and invertase activities in developing fruit of Lycopersicon esculentum Mill. and the sucrose accumulating Lycopersicon hirsutum Humb. and bonpl. Plant Physiology, 95(2), 623–627. DOI 10.1104/pp.95.2.623. [Google Scholar] [CrossRef]
33. Rosales, M. A., Rubio-Wilhelmi, M. M., Castellano, R., Castilla, N., Ruiz, J. M. et al. (2007). Sucrolytic activities in cherry tomato fruits in relation to temperature and solar radiation. Scientia Horticulturae, 113(3), 244–249. DOI 10.1016/j.scienta.2007.03.015. [Google Scholar] [CrossRef]
34. Duffus, C., Rosie, R. (1973). Starch hydrolysing enzymes in the developing barley grain. Planta, 109(2), 153–160. DOI 10.1007/BF00386123. [Google Scholar] [CrossRef]
35. Bhatia, S., Asthir, B. (2014). Calcium mitigates heat stress effect in wheat seeding growth by altering carbohydrate metabolism. Indian Journal of Plant Physiology, 19(2), 138–143. DOI 10.1007/s40502-014-0087-6. [Google Scholar] [CrossRef]
36. Rosa, M., Hilal, M., González, J. A., Prado, F. E. (2009). Low-temperature effect on enzyme activities involved in sucrose–starch partitioning in salt-stressed and salt-acclimated cotyledons of quinoa (Chenopodium quinoa Willd.) seedlings. Plant Physiology and Biochemistry, 47(4), 300–307. DOI 10.1016/j.plaphy.2008.12.001. [Google Scholar] [CrossRef]
37. Griffiths, C. A., Paul, M. J., Foyer, C. H. (2016). Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1857(10), 1715–1725. DOI 10.1016/j.bbabio.2016.07.007. [Google Scholar] [CrossRef]
38. Li, C., Liu, Y., Tian, J., Zhu, Y. S., Fan, J. J. (2020). Changes in sucrose metabolism in maize varieties with different cadmium sensitivities under cadmium stress. PLoS One, 15(12), e0243835. DOI 10.1371/journal.pone.0243835. [Google Scholar] [CrossRef]
39. Hutsch, B. W., Jung, S., Schubert, S. (2015). Comparison of salt and drought-stress effects on maize growth and yield formation with regard to acid invertase activity in the kernels. Journal of Agronomy and Crop Science, 201(5), 353–367. DOI 10.1111/jac.12111. [Google Scholar] [CrossRef]
40. Nemati, F., Ghanati, F., Gavlighi, H. A., Sharifi, M. (2018). Comparison of sucrose metabolism in wheat seedlings during drought stress and subsequent recovery. Biologia Plantarum, 62(3), 595–599. DOI 10.1007/s10535-018-0792-5. [Google Scholar] [CrossRef]
41. Gibson, S. I. (2005). Control of plant development and gene expression by sugar signaling. Current Opinion in Plant Biology, 8(1), 93–102. DOI 10.1016/j.pbi.2004.11.003. [Google Scholar] [CrossRef]
42. Rolland, F., Baena-Gonzalez, E., Sheen, J. (2006). Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annual Review of Plant Biology, 57, 675–709. DOI 10.1146/annurev.arplant.57.032905.105441. [Google Scholar] [CrossRef]
43. Liu, Z. Q., Liu, T., Liang, L. L., Li, Z., Hassan, M. J. et al. (2020). Enhanced photosynthesis, carbohydrates, and energy metabolism associated with chitosan-induced drought tolerance in creeping bentgrass. Crop Science, 60(2), 1064–1076. DOI 10.1002/csc2.20026. [Google Scholar] [CrossRef]
44. van den Ende, W., Valluru, R. (2008). Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? Journal of Experimental Botany, 60(1), 9–18. DOI 10.1093/jxb/ern297. [Google Scholar] [CrossRef]
45. Li, T., Heuvelink, E., Marcelis, L. F. M. (2015). Quantifying the source–sink balance and carbohydrate content in three tomato cultivars. Frontiers in Plant Science, 6. DOI 10.3389/fpls.2015.00416. [Google Scholar] [CrossRef]
46. Tetlow, I. J., Morell, M. K., Emes, M. J. (2004). Recent developments in understanding the regulation of starch metabolism in higher plants. Journal of Experimental Botany, 55(406), 2131–2145. DOI 10.1093/jxb/erh248. [Google Scholar] [CrossRef]
47. Thalmann, M., Pazmino, D., Seung, D., Horrer, D., Nigro, A. et al. (2016). Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. The Plant Cell, 28(8), 1860–1878. DOI 10.1105/tpc.16.00143. [Google Scholar] [CrossRef]
48. Kaplana, F., Dong, Y. S., Guy, C. L. (2010). Roles of ß-amylase and starch breakdown during temperatures stress. Physiologia Plantarum, 126(1), 120–128. DOI 10.1111/j.1399-3054.2006.00604.x. [Google Scholar] [CrossRef]
49. Sulpice, R., Pyl, E. T., Ishihara, H., Trenkamp, S., Steinfath, M. et al. (2009). Starch as a major integrator in the regulation of plant growth. Proceedings of the National Academy of Sciences, 106(25), 10348–10353. DOI 10.1073/pnas.0903478106. [Google Scholar] [CrossRef]
50. Müller-Röber, B. T., Kossmann, J., Hannah, L. C., Willmitzer, L., Sonnewald, U. (1990). One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Molecular & General Genetics, 224(1), 136. DOI 10.1007/BF00259460. [Google Scholar] [CrossRef]
51. Todaka, D., Matsushima, H., Morohashi, Y. (2000). Water stress enhances β-amylase activity in cucumber cotyledons. Journal of Experimental Botany, 51(345), 739–745. DOI 10.1093/jexbot/51.345.739. [Google Scholar] [CrossRef]
52. Wei, W., Li, Q. T., Chu, Y. N., Reiter, R. J., Yu, X. M. et al. (2015). Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. Journal of Experimental Botany, 66(3), 695–707. DOI 10.1093/jxb/eru392. [Google Scholar] [CrossRef]
53. Richter, J. A., Erban, A., Kopka, J., Zörb, C. (2015). Metabolic contribution to salt stress in two maize hybrids with contrasting resistance. Plant Science, 233, 107–115. DOI 10.1016/j.plantsci.2015.01.006. [Google Scholar] [CrossRef]
54. Pan, J. F., Cui, K. H., Wei, D., Huang, J. L., Xiang, J. et al. (2011). Relationships of non-structural carbohydrates accumulation and translocation with yield formation in rice recombinant inbred lines under two nitrogen levels. Physiol Plant, 141(4), 321–331. DOI 10.1111/j.1399-3054.2010.01441.x. [Google Scholar] [CrossRef]
55. Wang, D. R., Han, R. K., Wolfrum, E. J., McCouch, S. R. (2017). The buffering capacity of stems: Genetic architecture of nonstructural carbohydrates in cultivated Asian rice, Oryza sativa. New Phytologist, 215(2), 658–671. DOI 10.1111/nph.14614. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |