

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.018396
ARTICLE
Effect of Reaction-Finished Solution of Hydrochar (HRFS) Application on Rice Grain Yield and Nitrogen Use Efficiency in Saline Soil
1Co-Innovation Center for Sustainable Forestry in Southern China, College of Forestry, Nanjing Forestry University, Nanjing, 210037, China
2Environmental Sciences, School of Agriculture and Environment, Massey University, Palmerston North, 4442, New Zealand
3Zhejiang Academy of Forestry, Hangzhou, 310023, China
*Corresponding Author: Haijun Sun. Email: hjsun@njfu.edu.cn
Received: 21 July 2021; Accepted: 11 October 2021
Abstract: We conducted a pot experiment to examine the feasibility of applying a reaction-finished solution of hydrochar (HRFS) to enhance rice production in a saline soil. With this purpose, HRFS was applied (0, 10, 20, 40, 60, 80 and 100 mL/pot) and rice yield and nitrogen (N) use efficiency (NUE) were determined. HRFS application significantly (P < 0.05) increased rice grain yield by 19.6%–30.0% compared to the control treatment (CKU, with N but without HRFS addition). Moreover, HRFS application promoted plant height and straw biomass of rice. Increases of rice yield were mainly achieved by increases in the number of panicles and grains per panicle. Compared with the CKU treatment, the NUE of HRFS amendments significantly (P < 0.05) increased by 56.3%–71.7%. This indicated that the improvement of NUE was one of the mechanisms to improve rice grain yield with HRFS amendment. The results of regression analysis showed that there was a positive relationship (R2 = 0.8332) between rice yield and HRFS application rate within an appropriate range. The highest rice yield was recorded with the HRFS application of 40 mL/pot, but a further increase in HRFS application rate appeared to reduce rice yield. Based on the results of this pot study, HRFS application can increase rice yield in a saline soil by regulating its yield components and enhancing NUE. However, impact of HRFS on these variables showed a “dose effect”.
Keywords: Biochar; saline soil; nitrogen fertilizer; yield component; sustainable agriculture
Soil salinization is a global issue and a major limiting factor in land resource management and ecosystem protection [1,2]. The total area of salt-affected soils in the world is 831 million hectares, which includes 397 and 434 million hectares of saline and sodic soils, respectively [3]. Agricultural lands are decreasing constantly due to population growth and adverse environmental conditions [4,5]. Particularly in China, the arable land area continues to decrease gradually but the demand for food continues to increase. Therefore, it is essential to improve the saline-alkali soils and adopt sustainable agricultural practices to increase the arable land area and ensure national food security [6,7].
At present, measures for improving saline-alkali soils mainly include physical, chemical and biological measures [8,9]. Since the 21st Century, biochar, including pyrolysis carbon and hydrothermal carbon, has become one of the research hotspots as a soil additive or modifier applied to degraded or infertile soils [10–12]. In particular, hydrothermal carbon is a kind of biochar obtained by carbonizing biomass with water as a solvent and reaction medium at autogenous pressure and temperatures ranging from 180 to 375°C. Hydrochar has attracted many researchers’ attention due to its advantages for obtaining higher product yields but lower preparation energy consumption and cost, and lower flue gas emission [13–16]. Previous research indicated that adding hydrochar could improve soil physical and chemical properties and regulate both the structure and function of microbial communities [17,18]. In addition, Scheifele et al. [19] found that the dry matter weight and biological nitrogen (N) fixation of soybean nodules treated with hydrochar increased by 3.4 and 2.3 times, respectively, compared with the control. Further, the inorganic phase of hydrochar enriched essential nutrients for plant growth, which could promote the growth of soybean [19].
The application prospect of hydrochar is very broad in the near future, including the harmless disposal of waste and preparation of biomass nano-materials [20,21]. Consequently, a large amount of reaction finished solution of hydrochar (HRFS) wouldbe produced in the production process of hydrochar with agricultural and forest waste. A large number of organic compounds (such as organic acids and phenols) with different properties are often determined in the tail fluid, which are often acidic [22,23]. In addition, it is reported that organic acids like on humus and other organic substances in the HRFS can provide nutrients, which could promote crop growth and improve economic benefits when applied at reasonable concentration [24]. In contrast, an excessive application of HRFS could affect the soil microbial biomass and enzyme activity, and produce stress-effects on crop growth. This will not be conducive to the growth and development of crops and the resource utilization of agricultural and forestry wastes [25]. Therefore, an environmentally safe and efficient disposal of HRFS is an immediate need in the hydrothermal carbonization technology system. Application of HRFS in agricultural soils is an option to improve crop yields, especially in soils of a saline-alkali nature. However, to our knowledge, there is no detailed research evidence available in the literature to support the HRFS application program.
We hypothesized that the acid characteristics of HRFS and its organic matter and nutrients could adjust the pH of saline alkali soils, which will promote N uptake and yield of rice. Therefore, we conducted a pot experiment to study the effects of different amounts of HRFS application on rice N use efficiency (NUE) and yield in a saline alkali soil. This study will provide scientific theoretical and technical support for the application of HRFS to rice production systems on saline alkali soils.
2.1 Experimental Materials and Design
The soil samples were collected from the cultivated soil profile (0–20 cm) of coastal reclamation saline alkali soils in Sheyang County, Yancheng City, Jiangsu Province (33°46’ N and 120°15’ E), China. The basic physical and chemical properties of the tested soil were as follows: pH 8.0 (soil water ratio 1:5), bulk density 1.38 g/cm3, organic matter 5.65 g/kg, total salt content 4.4 g/kg, total N 1.02 g/kg. The size of the pot was 20 cm in diameter and 20 cm in height. A total of 6.4 kg soil was repacked into each pot, and the pot was submerged in water and kept for one week. Thereafter, the basal fertilizer was applied. Twenty eight-day-old seedlings of Dongzhi 14, a hybrid rice variety, were transplanted on June 04, 2019 with six seedlings in each pot. HRFS was applied on the soil surface when the rice seedlings turned green. A total of eight treatments were tested: (1) blank control treatment (CK0) with neither N nor HRFS; (2) control treatment (CKU) with 240 kg N/ha but no HRFS; and (3) six treatments with 240 kg N/ha and six rates of HRFS (10, 20, 40, 60, 80, and 100 mL HRFS/pot, labelled as HRFS10, HRFS20, HRFS40, HRFS60, HRFS80, and HRFS100, respectively). Each experimental treatment was repeated 3 times and the experiment was arranged in a random complete block design. Nitrogen was applied three times as urea (46%N): (1) as a base fertilizer (04 June); (2) fertilization at the tillering stage of morphological development (17 June), and (3) fertilization at the panicle stage of morphological development (12 August) according to the ratio of 30%: 30%: 40%. Phosphorus and potassium fertilizers were also applied as basal applications at the rates of 100 kg P2O5/ha (in form of superphosphate) and 100 kg K2O/ha (in form of potassium chloride), respectively. The hydrothermal carbonization experiment was carried out in a small batch high-pressure reactor. The specific experimental process was as follows 30 g of Chinese fir leaf litter and 300 g of water were placed into the reactor to ensure the litter material was completely immersed in water. Thereafter, the reactor body was closed and heated at 200°C. At the same time, a magnetic stirring device was turned on to heat the materials evenly. When the set temperature was reached, stirring was stopped. After the end of the reaction, the small batch of the reactor was quickly filled in with cooling water. When the reactor was cooled to room temperature and ambient pressure, the material was taken out, filtered and separated, and its tail liquid (i.e., HRFS) was collected. The properties of HRFS were as follows: pH 5.01, NH4+–N 290.9 mg/L, NO3––N 184.9 mg/L, TOC 9.0 g/L, humus 183 mg/L, small molecule organic acid 9.09 mg/L. Tap water was used for rice irrigation, and a water level of 3–4 cm was kept throughout the growth period of rice.
2.2 Determination Items and Methods
Rice plants were manually harvested at the maturity stage. Plant height was measured with a metric tape, the number of panicles per plant was counted, and the number of grains per panicle after manual threshing was counted with a grain counting plate. Sub-samples were placed in the oven at 105°C for 30 min. Thereafter, the temperature of the oven was adjusted to 80°C and the samples were dried to constant weight. Then, they were brought to room temperature (at the drying environment). The dry weight of stems, sheaths, leaves and grains was measured. The dried plant sub-samples were ground into powder with a high-speed crusher, passed through a 0.2 mm sieve, and kept in a vacuum bag for storage at 4°C. About 0.25 g of rice straw and grain powder sub-samples were digested in a H2SO4–H2O2 system, and the N concentrations of rice straw and grain were determined by the Kjeldahl method [26]. The rice NUE was estimated by the following formula:
where NF and N0 denote the N uptake as measured at harvest in the N fertilization and the CK0 treatments (kg/ha), respectively, while N denotes the inorganic N fertilizer added rate (240 kg/ha).
All statistical analyses were performed using SPSS 18.0 (SPSS Inc. Chicago, IL, USA). A one-way analysis of variance (ANOVA) was used to determine if either there were or not significant differences among treatments. Mean comparisons were made using the Duncan’s multiple-comparison test (P < 0.05). Regression analyses were conducted using the HRFS concentrations as the independent variable and grain yield and biomass of straw, and rice NUE as the dependent variables.
3.1 Plant Height and Straw Biomass of Rice
The average plant height and straw biomass of rice were 58.2 cm and 10.2 g/pot, respectively, for the CK0 treatment (with neither N fertilizer nor HRFS). The average plant height and straw of rice treated with urea N (i.e., CKU) increased to 62.9–69.5 cm and 14.3–26.3 g/pot, respectively (Fig. 1). However, these values were not significantly different (P>0.05) to those found in the control treatment. The average plant height on the HRFS added treatments increased by 6.0%–10.5% in comparison to CKU, but the differences were not statistically significant (Fig. 1A). These results showed that the HRFS treatments had no significant effects on the plant height of rice compared to the CKU treatment.
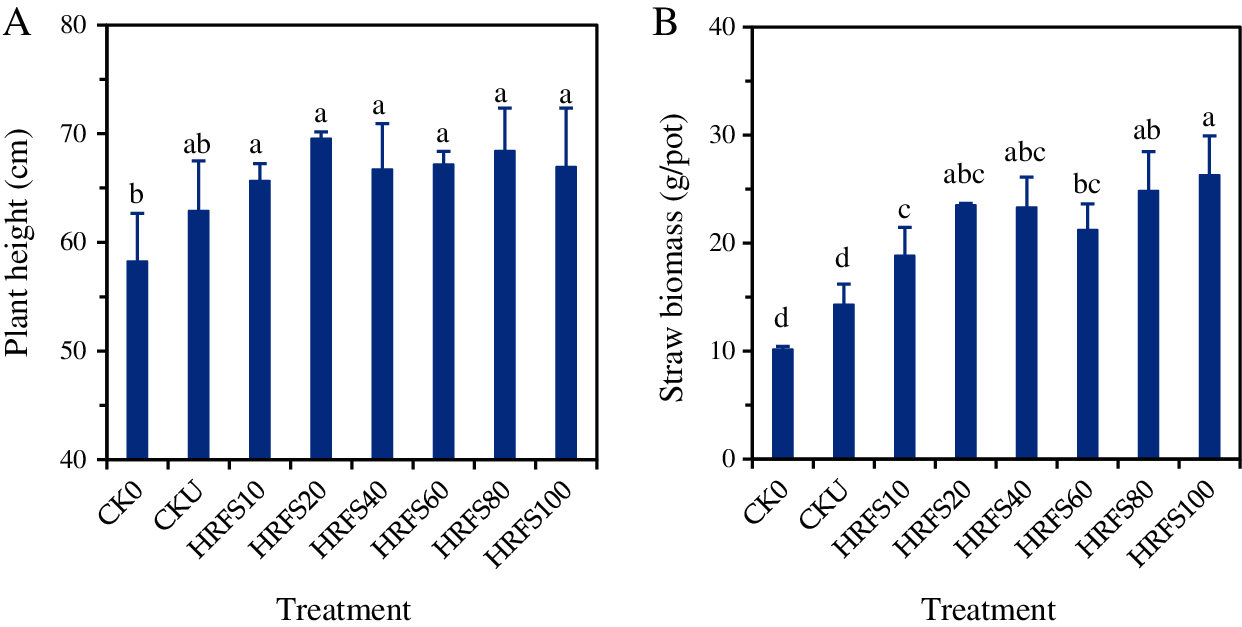
Figure 1: Effects of either N or HRFS application on plant height (A) and straw biomass (B) of rice. See the Materials and Methods section for meaning of the abbreviations of treatments. Error bars represent the standard deviation (SD) of three replications. Different letters above columns indicate significant differences among treatments according to the Duncan’s multiple-comparison test at P < 0.05
The HRFS treatments significantly increased (P < 0.05) the dry matter weight of rice straw by 24.0%–45.5% in comparison to the CKU treatment (Fig. 1B). This later treatment, however, was not significantly different (P > 0.05) from the CK0 treatment. The rice straw biomass on the HRFS80 and HRFS100 treatments was significantly (P < 0.05) increased by 31.9% and 39.5%, respectively, compared to that in the HRFS10 treatment. Also, the straw biomass under the HRFS100 treatment was 23.8% higher (P < 0.05) than that on the HRFS60 treatment.
3.2 Grain Yield and Its Components and Rice NUE
The mean rice grain yields on the CKU and HRFS treatments varied significantly (P < 0.05) from 12.7 g/pot to 18.1 g/pot, respectively. Compared with the CKU treatment, grain yield at all HRFS treatments significantly increased (P< 0.05) by 19.6%–30.0%. Values on the HRFS treatments were significantly higher (P < 0.05) than those under the CK0 treatment (Fig. 2A). There were no significant differences (P > 0.05) in rice yield among the different HRFS application rates (Fig. 2A).
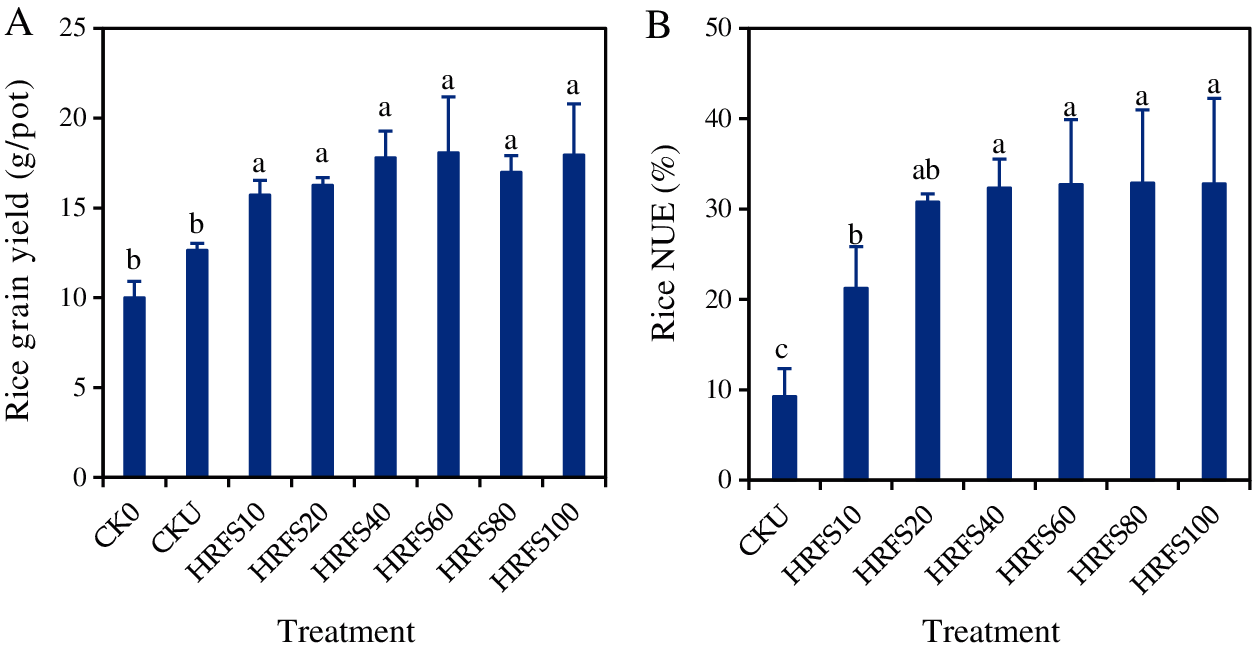
Figure 2: Effects of HRFS application with various rates on grain yield (A) and NUE (B) of rice. See the Materials and Methods section for meaning of the abbreviations of treatments. Error bars represent the standard deviation (SD) of three replications. Different letters above the columns indicate significant differences among treatments according to the Duncan’s multiple-comparison test at P < 0.05
The rice NUE on the HRFS treatments ranged from 21.3% to 32.9%, and significantly (P < 0.05) increased by 56.3%–71.7% compared with the CKU treatment (Fig. 2B). This indicates that application of HRFS could improve the N uptake capacity of rice. The rice NUE increased with increasing applications of HRFS rising up to 40 mL/pot. The NUE on the HRFS40 treatment was 51.6% significantly higher (P < 0.05) than that on the HRFS10 treatment. However, the NUE of rice did not increase (P > 0.05) when the HRFS application rate was beyond 40 mL/pot.
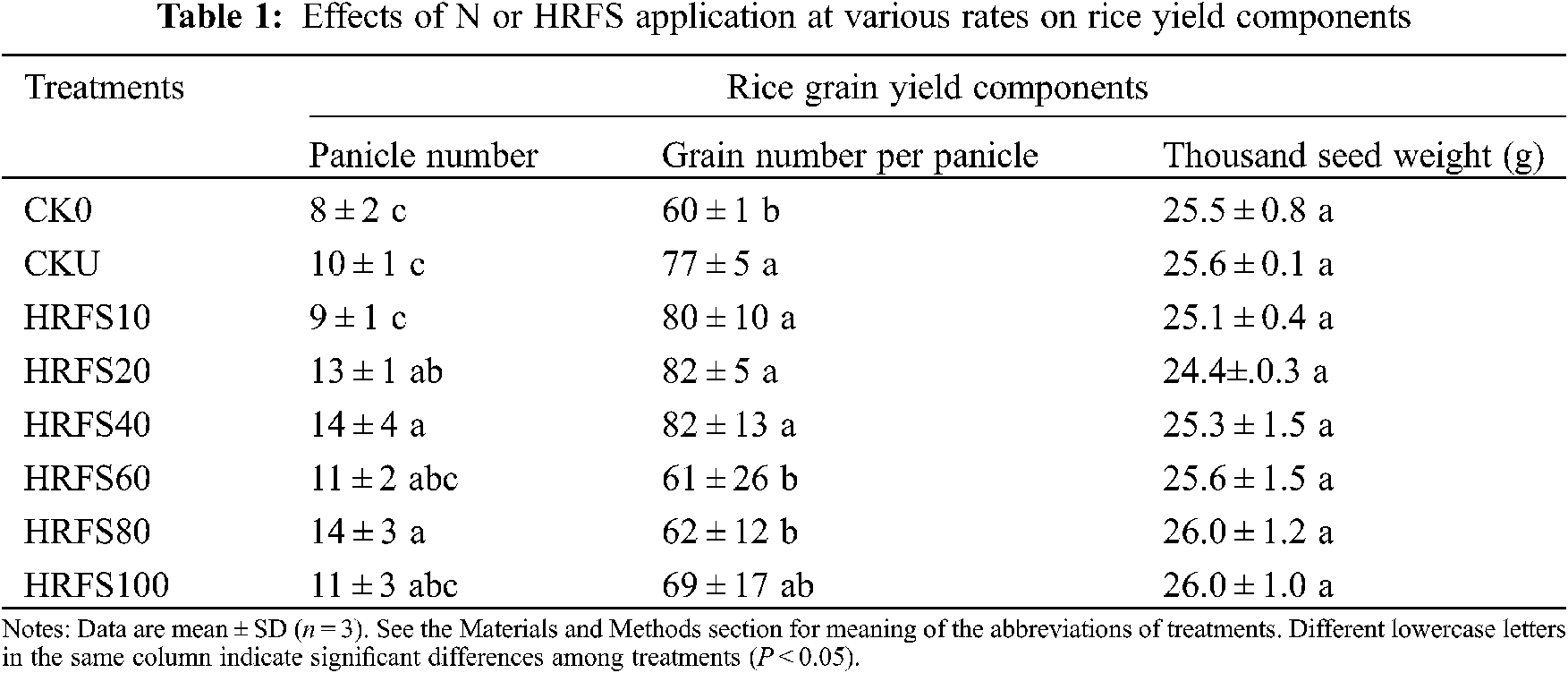
Panicle numbers on HRFS20, HRFS40, and HRFS80 treatments were significantly (P < 0.05) higher than those on the CKU treatment by 3–4 panicles/pot (Table 1). In addition, the number of grains per panicle increased when the HRFS application rate increased from 10 mL/pot to 40 mL/pot, but differences were not statistically significant (P > 0.05). The number of grains per panicle decreased (P < 0.05) when the HRFS application rate was above 40 mL/pot, except on the HRFS100 treatment (Table 1). Also, the number of grains per panicle in the HRFS60 and HRFS80 treatments was 20% significantly lower (P < 0.05) than that in the CKU treatment. However, there were no significant differences (P > 0.05) in the thousand grain weight among any of the tested treatments (Table 1).
There was no difference in soil pH on the CK0, CKU and HRFS10 treatments (Fig. 3). Interestingly, HRFS incorporation effectively decreased (P < 0.05) the soil pH after rice harvesting, in particular when the usage dose was over 20 mL/pot (Fig. 3). When applied with more than 20 mL/pot, HRFS significantly decreased (P < 0.05) the soil pH by 0.14–0.75 in comparison to the CKU treatment.
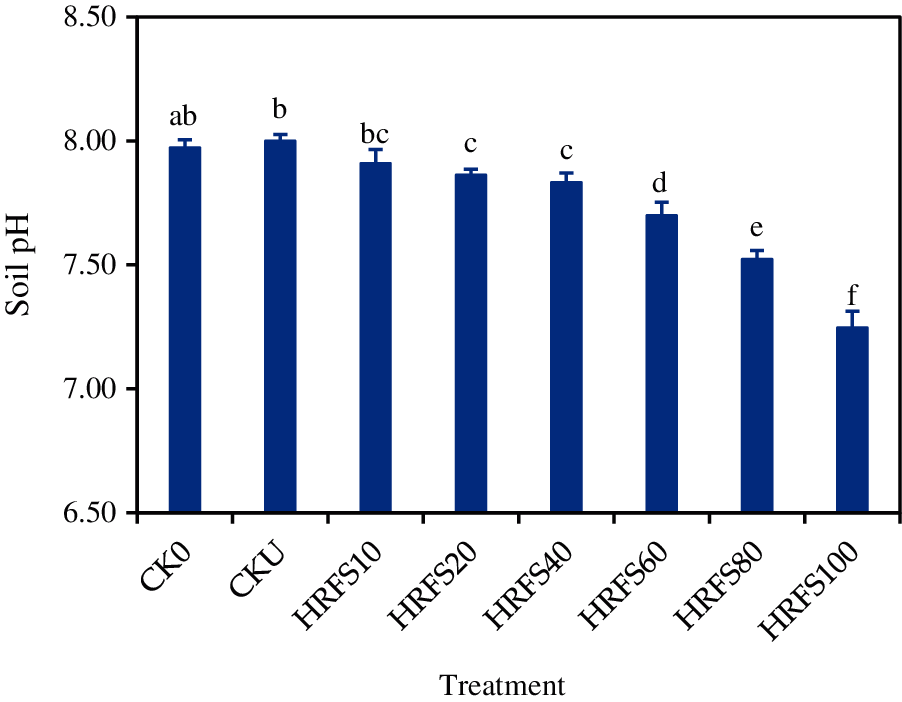
Figure 3: Effects of N or HRFS application at various rates on the pH of soil sampled after rice harvesting. See the Materials and Methods section for meaning of the abbreviations of the treatments. Error bars represent the standard deviation (SD) of three replications. Different letters above the columns indicate significant differences among treatments according to the Duncan’s multiple-comparison test at P < 0.05
Further analysis of the data showed that the foregoing effect increased with the increase of HRFS application rates up to an optimal level, namely a “dose effect” (Fig. 4). The regression analysis of the straw biomass, rice grain yield and NUE vs. the HRFS application rate showed that the relationships were positive, and the determination coefficients R2 were 0.7108, 0.8332 and 0.8492, respectively. The corresponding P values were 0.0658, 0.0434 and 0.1022, respectively. However, the parameters did not continue to increase with the increase of HRFS application rate.
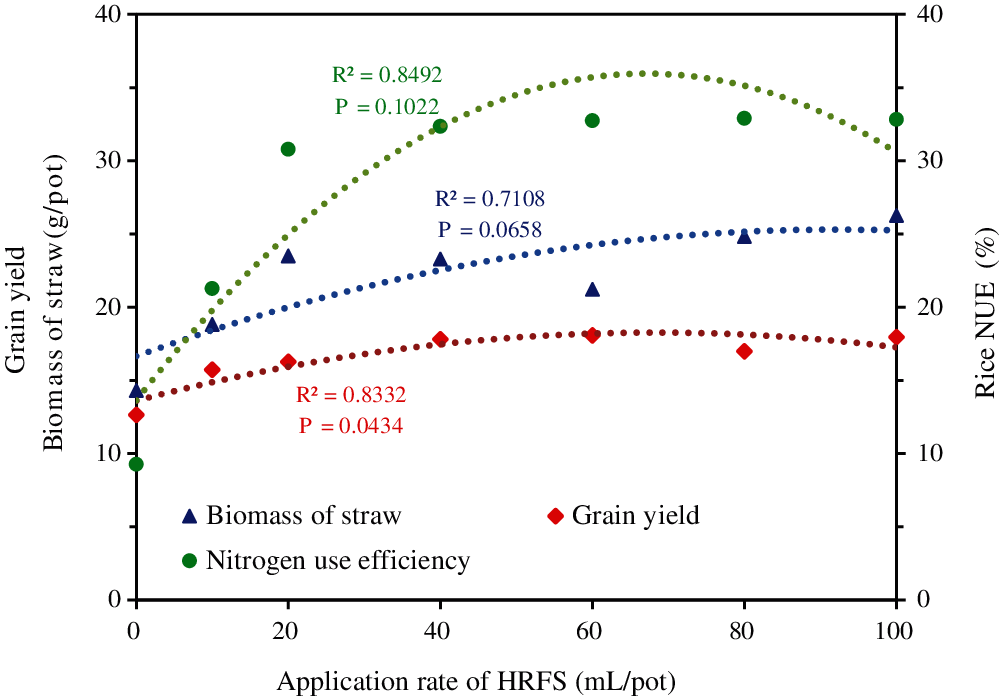
Figure 4: Regression analysis of the straw biomass, grain yield, and NUE of rice versus the HRFS application rate
The results showed that appropriate application of HRFS could (1) promote rice growth planted in a saline soil receiving a chemical N fertilizer with 240 kg/ha (Fig. 1), (2) improve the NUE (Fig. 2B), and (3) increase the grain yield of rice (Fig. 2A). The improvement of NUE may be the key mechanism for rice yield improvement in this experiment. HRFS significantly increased (P < 0.05) the NUE of rice by 56.3%–71.7% in comparison to the control treatment (Fig. 2B). Previous studies have shown that humic acid liquid fertilizer can (1) regulate the soil N cycle, (2) improve crop NUE and (3) promote crop yield [27–29]. Humus and a variety of small molecular organic acids probably play a major role in stimulating nutrient and organic carbon contents in the soil [24]. Research studies have shown that humic acid and organic acids can stimulate crop growth, improve crop nutrient utilization, increase crop yield and improve product quality [30]. Similar to liquid fertilizers, HRFS application in this study may change the physical and chemical properties of the tested soil. In particular, our results suggested that HRFS amendment decreased the pH of the tested saline soil (Fig. 3), as a result of the HRFS acidic property (with pH of 5.01). Therefore, appropriate application of HRFS can change the physical and chemical properties of saline soils, and promote growth, NUE and grain yield of rice.
In addition, some components of HRFS itself could influence rice growth and final grain production. Zhou et al. [31] showed that the application of wood vinegar (by-product of biochar pyrolysis, with acidic property) to the soil could significantly reduce the pH and increase the contents of SOM and total N, NH4+–N and NO3––N. Further, most of the organic compounds derived from biochar are ligands containing carboxyl, phenol, alcohol and enol functional groups, which can form stable metal organic complexes with various valence metal elements. This led to improve the content of some beneficial elements in the aqueous phase and promote their bioavailability [32]. Organic compounds can form chelates with some cations, which is conducive to nutrient movement, promotion of nutrient bioavailability, and therefore promotion of crop productivity [33]. The TOC, NH4+–N and NO3––N concentrations in the HRFS of the present study were 9 g/L, 290.9 mg/L and 184.9 mg/L, respectively. This could increase the SOM content and available N, leading to an increasing rice yield in the saline soil. In addition, other organic appropriate matters contained in HRFS may be beneficial to rice development. However, the promotion of HRFS on rice biomass was not completely transformed into rice grain yield. When the application of HRFS was more than 60 mL/pot, the number of grains per panicle decreased, indicating that HRFS application may lead to negative effects on rice growth and hinder its late reproductive growth. The increase of rice yield was mainly due to the increase of panicles and grains per panicle (Table 1). Remarkably, the panicle number and grain number per panicle were the highest when the HRFS rate was 40 mL/pot. Asai et al. [34] also showed that the application of soil additives such as biochar to promote rice yield was mainly achieved by increasing the number of panicles and grains per ear. In conclusion, HRFS application can improve NUE, growth and grain yield of rice in a saline soil.
From the resource utilization perspective, there is a more appropriate range of dosage that could save HRFS resources while promoting rice production (Fig. 4). Excessive application of other exogenous soil additives (such as hydrothermal carbon, 2 g/kg) also led to the reduction of rice yield [35]. Therefore, it is necessary to underpin the appropriate rate of application of HRFS when cultivating rice in saline soils. Considering the effect of HRFS on various growth and yield indexes of rice, the HRFS application rate of 40 mL/pot was the best from our pot experiment. This experiment conducted in pots only had a short research period. Therefore, long-term field experiments are highly recommended in future studies.
The application of HRFS could enhance rice growth and grain production in saline soils with a recommended N application rate of 240 kg/ha in the main rice-producing areas of Jiangsu Province. The rice grain yields increased by 19.6%–30.0% with the application of HRFS in comparison to the CKU treatment. This was mainly due to the increase of NUE, an increasing the number of panicles and grains per panicle, and balancing of the soil pH. In addition, the application of HRFS also had a “dose effect” on rice grain yield and other parameters. The treatment with 40 mL HRFS/pot was the appropriate application rate to achieve a higher yield and NUE of rice based on the current pot experiment. This study preliminarily proved that HRFS application was beneficial to rice production in a saline soil. More detailed field-scale research is recommended to confirm the results obtained in the present study.
Funding Statement: The Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21-0926), the National Natural Science Foundation of China (31972518) and the Qing Lan Project of Jiangsu Province financially supported this work.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ivits, E., Cherlet, M., Tóth, T., Lewińska, K. E., Tóth, G. (2013). Charactersation of productivity limitation of salt-affected lands in different climatic regions of Europe using remote sensing derived productivity indicators. Land Degradation & Development, 24(5), 438–452. DOI 10.1002/ldr.1140. [Google Scholar] [CrossRef]
2. Wu, J. H., Li, P. Y., Qian, H., Fang, Y. (2014). Assessment of soil salinization based on a low-cost method and its influencing factors in a semi-arid agricultural area, Northwest China. Environmental Earth Sciences, 71, 3465–3475. DOI 10.1007/s12665-013-2736-x. [Google Scholar] [CrossRef]
3. Zhang, Z. Y., Abuduwaili, J., Yimit, H. (2014). The occurrence, sources and spatial characteristics of soil salt and assessment of soil salinization risk in yanqi basin, Northwest China. PLoS One, 9, e106079. DOI 10.1371/journal.pone.0106079. [Google Scholar] [CrossRef]
4. Bai, X. L., Zhang, Z. B., Cui, J. J., Liu, Z. J., Chen, Z. J. et al. (2020). Strategies to mitigate nitrate leaching in vegetable production in China: A meta-analysis. Environmental Science and Pollution Research, 27, 18328–18391. DOI 10.1007/s11356-020-08322-1. [Google Scholar] [CrossRef]
5. Shi, W. M., Yao, J., Yan, F. (2009). Vegetable cultivation under greenhouse conditions leads to rapid accumulation of nutrients, acidification and salinity of soils and groundwater contamination in South-Eastern China. Nutrient Cycling in Agroecosystems, 83, 73–84. DOI 10.1007/s10705-008-9201-3. [Google Scholar] [CrossRef]
6. Yue, J. M., Fu, Z. Y., Zhang, L., Zhang, Z. H., Zhang, J. C. (2019). The positive effect of different 24-epiBL pretreatments on salinity tolerance in Robinia pseudoacacia L. seedlings. Forests, 10, 4. DOI 10.3390/f10010004. [Google Scholar] [CrossRef]
7. Wu, Y. P., Li, Y. F., Zheng, C. Y., Zhang, Y. F., Sun, Z. J. (2013). Organic amendment application influence soil organism abundance in saline alkali soil. European Journal of Soil Biology, 54, 32–40. DOI 10.1016/j.ejsobi.2012.10.006. [Google Scholar] [CrossRef]
8. Sun, H. J., Lu, H. Y., Chu, L., Shao, H. B., Shi, W. M. (2017). Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Science of the Total Environment, 575, 820–825. DOI 10.1016/j.scitotenv.2016.09.137. [Google Scholar] [CrossRef]
9. Tejada, M., Garcia, C., Gonzalez, J. L., Hernández, M. T. (2006). Use of organic amendment as a strategy for saline soil remediation: Influence on the physical, chemical and biological properties of soil. Soil Biology and Biochemistry, 38(6), 1413–1421. DOI 10.1016/j.soilbio.2005.10.017. [Google Scholar] [CrossRef]
10. Barrow, C. J. (2012). Biochar: Potential for countering land degradation and for improving agriculture. Applied Geography, 34, 21–28. DOI 10.1016/j.apgeog.2011.09.008. [Google Scholar] [CrossRef]
11. Chu, Q. N., Xu, S., Xue, L. H., Liu, Y., Feng, Y. F. et al. (2020). Bentonite hydrochar composites mitigate ammonia volatilization from paddy soil and improve nitrogen use efficiency. Science of the Total Environment, 718, 137301. DOI 10.1016/j.scitotenv.2020.137301. [Google Scholar] [CrossRef]
12. Sun, H. J., Zhang, Y., Yang, Y. T., Chen, Y. D., Jeyakumar, P. et al. (2021). Effect of biofertilizer and wheat straw biochar application on nitrous oxide emission and ammonia volatilization from paddy soil. Environmental Pollution, 275, 116640. DOI 10.1016/j.envpol.2021.116640. [Google Scholar] [CrossRef]
13. Feng, Y. F., Lu, H. Y., Liu, Y., Xue, L. H., Dionysiou, D. D. et al. (2017). Nano-cerium oxide functionalized biochar for phosphate retention: Preparation, optimization and rice paddy application. Chemosphere, 185, 816–825. DOI 10.1016/j.chemosphere.2017.07.107. [Google Scholar] [CrossRef]
14. Funke, A., Ziegler, F. (2010). Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioproducts & Biorefining, 4(2), 160–177. DOI 10.1002/bbb.198. [Google Scholar] [CrossRef]
15. Feng, Y. F., Sun, H. J., Han, L. F., Xue, L. H., Chen, Y. D. et al. (2019). Fabrication of hydrochar based on food waste (FWHTC) and its application in aqueous solution rare earth ions adsorptive removal: Process, mechanisms and disposal methodology. Journal of Cleaner Production, 212, 1423–1433. DOI 10.1016/j.jclepro.2018.12.094. [Google Scholar] [CrossRef]
16. Yu, S., Feng, Y. F., Xue, L. H., Sun, H. J., Han, L. F. et al. (2019). Biowaste to treasure: Application of microbial-aged hydrochar in rice paddy could improve nitrogen use efficiency and rice grain free amino acids. Journal of Cleaner Production, 240, 118–180. DOI 10.1016/j.jclepro.2019.118180. [Google Scholar] [CrossRef]
17. Li, Y. F., Zhang, J. J., Chang, S. X., Jiang, P. K., Zhou, G. M. et al. (2014). Converting native shrub forests to Chinese chestnut plantations and subsequent intensive management affected soil C and N pools. Forest Ecology and Management, 312, 161–169. DOI 10.1016/j.foreco.2013.10.008. [Google Scholar] [CrossRef]
18. Omil, B., Pineiro, V., Merino, A. (2013). Soil and tree responses to the application of wood ash containing charcoal in two soils with contrasting properties. Forest Ecology and Management, 295, 199–212. DOI 10.1016/j.foreco.2013.01.024. [Google Scholar] [CrossRef]
19. Scheifele, M., Hobia, A., Buegger, F., Gattinger, A., Schulin, R. et al. (2017). Impact of pyrochar and hydrochar on soybean (Glycine max L.) root nodulation and biological nitrogen fixation. Journal of Plant Nutrition and Soil Science, 180, 199–211. DOI 10.1002/jpln.201600419. [Google Scholar] [CrossRef]
20. Libra, J. A., Ro, K. S., Kammann, C., Funke, A., Berge, N. D. et al. (2014). Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels, 2(1), 71–106. DOI 10.4155/bfs.10.81. [Google Scholar] [CrossRef]
21. Yang, Y. H., Cui, J. H., Zheng, M. T., Hu, C. F., Tan, S. Z. (2012). One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chemical Communications, 48(3), 380–382. DOI 10.1039/c1cc15678k. [Google Scholar] [CrossRef]
22. Busch, D., Stark, A., Kammann, C. I., Glaser, B. (2013). Genotoxic and phytotoxic risk assessment of fresh and treated hydrochar from hydrothermal carbonization compared to biochar from pyrolysis. Ecotoxicology & Environmental Safety, 97, 59–66. DOI 10.1016/j.ecoenv.2013.07.003. [Google Scholar] [CrossRef]
23. Bargmann, I., Rillig, M. C., Buss, W., Kruse, A., Kuecke, M. (2013). Hydrochar and biochar effects on germination of spring barley. Journal of Agronomy and Crop Science, 199, 360–373. DOI 10.1111/jac.12024. [Google Scholar] [CrossRef]
24. Lin, Y., Munroe, P., Joseph, S., Henderson, R. (2012). Migration of dissolved organic carbon in biochars and biochar-mineral complexes. Brazilian Agricultural Research, 47(5), 677–686. DOI 10.1590/S0100-204X2012000500007. [Google Scholar] [CrossRef]
25. Jandl, G., Eckhardt, K. U., Bargmann, I., Kücke, M., Greef, J. et al. (2013). Hydrothermal carbonization of biomass residues: Mass spectrometric characterization for ecological effects in the soil-plant system. Journal of Environmental Quality, 42(1), 199–207. DOI 10.2134/jeq2012.0155. [Google Scholar] [CrossRef]
26. Sun, H. J., Zhang, H. L., Powlson, D., Min, J., Shi, W. M. (2015). Rice production, nitrous oxide emission and ammonia volatilization as impacted by the nitrification inhibitor 2-chloro-6-(trichloromethyl)-pyridine. Field Crops Research, 173, 1–7. DOI 10.1016/j.fcr.2014.12.012. [Google Scholar] [CrossRef]
27. Liu, N., Hou, T., Yin, H. J., Han, L. J., Huang, G. Q. (2019). Effects of amoxicillin on nitrogen transformation and bacterial community succession during aerobic composting. Journal of Hazardous Materials, 362, 258–265. DOI 10.1016/j.jhazmat.2018.09.028. [Google Scholar] [CrossRef]
28. She, Y. W., Jiao, S. Y., Ma, Z., Lin, H. T., Gao, W. S. et al. (2020). Humic acid-modified bentonite composite material enhances urea-nitrogen use efficiency. Chemosphere, 255, 126976. DOI 10.1016/j.chemosphere.2020.126976. [Google Scholar] [CrossRef]
29. Benzon, H. R. L., Rubenecia, M. R. U., Ultra, Jr., V. U., Lee, S. C. (2015). Chemical and biological properties of paddy soil treated with herbicides and pyroligneous acid. Journal of Agricultural Science, 7(4), 20–29. DOI 10.5539/jas.v7n4p20. [Google Scholar] [CrossRef]
30. Graber, E. R., Tsechansky, L., Lew, B., Cohen, E. (2014). Reducing capacity of water extracts of biochar and their solubilization of soil Mn and Fe. European Journal of Soil Science, 65(1), 162–172. DOI 10.1111/ejss.12071. [Google Scholar] [CrossRef]
31. Zhou, H. J., Geng, Y. Q., Cong, R. C., Li, Y. F., Liu, D. (2016). Effects of wood vinegar on chemical properties, enzyme activities and their correlation in saline alkali soil. Chinese Journal of Soil Science, 47(1), 105–111. DOI 10.19336/j.cnki.trtb.2016.01.017. [Google Scholar] [CrossRef]
32. Graber, E. R., Meller, H. Y., Kolton, M., Cytryn, E., Silber, A. et al. (2010). Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant and Soil, 337, 481–496. DOI 10.1007/s11104-010-0544-6. [Google Scholar] [CrossRef]
33. Lin, Y., Munroe, P., Joseph, S., Henderson, R., Ziolkowski, A. (2011). Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere, 287(2), 151–157. DOI 10.1016/j.chemosphere.2011.12.007. [Google Scholar] [CrossRef]
34. Asai, H., Samson, B. K., Stephan, H. M., Songyikhangsuthor, K., Homma, K. et al. (2009). Biochar amendment techniques for upland rice production in Northern laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Research, 111, 81–84. DOI 10.1016/j.fcr.2008.10.008. [Google Scholar] [CrossRef]
35. Shalaby, T. A., El-Ramady, H. (2014). Effect of foliar application of bio-stimulants on growth, yield, components, and storability of garlic (Allium sativum L.). Australian Journal of Crop Science, 8(2), 271–275. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |