

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.018552
ARTICLE
Changes in Germination and Seedling Traits of Sesame under Simulated Drought
1Department of Agronomy, PMAS-Arid Agriculture University, Rawalpindi, 46300, Pakistan
2International Center for Biosaline Agriculture, Directorate of Programs, Dubai, 14660, United Arab Emirates
3Soils, Water and Environment Research Institute, Agricultural Research Center, Cairo University, Giza, 12613, Egypt
4Department of Agronomy, Bahauddin Zakariya University, Multan, 60800, Pakistan
5Department of Agronomy, Sher-e-Bangla Agricultural University, Dhaka, 1207, Bangladesh
*Corresponding Authors: Mukhtar Ahmed. Email: ahmadmukhtar@uaar.edu.pk; Mirza Hasanuzzaman. Email: mhzsauag@yahoo.com
Received: 02 August 2021; Accepted: 20 October 2021
Abstract: Drought is considered one of the leading abiotic constraints to agricultural crop production globally. Present study was conducted to assess the effects of different drought treatments (viz. Control, 10% PEG, and 20% PEG) on seed germination, germination indices, seedling traits, and drought tolerance indices of sesame. Our results showed that maximum reduction in the studied parameters was observed at higher PEG concentration (i.e., 20% PEG). As compared to control, the drought treatments viz. 10% and 20% PEG decreased the values for germination indices, such as germination percentage, coefficient of variation of germination time, germination index, and seedling vigor index. Similarly, for seedling traits, the values were decreased for root length, shoot length, root shoot ratio, root fresh weight, shoot fresh weight, root dry weight and shoot dry weight under 10% and 20% PEG treatments significantly in comparison with control. Furthermore, relative to control, the values for drought tolerance indices, such as germination drought tolerance index, root length drought tolerance index, shoot length drought tolerance index, total seedling length drought tolerance index, root fresh weight drought tolerance index, shoot fresh weight drought tolerance index, total fresh weight drought tolerance index, root dry weight drought tolerance index, shoot dry weight drought tolerance index and total dry weight drought tolerance index were also reduced under 10% and 20% PEG treatments, respectively. Our results confirms that drought impact on seed germination and seedling traits could be quantified by using different indices which can further help to design drought adaptation and mitigation strategies. Based on these results it can be concluded that germination indices, seedling traits, and drought tolerance indices have great potential to simulate drought stress impacts on different crop traits thus they should be used in all kinds of stress related studies.
Keywords: Sesame; drought stress; PEG; germination indices; seedling traits; drought tolerance indices
Plants are exposed to several biotic and abiotic stresses that may prevent the plants to reach their full potential performance and threaten their survival [1]. Drought has been regarded as the most crucial and damaging abiotic constraint for the global crop production [2]. Drought stress adversely affects the plants due to decreased water availability and increased water losses (through evapotranspiration) as a result of warmer and drier climate [3]. Moreover, in the future, global climate change is expected to increase the severity and intensity of drought in different regions (due to decreased precipitation and increased evapotranspiration) which may further exacerbate the situation by intensifying the competition for water between crops and people [4]. For instance, recent droughts such as the California Drought (2011–2017) in California [5] and the Millennium Drought (1997–2009) in Southern Australia [6] are the key evidence of increased frequency and severity of droughts limiting the water availability. Pakistan is also experiencing the brunt of changing climate in the form of hydrological reserves shrinkage and droughts [7]. At present, water availability is reduced to 1,000 m3 per capita which was 5,600 m3 in 1950 [8]. Pakistan has faced the worst drought during 1998–2004 and during this drought period, major crops showed a decline in their production [9]. The changing climate and increasing drought issues are expected to induce detrimental effects on the crop’s ecological fitness and reduce crop productivity which will threaten food security [10]. According to Hussain et al. [11], it is expected that by 2050 wheat, rice, and maize yield in Pakistan would be reduced by 11%, 0.8%, and 3.3%. Hence, the understanding of drought impacts on plant growth and development is critical in crop production sciences to sustain crop productivity for future generations by improving the drought tolerance of plants.
The most common method to examine the plant responses to drought stress is the application of controlled water deficit conditions in the laboratory or glasshouse [3]. This method of applying controlled moisture stress dates back to more than the last 50 years, while there is limited information available on the agreement of best practice to induce water deficit in drought studies [12]. The most commonly used practices to generate moisture stress are; withholding irrigation to pots [3], using osmotically-active substances such as polyethylene glycol (PEG) to soil or solution [13], attaching vacuum pumps to pots [14], reducing water pressure in tubes [15], and adding calculated precise amounts of water to maintain different field capacity levels in pots [16]. Among these practices, the creation of osmotic potential using different osmotic substances is considered the more feasible approach to study the drought effects on germination and stress tolerance. Since PEG has a higher molecular weight among the osmotic substances used and cannot pass the plant cell wall (especially PEG-6000). Hence, it is widely used to regulate osmotic potential in germination and drought studies [17].
Sesame (Sesamum indicum L.) is one of the major conventional oilseed crops, particularly grown in tropical and subtropical areas of Asia, Africa, and South America [18]. According to FAO [19], 6,549,725 tonnes of sesame was produced globally on an area of 12,821,752 hectares in 2019, and the top three leading producers were India, Sudan, and China. Sesame seeds have high amounts of oil ranging from 50%–60% depending on cultivar and environmental conditions [20]. Its oil contains the important unsaturated fatty acids, i.e., oleic and linolenic acids [21], and antioxidants, i.e., sesamin, sesamol, and sesamolin [22], which greatly improves the oil quality. Additionally, sesame seeds contain minerals, i.e., calcium, iron, zinc, and iodine [23], and vitamins, i.e., E, B6, thiamin, riboflavin, niacin, and folic acid [24], which are highly nutritious for the human diet.
Several factors such as plant species, cultivar, growth stage, and duration or intensity of stress affect the plant responses to drought [25]. However, seed germination and seedling establishment are the most critical stages for plants’ life cycle and drought stress at these stages is one of the major limiting factors that restrict the successful crop establishment [26]. Drought significantly affects the germination indices and seedling characteristics and severe drought at these stages even leads to total crop failure [27]. Hence, understanding the plant responses to drought is important for the determination of germination and crop establishment under limited water availability. Although sesame is an important oilseed crop, there is very limited quantitative information about the sesame responses to drought stress during seed germination and seedling establishment. Thus, a comprehensive study was required to exploit the sesame potential to drought stress using germination indices, seedling characteristics, and drought tolerance indices.
Measures of drought responses based on germination indices and seedling traits under controlled and stress conditions have been previously used by researchers to quantify the drought tolerance in different plant species [28]. Recently, stress tolerance and drought susceptibility indices were reported to be the most useful indicators to measure the seed germination and seedling development responses to the drought [29]. Thus, drought responses can effectively be studied using the germination indices, seeding characteristics, and drought indices in combination. However, there is insufficient knowledge about the potential use of these indices to study the drought responses in sesame.
Therefore, this experiment was conducted to investigate the effects of PEG-induced drought on germination indices and seedling characteristics of sesame to verify how drought may limit crop establishment during the initial plant growth stages. Moreover, drought tolerance indices were also determined under stress conditions to quantify the drought tolerance of sesame.
2.1 Plant Material and Treatments
This experiment was carried out during the summer season of 2018 in the seed testing laboratory (33°64′9′′ N, 73°08′19′′ E) of the Agronomy Department, PMAS-Arid Agriculture University Rawalpindi, Pakistan. The experiment was performed in a controlled growth chamber at 29°C/26°C for 16 h of light and 8 h of dark periods. Seeds of two sesame cultivars (TS-5 and TS-3) were obtained from Ayub Agriculture Research Institute (AARI), Faisalabad. Before sowing sesame seeds were surface sterilized using ethanol (75%) for 10-min and then thoroughly rinsed with distilled water. We used eighteen Petri plates and fifteen healthy sterilized seeds were placed on blotter paper in each Petri plate. For the creation of drought conditions, PEG-6000 was used. Three drought treatments consisting of control, 10% PEG, and 20% PEG with corresponding osmotic potentials of 0.0, −0.2, and −0.6 Mpa were used in the experiment. The osmotic potential was determined using the equation given by Michel et al. [30]. Each Petri plate was served with a corresponding solution (5 ml) of either control, 10% PEG or 20% PEG drought stress treatment.
2.2 Measurements of Germination Indices
For germination evaluation, an incubation period of 4 days was set. Germination was recorded on each day from the 4th day of the experiment and the seeds were considered germinated having a radicle length of 2 mm. After the germination evaluation, germination indices viz. germination percentage (GP), Coefficient of variation of germination time (CVt), germination index (GI), and seedling vigor index (SVI) were determined according to the International Seed Testing Association (ISTA) protocols [31]. The description of germination indices calculations is given in Table 1.
2.3 Measurements of Seedling Traits
Measurements of seedling traits were done at the end of the germination evaluations. These measurements were taken on the 7th day of the experiment. The seedlings traits viz. root length (RL), shoot length (SL), root shoot ratio (RSR), root fresh weight (RFW), shoot fresh weight (SFW), root dry weight (RDW), and shoot dry weight (SDW) were recorded. The description of methods used for seedling trait measurements is given in Table 1.
2.4 Measurements of Drought Tolerance Indices
Drought tolerance indices (DTIs) were calculated at the end of the experiment, after the determination of germination indices and seedling traits. DTIs were calculated for both the germination indices and seedling traits, such as GP, RL, SL, TSL (total seedling length), RFW, SFW, TFW (total fresh weight), RDW, SDW, and TDW (total dry weight). The description of DTIs calculations is given in Table 1.
Statistical analysis was carried out using Statistix 8.1 Software. Significant differences among cultivars and drought treatments were determined by using a two-way [ANOVA (Analysis of Variance)] method combined with the [LSD (Least Significant Difference)] test. The significance of the results was evaluated at a 5% probability level. Moreover, linear regression analysis was performed to see the relationship among studied treatments and parameters.
3.1 Effects of Drought Stress on Germination Indices
PEG-induced drought stress significantly (P < 0.05) affected the number of germinated seeds and studied germination indices of sesame cultivars (Table 2). Overall, results revealed that the number of germinated seeds and drought indices was negatively affected by the increasing percentage of PEG in the drought treatments (Table 2 and Fig. 1). The maximum number of germinated seeds (5.00, 7.17, 7.67, and 8.83) were recorded for the control, whereas the minimum number of germinated seeds (0.83, 3.50, 4.00, and 4.50) were observed in the 20% PEG treatment, after 4, 5, 6 and 7 days of germination, respectively. Overall, the percentage of the number of seeds germinated was reduced by 40% and 83% after 4 days of germination, 26% and 51% after 5 days of germination, 39% and 48% after 6 days of germination, and 24% and 49% after 7 days of germination under 10% PEG and 20% PEG treatments, respectively.
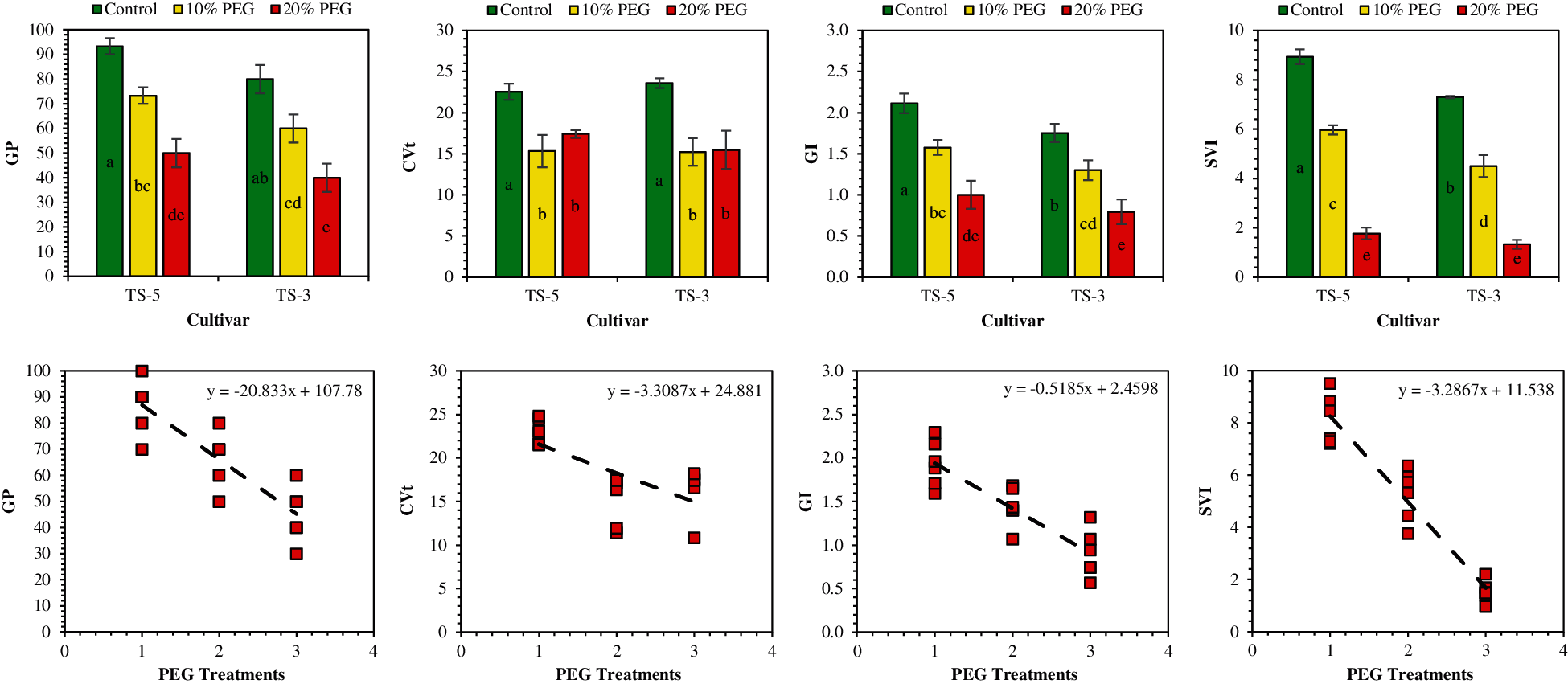
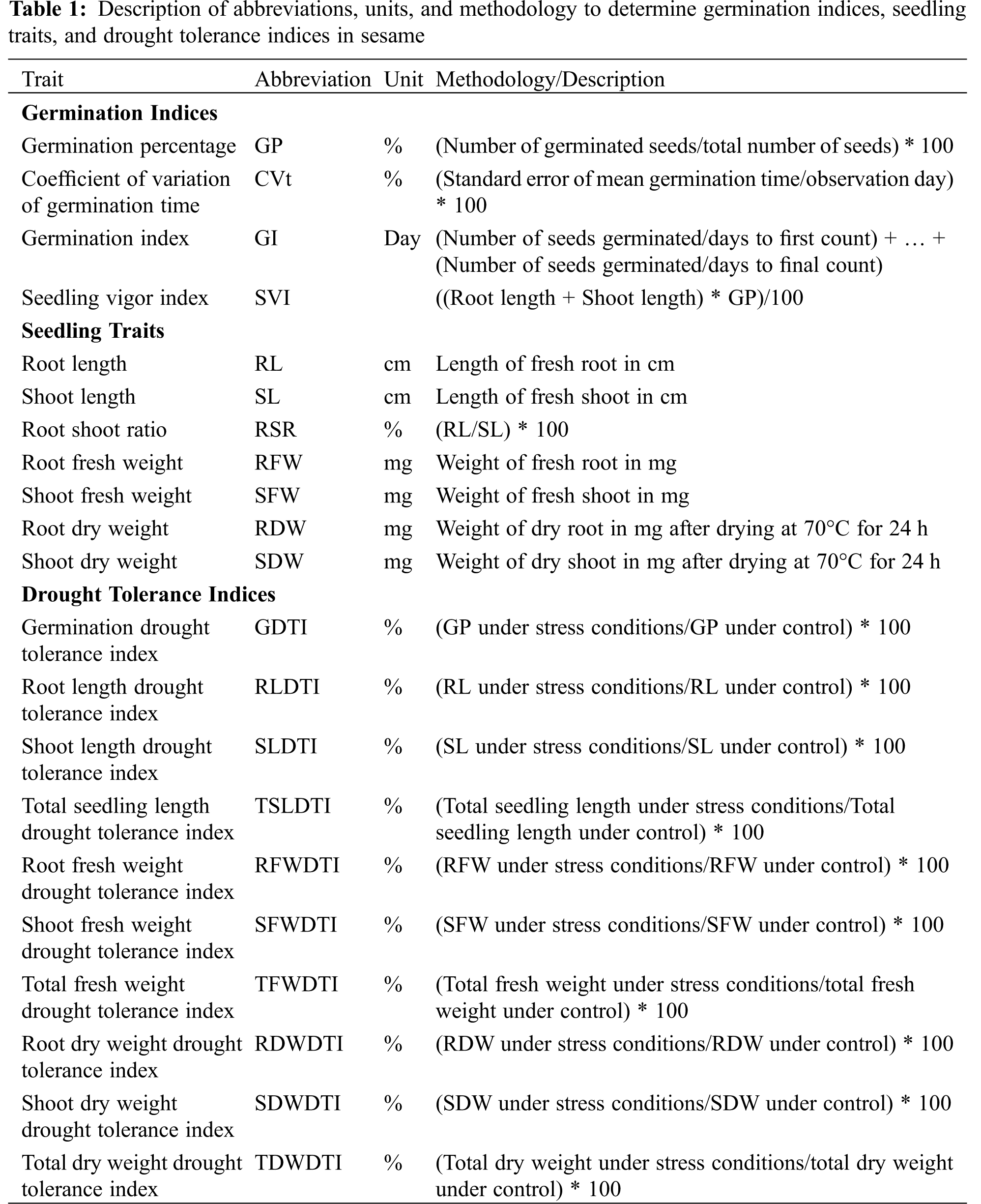
Figure 1: Effects of PEG on germination indices and relationship between PEG levels and germination indices. The GP, CVt, GI, and SVI represent the germination percentage, coefficient of variation of germination time, germination index, and seedling vigor index, respectively. Means are averaged over three replicates and means with a different letter varied significantly at P < 0.05
Germination indices were also affected by the PEG-induced drought conditions (Fig. 1). Germination percentage was recorded at 87%, 67% and 45% under control, 10% PEG and 20% PEG treatments, respectively. As compared to control, germination percentage was reduced by 23% under 10% PEG, and 48% under 20% PEG treatments. The coefficient of variation of germination time was reduced by 29% under 10% PEG treatment, and 34% under 20% PEG treatment, in comparison with control (Table 2). However, it suggested that at higher concentration, reduced number of germinated seeds also reduced the variation in the number of germinated seeds which ultimately decreased the value of the coefficient of variations (Fig. 1). For germination index, 25% and 53% reduction was noticed under 10% PEG and 20% PEG treatments, respectively, as compared with control. Similarly, seedling vigor index was also reduced in comparison with control by 36% under 10% PEG, and 81% under 20% PEG treatments.
3.2 Effects of Drought Stress on Seedling Traits
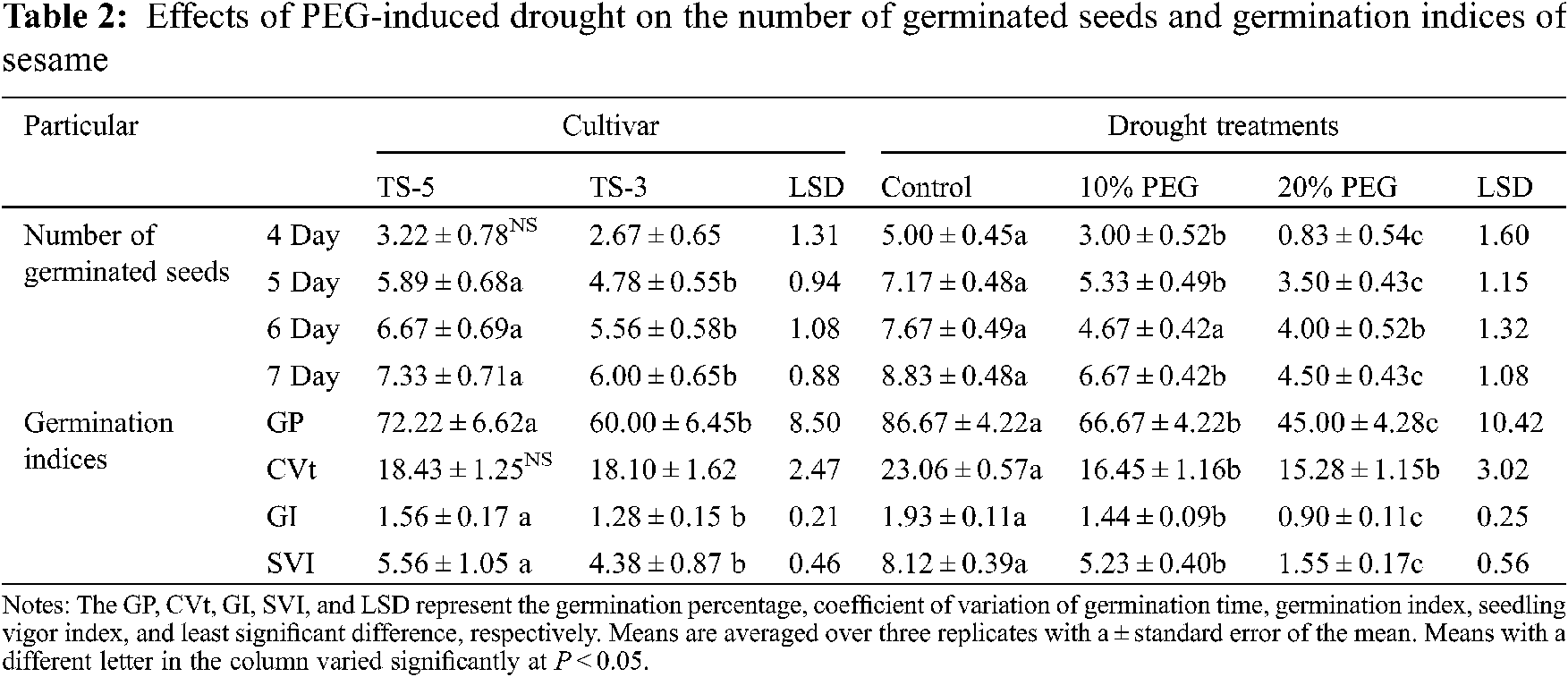
Seedling traits of the sesame were significantly impacted by the drought stress induced by the PEG application (Figs. 2 and 3). The maximum root length (4.5 cm) was measured in control, which was followed by the 3.2 cm root length in 10% PEG, and 1.1 cm in 20% PEG treatment. Similarly, maximum shoot length (4.9 cm) was recorded in control and minimum (2.3 cm) in 20% PEG treatment. Overall, drought stress reduced the root length by 28% and 76% while the shoot length by 6% and 53% under 10% PEG and 20% PEG treatments, respectively. Furthermore, compared with control, the root shoot ratio was also reduced by 24% under 10% PEG treatment and 49% under 20% PEG treatment (Table 3).
Figure 2: Effects of PEG on seedling traits and relationship between PEG levels and seedling traits. The RL, RFW, and RDW represent the root length, root fresh weight, and root dry weight, respectively. Means are averaged over three replicates and means with a different letter varied significantly at P < 0.05
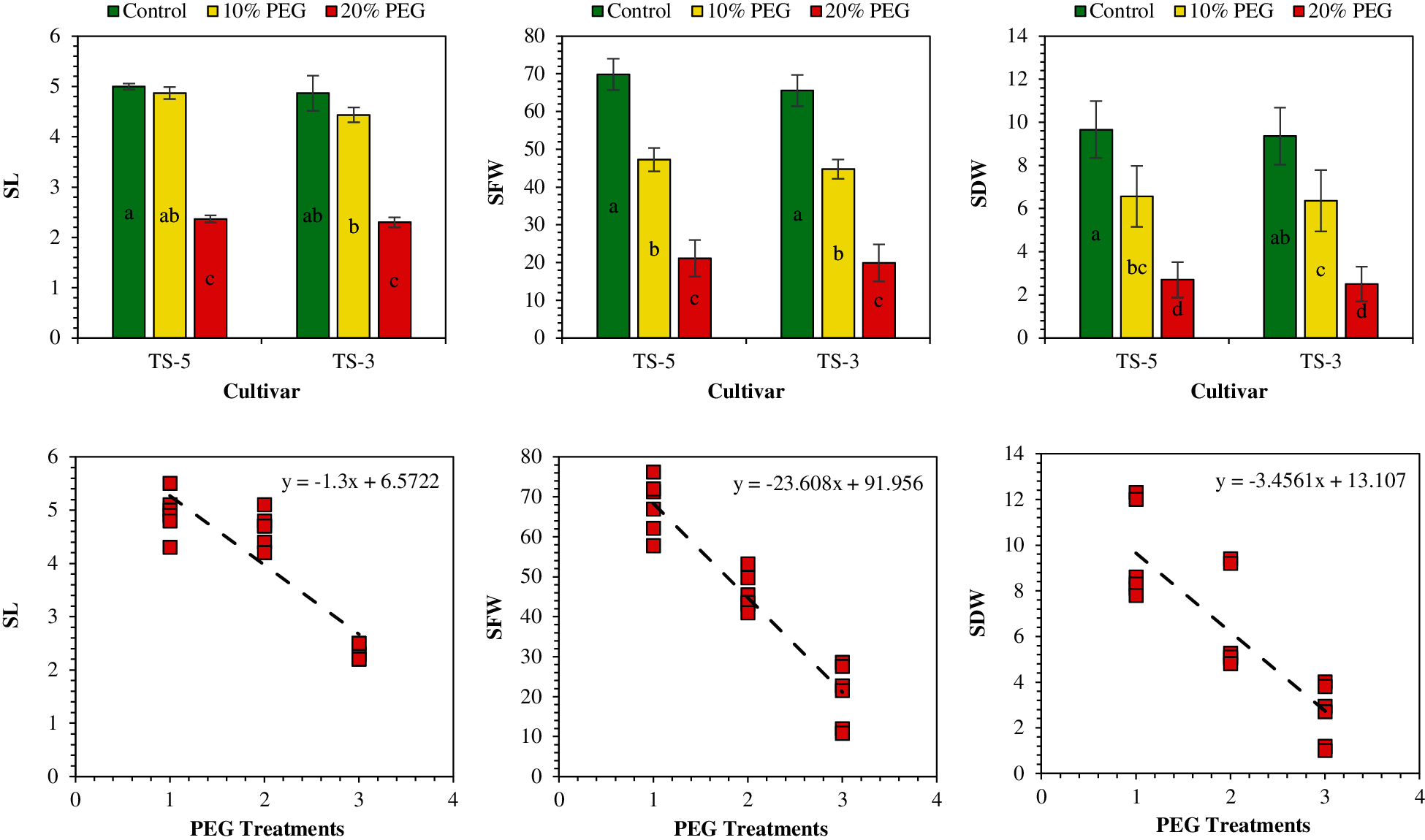
Figure 3: Effects of PEG on seedling traits and relationship between PEG levels and seedling traits. The SL, SFW, and SDW represent the shoot length, shoot fresh weight, and shoot dry weight, respectively. Means are averaged over three replicates and means with a different letter varied significantly at P < 0.05
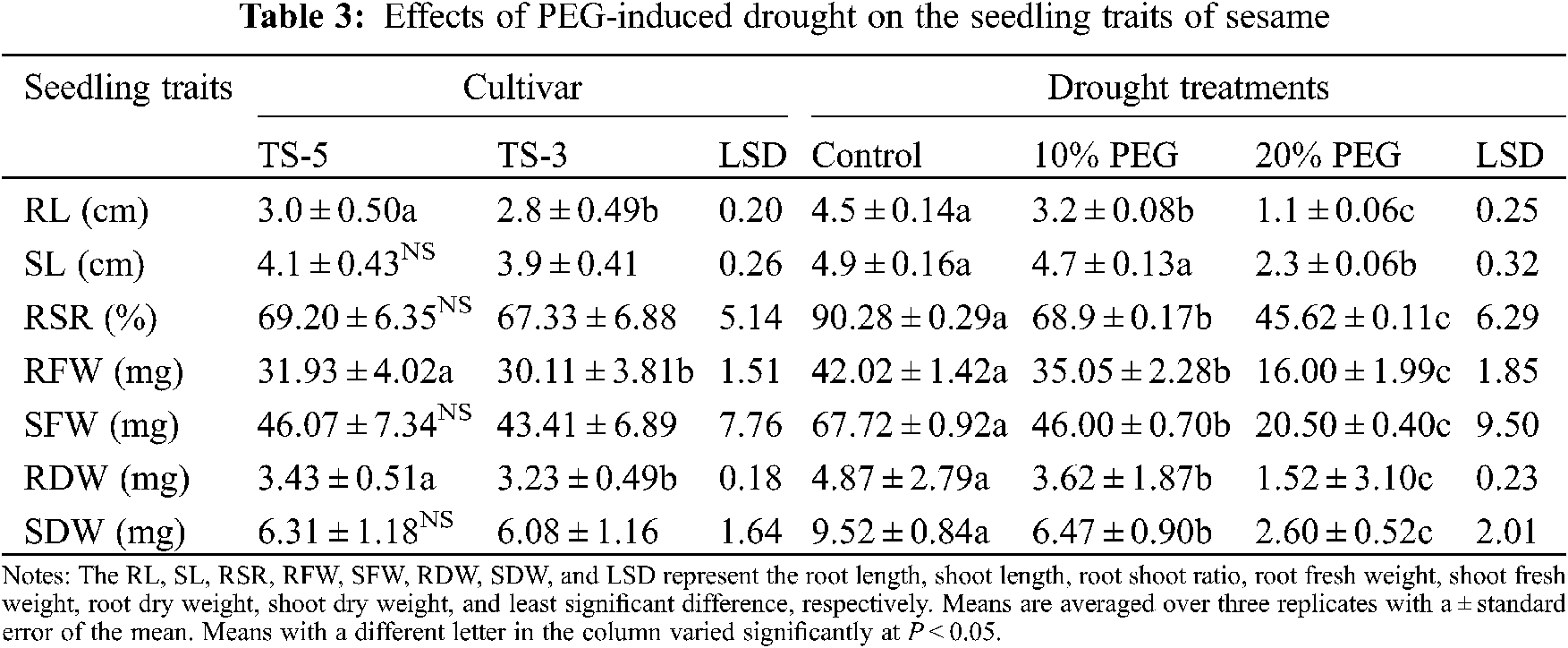
The fresh and dry weight of sesame seedlings was significantly (P < 0.05) influenced under drought caused by the PEG treatments. The highest fresh weight of root (42.02 mg) and shoot (67.72 mg) was obtained in control while the lowest fresh weight of root (16.00 mg) and shoot (20.50 mg) was measured under 20% PEG treatment. Similarly, the highest dry weight of root (4.87 mg) and shoot (9.52 mg) was attained in control whereas the lowest dry weight of root (1.52 mg) and shoot (2.60 mg) was recorded under 20% PEG treatment. On average, compared to control, the maximum reduction in fresh weight of root and shoot by 62% and 70%, and dry weight of root and shoot by 69% and 73% was observed under 20% PEG treatment (Table 3).
3.3 Effects of Drought Stress on Drought Tolerance Indices
The PEG treatments adversely affected the germination indices and seedling traits that ultimately impacted the drought tolerance of sesame seedlings (Table 4). Results of this experiment suggested that the drought tolerance of sesame seedlings is significantly reduced under drought conditions. The mean GDTI showed a successive decrease from 25% under 10% PEG to a 48% under 20% PEG treatment. The RLDTI and SLDTI were reduced by 28% and 5% under 10% PEG and by 76% and 53% under 20% PEG treatment. For TSLDTI, a significant reduction from 16% to 64% was recorded for 10% PEG and 20% PEG treatments, respectively (Fig. 4).
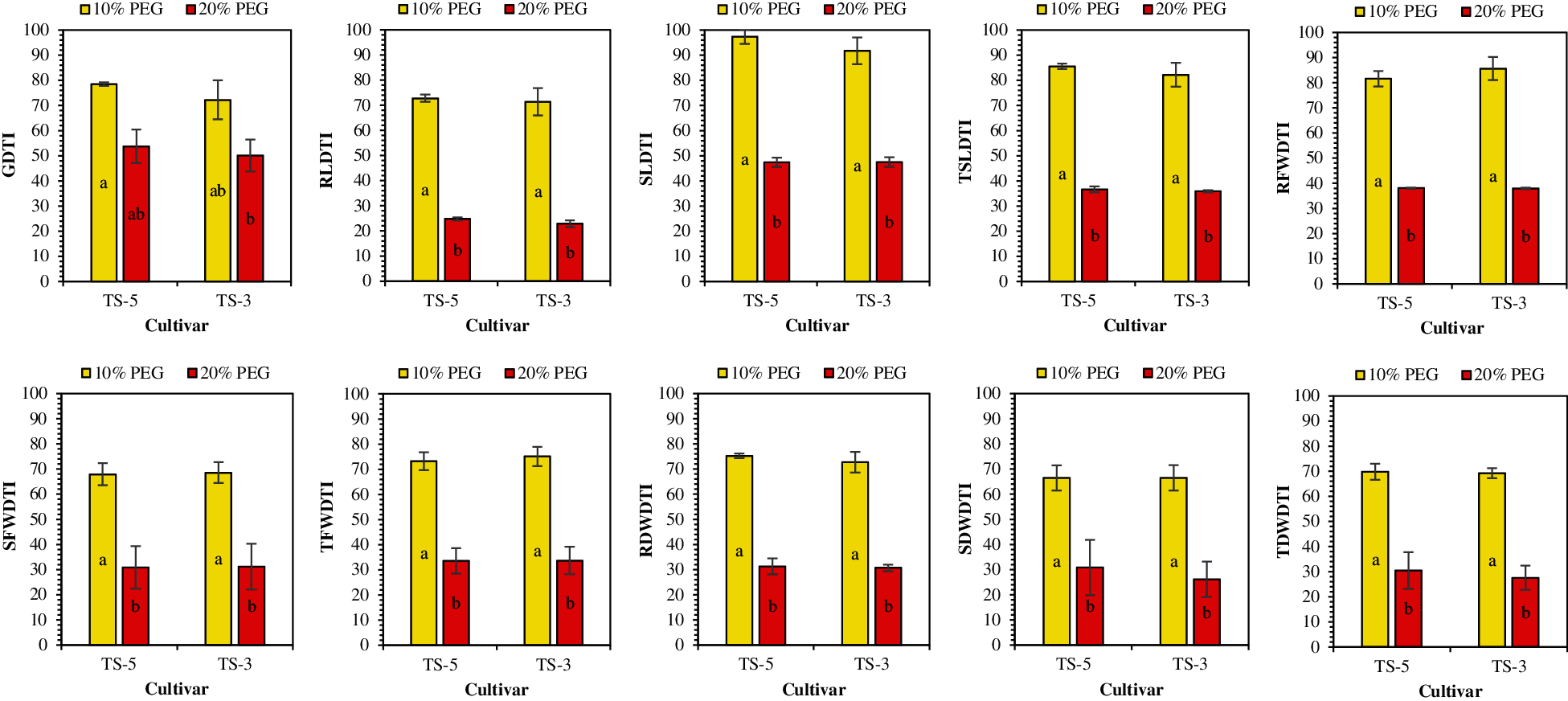
Figure 4: Effects of PEG on drought tolerance indices under different PEG levels, as compared with control (considering 100%). The GDTI, RLDTI, SLDTI, TSLDTI, RFWDTI, SFWDTI, TFWDTI, RDWDTI, SDWDTI, and TDWDTI represent the germination drought tolerance index, root length drought tolerance index, shoot length drought tolerance index, total shoot length drought tolerance index, root fresh weight drought tolerance index, shoot fresh weight drought tolerance index, total fresh weight drought tolerance index, root dry weight drought tolerance index, shoot dry weight drought tolerance index, and total dry weight drought tolerance index, respectively. Means are averaged over three replicates and means with a different letter varied significantly at P < 0.05
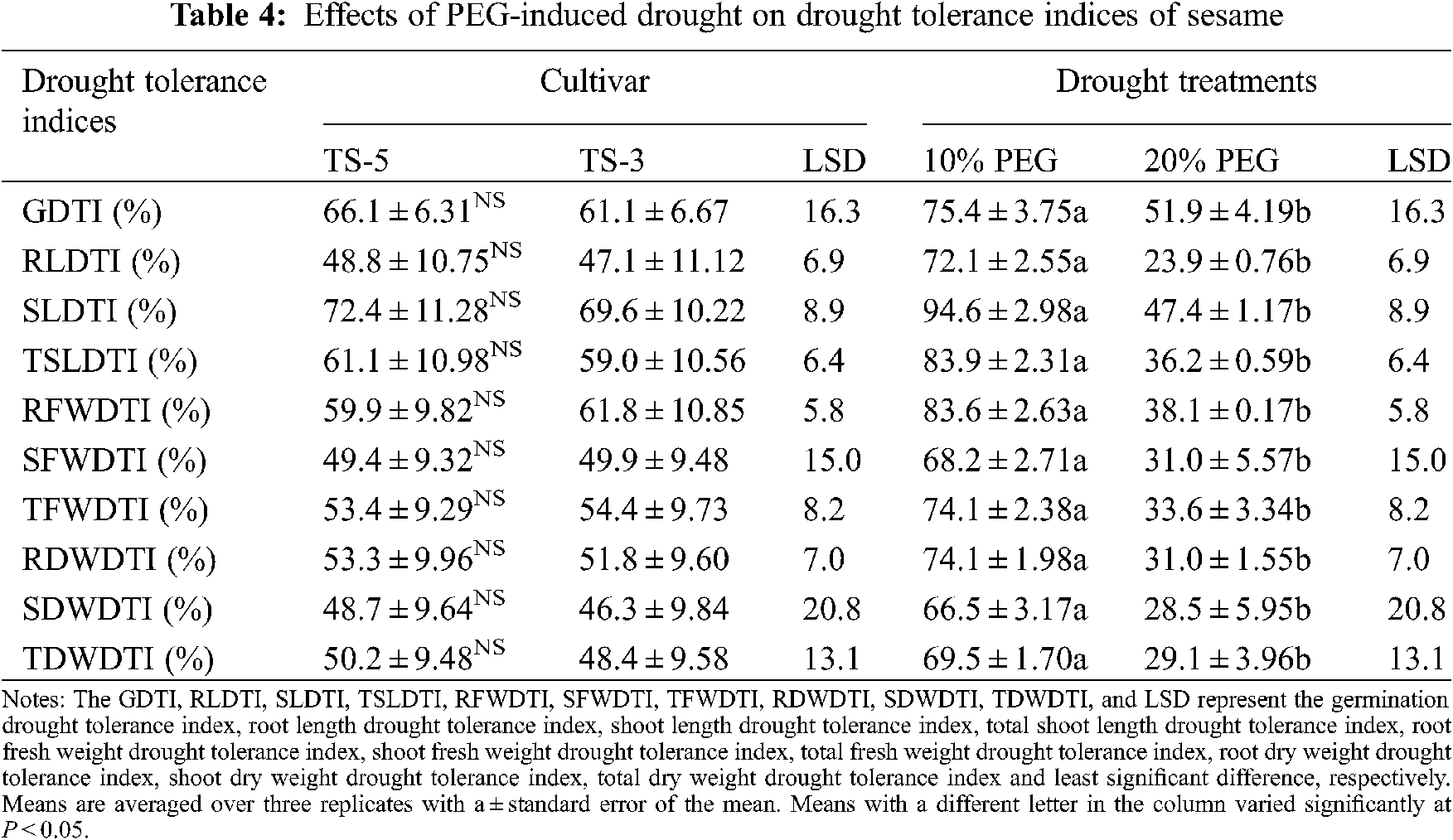
Drought tolerance for fresh weight and dry weight production of sesame was also influenced by the drought treatments. For fresh weight drought tolerance indices, RFWDTI and SFWDTI showed the 16% and 32% reduction under 10% PEG while 62% and 69% under 20% PEG treatment. However, TFWDTI has decreased by 26% and 66% under 10% PEG and 20% PEG treatments, respectively. Likewise, drought tolerance of sesame for dry weight of seedling was also affected under PEG application. The RDWDTI was declined by 26% and 69% whereas SDWDTI decreased by 34% and 72% under 10% PEG and 20% PEG treatments, respectively. Furthermore, TDWDTI decreased by 31% under 10% PEG and 71% under 20% PEG treatment (Fig. 4).
Drought is a potent abiotic stress that causes significant reductions in plant growth which results in substantial yield losses among different crops [32]. Several studies showed that the PEG has been widely used to simulate the drought impacts, especially on seed germination [17]. Germination is the critical period during the life cycle of plants, and it is controlled by several factors especially hormones, light, temperature, and moisture availability [33]. However, water availability is the most limiting factor in the germination of seeds and the growth of plants. During the period of germination, drought adversely affects the metabolic processes which reduce seed germination and ultimately delay the seedling establishment [34]. Low water potential under drought conditions is the most dominant factor inhibiting the process of seed germination [35]. Furthermore, a significant decrease in the germination characteristics was observed when the seeds were exposed to drought conditions. Significant reduction in germination percentage and germination indices was recorded under the drought condition that could be due to the reduced metabolic processes viz. synthesis of hydrolytic enzymes, hydrolysis of food material, radicle protrusion, and cell division and elongation [36–38]. Similar findings were reported in several studies where PEG-induced drought reduced the germination characteristics of different plants [39–42]. Furthermore, the seedling vigor index was also affected by different PEG concentrations. The possible reason for this decrease was dryness and reduced osmotic potential which affected the germination percentage and length of radicle and plumule [43]. Results of this study were consistent with the earlier reported trends of germination indices under drought conditions caused by PEG [17].
The presence of higher PEG concentrations, during the seedling growth, inhibits the developmental processes and negatively affects the seedling traits. At all the studied stress levels, root length, shoot length, root shoot ratio, root and shoot fresh and dry weight were decreased due to drought exposure. Similar observations were reported in earlier investigations by Radhouane [44], where the seedling traits were adversely affected under drought conditions due to reduced osmotic potential. Plants’ root system plays a significant role in water and nutrient uptake as well as strongly associated with drought tolerance [45]. Several studies suggested that a well-established root system shows a strong drought resistance in plants [46–48]. However, changes in seedling characteristics such as root length, root biomass, root volume, and root surface areas greatly depend on plant type, drought intensity, and duration [49,50]. In this experiment, root and shoot length showed a significant reduction, suggesting that under 10% PEG and 20% PEG, sesame seedling growth was inhibited. The possible reason for this reduction in root and shoot length might be due to the impeded cell division and elongation resulting in some sort of tuberization and lignification which ultimately slowed down the plant growth process under the stressed environment [51]. Likewise, the fresh and dry weight of root and shoot of sesame seedling progressively decreased with increasing PEG concentration. Based on our results, maximum fresh and dry weight was obtained at control and minimum at 20% PEG treatment (Table 3). Results of this experiment were in line with earlier findings where the fresh and dry weight of seedlings were reduced due to the declined water potential, however, the extent of reduction was greater in shoot than the roots [52,53]. Similar results were reported for other crops under water stress conditions and seedlings had reduced root and shoot length as well as fresh and dry biomass [17,41]. This decrease could be due to the damage caused to meristem cells of root and shoot by drought which disrupted the cell division and elongation process. Moreover, another possible reason might be that lowered water absorption by cells under drought conditions decreased the turgor pressure of cells which accelerated the growth retardation [3].
In this experiment, the drought tolerance of sesame was significantly reduced under the studied drought treatments. Furthermore, we also noted that different levels of drought stress significantly reduced the drought tolerance of seedling traits. However, the highest reduction in drought tolerance for all the studied parameters was observed under 20% PEG application. The reason for this decline may be ascribed to the reduced water potential which negatively impacts the germination process and seedling growth [29]. Another possible reason might be due to the reduced activity of enzymes involved in the germination and seedling establishment process due to drought stress. Muscolo et al. [54] reported that under PEG application, the activity of enzymes involved in the germination process (viz. α-amylase, β-amylase, and α-glucosidase) was reduced. Luo et al. [55] summarized in their work that stress enhances the reactive oxygen species accumulation through transcription of NADPH oxidase genes which leads to the mediation in plant developmental processes. Similarly, drought and ABA crosstalk during diverse biological pathways, also including seed germination and seedling establishment [56]. Our results further confirmed that PEG application adversely affects the drought tolerance characteristics of different plants [21,35,41]. Thus, these indices could be considered as the best criterion to evaluate the drought stress tolerance in different crops.
This study provided evidence that drought can significantly impact the seed germination process, seedling establishment, and drought tolerance of sesame that may result in a poor crop stand or even lead to crop failure under extreme drought conditions. Moreover, this study also revealed that germination and drought tolerance indices can be considered as best indicators to study the drought effects on plants. Therefore, it is concluded that these indices could be used for the evaluation of drought tolerance in different crops. To the best of our knowledge, this study is the first to provide comprehensive information on germination indices, seedling traits, and drought tolerance indices under the drought conditions in sesame crop.
Acknowledgement: All the authors are thankful to PMAS-Arid Agriculture University Rawalpindi, Pakistan for providing the resources and facilities to carry out this research.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Suzuki, N., Rivero, R. M., Shulaev, V., Blumwald, E., Mittler, R. (2014). Abiotic and biotic stress combinations. New Phytologist, 203(1), 32–43. DOI 10.1111/nph.12797. [Google Scholar] [CrossRef]
2. Marchin, R. M., Ossola, A., Leishman, M. R., Ellsworth, D. S. (2020). A simple method for simulating drought effects on plants. Frontiers in Plant Science, 10, 1715. DOI 10.3389/fpls.2019.01715. [Google Scholar] [CrossRef]
3. Hellal, F., El-Shabrawi, H., Abd El-Hady, M., Khatab, I., El-Sayed, S. et al. (2018). Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. Journal of Genetic Engineering and Biotechnology, 16(1), 203–212. DOI 10.1016/j.jgeb.2017.10.009. [Google Scholar] [CrossRef]
4. Naumann, G., Alfieri, L., Wyser, K., Mentaschi, L., Betts, R. et al. (2018). Global changes in drought conditions under different levels of warming. Geophysical Research Letters, 45(7), 3285–3296. DOI 10.1002/2017GL076521. [Google Scholar] [CrossRef]
5. Lambrecht, S. C., Gujral, A. K., Renshaw, L. J., Rosengreen, L. T. (2020). Evolutionary and plastic changes in a native annual plant after a historic drought. Ecology and Evolution, 10(11), 4570–4582. DOI 10.1002/ece3.6156. [Google Scholar] [CrossRef]
6. Freund, M., Henley, B. J., Karoly, D. J., Allen, K. J., Baker, P. J. (2017). Multi-century cool-and warm-season rainfall reconstructions for Australia’s major climatic regions. Climate of the Past, 13(12), 1751–1770. DOI 10.5194/cp-13-1751-2017. [Google Scholar] [CrossRef]
7. Chaudhry, Q. U. Z., Mahmood, A., Rasul, G., Afzaal, M. (2009). Climate change indicators of Pakistan. Pakistan Meterological Department. http://www.pmd.gov.pk/CC%20Indicators.pdf,1-42. [Google Scholar]
8. Bakhsh, A., Shahid, M. A. (2018). Water: Issues and remedies. In: Khan I. A., Khan M. S. (Eds.Developing sustainable agriculture in Pakistan, pp. 59. FL, USA: CRC Press. [Google Scholar]
9. Hussain, M., Mumtaz, S. (2014). Climate change and managing water crisis: Pakistan’s perspective. Reviews on Environmental Health, 29(1–2), 71–77. DOI 10.1515/reveh-2014-0020. [Google Scholar] [CrossRef]
10. Ferguson, J. N. (2019). Climate change and abiotic stress mechanisms in plants. Emerging Topics in Life Sciences, 3(2), 165–181. DOI 10.1042/ETLS20180105. [Google Scholar] [CrossRef]
11. Hussain, M., Butt, A. R., Uzma, F., Ahmed, R., Irshad, S. et al. (2020). A comprehensive review of climate change impacts, adaptation, and mitigation on environmental and natural calamities in Pakistan. Environmental Monitoring and Assessment, 192(1), 1–20. DOI 10.1007/s10661-019-7956-4. [Google Scholar] [CrossRef]
12. Munns, R., James, R. A., Sirault, X. R., Furbank, R. T., Jones, H. G. (2010). New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. Journal of Experimental Botany, 61(13), 3499–3507. DOI 10.1093/jxb/erq199. [Google Scholar] [CrossRef]
13. Zur, B. (1966). Osmotic control of the matric soil-water potential: I. Soil-water system. Soil Science, 102(6), 394–398. DOI 10.1097/00010694-196612000-00007. [Google Scholar] [CrossRef]
14. Bunce, J. A., Nasyrov, M. (2012). A new method of applying a controlled soil water stress, and its effect on the growth of cotton and soybean seedlings at ambient and elevated carbon dioxide. Environmental and Experimental Botany, 77, 165–169. DOI 10.1016/j.envexpbot.2011.11.015. [Google Scholar] [CrossRef]
15. Steinberg, S., Henninger, D. (1997). Response of the water status of soybean to changes in soil water potentials controlled by the water pressure in microporous tubes. Plant, Cell & Environment, 20(12), 1506–1516. DOI 10.1046/j.1365-3040.1997.d01-46.x. [Google Scholar] [CrossRef]
16. Earl, H. J. (2003). A precise gravimetric method for simulating drought stress in pot experiments. Crop Science, 43(5), 1868–1873. DOI 10.2135/cropsci2003.1868. [Google Scholar] [CrossRef]
17. Badr, A., El-Shazly, H. H., Tarawneh, R. A., Börner, A. (2020). Screening for drought tolerance in maize (Zea mays L.) germplasm using germination and seedling traits under simulated drought conditions. Plants, 9(5), 565. DOI 10.3390/plants9050565. [Google Scholar] [CrossRef]
18. Mehmood, M. Z., Afzal, O., Ahmed, M., Qadir, G., Kheir, A. M. et al. (2021). Can sulphur improve the nutrient uptake, partitioning, and seed yield of sesame? Arabian Journal of Geosciences, 14(10). DOI 10.1007/s12517-021-07229-6. [Google Scholar] [CrossRef]
19. FAO (2019). Food and agriculture organization statistical databases (FAOSTAT). http://www.fao.org/faostat/en/#data/QC. [Google Scholar]
20. Wei, X., Liu, K., Zhang, Y., Feng, Q., Wang, L. et al. (2015). Genetic discovery for oil production and quality in sesame. Nature Communications, 6(1), 1–10. DOI 10.1038/ncomms9609. [Google Scholar] [CrossRef]
21. Raza, M. A., Feng, L. Y., Iqbal, N., Manaf, A., Khalid, M. H. B. et al. (2018). Effect of sulphur application on photosynthesis and biomass accumulation of sesame varieties under rainfed conditions. Agronomy, 8(8), 149. DOI 10.3390/agronomy8080149. [Google Scholar] [CrossRef]
22. Amoo, S., Okorogbona, A., Du Plooy, C., Venter, S. (2017). Sesamum indicum. In: Medicinal spices and vegetables from Africa. pp. 549–579. New York: Elsevier. [Google Scholar]
23. Pickersgill, B., Bedigian, D. (2011). Sesame: The genus sesamum. Medicinal and aromatic plants-industrial profiles. In: Experimental agriculture, vol. 47, no. 4, pp. 733–734. Fl, USA: CRC Press. DOI 10.1017/S0014479711000627. [Google Scholar] [CrossRef]
24. Tripathy, S. K., Kar, J., Sahu, D. (2019). Advances in sesame (Sesamum indicum L.) breeding. In: Al-Khayri, J. M., Jain, S. M., Johnson, D. V. (Eds.Advances in plant breeding strategies: Industrial and food crops, vol. 6, pp. 577–635. Springer. [Google Scholar]
25. Zuffo, A. M., Steiner, F., Sousa, T. D. O., Aguilera, J. G., Teodoro, P. E. et al. (2020). How does water and salt stress affect the germination and initial growth of Brazilian soya bean cultivars? Journal of Agronomy and Crop Science, 206(6), 837–850. DOI 10.1111/jac.12434. [Google Scholar] [CrossRef]
26. Pushpavalli, R., Berger, J. D., Turner, N. C., Siddique, K. H., Colmer, T. D. et al. (2020). Cross-tolerance for drought, heat and salinity stresses in chickpea (Cicer arietinum L.). Journal of Agronomy and Crop Science, 206(3), 405–419. DOI 10.1111/jac.12393. [Google Scholar] [CrossRef]
27. Petrović, G., Jovičić, D., Nikolić, Z., Tamindžić, G., Ignjatov, M. et al. (2016). Comparative study of drought and salt stress effects on germination and seedling growth of pea. Genetika, 48(1), 373–381. DOI 10.2298/GENSR1601373P. [Google Scholar] [CrossRef]
28. Patanè, C., Saita, A., Sortino, O. (2013). Comparative effects of salt and water stress on seed germination and early embryo growth in two cultivars of sweet sorghum. Journal of Agronomy and Crop Science, 199(1), 30–37. DOI 10.1111/j.1439-037X.2012.00531.x. [Google Scholar] [CrossRef]
29. Grzesiak, S., Hordyńska, N., Szczyrek, P., Grzesiak, M. T., Noga, A. et al. (2019). Variation among wheat (Triticum aesativum L.) genotypes in response to the drought stress: I–Selection approaches. Journal of Plant Interactions, 14(1), 30–44. DOI 10.1080/17429145.2018.1550817. [Google Scholar] [CrossRef]
30. Michel, B. E., Kaufmann, M. R. (1973). The osmotic potential of polyethylene glycol 6000. Plant Physiology, 51(5), 914–916. DOI 10.1104/pp.51.5.914. [Google Scholar] [CrossRef]
31. ISTA (2019). International rules for seed testing. International Seed Testing Association, Sapporo, Japan. [Google Scholar]
32. Khan, A., Tan, D. K. Y., Afridi, M. Z., Luo, H., Tung, S. A. et al. (2017). Nitrogen fertility and abiotic stresses management in cotton crop: A review. Environmental Science and Pollution Research, 24(17), 14551–14566. DOI 10.1007/s11356-017-8920-x. [Google Scholar] [CrossRef]
33. Finch-Savage, W., Phelps, K., Steckel, J., Whalley, W., Rowse, H. (2001). Seed reserve-dependent growth responses to temperature and water potential in carrot (Daucus carota L.). Journal of Experimental Botany, 52(364), 2187–2197. DOI 10.1093/jexbot/52.364.2187. [Google Scholar] [CrossRef]
34. Bayoumi, T., Eid, M. H., Metwali, E. (2008). Application of physiological and biochemical indices as a screening technique for drought tolerance in wheat genotypes. African Journal of Biotechnology, 7(142341–2352. [Google Scholar]
35. Wang, J. G., Chen, G. C., Zhang, C. L. (2002). The effects of water stress on soluble protein content, the activity of SOD, POD and CAT of two ecotypes of reeds (Phragmites communis). Acta Botanica Boreali-Occidentalia Sinica, 22(3), 561–565. [Google Scholar]
36. Bewley, J. D. (1997). Seed germination and dormancy. The Plant Cell, 9(7), 1055. DOI 10.1105/tpc.9.7.1055. [Google Scholar] [CrossRef]
37. Bewley, J. D., Bradford, K., Hilhorst, H. (2012). Seeds: Physiology of development, germination and dormancy. New York, NY: Springer. DOI 10.1007/978-1-4614-4693-4. [Google Scholar] [CrossRef]
38. Ali, A. S., Elozeiri, A. A. (2017). Metabolic processes during seed germination, advances in seed biology. Jose C. Jimenez-Lopez, IntechOpen. DOI 10.5772/intechopen.70653. [Google Scholar] [CrossRef]
39. Salehi, F. (2010). The Effect of Drought Stress on Seedling Germination and Growth in 8 Genotypes of Bean. 11th Congress on Iranian Agronomy and Plant Breeding, Shahid Beheshti University. Gorgan Agriculture and Natural Resources, Gorgan, Iran. [Google Scholar]
40. Kafi, M., Nezami, A., Hoseyni, H., Masoomi, A. (2005). Physiological effects of drought stress by polyethylene glycol on germination of lentil (Lens culinaris medik.) genotypes. Iranian Journal of Field Crops Research, 3(1), 69–80. [Google Scholar]
41. El-Harfi, M., Hanine, H., Rizki, H., Latrache, H., Nabloussi, A. (2016). Effect of drought and salt stresses on germination and early seedling growth of different color-seeds of sesame (Sesamum indicum). International Journal of Agriculture & Biology, 18(61088–1094. [Google Scholar]
42. Boureima, S., Eyletters, M., Diouf, M., Diop, T., van Damme, P. (2011). Sensitivity of seed germination and seedling radicle growth to drought stress in sesame Sesamum indicum L. Research Journal of Environmental Sciences, 5(6), 557–564. DOI 10.3923/rjes.2011.557.564. [Google Scholar] [CrossRef]
43. Duman, I. (2006). Effects of seed priming with PEG or K3PO4 on germination and seedling growth in lettuce. Pakistan Journal of Biological Sciences, 9(5), 923–928. DOI 10.3923/pjbs.2006.923.928. [Google Scholar] [CrossRef]
44. Radhouane, L. (2013). Response of Tunisian autochthonous pearl millet (Pennisetum glaucum (L.) R. Br.) to drought stress induced by polyethylene glycol (PEG) 6000. International Research Journal of Genetic Engineering, 1(4), 51–53. [Google Scholar]
45. Ding, H., Zhang, Z., Dai, L., Song, W., Kang, T. et al. (2013). Responses of root morphology of peanut varieties differing in drought tolerance to water-deficient stress. Acta Ecologica Sinica, 33, 5169–5176. DOI 10.5846/stxb. [Google Scholar] [CrossRef]
46. Blum, A., Sullivan, C. (1997). The effect of plant size on wheat response to agents of drought stress. I. Root Drying. Australian Journal of Plant Physiology, 24(1), 35–41. DOI 10.1071/PP96022. [Google Scholar] [CrossRef]
47. Matsui, T., Singh, B. (2003). Root characteristics in cowpea related to drought tolerance at the seedling stage. Experimental Agriculture, 39(1), 29–38. DOI 10.1017/S0014479703001108. [Google Scholar] [CrossRef]
48. Songsri, P., Jogloy, S., Vorasoot, N., Akkasaeng, C., Patanothai, A. et al. (2008). Root distribution of drought-resistant peanut genotypes in response to drought. Journal of Agronomy and Crop Science, 194(2), 92–103. DOI 10.1111/j.1439-037X.2008.00296.x. [Google Scholar] [CrossRef]
49. Han, X., Song, F. (2006). Effect of drought stress on root growth and rhizosphere nutrients of maize (Zea mays L.). Journal of Soil Water Conservation, 20(3), 170–172. [Google Scholar]
50. Shang, X., Liu, H., Zhang, X., Lin, J., Duan, W. et al. (2010). Growth and physiological characteristics of roots in different flue-cured tobacco varieties under drought stress. Acta Botanica Boreali-Occidentalia Sinica, 30(2), 357–361. [Google Scholar]
51. Fraser, T. E., Silk, W. K., Rost, T. L. (1990). Effects of low water potential on cortical cell length in growing regions of maize roots. Plant Physiology, 93(2), 648–651. DOI 10.1104/pp.93.2.648. [Google Scholar] [CrossRef]
52. Baalbaki, R., Zurayk, R., Bleik, M., Talhouk, S. (1999). Germination and seedling development of drought tolerant and susceptible wheat under moisture stress. Seed Science and Technology, 27(1), 291–302. [Google Scholar]
53. El-Denary, M., El-Shawy, E. (2014). Molecular and field analysis of some barley genotypes for water stress tolerance. Egyptian Journal of Genetics and Cytology, 43(1), 187–198. DOI 10.21608/ejgc.2014.9940. [Google Scholar] [CrossRef]
54. Muscolo, A., Sidari, M., Anastasi, U., Santonoceto, C., Maggio, A. (2014). Effect of PEG-induced drought stress on seed germination of four lentil genotypes. Journal of Plant Interactions, 9(1), 354–363. DOI 10.1080/17429145.2013.835880. [Google Scholar] [CrossRef]
55. Luo, X., Dai, Y., Zheng, C., Yang, Y., Chen, W. et al. (2021). The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol, 229, 950–962. [Google Scholar]
56. Dang, H., Zhang, T., Li, G., Mu, Y., Lv, X. et al. (2020). Root-associated endophytic bacterial community composition and structure of three medicinal licorices and their changes with the growing year. BMC Microbiology, 20, 291. DOI 10.1186/s12866-020-01977-3. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |