

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.018659
ARTICLE
Genotype Screening of Recipient Resources with High Regeneration and Transformation Efficiency in Chrysanthemum
1Beijing Advanced Innovation Center for Tree Breeding by Molecular Design, Beijing Key Laboratory of Ornamental Plants Germplasm Innovation & Molecular Breeding, National Engineering Research Center for Floriculture, Beijing Laboratory of Urban and Rural Ecological Environment, Key Laboratory of Genetics and Breeding in Forest Trees and Ornamental Plants of Education Ministry, School of Landscape Architecture, Beijing Forestry University, Beijing, 100083, China
2Beijing Botanical Garden, Beijing, 100093, China
*Corresponding Author: He Huang. Email: 101navy@163.com
#Contributed equally to this work
Received: 08 August 2021; Accepted: 21 October 2021
Abstract: Genetic transformation is one of the key steps in the molecular breeding of chrysanthemum, which relies on an optimal regeneration and transformation system. However, the regeneration system of different chrysanthemum cultivars varies, and the regeneration time of most cultivars is long. To screen cultivars with highly efficient regeneration, leaves and shoot tip thin cell layers (tTCL) from eight chrysanthemum cultivars with different flower colors and flower types were cultured on Murashige and Skoog media (MS) supplemented with 1.0–5.0 mg L−1 6-benzylaminopurine (6-BA) and 0.1–1.0 mg L−1 α-naphthaleneacetic (NAA). The results showed that the most efficient regeneration media were MS + 6-BA 1.0 mg L−1 + NAA 0.5 mg L−1 for leaf explants and MS + 6-BA 5.0 mg L−1 + NAA 0.1 mg L−1 for tTCL explants. Subsequently, another 13 chrysanthemum cultivars were screened by using the media, and finally, three cultivars with high regeneration efficiency were obtained from 21 cultivars. Among these, C1 had the highest regeneration efficiency: the regeneration rate of leaf explants reached 80.0% after 42 days of culture, and the regeneration rate of tTCL explants reached 100% after 31 days of culture. Furthermore, we also established the transformation system for C1 as follows: preculturing for one day, infecting with Agrobacterium suspension (OD600 = 0.6) for 10 min, and cultivating in the regeneration medium with 350 mg L−1 carbenicillin and 10 mg L−1 kanamycin, thus ultimately achieving a transformation rate of 4.0%. In this study, a new chrysanthemum cultivar with an efficient regeneration and transformation system was screened, which is beneficial to enrich the flower color of chrysanthemum transgenic plant recipients and to the functional research of flower color or type-related genes.
Keywords: Chrysanthemum; tissue culture; regeneration system; genetic transformation; cultivars screening
Chrysanthemum (Chrysanthemum × morifolium Ramat.) is a perennial herb belonging to the Asteraceae family and is one of the most economically important and favored floricultural crops [1]. Crossbreeding was the most common approach to developing novel chrysanthemum cultivars in past decades, but it was laborious and had a long breeding period. Additionally, with the increase in breeding objectives, some desired traits cannot be produced by hybridization due to the deficiency of traditional techniques. In recent years, transgenic molecular breeding has been widely employed as Agrobacterium-mediated transformation into plants to change the ornamental traits of the plants, which has constituted a trend in ornamental plant breeding [2]. Agrobacterium-mediated transformation, as an effective means to transfer exogenous genes to ornamental plants, has been widely used in flower color modification, flowering regulation, resistance improvement, etc. [3]. Noda et al. [4] introduced CtA3′5′GT from Clitoria ternatea and CamF3′5′H from Campanula medium to chrysanthemum ‘Taihei’ by Agrobacterium-mediated transformation, and the two genes were expressed under the control of the CmF3H promoter from chrysanthemum, producing a true blue chrysanthemum flower. Furthermore, CmMYB8 was transferred into chrysanthemum ‘Jinba’ by the Agrobacterium-mediated method, and it was found that the lignin content accumulation in the plants was reduced, with the CmMYB8 overexpressing, thus verifying the function of this gene [5]. However, the genetic transformation of chrysanthemum is still limited by many factors, leading to a low transformation efficiency. Therefore, it is crucial to evaluate the factors that affect transformation efficiency and to construct a suitable genetic transformation system.
As the major factor of transgenic breeding, an efficient regeneration and transformation system can shorten the regeneration time of transgenic plants and improve the transformation efficiency. In vitro shoot regeneration of plants is affected by many factors, including explant type and age, genotype, culture conditions, and plant growth regulatory factors [6]. There are many reports on the regeneration system of chrysanthemum, however, the periods of these systems were generally too long, approximately 45–60 days. For example, the leaf explants of cultivar ‘Jinba’ started to differentiate after 45 days of culture, the leaves and internodes of ‘Satinbleu’ needed 8 weeks, and the leaves of ‘Resomee Splendid’ needed 7 weeks to differentiate [6–8]. An important reason for the long period is that most cultivars may not differentiate in the same medium due to browning after callus induction Hence, it is necessary to change the medium frequently in the regeneration of chrysanthemum, which makes the regeneration process complicated and time-consuming [9]. It was reported that the callus of chrysanthemum cultivar ‘White ND’ could be induced on MS medium supplemented with 1 mg L−1 6-BA and 2 mg L−1 NAA, but it needed to be changed onto medium containing 2 mg L−1 6-BA and 0.5 mg L−1 NAA to continue to differentiate [10]. Furthermore, most reports currently use cultivar ‘Jinba’ as the material for gene function verification [5,11], but it is a white, double-petaled cut chrysanthemum cultivar, which is not suitable for flower color and flower development research. Therefore, it is important to screen more chrysanthemum cultivars with high regeneration rates and diverse genotypes, and establish an efficient regeneration system for subsequent research.
In the present study, we exploit leaves and tTCLs of eight chrysanthemum cultivars as explants to screen high-efficiency regeneration cultivars and regeneration media by the comparison of their callus induction, differentiation and regeneration rates in twelve different media. Subsequently, another 13 cultivars were cultured on the high-efficiency regeneration media to obtain more efficient cultivars. On this basis, we constructed the Agrobacterium-mediated transformation system using the cultivar with the highest regeneration efficiency as the material. Our study will facilitate the research of molecular breeding and gene function of chrysanthemum.
Twenty-one potted multiflora chrysanthemum cultivars provided by artificial cross-breeding and introduction were transplanted in the greenhouse (Appendix Table S1; Fig. S1). Among them, 8 cultivars, namely, A13, A63, C1, C23, C55, 404, A12, and D12, were used to establish an efficient regeneration system, and 13 cultivars, namely, 99-136-1, 91-4-3, 98-5-1, 82-81-19, 14-42-1, baby tears (BT), 03-70-1, A20, C27, C60, D64, D78, and E8, were used to verify the efficient regeneration system. Cuttings were taken and thoroughly washed under running tap water before the flowering stage. They were then subsequently surface-sterilized with a 70% (v/v) ethanol solution for 30 s and with 1% sodium hypochlorite for 3–5 min, followed by five rinses with sterilized deionized water. The sterilized shoot tips were cultured on MS media [12] containing 3% (w/v) sucrose and 7% (w/v) agar. The chrysanthemums were maintained in the tissue culture room under the following conditions: light of 2000 lx for 16 h/d and 25 ± 1°C for 40 days.
2.2 Establishment of an Efficient Chrysanthemum Regeneration System
2.2.1 Effects of Explants on Shoot Regeneration
Leaves were collected from healthy tissue culture plantlets of 8 cultivars (A13, A63, C1, C23, C55, 404, A12, and D12), cut into leaf discs of 0.5 cm × 0.5 cm, and 1–2 stem segments below the shoot tips were cut into a shoot-tip thin cell layer (tTCL) of 0.1 cm. Explants were placed with the adaxial surface down, and there were 10 leaf discs or 10 tTCLs per treatment, with three replicates.
2.2.2 Effects of Plant Growth Regulators on Shoot Regeneration
Different concentrations of plant growth regulators were set to 12 treatments (Y1–Y12) with a randomized complete block design, including 4 concentrations of 6-benzyladenine (BA) and 3 concentrations of α-naphthaleneacetic (NAA) (Table 1). The calli of 8 cultivars (A13, A63, C1, C23, C55, 404, A12, and D12) were induced, redifferentiated and regenerated in these 12 treatments.
2.2.3 Effects of Plant Growth Regulators on Rooting
To evaluate the effects of different auxins on root induction, adventitious buds above 1.0 cm were transferred onto 1/2 MS medium supplemented with four concentrations of indolebutyric acid (IBA) and NAA (G1–G4) (Appendix Table S2). There were 10 adventitious buds in each treatment, with three replicates.
Callus induction rate = Number of explants forming callus/Number of explants inoculated × 100%;
Differentiation rate = Number of seedlings obtained from callus/Number of explants inoculated × 100%;
Regeneration rate = Number of explants with adventitious buds higher than 1.0 cm/Number of explants inoculated × 100%;
Number of shoots/explant = Total number of adventitious buds higher than 1.0 cm/Number of explants inoculated × 100%;
Differentiation time = Number of days from when the explants were transplanted to the medium to adventitious bud differentiation;
Regeneration time = Number of days from when the explants were transplanted to the medium to when the adventitious buds grew to 1.0 cm;
Rooting rate = Number of rooted explants/the total number of explants × 100%.
The experimental results were subjected to one-way analysis of variance (ANOVA) with Duncan’s multiple range test (with statistical significance at P < 0.05) using SPSS Statistics 25.0 (IBM). Different lowercases denote significant differences in the differentiation rates or differentiation time at the P < 0.05 level, and different capitals denote significant differences in the regeneration rates, regeneration time or no. of shoots/explant at the P < 0.05 level.
2.3 Verification of Efficient Regeneration System
Leaf explants and tTCL explants from 13 cultivars (99-136-1, 91-4-3, 98-5-1, 82-81-19, 14-42-1, BT, 03-70-1, A20, C27, C60, D64, D78, and E8) were cultured on the high-efficiency regeneration medium screened in Section 2.2 (Y2 and Y10). There were 10 leaf discs or 10 tTCLs per treatment, with three replicates. We calculated the callus induction rate, differentiation rate and time, regeneration rate and time and number of shoots/explant each cultivar in the same manner as in Section 2.2.4.
2.4 Establishment of the Transformation System of Chrysanthemum Cultivar C1
2.4.1 Sensitivity of Leaf Explants and tTCL Explants to Kanamycin
Leaf explants and tTCL explants of cultivar C1 were inoculated onto high-efficiency regeneration media with different concentrations of kanamycin (0, 2, 5, 8, 10, and 15 mg L−1). There were 10 leaf discs or 10 tTCLs per treatment. After the explants were incubated, the numbers of calli were recorded to calculate the regeneration rate. The adventitious shoots that grew above 1.0 cm were transplanted onto rooting media containing different concentrations of kanamycin (0, 5, 8, 10, 15, and 20 mg L−1). After the shoots were incubated for 30 days, the number of rooted explants was recorded to calculate the rooting rate.
2.4.2 Assessments of Factors that Affect the Transformation Efficiency
To screen the best combination of cocultivation time, microbial concentration, infection time, and antibiotic concentration, 9 treatments (S1–S9) were set according to four factors and three levels of the orthogonal table (Appendix Table S3). There were 20 leaf discs per treatment, with three replicates. The growth of leaf discs and regeneration rate were recorded after 30 days.
2.4.3 Plasmid Construction, DNA Isolation and PCR Amplification
The Agrobacterium tumefacians strain GV3101 carrying the overexpression vector pBI121 with ClbHLH62 was isolated from petals of Chrysanthemum lavandulifolium under the control of the cauliflower mosaic virus (CaMV) 35S promotor used for transformation (Appendix Fig. S2).
Total genomic DNA from the leaves of transgenic and wild-type plants was extracted using the TIANGEN™ DNA secure Plant Kit, and PCR was performed using specific primers and the following PCR conditions: forward 5′-GACGCACAATCCCACTATCC-3′ and reverse 5′-CATCTAATATGAAAATCGAGC-3′; PCR conditions: 95°C for 5 min; followed by 35 cycles of 95°C for 40 s, 57°C for 30 s, and 72°C for 1 min; followed by 72°C for 10 min. The amplified products were assessed by 1.0% (w/v) agarose gel electrophoresis and visualized using a gel imager.
3.1 Effects of Different Auxin and Cytokinin Concentrations on Leaf Explant Regeneration of Eight Chrysanthemum Cultivars
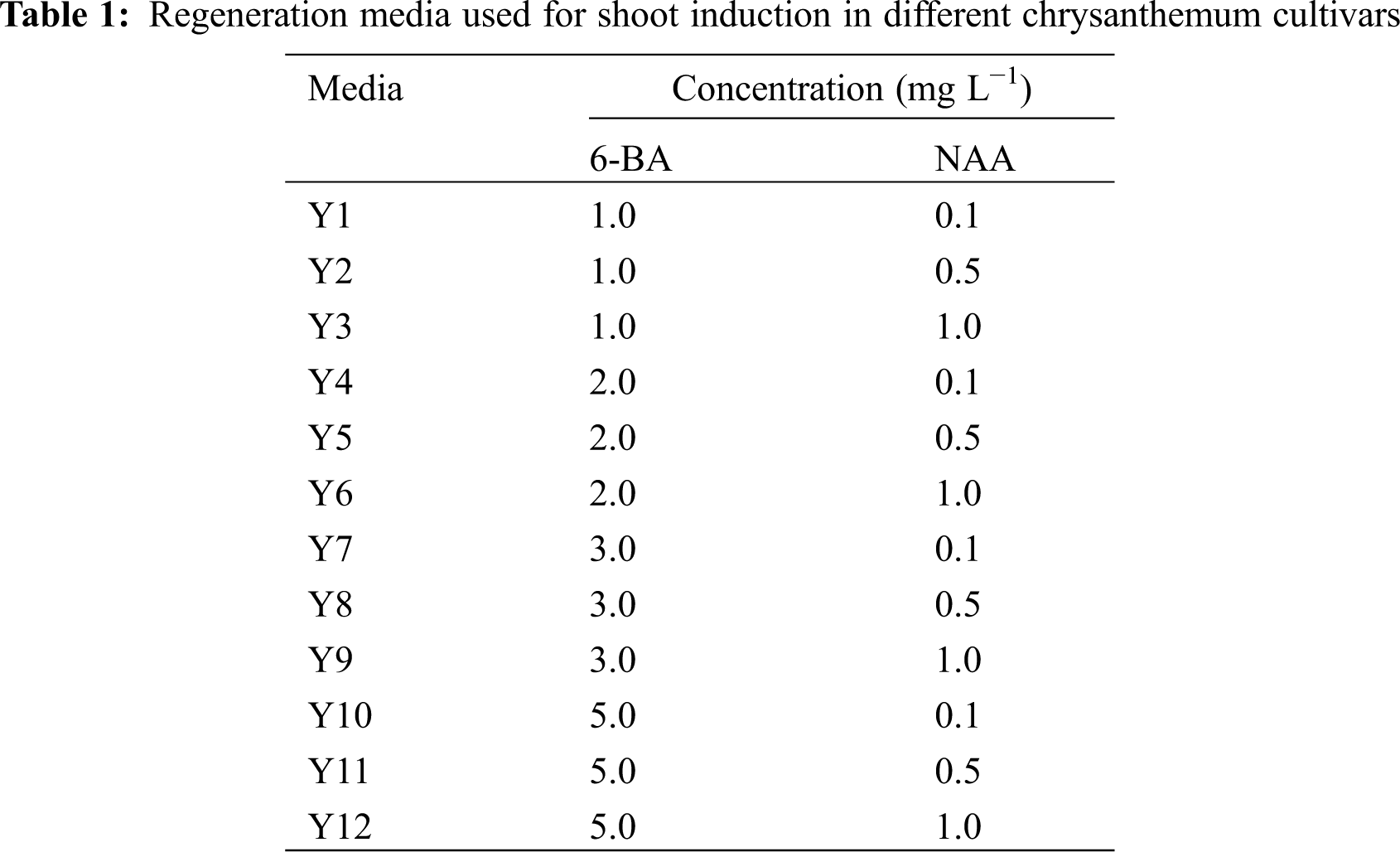
Leaf explants of eight cultivars were cultured on MS media supplemented with different concentrations of 6-BA and NAA (Y1–Y12). After 10 days, the edges of the leaf explants began to swell and calli were visible. The average callus rates of eight cultivars reached more than 87.7% in 12 treatments, indicating that it was relatively easy to induce calli from leaf explants of chrysanthemum (Appendix Table S4).
There were significant differences in the differentiation rates and regeneration rates between the 12 treatments (Fig. 1a). Explants of most cultivars achieved the highest differentiation rate and regeneration rate in the Y2 or Y3 treatment, and the differentiation time and regeneration time were the shortest in the Y2 treatment (Fig. 1b). In contrast, in Y7-Y12, most of the calli did not differentiate, or the differentiation rates were low, indicating that the low concentration of 6-BA and the high concentration of NAA might be beneficial to the callus differentiation of leaf explants.
Furthermore, the differentiation and regeneration of each cultivar were also quite different (Fig. 1a), and according to this characteristic, eight cultivars were divided into three types. The differentiation rate and regeneration rate of the first type were both relatively low, including D12, A12 and C23. After the calli were formed, they were almost unable to differentiate and regenerate in the same treatments. For example, the leaf explants of A12 showed severe browning 40 days after inoculation, resulting in failure to regenerate (Figs. 1a–1d). The second type had a high differentiation rate and low regeneration rate, including A13, A63 and C55. In the optimal treatment, differentiation from leaf explants of these cultivars reached 80.0%–100.0%, but then the browning and death of adventitious buds caused a relatively low regeneration rate (Figs. 1a–1d). The differentiation and regeneration rates of the third type were the highest, including C1 and 404. The differentiation rates from leaf explants reached more than 90.0% in the optimal treatment, the regeneration rates were more than 80.0%, and the number of shoots per explant was 8.3 and 4.2, respectively (Figs. 1a–1c). Among them, leaf explants of C1 began to differentiate after 20 days of inoculation, and the adventitious buds grew to 1.0 cm after approximately 40 days (Fig. 1b). Hence, leaf explants of C1 and 404 had high regeneration rates and short regeneration time, which were suitable for establishing an efficient regeneration system.
3.2 Effects of Different Auxin and Cytokinin Concentrations on tTCL Explant Regeneration of Eight Chrysanthemum Cultivars
After tTCL explants of eight cultivars were cultured in the Y1–Y12 treatments for 10–15 days, the average callus induction rates of eight cultivars reached 100.0% in the optimal treatment (Appendix Table S5). There were some differences in the differentiation rate and regeneration rate between the 12 treatments, and most of the cultivars had higher regeneration efficiency in the Y10 treatment (Fig. 2a). Similar to the leaf explants, they were divided into three types according to the differentiation and regeneration rate (Fig. 2a). C55, D12, A12 and C23 belonged to the first type, characterized by a low differentiation rate and regeneration rate in all treatments. For example, after 40 days of growth on the media, only 19.8% of adventitious buds of A12 regenerated. A large number of explants exhibited browning after 60 days, and only a small portion of them induced adventitious buds (Figs. 2a–2d). The second type including A13 and A63, had a high differentiation rate but a low regeneration rate and a long regeneration time (Figs. 2a, 2b). For example, the differentiation rate of A13 reached 89.7%, but the explants gradually exhibited browning and vitrification. Similar to the leaf explants, the differentiation rate and regeneration rate from tTCL explants of the third type were relatively high. Among them, the differentiation rate of C1 reached 100.0% after 12 days, and the mean number of shoots per explant reached 10.3 on the 30th day (Fig. 2c). In addition, tTCL explants of C1 and 404 could regenerate in a short time in the optimal treatment. Therefore, they were suitable for the establishment of an efficient regeneration system. Furthermore, the differentiation ability of A13, A63, C1, C23, D12, and A12 was better than that from the leaf explants (Figs. 1a, 1b).
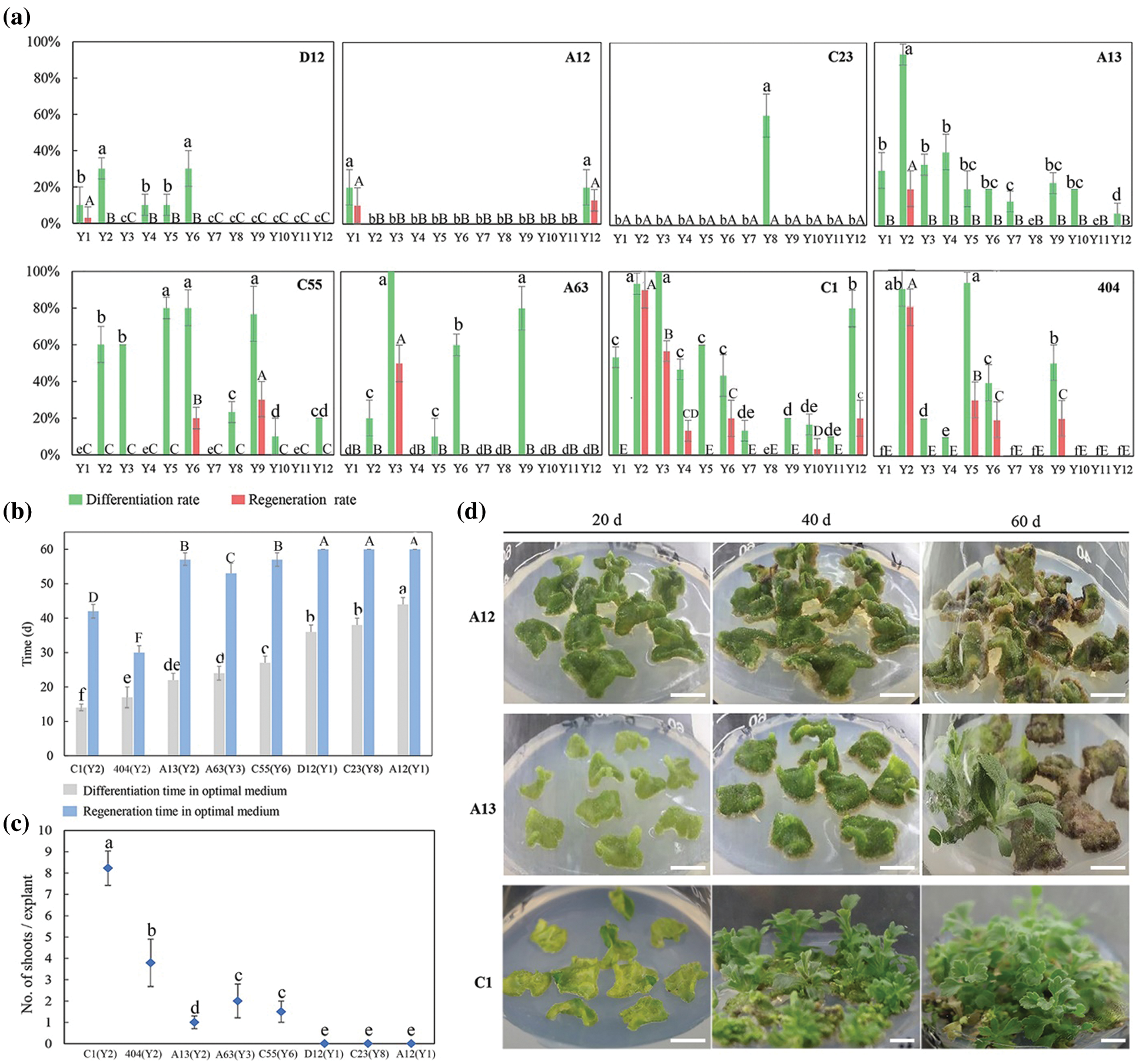
Figure 1: Influence of different auxin and cytokinin concentrations on leaf explants differentiation and regeneration of different cultivars. (a) Differentiation rates and regeneration rates from leaf explants of eight chrysanthemum cultivars in Y1–Y12 treatments; (b) Differentiation time and regeneration time from leaf explants of eight cultivars in the optimal treatment (the optimal treatment shown in brackets); (c) Mean number of shoots per leaf explant of eight cultivars in the optimal treatment (the optimal treatment shown in brackets); (d) The regeneration process from leaf explants of A12, A13 and C1 in the optimal treatment. Scale bar: 1 cm. Different lowercases denote significant differences in the differentiation rates or differentiation time at the P < 0.05 level by ANOVA; different capitals denote significant differences in the regeneration rates, regeneration time or proliferation coefficient at the P < 0.05 level by ANOVA
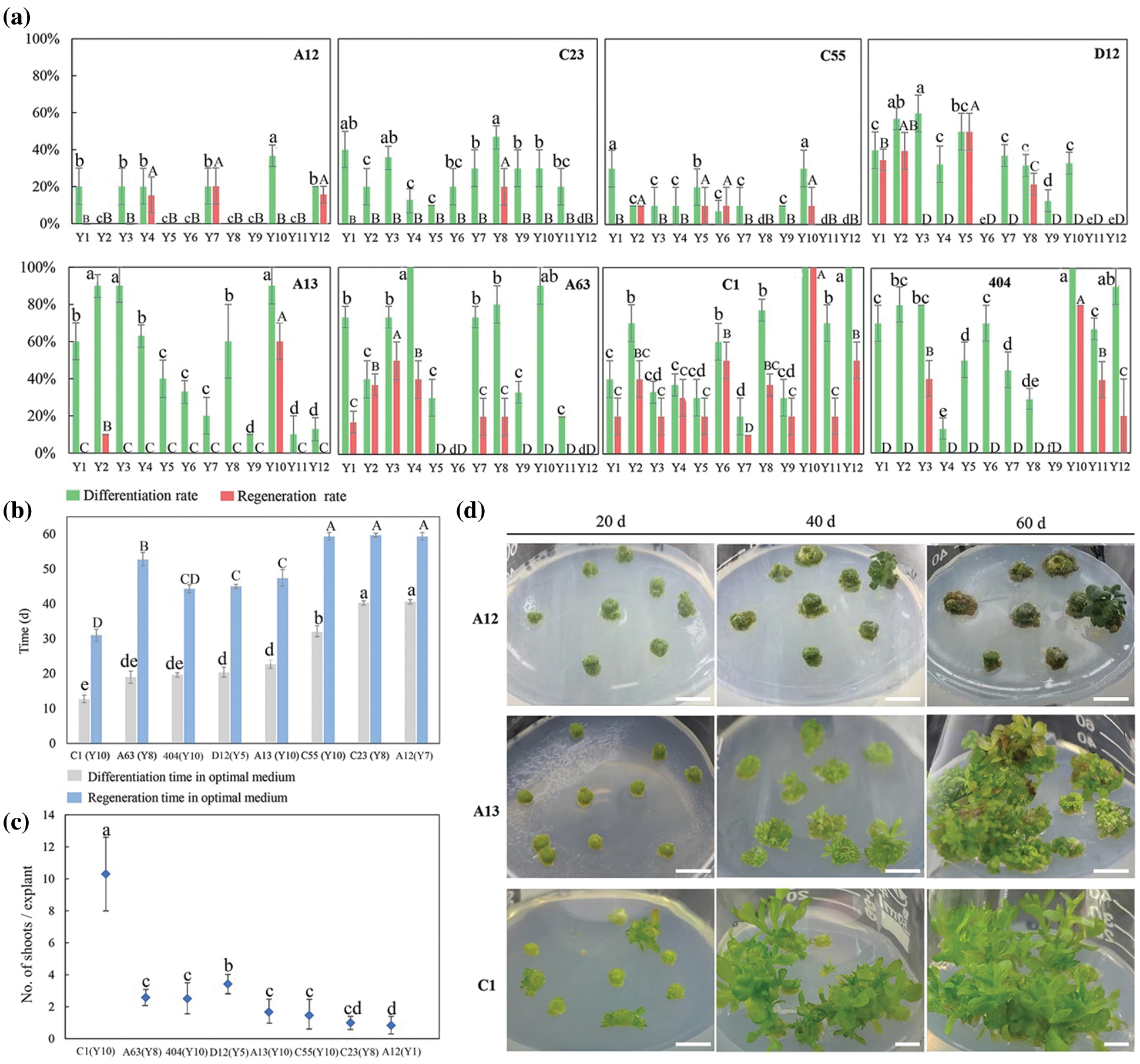
Figure 2: Influence of different auxin and cytokinin concentrations on tTCL explants differentiation and regeneration of different cultivars. (a) Differentiation rates and regeneration rates from tTCL explants of eight chrysanthemum cultivars in Y1–Y12 treatments; (b) Differentiation time and regeneration time from tTCL explants of eight cultivars in the optimal treatment (the optimal treatment shown in brackets); (c) Mean number of shoots per tTCL explant of eight cultivars in the optimal treatment (the optimal treatment shown in brackets); (d) The regeneration process from tTCL explants of A12, A13 and C1 in the optimal treatment. Scale bar: 1 cm. Different lowercases denote significant differences in the differentiation rates or differentiation time at the P < 0.05 level by ANOVA; different capitals denote significant differences in the regeneration rates, regeneration time or proliferation coefficient at the P < 0.05 level by ANOVA
3.3 Verification of the Efficient Regeneration System with 13 Chrysanthemum Cultivars
The research screened the high-efficiency regeneration treatment Y2 for leaf explants and Y10 for tTCL explants. To examine the applicability of the two efficient regeneration systems, leaf explants and tTCL explants from another 13 chrysanthemum cultivars with different flower colors or types were induced, and differentiated calli were induced in Y2 or Y10 (99-136-1, 91-4-3, 98-5-1, 82-81-19, 14-42-1, BT, 03-70-1, A20, C27, C60, D64, D78, and E8). The callus induction of all cultivars was efficient in the Y2 or Y10 treatment, but only seven cultivars were able to complete the regeneration process (Fig. 3). Taking 91-4-3 as an example, 90.0% of the leaf and tTCL explants induced adventitious buds, but the final regeneration rates were only 6.7%–20.0%, and the differentiation time and regeneration time were relatively long (30–35 d and 55–60 d, respectively). In contrast, the differentiation rates from leaf and tTCL explants of BT were both 100.0%, and the final regeneration rate reached 90.0%. The differentiation and regeneration times were short, approximately 23 days and 45 days, respectively. Leaf explants of C60 had a high differentiation rate (100.0%) and regeneration rate (96.7%), but the calli failed to be differentiated.
In conclusion, we screened three highly efficient regeneration cultivars: C1, 404 and BT. The leaf and tTCL explants of these cultivars could quickly complete the process of callus induction, differentiation and regeneration on the same media. For the two types of explants, we obtained two high-efficiency regeneration media. The optimal medium for leaf explants of the three cultivars was MS + 1 mg L−1 6-BA + 0.5 mg L−1 NAA (Y2). The regeneration periods were 42 d, 30 d and 45 d (Figs. 1b, 3a), and the proliferation coefficients were 8.2, 4.3 and 5.2 (Figs. 1c, 3a), respectively. The optimal medium for tTCL explants of the three cultivars was MS + 5 mg L−1 6-BA + 0.1 mg L−1 NAA (Y10). The regeneration periods were 30 d, 45 d and 50 d (Figs. 2b, 3b), and the proliferation coefficients were 10.3, 1.7, and 3.2, respectively (Figs. 2c, 3b).
Figure 3: Regeneration of 13 chrysanthemum cultivars in Y2 and Y10 treatments. (a) The differentiation rates, regeneration rates, differentiation time, regeneration time from leaf explants and number of shoots/leaf explant of 13 cultivars in Y2 treatment; (b) The differentiation rates, regeneration rates, differentiation time, regeneration time from tTCL explants and number of shoots/tTCL explant of 13 cultivars in Y10 treatment; (c) Adventitious bud development from leaf explants or tTCL explants of 6 cultivars. Scale bar: 1 cm. Different lowercases denote significant differences in the differentiation rates or differentiation time at the P < 0.05 level by ANOVA; different capitals denote significant differences in the regeneration rates, regeneration time or proliferation coefficient at the P < 0.05 level by ANOVA
3.4 Effects of Growth Regulators on the Rooting of Two High-Efficiency Regenerative Chrysanthemum Cultivars
To obtain complete plantlets, individual shoots higher than 1.0 cm from the explants of C1 and 404 were transferred onto 1/2 MS media containing different concentrations of IBA and NAA (Appendix Table S2). The rooting rates of C1 and 404 in G1–G4 treatments reached 86.7% after 15 days. The roots developed well after 30 days, and the rooting rates reached 100%. The result showed that the number of roots and root length were dependent on the type and concentration of auxin (Fig. 4). On the 20th day, the number of roots and root length in the G2 and G3 treatments were significantly greater than those in G1 (without exogenous auxin), indicating that a proper amount of IBA or NAA was beneficial to the rooting of chrysanthemum in vitro. In addition, the rooting of adventitious buds in the G2 and G3 treatments was better than that in G4, indicating that the combined effect of IBA and NAA was lower than that of a single hormone for C1 and 404. Taking the rooting rate and root length as indicators, we screened the best rooting medium for C1 and 404: 1/2 MS + 0.2 mg L−1 IBA (G3). Among them, the adventitious bud of 404 obtained the maximum root number (15.7) and root length (11.0 cm) in the G3 treatment.
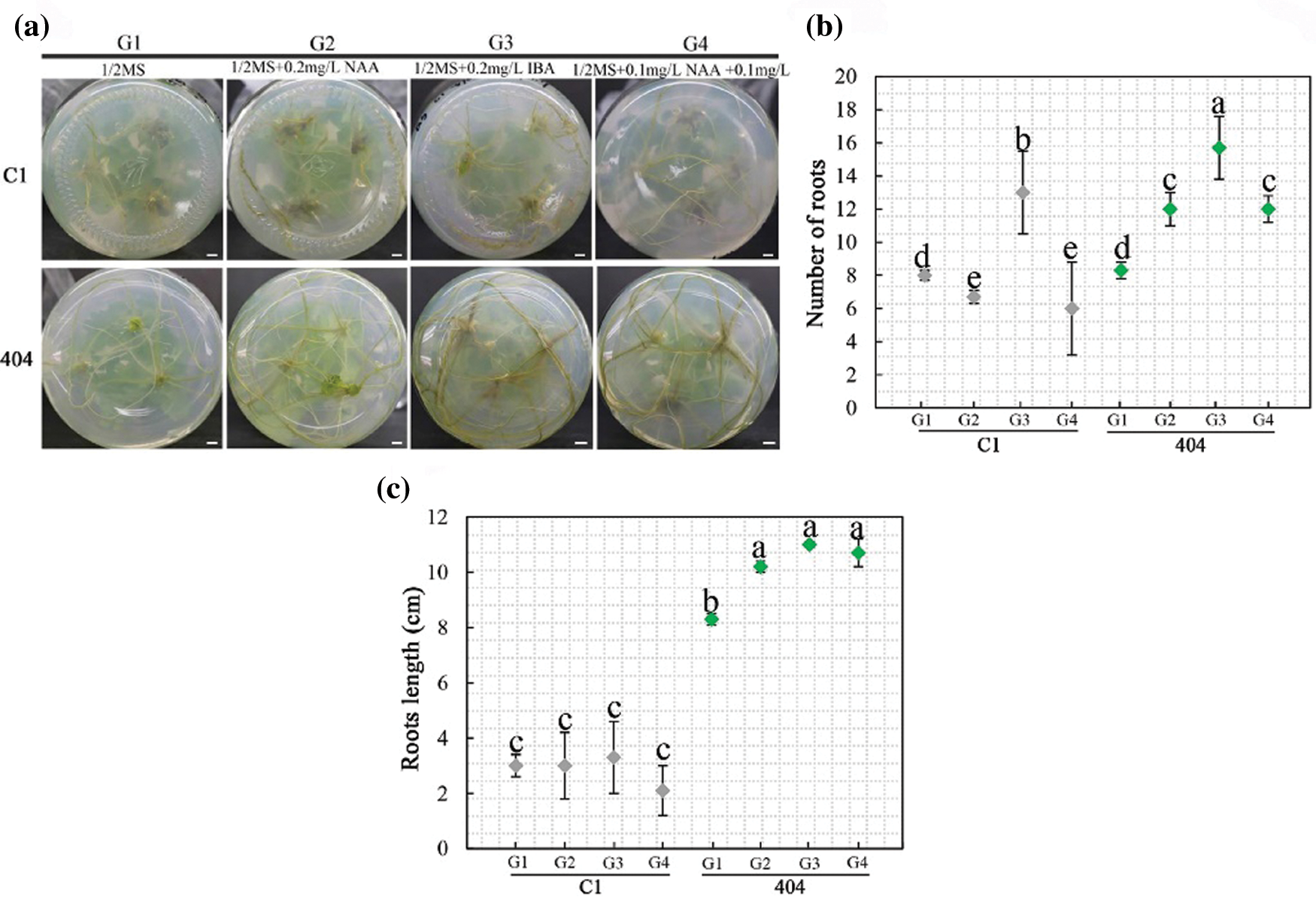
Figure 4: Effect of growth regulators on rooting. (a) Rooting status of C1 and 404 on different rooting media for 30 d. Scale bar: 1 cm; (b–c) Root number and length on different rooting media. Different letters denote significant differences in the number or length of roots at the P < 0.05 level by ANOVA
3.5 Establishment of the Transformation System of High-Efficiency Regeneration C1
Among the 21 chrysanthemum cultivars, leaf explants and tTCL explants of C1 had a relatively high regeneration rate and short regeneration time. Leaf explants and tTCL explants of C1 were used to determine sensitivity to kanamycin. Two kinds of explants showed different responses to kanamycin dosage (Fig. 5a). Approximately 30 days after cultivation, as the concentration of kanamycin increased, the regeneration ability of leaf explants decreased. When the concentration of kanamycin was 5 mg L−1 or 8 mg L−1, only a few explants were differentiated. The adventitious buds grew slowly, and the regeneration rate fell to 60.0%. When the concentration reached 10 mg L−1, the calli could be induced, but its differentiation was completely inhibited. When the concentration was 15 mg L−1, the formation of calli was inhibited, and the leaf explants all bleached (Fig. 5d). Therefore, 10 mg L−1 was used as the critical concentration of kanamycin for screening resistant adventitious buds from leaf explants. Compared with leaf explants, tTCL explants were more sensitive to kanamycin (Appendix Fig. S3a). When the concentration of kanamycin was 2 mg L−1, the calli began to brown, and the differentiated adventitious buds had severe vitrification and could not regenerate. Even on the medium with a low concentration of kanamycin, the calli from tTCL explants also exhibited vitrification, which indicated that the tTCL explant was probably susceptible to antibiotics and is not suitable as a stable receptor of the transformation system. In summary, we chose leaves as explants, and the kanamycin concentration was 10 mg L−1 to establish a genetic transformation system of C1.
The adventitious shoots differentiated from leaf explants were transferred onto rooting media with different concentrations of kanamycin (Fig. 5a). When the concentration of kanamycin was 5 mg L−1, the rooting of adventitious shoots was delayed, and the rooting rate fell to 60.0%. When the concentration of kanamycin was 8 mg L−1, adventitious shoots were completely inhibited from rooting, and the apical buds were partially etiolated (Appendix Fig. S3b). As the concentration of kanamycin continued to increase, the growth of explants was completely inhibited. Therefore, we used 8 mg L−1 as the minimum concentration to completely inhibit rooting.
Leaf explants were precultured for 1–3 days in the Y2 treatment (MS + 6-BA 1.0 mg L−1 + NAA 0.5 mg L−1). Then, the leaf explants were infected with Agrobacterium suspension carrying the recombinant vector pBI121-ClbHLH62 of different optical densities (OD600 = 0.4–0.8) for 5–15 min. After infection, they were cocultured for 3 days in the dark and inoculated in the Y2 treatment containing 200–500 mg L−1 carbenicillin. The result showed that the regeneration and differentiation rates from leaf explants in the S2 and S3 treatments were high, and explants differentiated into adventitious buds and regenerated fastest in the S2 treatment (Figs. 5b–5c). Therefore, the transformation system of C1 was as follows: the leaf explants were precultured for 1 day, infected with Agrobacterium suspension (OD600 = 0.6) for 10 min, and then cultivated with the Y2 treatment with 350 mg L−1 carbenicillin and 10 mg L−1 kanamycin. One hundred leaf explants were infected, 17 putative transformants regenerated from the selection medium, and finally, 4 transgenic plants were positive for integration of ClbHLH62 when analyzed by PCR (Fig. 5e). Therefore, the transformation efficiency (transformants/explants) was 4.0%.
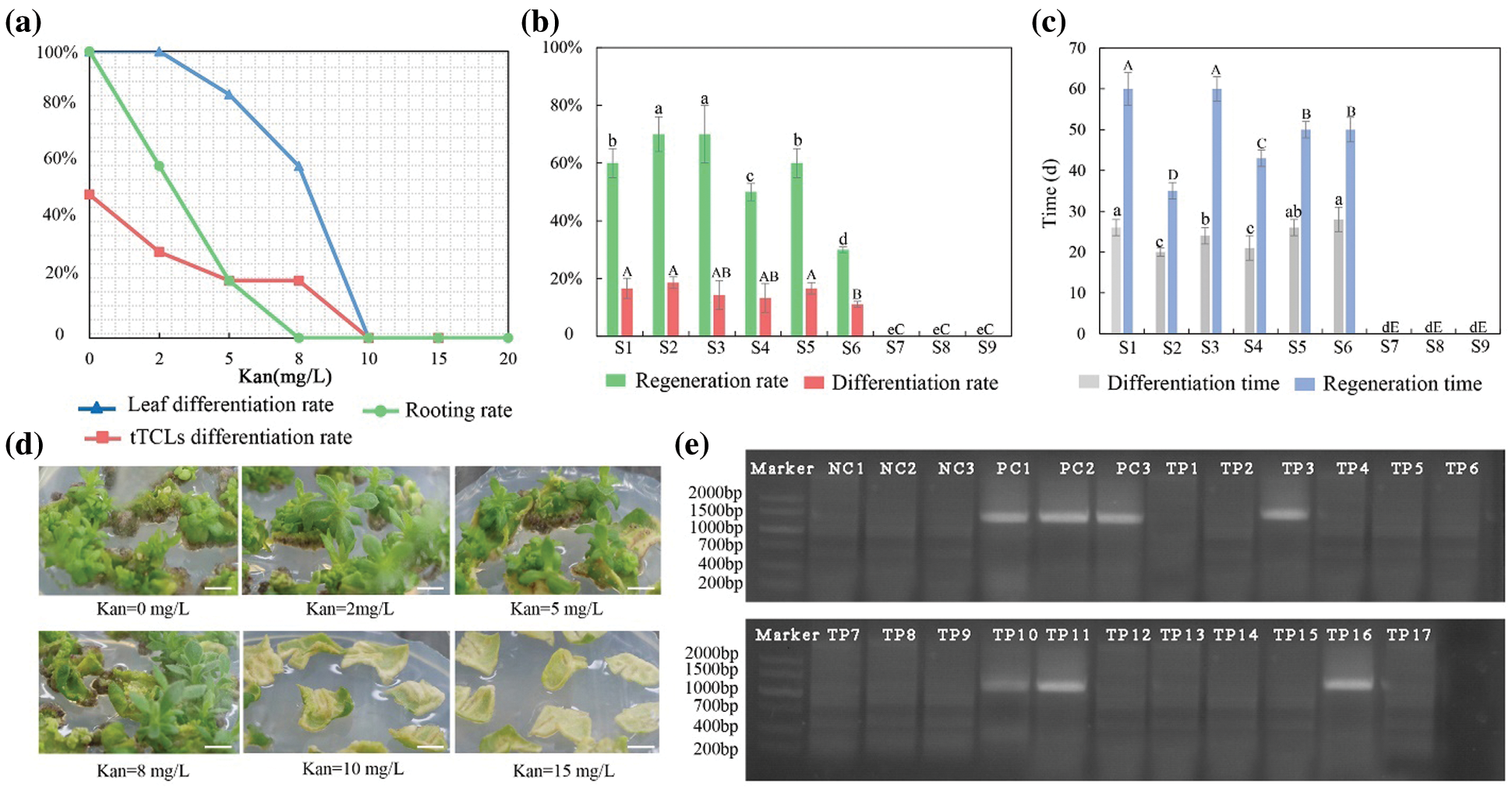
Figure 5: Establishment of transformation system for high-efficiency regeneration C1. (a) Determining the optimal concentration of kanamycin for differentiation of leaf and tTCL explants and rooting; (b) Effects of different transformation combinations on differentiation rate and regeneration rate of leaf explants; (c) Effects of different transformation combinations on differentiation time and regeneration time of leaf explants; (d) Regeneration of leaf explants in different kanamycin concentrations. Scale bar: 1 cm; (e) PCR detection of the target gene (ClbHLH62) in transgenic chrysanthemum C1 lines. M, DL2000 DNA marker; NC1-3, negative control; PC, positive control; TP1-TP17, Amplification of the ClbHLH62 gene in transformed lines. Different lowercases denote significant differences in the differentiation rate or differentiation time at the P < 0.05 level by ANOVA; different capitals denote significant differences in the regeneration rates or time at the P < 0.05 level by ANOVA
4.1 Effects of Genotype on the Regeneration of Chrysanthemum
Diverse genotypes are the main reason for the differences in chrysanthemum regeneration ability. Jan et al. [13] compared the regeneration frequency of 60 chrysanthemum cultivars, and only seven cultivars could regenerate. Lim et al. [14] studied the regeneration of 11 chrysanthemum cultivars and found that there were significant differences in the number of callus formation, bud germination and bud formation. In this study, we selected three chrysanthemum cultivars that could regenerate efficiently. On the best regeneration medua, the three cultivars could directly complete the process from callus induction to differentiation, and the callus induction rate and differentiation rate were both close to 100.0%. From inoculation to differentiation, it took approximately 30–45 days for adventitious buds to be higher than 1.0 cm, which significantly saved time and labor costs. Among them, C1 reached the maximum average shoot number per explant (10.3) with tTCL explants (Fig. 2c). Although other cultivars were able to induce calli, they could not differentiate into shoots or the regeneration rate was low without changing the media, so they were not suitable for establishing an efficient regeneration system.
There have been many reports on chrysanthemum regeneration systems, but unstable systems and low transformation efficiency are still problems to be solved. In previous studies, the differentiation rate was commonly used to measure the regeneration ability of species [15], but we found that it could not represent the regeneration ability of a cultivar in vitro. For example, the differentiation rates of A13, 91-4-3, A20, and D64 were more than 80.0%, but as time went by, the browning of explants would make the differentiated buds lose their growth ability and die and cause the actual regeneration rate to be less than 20.0%. In this study, after calli were induced from leaves and tTCL explants of A13, they were changed to nine differentiation media supplemented with 6-BA (1.0–3.0 mg L−1) and NAA (0.1–1.0 mg L−1) (Appendix Table S6). The result showed that both leaf and tTCL explants obtained a higher regeneration rate, and the degree of browning was reduced (Appendix Fig. S4). The regeneration rate of leaf explants in MS + 6-BA 1.0 mg L−1+ NAA 0.1 mg L−1 increased from 0% to 80.0%, and the regeneration rate of tTCL explants in MS + 6-BA 1.0 mg L−1 + NAA 0.1 mg L−1 increased from 60.0% to 100.0%. Growth regulators and other additives in the medium could affect the degree of browning of explants. Cytokinins KT, 6-BA, etc., could not only promote the synthesis of phenolic compounds but also stimulate the activity of polyphenol oxidase to intensify browning [16]. Therefore, it was speculated that changing the medium with a low concentration of 6-BA could reduce the degree of browning during callus differentiation. Furthermore, the study defined the regeneration rate as the number of explants with adventitious buds greater than 1.0 cm/the number of explants that differentiate into adventitious buds. The regeneration rate is a supplement to the induction rate of adventitious buds, and the combination of the two can better reflect the true regeneration ability of chrysanthemum calli.
Furthermore, ploidy has a significant effect on regeneration efficiency. In cucumber, pea and other plants, the proliferation of the cell cultures with higher ploidy level was faster than those retaining original ploidy levels [17–19]. In purple coneflower, explants with higher ploidy levels were more sensitive to the plant growth regulator, and the application of a concentration that was conducive to the regeneration of explants with lower ploidy levels was inhibitory to the regeneration of explants with higher ploidy levels [20]. Chrysanthemum is highly heterozygous and polyploid, and most potted multiflora cultivars are complex hexaploidy [21]. Few reports prove that the ploidy of explant affects regeneration and transformation efficiency of chrysanthemum, which may provide a new direction for future research.
4.2 Effects of Growth Regulators on the Regeneration of Chrysanthemum
It is a commonly used method to change the type and concentration of growth regulators to promote plant regeneration in plant tissue culture. 6-BA and NAA are widely used in the healing and differentiation of chrysanthemum explants, and the commonly used concentration ranges are 0.5–5 mg L−1 and 0.1–2 mg L−1, respectively [7,10,22]. In this study, we used different concentrations of 6-BA (1.0–5.0 mg L−1) and NAA (0.1–1.0 mg L−1) for callus induction, differentiation and regeneration of different varieties and found that most chrysanthemum varieties regenerated adventitious buds in 12 treatments.
Cytokines and auxin must be balanced during shoot regeneration from explants, and the ratio of these two anxins is species dependent [23]. A high cytokinin/auxin ratio promotes bud induction in chrysanthemum [24,25]. In this study, we found that when the concentration ratio of cytokinin to auxin was 2:1, it was beneficial to the formation and differentiation of leaf explants, while a high concentration of 6-BA (5 mg L−1) and a low concentration of NAA (0.1 mg L−1) were conducive to callus induction and differentiation of tTCL explants.
Studies of the effects of different auxins on root induction and development have been previously performed on chrysanthemums and have found superior effectiveness of IBA compared to IAA and NAA [26]. In this study, C1 and 404 could grow more and longer roots in the rooting medium supplemented with IBA. We found that 0.2 mg L−1 might be most suitable for root induction and development, and concentrations higher than the optimal concentration inhibit rooting, in accordance with the results of Naing et al. [7].
4.3 Effects of Explants on the Regeneration of Chrysanthemum
In this study, leaf explants and tTCL explants of chrysanthemum were tested to induce calli, and the callus induction rates were all higher than 80.0% in the Y1–Y12 treatments. However, the differentiation and regeneration of the same species were different under the same condition. The differentiation time, differentiation rate and regeneration rate from tTCL explants of A13, A63, C1, C23, D12 and A12 were better than those from the leaf explants. Furthermore, different explants responded differently to the same treatment, which may be due to the tissue specificity of the plant hormone receptor or the interaction between growth regulators and the endogenous hormone in the tissue [27].
There are relatively few studies on the regeneration of chrysanthemum with tTCL explants, but they have been used in the regeneration system of the lily, sorghum, etc. [28,29]. Teixeira de Silva et al. [30] used tTCLs of chrysanthemum ‘Shuhou-no-chikara’ to efficiently induce adventitious buds, but internode shortening, vitrification, and multiple branches often occurred. Similarly, we observed that in the same treatment, the degree of vitrification of tTCL explants was more serious than that of leaf explants. This might be due to the small size of tTCL explants and the larger wound area and contact area with the medium, which could make it easy to absorb too much water from the growth medium. In other words, this characteristic of tTCL explants also provides them with more morphogenetic cells and, therefore, a generally stronger ability to regenerate [31].
It was found that tTCL explants of C1 had a higher regeneration efficiency than leaf explants, and the regeneration ratio of which is 1.45 times faster than the commonly used cultivar ‘Jinba’. But tTCLs were greatly affected by the environment and were susceptible to kanamycin, which made it unsuitable as the transformation system in this study. Therefore, continuing to explore the transformation system of tTCL explants will further shorten the time for chrysanthemum gene transformation.
4.4 Agrobacterium-Mediated Transformation of Chrysanthemum
Compared with conventional breeding, transgenic technology does not need long-term periods of selection [32]. However, due to the influence of many factors, such as cocultivation duration, antibiotics, and genotype, the genetic transformation of chrysanthemum is limited, and the transformation efficiency is relatively low [11]. Therefore, it is necessary to determine the factors that affect the transformation efficiency. The genotype of chrysanthemum has a great impact on the transformation efficiency, including its susceptibility to Agrobacterium infection and its ability to regenerate plants in vitro [33,34]. The transformation efficiency also depends on the cell density of Agrobacterium. The study of Corredoira et al. [35] showed that at OD600 = 0.6 or higher, the growth curve of Agrobacterium corresponded to the middle of the exponential phase, and infection at this stage could increase the transfection efficiency. In this study, the optical density (OD600 = 0.6) was found to be an optimal inoculation density, and the early growth phase of Agrobacterium (OD600 = 0.4) was probably not active enough for explant infection, which was similar to the research of Naing et al. [2]. The cocultivation period and temperature in the dark environment affected the transformation efficiency. The maximum number of putative shoots was observed when the temperature was maintained at 22°C during the cocultivation period of leaf explants from the cultivar ‘Jinba’, and the transformation efficiency reached 4.0% after 2 days of cocultivation [11].
Furthermore, there are few reports about the effect of antibiotics on the Agrobacterium-mediated transformation of chrysanthemum. Carbenicillin, as an antibiotic to inhibit Agrobacterium, can prevent plantlet death caused by the long-term coexistence of Agrobacterium with the explants [36]. In this study, however, it was found that when the concentration of carbenicillin was 350 mg L−1, it could effectively inhibit the damage from excess Agrobacterium to seedlings; however, the leaf explants were necrotic in the culture medium when the concentration of carbenicillin was 500 mg L−1, which was similar to the research of Haider et al. [11].
Taken together, three chrysanthemum cultivars (C1, 404 and BT) with high-efficiency regeneration were screened, and an efficient regeneration system was established (MS + 6-BA 1.0 mg L−1 + NAA 0.5 mg L−1 for leaf explants and MS + 6-BA 5.0 mg L−1 + NAA 0.1 mg L−1 for tTCL explants). These cultivars only needed 30–45 days from inoculation to regeneration without changing the medium. The adventitious shoot rooting medium for C1 and 404 was established (1/2 MS + 0.2 mg L−1 IBA). In addition, we established an Agrobacterium-mediated genetic transformation system for C1, which finally differentiated into adventitious buds 30 days after culture. The positive transformation rate of gene detection was 4.0%.
Authors’ Contributions: HH and SLD conceived and designed this study. YJL, BJW and YL performed the experiments. YJL, BJW, YL, YMC and MMZ carried out the data analysis. YMC wrote this manuscript. All authors read and approved the final manuscript.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Acknowledgement: We thank the Beijing Botanical Garden for providing the greenhouse for the cultivation of different chrysanthemum cultivars.
Funding Statement: This work is supported by Fundamental Research Funds for the Central Universities (Grant No. 2021ZY34) and the National Natural Science Foundation of China (Grant No. 31870693).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Kim, S. J., Lee, C. H., Kim, J., Kim, K. S. (2014). Phylogenetic analysis of Korean native Chrysanthemum species based on morphological characteristics. Scientia Horticulturae, 175, 278–289. DOI 10.1016/j.scienta.2014.06.018. [Google Scholar] [CrossRef]
2. Naing, A. H., Ai, T. N., Jeon, S. M., Lim, S. H., Kim, C. K. (2016). An efficient protocol for Agrobacterium-mediated genetic transformation of recalcitrant chrysanthemum cultivar Shinma. Acta Physiologiae Plantarum, 38, 1–9. DOI 10.1007/s11738-015-2059-5. [Google Scholar] [CrossRef]
3. Kishi-Kaboshi, M., Aida, R., Sasaki, K. (2018). Genome engineering in ornamental plants: Current status and future prospects. Plant Physiology and Biochemistry, 131, 47–52. DOI 10.1016/j.plaphy.2018.03.015. [Google Scholar] [CrossRef]
4. Noda, N., Yoshioka, S., Kishimoto, S., Nakayama, M., Douzono, M. et al. (2017). Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism. Science Advances, 3, 1–11. DOI 10.1126/sciadv.1602785. [Google Scholar] [CrossRef]
5. Zhu, L., Guan, Y. X., Zhang, Z. H., Song, A. P., Chen, S. M. et al. (2020). CmMYB8 encodes an R2R3 MYB transcription factor which represses lignin and flavonoid synthesis in chrysanthemum. Plant Physiology and Biochemistry, 149, 217–224. DOI 10.1016/j.plaphy.2020.02.010. [Google Scholar] [CrossRef]
6. Kazeroonian, R., Mousavi, A., Jari, S. K., Tohidfar, M. (2018). Factors influencing in vitro organogenesis of Chrysanthemum morifolium cv. ‘Resomee Splendid’. Iranian Journal of Biotechnology, 16, 132–139. DOI 10.21859/ijb.1454. [Google Scholar] [CrossRef]
7. Naing, A. H., Park, K. I., Chung, M. Y., Lim, K. B., Kim, C. K. (2016). Optimization of factors affecting efficient shoot regeneration in chrysanthemum cv. Shinma. Brazilian Journal of Botany, 39, 975–984. DOI 10.1007/s40415-015-0143-0. [Google Scholar] [CrossRef]
8. Zalewska, M., Lema-Ruminska, J., Miler, N., Gruszka, M., Dabal, W. (2011). Induction of adventitious shoot regeneration in chrysanthemum as affected by the season. In Vitro Cellular and Developmental Biology-Plant, 47, 375–378. DOI 10.1007/s11627-010-9330-7. [Google Scholar] [CrossRef]
9. Nencheva, D. (2010). In vitro propagation of chrysanthemum. Methods in Molecular Biology, 589, 177–185. DOI 10.1007/978-1-60327-114-1_17. [Google Scholar] [CrossRef]
10. Adedeji, O. S., Naing, A. H., Kim, C. K. (2020). Protoplast isolation and shoot regeneration from protoplast-derived calli of chrysanthemum cv. White ND. Plant Cell, Tissue and Organ Culture, 141, 571–581. DOI 10.1007/s11240-020-01816-3. [Google Scholar] [CrossRef]
11. Haider, S., Gao, Y. H., Gao, Y. K. (2020). Standardized genetic transformation protocol for chrysanthemum cv. ‘Jinba’ with TERMINAL FLOWER 1 homolog CmTFL1a. Genes, 11, 860. DOI 10.3390/genes11080860. [Google Scholar] [CrossRef]
12. Murashige, T., Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497. DOI 10.1111/j.1399-3054.1962.tb08052.x. [Google Scholar] [CrossRef]
13. Jan, D. J., Wim, R., Monique, F. W. (1993). Restoring adventitious shoot formation on chrysanthemum leaf explants following cocultivation with Agrobacterium tumefaciens. Plant Cell, Tissue and Organ Culture, 32, 263–270. DOI 10.1007/BF00042287. [Google Scholar] [CrossRef]
14. Lim, K. B., Kwon, S. J., Lee, S. I., Hwang, Y. J., Naing, A. H. (2012). Influence of genotype, explant source, and gelling agent on in vitro shoot regeneration of chrysanthemum. Horticulture Environment and Biotechnology, 53, 329–335. DOI 10.1007/s13580-012-0063-x. [Google Scholar] [CrossRef]
15. Zhang, Y., Bozorov, T. A., Li, D. X., Zhou, P., Wen, X. J. et al. (2020). An efficient in vitro regeneration system from different wild apple (Malus sieversii) explants. Plant Methods, 16, 56. DOI 10.1186/s13007-020-00599-0. [Google Scholar] [CrossRef]
16. Kastner, U., Klocke, E., Abel, S. (2017). Regeneration of protoplasts after somatic hybridisation of Hydrangea. Plant Cell, Tissue and Organ Culture, 129, 359–373. DOI 10.1007/s11240-017-1183-x. [Google Scholar] [CrossRef]
17. Mahoney, J. D., Apicella, P. V., Apicella, M. H. (2018). Adventitious shoot regeneration from in vitro leaves of Aronia mitschurinii and cotyledons of closely related Pyrinae taxa. Scientia Horticulturae, 237, 135–141. DOI 10.1016/j.scienta.2018.03.062. [Google Scholar] [CrossRef]
18. Sarreb, D. A., Ładyżyński, M., Malepszy, S. (2002). Comparison of triploid and diploid cucumber in long-term liquid cultures. Plant Cell Tissue Organ Culture, 71, 231–235. DOI 10.1023/A:1020329720366. [Google Scholar] [CrossRef]
19. Ochatt, S. J., Mousset-Déclas, C., Rancillac, M. (2000). Fertile pea plants regenerate from protoplasts when calluses have not undergone endoreduplication. Plant Science, 156, 177–183. DOI 10.1016/S0168-9452(00)00250-8. [Google Scholar] [CrossRef]
20. Chen, X. L., Zhang, J. J., Zhang, R., Li, Q. L., Yang, Y. S. et al. (2013). An uncommon plant growth regulator, diethyl aminoethyl hexanoate, is highly effective in tissue cultures of the important medicinal plant purple coneflower (Echinacea purpurea L.). Biomed Research International, 2013, 4495–4500. DOI 10.1155/2013/540316. [Google Scholar] [CrossRef]
21. Su, J. S., Jiang, J. F., Zhang, F., Liu, Y., Ding, L. et al. (2019). Current achievements and future prospects in the genetic breeding of chrysanthemum: A review. Horticulture Research, 6, 109. DOI 10.1038/s41438-019-0193-8. [Google Scholar] [CrossRef]
22. Imtiaz, M., Khattak, A. M., Khan, M. A., Jalal, F., Hussain, S. et al. (2019). Rapid in-vitro propagation of Chrysanthemum morifolium through shoot bud explants. Pakistan Journal of Botany, 51, 1093–1098. DOI 10.30848/PJB2019-3(11). [Google Scholar] [CrossRef]
23. Hesami, M., Naderi, R., Tohidfar, M. (2019). Modeling and optimizing medium composition for shoot regeneration of chrysanthemum via radial basis function-non-dominated sorting genetic Algorithm-II (RBF-NSGAII). Scientific Reports, 9, 18237. DOI 10.1038/s41598-019-54257-0. [Google Scholar] [CrossRef]
24. Song, J. Y., Mattson, N. S., Jeong, B. R. (2011). Efficiency of shoot regeneration from leaf, stem, petiole and petal explants of six cultivars of Chrysanthemum morifolium. Plant Cell, Tissue and Organ Culture, 107, 295–304. DOI 10.1007/s11240-011-9980-0. [Google Scholar] [CrossRef]
25. Imtiaz, M., Khattak, A. M., Ara, N., Iqbal, A., Rahman, H. U. (2014). Micropropagation of Jartorpha curcas L. through shoot tip explants using different concentrations of phytohormones. Journal of Animal and Plant Sciences, 24, 229–233. DOI 10.1002/2013GB004685. [Google Scholar] [CrossRef]
26. Waseem, K., Jilani, M. S., Khan, M. S., Kiran, M., Khan, G. (2011). Efficient in vitro regeneration of chrysanthemum (Chrysanthemum morifolium L.) plantlets from nodal segments. African Journal of Biotechnology, 10, 1477–1484. DOI 10.13140/RG.2.2.15980.67208. [Google Scholar] [CrossRef]
27. Phillips, G. C., Garda, M. (2019). Plant tissue culture media and practices: An overview. In Vitro Cellular and Developmental Biology–Animal, 55, 242–257. DOI 10.1007/s11627-019-09983-5. [Google Scholar] [CrossRef]
28. Bakhshaie, M., Babalar, M., Mirmasoumi, M., Khalighi, A. (2010). Somatic embryogenesis and plant regeneration of Lilium ledebourii (Baker) Boiss., an endangered species. Plant Cell, Tissue and Organ Culture, 102, 229–235. DOI 10.1007/s11240-010-9726-4. [Google Scholar] [CrossRef]
29. Baskaran, P., Rajeswari, B. R., Jayabalan, N. (2014). Development of an in vitro regeneration system in sorghum [Sorghum bicolor (L.) Moench] using root transverse thin cell layers (tTCLs). Turkish Journal of Botany, 30, 1–9. [Google Scholar]
30. Teixeira de Silva, J. A., Seiichi, F. (2003). Chrysanthemum organogenesis through thin cell layer technology and plant growth regulator control. African Journal of Agricultural Research, 2, 504–514. DOI 10.3923/ajps.2003.505.514. [Google Scholar] [CrossRef]
31. Tripathi, D., Rai, K. K., Rai, S. K., Rai, S. P. (2018). An improved thin cell layer culture system for efficient clonal propagation and in vitro withanolide production in a medicinal plant Withania coagulans Dunal. Industrial Crops and Products, 119, 172–182. DOI 10.1016/j.indcrop.2018.04.012. [Google Scholar] [CrossRef]
32. Su, J. S., Jiang, J. F., Zhang, F., Liu, Y., Ding, L. et al. (2019). Current achievements and future prospects in the genetic breeding of chrysanthemum: A review. Horticulture Research, 6, 109. DOI 10.1038/s41438-019-0193-8. [Google Scholar] [CrossRef]
33. Suh, E. J., Han, B. H., Lee, Y. H., Lee, S. K., Hong, J. K. et al. (2015). The selection of domestically bred cultivars for spray-type chrysanthemum transformation. Korean Journal of Horticultural Science and Technology, 33, 947–954. DOI 10.7235/hort.2015.15017. [Google Scholar] [CrossRef]
34. Teixeira de Silva, J. A. (2004). Ornamental chrysanthemums: Improvement by biotechnology. Plant Cell, Tissue and Organ Culture, 79, 1–18. DOI 10.1023/b:ticu.0000049444.67329.b9. [Google Scholar] [CrossRef]
35. Corredoira, E., San-José, M. C., Ballester, A., Vieitez, A. M. (2005). Genetic transformation of Castanea sativa Mill. by Agrobacterium tumefaciens. Acta Horticulturae, 693, 387–393. DOI 10.17660/ActaHortic.2005.693.48. [Google Scholar] [CrossRef]
36. Sjahril, R., Mii, M. (2006). High-efficiency Agrobacterium-mediated transformation of Phalaenopsis using meropenem, a novel antibiotic to eliminate Agrobacterium. Journal of Horticultural Science and Biotechnology, 81, 458–464. DOI 10.1080/14620316.2006.11512088. [Google Scholar] [CrossRef]
Appendix
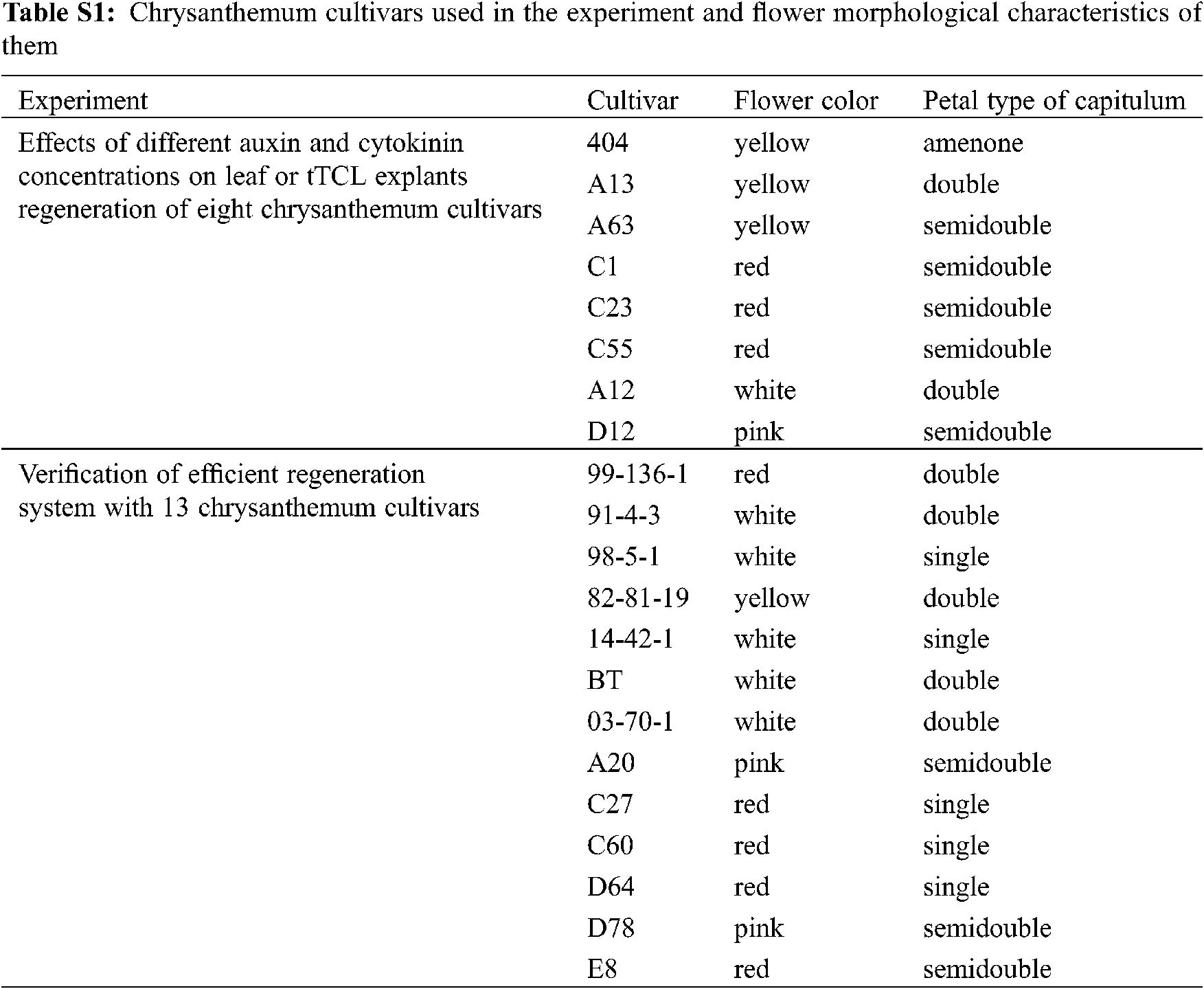

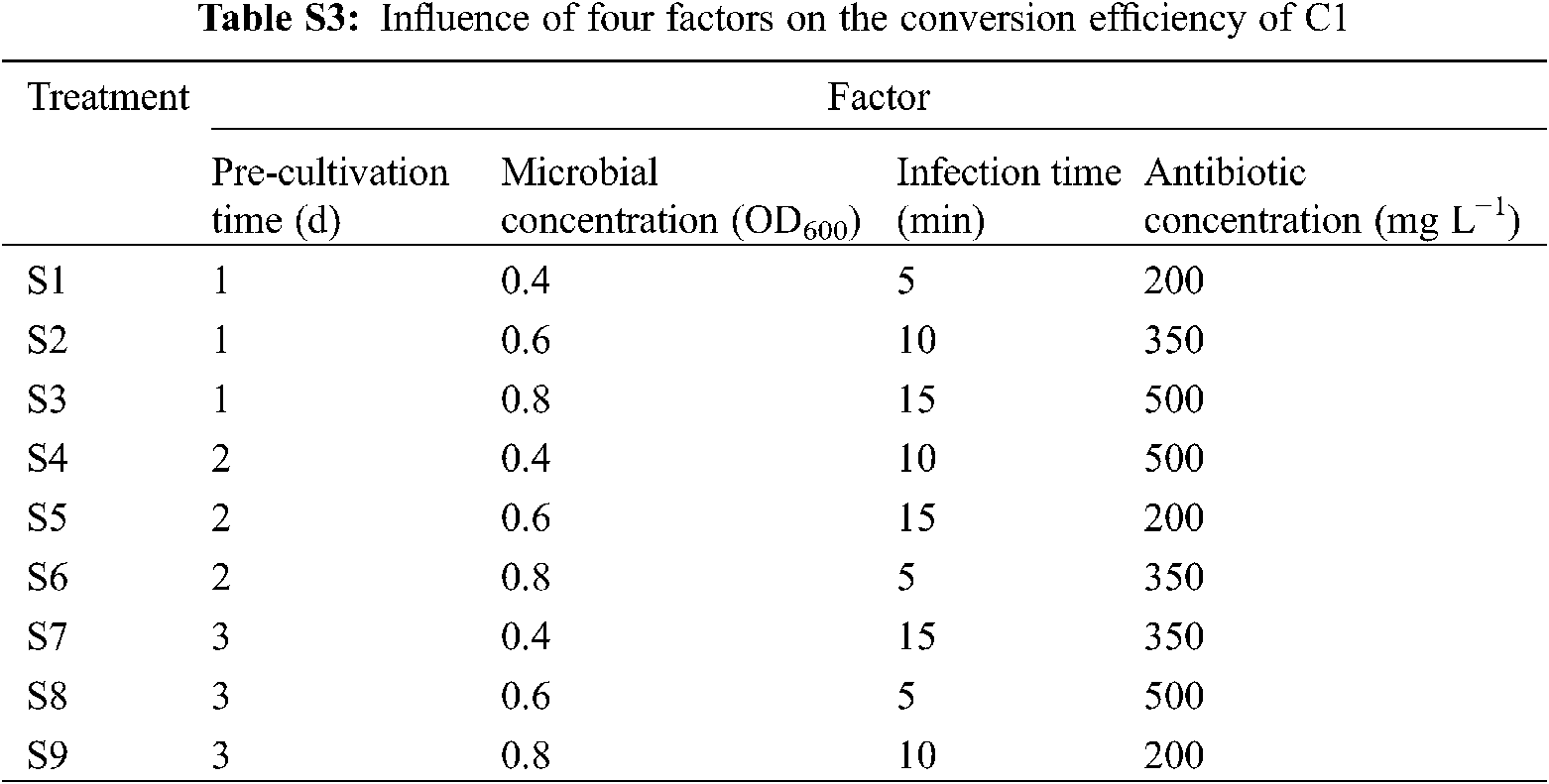
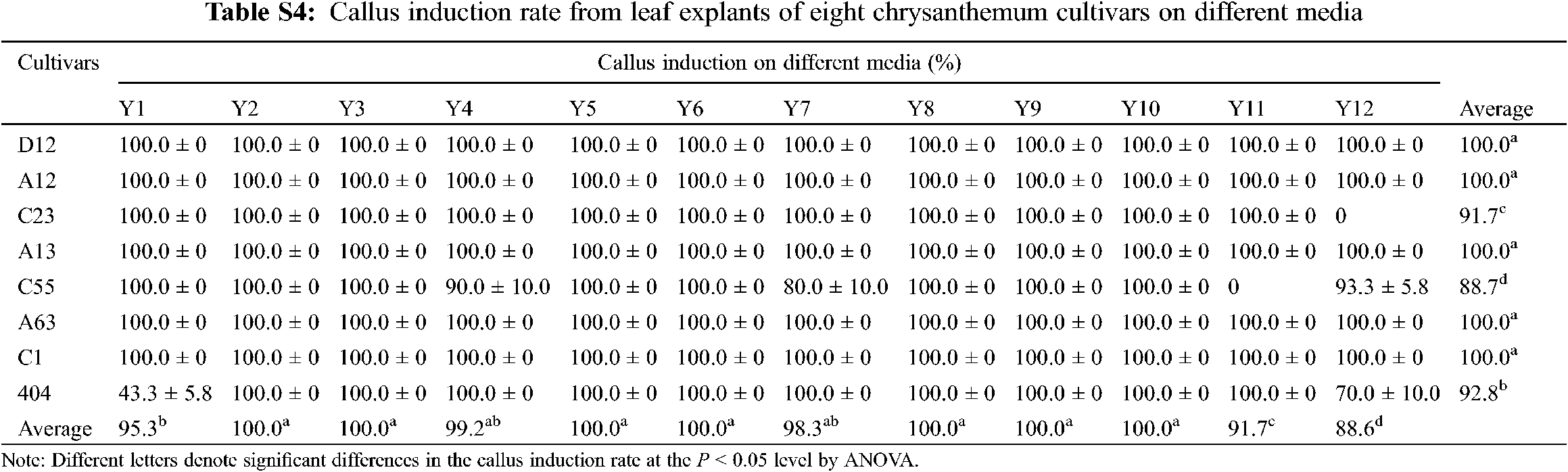
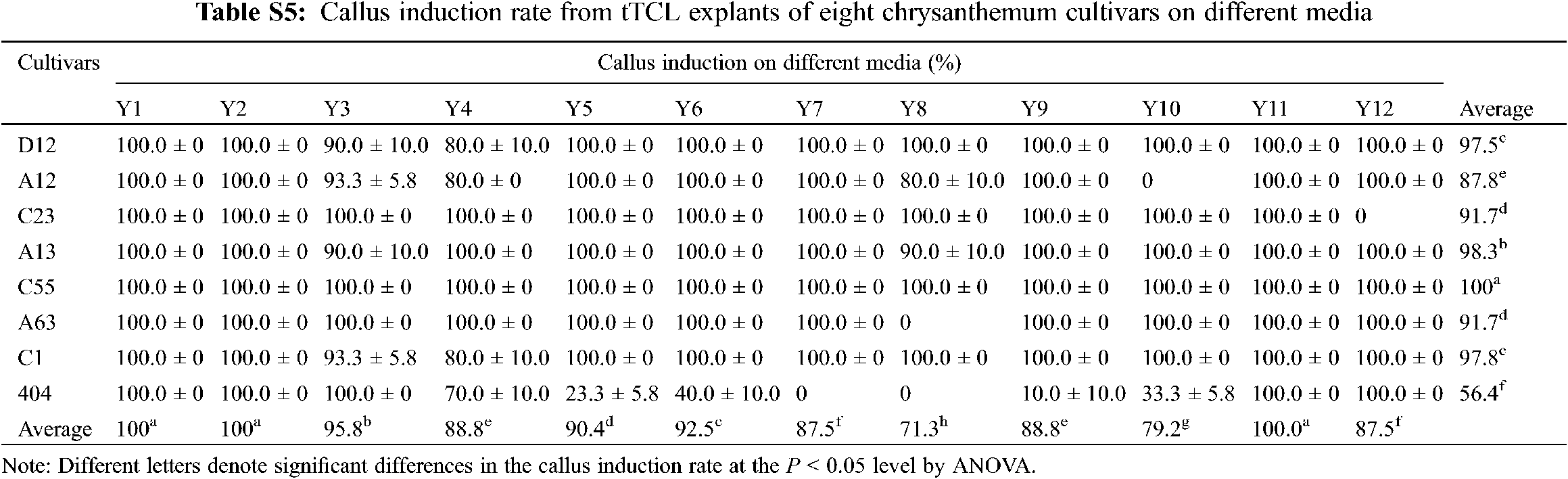
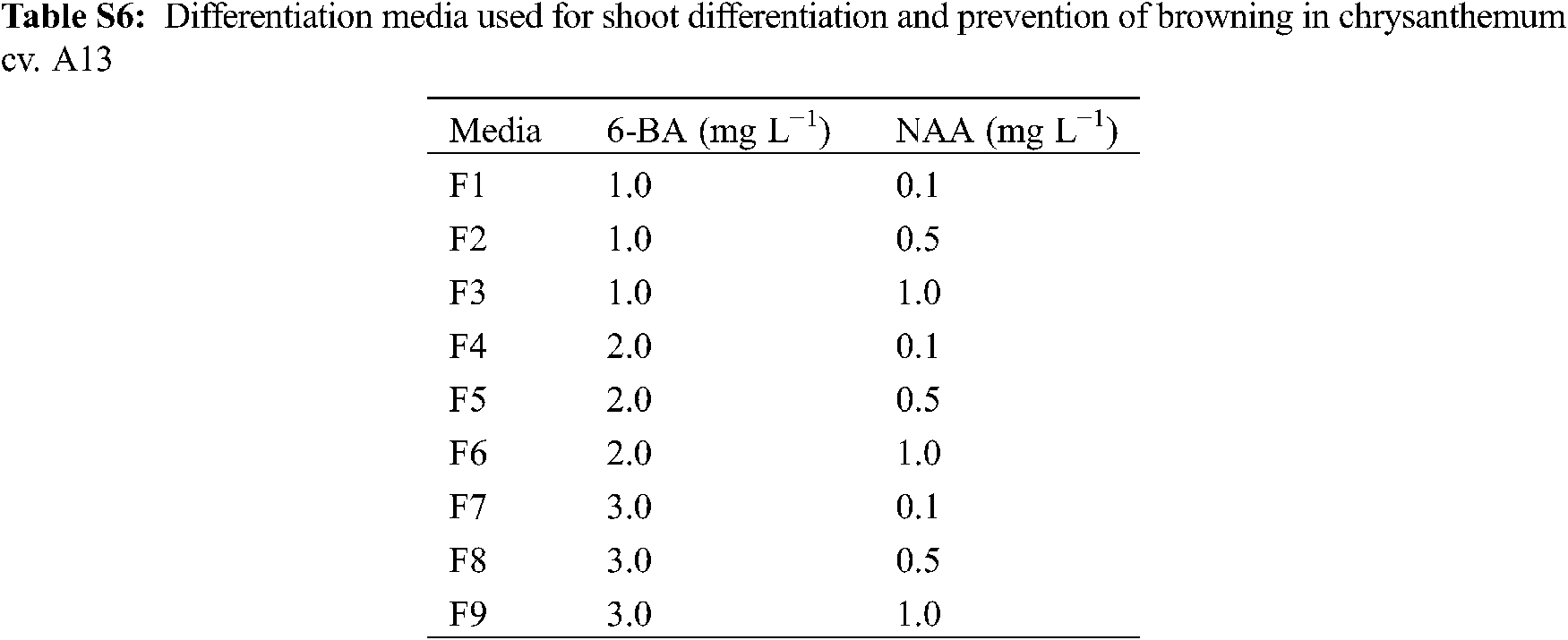
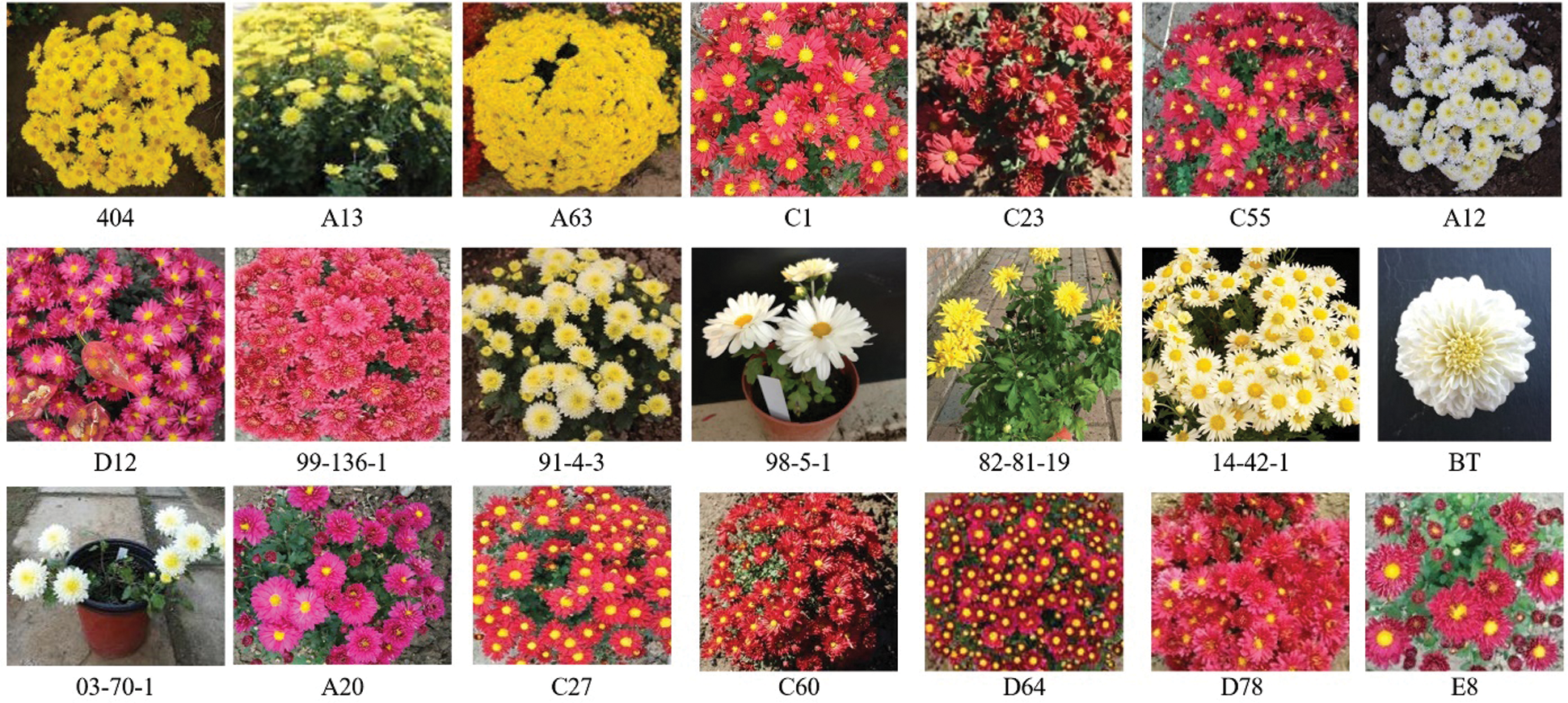
Figure S1: The photos of chrysanthemum cultivars used in the experiment
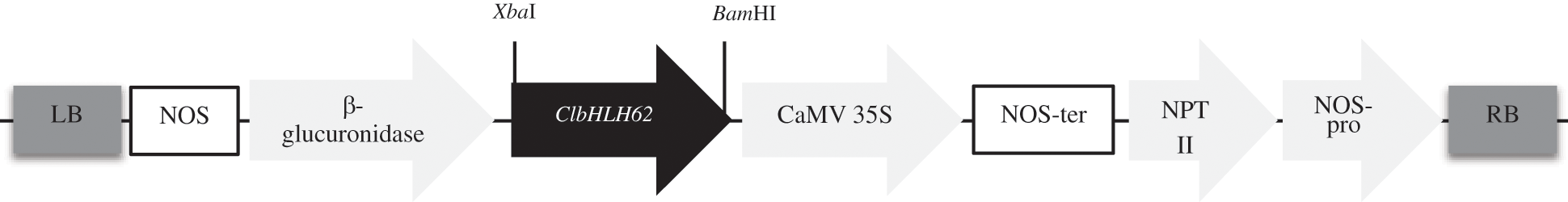
Figure S2: Diagram of the construction of the over-expression vector. ClbHLH62 gene was inserted between the CaMV 35S promoter and the β-glucuronidase gene. RB right border; LB left border
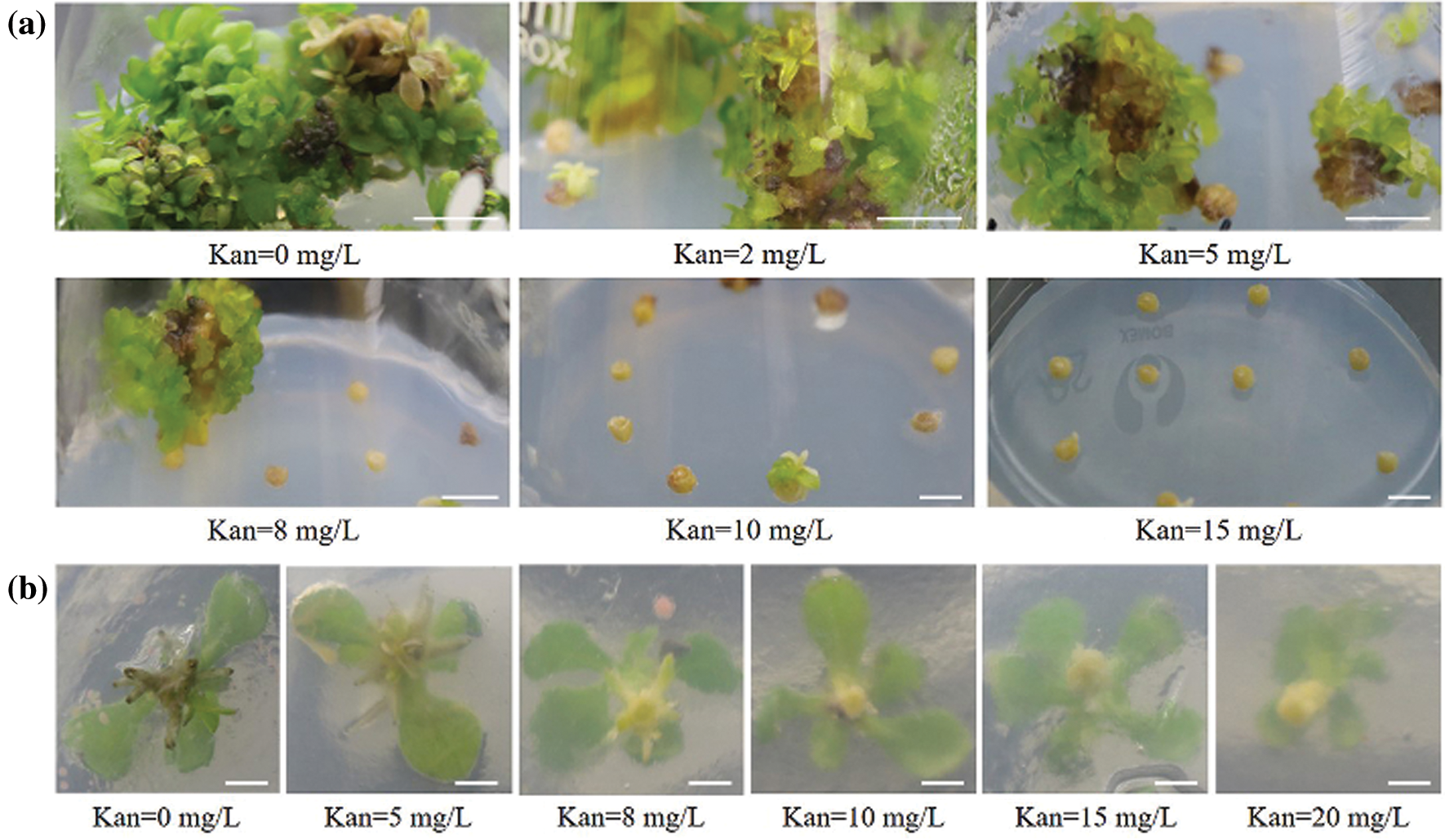
Figure S3: Determining the optimal concentration of kanamycin for tTCL explants differentiation and rooting of cv. C1. (a) Regeneration of tTCL explants in different kanamycin concentrations; (b) Rooting of cv. C1 in different kanamycin concentrations. Scale bar: 1 cm
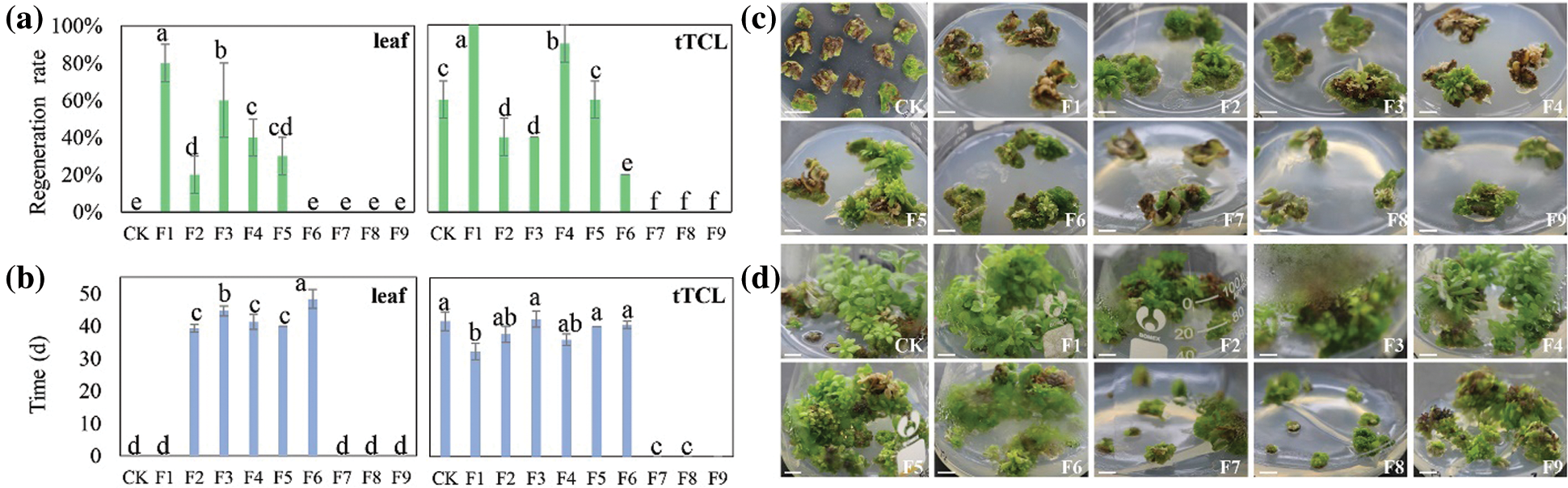
Figure S4: Regeneration of cv. A13 in the changed differentiation medium. (a) Regeneration rate of leaf explants or tTCL explants; (b) Regeneration time of leaf explants or tTCL explants; (c) Browning and regeneration of leaf explants on 9 changed differentiation media (F1–F9). CK, leaf explants of cv. A13 in Y2 treatment; (d) Browning and regeneration of tTCL explants on 9 changed differentiation media (F1–F9). CK, leaf explants of cv. A13 in Y10 treatment. Scale bar: 1 cm. Different letters significant differences in the regeneration rate or time of cv. A13 at the P < 0.05 level by ANOVA
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |